Abstract
Obesity is a global epidemic, accompanied by increased risk of type 2 diabetes and cardiovascular disease. Adipose tissue hypertrophy is associated with adipose tissue inflammation, which alters the secretion of adipose tissue‐derived bioactive products, known as adipokines. Adipokines determine vessel wall properties such as smooth muscle tone and vessel wall inflammation. Exercise is a mainstay of prevention of chronic, non‐communicable diseases, type 2 diabetes and cardiovascular disease in particular. Aside from reducing adipose tissue mass, exercise has been shown to reduce inflammatory activity in this tissue. Mechanistically, contracting muscles release bioactive molecules known as myokines, which alter the metabolic phenotype of adipose tissue. In adipose tissue, myokines induce browning, enhance fatty acid oxidation and improve insulin sensitivity. In the past years, the perivascular adipose tissue (PVAT) which surrounds the vasculature, has been shown to control vascular tone and inflammation through local release of adipokines. In obesity, an increase in mass and inflammation of PVAT culminate in dysregulation of adipokine secretion, which contributes to vascular dysfunction. This review describes our current understanding of the mechanisms by which active muscles interact with adipose tissue and improve vascular function. Aside from the exercise‐dependent regulation of canonical adipose tissue function, we will focus on the interactions between skeletal muscle and PVAT and the role of novel myokines, such as IL‐15, FGF21 and irisin, in these interactions.
Linked Articles
This article is part of a themed section on Molecular Mechanisms Regulating Perivascular Adipose Tissue – Potential Pharmacological Targets? To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.20/issuetoc
Abbreviations
- AMPK
AMP‐activated kinase
- AT
adipose tissue
- atNPs
angiogenesis‐targeted nanoparticles
- BAT
brown adipose tissue
- CLS
crown‐like structures
- CVD
cardiovascular diseases
- HFD
high‐fat diet
- METRNL
meteorin‐like
- NPR
natriuretic peptide receptor
- PGC1‐α
PPARγ co‐activator 1α
- PRDM16
PR domain‐containing 16
- PVAT
perivascular adipose tissue
- RT
resistance training
- SKM
skeletal muscle
- SNS
sympathetic nerve system
- SVF
stromal vascular fraction
- TLR4
toll‐like receptor 4
- UCP‐1
uncoupling protein 1
- VCAM‐1
vascular cell adhesion protein 1
- WAT
white adipose tissue
Tables of Links
| TARGETS | |
|---|---|
| Nuclear hormone receptors a | Enzymes e |
| PPARγ | Acetyl CoA carboxylase |
| Transporters b | Adenylate cyclase |
| GLUT4 | Akt (PKB) |
| SERCA2 | AMPKα1 |
| UCP1, SLC25A7 | AMPKα2 |
| Catalytic receptors c | eNOS, NOS3 |
| NPR | ERK1 |
| IL‐15Rα | ERK2 |
| TLR4 | PKA |
| GPCRs d | |
| β‐adrenoceptors |
| LIGANDS | |
|---|---|
| ACh | IL‐6 |
| Adiponectin | IL‐13 |
| ATP | IL‐15 |
| CCL2 | Insulin |
| Hydrogen peroxide | Noradrenaline |
| ICAM‐1 | NO |
| IL‐1β | TNF‐α |
| IL‐4 | VCAM‐1 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,d,eAlexander et al., 2015a,b,c,d,e).
Introduction
Mankind has experienced profound lifestyle changes during the past decades, changes such as high consumption of foods rich in sugars, fat and salt in combination with low physical activity, leading to increased rates of obesity. Obesity increases risk of type 2 diabetes, hypertension and cardiovascular diseases (CVD) (Malnick and Knobler, 2006). Adipose tissue (AT) functions as an endocrine organ that releases bioactive molecules known as adipokines (Lago et al., 2009). Despite the fact that excess of AT in obesity is generally viewed as harmful to individual's health, an adequate amount of AT and physiological levels of adipokines contribute to normal whole‐body metabolic homeostasis (Lago et al., 2009). In obesity, the balance between cardioprotective and pro‐inflammatory adipokines shifts towards pro‐inflammatory ones, most of which are risk factors for CVD (Coles, 2016).
Nearly 40% of the U.S. adult population performs less than 10 min·per week of continuous physical activity (Carlson et al., 2015). These statistics contrast sharply with the American Heart Association recommendation of at least 150 min per week of moderate‐intensity activity (Eckel et al., 2014) to improve healthy life span and prevent chronic diseases such as CVD and type 2 diabetes (Allender et al., 2007). Moreover, inactivity is related to increased central adiposity, oxidative stress, endothelial dysfunction and increased atherosclerotic lesion size in both humans (Hamburg et al., 2007) and animal models (Laufs et al., 2005; Pedersen, 2009). Similarly, exercise improves mood, endothelial function and arteriogenesis in the myocardium, lowers blood pressure and normalizes fat mass and inflammatory markers (Lee et al., 2005; Piepoli et al., 2010; McDowell et al., 2016). Recent research has shown that skeletal muscle (SKM) exhibits an endocrine function similar to that of AT (Pedersen and Febbraio, 2008). A contracting muscle releases soluble molecules, called myokines, which partly explain how muscles interact with other organs. Muscle activity increases laminar shear stress in the vascular system, which is a mechanical‐biological stimulus for the expression of anti‐thrombotic, anti‐atherogenic and vasodilator signals from the vasculature (Zhang and Friedman, 2012). Furthermore, exercise can directly modulate adipocyte physiology. Physical activity stimulates the CNS and, through noradrenalin and adrenalin, increases thermogenic activity within adipocytes by inducing mitochondrial biogenesis and uncoupling (Sutherland et al. 2009). In parallel, exercise ameliorates inflammation within AT by interacting with the immune cells inside AT.
Among AT depots, the perivascular adipose tissue (PVAT) displays a unique physiological role, that is, paracrine regulation of vascular function (Yudkin et al., 2005). PVAT carries an anti‐contractile property that influences arteriolar responses to agonists such as insulin, thereby contributing to the regulation of blood flow, nutrient uptake and tissue homeostasis (Meijer et al., 2011). On the other hand, PVAT is susceptible to inflammation, and in obesity, infiltration of immune cells into the PVAT is aggravated (Greenstein et al., 2009; Kranendonk et al., 2015). Alterations in the properties of PVAT have been linked to insulin resistance (Rittig et al., 2008), hypertension and impaired myocardial perfusion (Reifenberger et al., 2007).
In this review, we will cover the effects of exercise on PVAT metabolism, structure and functional phenotype, exploring central and local influences of muscle activity, and their consequences for vascular function. Firstly, we will discuss whole‐body alterations in AT and the mechanisms causing its dysfunction. Secondly, effects of exercise on PVAT and its inflammation will be highlighted. Finally, we will describe how new myokines such as FGF21, irisin and IL‐15 may affect AT.
Systemic adipose tissue dysfunction in obesity
In obesity, an increased fat mass is accompanied by alterations in the cellular composition and physiology of AT. Anatomical changes (i.e. AT hypertrophy and distribution), inflammation and dysregulated adipokine secretion characterize a dysfunctional adipose organ, which contributes to cardiovascular pathology (Fuster et al., 2016).
In obesity, the interplay between adipocytes and components of the immune system changes. Immune cells secrete cytokines that enhance AT inflammation, and at the same time, adipocytes express classical macrophage features (Kopp et al., 2009). As AT expansion occurs, macrophages, mast cells, B cells and T‐cell populations increase considerably (Huh et al., 2014). Macrophages comprise the largest population of immune cells within white adipose tissues (WAT) (i.e. subcutaneous, visceral and perivascular), and a hallmark of its dysfunction is the infiltration of pro‐inflammatory M1‐macrophages. This phenomenon has been observed in both humans (Kranendonk et al., 2015) and rodents (Kawanishi et al., 2013). Canonical ATs such as visceral (i.e. omental and mesenteric) and subcutaneous AT of obese humans present similar crown‐like structures (CLS), which consist of organized macrophage structures around necrotic adipocytes (Bigornia et al., 2012). The macrophage number differs between these depots and visceral and PVAT display distinct abnormalities in inflammation and morphology (Kranendonk et al., 2015).
In parallel, T‐cell populations shift from a predominantly anti‐inflammatory Treg and Th2 population to pro‐inflammatory CD4+ Th1, Th17 and CD8+ cells (Travers et al., 2015). As a consequence, there is an increased secretion of the inflammatory adipokines TNF‐α and WNT5a and a reduction of anti‐inflammatory adipokines such as secreted frizzled‐related protein 5 (Ouchi et al., 2010), adiponectin (Samaras et al., 2010) and Th2 cytokines (IL‐4, IL‐13 and IL‐10) (Feuerer et al., 2009). These phenomena have been observed in gonadal and visceral fat of high‐fat diet (HFD)‐fed mice and in subcutaneous (Feuerer et al., 2009; Zeyda et al., 2011) and visceral AT of obese humans (Zeyda et al., 2011). Other immune cells such as mast cells, NK‐cells and B‐cells also participate in this pro‐inflammatory phenotype by reducing the Th2 cytokines IL‐4 and IL‐13, increasing release of IFN‐γ and labelling necrotic adipocytes for macrophage phagocytosis (Huh et al., 2014). Subsequently, the elevated inflammatory activity features activation of IKKβ/NFκB and JNK pathways, which are known to be related to insulin resistance (Zeyda et al., 2011) (Figures 1 and 2).
Figure 1.
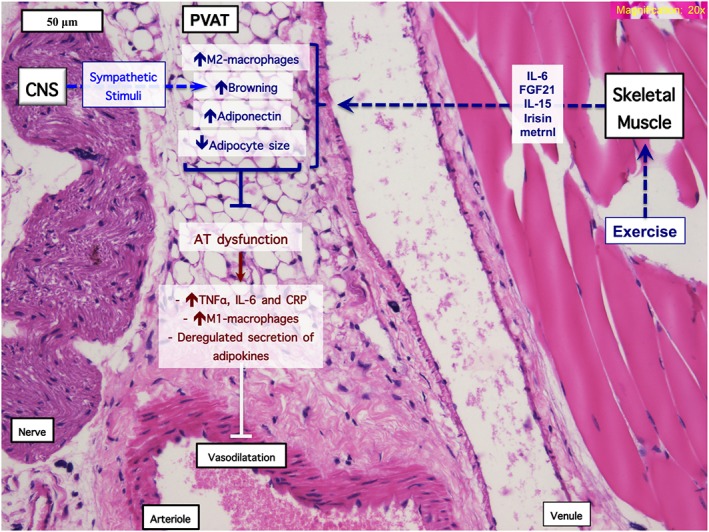
Central and paracrine control of the PVAT by exercise. Juxtaposition of PVAT, nerves CNS, SKM and microvessels in a transverse section of the mouse hindlimb and mechanisms of interaction with AT. First, central effects of exercise are exerted through noradrenaline from sympathetic nerve fibres, with subsequent beiging and vasodilator function of PVAT. Second, muscle activity regulates (perivascular) AT function through myokines such as FGF‐21, metrnl, irisin, IL‐15, IL‐6, acting in a paracrine fashion to antagonize dysfunction of AT (i.e. inflammation and dysregulated secretion of adipokines).
Figure 2.
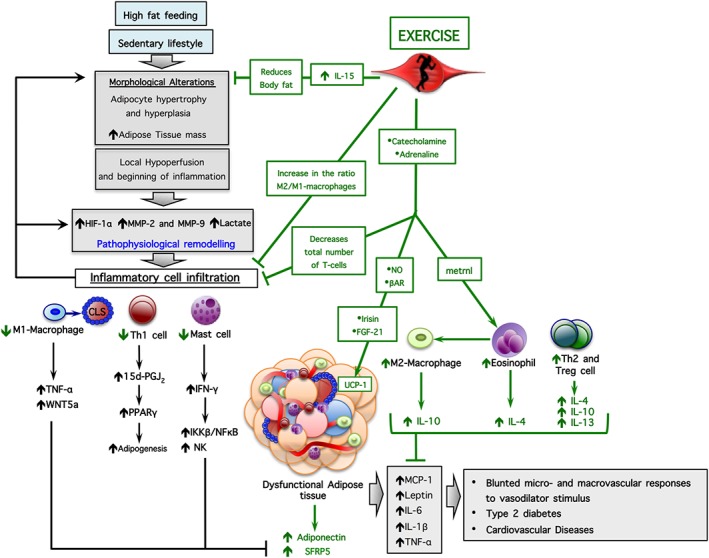
Signalling pathways involved in dysfunction of AT and effects of exercise. HFD feeding and a sedentary lifestyle change AT morphology, such as adipocyte hypertrophy and hyperplasia. Subsequently, AT shows hypoperfusion, inflammation and remodelling, with consequent inflammatory cell infiltration and abnormal secretion of inflammatory adipokines. Exercise exerts an anti‐inflammatory effect on dysfunctional AT. It activates the CNS and stimulates release of myokines, resulting in a reduced AT mass and an improved functional and metabolic phenotype. βAR, β‐adrenoceptors.
Although it is not known whether inflamed macrophages cause AT hyperplasia, they do release cytokines responsible for altering the secretion of adipokines (Lumeng et al., 2007). In addition, AT expansion requires an extensive vascular network (Sun et al., 2011), and during pathological expansion, angiogenesis does not match expansion requirements (Nishimura et al., 2007; Daquinag et al., 2011). In monocytes of HIF‐1α‐deficient mice, the inflammatory marker genes IL‐6, F4/80, Cd11c, Il‐1b and Nos2 and hypoxia‐related genes such as Vegfa and Glut 1 are markedly reduced. In epidydimal fat, metabolic genes such as adiponectin, Pparg and Pgcta are increased in these mice (Takikawa et al., 2016). In further studies, vascularization was also increased in eWAT of these KO‐mice, with higher expression of the endothelial cell markers, Cd31 and VE‐cad and the pericyte marker Cspg4. Collectively, these data suggest that hypoxia‐induced HIF‐1α contributes to the pathological expansion of AT (Takikawa et al., 2016).
In summary, communication between adipocytes and immune system cells increases low‐grade inflammation of AT, coinciding with morphological alterations, which culminates in altered AT physiological function.
PVAT and the control of vascular tone
The PVAT resides alongside large and small vessels, exerting anti‐contractile effects on conduit and resistance arteries, which are vital for maintenance of normal arterial vascular tone (Yudkin et al., 2005; Meijer et al., 2016). It may have a regulatory effect on metabolism (Chang et al., 2012), insulin sensitivity (Meijer et al., 2013) and inflammatory responses (Greenstein et al., 2009) via local release of hormones, cytokines and reactive oxygen and nitrogen species. These effects are reported to be endothelium‐dependent, via release of NO and alternatively through hydrogen peroxide (Gao et al., 2007; Malinowski et al., 2008). Although PVAT secretes adipokines, their plasma concentrations are generally insufficient to provoke systemic vascular actions. This, in combination with proven ex vivo effects of PVAT (Figure 1), suggests that PVAT‐derived adipokines work in a paracrine fashion, through outside‐to‐inside intercellular crosstalk. Apparently, this crosstalk is partly mediated by adiponectin, as has been shown by a number of studies (Eringa et al., 2007; Greenstein et al., 2009; Meijer et al., 2015). For example, globular adiponectin elicits vasodilator responses similar to the effect of PVAT incubation on mouse muscle resistance arteries (Meijer et al., 2013; de Boer et al., 2016). In obesity, reduced adiponectin release by PVAT is partially responsible for blunted vasodilatation in the muscle vasculature (Meijer et al., 2013). Similarly, local adiponectin secretion from peri‐coronary fat is relevant to myocardial perfusion in lean individuals (Date et al., 2006) (Figure 1). In conclusion, these data indicate PVAT as a promising candidate for explaining how impaired AT homeostasis affects vascular function.
PVAT and exercise
PVAT has been reported to release substances responsible for affecting vascular tone, and it seems that exercise contributes to this process. First, in obesity, the microarchitecture of PVAT is altered, and therefore, the secretion of vasodilator signals to the vascular wall is altered. In obese subjects with metabolic syndrome, increased adipocyte size accompanies the loss of the anti‐contractile effect of PVAT (Greenstein et al., 2009). Similar findings were seen in healthy obese women, in which PVAT adipocyte size was statistically related to disturbed insulin‐induced muscle microvascular perfusion but not to body mass index (Meijer et al., 2015). Exercise reduced body fat (i.e. reducing PVAT depots) and, at the same time, reducing PVAT inflammation (Lee et al., 2016). In obese mice, chronic exercise limits adipocyte size and reduces CLS and inflammatory cell recruitment in mesenteric fat (Haczeyni et al., 2015).
Changes in PVAT morphology and inflammation occur simultaneously and may be directly related. Infiltration of T cells and macrophages has been observed in periaortic AT in both obesity and atherosclerosis (Chatterjee et al., 2009). Accumulation of T lymphocytes may provoke an increase of PVAT mass, via production of 15d‐PGJ2 (15‐deoxy‐Δ12,14‐PGJ2) and activation of PPARγ (Feldon et al., 2006). In subsequent studies, PPARγ was confirmed as crucial for the regulation of PVAT mass (Chang et al., 2012), since its deletion from PVAT precursor cells triggered a complete loss of PVAT, impaired thermoregulation and endothelial dysfunction (Chang et al., 2012) (Figure 2).
Hypoxia might play a role in PVAT dysfunction (Bays, 2009). In rat mesenteric artery segments exposed to hypoxia, the anti‐contractile effect of PVAT was substantially attenuated (Greenstein et al., 2009). In fact, inflammation seems to precede hypoxia, and the consequent alteration of the secretory profile might be worse in PVAT compared with other depots (Chatterjee et al., 2009). After a period of hypoxia, incubation of rat mesenteric arteries with anti‐TNF‐α or anti‐IL‐6 antibody restored the anti‐contractile ability lost by overexpression of these inflammatory markers (Greenstein et al., 2009). Although exercise has anti‐inflammatory properties, its role on hypoxia‐induced inflammation and free radical scavenging is still uncertain.
Collectively, these data provide evidence that exercise‐induced improvements on PVAT might be achieved through (1) improvements of PVAT morphology, (2) reduced recruitment of inflammatory cells and (3) enhanced secretion of vasodilators by PVAT.
Effects of exercise on inflammation in PVAT
Inflammation of AT is an important event for the development of vascular dysfunction, destabilization of atherosclerotic plaques and increased formation of pro‐thrombotic products (Deanfield et al., 2007; Libby et al., 2009). As mentioned above, macrophages, especially the M1 subclass, play a role in this process. A wide variety of macrophages have been characterized (Martinez and Gordon, 2014), which can be broadly divided into two subsets: M1‐macrophages with predominantly inflammatory features (Hirata et al., 2011) and M2‐macrophages with a more immunosuppressive profile. Kranendonk and collaborators showed in patients undergoing surgery on their abdominal aorta that aortic PVAT presents a high secretion of inflammatory adipokines, such as CCL2, PAI‐1 and resistin, even compared with visceral AT (Kranendonk et al., 2015). This connection between vascular function and AT inflammation has also been observed in epicardial fat of coronary artery disease (Moncada and Higgs, 1993) patients. In these patients, epicardial AT showed high expression of inflammatory markers such as the M1 macrophage marker CD11c and CD68 and the cytokines IL‐6, TNF‐α and CCL2, compared with subcutaneous AT (Hirata et al., 2011).
Exercise can be considered an anti‐inflammatory treatment (Ruffino et al., 2016). Firstly, exercise decreases body fat and adipocyte hypertrophy. By decreasing AT mass (Speretta et al., 2012), exercise also decreases the number of inflammatory cells contained within the AT (Kawanishi et al., 2013). Secondly, in moderately obese adults, chronic exercise reduces inflammatory markers (i.e. TNF‐α, IL‐6) and leptin in mesenteric AT (Jung et al., 2008; Fritzen et al., 2015). Thirdly, even after a single bout of swimming, exercise was shown to improve insulin action and inflammation in the stromal vascular fraction (SVF) of AT (Oliveira et al., 2013), which are the connective tissue cells of AT. One possible mechanism by which exercise may reduce PVAT inflammation is the down‐regulation of toll‐like receptor 4 (TLR4) expression on monocytes and macrophages (Gleeson et al., 2011). Although not demonstrated in PVAT, this down‐regulation reduces mRNA for TNF‐α, IL‐1β and CCL2 in gonadal AT (Oliveira et al., 2013).
Furthermore, exercise diminishes the number of CD4+ and CD8+ T‐cells in both SVF and splenocytes (Kawanishi et al., 2013). Exercise effects on T cell populations and cytokine secretion may be mediated through increased catecholamine secretion (Alvarenga‐Filho et al., 2016; Kruger et al., 2016). High intensity interval training (Rao et al., 2014) in lean subjects increases the mobilization of the anti‐inflammatory regulatory T cells (Tregs) (Kruger et al., 2016), as treatment of cultured human T‐cells with catecholamine reduced their secretion of inflammatory cytokines (TNF‐α, IL‐6, IL‐17, IL‐21 and IL‐22) (Alvarenga‐Filho et al., 2016). Furthermore, exercise prevents TCD4+ and TCD8+ apoptosis, by increasing telomere length and decreasing annexin V, a marker of apoptosis (Silva et al., 2016). Further investigations are necessary to address how exercise modulates survival and T‐cell subpopulations inside PVAT, as well as their cytokine release.
Exercise has been shown to induce a phenotype switch from M1‐ to M2‐macrophages. In HFD‐fed mice, regardless of effects on body fat, exercise decreases TNF‐α, IL‐6, the adhesion molecules ICAM1 and VCAM1, CD11c and TLR4 expression and increases expression of the M2 marker CD163 (Kawanishi et al., 2010) in gonadal AT. In another study, exercise prevented infiltration of M1‐macrophages and reduced the total macrophage number in the stromal vascular fraction of 16 weeks HFD‐fed mice (Kawanishi et al., 2013). Nevertheless, similar questions need testing in PVAT as well.
Generally, resistance training (RT) changes body composition without alterations in body weight of both lean and obese subjects (Dias et al., 2015). RT changes the vascular system in concert with alterations in body fat in obese/overweight patients. In both obese adolescents and in older men, RT improved endothelium‐dependent vasodilatation after ACh (Dias et al., 2015) and increased femoral artery blood flow, microvascular blood flow and volume of the vastus lateralis muscle (Phillips et al., 2015). However, data on the influence of different exercise characteristics (i.e. intensity and volume) on AT properties such as inflammation are scarce, and this relationship needs to be further elucidated.
Collectively, these data suggest that exercise prevents or attenuates infiltration of immune cells into PVAT, improving insulin sensitivity and vascular function.
Mechanisms of exercise‐dependent control of adiponectin secretion by PVAT
Circulating adiponectin exerts pleiotropic control over genes that regulate plasma HDL and triglyceride levels (Cnop et al., 2003; Tschritter et al., 2003), as plasma adiponectin is positively related to plasma levels of HDL and negatively correlated to those of triglyceride.
Exercise, alone (Bluher et al., 2006) or combined with a dietary intervention, (Esposito et al., 2003) increases adiponectin in obese subjects. Although exercise incorporated in weight loss programs showed the most favourable changes in adiponectin (Hara et al., 2005), there are conflicting data concerning the effects of acute versus chronic exercise protocols (Simpson and Singh, 2008; Pop et al., 2010; Saunders et al., 2012).
Exercise influences on PVAT physiology may be concomitantly mediated by three different tissues: PVAT, SKM and the blood vessels. Adiponectin released by PVAT acts on the vessel wall through up‐regulation of AMP‐activated kinase (AMPK) activity (depending on its α subunit) (Fentz et al., 2015). Resistance arteries obtained from AMPKα2−/− mice presented a blunted effect of PVAT on insulin‐induced vasodilatation (Meijer et al., 2013). Therefore, it is likely that exercise locally improves vascular function by increasing adiponectin secretion by PVAT, which acts directly on the vascular wall. By increasing expression of the intracellular mediators of adiponectin signalling (α1 and α2), exercise also increases NO bioavailability, ROS scavenging, reduces adhesion molecules and attenuates endothelial activation (Cao et al., 2012; Kroller‐Schon et al., 2012). Adiponectin also acts indirectly on the vascular endothelium, protecting it against inflammation by inhibiting lipid accumulation in macrophages (Ouchi et al., 2001), promoting macrophage M2‐polarization and attenuating TLR4‐mediated activation of endothelial cells (Ohashi et al., 2010). Due to the heterogeneity of exercise regarding frequency, intensity and effects on body composition, a conclusion on how adiponectin is affected by exercise requires further investigation.
Exercise effects on metabolism of AT
In general, there are three types of AT: WAT (Huang et al., 2007), which stores fat and produces adipokines; brown adipose tissue (BAT) (Abate and Garg, 1995) that dissipates energy through heat, a function mediated by the uncoupling protein 1 (UCP‐1); and ‘beige’ AT which, like BAT, has thermogenic functions and expresses UCP‐1, albeit to a lesser extent. In humans, a higher number of brown/beige adipocytes are related to a metabolically healthy phenotype (Min et al., 2016).
In obesity, infiltration of macrophages into visceral AT is related to a reduction of the thermogenic capability of BAT (i.e. lower UCP‐1 expression in BAT) (Xu et al., 2011). Within the PVAT, loss of thermogenic activity decreased its anti‐atherogenic properties and endothelial effects (Chang et al., 2012). Notably, implantation of beige adipocytes in HFD‐fed mice improved glucose tolerance, reduced liver steatosis and normalized adiponectin levels (Min et al., 2016). Furthermore, angiogenesis‐targeted nanoparticles (atNP) prevented obesity in mice by promoting the transformation from white to beige adipocytes (Xue et al., 2016), suggesting that previous angiogenesis is required for the ‘beigeing’ processes (De Matteis et al., 2013; Frontini et al., 2013; Disanzo and You, 2014). Based on this information, atNPs could be used to target dysfunctional PVAT.
Nevertheless the process of browning or beigeing must be accompanied by vascularization, which is required to fulfil the increased oxygen demand of beige AT (Tran et al., 2012). These events will involve the up‐regulation of browning‐associated genes (i.e. Ucp‐1) and organelles (i.e. mitochondrial biogenesis). However, it is not fully clear whether exercise interacts with adipocytes through the vasculature, with the vasculature through PVAT, or with both.
Subsequently and concomitantly to the vascularization and perfusion of AT, there is the contribution of the CNS. BAT is more densely innervated than WAT (Lee and Tontonoz, 2014). Therefore, sympathetic activity and perfusion will be two contributors of thermogenesis in BAT (Morrison and Nakamura, 2011). In WAT, myokines and activation of natriuretic peptide receptors (NPR) mediate AT beigeing (Morrison and Nakamura, 2011). Interestingly, it has been shown that PVAT is not only autonomically innervated but also expresses β1, β2 and β3 adrenoceptors (Bulloch and Daly, 2014). Recently, electrical stimulation of murine mesenteric arteries showed functional effects of the sympathetic nervous system (SNS) activity on anti‐contractile properties of PVAT. SNS determines anti‐contractile properties of PVAT by triggering the release of vasodilators upon adrenergic stimulation (Saxton et al., 2016). Moreover, exercise increases the production of NO, which synergistically interacts with adrenaline to trigger the expression of NPR and β‐adrenoceptors. Thermogenesis will be stimulated with increased expression of BAT‐related genes such as Ucp‐1. By increasing BAT‐sites inside WAT, and enhancing intrinsic thermogenesis of BAT, obesity development is prevented, as lipid and carbohydrate metabolism is modulated in HFD‐mice (Zed and James, 1986; Wijers et al., 2011).
In addition to direct SNS effects, exercise‐induced AT browning may occur through autonomic stimulation of the release of browning‐related myokines, such as irisin and FGF‐21, which will be discussed below. Both chronic voluntary wheel exercise (Jenkins et al., 2013) and swimming (Sutherland et al., 2009), in rats, increase expression of NPRs, β2 and β3 adrenoceptors and mitochondrial genes, such as those for PPARγ co‐activator 1 α (PGC1‐α) and citrate synthase (mRNA and protein) in WAT. Nevertheless, conflicting data have been obtained in gonadal and retroperitoneal fat, suggesting different responses to exercise stimuli in different fat depots (Sutherland et al., 2009; Jenkins et al., 2013). For instance, chronic exercise increased Ucp‐1 gene expression in abdominal subcutaneous AT, but not in retroperitoneal and gonadal fat pads (Wu et al., 2014). In a study on the full genome DNA of thoracic aortic PVAT and BAT, the first had a brown‐like phenotype, as expression of uniquely BAT genes was identical in BAT (Fitzgibbons et al., 2011). This phenotype provided protection against inflammation, which was lower after HFD.
Aerobic exercise (1 or 6 weeks) in Sprague Dawley rats increased sympathetic noradrenergic tone, a more uniform distribution of lipid droplets and a more intense labelling of UCP‐1 in brown adipocytes, regardless of the duration of exercise (De Matteis et al., 2013). Interestingly, exercise increased sympathetic tone in BAT in a time‐dependent fashion, as 6 week exercised rats showed more tyrosine hydroxylase‐positive fibres and capillaries per adipocyte and a lower average size of lipid droplets compared with 1 week exercised rats.
Another exercise‐related stimulus of adipocyte browning is the protein, PR domain‐containing 16 (PRDM16). PRDM16 acts as a transcriptional cofactor, which is present in SKM and in both white and BATs (Seale et al., 2011). It controls a bidirectional cell fate switch between skeletal myoblasts and brown adipocytes (Seale et al., 2008) and participates in brown adipogenesis by binding and activating PPARγ. This feature was proven in PRDM16 knockout mice, which displayed not only an abnormal brown fat tissue morphology but also completely ablated gene expression of several BAT‐specific genes (i.e. Ucp‐1, Cidea and Cig30) (Seale et al., 2008). PRDM16 is up‐regulated by exercise and becomes more expressed during the transformation from white to brown‐like adipocytes in the mesentery (Xu et al., 2011). Nonetheless, further investigations that elucidate the role of PRDM16 in exercise‐induced browning are important.
Local control of exercise on AT: myokines
As mentioned in the previous section, exercise alters AT physiology centrally through the CNS and locally through myokine secretion by muscles. Myokines are bioactive molecules released by contracting muscles, which mediate exercise‐induced metabolic alterations in organs such as the AT (Pedersen et al., 2007). Here, we will discuss some important myokines, such as irisin, meteorin‐like (metrnl), FGF21, IL‐15 and the classical myokine IL‐6 to discuss their effects on AT physiology. The summarized findings of myokines studies on AT physiology discussed here, are shown in Table 1.
Table 1.
Summary of myokine studies: effects of exercise training
| Study | Subjects/animals | Intervention | Tissue examined | Main findings |
|---|---|---|---|---|
| Irisin | ||||
| Bostrom et al., 1995 | Muscle creatine kinase promoter (MCK)‐PGC1‐α transgenic and wild type BALB/c mice | 3 weeks of swimming | – | Increase of brown‐like genes on the WAT |
| Epididymal fat | Twofold increase in Ucp1 mRNA | |||
| Subcutaneous inguinal | 65‐fold increase in Ucp1 mRNA | |||
| 3 weeks of wheel running | Visceral, epididymal | Twofold increase in Ucp1 mRNA | ||
| Subcutaneous inguinal | 25‐fold increase in Ucp1 mRNA | |||
| Brown adipose tissue | Increase whole‐body energy expenditure | |||
| Non‐diabetic men | 10 weeks of endurance training at ~65% of VO2max | Muscle biopsies |
 FNDC5, VEGFB and TIMP4, LRG1 and mRNAs
FNDC5, VEGFB and TIMP4, LRG1 and mRNAs |
|
| Blood |
 Circulating Irisin Circulating Irisin |
|||
| BALB/c mice, primary adipocytes | Incubation with FNDC5 | Cell culture | 7 to 500‐fold increase of Ucp1; threefold in PPARγ mRNA | |
| Kim et al., 2013 | Lean and overweight/obese adults | 8 weeks of resistance training at 65–80% of 1RM | Blood |
 ~17.5% irisin plasma levels ~17.5% irisin plasma levels |
| 8 weeks of endurance training at 65–80% of HRmax |
 body fat and BMI body fat and BMI |
|||
| Huh et al., 2005 | Young males(± 20 years) | 8 weeks or 30 min of endurance training | Blood |
 ~18% circulating irisin ~18% circulating irisin |
| Metrnl | ||||
| Rao et al., 2010 | Young men | RT at 80% 1RM + 30 min of cycling at 70% VO2peak | Vastus lateralis muscle biopsies |
 metrnl up to 4 h post exercise. mRNA peak expression was at 1 h
metrnl up to 4 h post exercise. mRNA peak expression was at 1 h |
| Wild‐type C57/BL6J, BALB/cJ Myo‐PGC‐1α4, ΔdbGATA mice, metrnl KO mice | Subcutaneous fat; epididymal fat; |
 M2‐macrophages gene markers; M2‐macrophages gene markers;  inflammation genes in the AT (TNF‐α, IFN‐γ and IL‐1β) inflammation genes in the AT (TNF‐α, IFN‐γ and IL‐1β) |
||
| 60 min of downhill running exercise at 15 m·min−1 |
 Thermogenesis; mitochondrial gene programme; UCP‐1 ( Thermogenesis; mitochondrial gene programme; UCP‐1 ( ~3.5), DIO2, PGC‐1α, ERR‐α mRNA ~3.5), DIO2, PGC‐1α, ERR‐α mRNA |
|||
| Brown adipose tissue |
 metrnl mRNA and thermogenic gene programme;
metrnl mRNA and thermogenic gene programme;  UCP‐1 mRNA in BAT
UCP‐1 mRNA in BAT |
|||
| Quadriceps and triceps muscle | ~fourfold increase of metrnl mRNA | |||
| Blood | ~twofold increase in circulating metrnl | |||
| Adenovirus injection to deliver full‐length metrnl to the liver | Liver and blood | ~20‐fold increase in liver mRNA and ~five to sixfold in plasma | ||
Culture of myotubes  PGC‐1α4 expression PGC‐1α4 expression |
~eightfold increase of metrnl mRNA | |||
| Fibroblast growth factor 21 | ||||
| Cuevas‐Ramos et al., 2009 | Sedentary young women | 2 weeks of endurance exercise at 85% of HR | Blood |
 ~66% circulating levels of FGF‐21; ~66% circulating levels of FGF‐21;  ~25% triglycerides; ~25% triglycerides;  Epinephrine; Epinephrine;  FFA (~50%) FFA (~50%) |
| Tanimura et al., 2015 | Male ICR mice | 60 min of endurance exercise at 10–30 m·min−1 | Liver |
 mRNA and protein levels of FGF‐21 mRNA and protein levels of FGF‐21 |
| Blood |
 Serum FGF‐21 Serum FGF‐21 |
|||
| Gastrocnemius muscle |
 mRNA and protein of FGF‐21; mRNA and protein of FGF‐21;  p‐Akt/Akt protein p‐Akt/Akt protein |
|||
| Young sedentary men | Single bout for 60 min at 75% of VO2max | Blood |
 Serum FGF‐21, NEFA, 3‐hydroxybutyric acid Serum FGF‐21, NEFA, 3‐hydroxybutyric acid |
|
| Kim et al., 2015 | Male C57/BL6 mice | 30 min of endurance exercise at 25 m·min−1 | Blood |
 FGF‐21 serum levels FGF‐21 serum levels |
| 30 min of endurance exercise at 50 or 80% of VO2max | Liver |
 FGF‐21, PPARα, ATF4 mRNA
FGF‐21, PPARα, ATF4 mRNA |
||
| Male adults | Blood |
 FGF‐21 serum levels only 1 h after end of bout FGF‐21 serum levels only 1 h after end of bout |
||
| Slusher et al., 2016 | Obese and lean adults | 30 min of endurance training at 75% of VO2max | Blood |
 FGF‐21 in lean subjects regardles the time and in the obese only after 1 h post bout FGF‐21 in lean subjects regardles the time and in the obese only after 1 h post bout |
| Increased circulatory FGF‐21 correlates to total relative energy expenditure | ||||
| Hansen et al., 2016 | Young lean and type 2 diabetic men | 2 h of endurance exercise at 60% of VO2max + Patients that underwent through pancreatic clamp | Blood |
 Glucagon/Insulin ratio; Glucagon/Insulin ratio;  Plasma FGF‐21 in controls; Plasma FGF‐21 in controls; |
| In type 2 diabetic patients and Pancreatic clamp FGF‐21 is abolished in plasma | ||||
| IL‐15 | ||||
| Kim et al., 2015 | Male Zucker diabetic fatty rats | 12 weeks – 60 min of endurance training at 15–20 m·min−1 | Soleus and gastrocnemius muscles |
 IL‐15 protein levels in S. muscle IL‐15 protein levels in S. muscle |
| Bazgir et al., 2015e | Young male non‐ and athletes | Two bouts of RT, one focusing on CON at 70–80% 1RM and the second on ECC at 90–100% 1RM ‐with 8 to 10 repetitions | Blood |
 IL‐15 serum levels IL‐15 serum levels |
 hs‐CRP levels in athletes hs‐CRP levels in athletes | ||||
 hs‐CRP and TNF‐α levels in non‐athletes hs‐CRP and TNF‐α levels in non‐athletes | ||||
| Quinn et al., 2015 | IL‐15‐KO and IL‐15Rα KO mice | Endurance training at 15‐17 m·min−1 until exhaustion | Gastrocnemius muscle |
 Endurance capacity in KO mice; Endurance capacity in KO mice; MHC mRNA MHC mRNA |
 IL‐15 mRNA in G. muscle;
IL‐15 mRNA in G. muscle;  Serum IL‐15 in KO mice Serum IL‐15 in KO mice | ||||
 PPARδ and SIRT1 mRNA and protein after IL‐15 injection and 3 h post bout
PPARδ and SIRT1 mRNA and protein after IL‐15 injection and 3 h post bout | ||||
| Acute injection of IL‐15 | Blood |
 IL‐15Rα in Control and KO‐mice IL‐15Rα in Control and KO‐mice |
||
 IL‐15 circulating levels in IL‐15Rα‐KO mice IL‐15 circulating levels in IL‐15Rα‐KO mice | ||||
 PGC‐1α and 1β in SKM of IL‐15KO PGC‐1α and 1β in SKM of IL‐15KO | ||||
 IL15Rα after an acute bout of exercise IL15Rα after an acute bout of exercise | ||||
| Pistilli et al., 2008 | IL‐15Rα KO mice | 14 h data from Voluntary wheel exercise | EDL, gastrocnemius and quadriceps muscle |
 Ambulatory activity, fatigue resistance and wheel revolutions Ambulatory activity, fatigue resistance and wheel revolutions |
 IL‐15 mRNA in muscles of IL‐15Rα‐KO mice
IL‐15 mRNA in muscles of IL‐15Rα‐KO mice IL‐15, Ppard, Pgc‐1α, citrate synthase, SERCAII and
IL‐15, Ppard, Pgc‐1α, citrate synthase, SERCAII and  calsequestrin mRNA in EDL and gastrocnemius of IL‐15Rα‐KO mice
calsequestrin mRNA in EDL and gastrocnemius of IL‐15Rα‐KO mice | ||||
| IL‐6 | ||||
| McGinnis et al., 2015 | C57/Bl6 or IL‐6−/− mice | 3 days of 60 min of treadmill exercise at 18 m·min−1 prior I/R surgery procedure | Serum |
 ~4.5‐fold in IL‐6 and sIL‐6R serum concentration after 30 min exercise in controls; ~4.5‐fold in IL‐6 and sIL‐6R serum concentration after 30 min exercise in controls;  IL‐6R in G. muscle and heart after exercise in C57/Bl6 mice IL‐6R in G. muscle and heart after exercise in C57/Bl6 mice |
| Gastrocnemius muscle |
 COX‐2 mRNA and protein post exercise; iNOS and p‐STAT protein before and after exercise in IL‐6−/− in G. muscle
COX‐2 mRNA and protein post exercise; iNOS and p‐STAT protein before and after exercise in IL‐6−/− in G. muscle |
|||
 of ~65% of necrotic area, ECG score protection against arrhythmias in C57/Bl6 of ~65% of necrotic area, ECG score protection against arrhythmias in C57/Bl6 | ||||
| Heart |
 p‐p44/42 MAPK and p‐p38 MAPK proteins in the myocardium after the exercise in C57/Bl6 mice p‐p44/42 MAPK and p‐p38 MAPK proteins in the myocardium after the exercise in C57/Bl6 mice |
|||
Browning myokines
Irisin and meteorin‐like (metrnl)
Irisin is a hormone secreted by SKM that has been proposed as a novel preventive and therapeutic target for obesity and type 2 diabetes. It has been linked to browning and increased thermogenesis of AT (Bostrom et al., 2012), being induced by PGC1‐α (Kim et al., 2016). However, inconsistent data concerning serum concentrations of irisin in human studies and incompatibility with rodent data are required to elucidate its physiological role (Albrecht et al., 2015). First, the starting codon from the human gene encoding irisin, FNDC5 (ATG to ATA), is different between humans and rodents. As there may be transcription of a different protein, there are doubts concerning the translational value of rodent evidence to human physiology (Bostrom et al., 2012). Both in vitro and in vivo data from mice have shown that irisin increases UCP‐1. Secondly, effects of exercise on irisin levels are quite variable. Acute exercise interventions normally increase the levels of irisin, whereas the effects of chronic exercise vary between neutral and increased irisin (Albrecht et al., 2015; Kim et al., 2016). Interestingly, irisin levels are negatively related to key muscle metabolites (i.e. ATP) after a chronic exercise, suggesting that altered muscle metabolism triggers irisin secretion (Huh et al., 2012).
Initially irisin was proposed to enhance metabolism. However, serum levels of irisin were negatively associated with adiponectin in subjects with metabolic syndrome, while being positively related to body mass index, blood pressure and fasting glucose (Park et al., 2013). These results reinforce the inconsistency between human and rodent data.
Collectively, the data show that irisin increases after exercise, is linked to browning of AT, increases energy expenditure (Bostrom et al., 2012) and is associated with improvements of vascular function and insulin sensitivity in mice (Jiang et al., 2015; Lu et al., 2015). Nonetheless, clarification of the regulatory mechanisms of irisin levels and its physiological consequences in obese and lean human subjects are questions to future interventional studies.
Metrnl is a novel myokine, with features similar to those of irisin. Although studies on effects of exercise on metrnl are scarce, resistance exercise and cold stimuli are likely to up‐regulate metrnl (Lee and Tontonoz, 2014). Metrnl can incite the browning process in adipocytes but it may act indirectly through enhancing the number of eosinophils in AT. Recent reports showed that overexpression of metrnl is associated with eosinophil accumulation in the AT (Lee and Tontonoz, 2014), and observations in mice suggest that these cells are required for metrnl‐mediated browning (Rao et al., 2014). Metrnl induces the overexpression and secretion of type II cytokines IL‐4 and IL‐13 by eosinophils (Qiu et al., 2014), which in turn activate the cascade of alternative‐activated macrophages (M2‐), responsible for the secretion of catecholamine that promotes browning (Rao et al., 2014). Nevertheless, further studies in humans and mice are required to establish metrnl as an AT ‘browning’ myokine.
Other myokines
FGF21
Fibroblast growth factor 21 (FGF21) is mainly produced by the liver (Nishimura et al., 2000) and acts as a nutrient stress sensor (Zhang et al., 2015) that regulates the metabolism of carbohydrates and lipids. Changes in the level of FGF21 are related to insulin resistance, glucose intolerance and dyslipidemia (Coskun et al., 2008). Muscle production of FGF21 is regulated by insulin via the PI3kinase‐Akt pathway (Izumiya et al., 2008). In young subjects, hyperinsulinaemia increases both plasma FGF21 and its mRNA in SKM (Hojman et al., 2009). FGF21 acts through the βKlotho co‐receptor (βKR), which is strongly expressed in AT, but not in SKM (Ogawa et al., 2007). Overexpression of βKR in the AT protected mice against diet‐induced obesity and preserved insulin sensitivity (Samms et al., 2016). Consistent with a role in exercise effects, FGF21 treatment prevented weight gain, enhanced fat excretion and energy expenditure, thus decreasing insulin levels and fat mass, all in a dose‐dependent fashion in db/db and obese mice (Coskun et al., 2008).
On the other hand, AT‐derived FGF21 might be implicated in CVD, as it was positively associated with cardiometabolic risk factors, increased intima‐media thickness on both carotid and iliac arteries, high levels of C‐reactive protein, dysglycaemia, dyslipidaemia and decreased adiponectin levels (Chow et al., 2013; Xiao et al., 2015). These apparently conflicting findings on different tissue sources of FGF‐21 indicate the existence of tissue‐specific physiological functions of FGF21, which require further investigation.
Acute exercise is the most used intervention for raising FGF21 and indeed increases serum FGF21 in both healthy humans and mice (Cuevas‐Ramos et al., 2012), in addition to increased expression in SKM and liver (Kim et al., 2013b; Tanimura et al., 2016). Nevertheless, acute exercise does not increase FGF21 levels in either obese subjects (Slusher et al., 2015) or patients with type 2 diabetic (Hansen et al., 2016). Therefore, chronic studies are necessary to elucidate whether FGF21 expression and circulatory levels might be up‐regulated in obese and diabetic subjects.
Similar to the myokines mentioned above, FGF21 enhances the thermogenic capacity of the AT by switching on a brown‐fat gene programme in WAT (Lee et al., 2013). In BAT‐positive men, incubation of primary adipocytes from the neck with FGF21, increased BAT markers (i.e. UCP1 protein), oxygen consumption and infrared thermogenic images in a dose‐dependent manner (Lee et al., 2014). Moreover, BAT was an important source of FGF21 in men. Intriguingly, these effects were more robust during co‐incubation with irisin, suggesting a synergetic action of FGF21 and irisin on WAT browning. Additionally, the correlation between serum FGF21 and sympathetic nervous system activity supports a role of this protein as an exercise‐activated enhancer of whole‐body thermogenesis (Cuevas‐Ramos et al., 2012; Lee et al., 2013).
Together, these findings demonstrate FGF21 is a potential druggable target for obesity and diabetes. However, incomplete knowledge of its intracellular signalling pathways and tissue‐specific effects require studies to discriminate FGF21 actions on AT, muscle and liver.
IL‐15
IL‐15 is a cytokine that supports survival and proliferation of T lymphocytes (Grabstein et al., 1994). The plasma membrane receptor for IL‐15 is a trimer and this receptor is distributed throughout the body. It is composed of IL‐2 receptor β (IL‐2Rβ), a common γ chain (γc), and a specific IL‐15Rα chain (Giri et al., 1995). Interestingly, IL‐15 is found in abundance in SKM, but at very low levels in the AT (Pistilli et al., 2011). Additionally, it has been suggested that the α subunit of IL‐15 receptor (IL‐15Rα) regulates IL‐15 release from SKM (O'Connell and Pistilli, 2015). Paradoxically, lack of the α‐receptor (IL‐15Rα−/− mice) increased IL‐15 serum levels and at the same time driving a change in SKM to an oxidative phenotype (Pistilli et al., 2011; Quinn et al., 2014). These mice show increased activity and exercise endurance, accompanied by molecular (i.e. increased mRNA from Pgc‐1α and citrate synthase) and morphological signs (i.e. increased fibre number and single‐fibre area) of a beneficial change in muscle phenotype.
Although IL‐15 is considered a pro‐inflammatory cytokine, the fact that its circulating levels are negatively related to obesity, WAT mass and type 2 diabetes is also consistent with a role as a myokine (Quinn et al., 2009). Moreover, both endurance training in Zucker rats (Kim et al., 2013a) and RT in controls and athletes increased circulating IL‐15 levels (Bazgir et al., 2015). In both cases, exercise was negatively related to body fat and inflammatory markers.
Nevertheless, data from knockout models show the necessity of further studies concerning the differential action of IL‐15 on AT. For instance, IL‐15Rα−/− mice display an obesity‐resistant phenotype, with less body and liver fat accumulation (Loro et al., 2015). It seems that these effects of IL‐15 result from increased whole‐body energy metabolism, as IL‐15Rα−/− mice display increased oxygen consumption and utilization of muscle fatty acids (He et al., 2010). These data were corroborated by findings that IL‐15−/− mice presented decreased lipid accumulation in both white and BAT depots (Lacraz et al., 2016).
The effects of IL‐15 on vascular health are not known at present, but in vitro data showed that IL‐15 treatment of 3T3‐L1 pre‐adipocytes increased adiponectin production (Quinn et al., 2005), an important contributor to the anti‐contractile properties of PVAT (as already discussed). On the other hand, intravenous injections of IL‐15 provoked vasoconstriction in larger order arterioles (A1 and A2) in the microvascular bed of the cremaster muscle (Baker and Abel, 1995).
In conclusion, IL‐15 seems promising as a therapeutic approach not only to chronic diseases, such as obesity and type 2 diabetes, but also to improvements of muscle function. However, further investigations on metabolic and molecular changes linked to the expression of the IL15 receptor α are necessary.
IL‐6
In most physiological circumstances, IL‐6 is a pro‐inflammatory adipokine, which is abundantly secreted by PVAT, compared with retroperitoneal and subcutaneous fat (Gonzalez et al., 2016). IL‐6 is a mediator of inflammation and therefore related to AT dysfunction (Bays et al., 2009). Circulating IL‐6 levels are related to vascular dysfunction in men (Esposito et al., 2003), and IL‐6 is primarily viewed as an indirect mediator of vascular inflammation (Yudkin et al., 2000). IL‐6 levels are positively related to other inflammatory factors such as C‐reactive protein (Yudkin et al., 2000) and TNF‐α, which in turn are involved in endothelial dysfunction. IL‐6 derived from aortic PVAT is related to arterial stiffness in mice (Du et al., 2015). Nevertheless, there is no solid evidence at present pointing to IL‐6 itself as a major cause of vascular injury.
On the other hand, Pedersen and collaborators have shown an endocrine role of IL‐6 in active muscles, as part of the group of myokines (Pedersen et al., 2007). Circulating levels of IL‐6 are among the most markedly increased myokines during exercise (Pedersen and Febbraio, 2008), and muscle‐derived IL‐6 increased insulin sensitivity, metabolism and triggered muscle hypertrophy (Pedersen and Febbraio, 2008). In muscle‐specific IL‐6 knockout mice, there was decreased GLUT4 protein content in WAT and more inguinal WAT after HFD (Knudsen et al., 2015). These data strongly indicate that SKM IL‐6 exerts positive effects on WAT metabolism (Knudsen et al., 2015). Further investigations are crucial to differentiate between physiological effects of IL‐6 derived from AT and that derived from muscle.
Future perspectives and conclusions
In the context of worldwide obesity, there is strong evidence that the properties of AT determine vascular health. Specifically, PVAT produces vasoactive substances that can either impair or improve vascular function. In obesity, AT homeostasis is greatly changed, and exercise has been shown to normalize this process. Despite a number of unanswered questions, muscle‐derived hormones might be one group of substances holding great promise for future therapy. Therefore, goals for future research are first to perform careful analysis to separate effects mediated by muscle‐ and AT‐derived hormones, secondly, to further characterize specific myokine effects on human physiology and, finally, to clarify the effects of exercise on different AT depots.
Conflict of interest
The authors declare no conflicts of interest.
Boa, B. C. S. , Yudkin, J. S. , van Hinsbergh, V. W. M. , Bouskela, E. , and Eringa, E. C. (2017) Exercise effects on perivascular adipose tissue: endocrine and paracrine determinants of vascular function. British Journal of Pharmacology, 174: 3466–3481. doi: 10.1111/bph.13732.
References
- Abate N, Garg A (1995). Heterogeneity in adipose tissue metabolism: causes, implications and management of regional adiposity. Prog Lipid Res 34: 53–70. [DOI] [PubMed] [Google Scholar]
- Albrecht E, Norheim F, Thiede B, Holen T, Ohashi T, Schering L et al. (2015). Irisin – a myth rather than an exercise‐inducible myokine. Sci Rep 5: 8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015e). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allender S, Foster C, Scarborough P, Rayner M (2007). The burden of physical activity‐related ill health in the UK. J Epidemiol Community Health 61: 344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarenga‐Filho H, Sacramento PM, Ferreira TB, Hygino J, Abreu JE, Carvalho SR et al. (2016). Combined exercise training reduces fatigue and modulates the cytokine profile of T‐cells from multiple sclerosis patients in response to neuromediators. J Neuroimmunol 293: 91–99. [DOI] [PubMed] [Google Scholar]
- Baker CH, Abel FL (1995). Macro‐ and microcirculatory effects of IL‐15. Shock 4: 307–310. [DOI] [PubMed] [Google Scholar]
- Bays HE (2009). ‘Sick fat’, metabolic disease, and atherosclerosis. Am J Med 122: S26–S37. [DOI] [PubMed] [Google Scholar]
- Bazgir B, Salesi M, Koushki M, Amirghofran Z (2015). Effects of eccentric and concentric emphasized resistance exercise on IL‐15 serum levels and its relation to inflammatory markers in athletes and non‐athletes. Asian J Sports Med 6: e27980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigornia SJ, Farb MG, Mott MM, Hess DT, Carmine B, Fiscale A et al. (2012). Relation of depot‐specific adipose inflammation to insulin resistance in human obesity. Nutr Diabetes 2: e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluher M, Bullen JW Jr, Lee JH, Kralisch S, Fasshauer M, Kloting N et al. (2006). Circulating adiponectin and expression of adiponectin receptors in human skeletal muscle: associations with metabolic parameters and insulin resistance and regulation by physical training. J Clin Endocrinol Metab 91: 2310–2316. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC et al. (2012). A PGC1‐alpha‐dependent myokine that drives brown‐fat‐like development of white fat and thermogenesis. Nature 481: 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulloch JM, Daly CJ (2014). Autonomic nerves and perivascular fat: interactive mechanisms. Pharmacol Ther 143: 61–73. [DOI] [PubMed] [Google Scholar]
- Cao S, Li B, Yi X, Chang B, Zhu B, Lian Z et al. (2012). Effects of exercise on AMPK signaling and downstream components to PI3K in rat with type 2 diabetes. PLoS One 7: e51709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SA, Fulton JE, Pratt M, Yang Z, Adams EK (2015). Inadequate physical activity and health care expenditures in the United States. Prog Cardiovasc Dis 57: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C et al. (2012). Loss of perivascular adipose tissue on peroxisome proliferator‐activated receptor‐gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation 126: 1067–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G et al. (2009). Proinflammatory phenotype of perivascular adipocytes: influence of high‐fat feeding. Circ Res 104: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow WS, Xu A, Woo YC, Tso AW, Cheung SC, Fong CH et al. (2013). Serum fibroblast growth factor‐21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arterioscler Thromb Vasc Biol 33: 2454–2459. [DOI] [PubMed] [Google Scholar]
- Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ et al. (2003). Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 46: 459–469. [DOI] [PubMed] [Google Scholar]
- Coles CA (2016). Adipokines in healthy skeletal muscle and metabolic disease. Adv Exp Med Biol 900: 133–160. [DOI] [PubMed] [Google Scholar]
- Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y et al. (2008). Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149: 6018–6027. [DOI] [PubMed] [Google Scholar]
- Cuevas‐Ramos D, Almeda‐Valdes P, Meza‐Arana C, Brito‐Cordova G, Gomez‐Perez F, Mehta R et al. (2012). Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One 7: e38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daquinag AC, Zhang Y, Kolonin MG (2011). Vascular targeting of adipose tissue as an anti‐obesity approach. Trends Pharmacol Sci 32: 300–307. [DOI] [PubMed] [Google Scholar]
- Date H, Imamura T, Ideguchi T, Kawagoe J, Sumi T, Masuyama H et al. (2006). Adiponectin produced in coronary circulation regulates coronary flow reserve in nondiabetic patients with angiographically normal coronary arteries. Clin Cardiol 29: 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer MP, Meijer RI, Richter EA, Van Nieuw Amerongen GP, Sipkema P, Van Poelgeest EM et al. (2016). Globular adiponectin controls insulin‐mediated vasoreactivity in muscle through AMPKalpha2. Vascul Pharmacol 78: 24–35. [DOI] [PubMed] [Google Scholar]
- De Matteis R, Lucertini F, Guescini M, Polidori E, Zeppa S, Stocchi V et al. (2013). Exercise as a new physiological stimulus for brown adipose tissue activity. Nutr Metab Cardiovasc Dis 23: 582–590. [DOI] [PubMed] [Google Scholar]
- Deanfield JE, Halcox JP, Rabelink TJ (2007). Endothelial function and dysfunction: testing and clinical relevance. Circulation 115: 1285–1295. [DOI] [PubMed] [Google Scholar]
- Dias I, Farinatti P, De Souza MG, Manhanini DP, Balthazar E, Dantas DL et al. (2015). Effects of resistance training on obese adolescents. Med Sci Sports Exerc 47: 2636–2644. [DOI] [PubMed] [Google Scholar]
- Disanzo BL, You T (2014). Effects of exercise training on indicators of adipose tissue angiogenesis and hypoxia in obese rats. Metabolism 63: 452–455. [DOI] [PubMed] [Google Scholar]
- Du B, Ouyang A, Eng JS, Fleenor BS (2015). Aortic perivascular adipose‐derived interleukin‐6 contributes to arterial stiffness in low‐density lipoprotein receptor deficient mice. Am J Physiol Heart Circ Physiol 308: H1382–H1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Jakicic JM, Ard JD, De Jesus JM, Houston Miller N, Hubbard VS et al. (2014). 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 63: 2960–2984. [DOI] [PubMed] [Google Scholar]
- Eringa EC, Bakker W, Smulders YM, Serne EH, Yudkin JS, Stehouwer CD (2007). Regulation of vascular function and insulin sensitivity by adipose tissue: focus on perivascular adipose tissue. Microcirculation 14: 389–402. [DOI] [PubMed] [Google Scholar]
- Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R et al. (2003). Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA 289: 1799–1804. [DOI] [PubMed] [Google Scholar]
- Feldon SE, O'loughlin CW, Ray DM, Landskroner‐Eiger S, Seweryniak KE, Phipps RP (2006). Activated human T lymphocytes express cyclooxygenase‐2 and produce proadipogenic prostaglandins that drive human orbital fibroblast differentiation to adipocytes. Am J Pathol 169: 1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentz J, Kjobsted R, Kristensen CM, Hingst JR, Birk JB, Gudiksen A et al. (2015). AMPKalpha is essential for acute exercise‐induced gene responses but not for exercise training‐induced adaptations in mouse skeletal muscle. Am J Physiol Endocrinol Metab 309: E900–E914. [DOI] [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A et al. (2009). Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 15: 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP (2011). Similarity of mouse perivascular and brown adipose tissues and their resistance to diet‐induced inflammation. Am J Physiol Heart Circ Physiol 301: H1425–H1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzen AM, Lundsgaard AM, Jordy AB, Poulsen SK, Stender S, Pilegaard H et al. (2015). New nordic diet‐induced weight loss is accompanied by changes in metabolism and AMPK signaling in adipose tissue. J Clin Endocrinol Metab 100: 3509–3519. [DOI] [PubMed] [Google Scholar]
- Frontini A, Vitali A, Perugini J, Murano I, Romiti C, Ricquier D et al. (2013). White‐to‐brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochim Biophys Acta 1831: 950–959. [DOI] [PubMed] [Google Scholar]
- Fuster JJ, Ouchi N, Gokce N, Walsh K (2016). Obesity‐induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res 118: 1786–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Lu C, Su LY, Sharma AM, Lee RM (2007). Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol 151: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K et al. (1995). Identification and cloning of a novel IL‐15 binding protein that is structurally related to the alpha chain of the IL‐2 receptor. EMBO J 14: 3654–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA (2011). The anti‐inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 11: 607–615. [DOI] [PubMed] [Google Scholar]
- Gonzalez AM, Hoffman JR, Townsend JR, Jajtner AR, Boone CH, Beyer KS et al. (2016). Intramuscular MAPK signaling following high volume and high intensity resistance exercise protocols in trained men. Eur J Appl Physiol 116: 1663–1670. [DOI] [PubMed] [Google Scholar]
- Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V et al. (1994). Cloning of a T cell growth factor that interacts with the beta chain of the interleukin‐2 receptor. Science 264: 965–968. [DOI] [PubMed] [Google Scholar]
- Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M et al. (2009). Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119: 1661–1670. [DOI] [PubMed] [Google Scholar]
- Haczeyni F, Barn V, Mridha AR, Yeh MM, Estevez E, Febbraio MA et al. (2015). Exercise improves adipose function and inflammation and ameliorates fatty liver disease in obese diabetic mice. Obesity (Silver Spring) 23: 1845–1855. [DOI] [PubMed] [Google Scholar]
- Hamburg NM, Mcmackin CJ, Huang AL, Shenouda SM, Widlansky ME, Schulz E et al. (2007). Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol 27: 2650–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JS, Pedersen BK, Xu G, Lehmann R, Weigert C, Plomgaard P (2016). Exercise‐induced secretion of FGF21 and follistatin are blocked by pancreatic clamp and impaired in type 2 diabetes. J Clin Endocrinol Metab 101: 2816–2825. [DOI] [PubMed] [Google Scholar]
- Hara T, Fujiwara H, Nakao H, Mimura T, Yoshikawa T, Fujimoto S (2005). Body composition is related to increase in plasma adiponectin levels rather than training in young obese men. Eur J Appl Physiol 94: 520–526. [DOI] [PubMed] [Google Scholar]
- He Y, Wu X, Khan RS, Kastin AJ, Cornelissen‐Guillaume GG, Hsuchou H et al. (2010). IL‐15 receptor deletion results in circadian changes of locomotor and metabolic activity. J Mol Neurosci 41: 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y, Tabata M, Kurobe H, Motoki T, Akaike M, Nishio C et al. (2011). Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J Am Coll Cardiol 58: 248–255. [DOI] [PubMed] [Google Scholar]
- Hojman P, Pedersen M, Nielsen AR, Krogh‐Madsen R, Yfanti C, Åkerstrom T et al. (2009). Fibroblast growth factor‐21 is induced in human skeletal muscles by hyperinsulinemia. Diabetes 58: 2797–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA et al. (2007). Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol 27: 2113–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE et al. (2012). FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 61: 1725–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JY, Park YJ, Ham M, Kim JB (2014). Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells 37: 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K (2008). FGF21 is an Akt‐regulated myokine. FEBS Lett 582: 3805–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins NT, Padilla J, Rector RS, Laughlin MH (2013). Influence of regular physical activity and caloric restriction on beta‐adrenergic and natriuretic peptide receptor expression in retroperitoneal adipose tissue of OLETF rats. Exp Physiol 98: 1576–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Wan F, Wang F, Wu Q (2015). Irisin relaxes mouse mesenteric arteries through endothelium‐dependent and endothelium‐independent mechanisms. Biochem Biophys Res Commun 468: 832–836. [DOI] [PubMed] [Google Scholar]
- Jung SH, Park HS, Kim KS, Choi WH, Ahn CW, Kim BT et al. (2008). Effect of weight loss on some serum cytokines in human obesity: increase in IL‐10 after weight loss. J Nutr Biochem 19: 371–375. [DOI] [PubMed] [Google Scholar]
- Kawanishi N, Mizokami T, Yano H, Suzuki K (2013). Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Med Sci Sports Exerc 45: 1684–1693. [DOI] [PubMed] [Google Scholar]
- Kawanishi N, Yano H, Yokogawa Y, Suzuki K (2010). Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high‐fat‐diet‐induced obese mice. Exerc Immunol Rev 16: 105–118. [PubMed] [Google Scholar]
- Kim HJ, Lee HJ, So B, Son JS, Yoon D, Song W (2016). Effect of aerobic training and resistance training on circulating irisin level and their association with change of body composition in overweight/obese adults: a pilot study. Physiol Res 65: 271–279. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Park JY, Oh SL, Kim YA, So B, Seong JK et al. (2013a). Effect of treadmill exercise on interleukin‐15 expression and glucose tolerance in zucker diabetic Fatty rats. Diabetes Metab J 37: 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Kim SH, Min YK, Yang HM, Lee JB, Lee MS (2013b). Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS One 8: e63517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen JG, Bertholdt L, Joensen E, Lassen SB, Hidalgo J, Pilegaard H (2015). Skeletal muscle interleukin‐6 regulates metabolic factors in iWAT during HFD and exercise training. Obesity (Silver Spring) 23: 1616–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp A, Buechler C, Neumeier M, Weigert J, Aslanidis C, Scholmerich J et al. (2009). Innate immunity and adipocyte function: ligand‐specific activation of multiple toll‐like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obesity (Silver Spring) 17: 648–656. [DOI] [PubMed] [Google Scholar]
- Kranendonk ME, Van Herwaarden JA, Stupkova T, De Jager W, Vink A, Moll FL et al. (2015). Inflammatory characteristics of distinct abdominal adipose tissue depots relate differently to metabolic risk factors for cardiovascular disease: distinct fat depots and vascular risk factors. Atherosclerosis 239: 419–427. [DOI] [PubMed] [Google Scholar]
- Kroller‐Schon S, Jansen T, Hauptmann F, Schuler A, Heeren T, Hausding M et al. (2012). alpha1AMP‐activated protein kinase mediates vascular protective effects of exercise. Arterioscler Thromb Vasc Biol 32: 1632–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger K, Alack K, Ringseis R, Mink L, Pfeifer E, Schinle M et al. (2016). Apoptosis of T‐cell subsets after acute high‐intensity interval exercise. Med Sci Sports Exerc 48: 2021–2029. [DOI] [PubMed] [Google Scholar]
- Lacraz G, Rakotoarivelo V, Labbe SM, Vernier M, Noll C, Mayhue M et al. (2016). Deficiency of interleukin‐15 confers resistance to obesity by diminishing inflammation and enhancing the thermogenic function of adipose tissues. PLoS One 11 e0162995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago F, Gomez R, Gomez‐Reino JJ, Dieguez C, Gualillo O (2009). Adipokines as novel modulators of lipid metabolism. Trends Biochem Sci 34: 500–510. [DOI] [PubMed] [Google Scholar]
- Laufs U, Wassmann S, Czech T, Munzel T, Eisenhauer M, Bohm M et al. (2005). Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol 25: 809–814. [DOI] [PubMed] [Google Scholar]
- Lee P, Brychta RJ, Linderman J, Smith S, Chen KY, Celi FS (2013). Mild cold exposure modulates fibroblast growth factor 21 (FGF21) diurnal rhythm in humans: relationship between FGF21 levels, lipolysis, and cold‐induced thermogenesis. J Clin Endocrinol Metab 98: E98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C et al. (2014). Irisin and FGF21 are cold‐induced endocrine activators of brown fat function in humans. Cell Metab 19: 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SD, Tontonoz P (2014). Eosinophils in fat: pink is the new brown. Cell 157: 1249–1250. [DOI] [PubMed] [Google Scholar]
- Lee S, Kuk JL, Davidson L, Hudson R, Kilpatrick K, Graham TE et al. (2005). Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without type 2 diabetes. J Appl Physiol (1985) 99: 1220–1225. [DOI] [PubMed] [Google Scholar]
- Lee S, Norheim F, Langleite TM, Noreng HJ, Storas TH, Afman LA et al. (2016). Effect of energy restriction and physical exercise intervention on phenotypic flexibility as examined by transcriptomics analyses of mRNA from adipose tissue and whole body magnetic resonance imaging. Physiol Rep 4: e13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK (2009). Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 54: 2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loro E, Seifert EL, Moffat C, Romero F, Mishra MK, Sun Z et al. (2015). IL‐15R alpha is a determinant of muscle fuel utilization, and its loss protects against obesity. Am J Physiol Regul Integr Comp Physiol 309: R835–R844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Xiang G, Liu M, Mei W, Xiang L, Dong J (2015). Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E‐Null diabetic mice. Atherosclerosis 243: 438–448. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR (2007). Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowski M, Deja MA, Golba KS, Roleder T, Biernat J, Wos S (2008). Perivascular tissue of internal thoracic artery releases potent nitric oxide and prostacyclin‐independent anticontractile factor. Eur J Cardiothorac Surg 33: 225–231. [DOI] [PubMed] [Google Scholar]
- Malnick S, Knobler H (2006). The medical complications of obesity. Qjm 99: 565–579. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S (2014). The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6: 13. [DOI] [PMC free article] [PubMed]
- Mcdowell CP, Campbell MJ, Herring MP (2016). Sex‐related differences in mood responses to acute aerobic exercise. Med Sci Sports Exerc 48: 1798–1802. [DOI] [PubMed] [Google Scholar]
- McGinnis GR, Ballmann C, Peters B, Nanayakkara G, Roberts M, Amin R et al. (2015). Interleukin‐6 mediates exercise preconditioning against myocardial ischemia reperfusion injury. Am J Physiol Heart Circ Physiol 308: H1423–H1433. [DOI] [PubMed] [Google Scholar]
- Meijer RI, Bakker W, Alta CL, Sipkema P, Yudkin JS, Viollet BC et al. (2013). Perivascular adipose tissue control of insulin‐induced vasoreactivity in muscle is impaired in db/db mice. Diabetes 62: 590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer RI, Serne EH, Korkmaz HI, Van Der Peet DL, De Boer MP, Niessen HW et al. (2015). Insulin‐induced changes in skeletal muscle microvascular perfusion are dependent upon perivascular adipose tissue in women. Diabetologia 58: 1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer RI, Serne EH, Smulders YM, van Baak Hinsbergh VW, Yudkin JS, Eringa EC (2011). Perivascular adipose tissue and its role in type 2 diabetes and cardiovascular disease. Curr Diab Rep 11: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer RI, Serne EH, Yudkin JS, van Baak Hinsbergh VW, Smulders YM, Eringa EC (2016). Perivascular fat in human muscle. Lancet Diabetes Endocrinol 4: 958. [DOI] [PubMed] [Google Scholar]
- Min SY, Kady J, Nam M, Rojas‐Rodriguez R, Berkenwald A, Kim JH et al. (2016). Human 'brite/beige' adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat Med 22: 312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Higgs A (1993). The L‐arginine‐nitric oxide pathway. N Engl J Med 329: 2002–2012. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K (2011). Central neural pathways for thermoregulation. Front Biosci (Landmark Ed) 16: 74–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H et al. (2007). Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes 56: 1517–1526. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Nakatake Y, Konishi M, Itoh N (2000). Identification of a novel FGF, FGF‐21, preferentially expressed in the liver. Biochim Biophys Acta 1492: 203–206. [DOI] [PubMed] [Google Scholar]
- O'Connell GC, Pistilli EE (2015). Interleukin‐15 directly stimulates pro‐oxidative gene expression in skeletal muscle in‐vitro via a mechanism that requires interleukin‐15 receptor alpha. Biochem Biophys Res Commun 458: 614–619. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R et al. (2007). BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci U S A 104: 7432–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N et al. (2010). Adiponectin promotes macrophage polarization toward an anti‐inflammatory phenotype. J Biol Chem 285: 6153–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AG, Araujo TG, Carvalho BM, Guadagnini D, Rocha GZ, Bagarolli RA et al. (2013). Acute exercise induces a phenotypic switch in adipose tissue macrophage polarization in diet‐induced obese rats. Obesity (Silver Spring) 21: 2545–2556. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R et al. (2010). Sfrp5 is an anti‐inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 329: 454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y et al. (2001). Adipocyte‐derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte‐derived macrophages. Circulation 103: 1057–1063. [DOI] [PubMed] [Google Scholar]
- Park KH, Zaichenko L, Brinkoetter M, Thakkar B, Sahin‐Efe A, Joung KE et al. (2013). Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab 98: 4899–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA (2008). Muscle as an endocrine organ: focus on muscle‐derived interleukin‐6. Physiol Rev 88: 1379–1406. [DOI] [PubMed] [Google Scholar]
- Pedersen BK (2009). The diseasome of physical inactivity – and the role of myokines in muscle – fat cross talk. J Physiol 587: 5559–5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP (2007). Role of myokines in exercise and metabolism. J Appl Physiol (1985) 103: 1093–1098. [DOI] [PubMed] [Google Scholar]
- Phillips BE, Atherton PJ, Varadhan K, Limb MC, Wilkinson DJ, Sjoberg KA et al. (2015). The effects of resistance exercise training on macro‐ and micro‐circulatory responses to feeding and skeletal muscle protein anabolism in older men. J Physiol 593: 2721–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepoli MF, Corra U, Benzer W, Bjarnason‐Wehrens B, Dendale P, Gaita D et al. (2010). Secondary prevention through cardiac rehabilitation: from knowledge to implementation. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil 17: 1–17. [DOI] [PubMed] [Google Scholar]
- Pistilli EE, Bogdanovich S, Garton F, Yang N, Gulbin JP, Conner JD et al. (2011). Loss of IL‐15 receptor alpha alters the endurance, fatigability, and metabolic characteristics of mouse fast skeletal muscles. J Clin Invest 121: 3120–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop D, Bodisz G, Petrovai D, Borz B, Zdrenghea V, Zdrenghea D (2010). The effect of very short duration acute physical exercise upon adiponectin and leptin in overweight subjects. Rom J Intern Med 48: 39–45. [PubMed] [Google Scholar]
- Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM et al. (2014). Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157: 1292–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn LS, Anderson BG, Conner JD, Wolden‐Hanson T, Marcell TJ (2014). IL‐15 is required for postexercise induction of the pro‐oxidative mediators PPARdelta and SIRT1 in male mice. Endocrinology 155: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn LS, Anderson BG, Strait‐Bodey L, Stroud AM, Argiles JM (2009). Oversecretion of interleukin‐15 from skeletal muscle reduces adiposity. Am J Physiol Endocrinol Metab 296: E191–E202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn LS, Strait‐Bodey L, Anderson BG, Argiles JM, Havel PJ (2005). Interleukin‐15 stimulates adiponectin secretion by 3T3‐L1 adipocytes: evidence for a skeletal muscle‐to‐fat signaling pathway. Cell Biol Int 29: 449–457. [DOI] [PubMed] [Google Scholar]
- Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I et al. (2014). Meteorin‐like is a hormone that regulates immune‐adipose interactions to increase beige fat thermogenesis. Cell 157: 1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifenberger MS, Turk JR, Newcomer SC, Booth FW, Laughlin MH (2007). Perivascular fat alters reactivity of coronary artery: effects of diet and exercise. Med Sci Sports Exerc 39: 2125–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittig K, Staib K, Machann J, Bottcher M, Peter A, Schick F et al. (2008). Perivascular fatty tissue at the brachial artery is linked to insulin resistance but not to local endothelial dysfunction. Diabetologia 51: 2093–2099. [DOI] [PubMed] [Google Scholar]
- Ruffino JS, Davies NA, Morris K, Ludgate M, Zhang L, Webb R et al. (2016). Moderate‐intensity exercise alters markers of alternative activation in circulating monocytes in females: a putative role for PPARgamma. Eur J Appl Physiol 116: 1671–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaras K, Botelho NK, Chisholm DJ, Lord RV (2010). Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring) 18: 884–889. [DOI] [PubMed] [Google Scholar]
- Samms RJ, Cheng CC, Kharitonenkov A, Gimeno RE, Adams AC (2016). Overexpression of beta‐Klotho in adipose tissue sensitizes male mice to endogenous FGF21 and provides protection from diet‐induced obesity. Endocrinology 157: 1467–1480. [DOI] [PubMed] [Google Scholar]
- Saunders TJ, Palombella A, Mcguire KA, Janiszewski PM, Despres J, Ross R (2012). Acute exercise increases adiponectin levels in abdominally obese men. J Nutr Metab 2012: 148729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton S, Withers S, Ohanian J, Heagerty AM (2016). [OP.5A.07] The role of sympathetic nerves and noradrenertic transmission in perivascular adipose tissue function. J Hypertens 34 (Suppl 2): e55–e56.27799659 [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S et al. (2008). PRDM16 controls a brown fat/skeletal muscle switch. Nature 454: 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J et al. (2011). Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 121: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva LC, De Araujo AL, Fernandes JR, Matias Mde S, Silva PR, Duarte AJ et al. (2016). Moderate and intense exercise lifestyles attenuate the effects of aging on telomere length and the survival and composition of T cell subpopulations. Age (Dordr) 38: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson KA, Singh MA (2008). Effects of exercise on adiponectin: a systematic review. Obesity (Silver Spring) 16: 241–256. [DOI] [PubMed] [Google Scholar]
- Slusher AL, Whitehurst M, Zoeller RF, Mock JT, Maharaj M, Huang CJ (2015). Attenuated fibroblast growth factor 21 response to acute aerobic exercise in obese individuals. Nutr Metab Cardiovasc Dis 25: 839–845. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speretta GF, Rosante MC, Duarte FO, Leite RD, Lino AD, Andre RA et al. (2012). The effects of exercise modalities on adiposity in obese rats. Clinics (Sao Paulo) 67: 1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Kusminski CM, Scherer PE (2011). Adipose tissue remodeling and obesity. J Clin Invest 121: 2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC (2009). Exercise and adrenaline increase PGC‐1α mRNA expression in rat adipose tissue. J Physiol 587: 1607–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takikawa A, Mahmood A, Nawaz A, Kado T, Okabe K, Yamamoto S et al. (2016). HIF‐1alpha in myeloid cells promotes adipose tissue remodeling toward insulin resistance. Diabetes 65: 3649–3659. [DOI] [PubMed] [Google Scholar]
- Tanimura Y, Aoi W, Takanami Y, Kawai Y, Mizushima K, Naito Y et al. (2016). Acute exercise increases fibroblast growth factor 21 in metabolic organs and circulation. Physiol Rep 4: 12 pii; e12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran KV, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A et al. (2012). The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab 15: 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers RL, Motta AC, Betts JA, Bouloumie A, Thompson D (2015). The impact of adiposity on adipose tissue‐resident lymphocyte activation in humans. Int J Obes (Lond) 39: 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschritter O, Fritsche A, Thamer C, Haap M, Shirkavand F, Rahe S et al. (2003). Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes 52: 239–243. [DOI] [PubMed] [Google Scholar]
- Wijers SL, Schrauwen P, van Baak MA, Saris WH, van Markenl Lichtenbelt WD (2011). Beta‐adrenergic receptor blockade does not inhibit cold‐induced thermogenesis in humans: possible involvement of brown adipose tissue. J Clin Endocrinol Metab 96: E598–E605. [DOI] [PubMed] [Google Scholar]
- Wu MV, Bikopoulos G, Hung S, Ceddia RB (2014). Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats: impact on whole‐body energy expenditure. J Biol Chem 289: 34129–34140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Liu L, Xu A, Zhou P, Long Z, Tu Y et al. (2015). Serum fibroblast growth factor 21 levels are related to subclinical atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol 14: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ying Z, Cai M, Xu Z, Li Y, Jiang SY et al. (2011). Exercise ameliorates high‐fat diet‐induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 300: R1115–R1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Xu X, Zhang XQ, Farokhzad OC, Langer R (2016). Preventing diet‐induced obesity in mice by adipose tissue transformation and angiogenesis using targeted nanoparticles. Proc Natl Acad Sci U S A 113: 5552–5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin JS, Eringa E, Stehouwer CD (2005). ‘Vasocrine’ signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet 365: 1817–1820. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Kumari M, Humphries SE, Mohamed‐Ali V (2000). Inflammation, obesity, stress and coronary heart disease: is interleukin‐6 the link? Atherosclerosis 148: 209–214. [DOI] [PubMed] [Google Scholar]
- Zed C, James WP (1986). Dietary thermogenesis in obesity. Response to carbohydrate and protein meals: the effect of beta‐adrenergic blockade and semistarvation. Int J Obes (Lond) 10: 391–405. [PubMed] [Google Scholar]
- Zeyda M, Huber J, Prager G, Stulnig TM (2011). Inflammation correlates with markers of T‐cell subsets including regulatory T cells in adipose tissue from obese patients. Obesity (Silver Spring) 19: 743–748. [DOI] [PubMed] [Google Scholar]
- Zhang F, Yu L, Lin X, Cheng P, He L, Li X et al. (2015). Minireview: roles of fibroblast growth factors 19 and 21 in metabolic regulation and chronic diseases. Mol Endocrinol 29: 1400–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Friedman MH (2012). Adaptive response of vascular endothelial cells to an acute increase in shear stress magnitude. Am J Physiol Heart Circ Physiol 302: H983–H991. [DOI] [PMC free article] [PubMed] [Google Scholar]


