Abstract
Schizophrenia, depression and posttraumatic stress disorder (PTSD) are severe mental disorders and complicated diagnostic entities, due to their phenotypic, biological and genetic heterogeneity, unknown etiology, and poorly understood alterations in biological pathways and biological mechanisms. Disturbed homeostasis between overproduction of oxidant species, overcoming redox regulation and a lack of cellular antioxidant defenses, resulting in free radical-mediated pathology and subsequent neurotoxicity contributes to development of depression, schizophrenia and PTSD, their heterogeneous clinical presentation and resistance to treatment. Metabolomics is a discipline that combines different strategies with the aim to extract, detect, identify and quantify all metabolites that are present in a biological sample and might provide mechanistic insights into the etiology of various psychiatric disorders. Therefore, oxidative stress research combined with metabolomics might offer a novel approach in dissecting psychiatric disorders, since these data-driven but not necessarily hypothesis-driven methods might identify new targets, molecules and pathways responsible for development of schizophrenia, depression or PTSD. Findings from the oxidative research in psychiatry together with metabolomics data might facilitate development of specific and validated prognostic, therapeutic and clinical biomarkers. These methods might reveal bio-signatures of individual patients, leading to individualized treatment approach. In reviewing findings related to oxidative stress and metabolomics in selected psychiatric disorders, we have highlighted how these novel approaches might make a unique contribution to deeper understanding of psychopathological alterations underlying schizophrenia, depression and PTSD.
Keywords: Schizophrenia, Depression, Posttraumatic stress disorder, Oxidative stress, Lipid peroxidation, Metabolomics, Biomarkers
Graphical abstract
1. Psychiatric disorders
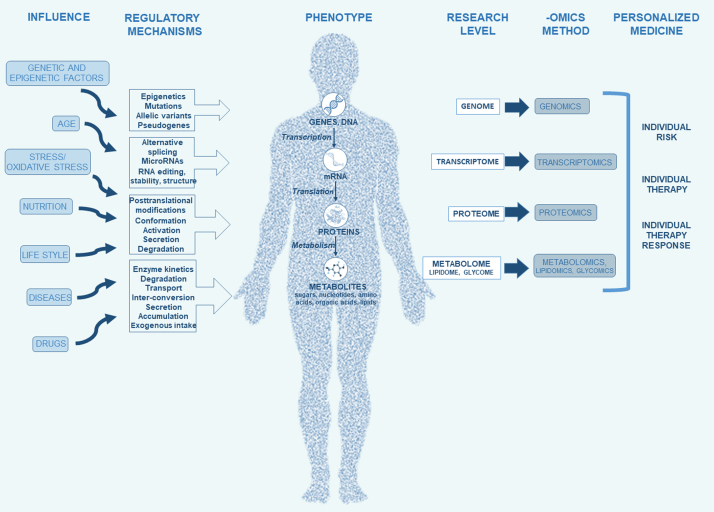
Psychiatric disorders such as schizophrenia, depression and posttraumatic stress disorder (PTSD) are severe mental disorders and complicated diagnostic entities, due to their phenotypic, biological and genetic heterogeneity and complex interactions between biological, environmental and genetic factors that cause development of these disorders. These disorders have usually unknown etiology, and their classification depends on the particular characteristic symptoms or the course of illness. Since the biological mechanisms and pathways underlying risk for these disorders are still not completely identified, there are no validated, selective and specific biomarkers for these diseases. To overcome these obstacles, „omics“ approach was proposed and used. They were promising approaches, especially genomics, since genome-wide association studies (GWAS) identified multiple genetic associations with psychiatric disorders, indicating a significant effect of heritability [70]. However, due to the polygenic nature of psychiatric disorders, GWAS indicated many genetic loci but with a ”small effect”, a questionable clinical significance, and limited biological insight [76], since numerous genetic loci associated with these disorders, biological pathways and mechanisms are yet not known. Namely, some substantial loci that might be important as risk factors or contributors for psychiatric disorders were not detected due to statistical corrections and cutoffs [70]. Besides genomics, other “omics” approaches include transcriptomics, proteomics and metabolomics (Fig. 1). These important approaches offer modern state-of-the-art techniques for understanding biological systems underlying vulnerability to psychiatric disorders and their interaction with genetic and environmental factors [83]. These methods use easy available body fluids such as blood, cerebrospinal fluid (CSF) or urine with a potential clinical utility of selected molecules as prognostic, therapeutic and clinical biomarkers [103].
Fig. 1.
Omics approach to psychiatric disorders.
2. Metabolomics
Metabolomics is a scientific discipline dealing with small (up to 1.5 kDa) molecules called metabolites. Metabolites are endogenous low-molecular weight substances synthesized in the cells during metabolic process [26]. Metabolites, such as amino acids, lipids, nucleic acids, vitamins, carbohydrates and organic acids are the final products of the controlled cellular processes [80], [101], [106] that are catalyzed by various enzymes [26]. Similarly to transcriptome and proteome, the set of these endogenous biomolecules constitute the metabolome [22] that can be determined on the cellular, tissue, organ or the whole organism level [34], [80]. Unlike genes and proteins, metabolites are much more diverse, and their variability depends on various genetic and environmental factors [22]. Different biological fluids or cell types have a characteristic set and concentration of metabolites [34]. Therefore, the balance between metabolites and all enzymes, cofactors, intermediates and substrates through metabolic pathways is needed to preserve homeostasis [26]. Metabolites highly reflect environmental influence, nutrition needs, effects of xenobiotics and medication, stress as well as different pathologies or internal changes in biochemical pathways of the studied system [76], [82]. The metabolome includes all metabolites that are present in biological samples, such as blood, urine, cell, tissue, organ or organism, and is a result of the gene x environment interaction and/or genotype x phenotype interaction [76]. Metabolic processes reveal the activities of the gene expression and proteins, and metabolome is therefore a product of genome and proteome interacting with environmental factors, and a regulator of the metabolic homeostasis. Altered balance in some metabolic pathways leads to metabolic diseases, such as diabetes, atherosclerosis, hypertension and obesity, and causes a major health problem worldwide [26]. Due to the great importance of metabolites in biological systems, metabolite analysis as a method is increasingly used in various fields of research, since it provides improved understanding of many pathological processes through altered metabolic pathways [106].
Metabolomics offers mechanistic insights into the etiology of various diseases [103], providing comprehension of complex interactions between phenotype and genotype, and it is useful tool for understanding how cells and therefore the whole organisms function in health and diseases [83]. Therefore, metabolomics has a major impact in pharmacology, environmental sciences, toxicology, cancer, nutrition, and neuropsychiatry, for detecting potential disease biomarkers [80], [106]. Biofluids used in metabolomics are CSF, plasma, urine, saliva, bronchoalveolar lavage fluid, digestive fluids, synovial fluid, cyst fluids and amniotic fluids, while platelets and CSF are the most commonly used for human metabolomic studies of psychiatric and neurological diseases [5], [80], [106].
There are two main approaches to metabolite analysis: i) untargeted (or non-targeted) metabolomics is the hypothesis generating, global unbiased analysis of all the small-molecule metabolites present within a biological system, under a given set of conditions [63]. It usually works by differential analysis of two or more groups in a semi-quantitative manner. This is strict sense what metabolomics means; ii) targeted metabolomics refers to studies aiming to measure specific known molecules, focusing on one or more related metabolic pathways which have been defined as biologically relevant in previous studies. Therefore, methods with high levels of specificity, precision and accuracy are applied. The potential of metabolomics lies in disease marker discovery and detection, but also in drug discovery, treatment response and in markers of toxicity. Altered metabolite levels can be detected at baseline and after treatment, with the aim to identify specific metabolites associated with good or poor therapeutic response, or development of medication side effects. The extraction, quantification, identification and interpretation of different metabolites require sophisticated analytical technologies, statistical methods for data interpretation, as well as bio-statistical and multi-variant methods [9], [43], [82]. Analytical techniques, including mass spectrometry (MS), coupled to different separation techniques and nuclear magnetic resonance (NMR) produce data on a large number of metabolites (Table 1), although additional technologies are required for metabolites that appear and act at lower concentrations [26]. Therefore, most data on all metabolites from a single sample are obtained from different analytical platforms. MS/chromatography and NMR are basic strategies for metabolomics [26], [34], [91].
Table 1.
Comparison of different analytical techniques in metabolomics.
|
ANALYTICAL TECHNIQUES |
||||
|---|---|---|---|---|
| NMR |
MS |
|||
| Principle of detection | Magnetic properties of atomic nuclei (1H, 13C, 31P) | Mass to charge ratio of ionized particles |
||
| LC | GC | CE | ||
| Sample preparation | + | + | - | + |
| Reproducibility/ robustness | + | - | + | - |
| Sensitivity | - | + | + | - |
| Types of components | Polar and non-polar | Broad use depending on LC type | Thermostable and volatile molecules | Polar charged molecules |
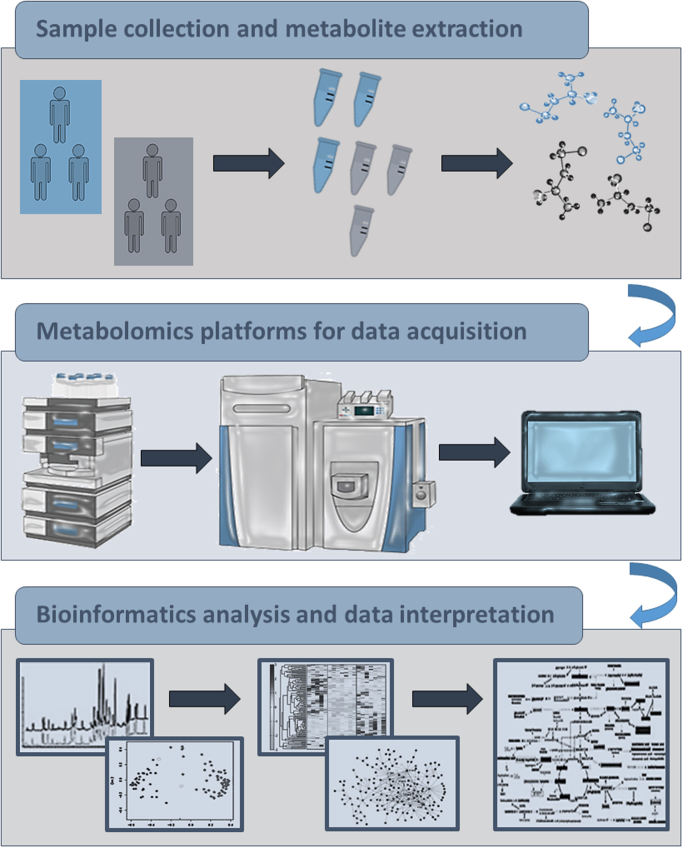
While MS relies on mass to charge ratio of ionized particles, NMR spectroscopy uses magnetic properties of certain nuclei, such as 1H, 13C, 31P and some others [106]. NMR spectroscopy is a reproducible and quantitative technique with low sensitivity. Mass spectrometers can detect low-concentration metabolites, but unlike NMR, MS quantitation is not so straightforward. MS is a powerful analytical platform whose efficiency is increased by coupling with the separation techniques, GC or LC or capillary electrophoresis. The main advantage of GC-MS lies in the fact that it enables compound identification according to both, retention time and mass spectrum [53]. Namely, it gives highly reproducible retention time profiles and fragmentation spectra. This is a valuable feature which enables users to use standard libraries, such as National Institute of Standard Technologies library (NIST) or Fiehn retention time locked library [46], as well as to build and share their own databases making the identification of compounds less demanding [74]. There are also some limitations of GC-MS. Since volatility of compounds is obligatory for GC-MS, this method usually starts with demanding sample treatment, including the removing of non-volatile compounds and derivatization process, which enables non-volatile compounds to become volatile [17]. At the end, a limited subset of compounds, usually organic acids, amino acids, mono- and di-saccharides, various clases of lipids and sterols can be analyzed by this method [37]. Sample treatment for LC-MS is usually less demanding and this analytical method can be also used for the analysis of non-volatile and thermally fragile molecules [113]. For example, semi-polar compounds, such as phenolic acids, flavonoids and glycosylated species are separated using reverse phase liquid chromatography (RPLC), but also non-polar compounds as most of lipids. Hydrophilic interaction liquid chromatography (HILIC) is used for analyzing polar compounds like sugars, amino acids, vitamins, carboxylic acids and nucleotides. Although LC-MS has broader use than GC-MS, the identification of compounds in LC-MS is more challenging. It is mostly putative identification starting with mass-based search against databases [27] often followed by tandem MS experiments or MS experiments with sample and authentic compound whose presence in the sample needs to be confirmed [92]. In capillary electrophoresis, separation of compounds happens according to the differences in their intrinsic electrophoretic mobility, depending on their charge and size [44]. Therefore, it is more suitable for the analysis of polar and charged metabolites such as amino acids. Although it is technically more demanding, with lower concentration sensitivity and higher migration time variability, it is still highly used for reproducible profiling of native peptides [75] and secondary metabolites. However, in order to expand metabolite coverage for untargeted metabolomics a combination of different analytical platforms is highly recommended [62]. Beside compound detection, identification and quantification, univariate (t-test, fold-change analysis, Wilcoxon rank sum test, analysis of variance) and multivariate, both unsupervised (principal component analysis) and supervised (partial least square-discriminant analysis), methods for statistical analysis are applied to detect those compounds whose levels are significantly different between distinct biological groups [98], [102]. In the last step, the compounds of interest should be placed in right biological context by performing a pathway analysis which implies assembling of metabolite sets according to available public databases such as Human Metabolome Data Base, The Small Molecule Pathway Database, Kyoto Encyclopedia of Genes and Genomes and others [39], [99] and text-mining of literature. At the end, the main goal is to identify a biological pathway included in disease condition or potential reliable biomarker, which can be further investigated (Fig. 2).
Fig. 2.
Metabolomics workflow.
2.1. Oxidative stress
Oxidative stress is characterized by the loss of homeostasis between overproduction of reactive oxygen species (ROS), and a lack of cellular antioxidant defenses going beyond the normal redox regulation [20], [89]. Oxidative stress cascade results in oxidative stress damage, which targets proteins, lipids and DNA. This process contributes to numerous pathological conditions, such as cancer, cardiovascular, respiratory and metabolic diseases, neurodegenerative and psychiatric disorders. Normal cellular metabolism results in production of ROS, which are under control of major antioxidants including superoxide dismutase, catalase, glutathione peroxidase, glutathione, uric acid, vitamin C, vitamin E, carotene and albumin [20]. However, under chronic oxidative stress, ROS-damaged biomolecules accumulate in the affected cells and in the entire organism, consequently affecting the metabolism [30], [68]. Thus, oxidative stress results in protein, lipid, carbohydrate and nucleic acid alterations leading to apoptosis, necrosis and other structural cell damages and modifications [66]. Metabolites, intermediates, enzymes and their activity in many metabolic pathways are altered due to high rates of ROS that can have both beneficial and harmful effects on target cells or tissues [35], [48]. ROS and reactive nitrogen species (RNS) are associated with various processes in the organism, such as lipid peroxidation, activation of phagocytes, trauma, oxidative phosphorylation, ischemia, etc. [35], [99]. Oxidative status of the organism and levels of ROS can be evaluated by indirect measurement of the oxidative products, enzymes and antioxidant levels in the brain or on the periphery. Oxidative stress induces neurochemical and neuroanatomical changes and it is involved in many psychiatric and neurodegenerative diseases, such as schizophrenia, Alzheimer's, Parkinson's and Huntington's disease [12], [57], [66].
Among specific pathophysiological features of oxidative stress, in particular of acute disorders, are alterations of the blood: brain barrier, which becomes permeable upon influences of the lipid peroxidation product 4-hydroxynonenal (HNE) causing inflammatory response resulting in systemic stress response [55], [84], [105]. However, while neuropathological aspects of oxidative stress and in particular of lipid peroxidation in neurodegenerative diseases were intensively studied, thus contributing to the modern concepts of major human diseases [104], the association of neuronal and systemic oxidative stress and lipid peroxidation with psychiatric diseases are less known and need more intense studies implementing modern concepts of integrative, personalized medicine [85].
2.2. Schizophrenia
Schizophrenia is a severe chronic mental, but also neurodevelopmental disorder, with a complex presentation and complicated and diverse symptoms. It is assumed that ~1% of the people worldwide have schizophrenia [8]. The disorder is characterized by different clusters of symptoms that might be subdivided into positive, negative, cognitive and mood (depressive) symptoms [2], [3], [24]. It is highly heritable and polygenic, with heterogeneous underlying neurobiology, and with poorly understood etiology [67]. Combination of various environmental factors (early life experience, exposure to violence, cannabis, drug abuse, migration, etc.) and their interactions with different genetic variations, but also epigenetic influences contribute to schizophrenia development [67], [76]. Although GWAS offered some novel genetic loci associated with schizophrenia, the problem is frequent non-replication and limited reproducible findings of these data, due to schizophrenia's phenotypic, genotypic, biological, but also clinical heterogeneity.
2.3. Schizophrenia and oxidative stress
Oxidative stress has been revealed as one of the biological underpinning of the pathophysiology of schizophrenia [20], [7]. Namely, it is tightly associated with inflammation, which generates ROS. Dysregulation of the redox balance during neurodevelopment might damage neurons, compromise neuronal survival, induce interneuron deficits via nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, elicit oligodendrocyte abnormalities, impair GABA interneurons, and affect biochemical processes that cause neuronal dysfunction, mitochondrial dysfunction, affect activity of the N-methyl-D-aspartate receptors and produce aberrant inflammatory responses [20], [7]. Markers of oxidative stress damage could be detected early in prodromal stage of schizophrenia; therefore, oxidative stress markers might be used to improve early diagnostic procedures, detection and interventions, and to advance treatment outcome [20], [7]. In patients with schizophrenia, homocysteine, a marker of the oxidative stress, was found to be associated with the severity of the positive, negative, cognitive and depressive symptoms, and with poor functioning [29].
2.4. Metabolomics in schizophrenia
Metabolomic studies in schizophrenia are complicated by the clinical heterogeneity of the schizophrenia symptoms and different treatment options. To avoid these confounders, the first episode of psychosis (i.e. in drug naive patients) could be evaluated. In this group, several metabolic disturbances in plasma or urine were found [11]. These metabolites were associated with neurotransmitter, amino acid, glucose and energy metabolism imbalances, oxidative stress and lipid metabolism disturbances, disruptions in antioxidant defense system, bowel microflora and endocrine system in schizophrenia patients [11]. Detailed metabolomic analysis revealed altered levels of noradrenergic metabolites (noradrenaline, vanil-mandelic acid, 3-methoxy-4-hydroxyphenylglycol), increased levels of plasma alanine, glycine and urine valine and glycine levels, as well as reduced levels of fasting plasma glucose, phosphatidylcholine, high-, low- and very low-density lipoproteins, unsaturated fatty acids and lipids; decreased levels of citrate, α-KG, creatine and creatinine, acetoacetate and 3-hydroxybutyrate and elevated levels of lactate and lysophosphatidylcholines in first-episode neuroleptic-naïve schizophrenia patients [11]. Biomarkers of the elevated oxidative stress were lower levels of antioxidants uric acid and taurine, but also increased lysophosphatidylcholines that transfer to phosphatidylcholines during the oxidation of LDL cholesterol, contributing to the development of cardiovascular and metabolic diseases and metabolic syndrome in schizophrenia [11]. Targeted metabolomic approach to schizophrenia revealed five metabolites: higher concentration of ornithine and lower concentration of arginine, glutamine, histidine and one lipid (PC ae C38:6), which could be used as biomarkers of schizophrenia since they were significantly different from values in controls and were not affected by antipsychotic medication [32]. In addition, these metabolites were associated with 13 risk genes for schizophrenia including gene coding for nitric oxide synthase 1, transcription factor 4, neurotrophin receptor kinase 3, catechol-O-methyltransferase, mitochondrial proline dehydrogenase, leucine carboxyl methyltransferase 1, cytosolic purine 50-nucleotidase, DNA-directed RNA polymerase III subunit RPC3, integrin beta-1, integrin alpha-10, clathrin, heavy chain-like 1, cell division control protein 42 homolog and cut-like homeobox 1 [32]. These associations are in line with studies suggesting genetic susceptibility of schizophrenia. Observed differentiating metabolites were primarily related to altered glutamine and arginine metabolism, disturbed biosynthetic process of nitrogen compounds, impaired learning memory, immune and immune-related signaling and neurotrophin signaling pathways [32]. Therefore, these metabolomic markers might be used as theranostic biomarkers of schizophrenia [32]. A detailed metabolomic study indicated that pathways involved in glucoregulation and proline metabolism were affected in schizophrenia [71]. Namely, the combination of selected metabolites associated with proline- and insulin-related changes were confirmed with higher metabolite levels of saturated triglycerides, branched chain amino acids, phenylalanine and tyrosine, and proline, glutamic, lactic and pyruvic acids found in schizophrenia patients in comparison to controls [71]. This study confirmed the usefulness of metabolomics as a powerful tool in dissecting illness pathways of schizophrenia. Changes, such as increases and decreases of various metabolite levels in schizophrenia are presented in Table 2.
Table 2.
Altered metabolite levels in schizophrenia.
| PSYCHIATRIC DISEASE |
METABOLOMICS |
REFERENCES | |
|---|---|---|---|
| Increased levels ↑ | Decreased levels ↓ | ||
| Schizophrenia | Alanine | Glucose | [11,32,71] |
| Glycine | Phosphatidylcholine | ||
| Valine (urine) | HDL | ||
| Glycine (urine) | LDL | ||
| Lactate | VLDL | ||
| Lysophosphatidylcholines | Unsaturated fatty acids | ||
| Ornithine | Lipids | ||
| 3-Indolebutyrate (microflora) | Citrate | ||
| Phenylalanine | α-KG | ||
| Tyrosine | Creatine | ||
| Proline | Creatinine | ||
| Glutamic acid | Acetoacetate | ||
| Pyruvic acid | 3-Hydroxybutyrate | ||
| Saturated triglycerides | Arginine | ||
| Glutamine | |||
| Histidine | |||
| Ketone bodies | |||
| Hippurate (microflora) | |||
| Trimethylamine-N-oxide (microflora) | |||
3. Depression
Depression is complex and clinically heterogeneous psychiatric disorder affecting 10–15% of people during lifetime and representing one of the main causes of disability and suicide [15]. Relatively low efficacy of treatment response (< 50%) to antidepressant drugs is probably due to a lack of well-characterized depression biomarkers. Although GWAS failed to identify genetic loci associated with depression [90], various research suggests strong interaction between genes and environment. The factors affecting nervous, endocrine, metabolic and immune systems and consequently influencing the development of depression include genetic and epigenetic factors, sex, hypothalamic-pituitary-adrenal (HPA) axis reactivity, as well as environmental influences such as toxins, early life adversities, socioeconomic status and stress [52]. Therefore, the analysis of metabolomics data might contribute to a better stratification of depressive subtypes and elucidation of complex interactions between genes and environment [76].
3.1. Depression and oxidative stress
Various studies demonstrated the involvement of oxidative stress in depression, as evidenced by observed elevated lipid peroxidation, higher generation of ROS and dysfunction of mitochondria [1], [47]. In patients with depression, both increased ROS production and its impaired neutralization by antioxidant and detoxification mechanisms were found [54]. This oxidant-antioxidant imbalance in depression may result in higher damage of various biomolecules, including DNA [14]. However, in addition to oxidative stress, increased DNA damage might be due to a less efficient DNA damage repair [14]. Further studies including metabolomics approaches could help to elucidate the exact mechanisms underlying altered oxidative status as well as usefulness of oxidative stress biomarkers in depression.
3.2. Metabolomics in depression
Increased plasma lysophospholipids, monoglycerophospholipids and phosphatidyle-thanolamines, which are involved in important cell processes such as energy storage, membrane integrity, as well as cell signaling, survival and apoptosis, have been found in depressive patients [49], together with decreased levels of free fatty acids. On the other hand, treatment with antidepressant drugs produced increased release of lysophospholipids in the mouse brain (Lee et al., 2009). Lower levels of different acyl carnitine molecules, observed in plasma of subjects with depression, could be used as possible biomarkers of depression [49]. Acetyl-l-carnitine might be effective for treatment of depression [95]. Carnitine molecules are very important for the transport of a long chain fatty acyl group across the inner mitochondrial membrane. Reduced plasma concentrations of stearic and palmitic amide, as well as lithocholic, deoxycholic, glycodesoxycholic, glycoursodeoxycholic and taurochenodeoxycholic acid were found in depression [49]. Palmitic acid induces anxiety-like behaviors in animals [59], whereas decreased palmitic acid brain levels are associated with anxiolytic effects [86]. In contrast to palmitic acid-induced reduction of proliferation of neuronal progenitor cells [73], stearic, glycoursodeoxycholic and taurochenodeoxycholic acids were shown to exert neuroprotective effects [69], [94], [96]. Results demonstrating higher serum glutamate and aspartate levels in depression suggest possible abnormalities in amino-acid pathway of the urea cycle in the liver or the disruption of NMDA receptor function [49]. The elevated levels of alanine, taurine, citrate, formate, glycine, isobutyrate and nicotinate, involved in the gluconeogenesis and fatty acid synthesis, have been observed in the urine of patients with depression [112]. In addition to the increase of these amino acids, lower levels of plasma or urine glucose, lactate, and pyruvate as well as increased levels of α-ketoglutarate, succinate, malonate, methylmalonate, and succinyl-CoA further supported the imbalance between glycolysis and gluconeogenesis and shift toward fatty acid and/or amino acid catabolism in depression [111], [112]. A decrease in tryptophan and tyrosine levels found in depression [49], [52], [60], might also suggest increased conversion to serotonin and cateholamines such as dopamine, norepinephrine and epinephrine or deficit in protein catabolism. Lower CSF levels of 5-hydroxyindoleacetic acid (5-HIAA) and homovanillic acid (HVA), which are serotonin, dopamine, and norepinephrine metabolites, implied altered serotonin and dopamine functions in depressed patients [4]. Metabolites from tryptophan, tyrosine and methionine pathways might be useful for differentiation between remitted and non-remitted depression [38]; however, the results regarding methionine plasma levels in depressive patients are contradictory [49], [100]. Decreased concentrations of N-methylnicotinamide in depression [112] might suggest the deficiency of nicotinamide, important for coenzymes in oxidation-reduction reactions. [111] and 23 urinary metabolites (2013b) altered in depressed patients, and proposed a panel of 6 urinary metabolites (sorbitol, uric acid, azelaic acid, quinolinic acid, hippuric acid, and tyrosine) as candidate diagnostic biomarkers for depression [109]. Finally, the same group of authors [110] also proposed metabolite signature from peripheral blood mononuclear cells composed from 17 metabolites mainly involved in disturbances of energy and neurotransmitter metabolism, which could distinguish depressed patients not only from healthy controls but also from schizophrenia subjects (Table 3).
Table 3.
Altered metabolite levels in depression.
| PSYCHIATRIC DISEASE |
METABOLOMICS |
REFERENCES | |
|---|---|---|---|
| Increased levels ↑ | Decreased levels ↓ | ||
| Depression |
|
|
|
3.3. Posttraumatic stress disorder
Posttraumatic stress disorder (PTSD) is a common, prevalent, severe, disabling and debilitating mental disorder causing a response of helplessness, intense fear and horror. It develops in some, but not all persons after traumatic exposure. PTSD is trauma- and stressor-related disorder in which direct exposure of traumatic experience or stressful event leads to an onset of symptoms: re-experiencing, avoidance, numbing and hyper-arousal. Such psychological trauma has impact on molecular, cellular and organic systems of the individual ([6]; [107]; [78]). PTSD is frequent worldwide, with varying lifetime prevalence from 8% in USA [45] to 18% in Croatia [81]. Its etiology is still not clear, but psychological trauma impacts molecular, cellular and organic systems of the individual ([6]; [107]; [16], [78], [87]). Development of PTSD is facilitated by the presence of different risk factors such as female gender, severity, duration and number of traumatic incidents, childhood abuse and neglect, lack of family and social support and existence of previous mental disorders [114]. Since not all subjects exposed to a traumatic event develop PTSD, the extent to which individuals are vulnerable or resilient to trauma depends on a number of factors, primarily trauma related, psychosocial, biological, environmental, genetic and epigenetic factors, and the interaction between them. Biological changes in PTSD are systemic [56] and include altered HPA axis function and immune, neurotransmitter and neurotrophic functions, increased thyroid activity, high sensitization of the nervous system, reduction in prefrontal brain regions, lower hippocampal volume, increased activity of amygdala, enhanced risk of cardiovascular, metabolic and autoimmune diseases, but also accelerated age-related processes, such as altered N-glycosylation, increased DNA damage and shortened telomeres [42], [56], [61], [78], [88].
3.4. PTSD and oxidative stress
Biochemical data and animal studies point to possible role of oxidative stress in stress-related and anxiety disorders. Therefore, it is assumed that changes induced by the oxidative stress can be found in PTSD. Traumatic stress causes changes in physical appearance, and alterations on skin and hair appear in affected subjects, similarly as after the effects of oxidative stress [57]. While sleep disturbances are characteristic features of PTSD, animal studies have shown that sleep deprivation among rats causes oxidative stress, and has a major impact on memory and anxiety behavior [57]. Human studies failed to find a clear association between oxidative stress biomarkers and PTSD [12,92]. In Croatian war veterans with combat related PTSD, a lack of significant association between PTSD and urinary concentrations of 8-OHdG, serum thromboxane B2, and serum urates. However, significantly lower levels of protein carbonyls were found in the PTSD group than in the control group [12]. Although the nominal differences in serum concentration of protein carbonyls, albumins and total proteins were found between subjects with PTSD and control subjects, since subjects with PTSD had significantly lower concentrations than control group, these differences disappeared when evaluated using the receiver operating characteristic ROC analysis as a graphical plot that illustrates the diagnostic ability for the binary system which has variable discrimination threshold. The ROC curves did not separate the groups satisfactory, and ROC analysis revealed excess of false negative and false positive results for protein carbonyls, total proteins and albumin [12]. Another study found that a product of lipid peroxidation, malondialdehyde (MDA) is not associated with PTSD [92]. Hence, further studies are needed to clarify the role of oxidative stress in PTSD [57]. Such a confusing findings indicating possible negative association of oxidative protein modifications and no MDA and 8-OHdG changes in PTSD indicate the need to study in particular the metabolism of HNE and the levels of HNE-His adducts in PTSD patients. Namely, the association of HNE and in particular its adducts to proteins, such as albumin, were found to be potentially very reliable biomarker of tissue specific and systemic lipid peroxidation associated with metabolic disorders, cancer and inflammatory processes including neuroborreliosis and encephalitis, which were found to be associated with systemic metabolic alterations, both reflecting like HNE-His disease progression and the efficacy of therapies used [19], [25], [36], [50], [51], [58], [77], [97].
3.5. Metabolomics in PTSD
Animal studies have shown altered citric acid cycle and myelination pathway, i.e. glutamate, alanine, aspartate, glyoxylate, citric acid cycle and dicarboxylate metabolism pathways were involved in the hyperarousal among shocked mice [41]. Furthermore, metabolites, such as sarcosine, kynurenic acid, nicotinate and xanthurenic acid were correlated with behavioral changes in stressed mice [40], while intermediates involved in the process of citric acid cycle, such as citric, isocitric, aconitic, succinic and oxalacetic acids were decreased in shocked mice [41]. To our knowledge, only one preliminary study [42] analyzed metabolites in a small number of PTSD subjects: in 20 PTSD patients and 18 controls. They discovered 13 changed metabolites, including one metabolite in endocannabinoid signaling and four glycerophospholipids among PTSD patients. Metabolites were classified as monosaccharides, fatty acids metabolites, glycerophospholipids, bile acids, nucleosides, anti-oxidants and phospholipids [42]. N-acetylglucosamine-6-phosphate, palmitoylethanolamide, palmitic amide, guanosine, inosine and pantothenic acid were down-regulated among PTSD patients, while concentrations of glycerophosphoethanolamines in serum were increased in subjects with PTSD compared to control subjects. Guanosine and inosine showed the strong relationship with PTSD symptomatology, suggesting that these altered metabolites have dose-dependent relationship with PTSD symptoms. In this study [42] four glycerophospholipids that are involved in neuro-inflammation and alterations in cell membrane dynamics and metabolism, were increased in PTSD. Fatty acid metabolites palmitoylethanolamide and palmitic amide, responsible for the energy metabolism, signaling, endocannabinoid system and stress responses, were decreased in PTSD. Nucleosides, guanosine and inosine were reduced in PTSD, and these metabolites have important roles in central nervous system, sleep and memory. Key signaling molecules, regulators of lipid, glucose and energy metabolism and bile acids were also altered in PTSD: 3α-hydroxy-5β-cholan-24-oic acid and glycocholic acid levels were lower, while 7α,12α-dihydroxy-3-oxocholest-4-en-26-oic acid was higher in PTSD. Out of monosaccharides, N-acetylglucosamine-6-phosphate, a precursor of uridine diphosphate N-acetylglucosamine, was decreased in PTSD [42], and this finding might explain why subjects with PTSD develop frequently autoimmune and inflammatory diseases [10]. In line with these findings, anti-oxidant, pantothenic acid, was decreased in PTSD. Antioxidants are important as they protect from oxidative processes and oxidative damage or age-induced damages. Metabolites associated with PTSD (Table 4) are also involved in several biological processes, such as biological aging, oxidative stress, inflammation, metabolism, autoimmune reactions and cellular signaling.
Table 4.
Altered metabolite levels in PTSD.
| PSYCHIATRIC DISEASE | METABOLOMICS | REFERENCES | |
|---|---|---|---|
| Increased levels ↑ | Decreased levels ↓ | ||
| PTSD | Glycerophosphoethanolamines (serum) | Citric acid (mice) Isocitric acid (mice) | [42,41,31,33,64] |
| Glycerophospholipids | Aconitic acid (mice) | ||
| 7α,12α-dihydroxy-3-oxocholest-4-en-26-oic acid | Succinic acid (mice) | ||
| Oxalacetic acid (mice) | |||
| N-acetylglucosamine-6-Phosphate, | |||
| Palmitoylethanolamide | |||
| Palmitic amide | |||
| Guanosine | |||
| Inosine | |||
| Pantothenic acid | |||
| 3α-hydroxy-5β-cholan-24-Oic acid | |||
| Glycocholic acid | |||
In addition, metabolites have a major impact as signaling molecules, energy sources and membrane components on many psychopathological processes. Down-regulation of these metabolites is linked with specific symptoms and proposed theory that endocannabinoid system is associated with PTSD [42]. PTSD has been also associated with increased inflammatory response and production of pro-inflammatory cytokines [28], [56], [72], while palmitoylethanolamide has been negatively associated with PTSD [31], [33], [64]. Therefore, all these data reveal that detected altered metabolites might have important role in development of PTSD and that metabolomics-related multidimensional data should be used to discover perturbed biological networks affected by traumatic experiences [65].
Common metabolites that are altered in depression, schizophrenia and PTSD are shown in Table 5. As can be concluded from this short overview, there are (as far as we are aware) no common metabolites for all three psychiatric disorders (depression, schizophrenia and PTSD). However, depression and schizophrenia share only two metabolites, glutamate and glycine, which are both increased in these disorders. Palmitic amide was the only shared metabolite decreased in both depression and PTSD (Table 5).
Table 5.
Common metabolites altered in schizophrenia, depression and PTSD.
| METABOLITES |
PSYCHIATRIC DISEASE |
||
|---|---|---|---|
| Schizophrenia | Depression | PTSD | |
| Alanine | - | - | ND |
| α-KG | - | + | ND |
| Citrate | - | + | - (mice) |
| Glucose | - | - | ND |
| Glutamate | + | + | ND |
| Glycerophospholipids | + | ND | + |
| Glycine | + | + | ND |
| Lactate | + | - | ND |
| Palmitic amide | - | + | + |
| Pyruvate | + | - | ND |
| Succinate | ND | + | - (mice) |
| Tyrosine | + | - | ND |
| Valine | + | - | ND |
+ (increased levels); - (decreased levels); ND (not detected).
4. Conclusion
Novel evidence reveals that disruption of the homeostasis mechanisms, the onset of chronic oxidative stress, free radical-mediated pathology and subsequent neurotoxicity contributes to development of depression, schizophrenia and PTSD, their diverse clinical symptoms and poor treatment response [108]. Add-on treatments based on strategies aimed to target oxidative/nitrosative stress and to provide antioxidant supplementation may improve treatment options and confirm the significant role of oxidative stress in the biological/biochemical underpinning of these disorders [108]. Oxidative stress research, combined with metabolomics, is a promising approach in dissecting psychiatric disorders, since these data-driven, but not necessarily hypothesis-driven methods might identify new molecules and molecular pathways and circuits associated with complex phenotypes such as schizophrenia, depression or PTSD [65]. Metabolomics might facilitate development of individualized treatment approach and help in identifying medication that would normalize dysregulated molecular networks in these disorders, predict good or poor response to treatment or development of side effects as well as offer theranostic biomarkers and reveal bio-signatures of individual patients, leading to a personalized medicine approach [65], thus supporting also the novel paradigm of pathophysiology of lipid peroxidation [21], [79] and modern concepts for the use of oxidative stress biomarkers to monitor major human diseases [13], [18], [23]. In all these considerations it has to be taken into account that for most of the psychiatric disorders metabolomic approaches can only be performed in blood or CSF specimen. Rarely do we get a direct insight into the cellular metabolites during psychiatric processes. Therefore, only a combination of human studies and representative animal models, combined with “omics” approaches can lead to further insight into such diseases and advance novel treatment strategies.
Conflicts of interest
None.
Acknowledgments
This work has been supported by offset project CRO_A-00033 “Technology & Know-how Transfer in Metabolomics and Establishment of Latest Scientific Equipment in Zagreb”, funded by Patria, PI: Neven Zarkovic, and by Croatian Science Foundation, project no. IP-2014-09-4289 “Glycogene-PTSD: Genomic and glycomic biomarkers for PTSD”; PI: Nela Pivac.
References
- 1.Anderson G., Maes M. Oxidative/nitrosative stress and immuno-inflammatory pathways in depression: treatment implications. Curr. Pharm. Des. 2014;20:3812–3847. doi: 10.2174/13816128113196660738. [DOI] [PubMed] [Google Scholar]
- 2.APA . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4th ed. American Psychiatric Association; Washington, DC: 1994. (886 p.) [Google Scholar]
- 3.APA . Diagnostic and Statistical Manual of Mental Disorders (DSMV) 5th ed. American Psychiatric Association; Washington, DC: 2013. (947 p.) [Google Scholar]
- 4.Asberg M., Bertilsson L., Mårtensson B., Scalia-Tomba G.P., Thorén P., Träskman-Bendz L. CSF monoamine metabolites in melancholia. Acta Psychiatr. Scand. 1984;69:201–219. doi: 10.1111/j.1600-0447.1984.tb02488.x. [DOI] [PubMed] [Google Scholar]
- 5.Beckonert O., Keun H.C., Ebbels T.M.D., Bundy J., Holmes E., Lindon J.C. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007;2:2692–2703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 6.Bisson J.I. Post-traumatic stress disorder. Occup. Med-C. 2007;57:399–403. doi: 10.1093/occmed/kqm069. [DOI] [PubMed] [Google Scholar]
- 7.Bitanihirwe B.K.Y., Woo T.U.W. Oxidative stress in schizophrenia: an integrated approach. Neurosci. Biobehav. Rev. 2011;35:878–893. doi: 10.1016/j.neubiorev.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bray N.J., Leweke F.M., Kapur S., Meyer-Lindenberg A. The neurobiology of schizophrenia: new leads and avenues for treatment. Curr. Opin. Neurobiol. 2010;20:810–815. doi: 10.1016/j.conb.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Brennan L. NMR-based metabolomics: from sample preparation to applications in nutrition research. Prog. Nucl. Mag. Res. Spectrosc. 2014;83:42–49. doi: 10.1016/j.pnmrs.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Britvic D., Anticevic V., Kaliterna M., Lusic L., Beg A., Brajevic-Gizdic I. Comorbidities with Posttraumatic Stress Disorder (PTSD) among combat veterans: 15 years postwar analysis. Int. J. Clin. Health Psychol. 2015;15:81–92. doi: 10.1016/j.ijchp.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai H.L., Li H.D., Yan X.Z., Sun B., Zhang Q., Yan M. Metabolomic analysis of biochemical changes in the plasma and urine of first-episode neuroleptic-naïve schizophrenia patients after treatment with risperidone. J. Proteome Res. 2012;11:4338–4350. doi: 10.1021/pr300459d. [DOI] [PubMed] [Google Scholar]
- 12.Ceprnja M., Derek L., Unic A., Blazev M., Fistonic M., Kozaric-Kovacic D. Oxidative stress markers in patients with post-traumatic stress disorder. Coll. Antropol. 2011;35:1155–1160. [PubMed] [Google Scholar]
- 13.Cipak Gasparovic A., Zarkovic N., Zarkovic K., Semen K., Kaminskyy K., Yelisyeyeva O., Bottary S.P. Biomarkers of Redox signalling, oxidative and nitro-oxidative stress: conventional and novel approaches. Br. J. Phamacol. 2017;174:1771–1783. doi: 10.1111/bph.13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czarny P., Wigner P., Galecki P., Sliwinski T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017;S0278–5846(16) doi: 10.1016/j.pnpbp.2017.06.036. (30298-6) [DOI] [PubMed] [Google Scholar]
- 15.Dean J., Keshavan M. The neurobiology of depression: an integrated view. Asian J. Psychiatrry. 2017;27:101–111. doi: 10.1016/j.ajp.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Domschke K. Patho-genetics of posttraumatic stress disorder. Psychiatr. Danub. 2012;24:267–273. [PubMed] [Google Scholar]
- 17.Dunn W.B., Broadhurst D., Ellis D.I., Brown M., Halsall A., O’Hagan S. A GC-TOF-MS study of the stability of serum and urine metabolomes during the UK Biobank sample collection and preparation protocols. Int. J. Epidemiol. 2008;37:23–30. doi: 10.1093/ije/dym281. [DOI] [PubMed] [Google Scholar]
- 18.Egea J., Fabregat I., Frapart Y.M., Ghezzi P., Görlach A. European contribution to the study of ROS: a summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS) Redox Biol. 2017;13:94–162. doi: 10.1016/j.redox.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elrayess M.A., Almuraikhy S., Kafienah W., Al-Menhali A., Alkhelaifi F., Bashah M., Zarkovic K., Zarkovic N., Waeg G., Alsayrafi M., Jaganjac M. 4-Hydroxynonenal causes impairment of human subcutaneous adipogenesis and induction of adipocyte insulin resistance. Free Radic. Biol. Med. 2017;104:129–137. doi: 10.1016/j.freeradbiomed.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Emiliani F.E., Sedlak T.W., Sawa A. Oxidative stress and schizophrenia: recent breakthroughs from an old story. Curr. Opin. Psychiatry. 2014;27:185–190. doi: 10.1097/YCO.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedorova M., Zarkovic N. Preface to the special issue on 4-Hydroxynonenal and related lipid oxidation products. Free Radic. Biol. Med. 2017;111:1. doi: 10.1016/j.freeradbiomed.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Fiehn O. Metabolomics – the link between genotypes and phenotypes. Plant Mol. Biol. 2002;48:155–171. [PubMed] [Google Scholar]
- 23.Frijhoff J., Winyard P.G., Zarkovic N., Davies S. Clinical relevance of biomarkers of oxidative stress. Antioxid. Redox Signal. 2015;23:1144–1170. doi: 10.1089/ars.2015.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Alvarez L., Paz Garcia-Portilla M., Gonzalez-Blanco L., Saiz Martinez P.A., de la Fuente-Tomas L., Menendez-Miranda I. Differential blood-based biomarkers of psychopathological dimensions of schizophrenia. Rev. Psiquiatr. Salud Ment. 2016;9:219–227. doi: 10.1016/j.rpsm.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Gęgotek A., Nikliński J., Zarkovic N., Zarkovic K., Waeg G., Łuczaj W., Charkiewicz R., Skrzydlewska E. Lipid mediators involved in the oxidative stress and antioxidant defence of human lung cancer cells. Redox Biol. 2016;9:210–219. doi: 10.1016/j.redox.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.German J.B., Hammock B.D., Watkins S.M. Metabolomics: building on a century of biochemistry to guide human health. Metabolomics. 2005;1:3–9. doi: 10.1007/s11306-005-1102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Go E.P. Database resources in metabolomics: an overview. J. Neuroimmune Pharmacol. 2010;5:18–30. doi: 10.1007/s11481-009-9157-3. [DOI] [PubMed] [Google Scholar]
- 28.Gola H., Engler H., Sommershof A., Adenauer H., Kolassa S., Schedlowski M. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013;13:40. doi: 10.1186/1471-244X-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Blanco L., Garcia-Portilla M.P., Garcia-Alvarez L., Iglesias C., Saiz P., Coto A. Inflammatory and metabolic biomarkers of psychopathological dimensions of schizophrenia. Eur. Psychiatry. 2016;33:250. [Google Scholar]
- 30.Greenberg M.S., Tanev K., Marin M.F., Pitman R.K. Stress, PTSD, and dementia. Alzheimers Dement. 2014;10:155–165. doi: 10.1016/j.jalz.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Hauer D., Schelling G., Gola H., Campolongo P., Morath J., Roozendaal B. Plasma concentrations of endocannabinoids and related primary fatty acid amides in patients with post-traumatic stress disorder. PLoS ONE. 2013;8:62741. doi: 10.1371/journal.pone.0062741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Y., Yu Z., Giegling I., Xie L., Hartmann A.M., Prehn C. Schizophrenia shows a unique metabolomics signature in plasma. Transl. Psychiat. 2012;2:149. doi: 10.1038/tp.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill M.N., Bierer L.M., Makotkine I., Golier J.A., Galea S., McEwen B.S. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the world trade center attacks. Psychoneuroendocrinology. 2013;38:2952–2961. doi: 10.1016/j.psyneuen.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmes E., Wilson I.D., Nicholson J.K. Metabolic phenotyping in health and disease. Cell. 2008;134:714–717. doi: 10.1016/j.cell.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 35.Hovatta I., Juhila J., Donner J. Oxidative stress in anxiety and comorbid disorders. Neurosci. Res. 2010;68:261–275. doi: 10.1016/j.neures.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Jaganjac M., Almuraikhy S., Al-Khelaifi M., Al-Jaber M., Bashah M., Mazloum N.A., Zarkovic K., Zarkovic N., Waeg G., Kafienah W. Combined metformin and insulin treatment reverses metabolically impaired omental adipogenesis and accumulation of 4-hydroxynonenal in obese diabetic patients. Redox Biol. 2017;12:483–490. doi: 10.1016/j.redox.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaal E., Janssen H.G. Extending the molecular application range of gas chromatography. J. Chromatogr. A. 2008;1184:43–60. doi: 10.1016/j.chroma.2007.11.114. [DOI] [PubMed] [Google Scholar]
- 38.Kaddurah-Daouk R., Yuan P., Boyle S.H., Matson W., Wang Z., Zeng Z.B. Cerebrospinal fluid metabolome in mood disorders-remission state has a unique metabolic profile. Sci. Rep. 2012;2:667. doi: 10.1038/srep00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanehisa M., Goto S., Sato Y., Kawashima M., Furumichi M., Tanabe M. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res. 2014;42:199–205. doi: 10.1093/nar/gkt1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kao C.Y., Anderzhanova E., Asara J.M., Wotjak C.T., Turck C.W. NextGen brain microdialysis: applying modern metabolomics technology to the analysis of extracellular fluid in the central nervous system. Mol. Neuropsychiatry. 2015;1:60–67. doi: 10.1159/000381855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kao C.Y. Graduate School of Systemic Neurosciences der Ludwig-Maximilians-Universität München; Munich, Germany: 2015. Pathway and Biomarker Discovery in a Posttraumatic Stress Disorder Mouse Model (Doctoral disertation) [Google Scholar]
- 42.Karabatsiakis A., Hamuni G., Wilker S., Kolassa S., Renu D., Kadereit S. Metabolite profiling in posttraumatic stress disorder. J. Mol. Psychiatry. 2015;3:1–11. doi: 10.1186/s40303-015-0007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasture V.S., Musmade D.S., Vakte M.B., Sonawane S.B., Patil P.P. Metabolomics: current technologies and future trends. Int J. Res Dev. Pharm. Life Sci. 2012-13;2:206–217. [Google Scholar]
- 44.Kemp G. Capillary electrophoresis. Biotechnol. Appl. Biochem. 1998;27:9–17. doi: 10.1111/j.1470-8744.1998.tb01369.x. [DOI] [PubMed] [Google Scholar]
- 45.Kilpatrick D.G., Resnick H.S., Milanak M.E., Miller M.W., Keyes K.M., Friedman M.J. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-V criteria. J. Trauma Stress. 2013;26:537–547. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kind T., Wohlgemuth G., Lee D.Y., Lu Y., Palazoglu M., Shahbaz S. FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009;81:10038–10048. doi: 10.1021/ac9019522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klinedinst N.J., Regenold W.T. A mitochondrial bioenergetic basis of depression. J. Bioenergy Biomembr. 2015;47:155–171. doi: 10.1007/s10863-014-9584-6. [DOI] [PubMed] [Google Scholar]
- 48.Liu J., Litt L., Segal M.R., Kelly M.J.S., Pelton J.G., Kim M. Metabolomics of oxidative stress in recent studies of endogenous and exogenously administered intermediate metabolites. Int. J. Mol. Sci. 2011;12:6469–6501. doi: 10.3390/ijms12106469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X., Zheng P., Zhao X., Zhang Y., Hu C., Li J., Zhao J., Zhou J., Xie P., Xu G. Discovery and validation of plasma biomarkers for major depressive disorder classification based on liquid chromatography-mass spectrometry. J. Proteome Res. 2015;14:2322–2330. doi: 10.1021/acs.jproteome.5b00144. [DOI] [PubMed] [Google Scholar]
- 50.Łuczaj W., Moniuszko A., Jarocka-Karpowicz I., Pancewicz S., Andrisic L., Zarkovic N., Skrzydlewska E. Tick-borne encephalitis - lipid peroxidation and its consequences. Scand. J. Clin. Lab Investig. 2016;28:1–9. doi: 10.3109/00365513.2015.1084040. [DOI] [PubMed] [Google Scholar]
- 51.Łuczaj W., Gindzienska-Sieskiewicz E., Jarocka-Karpowicz I., Andrisic L., Sierakowski S., Zarkovic N., Waeg G., Skrzydlewska E. The onset of lipid peroxidation in rheumatoid arthritis: consequences and monitoring. Free Radic. Res. 2016;50:304–313. doi: 10.3109/10715762.2015.1112901. [DOI] [PubMed] [Google Scholar]
- 52.Martins-de-Souza D. Proteomics, metabolomics, and protein interactomics in the characterization of the molecular features of major depressive disorder. Dialog. Clin. Neurosci. 2014;16:63–73. doi: 10.31887/DCNS.2014.16.1/dmartins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mastrangelo A., Ferrarini A., Rey-Stolle F., Garcia A., Barbas C. From sample treatment to biomarker discovery: a tutorial for untargeted metabolomics based on GC-(EI)-Q-MS. Anal. Chim. Acta. 2015;900:21–35. doi: 10.1016/j.aca.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Maurya P.K., Noto C., Rizzo L.B., Rios A.C., Nunes S.O., Barbosa D.S. The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;65:134–144. doi: 10.1016/j.pnpbp.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 55.Mertsch K., Blasig I., Grune T. 4-Hydroxynonenal impairs the permeability of an in vitro rat blood–brain barrier. Neurosci. Lett. 2001;314:135–138. doi: 10.1016/s0304-3940(01)02299-6. [DOI] [PubMed] [Google Scholar]
- 56.Michopoulos V., Norrholm S.D., Jovanovic T. Diagnostic biomarkers for posttraumatic stressdisorder: promising horizons from translational neuroscience research. Biol. Psychiatry. 2015;78:344–353. doi: 10.1016/j.biopsych.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller M.W., Sadeh N. Traumatic stress, oxidative stress and post-traumatic stress disorder: neurodegeneration and the accelerated-aging hypothesis. Mol. Psychiatry. 2014;19:1156–1162. doi: 10.1038/mp.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moniuszko-Malinowska A., Łuczaj W., Jarocka-Karpowicz I., Pancewicz S., Zajkowska J., Andrisic L., Zarkovic N., Skrzydlewska E. Lipid peroxidation in the pathogenesis of neuroborreliosis. Free Radic. Biol. Med. 2016;96:255–263. doi: 10.1016/j.freeradbiomed.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 59.Moon M.L., Joesting J.J., Lawson M.A., Chiu G.S., Blevins N.A., Kwakwa K.A. The saturated fatty acid, palmitic acid, induces anxiety-like behavior in mice. Metabolism. 2014;63:1131–1140. doi: 10.1016/j.metabol.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moreno F.A., Parkinson D., Palmer C., Castro W.L., Misiaszek J., El Khoury A. CSF neurochemicals during tryptophan depletion in individuals with remitted depression and healthy controls. Eur. Neuropsychopharmacol. 2010;20:18–24. doi: 10.1016/j.euroneuro.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moreno-Villanueva M., Morath J., Vanhooren V., Elbert T., Kolassa S., Libert C. N-glycosylation profiling of plasma provides evidence for accelerated physiological aging in post-traumatic stress disorder. Transl. Psychiatry. 2013;3:320. doi: 10.1038/tp.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naz S., Garcia A., Barbas C. Multiplatform analytical methodology for metabolic fingerprinting of lung tissue. Anal. Chem. 2013;85:10941–10948. doi: 10.1021/ac402411n. [DOI] [PubMed] [Google Scholar]
- 63.Naz S., Moreira dos Santos D.C., García A., Barbas C. Analytical protocols based on LC-MS, GC-MS and CE-MS for nontargeted metabolomics of biological tissues. Bioanalysis. 2014;6:1657–1677. doi: 10.4155/bio.14.119. [DOI] [PubMed] [Google Scholar]
- 64.Neumeister A., Normandin M.D., Pietrzak R.H., Piomelli D., Zheng M.Q., Gujarro-Anton A. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol. Psychiatry. 2013;18:1034–1040. doi: 10.1038/mp.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neylan T.C., Schadt E.E., Yehuda R. Biomarkers for combat-related PTSD: focus on molecular networks from high-dimensional data. Eur. J. Psychotraumatol. 2014;5:23938. doi: 10.3402/ejpt.v5.23938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ng F., Berk M., Dean O., Bush A.I. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008;11:851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 67.Nikolac Perkovic M., Nedic Erjavec G., Svob Strac D., Uzun S., Kozumplik O., Pivac N. Theranostic biomarkers for schizophrenia. Int. J. Mol. Sci. 2017;18:733. doi: 10.3390/ijms18040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Noctor G., Lelarge-Trouverie C., Mhamdi A. The metabolomics of oxidative stress. Phytochemistry. 2015;112:33–53. doi: 10.1016/j.phytochem.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Nunes A.F., Amaral J.D., Lo A.C., Fonseca M.B., Viana R.J., Callaerts-Vegh Z. TUDCA, a bile acid, attenuates amyloid precursor protein processing and amyloid-β deposition in APP/PS1 mice. Mol. Neurobiol. 2012;45:440–454. doi: 10.1007/s12035-012-8256-y. [DOI] [PubMed] [Google Scholar]
- 70.O'Dushlaine C., Rossin L., Lee P.H., Duncan L., Parikshak N.N., Newhouse S. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat. Neurosci. 2015;18:199–209. doi: 10.1038/nn.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oresic M., Tang J., Seppänen-Laakso T., Mattila I., Saarni S.E., Saarni S.I. Metabolome in schizophrenia and other psychotic disorders: a general population-based study. Genome Med. 2011;3:19. doi: 10.1186/gm233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pace T.W., Heim C.M. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav. Immun. 2011;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Park H.R., Kim J.Y., Park K.Y., Lee J. Lipotoxicity of palmitic acid on neural progenitor cells and hippocampal neurogenesis. Toxicol. Res. 2011;27:103–110. doi: 10.5487/TR.2011.27.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pasikanti K.K., Ho P.C., Chan E.C.Y. Gas chromatography/mass spectrometry in metabolic profiling of biological fluids. J. Chromatogr. B. 2008;871:202–211. doi: 10.1016/j.jchromb.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 75.Pejchinovski M., Klein J., Ramírez-Torres A., Bitsika V., Mermelekas G., Vlahou A. Comparison of higher energy collisional dissociation and collision-induced dissociationMS/MS sequencing methods for identification of naturally occurring peptides in human urine. Proteom. Clin. Appl. 2015;9:531–542. doi: 10.1002/prca.201400163. [DOI] [PubMed] [Google Scholar]
- 76.Petrovchich I., Sosinsky A., Konde A., Archibald A., Henderson D., Maletic-Savatic M. Metabolomics in schizophrenia and major depressive disorder. Front Biol. 2016;11:222–231. [Google Scholar]
- 77.Piskac Zivkovic N., Petrovecki M., Tomasovic Loncaric C., Nikolic I., Waeg G., Jaganjac M., Zarkovic K., Zarkovic N. Positron emission tomography-computed tomography and 4-hydroxynonenal-histidine immunohistochemistry reveal differential onset of lipid peroxidation in primary lung cancer and in pulmonary metastasis of remote malignancies. Redox Biol. 2017;11:600–605. doi: 10.1016/j.redox.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poli G., Zarkovic N. Editorial introduction to the special issue on 4-hydroxynonenal and related lipid oxidation products. Free Radic. Biol. Med. 2017;111:2–5. doi: 10.1016/j.freeradbiomed.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 80.Pontes J.G.M., Brasil A.J.M., Cruz G.C.F., deSouza R.N., Tasic L.J. NMR-based metabolomics strategies: plants, animals and humans. Anal. Methods. 2016;9:1078–1096. [Google Scholar]
- 81.Priebe S., Bogic M., Ajdukovic D., Franciskovic T., Galeazzi G.M., Kucukalic A. Mental disorders following war in the Balkans: a study in 5 countries. Arch. Gen. Psychiatry. 2010;67:518–528. doi: 10.1001/archgenpsychiatry.2010.37. [DOI] [PubMed] [Google Scholar]
- 82.Quinones M.P., Kaddurah-Daouk R. Metabolomics tools for identifying biomarkers for neuropsychiatric diseases. Neurobiol. Dis. 2009;35:165–176. doi: 10.1016/j.nbd.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 83.Roessner U., Bowne J. What is metabolomics all about? Biotechniques. 2009;46:363–365. doi: 10.2144/000113133. [DOI] [PubMed] [Google Scholar]
- 84.Rojo A.I., McBean G., Cindric M., Egea J., López M.G., Rada P., Zarkovic N., Cuadrado A. Redox control of microglial function: molecular mechanisms and functional significance. Antioxid. Redox Signal. 2014;21:1766–1801. doi: 10.1089/ars.2013.5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Romano A., Serviddio G., Calcagnini S., Villani R., Giudetti A.M., Cassano T., Gaetani S. Linking lipid peroxidation and neuropsychiatric disorders: focus on 4-hydroxy-2-nonenal. Free Radic. Biol. Med. 2017;111:281–293. doi: 10.1016/j.freeradbiomed.2016.12.046. [DOI] [PubMed] [Google Scholar]
- 86.Santos-Soto I.J., Chorna N., Carballeira N.M., Vélez-Bartolomei J.G., Méndez-Merced A.T., Chornyy A.P. Voluntary running in young adult mice reduces anxiety-like behavior and increases the accumulation of bioactive lipids in the cerebral cortex. PLoS ONE. 2013;8:81459. doi: 10.1371/journal.pone.0081459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schmidt U., Holsboer F., Rein T. Epigenetic aspects of posttraumatic stress disorder. Dis. Markers. 2011;30:77–87. doi: 10.3233/DMA-2011-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schuff N., Neylan T.C., Lenoci M.A., Du A.T., Weiss D.S., Marmar C.R. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol. Psychiatry. 2001;50:952–959. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smoller J.W. The genetics of stress-related disorders: PTSD, depression, and anxiety disorders. Neuropsychopharmacology. 2016;41:297–319. doi: 10.1038/npp.2015.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sparkman O.D., Penton Z.E., Kitson F.G. 2nd ed. Academic Press; Cambridge Massachusetts, USA: 2011. Gas Chromatography and Mass Spectrometry: a Practical Guide; p. 632. [Google Scholar]
- 92.Sumner L.W., Amberg A., Barrett D., Beale M.H., Beger R., Daykin C.A. Proposed minimum reporting standards for chemical analysis. Metabolomics. 2007;3:211–221. doi: 10.1007/s11306-007-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tezcan E., Atmaca M., Kuloglu M., Ustundag B. Free radicals in patients with post-traumatic stress disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2003;253:89–91. doi: 10.1007/s00406-003-0413-x. [DOI] [PubMed] [Google Scholar]
- 94.Vaz A.R., Cunha C., Gomes C., Schmucki N., Barbosa M., Brites D. Glycoursodeoxycholic acid reduces matrix metalloproteinase-9 and caspase-9 activation in a cellular model of superoxide dismutase-1 neurodegeneration. Mol. Neurobiol. 2015;51:864–877. doi: 10.1007/s12035-014-8731-8. [DOI] [PubMed] [Google Scholar]
- 95.Wang S.M., Han C., Lee S.J., Patkar A.A., Masand P., Pae C.U. A review of current evidence for acetyl-l-carnitine in the treatment of depression. J. Psychiatr. Res. 2014;53:30–37. doi: 10.1016/j.jpsychires.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 96.Wang Z.J., Li G.M., Tang W.L., Yin M. Neuroprotective effects of stearic acid against toxicity of oxygen/glucose deprivation or glutamate on rat cortical or hippocampal slices. Acta Pharmacol. Sin. 2006;27:145–150. doi: 10.1111/j.1745-7254.2006.00259.x. [DOI] [PubMed] [Google Scholar]
- 97.Weber D., Milkovic L., Bennett S.J., Griffiths H.R., Zarkovic N., Grune T. Measurement of HNE protein adducts in human plasma and serum by ELISA - Comparison of two primary antibodies. Redox Biol. 2013;1:226–233. doi: 10.1016/j.redox.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Westerhuis J.A., Hoefsloot H.C.J., Smit S., Vis D.J., Smilde A.K., van Velzen E.J.J. Assessment of PLSDA cross validation. Metabolomics. 2008;4:81–89. [Google Scholar]
- 99.Wishart D.S., Tzur D., Knox C., Eisner R., Guo A.C., Young N. HMDB: the human metabolome database. Nucleic Acids Res. 2007;35:521–526. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Woo H.I., Chun M.R., Yang J.S., Lim S.W., Kim M.J., Kim S.W. Plasma amino acid profiling in major depressive disorder treated with selective serotonin reuptake inhibitors. CNS Neurosci. Ther. 2015;21:417–424. doi: 10.1111/cns.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wood P.L. Mass spectrometry strategies for clinical metabolomics and lipidomics in psychiatry, neurology, and neuro-oncology. Neuropsychopharmacology. 2014;39:24–33. doi: 10.1038/npp.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Worley B., Powers R. Multivariate analysis in metabolomics. Curr. Metabol. 2013;1:92–107. doi: 10.2174/2213235X11301010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang J., Chen T., Sun L., Zhao Z., Qi X., Zhou K. Potential metabolite markers of schizophrenia. Mol. Psychiatry. 2013;18:67–78. doi: 10.1038/mp.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zarkovic K., Jakovcevic A., Zarkovic N. Contribution of the HNE-immunohistochemistry to modern pathological concepts of major human diseases. Free Radic. Biol. Med. 2017;111:110–126. doi: 10.1016/j.freeradbiomed.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 105.Zarkovic N., Zarkovic K., Schaur R.J., Štolc S., Schlag G., Redl H., Waeg G., Borovic S. 4-Hydroxynonenal as a second messenger of free radicals and growth modifying factor. Life Sci. 1999;65:1901–1904. doi: 10.1016/s0024-3205(99)00444-0. [DOI] [PubMed] [Google Scholar]
- 106.Zhang A., Sun H., Wang X. Serum metabolomics as a novel diagnostic approach for disease: a systematic review. Anal. Bioanal. Chem. 2012;404:1239–1245. doi: 10.1007/s00216-012-6117-1. [DOI] [PubMed] [Google Scholar]
- 107.Zhang L., Li H., Benedek D., Li X., Ursano R. A strategy for the development of biomarker tests for PTSD. Med. Hypotheses. 2009;73:404–409. doi: 10.1016/j.mehy.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 108.Zhang X.Y., Yao J.K. Oxidative stress and therapeutic implications in psychiatric disorders. Prog. Neuro-Psychophopharmacol Biol. Psychiatry. 2013;46:197–199. doi: 10.1016/j.pnpbp.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 109.Zheng P., Chen J.J., Huang T., Wang M.J., Wang Y., Dong M.X. A novel urinary metabolite signature for diagnosing major depressive disorder. J. Proteome Res. 2013;12:5904–5911. doi: 10.1021/pr400939q. [DOI] [PubMed] [Google Scholar]
- 110.Zheng P., Fang Z., Xu X.J., Liu M.L., Du X., Zhang X. Metabolite signature for diagnosing major depressive disorder in peripheral blood mononuclear cells. J. Affect. Disord. 2016;195:75–81. doi: 10.1016/j.jad.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 111.Zheng P., Gao H.C., Li Q., Shao W.H., Zhang M.L., Cheng K. Plasma metabonomics as a novel diagnostic approach for major depressive disorder. J. Proteome Res. 2012;11:1741–1748. doi: 10.1021/pr2010082. [DOI] [PubMed] [Google Scholar]
- 112.Zheng P., Wang Y., Chen L., Yang D., Meng H., Zhou D. Identification and validation of urinary metabolite biomarkers for major depressive disorder. Mol. Cell Proteom. 2013;12:207–214. doi: 10.1074/mcp.M112.021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou B., Xiao J.F., Tulia L., Ressom H.W. LC-MS-based metabolomics. Mol. Biosyst. 2012;8:470–481. doi: 10.1039/c1mb05350g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zoladz P.R., Diamond D.M. Current status on behavioral and biological markers of PTSD: a search for clarity in a conflicting literature. Neurosci. Biobehav. 2013;37:860–895. doi: 10.1016/j.neubiorev.2013.03.024. [DOI] [PubMed] [Google Scholar]