Abstract
Unlike relapsing remitting multiple sclerosis, there are very few therapeutic options for patients with progressive forms of multiple sclerosis. While immune mechanisms are key participants in the pathogenesis of relapsing remitting multiple sclerosis, the mechanisms underlying the development of progressive multiple sclerosis are less well understood. Putative mechanisms behind progressive multiple sclerosis have been put forth: insufficient energy production via mitochondrial dysfunction, activated microglia, iron accumulation, oxidative stress, activated astrocytes, Wallerian degeneration, apoptosis, etc. Furthermore, repair processes such as remyelination are incomplete. Experimental therapies that strive to improve metabolism within neurons and glia, e.g., oligodendrocytes, could act to counter inadequate energy supplies and/or support remyelination. Most experimental approaches have been examined as standalone interventions; however, it is apparent that the biochemical steps being targeted are part of larger pathways, which are further intertwined with other metabolic pathways. Thus, the potential benefits of a tested intervention, or of an established therapy, e.g., ocrelizumab, could be undermined by constraints on upstream and/or downstream steps. If correct, then this argues for a more comprehensive, multifaceted approach to therapy. Here we review experimental approaches to support neuronal and glial metabolism, and/or promote remyelination, which may have potential to lessen or delay progressive multiple sclerosis.
Keywords: acetyl-coenzyme A carboxylase, biotin, estrogen, iron, Kynurenine pathway, mitochondria, thyroid hormone, remyelination, stem cells, vitamin D
Introduction
Disease modifying therapies act by disrupting various facets of the immune response to ameliorate disease activity in patients with relapsing remitting multiple sclerosis (RRMS). These therapies can slow the development of disability and as a corollary, they may possibly diminish the conversion of RRMS to secondary progressive multiple sclerosis (SPMS) (Trojano et al., 2007, 2009; Gold et al., 2010; Kappos et al., 2015; Giovannoni et al., 2016; Wingerchuk and Weinshenker, 2016). However, once a patient develops SPMS, or if a patient has primary progressive multiple sclerosis (PPMS), then immunotherapies are generally not effective in lessening the disease course (Wiendl and Hohlfeld, 2009; Wingerchuk and Weinshenker, 2016; Sartori et al., 2017). Two exceptions are ocrelizumab, an anti-CD20 antibody that depletes B cells, and siponimod, a modulator of the sphingosine 1-phosphate receptor. Ocrelizumab, which is approved for use in PPMS patients, lessens the probability of disease progression (Montalban et al., 2017) while siponimod has been shown to reduce the risk of disability progression in SPMS patients (Kappos et al., 2016; Mao-Draayer et al., 2017). However, disease progression still occurs in most PPMS or SPMS patients treated with these medications, and when viewed in combination with negative results from other immunotherapies, it suggests that there are additional mechanisms outside of the immune system that play a role in advancing multiple sclerosis (MS) pathology. Although we do not have a complete understanding of the pathogenesis of the progressive forms of this disease, putative mechanisms have been put forth. It has been proposed that inflammatory demyelination eventually leads to neurodegeneration by various mechanisms including, but not limited to, energy depletion through mitochondrial dysfunction and/or hypoxia related processes, oxidative stress, activated microglia, activated astrocytes, Wallerian degeneration, iron accumulation, and apoptosis (Weigel et al., 2014; Mahad et al., 2015; Kawachi and Lassmann, 2017). While these mechanisms are associated with progressive MS, they likely begin during RRMS prior to the full conversion to SPMS. Interventions that act on one or more of these proposed mechanisms, or that assist in the repair of the central nervous system (CNS), are worth exploring for their potential as a treatment, or adjunct therapy, for patients with progressive MS. This paper will focus on a subset of these potential interventions, particularly those that facilitate metabolism to support neuronal and oligodendrocyte functions, and that possibly promote remyelination efforts (Table 1).
Table 1.
Potential approaches to support metabolism and/or remyelination for progressive multiple sclerosis
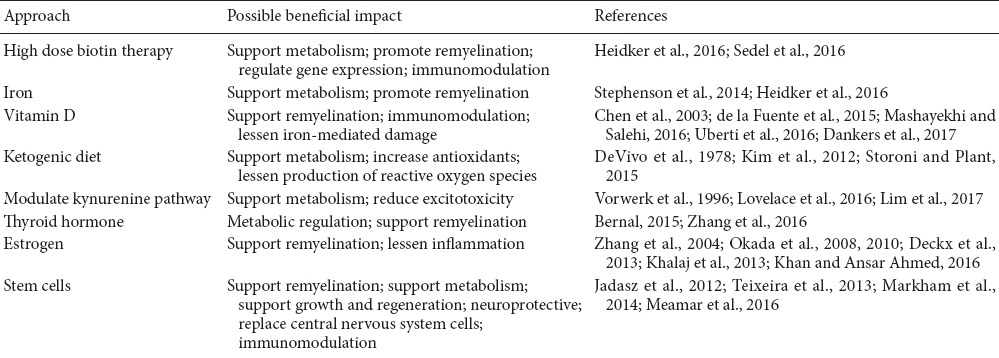
The Intertwined Roles of Biotin, Iron and Vitamin D for Metabolic Support
Adenosine triphosphate (ATP) is necessary in the CNS for maintenance of membrane potentials, synaptic activities, metabolic functions of neuronal and glial cells, etc. Maintenance of membrane potentials by Na+/K+ ATPases in healthy neurons accounts for a substantial portion of a neuron’s energy consumption (Howarth et al., 2012), which can be altered by inflammation and demyelination (Trapp and Stys, 2009). This can cause a disparity of energy production versus expenditure known as “virtual hypoxia” that could lead to events that result in axonal degeneration and neuronal dysfunction or degeneration (Stys, 2004; Trapp and Stys, 2009; Sedel et al., 2016; Kawachi and Lassmann, 2017).
Recently, an early study indicated that high dose biotin therapy (HDBT) improved results for the expanded disability status scale and/or the timed 25-foot walk in 12.6% of patients with progressive MS (Tourbah et al., 2016), but these positive results require additional verification (Chataway, 2016). The putative mechanisms of action that were proposed for HDBT focused on metabolic support, i.e., promoting remyelination by enhancing fatty acid synthesis via increasing the functionality of biotin containing carboxylases and facilitating energy production in neurons, potentially countering virtual hypoxia (Sedel et al., 2016). Other mechanisms, e.g., regulation of gene expression or limiting immune responses, may also be involved (Heidker et al., 2016; Sedel et al., 2016) (Table 1).
Upon examination of the metabolic pathways that involve biotin, it is apparent that other nutrients such as iron are utilized by these pathways. For example, both biotin and iron participate in biochemical reactions involved in energy production (Heidker et al., 2016). Multiple biotin-containing enzymes are involved with the generation of intermediates of the tricarboxylic acid (TCA) cycle that supply the electron transport chain (ETC), which utilizes iron containing enzymes (e.g., iron-sulfur clusters, cytochrome C which contains heme) for the further production of ATP. Heme synthesis is an additional interrelationship, requiring iron as well as metabolites generated by the biotin-dependent pyruvate and methylcrotonyl carboxylases (Atamna, 2004; Atamna et al., 2007; Voet and Voet, 2011).
Lipid synthesis is another example of intertwined roles for biotin and iron. The biotin-dependent acetyl-coenzyme A carboxylase 1 initiates lipid synthesis, which requires heme and iron-sulfur clusters as cofactors for several enzymes, as does cholesterol synthesis (Voet and Voet, 2011). As lipids and cholesterol are key components of myelin, increased synthesis of these compounds in conjunction with remyelination has been proposed as a therapeutic mechanism of HDBT (Sedel et al., 2016). Interestingly, this hypothesis is somewhat in contrast with the usage of simvastatin, which inhibits cholesterol synthesis, to treat SPMS. Simvastatin was shown in a phase II clinical trial to potentially have value for treating SPMS, with the putative therapeutic mechanisms including immunomodulative, neuroprotective or vascular effects (Chataway et al., 2014). However, simvastatin can hinder myelination during development (Xiang and Reeves, 2009) and potentially impair remyelination efforts (Miron et al., 2009), which in theory could limit its usefulness as a treatment for progressive MS.
While HDBT likely supports metabolism, it may be acting to correct a biotin deficiency that is present in a subgroup of MS patients that developed consequentially to have a prolonged chronic disease or that occurred independent of MS (Heidker et al., 2016; Siddiqui et al., 2017). In addition, the prolonged inflammation associated with MS could, in theory, cause a reduction in the availability of iron for biochemical pathways in neurons and oligodendrocytes (Heidker et al., 2016). Thus, a localized deficiency of iron (or heme) at the biochemical level, but not necessarily at the tissue or systemic level, would act to lessen support for energy production, remyelination and/or the maintenance of myelin (Heidker et al., 2016). Interestingly, in patients with chronic progressive MS, normally appearing white matter areas have reduced levels of iron together with low levels of inflammation (Hametner et al., 2013). Additionally, anemia has been identified as a comorbidity in MS, and it may aggravate disease, e.g., increasing the risk of a relapse (Tettey et al., 2016). However, supplying neurons and oligodendrocytes with optimal iron levels could be complicated because iron is present in excess in some brain regions (e.g., thalamus, caudate, globus pallidus, putamen) and it can become deposited around damaged vessels in patients with MS, e.g., due to microhemorrhaging (Weigel et al., 2014). In theory, this excess iron could promote pathology by catalyzing oxidative or nitrative tissue damage, increasing proinflammatory cytokine production, supporting increased glutamate release potentially leading to excitotoxicity, etc. (Weigel et al., 2014). Microinjection of ferritin, which can bind copious amounts of iron, into the rat spinal cord resulted in oligodendrocyte and neuronal toxicity, but the injected ferritin also stimulated the proliferation of progenitor cells that differentiated into oligodendrocytes (Schonberg et al., 2012). Thus, identifying a means to achieve an appropriate level of iron for optimal support of metabolism and remyelination efforts while limiting the effects of excess iron contributing to pathogenesis would be a desired goal as part of an intervention strategy.
Interestingly, vitamin D has been shown to lessen iron induced toxicity in the locus coeruleus and cultured neuroblastoma cells (Chen et al., 2003; Uberti et al., 2016), suggesting that vitamin D could help protect against iron related damage in MS. Low levels of vitamin D have also been correlated with an increased risk of developing MS, and levels may be inversely associated with progression of disease activity in RRMS patients (Ascherio and Munger, 2016; Hempel et al., 2017). Iron, as part of heme, is necessary for the metabolism of vitamin D (Toxqui and Vaquero, 2015), and as stated above, products of the biotin-containing enzymes, pyruvate carboxylase and methylcrotonyl carboxylase, are used in the synthesis of heme. Vitamin D plays a role in myelination and remyelination by increasing differentiation of oligodendrocyte precursor cells (OPCs), presumably via activation of the vitamin D receptor and its heterodimer partner, the retinoid X receptor gamma (de la Fuente et al., 2015). Additionally, vitamin D increased the expression of oligodendrocyte/myelin proteins (i.e., myelin oligodendrocyte glycoprotein and CNPase) when administered during remyelination following demyelination induced by cuprizone (Mashayekhi and Salehi, 2016). These effects likely occur in conjunction with vitamin D’s immunomodulatory role (Dankers et al., 2017). Vitamin D supplementation has been tested in various clinical trials that focused mostly on RRMS or clinically isolated syndrome, but should also be thoroughly evaluated in patients with progressive MS (Pozuelo-Moyano et al., 2013), especially given its putative action on remyelination and possible ability to lesson iron mediated CNS damage.
Vitamin D, biotin and iron may all work together to support myelination and facilitate remyelination efforts (Table 1). Maintaining optimal levels of these nutrients is predicted to enable the best outcome for patients with progressive MS since a deficiency in one could affect or undermine the functions of the others.
Additional Experimental Approaches to Promote Metabolism
Heightened ATP consumption is thought to occur following demyelination due to the redistribution of channels along the axon to support the propagation of impulses (Hollingsworth et al., 2017). This is contrasted with healthy myelin which helps maintain the distribution of sodium channels necessary for saltatory conduction thereby resulting in efficient energy utilization, while oligodendrocytes also provide trophic support for axons (Hollingsworth et al., 2017). The repair process of remyelination depends on the migration and differentiation of OPCs into oligodendrocytes capable of forming myelin, but remyelination is an incomplete and heterogenous process (Hollingsworth et al., 2017). As in myelination, proper metabolic support should facilitate remyelination efforts while also supporting axonal processes and other neuronal functions (Heidker et al., 2016). Along these lines, additional therapies that support ATP production and/or drive remyelination in the CNS are being explored, e.g., ketogenic diet, thyroid hormone, estrogen, and stem cells (SCs) (Table 1). While many of these studies have been performed in the context of RRMS, or related models, they may provide important insight into therapeutic mechanisms that may help ameliorate progressive forms of MS.
Mitochondria generate ATP within neurons via oxidative metabolism (Bélanger et al., 2011), with decreased mitochondrial functionality contributing to neurodegeneration (Storoni and Plant, 2015). Glucose which is metabolized into pyruvate via glycolysis, supports ATP generation via the oxidative pathways of the TCA cycle and the ETC (Voet and Voet, 2011; Mathur et al., 2014). Disturbances of glucose and pyruvate metabolism in MS patients are indicated by elevated blood or cerebrospinal fluid levels of pyruvate, alpha-ketoglutarate, and citrate, while SPMS patients exhibit increased CSF levels of metabolites from both extra-mitochondrial glucose and anaerobic metabolism (Mathur et al., 2014). A ketogenic diet, which can increase ATP levels, has been proposed as a possible supportive treatment for progressive MS (Storoni and Plant, 2015). In rats, a ketogenic diet produced higher ATP/adenosine diphosphate ratios in the cerebrum than normal diets, making it conceivable that a ketogenic diet might assist in providing more adequate levels of ATP for neuronal support (DeVivo et al., 1978). Additionally, ketogenic diets may increase antioxidants and promote the expression of uncoupling proteins, which may lessen the production of reactive oxygen species (ROS) (Storoni and Plant, 2015). When tested in murine experimental autoimmune encephalomyelitis (EAE), a model of MS, a ketogenic diet caused decreased levels of ROS and inflammatory cytokines (Kim et al., 2012).
Rats exposed to a ketogenic diet for 3 weeks had an increased production of kynurenic acid (KA) in certain areas of the brain (Żarnowski et al., 2012). Systemic administration of L-kynurenine, a precursor of KA, has been shown to have neuroprotective properties, i.e., reducing N-methyl-D-aspartic acid induced death of retinal ganglion cells in rats (Vorwerk et al., 1996). KA, which can block excitotoxicity, is generated by the kynurenine pathway (KP), through the degradation of the amino acid tryptophan (Lovelace et al., 2016; Lim et al., 2017). This process also results in the production of nicotinamide adenine dinucleotide (NAD+), which is involved in multiple processes, e.g., transcription, signaling and cellular metabolism including glycolysis, TCA, and beta oxidation (Cantó et al., 2015; Lovelace et al., 2016; Lim et al., 2017). Chronic inflammation, such as that found in conditions like MS, can cause the KP to become activated and dysregulated (Lovelace et al., 2016; Lim et al., 2017). This dysregulation leads to lower NAD+ levels and the possible depletion of energy (Lovelace et al., 2016; Lim et al., 2017). Paradoxically, two other metabolites of the KP, 3-hydroxykynurenine and quinolinic acid (QA), are both excitotoxins and are elevated in the spinal cord of EAE rats (Chiarugi et al., 2001; Lovelace et al., 2016). Excessive QA with insufficient KA leads to excitotoxicity, and a recent study has shown the ratio of QA to KA is elevated in progressive forms of MS, but not RRMS, making it a potential biomarker of disease progression (Lim et al., 2017). Together, these factors make regulation of the KP an attractive therapeutic target for progressive MS (Lim et al., 2017).
Hormones, such as TH which is well known for its role in metabolic regulation, may play a supportive role in remyelination. As developmental myelination is driven in part by TH stimulating differentiation of OPCs (Bernal, 2015), researchers have begun exploring the possibility that TH may increase remyelination in MS. Positive results, including lessening impulse propagation deficits, decreasing myelin loss, and lowering axonal degeneration were seen in rats with EAE that were treated with TH starting at the presentation of clinical signs, i.e., 10 days post immunization (Dell’Acqua et al., 2012). Similarly, injections of TH after cuprizone administration in murine models, led to increased differentiation of OPCs and enhanced remyelination (Zhang et al., 2016). These effects may be mediated by increased transcription of Kruppel-like factor 9 (KLF9), which encourages differentiation of OPCs (Zhang et al., 2016). The positive results in animal studies have led to a phase I clinical trial (NCT02760056) which is underway to investigate the dosage and safety of Liothyronine (T3) in patients with MS.
Estrogen, which regulates reproduction, energy balance, and immune function (López and Tena-Sempere, 2015; Khan and Ansar Ahmed, 2016), is also being considered as a therapeutic approach for MS. Remission rates of RRMS approach nearly 80% during the later stages of pregnancy when estrogen is elevated (Vukusic et al., 2004; Gold and Voskuhl, 2009; Deckx et al., 2013). A phase II trial comparing women treated with either estriol (a form of estrogen secreted by the placenta during pregnancy) or a placebo, each in combination with glatiramer acetate, showed decreased relapse rates of MS with estriol treatment as compared to placebo (Voskuhl et al., 2016). It is thought that activation of estrogen receptors decreases the expression of inflammatory signaling molecules, thereby diminishing inflammation and the associated demyelination seen in MS (Deckx et al., 2013; Khan and Ansar Ahmed, 2016). Estrogen also appears to support the proliferation, differentiation, and process formation of oligodendrocytes (Zhang et al., 2004; Okada et al., 2008, 2010; Khalaj et al., 2013), possibly promoting remyelination. In addition to estrogen, a selective estrogen receptor modulator, Tamoxifen, has recently been shown to increase rates of remyelination both in vitro and in rats following focal demyelination induced by ethidium bromide (Gonzalez et al., 2016). The efficacy of estrogen receptor agonists is being explored using mouse models (Kumar et al., 2013; Moore et al., 2014; Itoh et al., 2017).
Another area of active research has been exploring the use of SCs as a possible treatment for MS. Autologous hematopoietic SCs have been used in the regeneration of the immune system following immunoablation with some success in MS patients with aggressive disease (Mancardi and Saccardi, 2008; Muraro et al., 2017). Experimental studies suggest that neural and mesenchymal SCs could act to directly replace cells (e.g., oligodendrocytes and neurons) and myelin (Jadasz et al., 2012; Meamar et al., 2016). Additionally, SCs can also produce immunomodulatory and trophic factors, with neuroprotective, regenerative, and growth properties (Reekmans et al., 2012; Teixeira et al., 2013; Meamar et al., 2016). The exact mechanisms by which SCs improve EAE outcomes are unclear, but may favor activation of endogenous repair mechanisms (Reekmans et al., 2012). For example, SCs can activate endogenous cells to undergo growth or differentiation, and support other functions such as remyelination (Jadasz et al., 2012; Meamar et al., 2016). Among the many factors produced by mesenchymal SCs, brain derived neurotrophic factor is thought to support remyelination (KhorshidAhmad et al., 2016), promote survival of neurons/terminals, enhance neurite outgrowth (Crigler et al., 2006; Cova et al., 2010; Hsieh et al., 2013), and promote mitochondrial function (Markham et al., 2004, 2012, 2014). These functions fit nicely into the category of providing metabolic support for neurons and/or oligodendrocytes as a potential therapeutic mechanism. Thus, these multiple properties of SCs would be predicted to ameliorate the putative pathogenic mechanisms of progressive MS.
Conclusions
Therapies that support myelin maintenance, facilitate remyelination, and/or promote metabolism could in theory act to counter disease progression and possibly even help restore some small levels of function in some patients with progressive MS. Maximal benefit would be expected to come from an intervention that is started early in the course of progressive disease, including during the transition period from RRMS to SPMS, which may even help forestall the full conversion to SPMS. In patients with advanced progressive MS, where considerable pathology has accumulated (e.g., extensive loss of axons and neurons), it will be more challenging for interventions targeting metabolism or remyelination to make a meaningful impact.
To achieve maximum benefit from metabolic therapies, multiple steps along biochemical pathways likely will need to be targeted as a deficiency in one step could undermine the effects of the intervention at a different step. A sub-optimal level of a nutrient could also limit responses in a trial of a test compound directed at a different facet of the pathogenic process, and a multifaceted approach with therapies that target other pathogenic mechanisms, e.g., ocrelizumab acting on B cells, is predicted to give a better outcome than a therapy limited to only metabolic support.
In summary, therapies that support metabolism may be useful for treating patients with progressive MS. Maximal gains in the battle against this disease will likely come from a combination of therapies that are started early in the course of disease and promote repair, counter the disease process, and provide optimal support of oligodendrocyte and neuronal structures and functions.
Footnotes
Conflicts of interest: RMH is currently employed by Employer Solutions, a division of Quest Diagnostics. SML has received funding from Apo-Pharma, Inc.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Mohammad Khalaj-Kondori, University of Tabriz, Iran.
References
- Ascherio A, Munger KL. Epidemiology of multiple sclerosis: from risk factors to prevention-an update. Semin Neurol. 2016;36:103–114. doi: 10.1055/s-0036-1579693. [DOI] [PubMed] [Google Scholar]
- Atamna H. Heme, iron, and the mitochondrial decay of ageing. Ageing Res Rev. 2004;3:303–318. doi: 10.1016/j.arr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Atamna H, Newberry J, Erlitzki R, Schultz CS, Ames BN. Biotin deficiency inhibits heme synthesis and impairs mitochondria in human lung fibroblasts. J Nutr. 2007;137:25–30. doi: 10.1093/jn/137.1.25. [DOI] [PubMed] [Google Scholar]
- Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Bernal J. Thyroid Hormones in Brain Development and Function. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, editors. Endotext. South Dartmouth (MA): MDText.com, Inc; 2015. [Google Scholar]
- Cantó C, Menzies K, Auwerx J. NAD(+) metabolism and the control of energy homeostasis: A balancing act between mitochondria and the nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chataway J. Biotin in progressive multiple sclerosis: A new lead? Mult Scler. 2016;22:1640–1641. doi: 10.1177/1352458516676387. [DOI] [PubMed] [Google Scholar]
- Chataway J, Schuerer N, Alsanousi A, Chan D, MacManus D, Hunter K, Anderson V, Bangham CR, Clegg S, Nielsen C, Fox NC, Wilkie D, Nicholas JM, Calder VL, Greenwood J, Frost C, Nicholas R. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet. 2014;383:2213–2221. doi: 10.1016/S0140-6736(13)62242-4. [DOI] [PubMed] [Google Scholar]
- Chen KB, Lin AM, Chiu TH. Systemic vitamin D3 attenuated oxidative injuries in the locus coeruleus of rat brain. Ann N Y Acad Sci. 2003;993:313–324. doi: 10.1111/j.1749-6632.2003.tb07539.x. [DOI] [PubMed] [Google Scholar]
- Chiarugi A, Cozzi A, Ballerini C, Massacesi L, Moroni F. Kynurenine 3-mono-oxygenase activity and neurotoxic kynurenine metabolites increase in the spinal cord of rats with experimental allergic encephalomyelitis. Neuroscience. 2001;102:687–695. doi: 10.1016/s0306-4522(00)00504-2. [DOI] [PubMed] [Google Scholar]
- Cova L, Armentero MT, Zennaro E, Calzarossa C, Bossolasco P, Busca G, Lambertenghi Deliliers G, Polli E, Nappi G, Silani V, Blandini F. Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson’s disease. Brain Res. 2010;1311:12–27. doi: 10.1016/j.brainres.2009.11.041. [DOI] [PubMed] [Google Scholar]
- Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;198:54–64. doi: 10.1016/j.expneurol.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Dankers W, Colin EM, van Hamburg JP, Lubberts E. Vitamin D in autoimmunity: molecular mechanisms and therapeutic potential. Front Immunol. 2017;7:697. doi: 10.3389/fimmu.2016.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente AG, Errea O, van Wijngaarden P, Gonzalez GA, Kerninon C, Jarjour AA, Lewis HJ, Jones CA, Nait-Oumesmar B, Zhao C, Huang JK, ffrench-Constant C, Franklin RJ. Vitamin D receptor-retinoid X receptor heterodimer signaling regulates oligodendrocyte progenitor cell differentiation. J Cell Biol. 2015;211:975–985. doi: 10.1083/jcb.201505119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckx N, Lee WP, Berneman ZN, Cools N. Neuroendocrine immunoregulation in multiple sclerosis. Clin Dev Immunol. 2013;2013:705232. doi: 10.1155/2013/705232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Acqua ML, Lorenzini L, D’Intino G, Sivilia S, Pasqualetti P, Panetta V, Paradisi M, Filippi MM, Baiguera C, Pizzi M, Giardino L, Rossini PM, Calzà L. Functional and molecular evidence of myelin- and neuroprotection by thyroid hormone administration in experimental allergic encephalomyelitis. Neuropathol Appl Neurobiol. 2012;38:454–470. doi: 10.1111/j.1365-2990.2011.01228.x. [DOI] [PubMed] [Google Scholar]
- DeVivo DC, Leckie MP, Ferrendelli JS, McDougal DB., Jr Chronic ketosis and cerebral metabolism. Ann Neurol. 1978;3:331–337. doi: 10.1002/ana.410030410. [DOI] [PubMed] [Google Scholar]
- Giovannoni G, Cohen JA, Coles AJ, Hartung HP, Havrdova E, Selmaj KW, Margolin DH, Lake SL, Kaup SM, Panzara MA, Compston DA. CARE-MS II Investigators (2016) Alemtuzumab improves preexisting disability in active relapsing-remitting MS patients. Neurology. 87:1985–1992. doi: 10.1212/WNL.0000000000003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R, Wolinsky JS, Amato MP, Comi G. Evolving expectations around early management of multiple sclerosis. Ther Adv Neurol Disord. 2010;3:351–367. doi: 10.1177/1756285610385608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SM, Voskuhl RR. Estrogen and testosterone therapies in multiple sclerosis. Prog Brain Res. 2009;175:239–251. doi: 10.1016/S0079-6123(09)17516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez GA, Hofer MP, Syed YA, Amaral AI, Rundle J, Rahman S, Zhao C, Kotter MR. Tamoxifen accelerates the repair of demyelinated lesions in the central nervous system. Sci Rep. 2016;6:31599. doi: 10.1038/srep31599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hametner S, Wimmer I, Haider L, Pfeifenbring S, Brück W, Lassmann H. Iron and neurodegeneration in the multiple sclerosis brain. Ann Neurol. 2013;74:848–861. doi: 10.1002/ana.23974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidker RM, Emerson MR, LeVine SM. Intersections of pathways involving biotin and iron relative to therapeutic mechanisms for progressive multiple sclerosis. Discov Med. 2016;22:381–387. [PubMed] [Google Scholar]
- Hempel S, Graham GD, Fu N, Estrada E, Chen AY, Miake-Lye I, Miles JN, Shanman R, Shekelle PG, Beroes JM, Wallin MT. A systematic review of modifiable risk factors in the progression of multiple sclerosis. Mult Scler. 2017;23:525–533. doi: 10.1177/1352458517690270. [DOI] [PubMed] [Google Scholar]
- Hollingsworth E, Khouri J, Imitola J. Endogenous repair and development inspired therapy of neurodegeneration in progressive multiple sclerosis. Expert Rev Neurother. 2017;17:611–629. doi: 10.1080/14737175.2017.1287564. [DOI] [PubMed] [Google Scholar]
- Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab. 2012;32:1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JY, Wang HW, Chang SJ, Liao KH, Lee IH, Lin WS, Wu CH, Lin WY, Cheng SM. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS One. 2013;8:e72604. doi: 10.1371/journal.pone.0072604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Kim R, Peng M, DiFilippo E, Johnsonbaugh H, MacKenzie-Graham A, Voskuhl RR. Bedside to bench to bedside research: Estrogen receptor beta ligand as a candidate neuroprotective treatment for multiple sclerosis. J Neuroimmunol. 2017;304:63–71. doi: 10.1016/j.jneuroim.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadasz JJ, Aigner L, Rivera FJ, Küry P. The remyelination Philosopher’s Stone: stem and progenitor cell therapies for multiple sclerosis. Cell Tissue Res. 2012;349:331–347. doi: 10.1007/s00441-012-1331-x. [DOI] [PubMed] [Google Scholar]
- Kappos L, Kuhle J, Multanen J, Kremenchutzky M, Verdun di Cantogno E, Cornelisse P, Lehr L, Casset-Semanaz F, Issard D, Uitdehaag BM. Factors influencing long-term outcomes in relapsing-remitting multiple sclerosis: PRISMS-15. J Neurol Neurosurg Psychiatry. 2015;86:1202–1207. doi: 10.1136/jnnp-2014-310024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L, Bar-Or AC, B, Fox R, Giovannoni G, Gold R, Vermersch P, Arnould S, Sidorenko T, Wolf C, Wallstroem E, Dahlke F. Efficacy and safety of siponimod in secondary progressive multiple sclerosis - Results of the placebo controlled, doubleblind, Phase III EXPAND study. Proceedings of the 32nd congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), London, 14–17 September. 2016 [Google Scholar]
- Kawachi I, Lassmann H. Neurodegeneration in multiple sclerosis and neuromyelitis optica. J Neurol Neurosurg Psychiatry. 2017;88:137–145. doi: 10.1136/jnnp-2016-313300. [DOI] [PubMed] [Google Scholar]
- Khalaj AJ, Yoon J, Nakai J, Winchester Z, Moore SM, Yoo T, Martinez-Torres L, Kumar S, Itoh N, Tiwari-Woodruff SK. Estrogen receptor (ER) β expression in oligodendrocytes is required for attenuation of clinical disease by an ERβ ligand. Proc Natl Acad Sci U S A. 2013;110:19125–19130. doi: 10.1073/pnas.1311763110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan D, Ansar Ahmed S. The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Front Immunol. 2016;6:635. doi: 10.3389/fimmu.2015.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KhorshidAhmad T, Acosta C, Cortes C, Lakowski TM, Gangadaran S, Namaka M. Transcriptional regulation of brain-derived neurotrophic factor (BDNF) by methyl CpG binding protein 2 (MeCP2): a novel mechanism for re-myelination and/or myelin repair involved in the treatment of multiple sclerosis (MS) Mol Neurobiol. 2016;53:1092–1107. doi: 10.1007/s12035-014-9074-1. [DOI] [PubMed] [Google Scholar]
- Kim DY, Hao J, Liu R, Turner G, Shi FD, Rho JM. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS One. 2012;7:e35476. doi: 10.1371/journal.pone.0035476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Patel R, Moore S, Crawford DK, Suwanna N, Mangiardi M, Tiwari-Woodruff SK. Estrogen receptor beta ligand therapy activates PI3K/Akt/mTOR signaling in oligodendrocytes and promotes remyelination in a mouse model of multiple sclerosis. Neurobiol Dis. 2013;56:131–144. doi: 10.1016/j.nbd.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López M, Tena-Sempere M. Estrogens and the control of energy homeostasis: a brain perspective. Trends Endocrinol Metab. 2015;26:411–421. doi: 10.1016/j.tem.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Lim CK, Bilgin A, Lovejoy DB, Tan V, Bustamante S, Taylor BV, Bessede A, Brew BJ, Guillemin GJ. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci Rep. 2017;7:41473. doi: 10.1038/srep41473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelace MD, Varney B, Sundaram G, Franco NF, Ng ML, Pai S, Lim CK, Guillemin GJ, Brew BJ. Current evidence for a role of the kynurenine pathway of tryptophan metabolism in multiple sclerosis. Front Immunol. 2016;7:246. doi: 10.3389/fimmu.2016.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- Mancardi G, Saccardi R. Autologous haematopoietic stem-cell transplantation in multiple sclerosis. Lancet Neurol. 2008;7:626–636. doi: 10.1016/S1474-4422(08)70138-8. [DOI] [PubMed] [Google Scholar]
- Mao-Draayer Y, Sarazin J, Fox D, Schiopu E. The sphingosine-1-phosphate receptor: A novel therapeutic target for multiple sclerosis and other autoimmune diseases. Clin Immunol. 2017;175:10–15. doi: 10.1016/j.clim.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham A, Cameron I, Franklin P, Spedding M. BDNF increases rat brain mitochondrial respiratory coupling at complex I, but not complex II. Eur J Neurosci. 2004;20:1189–1196. doi: 10.1111/j.1460-9568.2004.03578.x. [DOI] [PubMed] [Google Scholar]
- Markham A, Bains R, Franklin P, Spedding M. Changes in mitochondrial function are pivotal in neurodegenerative and psychiatric disorders: how important is BDNF? Br J Pharmacol. 2014;171:2206–2229. doi: 10.1111/bph.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham A, Cameron I, Bains R, Franklin P, Kiss JP, Schwendimann L, Gressens P, Spedding M. Brain-derived neurotrophic factor-mediated effects on mitochondrial respiratory coupling and neuroprotection share the same molecular signalling pathways. Eur J Neurosci. 2012;35:366–374. doi: 10.1111/j.1460-9568.2011.07965.x. [DOI] [PubMed] [Google Scholar]
- Mashayekhi F, Salehi Z. Administration of vitamin D3 induces CNPase and myelin oligodendrocyte glycoprotein expression in the cerebral cortex of the murine model of cuprizone-induced demyelination. Folia Neuropathol. 2016;54:259–264. doi: 10.5114/fn.2016.62535. [DOI] [PubMed] [Google Scholar]
- Mathur D, López-Rodas G, Casanova B, Marti MB. Perturbed glucose metabolism: insights into multiple sclerosis pathogenesis. Front Neurol. 2014;5:250. doi: 10.3389/fneur.2014.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meamar R, Nematollahi S, Dehghani L, Mirmosayyeb O, Shayegannejad V, Basiri K, Tanhaei AP. The role of stem cell therapy in multiple sclerosis: An overview of the current status of the clinical studies. Adv Biomed Res. 2016;5:46. doi: 10.4103/2277-9175.178791. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Miron VE, Zehntner SP, Kuhlmann T, Ludwin SK, Owens T, Kennedy TE, Bedell BJ, Antel JP. Statin therapy inhibits remyelination in the central nervous system. Am J Pathol. 2009;174:1880–1890. doi: 10.2353/ajpath.2009.080947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, de Seze J, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Rammohan KW, Selmaj K, Traboulsee A, Sauter A, Masterman D, Fontoura P, Belachew S, Garren H, Mairon N, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- Moore SM, Khalaj AJ, Kumar S, Winchester Z, Yoon J, Yoo T, Martinez-Torres L, Yasui N, Katzenellenbogen JA, Tiwari-Woodruff SK. Multiple functional therapeutic effects of the estrogen receptor beta agonist indazole-Cl in a mouse model of multiple sclerosis. Proc Natl Acad Sci U S A. 2014;111:18061–18066. doi: 10.1073/pnas.1411294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraro PA, Pasquini M, Atkins HL, Bowen JD, Farge D, Fassas A, Freedman MS, Georges GE, Gualandi F, Hamerschlak N, Havrdova E, Kimiskidis VK, Kozak T, Mancardi GL, Massacesi L, Moraes DA, Nash RA, Pavletic S, Ouyang J, Rovira M, et al. Long-term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol. 2017;74:459–469. doi: 10.1001/jamaneurol.2016.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Makino A, Nakajima M, Okuyama S, Furukawa S, Furukawa Y. Estrogen stimulates proliferation and differentiation of neural stem/progenitor cells through different signal transduction pathways. Int J Mol Sci. 2010;11:4114–4123. doi: 10.3390/ijms11104114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Murase K, Makino A, Nakajima M, Kaku T, Furukawa S, Furukawa Y. Effects of estrogens on proliferation and differentiation of neural stem/progenitor cells. Biomed Res. 2008;29:163–170. doi: 10.2220/biomedres.29.163. [DOI] [PubMed] [Google Scholar]
- Pozuelo-Moyano B, Benito-León J, Mitchell AJ, Hernández-Gallego J. A systematic review of randomized, double-blind, placebo-controlled trials examining the clinical efficacy of vitamin D in multiple sclerosis. Neuroepidemiology. 2013;40:147–153. doi: 10.1159/000345122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reekmans K, Praet J, De Vocht N, Daans J, Van der Linden A, Berneman Z, Ponsaerts P. Stem cell therapy for multiple sclerosis: preclinical evidence beyond all doubt? Regen Med. 2012;7:245–259. doi: 10.2217/rme.12.5. [DOI] [PubMed] [Google Scholar]
- Sartori A, Fantini J, Manganotti P. How far away from having an effective treatment option for progressive multiple sclerosis are we? Expert Opin Pharmacother. 2017;18:953–955. doi: 10.1080/14656566.2017.1326909. [DOI] [PubMed] [Google Scholar]
- Schonberg DL, Goldstein EZ, Sahinkaya FR, Wei P, Popovich PG, McTigue DM. Ferritin stimulates oligodendrocyte genesis in the adult spinal cord and can be transferred from macrophages to NG2 cells in vivo. J Neurosci. 2012;32:5374–5384. doi: 10.1523/JNEUROSCI.3517-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedel F, Bernard D, Mock DM, Tourbah A. Targeting demyelination and virtual hypoxia with high-dose biotin as a treatment for progressive multiple sclerosis. Neuropharmacology. 2016;110:644–653. doi: 10.1016/j.neuropharm.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Siddiqui U, Egnor E, Sloane JA. Biotin supplementation in MS clinically valuable but can alter multiple blood test results. Mult Scler. 2017;23:619–620. doi: 10.1177/1352458516680751. [DOI] [PubMed] [Google Scholar]
- Stephenson E, Nathoo N, Mahjoub Y, Dunn JF, Yong VW. Iron in multiple sclerosis: roles in neurodegeneration and repair. Nat Rev Neurol. 2014;10:459–468. doi: 10.1038/nrneurol.2014.118. [DOI] [PubMed] [Google Scholar]
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi: 10.1155/2015/681289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys PK. Axonal degeneration in multiple sclerosis: is it time for neuroprotective strategies? Ann Neurol. 2004;55:601–603. doi: 10.1002/ana.20082. [DOI] [PubMed] [Google Scholar]
- Teixeira FG, Carvalho MM, Sousa N, Salgado AJ. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol Life Sci. 2013;70:3871–3882. doi: 10.1007/s00018-013-1290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettey P, Siejka D, Simpson S, Jr, Taylor B, Blizzard L, Ponsonby AL, Dwyer T, van der Mei I. Frequency of comorbidities and their association with clinical disability and relapse in multiple sclerosis. Neuroepidemiology. 2016;46:106–113. doi: 10.1159/000442203. [DOI] [PubMed] [Google Scholar]
- Tourbah A, Lebrun-Frenay C, Edan G, Clanet M, Papeix C, Vukusic S, De Seze J, Debouverie M, Gout O, Clavelou P, Defer G, Laplaud DA, Moreau T, Labauge P, Brochet B, Sedel F, Pelletier J. MS-SPI study group (2016) MD1003 (high-dose biotin) for the treatment of progressive multiple sclerosis: A randomised, double-blind, placebo-controlled study. Mult Scler. 22:1719–1731. doi: 10.1177/1352458516667568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toxqui L, Vaquero MP. Chronic iron deficiency as an emerging risk factor for osteoporosis: a hypothesis. Nutrients. 2015;7:2324–2344. doi: 10.3390/nu7042324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009;8:280–291. doi: 10.1016/S1474-4422(09)70043-2. [DOI] [PubMed] [Google Scholar]
- Trojano M, Pellegrini F, Fuiani A, Paolicelli D, Zipoli V, Zimatore GB, Di Monte E, Portaccio E, Lepore V, Livrea P, Amato MP. New natural history of interferon-beta-treated relapsing multiple sclerosis. Ann Neurol. 2007;61:300–306. doi: 10.1002/ana.21102. [DOI] [PubMed] [Google Scholar]
- Trojano M, Pellegrini F, Paolicelli D, Fuiani A, Zimatore GB, Tortorella C, Simone IL, Patti F, Ghezzi A, Zipoli V, Rossi P, Pozzilli C, Salemi G, Lugaresi A, Bergamaschi R, Millefiorini E, Clerico M, Lus G, Vianello M, Avolio C, et al. Real-life impact of early interferon beta therapy in relapsing multiple sclerosis. Ann Neurol. 2009;66:513–520. doi: 10.1002/ana.21757. [DOI] [PubMed] [Google Scholar]
- Uberti F, Morsanuto V, Bardelli C, Molinari C. Protective effects of 1alpha, 25-Dihydroxyvitamin D3 on cultured neural cells exposed to catalytic iron. Physiol Rep. 2016;4:e12769. doi: 10.14814/phy2.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voet D, Voet JG. Biochemistry. Hoboken, NJ: John Wiley & Sons; 2011. [DOI] [PubMed] [Google Scholar]
- Vorwerk CK, Kreutz MR, Dreyer EB, Sabel BA. Systemic L-kynurenine administration partially protects against NMDA, but not kainate-induced degeneration of retinal ganglion cells, and reduces visual discrimination deficits in adults rats. Invest Ophthalmol Vis Sci. 1996;37:2382–2392. [PubMed] [Google Scholar]
- Voskuhl RR, Wang H, Wu TC, Sicotte NL, Nakamura K, Kurth F, Itoh N, Bardens J, Bernard JT, Corboy JR, Cross AH, Dhib-Jalbut S, Ford CC, Frohman EM, Giesser B, Jacobs D, Kasper LH, Lynch S, Parry G, Racke MK, et al. Estriol combined with glatiramer acetate for women with relapsing-remitting multiple sclerosis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:35–46. doi: 10.1016/S1474-4422(15)00322-1. [DOI] [PubMed] [Google Scholar]
- Vukusic S, Hutchinson M, Hours M, Moreau T, Cortinovis-Tourniaire P, Adeleine P, Confavreux C. Pregnancy In Multiple Sclerosis Group (2004) Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 127:1353–1360. doi: 10.1093/brain/awh152. [DOI] [PubMed] [Google Scholar]
- Weigel KJ, Lynch SG, LeVine SM. Iron chelation and multiple sclerosis. ASN Neuro. 2014;6:e00136. doi: 10.1042/AN20130037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiendl H, Hohlfeld R. Multiple sclerosis therapeutics: unexpected outcomes clouding undisputed successes. Neurology. 2009;72:1008–1015. doi: 10.1212/01.wnl.0000344417.42972.54. [DOI] [PubMed] [Google Scholar]
- Wingerchuk DM, Weinshenker BG. Disease modifying therapies for relapsing multiple sclerosis. BMJ. 2016;354:i3518. doi: 10.1136/bmj.i3518. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Reeves SA. Simvastatin induces cell death in a mouse cerebellar slice culture (CSC) model of developmental myelination. Exp Neurol. 2009;215:41–47. doi: 10.1016/j.expneurol.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Żarnowski T, Chorągiewicz T, Tulidowicz-Bielak M, Thaler S, Rejdak R, Żarnowska I, Turski WA, Gasior M. Ketogenic diet increases concentrations of kynurenic acid in discrete brain structures of young and adult rats. J Neural Transm. 2012;119:679–684. doi: 10.1007/s00702-011-0750-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Ma Z, Qin H, Yao Z. Thyroid hormone potentially benefits multiple sclerosis via facilitating remyelination. Mol Neurobiol. 2016;53:4406–4416. doi: 10.1007/s12035-015-9375-z. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Cerghet M, Mullins C, Williamson M, Bessert D, Skoff R. Comparison of in vivo and in vitro subcellular localization of estrogen receptors alpha and beta in oligodendrocytes. J Neurochem. 2004;89:674–684. doi: 10.1111/j.1471-4159.2004.02388.x. [DOI] [PubMed] [Google Scholar]


