Abstract
A high-fat (HF) diet alters gut microbiota and promotes obesity related inflammation and cognitive impairment. Teasaponin is the major active component of tea, and has been associated with anti-inflammatory effects and improved microbiota composition. However, the potential protective effects of teasaponin, against HF diet-induced obesity and its associated alteration of gut microbiota, inflammation and cognitive decline have not been studied. In this study, obesity was induced in C57BL/6 J male mice by feeding a HF diet for 8 weeks, followed by treatment with oral teasaponin (0.5%) mixed in HF diet for a further 6 weeks. Teasaponin treatment prevented the HF diet-induced recognition memory impairment and improved neuroinflammation, gliosis and brain-derived neurotrophic factor (BDNF) deficits in the hippocampus. Furthermore, teasaponin attenuated the HF diet-induced endotoxemia, pro-inflammatory macrophage accumulation in the colon and gut microbiota alterations. Teasaponin also improved glucose tolerance and reduced body weight gain in HF diet-induced obese mice. The behavioral and neurochemical improvements suggest that teasaponin could limit unfavorable gut microbiota alterations and cognitive decline in HF diet-induced obesity.
Introduction
Obesity increases the incidence of insulin resistance, type 2 diabetes, and cognitive decline in neurodegenerative diseases such as vascular dementia and Alzheimer’s disease (AD)1,2. Obesity is characterized by low grade systemic inflammation, which is associated with an alteration of gut microbiota and brain function3. Empirical evidence has linked a high-fat (HF) diet with alterations of gut microbiota4 and impairments in cognition, including a decline in recognition memory in both animal and human studies5,6. The hippocampus plays an important role in improving recognition memory7–9. Lesions in the hippocampus severely disrupt object recognition7, object-in-place memory10, and temporal order recognition memory11 in rodent studies.
Previous studies have provided compelling evidence to suggest an association between the gut microbiota, HF diet and body weight regulation4,12. Firmicutes and Bacteriodetes account for more than 90% of the total gut microbiota13. An 8-week HF diet increased Firmicutes and reduced Bacteriodetes in mice4. Consistent with animal models, a similar difference of an increased ratio of Firmicutes/Bacteroidetes in the gut microbiota has also been reported in obese humans14. Moreover, increased fat intake has been strongly correlated with increased plasma lipopolysaccharide (LPS), the endotoxin which is a major component of the outer membrane in Gram-negative bacteria10. In HF diet-induced obesity, endotoxemia has been linked with alterations of gut microbiota and increased intestinal permeability11. Currently, obesity and the related cognitive decline are associated with microbiota alterations and low-grade systemic and central inflammation9,10, which contribute to interruption of the gut-brain axis3.
It is known that endotoxemia can trigger systemic inflammation including neuroinflammation, and cognitive impairment15. For example, systemic LPS administration activated microglia and increased expression of pro-inflammatory factors in the hippocampus of mice16. Microglia and astrocytes are the principal immune cells in the central nervous system (CNS). The activation of microglia promotes the release of pro-inflammatory cytokines, which contribute to the neurodegenerative process15. Microglia and astrocytes express numerous members of the Toll-like receptor (TLR) family17, which recognize conserved microbial motifs expressed by a wide array of pathogens. For example, LPS binds to TLR4 mediated by myeloid differentiation primary-response protein 88 (MyD88), and activates c-Jun N-terminal kinase (JNK) and nuclear factor-kappa B (NFκB), two important inflammatory signaling molecules17. The activation of the TLR4-MyD88-JNK/NFκB signaling pathway leads to the production of pro-inflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), and contributes to the development of neurodegenerative diseases18–21. Adult hippocampal neurogenesis and its related learning and memory are influenced negatively by microglial activation and neuroinflammation18. Furthermore, intestinal microbiota affect central levels of brain-derived neurotropic factor (BDNF) and cognitive behavior. Administration of oral antimicrobials to SPF mice transiently alter the composition of intestinal microbiota and increase hippocampal expression of BDNF19.
Tea has been cultivated for thousands of years and used for beverage and medicinal purposes. Previous studies have found tea can prevent obesity and abnormal glucose and lipid metabolism20. Recently, research on tea has shown it possesses anti-inflammatory properties, and improves microbiota and cognition21–25. Phenolics and saponin are the two major active components of tea extract. Most research to date has focused on the interaction of dietary polyphenols and intestinal microbiota26–28. However, the effects of teasaponin on the modulation of the intestinal microbiota and cognition remain poorly investigated and understood. This study used a chronic HF diet-induced obese mouse model to assess the ability of dietary teasponin to regulate the gut-brain axis and ultimately prevent cognitive impairment, glucose intolerance and body weight gain. The gut microbiota, endotoxinemia, colonic macrophage shift, hippocampal inflammation and gliosis, recognition memory, body weight gain, and glucose tolerance were examined in these mice.
Results
Teasaponin reversed the alteration of gut microbiota and systemic inflammation induced by a HF diet in obese mice
To investigate the effect of high-fat diet on intestinal microbiota and whether teasaponin could reverse this effect, qRT-PCR was used to evaluate the abundance of several vital species of gut flora in the cecal content, including Bacteroides-Prevotella spp., Desulfovibrios spp., Bifidobacterium spp. and Lactobacillus spp. The HF diet significantly decreased the amount of Bacteroides-Prevotella spp. (p = 0.016) and Desulfovibrios spp. DNA (p < 0.001), and increased Bifidobacterium spp. (p = 0.002) and Lactobacillus spp. DNA (p = 0.001) compared with the control mice (Fig. 1A). A 6-week teasaponin oral treatment prevented the alteration of gut microbiota induced by the HF diet. The amount of Bacteroides-Prevotella spp. DNA and Desulfovibrios spp. DNA were significantly increased in the HF + TS group compared to the HF group (p = 0.007, p = 0.013 respectively). The amount of Bacteroides-Prevotella spp. DNA in the HF + TS group was similar to the control mice (p = 0.488), while the amount of Desulfovibrios spp. DNA in the HF + TS group was still significantly lower than the control mice (p < 0.001). Meanwhile, the teasaponin treatment also prevented the HF diet-induced alteration of the amount of Bifidobacterium spp. DNA (p = 0.003) and Lactobacillus spp. DNA (p = 0.001) in the HF + TS group compared to the HF group. There was no statistical difference between the HF + TS group and the control group in Lactobacillus spp. DNA (p = 0.992) and Bifidobacterium spp. DNA (p = 0.701) (Fig. 1A).
Figure 1.
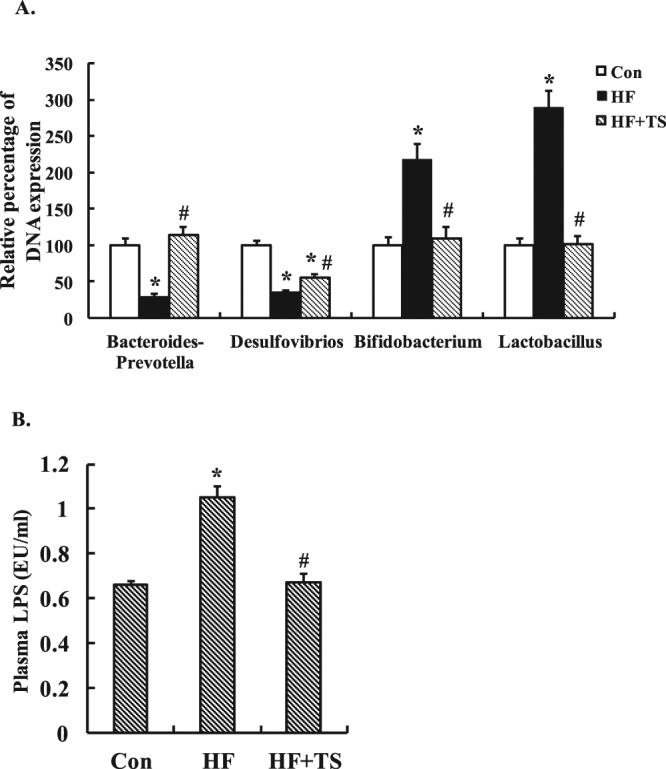
(A) Bacteroides-Prevotella, Desulfovibrios, Bifidobacterium and Lactobacillus DNA expressions in gut microbiota of the control group (Con), high-fat diet group (HF), and HF with teasaponin treatment group (HF + TS) (n = 12 per group). *p < 0.05 compared to the Con group, # p < 0.05 compared to the HF group, values are means ± SEM. (B) The plasma LPS level of control group (Con), high-fat diet group (HF), and HF with teasaponin treatment group (HF + TS) (n = 12 per group).
To further determine whether teasaponin also decreased systemic inflammation with improving gut microbiota, the plasma concentration of LPS was measured. Teasaponin prevented an increase of plasma LPS induced by the HF diet after the 6-week treatment (Control: 0.66 ± 0.03 EU/ml, HF: 1.05 ± 0.08 EU/ml, HF + TS: 0.67 ± 0.04 EU/ml, p = 0.001). There was no difference between the control and the HF + TS groups in the plasma LPS concentrations (p = 0.805) (Fig. 1B).
Teasaponin reduced M1 macrophage accumulation in the colon of HF diet-induced obese mice
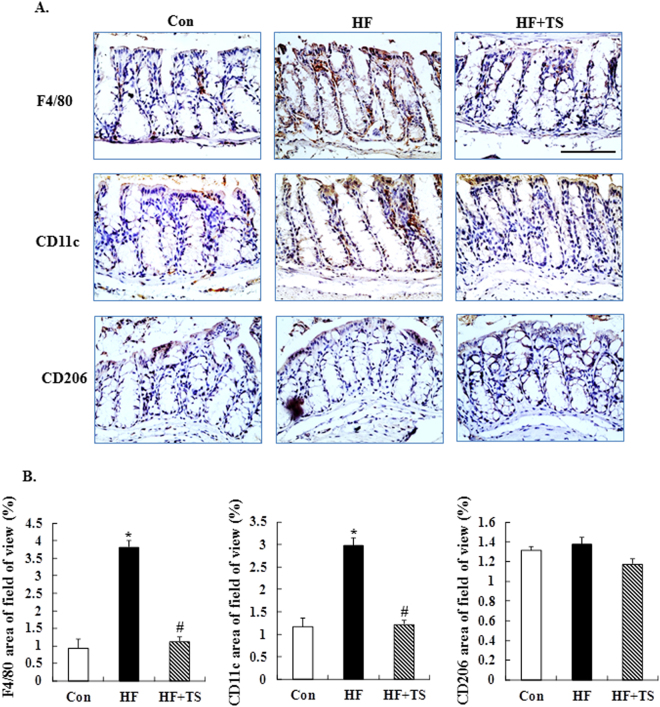
To investigate the effect of teasaponin on macrophage accumulation in the colon of HF diet-induced obese mice, macrophages were stained with F4/80 antibody. The positive immunoreactivity of F4/80 was significantly increased in the colon of obese mice (p < 0.001), while it was ameliorated by the teasaponin treatment (p < 0.001) (Fig. 2A and B). Furthermore, the types of macrophages were characterized. CD11c was used to detect M1 macrophages, which produce pro-inflammatory cytokines, and CD206 was used to detect M2 macrophages which produce anti-inflammatory cytokines. Teasaponin significantly reduced the CD11c positive staining (p < 0.001), but there was no significant difference in the CD206 staining of the colon among the control group, the HF group and the HF + TS group (Fig. 2A and B).
Figure 2.
The expression of F4/80, CD11c, and CD206 macrophages in the colon. (A) Immunohistochemical staining, scale bar = 100 μm. (B) Quantification of the F4/80, CD11c, and CD206-positive areas (%). *p < 0.05 compared to the Con group, # p < 0.05 compared to the HF group, values are means ± SEM.
Teasaponin suppressed the hippocampal inflammation in HF diet-induced obese mice
To investigate whether LPS (endotoxemia) could be related to hippocampal inflammation, the TLR4-My88-NFκB/JNK signaling pathway and pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β) were examined in hippocampal tissue. Western blotting revealed that the TLR4 (p = 0.036) and MyD88 (p < 0.001) levels in the hippocampus were significantly higher in the HF group than the control group. The TLR4 and MyD88 levels were significantly lower in the HF + TS group compared to the HF group (p = 0.032, p < 0.001 respectively), with the teasaponin treatment restoring the TLR4 and MyD88 levels to control levels (Fig. 3). Furthermore, the p-JNK (p < 0.001) and NFκB (p = 0.005) levels were significantly higher in the HF group compared to the control group, while the teasaponin treatment prevented an increase of p-JNK and NFκB (p < 0.001, p = 0.008) (Fig. 3). Levels of pro-inflammatory cytokines, IL-1β (p = 0.015), IL-6 (p = 0.006) and TNF-α (p = 0.009) were significantly higher in the HF group compared to the control group (Fig. 3). Teasaponin significantly decreased IL-1β (p = 0.003) and IL-6 (p = 0.015) levels compared to the HF group, with the HF + TS group showing no difference in IL-1β and IL-6 levels compared the control group.
Figure 3.
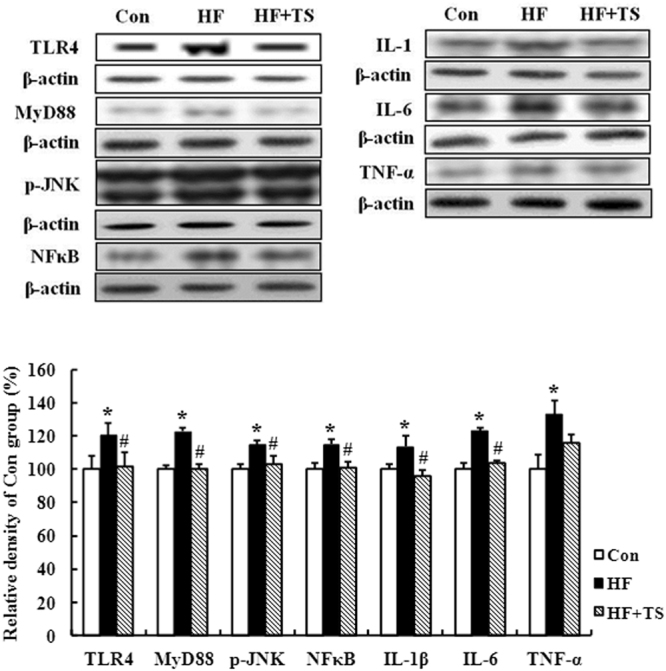
Protein expression levels of TLR4, MyD88, p-JNK, NFκB, IL-1β, IL-6, and TNF-α in the hippocampus of the control group (Con), high-fat diet group (HF), and HF with teasaponin treatment group (HF + TS) (n = 12 per group). *p < 0.05 compared to the Con group, # p < 0.05 compared to the HF group, values are means ± SEM.
Teasaponin ameliorated microglial and astrocyte accumulation in the hippocampus of HF diet-induced obese mice
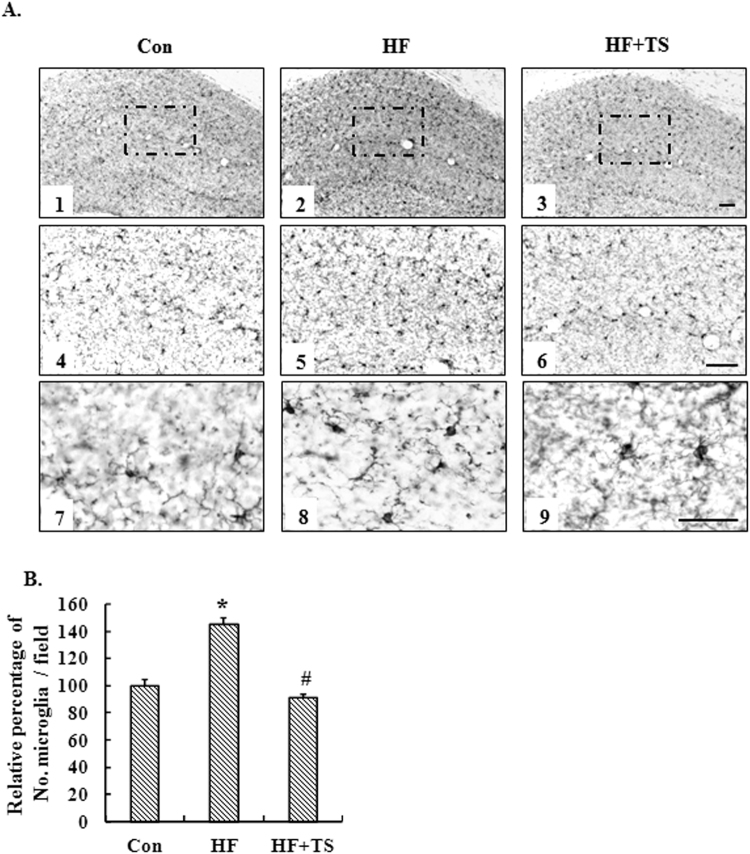
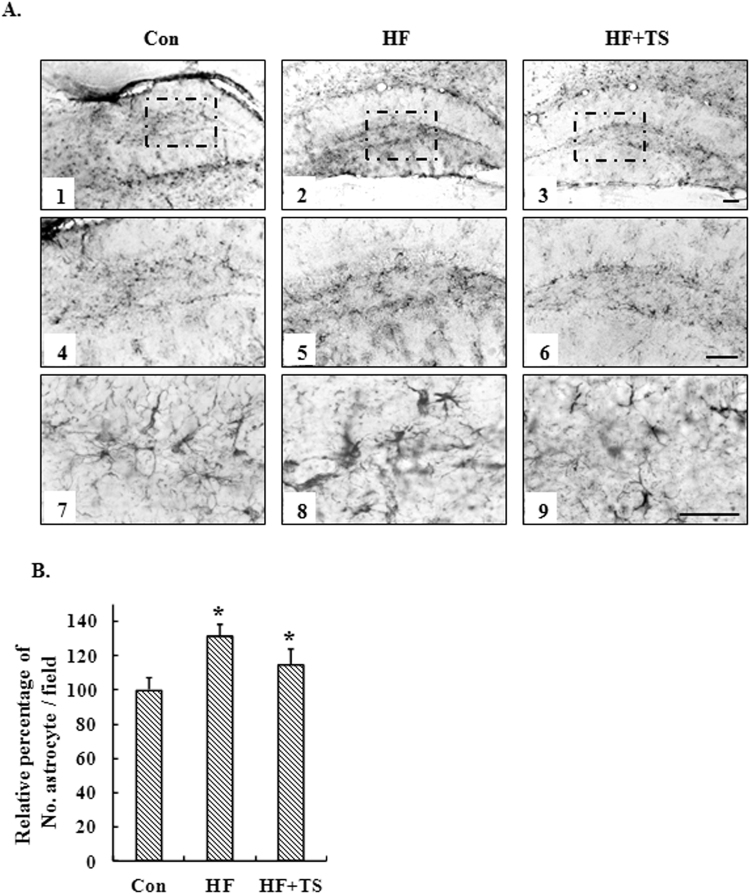
Activation, recruitment, and proliferation of microglia and astrocytes are hallmarks of the brain response to neuronal injury. We therefore assessed the expression of a microglial cell-specific marker, Iba-1 and an astrocyte activation marker, GFAP, in the hippocampus to investigate the effects of a HF diet on inflammatory responses and to examine whether teasaponin mitigates the activation and infiltration of glial cells in the brain. Immunohistochemistry revealed the number of hippocampal microglia in the HF group increased nearly 50% compared to the control group (p < 0.001), while teasaponin significantly ameliorated microglial accumulation in the hippocampus of high-fat diet-induced obese mice after 6 weeks treatment (p < 0.001) (Fig. 4). Microglial number in the HF + TS group was similar to the control group (p = 0.214). Meanwhile, the HF diet increased the number of astrocytes in the hippocampus of obese mice by nearly 25% compared to the control mice fed lab chow (p = 0.002). Teasaponin, to some degree, ameliorated astrocyte accumulation in the hippocampus of the HF group, but there was no statistical difference (p = 0.147). The number of astrocytes in the hippocampus of the HF + TS group was still higher than the control group (p = 0.044) (Fig. 5).
Figure 4.
(A) Microglia accumulation in the hippocampus of the control group (Con), high-fat diet group (HF) and HF with teasaponin treatment group (HF + TS) shown at ×5 (A1-3), ×10 (A4-6) and ×40 (A7-9) magnification; Scale bar = 20 μm. (B) Relative percentage of No. microglia in the hippocampus (%). *p < 0.05 compared to the Con group, # p < 0.05 compared to the HF group, values are means ± SEM.
Figure 5.
(A) Astrocyte accumulation in the hippocampus of the control group (Con), high-fat diet group (HF) and HF with teasaponin treatment group (HF + TS) shown at ×5 (A1-3), ×10 (A4-6) and ×40 (A7-9) magnification; Scale bar = 20 μm. (B) Relative percentage of No. astrocyte in the hippocampus (%). *p < 0.05 compared to the Con group, # p < 0.05 compared to the HF group, values are means ± SEM.
Teasaponin improved recognition memory and increased BDNF levels in the hippocampus of HF diet-induced obese mice
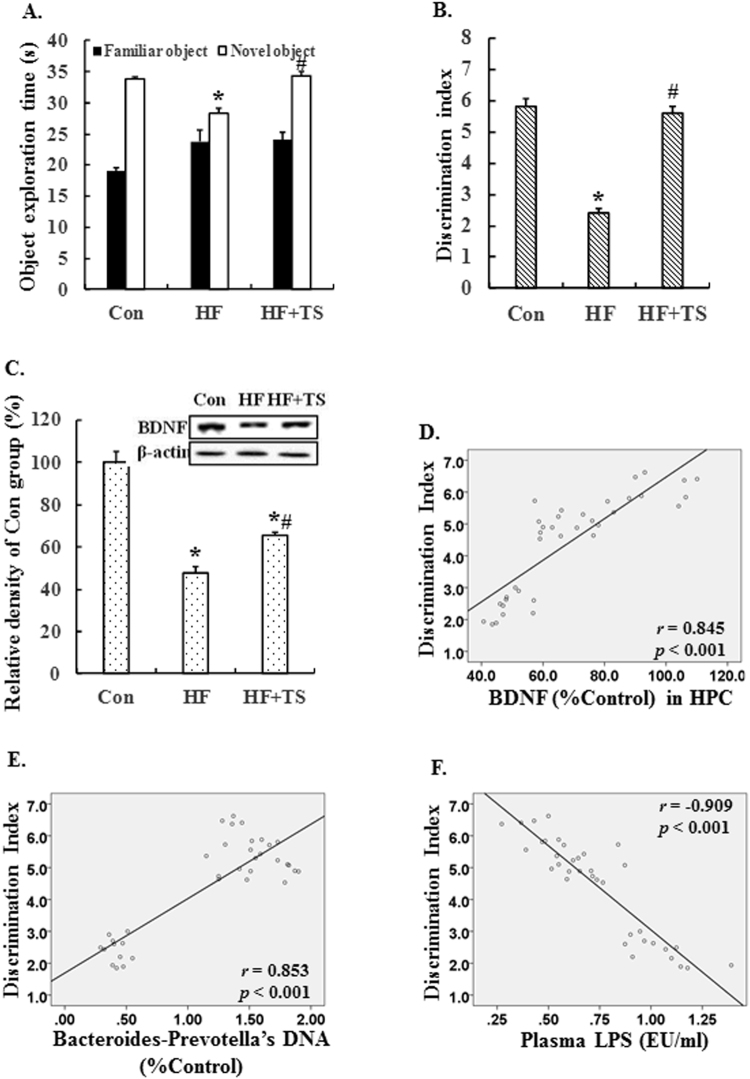
To assess whether teasaponin treatment can prevent HF diet-induced recognition memory deficits, we performed a novel object recognition test in mice. During the training session of the test, there was no significant difference in the percentage of time spent exploring the objects in the open-field among the control group, the HF group and the HF + TS group (p > 0.05). During the retention session, HF mice showed reduced novel object exploration compared to controls, while the teasaponin treatment restored this to control levels (Control: 33.84 ± 2.58 seconds, HF: 28.25 ± 1.55 seconds, HF + TS: 34.36 ± 2.57 seconds, p = 0.001) (Fig. 6A). The discrimination index was further analyzed to assess recognition memory. The HF diet decreased the discrimination index by 58.62% compared to the control group (p < 0.001), while the teasaponin treatment increased the discrimination index by 133.33% compared with the HF group (p < 0.001) without significant difference compared with control group (Fig. 6B). These results show that teasaponin treatment improved recognition memory deficits caused by a HF diet.
Figure 6.
The object exploration time (A) and the discrimination index (B) in the novel object recognition test of the control group (Con), high-fat diet group (HF) and HF with teasaponin treatment group (HF + TS). (C) The level of BDNF expression in the hippocampus of mice (n = 12 per group). The discrimination index in novel object recognition was positively correlated with BDNF in the hippocampus (D) and Bacteroides-Prevotella spp. DNA in cecal contents (E), and was negatively correlated with plasma LPS (F). *p < 0.05 compared to the Con group, # p < 0.05 compared to the HF group, values are means ± SEM.
We then measured hippocampal BDNF levels, which are important for synaptic plasticity and learning and memory. Compared with the control group, the BDNF level of the HF group was significantly lower (p < 0.001) (Fig. 6C). Importantly, the teasaponin treatment significantly increased the BDNF level in the hippocampus of the HF + TS group compared to the HF group (p = 0.011), but the BDNF level in the HF + TS group was still lower (p < 0.001) than the control group (Fig. 6C). There was a significant positive correlation between the hippocampal BDNF level and the discrimination index of the novel object recognition test (r = 0.845, p < 0.001) (Fig. 6D). Furthermore, the discrimination index was also positively correlated with Bacteroides-Prevotella spp. DNA in cecal contents (r = 0.853, p < 0.001) (Fig. 6E) and was negatively correlated with the plasma LPS level (r = −0.909, p < 0.001) (Fig. 6F).
Teasaponin reduced body weight and improved glucose tolerance in HF diet-induced obese mice
The body weight in HF groups was significantly higher than that of the control group (HF: 33.11 ± 2.41 g, Control: 25.80 ± 1.56 g, p < 0.05) after 8 weeks of HF diet (Fig. 7A). After one week of treatment, the teasaponin group began to show a decrease in body weight. After the teasaponin treatment for 6 weeks, the final body weight was significantly lower in the HF + TS group than the HF group (HF + TS: 31.32 ± 1.87 g, HF: 39.14 ± 3.67 g, p = 0.038), however the body weight in the HF + TS group remained higher than the control group (HF + TS: 31.32 ± 1.87 g, Control: 27.33 ± 1.54 g, p < 0.001) (Fig. 7A). There was no difference in energy intake during the teasaponin treatment period between HF + TS and HF group (all p > 0.05) (Fig. 7B). Furthermore, teasaponin significantly improved glucose tolerance in the HF diet-induced obese mice (Fig. 7C). The fasting glucose level of the HF + TS group and the control group was significantly lower than the HF group (all p < 0.05), while there was no difference between the HF + TS group and the control group (p = 0.326). The blood glucose level of the teasaponin treatment group significantly decreased at 15, 30, 60, and 120 minutes compared with the HF group (all p < 0.05), but they were still higher than those of the control group (all p < 0.05).
Figure 7.
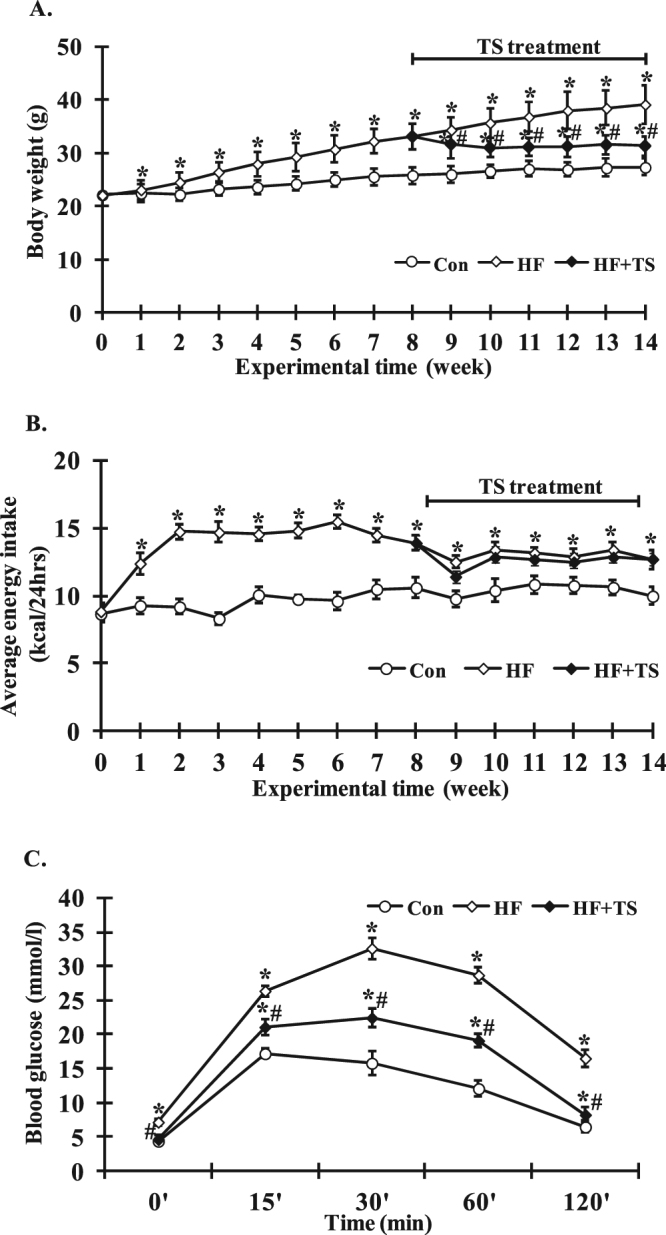
Body weight (A), energy intake (B) and glucose tolerance (C) of the control group (Con), high-fat diet group (HF) and HF with teasaponin treatment group (HF + TS). *p < 0.05 compared to the Con group, # p < 0.05 compared to the HF group, values are means ± SEM.
Discussion
Our results show that a long-term teasaponin treatment improved gut microbiota, suppressed endotoxin in plasma, enhanced object recognition memory, inhibited hippocampal inflammation and gliosis, and increased BDNF in the hippocampus in HF diet fed mice. Previously, tea or derivative powders have been shown to improve fecal microbiota and have anti-inflammatory benefits in both human and animal studies21,22. Moreover, clinical and animal studies have shown that tea improved cognitive function and prevented impairment of learning and memory23–25. In the present study, treatment with teasaponin, an important active ingredient of tea, improved microbiota and enhanced memory for object recognition in HF diet fed mice. Therefore, the effect of teasaponin on manipulation of the gut microbiota may contribute to the ability of tea to improve cognitive function and memory.
Compelling evidence suggests there is an association between gut microbiota, HF diet and body weight regulation4,12. The ratio of the two dominant gut phyla, Firmicutes and Bacteroidetes, increases with obesity in human studies and animal experiments4,14,29. A HF diet for 8 weeks post-weaning has been shown to change the microbiota, decrease the overall bacterial abundance, and increase the ratio of Firmicutes to Bacteriodetes in mice4. Furthermore, alteration of gut microbiota induced by increased nutrient load has been directly correlated with stool energy loss in lean individuals, as evidenced by an increase in Firmicutes and a decrease in Bacteroidetes which were associated with an increased energy harvest in subjects with a greater fractional decrease in stool energy loss12. In our study, we found that teasaponin prevented a HF diet induced alteration of microbiota at the species level, especially increasing Bacteroides-Prevotella spp. and decreasing Lactobacillus spp belonging to Bacteroidetes and Firmicutes respectively30. Overall these findings suggest that the prebiotic-like effects of teasaponin on microbiota may contribute to decreased energy accumulation and body weight gain with stool energy loss following improving microbiota.
In the present study, teasaponin treatment alleviated the hyper-endotoxemia in HF diet-induce obese mice. Previously, the plasma LPS level was closely correlated with changes in the Gram-negative Bacteroides-like intestinal bacteria that reside within the Bacteroidetes phylum31. We found elevated plasma LPS and decreased Gram-negative bacterium Bacteroides-Prevotella spp. and Desulfovibrios spp. DNA levels in HF diet-induced obese mice. An elevated plasma LPS level has been related to the over-production of LPS in the gut by the death of Gram-negative bacteria and their translocation into the bloodstream via an increased intestinal permeability in HF diet-induced obesity32. Therefore, a reduction in Gram-negative bacterium, as observed in the present study, may over-release LPS leading to hyperendotoxemia in the HF diet-induced obese mice. Importantly, dietary teasaponin decreased plasma LPS in these obese mice, perhaps by preventing the decrease of Gram-negative bacterium (including Bacteroides-Prevotella spp. and Desulfovibrios spp.) induced by a HF diet at the same time. These data strongly suggest that teasaponin affected the intestinal microbiota and was responsible for the attenuation of metabolic endotoxemia in HF diet-induced obesity. A HF diet has previously been shown to elevate LPS levels in colonic fluid to activate pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β) in the colon33. Our study showed that a chronic high-fat diet induced pro-inflammatory M1 macrophage accumulation in the colon of obese mice accompanied by increased levels of TNF-α, IL-6 and IL-1β, M1 macrophage produced pro-inflammatory cytokines34,35. Importantly, this present study shows that dietary teasaponin significantly ameliorated M1 macrophage accumulation in the colon of HF diet-induced obese mice.
We report that Lactobacillus spp. and Bifidobacterium spp. were increased and Desulfovibrios spp. was decreased following a HF diet. While Desulfovibrios spp. has been found to be increased in a previous study of HF diet-induced obesity36, Lactobacillus spp. and Bifidobacterium spp have been reported to decrease following HF diet in rodents13,36–38. This contrast may be due to differences in food composition (percentage of fat, type of fatty acids or feeding period) or experimental methods (feeding HF diet or inoculating fresh feces from HF diet fed mice) between the studies. For example in P. D. Cani’s study, the HF diet contains 72% of the energy from fat which is higher than that used in our study (60%)37. In Kim’s study, inoculation with HF diet faeces resulted in a reduced number of Bifidobacteria colonies in mice13. Our finding of increased Lactobacillus spp in the gut microbiome of mice after HF diet, is consistent with a human study which reported a significantly higher concentration of Lactobacillus species in obese patients than in lean controls or anorexic patients39. Further, it was reported that specific Lactobacillus in human feces, such as Lactobacillus reuteri or Lactobacillus sakei, were positively correlated with body mass index in 263 individuals, including 134 obese, 38 overweight, 76 lean and 15 anorexic subjects40, proving support for an association between increased Lactobacilius and increased body weight. It is reported that Bifidobacterium spp is significantly increased in HF diet for 4 weeks in mice and ob/ob mice41, which is in agreement with our observations. Furthermore, many different species and strains of Lactobacillus spp and Bifidobacterium spp contribute to weight gain in infants. For example, Bifidobacterium lactis was significantly associated with increased weight gain in infants born to mothers with HIV infection42. Lactobacillus acidophilus, Lactobacillus fermentum and Lactobacillus ingluviei administration significantly increases weight gain in infants43. Therefore, the role of different species or strain of Lactobacillus spp. and Bifidobacterium spp. in weight gain require further clarification.
Consistent with a previous study that teasaponin affects the rumen fermentation and increases microbial mass yield in vitro 44, the present study showed that teasaponin affects gut microbiota in HF diet-induced obese mice. The exact mechanism underlying this is not clear, however, teasaponin improves the enzymatic hydrolysis of dietary fibre (such as glucan and lignin), which are microbiota-accessible carbohydrates45. Teasaponin can also be degraded by cellulolytic enzymes and release glucose, which could be utilized by microbiota to promote saccchrification and fermentation45.
Previously, systemic LPS administration activated microglia and increased expression of pro-inflammatory factors in the hippocampus of mice16. We found that HF diet induced hippocampal inflammation in obese mice with hyper-endotoxemia. Therefore, the systemic accumulation of toxins, induced by a HF diet may contribute to inflammation in the hippocampus. It has been reported that activated microglial cells release pro-inflammatory cytokines IL-1 and TNF-α, which contribute to the neurodegenerative process46. The activation of astrocytes is known to induce innate and adaptive immunity47 and astroglial responses were assessed in the present study by GFAP immunostaining. Our findings showed that HF diet promoted the expression of GFAP and Iba-1 in hippocampus suggesting an excessive infiltration of glial cells and possible gliosis. Importantly, dietary teasaponin constrained the activation of Iba-1, but not GFAP, induced by the HF diet. It is known that astrocytes contain glycogen and are capable of glycogenesis. Therefore, teasaponin only inhibited the inflammatory response in microglia, but did not affect astrocytes in the hippocampus. Hippocampal inflammation is highly associated with recognition memory impairment48. In the present study, teasaponin significantly improved recognition memory in a novel object testing procedure with inhibition of microgliosis and inflammation in the hippocampus. Therefore, teasaponin, in preventing hyperendotoxemia and hippocampal inflammation, may contribute to recognition memory improvement.
TLR4 is the receptor for LPS and expressed in microglia, astrocytes and neurons49,59. Activation of TLR4 signaling inhibits neurogenesis50, while deficiency of TLR4 dramatically ameliorates neuroinflammation in the hippocampus of C57BL/6 mice51. As a critical adapter protein for TLR4, MyD88 leads to an activation of downstream nuclear factor-κB (NFκB)/JNK and subsequent production of pro-inflammatory cytokines implicated in neurotoxicity52–54. In this study, dietary teasaponin prevented the activation of the TLR4-My88-JNK/NFκB inflammatory signaling pathway and an over-expression of pro-inflammatory cytokines in the hippocampus induced by a chronic HF diet. Previous studies have demonstrated that inflammation decreases the expression of BDNF in the rat hippocampus55,56 and reduced hippocampal BDNF is associated with recognition memory impairment; increasing hippocampal BDNF has been shown to improve recognition memory57. In the present work, teasaponin increased BDNF levels in the hippocampus of obese mice, which may contribute to the improvement in recognition memory in these mice. Furthermore, in the present study, there was a significant positive correlation between the level of Bacteroides-Prevotella spp. and cognitive ability as measured by the discrimination index in the novel object recognition test. Indeed, a recent study reports that infants with high levels of gut Bacteroides at 1 year of age show higher cognitive ability at 2 years old58. Overall, dietary teasaponin may improve microbiota-gut-brain function and cognition.
In conclusion, this study demonstrated that dietary teasaponin reinstalls gut microbiota, and reduces gut mucosal inflammation and hyperendotoxinemia in chronic HF diet-induced obese mice. These peripheral beneficial effects are accompanied by a reduction of CNS hippocampal microglia activation, an increase of BDNF production, and an improvement of cognitive function in obese mice. The behavioral and neurochemical improvements suggest that teasaponin could be a promising strategy to improve gut-brain axis for the prevention of HF diet-induced obesity and associated cognitive decline. This study demonstrated the beneficial effect of teasaponin on gut microbiota using qRT-PCR. To further characterize detailed changes of gut microbiota composition, future studies should use 16S rRNA sequencing or metagenomic sequencing. Furthermore, to elucidate the effects on the brain-gut axis, more candidate species of microbiota, which may be involved in processes such as amyloid secreting, γ-aminobutyric acid biosynthesis, short-chain fatty acids biosynthesis, histone deacetylation (HDAC) and G-protein-coupled receptors, should be analysed.
Methods
Animals and treatments
Thirty-six C57BL/6 J male mice (8 weeks old) were purchased from the Australian Bio-Resource Centre (Moss Vale, NSW), and housed in environmentally controlled conditions (temperature 22 °C, 12 hour light/dark cycle). Twelve mice received a lab chow diet as a control (Con group). Twenty-four mice received a HF diet (HF group) containing 60% of energy from fat (SF13-092; Specialty Feeds, Glen Forrest, WA). After 8 weeks, the twenty-four mice fed a HF diet were divided into two groups: twelve mice continued to be fed the HF diet, and the other twelve mice were fed this diet with the addition of 0.5% teasaponin60 (HF + TS group) for 6 weeks. Teasaponin (96%, C57H90O26, MW = 1200) was purchased from the Aladdin Chemistry Co. Ltd, China. Body weight was measured on the last day in every week. Food intake was recorded on the first day in every week. A weighed amount of fresh diet was given at the beginning of the dark cycle. The remaining food in the cage plus spillage were collected and weighed 24 hours later. After 6 weeks of treatment, the novel object recognition test and intraperitoneal glucose tolerance test (IPGTT described below) were carried out. All mice were asphyxiated in chambers prefilled with CO2 four days after the tests were carried out. Plasma, cecal contents, colon tissue and hippocampus tissues were collected, snap frozen and stored at −80 °C for further analyses as detailed below. The study was approved by the University of Wollongong Animal Ethics Committee (AE13/11) and all animal experiments were conducted in compliance with the National Health and Medical Research Council Australian, Code of Practice for the Care and Use of Animals for Scientific Purposes (2004).
Real-time PCR (qRT-PCR) to quantify microbial species from cecal content
DNA from cecal contents were extracted by the QIAamp DNA Stool Minikit (QIAGEN, Germany) according to the manufacturer’s instructions. Group-specific primers based on 16S rDNA sequences PCR assay were forward Bacteroides-Prevotella, GAGAGGAAGGTCCCCCAC; reverse Bacteroides-Prevotella, CGCTACTTGGCTGGTTCAG; forward Desulfovibrios, CCGTAGATATCTGGAGGAACATCAG; reverse Desulfovibrios, ACATCTAGCATCCATCGTTTACAGC; forward Lactobacillus, GAGGCAGCAGTAGGGAATCTTC; reverse Lactobacillus, GGCCAGTTACTACCTCTATCCTTCTTC; forward Bifidobacterium, CGCGTCTGGTGTGAAAG; reverse Bifidobacterium, CCCCACATCCAGCATCCA. Quantitative real-time PCR was performed in a 20-μL final reaction volume using a SYBR green I master in a Lightcycler 480 (F. Hoffmann-La Roche Ltd, Switzerland). Amplification was carried out with 40 cycles of 95 °C for 5 seconds, 60 °C for 10 seconds, and 72 °C for 10 seconds. Each assay was performed in duplicate in the same run. The level of expression for each gene was calculated using the comparative threshold cycle value (Ct) method, using the formula 2−ΔΔCt (where ΔΔCt = ΔCt sample–ΔCt reference). The final results were expressed as normalized fold values relative to the normal group as described previously61.
Lipopolysaccharide (LPS) determination
The concentration of plasma LPS was measured by enzyme-linked immunosorbent assay (LAL assay kit, Hycult Biotech, The Netherlands). The absorbance at 405 nm was measured with a spectrophotometer. The measurable concentration ranges was from 0.04 to 10 EU/ml. All samples for LPS measurements were performed in duplicate.
Western blotting
Hippocampi were dissected and homogenized in NP-40 lysis buffer. The following antibodies were used: MyD88 (1:1000 dilution; sc-74532), NFκB (1:1000 dilution; sc-7178), p-JNK (1:1000 dilution; sc-81502), IL-1β (1:1000 dilution; sc-7884), BDNF (1:1000 dilution; sc-20981) and IL-6 (1:1000 dilution; sc-7920) all purchased from Santa Cruz Biotechnology (Santa Cruz, CA); TNF-α (1:1000 dilution; #11948) and TLR4 (1:1000 dilution; #2219) were purchased from Cell Signaling Technology (Beverly, MA). The bands corresponding to the proteins of interest were scanned and band densities were analyzed using the automatic imaging analysis system, Quantity One (Bio-Rad Laboratories, Hercules, CA). All quantitative analyses were compared with the control group.
Immunohistochemistry
As described previously62, fixed colon tissues were sectioned at 5 µm and incubated overnight at 4 °C with primary antibodies. Sections were then incubated consecutively with secondary antibodies for 2 hours at room temperature. The sections were then washed and incubated with streptavidin-HRP polymer conjugate (Sigma-Aldrich, Sydney, Australia). The sections were then washed and developed using the ImmPACT DAB peroxidase substrate kit (Vector laboratories Inc., Burlingame, USA) and counterstained with haematoxylin (POCD Scientific, Artarmon, Australia). Six fields from three sections of each mouse were captured. The immunohistochemical staining F4/80, CD11c, and CD206 were quantified as a percentage of positive area per image by Image J software. The immunoreactivity is quantified as the percentage of pixels in an area of interest that have intensity greater than the background.
Fixed hippocampus tissues were sectioned at 35 µm. The sections were washed 3 times with Tris-Buffered Saline (TBS) for 10 minutes, and then washed in 1% H2O2 in phosphate buffer saline (PBS) for 30 minutes. After three washings with PBS for 5 minutes, the sections were blocked with 5% normal horse serum for 1 hour and incubated overnight at 4 °C with primary antibodies. Then sections were washed 3 times with PBS and Tween 20 (PBST) and incubated consecutively with secondary antibodies for 2 hours at room temperature. The sections were then washed and incubated with streptavidin-HRP polymer conjugate (Sigma-Aldrich Pty. Ltd, Sydney, Australia) for 1 hour at room temperature. The sections were then washed and developed using the ImmPACT DAB peroxidase substrate kit. Six fields from three sections of each mouse were viewed under a Leica microscope and digital photographs were captured. Iba1 and GFAP were used to evaluate the activated phenotype of microglia and astrocytes according to Iba1 and GFAP positive staining and cell morphology. Positive cells were manually counted using Photoshop (Adobe).
Novel object recognition test
Recognition memory was assessed by performing a novel object recognition test based on our group’s previous studies5. Briefly, mice received a 5 minutes habituation in an empty open field arena (a white open-field square box, 55 cm × 55 cm × 35 cm). During the training session, two identical objects (A) were placed at opposing corners of the box, 5 cm from the adjacent wall. Each mouse was then placed in the middle of the open-field box and left to explore the objects for 5 minutes, and the percentage of time spent exploring the two identical objects in the open-field box was calculated (time spent on the two identical objects divided by 5 minutes). For the retention session, one familiar object (A) was replaced with one novel object (B) and measurements were taken according to how much time each mouse spent at each object as per the training session. Following a 90-minute inter-trial interval, we returned mice to explore for another 5 minutes. Novel object exploration time and the discrimination index (DI = [(Novel Object Exploration Time/Total Exploration Time) − (Familiar Object Exploration Time/Total Exploration Time)] × 100) were used to evaluate the recognition memory of the mice63.
Intraperitoneal glucose tolerance test
All mice were fasted overnight before a glucose tolerance test was performed to assess glucose clearance, following an intraperitoneal injection of glucose (0.5 g/kg; Sigma-Aldrich, St Louis, MO, USA). Blood samples were taken from the tail vein at 0, 15, 30, 60 and 120 minutes following the injection of glucose. Blood glucose was measured using an Accu-Chek glucometer (Roche Diagnostics GmbH Mannheim, Germany).
Statistical analysis
The statistical package SPSS 20 (SPSS, Chicago, IL) was used to analyze data. Data was first tested for normality before differences among the Con, HF and HF + TS groups were determined using one-way analysis of variance (ANOVA). This was followed by the post hoc Tukey-Kramer honestly significant difference (HSD) test for multiple comparisons among the groups. A p value of <0.05 was considered to be statistically significant. Values were expressed as mean ± SEM. Pearson’s correlations were used to examine the relationship between the discrimination index in the novel object recognition test and plasma LPS and BDNF levels in the hippocampus.
Acknowledgements
The authors wish to thank the following individuals for their contributions. A/Prof. K. Russell (Department of Applied Statistics, University of Wollongong) for his suggestions regarding the statistical analysis, A/Prof. Eleanor Beck for her editorial revision of the manuscript. This study was supported by Diabetes Australia Research Trust Research Projects to Prof XF Huang. Y.H.Y. is supported by the National Health and Medical Research Council of Australia (NHMRC 573441).
Author Contributions
S.W. and Y.H.Y. researched data and wrote the manuscript. P.Z., Q.W. and K.Y.Z. collected parts of researched data. K.N. edited the manuscript. Y.H.Y. and X.F.H. contributed to the experimental design and reviewed and edited the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xu-Feng Huang, Email: xhuang@uow.edu.au.
Yinghua Yu, Email: yinghua@uow.edu.au.
References
- 1.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–81. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh-Manoux A, et al. Obesity phenotypes in midlife and cognition in early old age: the Whitehall II cohort study. Neurology. 2012;79:755–62. doi: 10.1212/WNL.0b013e3182661f63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith PA. The tantalizing links between gut microbes and the brain. Nature. 2015;526:312–4. doi: 10.1038/526312a. [DOI] [PubMed] [Google Scholar]
- 4.Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–23. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camer D, et al. Bardoxolone methyl prevents high-fat diet-induced alterations in prefrontal cortex signalling molecules involved in recognition memory. Prog Neuropsychopharmacol Biol Psychiatry. 2015;59:68–75. doi: 10.1016/j.pnpbp.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Proctor C, Thiennimitr P, Chattipakorn N, Chattipakorn SC. Diet, gut microbiota and cognition. Metab Brain Dis. 2017;32:1–17. doi: 10.1007/s11011-016-9917-8. [DOI] [PubMed] [Google Scholar]
- 7.De Luca SN, et al. Early life overfeeding impairs spatial memory performance by reducing microglial sensitivity to learning. J Neuroinflammation. 2016;13:112. doi: 10.1186/s12974-016-0578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luine V. Recognition memory tasks in neuroendocrine research. Behav Brain Res. 2015;285:158–64. doi: 10.1016/j.bbr.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA. 2004;101:14515–20. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kentner AC, Khoury A, Lima Queiroz E, MacRae M. Environmental enrichment rescues the effects of early life inflammation on markers of synaptic transmission and plasticity. Brain Behav Immun. 2016;57:151–60. doi: 10.1016/j.bbi.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Picot M, et al. Neural Androgen Receptor Deletion Impairs the Temporal Processing of Objects and Hippocampal CA1-Dependent Mechanisms. PLoS One. 2016;11:e0148328. doi: 10.1371/journal.pone.0148328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jumpertz R, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High Fat Diet-Induced Gut Microbiota Exacerbates Inflammation and Obesity in Mice via the TLR4 Signaling Pathway. PLoS One. 2012;7:e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–30. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Podbielska M, et al. Neuron-microglia interaction induced bi-directional cytotoxicity associated with calpain activation. J Neurochem. 2016;139:440–455. doi: 10.1111/jnc.13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin L, et al. Systemic LPS Causes Chronic Neuroinflammation and Progressive Neurodegeneration. Glia. 2007;55:453–62. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurley C, et al. Microglia and Astrocyte Activation by Toll-Like Receptor Ligands: Modulation by PPAR-gamma Agonists. PPAR Res. 2008;2008:453120. doi: 10.1155/2008/453120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sierra A, et al. Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis. Neural Plast. 2014;2014:610343. doi: 10.1155/2014/610343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bercik P, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 20.Wolfram S, Wang Y, Thielecke F. Anti-obesity effects of green tea: from bedside to bench. Mol Nutr Food Res. 2006;50:176–87. doi: 10.1002/mnfr.200500102. [DOI] [PubMed] [Google Scholar]
- 21.Axling U, et al. Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6 J mice. Nutr Metab (Lond) 2012;9:105. doi: 10.1186/1743-7075-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin JS, Touyama M, Hisada T, Benno Y. Effects of green tea consumption on human fecal microbiota with special reference to Bifidobacterium species. Microbiol Immunol. 2012;56:729–39. doi: 10.1111/j.1348-0421.2012.00502.x. [DOI] [PubMed] [Google Scholar]
- 23.Kuriyama S, et al. Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project 1. Am J Clin Nutr. 2006;83:355–61. doi: 10.1093/ajcn/83.2.355. [DOI] [PubMed] [Google Scholar]
- 24.Ng TP, Feng L, Niti M, Kua EH, Yap KB. Tea consumption and cognitive impairment and decline in older Chinese adults. Am J Clin Nutr. 2008;88:224–31. doi: 10.1093/ajcn/88.1.224. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, et al. Long-term green tea catechin administration prevents spatial learning and memory impairment in senescence-accelerated mouse prone-8 mice by decreasing Abeta1-42 oligomers and upregulating synaptic plasticity-related proteins in the hippocampus. Neuroscience. 2009;163:741–9. doi: 10.1016/j.neuroscience.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 26.van Duynhoven J, et al. Interactions of black tea polyphenols with human gut microbiota: implications for gut and cardiovascular health. Am J Clin Nutr. 2013;98:1631S–1641S. doi: 10.3945/ajcn.113.058263. [DOI] [PubMed] [Google Scholar]
- 27.Wang P, et al. Oat avenanthramide-C (2c) is biotransformed by mice and the human microbiota into bioactive metabolites. J Nutr. 2015;145:239–45. doi: 10.3945/jn.114.206508. [DOI] [PubMed] [Google Scholar]
- 28.Duda-Chodak A, Tarko T, Satora P, Sroka P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur J Nutr. 2015;54:325–41. doi: 10.1007/s00394-015-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hildebrandt MA, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–24. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med. 2013;34:39–58. doi: 10.1016/j.mam.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Cani PD, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 32.Neal MD, et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176:3070–9. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 33.Lim SM, Jeong JJ, Woo KH, Han MJ, Kim DH. Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr Res. 2016;36:337–48. doi: 10.1016/j.nutres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Karuppagounder V, et al. Modulation of Macrophage Polarization and HMGB1-TLR2/TLR4 Cascade Plays a Crucial Role for Cardiac Remodeling in Senescence-Accelerated Prone Mice. PLoS One. 2016;11:e0152922. doi: 10.1371/journal.pone.0152922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singla DK, Singla R, Wang J. BMP-7 Treatment Increases M2 Macrophage Differentiation and Reduces Inflammation and Plaque Formation in Apo E−/− Mice. PLoS One. 2016;11:e0147897. doi: 10.1371/journal.pone.0147897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin H, An Y, Hao F, Wang Y, Tang H. Correlations of Fecal Metabonomic and Microbiomic Changes Induced by High-fat Diet in the Pre-Obesity State. Sci Rep. 2016;6:21618. doi: 10.1038/srep21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cani PD, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–83. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 38.Moreira AP, et al. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr. 2012;108:801–9. doi: 10.1017/S0007114512001213. [DOI] [PubMed] [Google Scholar]
- 39.Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS One. 2009;4:e7125. doi: 10.1371/journal.pone.0007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Million M, et al. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int J Obes (Lond) 2013;37:1460–6. doi: 10.1038/ijo.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Cani PD, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 42.Steenhout PG, Rochat F, Hager C. The effect of Bifidobacterium lactis on the growth of infants: a pooled analysis of randomized controlled studies. Ann Nutr Metab. 2009;55:334–40. doi: 10.1159/000248992. [DOI] [PubMed] [Google Scholar]
- 43.Million M, et al. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog. 2012;53:100–8. doi: 10.1016/j.micpath.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Hu WL, Wu YM, Liu JX, Guo YQ, Ye JA. Tea saponins affect in vitro fermentation and methanogenesis in faunated and defaunated rumen fluid. J Zhejiang Univ Sci B. 2005;6:787–92. doi: 10.1631/jzus.2005.B0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng Y, et al. Effects of tea saponin on glucan conversion and bonding behaviour of cellulolytic enzymes during enzymatic hydrolysis of corncob residue with high lignin content. Biotechnol Biofuels. 2013;6:161. doi: 10.1186/1754-6834-6-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang T, et al. LXW7 ameliorates focal cerebral ischemia injury and attenuates inflammatory responses in activated microglia in rats. Braz J Med Biol Res. 2016;49:e5287. doi: 10.1590/1414-431X20165287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Legido A, Katsetos CD. Experimental studies in epilepsy: immunologic and inflammatory mechanisms. Semin Pediatr Neurol. 2014;21:197–206. doi: 10.1016/j.spen.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Balducci C, et al. Toll-like receptor 4-dependent glial cell activation mediates the impairment in memory establishment induced by β-amyloid oligomers in an acute mouse model of Alzheimer’s disease. Brain Behav Immun. 2017;60:188–197. doi: 10.1016/j.bbi.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Tang SC, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA. 2007;104:13798–803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rolls A, et al. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9:1081–8. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- 51.Chen T, et al. Toll-like receptor 4 knockout ameliorates neuroinflammation due to lung-brain interaction in mechanically ventilated mice. Brain Behav Immun. 2016;56:42–55. doi: 10.1016/j.bbi.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Li GZ, et al. Expression of myeloid differentiation primary response protein 88 (Myd88) in the cerebral cortex after experimental traumatic brain injury in rats. Brain Res. 2011;1396:96–104. doi: 10.1016/j.brainres.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, et al. Lipopolysaccharide sensitizes neonatal hypoxic-ischemic brain injury in a MyD88-dependent manner. J Immunol. 2009;183:7471–7. doi: 10.4049/jimmunol.0900762. [DOI] [PubMed] [Google Scholar]
- 54.Tao L, et al. 1-O-tigloyl-1-O-deacetyl-nimbolinin B inhibits LPS-stimulated inflammatory responses by suppressing NF-κB and JNK activation in microglia cells. J Pharmacol Sci. 2014;125:364–74. doi: 10.1254/jphs.14025FP. [DOI] [PubMed] [Google Scholar]
- 55.Zhang JC, Yao W, Hashimoto K. Brain-derived Neurotrophic Factor (BDNF)-TrkB Signaling in Inflammation-related Depression and Potential Therapeutic Targets. Curr Neuropharmacol. 2016;14:721–31. doi: 10.2174/1570159X14666160119094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Motaghinejad M, Motevalian M, Shabab B. Neuroprotective effects of various doses of topiramate against methylphenidate induced oxidative stress and inflammation in rat isolated hippocampus. Clin Exp Pharmacol Physiol. 2016;43:360–71. doi: 10.1111/1440-1681.12538. [DOI] [PubMed] [Google Scholar]
- 57.Jeon SJ, et al. Oleanolic acid ameliorates cognitive dysfunction caused by cholinergic blockade via TrkB-dependent BDNF signaling. Neuropharmacology. 2016;113(Pt A):100–109. doi: 10.1016/j.neuropharm.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 58.Carlson, A. L. et al. Variation in the infant gut microbiome is associated with cognitive development and brain morphometry. In Annual Meeting of the American College of Neuropsychoparmacology. ACNP (2016).
- 59.Okun E, et al. Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis. Proc Natl Acad Sci USA. 2010;107:15625–30. doi: 10.1073/pnas.1005807107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han LK, et al. Anti-obesity effects in rodents of dietary teasaponin, a lipase inhibitor. Int J Obes Relat Metab Disord. 2001;25:1459–64. doi: 10.1038/sj.ijo.0801747. [DOI] [PubMed] [Google Scholar]
- 61.Yu Y, et al. Teasaponin reduces inflammation and central leptin resistance in diet-induced obese male mice. Endocrinology. 2013;154:3130–40. doi: 10.1210/en.2013-1218. [DOI] [PubMed] [Google Scholar]
- 62.Dinh CH, et al. Bardoxolone Methyl Prevents High-Fat Diet-Induced Colon Inflammation in Mice. J Histochem Cytochem. 2016;64:237–55. doi: 10.1369/0022155416631803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arqué G, et al. Impaired spatial learning strategies and novel object recognition in mice haploinsufficient for the dual specificity tyrosine-regulated kinase-1A (Dyrk1A) PLoS One. 2008;3:e2575. doi: 10.1371/journal.pone.0002575. [DOI] [PMC free article] [PubMed] [Google Scholar]