Abstract
Background: Parkinson disease (PD) is a neurodegenerative disorder that has been associated with many factors, including oxidative stress, inflammation, and iron accumulation. The antioxidant, anti-inflammatory, and iron-chelating properties of epigallocatechin gallate (EGCG), a major polyphenol in green tea, may offer protection against PD.
Objective: We sought to determine the neurorescue effects of EGCG and the role of iron in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD.
Methods: We evaluated the neurorescue effect of EGCG (25 mg/kg, 7 d, oral administration) against MPTP-induced (20 mg/kg, 3 d, intraperitoneal injection) neurodegeneration in C57 male black mice. Thirty mice weighing ∼25 g were divided into 3 groups: control, MPTP, and MPTP + EGCG. The neurorescue effect of EGCG was assessed with the use of motor behavior tests, neurotransmitter analysis, oxidative stress indicators, and iron-related protein expression.
Results: Compared with the control group, MPTP treatment shortened the mice’s latency to fall from the rotarod by 16% (P < 0.05), decreased the striatal dopamine concentration by 58% (P < 0.001) and dihydroxyphenylacetic acid by 35% (P < 0.05), and increased serum protein carbonyls by 71% (P = 0.07). However, EGCG rescued MPTP-induced neurotoxicity by increasing the rotational latency by 17% (P < 0.05) to a value similar to the control group. Striatal dopamine concentrations were 40% higher in the MPTP + EGCG group than in the MPTP group (P < 0.05), but the values were significantly lower than in the control group. Compared with the MPTP and control groups, mice in the MPTP + EGCG group had higher substantia nigra ferroportin expression (44% and 35%, respectively) (P < 0.05) but not hepcidin and divalent metal transporter 1 expression.
Conclusion: Overall, our study demonstrated that EGCG regulated the iron-export protein ferroportin in substantia nigra, reduced oxidative stress, and exerted a neurorescue effect against MPTP-induced functional and neurochemical deficits in mice.
Keywords: Parkinson disease, EGCG, MPTP, ferroportin, oxidative stress
Introduction
Parkinson disease (PD) is a chronic neurodegenerative disorder characterized primarily by the progressive degeneration of dopaminergic neurons in the substantia nigra (SN), resulting in irreversible motor dysfunction such as resting tremor, bradykinesia, and postural instability (1). The exact causes and mechanisms of pathogenesis of PD remain unknown; however, the involvement of oxidative stress, chronic inflammation, and iron accumulation has been the focus of attention over the past 10 years (2, 3).
The role of oxidative stress in initiating or promoting neurodegeneration is demonstrated by postmortem brain analyses that have shown increased levels of lipid peroxidation, carbonyl modifications of proteins, and DNA and RNA oxidation (4). Neuroinflammation is also considered a major component in PD pathogenesis because the activation of microglia and accumulation of cytokines are found in both postmortem PD brains and most experimental models of PD (5). Iron accumulation is thought to be involved in PD pathogenesis because free iron can enhance oxidative stress by generating highly toxic hydroxyl radicals through the Fenton reaction. Iron accumulation can also induce neuroinflammation by stimulating microglial activation and enhancing the release of proinflammatory cytokines (6, 7). MRI has substantiated abnormal iron accumulation in the SN of PD patients as well as in postmortem brains, and this accumulation is considered to be an invariable pathologic feature of PD (8–10). It has been suggested that iron accumulation in the brain may be caused by several factors, including a disturbed blood-brain barrier, occupational exposure, or misregulation of iron-related proteins (11, 12). The hepcidin-ferroportin axis is a key regulator for cellular iron metabolism. Hepcidin is a peptide secreted primarily by the liver that regulates cellular iron efflux by binding to the iron exporter ferroportin on the cell surface and inducing its internalization and degradation (13). It has been suggested that the hepcidin-ferroportin axis is widely expressed in the brain and may play an important role in brain iron homeostasis (14, 15).
For the past several decades, several animal models of PD have been developed to study the pathophysiology and to assess the potential of neuroprotective therapies. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a widely used neurotoxin that crosses the blood-brain barrier, converts to its metabolite 1-methyl-4-phenylpyridinium (MPP+), and induces neurodegeneration by inhibiting mitochondrial complex I activity and generating reactive oxygen species (16). Studies have also demonstrated nigral iron accumulation in MPTP-induced animal models that may be associated with the altered expression of iron-related proteins, such as the increased expression of iron importer divalent metal transporter 1 (DMT1), or the decreased expression of the iron exporter ferroportin (17–19). Moreover, the effectiveness of feeding an iron-deficient diet or pharmacologic iron chelation therapy to prevent MPTP-induced neurotoxicity further supports iron participation in MPTP-induced neurodegeneration (20, 21).
Green tea has been widely consumed in Asian countries, and an inverse relation between tea consumption and the incidence of dementia, PD, or Alzheimer disease has been reported (22–24). Epigallocatechin gallate (EGCG), the most abundant green tea polyphenol, has free-radical scavenging, iron-chelating, and anti-inflammatory properties (25). Several studies have demonstrated the neuroprotective effects of EGCG against MPP+- or MPTP-induced neurodegeneration in both cell culture and animal models of PD (26–28). One study showed that EGCG suppressed MPP+-induced oxidative stress in PC12 cells via the sirtuin 1/PPARγ coactivator 1α signaling pathway and increased antioxidant enzymes (26). Another study showed that EGCG at a low concentration (1 mg/kg) provided important protection against MPTP-induced neuronal loss through the inhibition of microglial activation (27). However, the neurorescue potential of EGCG to restore neuronal damage in post-MPTP–induced Parkinsonism has not been well studied. One study suggested that oral EGCG administration resulted in a substantial recovery of tyrosine hydroxylase–positive neurons in post-MPTP treatment (22), but studies on the neuroprotective effects of EGCG through iron-related proteins are limited. We sought herein to determine the neurorescue effects of EGCG in MPTP-induced PD and to examine the involvement of iron-related proteins in the protective effect.
Methods
Chemicals.
MPTP, EGCG, and mouse β-actin antibody were purchased from Sigma-Aldrich. Perchloric acid and sodium metabisulfite were purchased from Fisher Scientific. The rabbit polyclonal antibodies for ferroportin, DMT1 with and without the iron response element, and hepcidin were purchased from Abcam. Alexa Fluor 680 conjugated anti-mouse IgG was purchased from Invitrogen. IRdye 800 conjugated anti-rabbit IgG and Western blot blocking buffer were purchased from Rockland. The commercial assay kit for protein carbonyl was purchased from Cayman Chemical Company.
Animals and treatment.
Male C57 black mice weighing ∼25 g were purchased from Charles River and housed individually in a temperature- and humidity-controlled room with a 12-h light-dark cycle. Food and water were provided ad libitum. All mice were given Teklad traditional rodent diets (Teklad S-2335 #7004 mouse breeder sterilizable diet containing 172 g protein/kg, 114 g fat/kg, 452 g carbohydrate/kg, and 27 g crude fiber/kg) purchased from Envigo. Mice were divided into 3 groups: control (n = 10), MPTP (n = 10), and MPTP + EGCG (n = 10). The mice in the last 2 groups were given 20 mg MPTP/kg intraperitoneally for the first 3 d to induce neurodegeneration. On day 4, the MPTP + EGCG mice were given 25 mg EGCG/kg orally for an additional 7 d; those in the control and MPTP groups were given distilled water by gavage. Mice in the control group were given an equal volume of PBS. We did not include an EGCG-only group because a previous study (Q Xu, AG Kanthasamy, MB Reddy, unpublished results, 2007) showed that the EGCG-only treatment did not significantly affect the striatal dopamine concentration compared with the control. All animals were killed by decapitation 3 d after the last dose of EGCG. The experimental protocol was approved by the Iowa State University Institutional Animal Care and Use Committee.
Accelerated rotarod test.
Motor coordination and balance alterations were measured with the use of the accelerating rotarod test as described previously (29). The mice were assessed 5 times at an accelerating speed of 4–60 × g for 3 min. The length of time each mouse was able to stay on the rotating rod was recorded with computer software (AccuRotor version 6.2; Omnitech Electronics Inc.) and averaged for the analysis. The trials during which the mice jumped off the rod were excluded.
Protein carbonyl assay.
Blood samples from mice were collected by cardiac puncture, and serum was used to assess protein carbonyls following the instructions provided in the commercial kit as described previously (30).
Analysis of striatal dopamine and its metabolite.
Concentrations of striatal dopamine and its metabolites were determined from striatal extracts containing 0.1 M perchloric acid, 0.05% Na2 EDTA, and 0.1% sodium metabisulfite with the use of HPLC with electrochemical detection as described previously (31). Dopamine and DOPAC were separated isocratically on a C-18 reversed-phase column (100 × 4.6 mm) (Agilent Technologies) at a flow rate of 0.6 mL/min with the use of a Dionex Ultimate 3000 HPLC system (pump ISO-3100SD; Thermo Scientific) equipped with a refrigerated autosampler (model WPS-3000TSL) and electrochemical detection system. (CoulArray model 5600A coupled with microdialysis cell 5014B and a guard cell model 5020). The integration and data analysis were performed with the use of CoulArray version 3.10 software (ESA Inc.). Dopamine and DOPAC concentrations were presented as nanograms per milligram wet tissue.
Western blot analysis.
SN tissue was lysed with a modified radioimmunoprecipitation assay buffer, and the lysates were loaded and separated on 12% SDS-PAGE gels or 16% Tricine SDS-PAGE gels as described previously (32). After separation, the proteins were transferred onto a nitrocellulose or polyvinylidene difluoride membrane, and nonspecific binding sites were blocked with Western blot blocking buffer for 1 h. The membranes with transferred proteins were probed with primary antibodies directed against DMT1 ± iron response element rabbit polyclonal (1:1000), hepcidin rabbit polyclonal (1:500), or ferroportin rabbit polyclonal (1:1000) followed by incubation with infrared dye 800 anti-rabbit secondary antibody (1:5000) and then visualized on an Odyssey infrared imaging system (LI-COR) and quantified with ImageJ with the use of β-actin as an internal control.
Statistics.
Data were analyzed with the use of Prism version 5.0 (GraphPad Software). Values for each treatment group were presented as relative to the control group (without treatment). Differences between the treatments were compared with the use of ANOVA with Tukey’s multiple comparison test and considered significant at P ≤ 0.05. The differences (final – initial weight) in weight gain were compared between treatment groupswith the use of ANOVA with Tukey’s multiple comparison test.
Results
Weight gain.
Weight gain over 12 d did not differ between the control (mean ± SD: 1.5 ± 0.37 g), MPTP (2.5 ± 0.34 g), and MPTP + EGCG groups (1.9 ± 0.31 g).
EGCG reversed MPTP-induced reduction of rotarod activity.
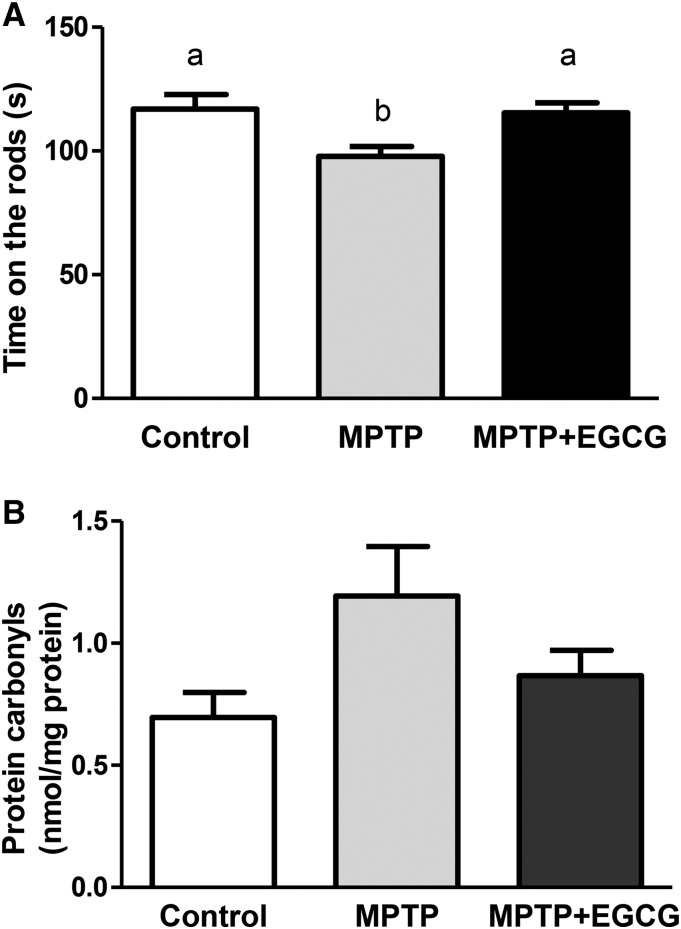
The protection of EGCG against MPTP-induced behavioral deficits, as evaluated by the accelerated rotarod test, is shown in Figure 1A. The MPTP-treated mice showed an impaired ability to remain on the rod, exhibiting a 16% reduction in the mean time spent on the rotarod (P < 0.05) compared with the control group. However, rotarod activity of the MPTP + EGCG mice was similar to those in the control group and significantly improved (P < 0.05) compared with those in the impaired MPTP treatment group.
FIGURE 1.
Effects of EGCG on MPTP-induced motor deficits in an accelerated rotarod test (A) and on oxidative stress assessed as serum protein carbonyl concentration (B) in a mouse model of Parkinson disease. Values are means ± SEMs [n = 10 (A) or 7–8 (B)]. Labeled means without a common letter differ, P < 0.05. EGCG, epigallocatechin gallate; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
EGCG protected against MPTP-induced oxidative stress.
EGCG treatment reduced MPTP-induced oxidative stress measured as protein carbonyls in serum (Figure 1B). Although not significant (P = 0.07), the serum protein carbonyl concentrations in the MPTP-treated mice was 71% higher than that in the control group. However, when data were normalized to the control group (100%), the difference was significant (P < 0.05; data not shown). There was no significant difference between MPTP + EGCG and control groups either using absolute or normalized values, indicating EGCG might partially reduce MPTP-induced oxidative stress.
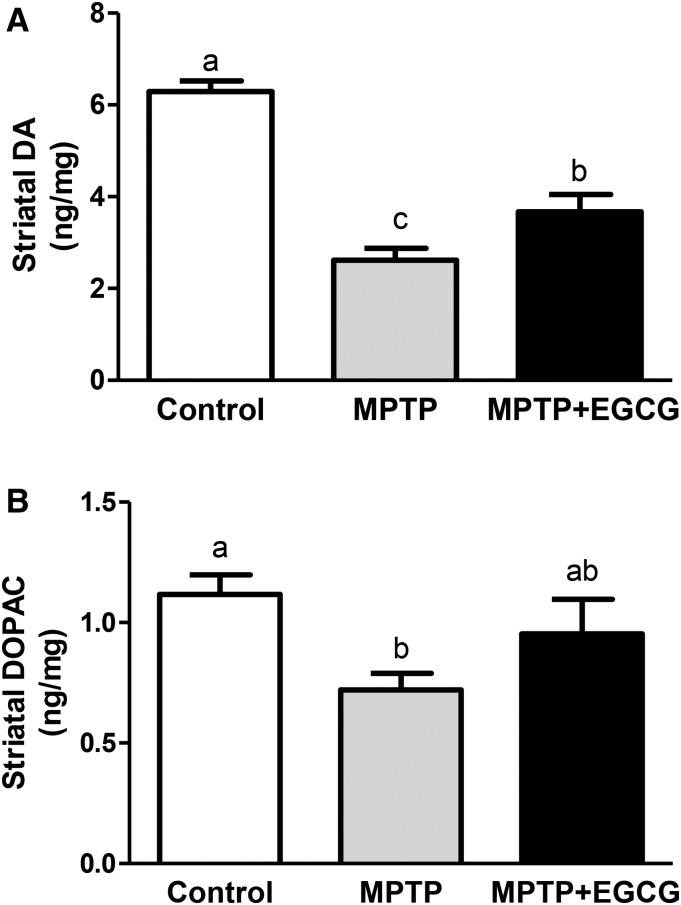
EGCG preserved MPTP-induced striatal dopamine reduction.
Striatal dopamine and DOPAC concentrations were 58% (P < 0.001) and 35% (P < 0.05) lower, respectively, in MPTP-treated mice than in the control mice. The mean striatal dopamine concentration was significantly higher (P < 0.05) for mice in the EGCG + MPTP group than in the MPTP group but still significantly lower than the control group, suggesting only partial protection from ECGG in restoring dopamine concentrations (Figure 2A). The mean DOPAC concentration in the MPTP + EGCG group was intermediate to the other 2 groups and not significantly different from either group (Figure 2B).
FIGURE 2.
Neurorescue effects of EGCG on MPTP-induced changes in striatal DA (A) and DOPAC (B) concentrations in a mouse model of Parkinson disease. Values are means ± SEMs (n = 10). Labeled means without a common letter differ, P < 0.05. DA, dopamine; DOPAC, dihydroxyphenylacetic acid; EGCG, epigallocatechin gallate; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
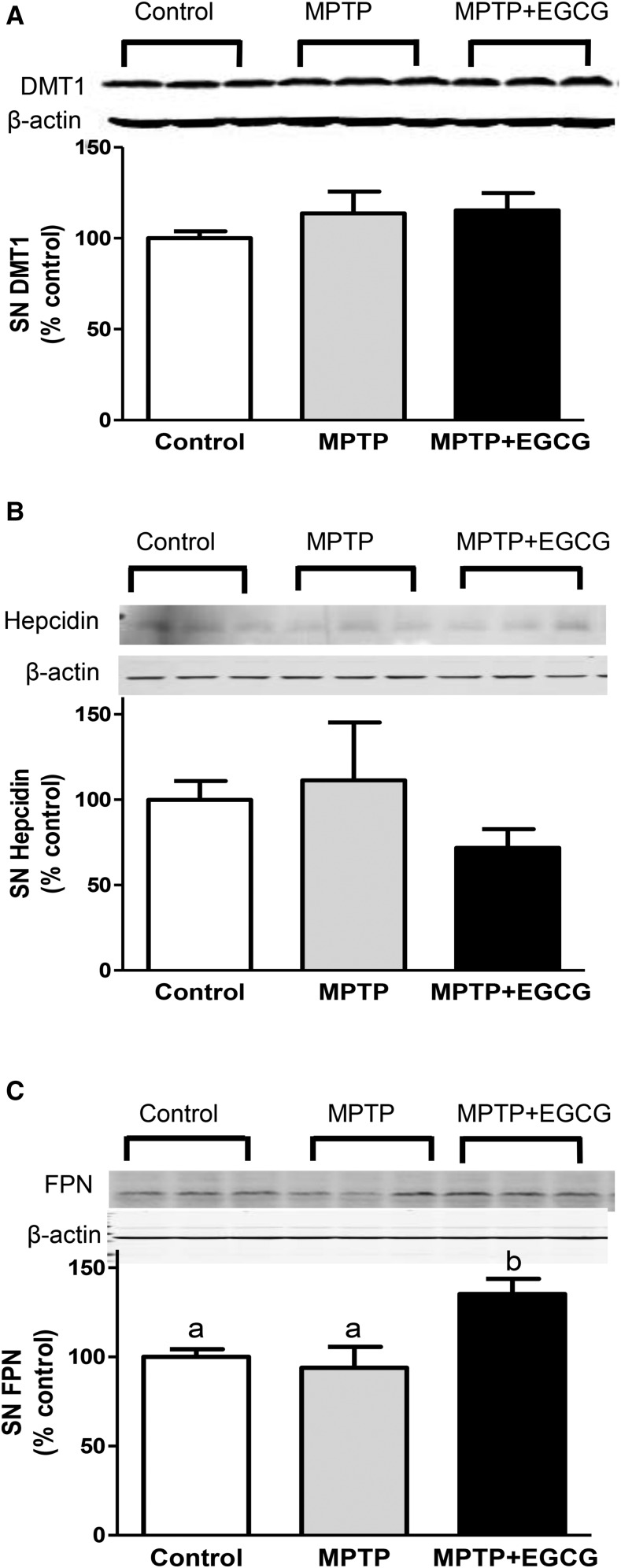
EGCG altered iron-related protein expression.
To further study the mechanisms involved in the neurorescue effect of EGCG, we assessed the expression of iron-related proteins DMT1, hepcidin, and ferroportin in the SN. SN DMT1 and hepcidin expression did not differ between the groups (Figure 3A, B), but ferroportin expression was 44% and 35% greater in the MPTP + EGCG group than in the MPTP and control groups (P < 0.05), respectively (Figure 3C).
FIGURE 3.
Effects of MPTP alone or with EGCG on SN DMT1 (A), hepcidin (B), and FPN (C) in a mouse model of Parkinson disease. Values are means ± SEMs (n = 6). Labeled means without a common letter differ, P < 0.05. Representative Western blots are shown above each panel (n = 3). DMT1, divalent metal transporter 1; EGCG, epigallocatechin gallate; FPN, ferroportin; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; SN, substantia nigra.
Discussion
PD is the second most common neurodegenerative disorder, affecting ∼5.2 million people worldwide (33). However, to date, there is no strategy available for curing PD patients. Traditional therapies, such as levodopa, provide only symptomatic relief and cause substantial motor complications. Moreover, these therapies are not able to reverse or stop the progression of the disease (34). Based on the involvement of iron accumulation and oxidative stress in the pathogenesis of PD, compounds with free-radical scavenging and iron-chelating properties have been considered promising candidates for treating PD. The iron chelator desferoxamine is reported to reduce iron accumulation and oxidative stress and protect against MPTP-induced neurotoxicity in mice (35). The metal chelator clioquinol has also been demonstrated to chelate both ferrous and ferric iron and protect against the MPTP-induced loss of striatal dopamine in vivo (21). We have also found previously that the natural iron chelator phytic acid could protect against both MPP+- and 6-hydroxydopamine (6-OHDA)-induced dopaminergic neuron apoptosis in normal and excess iron conditions (36, 37). Although these iron chelators may be effective in providing neuroprotection in PD, their therapeutic use in PD patients is limited because of their inability to cross the blood-brain barrier or ability to cause severe side effects, or both. Deferoxamine has limited ability to cross the blood-brain barrier because of its hydrophilic nature and may cause neurotoxicity with high doses (38, 39). The safety of clioquinol has also been questioned because it may cause serum vitamin B-12 deficiency (40). Clinical studies with the use of deferiprone have shown decreased brain iron content and a slight improvement in motor symptoms, suggesting the importance of treating PD patients with iron chelation (41, 42).
EGCG is the major green tea polyphenol and has gained attention in PD because of its free-radical scavenging, iron-chelating, and anti-inflammatory properties (25). In vitro studies have shown that EGCG may protect against both MPP+- and 6-OHDA-induced neurotoxicity (26, 43). In agreement, in vivo studies in mice also have shown that EGCG may prevent striatal dopamine depletion and the loss of tyrosine hydroxylase–positive neurons induced by MPTP (28, 44). The natural origin of EGCG and its ability to cross the blood-brain barrier also makes it an appealing clinical approach for PD treatment (25). EGCG has been reported to be easily absorbed in the digestive tract and widely distributed to various organs, including the brain, with similar concentrations found in the liver, kidney, lung, heart, spleen, and pancreas (44).
Previous studies have examined the neuroprotective effect of EGCG (28, 44), but studies in which the neurorescue effects were evaluated after inducing neurotoxicity as well as those that evaluated the effect of EGCG on iron-related proteins in the brain that are perturbed in PD are limited. One study showed that administering 5 mg EGCG/kg orally for 2 wk after MPTP treatment (20 mg/kg for 4 d) resulted in a substantial recovery of the nigral dopaminergic neurons (22). Concordantly, our results also showed that EGCG posttreatment (25 mg/kg for 7 d) not only rescued MPTP-induced dopamine depletion but also improved motor deficits caused by MPTP, as assessed with the use of the accelerated rotarod test. Striatal dopamine depletion found in MPTP mice was attenuated by EGCG treatment, although concentrations were still lower than in the control group. Because dopamine depletion is the major cause of motor dysfunction in PD, it was encouraging to see the improvement with EGCG because of its future potential for use in humans. The accelerated rotarod test is a behavior test used to measure animals’ innate motor skills, resembling akinesia and bradykinesia in human Parkinsonism (45). MPTP administration resulted in decreased rotarod duration, and EGCG posttreatment completely corrected motor deficits, suggesting its ability to alleviate PD symptoms. In addition, our results also showed that EGCG posttreatment reduced serum protein carbonyls, which were elevated by MPTP, a finding consistent with our previously published study that showed that 3 cups of green tea (∼720 mL) consumed for 3 mo improved antioxidant enzymes and reduced oxidative damage to lipids and proteins in PD patients (46). Furthermore, our unpublished data (Q Xu, AG Kanthasamy, MB Reddy, unpublished results, 2017) showed that EGCG may prevent neurotoxicity induced by proinflammatory cytokine TNF-α, suggesting that the anti-inflammatory activity of EGCG may also be involved in neuroprotection.
In this study, MPTP treatment did not significantly affect DMT1, hepcidin, or ferroportin expression in the SN. These results are inconsistent with a previous study that showed nigral iron accumulation with increased DMT1 expression and decreased ferroportin expression in a chronic MPTP-induced PD model (19). However, our study used a subacute MPTP model (20 mg/kg for 3 d) rather than a chronic MPTP model (10 doses of 30 mg/kg for 5 wk), and the treatment in the chronic model may have accounted for the observed nigral iron accumulation by altering iron transporters. We found that EGCG did not affect hepcidin or DMT1 expression but did significantly affect nigral ferroportin expression, which may be because ferroportin can be regulated in a hepcidin-independent manner. Research has shown that ferroportin is regulated by iron-regulatory protein or iron-responsive elements at the posttranscriptional level or degraded at the posttranslational level because of the absence of ceruloplasmin (47). These proteins are also associated with oxidative stress or inflammation (48, 49). Based on the antioxidant and anti-inflammatory properties of EGCG, we expect EGCG to downregulate ferroportin, alleviating an excess iron condition via these proteins.
To our knowledge, this is the first in vivo study that has shown the substantial upregulation of ferroportin after EGCG treatment. These results do not support our previous study regarding EGCG’s effect on hepcidin and DMT1, but they are in agreement with our study that showed protection against 6-OHDA-induced neurotoxicity by alleviating the intracellular iron concentration and upregulating ferroportin in a cell-culture model of PD (50). However, different neurotoxins and different models (in vivo compared with in vitro) were used in the 2 studies. Because iron accumulation can exacerbate MPTP-induced dopaminergic neurodegeneration (51), the upregulation of ferroportin may be an underlying neuroprotective mechanism of EGCG by reducing nigral iron. Future studies are needed to measure iron concentrations, especially the labile iron pool, in SN and confirm that EGCG provides protection by lowering the nigral iron concentration. The 25-mg EGCG/kg dose used in our study equates to ∼2 mg/kg in humans with the use of the body-surface area normalization method (52) or 140 mg EGCG consumed daily by a 70-kg person. Based on a previous study that showed that a cup of green tea (2.5 g green tea leaves/200mL water) may contain ≤90 mg EGCG (53), the habitual consumption of green tea (3 cups/d) can exceed the targeted amount of 140 mg (46).
Overall, our study demonstrated that EGCG can not only restore MPTP-induced functional and neurochemical deficits but also regulate the iron-export protein ferroportin in the SN and reduce oxidative stress. Future clinical studies are needed to confirm the protective effect of EGCG; however, our findings suggest its potential therapeutic use after the onset of PD.
Acknowledgments
We thank Denise Rothschild for editing the manuscript. The authors’ responsibilities were as follows—AGK and MBR: designed and supervised the research; QX: conducted the research, analyzed the data, and wrote and revised the paper; ML: conducted the experiments; AGK: provided the laboratory and essential materials necessary for the research and supervised the PD-associated assays; MBR: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Footnotes
Abbreviations used: DMT1, divalent metal transporter 1; DOPAC, dihydroxyphenylaceticacid; EGCG, epigallocatechin gallate; MPP+, 1-methyl-4-phenylpyridinium; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PD, Parkinson disease; SN, substantia nigra; 6-OHDA, 6-hydroxydopamine.
References
- 1.Litteljohn D, Mangano E, Clarke M, Bobyn J, Moloney K, Hayley S. Inflammatory mechanisms of neurodegeneration in toxin-based models of Parkinson’s disease. Parkinsons Dis 2011;2011:713517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radad K, Gille G, Rausch WD. Short review on dopamine agonists: insight into clinical and research studies relevant to Parkinson’s disease. Pharmacol Rep 2005;57:701–12. [PubMed] [Google Scholar]
- 3.Urrutia PJ, Menam NP, Núñez MT. The interplay between iron accumulation, mitochondrial dysfunction, and inflammation during the execution step of neurodegenerative disorders. Front Pharmacol 2014;5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis 2013;3:461–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis 2010;37:510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rathnasamy G, Ling EA, Kaur C. Consequences of iron accumulation in microglia and its implications in neuropathological conditions. CNS Neurol Disord Drug Targets 2013;12:785–98. [DOI] [PubMed] [Google Scholar]
- 7.Lin M, Rippe RA, Niemela O, Brittenham G, Tsukamoto H. Role of iron in NF-kappa B activation and cytokine gene expression by rat hepatic macrophages. Am J Physiol 1997;272:G1355–64. [DOI] [PubMed] [Google Scholar]
- 8.Ayton S, Lei P. Nigral iron elevation is an invariable feature of Parkinson’s disease and is a sufficient cause of neurodegeneration. BioMed Res Int 2014;2014:581256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelman N, Gorell JM, Barker PB, Savage RM, Spickler EM, Windham JP, Knight RA. MR imaging of human brain at 3.0 T: preliminary report on transverse relaxation rates and relation to estimated iron content. Radiology 1999;210:759–67. [DOI] [PubMed] [Google Scholar]
- 10.Berg D, Becker G, Riederer P, Riess O. Iron in neurodegenerative disorders. Neurotox Res 2002;4:637–53. [DOI] [PubMed] [Google Scholar]
- 11.Mounsey RB, Teismann P. Chelators in the treatment of iron accumulation in Parkinson’s disease. Int J Cell Biol 2012;2012:983245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le W. Role of iron in UPS impairment model of Parkinson’s disease. Parkinsonism Relat Disord 2014;20(Suppl 1):S158–61. [DOI] [PubMed] [Google Scholar]
- 13.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004;306:2090–3. [DOI] [PubMed] [Google Scholar]
- 14.Raha-Chowdhury R, Raha AA, Forostyak S, Zhao JW, Stott SR, Bomford A. Expression and cellular localization of hepcidin mRNA and protein in normal rat brain. BMC Neurosci 2015;16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang SM, Fu LJ, Duan XL, Crooks DR, Yu P, Qian ZM, Di XJ, Li J, Rouault TA, Chang YZ. Role of hepcidin in murine brain iron metabolism. Cell Mol Life Sci 2010;67:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meredith GE, Rademacher DJ. MPTP mouse models of Parkinson’s disease: an update. J Parkinsons Dis 2011;1:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He Y, Thong PS, Lee T, Leong SK, Mao BY, Dong F, Watt F. Dopaminergic cell death precedes iron elevation in MPTP-injected monkeys. Free Radic Biol Med 2003;35:540–7. [DOI] [PubMed] [Google Scholar]
- 18.Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arredondo M, Duyckaerts C, Sazdovitch V, Zhao L, Garrick LM, et al. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proc Natl Acad Sci USA 2008;105:18578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv Z, Jiang H, Xu H, Song N, Xie J. Increased iron levels correlate with the selective nigral dopaminergic neuron degeneration in Parkinson’s disease. J Neural Transm 2011;118:361–9. [DOI] [PubMed] [Google Scholar]
- 20.Levenson CW, Cutler RG, Ladenheim B, Cadet JL, Hare J, Mattson MP. Role of dietary iron restriction in a mouse model of Parkinson’s disease. Exp Neurol 2004;190:506–14. [DOI] [PubMed] [Google Scholar]
- 21.Kaur D, Yantiri F, Rajagopalan S, Kumar J, Mo JQ, Boonplueang R, Viswanath V, Jacobs R, Yang L, Beal MF, et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson’s disease. Neuron 2003;37:899–909. [DOI] [PubMed] [Google Scholar]
- 22.Mandel SA, Amit T, Kalfon L, Reznichenko L, Youdim MB. Targeting multiple neurodegenerative diseases etiologies with multimodal-acting green tea catechins. J Nutr 2008;138:1578S–83S. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka K, Miyake Y, Fukushima W, Sasaki S, Kiyohara C, Tsuboi Y, Yamada T, Oeda T, Miki T, Kawamura N, et al. Intake of Japanese and Chinese teas reduces risk of Parkinson’s disease. Parkinsonism Relat Disord 2011;17:446–50. [DOI] [PubMed] [Google Scholar]
- 24.Tomata Y, Sugiyama K, Kaiho Y, Honkura K, Watanabe T, Zhang S, Sugawara Y, Tsuji I. Green tea consumption and the risk of incident dementia in elderly Japanese: the Ohsaki Cohort 2006 Study. Am J Geriatr Psychiatry 2016;24:881–9. [DOI] [PubMed] [Google Scholar]
- 25.Mandel S, Amit T, Reznichenko L, Weinreb O, Youdim MB. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Mol Nutr Food Res 2006;50:229–34. [DOI] [PubMed] [Google Scholar]
- 26.Ye Q, Ye L, Xu X, Huang B, Zhang X, Zhu Y, Chen X. Epigallocatechin-3-gallate suppresses 1-methyl-4-phenyl-pyridine-induced oxidative stress in PC12 cells via the SIRT1/PGC-1alpha signaling pathway. BMC Complement Altern Med 2012;12:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li R, Peng N, Du F, Li XP, Le WD. Epigallocatechin gallate protects dopaminergic neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity by inhibiting microglial cell activation. Nan Fang Yi Ke Da Xue Xue Bao 2006;26:376–80. [PubMed] [Google Scholar]
- 28.Choi JY, Park CS, Kim DJ, Cho MH, Jin BK, Pie JE, Chung WG. Prevention of nitric oxide-mediated 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease in mice by tea phenolic epigallocatechin 3-gallate. Neurotoxicology 2002;23:367–74. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh A, Kanthasamy A, Joseph J, Anantharam V, Srivastava P, Dranka BP, Kalyanaraman B, Kanthasamy AG. Anti-inflammatory and neuroprotective effects of an orally active apocynin derivative in pre-clinical models of Parkinson’s disease. J Neuroinflammation 2012;9:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynch TL IV, Sivaguru M, Velayutham M, Cardounel AJ, Michels M, Barefield D, Govindan S, Dos Remedios C, van der Velden J, Sadayappan S. Oxidative stress in dilated cardiomyopathy caused by MYBPC3 mutation. Oxid Med Cell Longev 2015;2015:424751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ngwa HA, Kanthasamy A, Jin H, Anantharam V, Kanthasamy AG. Vanadium exposure induces olfactory dysfunction in an animal model of metal neurotoxicity. Neurotoxicology 2014;43:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon R, Anantharam V, Kanthasamy AG, Kanthasamy A. Proteolytic activation of proapoptotic kinase protein kinase Cdelta by tumor necrosis factor alpha death receptor signaling in dopaminergic neurons during neuroinflammation. J Neuroinflammation 2012;9:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter E, Odin P. Cost-effectiveness of continuous subcutaneous apomorphine in the treatment of Parkinson’s disease in the UK and Germany. J Med Econ 2015;18:155–65. [DOI] [PubMed] [Google Scholar]
- 34.Jankovic J, Aguilar LG. Current approaches to the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat 2008;4:743–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lan J, Jiang DH. Desferrioxamine and vitamin E protect against iron and MPTP-induced neurodegeneration in mice. J Neural Transm 1997;104:469–81. [DOI] [PubMed] [Google Scholar]
- 36.Xu Q, Kanthasamy AG, Reddy MB. Neuroprotective effect of the natural iron chelator, phytic acid in a cell culture model of Parkinson’s disease. Toxicology 2008;245:101–8. [DOI] [PubMed] [Google Scholar]
- 37.Xu Q, Kanthasamy AG, Reddy MB. Phytic acid protects against 6-hydroxydopamine-induced dopaminergic neuron apoptosis in normal and iron excess conditions in a cell culture model. Parkinsons Dis 2011;2011:431068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu G, Men P, Perry G, Smith MA. Nanoparticle and iron chelators as a potential novel Alzheimer therapy. Methods Mol Biol 2010;610:123–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine JE, Cohen A, MacQueen M, Martin M, Giardina PJ. Sensorimotor neurotoxicity associated with high-dose deferoxamine treatment. J Pediatr Hematol Oncol 1997;19:139–41. [DOI] [PubMed] [Google Scholar]
- 40.Kaur D, Andersen JK. Ironing out Parkinson’s disease: is therapeutic treatment with iron chelators a real possibility? Aging Cell 2002;1:17–21. [DOI] [PubMed] [Google Scholar]
- 41.Kwiatkowski A, Ryckewaert G, Jissendi Tchofo P, Moreau C, Vuillaume I, Chinnery PF, Destee A, Defebvre L, Devos D. Long-term improvement under deferiprone in a case of neurodegeneration with brain iron accumulation. Parkinsonism Relat Disord 2012;18:110–2. [DOI] [PubMed] [Google Scholar]
- 42.Abbruzzese G, Cossu G, Balocco M, Marchese R, Murgia D, Melis M, Galanello R, Barella S, Matta G, Ruffinengo U, et al. A pilot trial of deferiprone for neurodegeneration with brain iron accumulation. Haematologica 2011;96:1708–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levites Y, Amit T, Youdim MB, Mandel S. Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (−)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem 2002;277:30574–80. [DOI] [PubMed] [Google Scholar]
- 44.Levites Y, Weinreb O, Maor G, Youdim MB, Mandel S. Green tea polyphenol (−)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. J Neurochem 2001;78:1073–82. [DOI] [PubMed] [Google Scholar]
- 45.Potashkin JA, Blume SR, Runkle NK. Limitations of animal models of Parkinson’s disease. Parkinsons Dis 2010;2011:658083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen D, Zhou Y, Lyons KE, Reddy MB. Green tea consumption reduces oxidative stress in Parkinson’s disease patients. JBBS 2015;5:194–202. [Google Scholar]
- 47.Ward DM, Kaplan J. Ferroportin-mediated iron transport: expression and regulation. Biochim Biophys Acta 2012;1823: 1426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pantopoulos K, Hentze MW. Rapid responses to oxidative stress mediated by iron regulatory protein. EMBO J 1995;14:2917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lazzaro M, Bettegazzi B, Barbariga M, Codazzi F, Zacchetti D, Alessio M. Ceruloplasmin potentiates nitric oxide synthase activity and cytokine secretion in activated microglia. J Neuroinflammation 2014;11:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen D, Kanthasamy A, Reddy MB. EGCG protects against 6-OHDA-induced neurotoxicity in a cell culture model. Parkinsons Dis 2015;2015:843906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.You LH, Li F, Wang L, Zhao SE, Wang SM, Zhang LL, Zhang LH, Duan XL, Yu P, Chang YZ. Brain iron accumulation exacerbates the pathogenesis of MPTP-induced Parkinson’s disease. Neuroscience 2015;284:234–46. [DOI] [PubMed] [Google Scholar]
- 52.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008;22:659–61. [DOI] [PubMed] [Google Scholar]
- 53.Morin MP, Bedran TB, Fournier-Larente J, Haas B, Azelma J, Grenier D. Green tea extract and its major constituent epigallocatechin-3-gallate inhibit growth and halitosis-related properties of Solobacterium moorei. BMC Complement Altern Med 2015;15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]