Abstract
Anterior cruciate ligament reconstruction (ACLR) provides an established surgical intervention to control pathological tibiofemoral translational and rotational movement. ACLR is a safe and reproducible intervention, but there remains an underlying rate of failure to return to preinjury sporting activity levels. Postoperative pathological laxity and graft reinjury remain concerns. Previously, unrecognized meniscal lesions, disruption of the lateral capsule, and extracapsular structures offer potential avenues to treat and to therefore improve kinematic outcome and functional results, following reconstruction. Addressing laterally based injuries may also improve the durability of intraarticular ACLR. Improving the anterior cruciate ligament (ACL) graft replication of the normal ACL attachment points on the femur and the tibia, using either double bundle or anatomical single bundle techniques, improves kinematics, which may benefit outcome and functionality, following reconstruction.
Keywords: Anterior cruciate ligament, graft injury, lateral tenodesis, ramp lesion
MeSH terms: Anterior cruciate ligament, Anterior cruciate ligament reconstruction, sports injuries, athletic injuries
Introduction
The anterior cruciate ligament (ACL) is a key ligamentous structure guiding rotation in the normal knee joint. Its disruption changes the ability of the knee joint to maintain stability during rotational, accelerative and decelerative activities, producing instability of the knee during these loaded activities, which may result in mechanical failure and giving way.
At the time of primary injury, there is often an accompanying impact to the articular aspect of the lateral femoral condyle against the lateral and posterolateral tibia, producing a characteristic distribution of bone edema. In addition, the menisci are often injured, with the medial meniscus being more commonly injured acutely, and the lateral meniscus becoming increasingly vulnerable to injury in the chronic setting, as a consequence of chronic pathological lateral tibiofemoral compartment rotational and translational movement.
In the USA alone over 200,000 ACL injuries occur per year, with the overwhelming majority undergoing surgical reconstruction.
In addition to the common initiator of a sporting injury causing ACL disruption, ligament disruption is a common occurrence within manual laboring occupations and road traffic accidents (RTAs), producing significant incapacity in the working environment. The etiology underlying ACL injury varies significantly by country, with the vast majority being related to sporting injury in the USA, Western Europe and Scandanavia1 whereas 58% were associated with a sporting injury, 26% with an RTA, and 16% were related to other nonsporting injuries in an Indian cohort.2 The mean age of an individual sustaining an ACL rupture being 28 years, in a Swedish cohort, with up to 80 ACL disruptions per year, per 100,000 population,1 with a US population study identifying an annual incidence of 68.6 per 100,000 person years.3
Activity, Sports, and Gender-specific Risks of Anterior Cruciate Ligament Injury
There is significant variability in the incidence of ACL disruption relating to participation in differing sports and occupations. Predisposing occupations to ACL disruption include any, where loading of the knee occurs on uneven surfaces, such as farming, construction work, mining and the military, with an incidence of 3.79 ACL injuries per 1000 person years in US military recruits, compared to 2.41 per 1000 years peak incidence for 19–25 year old males in the general population.3,4
ACL disruption rates are generally considered in relation to 1000 h of sports-specific exposure, including both competition and sports-specific training.5 There is not only considerable variability within differing sports but also when identifying experience and seniority within the same given sport, in addition to gender differences.
Using 1000 h of sports-specific exposure, ACL disruption rates are 0.07 for female and male adult recreational soccer players.5,6 A rate of 0.21 is documented for collegiate soccer players, with a rate of 0.32 for women and 0.12 for men.6,7,8 College basketball studies identify a combined risk of 0.18 per 1000 exposures, with a male figure of 0.08 and a female figure of 0.30.6 Alpine skiing demonstrates an incidence of 0.63 for an adult population but highlights the effect of experience and training in that professional skiers, and ski school employees have a significantly lower incidence at 0.04 per 1000 exposures, with no gender difference, compared to the general skiing population.9 Participation in Lacross identifies an annual ACL injury rate of 0.23 per 1000 athletic exposures, for female participants.10 Across all sports, there are specific risks unique to that sport, but with a higher incidence of ACL injury in women than men, irrespective of seniority, or training level, with the exception of Alpine skiing, when studying professional participants. Accordingly, ACL disruption is an increasing epidemic worldwide, consequent to increasing sporting participation and an increasing female participation in sporting activity at all levels.
The explanation of greater female over male incidence of ACL injury is incompletely understood and is likely to be multifactorial, including hip rotational profile, tibiofemoral coronal alignment, and gender differences in knee anatomy, but particularly centering around neuromuscular control of coronal and sagittal balance in loading, combined with differences in lower limb muscle strength and fatigue tolerance.11,12,13,14,15 Many of the dynamic, neuromuscular predispositions to injury can be optimized with training.16
Successful Outcome of Surgical Treatment as Defined by Return to Sport
The objective of ACL reconstruction (ACLR) is to enable a return to preinjury activity levels, whether they relate to occupation, or (more usually) return to prior sporting participation levels. Therefore, before considering the influences on surgical success, and how techniques might be altered to improve successful intervention, what are the current, established outcomes for return to play (RTP)?
Return to sporting activity following ACLR demonstrates a mismatch between the restoration of function and kinematic stability in the knee, against the actual sporting level re-achieved.
In a meta-analysis of 5,770 patients following ACLR, over 82% recommended sports, with 90% of the knees being rated as normal or near normal, on strength and laxity assessment, and 85% scoring normal or near normal on the International Knee Documentation Committee (IKDC) activity scores. However, only 62% returned to their preinjury sport.17
Negative influences on returning to same sport preinjury levels include increasing age at reconstruction, female gender, and smoking. These factors also predict lower outcome scores 10 years following reconstruction.18 Professional athletes have a greater likelihood of returning to preinjury same sport levels than amateur and recreational participants. The likelihood of successful RTP, following ACLR in professional American football players (NFL), is 92% in the quarterback position, but with only 64.3% of linemen returning to play, highlighting differing demands and loading between playing positions, within the same sport.19,20
Increasing chronicity of injury, even beyond 3 months, before reconstruction, has an adverse influence on return to same sport participation.21 These influences are independent of other issues associated with chronicity, such as meniscal and osteochondral injury.22,23
One significant cause for an athlete not returning to their preinjury sporting level is a concern over reinjury, and this may override any objective evidence of reconstruction having restored knee stability. This may partly explain the influence of chronicity in return to sport figures, with multiple instability events before stabilization, producing longer-term illness patterning.
Time taken to RTP is usually based around surgeon preference and may be simply related to time from reconstruction, particularly in relation to the specific sports season.19,20 Strength testing of quadriceps, hamstrings or both can provide quantitative data, in relation to the recommencement of sports.24 Functional activity assessments, including maintaining rotational control on single-leg jump, serve to guide the return to sports-specific training,25 with some researchers focusing that the successful return to sports should be characterized as the same sport, at the same intensity and activity level as before injury.25
Anterior Cruciate Ligament Graft Selection and the Influence on Outcome
Overall, there is no clinically significant difference in outcome between autograft materials, in reconstructing the ACL in the skeletally mature patient26 although there is a slightly increased likelihood of revision in hamstring autograft, as opposed to patellar tendon autograft, in a series of 17,436 ACLRs, of 5 years duration.27 Skeletally immature cases show an overwhelming utilization of soft-tissue autograft, usually using hamstring tendons.
The predominant autograft sources are the tendons of semitendinosus and gracilis muscles, or else the central region of the patellar tendon, incorporating attached bone blocks from the patellar and tibial tuberosity (bone-patellar-tendon-bone [BPTB]). Historically, the ease of securing graft bone blocks using interference screws in tibial and femoral bone tunnels has enabled earlier mobilization of patellar tendon autograft reconstruction. However, developments in soft-tissue fixation devices particularly as regards suspensory techniques, has promoted soft tissue grafts, as the mechanical construct can resist loads to enable early rehabilitation.
Less frequently utilized autograft options include quadriceps tendon, with or without attached superior pole patellar bone block, with quadriceps tendon autograft demonstrating less postoperative patholaxity than a matched hamstring cohort.28
Metaanalysis data demonstrate a marginal benefit in biomechanical results of BPTB over medial hamstring autografts, but these minimal differences do not materialize into a benefit in outcome.26 This minimal biomechanical preference is in contradiction to mechanical strength testing of harvested tissue, which demonstrates a higher load to failure and stiffness of multiple strand hamstring tendon graft over harvested 10 mm width patellar tendon.
Patellar tendon autograft increases the risk of early patellar fracture, with an incidence reported between 1.3% and 0.12%, limitation in end range extension,29,30,31 anterior knee pain, and potentially a higher rate of long term osteoarthrosis, over hamstring graft utilization.31 In contrast, hamstring harvesting produces some loss of active knee flexion strength, particularly in deep flexion, with tibial internal rotation.31,32,33 Accordingly, the author prefers to avoid hamstring harvesting in heavily hamstring dependent sports, including hurdling and some forms of gymnastics.
ACLR graft selection is therefore predominantly influenced by surgeon preference, patient age, and specific sports participation, with autograft medial hamstrings or patellar tendon being the selection for the majority of surgeons.
Allograft utilization varies greatly by geographical region, depending on availability of the resource and cost implications. Allograft selection influences include both donor site and methods of preparation and sterilization. The advantages of allograft include an absence of donor site morbidity and associated complications, along with the availability of differing size options, particularly in relation to ligament revision applications. Concerns over allograft utilization include the high cost of processing, higher rates of rerupture, compared with size-matched autograft, and potential cadaveric donor to recipient disease transmission, including viral and prion disease.
Results of nonirradiated allograft ACLRs are comparable to those of autograft, in some studies,34 when utilizing patient reported outcome measures (PROM) data and reinjury rates. However, the majority of studies identifies a greater patholaxity, and higher rerupture rate in allograft reconstruction than autograft, with a failure rate of up to three times greater in allograft cases, over a 10-year period. In a study of the National Collegiate Athletic Association participants, in division 1, a revision rate of 62% was noted for irradiated allograft ACLR, versus 0% in autograft cases.35 An overall revision rate of 20% was noted for cases of under 25 years of age, at the time of reconstruction.
Allograft tissue processing has a significant effect on graft behavior, with a direct relationship between sterilization radiation levels, postoperative persistent laxity, and rerupture rates. In a study comparing nonirradiated patellar tendon allograft, 2.5 Mrad irradiated BPTB allograft and BPTB autografts, over a mean of 31 months, the rerupture rates were 8.8%, 34.4%, and 6.1%, respectively.36 These data are broadly in line with a number of other studies, such that grafts undergoing exposure of 2.5 Mrad or greater are no longer recommended, due to concerns over high levels of rerupture.
Low-dose irradiated, or nonirradiated grafts have a greater probability of rerupture than autograft options, but are subject to risks of viral and prion transfer to recipient, even though these risks are theoretically low (0.00015%). High-dose irradiation eliminates the remote possibility of disease transmission, at the cost of a significant deterioration in allograft mechanical properties and significantly elevated reinjury rates.
Nonbiological grafts have proven unreliable in providing long term durability and are not recommended, although the LARS device (polyethylene terephthalate) may provide an alternative nonbiological reconstruction, depending on longer term outcome measures.
Nonbiological grafts may have an application in two expanding settings; in the support of a repaired native ACL during early and intermediate stages of healing, and when used as a load sharing device to support and prevent graft overload following ACLR (InternalBrace, Arthrex, Naples, FL, USA).
Complications of Anterior Cruciate Ligament Reconstruction
Although a common procedure, ACLR surgery remains a complex and technically demanding surgical intervention, depending both on accurate preoperative identification of the nature of the ligament compromise, and on reproducible surgical technique.
Causes of failure of reconstruction include ongoing pathological laxity in the knee, consequent to poor operative technique, fixation implant failure, and incorrect graft positioning. Failure to recognize and treat more complex instability patterns results in ongoing pathological laxity, most commonly in relation injury to the posterolateral constraints of the knee, centered on the popliteus musculo-tendinous unit, the popliteofibular ligament, and the posterolateral capsule.
Documented rerupture rates vary, influenced by gender, age, generalized laxity, lower limb alignment and sporting exposure, among a number of independent variables.18
Primary ACL rerupture rates, following autograft hamstring or patellar tendon graft usage, are approximately 3% at 2 years following reconstruction (MOON cohort) and up to 12% at 5 years, which are similar to the incidence of contra lateral ACL injury, over the same time points.33 Comparing BPTB against 4 strand hamstring autograft over a 15-year period identified a 17% re-rupture rate in hamstring graft, against 8% in BPTB, with no difference in IKDC scores.31 Differing rerupture rates related to age and graft treatment are discussed elsewhere.18
Infection is relatively rare in primary ACLR, as a secondary effect of the patient population generally being younger and of good general medical health, with a low incidence of medical comorbidities, including diabetes and peripheral vascular disease. Overall infection rates for primary ACL surgery are 0.5%,37,38 with the majority of infections becoming clinically apparent within 3 weeks of intervention. Primary graft infections mostly occur in cases with associated meniscal repair, or in knees having undergone multiple prior interventions, before reconstruction.
Additional complications include limitation in range of movement, which may be related to hardware or graft malposition, or arthrofibrosis. Arthrofibrosis, should not be considered as a cause for loss of range of motion until infection and chronic regional pain syndrome has been excluded. Arthroscopic arthrolysis, by clearing the medial and lateral gutters and suprapatellar pouch can be effective in restoring range of movement;39 with anterior decompression, suprapatellar recess release, and fat pad release improving fixed flexion.
Synovitis may result in increased pain levels, accompanied by effusion, which in turn limits range of movement. Once infection has been discounted, then arthroscopic synovectomy may help to control synovial response, but commonly synovitis relates to unrecognized intraarticular infection.
Pain following ACLR may relate to osteochondral damage and progressive arthrosis, meniscal pathology, prominent hardware (including meniscal repair implants), infection, synovitis, and neural injury. Careful reexamination, imaging, and blood assay are indicated to identify the cause of ongoing pain. Potential neural injury, particularly related to the infrapatellar branch of the saphenous nerve, at the time of medial hamstring graft harvest, should be discussed as part of the preoperative consenting process. The neural injury may promote chronic regional pain syndrome, particularly if the reconstructed knee has undergone multiple surgical interventions before ACLR.
Developments in Anterior Cruciate Ligament Reconstruction
Reconstruction of the ACL aims to restore knee function, with the elimination of significant laxity and consequent instability, in addition to decreasing the incidence of secondary meniscal and osteochondral injury. However, most reconstruction techniques do not return the knee to an entirely normal biomechanical structure as is demonstrated by an increased reinjury rate (when compared to a knee with a native ACL), return to sports data, and patient satisfaction rates.
Current developments in sports medicine knee surgery and ACLR center around two main areas. First, an improved appreciation in graft attachment points, particularly in relation to the femur and second, the recognition that ACL disruption is a heterogenous injury, with variable involvement of the lateral capsular structures.
Developments over the last two decades focus around the aim of reproducing a more anatomically representative reconstruction, with recognition that the anatomical foot print, or attachment point on the medial wall of the lateral femoral condyle, represented a broader insertion point, extending anteriorly to beyond the midpoint of the lateral wall, when arthroscopically viewed with the knee at 90° of flexion [Figure 1].
Figure 1.
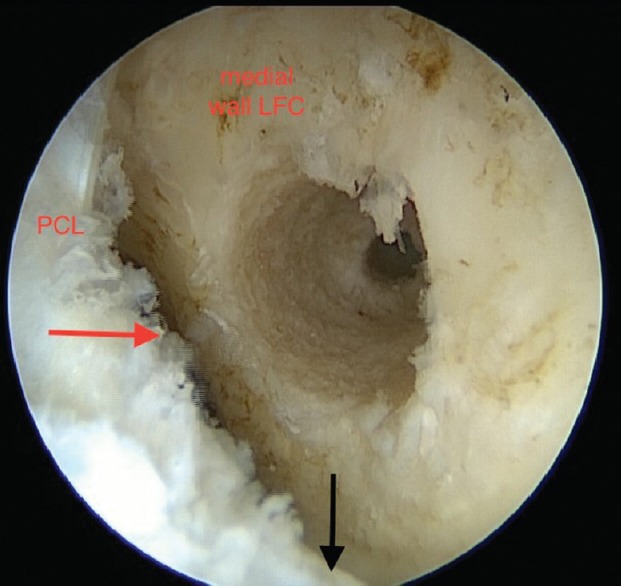
Arthroscopic view showing 9 mm femoral drill hole positioning. PCL: Posterior cruciate ligament, Medial wall LFC: Medial wall lateral femoral condyle. Red arrow: Posterior intercondylar border lateral femoral condyle. Black arrow: Tibiofemoral joint line
Appreciation of the broad nature of the femoral footprint promoted double-bundle ACLR techniques, with the anteromedial bundle of the reconstruction running from a more anterior orientation on the tibial footprint, to a more medial, or proximal orientation on the femoral footprint. The posterolateral bundle is based with a posterior (and medial) tibial orientation on the tibia, passing to a more distal and lateral aspect on the femoral condyle.
Biomechanical cadaveric and clinical studies demonstrate improved mechanical stability, in both rotation and translation, with double-bundle ACLR techniques.40,41,42,43 However, thus far, clinical studies have not convincingly confirmed improved outcome as predicted by biomechanical studies, over more evolved single-bundle reconstruction,44 and therefore, double-bundle reconstruction methods have yet to be universally adopted.43
The disadvantages of using double-bundle reconstruction techniques include increased operating time and complexity, bone loss should revision surgery be required and implant costs. In addition, the placing of twin drill holes on the tibial and femoral footprints may not be viable, in patient populations of smaller stature, or when the intercondylar notch morphology is narrow.
Improved understanding of the biomechanical advantages of double-bundle reconstruction has directed modification in tunnel positioning and graft orientation when using a single bundle, placing the tibial graft tunnel more anterior and central on the tibial ACL footprint [Figure 2]. In turn, the femoral tunnel graft drill hole is brought more distal on the lateral femoral condyle, or anterior, if the femur is viewed arthroscopically, with the knee at 90° of flexion. This evolution in ACL graft positioning has been referred to as “anatomical positioning” and provides improved rotational constraint, compared to a more “vertical” orientation of the ACLR, with the site of femoral attachment of the graft in the posterior aspect of the intercondylar notch, in a more proximal and medial orientation.
Figure 2.
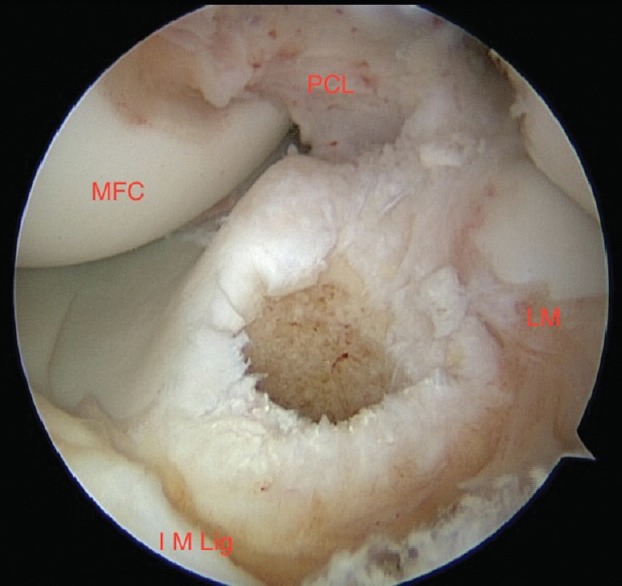
Arthroscopic view showing tibial anterior cruciate ligament tunnel. PCL: Anterior border posterior cruciate ligament, MFC: Medial femoral condyle, IM Lig: Anterior intermeniscal ligament, LM: Posterior border of anterior horn of lateral meniscus
By increasing the size of the femoral and tibial ACL attachment points, with both double-tunnel reconstruction methods or using larger grafts, when utilizing an anatomic single-bundle technique, increased native footprint coverage results in increased biomechanical rotational and translational control, following ligament reconstruction.
Increasing footprint coverage and graft size may ultimately decrease reinjury rates. However, anatomical positioning of the femoral graft, placing the bone tunnel in a more distal and posterior site (or anterior and toward the joint line, when arthroscopically viewed at 90° of flexion), is associated with a rise in graft loading45 and possible clinical failure, due to over loading of the graft in the healing phase, in contrast to the techniques of more vertical graft orientation on the femoral footprint, which were less able to control rotation, but which generated a more mature interface between bone and graft, due to lower graft tension during the healing phase.
An ACL disruption is often accompanied by meniscal or osteochondral injury. Tearing of the body or root of the menisci has long been identified as part of the primary knee injury, particularly of the osseous attachment of the posterior horn of the lateral meniscus. However, attention has focused on a variant of injury to the posterior horn of the medial meniscus, first identified by Strobel in 1988. Disruption of the attachment of the posterior horn of the medial meniscus from the capsular junction, with disruption of the posterior aspect of the meniscotibial ligament, produces an injury referred to as a ramp lesion, by Strobel [Figure 3]. Further highlighted by Bollen,46 this lesion occurs in between 9% and 17% of ACL disruptions.46,47 This meniscocapsular tear requires surgical repair [Figure 4], to decrease ACL graft loads, particularly in the sagittal plane.
Figure 3.
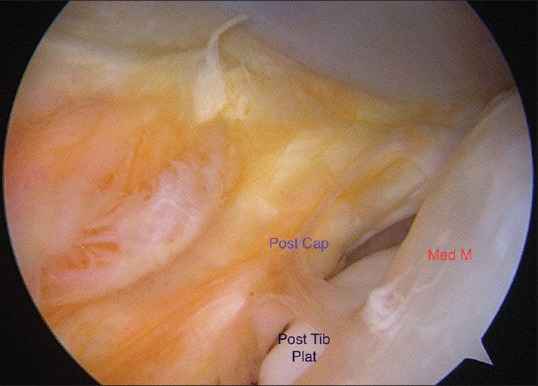
Arthroscopic view showing ramp lesion. Post cap: Posteromedial capsular detachment from meniscus, Med M: Posterior region of medial meniscus, Post tib plat: Posterior region of medial tibial plateau visible through ramp tear
Figure 4.
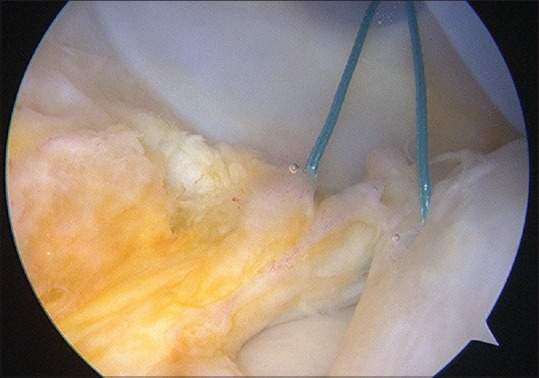
Arthroscopic view showing suture repair of Ramp lesion of posteromedial capsule
In addition to the intraarticular injury pattern, there is a variable degree of injury to capsular and extracapsular lateral soft tissues. With attempts to refine the results of ACLR, recognizing and treating lateral soft-tissue injuries decreases persistent pivot shift, following reconstruction and may decrease reinjury rate.
Segond first described a lateral tibial pericapsular bone avulsion related to ACL injury, in 1879. Since this original identification, it has been recognized that ACL disruption is often accompanied by a variable injury to the lateral capsule, including tissues attached to the lateral meniscus,48 and more superficially, to the level of the deep surface of the IT band. Dissection and subsequent biomechanical studies have confirmed the presence of lateral-sided constraints that resist internal tibial torsion and translation.
Open lateral tenodesis, as proposed Lemaire, Macintosh, Ellison, and Andrews, among others, aims to utilize the tissue of the iliotibial band to retension and reinforce the lateral aspect of the knee, superficial to the lateral capsule, to resist internal rotation of the tibia.49,50,51,52 Lateral tenodesis, both in isolation and when combined with intraarticular ACLR, provides improved internal tibial torsional control. Dejour, in utilizing BPTB intraarticular reconstruction, in association with Lemaire extracapsular lateral tenodesis in 251 procedures, with a minimum of 3 years followup, noted good or excellent results in 83%.53 The popularity of extracapsular lateral tenodesis diminished with concerns over the extent of lateral incision size, loss of flexion, with some techniques in particular, and postoperative lateral irritability. Over-constraint of the lateral tibiofemoral compartment with loss of internal tibial rotation and lateral compartmental arthrosis were also concerns relating to open lateral tenodesis.
However, outcome evidence does not suggest that lateral compartmental arthrosis is, in fact, a concern when incorporating lateral soft-tissue tenodesis, as an augmentation to ACLR.54 In addition, improved understanding of the requirements of lateral augmentation has enabled the surgical procedure to be carried out through smaller laterally based incisions, so improving cosmesis and reducing lateral discomfort postoperatively. Avoiding over constraint, by fixing the lateral augmentation with the knee toward extension, rather than in significant flexion, with the tibia in neutral rotation and without over-tensioning the graft at fixation, has reawakened interest in lateral tenodesis, in combination with intraarticular ACLR,55 with reduction in intraarticular graft forces of up to 47%, when combining a lateral tenodeis with anatomic ACLR,56 which may protect intraarticular graft tissue from excess loading, during early graft healing.
In conjunction with the revisiting of the outcome benefits of mini-open lateral tenodesis is interest in augmentation and reconstruction of the anterolateral ligament (ALL), as repopularized by Claes et al.57 The ALL is one component of a variable lateral fascial condensation, situated at the level of, and deep to, the iliotibial band region, and the lateral intermuscular septum. The ALL component of this lateral fibrous tissue is described as an extracapsular band running from a site just posterior and proximal to the lateral collateral ligament attachment point on the femur, passing distally and anteriorly, superficial to the fibular collateral ligament, to a point 1 cm distal to the lateral joint line, midway between the center on Gerdy's tubercle, and the attachment of the fibular collateral ligament to the fibular head. This tibial attachment point closely relates to the site of a Segond avulsion fracture and has been noted in up to 40 out of 41 dissection specimens.57
With ongoing debate on the precise femoral origin of the ALL,57,58,59 attention continues to be directed to the capsule and extracapsular lateral structures injured at the point of ACL rupture, by pathological tibial internal rotation and translation.60
Conclusion
Accordingly, although kinematic studies suggest improved constraint by the adoption of double-bundle intraarticular reconstruction techniques, such techniques have yet to demonstrate a significant improvement in the clinical outcome of ACLR, as defined by return to previous sporting levels and improved PROMs and activity levels.
Adoption of more anatomically oriented ACLR methods, combined with addressing previously undertreated meniscal injuries, both of the osseous attachment of the posterior horns of the menisci and of the posterior body of the medial meniscus to the posteromedial capsule (ramp lesion) may improve outcome following ACLR.
Repair or augmentation of lateral pericapsular structures, through reconstruction of the ALL, or else by more traditional open lateral tenodesis, aimed to resist pathological internal tibial torsion and intraarticular graft overload, during early healing, would seem to offer the potential for improved biomechanical control, decreased patholaxity, and ACL graft reinjury rates.31,33,34
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Englund M, Eriksson K, Forssbland M, Frobell R, Kvist J, Herbertsson P. Swedish ACL Register. Annual Report. The Swedish National Knee Ligament Register. X Base. 2014 [Google Scholar]
- 2.Jacob KM, Oommen AT. A retrospective analysis of risk factors for meniscal co-morbidities in anterior cruciate ligament injuries. Indian J Orthop. 2012;46:566–9. doi: 10.4103/0019-5413.101038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanders TL, Maradit Kremers H, Bryan AJ, Larson DR, Dahm DL, Levy BA, et al. Incidence of anterior cruciate ligament tears and reconstruction: A 21-year population-based study. Am J Sports Med. 2016;44:1502–7. doi: 10.1177/0363546516629944. [DOI] [PubMed] [Google Scholar]
- 4.Owens BD, Mountcastle SB, Dunn WR, DeBerardino TM, Taylor DC. Incidence of anterior cruciate ligament injury among active duty U.S. military servicemen and servicewomen. Mil Med. 2007;172:90–1. doi: 10.7205/milmed.172.1.90. [DOI] [PubMed] [Google Scholar]
- 5.Prodromos CC, Han Y, Rogowski J, Joyce BT, Shi K. The incidence of anterior cruciate ligament injury as a function of gender, sport, and injury-reduction programs. In: Prodromos CC, Brown CH, Fu FH, Georgoulis A, Gobbi A, Howell SM, Johnson D, Paulos LE, Shelbourne KD, editors. The Anterior Cruciate Ligament. Philadelphia: Saunders Elsevier; 2008. pp. 28–41. [Google Scholar]
- 6.Harmon KG, Dick R. The relationship of skill level to anterior cruciate ligament injury. Clin J Sport Med. 1998;8:260–5. doi: 10.1097/00042752-199810000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Mihata LC, Beutler AI, Boden BP. Comparing the incidence of anterior cruciate ligament injury in collegiate lacrosse, soccer, and basketball players: Implications for anterior cruciate ligament mechanism and prevention. Am J Sports Med. 2006;34:899–904. doi: 10.1177/0363546505285582. [DOI] [PubMed] [Google Scholar]
- 8.Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23:694–701. doi: 10.1177/036354659502300611. [DOI] [PubMed] [Google Scholar]
- 9.Viola RW, Steadman JR, Mair SD, Briggs KK, Sterett WI. Anterior cruciate ligament injury incidence among male and female professional alpine skiers. Am J Sports Med. 1999;27:792–5. doi: 10.1177/03635465990270061701. [DOI] [PubMed] [Google Scholar]
- 10.Agel J, Rockwood T, Klossner D. Collegiate ACL injury rates across 15 sports: National collegiate athletic association injury surveillance system data update (2004-2005 Through 2012-2013) Clin J Sport Med. 2016;26:518–23. doi: 10.1097/JSM.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 11.Chappell JD, Creighton RA, Giuliani C, Yu B, Garrett WE. Kinematics and electromyography of landing preparation in vertical stop-jump: Risks for noncontact anterior cruciate ligament injury. Am J Sports Med. 2007;35:235–41. doi: 10.1177/0363546506294077. [DOI] [PubMed] [Google Scholar]
- 12.Ireland ML. The female ACL: Why is it more prone to injury? Orthop Clin North Am. 2002;33:637–51. doi: 10.1016/s0030-5898(02)00028-7. [DOI] [PubMed] [Google Scholar]
- 13.Al-Saeed O, Brown M, Athyal R, Sheikh M. Association of femoral intercondylar notch morphology, width index and the risk of anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2013;21:678–82. doi: 10.1007/s00167-012-2038-y. [DOI] [PubMed] [Google Scholar]
- 14.Cheung EC, Boguszewski DV, Joshi NB, Wang D, McAllister DR. Anatomic factors that may predispose female athletes to anterior cruciate ligament injury. Curr Sports Med Rep. 2015;14:368–72. doi: 10.1249/JSR.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 15.Wojtys EM, Ashton-Miller JA, Huston LJ. A gender-related difference in the contribution of the knee musculature to sagittal-plane shear stiffness in subjects with similar knee laxity. J Bone Joint Surg Am. 2002;84-A:10–6. doi: 10.2106/00004623-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Nyman E, Jr, Armstrong CW. Real-time feedback during drop landing training improves subsequent frontal and sagittal plane knee kinematics. Clin Biomech (Bristol, Avon) 2015;30:988–94. doi: 10.1016/j.clinbiomech.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Ardern CL, Webster KE, Taylor NF, Feller JA. Return to sport following anterior cruciate ligament reconstruction surgery: A systematic review and meta-analysis of the state of play. Br J Sports Med. 2011;45:596–606. doi: 10.1136/bjsm.2010.076364. [DOI] [PubMed] [Google Scholar]
- 18.Spindler KP, Huston LJ MOON Knee Group. O’Donoghue Sports Injury Award. 10 Year Outcomes and Risk Factors after ACL Reconstruction: A Multicentre Cohort Study. AOSSM Annual Meeting, Toronto. 2017 Jul [Google Scholar]
- 19.Cinque ME, Hannon CP, Bohl DD, Erickson BJ, Verma NN, Cole BJ, et al. Return to sport and performance after anterior cruciate ligament reconstruction in national football league linemen. Orthop J Sports Med. 2017;5:2325967117711681. doi: 10.1177/2325967117711681. eCollection June 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erickson BJ, Harris JD, Heninger JR, Frank R, Bush-Joseph CA, Verma NN, et al. Performance and return-to-sport after ACL reconstruction in NFL quarterbacks. Orthopedics. 2014;37:e728–34. doi: 10.3928/01477447-20140728-59. [DOI] [PubMed] [Google Scholar]
- 21.Feller J, Webster KE. Return to sport following anterior cruciate ligament reconstruction. Int Orthop. 2013;37:285–90. doi: 10.1007/s00264-012-1690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox CL, Huston LJ, Dunn WR, Reinke EK, Nwosu SK, Parker RD, et al. Are articular cartilage lesions and meniscus tears predictive of IKDC, KOOS, and Marx activity level outcomes after anterior cruciate ligament reconstruction? A 6-year multicenter cohort study. Am J Sports Med. 2014;42:1058–67. doi: 10.1177/0363546514525910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westermann RW, Jones M, Wasserstein D, Spindler KP. Clinical and radiographic outcomes of meniscus surgery and future targets for biologic intervention: A review of data from the MOON Group. Connect Tissue Res. 2017;58:366–72. doi: 10.1080/03008207.2017.1297808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams D, Logerstedt DS, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current concepts for anterior cruciate ligament reconstruction: A criterion-based rehabilitation progression. J Orthop Sports Phys Ther. 2012;42:601–14. doi: 10.2519/jospt.2012.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29:213–8. doi: 10.1177/03635465010290021601. [DOI] [PubMed] [Google Scholar]
- 26.Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q. A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2015;22:100–10. doi: 10.1016/j.knee.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Maletis GB, Inacio MC, Funahashi TT. Risk factors associated with revision and contralateral anterior cruciate ligament reconstructions in the Kaiser Permanente ACLR registry. Am J Sports Med. 2015;43:641–7. doi: 10.1177/0363546514561745. [DOI] [PubMed] [Google Scholar]
- 28.Cavaignac E, Coulin B, Tscholl P, Nik Mohd Fatmy N, Duthon V, Menetrey J. Is quadriceps tendon autograft a better choice than hamstring autograft for anterior cruciate ligament reconstruction? A comparative study with a mean follow-up of 3.6 years. Am J Sports Med. 2017;45:1326–32. doi: 10.1177/0363546516688665. [DOI] [PubMed] [Google Scholar]
- 29.Stein DA, Hunt SA, Rosen JE, Sherman OH. The incidence and outcome of patella fractures after anterior cruciate ligament reconstruction. Arthroscopy. 2002;18:578–83. doi: 10.1053/jars.2002.30658. [DOI] [PubMed] [Google Scholar]
- 30.Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR., Jr The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24:162–6. doi: 10.1016/j.arthro.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Leys T, Salmon L, Waller A, Linklater J, Pinczewski L. Clinical results and risk factors for reinjury 15 years after anterior cruciate ligament reconstruction. Prospective study of hamstrings and patellar tendon grafts. Am J Sports Med. 2012;3:595–605. doi: 10.1177/0363546511430375. [DOI] [PubMed] [Google Scholar]
- 32.Mohtadi NG, Chan DS, Dainty KN, Whelan DB. Patellar tendon versus hamstring tendon autograft for anterior cruciate ligament rupture in adults. Cochrane Database Syst Rev. 2011 Sep;7(9):CD005960. doi: 10.1002/14651858.CD005960.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salmon L, Russell V, Musgrove T, Pinczewski L, Refshauge K. Incidence and risk factors for graft rupture and contralateral rupture after anterior cruciate ligament reconstruction. Arthroscopy. 2005;21:948–57. doi: 10.1016/j.arthro.2005.04.110. [DOI] [PubMed] [Google Scholar]
- 34.Mariscalco MW, Magnussen RA, Mehta D, Hewett TE, Flanigan DC, Kaeding CC. Autograft versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: A systematic review. Am J Sports Med. 2014;42:492–9. doi: 10.1177/0363546513497566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lenehan EA, Payne WB, Askam BM, Grana WA, Farrow LD. Long-term outcomes of allograft reconstruction of the anterior cruciate ligament. Am J Orthop (Belle Mead NJ) 2015;44:217–22. [PubMed] [Google Scholar]
- 36.Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: A prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17:464–74. doi: 10.1007/s00167-008-0714-8. [DOI] [PubMed] [Google Scholar]
- 37.McAllister DR, Parker RD, Cooper AE. Outcomes of postoperative sepsis after anterior cruciate ligament reconstruction. Am J Sports Med. 1999;27:562–70. doi: 10.1177/03635465990270050301. [DOI] [PubMed] [Google Scholar]
- 38.Zalavras CG, Patzakis MJ. Infections in anterior cruciate ligament surgery. In: Prodromos CC, Brown CH, Fu FH, Georgoulis A, Gobbi A, Howell SM, Johnson D, Paulos LE, Shelbourne KD, editors. The Anterior Cruciate Ligament. Philadelphia: Saunders Elsevier; 2008. pp. 551–60. [Google Scholar]
- 39.Ekhtiari S, Horner NS, de Sa D, Simunovic N, Hirschmann MT, Ogilvie R, et al. Arthrofibrosis after ACL reconstruction is best treated in a step-wise approach with early recognition and intervention: A systematic review. Knee Surg Sports Traumatol Arthrosc. 2017 Mar 4; doi: 10.1007/s00167-017-4482-1. doi: 10.1007/s00167-017-4482-1. [DOI] [PubMed] [Google Scholar]
- 40.Tsai AG, Wijdicks CA, Walsh MP, Laprade RF. Comparative kinematic evaluation of all-inside single-bundle and double-bundle anterior cruciate ligament reconstruction: A biomechanical study. Am J Sports Med. 2010;38(2):263–72. doi: 10.1177/0363546509348053. [DOI] [PubMed] [Google Scholar]
- 41.Järvelä T, Moisala AS, Sihvonen R, Järvelä S, Kannus P, Järvinen M. Double-bundle anterior cruciate ligament reconstruction using hamstring autografts and bioabsorbable interference screw fixation: Prospective, randomized, clinical study with 2-year results. Am J Sports Med. 2008;36:290–7. doi: 10.1177/0363546507308360. [DOI] [PubMed] [Google Scholar]
- 42.Siebold R, Dehler C, Ellert T. Prospective randomized comparison of double-bundle versus single-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2008;24:137–45. doi: 10.1016/j.arthro.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 43.Hussein M, van Eck CF, Cretnik A, Dinevsku D, Fu FH. Prospective randomised clinical evaluation of conventional single-bundle, anatomic single-bundle and anatomic double-bundle anterior cruciate ligament reconstruction. 281 cases with 3-5 year follow up. Am J Sports Med. 2012;3:512–20. doi: 10.1177/0363546511426416. [DOI] [PubMed] [Google Scholar]
- 44.Mohtadi NG, Chan DS. A Randomised Trail Comparing Patellar, Hamstring and Double-Bundle ACL Reconstruction at 5 Years. AOSSM Annual Meeting. 2017 Jul;:139. [Google Scholar]
- 45.Nawabi DH, Tucker S, Schafer KA, Zuiderbaan HA, Nguyen JT, Wickiewicz TL, et al. ACL fibers near the lateral inter-condylar ridge are the most load bearing during stability examinations and isometric through passive flexion. Am J Sports Med. 2016;44:2563–71. doi: 10.1177/0363546516652876. [DOI] [PubMed] [Google Scholar]
- 46.Bollen SR. Posteromedial meniscocapsular injury associated with rupture of the anterior cruciate ligament: A previously unrecognized association. J Bone Joint Surg Br. 2010;92:222–3. doi: 10.1302/0301-620X.92B2.22974. [DOI] [PubMed] [Google Scholar]
- 47.Chahla J, Dean CS, Moatshe G, Mitchell JJ, Cram TR, Yacuzzi C, et al. Meniscal ramp lesions: Anatomy, incidence, diagnosis, and treatment. Orthop J Sports Med. 2016;4(7) doi: 10.1177/2325967116657815. 325967116657815 on line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terry GC, Norwood LA, Hughston JC, Caldwell KM. How iliotibial tract injuries of the knee combine with acute anterior cruciate ligament tears to influence abnormal anterior tibial displacement. Am J Sports Med. 1993;21:55–60. doi: 10.1177/036354659302100110. [DOI] [PubMed] [Google Scholar]
- 49.Ellison AE. Distal iliotibial-band transfer for anterolateral rotatory instability of the knee. J Bone Joint Surg Am. 1979;61:330–7. [PubMed] [Google Scholar]
- 50.Ireland J, Trickey EL. Macintosh tenodesis for anterolateral instability of the knee. J Bone Joint Surg Br. 1980;62:340–5. doi: 10.1302/0301-620X.62B3.7410466. [DOI] [PubMed] [Google Scholar]
- 51.Andrews JR, Sanders R. A “mini-reconstruction” technique in treating anterolateral rotatory instability (ALRI) Clin Orthop Relat Res. 1983 Jan-Feb;172:93–6. [PubMed] [Google Scholar]
- 52.Dandy DJ. Some clinical aspects of reconstruction for chronic anterior cruciate ligament deficiency. Ann R Coll Surg Engl. 1995;77:290–8. [PMC free article] [PubMed] [Google Scholar]
- 53.Dejour H, Walch G, Neyret P, Adeleine P. Results of surgically treated chronic anterior laxities. Apropos of 251 cases reviewed with a minimum follow-up of 3 years. Rev Chir Orthop Reparatrice Appar Mot. 1988;74:622–36. [PubMed] [Google Scholar]
- 54.Marcacci M, Zaffagnini S, Giordano G, Iacono F, Presti ML. Anterior cruciate ligament reconstruction associated with extra-articular tenodesis: A prospective clinical and radiographic evaluation with 10- to 13-year follow-up. Am J Sports Med. 2009;37:707–14. doi: 10.1177/0363546508328114. [DOI] [PubMed] [Google Scholar]
- 55.Lording TD, Lustig S, Servien E, Neyret P. Lateral reinforcement in anterior cruciate ligament reconstruction. Asia Pac J Sports Med Arthrosc Rehabitiation Technol. 2014;1:3–10. [Google Scholar]
- 56.Engebretsen L, Lew WD, Lewis JL, Hunter RE. The effect of an iliotibial tenodesis on intraarticular graft forces and knee joint motion. Am J Sports Med. 1990;18:169–76. doi: 10.1177/036354659001800210. [DOI] [PubMed] [Google Scholar]
- 57.Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223:321–8. doi: 10.1111/joa.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dodds AL, Gupte CM, Neyret P, Williams AM, Amis AA. Extra-articular techniques in anterior cruciate ligament reconstruction: A literature review. J Bone Joint Surg Br. 2011;93:1440–8. doi: 10.1302/0301-620X.93B11.27632. [DOI] [PubMed] [Google Scholar]
- 59.Chahla J, Menge TJ, Mitchell JJ, Dean CS, LaPrade RF. Anterolateral ligament reconstruction technique: An anatomic-based approach. Arthrosc Tech. 2016;5:e453–7. doi: 10.1016/j.eats.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sonnery-Cottet B, Thaunat M, Freychet B, Pupim BH, Murphy CG, Claes S. Outcome of a Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction Technique With a Minimum 2-Year Follow-up. Am J Sports Med. 2015;43:1598–605. doi: 10.1177/0363546515571571. [DOI] [PubMed] [Google Scholar]


