Abstract
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide. The aim of the present study was to reveal the prognostic significance of CD147 and to preliminarily explore the molecular mechanisms involved. Blood and tumor tissue specimens were obtained from 133 HCC patients. All patients were followed up for 4 years. The serum and tissue levels of CD147 were analyzed using ELISA and immunohistochemistry, respectively. The SMMC-7721 hepatoma carcinoma cell line was transfected with CD147 overexpression vector and cell migration was evaluated using a wound healing assay. Extracellular signal-regulated kinase (ERK) inhibitor UO126 was applied to study the role of the ERK pathway in cell migration. CD147 expression in HCC tissue was associated with poor prognosis of patients [odds ratio (OR): 3.13, 95% confidence interval (CI): 1.52–6.43], and patients with no CD147 expression had a significantly survival advantage (P=0.016). However, serum CD147 levels had no such prognostic significance (OR: 1.94, 95% CI: 0.96–3.91; P=0.097). In the wound healing assay, the wound distance in the non-transfected cell group was wider than that in the transfected cell group without UO126 treatment (178.0±31.1 vs. 106.0±20.7 µm; P=0.003), but similar to that in the transfected cell group with 10 µM UO126 treatment (170.4±13.2 µm; P=0.629). The present study revealed that the expression of CD147 in HCC tissue is an independent prognostic indicator. In addition CD147 overexpression may be associated with tumor cell migration and ERK signaling pathway activation.
Keywords: hepatocellular carcinoma, CD147, prognosis, migration, ERK signaling pathway
Introduction
In the past few decades, the incidence of hepatocellular carcinoma (HCC) has been rapidly increasing (1,2). HCC has become one of the most common malignancies in the world (3,4). Although multiple novel diagnostic and therapeutic methods have been developed, HCC remains a fatal disease, and >500,000 patients die from HCC each year (5). Malignancies including HCC have unique biological behaviors, such as metastasis and migration, which usually cause treatment failure and poor prognosis (6). While numerous studies have assessed the mechanisms of metastasis and migration in HCC, they have remained to be fully elucidated.
CD147 is a transmembrane glycoprotein, which is widely distributed throughout the human body (7). CD147 itself has no obvious biological activity, while it is converted into its active form by N-glycosylation (8). Activated CD147 has an essential role in several biochemical processes (9). Studies have focused on its potential biological functions in malignant tumors and have revealed that upregulation of CD147 may contribute to carcinogenesis and tumor cell migration in several cancer types, such as colorectal adenocarcinoma (10–12) and head and neck squamous cell carcinoma (13). Sato et al (14) further reported that CD147 expression levels are predictors of the outcome for renal cell carcinoma patients. A mechanistic assessment by Iacono et al (15) suggested that CD147 elevated the expression levels of various metalloproteinases to promote carcinogenesis, angiogenesis and metastasis (16). However, the clinical and biological significance of CD147 overexpression in HCC has remained to be fully elucidated.
Therefore, the present study aimed to determine the serum levels and the tumor tissue expression levels of CD147 in HCC, and to reveal the prognostic significance of CD147 in patients. An in vitro study was also performed to assess the role of CD147 in tumor cell migration associated with extracellular signal-regulated kinase (ERK) pathway activation.
Materials and methods
Patients and samples
The present study was approved by the ethics committees of the Affiliated Hospital of Qingdao University (Qingdao, China). A total of 133 patients with pathologically confirmed HCC who were treated at the Affiliated Hospital of Qingdao University (Qingdao, China) between January 2008 and December 2009 were enrolled in the present study. All patients provided written informed consent, donated blood specimens and underwent HCC resection. During the operation, 133 HCC tissue specimens were obtained for analyses. None of the patients had received any pre-operative antitumor therapy. Demographic and medical information on the patients was collected from medical records and face-to-face interviews. All patients were followed up by telephone for 48 months or until they died during the period. The tumor-nodes-metastasis (TNM) stage was determined according to the classification of the American Joint Committee on Cancer (17).
Serum analysis of CD147
Blood specimens were adequately centrifuged for extracting serum specimens. Resulting serum specimens were stored at −80°C until analysis. Serum levels of CD147 were detected using a commercial human EMMPRIN/CD147 Quantikine ELISA Kit (catalogue no. DEMP00; R&D Systems, Minneapolis, MN, USA) according to a manufacturer's instructions (18). The experiment was performed twice for each specimen, and the arithmetic mean of two results was reported.
Immunohistochemistry
Fresh HCC tissue specimens were stored in liquid nitrogen for no more than three months. Expression levels of CD147 in HCC tissues were evaluated using immunohistochemistry according to standard operating procedures (19,20). Following thawing tissues were fixed in 10% neutral buffered formalin, paraffin-embedded and cut into 4-µm sections. Subsequent to conventional de-waxing with xylene and rehydration, followed by rinsing in PBS, samples were immersed in sodium citrate-buffered saline (0.001 mol/l, pH 6.0) and heated in the microwave. Following cooling at 37°C for 20 min, samples were treated with 3% hydrogen peroxide for 20 min and incubated with normal goat serum at 37°C for 20 min. Slices were then incubated with anti-EMMPRIN/CD147 antibody (catalogue no. AF972; R&D Systems; 5 µg/ml) overnight at 4°C, followed by incubation at 37°C for 30 min with biotin-labeled anti-goat IgG secondary antibody (catalogue no. CTS008; R&D Systems; 5 µg/ml). Visualization was performed with 3,3′-diaminobenzidine and hematoxylin. A positive result was defined as >20% of HCC cells exhibiting positive staining in one tissue specimen.
Cell line and culture
The SMMC-7721 human HCC cell line was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). SMMC-7721 cells were routinely cultured in RPMI-1640 medium containing 10% fetal bovine serum (Hyclone Laboratories; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C in a humidified atmosphere containing 5% CO2 (21). Monolayers of cells were cultured on 6-well plates for analysis.
Transfection and ERK inhibition
SMMC-7721 cells were divided into overexpression of CD147 group (OC group), ERK inhibition group 1 (EI group 1), ERK inhibition group 2 (EI group 2) and negative control group (NC group). Each group contained 15 wells of SMMC-7721 cells. Plasmids carrying CD147 complementary DNA (cDNA), which were purchased from Genepharma Co., Ltd. (Shanghai, China) and transfected into HCC cells in the OC group, EI group 1 and EI group 2 using Lipofectamine® 2000 transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Detailed steps were as follows: i) Cell culture for 24 h (65–70% confluence); ii) transfection with CD147 cDNA plasmid; and iii) selection of stable clones by G418 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) (22). Cells in the EI group 1 and EI group 2 were treated with 5 and 10 µM UO126 (ERK inhibitor; Alexis Biochemicals; Enzo Life Sciences, Inc., Farmingdale, NY, USA), respectively.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Expression levels of CD147 mRNA in SMMC-7721 cells of the OC and NC groups were detected by RT-qPCR using a SYBR Premix Ex Taq kit (catalogue no. RR820A; Takara Bio, Inc., Otsu, Japan) on an ABI 7300 (Thermo Fisher Scientific, Inc.). Total RNA was extracted with TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and transcribed into cDNA using a PrimeScript RT reagent kit with gDNA Eraser (catalogue no. RR047a; Takara Bio, Inc.). Forward and reverse primer sequences of CD147 were 5′-CAGCGGTTGGAGGTTGT-3′ and 5′-TTTGAGGGTGGAGGTGG-3′, respectively. β-actin was used as endogenous control, and forward and reverse primer sequences were as follows: 5′-AAAGACCTGTACGCCAACAC-3′ and 5′-GTCATACTCCTGCTTGCTGAT-3′, respectively. The PCR cycling program was as follows: Denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec and elongation at 72°C for 30 sec for 40 cycles (23). In each group, detection was performed five times using 5 wells of cells.
Western blot analysis
The expression levels of CD147 protein in the OC and NC groups were detected by western blot analysis. Cells were lysed in RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) and in protease and phosphatase inhibitor cocktail (catalogue no. 78443; Thermo Fisher Scientific, Inc.). The cell lysates were incubated on ice for 10 min. The supernatants underwent centrifugation at 14,000 × g for 15 min at 4°C. Total protein concentrations were assayed using BCA Protein Assay kit (catalogue no. P0010; Beyotime Institute of Biotechnology) according to the manufacturers protocol. Equal amounts of cell lysate (50 µg) were separated by 12% SDS-PAGE and transferred onto a nitrocellulose membrane. The membrane was blocked with 5% skimmed milk in Tris-buffered saline, followed by incubation overnight at 4°C with rabbit anti-human EMMPRIN/CD147 antibody (catalogue no. PB0239; 1:1,000 dilution; Boster Biotechnology, Ltd., Wuhan, China) and β-actin antibody (catalogue no. A2066; 1:5,000 dilution; Sigma-Aldrich; Merck KGaA). Subsequently, the membranes were incubated overnight at 4°C with horseradish peroxidase-conjugated goat anti-rabbit antibodies (catalogue no. ab6721; 1:10,000 dilution; Abcam, Cambridge, UK). The chemiluminescent substrate SuperSignal West Femto trial kit (catalogue no. PI34094; Thermo Fisher Scientific, Inc.) was adopted for visualizing the antibodies (24). Relative intensities of CD147 vs. β-actin bands were quantified using Totallab v2.01 software (Totallab Ltd., Newcastle upon Tyne, UK). In each group, detection was performed five times using five wells of cells.
Wound healing assay
The migratory capacity of SMMC-7721 cells in all four groups was evaluated using a wound healing assay. Detailed steps were as follows: i) Incubation until formation of a confluent monolayer; ii) scratching cell wounds with Eppendorf tips (0.1–10 µl); iii) rinsing with PBS; and iv) incubating in serum-free RPMI-1640 medium for 24 h (25). In each group, detection was performed five times using five wells of cells.
Statistical analysis
SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. An independent sample t-test was used for detecting differences between continuous variables. P<0.05 was considered to indicate a statistically significant difference. The association between patient prognosis and CD147 levels was evaluated by uni- and multivariate logistic regression analysis. The odds ratio (OR) with 95% confidence interval (CI) was calculated. If the CI did not include value 1, it was considered to indicate statistical significance. The difference in survival between patients stratified by CD147 levels was evaluated by Kaplan-Meyer survival analysis.
Results
Patient data
As presented in Table I, a total of 133 patients with HCC were included in the present study. Among them, there were 110 men and 23 women. The majority of patients had been infected with hepatitis B virus (85.7%), and had suffered from liver cirrhosis (79.0%). There were no TNM stage-IV patients in the study. ELISA and immunohistochemistry were adopted for detecting of CD147 levels in human serum and tumor tissue, respectively. The serum levels of CD147 were 21.2±10.9 ng/ml and positive expression of CD147 protein was observed in 77 HCC tissue specimens. During the 48-month follow-up period, 78 of the patients died of HCC and 55 survived.
Table I.
Characteristics of patients with hepatocellular carcinoma (n=133).
| Parameter | Value |
|---|---|
| Males | 110 (82.7) |
| Age (years) | 54.6±8.1 |
| Han ethnicity | 127 (95.5) |
| AFP (ng/ml) | 26.1±4.7 |
| HBsAg positive | 114 (85.7) |
| Cirrhosis | 105 (79.0) |
| Tumor size ≥3 cm | 84 (63.2) |
| Multifocal tumor | 57 (42.9) |
| TNM stage | |
| I | 33 (24.8) |
| II | 56 (42.1) |
| III | 44 (33.1) |
| Serum levels of CD147 (ng/ml) | 21.2±10.9 |
| Tissue expression of CD147 | |
| Positive | 77 (57.9) |
| Negative | 56 (42.1) |
| Prognosis at the end of follow-up period, 48 months | |
| Survival | 55 (41.4) |
| Death | 78 (58.6) |
Values are expressed as n (%) or as mean ± standard deviation. AFP, α-fetoprotein; HbsAg, hepatitis B virus surface antigen; TNM, tumor-nodes-metastasis.
CD147 in HCC tissues is an independent predictor of survival
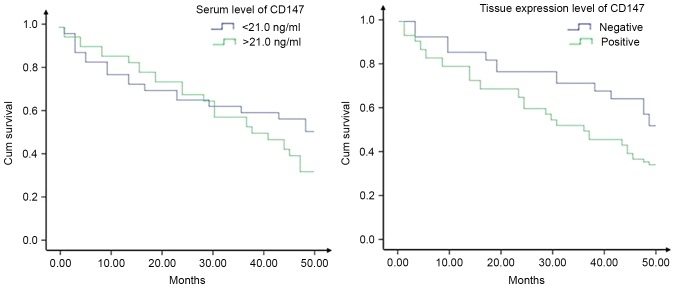
As displayed in v II, elevated CD147 levels in serum and tumor tissue were separately associated with poor prognosis in HCC patients according to univariate regression analysis (OR: 2.21, 95% CI: 1.09–4.47 and OR: 2.11, 95% CI: 1.04–4.27). After adjustment for multiple potential confounding factors, the association between serum levels of CD147 and prognosis in HCC patients became insignificant (OR: 1.94, 95% CI: 0.96–3.91), but positive expression of CD147 in HCC tissues was still highly correlated with poor prognosis in patients (OR: 3.13, 95% CI: 1.52–6.43). As presented in Fig. 1, patients with negative expression of CD147 in their HCC tissues had a significant survival advantage (P=0.016), but such a survival advantage was not discovered in patients with low serum levels of CD147 (<21.0 ng/ml; P=0.097).
Figure 1.
Survival analysis of patients with hepatocellular carcinoma according to serum levels (left; P=0.097) and tissue expression levels (right; P=0.016) of CD147. Cum, cumulative.
Confirmation of ectopic CD147 expression in HCC cells
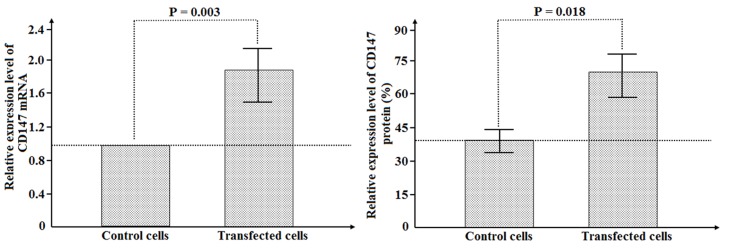
As displayed in Fig. 2, mRNA and protein levels of CD147 were detected by RT-qPCR and western blot analysis, respectively. The mRNA expression levels of CD147 were significantly increased by 84.0±43.9% in transfected SMMC-7721 cells compared with those in control SMMC-7721 cells (P=0.003). Similarly, the protein levels were upregulated in transfected cells compared with those in control cells (70.8±20.0 vs. 40.8±10.8% of control; P=0.018).
Figure 2.
Expression of CD147 mRNA and protein in SMMC-7721 cell line after transfection of CD147 overexpression vector.
CD147 overexpression enhances HCC cell migration via ERK pathway
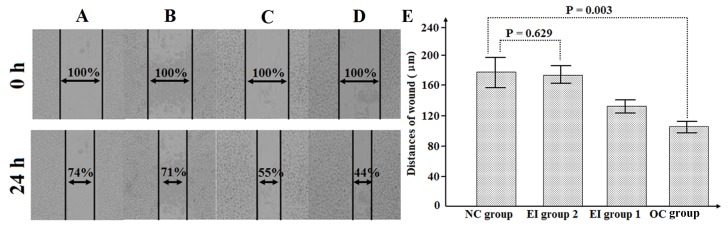
As displayed in Fig. 3, the wound distance in the OC group was significantly shorter than that in the NC group after 24 h of incubation (106.0±20.7 vs. 178.0±31.1 µm; P=0.003). With increasing of UO126 concentration, wound distances in EI groups 1 and 2 gradually widened (131.2±15.5 and 170.4±13.2 µm), resulting in an equivalent wound distance between NC group and EI group 2 (P=0.629).
Figure 3.
Differences in SMMC-7721 cell migration abilities in (A) NC group, (B) EI group 1 (CD1475 overexpression and treatment with 5 µM UO126), (C) EI group 2 (CD1475 overexpression and treatment with 10 µM UO126) and (D) OC group. (E) Wound sizes for the NC, EI group 2, EI group 1 and OC groups. NC, negative control; EI, extracellular signal-regulated kinase inhibitor; OC, overexpression of CD147.
Discussion
The present study, revealed a difference in prognostic significance between serum levels and tumor tissue expression levels of CD147 in HCC patients. To the best of our knowledge, the present study was the first to report this.
Blood and tumor tissue specimens were obtained from 133 HCC patients. For all patients the diagnosis was pathologically confirmed, which ensured diagnostic accuracy. Previous studies suggested that the expression levels of CD147 in a variety of malignant tumors may be correlated with chemoradiotherapy resistance (26,27), and the possibility of chemoradiotherapy affecting CD147 levels and their prognostic value could not be completely ruled out. Therefore, patients who received pre-operative radiotherapy and chemotherapy were excluded from the study to avoid potential bias.
Based on the results of CD147 detection and long-term follow-up, logistic regression analyses were performed, which separately revealed a 200% increased risk of death in HCC patients with high serum levels (≥21.0 ng/ml) and positive tissue expression of CD147. Multiple clinical pathological indicators, such as TNM stage, tumor size and serum α-fetoprotein (AFP) levels, were also potential recurrent and prognostic factors (28–30), and they may not be evenly distributed between surviving and dead patients. Therefore, multivariate logistic regression analyses were well adjusted for these potential confounders, revealing that poor prognosis during the follow-up period was still associated with positive tissue expression of CD147, but the association between increased risk of mortality and high serum CD147 levels lost its significance.
Furthermore, Kaplan-Meyer survival analyses were performed, which provided results consistent with the above. Significant survival advantages were observed in patients with negative tissue expression of CD147, but not in patients with low serum CD147 levels. In summary, tumor tissue expression levels of CD147, but not serum levels of CD147, may be an independent prognostic indicator in HCC patients.
The present study also performed in vitro tests in order to determine the association between expression levels of CD147 and the migratory ability of SMMC-7721 cells. Using a vector transfection technique, CD147-overexpressing SMMC-7721 cells were generated, while non-transfected SMMC-7721 cells were used as control cells. RT-qPCR and western blot analysis confirmed that the mRNA and protein expression levels of CD147 were significantly elevated in transfected cells compared with those in control cells, proving that transfection had been successful.
In the wound healing assay, CD147-overexpressing SMMC-7721 cells demonstrated a greater migratory ability compared with that of the control SMMC-7721 cells. Therefore, CD147 overexpression may have a role in SMMC-7721 cell migration and metastasis. This molecular mechanism partly explains for the prognostic significance of CD147 in HCC patients.
CD147-oveexpressing SMMC-7721 cells were also treated with UO126 (ERK inhibitor). The concentration of UO126 was inversely correlated with the migratory ability of CD147-overexpressing SMMC-7721 cells. As blocking of this pathway inhibited CD147-mediated cell migration, the ERK signaling pathway is likely to be activated in CD147-induced cell migration.
Two potential limitations of the study were follows: First, only 133 patients were enrolled, and subject selection did not fully comply with principles of randomization. Therefore, the representation of patients may be statistically insufficient. Furthermore, no in vivo study was performed. While the present study was of a preliminary nature, it provided information encouraging future research.
In conclusion, the present study discovered that upregulated CD147 expression in tumor tissues, but not elevated serum levels of CD147, was associated with poor prognosis of HCC patients, and revealed that tissue CD147 expression levels are an independent prognostic indicator in HCC. Furthermore CD147 overexpression in SMMC-7721 cells was found to be associated with enhancement of their migratory ability, which was inversed by inhibition of ERK, indicating that upregulated CD147 expression may promote tumor cell migration through the ERK signaling pathway. However, the precise underlying mechanisms require further study.
Table II.
Association between poor prognosis and CD147 in patients with hepatocellular carcinoma.
| Parameter | Univariate regression, OR (95% CI) | Multivariate regressiona, OR (95% CI) |
|---|---|---|
| Serum levels of CD147 (≥21.0 vs. <21.0 ng/ml) | 2.21 (1.09, 4.47) | 1.94 (0.96, 3.91) |
| Tissue expression of CD147 (positive vs. negative) | 2.11 (1.04, 4.27) | 3.13 (1.52, 6.43) |
Multivariate-adjusted ORs were matched for gender, age, ethnicity, serum levels of α-fetoprotein, onset of hepatitis B virus surface antigen, onset of cirrhosis, tumor size, number of tumors and tumor-nodes-metastasis stage. OR, odds ratio; CI, confidence interval.
References
- 1.El-Serag HB. Hepatocellular carcinoma: Recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. (5 Suppl 1) [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases: Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 5.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–399. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 6.Wei C, Pan Q, Wu K, Li R. Clinical characterization for proliferation and metastasis in advanced hepatocellular carcinoma patients. Int J Clin Exp Pathol. 2015;8:13429–13431. [PMC free article] [PubMed] [Google Scholar]
- 7.Weidle UH, Scheuer W, Eggle D, Klostermann S, Stockinger H. Cancer-related issues of CD147. Cancer Genomics Proteomics. 2010;7:157–169. [PubMed] [Google Scholar]
- 8.Bai Y, Huang W, Ma LT, Jiang JL, Chen ZN. Importance of N-glycosylation on CD147 for its biological functions. Int J Mol Sci. 2014;15:6356–6377. doi: 10.3390/ijms15046356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fadool JM, Linser PJ. 5A11 antigen is a cell recognition molecule which is involved in neuronal-glial interactions in avian neural retina. Dev Dyn. 1993;196:252–262. doi: 10.1002/aja.1001960406. [DOI] [PubMed] [Google Scholar]
- 10.Zheng HC, Wang W, Xu XY, Xia P, Yu M, Sugiyama T, Takano Y. Up-regulated EMMPRIN/CD147 protein expression might play a role in colorectal carcinogenesis and its subsequent progression without an alteration of its glycosylation and mRNA level. J Cancer Res Clin Oncol. 2011;137:585–596. doi: 10.1007/s00432-010-0919-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li R, Pan Y, He B, Xu Y, Gao T, Song G, Sun H, Deng Q, Wang S. Downregulation of CD147 expression by RNA interference inhibits HT29 cell proliferation, invasion and tumorigenicity in vitro and in vivo. Int J Oncol. 2013;43:1885–1894. doi: 10.3892/ijo.2013.2108. [DOI] [PubMed] [Google Scholar]
- 12.Xu T, Zhou M, Peng L, Kong S, Miao R, Shi Y, Sheng H, Li L. Upregulation of CD147 promotes cell invasion, epithelial-to-mesenchymal transition and activates MAPK/ERK signaling pathway in colorectal cancer. Int J Clin Exp Pathol. 2014;7:7432–7441. [PMC free article] [PubMed] [Google Scholar]
- 13.Sweeny L, Liu Z, Bush BD, Hartman Y, Zhou T, Rosenthal EL. CD147 and AGR2 expression promote cellular proliferation and metastasis of head and neck squamous cell carcinoma. Exp Cell Res. 2012;318:1788–1798. doi: 10.1016/j.yexcr.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato M, Nakai Y, Nakata W, Yoshida T, Hatano K, Kawashima A, Fujita K, Uemura M, Takayama H, Nonomura N. EMMPRIN promotes angiogenesis, proliferation, invasion and resistance to sunitinib in renal cell carcinoma, and its level predicts patient outcome. PLoS One. 2013;8:74313. doi: 10.1371/journal.pone.0074313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iacono KT, Brown AL, Greene MI, Saouaf SJ. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp Mol Pathol. 2007;83:283–295. doi: 10.1016/j.yexmp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jodele S, Blavier L, Yoon JM, DeClerck YA. Modifying the soil to affect the seed: Role of stromal-derived matrix metalloproteinases in cancer progression. Cancer Metastasis Rev. 2006;25:35–43. doi: 10.1007/s10555-006-7887-8. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 18.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971;8:871–874. doi: 10.1016/0019-2791(71)90454-X. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Xu J, Chen L, Zhong WD, Zhang Z, Mi L, Zhang Y, Liao CG, Bian HJ, Jiang JL, et al. HAb18G (CD147), a cancer-associated biomarker and its role in cancer detection. Histopathology. 2009;54:677–687. doi: 10.1111/j.1365-2559.2009.03280.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen ZN. Significance and application of anti-malignant hepatoma MAb HAb18 in radioimmunal diagnosis of human hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi. 1992;14:9–12. (In Chinese) [PubMed] [Google Scholar]
- 21.Ji Y, Wang Z, Li Z, Huang N, Chen H, Li B, Hui B. Silencing IGF-II impairs C-myc and N-ras expressions of SMMC-7721 cells via suppressing FAK/PI3K/Akt signaling pathway. Cytokine. 2017;90:44–53. doi: 10.1016/j.cyto.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Jiang JL, Zhou Q, Yu MK, Ho LS, Chen ZN, Chan HC. The involvement of HAb18G/CD147 in regulation of store-operated calcium entry and metastasis of human hepatoma cells. J Biol Chem. 2001;276:46870–46877. doi: 10.1074/jbc.M108291200. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Ru NY, Zhang Y, Li Y, Wei D, Ren Z, Huang XF, Chen ZN, Bian H. HAb18G/CD147 promotes epithelial-mesenchymal transition through TGF-β signaling and is transcriptionally regulated by Slug. Oncogene. 2011;30:4410–4427. doi: 10.1038/onc.2011.149. [DOI] [PubMed] [Google Scholar]
- 24.Sun Z, Chen R, Cheng K, Liu H, Qin H, Ye M, Zou H. A new method for quantitative analysis of cell surface glycoproteome. Proteomics. 2012;12:3328–3337. doi: 10.1002/pmic.201200150. [DOI] [PubMed] [Google Scholar]
- 25.Liu YH, Jin JL, Wang YZ, Tan Y, Zhou YY, Peng T, Li F, Liang WD, Chartrand P, Jiang YY, Shen ZF. Protrusion-localized STAT3 mRNA promotes metastasis of highly metastatic hepatocellular carcinoma cells in vitro. Acta Pharmacol Sin. 2016;37:805–813. doi: 10.1038/aps.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng HZ, Qu YQ, Liang AB, Deng AM, Zhang WJ, Xiu B, Wang H, Wang H. Expression of CD147 in advanced non-small cell lung cancer correlated with cisplatin-based chemotherapy resistance. Neoplasma. 2011;58:449–454. doi: 10.4149/neo_2011_05_449. [DOI] [PubMed] [Google Scholar]
- 27.Wu J, Li Y, Dang YZ, Gao HX, Jiang JL, Chen ZN. HAb18G/CD147 promotes radioresistance in hepatocellular carcinoma cells: A potential role for integrin β1 signaling. Mol Cancer Ther. 2015;14:553–563. doi: 10.1158/1535-7163.MCT-14-0618. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Duan LG, Lu WS, Yan LN, Xiao GQ, Jiang L, Yang J, Yang JY. Prognosis evaluation in patients with hepatocellular carcinoma after hepatectomy: Comparison of BCLC, TNM and Hangzhou criteria staging systems. PLoS One. 2014;9:e103228. doi: 10.1371/journal.pone.0103228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsh JW, Dvorchik I, Bonham CA, Iwatsuki S. Is the pathologic TNM staging system for patients with hepatoma predictive of outcome? Cancer. 2000;88:538–543. doi: 10.1002/(SICI)1097-0142(20000201)88:3<538::AID-CNCR7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 30.Wang NY, Wang C, Li W, Wang GJ, Cui GZ, He H, Zhao HJ. Prognostic value of serum AFP, AFP-L3 and GP73 in monitoring short-term treatment response and recurrence of hepatocellular carcinoma after radiofrequency ablation. Asian Pac J Cancer Prev. 2014;15:1539–1544. doi: 10.7314/APJCP.2014.15.4.1539. [DOI] [PubMed] [Google Scholar]