ABSTRACT
Widespread antibiotic use in clinical medicine and the livestock industry has contributed to the global spread of multidrug-resistant (MDR) bacterial pathogens, including Acinetobacter baumannii. We report on a method used to produce a personalized bacteriophage-based therapeutic treatment for a 68-year-old diabetic patient with necrotizing pancreatitis complicated by an MDR A. baumannii infection. Despite multiple antibiotic courses and efforts at percutaneous drainage of a pancreatic pseudocyst, the patient deteriorated over a 4-month period. In the absence of effective antibiotics, two laboratories identified nine different bacteriophages with lytic activity for an A. baumannii isolate from the patient. Administration of these bacteriophages intravenously and percutaneously into the abscess cavities was associated with reversal of the patient's downward clinical trajectory, clearance of the A. baumannii infection, and a return to health. The outcome of this case suggests that the methods described here for the production of bacteriophage therapeutics could be applied to similar cases and that more concerted efforts to investigate the use of therapeutic bacteriophages for MDR bacterial infections are warranted.
KEYWORDS: Acinetobacter, bacteriophage therapy, multidrug resistance
INTRODUCTION
Increased antibiotic resistance is an important global issue. The World Health Organization (WHO), the Centers for Disease Control (CDC), the National Institutes of Health (NIH), the Gates Foundation, and other entities have tried to draw public attention to the growing crisis. The CDC has termed the present time the “postantibiotic” era (1) because resistance to almost every available antibiotic abounds, and multidrug-resistant (MDR) infections are increasingly more common. This problem stems from a variety of factors, including widespread agricultural use of antibiotics, inappropriate prescription of antibiotics, a decrease in the number of new antibiotics entering the market, and the increased positive selection of multidrug resistance when gained through the natural prokaryotic exchange of genetic material (reviewed in reference 2).
The ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) pathogens are a group of commonly MDR organisms at the heart of the antibiotic resistance crisis. As one of the ESKAPE pathogens, Acinetobacter baumannii is a frequently isolated organism from infections in clinical settings and has been of particular concern to active-duty military service members injured in combat (3). A. baumannii is a Gram-negative, naturally competent organism that is highly adept at acquiring and maintaining multiple genetic elements encoding antimicrobial resistance determinants (4–6). The growing impact of MDR pathogens has increased initiatives to discover infectious disease therapeutics with novel mechanisms of action (7, 8). Recently, as part of a collaboration between the U.S. Army and the U.S. Navy, we rescued mice from an MDR A. baumannii wound infection using a mixture of natural phages that were selected specifically for their synergistic activity against the infecting A. baumannii strain (9). Even with these positive results, there are relatively few studies of phage efficacy and/or safety in the current medical literature (relevant examples include references 10, to ,18). In addition to demonstrating that phage treatment can be efficacious when a panel of phages are isolated from the environment against the particular strain causing the infection, the A. baumannii study also demonstrated that the surviving A. baumannii bacteria had decreased virulence, as measured in the Galleria mellonella (wax worm) model (9). This decreased virulence of phage-resistant A. baumannii was correlated with decreased capsule production, as measured by Raman spectroscopy (9). In the present study, a bacterial isolate was provided from a patient suffering from necrotizing pancreatitis complicated by an MDR A. baumannii-infected pancreatic pseudocyst. In two independent laboratories, the patient's initial isolate was used to screen previously isolated phages and to select new phages for incorporation into phage mixtures to make specific phage cocktails. Food and Drug Administration (FDA) authorization to administer the cocktails as an emergency investigational new drug (eIND) was obtained, and the patient's condition improved dramatically following phage therapy. Here, we present our investigation of certain key aspects of the phage therapy in vivo, such as cocktail development, pharmacokinetics, immune response, and phage resistance. Despite an eventual rise of phage resistance, an iterative process of phage cocktail formulation resulted in resolution of the patient's infection. Interestingly, the phage-resistant phenotype that arose over time was associated with increased antibiotic sensitivity when phage and antibiotics were simultaneously administered.
RESULTS
A. baumannii susceptibility to bacteriophages.
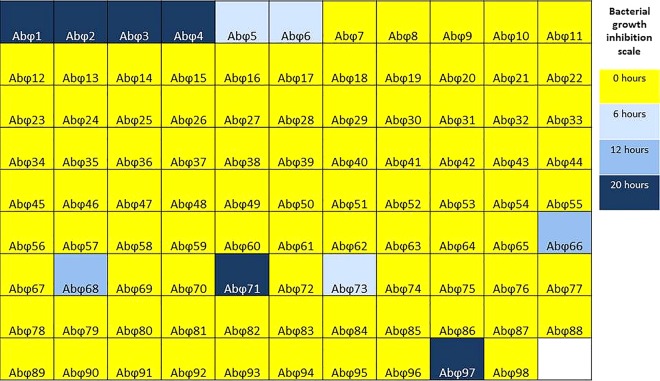
The clinical course of the patient, a 68-year-old diabetic man who developed necrotizing pancreatitis complicated by an MDR A. baumannii-infected pancreatic pseudocyst, is illustrated in Fig. 1. Due to the patient's rapidly deteriorating condition and the inability to control his infection with antibiotics, a bacteriophage therapy process was embarked upon as an eIND. A subset of 98 A. baumannii-specific lytic bacteriophages (out of 200 total) that were previously harvested from environmental sources by the Biological Defense Research Directorate (BDRD) of the Naval Medical Research Center (NMRC) were screened for activity against the three clinical isolates in this study (Fig. 2). This subset of 98 phages was initially selected for screening because previous work had demonstrated that, collectively, these bacteriophages are highly active against a broad range of MDR A. baumannii isolates (data not shown). Within 18 h of receipt of the first clinical isolate, selection of an appropriate therapeutic cocktail for the original isolate, named TP1, was achieved via an OmniLog-based time-kill assay system (19, 20). The bacterial isolates from this study are summarized in Table 1. The assay revealed that most of the phage screened have almost no discernible effect against this clinical isolate. However, phage AbΦ5, AbΦ6, and AbΦ73 were able to inhibit growth for up to 6 h. Phage AbΦ66 and AbΦ68 were able to inhibit growth for 12 h. Most notably, phages AB-Navy1, AbΦ2, AbΦ3, AB-Navy4, AB-Navy71, and AB-Navy97 were able to inhibit growth for over 20 h. Phage AB-Navy1, AB-Navy4, AB-Navy71, and AB-Navy97 were used in the phage cocktail based on previous host range analysis (data not shown) that indicated that each phage was different from the others. TP1 growth inhibition effects were more pronounced when a cocktail of these bacteriophages was used than when individual bacteriophages were used separately (Fig. 3).
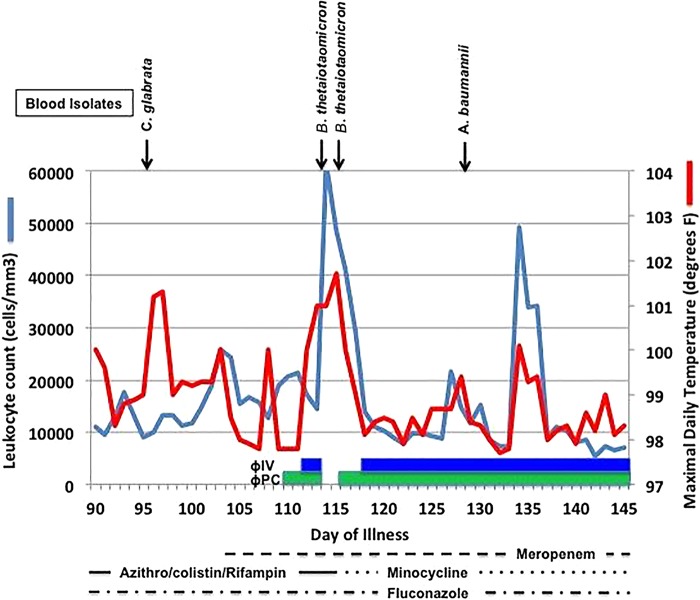
FIG 1.
Clinical course before and during the initial phase of bacteriophage therapy. Positive blood cultures are depicted above the graphic data, whereas antibiotic and phage administration are indicated below.
FIG 2.
Screening of A. baumannii phage library against TP1 clinical isolate. A subset of 98 phages from the Navy phage library were individually tested against the A. baumannii TP1 strain using the OmniLog system (Biolog, Hayward, CA) as described in references 19 and 20. Briefly, growth of bacteria or lack of growth due to lysis from phage infection was monitored every 15 min via a redox chemical reaction employing cellular respiration as a universal reporter, where cellular respiration from growth reduces a tetrazolium-based dye and produces a color change. If the growth is weakly positive or is negative, then respiration is slow or absent and so little to no color change is observed. The results of this assay after 20 h are summarized here, where the color gradient indicates the duration of bacterial growth inhibition.
TABLE 1.
Bacterial isolates from this study
| Strain | Isolation date | Site of isolation | Phenotype |
|---|---|---|---|
| TP1 | 10 March 2016 | Pancreatic drainage of patient | Capsulated, susceptible to ΦIV |
| TP2 | 21 March 2016 | Pancreatic drainage of patient | Capsulated, reduced susceptibility to ΦIV |
| TP3 | 23 March 2016 | Pancreatic drainage of patient | Unencapsulated, resistant to ΦIV |
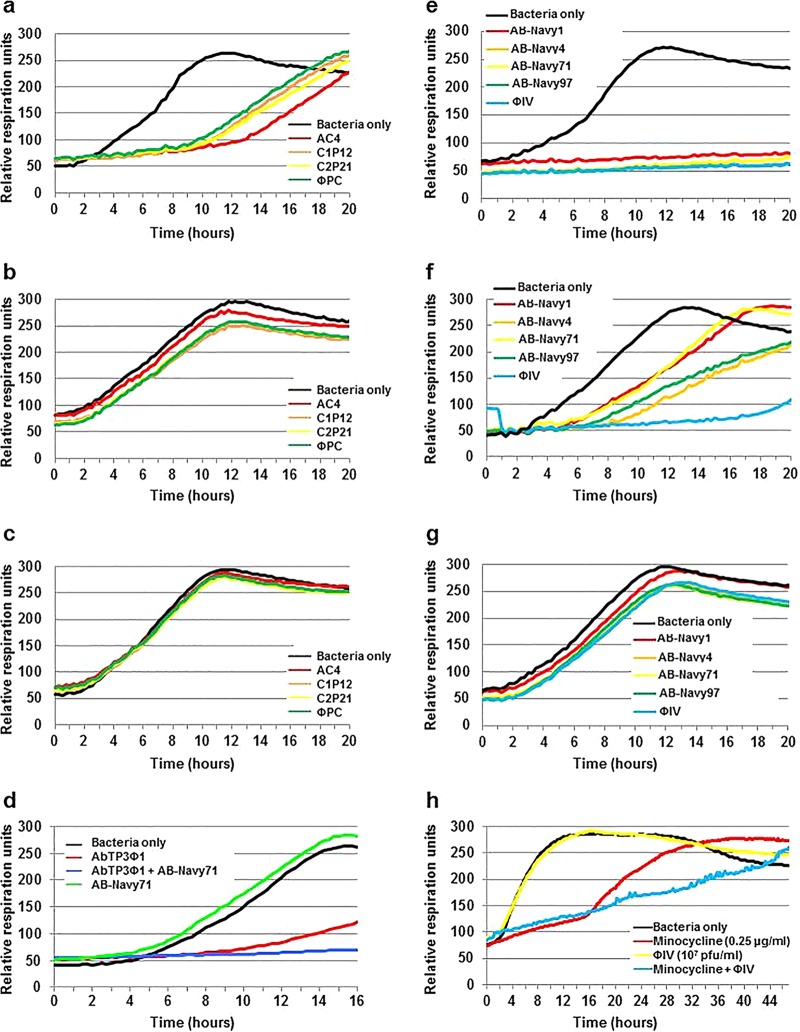
FIG 3.
Activity of bacteriophage cocktails. Activity of cocktails ΦPC and ΦIV against serial isolates of A. baumannii isolated from intra-abdominal drains before bacteriophage therapy (strain TP1) (a and e), 4 days after initiation of antibiotic therapy (strain TP2) (b and f), and 8 days after initiation of bacteriophage therapy (strain TP3) (c and g). Panel d demonstrates the derivation of a second-generation bacteriophage cocktail directed at the TP3 A. baumannii strain, a mixture of AB-Navy71 and AbTP3Φ1. Panel h demonstrates the additive activity of the ΦIV bacteriophage cocktail (107 PFU) and a sublethal concentration of minocycline (0.25 μg/ml) against A. baumannii strain TP3. The 50% inhibitory concentrations of minocycline for A. baumannii strains TP1, TP2, and TP3 were 1, 2, and 4 μg/ml, respectively.
Initial screening of 37 A. baumannii-specific bacteriophages against the TP1 isolate at the Center for Phage Technology (CPT) at Texas A & M University revealed that one of these bacteriophages, AC4 (originally obtained from AmpliPhi Corporation) inhibited TP1 growth. Subsequently, three more bacteriophages (C1P12, C2P21, and C2P24) were identified after additional screening of 100 environmental samples available at the CPT. The capability of these three new bacteriophage isolates (C1P12, C2P21, and C2P24) to inhibit TP1 growth was similar to that of bacteriophage AC4 (Fig. 3). The individual bacteriophages used in this study are detailed in Table 2, and the composition and administration of the bacteriophage cocktails are detailed in Table 3. With continued treatment, in vitro susceptibility studies of serial A. baumannii isolates demonstrated stepwise selection of resistance to the eight phages present in the original therapeutic cocktails. Representative data related to the antimicrobial activity of the bacteriophages comprising the ΦIV and ΦPC cocktails over the initial 3 weeks of therapy are presented in Fig. 3a to c and Fig. 3e to g, respectively. It was determined retrospectively on day 19 of phage treatment by use of the OmniLog-based time-kill assay system that by day 116 (which was the 8th day of bacteriophage therapy), each of the phages had lost activity individually and in their respective original mixtures against the A. baumannii isolates that emerged in the presence of the phages. In other words, the TP3 isolate was found in vitro to be resistant to both of the two original bacteriophage cocktails. Therefore, an additional phage, AbTP3Φ01, was selected for its activity against TP3 and combined with one of the original phages (AB-Navy71) to produce a third phage cocktail, called ΦIVB (Table 3), which was then administered to the patient.
TABLE 2.
Phages used in this study
| Phage | Activity against indicated isolate(s) | Source | Source of isolate | Family |
|---|---|---|---|---|
| AB-Navy1 | TP1 | U.S. Navy phage library | Sewage water | Myoviridae |
| AB-Navy4 | TP1 | U.S. Navy phage library | Sewage water | Myoviridae |
| AB-Navy71 | TP1 | U.S. Navy phage library | Sewage water | Myoviridae |
| AB-Navy97 | TP1 | U.S. Navy phage library | Sewage water | Myoviridae |
| AbTP3Φ1 | TP1, TP2, TP3 | U.S. Navy phage library | Sewage water | Podoviridae |
| AC4 | TP1 | AmpliPhi Corporation, CA | Environmental sample | Myoviridae |
| C1P12 | TP1 | Texas A&M University | Environmental sample | Myoviridae |
| C2P21 | TP1 | Texas A&M University | Environmental sample | Myoviridae |
| C2P24 | TP1 | Texas A&M University | Environmental sample | Myoviridae |
TABLE 3.
Composition and details of administration of each phage cocktail
| Phage cocktail | Cocktail composition | Route of administration | Duration of administration | Therapeutic dose |
|---|---|---|---|---|
| ΦPC | AC4, C1P12, C2P21, C2P24 | Intracavitarya | 18 wks, beginning day 109 | NAb |
| ΦIV | AB-Navy1, AB-Navy4, AB-Navy71, AB-Navy97 | Intravenous | 16 wks, beginning day 111 | 5 × 109 PFU |
| ΦIVB | AB-Navy71, AbTP3Φ1 | Intravenous | 2 wks, beginning day 221 | 5 × 109 PFU |
Through percutaneous catheters draining the pseudocyst cavity, the gallbladder, and a third intra-abdominal cavity.
NA, not applicable (used for intracavitary washes).
Endotoxin level in bacteriophage preparation.
As potential residual endotoxin from the bacterial host cells could be harmful to the patient, each separate bacteriophage cocktail was assessed for residual endotoxin using a commercial assay. Average endotoxin levels of bacteriophage cocktails ΦPC, ΦIV, and ΦIVB were 2.4 × 103 endotoxin units (EU)/ml, 5.89 × 103 EU/ml, and 1.64 × 103 EU/ml, respectively. Therefore, each bacteriophage cocktail preparation was diluted accordingly in lactated Ringer's solution to meet the FDA-recommended endotoxin limitation for intravenous (i.v.) application of 5 EU/kg of body weight/h.
Outcome of bacteriophage therapy.
The patient's prognosis was grave when bacteriophage therapy was first initiated with cocktail ΦPC through percutaneous catheters draining the pseudocyst cavity, the biliary cavity, and a third intra-abdominal cavity. During this time, the patient was unresponsive to commands and had developed renal failure with a creatinine level of 3.68 mg/dl. Over the next 36 h, his clinical condition was stable, but he remained comatose, intubated, and on three pressors with worsening renal and hepatic function. In view of his ongoing critical clinical condition and since it was clear that his A. baumannii infection included anatomic sites well beyond the intra-abdominal cavities, an additional systemic administration of the bacteriophage therapy was instigated through intravenous administration of a new bacteriophage cocktail (ΦIV). The intravenous bacteriophage administration was well tolerated and was repeated at increasingly frequent intervals over the next 2 days. The patient's pressor requirements diminished, and he abruptly awoke from his coma and became conversant with his family for the first time in several weeks. It was then noted that a recent A. baumannii isolate was susceptible to minocycline, and that antibiotic was added to his regimen 4 days after the initial administration of cocktail ΦPC. Over the ensuing 3 weeks, the course remained complex, but the patient generally demonstrated ongoing improvement on all fronts. His mental status continued to improve, and he was fully conversant and lucid. He was weaned off the ventilator, his pressors were gradually weaned and discontinued, and his renal function gradually improved. Bacteriophage therapy was continued for an additional 8 weeks, during which time he demonstrated continued clinical improvement. All drains were removed and he was discharged home on day 245. He has subsequently returned to work.
Emergence of bacteriophage-resistant bacteria during bacteriophage therapy.
During the course of treatment, the bacteriophages used in this study were examined for in vitro activity against successive patient isolates as those isolates became available. Consequently, it was discovered that an A. baumannii isolate, TP3, which was isolated 8 days after initiation of bacteriophage therapy, was resistant to both of the initially used bacteriophage cocktails (ΦPC and ΦIV). Therefore, TP3 was used to rapidly (within 72 h) select for additional bacteriophages with lytic activity against this isolate. Using conventional bacteriophage enrichment techniques, an additional bacteriophage, AbTP3Φ01, was isolated from raw sewage that inhibited growth of isolate TP3. Interestingly, this effect was enhanced when AbTP3Φ01 was combined with bacteriophage AB-Navy71 from the original ΦIV cocktail (Fig. 3d). The activity of the ΦIV cocktail was tested in combination with different concentrations of minocycline to determine whether the bacteriophage cocktail affected the activity of sub-MICs of the drug. As shown in Fig. 3h, although the bacteriophage cocktail itself had lost activity against the organism, the bacteriophage appeared to prevent the outgrowth of bacteria with enhanced minocycline resistance after extended culture.
Bacteriophage pharmacokinetics.
To better understand the pharmacokinetics of intravenous administration of therapeutic bacteriophages, we examined the titer of active phage in plasma samples after i.v. administration of the ΦIVB phage cocktail. We found bacteriophage concentrations of 18,000 PFU/ml in plasma 5 min after an i.v. bolus of 4 × 109 PFU of bacteriophages. These levels fell over the 6-h dosing interval (Fig. 4). An in vitro study of phage inactivation in plasma was subsequently conducted using plasma collected 90 days following cessation of phage therapy. The study demonstrated that the titer of each phage in the ΦIVB phage cocktail as well as phage AbTP3Φ1 declined at a higher rate when suspended in the patient's plasma than when suspended in normal saline. This supports the possibility that phage neutralization by plasma might be one of the contributors to the decay of phage activity in plasma in the pharmacokinetic studies (see the supplemental material).
FIG 4.
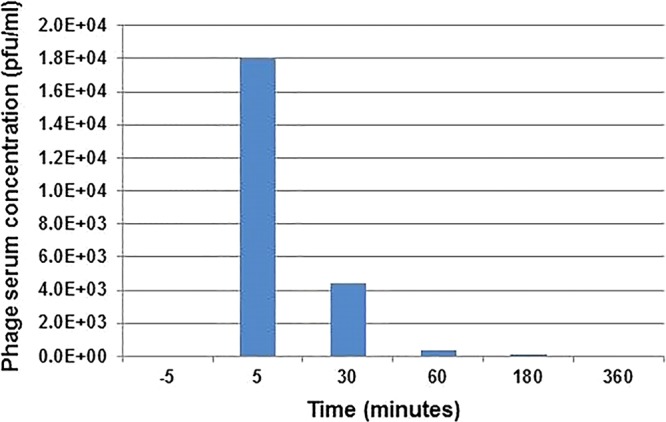
Bacteriophage titer from plasma samples during bacteriophage therapy. Plasma samples collected 5 min prior to and following administration of 5 × 109 PFU of bacteriophage via intravenous injection indicated that bacteriophage titers in systemic circulation increase rapidly from 0 PFU/ml to 1.8 × 104 PFU/ml. The bacteriophage titer dropped to 4.4 × 103 PFU/ml by 30 min, 3.3 × 102 PFU/ml by 60 min, and 20 PFU/ml by 120 min postinjection. Plasma samples collected 6 h following the initial injection contained no detectable bacteriophage titer (limit of bacteriophage detection, 20 PFU/ml).
Morphological characteristics of bacteriophages and the hosts.
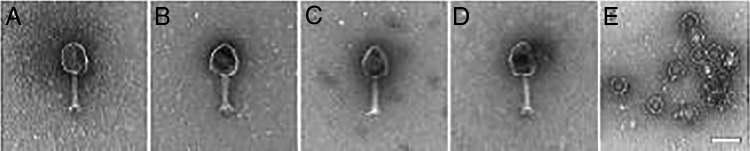
Electron micrographs of the ΦIV and ΦPC cocktail bacteriophages indicated that these bacteriophages display a short tail with well-defined head structure. Morphologically, these eight bacteriophages are all consistent with the Myoviridae family of bacteriophage, whereas bacteriophage AbTP3Φ1 is comparatively small and consistent with the Podoviridae family (Fig. 5).
FIG 5.
Transmission electron micrographs of A. baumannii-specific bacteriophages. Electron micrographs of the ΦIV cocktail phages showed large prolate myobacteriophage morphology (a to d) and short small podophage morphology (e). Bacteriophage AbTP3Φ1, isolated against strain TP3, is a small podophage. (a) AB-Navy1; (b) AB-Navy4; (c) AB-Navy71; (d)AB-Navy97; (e) AbTP3Φ1.
In addition, we sought to investigate the morphological characteristics of the bacterial isolates and some phenotypic differences observed among them. First, following cultivation of the patient's isolates in the laboratory, a “sticky” phenotype was observed only for TP3. As organisms absorbed water, a mucinous overlayer was formed.
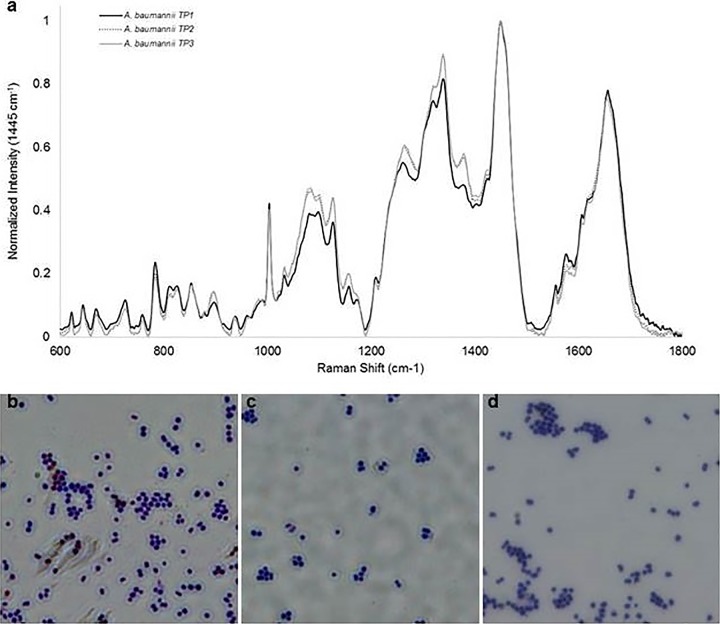
Second, Raman spectroscopy was conducted on TP1, TP2, and TP3 with the purpose of examining alterations in molecular composition of bacterial isolates during the evolution of phage resistance. Mean Raman spectra of each isolate are shown in Fig. 6a. Raman spectra are dominated by polysaccharide and protein contributions. The Raman spectral band at 979 cm−1 previously reported to be associated with capsule-related colony morphology and phage susceptibility in AB5075 strains (9) was not observed in any of the interrogated strains, suggesting alterations to capsule structure in the encapsulated TP strains. When the spectra of TP1 (capsulated, susceptible to first cocktail), TP2 (capsulated, reduced susceptibility to first cocktail), and TP3 (unencapsulated, resistant to first cocktail) are compared, the intensity within the 1,030 to 1,380 cm−1 region is significantly increased for TP2 and TP3 spectra and only minor alterations in relative peak intensities are observed across all thee strains. For bacterial isolates, the 1,030 to 1,380 cm−1 spectral region is composed of contributions from both proteins and polysaccharides. There are no observed changes within the amide I region (1,600 to 1,700 cm−1), a spectral region sensitive to changes in protein contribution and secondary structure and not associated with polysaccharides (21, 22). The marked increase in peak intensity within 1,030 to 1,380 cm−1 can therefore be attributed to increased polysaccharide content in TP2 and TP3 samples. Loss of the bacterial capsule in TP3 with an observed increase in polysaccharide content suggests increased production of extracellular polymeric substance in response to phage treatment and may contribute to the transition in phage susceptibility observed from TP1 to TP3. Finally, given these results, we performed capsular staining of TP1, TP2, and TP3 isolates. Upon staining and microscopy, TP1 and TP2 were both found to exhibit a typical capsule, whereas TP3 did not (Fig. 6b to d; see Fig. S3 to S5 in the supplemental material), consistent with these Raman spectroscopy data interpretations.
FIG 6.
Investigation into morphological characteristics of A. baumannii isolates. The apparent morphological differences of the three A. baumannii isolates were examined by Raman spectroscopy using a PhAT Probe 830-nm system (a) and by capsule staining with crystal violet (b to d). Capsule staining of isolates TP1 (b), TP2 (c), and TP3 (d) is shown.
DISCUSSION
Here we demonstrated the successful application of a novel approach to the preparation of personalized therapeutic bacteriophage cocktails to rescue a patient from a life-threatening MDR A. baumannii infection. This case supports further study of the use of phage therapy in treating patients suffering from MDR bacterial infections with limited therapeutic options. As with any uncontrolled clinical observation, there are a number of important caveats; primarily, we cannot exclude the possibility that reversal of his clinical deterioration was unrelated to the phage therapy. However, after an inexorably downhill clinical course over the prior 3 months to the point that discussions about clinical futility had been initiated, a clear turning point was observed within 48 h of starting intravenous bacteriophage therapy. The potential interplay between the bacteriophages used in the patient's therapy and minocycline is also complex. His A. baumannii infection rapidly became resistant to colistin and tigecycline early in the course of treatment, but the activity of minocycline was maintained for several weeks when it was added to his bacteriophage therapy 5 days after bacteriophage therapy was initiated. We were able to demonstrate an additive in vitro activity between the bacteriophages and subinhibitory concentrations of minocycline when used in combination against a bacteriophage-resistant A. baumannii isolate. Additive or synergistic activity between bacteriophages and traditional antibiotics has been previously demonstrated both in vitro and in animal model systems (for examples, see references 9, 18, and 23 to 25 and references therein). Of particular relevance is a recent paper by Oechslin et al. (18) describing the interaction between phage and antibiotics in a Pseudomonas aeruginosa endocarditis infection model which includes unprecedented practical evidence for the idea that the most significant benefits of phage therapy will emerge synergistically and that the emergence of phage-resistant bacteria can be avoided or delayed through combination therapy. It is worth noting the significantly different pharmacokinetic data between our study and the report by Oechslin et al. Where phage clearance was on the order of hours in the rat model, it was on the order of minutes in our human patient. Differences between the two studies could contribute to the differences observed. In the Oechslin study, the phage plasma longevity was measured using a cocktail consisting of 12 phages in uninfected rats rather than 4 phages in the presence of infection. In addition, the phage titer-to-body weight ratio differed greatly. In the animal study 1 × 1010 PFU/ml was injected into the small rat body whereas 5 × 109 phages were injected into a much larger human body in our study. It is also possible that differences in plasma clearance kinetics reflect antibody-mediated neutralization or other clearance mechanisms that were operative in our patient that were not present in the rat model. A fuller understanding of peripheral blood phage kinetics in humans will require more systematic studies in additional patients.
Bacterial mutation to phage resistance has also been associated with significant fitness costs for the bacterium. Surface features such as capsule, lipopolysaccharide, or pili may be used as bacteriophage receptors but can also be pathogenicity factors, and their loss may result in attenuated virulence (29). Although A. baumannii was not immediately cleared from abscess cavity drainage or bronchial washings, strains with substantially reduced susceptibility to the phages administered subsequently emerged. This strongly suggests that the A. baumannii population evolved in response to selection pressure exerted by the phages. In fact, we observed differences in colony morphology among these isolates, which could possibly be due to differences in capsular production and a loss of virulence, consistent with previous results obtained from phage treatment in a mouse model of A. baumannii infection (9). Results obtained from Raman spectroscopy and capsular staining are in support of this idea, with Raman spectroscopy showing subtle differences among all three isolates and what could potentially be an extracellular polymeric substance associated with TP3 and capsular staining indicating lack of capsule around TP3 cells. It has been shown in other studies that culturing A. baumannii with antibiotics at concentrations below the MIC can result in differences in capsular production (26). The lack of capsule on TP3 cells may also contribute to the enhancement of their phage-antibiotic synergy, possibly because antibiotics may more easily penetrate TP3's outer membrane (Fig. 3h). Although A. baumannii with putative reduced fitness could be isolated from open abscess cavities and bronchial washings for some time after the initiation of phage therapy, the only time phage was documented in the patient's bloodstream after therapy was initiated was in association with migration of his cholecystostomy tube into his hepatic parenchyma on day 127. The bacterial isolates from this study, as well as the bacteriophages used in treatment, are currently being characterized at the genetic level to facilitate our understanding of the mechanisms involved in the loss of capsule, the enhancement of antibiotic synergy, and the rise of bacteriophage resistance during the course of this patient's treatment with phages.
A number of concerns have been raised about the potential toxicities and the practicality of bacteriophage therapy for MDR bacterial infections. However, in this particular case, we overcame these hurdles and did not observe any discernible adverse clinical events. For example, concerns have arisen about the possibility that an accelerated lysis of Gram-negative bacterial pathogens could release clinically significant levels of endotoxin (27, 28). A parallel concern can be made for administration of some antibiotics or any other alternative treatment that could lead to rapid, widespread cell lysis. While the patient's clinical instability at the time therapy was initiated made it difficult to detect all but the most dramatic deleterious clinical effects of phage therapy, no adverse effects of phage administration were evident in association with either the intracavitary or i.v. phage administrations.
It has also been posited that phages could facilitate the transfer of genetic elements conferring drug resistance and pathogenicity among bacteria; however, by producing phage on the same bacterial isolate already present in the patient, the risk of introducing exogenous genetic information conferring increased virulence or antibiotic resistance is minimized. The inherent specificity of bacteriophage to the species if not strain level minimizes the potential of horizontal gene transfer, in comparison to more promiscuous plasmid conjugation or the uptake of exogenous DNA in naturally transformable hosts. Counter to the concerns for increased antibiotic resistance following phage therapy, in the current case, we also consistently observed decreased resistance to antibiotics after phage therapy. Additionally, the narrow bacterial host range of bacteriophages can also be a potential advantage in the treatment of MDR organisms, as their specificity would be less likely to perturb the commensal microbiome of the patient.
Since A. baumannii phages have narrow host ranges, it was necessary to identify phages capable of propagating on the TP1 strain isolated from the patient. Through a labor-intensive enterprise, two laboratories were each able to independently identify, propagate, and purify four bacteriophages with lytic activity directed at the isolate within 10 days of receiving the strain. A. baumannii isolates characterized after the start of bacteriophage therapy had reduced susceptibility to the initial bacteriophages. The emergence of bacteriophage-resistant populations of A. baumannii was likely delayed by the use of combinations of bacteriophages (see Fig. 3f for an example). When the resistant bacteria were isolated, it was possible to compose a new bacteriophage cocktail that was active against the bacteriophage-resistant A. baumannii strain (TP3) to counter the emergence of phage-resistant subpopulations. The specificity of anti-bacteriophage systems makes it unlikely that broad anti-bacteriophage mechanisms will arise, as opposed to the spread of multidrug-resistant efflux pump mechanisms in response to conventional broad-spectrum antibiotic therapy. Finally, other reported benefits of bacteriophage therapy include an ability to exhibit synergy with or restore susceptibility to conventional antimicrobial agents, as observed in this case, and the potential ability to disrupt biofilms (24, 25).
Many questions remain about the clinical potential of bacteriophage therapy for serious MDR organisms. Future studies should focus on delineating and optimizing safety, pharmacokinetics and pharmacodynamics, multiplicity of infection and valency, efficiency of bacteriophage identification and deployment, modes of administration, and methodologies for monitoring the emergence of bacteriophage resistance once therapy is initiated. In addition, the impact of phage on biofilms and on the microbiome of the host is of interest, as is how best to manage concomitant antimicrobial therapy. These trials will face the same challenges posed by those who seek to evaluate novel small-molecule antimicrobial agents in the treatment of patients with severe multidrug-resistant infections. Nonetheless, while it is clear that administering phage as a monotherapy is unlikely in cases of serious illness, with the increasing threats posed by MDR bacterial pathogens and the slow progress in the development of novel classes of traditional antimicrobial agents, novel approaches are required. In this context, clinical trials focusing on delineating the extent to which bacteriophage-based therapeutics could be used on their own as a last resort or as an adjunct to traditional antibiotics are warranted.
MATERIALS AND METHODS
A. baumannii clinical isolates.
A. baumannii clinical isolates were cultured from multiple drains, peritoneal fluid, and respiratory secretions of the patient. The three isolates used in this study were designated TP1, TP2, and TP3 for the first, second, and third temporal variants, respectively. A. baumannii isolates originally used to harvest natural phages from various environmental samples were genetically diverse and obtained from the Navy's Wound Infections Culture Collection, originally received from the Army's Multidrug-Resistant Organism Repository and Surveillance Network (MRSN) and the Naval Medical Research Unit 6 in Lima, Peru. All A. baumannii isolates were maintained on tryptic soy broth (TSB; Becton, Dickinson and Company, Sparks, MD) and stored in 15% glycerol at −80°C.
Selection of therapeutic phages.
Phages used for this treatment were selected and prepared for clinical use by two different groups. Phages provided by the Biological Defense Research Directorate (BDRD) of the Naval Medical Research Center (AB-Navy1, AB-Navy4, AB-Navy71, AB-Navy97, and AbTP3Φ1) were isolated from various environmental samples by using routine isolation techniques, as previously described (9). Briefly, A. baumannii clinical isolates from the Navy's Wound Infections Culture Collection were used to isolate and store pathogen-specific phages. Following isolation, the phages were triple plaque-purified on their respective host bacterium. Finally, small-scale phage amplifications on their corresponding host bacterium were performed to prepare the A. baumannii-specific phage library, which was subsequently stored at 4°C until required. The growth of A. baumannii clinical isolates TP1, TP2, and TP3 in the presence of phage was evaluated via spot testing and also in a Biolog imaging system (Biolog, Hayward, CA) (19, 20). In this case, one covered 96-well plate was used per phage strain and incubated at 37°C for 24 h, with wells positive only for bacteria and test wells with a single phage added at a multiplicity of infection (MOI) of approximately 100. The phage candidates that showed the strongest antibacterial activity as measured by this assay were selected for inclusion in the therapeutic cocktails: AB-Navy1, AB-Navy4, AB-Navy71, AB-Navy97 against strain TP1, and, later, AbTP3Φ1 against strain TP3.
At the Center for Phage Technology (CPT), 10 A. baumannii phages from the CPT collection and 30 more solicited from multiple academic, clinical, and corporate sources were screened for activity on TP1 by spot assays using crude lysates. A phage from AmpliPhi Corporation, AC4, was found to form plaques on lawns of TP1. Another 100 environmental samples available at the CPT were screened by enrichment, yielding three new isolates (C1P12, C2P21, and C2P24) that inhibited growth of TP1.
Propagation and purification of therapeutic bacteriophages.
Large-scale bacteriophage amplification of each BDRD bacteriophage isolate was performed in two steps before purification. First, initial amplifications (via a plate lysate method) of the phage were used to inoculate 3.6-liter large-scale liquid lysates (10). These lysates were centrifuged at 10,000 × g for 20 min to remove bacterial debris, vacuum filtered through 0.22-μm filters, and then concentrated using a Millipore Pelican 2 cassette 300,000-molecular-weight-cutoff (MWCO) tangential-flow filtration system to a volume of approximately 0.5 liter. Following concentration, the culture medium was replaced with phosphate-buffered saline (PBS) via diafiltration. The resulting lysate was further concentrated via diafiltration to a final volume of 0.2 liter prior to collection. Finally, the concentrated bacteriophage mixture was purified using CsCl isopycnic gradient centrifugation as previously described (10) and dialyzed in PBS to remove cesium chloride. Purified bacteriophages were combined into a four-bacteriophage cocktail designated ΦIV. After the patient's A. baumannii isolate became insensitive to the ΦIV bacteriophage cocktail, a second bacteriophage cocktail designated ΦIVB was prepared by combining AB-Navy71 from the original cocktail with a newly isolated bacteriophage (AbTP3Φ01) that was capable of forming plaques on lawns of A. baumannii TP3.
At the CPT, large-scale propagation and purification of phages C1P12, C2P21, C2P24, and AC4 was performed using plate lysates grown from single plaques to inoculate 1-liter logarithmic cultures of the host at an input MOI of <102 in the presence of 5 mM MgSO4. The infected cultures were grown until the onset of lysis, as measured by optical density at 550 nm (OD550), at which time sodium citrate was added to a final concentration of 10 mM. The infected culture was aerated until lysis was complete. Lysates were cleared by centrifugation (6,000 × g, 40 min, 4°C) and sterilized by filtration through 0.22-μm membranes. Bacteriophages were concentrated by centrifugation at 6,000 × g for 10 h at 4°C, and phage pellets were gently resuspended in 10 ml of Dulbecco’s phosphate-buffered saline and sterilized again by filtration. The sterilized phage suspensions were subjected to ultrafiltration and 1-octanol extraction to remove lipopolysaccharide (LPS) as previously described (30). In most cases, this final treatment was performed at the Laboratory for Viral Information at San Diego State University. The four phages were combined to comprise a cocktail (ΦPC) that was used for intracavitary administration. Phage were introduced into each cavity every 6 h.
Efficiency of plating of selected bacteriophages on clinical isolates.
To evaluate the killing efficacy of each phage on clinical isolates, a dilution series of bacteriophage preparation was spotted on a bacterial lawn to observe plaque formation (25). Briefly, 100 μl of an overnight culture of each A. baumannii isolate was used to individually inoculate 2.5 ml of 0.7% molten top agar (temperature, 50°C). The inoculated agar was spread onto tryptic soy (TS) agar plates. Top agar was allowed to cool at room temperature, and then 10-μl aliquots of 10-fold serial dilutions of each selected bacteriophage were spotted on the plate surface. All spots were allowed to fully absorb into the top agar, and the plates were incubated at 37°C for 24 h in a humidified chamber for plaque formation.
Electron microscopy.
The A. baumannii phages were grown in their corresponding host by standard procedures and purified via CsCl density gradient centrifugation. The phage preparation was fixed using a solution of 4% paraformaldehyde and 1.0% glutaraldehyde, spread onto electron microscope grids, negatively stained with uranyl acetate, and imaged in an FEI Tecnai T12 transmission electron microscope.
Endotoxin assays in phage preparations.
Endotoxin levels of each ΦIV cocktail were estimated using the QCL-1000 endpoint chromogenic LAL assay, and ΦPC endotoxin levels were determined by the PyroGene recombinant factor C assay (Lonza, Walkersville, MD), according to the manufacturer's directions.
The MDR A. baumannii infection and pathophysiology of the patient.
The patient, a 68-year-old diabetic man, developed gallstone-induced acute pancreatitis. An abdominal computed tomography (CT) scan revealed a pancreatic pseudocyst (see Fig. S1 in the supplemental material). The pseudocyst was drained through two pigtail cystogastrostomy tubes, and a pancreatic stent was placed. Cultures of the pseudocyst aspirate grew Candida albicans and MDR A. baumannii. The patient received courses of vancomycin, meropenem, colistin, and tigecycline. A subsequent culture from the pseudocyst fluid grew A. baumannii that was resistant to cephalosporins, meropenem, gentamicin, amikacin, trimethoprim-sulfamethoxazole, tetracycline, ciprofloxacin, and colistin. Susceptibility testing revealed synergy between colistin and azithromycin against the MDR A. baumannii, and treatment with these two antibiotics was initiated on day 36 after the initial infection. MDR A. baumannii was repeatedly isolated from multiple abdominal drains, the patient's clinical condition further deteriorated, and on day 51, he developed respiratory failure and hypotension requiring intubation, fluid resuscitation, pressors, and ultimately transfer to the intensive care unit. Rifampin was determined in vitro to provide added antibiotic synergy against the MDR A. baumannii isolate, and it was therefore included in his regimen. During the remainder of the second and third months of hospitalization, the patient's clinical course further deteriorated. He developed emphysematous cholecystitis and became increasingly delirious, with declining renal function and increasing leukocyte counts. Cultures of multiple drains, peritoneal fluid, and respiratory secretions all produced MDR A. baumannii. Renal and hepatic function worsened. By day 108, the patient was on multiple pressors and was unresponsive, with a plasma creatinine level of 3.68 mg/dl. Due to the unavailability of any additional effective antimicrobial agents, an eIND application was submitted to the FDA requesting authorization to treat his uncontrolled A. baumannii infection with a combination of phages.
Treatment with phage.
Phage therapy was initiated on day 109 (after the initial infection) with the installation of a cocktail containing four of the anti-A. baumannii phages (∼109 PFU/dose) described previously as ΦPC through percutaneous catheters draining the pseudocyst cavity, the gallbladder, and a third intra-abdominal cavity. Intracavitary instillations of this cocktail were continued at 6- to 12-h intervals. Thirty-six hours after the initiation of intracavitary instillations of the bacteriophage cocktail, bacteriophage therapy was intensified and broadened through the intravenous administration of an additional bacteriophage cocktail (∼109 PFU/dose) consisting of the Navy's four anti-A. baumannii bacteriophages (described previously as ΦIV). Since this was well tolerated, i.v. bacteriophage therapy was repeated 12 h later and then at increasingly frequent intervals over the next 2 days to reach a dosing frequency of every 2 h. Azithromycin, colistin, and rifampin were discontinued; meropenem and fluconazole were continued. However, 2 days following initiation of intravenous phage therapy, the patient's pressor requirements abruptly increased and bacteriophage therapy was temporarily withheld. The meropenem dose was increased, and intravenous catheters were changed. It was subsequently demonstrated that the clinical deterioration was accompanied by a transient septic episode with Bacteroides thetaiotaomicron bacteria that were felt to have arisen from his pancreas. On day 115, the patient's A. baumannii isolate was found to be susceptible to minocycline (3 μg/ml), which was added to the patient's treatment regimen. Intracavitary and intravenous bacteriophage therapies were resumed on days 116 and 118, respectively. Subsequently, the combinations of intracavitary and intravenous therapy with the ΦPC and ΦIV cocktails, respectively, were continued (generally at 6- to 8-h intervals) until day 167. When reduced phage susceptibility of serial isolates of the patient's A. baumannii was demonstrated in vitro, a third bacteriophage cocktail consisting of one new bacteriophage and one of the initially selected phages (designated ΦIVB) was developed to effectively target the phage-resistant bacterial isolate and administered during the last 2 weeks of therapy. With ongoing clinical improvement, minocycline and meropenem were discontinued on days 168 and 179, respectively. Combinations of intracavitary and intravenous bacteriophage therapy were continued for a total of 59 days.
Pharmacokinetic studies.
Plasma and serum samples were collected after bacteriophage therapy and filtered through 0.22-μm Corning Spin-X filters. An aliquot of each filtrate was further diluted 1:10 and 1:100 in SM buffer, and 10 μl of each concentration of plasma/serum dilutions was mixed with 100 μl of cultured A. baumannii TP1 at an OD of 0.5. The bacteriophage-bacterium mixtures were incubated at 37°C for 20 min before being plated via soft agar overlay (31). The plates were incubated at 37°C overnight in a humidified chamber, and bacteriophage plaques were counted the next day.
Raman spectroscopic analysis of A. baumannii strains.
A. baumannii isolates were examined for modifications in cellular and extracellular composition via Raman spectroscopy using a Kaiser Rxn1 PhAT probe 830-nm system (Kaiser Optical Systems, Inc., Ann Arbor, MI) and a 1-mm diameter excitation spot size as previously described (9). Prior to spectral data acquisition, each sample was obtained from LB agar plates and directly transferred to an aluminum foil-covered disposable weigh dish for spectral data collection. Dark spectrum-subtracted and intensity-corrected spectra were each acquired using 5-s acquisitions, 10 accumulations, and the cosmic ray removal feature selected for a total laser exposure of 100 s. Each sample was assayed in three locations, one spectrum per location. Per sample, the three localized spectra were examined to ensure steady sample hydration and spectral consistency across locations. Once verified, spectra were averaged per sample, truncated to 600 to 1,800 cm−1, baseline subtracted using a sixth-order polynomial fitting routine, and normalized to the methyl/methylene scissoring band at 1,445 cm−1 (see Fig. S2 in the supplemental material for a schematic).
Capsule staining.
A. baumannii isolates were examined for potential capsule expression using crystal violet staining. For each isolate, a loopful of bacteria in PBS was used to smear a glass slide and then allowed to air dry. Each slide was stained with crystal violet for 1 min, rinsed with 20% copper sulfate, and dried prior to imaging at ×100 magnification under oil immersion using an Olympus BX51TRF microscope equipped with an Olympus DP72 camera (Olympus Corporation, Waltham, MA).
Supplementary Material
ACKNOWLEDGMENTS
The wise advice and counsel provided by Dr. Carl Merril regarding phage therapy is gratefully acknowledged. The expert advice of Dr. Cara Fiore and her colleagues at the Center for Biologics Evaluation and Research of the FDA is also acknowledged. We are indebted to Panacea Pharmaceuticals (Gaithersburg, MD) for the gift of endotoxin assay reagents and for the use of their production facilities for the preparation of phages imaged by the National Biodefense Analysis and Countermeasures Center. The authors gratefully acknowledge the many people in the phage research community who selflessly provided phage isolates for potential use in this clinical intervention, the UCSD Medical Center Research Pharmacy for its assistance in compounding and administering the phages, and the Thornton ICU staff for their expert care for this patient. Finally, T.H. would like to acknowledge Captain (U.S. Navy) Eric R. Hall, Ph.D., for early contributions to and current support of the Navy's phage program and Commander (U.S. Navy) Matthew Doan and Captain (U.S. Navy) Jacqueline D. Rychnovsky, Ph.D., for their support related to this case.
The views expressed in this manuscript are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, or the U.S. Government. T.H., L.A.E., J.M.R., and D.M.W. are U.S. military service members. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that copyright protection under this title is not available for any work of the United States Government. Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00954-17.
REFERENCES
- 1.Anonymous. 2013. Antibiotic resistance threats in the United States. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 2.Ventola CL. 2015. The antibiotic resistance crisis: part 1: causes and threats. P T 40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 3.Calhoun JH, Murray CK, Manring MM. 2008. Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin Orthop Relat Res 466:1356–1362. doi: 10.1007/s11999-008-0212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arias CA, Murray BE. 2009. Antibiotic-resistant bugs in the 21st century—a clinical super-challenge. N Engl J Med 360:439–443. doi: 10.1056/NEJMp0804651. [DOI] [PubMed] [Google Scholar]
- 5.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pendleton JN, Gorman SP, Gilmore BF. 2013. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther 11:297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- 7.Brown ED, Wright GD. 2016. Antibacterial drug discovery in the resistance era. Nature 529:336–343. doi: 10.1038/nature17042. [DOI] [PubMed] [Google Scholar]
- 8.Spellberg B, Bartlett JG, Gilbert DN. 2013. The future of antibiotics and resistance. N Engl J Med 368:299–302. doi: 10.1056/NEJMp1215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regeimbal JM, Jacobs AC, Corey BW, Henry MS, Thompson MG, Pavlicek RL, Quinones J, Hannah RM, Ghebremedhin M, Crane NJ, Zurawski DV, Teneza-Mora NC, Biswas B, Hall ER. 2016. Personalized therapeutic cocktail of wild environmental phages rescues mice from Acinetobacter baumannii wound infections. Antimicrob Agents Chemother 60:5806–5816. doi: 10.1128/AAC.02877-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biswas B, Adhya S, Washart P, Paul B, Trostel AN, Powell B, Carlton R, Merril CR. 2002. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect Immun 70:204–210. doi: 10.1128/IAI.70.1.204-210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merril CR, Biswas B, Carlton R, Jensen NC, Creed GJ, Zullo S, Adhya S. 1996. Long-circulating bacteriophage as antibacterial agents. Proc Natl Acad Sci U S A 93:3188–3192. doi: 10.1073/pnas.93.8.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhoads DD, Wolcott RD, Kuskowski MA, Wolcott BM, Ward LS, Sulakvelidze A. 2009. Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial. J Wound Care 18:237–238, 240–233. doi: 10.12968/jowc.2009.18.6.42801. [DOI] [PubMed] [Google Scholar]
- 13.Smith HW, Huggins MB. 1983. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J Gen Microbiol 129:2659–2675. [DOI] [PubMed] [Google Scholar]
- 14.Smith HW, Huggins MB. 1982. Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. J Gen Microbiol 128:307–318. [DOI] [PubMed] [Google Scholar]
- 15.Wright A, Hawkins CH, Anggard EE, Harper DR. 2009. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin Otolaryngol 34:349–357. doi: 10.1111/j.1749-4486.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 16.Sarker SA, Berger B, Deng Y, Kieser S, Foata F, Moine D, Descombes P, Sultana S, Huq S, Bardhan PK, Vuillet V, Praplan F, Brussow H. 2017. Oral application of Escherichia coli bacteriophage: safety tests in healthy and diarrheal children from Bangladesh. Environ Microbiol 19:237–250. doi: 10.1111/1462-2920.13574. [DOI] [PubMed] [Google Scholar]
- 17.Sarker SA, Sultana S, Reuteler G, Moine D, Descombes P, Charton F, Bourdin G, McCallin S, Ngom-Bru C, Neville T, Akter M, Huq S, Qadri F, Talukdar K, Kassam M, Delley M, Loiseau C, Deng Y, El Aidy S, Berger B, Brussow H. 2016. Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: a randomized trial in children from Bangladesh. EBioMedicine 4:124–137. doi: 10.1016/j.ebiom.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oechslin F, Piccardi P, Mancini S, Gabard J, Moreillon P, Entenza JM, Resch G, Que YA. 2017. Synergistic interaction between phage therapy and antibiotics clears Pseudomonas aeruginosa infection in endocarditis and reduces virulence. J Infect Dis 215:703–712. doi: 10.1093/infdis/jiw632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry M, Biswas B, Vincent L, Mokashi V, Schuch R, Bishop-Lilly KA, Sozhamannan S. 2012. Development of a high throughput assay for indirectly measuring phage growth using the OmniLog(TM) system. Bacteriophage 2:159–167. doi: 10.4161/bact.21440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estrella LA, Quinones J, Henry M, Hannah RM, Pope RK, Hamilton T, Teneza-Mora N, Hall E, Biswajit B. 2016. Characterization of novel Staphylococcus aureus lytic phage and defining their combinatorial virulence using the OmniLog(R) system. Bacteriophage 6:e1219440. doi: 10.1080/21597081.2016.1219440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Gelder J, De Gussem K, Vandenabeele P, Moens L. 2007. Reference database of Raman spectra of biological molecules. J Raman Spectrosc 38:1133–1147. doi: 10.1002/jrs.1734. [DOI] [Google Scholar]
- 22.Socrates G. 2004. Infrared and Raman characteristic group frequencies, 3rd ed John Wiley & Sons Ltd, New York, NY. [Google Scholar]
- 23.Stratton CW. 2017. Phages, fitness, virulence, and synergy: a novel approach for the therapy of infections caused by Pseudomonas aeruginosa. J Infect Dis 215:668–670. [DOI] [PubMed] [Google Scholar]
- 24.Knezevic P, Curcin S, Aleksic V, Petrusic M, Vlaski L. 2013. Phage-antibiotic synergism: a possible approach to combatting Pseudomonas aeruginosa. Res Microbiol 164:55–60. doi: 10.1016/j.resmic.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Nouraldin AAM, Baddour MM, Harfoush RAH, Essa SAM. 2016. Bacteriophage-antibiotic synergism to control planktonic and biofilm producing clinical isolates of Pseudomonas aeruginosa. Alexandria Med J 52:99–105. doi: 10.1016/j.ajme.2015.05.002. [DOI] [Google Scholar]
- 26.Geisinger E, Isberg RR. 2015. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog 11:e1004691. doi: 10.1371/journal.ppat.1004691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dufour N, Delattre R, Ricard JD, Debarbieux L. 2017. The lysis of pathogenic Escherichia coli by bacteriophages releases less endotoxin than by beta-lactams. Clin Infect Dis 64:1582–1588. doi: 10.1093/cid/cix184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cisek AA, Dabrowska I, Gregorczyk KP, Wyzewski Z. 2017. Phage therapy in bacterial infections treatment: one hundred years after the discovery of bacteriophages. Curr Microbiol 74:277–283. doi: 10.1007/s00284-016-1166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alonso AN, Perry KJ, Regeimbal JM, Regan PM, Higgins DE. 2014. Identification of Listeria monocytogenes determinants required for biofilm formation. PLoS One 9:e113696. doi: 10.1371/journal.pone.0113696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonilla N, Rojas MI, Netto Flores Cruz G, Hung SH, Rohwer F, Barr JJ. 2016. Phage on tap-a quick and efficient protocol for the preparation of bacteriophage laboratory stocks. PeerJ 4:e2261. doi: 10.7717/peerj.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams MH. 1959. Bacteriophages, 2nd ed. Interscience Publishers Inc, New York, NY. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.