Abstract
While traditional forensic genetics has been oriented towards using human DNA in criminal investigation and civil court cases, it currently presents a much wider application range, including not only legal situations sensu stricto but also and, increasingly often, to preemptively avoid judicial processes. Despite some difficulties, current forensic genetics is progressively incorporating the analysis of nonhuman genetic material to a greater extent. The analysis of this material—including other animal species, plants, or microorganisms—is now broadly used, providing ancillary evidence in criminalistics in cases such as animal attacks, trafficking of species, bioterrorism and biocrimes, and identification of fraudulent food composition, among many others. Here, we explore how nonhuman forensic genetics is being revolutionized by the increasing variety of genetic markers, the establishment of faster, less error-burdened and cheaper sequencing technologies, and the emergence and improvement of models, methods, and bioinformatics facilities.
Introduction
Forensic genetics derives from a late offshoot of the big tree resulting from the conjunction between legal medicine and criminalistics (for the distinction between forensic genetics and other forensic sciences, see [1–3]). Its historical evolution shows substantial theoretical and technological developments and has, meanwhile, turned this discipline into a broad and independent scientific area for which it is becoming more and more difficult to identify its most remote ancestors. The evolution of modern societies substantially broadened the forensic framework by introducing new forms of resolution of disputes, allowing space for prevention, and regulating more restrictively the prosecution investigations. This means that a potentially forensic situation is the one for which 2 or more sides (individual persons or institutions) agree on the reality of the facts but do disagree on the causes or authorship (thereafter, the term “forensic” is used for these scenarios). Thus, civil litigations (and not just criminal) are common but also conflicts (which are increasing with time) that are attempted to be solved outside a formal court environment [4].
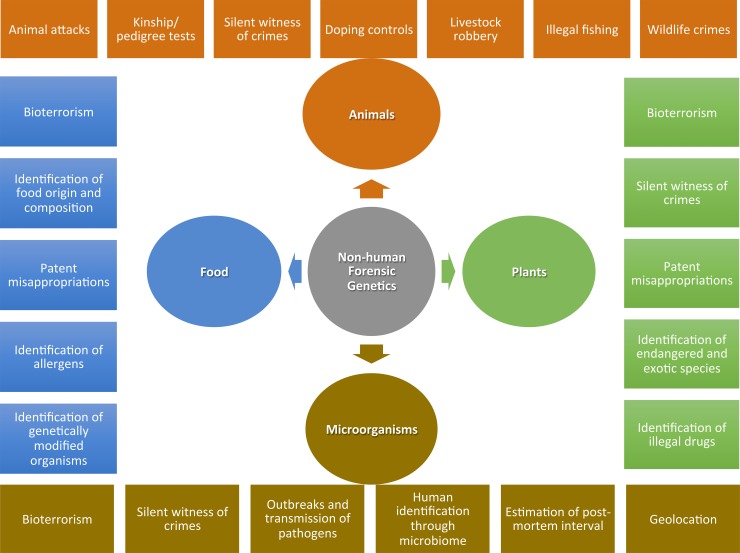
It is surprising that most of the life span of the discipline has been devoted to human genetics [e.g., 5], since a number of disagreements on questions intrinsically related to nonhuman materials always existed and, even when strictly human issues are at stake (such as the identification of a murderer), evidence from nonhuman sources can be crucial or are just the sole type of available evidence [e.g., 6]. This has been recognized by the first scientific journal explicitly devoted to forensic genetics (Forensic Science International: Genetics), when defining it as “The application of genetics to human and nonhuman material (in the sense of a science with the purpose of studying inherited characteristics for the analysis of inter- and intraspecific variations in populations) for the resolution of legal conflicts” [7]. Consequently, the division between human and nonhuman forensic genetics (HFG and NHFG, respectively) is not just the result of an anthropocentric historical tradition; rather, it could be derived from the different genomic architectures of the involved organisms [8]. Importantly, a number of forensically relevant questions are unthinkable in purely human terms (Fig 1), and in this review, we highlight their relevance.
Fig 1. Most relevant applications of the zoology, botany, microbiology, and food analysis and traceability sciences to NHFG.
Diverse examples for each of these applications are shown in S1 Table and S2 Table (see also S1 Text) and described in the section applications of NHFG. NHFG, nonhuman forensic genetics.
Below, we begin by describing the commonly used methodologies, including genotyping and sequencing strategies, evolutionary frameworks, and statistical approaches. Next, we broadly describe applications of NHFG based on diverse biological sources. Finally, we discuss the future of the discipline, including needs and recommendations.
Experimental methodologies in NHFG
The techniques of forensic genetics originally developed for humans were rapidly adapted to other sources of genetic material. The experimental pipeline used in NHFG (Fig 2) starts with a request for a genetic testing. Next, samples are collected using a sampling kit (either commercial or assembled in the laboratory) and transported to the laboratory under proper conditions. An accurate description of the biological nature of the sample is usually included, and a unique code must be assigned to each collected sample. If the request is part of a legal procedure, not only traceability but also the strict maintenance of the chain of custody (chronological documentation of the evidence) are key issues. The procedure continues in the laboratory, where the genetic material is extracted from the samples using an appropriate and validated protocol. However, certain urgent situations (e.g., bioterrorism) may require the use of methods that were not previously validated. The laboratory may have to deal with new kinds of biological material or taxonomic groups never studied before. In such cases, the laboratory has to be able to develop a valid strategy to extract DNA with sufficient quality and quantity for downstream analyses.
Fig 2. Pipeline showing the main steps usually involved in processing forensic nonhuman DNA samples.
The exact procedure will depend on the conditions available at each laboratory. The process starts with the evaluation of the case and sample collection (green boxes). The procedure continues in the laboratory, where the DNA is extracted from the biological source material and analyzed according to an appropriate protocol (blue boxes). The genetic information is then compared with reference databases and the results are described in a written report (red boxes).
Care must be taken when extracting and storing the genetic material to maintain integrity. Storage of nonhuman evidence does not create any specific problem (except required space) that is not common to HFG, and it must be handled in the same way as human material, following the same rules in labelling, chain of custody recording, etc. However, reproducibility in NHFG is clearly a major issue, especially when dealing with wildlife and environmental materials, due to inherent sampling difficulties. Therefore, validation studies cannot be performed in the same strict sense as they are in HFG (for a guideline on these problems, see [9]).
The selection of the genetic test depends on the question to be addressed (see next subsection). For instance, the sequencing of a PCR-amplified genetic region (e.g., cytochrome b [CYTB], cytochrome c oxidase I [COI], and ribosomal RNA [rRNA] genes) is often used for species identification. The identification of individuals can be achieved using a number of markers sufficient to provide high power of discrimination. In any case, it is important to check the quality of profiles or DNA sequences before analysing the results. The genetic information obtained is then compared with other genetic information (i.e., derived from reliable databases [e.g., 10] or reference sample[s]) considering statistical analyses. The experimental workflow ends with a report describing the technical procedures applied and the answers to the question(s) of the request.
Genotyping and sequencing
Genetic identification is based on polymorphic DNA markers that can provide sufficient discriminatory resolution. Traditionally, PCR-based methodologies designed to generate short amplicons, such as Rapid Amplification of Random DNA (RAPD) [11], Inter Simple Sequence Repeats (ISSR) [12], and Amplified Fragment Length Polymorphism (AFLP) [13], were applied in NHFG analyses. Two relevant forensic cases applying RADP are the analysis of plant (seed pods) DNA in a murder case in Phoenix [14], and the analysis of the outbreak of human anthrax occurred in Sverdlovsk (Ekaterinburg, Russia) [15].
However, due to their limitations, these techniques were rapidly replaced by Simple Sequence Repeats (SSRs, Short Tandem Repeats [STRs], or microsatellites) and Single Nucleotide Polymorphisms (SNPs) [e.g., 16]. The development of reduced-size STR amplicons (miniSTRs) can provide easy PCR amplification of degraded DNA samples, better estimation of mutation rates and allele frequencies, and construction of allelic ladders for accurate classification of alleles. Alternative methods to PCR include technologies such as nucleic acid sequence-based amplification (NASBA) and loop-mediated isothermal amplification (LAMP) [17, 18]. Later, a more advanced post-PCR technique, high-resolution DNA melting (HRM) analysis, which is based on the detection of small differences in amplicon melting (dissociation) curves, was also considered for NHFG [e.g., 19]. On the other hand, for DNA barcoding, a technique widely used in species identification [e.g., 20], is necessary to determine the DNA base composition by targeting specific regions with the Sanger sequencing method. Recently, the arrival of next generation sequencing (NGS) has also revolutionized forensic genetics [21]. These new technologies provide clear advantages regarding high-throughput due to an extensive multiplexing capacity and parallel sequencing of millions of molecules (Multiple Parallel Sequencing, MPS), allowing a faster and more informative analysis (i.e., characterization of allelic and copy-number variation, CNV) of the genomic material in a sample. Concerning NHFG, MPS is particularly useful for the analysis of samples of complex mixtures since untargeted approaches can be used without prior knowledge about the source. MPS presents additional advantages for NHFG such as the detection of rare polymorphisms, high resolution of genetic analysis, and informative power.
Methodologies for the evaluation of statistical evidence and for evolutionary analysis
Advances in NHFG are also caused by the progress of bioinformatics and statistical tools. A clear example is the emergence and evolution of the bioinformatics pipeline for the assembly of reads generated in MPS [see for a review, 22]. We next describe 2 analytic facets of crucial importance for NHFG: the quantitative evaluation of DNA evidence in the context of identification kinship and population/species assignment (i.e., in shallow evolutionary timescales) and the evolutionary analysis of genetic data (e.g., in transmission of fast evolving pathogens).
Statistical evaluation of evidence
Here, we overview the use of nonhuman genetic material (NHGM) as ancillary evidence to solve classical forensic problems and in cases that fall outside the civil and criminal human authorship or responsibility.
NHGM used as auxiliary evidence in litigations of human authorship
NHGM can play a crucial role in the investigation of diverse criminal disputes with the aim of identifying the human individual(s) who committed a crime or is/are responsible for some liability or damage. Historically, the first contributions correspond to situations in which NHGM is used as a silent witness resulting from involuntary transfer and leading to the so-called transfer or associative evidence. This type of NHGM usage is best illustrated in criminalistics where it is increasingly important, as perpetrators are progressively avoiding carefully leaving their biological traces in the crime scene. However, they can, for example, inadvertently leave their pets’ hairs at the crime scene or, inversely, to carry the victims’ pet biological material [e.g., 6]. Although pets are exceedingly common in modern households, many more exotic situations fit with this silent witness type of NHGM use (i.e., knotgrass [23], mosses [24], oak [25], and soil DNA [26]; see S1 Table and section applications of NHFG). Besides the existing variety of applications, new developments are already at sight such as the genetic profiling of microbiomes and microbial metagenomics [e.g., 27, 28–30], or in a not too distant future, the identification of transmitted strains of pathogens or commensals even many years after the crime (as it has been already done for viral transmissions [e.g., 31, 32–36]).
NHGM used as evidence in other litigations
There are several cases for which the expert evidence is used to deal with a law/regulation infringement, irrespectively of the human authorship or responsibility (which may be investigated separately). Here, a comprehensive classification is complex, given the dynamic evolution of the applications and the diversity of ever-growing fields for which laws are constantly being issued. Nevertheless, we must note a clear difference between the applications in which the litigation treats the nonhuman in a framework similar to human cases (individualization and kinship) and a plethora of other applications.
Concerning the former, both theoretical frameworks and technological platforms developed for humans can be almost directly translated. This category includes several scenarios: (i) an individual living being (e.g., an animal) is the direct causation of an injury to another living being or causes property damages [e.g., 37, 38]; (ii) the genetic relationship (e.g., paternity) of a living being to another one is unsettled [e.g., 39, 40]; (iii) the identity of the donor of a sample is under dispute (e.g., doping controls in horse races) [e.g., 41] (S2 Table).
As for the latter, a wide range of examples considering NHGM as auxiliary or direct evidence in litigations are derived from diverse subdisciplines such as forensics zoology, botany, microbiology, and food analysis (see Fig 1 and section applications of NHFG).
In any case, statistical analysis should provide likelihoods of observations, rather than categorical answers, and at least 2 alternative, mutually exclusive hypotheses should be formulated. Broadly speaking, statistical evaluation in NHFG can be required for 3 major scenarios:
Individual identification or kinship. It involves cases such as “was a given dog the perpetrator of the attack?” or “is a given foal the offspring of a given highly prized horse?”
Species identification. It involves interspecies cases such as “does the label of a processed fish product agree with the species of origin?”
Subspecific assignment/identification. It involves cases related with breed, variety, or populations such as “was the attack perpetrated by a dog or by a wolf?”
Regarding scenario A, similarly to what has been established for humans, autosomal STRs are preferentially considered and analysed with a Bayesian approach (in which prior odds are combined with probabilities of the genotypic observations assuming the alternative hypotheses). Nevertheless, several difficulties can arise in practice, especially when dealing with small sized and/or poorly studied populations, as in endangered species. The lack of knowledge in the population structure and sampling errors obviously has a serious impact on the confidence of the parameter estimates. The software developed in HFG for kinship and identification can be used in this scenario. For instance, the computer programs GDA [42] and GenePop [43] can be applied to test Hardy-Weinberg Equilibrium and to estimate population genetics parameters, while the program Familias [44] can be used to compute kinship likelihood ratios.
Regarding scenario B, the traditional procedure consists of comparing sequences that are highly variable among species but highly conserved within species, in the so-called DNA barcoding [45]. An alternative approach relies on comparing lengths of insertion and deletion polymorphisms without requiring DNA sequencing [46, 47]. Importantly, these approaches are only possible due to the existence and maintenance of reliable and public databases such as GenBank, EMBL, and Bold. Note that the increasing number and length of sequences existing in databases and the development of automated mechanisms to prevent misclassified sequences would allow more confidence in species identification. Finally, the statistical significance of sequence comparison should be computed [48] and reported.
Regarding scenario C, the selection of the genetic marker depends on the investigated species. For metazoan, the most used markers are regions of the mitochondrial genome (and plastid for Plantae) that can provide accurate distinction between subspecies [e.g., 49] and autosomal regions of nuclear DNA [e.g., 50]. The program STRUCTURE [51] is widely used for the Bayesian assignment of an individual to a population (or subspecies). Again, the statistical evaluation should be performed and reported.
Evolutionary analysis of genetic data in forensic genetics
Diverse organisms involved in forensic studies present short generation times and belong to large populations (as most pathogens). Therefore, relationships between queried and control samples are usually obtained under the light of evolutionary analyses since those samples most likely belong to distant generations [52]. The evolutionary analysis not only provides the identification of genetic relationships (dealing with questions like, “is the suspect the cause of the studied transmission or outbreak?” or “which individuals were infected by the suspect and which individuals were infected or coinfected from other sources?”) [e.g., 31–36, 53, 54, 55], but also allows the estimation of the timing of transmission events (i.e., infection date of each individual, including the individual that generated the outbreak) [e.g., 53, 54].
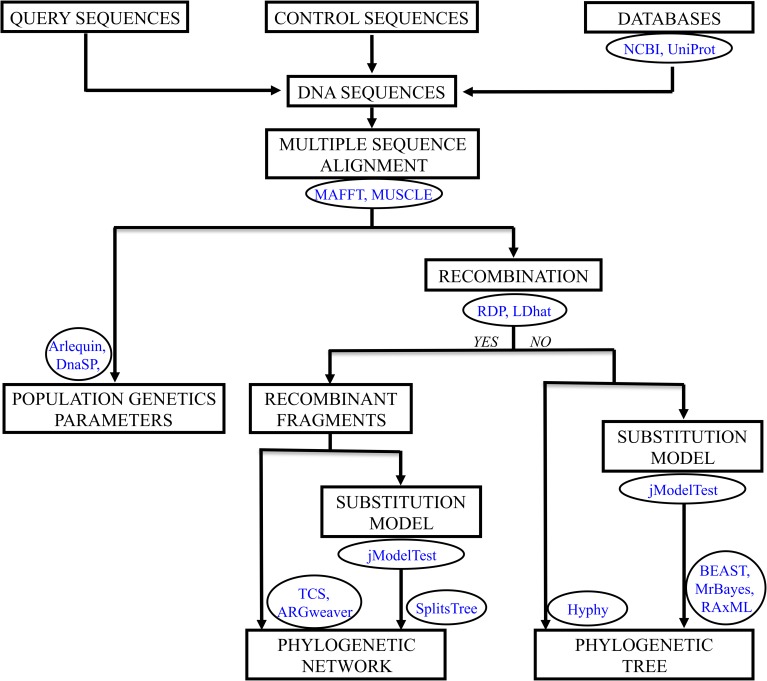
The computational pipeline for the evolutionary analysis of genetic data in NHFG follows well-established methodologies (Fig 3); however, several steps must be carefully performed. First, query, control (from local and background regions), and external (i.e., from reliable databases) sequences must be aligned. Next, population genetics statistics such as genetic diversity and genetic differentiation (i.e., between query and control sequences) can be estimated [e.g., 54, 55, 56]. The alignment can also be used to infer a phylogenetic history that depicts genetic relationships between the sample sequences and provides the timing of common ancestors (i.e., transmission events). A large number of pathogens (including those involved in most of NHFG cases, Human Immunodeficiency Virus [HIV] and Hepatitis C Virus [HCV]) evolve with processes of exchange of genetic material such as recombination [57, 58] and horizontal gene transfer [59]. Importantly, ignoring these processes can bias phylogenetic tree inferences by generating incorrect branch lengths and topologies [60, 61]. Therefore, under the presence of these processes, a phylogenetic network, which may have embedded a phylogenetic tree for each exchanged fragment [62], should be inferred [35, 61, 63, 64]. Indeed, a substitution model of evolution that fits the data best should be selected and considered in sophisticated phylogenetic inferences (i.e., based on maximum-likelihood or Bayesian approaches) [61, 65]. Importantly, phylogenetic approaches usually implement statistical confidence of the inferred evolutionary relationships through a bootstrap analysis [66]. In NHFG, this statistical parameter can provide a measure of the reliability of relationships between the pathogen genetic sequences of the investigated individuals. For example, a number of forensic studies based on phylogenetic inferences showed a classification of all control individuals in significantly separated clades, whereas individuals related with the studied outbreak or transmission clustered in a unique clade [e.g., 31, 32, 34, 35, 53, 54, 55]. Likelihood ratio tests can also be useful for hypothesis testing (i.e., testing if control sequences group or not with the studied outbreak) [e.g., 54].
Fig 3. Pipeline showing the evolutionary analysis of genetic data oriented to NHFG.
Data and tasks are shown in boxes, and databases and computer frameworks are shown in circles. Population genetic parameters include measures of genetic diversity, genetic differentiation, and demographics. The phylogenetic analysis requires the previous identification of recombination and can be performed ignoring or considering a substitution model of evolution. NHFG, nonhuman forensic genetics.
As noted above, the estimated time of internal nodes of the inferred phylogeny can be useful in forensic litigations by revealing the timing of infections [52]. These times can be estimated with Bayesian approaches [e.g., 67] accounting for longitudinal sampling (the tips are dated with the corresponding sampling times) to calibrate the (often relaxed) molecular clock and can provide accurate confidence intervals [e.g., 54].
Applications of NHFG
NHFG is expanding to more and more biological areas due to the increasing emergence of forensic cases based on NHGM. In this section, we revise the most relevant areas of NHFG, including zoology, botany, microbiology, and food analysis and traceability.
Zoology
The relevance and close presence of animals in a variety of human activities explain why they are among the first targets of NHFG [6, 68–70]. The number of animal species studied from a forensic genetics perspective has increased significantly, and different testing protocols have been developed for determining the identity of a sample at different biological levels such as individual, population, breed, species, or higher taxonomic classifications.
The preferential DNA markers used for individual identification in animals are autosomal STRs, as established in HFG. For example, STR kits have been developed for individual identification and kinship testing in dogs [71–78], cats [79–81], horses [40, 82, 83], cattle [39, 84], bears [85], deer [86, 87], badgers [88], birds [89, 90], and koi carps [91]. They have also been employed in resolving criminal and civil cases, such as dog or bear attacks [37, 38, 92], silent witnesses of crimes [6], identification of samples from sport horses [41, 93], and in wildlife crime investigations (wildlife forensics), including big cats [94], mouflons [95, 96], wild boars [97, 98], and elephants [99], among others. Concerning the latter, we want to highlight the application of forensic genetics to the illegal wildlife trade (IWT), since this is one of the biggest threats to a variety of species and habitats, with a consequent loss of biodiversity [100, 101]. In addition, IWT is a large-scale business estimated in billions of euros that generate negative socioeconomic impacts [100, 101]. Importantly, forensic genetics plays a crucial role in wildlife law enforcement [101].
Pioneer works endured tremendous efforts trying to reach the quality standards of human genetic testing. Difficulties in developing a new genotyping system for animals are various, including the collection of representative samples (especially problematic in wild species), the access to high-quality genomic sequences (not available for several species) and obtaining funding for the experiments (often focused on human research). Therefore, some of these STR kits are still a few steps behind those developed for human identification. For example, dinucleotide repeats are still used in nonhuman DNA testing [e.g., 37, 88, 91, 94], making it difficult to interpret sample mixtures and heterozygotes due to stutter product formation [102]. The most advanced nonhuman profiling kits are those developed for domesticated animals, including several STRs with tetranucleotide repeats [e.g., 72, 80, 81, 103]. Indeed, sex chromosome STR markers can also be useful for NHFG, however they still remain uncharacterized for many animal species. The mammalian Y-chromosome is used for gender identification, resolving paternity and family structures with application in forensic investigations [e.g., 104, 105, 106]. The development of an X-chromosome STR kit for dogs in 2010 [107] was a promising step in this field but, unfortunately, it was not followed by similar works in other species. The determination of the sex in birds has been possible using markers located in the W and Z chromosomes [e.g., 108, 109, 110].
A few panels of autosomal SNPs have also been developed for individual identification in different animal species [111–117]. These genetic markers may have some technical advantages over STRs [e.g., 102, 118] and can provide information about physical traits.
Forensic zoology often has to deal with degraded samples. In such cases, mtDNA may be the only source of genetic information that can be used. The high copy number of mtDNA in cells increases the probability of obtaining results from degraded/low-copy DNA samples such as hair, bones, and scat [119, 120]. Importantly, the same mtDNA sequence can be found in many individuals of a population and therefore cannot be used for individual identification. However, it can be used to exclude an individual as a source of a casework sample, and its utility has been demonstrated for a variety of animal species [e.g., 70, 121, 122–124]. Nevertheless, the most successful use of mtDNA in forensic zoology has been in species identification. Different mtDNA regions have been tested and validated for use in a forensic context, including CYTB [125–127], COI [128–130], and rRNA genes [131, 132]. The procedure usually involves the sequencing of a variable region amplified with conserved PCR primers followed by database searches and phylogenetic analyses. This strategy was applied in different forensic investigations such as identification of rhinoceros horns [133], ivory [134], turtle shells [135], endangered snake species [136], tigers [137], forensically important insect species [138–140], illegally smuggled eggs [141], or fish and fish products [142–144]. A few multiplex PCR/primer extension assays to genotype mtDNA SNPs have also been developed for species and subspecies identification (i.e., tiger [49], elephant [145], and other animals [146, 147]).
While the genetic identification of an individual or a species is not problematic in most situations, defining animal breeds or geographical populations has been considerably more difficult. Most breeds had a recent origin and are often defined by a few morphological features arbitrarily defined. For instance, cat breeds are defined by phenotypic characteristics (e.g., hair length, coat patterning, and colours) that are single-gene traits. Nevertheless, most cats can be assigned to their proper breed or population of origin using genetic data [148].
A crucial aspect for some forensic cases (i.e., poaching or illegal logging) is the identification of the origin of the sample. This identification depends on the existence of genetic data in different regions (including the region of the “real” origin), enough genetic differentiation among regions and the quality of the analytical method. The recent origin, intensive inbreeding, and genetic drift make difficult-to-use neutral genetic markers for rigorous identification of breed or populations. In such ambiguous cases, genetic tests should assess the genetic variants of the morphological traits that define the breed. However, our understanding of the genetics underlying such complex traits is still very limited, although significant progress is expected [149]. A famous case of origin identification was the mad cow disease between the United States and Canada [details in 150, 151], where a novel parentage testing was developed by combining prions and kinship [151].
Botany
Plant evidence can provide crucial information for the reconstruction of forensically relevant events or in cases where the crime scene and autopsy reports are not compelling [152].
Conventional taxonomic identification (using morphological methods) has a reduced application since botanical forensic evidences are often very fragmented (e.g., pieces of leaves or seeds) limiting the use of dichotomous keys. However, molecular markers can be applied to identify samples, regardless of their state, morphology, and development phase. In this concern, in the last couple of decades, diverse molecular markers have been applied for the forensic identification of species and individuals (i.e., HRM coupled with specific barcodes or real-time PCR to analyze chloroplast DNA regions) [e.g., 153, 154]. Importantly, second- and third-generation sequencing methodologies are providing affordable analysis of complex and degraded plant samples [155, 156].
Forensic botany presents numerous applications such as the identification of the origin of seized illegal drugs (marijuana [157], kratom [158], or opium [159]), detection of illegal logging [160, 161], importation and commercialization of endangered and exotic species [162, 163], or bioterrorism (abrin and ricin attacks [164]). It can also provide useful supporting evidence in crime scene investigations, allowing us to establish a link between the victim and the suspect, placing the suspect at a crime scene or estimating the time of death [e.g., 23, 25, 165–167].
Microbiology
Although microbes have long been recognized as important players in our daily life, present in areas such as medicine and public health, ecology, and in industrial applications, microbial forensics (MF) is still a relatively recent scientific field [168, 169].
MF aims to identify a target microorganism and its source. Although culture in selective growth media remains as the preferred standard for characterization of microbial agents at the resolution of genus/species level, complementary detection methods based on diverse molecular markers are increasingly applied [170]. Indeed, NGS technologies profoundly improved the ability to detect microorganisms, even when present in low abundance or in degraded or mixture samples, and to differentiate at strain/isolate level, using diagnostic genomic signatures [171].
Applications of MF involve diverse areas such as biocrimes, bioterrorism, frauds, outbreaks and transmission of pathogens, or accidental release of a biological agent or a toxin [e.g., 54, 172]. Additionally, the recent breakthroughs derived from NGS technologies allowed the analysis of microbial evidence to be expanded to cases related with geolocation, body fluid characterization, or postmortem interval estimation [168].
Some biological agents can be used as weapons or threats. The best well-known example is the Amerithrax case (2001), where letters laden with Bacillus anthracis spores were sent through the U.S. Postal Service to several media offices in New York and Florida and to U.S. senators in Washington [173, 174]. In this case, DNA evidence was found in the suspect’s laboratory.
Under the scope of epidemiological investigation, MF also helps to determine whether a pathogen outbreak was natural or human-driven. Therefore, MF is intimately associated with epidemiological surveys, allowing studying and following disease outbreak dynamics, mainly concerning the identification of the agent or toxin, origin and natural reservoirs, genetic diversity and evolution, and possible transmission routes. Some well-known cases of the epidemiological studies are the swine-origin influenza A virus (H1N1; 2009) [175], the Haitian cholera (2010) [176], the haemolytic-uremic syndrome (Escherichia coli O104:H4; 2011) [177], the Coronavirus Middle East respiratory syndrome (2012) [178], the avian-origin Influenza A virus (H7N9; 2013) [179], the West African Ebola virus (2013/2015) [180], the Middle Eastern poliomyelitis (2014) [181], the Portuguese Legionnaires’ disease (2014) [182], and the Zika virus outbreaks [183]. Note that most of the cases indicated above applied NGS approaches to identify and study the different biological agents.
Applications of MF in biocrimes also include the tracking of sexually transmitted diseases and healthcare malpractice linked to the transmission of HIV [e.g., 31–36, 53, 184] and HCV [e.g., 54, 55]. Moreover, this discipline is also used to determine responsibilities in cases of hospital-acquired infections [e.g., 185, 186] or sudden death syndrome [e.g., 187, 188].
The human microbiome is starting to be a focus of interest for identification purposes. The rational is to trace human microbiomes on our skin on the surfaces and objects we interact with the potential to supplement the use of human DNA for associating people with evidence and environments. The Human Microbiome Project has significantly improved the scientific knowledge in the field [189, 190]. Note that there are 10 times more bacteria than human cells in our body [191], and a number of them appears to be unique to each person [192], offering an opportunity for new identification biomarkers [193]. Thus, the human microbiome could be used to identify suspects [e.g., 27, 28, 29] and to estimate the postmortem interval [194]. For example, the origin of human remains from the Second World War was ascertained with the parvovirus B19V [195]. Although these are promising findings, we consider that we are still far from a foundational validation of this approach to be used in legal cases.
One of the main constraints associated with the use of MF is the lack of standards and guidelines, although phylogenetic analyses have supported associations and have successfully been admitted as evidence in legal criminal cases [196]. Another limitation is the insufficiency of reference databases lacking endemic data or microorganism source tracing, reference genome sequences, metadata, and representative genetic diversity coverage [197].
Food analysis and traceability
The investigation of the biological composition of food products regarding the species, variety or cultivar, and geographic origin is of major forensic interest. Such investigations are relevant for guaranteeing consumer choices according to health concerns (e.g., sensitivities or allergies), dietary preferences (e.g., vegetarian, nongenetically modified organisms), religious beliefs (e.g., halal and kosher specifications), and to detect fraudulent substitution of a given species by a similar one with lower economic value [198, 199]. Labelling is indispensable for producers, retailers, and consumers to recognize and validate components of foodstuffs [200]. Unfortunately, labels of products often provide insufficient and erroneous information concerning the exact contents.
The methodology used in food forensics is similar to that used in classical crime investigations, facing the same demands of dealing with potentially degraded DNA samples [201]. Several DNA-based methods have become remarkably valuable for protecting and certifying the quality and source of food [202, 203]. The first studies performed in the 90’s resorted to classical techniques (i.e., RADP and ISSR) but nowadays, real-time PCR [204], HRM [205–207], and MPS [208] are widely applied for food traceability with the advantage of quantifying each particular component in a faster and affordable procedure.
These genetic markers have been applied to perform identification in a variety of food products such as olive oil [e.g., 209, 210], grapevine cultivars [e.g., 211, 212, 213], composition of honey [e.g., 214, 215, 216], mushrooms [e.g., 217, 218, 219], dairy products [e.g., 220, 221, 222], seafood products [20], or meat species adulteration [223]. Additional documented cases include: i) identification of cultivars of basmati rice [224], pome [225] and stone fruits [226], leguminosae [227, 228], coffee [229], and tea and infusions [230]; ii) patent misappropriation of strawberry cultivars [231]; iii) confirmation of Protected Designation of Origin (PDO), Protected Geographical Indication (PGI), or Traditional Speciality Guaranteed (TSG) in olive [232] and grape [213, 233] products; iv) adulteration of traditional medicines [234, 235] and herbs or spices [236]; v) insufficient and erroneous food labelling, including the presence of some hidden allergens [237, 238] or genetically modified organisms [239] (GMOs; see section Genetically modified organisms).
Food microbiology
Over the last 2 decades, the prevalence of foodborne diseases has drastically increased, becoming a worldwide major public health concern. Foodborne diseases are often triggered by the consumption of food or water contaminated either by pathogens (bacteria, viruses, fungi, and parasites) or derived toxins. The most common pathogens responsible for foodborne disease outbreaks are Listeria monocytogenes, Escherichia coli O157:H7, Staphylococcus aureus, Salmonella enterica, Bacillus cereus, Vibrio spp., Campylobacter jejuni, Clostridium perfringens, and Shigella dysenteriae. These pathogens are often associated with consumption of raw (e.g., fruits and vegetables) or undercooked foods (e.g., seafood, meat, and poultry) [240]. To overcome the limitations of the traditional culture of microorganisms (e.g., may disallow the cultivation of the major foodborne pathogen or may present a slow growth leading to long periods of time cultivation), DNA/RNA-based methods (i.e., STR, NASBA, LAMP, and NGS) are usually applied [e.g., 241].
Genetically modified organisms
An area of growing interest is the detection of GMOs. The number of genetically modified plants has been growing in recent years despite the intense discussion about the benefits or damage that these organisms may have on humans and ecosystems. The detection of a GMO is carried out by targeting the genetic elements (promotors, protein-coding regions or terminators) that have been introduced artificially in the genome of the transgenic organism in order to improve a particular trait [242]. A curated list of transgenic reference sequences has been recently made available and is expected to facilitate the development of methods for testing GMOs and the implementation of regulatory policies [243]. The labelling and traceability of GMOs are important issues that are highly regulated. If the content exceeds a certain threshold, the product must be labeled accordingly. The most commonly used DNA-based methodology for GMO testing is PCR, although other techniques have been proposed [244–246]. The quantification of DNA targets is usually done by real-time PCR, where the copy number of the transgenic element detected is correlated to a common plant marker, allowing the determination of the GMO proportion in the sample [247, 248]. The correct detection of genetically modified materials is of forensic relevance not only due to strict legislation regarding the labelling of food products but also due to the type of materials from which DNA has to be extracted. For example, transgenic constructs have to be identified in DNA extracted from products like corn germ, flour, pasta, corn flakes, cookies, baked products, sugars derived from corn starch, soy cream or milk (liquid or lyophilized), tofu, meat products, lecithin, and even oil. Although most of the currently available GMOs are plants, the picture is expected to change soon. The first genetically modified animal (AquAdvantage salmon) is on the verge of being approved for human consumption in different countries [249]. New methods are being developed to detect the genetically modified salmon in food products [250, 251]. Strong legislation is expected to regulate the presence of this transgenic animal in foods and environmental samples [252, 253] and, consequently, reliable and sensitive methods for its detection will be required by regulatory and scientific agencies worldwide [245, 254].
The future of NHFG
Within the enormous variety of applications, methods, and sources of NHFG, the forensic use of NHGM is still limited and faces enormous difficulties due to diverse causes. Among them, and perhaps the most important, is the sheer amount of biodiversity and the current poor knowledge about it, with an impact not only on the species identification problem but also at the intraspecific level where, for most wildlife organisms, population genetics data are nonexistent or extremely poor. This makes relevant parameters difficult to estimate with acceptable accuracy and thus inhibits solid statistical evaluation of the evidence [255]. In this concern, the impact of the International Barcode of Life project (iBOL, http://www.ibol.org/) on forensics has been much less than desired and several difficulties have been raised on its power, limitations, and governance [256, 257]. In fact, most biodiversity studies do not meet classical forensic standards (demanded in forensic routine casework), due to the inherently limited sampling, references and controls. Moreover, there is a lack of agreement and concerted actions between the scientific societies aiming at the forensic use of NHGM (ISAG, International Society for Animal Genetics; ISFG, International Society for Forensic Genetics; SWSF, The Society for Wildlife Forensic Science; ISEF, International Society of Environmental Forensics) that is reflected in nonreconcilable or even contradictory recommendations and guidelines (particularly between ISFG [9] and ISAG/FAO [258]). Given the increasing use of NHFG, we do hope for some progress in joining efforts between scientific communities for a mutual benefit.
On the other hand, less error-burdened, cheaper, and faster MPS, together with progress in bioinformatics frameworks and computational resources, now allow the analysis of complex samples (i.e., commingled samples with DNA from more than one contributor/species) with more accurate and reliable results [e.g., 177, 186]. With third generation sequencing technologies, single DNA molecules can be analyzed individually [e.g., 259] and, therefore, haplotypes can be determined. These advances are expected to revolutionize NHFG. Among other examples, MPS was already applied to the identification of species for quality control in the development and authentication of herbal and traditional medicines [260] and for the discrimination of soils and other detritus from alternative environments and locations, based on the composition of the microflora, plants, metazoan, and protozoa DNA sequences [21, 261–265]. As noted in MF, the implementation of MPS is particularly useful for epidemiological studies. However, more research is necessary for the improvement of libraries (i.e., reference sequences reflecting the coverage of the entire genome of diverse organisms), development of bioinformatics platforms (i.e., for decreasing memory requirements and implementing algorithms for parallel computing) and for reproducibility and assignment of general quality of the results. Moreover, current MPS technologies still present relatively high sequencing errors [e.g., 266] which, although could be assumed for other disciplines, may not meet forensic standards [267]. Therefore, strict MPS validation studies are mandatory but they are still very scarce, even in human applications.
In this regard, it is clear that genetic analyses based on very large datasets (ideally, whole genomes) can provide high statistical confidence that can be useful for forensic cases [268]. However, systematic biases in methods applied to the analysis of large data can lead to precise but incorrect results [269–271]. Therefore, not only are large datasets required, less biased state-of-the-art methodologies should also be applied [272]. An example of this situation is the evolutionary analysis of genetic data. This analysis can be improved with the consideration of more complex substitution models of evolution (i.e., nonreversible and nonstationary) that can better fit the data [65, 273]. However, these models were not implemented yet into the traditional frameworks of the phylogenetic pipeline and, to our knowledge, all existing NHFG studies have ignored them. In addition, as noted above, evolutionary processes that exchange genetic material (e.g., recombination) can bias phylogenetic tree inferences [60]. However, to our knowledge, all existing NHFG studies including phylogenetic tree inferences from pathogens that usually evolve with high recombination rates (i.e., HIV and HCV) ignored recombination [e.g., 31–36, 53, 54, 55]. We strongly recommend considering these aspects in future NHFG studies.
The future of NHFG is dependent on the progress in removing current limitations (i.e., funding, adapting scientific methods into court [274], taking away from HFG and dealing with much smaller documented biodiversity being more complex to achieve forensic standards), but this is an emerging field of increasing importance. The number of papers in the top forensic journals on nonhuman DNA typing topics is increasing at a rate of 15% per year, especially on IWT [275]. As mentioned by Ogden and Linacre [101], perhaps the main difficulty in this field is the large proportion of traded products originated from underdeveloped countries where wildlife trade monitoring and the ability of the enforcement agencies to act are limited. That difficulty is caused by the lack of funding since the priorities of the majority of law enforcement agencies are crimes against humans and their properties.
The continuous incorporation of genomic data in reliable databases together with progress of experimental methodologies and analytical software are expected to further increase the application of NHFG. Assuming this direction, we believe that, in the future, NHFG could even overpass HFG in number of cases investigated, since the number of informative organisms is extremely large.
Supporting information
(PDF)
(PDF)
(PDF)
Acknowledgments
We also thank Christopher Phillips, Rui Pereira, Luis Alvarez, and Helena Machado for helpful comments on an earlier version of this study.
Funding Statement
MA, FP, MO, NP, AML and VG are supported by the Portuguese Foundation for Science and Technology (FCT), European Regional Development Fund (ERDF) and Programa Operacional Potencial Humano, through the grants IF/00955/2014, IF/01356/2012, SFRH/BPD/66071/2009, SFRH/BPD/97414/2013, IF/01262/2014 and SFRH/BPD/76207/2011, respectively. MA was also supported by the “Ramón y Cajal” grant RYC-2015-18241 from the Spanish Government. IPATIMUP integrates the i3S Research Unit, which is partially supported by FCT. The funders had no role in the preparation of the article.
References
- 1.Saks MJ, Koehler JJ. The coming paradigm shift in forensic identification science. Science. 2005;309(5736):892–5. doi: 10.1126/science.1111565 . [DOI] [PubMed] [Google Scholar]
- 2.Amorim A. Basic Principles In: Siegel JA, Saukko PJ, editors. Encyclopedia of Forensic Sciences. 1 2nd ed. Waltham: Academic Press; 2013. p. 211–3. [Google Scholar]
- 3.Amorim A. Introduction to the Special Issue on Forensic Genetics: Non-Human DNA. The Open Forensic Science Journal. 2010;3:6–8. [Google Scholar]
- 4.Almog J. Forensics as a proactive science. Sci Justice. 2014;54(5):325–6. doi: 10.1016/j.scijus.2014.05.008 . [DOI] [PubMed] [Google Scholar]
- 5.Kayser M, de Knijff P. Improving human forensics through advances in genetics, genomics and molecular biology. Nat Rev Genet. 2011;12(3):179–92. doi: 10.1038/nrg2952 . [DOI] [PubMed] [Google Scholar]
- 6.Menotti-Raymond MA, David VA, O'Brien SJ. Pet cat hair implicates murder suspect. Nature. 1997;386(6627):774 doi: 10.1038/386774a0 . [DOI] [PubMed] [Google Scholar]
- 7.Carracedo A, Sobrino B, Lareu MV. Forensic DNA typing technologies: A review In: Bogusz MJ, editor. Handbook of Analytical Separations. 7 Amsterdam: Elsevier; 2007. p. 560–623. [Google Scholar]
- 8.Coyle HM. Nonhuman DNA Typing: Theory and Casework Applications: CRC Press; 2007. [Google Scholar]
- 9.Linacre A, Gusmão L, Hecht W, Hellmann AP, Mayr WR, Parson W, et al. ISFG: Recommendations regarding the use of non-human (animal) DNA in forensic genetic investigations. Forensic Science International: Genetics. 2011;5(5):501–5. http://dx.doi.org/10.1016/j.fsigen.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Budowle B, Moretti TR, Niezgoda SJ, Brown BL, editors. CODIS and PCR-based short tandem repeat loci: law enforcement tools. Second European Symposium on Human Identification; 1998; Madison, WI: Promega Corporation.
- 11.Williams JG, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18(22):6531–5. ; PubMed Central PMCID: PMCPMC332606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godwin ID, Aitken EA, Smith LW. Application of inter simple sequence repeat (ISSR) markers to plant genetics. Electrophoresis. 1997;18(9):1524–8. doi: 10.1002/elps.1150180906 . [DOI] [PubMed] [Google Scholar]
- 13.Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, et al. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 1995;23(21):4407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon CK. Forensic science. Botanical witness for the prosecution. Science. 1993;260(5110):894. [DOI] [PubMed] [Google Scholar]
- 15.Jackson PJ, Hugh-Jones ME, Adair DM, Green G, Hill KK, Kuske CR, et al. PCR analysis of tissue samples from the 1979 Sverdlovsk anthrax victims: the presence of multiple Bacillus anthracis strains in different victims. Proc Natl Acad Sci U S A. 1998;95(3):1224–9. ; PubMed Central PMCID: PMCPMC18726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zietkiewicz E, Rafalski A, Labuda D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics. 1994;20(2):176–83. doi: 10.1006/geno.1994.1151 . [DOI] [PubMed] [Google Scholar]
- 17.Fakruddin M, Mannan KS, Chowdhury A, Mazumdar RM, Hossain MN, Islam S, et al. Nucleic acid amplification: Alternative methods of polymerase chain reaction. J Pharm Bioallied Sci. 2013;5(4):245–52. doi: 10.4103/0975-7406.120066 ; PubMed Central PMCID: PMCPMC3831736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ceuppens S, Li D, Uyttendaele M, Renault P, Ross P, Ranst MV, et al. Molecular Methods in Food Safety Microbiology: Interpretation and Implications of Nucleic Acid Detection. Comprehensive Reviews in Food Science and Food Safety. 2014;13(4):551–77. doi: 10.1111/1541-4337.12072 [DOI] [PubMed] [Google Scholar]
- 19.Malewski T, Draber-Monko A, Pomorski J, Los M, Bogdanowicz W. Identification of forensically important blowfly species (Diptera: Calliphoridae) by high-resolution melting PCR analysis. Int J Legal Med. 2010;124(4):277–85. doi: 10.1007/s00414-009-0396-x . [DOI] [PubMed] [Google Scholar]
- 20.Stern DB, Castro Nallar E, Rathod J, Crandall KA. DNA Barcoding analysis of seafood accuracy in Washington, D.C. restaurants. PeerJ. 2017;5:e3234 doi: 10.7717/peerj.3234 ; PubMed Central PMCID: PMCPMC5407275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borsting C, Morling N. Next generation sequencing and its applications in forensic genetics. Forensic Sci Int Genet. 2015;18:78–89. doi: 10.1016/j.fsigen.2015.02.002 . [DOI] [PubMed] [Google Scholar]
- 22.Horner DS, Pavesi G, Castrignano T, De Meo PD, Liuni S, Sammeth M, et al. Bioinformatics approaches for genomics and post genomics applications of next-generation sequencing. Brief Bioinform. 2010;11(2):181–97. doi: 10.1093/bib/bbp046 . [DOI] [PubMed] [Google Scholar]
- 23.Koopman WJ, Kuiper I, Klein-Geltink DJ, Sabatino GJ, Smulders MJ. Botanical DNA evidence in criminal cases: Knotgrass (Polygonum aviculare L.) as a model species. Forensic Sci Int Genet. 2012;6(3):366–74. doi: 10.1016/j.fsigen.2011.07.013 . [DOI] [PubMed] [Google Scholar]
- 24.Korpelainen H, Virtanen V. DNA fingerprinting of mosses. J Forensic Sci. 2003;48(4):804–7. . [PubMed] [Google Scholar]
- 25.Craft KJ, Owens JD, Ashley MV. Application of plant DNA markers in forensic botany: genetic comparison of Quercus evidence leaves to crime scene trees using microsatellites. Forensic Sci Int. 2007;165(1):64–70. doi: 10.1016/j.forsciint.2006.03.002 . [DOI] [PubMed] [Google Scholar]
- 26.Concheri G, Bertoldi D, Polone E, Otto S, Larcher R, Squartini A. Chemical elemental distribution and soil DNA fingerprints provide the critical evidence in murder case investigation. PLoS One. 2011;6(6):e20222 doi: 10.1371/journal.pone.0020222 ; PubMed Central PMCID: PMCPMC3108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao X, Liu W, Han J, Pei G, Tong Y, Luo Y. Analysis of Microbiome DNA on Frequently Touched Items and from Palm Prints. Journal of Forensic Science and Medicine. 2016;2(2):74–7. doi: 10.4103/2349-5014.184190 [Google Scholar]
- 28.Leake SL, Pagni M, Falquet L, Taroni F, Greub G. The salivary microbiome for differentiating individuals: proof of principle. Microbes Infect. 2016;18(6):399–405. doi: 10.1016/j.micinf.2016.03.011 . [DOI] [PubMed] [Google Scholar]
- 29.Xie H, Guo R, Zhong H, Feng Q, Lan Z, Qin B, et al. Shotgun Metagenomics of 250 Adult Twins Reveals Genetic and Environmental Impacts on the Gut Microbiome. Cell Syst. 2016;3(6):572–84 e3. doi: 10.1016/j.cels.2016.10.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fierer N, Lauber CL, Zhou N, McDonald D, Costello EK, Knight R. Forensic identification using skin bacterial communities. Proc Natl Acad Sci U S A. 2010;107(14):6477–81. doi: 10.1073/pnas.1000162107 ; PubMed Central PMCID: PMCPMC2852011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ou CY, Ciesielski CA, Myers G, Bandea CI, Luo CC, Korber BT, et al. Molecular epidemiology of HIV transmission in a dental practice. Science. 1992;256(5060):1165–71. . [DOI] [PubMed] [Google Scholar]
- 32.Albert J, Wahlberg J, Leitner T, Escanilla D, Uhlen M. Analysis of a rape case by direct sequencing of the human immunodeficiency virus type 1 pol and gag genes. J Virol. 1994;68(9):5918–24. ; PubMed Central PMCID: PMCPMC236997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machuca R, Jorgensen LB, Theilade P, Nielsen C. Molecular investigation of transmission of human immunodeficiency virus type 1 in a criminal case. Clin Diagn Lab Immunol. 2001;8(5):884–90. doi: 10.1128/CDLI.8.5.884-890.2001 ; PubMed Central PMCID: PMCPMC96165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metzker ML, Mindell DP, Liu XM, Ptak RG, Gibbs RA, Hillis DM. Molecular evidence of HIV-1 transmission in a criminal case. Proc Natl Acad Sci U S A. 2002;99(22):14292–7. doi: 10.1073/pnas.222522599 ; PubMed Central PMCID: PMCPMC137877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemey P, Van Dooren S, Van Laethem K, Schrooten Y, Derdelinckx I, Goubau P, et al. Molecular testing of multiple HIV-1 transmissions in a criminal case. AIDS. 2005;19(15):1649–58. . [DOI] [PubMed] [Google Scholar]
- 36.Scaduto DI, Brown JM, Haaland WC, Zwickl DJ, Hillis DM, Metzker ML. Source identification in two criminal cases using phylogenetic analysis of HIV-1 DNA sequences. Proc Natl Acad Sci U S A. 2010;107(50):21242–7. doi: 10.1073/pnas.1015673107 ; PubMed Central PMCID: PMCPMC3003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frosch C, Dutsov A, Georgiev G, Nowak C. Case report of a fatal bear attack documented by forensic wildlife genetics. Forensic Sci Int Genet. 2011;5(4):342–4. doi: 10.1016/j.fsigen.2011.01.009 . [DOI] [PubMed] [Google Scholar]
- 38.Tsuji A, Ishiko A, Kimura H, Nurimoto M, Kudo K, Ikeda N. Unusual death of a baby: a dog attack and confirmation using human and canine STRs. Int J Legal Med. 2008;122(1):59–62. doi: 10.1007/s00414-006-0150-6 . [DOI] [PubMed] [Google Scholar]
- 39.Liron JP, Ripoli MV, Garcia PP, Giovambattista G. Assignment of paternity in a judicial dispute between two neighbor Holstein dairy farmers. J Forensic Sci. 2004;49(1):96–8. . [PubMed] [Google Scholar]
- 40.van de Goor LH, van Haeringen WA, Lenstra JA. Population studies of 17 equine STR for forensic and phylogenetic analysis. Anim Genet. 2011;42(6):627–33. doi: 10.1111/j.1365-2052.2011.02194.x . [DOI] [PubMed] [Google Scholar]
- 41.Tobe SS, Reid SJ, Linacre AMT. Successful DNA typing of a drug positive urine sample from a race horse. Forensic Sci Int. 2007;173(1):85–6. doi: 10.1016/j.forsciint.2006.08.009 [Google Scholar]
- 42.Weir BS. Genetic Data Analysis. 2nd ed. Sunderland, MA: Sinauer Associates; 1996. [Google Scholar]
- 43.Rousset F. genepop'007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Resour. 2008;8(1):103–6. doi: 10.1111/j.1471-8286.2007.01931.x . [DOI] [PubMed] [Google Scholar]
- 44.Egeland T, Mostad PF, Mevag B, Stenersen M. Beyond traditional paternity and identification cases. Selecting the most probable pedigree. Forensic Sci Int. 2000;110(1):47–59. . [DOI] [PubMed] [Google Scholar]
- 45.Hebert PD, Ratnasingham S, deWaard JR. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc Biol Sci. 2003;270 Suppl 1:S96–9. doi: 10.1098/rsbl.2003.0025 ; PubMed Central PMCID: PMCPMC1698023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pereira F, Carneiro J, Matthiesen R, van Asch B, Pinto N, Gusmao L, et al. Identification of species by multiplex analysis of variable-length sequences. Nucleic Acids Res. 2010;38(22):e203 doi: 10.1093/nar/gkq865 ; PubMed Central PMCID: PMCPMC3001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carneiro J, Pereira F, Amorim A. SPInDel: a multifunctional workbench for species identification using insertion/deletion variants. Mol Ecol Resour. 2012;12(6):1190–5. doi: 10.1111/1755-0998.12011 . [DOI] [PubMed] [Google Scholar]
- 48.Tobe SS, Kitchener AC, Linacre AM. Reconstructing mammalian phylogenies: a detailed comparison of the cytochrome B and cytochrome oxidase subunit I mitochondrial genes. PLoS One. 2010;5(11):e14156 doi: 10.1371/journal.pone.0014156 ; PubMed Central PMCID: PMCPMC2994770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitpipit T, Tobe SS, Kitchener AC, Gill P, Linacre A. The development and validation of a single SNaPshot multiplex for tiger species and subspecies identification—implications for forensic purposes. Forensic Sci Int Genet. 2012;6(2):250–7. doi: 10.1016/j.fsigen.2011.06.001 . [DOI] [PubMed] [Google Scholar]
- 50.van Asch B, Alves C, Santos L, Pinheiro R, Pereira F, Gusmao L, et al. Genetic profiles and sex identification of found-dead wolves determined by the use of an 11-loci PCR multiplex. Forensic Sci Int Genet. 2010;4(2):68–72. doi: 10.1016/j.fsigen.2009.05.003 . [DOI] [PubMed] [Google Scholar]
- 51.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–59. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sajantila A. Molecular clocks ticking in the court room. Investig Genet. 2014;5(1):4 doi: 10.1186/2041-2223-5-4 ; PubMed Central PMCID: PMCPMC3938035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siljic M, Salemovic D, Cirkovic V, Pesic-Pavlovic I, Ranin J, Todorovic M, et al. Forensic application of phylogenetic analyses—Exploration of suspected HIV-1 transmission case. Forensic Sci Int Genet. 2017;27:100–5. doi: 10.1016/j.fsigen.2016.12.006 . [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez-Candelas F, Bracho MA, Wrobel B, Moya A. Molecular evolution in court: analysis of a large hepatitis C virus outbreak from an evolving source. BMC Biol. 2013;11:76 doi: 10.1186/1741-7007-11-76 ; PubMed Central PMCID: PMCPMC3717074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez-Candelas F, Bracho MA, Moya A. Molecular epidemiology and forensic genetics: application to a hepatitis C virus transmission event at a hemodialysis unit. J Infect Dis. 2003;187(3):352–8. doi: 10.1086/367965 . [DOI] [PubMed] [Google Scholar]
- 56.Zhivotovsky LA, Ahmed S, Wang W, Bittles AH. The forensic DNA implications of genetic differentiation between endogamous communities. Forensic Sci Int. 2001;119(3):269–72. . [DOI] [PubMed] [Google Scholar]
- 57.Perez-Losada M, Arenas M, Galan JC, Palero F, Gonzalez-Candelas F. Recombination in viruses: Mechanisms, methods of study, and evolutionary consequences. Infect Genet Evol. 2015;30C:296–307. doi: 10.1016/j.meegid.2014.12.022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arenas M, Lorenzo-Redondo R, Lopez-Galindez C. Influence of mutation and recombination on HIV-1 in vitro fitness recovery. Mol Phylogenet Evol. 2016;94(Pt A):264–70. doi: 10.1016/j.ympev.2015.09.001 . [DOI] [PubMed] [Google Scholar]
- 59.Juhas M. Horizontal gene transfer in human pathogens. Crit Rev Microbiol. 2015;41(1):101–8. doi: 10.3109/1040841X.2013.804031 . [DOI] [PubMed] [Google Scholar]
- 60.Schierup MH, Hein J. Consequences of recombination on traditional phylogenetic analysis. Genetics. 2000;156:879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mallo D, Sánchez-Cobos A, Arenas M. Diverse Considerations for Successful Phylogenetic Tree Reconstruction: Impacts from Model Misspecification, Recombination, Homoplasy, and Pattern Recognition In: Elloumi M, Iliopoulos C, Wang J, Zomaya A, editors. Pattern Recognition in Computational Molecular Biology: John Wiley & Sons, Inc; 2016. p. 439–56. [Google Scholar]
- 62.Arenas M. The Importance and Application of the Ancestral Recombination Graph. Front Genet. 2013;4:206 doi: 10.3389/fgene.2013.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arenas M, Posada D. The effect of recombination on the reconstruction of ancestral sequences. Genetics. 2010;184(4):1133–9. doi: 10.1534/genetics.109.113423 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arenas M, Patricio M, Posada D, Valiente G. Characterization of phylogenetic networks with NetTest. BMC Bioinformatics. 2010;11(1):268 doi: 10.1186/1471-2105-11-268 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lemmon AR, Moriarty EC. The importance of proper model assumption in bayesian phylogenetics. Syst Biol. 2004;53(2):265–77. doi: 10.1080/10635150490423520 . [DOI] [PubMed] [Google Scholar]
- 66.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–91. doi: 10.1111/j.1558-5646.1985.tb00420.x [DOI] [PubMed] [Google Scholar]
- 67.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214 doi: 10.1186/1471-2148-7-214 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burke T, Bruford MW. DNA fingerprinting in birds. Nature. 1987;327(6118):149–52. doi: 10.1038/327149a0 . [DOI] [PubMed] [Google Scholar]
- 69.Beamonte D, Guerra A, Ruiz B, Alemany J. Microsatellite DNA polymorphism analysis in a case of an illegal cattle purchase. J Forensic Sci. 1995;40(4):692–4. . [PubMed] [Google Scholar]
- 70.Schneider PM, Seo Y, Rittner C. Forensic mtDNA hair analysis excludes a dog from having caused a traffic accident. Int J Legal Med. 1999;112(5):315–6. . [DOI] [PubMed] [Google Scholar]
- 71.Ogden R, Mellanby RJ, Clements D, Gow AG, Powell R, McEwing R. Genetic data from 15 STR loci for forensic individual identification and parentage analyses in UK domestic dogs (Canis lupus familiaris). Forensic Sci Int Genet. 2012;6(2):e63–5. doi: 10.1016/j.fsigen.2011.04.015 . [DOI] [PubMed] [Google Scholar]
- 72.Dayton M, Koskinen MT, Tom BK, Mattila AM, Johnston E, Halverson J, et al. Developmental validation of short tandem repeat reagent kit for forensic DNA profiling of canine biological material. Croat Med J. 2009;50(3):268–85. doi: 10.3325/cmj.2009.50.268 ; PubMed Central PMCID: PMCPMC2702741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kanthaswamy S, Tom BK, Mattila AM, Johnston E, Dayton M, Kinaga J, et al. Canine population data generated from a multiplex STR kit for use in forensic casework. J Forensic Sci. 2009;54(4):829–40. doi: 10.1111/j.1556-4029.2009.01080.x . [DOI] [PubMed] [Google Scholar]
- 74.van Asch B, Alves C, Gusmao L, Pereira V, Pereira F, Amorim A. A new autosomal STR nineplex for canine identification and parentage testing. Electrophoresis. 2009;30(2):417–23. doi: 10.1002/elps.200800307 . [DOI] [PubMed] [Google Scholar]
- 75.Hellmann AP, Rohleder U, Eichmann C, Pfeiffer I, Parson W, Schleenbecker U. A proposal for standardization in forensic canine DNA typing: allele nomenclature of six canine-specific STR loci. J Forensic Sci. 2006;51(2):274–81. doi: 10.1111/j.1556-4029.2006.00049.x . [DOI] [PubMed] [Google Scholar]
- 76.Eichmann C, Berger B, Steinlechner M, Parson W. Estimating the probability of identity in a random dog population using 15 highly polymorphic canine STR markers. Forensic Sci Int. 2005;151(1):37–44. doi: 10.1016/j.forsciint.2004.07.002 . [DOI] [PubMed] [Google Scholar]
- 77.Eichmann C, Berger B, Parson W. A proposed nomenclature for 15 canine-specific polymorphic STR loci for forensic purposes. Int J Legal Med. 2004;118(5):249–66. doi: 10.1007/s00414-004-0452-5 . [DOI] [PubMed] [Google Scholar]
- 78.Berger B, Berger C, Hecht W, Hellmann A, Rohleder U, Schleenbecker U, et al. Validation of two canine STR multiplex-assays following the ISFG recommendations for non-human DNA analysis. Forensic Sci Int Genet. 2014;8(1):90–100. doi: 10.1016/j.fsigen.2013.07.002 . [DOI] [PubMed] [Google Scholar]
- 79.Menotti-Raymond M, David VA, Stephens JC, Lyons LA, O'Brien SJ. Genetic individualization of domestic cats using feline STR loci for forensic applications. J Forensic Sci. 1997;42(6):1039–51. . [PubMed] [Google Scholar]
- 80.Menotti-Raymond MA, David VA, Wachter LL, Butler JM, O'Brien SJ. An STR forensic typing system for genetic individualization of domestic cat (Felis catus) samples. J Forensic Sci. 2005;50(5):1061–70. . [PubMed] [Google Scholar]
- 81.Coomber N, David VA, O'Brien SJ, Menotti-Raymond M. Validation of a short tandem repeat multiplex typing system for genetic individualization of domestic cat samples. Croat Med J. 2007;48(4):547–55. ; PubMed Central PMCID: PMCPMC2080565. [PMC free article] [PubMed] [Google Scholar]
- 82.Bowling AT, Eggleston-Stott ML, Byrns G, Clark RS, Dileanis S, Wictum E. Validation of microsatellite markers for routine horse parentage testing. Anim Genet. 1997;28(4):247–52. . [DOI] [PubMed] [Google Scholar]
- 83.van de Goor LH, Panneman H, van Haeringen WA. A proposal for standardization in forensic equine DNA typing: allele nomenclature for 17 equine-specific STR loci. Anim Genet. 2010;41(2):122–7. doi: 10.1111/j.1365-2052.2009.01975.x . [DOI] [PubMed] [Google Scholar]
- 84.van de Goor LH, Panneman H, van Haeringen WA. A proposal for standardization in forensic bovine DNA typing: allele nomenclature of 16 cattle-specific short tandem repeat loci. Anim Genet. 2009;40(5):630–6. doi: 10.1111/j.1365-2052.2009.01891.x . [DOI] [PubMed] [Google Scholar]
- 85.Eiken HG, Andreassen RJ, Kopatz A, Bjervamoen SG, Wartiainen I, Tobiassen C, et al. Population data for 12 STR loci in Northern European brown bear (Ursus arctos) and application of DNA profiles for forensic casework. Forensic Science International: Genetics Supplement Series. 2009;2(1):273–4. doi: 10.1016/j.fsigss.2009.07.007 [Google Scholar]
- 86.Poetsch M, Seefeldt S, Maschke M, Lignitz E. Analysis of microsatellite polymorphism in red deer, roe deer, and fallow deer—possible employment in forensic applications. Forensic Sci Int. 2001;116(1):1–8. . [DOI] [PubMed] [Google Scholar]
- 87.Smith PF, DenDanto D, Smith KT, Palman D, Kornfield I. Allele frequencies for three STR loci RT24, RT09, and BM1225 in northern New England white-tailed deer. J Forensic Sci. 2002;47(3):673–5. . [PubMed] [Google Scholar]
- 88.Dawnay N, Ogden R, Thorpe RS, Pope LC, Dawson DA, McEwing R. A forensic STR profiling system for the Eurasian badger: a framework for developing profiling systems for wildlife species. Forensic Sci Int Genet. 2008;2(1):47–53. doi: 10.1016/j.fsigen.2007.08.006 . [DOI] [PubMed] [Google Scholar]
- 89.Dawnay N, Ogden R, Wetton JH, Thorpe RS, McEwing R. Genetic data from 28 STR loci for forensic individual identification and parentage analyses in 6 bird of prey species. Forensic Sci Int Genet. 2009;3(2):e63–9. doi: 10.1016/j.fsigen.2008.07.001 . [DOI] [PubMed] [Google Scholar]
- 90.Lee JC, Tsai LC, Kuan YY, Chien WH, Chang KT, Wu CH, et al. Racing pigeon identification using STR and chromo-helicase DNA binding gene markers. Electrophoresis. 2007;28(23):4274–81. doi: 10.1002/elps.200700063 . [DOI] [PubMed] [Google Scholar]
- 91.Grobler JP, Kotze A, Swart H, Hallerman EM. The application of microsatellite DNA markers for forensic analysis of koi carp (Cyprinus carpio). S Afr J Sci. 2005;101:19–21. [Google Scholar]
- 92.Eichmann C, Berger B, Reinhold M, Lutz M, Parson W. Canine-specific STR typing of saliva traces on dog bite wounds. Int J Legal Med. 2004;118(6):337–42. doi: 10.1007/s00414-004-0479-7 . [DOI] [PubMed] [Google Scholar]
- 93.Chen JW, Uboh CE, Soma LR, Li X, Guan F, You Y, et al. Identification of racehorse and sample contamination by novel 24-plex STR system. Forensic Sci Int Genet. 2010;4(3):158–67. doi: 10.1016/j.fsigen.2009.08.001 . [DOI] [PubMed] [Google Scholar]
- 94.Singh A, Gaur A, Shailaja K, Satyare Bala B, Singh L. A novel microsatellite (STR) marker for forensic identification of big cats in India. Forensic Sci Int. 2004;141(2–3):143–7. doi: 10.1016/j.forsciint.2004.01.015 . [DOI] [PubMed] [Google Scholar]
- 95.Barbanera F, Guerrini M, Beccani C, Forcina G, Anayiotos P, Panayides P. Conservation of endemic and threatened wildlife: molecular forensic DNA against poaching of the Cypriot mouflon (Ovis orientalis ophion, Bovidae). Forensic Sci Int Genet. 2012;6(5):671–5. doi: 10.1016/j.fsigen.2011.12.001 . [DOI] [PubMed] [Google Scholar]
- 96.Lorenzini R, Cabras P, Fanelli R, Carboni GL. Wildlife molecular forensics: identification of the Sardinian mouflon using STR profiling and the Bayesian assignment test. Forensic Sci Int Genet. 2011;5(4):345–9. doi: 10.1016/j.fsigen.2011.01.012 . [DOI] [PubMed] [Google Scholar]
- 97.Lorenzini R. DNA forensics and the poaching of wildlife in Italy: a case study. Forensic Sci Int. 2005;153(2–3):218–21. doi: 10.1016/j.forsciint.2005.04.032 . [DOI] [PubMed] [Google Scholar]
- 98.Caratti S, Rossi L, Sona B, Origlia S, Viara S, Martano G, et al. Analysis of 11 tetrameric STRs in wild boars for forensic purposes. Forensic Sci Int Genet. 2010;4(5):339–42. doi: 10.1016/j.fsigen.2010.07.001 . [DOI] [PubMed] [Google Scholar]
- 99.Wasser SK, Joseph Clark W, Drori O, Stephen Kisamo E, Mailand C, Mutayoba B, et al. Combating the illegal trade in African elephant ivory with DNA forensics. Conserv Biol. 2008;22(4):1065–71. 10.1111/j.1523-1739.2008.01012.x. doi: 10.1111/j.1523-1739.2008.01012.x . [DOI] [PubMed] [Google Scholar]
- 100.Alacs EA, Georges A, FitzSimmons NN, Robertson J. DNA detective: a review of molecular approaches to wildlife forensics. Forensic Sci Med Pathol. 2010;6(3):180–94. doi: 10.1007/s12024-009-9131-7 . [DOI] [PubMed] [Google Scholar]
- 101.Ogden R, Linacre A. Wildlife forensic science: A review of genetic geographic origin assignment. Forensic Sci Int Genet. 2015;18:152–9. doi: 10.1016/j.fsigen.2015.02.008 . [DOI] [PubMed] [Google Scholar]
- 102.Butler JM. Advanced Topics in Forensic DNA Typing: Methodology. San Diego: Academic Press; 2012. [Google Scholar]
- 103.van Hoppe MJ, Dy MA, van den Einden M, Iyengar A. SkydancerPlex: A novel STR multiplex validated for forensic use in the hen harrier (Circus cyaneus). Forensic Sci Int Genet. 2016;22:100–9. doi: 10.1016/j.fsigen.2016.02.003 . [DOI] [PubMed] [Google Scholar]
- 104.Gupta SK, Thangaraj K, Singh L. A simple and inexpensive molecular method for sexing and identification of the forensic samples of elephant origin. J Forensic Sci. 2006;51(4):805–7. doi: 10.1111/j.1556-4029.2006.00154.x . [DOI] [PubMed] [Google Scholar]
- 105.Wilson PJ, White BN. Sex identification of elk (Cervus elaphus canadensis), moose (Alces alces), and white-tailed deer (Odocoileus virginianus) using the polymerase chain reaction. J Forensic Sci. 1998;43(3):477–82. . [PubMed] [Google Scholar]
- 106.Aarnes SG, Hagen SB, Andreassen R, Schregel J, Knappskog PM, Hailer F, et al. Y-chromosomal testing of brown bears (Ursus arctos): Validation of a multiplex PCR-approach for nine STRs suitable for fecal and hair samples. Forensic Sci Int Genet. 2015;19:197–204. doi: 10.1016/j.fsigen.2015.07.018 . [DOI] [PubMed] [Google Scholar]
- 107.van Asch B, Pinheiro R, Pereira R, Alves C, Pereira V, Pereira F, et al. A framework for the development of STR genotyping in domestic animal species: characterization and population study of 12 canine X-chromosome loci. Electrophoresis. 2010;31(2):303–8. doi: 10.1002/elps.200900389 . [DOI] [PubMed] [Google Scholar]
- 108.Jensen T, Pernasetti FM, Durrant B. Conditions for rapid sex determination in 47 avian species by PCR of genomic DNA from blood, shell-membrane blood vessels, and feathers. Zoo Biol. 2003;22(6):561–71. doi: 10.1002/zoo.10101 [Google Scholar]
- 109.Ong AH, Vellayan S. An evaluation of CHD-Specific primer sets for sex typing of birds from feathers. Zoo Biol. 2008;27(1):62–9. doi: 10.1002/zoo.20163 . [DOI] [PubMed] [Google Scholar]
- 110.An J, Lee MY, Min MS, Lee MH, Lee H. A molecular genetic approach for species identification of mammals and sex determination of birds in a forensic case of poaching from South Korea. Forensic Sci Int. 2007;167(1):59–61. doi: 10.1016/j.forsciint.2005.12.031 . [DOI] [PubMed] [Google Scholar]
- 111.Heaton MP, Leymaster KA, Kalbfleisch TS, Kijas JW, Clarke SM, McEwan J, et al. SNPs for parentage testing and traceability in globally diverse breeds of sheep. PLoS One. 2014;9(4):e94851 doi: 10.1371/journal.pone.0094851 ; PubMed Central PMCID: PMCPMC3989260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heaton MP, Keen JE, Clawson ML, Harhay GP, Bauer N, Shultz C, et al. Use of bovine single nucleotide polymorphism markers to verify sample tracking in beef processing. J Am Vet Med Assoc. 2005;226(8):1311–4. . [DOI] [PubMed] [Google Scholar]
- 113.Heaton MP, Harhay GP, Bennett GL, Stone RT, Grosse WM, Casas E, et al. Selection and use of SNP markers for animal identification and paternity analysis in U.S. beef cattle. Mamm Genome. 2002;13(5):272–81. doi: 10.1007/s00335-001-2146-3 . [DOI] [PubMed] [Google Scholar]
- 114.Goffaux F, China B, Dams L, Clinquart A, Daube G. Development of a genetic traceability test in pig based on single nucleotide polymorphism detection. Forensic Sci Int. 2005;151(2–3):239–47. doi: 10.1016/j.forsciint.2005.02.013 . [DOI] [PubMed] [Google Scholar]
- 115.Rohrer GA, Freking BA, Nonneman D. Single nucleotide polymorphisms for pig identification and parentage exclusion. Anim Genet. 2007;38(3):253–8. doi: 10.1111/j.1365-2052.2007.01593.x . [DOI] [PubMed] [Google Scholar]
- 116.Brooks A, Creighton EK, Gandolfi B, Khan R, Grahn RA, Lyons LA. SNP Miniplexes for Individual Identification of Random-Bred Domestic Cats. J Forensic Sci. 2016;61(3):594–606. doi: 10.1111/1556-4029.13026 ; PubMed Central PMCID: PMCPMC5019183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Werner FA, Durstewitz G, Habermann FA, Thaller G, Kramer W, Kollers S, et al. Detection and characterization of SNPs useful for identity control and parentage testing in major European dairy breeds. Anim Genet. 2004;35(1):44–9. . [DOI] [PubMed] [Google Scholar]
- 118.Syvanen AC. Accessing genetic variation: genotyping single nucleotide polymorphisms. Nat Rev Genet. 2001;2(12):930–42. doi: 10.1038/35103535 . [DOI] [PubMed] [Google Scholar]
- 119.Budowle B, Allard MW, Wilson MR, Chakraborty R. Forensics and mitochondrial DNA: applications, debates, and foundations. Annu Rev Genomics Hum Genet. 2003;4:119–41. 10.1146/annurev.genom.4.070802.110352. doi: 10.1146/annurev.genom.4.070802.110352 . [DOI] [PubMed] [Google Scholar]
- 120.Pereira F, Carneiro J, Van Asch B. A guide for mitochondrial DNA analysis in non-human forensic investigations. Open Forensic Science Journal. 2010;3:33–44. [Google Scholar]
- 121.Himmelberger AL, Spear TF, Satkoski JA, George DA, Garnica WT, Malladi VS, et al. Forensic utility of the mitochondrial hypervariable region 1 of domestic dogs, in conjunction with breed and geographic information. J Forensic Sci. 2008;53(1):81–9. doi: 10.1111/j.1556-4029.2007.00615.x . [DOI] [PubMed] [Google Scholar]
- 122.Tarditi CR, Grahn RA, Evans JJ, Kurushima JD, Lyons LA. Mitochondrial DNA sequencing of cat hair: an informative forensic tool. J Forensic Sci. 2011;56 Suppl 1:S36–46. doi: 10.1111/j.1556-4029.2010.01592.x ; PubMed Central PMCID: PMCPMC3059515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Savolainen P, Rosen B, Holmberg A, Leitner T, Uhlen M, Lundeberg J. Sequence analysis of domestic dog mitochondrial DNA for forensic use. J Forensic Sci. 1997;42(4):593–600. . [PubMed] [Google Scholar]
- 124.Wu H, Wan QH, Fang SG, Zhang SY. Application of mitochondrial DNA sequence analysis in the forensic identification of Chinese sika deer subspecies. Forensic Sci Int. 2005;148(2–3):101–5. doi: 10.1016/j.forsciint.2004.04.072 . [DOI] [PubMed] [Google Scholar]
- 125.Branicki W, Kupiec T, Pawlowski R. Validation of cytochrome b sequence analysis as a method of species identification. J Forensic Sci. 2003;48(1):83–7. . [PubMed] [Google Scholar]
- 126.Parson W, Pegoraro K, Niederstatter H, Foger M, Steinlechner M. Species identification by means of the cytochrome b gene. Int J Legal Med. 2000;114(1–2):23–8. . [DOI] [PubMed] [Google Scholar]
- 127.Hsieh HM, Chiang HL, Tsai LC, Lai SY, Huang NE, Linacre A, et al. Cytochrome b gene for species identification of the conservation animals. Forensic Sci Int. 2001;122(1):7–18. . [DOI] [PubMed] [Google Scholar]
- 128.Hajibabaei M, Smith MA, Janzen DH, Rodriguez JJ, Whitfield JB, Hebert PDN. A minimalist barcode can identify a specimen whose DNA is degraded. Mol Ecol Notes. 2006;6(4):959–64. doi: 10.1111/j.1471-8286.2006.01470.x [Google Scholar]
- 129.Dawnay N, Ogden R, McEwing R, Carvalho GR, Thorpe RS. Validation of the barcoding gene COI for use in forensic genetic species identification. Forensic Sci Int. 2007;173(1):1–6. doi: 10.1016/j.forsciint.2006.09.013 . [DOI] [PubMed] [Google Scholar]
- 130.Wilson-Wilde L, Norman J, Robertson J, Sarre S, Georges A. Current issues in species identification for forensic science and the validity of using the cytochrome oxidase I (COI) gene. Forensic Sci Med Pathol. 2010;6(3):233–41. doi: 10.1007/s12024-010-9172-y . [DOI] [PubMed] [Google Scholar]
- 131.Melton T, Holland C. Routine forensic use of the mitochondrial 12S ribosomal RNA gene for species identification. J Forensic Sci. 2007;52(6):1305–7. doi: 10.1111/j.1556-4029.2007.00553.x . [DOI] [PubMed] [Google Scholar]
- 132.Balitzki-Korte B, Anslinger K, Bartsch C, Rolf B. Species identification by means of pyrosequencing the mitochondrial 12S rRNA gene. Int J Legal Med. 2005;119(5):291–4. doi: 10.1007/s00414-005-0537-9 . [DOI] [PubMed] [Google Scholar]
- 133.Hsieh HM, Huang LH, Tsai LC, Kuo YC, Meng HH, Linacre A, et al. Species identification of rhinoceros horns using the cytochrome b gene. Forensic Sci Int. 2003;136(1–3):1–11. . [DOI] [PubMed] [Google Scholar]
- 134.Lee JC, Hsieh HM, Huang LH, Kuo YC, Wu JH, Chin SC, et al. Ivory identification by DNA profiling of cytochrome b gene. Int J Legal Med. 2009;123(2):117–21. doi: 10.1007/s00414-008-0264-0 . [DOI] [PubMed] [Google Scholar]
- 135.Lee JC, Tsai LC, Liao SP, Linacre A, Hsieh HM. Species identification using the cytochrome b gene of commercial turtle shells. Forensic Sci Int Genet. 2009;3(2):67–73. doi: 10.1016/j.fsigen.2008.10.005 . [DOI] [PubMed] [Google Scholar]
- 136.Wong KL, Wang J, But PP, Shaw PC. Application of cytochrome b DNA sequences for the authentication of endangered snake species. Forensic Sci Int. 2004;139(1):49–55. . [DOI] [PubMed] [Google Scholar]
- 137.Wan QH, Fang SG. Application of species-specific polymerase chain reaction in the forensic identification of tiger species. Forensic Sci Int. 2003;131(1):75–8. . [DOI] [PubMed] [Google Scholar]
- 138.Wells JD, Stevens JR. Application of DNA-based methods in forensic entomology. Annu Rev Entomol. 2008;53:103–20. doi: 10.1146/annurev.ento.52.110405.091423 . [DOI] [PubMed] [Google Scholar]
- 139.Sperling FA, Anderson GS, Hickey DA. A DNA-based approach to the identification of insect species used for postmortem interval estimation. J Forensic Sci. 1994;39(2):418–27. . [PubMed] [Google Scholar]
- 140.Malgorn Y, Coquoz R. DNA typing for identification of some species of Calliphoridae. An interest in forensic entomology. Forensic Sci Int. 1999;102(2–3):111–9. . [DOI] [PubMed] [Google Scholar]
- 141.Coghlan ML, White NE, Parkinson L, Haile J, Spencer PB, Bunce M. Egg forensics: an appraisal of DNA sequencing to assist in species identification of illegally smuggled eggs. Forensic Sci Int Genet. 2012;6(2):268–73. doi: 10.1016/j.fsigen.2011.06.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.DeSalle R, Birstein VJ. PCR identification of black caviar. Nature. 1996;381(6579):197–8. [Google Scholar]
- 143.Abercrombie DL, Clarke SC, Shivji MS. Global-scale genetic identification of hammerhead sharks: Application to assessment of the international fin trade and law enforcement. Conserv Genet. 2005;6(5):775–88. doi: 10.1007/s10592-005-9036-2 [Google Scholar]
- 144.Chapman DD, Abercrombie DL, Douady CJ, Pikitch EK, Stanhopen MJ, Shivji MS. A streamlined, bi-organelle, multiplex PCR approach to species identification: Application to global conservation and trade monitoring of the great white shark, Carcharodon carcharias. Conserv Genet. 2003;4(4):415–25. doi: 10.1023/A:1024771215616 [Google Scholar]
- 145.Kitpipit T, Thongjued K, Penchart K, Ouithavon K, Chotigeat W. Mini-SNaPshot multiplex assays authenticate elephant ivory and simultaneously identify the species origin. Forensic Sci Int Genet. 2017;27:106–15. doi: 10.1016/j.fsigen.2016.12.007 . [DOI] [PubMed] [Google Scholar]
- 146.Sato I, Nakaki S, Murata K, Takeshita H, Mukai T. Forensic hair analysis to identify animal species on a case of pet animal abuse. Int J Legal Med. 2010;124(3):249–56. doi: 10.1007/s00414-009-0383-2 . [DOI] [PubMed] [Google Scholar]
- 147.Nakaki S, Hino D, Miyoshi M, Nakayama H, Moriyoshi H, Morikawa T, et al. Study of animal species (human, dog and cat) identification using a multiplex single-base primer extension reaction in the cytochrome b gene. Forensic Sci Int. 2007;173(2–3):97–102. doi: 10.1016/j.forsciint.2007.02.010 . [DOI] [PubMed] [Google Scholar]
- 148.Kurushima JD, Lipinski MJ, Gandolfi B, Froenicke L, Grahn JC, Grahn RA, et al. Variation of cats under domestication: genetic assignment of domestic cats to breeds and worldwide random-bred populations. Anim Genet. 2013;44(3):311–24. doi: 10.1111/age.12008 ; PubMed Central PMCID: PMCPMC3594446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Andersson L, Georges M. Domestic-animal genomics: deciphering the genetics of complex traits. Nat Rev Genet. 2004;5(3):202–12. doi: 10.1038/nrg1294 . [DOI] [PubMed] [Google Scholar]
- 150.O'Neill K. How Two Cows Make a Crisis: U.S.-Canada Trade Relations and Mad Cow Disease. American Review of Canadian Studies. 2005;35(2):295–319. doi: 10.1080/02722010509481374 [Google Scholar]
- 151.Coulthart MB, Mogk R, Rancourt JM, Godal DL, Czub S. Prion protein gene sequence of Canada's first non-imported case of bovine spongiform encephalopathy (BSE). Genome. 2003;46(6):1005–9. doi: 10.1139/g03-124 . [DOI] [PubMed] [Google Scholar]
- 152.Aquila I, Ausania F, Di Nunzio C, Serra A, Boca S, Capelli A, et al. The role of forensic botany in crime scene investigation: case report and review of literature. J Forensic Sci. 2014;59(3):820–4. doi: 10.1111/1556-4029.12401 . [DOI] [PubMed] [Google Scholar]
- 153.Ferri G, Corradini B, Ferrari F, Santunione AL, Palazzoli F, Alu M. Forensic botany II, DNA barcode for land plants: Which markers after the international agreement? Forensic Sci Int Genet. 2015;15:131–6. doi: 10.1016/j.fsigen.2014.10.005 . [DOI] [PubMed] [Google Scholar]
- 154.Linacre A, Thorpe J. Detection and identification of cannabis by DNA. Forensic Sci Int. 1998;91(1):71–6. . [DOI] [PubMed] [Google Scholar]
- 155.Prosser SW, Hebert PD. Rapid identification of the botanical and entomological sources of honey using DNA metabarcoding. Food Chem. 2017;214:183–91. doi: 10.1016/j.foodchem.2016.07.077 . [DOI] [PubMed] [Google Scholar]
- 156.Sharma S, Shrivastava N. Renaissance in phytomedicines: promising implications of NGS technologies. Planta. 2016;244(1):19–38. doi: 10.1007/s00425-016-2492-8 . [DOI] [PubMed] [Google Scholar]
- 157.Dufresnes C, Jan C, Bienert F, Goudet J, Fumagalli L. Broad-Scale Genetic Diversity of Cannabis for Forensic Applications. PLoS One. 2017;12(1):e0170522 doi: 10.1371/journal.pone.0170522 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sukrong S, Zhu S, Ruangrungsi N, Phadungcharoen T, Palanuvej C, Komatsu K. Molecular analysis of the genus Mitragyna existing in Thailand based on rDNA ITS sequences and its application to identify a narcotic species: Mitragyna speciosa. Biol Pharm Bull. 2007;30(7):1284–8. . [DOI] [PubMed] [Google Scholar]
- 159.Lee EJ, Hwang IK, Kim NY, Lee KL, Han MS, Lee YH, et al. An assessment of the utility of universal and specific genetic markers for opium poppy identification. J Forensic Sci. 2010;55(5):1202–8. doi: 10.1111/j.1556-4029.2010.01423.x . [DOI] [PubMed] [Google Scholar]
- 160.Nowakowska JA, Oszako T, Tereba A, Konecka A. Forest Tree Species Traced with a DNA-Based Proof for Illegal Logging Case in Poland In: Pontarotti P, editor. Evolutionary Biology: Biodiversification from Genotype to Phenotype. Cham: Springer International Publishing; 2015. p. 373–88. [Google Scholar]
- 161.Dormontt EE, Boner M, Braun B, Breulmann G, Degen B, Espinoza E, et al. Forensic timber identification: It's time to integrate disciplines to combat illegal logging. Biol Conserv. 2015;191:790–8. http://dx.doi.org/10.1016/j.biocon.2015.06.038. [Google Scholar]
- 162.Honjo M, Ueno S, Tsumura Y, Handa T, Washitani I, Ohsawa R. Tracing the origins of stocks of the endangered species Primula sieboldii using nuclear microsatellites and chloroplast DNA. Conserv Genet. 2008;9(5):1139–47. doi: 10.1007/s10592-007-9427-7 [Google Scholar]
- 163.Nazareno AG, dos Reis MS. Where did they come from? Genetic diversity and forensic investigation of the threatened palm species Butia eriospatha. Conserv Genet. 2014;15(2):441–52. doi: 10.1007/s10592-013-0552-1 [Google Scholar]
- 164.Felder E, Mossbrugger I, Lange M, Wolfel R. Simultaneous detection of ricin and abrin DNA by real-time PCR (qPCR). Toxins (Basel). 2012;4(9):633–42. doi: 10.3390/toxins4090633 ; PubMed Central PMCID: PMCPMC3475220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ward J, Gilmore SR, Robertson J, Peakall R. A grass molecular identification system for forensic botany: a critical evaluation of the strengths and limitations. J Forensic Sci. 2009;54(6):1254–60. doi: 10.1111/j.1556-4029.2009.01196.x . [DOI] [PubMed] [Google Scholar]
- 166.Virtanen V, Korpelainen H, Kostamo K. Forensic botany: usability of bryophyte material in forensic studies. Forensic Sci Int. 2007;172(2–3):161–3. doi: 10.1016/j.forsciint.2006.11.012 . [DOI] [PubMed] [Google Scholar]
- 167.Cardoso HF, Santos A, Dias R, Garcia C, Pinto M, Sergio C, et al. Establishing a minimum postmortem interval of human remains in an advanced state of skeletonization using the growth rate of bryophytes and plant roots. Int J Legal Med. 2010;124(5):451–6. doi: 10.1007/s00414-009-0372-5 . [DOI] [PubMed] [Google Scholar]
- 168.Schmedes SE, Sajantila A, Budowle B. Expansion of Microbial Forensics. J Clin Microbiol. 2016. doi: 10.1128/JCM.00046-16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shrivastava P, Jain T, Gupta MK. Microbial Forensics in Legal Medicine. SAS Journal of Medicine. 2015;1(1):33–40. [Google Scholar]
- 170.Budowle B, Johnson MD, Fraser CM, Leighton TJ, Murch RS, Chakraborty R. Genetic analysis and attribution of microbial forensics evidence. Crit Rev Microbiol. 2005;31(4):233–54. doi: 10.1080/10408410500304082 . [DOI] [PubMed] [Google Scholar]
- 171.Budowle B, Connell ND, Bielecka-Oder A, Colwell RR, Corbett CR, Fletcher J, et al. Validation of high throughput sequencing and microbial forensics applications. Investig Genet. 2014;5:9 doi: 10.1186/2041-2223-5-9 ; PubMed Central PMCID: PMCPMC4123828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Budowle B, Schutzer SE, Einseln A, Kelley LC, Walsh AC, Smith JA, et al. Public health. Building microbial forensics as a response to bioterrorism. Science. 2003;301(5641):1852–3. doi: 10.1126/science.1090083 . [DOI] [PubMed] [Google Scholar]
- 173.Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M, et al. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis. 2001;7(6):933–44. doi: 10.3201/eid0706.010604 ; PubMed Central PMCID: PMCPMC2631903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jernigan DB, Raghunathan PL, Bell BP, Brechner R, Bresnitz EA, Butler JC, et al. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis. 2002;8(10):1019–28. doi: 10.3201/eid0810.020353 ; PubMed Central PMCID: PMCPMC2730292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Baillie GJ, Galiano M, Agapow PM, Myers R, Chiam R, Gall A, et al. Evolutionary dynamics of local pandemic H1N1/2009 influenza virus lineages revealed by whole-genome analysis. J Virol. 2012;86(1):11–8. doi: 10.1128/JVI.05347-11 ; PubMed Central PMCID: PMCPMC3255882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chin CS, Sorenson J, Harris JB, Robins WP, Charles RC, Jean-Charles RR, et al. The origin of the Haitian cholera outbreak strain. N Engl J Med. 2011;364(1):33–42. doi: 10.1056/NEJMoa1012928 ; PubMed Central PMCID: PMCPMC3030187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Grad YH, Lipsitch M, Feldgarden M, Arachchi HM, Cerqueira GC, Fitzgerald M, et al. Genomic epidemiology of the Escherichia coli O104:H4 outbreaks in Europe, 2011. Proc Natl Acad Sci U S A. 2012;109(8):3065–70. doi: 10.1073/pnas.1121491109 ; PubMed Central PMCID: PMCPMC3286951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407–16. doi: 10.1056/NEJMoa1306742 ; PubMed Central PMCID: PMCPMC4029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hu Y, Zhang Y, Ren X, Liu Y, Xiao Y, Li L, et al. A case report demonstrating the utility of next generation sequencing in analyzing serial samples from the lung following an infection with influenza A (H7N9) virus. J Clin Virol. 2016;76:45–50. doi: 10.1016/j.jcv.2015.12.013 . [DOI] [PubMed] [Google Scholar]
- 180.Park DJ, Dudas G, Wohl S, Goba A, Whitmer SL, Andersen KG, et al. Ebola Virus Epidemiology, Transmission, and Evolution during Seven Months in Sierra Leone. Cell. 2015;161(7):1516–26. doi: 10.1016/j.cell.2015.06.007 ; PubMed Central PMCID: PMCPMC4503805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Aylward RB, Alwan A. Polio in Syria. The Lancet. 2014;383(9916):489–91. doi: 10.1016/S0140-6736(14)60132-X [DOI] [PubMed] [Google Scholar]
- 182.George F, Shivaji T, Pinto CS, Serra LAO, Valente J, Albuquerque MJ, et al. A large outbreak of Legionnaires’ Disease in an industrial town in Portugal. Revista Portuguesa de Saúde Pública. 2016;34(3):199–208. http://dx.doi.org/10.1016/j.rpsp.2016.10.001. [Google Scholar]
- 183.Faria NR, Azevedo Rdo S, Kraemer MU, Souza R, Cunha MS, Hill SC, et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science. 2016;352(6283):345–9. doi: 10.1126/science.aaf5036 ; PubMed Central PMCID: PMCPMC4918795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chen M, Ma Y, Yang C, Yang L, Chen H, Dong L, et al. The combination of phylogenetic analysis with epidemiological and serological data to track HIV-1 transmission in a sexual transmission case. PLoS One. 2015;10(3):e0119989 doi: 10.1371/journal.pone.0119989 ; PubMed Central PMCID: PMCPMC4373787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Eyre DW, Golubchik T, Gordon NC, Bowden R, Piazza P, Batty EM, et al. A pilot study of rapid benchtop sequencing of Staphylococcus aureus and Clostridium difficile for outbreak detection and surveillance. BMJ Open. 2012;2(3). doi: 10.1136/bmjopen-2012-001124 ; PubMed Central PMCID: PMCPMC3378946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Koser CU, Holden MT, Ellington MJ, Cartwright EJ, Brown NM, Ogilvy-Stuart AL, et al. Rapid whole-genome sequencing for investigation of a neonatal MRSA outbreak. N Engl J Med. 2012;366(24):2267–75. doi: 10.1056/NEJMoa1109910 ; PubMed Central PMCID: PMCPMC3715836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Neubauer J, Lecca MR, Russo G, Bartsch C, Medeiros-Domingo A, Berger W, et al. Post-mortem whole-exome analysis in a large sudden infant death syndrome cohort with a focus on cardiovascular and metabolic genetic diseases. Eur J Hum Genet. 2017. doi: 10.1038/ejhg.2016.199 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Alfelali M, Khandaker G. Infectious causes of sudden infant death syndrome. Paediatr Respir Rev. 2014;15(4):307–11. doi: 10.1016/j.prrv.2014.09.004 . [DOI] [PubMed] [Google Scholar]
- 189.Grice EA. The intersection of microbiome and host at the skin interface: genomic- and metagenomic-based insights. Genome Res. 2015;25(10):1514–20. doi: 10.1101/gr.191320.115 ; PubMed Central PMCID: PMCPMC4579337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. doi: 10.1038/nature11234 ; PubMed Central PMCID: PMCPMC3564958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016;14(8):e1002533 doi: 10.1371/journal.pbio.1002533 ; PubMed Central PMCID: PMCPMC4991899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhu A, Sunagawa S, Mende DR, Bork P. Inter-individual differences in the gene content of human gut bacterial species. Genome Biol. 2015;16:82 doi: 10.1186/s13059-015-0646-9 ; PubMed Central PMCID: PMCPMC4428241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hampton-Marcell JT, Lopez JV, Gilbert JA. The human microbiome: an emerging tool in forensics. Microb Biotechnol. 2017;10(2):228–30. doi: 10.1111/1751-7915.12699 ; PubMed Central PMCID: PMCPMC5328825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Javan GT, Finley SJ, Abidin Z, Mulle JG. The Thanatomicrobiome: A Missing Piece of the Microbial Puzzle of Death. Front Microbiol. 2016;7:225 doi: 10.3389/fmicb.2016.00225 ; PubMed Central PMCID: PMCPMC4764706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Toppinen M, Perdomo MF, Palo JU, Simmonds P, Lycett SJ, Soderlund-Venermo M, et al. Bones hold the key to DNA virus history and epidemiology. Sci Rep. 2015;5:17226 doi: 10.1038/srep17226 ; PubMed Central PMCID: PMCPMC4661702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bhattacharya S. Science in court: Disease detectives. Nature. 2014;506(7489):424–6. doi: 10.1038/506424a . [DOI] [PubMed] [Google Scholar]
- 197.Sjodin A, Broman T, Melefors O, Andersson G, Rasmusson B, Knutsson R, et al. The need for high-quality whole-genome sequence databases in microbial forensics. Biosecur Bioterror. 2013;11 Suppl 1:S78–86. doi: 10.1089/bsp.2013.0007 . [DOI] [PubMed] [Google Scholar]
- 198.Araujo R, Pereira F, van Asch B. Applications of DNA-Based Methods in Food Forensics In: Amorim A, Budowle B, editors. Handbook of Forensic Genetics: Biodiversity and Heredity in Civil and Criminal Investigation. New Jersey: World Scientific; 2017. p. 493–517. [Google Scholar]
- 199.Teletchea F, Maudet C, Hanni C. Food and forensic molecular identification: update and challenges. Trends Biotechnol. 2005;23(7):359–66. doi: 10.1016/j.tibtech.2005.05.006 . [DOI] [PubMed] [Google Scholar]
- 200.Pascal G, Mahe S. Identity, traceability, acceptability and substantial equivalence of food. Cell Mol Biol (Noisy-Le-Grand). 2001;47(8):1329–42. . [PubMed] [Google Scholar]
- 201.Primrose S, Woolfe M, Rollinson S. Food forensics: methods for determining the authenticity of foodstuffs. Trends in Food Science & Technology. 2010;21(12):582–90. http://dx.doi.org/10.1016/j.tifs.2010.09.006. [Google Scholar]
- 202.Galimberti A, De Mattia F, Losa A, Bruni I, Federici S, Casiraghi M, et al. DNA barcoding as a new tool for food traceability. Food Res Int. 2013;50(1):55–63. http://dx.doi.org/10.1016/j.foodres.2012.09.036. [Google Scholar]
- 203.Oliveira M, Amorim MI, L. A. Authentication of plant food products: Under the magnification of Botany Forensics. Nova Acta Científica Compostelana. 2017;24:45–62. [Google Scholar]
- 204.Martins-Lopes P, Gomes S, Pereira L, Guedes-Pinto H. Molecular markers for food traceability. Food Technology and Biotechnology. 2013;51(2):198–207. [Google Scholar]
- 205.Pereira L, Gomes S, Castro C, Eiras-Dias JE, Brazao J, Graca A, et al. High Resolution Melting (HRM) applied to wine authenticity. Food Chem. 2017;216:80–6. doi: 10.1016/j.foodchem.2016.07.185 . [DOI] [PubMed] [Google Scholar]
- 206.Osathanunkul M, Suwannapoom C, Osathanunkul K, Madesis P, de Boer H. Evaluation of DNA barcoding coupled high resolution melting for discrimination of closely related species in phytopharmaceuticals. Phytomedicine. 2016;23(2):156–65. doi: 10.1016/j.phymed.2015.11.018 . [DOI] [PubMed] [Google Scholar]
- 207.Xanthopoulou A, Ganopoulos I, Kalivas A, Osathanunkul M, Chatzopoulou P, Tsaftaris A, et al. Multiplex HRM analysis as a tool for rapid molecular authentication of nine herbal teas. Food Control. 2016;60:113–6. http://dx.doi.org/10.1016/j.foodcont.2015.07.021. [Google Scholar]
- 208.Ortola-Vidal A, Schnerr H, Rojmyr M, Lysholm F, Knight A. Quantitative identification of plant genera in food products using PCR and Pyrosequencing® technology. Food Control. 2007;18(8):921–7. http://dx.doi.org/10.1016/j.foodcont.2006.04.013. [Google Scholar]
- 209.Bazakos C, Dulger AO, Uncu AT, Spaniolas S, Spano T, Kalaitzis P. A SNP-based PCR-RFLP capillary electrophoresis analysis for the identification of the varietal origin of olive oils. Food Chem. 2012;134(4):2411–8. doi: 10.1016/j.foodchem.2012.04.031 . [DOI] [PubMed] [Google Scholar]
- 210.Pasqualone A, Montemurro C, Caponio F, Blanco A. Identification of virgin olive oil from different cultivars by analysis of DNA microsatellites. J Agric Food Chem. 2004;52(5):1068–71. doi: 10.1021/jf0348424 . [DOI] [PubMed] [Google Scholar]
- 211.Cipriani G, Marrazzo MT, Di Gaspero G, Pfeiffer A, Morgante M, Testolin R. A set of microsatellite markers with long core repeat optimized for grape (Vitis spp.) genotyping. BMC Plant Biol. 2008;8:127 doi: 10.1186/1471-2229-8-127 ; PubMed Central PMCID: PMCPMC2625351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cabezas JA, Ibanez J, Lijavetzky D, Velez D, Bravo G, Rodriguez V, et al. A 48 SNP set for grapevine cultivar identification. BMC Plant Biol. 2011;11:153 doi: 10.1186/1471-2229-11-153 ; PubMed Central PMCID: PMCPMC3221639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Santos S, Oliveira M, Amorim A, van Asch B. A forensic perspective on the genetic identification of grapevine (Vitis vinifera L.) varieties using STR markers. Electrophoresis. 2014;35(21–22):3201–7. doi: 10.1002/elps.201400107 . [DOI] [PubMed] [Google Scholar]
- 214.Laube I, Hird H, Brodmann P, Ullmann S, Schöne-Michling M, Chisholm J, et al. Development of primer and probe sets for the detection of plant species in honey. Food Chem. 2010;118(4):979–86. http://dx.doi.org/10.1016/j.foodchem.2008.09.063. [Google Scholar]
- 215.Bruni I, Galimberti A, Caridi L, Scaccabarozzi D, De Mattia F, Casiraghi M, et al. A DNA barcoding approach to identify plant species in multiflower honey. Food Chem. 2015;170:308–15. doi: 10.1016/j.foodchem.2014.08.060 [DOI] [PubMed] [Google Scholar]
- 216.Olivieri C, Marota I, Rollo F, Luciani S. Tracking plant, fungal, and bacterial DNA in honey specimens. J Forensic Sci. 2012;57(1):222–7. doi: 10.1111/j.1556-4029.2011.01964.x . [DOI] [PubMed] [Google Scholar]
- 217.Bonito G. Fast DNA-based identification of the black truffle Tuber melanosporum with direct PCR and species-specific primers. FEMS Microbiol Lett. 2009;301(2):171–5. doi: 10.1111/j.1574-6968.2009.01812.x . [DOI] [PubMed] [Google Scholar]
- 218.Gausterer C, Penker M, Krisai-Greilhuber I, Stein C, Stimpfl T. Rapid genetic detection of ingested Amanita phalloides. Forensic Sci Int Genet. 2014;9:66–71. doi: 10.1016/j.fsigen.2013.11.002 . [DOI] [PubMed] [Google Scholar]
- 219.Nugent KG, Saville BJ. Forensic analysis of hallucinogenic fungi: a DNA-based approach. Forensic Sci Int. 2004;140(2–3):147–57. doi: 10.1016/j.forsciint.2003.11.022 . [DOI] [PubMed] [Google Scholar]
- 220.Goncalves J, Pereira F, Amorim A, van Asch B. New method for the simultaneous identification of cow, sheep, goat, and water buffalo in dairy products by analysis of short species-specific mitochondrial DNA targets. J Agric Food Chem. 2012;60(42):10480–5. doi: 10.1021/jf3029896 . [DOI] [PubMed] [Google Scholar]
- 221.Mafra I, Roxo Á, Ferreira IMPLVO, Oliveira MBPP. A duplex polymerase chain reaction for the quantitative detection of cows’ milk in goats’ milk cheese. Int Dairy J. 2007;17(9):1132–8. http://dx.doi.org/10.1016/j.idairyj.2007.01.009. [Google Scholar]
- 222.De S, Brahma B, Polley S, Mukherjee A, Banerjee D, Gohaina M, et al. Simplex and duplex PCR assays for species specific identification of cattle and buffalo milk and cheese. Food Control. 2011;22(5):690–6. http://dx.doi.org/10.1016/j.foodcont.2010.09.026. [Google Scholar]
- 223.Fajardo V, González I, Rojas M, García T, Martín R. A review of current PCR-based methodologies for the authentication of meats from game animal species. Trends in Food Science & Technology. 2010;21(8):408–21. http://dx.doi.org/10.1016/j.tifs.2010.06.002. [Google Scholar]
- 224.Ganopoulos I, Argiriou A, Tsaftaris A. Adulterations in Basmati rice detected quantitatively by combined use of microsatellite and fragrance typing with High Resolution Melting (HRM) analysis. Food Chem. 2011;129(2):652–9. http://dx.doi.org/10.1016/j.foodchem.2011.04.109. [DOI] [PubMed] [Google Scholar]
- 225.Chagne D, Gasic K, Crowhurst RN, Han Y, Bassett HC, Bowatte DR, et al. Development of a set of SNP markers present in expressed genes of the apple. Genomics. 2008;92(5):353–8. doi: 10.1016/j.ygeno.2008.07.008 . [DOI] [PubMed] [Google Scholar]
- 226.Ahmad R, Parfitt DE, Fass J, Ogundiwin E, Dhingra A, Gradziel TM, et al. Whole genome sequencing of peach (Prunus persica L.) for SNP identification and selection. BMC Genomics. 2011;12:569 doi: 10.1186/1471-2164-12-569 ; PubMed Central PMCID: PMCPMC3253712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ganopoulos I, Madesis P, Darzentas N, Argiriou A, Tsaftaris A. Barcode High Resolution Melting (Bar-HRM) analysis for detection and quantification of PDO "Fava Santorinis" (Lathyrus clymenum) adulterants. Food Chem. 2012;133(2):505–12. doi: 10.1016/j.foodchem.2012.01.015 . [DOI] [PubMed] [Google Scholar]
- 228.Madesis P, Ganopoulos I, Anagnostis A, Tsaftaris A. The application of Bar-HRM (Barcode DNA-High Resolution Melting) analysis for authenticity testing and quantitative detection of bean crops (Leguminosae) without prior DNA purification. Food Control. 2012;25(2):576–82. http://dx.doi.org/10.1016/j.foodcont.2011.11.034. [Google Scholar]
- 229.Ferreira T, Farah A, Oliveira TC, Lima IS, Vitório F, Oliveira EMM. Using Real-Time PCR as a tool for monitoring the authenticity of commercial coffees. Food Chem. 2016;199:433–8. doi: 10.1016/j.foodchem.2015.12.045 [DOI] [PubMed] [Google Scholar]
- 230.Stoeckle MY, Gamble CC, Kirpekar R, Young G, Ahmed S, Little DP. Commercial teas highlight plant DNA barcode identification successes and obstacles. Sci Rep. 2011;1:42 doi: 10.1038/srep00042 ; PubMed Central PMCID: PMCPMC3216529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Congiu L, Chicca M, Cella R, Rossi R, Bernacchia G. The use of random amplified polymorphic DNA (RAPD) markers to identify strawberry varieties: a forensic application. Mol Ecol. 2000;9(2):229–32. . [DOI] [PubMed] [Google Scholar]
- 232.Giménez MJ, Pistón F, Martín A, Atienza SG. Application of real-time PCR on the development of molecular markers and to evaluate critical aspects for olive oil authentication. Food Chem. 2010;118(2):482–7. http://dx.doi.org/10.1016/j.foodchem.2009.05.012. [Google Scholar]
- 233.Zarouri B, Vargas AM, Gaforio L, Aller M, de Andrés MT, Cabezas JA. Whole-genome genotyping of grape using a panel of microsatellite multiplex PCRs. Tree Genetics & Genomes. 2015;11(2):17 doi: 10.1007/s11295-015-0843-4 [Google Scholar]
- 234.Moon BC, Kim WJ, Ji Y, Lee YM, Kang YM, Choi G. Molecular identification of the traditional herbal medicines, Arisaematis Rhizoma and Pinelliae Tuber, and common adulterants via universal DNA barcode sequences. Genet Mol Res. 2016;15(1). doi: 10.4238/gmr.15017064 . [DOI] [PubMed] [Google Scholar]
- 235.Ganie SH, Upadhyay P, Das S, Prasad Sharma M. Authentication of medicinal plants by DNA markers. Plant Gene. 2015;4:83–99. http://dx.doi.org/10.1016/j.plgene.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jiang C, Cao L, Yuan Y, Chen M, Jin Y, Huang L. Barcoding melting curve analysis for rapid, sensitive, and discriminating authentication of saffron (Crocus sativus L.) from its adulterants. Biomed Res Int. 2014;2014:809037 doi: 10.1155/2014/809037 ; PubMed Central PMCID: PMCPMC4274822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Linacero R, Ballesteros I, Sanchiz A, Prieto N, Iniesto E, Martinez Y, et al. Detection by real time PCR of walnut allergen coding sequences in processed foods. Food Chem. 2016;202:334–40. doi: 10.1016/j.foodchem.2016.01.132 . [DOI] [PubMed] [Google Scholar]
- 238.López-Calleja IM, de la Cruz S, González I, García T, Martín R. Duplex real-time PCR using TaqMan® for the detection of sunflower (Helianthus annuus) and poppy (Papaver rhoeas) in commercial food products. LWT—Food Science and Technology. 2016;65:999–1007. http://dx.doi.org/10.1016/j.lwt.2015.09.026. [Google Scholar]
- 239.Köppel R, Ganeshan A, van Velsen F, Bucher T. Five pentaplex real-time PCR systems for the efficient determination of 20 genetically modified maize traits in food. Eur Food Res Technol. 2017;243(2):215–25. doi: 10.1007/s00217-016-2737-6 [Google Scholar]
- 240.Law JW, Ab Mutalib NS, Chan KG, Lee LH. Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front Microbiol. 2014;5:770 doi: 10.3389/fmicb.2014.00770 ; PubMed Central PMCID: PMCPMC4290631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Schlaberg R, Chiu CY, Miller S, Procop GW, Weinstock G, Professional Practice C, et al. Validation of Metagenomic Next-Generation Sequencing Tests for Universal Pathogen Detection. Arch Pathol Lab Med. 2017. doi: 10.5858/arpa.2016-0539-RA . [DOI] [PubMed] [Google Scholar]
- 242.Žel J, Milavec M, Morisset D, Plan D, Van den Eede G, Gruden K. How to Reliably Test for GMOs: Springer US; 2012. [Google Scholar]
- 243.Moreira F, Carneiro J, Pereira F. A proposal for standardization of transgenic reference sequences used in food forensics. Forensic Sci Int Genet. 2017;29:e26–e8. doi: 10.1016/j.fsigen.2017.04.022 . [DOI] [PubMed] [Google Scholar]
- 244.Holst-Jensen A, Bertheau Y, de Loose M, Grohmann L, Hamels S, Hougs L, et al. Detecting un-authorized genetically modified organisms (GMOs) and derived materials. Biotechnol Adv. 2012;30(6):1318–35. doi: 10.1016/j.biotechadv.2012.01.024 . [DOI] [PubMed] [Google Scholar]
- 245.Holst-Jensen A. Testing for genetically modified organisms (GMOs): Past, present and future perspectives. Biotechnol Adv. 2009;27(6):1071–82. doi: 10.1016/j.biotechadv.2009.05.025 . [DOI] [PubMed] [Google Scholar]
- 246.Gachet E, Martin GG, Vigneau F, Meyer G. Detection of genetically modified organisms (GMOs) by PCR: a brief review of methodologies available. Trends in Food Science & Technology. 1998;9(11–12):380–8. http://dx.doi.org/10.1016/S0924-2244(99)00002-3. [Google Scholar]
- 247.Cankar K, Stebih D, Dreo T, Zel J, Gruden K. Critical points of DNA quantification by real-time PCR—effects of DNA extraction method and sample matrix on quantification of genetically modified organisms. BMC Biotechnol. 2006;6:37 doi: 10.1186/1472-6750-6-37 ; PubMed Central PMCID: PMCPMC1569826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Buh Gasparic M, Tengs T, La Paz JL, Holst-Jensen A, Pla M, Esteve T, et al. Comparison of nine different real-time PCR chemistries for qualitative and quantitative applications in GMO detection. Anal Bioanal Chem. 2010;396(6):2023–9. doi: 10.1007/s00216-009-3418-0 . [DOI] [PubMed] [Google Scholar]
- 249.Waltz E. GM salmon declared fit for dinner plates. Nat Biotechnol. 2016;34(1):7–9. doi: 10.1038/nbt0116-7a . [DOI] [PubMed] [Google Scholar]
- 250.Castro C, Amorim M, Moreira F, Pereira F. A method to assemble DNA fragments mimicking junctions of transgenic elements: Application to the AquAdvantage salmon. Food Control. 2017;82:179–83. http://dx.doi.org/10.1016/j.foodcont.2017.06.037. [Google Scholar]
- 251.Ben Hafsa A, Nabi N, Zellama MS, Said K, Chaouachi M. A new specific reference gene based on growth hormone gene (GH1) used for detection and relative quantification of Aquadvantage(R) GM salmon (Salmo salar L.) in food products. Food Chem. 2016;190:1040–5. doi: 10.1016/j.foodchem.2015.06.064 . [DOI] [PubMed] [Google Scholar]
- 252.Murray JD, Maga EA. Opinion: A new paradigm for regulating genetically engineered animals that are used as food. Proc Natl Acad Sci U S A. 2016;113(13):3410–3. doi: 10.1073/pnas.1602474113 ; PubMed Central PMCID: PMCPMC4822638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Vazquez-Salat N, Salter B, Smets G, Houdebine LM. The current state of GMO governance: are we ready for GM animals? Biotechnol Adv. 2012;30(6):1336–43. doi: 10.1016/j.biotechadv.2012.02.006 . [DOI] [PubMed] [Google Scholar]
- 254.Morisset D, Demsar T, Gruden K, Vojvoda J, Stebih D, Zel J. Detection of genetically modified organisms—closing the gaps. Nat Biotechnol. 2009;27(8):700–1. doi: 10.1038/nbt0809-700 . [DOI] [PubMed] [Google Scholar]
- 255.Budowle B, Schutzer SE, Morse SA, Martinez KF, Chakraborty R, Marrone BL, et al. Criteria for validation of methods in microbial forensics. Appl Environ Microbiol. 2008;74(18):5599–607. doi: 10.1128/AEM.00966-08 ; PubMed Central PMCID: PMCPMC2547046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Casiraghi M, Labra M, Ferri E, Galimberti A, De Mattia F. DNA barcoding: a six-question tour to improve users' awareness about the method. Brief Bioinform. 2010;11(4):440–53. doi: 10.1093/bib/bbq003 . [DOI] [PubMed] [Google Scholar]
- 257.Taylor HR, Harris WE. An emergent science on the brink of irrelevance: a review of the past 8 years of DNA barcoding. Mol Ecol Resour. 2012;12(3):377–88. doi: 10.1111/j.1755-0998.2012.03119.x . [DOI] [PubMed] [Google Scholar]
- 258.FAO. Molecular genetic characterization of animal genetic resources. FAO Animal Production and Health Guidelines. 9. Rome2011.
- 259.Gawad C, Koh W, Quake SR. Single-cell genome sequencing: current state of the science. Nat Rev Genet. 2016;17(3):175–88. doi: 10.1038/nrg.2015.16 . [DOI] [PubMed] [Google Scholar]
- 260.Cheng X, Chen X, Su X, Zhao H, Han M, Bo C, et al. DNA extraction protocol for biological ingredient analysis of Liuwei Dihuang Wan. Genomics Proteomics Bioinformatics. 2014;12(3):137–43. doi: 10.1016/j.gpb.2014.03.002 ; PubMed Central PMCID: PMCPMC4411345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Giampaoli S, Berti A, Di Maggio RM, Pilli E, Valentini A, Valeriani F, et al. The environmental biological signature: NGS profiling for forensic comparison of soils. Forensic Sci Int. 2014;240:41–7. doi: 10.1016/j.forsciint.2014.02.028 . [DOI] [PubMed] [Google Scholar]
- 262.Young JM, Weyrich LS, Cooper A. Forensic soil DNA analysis using high-throughput sequencing: a comparison of four molecular markers. Forensic Sci Int Genet. 2014;13:176–84. doi: 10.1016/j.fsigen.2014.07.014 . [DOI] [PubMed] [Google Scholar]
- 263.Lilje L, Lillsaar T, Rätsep R, Simm J, Aaspõllu A. Soil sample metagenome NGS data management for forensic investigation. Forensic Science International: Genetics Supplement Series. 2013;4(1):e35–e6. http://dx.doi.org/10.1016/j.fsigss.2013.10.017. [Google Scholar]
- 264.Young JM, Austin JJ, Weyrich LS. Soil DNA metabarcoding and high-throughput sequencing as a forensic tool: considerations, potential limitations and recommendations. FEMS Microbiol Ecol. 2017;93(2). doi: 10.1093/femsec/fiw207 . [DOI] [PubMed] [Google Scholar]
- 265.Yang Y, Xie B, Yan J. Application of next-generation sequencing technology in forensic science. Genomics Proteomics Bioinformatics. 2014;12(5):190–7. doi: 10.1016/j.gpb.2014.09.001 ; PubMed Central PMCID: PMCPMC4411420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wall JD, Tang LF, Zerbe B, Kvale MN, Kwok PY, Schaefer C, et al. Estimating genotype error rates from high-coverage next-generation sequence data. Genome Res. 2014;24(11):1734–9. doi: 10.1101/gr.168393.113 ; PubMed Central PMCID: PMCPMC4216915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Bandelt HJ, Salas A. Current next generation sequencing technology may not meet forensic standards. Forensic Sci Int Genet. 2012;6(1):143–5. doi: 10.1016/j.fsigen.2011.04.004 . [DOI] [PubMed] [Google Scholar]
- 268.Jager AC, Alvarez ML, Davis CP, Guzman E, Han Y, Way L, et al. Developmental validation of the MiSeq FGx Forensic Genomics System for Targeted Next Generation Sequencing in Forensic DNA Casework and Database Laboratories. Forensic Sci Int Genet. 2017;28:52–70. doi: 10.1016/j.fsigen.2017.01.011 . [DOI] [PubMed] [Google Scholar]
- 269.Kumar S, Filipski AJ, Battistuzzi FU, Kosakovsky Pond SL, Tamura K. Statistics and truth in phylogenomics. Mol Biol Evol. 2012;29(2):457–72. doi: 10.1093/molbev/msr202 ; PubMed Central PMCID: PMC3258035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Rodriguez-Ezpeleta N, Brinkmann H, Roure B, Lartillot N, Lang BF, Philippe H. Detecting and overcoming systematic errors in genome-scale phylogenies. Syst Biol. 2007;56(3):389–99. doi: 10.1080/10635150701397643 . [DOI] [PubMed] [Google Scholar]
- 271.Phillips MJ, Delsuc F, Penny D. Genome-scale phylogeny and the detection of systematic biases. Mol Biol Evol. 2004;21(7):1455–8. doi: 10.1093/molbev/msh137 . [DOI] [PubMed] [Google Scholar]
- 272.Anisimova M, Liberles DA, Philippe H, Provan J, Pupko T, von Haeseler A. State-of the art methodologies dictate new standards for phylogenetic analysis. BMC Evol Biol. 2013;13:161 doi: 10.1186/1471-2148-13-161 ; PubMed Central PMCID: PMCPMC3751533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Arenas M. Trends in substitution models of molecular evolution. Front Genet. 2015;6:319 doi: 10.3389/fgene.2015.00319 ; PubMed Central PMCID: PMCPMC4620419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Houck MM. Intellectual infrastructure: A modest critique of forensic science. Science and Justice. 2013;53(1):1 doi: 10.1016/j.scijus.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 275.FSI G. Publisher report for editors. Forensic Sci Int Genet. 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)