Abstract
Food addiction (FA) is loosely defined as hedonic eating behavior involving the consumption of highly palatable foods (ie, foods high in salt, fat, and sugar) in quantities beyond homeostatic energy requirements. FA shares some common symptomology with other pathological eating disorders, such as binge eating. Current theories suggest that FA shares both behavioral similarities and overlapping neural correlates to other substance addictions. Although preliminary, neuroimaging studies in response to food cues and the consumption of highly palatable food in individuals with FA compared to healthy controls have shown differing activation patterns and connectivity in brain reward circuits including regions such as the striatum, amygdala, orbitofrontal cortex, insula, and nucleus accumbens. Additional effects have been noted in the hypothalamus, a brain area responsible for regulating eating behaviors and peripheral satiety networks. FA is highly impacted by impulsivity and mood. Chronic stress can negatively affect hypothalamic–pituitary–adrenal axis functioning, thus influencing eating behavior and increasing desirability of highly palatable foods. Future work will require clearly defining FA as a distinct diagnosis from other eating disorders.
1. INTRODUCTION
The primary focus of this chapter is to discuss the current understanding of food addiction (FA) in light of neurobehavioral models based on findings from advanced neuroimaging tools. Recent literature suggests that biological, psychological, behavioral, and nutritional components play a part in the etiology and persistence of FA (Gearhardt et al., 2011; Parylak, Koob, & Zorrilla, 2011; Singh, 2014; Sinha & Jastreboff, 2013; Ziauddeen, Alonso-Alonso, Hill, Kelley, & Khan, 2015). In the United States, more than two-thirds of adults are considered overweight or obese, and obesity is linked to an array of health risks, ranging from type 2 diabetes and heart disease to breast, colon, endometrial, and kidney cancer (The National Institute of Diabetes and Digestive and Kidney Diseases, 2012). Although “food addiction” and “obesity” are often used interchangeably, these designations correspond to unique underlying concepts. FA is loosely defined as a behavior involving the uncontrollable urge or insatiable desire to continue eating past what would be considered a physical or metabolic need (Parylak et al., 2011) whereas obesity is defined by a body mass index (BMI) of 30 and above (National Institutes of Health & Department of Health and Human Services, n.d.). A person can have a FA and not be obese. Conversely, one can be obese and not have a FA, although obesity may be a consequence of FA, and therefore FA incidence may be higher among this population (Meule, 2011; Pursey, Stanwell, Gearhardt, Collins, & Burrows, 2014).
From a theoretical and research-based viewpoint, it is important to distinguish between FA and other eating disorders. The Diagnostic and Statistical Manual, 5th Edition (DSM-5) describes criteria for “Feeding and Eating Disorders” (American Psychiatric Association, 2013a) of which the three most prevalent are anorexia nervosa, bulimia nervosa, and binge-eating disorder. Anorexia nervosa is characterized by restriction of energy intake relative to requirements and significantly low body weight. Bulimia nervosa includes episodes of binge-eating and compensatory behaviors to prevent weight gain. Lastly, binge-eating disorder involves recurrent binge-eating episodes along with extreme distress surrounding the binge-eating behavior (American Psychiatric Association, 2013a). Community studies including participants with a diverse weight range showed that individuals with FA, but not binge-eating disorder, report significant levels of impairment and distress, including depressive symptoms, impulsivity, and negative affect (Gearhardt, Boswell, & White, 2014). Among individuals who meet criteria for FA, the frequency of binge-eating disorder ranges from 27% to 30%, suggesting a high comorbidity between the two conditions (Davis et al., 2011; Gearhardt, Boswell, & White, 2014). While there is a strong association between FA and eating disorders, predominantly bulimia nervosa and binge-eating disorder, they have distinctly different presentations, particularly related to the frequency and level of symptomology. FA appears to be marked by more severe depression, negative affect, and general levels of distress compared to other eating disorders.
As a concept, addiction to food is complex, and its meaning has evolved over time. It is necessary to operationalize this notion in order to provide a framework for further investigation. In a review of over 40 papers on the definition of addiction, Sussman and Sussman (2011) formulated a list of criteria: (1) partaking in behavior due to appetitive effects; (2) spending a great deal of time thinking about, obtaining, using, and recovering from the effects of the substance; (3) satiation, described as a period of time directly after the use of a substance when the addictive behavior temporarily subsides only to return quite quickly as the effects dissipate; (4) impulsivity or loss of control over the behavior; and (5) suffering negative psychosocial, emotional, and health consequences. The concept of FA will be evaluated using these criteria. Addiction, as defined by Sussman and Sussman (2011), closely resembles the DSM-5 criteria for substance use disorders (American Psychiatric Association, 2013b). The Yale Food Addiction Scale Version 2.0 (YFAS 2.0), the only currently existing operational measure of FA, is based on DSM-5 criteria (Gearhardt, Corbin, & Brownell, 2016). The YFAS 2.0 maps onto the DSM-5 criteria for addiction by asking questions about time spent on eating and the quantity of food consumed, unsuccessful efforts to cut down despite high motivation, time spent on obtaining food, presence of cravings, eating habits negatively impacting obligations and social relations, eating despite negative physical consequences, a need for greater food intake to achieve satiety (ie, tolerance), and negative physiological or psychological feelings when eating certain foods is stopped (ie, withdrawal) (American Psychiatric Association, 2013b; Gearhardt et al., 2016).
A meta-analysis including 28 studies found a weighted mean prevalence for FA of 19.9% among adults in the United States (Pursey et al., 2014). When looking at studies that addressed sex differences specifically, rates were greater in woman than in men (12.2% vs 6.4%). Overweight/obese subjects had higher rates of FA than those with a healthy BMI (24.9% vs 11.1%). Lastly, FA also appears to be more prevalent in individuals 35 years and older than in individuals younger than 35 (22.2% vs 17.0%). Although these findings should be interpreted with caution, because the majority of participants were female and obese, they provide initial insight into the number of people in the United States with FA (Pursey et al., 2014).
Neuroimaging permits researchers to study the effects of food-related stimuli on specific brain regions and their intrinsic functional connectivity. Specifically, functional magnetic resonance imaging (fMRI) can be used to measure the blood oxygenation level-dependent response to stimuli or at rest. A variety of eating behaviors, including the processing of tastes, appearances, and scents of food, and satiety, reward, motivation, reappraisal, craving, and consumption associated with food have been investigated with fMRI (Giuliani & Pfeifer, 2015; Huerta, Sarkar, Duong, Laird, & Fox, 2014; Jiang, Soussignan, Schaal, & Royet, 2015; Murdaugh, Cox, Cook, & Weller, 2012; Robinson, Fischer, Ahuja, Lesser, & Maniates, 2016; Rolls, 2015; Rolls, Kellerhals, & Nichols, 2015; Simmons et al., 2014; Thomas et al., 2015; van Bloemendaal et al., 2015; Wang, Smith, & Delgado, 2016). Evaluation of FA has demonstrated alterations in brain regions involved in reward processing, homeostatic regulation, emotional reactivity, and executive control (Gearhardt, Boswell, & Potenza, 2014; Lao-Kaim et al., 2015; Simon et al., 2016; Ziauddeen, Farooqi, & Fletcher, 2012). Because neuroimaging of FA is still in preliminary stages, only a few studies are currently available to review.
2. FACTORS THAT LEAD TO THE FORMATION OF FA
2.1 Hyperpalatable Foods
Specific types of foods, frequently referred to as “palatable” or “hyperpalatable,” due to their high content of salt, sugar, and fat (Gearhardt, Grilo, DiLeone, Brownell, & Potenza, 2011), are considered to have addictive potential (de Macedo, de Freitas, & da Silva Torres, 2016) because they contain a high density of nutrients or additives (Meule, 2015). Schulte, Avena, and Gearhardt (2015) asked a group of 120 undergraduate students to indicate which among a nutritionally varied list of 35 foods were most addictive. Analyses revealed that the most common foods chosen were processed, had a high-fat content, and a high glycemic load, a measure of the extent to which a carbohydrate affects blood sugar levels (Monro & Shaw, 2008). It is possible that diets high in palatable foods along with other risk factors can increase risk for developing a FA (Gearhardt, Davis, Kuschner, & Brownell, 2011). For example, it has been shown with fMRI that even in healthy young women, viewing pictures of highly palatable food or drinks activated brain regions of the reward circuitry (Gearhardt, Yokum, et al., 2011).
2.2 Operant Conditioning
Operant conditioning can help explain how an individual may transition from occasional, nonpathological consumption of hyperpalatable foods to problematic, addictive eating. The evolution of drug addiction may be explained by a learned process wherein a person is initially motivated by the rewarding aspects of a substance, such as feeling “high” or experiencing elevated mood (positive reinforcement) (Koob, 2000; Weiss et al., 2001). As time progresses, motivation shifts from desire for positive experiences to avoidance of aversive experiences, such as those that accompany withdrawal. This avoidance is achieved through continued use of the substance despite the loss of the original euphoric feeling (negative reinforcement) (Koob, 2000, 2003; Weiss et al., 2001). It has been hypothesized that this same model applies to hyperpalatable foods leading to FA (Meule & Kubler, 2012). Initially, one may be motivated to eat specific energy-dense foods due to the rewarding feelings elicited when eating such foods (Parylak et al., 2011). Over time, the consumption of highly palatable foods may lead to neuroadaptations in brain reward centers involving the downregulation of D2 receptors, as seen in other substance addictions: motivation switches to a desire to ameliorate negative emotional or physiological states, such as depression, anxiety, irritability, or other somatic symptoms related to absence of the highly palatable food (de Macedo et al., 2016; Meule & Kubler, 2012; Parylak et al., 2011). To date, most of the evidence for this type of neuroplasticity in FA comes from animal studies (Ahmed et al., 2014; Johnson & Kenny, 2010; van de Giessen et al., 2013).
2.3 Mood and Stress
There is evidence that those with FA or FA symptomology may be using addictive eating behaviors to cope with negative emotional states (Micanti et al., 2016). For example, binge-eating behavior is associated with psychosocial factors such as negative affect and mood dysregulation (Micanti et al., 2016), weight cycling (Zwaan, Engeli, & Muller, 2015), body dissatisfaction (Goldschmidt, Wall, Choo, Becker, & Neumark-Sztainer, 2016), fear of self-compassion (Kelly, Vimalakanthan, & Carter, 2014), and neuroticism (Womble et al., 2001). Individuals who are underweight or normal weight report lower food consumption when experiencing negative emotions than when experiencing positive emotions. The inverse is true for overweight individuals, who consume more food when experiencing negative emotions (Geliebter & Aversa, 2003). Similar results were seen in a group of women who scored high on the YFAS and showed significantly higher levels of depression than those whose scores did not indicate FA (Berenson, Laz, Pohlmeier, Rahman, & Cunningham, 2015. There is also substantial evidence to support the connection between stress and addiction (Cui et al., 2013; Koob, 2013; Spanagel, Noori, & Heilig, 2014; Taylor et al., 2014). Stress has been found to be associated with a change in eating patterns, including binging episodes and craving highly palatable foods (Sinha & Jastreboff, 2013). Negative affect and stress may also represent withdrawal in individuals with FA when consumption of highly palatable foods stops; thus, leading to increased motivation to continue addictive eating habits in an effort to mitigate the negative symptoms (Avena, Bocarsly, Hoebel, & Gold, 2011; Avena, Bocarsly, Rada, Kim, & Hoebel, 2008). This process is consistent with other substance addictions that maintain the addictive cycle through engagement in the addictive behavior, leading to satiation and tolerance, withdrawals and cravings, and continued use of the substance (Sussman & Sussman, 2011).
2.4 Food Cues
Mood states may differentially impact attention given to food cues in those with and without FA (Frayn, Sears, & von Ranson, 2016). Frayn et al. (2016) found that after inducing a sad mood, those with diagnosed FA (via YFAS) showed increased attention to unhealthy food cues compared to healthy controls. Functional neuroimaging studies consistently report that substance-related cues increase activation of reward networks involving the dorsolateral prefrontal cortex, orbitofrontal cortex, anterior cingulate cortex, amygdala, insula, and striatum and in addicted vs healthy subjects, assumed to contribute to continued use and relapse (Franklin et al., 2007; McBride, Barrett, Kelly, Aw, & Dagher, 2006). Conversely, when the desired substance is used, it typically leads to decreased reward circuit activation (Martinez et al., 2005, 2007; Volkow et al., 1997). This suggests that addicted individuals relative to healthy controls place a higher reward value on substance-related cues, but experience a lower level of satiation when the substance is used.
There is some indication that similar patterns exist in obese individuals when responding to food cues: these individuals show greater activation in the left dorsomedial prefrontal cortex, orbitofrontal cortex, right precentral gyrus, anterior cingulate cortex, amygdala, striatum, mediodorsal thalamus, and right parahippocampal gyrus (Brooks, Cedernaes, & Schioth, 2013; McBride et al., 2006). Like other drug-addicted individuals, those who are obese show less dorsal striatal and medial orbitofrontal cortex activation during the consumption of their “drug of choice” (Stice, Spoor, Bohon, & Small, 2008; Stice, Spoor, Bohon, Veldhuizen, & Small, 2008). As obesity is commonly associated with FA, it was hypothesized that similar activation patterns would be found in individuals who were diagnosed with a FA. Gearhardt, Yokum, et al. (2011) found that individuals who scored high on the YFAS (endorsed four or more items) showed activation in the left anterior cingulate cortex, left medial orbitofrontal cortex, and left amygdala when presented with a highly palatable food cue (ie, a chocolate milkshake solution administered through a syringe into the mouth of the subject while in the scanner), while those with low YFAS scores did not. Furthermore, the FA group showed decreased activation in the lateral orbitofrontal cortex during consumption of highly palatable food (Gearhardt, Yokum, et al., 2011). These results mirror those reported in research on other substance addictions, as well as those seen among obese individuals (Brooks et al., 2013). This provides evidence for a neurobiological mechanism underlying reward circuitry activation in individuals with FA.
2.5 Craving
Individuals with a high affinity for palatable foods have an increase in the strength of cravings when exposed to food cues (Stojek, Fischer, & MacKillop, 2015). Food craving for sweet or carbohydrate-rich foods was found to be a partial mediator between addictive eating and both elevated BMI and binge-eating episodes (Joyner, Gearhardt, & White, 2015), while cravings for high-fat foods appeared to mediate the relationship between addictive eating and elevated BMI (Joyner et al., 2015). Craving is a dynamic process, engaging reward, emotional, salience, self-referential, executive control, and memory networks (Ekhtiari, Nasseri, Yavari, Mokri, & Monterosso, 2016). A resting-state fMRI study showed increased functional connectivity between striatal reward network regions and self-referential default mode network regions (medial prefrontal cortex, posterior cingulate cortex, and angular gyrus), insula, and somatosensory cortex in overweight subjects (BMI > 25) relative to healthy controls. In addition, food-craving scores were positively correlated with the increased functional connectivity in the dorsal striatum (Contreras-Rodriguez, Martin-Perez, Vilar-Lopez, & Verdejo-Garcia, 2015). As suggested by the authors, the presence of increased connectivity in the dorsal striatal network observed in obese, but not normal weight subjects, provide evidence for neural adaptations in brain reward circuitry that may contribute to addictive eating. In addition to the striatum, brain reward regions usually associated with food cravings include the ventral tegmental area, nucleus accumbens, amygdala, and hippocampus (Frankort et al., 2014). Subjects given a monotonous diet for 1.5 days, consisting only of the complete nutrition drink “Boost,” had greater brain activation in the insula, caudate, and hippocampus in response to cues of their favorite foods vs the monotonous food, ie, “Boost” (Pelchat, Johnson, Chan, Valdez, and Ragland (2004). The brain regions observed during cravings for favored foods overlapped with those seen for drug cravings, indicating that activation patterns of reward circuitry related to cravings may be similar in both FA and drug addiction (Volkow, Fowler, Wang, & Goldstein, 2002).
2.6 Impulsivity
Impulsivity, defined as an unplanned response to internal or external stimuli, without prior forethought and a disregard for potential negative consequences (Bari & Robbins, 2013), may account for reward-seeking behavior, and is associated with higher rates of relapse among addicted individuals (Doran, Spring, McChargue, Pergadia, & Richmond, 2004; Miller, 1991). Impulsivity includes difficulties with response inhibition and the inability to delay gratification, as evidenced by choosing an immediate reward over a long-term benefit (Winstanley, Eagle, & Robbins, 2006). Higher levels of impulsivity may be associated with hedonic eating (ie, eating after energy requirements have been met) patterns (Nederkoorn, Smulders, Havermans, Roefs, & Jansen, 2006). Impairments in impulse control mechanisms contribute to many disorders, including binge eating, bulimia nervosa, drug addiction, alcoholism, and Internet gaming (Alhassoon, Sorg, Stern, Hall, & Wollman, 2015; Chen et al., 2016; Ding et al., 2014; Gearhardt, Boswell, & Potenza, 2014; Goldstein & Volkow, 2011).
Studies indicate that aberrant activities in regions such as the prefrontal cortex, anterior cingulate cortex, inferior frontal gyrus, and orbitofrontal cortex may be accompanied with impulsive behaviors (Davids et al., 2010; Tang, Posner, Rothbart, & Volkow, 2015; Uher et al., 2004). In a delay discounting task study, decreased activation of executive brain regions (ie, frontal gyri and inferior parietal lobule) in obese subjects was correlated to increased impulsivity as well as future weight gain (Kishinevsky et al., 2012; Stoeckel, Murdaugh, Cox, Cook, & Weller, 2013). These results suggest deficits of inhibitory functions in obese subjects. In addition, other self-regulatory control studies showed that people with bulimia nervosa had increased impulsivity with abnormal anterior cingulate and frontal cortical engagement (Marsh et al., 2011, 2009). Additional results suggest that those with disordered eating may have dysfunctional frontostriatal systems leading to a loss of control over feeding behavior. Deficient executive control, associated with impulsivity, as well as dysregulated craving and reward circuitry, may contribute to FA.
3. MAINTENANCE OF FA
3.1 Food Reward and Motivation
The mechanisms that contribute to maintaining FA are hypothesized to be similar to those in alcohol and illicit substance addictions (Avena et al., 2011; DiLeone, Taylor, & Picciotto, 2012; Hone-Blanchet & Fecteau, 2014). Similarities are primarily related to the effects these addictions have on the brain reward circuit (Parylak et al., 2011; Val-Laillet et al., 2015; Ziauddeen et al., 2015). The dopaminergic mesolimbic reward pathway connects the ventral tegmental area to the nucleus accumbens and is associated with positive reinforcement, food reward, and addiction (Volkow, Wang, Fowler, Tomasi, & Baler, 2012). Animal studies have shown that food has rewarding properties and can increase the firing rate of dopamine neurons and the release of dopamine in the nucleus accumbens (Hernandez & Hoebel, 1988; Norgren, Hajnal, & Mungarndee, 2006). Consumption of foods high in sugar content triggers the release of endogenous opioids in the nucleus accumbens, thus activating the dopaminergic reward system (Lerma-Cabrera, Carvajal, & Lopez-Legarrea, 2016). This mirrors the effects of other highly addictive substances, such as cocaine (Blum, Thanos, & Gold, 2014) and speaks to the addictive potential of specific types of foods.
Substance addictions also show evidence of reward deficiency syndrome (Blum et al., 2000), a neural adaptation in response to continued exposure to a substance (or behavior, eg, gambling), that occurs when repeated engagement stimulates reward mechanisms so powerfully that the population of dopamine D2 receptors (D2R) declines (Benton & Young, 2016; Blum et al., 2000, 2014; Gyollai et al., 2014). Due to this decrease in D2R, increased intake is necessary in order to elicit the same degree of reward that was previously achieved through a lower dose (Blum et al., 2000). A further indication of the reward deficiency syndrome is the presence of intense cravings and withdrawal symptoms (Benton & Young, 2016). Similar reductions in D2Rs are hypothesized to occur in FA.
Benton and Young (2016) performed a meta-analysis to determine the relationship between BMI and the presence of Taq1A polymorphism, an A1 allele found to be associated with a lower number of D2R in both healthy and alcoholic subjects (Jonsson et al., 1999; Noble, Blum, Ritchie, Montgomery, & Sheridan, 1991; Pohjalainen et al., 1998). They found no significant effects of the A1 allele on BMI, and therefore concluded that there was no support for the reward deficiency theory of FA (Benton & Young, 2016). However, another study reported a negative correlation between BMI and the dorsal striatal response (left caudate, bilateral putamen) to a taste of chocolate milkshake vs water (Stice, Spoor, Bohon, et al., 2008): the presence of the A1 allele significantly moderated the negative correlation between BMI and left caudate activation. While additional studies are necessary to evaluate the reward deficiency hypothesis in FA, these results do demonstrate differing response levels in brain reward circuits related to consumption of highly palatable food in those with low vs high BMI’s (Stice, Spoor, Bohon, et al., 2008). Specifically, the experience of reward from consuming food may not be as strong for those with higher BMI’s, which may lead to overeating in an attempt to compensate for a dampened reward system.
3.2 Energy Homeostasis and Regulatory Mechanisms
Food intake is necessary for normal bodily energy expenditure, and appetite is controlled by complex neuronal and hormonal systems including the hypothalamus and peripheral satiety networks (Behary & Miras, 2014; Camilleri, 2015; Joly-Amado et al., 2014). Through a process known as “adiposity negative feedback,” circulating signals from the body inform the brain of current energy stores. In response, the brain adjusts food intake (Kennedy, 1953). The hypothalamus is located directly below the thalamus in the limbic system and is responsible for a wide variety of regulatory functions, including body temperature, hunger, thirst, and the sleep–wake cycle (Snell, 2010). The peripheral satiety system includes hormonal inputs from the pancreas, liver, and adipose tissue (Morton, Meek, & Schwartz, 2014). Leptin is an adipocyte hormone that decreases appetite (as part of a negative feedback system) by acting on hypothalamic neurons that regulate energy homeostasis (Benoit, Clegg, Seeley, & Woods, 2004). Insulin, a pancreatic hormone much like leptin that it is sensitive to the amount of stored body fat, the levels of which increase when body fat is high and decrease when body fat is low, can also reduce appetite (Figlewicz, 2003). Ghrelin is a gustatory hormone that works to increase appetite (Chaudhri, Small, & Bloom, 2006; Kawahara et al., 2013; Wren & Bloom, 2007). It is believed that adiposity negative feedback works by increasing the brain’s sensitivity to satiety signals (Morton et al., 2014). For example, as weight loss lowers plasma levels of leptin and insulin, the satiating effect of food is decreased. Conversely, as weight increases, these hormone levels also increase, producing a higher sensitivity to satiety signals (Hulsey, Lu, Wang, Martin, & Baile, 1998; Kahler et al., 1998). Communication between the hypothalamus and brain reward circuitry regulate these appetitive responses. For example, the lateral hypothalamic area receives information from the nucleus accumbens, allowing for the integration of information from reward brain regions and satiety signals from other areas of the central and peripheral nervous systems (Morton et al., 2005). This information is then projected to areas such as the hindbrain and the nucleus of the solitary tract, which are responsible for regulating satiety (Grill et al., 2002).
While leptin, insulin, and ghrelin work within the adiposity negative feedback system, other gut peptides are also involved in the perception of satiety and act as signals to increase or decrease appetite (Morton et al., 2014). Peptides that are implicated in reducing appetite include peptide YY (Batterham & Bloom, 2003), glucagon-like peptide 1 (van Bloemendaal, Ten Kulve, la Fleur, Ijzerman, & Diamant, 2014), and cholecystokinin (D’Agostino et al., 2016), and are secreted in response to food ingestion (Wren & Bloom, 2007). Mechanoreceptors located in the smooth muscle layer of the gut are also involved in sending satiety signals to the central nervous system, via afferent fibers along the vagus nerve that project to the nucleus of the solitary tract in the caudal hindbrain (Chaudhri et al., 2006; Morton et al., 2014). Other important components of appetite regulation include agouti-related protein receptors, located in the hypothalamic arcuate nucleus, which release neuropeptide Y, agouti-related peptide, and the neurotransmitter γ-aminobutyric acid (Krashes, Shah, Koda, & Lowell, 2013). Together, these work to promote appetite, when activated by ghrelin (Shrestha, Wickwire, & Giraudo, 2006), and are inhibited by insulin and leptin (Varela & Horvath, 2012).
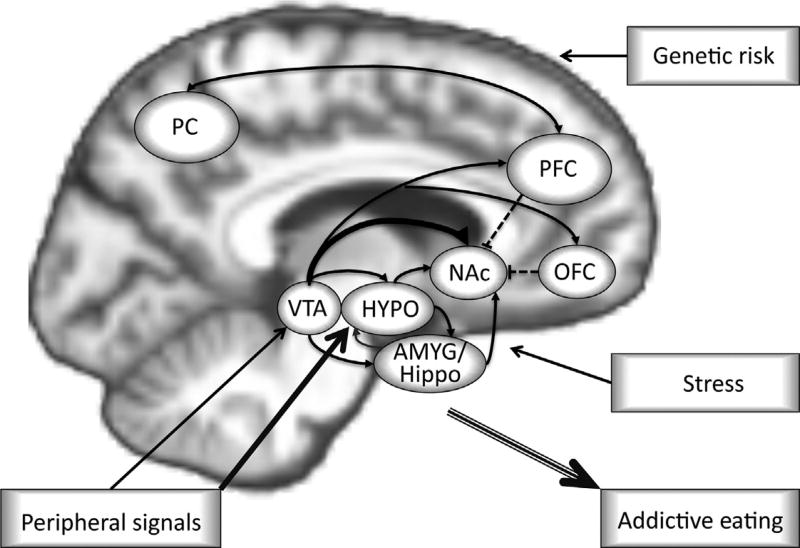
Hypothalamic centers and peripheral networks strive to maintain homeostatic eating, defined as a state of equilibrium between the amount of energy taken in through food consumption and the metabolic demands of the body (Farr, Li, & Mantzoros, 2016). Over time, highly palatable foods may disrupt these appetite regulatory mechanisms (de Macedo et al., 2016; Johnson & Wardle, 2014) and promote hedonic eating (Lerma-Cabrera et al., 2016; Pandit, Mercer, Overduin, la Fleur, & Adan, 2012). Specifically, there is evidence that highly palatable foods can dampen satiety signals, thus leading to overeating. This is further compounded by increased activation of the reward system, which turns eating into a reward-driven behavior instead of a homeostatic process (Erlanson-Albertsson, 2005) (Fig. 1).
Fig. 1.
Model of food addiction as a result of highly palpable food intake, stress, genetic risk, and overlapping circuits of the brain interacting with peripheral signals. The hypothalamus (HYPO) is part of the hypothalamic–pituitary–adrenal (HPA) axis, sensitive to stress, and critical for food intake. It is modulated by the ventral tegmental area (VTA) and amygdala (AMYG). In food addiction the strength of inhibitory control provided via orbitofrontal (OFC), prefrontal (PFC), and parietal cortices (PC) (dashed lines) is weakened and together with heightened food craving and seeking via reward VTA and nucleus accumbens (NAc) and addiction memory via limbic regions (AMYG/Hippocampus), and malfunctioning peripheral signals for appetite regulation (satiety), becomes a continued cycle of food addiction. Adapted from DiLeone, R. J., Taylor, J. R., & Picciotto, M. R. (2012). The drive to eat: Comparisons and distinctions between mechanisms of food reward and drug addiction. Nature Neuroscience, 15 (10), 1330–1335. http://dx.doi.org/10.1038/nn.3202.
Prader Willi syndrome (PWS), a genetic disease that causes hypothalamic dysfunction, results in hyperphagia, obesity, stunted growth, and sleep abnormalities (Di Lorenzo, Sberveglieri, Marrama, Landi, & Ferri, 2016). Of particular interest to FA is the fact that those with PWS suffer from an insatiable appetite that often leads to overeating (McAllister, Whittington, & Holland, 2011). In individuals with PWS, the consumption of high calorie food is associated with significantly elevated activation of the orbitofrontal cortex and hypothalamus (Dimitropoulos & Schultz, 2008; Key & Dykens, 2008). In contrast to what is observed in healthy individuals—a decrease in ghrelin after a meal, terminating the motivation to consume more food (Chaudhri et al., 2006; Wren & Bloom, 2007)— those with PWS have increased ghrelin levels both before and after meals leading to overeating past the point of homeostatic energy demands (Feigerlova et al., 2008). Individuals with PWS are also likely to suffer from comorbid conditions, including anxiety and mood disorders (Hiraiwa, Maegaki, Oka, & Ohno, 2007), which as previously discussed are also associated with addictive eating (Singh, 2014).
3.3 The Impact of Stress on Hypothalamic Functioning
Chronic stress can independently contribute to addictive eating behaviors (Torres & Nowson, 2007). Recent models explaining the neuroadaptive consequences of acute and chronic stress responses now help explain the association between stress, addiction, and abnormal eating behaviors (Sinha & Jastreboff, 2013). Two systems, including the hypothalamic– pituitary–adrenal axis (HPA) and the autonomic nervous system, are responsible for physiological responses to acute stress (McEwen, 2007; Sinha, 2008). The HPA axis response commences with the release of corticotropin-releasing factor (CRF) from the paraventricular nucleus of the hypothalamus, which then triggers a release of adrenocorticotropin hormone (ACTH) from the anterior pituitary, resulting in the peripheral secretion of glucocorticoids, also known as cortisol or corticosterone, from the adrenal glands (McEwen, 2007). The release of CRF and ACTH helps with gluco-neogenesis (Sinha & Jastreboff, 2013), which is a metabolic pathway responsible for generating glucose from noncarbohydrate substrates (Berg, Tymoczko, & Stryer, 2002). These hormones also help with energy mobilization (Dallman, Akana, Strack, Hanson, & Sebastian, 1995), which is the process of directing and prioritizing stored energy, in the form of glucose, to parts of the body where it is most needed (Duffy, 1951). The release of CRF and ACTH during acute stress is terminated by glucocorticoid negative feedback, thereby allowing the systems to return to homeostasis (Dallman et al., 1995; Sinha & Jastreboff, 2013). Evidence from animal models indicates that during acute stress, food consumption decreases (Marti, Marti, & Armario, 1994), a result that has been replicated in human studies (Dallman, Pecoraro, & la Fleur, 2005).
After periods of prolonged and uncontrollable stress, the HPA axis can become dysregulated, leading to changes in the release patterns of hormones, neuropeptides, and glucocorticoids (Lupien, McEwen, Gunnar, & Heim, 2009; McEwen, 2007). Specifically, HPA axis dysregulation can cause insulin resistance due to altered glucose metabolism and can lead to excess abdominal fat (Wilcox, 2005). As levels of plasma insulin and abdominal fat increase, HPA axis activity decreases, and an increased intake of highly palatable foods is observed (Chrousos, 2000; Dallman et al., 2005; Tataranni et al., 1996; Warne, 2009). Stress-induced hypothalamic dysregulation may help explain the maintenance of FA (Berenson et al., 2015; Frayn et al., 2016; Micanti et al., 2016) (Fig. 1).
3.4 Cognitive Control and Addictive Eating
Cognitive control regions interact with reward and emotional systems, and act as top-down regulators to reorient attentional resources, make decisions, appraise rewards, and initiate or inhibit motivation to consume food (Ekhtiari, Faghiri, Oghabian, & Paulus, 2016; Schulte, Grilo, & Gearhardt, 2016; Spechler et al., 2016; Val-Laillet et al., 2015; Zilverstand, Parvaz, Moeller, & Goldstein, 2016). In obese populations, the consumption of highly palatable foods can lead to downregulation of the reward network, and deficiencies in cortical top-down regulation of eating behavior (Volkow, Wang, & Baler, 2011). In FA, the strength of inhibitory control provided via orbitofrontal, prefrontal, and parietal cortices is weakened and together with heightened food craving and seeking via the ventral tegmental area and nucleus accumbens, addiction memory via limbic regions (amygdala and hippocampus), and malfunctioning peripheral signals for appetite regulation (satiety), this becomes a vicious cycle (DiLeone et al., 2012) (see Fig. 1 for model).
4. DISCUSSION
The concept of “food addiction” is relatively novel, requires a clear definition, additional research, and a general consensus (Albayrak, Wolfle, & Hebebrand, 2012; Hebebrand et al., 2014; Ziauddeen et al., 2012; Ziauddeen & Fletcher, 2013). Proponents of the concept suggest that similarities between FA and other substance addictions, in particular, operant conditioning, effects of mood and stress, cue reactivity, craving, and impulsivity support food as an addictive substance (Ahmed et al., 2014; Berenson et al., 2015; Frayn et al., 2016; Kishinevsky et al., 2012; Pelchat et al., 2004). There is also some neural evidence supporting food as an addiction because of observations of downregulation of reward circuitry and difficulties with top-down cognitive control (Blum et al., 2014; Stice, Spoor, Bohon, et al., 2008; Volkow et al., 2003, 1997). Furthermore, animal studies show evidence of withdrawal symptoms in rats when access to sucrose solutions is terminated, as well as binge-like eating and dopaminergic dysregulation after removal of a high-fat diet (Avena et al., 2008; Carlin et al., 2016; Colantuoni et al., 2002). Critics of the FA hypothesis discuss an inability to pinpoint the exact “substance” that is responsible for the neuroaddictive responses (Ziauddeen & Fletcher, 2013). The types of foods involved in addictive eating are varied and even within one type of food, ie, carbohydrates, there are multiple chemicals and components (Ziauddeen et al., 2012; Ziauddeen & Fletcher, 2013). Moreover, those who deny the existence of FA claim there is not currently enough evidence that FA resembles other substance addictions from a neurological standpoint (Benton & Young, 2016; Ziauddeen & Fletcher, 2013).
However, there is evidence to support the notion that FA is an addiction according to the criteria put forth by Sussman and Sussman (2011). FA includes eating due to appetitive effects, preoccupation with food, temporary satiation, loss of control, and suffering negative consequences due to eating behaviors (Gearhardt, Boswell, & White, 2014; Gearhardt et al., 2016; Gearhardt, Yokum, et al., 2011; Joyner et al., 2015; Schulte, Grilo, & Gearhardt, 2016). FA does not, however, map perfectly onto a substance addiction model as purported in the DSM-5. Part of the problem is one of nomenclature: Hebebrand et al. (2014) suggest that “eating addiction” may better describe problematic hedonic eating than “food addiction,” and in this sense, an eating addiction may be more similar to gambling disorder (American Psychiatric Association, 2013b). Gambling disorders have been found to affect brain reward circuitry in a manner similar to substance addiction, much like what is seen with FA. However, there is no “substance” causing the addiction (Blum et al., 2000; Gyollai et al., 2014).
While there may be considerable overlap with other abnormal eating patterns, there is a need to properly define FA criteria so as to better create representative experimental groups that will provide information on this condition in particular. The YFAS 2.0 has attempted to provide a framework for the diagnosis and grouping of individuals with FA. However, if FA were considered a behavioral addiction instead of a substance addiction, then the assessment would benefit from following a model more closely related to behavioral addictions in the DSM-5, such as gambling disorder, rather than the current model, which maps onto substance disorder criteria (American Psychiatric Association, 2013b; Hebebrand et al., 2014). Furthermore, the high prevalence of comorbid mental health disorders among addicted individuals, particularly involving mood dysregulation, may provide false positives on the YFAS 2.0 because many people eat in stressful situations or use eating to cope with negative affect (Albayrak et al., 2012). Efforts to disentangle FA from other eating disorders or health consequences related to eating, such as obesity and its associated negative influences on health, would help lend more credence to the concept of FA as a discrete condition.
Acknowledgments
We thank Eden Gallanter and Eva M. Müller-Oehring for their critical review and comments on this manuscript. NIH Grant R01 AA023165 funded this work.
Footnotes
The authors declare no conflict of interest.
References
- Ahmed S, Kashem MA, Sarker R, Ahmed EU, Hargreaves GA, McGregor IS. Neuroadaptations in the striatal proteome of the rat following prolonged excessive sucrose intake. Neurochemical Research. 2014;39(5):815–824. doi: 10.1007/s11064-014-1274-6. http://dx.doi.org/10.1007/s11064-014-1274-6. [DOI] [PubMed] [Google Scholar]
- Albayrak O, Wolfle SM, Hebebrand J. Does food addiction exist? A phenomenological discussion based on the psychiatric classification of substance-related disorders and addiction. Obesity Facts. 2012;5(2):165–179. doi: 10.1159/000338310. http://dx.doi.org/10.1159/000338310. [DOI] [PubMed] [Google Scholar]
- Alhassoon OM, Sorg SF, Stern MJ, Hall MG, Wollman SC. Neuro-imaging in alcohol-use disorders: Clinical implications and future directions. Future Neurology. 2015;10(4):345–356. http://dx.doi.org/10.2217/fnl.15.17. [Google Scholar]
- American Psychiatric Association. Feeding and eating disorders diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Association; 2013a. [Google Scholar]
- American Psychiatric Association. Substance-related and addictive disorders diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Association; 2013b. [Google Scholar]
- Avena NM, Bocarsly ME, Hoebel BG, Gold MS. Overlaps in the nosology of substance abuse and overeating: The translational implications of “food addiction”. Current Drug Abuse Reviews. 2011;4(3):133–139. doi: 10.2174/1874473711104030133. [DOI] [PubMed] [Google Scholar]
- Avena NM, Bocarsly ME, Rada P, Kim A, Hoebel BG. After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/ acetylcholine imbalance. Physiology & Behavior. 2008;94(3):309–315. doi: 10.1016/j.physbeh.2008.01.008. http://dx.doi.org/10.1016/j.physbeh.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: Behavioral and neural basis of response control. Progress in Neurobiology. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. http://dx.doi.org/10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Bloom SR. The gut hormone peptide YY regulates appetite. Annals of the New York Academy of Sciences. 2003;994:162–168. doi: 10.1111/j.1749-6632.2003.tb03176.x. [DOI] [PubMed] [Google Scholar]
- Behary P, Miras AD. Brain responses to food and weight loss. Experimental Physiology. 2014;99(9):1121–1127. doi: 10.1113/expphysiol.2014.078303. http://dx.doi.org/10.1113/expphysiol.2014.078303. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin as adiposity signals. Recent Progress in Hormone Research. 2004;59:267–285. doi: 10.1210/rp.59.1.267. [DOI] [PubMed] [Google Scholar]
- Benton D, Young HA. A meta-analysis of the relationship between brain dopa-mine receptors and obesity: A matter of changes in behavior rather than food addiction? International Journal of Obesity (London) 2016;40(Suppl. 1):S12–S21. doi: 10.1038/ijo.2016.9. http://dx.doi.org/10.1038/ijo.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson AB, Laz TH, Pohlmeier AM, Rahman M, Cunningham KA. Prevalence of food addiction among low-income reproductive-aged women. Journal of Women’s Health (2002) 2015;24(9):740–744. doi: 10.1089/jwh.2014.5182. http://dx.doi.org/10.1089/jwh.2014.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JM, Tymoczko JL, Stryer L. Biochemistry, glycolysis and gluconeogenesis. New York: WH Freeman; 2002. Retrieved from. http://www.ncbi.nlm.nih.gov/books/NBK21150/ [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Comings DE. Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. Journal of Psychoactive Drugs. 2000;32(Suppl. i–iv):1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Blum K, Thanos PK, Gold MS. Dopamine and glucose, obesity, and reward deficiency syndrome. Frontiers in Psychology. 2014;5:919. doi: 10.3389/fpsyg.2014.00919. http://dx.doi.org/10.3389/fpsyg.2014.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, Cedernaes J, Schioth HB. Increased prefrontal and para-hippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: A meta-analysis of fMRI studies. PloS One. 2013;8(4):e60393. doi: 10.1371/journal.pone.0060393. http://dx.doi.org/10.1371/journal.pone.0060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M. Peripheral mechanisms in appetite regulation. Gastroenterology. 2015;148(6):1219–1233. doi: 10.1053/j.gastro.2014.09.016. http://dx.doi.org/10.1053/j.gastro.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin JL, McKee SE, Hill-Smith T, Grissom NM, George R, Lucki I, Reyes TM. Removal of high-fat diet after chronic exposure drives binge behavior and dopaminergic dysregulation in female mice. Neuroscience. 2016;326:170–179. doi: 10.1016/j.neuroscience.2016.04.002. http://dx.doi.org/10.1016/j.neuroscience.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philosophical Transactions of the Royal Society of London. Series B. Biological Sciences. 2006;361(1471):1187–1209. doi: 10.1098/rstb.2006.1856. http://dx.doi.org/10.1098/rstb.2006.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Yen JY, Wang PW, Liu GC, Yen CF, Ko CH. Altered functional connectivity of the insula and nucleus accumbens in internet gaming disorder: A resting state fMRI study. European Addiction Research. 2016;22(4):192–200. doi: 10.1159/000440716. http://dx.doi.org/10.1159/000440716. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The stress response and immune function: Clinical implications. The 1999 Novera H. Spector lecture. Annals of the New York Academy of Sciences. 2000;917:38–67. doi: 10.1111/j.1749-6632.2000.tb05371.x. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obesity Research. 2002;10(6):478–488. doi: 10.1038/oby.2002.66. http://dx.doi.org/10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Contreras-Rodriguez O, Martin-Perez C, Vilar-Lopez R, Verdejo-Garcia A. Ventral and dorsal striatum networks in obesity: Link to food craving and weight gain. Biological Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.11.020. http://dx.doi.org/10.1016/j.biopsych.2015.11.020 (Epub ahead of print) [DOI] [PubMed]
- Cui C, Noronha A, Morikawa H, Alvarez VA, Stuber GD, Szumlinski KK, Wilcox MV. New insights on neurobiological mechanisms underlying alcohol addiction. Neuropharmacology. 2013;67:223–232. doi: 10.1016/j.neuropharm.2012.09.022. http://dx.doi.org/10.1016/j.neuropharm.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino G, Lyons DJ, Cristiano C, Burke LK, Madara JC, Campbell JN, Heisler LK. Appetite controlled by a cholecystokinin nucleus of the solitary tract to hypothalamus neurocircuit. Elife. 2016;5:e12225. doi: 10.7554/eLife.12225. http://dx.doi.org/10.7554/eLife.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Strack AM, Hanson ES, Sebastian RJ. The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at a site proximal to CRF neurons. Annals of the New York Academy of Sciences. 1995;771:730–742. doi: 10.1111/j.1749-6632.1995.tb44724.x. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: Self-medication and abdominal obesity. Brain, Behavior, and Immunity. 2005;19(4):275–280. doi: 10.1016/j.bbi.2004.11.004. http://dx.doi.org/10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Davids S, Lauffer H, Thoms K, Jagdhuhn M, Hirschfeld H, Domin M, Lotze M. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. International Journal of Obesity. 2010;34(1):94–104. doi: 10.1038/ijo.2009.193. http://dx.doi.org/10.1038/ijo.2009.193. [DOI] [PubMed] [Google Scholar]
- Davis C, Curtis C, Levitan RD, Carter JC, Kaplan AS, Kennedy JL. Evidence that ‘food addiction’ is a valid phenotype of obesity. Appetite. 2011;57(3):711–717. doi: 10.1016/j.appet.2011.08.017. http://dx.doi.org/10.1016/j.appet.2011.08.017. [DOI] [PubMed] [Google Scholar]
- de Macedo IC, de Freitas JS, da Silva Torres IL. The influence of palatable diets in reward system activation: A mini review. Advances in Pharmacological Sciences. 2016;2016:7238679. doi: 10.1155/2016/7238679. http://dx.doi.org/10.1155/2016/7238679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo R, Sberveglieri S, Marrama D, Landi G, Ferri P. Weight control and behavior rehabilitation in a patient suffering from Prader Willi syndrome. BMC Research Notes. 2016;9(1):199. doi: 10.1186/s13104-016-1981-y. http://dx.doi.org/10.1186/s13104-016-1981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLeone RJ, Taylor JR, Picciotto MR. The drive to eat: Comparisons and distinctions between mechanisms of food reward and drug addiction. Nature Neuroscience. 2012;15(10):1330–1335. doi: 10.1038/nn.3202. http://dx.doi.org/10.1038/nn.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitropoulos A, Schultz RT. Food-related neural circuitry in Prader-Willi syndrome: Response to high- versus low-calorie foods. Journal of Autism and Developmental Disorders. 2008;38(9):1642–1653. doi: 10.1007/s10803-008-0546-x. http://dx.doi.org/10.1007/s10803-008-0546-x. [DOI] [PubMed] [Google Scholar]
- Ding WN, Sun JH, Sun YW, Chen X, Zhou Y, Zhuang ZG, Du YS. Trait impulsivity and impaired prefrontal impulse inhibition function in adolescents with internet gaming addiction revealed by a Go/No-Go fMRI study. Behavioral and Brain Functions. 2014;10:20. doi: 10.1186/1744-9081-10-20. http://dx.doi.org/10.1186/1744-9081-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, Spring B, McChargue D, Pergadia M, Richmond M. Impulsivity and smoking relapse. Nicotine & Tobacco Research. 2004;6(4):641–647. doi: 10.1080/14622200410001727939. http://dx.doi.org/10.1080/14622200410001727939. [DOI] [PubMed] [Google Scholar]
- Duffy E. The concept of energy mobilization. Psychological Review. 1951;58(1):30–40. doi: 10.1037/h0054220. [DOI] [PubMed] [Google Scholar]
- Ekhtiari H, Faghiri A, Oghabian M-A, Paulus MP. Functional neuroimaging for addiction medicine: From mechanisms to practical considerations. In: Hamed E, Martin PP, editors. Progress in Brain Research. Vol. 224. Cambridge, MA: Elsevier; 2016. pp. 129–153. chapter 7. [DOI] [PubMed] [Google Scholar]
- Ekhtiari H, Nasseri P, Yavari F, Mokri A, Monterosso J. Neuroscience of drug craving for addiction medicine: From circuits to therapies. In: Hamed E, Martin P, editors. Progress in brain research. Vol. 223. Cambridge, MA: Elsevier; 2016. pp. 115–141. chapter 7. [DOI] [PubMed] [Google Scholar]
- Erlanson-Albertsson C. How palatable food disrupts appetite regulation. Basic & Clinical Pharmacology & Toxicology. 2005;97(2):61–73. doi: 10.1111/j.1742-7843.2005.pto_179.x. http://dx.doi.org/10.1111/j.1742-7843.2005.pto_179.x. [DOI] [PubMed] [Google Scholar]
- Farr OM, Li CS, Mantzoros CS. Central nervous system regulation of eating: Insights from human brain imaging. Metabolism. 2016;65(5):699–713. doi: 10.1016/j.metabol.2016.02.002. http://dx.doi.org/10.1016/j.metabol.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigerlova E, Diene G, Conte-Auriol F, Molinas C, Gennero I, Salles JP, Tauber M. Hyperghrelinemia precedes obesity in Prader-Willi syndrome. The Journal of Clinical Endocrinology and Metabolism. 2008;93(7):2800–2805. doi: 10.1210/jc.2007-2138. http://dx.doi.org/10.1210/jc.2007-2138. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP. Adiposity signals and food reward: Expanding the CNS roles of insulin and leptin. American Journal of Physiology. Regulatory. Integrative and Comparative Physiology. 2003;284(4):R882–R892. doi: 10.1152/ajpregu.00602.2002. http://dx.doi.org/10.1152/ajpregu.00602.2002. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: A perfusion fMRI study. Neuropsychopharmacology. 2007;32(11):2301–2309. doi: 10.1038/sj.npp.1301371. http://dx.doi.org/10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Frankort A, Roefs A, Siep N, Roebroeck A, Havermans R, Jansen A. The craving stops before you feel it: Neural correlates of chocolate craving during cue exposure with response prevention. Cerebral Cortex. 2014;24(6):1589–1600. doi: 10.1093/cercor/bht016. http://dx.doi.org/10.1093/cercor/bht016. [DOI] [PubMed] [Google Scholar]
- Frayn M, Sears CR, von Ranson KM. A sad mood increases attention to unhealthy food images in women with food addiction. Appetite. 2016;100:55–63. doi: 10.1016/j.appet.2016.02.008. http://dx.doi.org/10.1016/j.appet.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Boswell RG, Potenza MN. Neuroimaging of eating disorders, substance used is orders, and addictions: Overlapping and unique systems. In: Brewerton DT, Dennis A Baker, editors. Eating disorders, addictions and substance use disorders: Research, clinical and treatment perspectives. Berlin, Heidelberg: Springer; 2014a. pp. 71–89. [Google Scholar]
- Gearhardt AN, Boswell RG, White MA. The association of “food addiction” with disordered eating and body mass index. Eating Behaviors. 2014b;15(3):427–433. doi: 10.1016/j.eatbeh.2014.05.001. http://dx.doi.org/10.1016/j.eatbeh.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Corbin WR, Brownell KD. Development of the Yale Food Addiction Scale Version 2.0. Psychology of Addictive Behaviors. 2016;30(1):113–121. doi: 10.1037/adb0000136. http://dx.doi.org/10.1037/adb0000136. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Davis C, Kuschner R, Brownell KD. The addiction potential of hyperpalatable foods. Current Drug Abuse Reviews. 2011;4(3):140–145. doi: 10.2174/1874473711104030140. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Grilo CM, DiLeone RJ, Brownell KD, Potenza MN. Can food be addictive? Public health and policy implications. Addiction. 2011;106(7):1208–1212. doi: 10.1111/j.1360-0443.2010.03301.x. http://dx.doi.org/10.1111/j.1360-0443.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural correlates of food addiction. Archives of General Psychiatry. 2011;68(8):808–816. doi: 10.1001/archgenpsychiatry.2011.32. http://dx.doi.org/10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geliebter A, Aversa A. Emotional eating in overweight, normal weight, and underweight individuals. Eating Behaviors. 2003;3(4):341–347. doi: 10.1016/s1471-0153(02)00100-9. [DOI] [PubMed] [Google Scholar]
- Giuliani NR, Pfeifer JH. Age-related changes in reappraisal of appetitive cravings during adolescence. NeuroImage. 2015;108:173–181. doi: 10.1016/j.neuroimage.2014.12.037. http://dx.doi.org/10.1016/j.neuroimage.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, Wall M, Choo TH, Becker C, Neumark-Sztainer D. Shared risk factors for mood-, eating-, and weight-related health outcomes. Health Psychology. 2016;35(3):245–252. doi: 10.1037/hea0000283. http://dx.doi.org/10.1037/hea0000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nature Reviews. Neuroscience. 2011;12(11):652–669. doi: 10.1038/nrn3119. http://dx.doi.org/10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143(1):239–246. doi: 10.1210/endo.143.1.8589. http://dx.doi.org/10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- Gyollai A, Griffiths MD, Barta C, Vereczkei A, Urban R, Kun B, Demetrovics Z. The genetics of problem and pathological gambling: A systematic review. Current Pharmaceutical Design. 2014;20(25):3993–3999. doi: 10.2174/13816128113199990626. [DOI] [PubMed] [Google Scholar]
- Hebebrand J, Albayrak O, Adan R, Antel J, Dieguez C, de Jong J, Dickson SL. “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neuroscience and Biobehavioral Reviews. 2014;47:295–306. doi: 10.1016/j.neubiorev.2014.08.016. http://dx.doi.org/10.1016/j.neubiorev.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sciences. 1988;42(18):1705–1712. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- Hiraiwa R, Maegaki Y, Oka A, Ohno K. Behavioral and psychiatric disorders in Prader-Willi syndrome: A population study in Japan. Brain and Development. 2007;29(9):535–542. doi: 10.1016/j.braindev.2007.01.005. http://dx.doi.org/10.1016/j.braindev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Hone-Blanchet A, Fecteau S. Overlap of food addiction and substance use disorders definitions: Analysis of animal and human studies. Neuropharmacology. 2014;85:81–90. doi: 10.1016/j.neuropharm.2014.05.019. http://dx.doi.org/10.1016/j.neuropharm.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Huerta CI, Sarkar PR, Duong TQ, Laird AR, Fox PT. Neural bases of food perception: Coordinate-based meta-analyses of neuroimaging studies in multiple modalities. Obesity (Silver Spring) 2014;22(6):1439–1446. doi: 10.1002/oby.20659. http://dx.doi.org/10.1002/oby.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsey MG, Lu H, Wang T, Martin RJ, Baile CA. Intra-cerebroventricular (i.c.v.) administration of mouse leptin in rats: Behavioral specificity and effects on meal patterns. Physiology & Behavior. 1998;65(3):445–455. doi: 10.1016/s0031-9384(98)00180-2. [DOI] [PubMed] [Google Scholar]
- Jiang T, Soussignan R, Schaal B, Royet JP. Reward for food odors: An fMRI study of liking and wanting as a function of metabolic state and BMI. Social Cognitive and Affective Neuroscience. 2015;10(4):561–568. doi: 10.1093/scan/nsu086. http://dx.doi.org/10.1093/scan/nsu086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neuroscience. 2010;13(5):635–641. doi: 10.1038/nn.2519. http://dx.doi.org/10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F, Wardle J. Variety, palatability, and obesity. Advances in Nutrition. 2014;5(6):851–859. doi: 10.3945/an.114.007120. http://dx.doi.org/10.3945/an.114.007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly-Amado A, Cansell C, Denis RG, Delbes AS, Castel J, Martinez S, Luquet S. The hypothalamic arcuate nucleus and the control of peripheral substrates. Best Practice & Research. Clinical Endocrinology & Metabolism. 2014;28(5):725–737. doi: 10.1016/j.beem.2014.03.003. http://dx.doi.org/10.1016/j.beem.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall GC. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Molecular Psychiatry. 1999;4(3):290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Joyner MA, Gearhardt AN, White MA. Food craving as a mediator between addictive-like eating and problematic eating outcomes. Eating Behaviors. 2015;19:98–101. doi: 10.1016/j.eatbeh.2015.07.005. http://dx.doi.org/10.1016/j.eatbeh.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler A, Geary N, Eckel LA, Campfield LA, Smith FJ, Langhans W. Chronic administration of OB protein decreases food intake by selectively reducing meal size in male rats. The American Journal of Physiology. 1998;275(1 Pt. 2):R180–R185. doi: 10.1152/ajpregu.1998.275.1.R180. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Kaneko F, Yamada M, Kishikawa Y, Kawahara H, Nishi A. Food reward-sensitive interaction of ghrelin and opioid receptor pathways in mesolimbic dopamine system. Neuropharmacology. 2013;67:395–402. doi: 10.1016/j.neuropharm.2012.11.022. http://dx.doi.org/10.1016/j.neuropharm.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Kelly AC, Vimalakanthan K, Carter JC. Understanding the roles of self-esteem, self-compassion, and fear of self-compassion in eating disorder pathology: An examination of female students and eating disorder patients. Eating Behaviors. 2014;15(3):388–391. doi: 10.1016/j.eatbeh.2014.04.008. http://dx.doi.org/10.1016/j.eatbeh.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proceedings of the Royal Society of London - Series B: Biological Sciences. 1953;140(901):578–596. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- Key AP, Dykens EM. ‘Hungry eyes’: Visual processing of food images in adults with Prader-Willi syndrome. Journal of Intellectual Disability Research. 2008;52(Pt. 6):536–546. doi: 10.1111/j.1365-2788.2008.01062.x. http://dx.doi.org/10.1111/j.1365-2788.2008.01062.x. [DOI] [PubMed] [Google Scholar]
- Kishinevsky FI, Cox JE, Murdaugh DL, Stoeckel LE, Cook EW, 3rd, Weller RE. fMRI reactivity on a delay discounting task predicts weight gain in obese women. Appetite. 2012;58(2):582–592. doi: 10.1016/j.appet.2011.11.029. http://dx.doi.org/10.1016/j.appet.2011.11.029. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction. Toward the development of new therapies. Annals of the New York Academy of Sciences. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: Studies on the extended amygdala. European Neuropsychopharmacology. 2003;13(6):442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: Alcohol addiction as a reward deficit disorder. Current Topics in Behavioral Neurosciences. 2013;13:3–30. doi: 10.1007/7854_2011_129. http://dx.doi.org/10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metabolism. 2013;18(4):588–595. doi: 10.1016/j.cmet.2013.09.009. http://dx.doi.org/10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao-Kaim NP, Fonville L, Giampietro VP, Williams SC, Simmons A, Tchanturia K. Aberrant function of learning and cognitive control networks underlie inefficient cognitive flexibility in anorexia nervosa: A cross-sectional fMRI study. PloS One. 2015;10(5):e0124027. doi: 10.1371/journal.pone.0124027. http://dx.doi.org/10.1371/journal.pone.0124027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma-Cabrera JM, Carvajal F, Lopez-Legarrea P. Food addiction as a new piece of the obesity framework. Nutrition Journal. 2016;15:5. doi: 10.1186/s12937-016-0124-6. http://dx.doi.org/10.1186/s12937-016-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews. Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. http://dx.doi.org/10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Marsh R, Horga G, Wang Z, Wang P, Klahr KW, Berner LA, Peterson BS. An FMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. The American Journal of Psychiatry. 2011;168(11):1210–1220. doi: 10.1176/appi.ajp.2011.11010094. http://dx.doi.org/10.1176/appi.ajp.2011.11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Steinglass JE, Gerber AJ, Graziano O’Leary K, Wang Z, Murphy D, Peterson BS. Deficient activity in the neural systems that mediate self-regulatory control in bulimia nervosa. Archives of General Psychiatry. 2009;66(1):51–63. doi: 10.1001/archgenpsychiatry.2008.504. http://dx.doi.org/10.1001/archgenpsychiatry.2008.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti O, Marti J, Armario A. Effects of chronic stress on food intake in rats: Influence of stressor intensity and duration of daily exposure. Physiology & Behavior. 1994;55(4):747–753. doi: 10.1016/0031-9384(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biological Psychiatry. 2005;58(10):779–786. doi: 10.1016/j.biopsych.2005.04.044. http://dx.doi.org/10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Laruelle M. Amphetamine-induced dopamine release: Markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. The American Journal of Psychiatry. 2007;164(4):622–629. doi: 10.1176/ajp.2007.164.4.622. http://dx.doi.org/10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- McAllister CJ, Whittington JE, Holland AJ. Development of the eating behaviour in Prader-Willi syndrome: Advances in our understanding. International Journal of Obesity. 2011;35(2):188–197. doi: 10.1038/ijo.2010.139. http://dx.doi.org/10.1038/ijo.2010.139. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: An fMRI study. Neuropsychopharmacology. 2006;31(12):2728–2738. doi: 10.1038/sj.npp.1301075. http://dx.doi.org/10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. http://dx.doi.org/10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Meule A. How prevalent is “food addiction”? Frontiers in Psychology. 2011;2:61. doi: 10.3389/fpsyt.2011.00061. http://dx.doi.org/10.3389/fpsyt.2011.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meule A. Back by popular demand: A narrative review on the history of food addiction research. The Yale Journal of Biology and Medicine. 2015;88(3):295–302. [PMC free article] [PubMed] [Google Scholar]
- Meule A, Kubler A. Food cravings in food addiction: The distinct role of positive reinforcement. Eating Behaviors. 2012;13(3):252–255. doi: 10.1016/j.eatbeh.2012.02.001. http://dx.doi.org/10.1016/j.eatbeh.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Micanti F, Iasevoli F, Cucciniello C, Costabile R, Loiarro G, Pecoraro G, Galletta D. The relationship between emotional regulation and eating behaviour: A multidimensional analysis of obesity psychopathology. Eating and Weight Disorders. 2016 doi: 10.1007/s40519-016-0275-7. http://dx.doi.org/10.1007/s40519-016-0275-7. (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- Miller L. Predicting relapse and recovery in alcoholism and addiction: Neuropsy-chology, personality, and cognitive style. Journal of Substance Abuse Treatment. 1991;8(4):277–291. doi: 10.1016/0740-5472(91)90051-b. [DOI] [PubMed] [Google Scholar]
- Monro JA, Shaw M. Glycemic impact, glycemic glucose equivalents, glycemic index, and glycemic load: Definitions, distinctions, and implications. The American Journal of Clinical Nutrition. 2008;87(1):237S–243S. doi: 10.1093/ajcn/87.1.237S. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Schwartz MW. Leptin action in the forebrain regulates the hindbrain response to satiety signals. The Journal of Clinical Investigation. 2005;115(3):703–710. doi: 10.1172/JCI200522081. http://dx.doi.org/10.1172/JCI22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nature Reviews. Neuroscience. 2014;15(6):367–378. doi: 10.1038/nrn3745. http://dx.doi.org/10.1038/nrn3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaugh DL, Cox JE, Cook EW, 3rd, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. NeuroImage. 2012;59(3):2709–2721. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health & Department of Health and Human Services. Assessing your weight and health risk. Retrieved May 24, 2016, from http://www.nhlbi.nih.gov/health/educational/lose_wt/risk.htm.
- Nederkoorn C, Smulders FT, Havermans RC, Roefs A, Jansen A. Impulsivity in obese women. Appetite. 2006;47(2):253–256. doi: 10.1016/j.appet.2006.05.008. http://dx.doi.org/10.1016/j.appet.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Archives of General Psychiatry. 1991;48(7):648–654. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- Norgren R, Hajnal A, Mungarndee SS. Gustatory reward and the nucleus accumbens. Physiology & Behavior. 2006;89(4):531–535. doi: 10.1016/j.physbeh.2006.05.024. http://dx.doi.org/10.1016/j.physbeh.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit R, Mercer JG, Overduin J, la Fleur SE, Adan RA. Dietary factors affect food reward and motivation to eat. Obesity Facts. 2012;5(2):221–242. doi: 10.1159/000338073. http://dx.doi.org/10.1159/000338073. [DOI] [PubMed] [Google Scholar]
- Parylak SL, Koob GF, Zorrilla EP. The dark side of food addiction. Physiology & Behavior. 2011;104(1):149–156. doi: 10.1016/j.physbeh.2011.04.063. http://dx.doi.org/10.1016/j.physbeh.2011.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: Food-craving activation during fMRI. NeuroImage. 2004;23(4):1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. http://dx.doi.org/10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Nagren K, Lehikoinen P, Anttila K, Syvalahti EK, Hietala J. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Molecular Psychiatry. 1998;3(3):256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- Pursey KM, Stanwell P, Gearhardt AN, Collins CE, Burrows TL. The prevalence of food addiction as assessed by the Yale Food Addiction Scale: A systematic review. Nutrients. 2014;6(10):4552–4590. doi: 10.3390/nu6104552. http://dx.doi.org/10.3390/nu6104552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, Fischer AM, Ahuja A, Lesser EN, Maniates H. Roles of “wanting” and “liking” in motivating behavior: Gambling, food, and drug addictions. Current Topics in Behavioral Neurosciences. 2016;27:105–136. doi: 10.1007/7854_2015_387. http://dx.doi.org/10.1007/7854_2015_387. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Taste, olfactory, and food reward value processing in the brain. Progress in Neurobiology. 2015;127–128:64–90. doi: 10.1016/j.pneurobio.2015.03.002. http://dx.doi.org/10.1016/j.pneurobio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kellerhals MB, Nichols TE. Age differences in the brain mechanisms of good taste. NeuroImage. 2015;113:298–309. doi: 10.1016/j.neuroimage.2015.03.065. http://dx.doi.org/10.1016/j.neuroimage.2015.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte EM, Avena NM, Gearhardt AN. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PloS One. 2015;10(2):e0117959. doi: 10.1371/journal.pone.0117959. http://dx.doi.org/10.1371/journal.pone.0117959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte EM, Grilo CM, Gearhardt AN. Shared and unique mechanisms underlying binge eating disorder and addictive disorders. Clinical Psychology Review. 2016;44:125–139. doi: 10.1016/j.cpr.2016.02.001. http://dx.doi.org/10.1016/j.cpr.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha YB, Wickwire K, Giraudo SQ. Role of AgRP on Ghrelin-induced feeding in the hypothalamic paraventricular nucleus. Regulatory Peptides. 2006;133(1–3):68–73. doi: 10.1016/j.regpep.2005.09.021. http://dx.doi.org/10.1016/j.regpep.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Rapuano KM, Ingeholm JE, Avery J, Kallman S, Hall KD, Martin A. The ventral pallidum and orbitofrontal cortex support food pleasantness inferences. Brain Structure & Function. 2014;219(2):473–483. doi: 10.1007/s00429-013-0511-0. http://dx.doi.org/10.1007/s00429-013-0511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JJ, Skunde M, Walther S, Bendszus M, Herzog W, Friederich HC. Neural signature of food reward processing in bulimic-type eating disorders. Social Cognitive and Affective Neuroscience. 2016 doi: 10.1093/scan/nsw049. http://dx.doi.org/10.1093/scan/nsw049. (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- Singh M. Mood, food, and obesity. Frontiers in Psychology. 2014;5:925. doi: 10.3389/fpsyg.2014.00925. http://dx.doi.org/10.3389/fpsyg.2014.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141:105–130. doi: 10.1196/annals.1441.030. http://dx.doi.org/10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Jastreboff AM. Stress as a common risk factor for obesity and addiction. Biological Psychiatry. 2013;73(9):827–835. doi: 10.1016/j.biopsych.2013.01.032. http://dx.doi.org/10.1016/j.biopsych.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell RS. Clinical neuroanatomy. Philadelphia: Lippincott Williams; 2010. [Google Scholar]
- Spanagel R, Noori HR, Heilig M. Stress and alcohol interactions: Animal studies and clinical significance. Trends in Neurosciences. 2014;37(4):219–227. doi: 10.1016/j.tins.2014.02.006. http://dx.doi.org/10.1016/j.tins.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Spechler PA, Chaarani B, Hudson KE, Potter A, Foxe JJ, Garavan H. Response inhibition and addiction medicine: From use to abstinence. In: Hamed E, Martin P, editors. Progress in brain research. Vol. 223. Cambridge, MA: Elsevier; 2016. pp. 143–164. chapter 8. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008a;322(5900):449–452. doi: 10.1126/science.1161550. http://dx.doi.org/10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: A functional magnetic resonance imaging study. Journal of Abnormal Psychology. 2008b;117(4):924–935. doi: 10.1037/a0013600. http://dx.doi.org/10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Murdaugh DL, Cox JE, Cook EW, 3rd, Weller RE. Greater impulsivity is associated with decreased brain activation in obese women during a delay discounting task. Brain Imaging and Behavior. 2013;7(2):116–128. doi: 10.1007/s11682-012-9201-4. http://dx.doi.org/10.1007/s11682-012-9201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojek MK, Fischer S, MacKillop J. Stress, cues, and eating behavior. Using drug addiction paradigms to understand motivation for food. Appetite. 2015;92:252–260. doi: 10.1016/j.appet.2015.05.027. http://dx.doi.org/10.1016/j.appet.2015.05.027. [DOI] [PubMed] [Google Scholar]
- Sussman S, Sussman AN. Considering the definition of addiction. International. Journal of Environmental Research and Public Health. 2011;8(10):4025–4038. doi: 10.3390/ijerph8104025. http://dx.doi.org/10.3390/ijerph8104025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Posner MI, Rothbart MK, Volkow ND. Circuitry of self-control and its role in reducing addiction. Trends in Cognitive Sciences. 2015;19(8):439–444. doi: 10.1016/j.tics.2015.06.007. http://dx.doi.org/10.1016/j.tics.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. The American Journal of Physiology. 1996;271(2 Pt. 1):E317–E325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- Taylor SB, Anglin JM, Paode PR, Riggert AG, Olive MF, Conrad CD. Chronic stress may facilitate the recruitment of habit- and addiction-related neu-rocircuitries through neuronal restructuring of the striatum. Neuroscience. 2014;280:231–242. doi: 10.1016/j.neuroscience.2014.09.029. http://dx.doi.org/10.1016/j.neuroscience.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Institute of Diabetes and Digestive and Kidney Diseases. Overweight and obesity statistics. 2012 Retrieved from. http://www.niddk.nih.gov/health-information/health-statistics/Pages/overweight-obesity-statistics.aspx.
- Thomas JM, Higgs S, Dourish CT, Hansen PC, Harmer CJ, McCabe C. Satiation attenuates BOLD activity in brain regions involved in reward and increases activity in dorsolateral prefrontal cortex: An fMRI study in healthy volunteers. The American Journal of Clinical Nutrition. 2015;101(4):697–704. doi: 10.3945/ajcn.114.097543. http://dx.doi.org/10.3945/ajcn.114.097543. [DOI] [PubMed] [Google Scholar]
- Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23(11–12):887–894. doi: 10.1016/j.nut.2007.08.008. http://dx.doi.org/10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Uher R, Murphy T, Brammer MJ, Dalgleish T, Phillips ML, Ng VW, Treasure J. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. The American Journal of Psychiatry. 2004;161(7):1238–1246. doi: 10.1176/appi.ajp.161.7.1238. http://dx.doi.org/10.1176/appi.ajp.161.7.1238. [DOI] [PubMed] [Google Scholar]
- Val-Laillet D, Aarts E, Weber B, Ferrari M, Quaresima V, Stoeckel LE, Stice E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clinical. 2015;8:1–31. doi: 10.1016/j.nicl.2015.03.016. http://dx.doi.org/10.1016/j.nicl.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. Effects of glucagon-like peptide 1 on appetite and body weight: Focus on the CNS. The Journal of Endocrinology. 2014;221(1):T1–T16. doi: 10.1530/JOE-13-0414. http://dx.doi.org/10.1530/JOE-13-0414. [DOI] [PubMed] [Google Scholar]
- van Bloemendaal L, Veltman DJ, Ten Kulve JS, Groot PF, Ruhe HG, Barkhof F, Ijzerman RG. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. Diabetes, Obesity & Metabolism. 2015;17(9):878–886. doi: 10.1111/dom.12506. http://dx.doi.org/10.1111/dom.12506. [DOI] [PubMed] [Google Scholar]
- van de Giessen E, la Fleur SE, Eggels L, de Bruin K, van den Brink W, Booij J. High fat/carbohydrate ratio but not total energy intake induces lower striatal dopamine D2/3 receptor availability in diet-induced obesity. International Journal of Obesity. 2013;37(5):754–757. doi: 10.1038/ijo.2012.128. http://dx.doi.org/10.1038/ijo.2012.128. [DOI] [PubMed] [Google Scholar]
- Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Reports. 2012;13(12):1079–1086. doi: 10.1038/embor.2012.174. http://dx.doi.org/10.1038/embor.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: Insight from imaging studies. Neurobiology of Learning and Memory. 2002;78(3):610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: Implications for obesity. Trends in Cognitive Sciences. 2011;15(1):37–46. doi: 10.1016/j.tics.2010.11.001. http://dx.doi.org/10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386(6627):830–833. doi: 10.1038/386830a0. http://dx.doi.org/10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: Overlapping circuits in human obesity and addiction. Current Topics in Behavioral Neurosciences. 2012;11:1–24. doi: 10.1007/7854_2011_169. http://dx.doi.org/10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Jayne M, Fowler JS, Zhu W, Pappas N. Brain dopamine is associated with eating behaviors in humans. The International Journal of Eating Disorders. 2003;33(2):136–142. doi: 10.1002/eat.10118. http://dx.doi.org/10.1002/eat.10118. [DOI] [PubMed] [Google Scholar]
- Wang KS, Smith DV, Delgado MR. Using fMRI to study reward processing in humans: Past, present, and future. Journal of Neurophysiology. 2016;115(3):1664–1678. doi: 10.1152/jn.00333.2015. http://dx.doi.org/10.1152/jn.00333.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne JP. Shaping the stress response: Interplay of palatable food choices, glucocorticoids, insulin and abdominal obesity. Molecular and Cellular Endocrinology. 2009;300(1–2):137–146. doi: 10.1016/j.mce.2008.09.036. http://dx.doi.org/10.1016/j.mce.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Annals of the New York Academy of Sciences. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Wilcox G. Insulin and insulin resistance. Clinical Biochemist Reviews. 2005;26(2):19–39. [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Clinical Psychology Review. 2006;26(4):379–395. doi: 10.1016/j.cpr.2006.01.001. http://dx.doi.org/10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble LG, Williamson DA, Martin CK, Zucker NL, Thaw JM, Netemeyer R, Greenway FL. Psychosocial variables associated with binge eating in obese males and females. The International Journal of Eating Disorders. 2001;30(2):217–221. doi: 10.1002/eat.1076. [DOI] [PubMed] [Google Scholar]
- Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132(6):2116–2130. doi: 10.1053/j.gastro.2007.03.048. http://dx.doi.org/10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Ziauddeen H, Alonso-Alonso M, Hill JO, Kelley M, Khan NA. Obesity and the neurocognitive basis of food reward and the control of intake. Advances in Nutrition. 2015;6(4):474–486. doi: 10.3945/an.115.008268. http://dx.doi.org/10.3945/an.115.008268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziauddeen H, Farooqi IS, Fletcher PC. Obesity and the brain: How convincing is the addiction model? Nature Reviews. Neuroscience. 2012;13(4):279–286. doi: 10.1038/nrn3212. http://dx.doi.org/10.1038/nrn3212. [DOI] [PubMed] [Google Scholar]
- Ziauddeen H, Fletcher PC. Is food addiction a valid and useful concept? Obesity Reviews. 2013;14(1):19–28. doi: 10.1111/j.1467-789X.2012.01046.x. http://dx.doi.org/10.1111/j.1467-789X.2012.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand A, Parvaz MA, Moeller SJ, Goldstein RZ. Cognitive interventions for addiction medicine: Understanding the underlying neurobiological mechanisms. In: Hamed E, Martin PP, editors. Progress in brain research. Vol. 224. Cambridge, MA: Elsevier; 2016. pp. 285–304. chapter 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaan M, Engeli S, Muller A. Temperamental factors in severe weight cycling. A cross-sectional study. Appetite. 2015;91:336–342. doi: 10.1016/j.appet.2015.04.064. http://dx.doi.org/10.1016/j.appet.2015.04.064. [DOI] [PubMed] [Google Scholar]