Abstract
Clear-cell renal cell carcinoma (ccRCC) is an aggressive and malignant kidney cancer which has the worst prognosis. Although microRNAs (miRNAs) have recently been identified as a novel class of regulators in oncogenesis and metastasis, there are few studies on their participation in ccRCC. In the present study, we observed that miR-367 expression was increased in both human ccRCC tissues and cell lines. Cell proliferation was evaluated by MTT assay and 5-Ethynyl-2′-deoxyuridine (EdU) assay kit, which indicated that inhibition of miR-367 could suppress the ccRCC proliferation. Forced expression of miR-367 substantially induced cell migration and invasion evidenced by wound-healing and transwell assays, and this carcinogenesis could be abolished by miR-367 inhibitor treatment. Further analysis identified Metastasis-Associated Protein 3 (MTA3) as a direct target of miR-367. QRT-PCR and western blot results indicated the correlative expression of miR-367 and MTA3 in ccRCC tissue samples. Overexpression of MTA3 reversed miR-367-induced cell proliferation, migration and invasion. Our data uncovered a novel molecular interaction between miR-367 and MTA3, indicating a therapeutic strategy of miR-367 for ccRCC.
Keywords: miR-367, MTA3, ccRCC, proliferation, metastasis
INTRODUCTION
Renal cell carcinoma has the third highest mortality among all genitourinary cancers worldwide, of which clear-cell renal cell carcinoma (ccRCC) accounts for approximately 70% [1]. In 2013, approximately 65,150 new cases and 13,680 deaths were predicted to be associated with renal cell carcinoma in the United States [2]. Although early-stage ccRCC is curable by surgery resection, the prognosis for metastatic ccRCC patients is still poor. Researches have indicated that the 5-year survival rate of ccRCC patients remains no more than 10% [3]. Therefore, there is greater impetus to identify specific genes associated with tumor progression and migration so that novel therapeutic targets can optimize present prognostic systems.
MicroRNAs (miRNAs) are endogenous, small non-coding RNAs of 19–22 nucleotides in length that modulate fundamental cell processes at the post-transcriptional level [4]. Accumulating evidence has implicated miRNAs as essential regulators of ccRCC by targeting transcription factors or pivotal signal pathways [5–7]. MiR-335 can dictate ccRCC cells fate by inhibiting the proliferation and invasion through the repression of Bcl-w [8]. Nishikawa et al. clarify that tumor-suppressive miRNA-29s directly target LOXL2 inhibiting renal cell carcinoma progression [9]. In addition, researchers manifest that miR-367 elevation leads to poor prognosis in non-small cell lung cancer [10–11], accelerates breast cancer progression by binding with the 3′UTR of the calcium channel ryanodine receptor gene 3 (RYR3) [12] and promotes proliferation of hepatocellular carcinoma cell [13]. However, researches are lack in the potential role of miR-367 in ccRCC.
Metastasis-associated protein 3 (MTA3) is a kind of metastasis-associated protein, all of which serve as subunits of the Mi-2/NuRD nucleosome remodeling and deacetylase (NuRD) protein complex [14–16]. It is found that MTA3 expression level is significantly increased in hepatocellular carcinoma and identified to correlate with tumor progression and the poor prognosis [17]. Researchers also manifest that MTA3 can regulate the proteins of BAX, Cleved-Caspase-3, p-PARP and Bcl-2 to modify the progression of cellular apoptosis in NSCLCs [18]. Moreover, MTA3 is reported to be an independent and unfavorable prognostic marker [19]. However, till now, few studies focus on the expression of MTA3 and its potential association with microRNAs in ccRCC. In this study, we first describe potential roles of miR-367 in the proliferation and metastasis of ccRCC and then center on the underlying molecular mechanisms of which MTA3, one of the direct targets of miR-367, may contribute to ccRCC progression.
RESULTS
MiR-367 expression is elevated both in vivo and in vitro
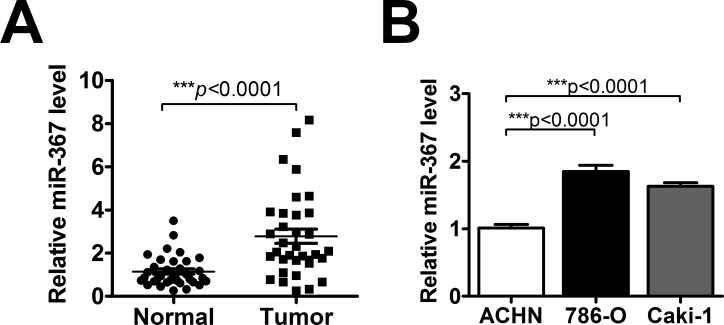
To explore the potential role of miR-367 in ccRCC carcinogenesis, we first measured the expression of miR-367 in 35 pairs of ccRCC tissues and their matched normal renal tissues using qRT-PCR. The result showed that miR-367 expression was increased in ccRCC tissues (Figure 1A). In addition, miR-367 was also dramatically upregulated both in 786-O and Caki-1 cell lines (Figure 1B). The above results suggested that miR-367 might be involved in the progression of ccRCC.
Figure 1. MiR-367 was highly expressed in ccRCC tissues and cells.
(A) QRT-PCR analysis of miR-367 in 35 pairs ccRCC tissues. Data are expressed as mean ± SEM. ***p<0.0001 vs. normal. (B) The expression of miR-367 was measured by qRT-PCR in human renal cell adenocarcinoma ACHN and two ccRCC cell lines (786-O and Caki-1). Data are expressed as mean ± SEM. ***p<0.0001 vs. ACHN.
MiR-367 contributes to cell proliferation in ccRCC
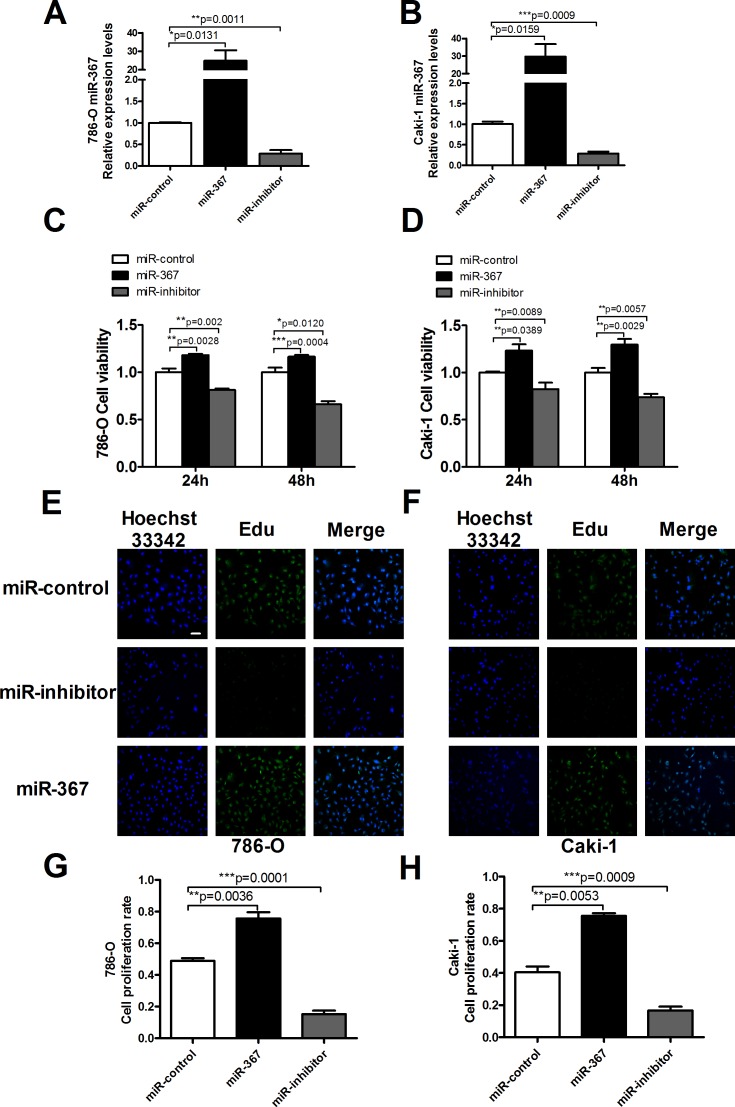
To identify the function of miR-367 in ccRCC cell proliferation, miR-367 mimics (miR-367) or inhibitor were employed both in Caki-1 and 786-O cell lines. The transfection efficiency was evaluated by qRT-PCR (Figure 2A and 2B). Our analysis indicated that miR-367 overexpression dominantly increased cell viability compared with its corresponding miR-control at 24 h and 48 h time points, whereas miR-367 inhibitor markedly decreased cell viability (Figure 2C and 2D). 5-Ethynyl-2′ -deoxyuridine (EdU) assay was performed to further explore the functional role of miR-367 in ccRCC cell proliferation. The results demonstrated that more proliferative cells double labeled with EdU and Hoechst 33342 were observed in the group treated with miR-367 mimics versus miR-control, whereas a markedly reduction was seen after transfection of miR-367 inhibitor (Figure 2G and 2H). Representative images were shown in Figure 2E and 2F. These data together suggested that miR-367 could facilitate ccRCC cell proliferation.
Figure 2. MiR-367 regulated cell proliferation in ccRCC cells.
(A and B) MiR-367 level was measured by qRT-PCR after transfection with miR-control (negative control), miR-367 mimics (miR-367) or miR-inhibitor in 786-O and Caki-1 cells. (C and D) Caki-1 and 786-O viabilities were detected using MTT assay. ccRCC cells were transfected with miR-367 or miR-367 inhibitor for 24 h and 48 h. (E and F) Representative images of Edu staining showing proliferation cells (stained in green). Nuclei that double labeled with EdU (green) and Hoechst 33342 (blue) were considered to be new proliferative cells. Scale bar indicates 100 μm. (G and H) Quantitative analysis of double labeled nuclei was performed. The number of double labeled nuclei was significantly increased in miR-367-treated group compared with miR-control-treated group. Data are expressed as mean ± SEM. **p<0.01 or *p<0.05 vs. miR-control.
MiR-367 promotes ccRCC cell migration and invasion
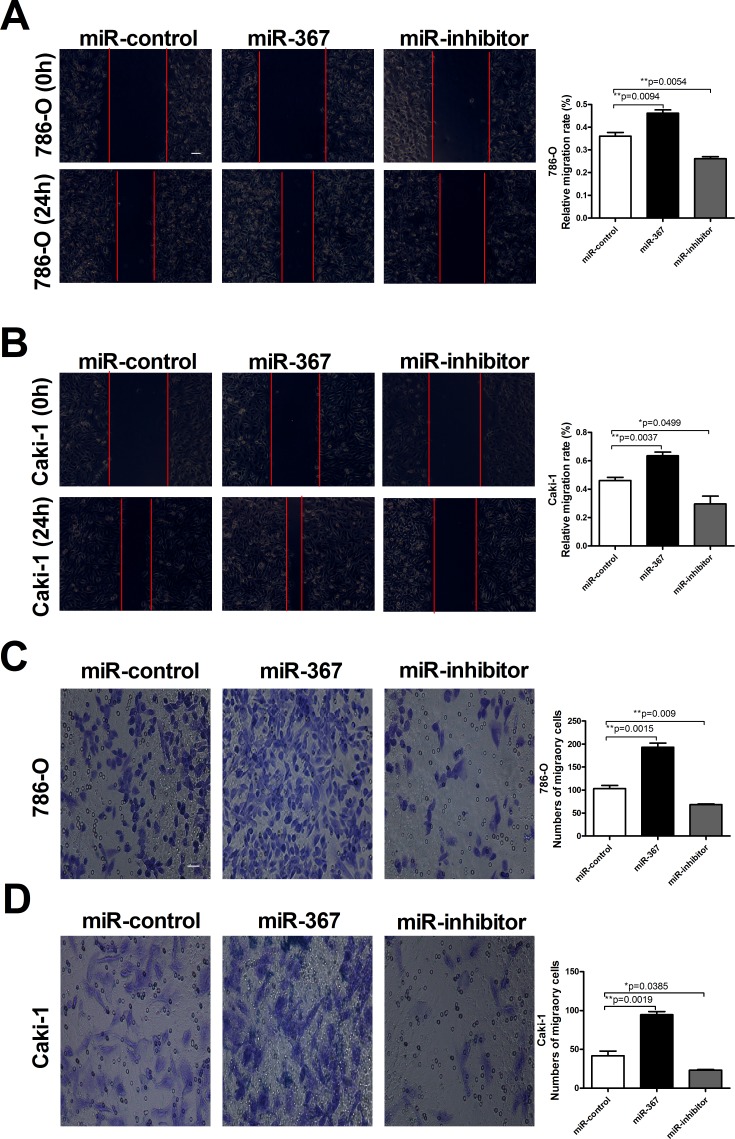
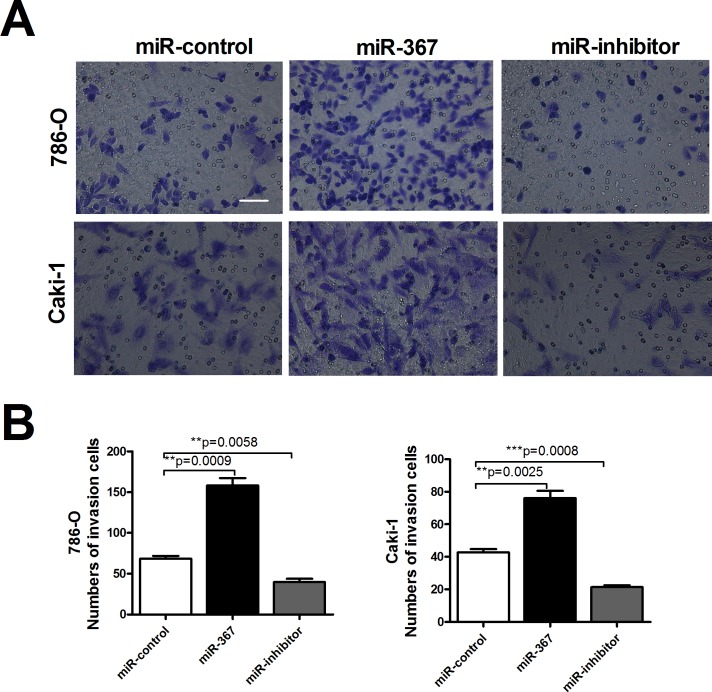
Since metastasis of cancer cells is considered as a critical aspect of cancer progression, we detected the effect of miR-367 on migration and invasion capacities of ccRCC cells. The relative migration rates of 786-O and Caki-1 cells were substantially reduced after treatment with miR-367 inhibitor at 24h time point using wound healing assay (Figure 3A and 3B). We further performed transwell assay to assess cell migration. The results also showed that the number of migratory cells were significantly reduced after miR-367 inhibitor administration (Figure 3C and 3D). Transwell assay with Matrigel was employed to evaluate the ability of cell invasion. Elevated level of miR-367 prominently increased cell invasion which could be neutralized by miR-367 inhibitor (Figure 4A and 4B). In addition, to further illuminate the effect of miR-367 on cell migration and invasion, we examined the involvement of miR-367 in Epithelial–mesenchymal transition (EMT) regulation in ccRCC cells. The specific overexpression of miR-367 significantly downregulated the E-cadherin protein level and upregulated the α-SMA protein level, whereas the suppression of miR-367 reversed the results (Supplementary Figure 1A and 1B). These findings confirmed that miR-367 could induce ccRCC cell migration and invasion.
Figure 3. MiR-367 elevation promoted ccRCC cells migration.
(A and B) Wound healing assay confirming migration ability of 786-O and Caki-1 cell lines transfected with miR-367 or miR-367 inhibitor. (C and D) Transwell assay showed the number of migratory cells in 786-O and Caki-1 cell lines in different treated groups. Scale bar indicates 100 μm. Data are expressed as mean ± SEM. **p<0.01 or *p<0.05 vs. miR-control.
Figure 4. MiR-367 increased the number of invasion cells in ccRCC.
(A) Transwell assays with Matrigel of 786-O and Caki-1 cells transfected with miR-367 or miR-367 inhibitor. Scale bar indicates 100 μm (B) Quantitative analysis of the number of invasive cells. Data are expressed as mean ± SEM. **p<0.01 vs. miR-control.
MTA3 transcription is negatively regulated by miR-367
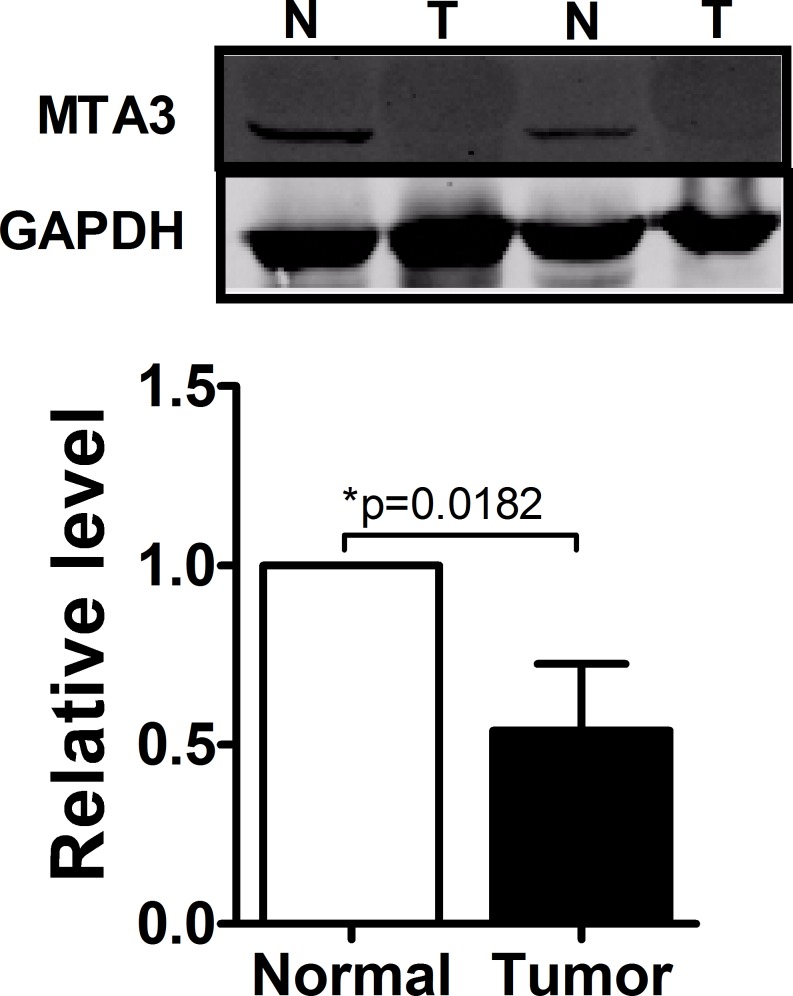
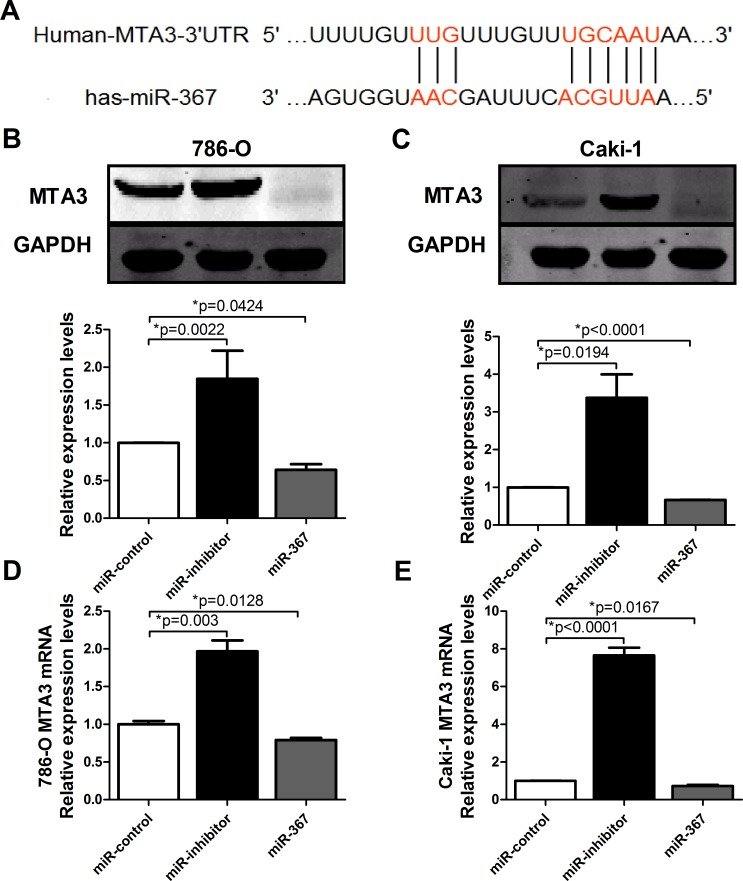
Having established the functional phenotype of miR-367 in ccRCC, we further wanted to identify its target genes to gain insights into the molecular mechanism. Towards this, several bioinformatics predictions were applied, such as TargetScan and miRanDa. All databases predict MTA3 as a potential target for miR-367. Detection of MTA3 expression in ccRCC patients showed that the protein level of MTA3 was substantially decreased in ccRCC patients (Figure 5). The MTA3-encoding mRNA contains a 3′UTR binding site for miR-367 as shown in Figure 6A. Inhibition of miR-367 increased MTA3 protein level both in 786-O and Caki-1 cells, while adverse results were observed when treated with miR-367 mimics (Figure 6B and 6C). As expected, the same results of the mRNA level of MTA3 in 786-O and Caki-1 cells were quantified by qRT-PCR (Figure 6D and 6E). Together, these indicated that MTA3 is a target of miR-367.
Figure 5. MTA3 was obviously decreased in ccRCC patients.
Western blot was performed to show that protein level of MTA3 was dominantly decreased in ccRCC patients.
Figure 6. MTA3 is the target of miR-367.
(A) Bioinformatics prediction was showed. (B and C) The protein levels of MTA3 in 786-O and Caki-1 cell lines were analyzed by western blotting. (D and E) The mRNA level of MTA3 was measured by qRT-PCR. Overexpression of miR-367 could inhibit the mRNA expression of MTA3. Data are expressed as mean ± SEM. *p<0.05 vs. miR-control.
MTA3 could reverse proliferation and metastasis in ccRCC cell
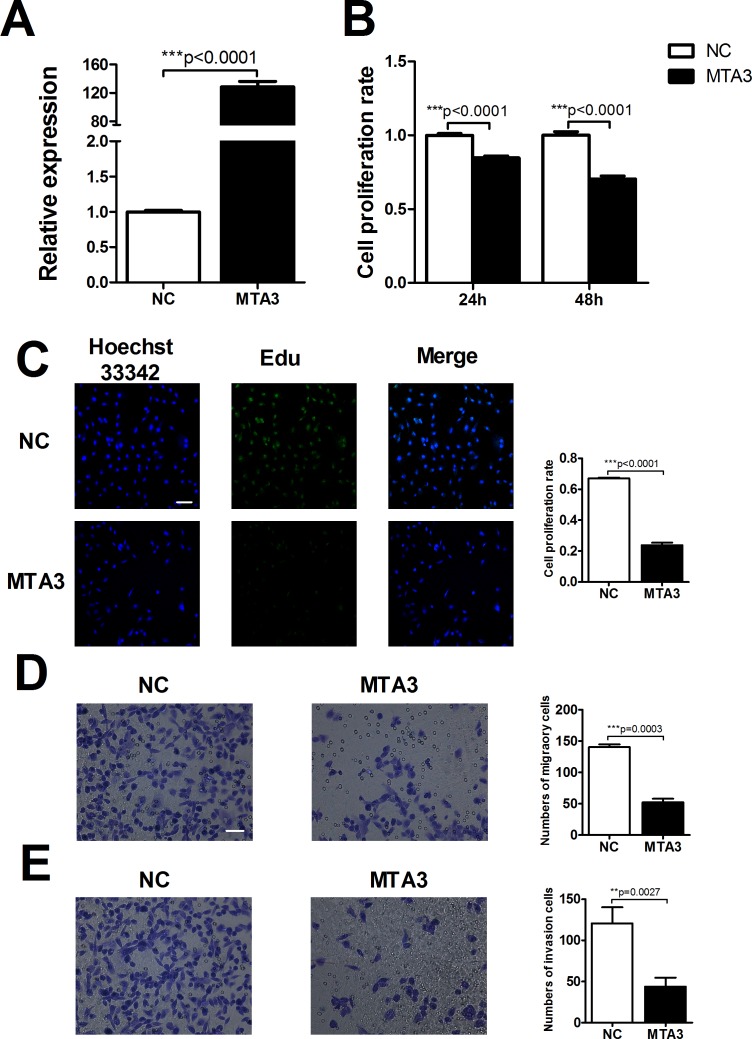
Emerging evidence suggested that MTA3 aberrantly expressed in multiple cancers, including carcinoma of the esophagus, breast, lung, uterus and nasopharynx. This raised the question that MTA3 might play a crucial role in tumors and our interest was directed to explore whether the abnormal of MTA3 was associated with occurrence and development of tumors. Transfection efficiency of MTA3 was first assessed by qRT-PCR suggesting the successful transfection in ccRCC cells (Figure 7A). Western blot was also performed to examine the levels of MTA3 in HK-2, 786-O and caki-1 cell lines. MTA3 was decreased in ccRCC cell lines compared with human renal proximal tubular cells (HK-2 cells) (Supplementary Figure 2). Next, we detected cell viability using MTT and EdU assays, respectively. As shown in Figure 7B and 7C, MTA3 elevation could dramatically decrease cell viability at 24 h and 48h time points. Furthermore, MTA3 elevation was found to significantly reduce migration and invasion of 786-O cells (Figure 7D and 7E). Wound Healing and MTT experiments were carried out to demonstrate inhibition effect of overexpressed-MTA3 on caki-1 cell proliferation and metastasis (Supplementary Figure 3A and 3B). Based on the above data, we concluded that miR-367 mediated proliferation, migration and invasion was, at least in part, attributable to regulate the level of transcriptional and translational MTA3 in ccRCC cells.
Figure 7. MTA3 is involved in ccRCC proliferation and metastasis.
(A) Transfection efficiency of MTA3 overexpression was assessed by qRT-PCR. (B) MTT assay to confirm the ability of MTA3 on ccRCC cell viability. (C) After treatment with MTA3 plasmid, the proliferation rate of 786-O cells was determined by EdU kit. (D and E) Representative micrographs (left) were showed. Relative quantification (right) of cell migration (top) and invasion (bottom) were measured after infected with negative control (NC) or MTA3 in 786-O cells. Scale bar indicates 100 μm. Data are expressed as mean ± SEM. **p<0.01 or *p<0.05 vs. NC.
DISCUSSION
In the present study, we found that the expression of miR-367 in tumor tissues was significantly higher than that in matched adjacent normal tissues (Figure 1). In addition, we illustrated that miR-367 played crucial roles in proliferation and metastasis of ccRCC cells. Inhibition of miR-367 could suppress the proliferation, migration and invasion both in 786-O and Caki-1 cells (Figure 2, 3 and 4). As EMT is a key process of tumor invasion, migration, and metastasis [22], the regulation of EMT process by miR-367 further established miR-367 as a pivotal promoter in cell migration (Supplementary Figure 1). Importantly, this is the first study to identify MTA3 as a direct target gene of miR-367. The protein expression of MTA3 was significantly reduced in ccRCC patients. Further studies showed that miR-367 elevation could substantially reduce the protein and mRNA expressions of MTA3 (Figure 5, 6 and 7). Thus, miR-367 may act as a novel type of oncogene by targeting MTA3 in pathophysiologic process of ccRCC.
MiRNAs regulate the expression of target genes at posttranscriptional levels, which induce mRNA degradation or inhibit translation via imperfect hybridization with the 3′-UTRs of target mRNAs [23]. Recent studies suggested that dysregulation of miRNAs was involved in various aspects of cancer progressions [24]. Aberrant expression of miR-367 has been described in multiple types of cancers, such as lung cancer [25], hepatocellular and esophagus cancers [26, 27]. However, little is known about the expression and exact mechanism of miR-367 in ccRCC. To explore the effect of miR-367 on ccRCC, we first analyzed the expression of miR-367 in ccRCC patients. Results showed that miR-367 was significantly upregulated in the tumor tissues. It implied that miR-367 dysregulation was involved in occurrence and development of kidney tumors.
The mechanisms underlying the regulation by miRNAs are often attributed to targeting crucial factors or key signaling pathways [28]. For example, miR-429 regulates cellular function by targeting VEGF [29]. miR-19a promotes ccRCC through downregulation the expression of PTEN, thereby regulating smad4 signaling pathway [30]. miR-30 enhances HIF-2α activity in ccRCC [31]. In our present study, we used two different types of prediction software to predict target genes for miR-367. We found that MTA3 could be a potential downstream target. MTA3 is the latest identified member of metastasis-associated protein family, which also includes MTA1 and MTA2. MTA3 was considered as an estrogen-dependent component of the Mi-2/NuRD transcriptional corepressor in breast epithelial cells [14]. Recently, accumulating evidence showed that MTA3 could regulate cell proliferation and differentiation in various cancers and act as an oncogene in breast cancer and non-small cell lung cancer (NSCLC). Researchers found that MTA3 was dominantly increased in the progress of the above mentioned tumors [32, 33]. In contrast, Dong et al. manifested that MTA3 expression was significantly reduced in both tumor tissues and cell lines in gastroesophageal junction (GEJ) adenocarcinoma [34]. Brüning et al. found that a significantly lower expression of MTA3 was obtained from 200 patients with endometrioid adenocarcinoma [35]. In this study, serious decrease of MTA3 was observed in ccRCC, indicating that MTA3 played a key role in pathophysiologic process of ccRCC. Further results indicated that overexpression of MTA3 suppressed the proliferation and migration of ccRCC cells and the abnormity of MTA3 was controlled by miRNA-367. Therefore, the potential molecular mechanism could be illustrated by the association between miR-367 and MTA3. However, the effect of miR-367 on ccRCC may be regulated by other genes and pathways as well, and the full mechanisms require our further investigations.
Overall, our study is the first to show induced expression of miR-367 and reduced expression of MTA3 in human ccRCC tissues and cell lines. Targeting MTA3 or inhibiting miR-367 could decrease cell proliferation, migration and invasion. Therefore, miR-367 might represent a novel therapeutic target for treatment of ccRCC patients.
MATERIALS AND METHODS
Patients and clinical specimens
Clinical specimens of carcinoma tissues and the corresponding para-carcinoma tissues were obtained from 35 ccRCC patients who had undergone nephrectomy from July 2015 to January 2016 at the Affiliated Tumor Hospital of the Harbin Medical University (Table 1). Written prior informed consent for tissue donation was acquired from each enrolled patient. The protocol was carried out in accordance with the approved guidelines by the Ethics and Scientific Committees of Harbin Medical University.
Table 1. Characteristics of ccRCC clinical specimens.
| n | (%) | |
|---|---|---|
| Sex | ||
| Male | 23 | (65.7) |
| Female | 12 | (34.3) |
| Age | ||
| ≤55 | 14 | (40.0) |
| >55 | 21 | (60.0) |
| Grade | ||
| G1 | 8 | (22.8) |
| G2 | 14 | (40) |
| G3 | 10 | (28.6) |
| G4 | 3 | (8.6) |
| Tumor size | ||
| ≤5 | 12 | (34.3) |
| >5 | 23 | (65.7) |
| T stage | ||
| T1a | 4 | (11.4) |
| T1b | 21 | (60) |
| T2a | 5 | (14.3) |
| T2b | 0 | (0.0) |
| T3 | 3 | (8.6) |
| T4 | 2 | (5.7) |
Cell culture and transfection
Two ccRCC cell lines (Caki-1 and 786-O) were obtained from the Chinese Academy of Sciences (Shanghai, China). Caki-1 cells were cultured in McCoy's 5A medium (Gibco, Grand Island, NY, U.S.) supplemented with 15% fetal bovine serum (FBS; Shanghai Sangon Biological Engineering Technology and Services Co., Ltd., Shanghai, China), and 786-O cells were cultured in RPMI 1640 (Wisent, Saint-Jean-Baptiste, Canada) supplemented with 10% FBS. All the cells were maintained at 37°C in humidified 5% CO2 atmosphere. For transfections, cells were starved in serum-free medium for 24 h and transiently transfected with miR-367 mimics, miR-367 inhibitors or negative controls (GenePharma Co., Ltd., Shanghai, China). MTA3 plasmid (RiboBio Co., Ltd., Guangzhou, Guangdong, China) was transferred into cells by using Lipofectamine 2000 reagent (Invitrogen, CA, Carlsbad, USA) according to the manufacturer's protocol. Cells were used for protein/RNA extraction after 24 hours of transfection with the maximum efficiency.
Cell viability assay
A 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay was performed to evaluate ccRCC cell viability. Briefly, cells were seeded into 96-well plates followed by miRNA or drug treatments for 24 h. A total of 20 μl of MTT solution was added to each well, and the cells were incubated for 4 h. Thereafter, a total 150 μl of DMSO was added to dissolve the formazan crystals. Absorbance at 490 nm was recorded [20].
EdU assays
Cells seeded on coverslips in 24-well culture plates were treated as experiment design. The proliferation of Caki-1 and 786-O cells were detected by EdU kit (RiboBio, China) according to the manufacturer's instructions [21]. The stained cells were examined under a confocal laser scanning microscope (FV300, Olympus, Japan). Nuclei that double labeled with EdU and Hoechst 33342 were considered to be positive cells.
Cell migration and invasion assays
Twenty-four hours after transfection, 1×105 cells in serum-free medium were seeded into the upper chambers of an insert (8-μm pore size; Corning, NY, USA) coated with or without Matrigel (BD Bioscience). The lower chambers were filled with McCoy's 5A/1640 medium containing 10% FBS as a chemoattractant. After 18 h of incubation, the upper side of membrane was cleaned and cells that had invaded through the membrane on the bottom were fixed with 0.1% paraformaldehyde and then stained with 0.1% crystal violet. The migrated cells were observed and counted under a microscope. Cells were seeded in six-well plate with McCoy's 5A/1640 medium to analyze wound healing. After 48 h, the cell monolayer was wounded using a plastic pipette tip and then rinsed with PBS and cultured with serum-free McCoy's 5A/1640 for 24 h. The wound closure was photographed using a microscope(Olympus, Japan).
Protein isolation and western blot analysis
Western blot analysis was carried out as previously described. The cells were lysed with RIPA buffer containing protease inhibitors phenylmethanesulfonyl fluoride (PMSF). After centrifugation, the supernatant was collected and quantified using the BCA kit (Beyotime, Shanghai, China). Protein samples were then separated by SDS-PAGE and transferred to nitrocellulose membranes which were blocked by 5% non-fat milk for two hours at room temperature. The membranes were rinsed and probed with anti-MTA3 (Abcam, Cambridge, MA, USA) overnight, followed by incubation with florescence-labeled secondary antibody for one hour. GAPDH (Kangcheng Inc., Shanghai, China) was used as an internal control.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
Total RNA was harvested from tissues and cells using TRIzol reagent (Invitrogen, CA, USA) according to the manufacturer's protocol. The extracted RNA was then reverse transcribed into cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA; Cat. no. 4368814). The SYBR Green PCR Master Mix Kit (Applied Biosystems) was used to quantify the relative levels of miR-367 and MTA3, with U6 and GAPDH serving as internal controls respectively. The sequences of primers are as follows:
has-MTA3:5′-TCCTCCAGCAACCCATACCT-3′ (forward) and 5′-TCGGTCAAGTCAGCCTCAAC-3′ (reverse);GAPDH:5′-AAGAAGGTGGTGAAGCAGGC-3′(forward) and5′-TCCACCACCCAGTTGCTGTA-3′ (reverse);has-miR-367:5′-CGAGCAATTGCACTTTAG CAAT-3′;andU6:5′-GCTTCGGCAGCACATATA CTAAAAT-3′ (forward)and5′-CGCTTCACGAATTTG CGTGTCAT -3′ (reverse).
Data analysis
All data in this study were expressed as the mean ± SEM. Statistical comparison between two groups were performed by two-tailed student's t-test and one-way ANOVA followed by Tukey's test were employed to compare three or more means. p < 0.05 was considered statistically significant.
SUPPLEMENTARY MATERIALS FIGURES
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81570399, 81270042 and 81503069) and the Program for New Century Excellent Talents In Heilongjiang Provincial University (1254-NCET-012).
Abbreviations
- miR
miRNA, microRNA
- MTA3
metastasis-associated protein 3
- ccRCC
clear-cell renal cell carcinoma
- NSCLC
non-small cell lung cancer
- LOXL2
lysyl oxidase like 2
- RYR3
ryanodine receptor gene 3
- qRT-PCR
quantificational real-time polymerase chain reaction
- NuRD
nucleosome remodeling and deacetylase
- EMT
epithelial-to-mesenchymal transition
- VEGF
vascular endothelial growth factor
- HIF2α
hypoxia-inducible factor 2α
- PTEN
phosphatase and tensin homolog
- GEJ
gastroesophageal junction
Footnotes
Author contributions
Yong.Z. and R.A. conceived and designed the study. D.D., Yue.Z., L.W., J.F., X.B., Y.L., H.D. and Q.L. performed the experiments. D.D., Yue.Z. and Y.F. analyzed the results. Yue.Z., Y.F. and Yong.Z. wrote the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 2.Cancer statistics. Jama. 2013;310:982. doi: 10.1001/jama.2013.5289. [DOI] [PubMed] [Google Scholar]
- 3.Krambeck AE, Dong H, Thompson RH, Kuntz SM, Lohse CM, Leibovich BC, Blute ML, Sebo TJ, Cheville JC, Parker AS, Kwon ED. Survivin and b7-h1 are collaborative predictors of survival and represent potential therapeutic targets for patients with renal cell carcinoma. Clinical cancer research. 2007;13:1749–1756. doi: 10.1158/1078-0432.CCR-06-2129. [DOI] [PubMed] [Google Scholar]
- 4.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Xiao H, Xiao W, Cao J, Li H, Guan W, Guo X, Chen K, Zheng T, Ye Z, Wang J, Xu H. miR-206 functions as a novel cell cycle regulator and tumor suppressor in clear-cell renal cell carcinoma. Cancer letters. 2016;374:107–116. doi: 10.1016/j.canlet.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Brannon AR, Reddy AR, Alexe G, Seiler MW, Arreola A, Oza JH, Yao M, Juan D, Liou LS, Ganesan S, Levine AJ, Rathmell WK, Bhanot GV. Identifying mRNA targets of microRNA dysregulated in cancer: with application to clear cell Renal Cell Carcinoma. BMC systems biology. 2010;4:51. doi: 10.1186/1752-0509-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juan D, Alexe G, Antes T, Liu H, Madabhushi A, Delisi C, Ganesan S, Bhanot G, Liou LS. Identification of a microRNA panel for clear-cell kidney cancer. Urology. 2010;75:835–841. doi: 10.1016/j.urology.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Li M, Zhang R, Wang Y, Zang W, Ma Y, Zhao G, Zhang G. Effect of miR-335 upregulation on the apoptosis and invasion of lung cancer cell A549 and H1299. Tumour biology. 2013;34:3101–3109. doi: 10.1007/s13277-013-0878-9. [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa R, Chiyomaru T, Enokida H, Inoguchi S, Ishihara T, Matsushita R, Goto Y, Fukumoto I, Nakagawa M, Seki N. Tumour-suppressive microRNA-29s directly regulate LOXL2 expression and inhibit cancer cell migration and invasion in renal cell carcinoma. FEBS letters. 2015;589:2136–2145. doi: 10.1016/j.febslet.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Campayo M, Navarro A, Vinolas N, Diaz T, Tejero R, Gimferrer JM, Molins L, Cabanas ML, Ramirez J, Monzo M, Marrades R. Low miR-145 and high miR-367 are associated with unfavourable prognosis in resected nonsmall cell lung cancer. The European respiratory journal. 2013;41:1172–1178. doi: 10.1183/09031936.00048712. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Z, Xu Y, Zhao J, Liu Q, Feng W, Fan J, Wang P. miR-367 promotes epithelial-to-mesenchymal transition and invasion of pancreatic ductal adenocarcinoma cells by targeting the Smad7-TGF-beta signalling pathway. British journal of cancer. 2015;112:1367–1375. doi: 10.1038/bjc.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Liu Y, Song F, Zheng H, Hu L, Lu H, Liu P, Hao X, Zhang W, Chen K. Functional SNP in the microRNA-367 binding site in the 3'UTR of the calcium channel ryanodine receptor gene 3 (RYR3) affects breast cancer risk and calcification. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13653–13658. doi: 10.1073/pnas.1103360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng X, Lu P, Fan Q. miR-367 promotes proliferation and invasion of hepatocellular carcinoma cells by negatively regulating PTEN. Biochemical and biophysical research communications. 2016;470:187–191. doi: 10.1016/j.bbrc.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Fujita N, Jaye DL, Kajita M, Geigerman C, Moreno CS, Wade PA. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell. 2003;113:207–219. doi: 10.1016/s0092-8674(03)00234-4. [DOI] [PubMed] [Google Scholar]
- 15.Roche AE, Bassett BJ, Samant SA, Hong W, Blobel GA, Svensson EC. The zinc finger and C-terminal domains of MTA proteins are required for FOG-2-mediated transcriptional repression via the NuRD complex. Journal of molecular and cellular cardiology. 2008;44:352–360. doi: 10.1016/j.yjmcc.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toh Y, Nicolson GL. The role of the MTA family and their encoded proteins in human cancers: molecular functions and clinical implications. Clinical & experimental metastasis. 2009;26:215–227. doi: 10.1007/s10585-008-9233-8. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Li G, Li J, Li J, Li T, Yu J, Qin C. Overexpression of the metastasis-associated gene MTA3 correlates with tumor progression and poor prognosis in hepatocellular carcinoma. Journal of gastroenterology and hepatology. 2016;32:1525–1529. doi: 10.1111/jgh.13680. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Wang Q, Zhang L, Bao H, Zhang H. Regulation Mechanism of MTA3 in the Apoptosis of NSCLC Cells. [Article in Chinese] Zhongguo fei ai za zhi. 2015;18:610–615. doi: 10.3779/j.issn.1009-3419.2015.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mylonas I, Bruning A. The metastasis-associated gene MTA3 is an independent prognostic parameter in uterine non-endometrioid carcinomas. Histopathology. 2012;60:665–670. doi: 10.1111/j.1365-2559.2011.04103.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Qin W, Zhang L, Wu X, Du N, Hu Y, Li X, Shen N, Xiao D, Zhang H, Li Z, Zhang Y, Yang H, et al. MicroRNA-26a prevents endothelial cell apoptosis by directly targeting TRPC6 in the setting of atherosclerosis. Scientific reports. 2015;5:9401. doi: 10.1038/srep09401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang T, Hu Y, Ju J, Hou L, Li Z, Xiao D, Li Y, Yao J, Wang C, Zhang Y, Zhang L. Downregulation of miR-522 suppresses proliferation and metastasis of non-small cell lung cancer cells by directly targeting DENN/MADD domain containing 2D. Scientific reports. 2016;6:19346. doi: 10.1038/srep19346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeung KT, Yang J. Epithelial-mesenchymal transition in tumor metastasis. Molecular oncology. 2017;11:28–39. doi: 10.1002/1878-0261.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nature reviews Molecular cell biology. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 24.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nature reviews Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Wu W, Wang J, Huang C, Wen W, Zhao F, Xu X, Pan X, Wang W, Zhu Q, Chen L. miR-367 promotes the proliferation and invasion of non-small cell lung cancer via targeting FBXW7. Oncology reports. 2017;37:1052–1058. doi: 10.3892/or.2016.5314. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Lin H, Li G, Sun Y, Chen J, Shi L, Cai X, Chang C. The miR-367-3p Increases Sorafenib Chemotherapy Efficacy to Suppress Hepatocellular Carcinoma Metastasis through Altering the Androgen Receptor Signals. EBioMedicine. 2016;12:55–67. doi: 10.1016/j.ebiom.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun J, Song K, Feng X, Gao S. MicroRNA-367 is a potential diagnostic biomarker for patients with esophageal squamous cell carcinoma. Biochemical and biophysical research communications. 2016;473:363–369. doi: 10.1016/j.bbrc.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 28.Xing T, He H. Epigenomics of clear cell renal cell carcinoma: mechanisms and potential use in molecular pathology. Chinese journal of cancer research. 2016;28:80–91. doi: 10.3978/j.issn.1000-9604.2016.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen D, Li Y, Li Y, Jin L, Su Z, Yu Z, Yang S, Mao X, Lai Y. Tumor suppressive microRNA429 regulates cellular function by targeting VEGF in clear cell renal cell carcinoma. Molecular medicine reports. 2016;13:1361–1366. doi: 10.3892/mmr.2015.4653. [DOI] [PubMed] [Google Scholar]
- 30.Campbell K, Casanova J. A common framework for EMT and collective cell migration. Development. 2016;143:4291–4300. doi: 10.1242/dev.139071. [DOI] [PubMed] [Google Scholar]
- 31.Mathew LK, Lee SS, Skuli N, Rao S, Keith B, Nathanson KL, Lal P, Simon MC. Restricted expression of miR-30c-2-3p and miR-30a-3p in clear cell renal cell carcinomas enhances HIF2alpha activity. Cancer discovery. 2014;4:53–60. doi: 10.1158/2159-8290.CD-13-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Sun L, Xu Y, Li Z, Luo W, Tang Z, Qiu X, Wang E. Overexpression of MTA3 Correlates with Tumor Progression in Non-Small Cell Lung Cancer. PloS one. 2013;8:e66679. doi: 10.1371/journal.pone.0066679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Si W, Huang W, Zheng Y, Yang Y, Liu X, Shan L, Zhou X, Wang Y, Su D, Gao J, Yan R, Han X, Li W, et al. Dysfunction of the Reciprocal Feedback Loop between GATA3- and ZEB2-Nucleated Repression Programs Contributes to Breast Cancer Metastasis. Cancer cell. 2015;27:822–836. doi: 10.1016/j.ccell.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Dong H, Guo H, Xie L, Wang G, Zhong X, Khoury T, Tan D, Zhang H. The metastasis-associated gene MTA3, a component of the Mi-2/NuRD transcriptional repression complex, predicts prognosis of gastroesophageal junction adenocarcinoma. PloS one. 2013;8:e62986. doi: 10.1371/journal.pone.0062986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruning A, Juckstock J, Blankenstein T, Makovitzky J, Kunze S, Mylonas I. The metastasis-associated gene MTA3 is downregulated in advanced endometrioid adenocarcinomas. Histology and histopathology. 2010;25:1447–1456. doi: 10.14670/HH-25.1447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.