Abstract
Purpose
To investigate the prevalence of visual field defects in glaucomatous eyes, glaucoma suspects, and ocular hypertensives with 24-2 and 10-2 visual fields.
Design
Prospective, cross-sectional study.
Participants
Patients with or suspected glaucoma.
Testing
24-2 and 10-2 visual fields. Patients were classified into 3 groups based upon the presence of glaucomatous optic neuropathy (GON) and 24-2 visual field abnormalities: early glaucoma (GON and abnormal visual field, mean deviation >−6 dB), glaucoma suspects (GON and normal visual field), and ocular hypertensives (normal disc, normal visual field, and intraocular pressure >22 mmHg). For the classification of visual field abnormalities, 24-2 and 10-2 tests performed on the same visit were analyzed.
Main outcome measure
Comparison of the prevalence of abnormal 24-2 versus 10-2 visual field results based upon cluster criteria in each diagnostic group.
Results
775 eyes (497 patients) were evaluated. 364 eyes had early glaucoma, 303 were glaucoma suspects, and 108 were ocular hypertensives. In the glaucoma group, 16 of the 26 (61.5%) eyes classified as normal based upon cluster criteria on 24-2 tests were classified as abnormal on 10-2 visual fields. In eyes with suspected glaucoma, 79 of the 200 (39.5%) eyes classified as normal on 24-2 were classified as abnormal on 10-2 visual fields. In ocular hypertensive eyes, 28 of the 79 (35.4%) eyes classified as normal on the 24-2 were abnormal on the 10-2. Patients of African descent were more likely to have an abnormal 10-2 result (67.3 vs. 56.8%, P=0.009).
Conclusions
Central visual field damage seen on 10-2 is often missed with 24-2 strategy in all groups. This finding has implications for the diagnosis of glaucoma and classification of severity.
Keywords: glaucoma, ocular hypertension, visual fields, macula, optic nerve
There is compelling structural and functional evidence that glaucomatous damage to the macula occurs even in early stages of the disease.1 For example, since Drance2 first pointed out that the central visual field could be affected even in early glaucoma, evidence has been mounting that macular damage, as seen with standard automated perimetry (SAP), is very common.1, 3–7 This information is clinically important since the macula (herewith defined as the central 8 degrees around the fovea) includes about 30% of all retinal ganglion cells (RGCs) 8 and supplies the information for 55–60% of the primary visual cortex.9 Given this high density of RGCs in the macula and their overwhelming representation in the visual cortex, it is not surprising that damage to the macula can substantially affect health-related quality of life (HRQoL).10 Glaucoma impacts patients’ HRQoL in multiple ways, including driving,11 walking and falls,12 and reading.13 Moreover, central vision – which correlates with macular function – is important when performing activities of daily life. The psychological burden increases as vision decreases, along with a growing fear of blindness, social withdrawal from impaired vision, and depression.14 Therefore, glaucoma care aims to enhance patients’ HRQoL by preserving visual function without causing untoward effects from treatment.15
However, glaucomatous damage to the macula will be missed in clinical practice if only 24-2 visual fields and peripapillary optical coherence tomography (OCT) scans are performed,16, 17 as is often the case. In particular, studies have shown that macular damage is prevalent among patients with early glaucoma if one employs the appropriate tools to assess it, namely 10-2 visual fields 4–6, 16 and OCT cube scans of the macula.1, 17–19
Notably, Traynis et al4 has shown that as many as 16% of eyes with a normal 24-2 visual field result have significant abnormalities on 10-2 in this sample of patients with early glaucomatous functional loss. This number is striking as many of the so-called “glaucoma suspects” or “pre-perimetric glaucoma” may in fact have central damage which now places them as “severe glaucoma” based on the clinical classification system currently widely employed.20 This information comes from a prospective, cross-sectional database in which patients underwent 24-2, 10-2, and sdOCT testing irrespective of their clinical status in order to minimize selection bias, as long as they had signs of glaucomatous optic neuropathy (GON) and their visual fields were not severely affected (i.e.: 24-2 visual field mean deviation (MD) better than −6 dB).4 One limitation of that study, however, is that all patients had GON, which by itself limits the generalizability of our conclusions. Similarly, Park et al5 found that 74% of eyes had a parafoveal scotoma detected on the 10-2 visual field test in a population with GON and abnormal 24-2 visual fields with MD better than −6 dB. In a population that included primary open-angle glaucoma (mild, moderate, and severe), ocular hypertensives, and glaucoma suspects, Sullivan-Mee et al6 reported that 6% of eyes without 24-2 field loss exhibited a 10-2 defect. However, the break-down of the prevalence of 10-2 abnormalities among ocular hypertensives and glaucoma suspects was not reported, as the group “without 24-2 field loss” represented pooled information from all three groups (i.e.: including so called “glaucoma patients with no loss on the 24-2”).
To address this issue, in the present paper we analyzed an independent database that includes subjects with and without GON, including eyes with early glaucoma field loss, glaucoma suspects, and ocular hypertensives. Participants of the African Descent and Evaluation Study (ADAGES),21 a multi-center, prospective, longitudinal study including the entire spectrum of glaucomatous damage were included. In ADAGES, all participants underwent a standardized frequency of visits and testing, including 24-2 and 10-2 visual field testing. Moreover, all participants had extensive experience with perimetry, which minimized the undesired effects of unreliable test results and learning effects. In this group of patients, we tested the hypothesis that central, 10-2 visual field defects are often missed on 24-2 tests not only in eyes with established glaucoma, but also among glaucoma suspects and ocular hypertensives.
METHODS
The three-site ADAGES collaboration includes the Hamilton Glaucoma Center at the Department of Ophthalmology, University of California-San Diego (UCSD) (data coordinating center), Edward S. Harkness Eye Institute at Columbia University Medical Center (site formerly located at New York Eye and Ear Infirmary), and the Department of Ophthalmology, University of Alabama-Birmingham (UAB). The institutional review boards at all sites approved the study methodology, which adheres to the tenets of the Declaration of Helsinki and to the Health Insurance Portability and Accountability Act. All participants gave written informed consent. ADAGES enrollment began in January 2003 and ended in July 2006, while follow-up continued until 2016.
Participants
Participants were asked to identify their race by self-report using the National Eye Institute inclusion/enrollment system describing ethnicity and race (http://orwh.od.nih.gov/pubs/outreach.pdf [pages 120–121]). Information regarding a family history of glaucoma (biological mother, father, sibling, aunt, uncle, and grandparent) was also obtained. Normal and patient participants were recruited from the glaucoma clinics and ophthalmic practices at each of the three recruiting sites, by advertisement and community presentations, and by referral from other ophthalmologists and optometrists in the community.
The ocular testing completed for ADAGES has been described elsewhere.21 In brief, participants underwent a comprehensive ophthalmic examination, including annual review of medical history, best-corrected visual acuity, slit-lamp biomicroscopy, intraocular pressure (IOP), dilated funduscopy examination, pachymetry, simultaneous stereoscopic optic disc photography, and standard automated perimetry (SAP) with 24-2 and 10-2 Swedish interactive threshold algorithm (Carl Zeiss Meditec, Inc, Dublin, California, USA). Both 24-2 and 10-2 visual fields were repeated every 6 months and optic disc photographs were performed every 12 months.
Inclusion criteria at baseline
All participants had open angles, a best-corrected visual acuity ≥ 20/40, and a refractive error <5.0 diopters sphere and <3.0 diopters cylinder. At least one high-quality stereophotograph and two reliable standard automated perimetry (SAP) Humphrey 24-2 field test results at baseline were required, defined as <33% false positives, false negatives, and fixation losses. Although 10-2 tests were not employed at baseline to define the diagnostic groups, they had to meet same reliability criteria as 24-2 tests. Both eyes were included, except in cases where only one eye met the study criteria. All participants were older than 18 years. Diabetic participants without evidence of retinopathy were included.
Exclusion criteria
Participants were excluded if they had a history of intraocular surgery (except for uncomplicated cataract surgery or glaucoma surgery), secondary causes of glaucoma (e.g., iridocyclitis, trauma), other systemic or ocular diseases known to affect the visual field (e.g., pituitary lesions, demyelinating diseases, etc.), significant cognitive impairment, history of stroke, Alzheimer disease, or dementia, problems other than glaucoma affecting color vision, an inability to perform visual field examinations reliably, or a life-threatening disease that precluded retention in the study.
Evaluation of the optic nerve complex
All data were processed through the ADAGES Coordinating Center, the VisFACT (Visual Field Assessment Center), and the IDEA (Imaging Data Evaluation and Analysis) Center housed at the Hamilton Glaucoma Center, UCSD. The IDEA Center processed and reviewed the quality of all simultaneous stereophotographs. These reading centers also handled all data from DIGS and other National Eye Institute or industry-sponsored trials. Both centers are responsible for certifying visual field and imaging technicians and photo graders, processing any data-related queries to and from each site, and requesting that tests be repeated when needed.
All color simultaneous stereophotographs were taken using a Nidek Stereo Camera Model 3-DX (Nidek Inc, Palo Alto, California) after maximal pupil dilation. All photograph evaluations were performed using a simultaneous stereoscopic viewer (Asahi Pentax Stereo Viewer II; Pentax, Tokyo, Japan) with a standard fluorescent light bulb. Certified photograph graders evaluated all photographs. To be certified, individuals were trained and then tested on separate standardized sets of stereophotographs depicting (1) glaucomatous and healthy eyes and (2) progressing and non-progressing eyes. Evidence from the Ocular Hypertension Treatment Study (OHTS) and the European Glaucoma Prevention Study (EGPS) indicated that reproducibility of stereophotograph assessment is good when graders have been trained using this type of formal protocol.22, 23
Glaucomatous optic neuropathy was defined as excavation, neuroretinal rim thinning or notching, localized or diffuse retinal nerve fiber layer defect, or vertical cup-disc ratio (VCDR) asymmetry > 0.2 between eyes (not explained by differences in disc size) based on masked grading of stereophotographs by two graders at the IDEA Center. Disagreement regarding GON status was resolved by adjudication by a third experienced grader or by consensus. Only photographs of adequate quality were used for evaluation. Disc photo grading closest to the date of the 24-2 and 10-2 visual fields were used for the definitions described below.
Standard automated perimetry 24-2 visual field results were considered abnormal if the pattern standard deviation (PSD) had a P< 5% or the Glaucoma Hemifield Test result was “outside normal limits.” Abnormality had to be confirmed with an additional visual field test.
There is little consensus among clinicians and researchers on how to define glaucoma, glaucoma suspect, and ocular hypertension. For instance, some clinicians require both visual field loss and evidence of GON for a diagnosis of glaucoma, referring to those with only one of these findings as having suspected glaucoma. Others consider the presence of GON, with or without repeatable visual field loss, sufficient to diagnose glaucoma. For the purposes of the present study, we opted to use the criteria set in the ADAGES design,21 which are described in Table 1. Moreover, we did not include participants with repeatable visual field loss and no signs of GON because there is even poorer consensus on what these subjects in fact represent. Some argue that a closer look would reveal that in reality they do have structural abnormalities on imaging devices such as OCT, or, if they do not, that their repeatable visual field loss actually consists of false-positive results. For simplicity, we refrained from this discussion and focused on the diagnostic groups more commonly encountered in clinical practice.
Table 1.
African Descent and Evaluation Study (ADAGES) classification system. Modified from: Sample PA, Girkin CA, Zangwill LM, et al. Archives of Ophthalmology 2009;127(9):1136–1145.
| Diagnostic category | IOP | Photo grade | 24-2 SAP visual field test result | ||
|---|---|---|---|---|---|
| Ocular hypertension | >22 mmHg* or taking IOP-lowering medication | AND | Normal | AND | Normal |
| Glaucoma suspect (GON only) | Not applicable | Glaucomatous | AND | Normal | |
| Glaucoma with 24-2 visual field loss** | Not applicable | Glaucomatous | AND | Glaucomatous (repeatable) | |
Abbreviations: IOP= intraocular pressure; GON= glaucomatous optic neuropathy; SAP: standard automated achromatic perimetry
Intraocular pressure 22 mmHg or higher measured as such on baseline examination or documented history of such
Standard automated perimetry 24-2 visual field results were considered abnormal if the pattern standard deviation (PSD) had a P< 5% or the Glaucoma Hemifield Test result was “outside normal limits.” Abnormality had to be confirmed with an additional visual field test.
For 10-2 visual fields, the same reliability and repeatability criteria were required. To avoid central field defects due to severe glaucoma, only eyes with MD values better than −6 dB on the 24-2 test were included. The visual field result of each eye was classified using a cluster rule we employed in our previous study.4 Specifically, a 24-2 visual field was considered abnormal if either hemifield on the total deviation (TD) or pattern deviation (PD) plot was abnormal. A hemifield was categorized as abnormal if there were a cluster of 3 contiguous points (5%, 5%, 1%; 5%, 2%, 2%, or worse) within the hemifield. However, only one point in each cluster was allowed to lie on the edge of the field. The same criteria were required for 10-2 visual fields, except that points in the cluster were allowed to lie on the edge of the field. Only 24-2 and 10-2 visual fields performed on the same day were analyzed. Given that all tests are expected to have false-positive results, we investigated the specificity of our cluster criteria in a sample of 301 healthy eyes of 233 subjects (all with normal optic discs, normal 24-2 visual fields, and IOP< 22 mmHg) who underwent at least two reliable 24-2 and 10-2 tests. We found that 14 (4.6%) had an abnormal 10-2 test results based on our cluster criteria. Therefore, we assumed the specificity of our cluster criteria to be approximately 95%. Of note, the above described cluster criteria were not used as inclusion/exclusion criteria for this study, as the ADAGES criteria are based upon the GHT and PSD results as described under “Evaluation of the optic nerve complex” and Table 1. The cluster criteria will be used to define glaucomatous visual field loss on 10-2 tests and as an ancillary definition of 24-2 abnormality based upon clusters of abnormal points, as opposed to global indices.
Statistical analyses
Continuous variables are described as means and standard deviation (SD). Two-by-two contingency tables were built to assess differences in frequency of normal vs. abnormal central fields between 24-2 and 10-2 test results. Individual tables were built for the 3 groups described in Table 1. We also investigated the 24-2 visual field characteristics that could predict the presence of an abnormal 10-2 result using binary logistic regression. The dependent variable was whether the 10-2 test was abnormal (based upon the cluster criteria described above) and the independent variables was the presence of central 24-2 abnormalities (defined based upon different probabilities (P-values <5%, 2%, 1%, or 0.5%) on the TD or PD plots). Statistical analyses were performed using commercially available software (STATA, version 14; StataCorp LP, College Station, TX). Statistical significance was defined at P<0.05.
RESULTS
We included 775 eyes (497 patients), 54.4% were women, and 50.9% had African ancestry. The median (10th and 90th percentiles) test duration was 5.0 (4.3 and 6.3) and 5.4 (4.7 to 7.1) minutes with 24-2 and 10-2 tests, respectively. 364 eyes had glaucoma with early visual field damage (24-2 MD better than −6 dB), 303 had suspected glaucoma, and 108 were ocular hypertensives (Table 2).
Table 2.
Demographic characteristics.
| Glaucomatous | Suspects | Ocular Hypertensives | |
|---|---|---|---|
| Number of eyes | 364 | 303 | 108 |
| Age (years) | 63.36 (20.1) | 60.39 (18.7) | 64.79 (12.4) |
| Sex (Female) | 196 (53.8) | 160 (52.8) | 66 (61.1) |
| Race (African descent) | 209 (57.4) | 153 (50.4) | 33 (30.5) |
| 24-2 MD (dB) | −2.27 (1.81) | −0.15 (1.41) | 0.02 (1.34) |
| 24-2 Test Duration (minutes) | 5.5 (0.8) | 4.9 (0.7) | 4.8 (0.6) |
| 10-2 MD (dB) | −2.16 (2.79) | −0.44 (1.43) | −0.15 (1.39) |
| 10-2 Test Duration (minutes) | 6.0 (1.0) | 5.3 (0.6) | 5.3 (0.6) |
Abbreviations: MD= mean deviation; dB= decibels
Values in parentheses are % for categorical variables and standard deviation for continuous variables.
As expected, the majority of eyes with glaucoma and early visual field damage had abnormal 24-2 tests based on cluster criteria (338 eyes of 364, 92.8%), while abnormal clusters on 10-2 results (297/364 eyes, 81.5%) were nearly as common (Table 3). However, 16 of the 26 (61.5%) eyes classified as normal based upon cluster criteria on 24-2 tests were classified as abnormal on 10-2 visual fields. Similarly, 57 of 67 (85.0%) eyes classified as normal based on 10-2 were classified as abnormal by 24-2. All these cases included abnormalities located outside the central 10 degrees of the 24-2, an area which by definition is not tested with 10-2 fields.
Table 3.
Eyes of patients with glaucomatous optic neuropathy and abnormal 24-2 visual fields (mean deviation better than −6 dB). Frequency of normal and abnormal central visual field defects on 24-2 vs. 10-2 test results based upon cluster criteria.
| 10-2 | ||||
|---|---|---|---|---|
| Normal | Abnormal | Total | ||
| 24-2 | Normal | 10 | 16 | 26 |
| Abnormal | 57 | 281 | 338 | |
| Total | 67 | 297 | 364 | |
In eyes with suspected glaucoma, abnormal 10-2 results (142 of 303 eyes, 46.8%) were also nearly as common as abnormal 24-2 results based upon cluster criteria (103 eyes, 33.9%) (Table 4). However, 79 of the 200 (39.5%) eyes classified as normal on 24-2 based upon cluster criteria were classified as abnormal on 10-2 visual fields. Similarly, 40 of 161 (24.8%) eyes classified as normal based on 10-2 were classified as abnormal by 24-2. All of such cases were outside the central 10 degrees of the 24-2.
Table 4.
Eyes of patients with suspected glaucoma. Frequency of normal and abnormal central visual field defects on 24-2 vs. 10-2 test results based upon cluster criteria.
| 10-2 | ||||
|---|---|---|---|---|
| Normal | Abnormal | Total | ||
| 24-2 | Normal | 121 | 79 | 200 |
| Abnormal | 40 | 63 | 103 | |
| Total | 161 | 142 | 303 | |
In ocular hypertensive eyes, 29 eyes of 108 (26.8%) eyes had an abnormal 24-2 result based upon cluster criteria, while 43 (39.8%) had an abnormal 10-2 test result (Table 5). However, 28 of the 79 (35.4%) eyes classified as normal on the 24-2 based upon cluster criteria were abnormal on the 10-2. Similarly, 14 of 65 (21.5%) eyes classified as normal based on 10-2 were classified as abnormal by 24-2. Again, all of these cases included abnormalities outside the central 10 degrees of the 24-2.
Table 5.
Eyes of patients with ocular hypertension. Frequency of normal and abnormal central visual field defects on 24-2 vs. 10-2 test results based upon cluster criteria.
| 10-2 | ||||
|---|---|---|---|---|
| Normal | Abnormal | Total | ||
| 24-2 | Normal | 51 | 28 | 79 |
| Abnormal | 14 | 15 | 29 | |
| Total | 65 | 43 | 108 | |
The prevalence of abnormal 10-2 results in eyes with normal 24-2 results in early glaucoma, glaucoma suspects, and ocular hypertensives was significantly higher than that seen in healthy eyes (4.6%, all P<0.001, Fisher’s exact test). Moreover, we investigated the characteristics of the 24-2 result that predicted the presence of an abnormal 10-2 result. In eyes with glaucoma and early field loss, the presence of at least one test location on the TD or PD plot at P<0.5% within the central 10 degrees (see blue crosses in Figure) was significantly associated with an abnormal 10-2 result (odds ratio, OR=7.52; 95% CI=3.47 to 16.29; P<0.001). Similar results were found in glaucoma suspects (OR=4.42; 95% CI=1.20 to 16.18; P=0.025) and ocular hypertensives (100% of cases). This effect was smaller as we changed the probabilities to 1%, 2%, and 5% (Table 6).
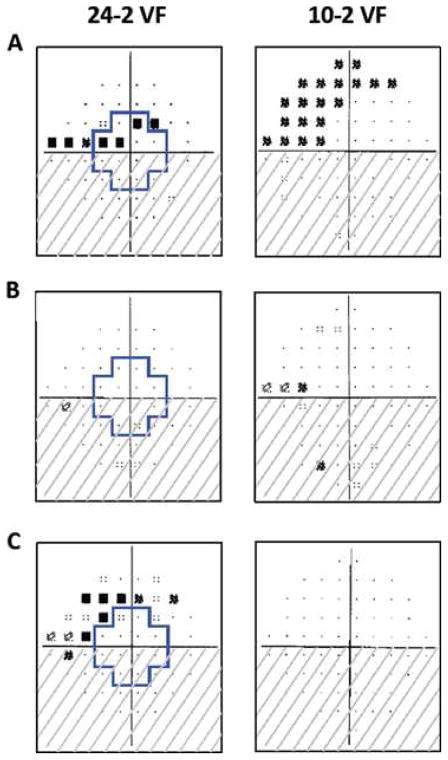
Figure.
The region within the blue outline on the 24-2 visual fields corresponds to the region in the central 10° tested by the 10-2 visual field test points. All fields are presented in right eye view. (A) Both superior hemifields are abnormal. (B) The superior hemifield is normal on the 24-2, but abnormal on the 10-2. (C) The superior hemifield is abnormal on the 24-2, but normal on the 10-2. Source: Traynis I, De Moraes CG, Raza AS, et al. JAMA Ophthalmology. 2014;132(3):291–297.4
Table 6.
Predictive value of 24-2 visual field abnormalities (within the central 10 degrees) on having an abnormal 10-2 results. The 24-2 abnormalities are defined based upon probabilities on total deviation or pattern deviation plots.
| Early Glaucoma | Glaucoma Suspects | Ocular Hypertension | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| P<0.5% | 7.52 | 3.47 to 16.29 | <0.001 | 4.42 | 1.20 to 16.18 | 0.025 | * | * | * |
| P<1% | 3.30 | 1.66 to 6.57 | 0.001 | 6.43 | 2.14 to 19.31 | 0.001 | 4.14 | 0.76 to 22.42 | 0.099 |
| P<2% | 3.50 | 1.83 to 6.70 | <0.001 | 5.71 | 2.73 to 11.96 | <0.001 | 1.91 | 0.59 to 6.13 | 0.276 |
| P<5% | 2.59 | 1.50 to 4.45 | 0.001 | 2.48 | 1.50 to 4.10 | <0.001 | 1.91 | 0.77 to 4.72 | 0.159 |
Abbreviations: OR= odds ratio; CI= confidence interval of the mean
Logistic model predicted perfect association (100%)
When combining all groups (n=775 eyes), patients of African descent were more likely to have an abnormal 10-2 result than those of European descent (67.3 vs. 56.8%, OR=1.56; 95% CI=1.12 to 2.18, P=0.009). Neither age nor sex were significantly associated with abnormal 10-2 results (P>0.200).
Because the proportion of eyes with visual field abnormalities depends upon the criteria employed, we performed an ancillary analysis using the above described cluster criteria (i.e., at least 3 contiguous points at 5%, 5%, 1%; 5%, 2%, 2%, or worse within the hemifield) as opposed to the ADAGES definition (i.e.: GHT “outside normal limits” or PSD< 5%) to define an abnormal 24-2 visual field. By applying these criteria, the number of eyes with early glaucoma, suspected glaucoma, and ocular hypertension changed to 338, 200, and 79 (note that these numbers can be obtained directly from Tables 3, 4, and 5). We then compared the number of eyes with a central abnormality on the 24-2 visual field (defined as any of the 12 central points at P<5% or worse) versus abnormal 10-2 results (defined the same way as above). Among early glaucoma eyes, 61 of 338 (18%) had a normal central 24-2, of which the 10-2 was abnormal in 37 (60.6%); among glaucoma suspects, 155 of 200 (77.5%) had a normal central 24-2, of which the 10-2 was abnormal in 57 (36.7%); among ocular hypertensives, 70 of 79 (88.6%) had a normal central 24-2, of which the 10-2 was abnormal in 24 (34.2%). Therefore, regardless of the criteria employed, a large proportion of eyes within the glaucoma continuum have abnormal 10-2 visual field tests despite normal 24-2 results.
DISCUSSION
We tested the hypothesis that 24-2 visual tests miss central damage detected with 10-2 tests in patients with ocular hypertension, suspected glaucoma, and early glaucomatous field loss. Despite a similar number of 24-2 and 10-2 tests detecting central defects, in many eyes the 10-2 tests revealed damage missed by 24-2 tests (35, 39, and 61%, respectively for ocular hypertensives, suspected glaucoma, and early glaucomatous field loss). Although these abnormalities detected with 10-2 but missed with 24-2 may not always represent true functional loss, these findings have implications not only on the definition of glaucoma, but also the classification of severity.
Despite the lack of consensus of how to define early glaucoma, most experts agree that the presence of structural damage is required, while functional loss, measured with SAP using 6-degree spacing, can be used to increase the likelihood of the disease and, for many, confirm the diagnosis of early glaucoma.24 According to the most recent World Glaucoma Association (WGA) Consensus on the Diagnosis of Open-angle Glaucoma, the combination of GON and a GHT outside normal limits on 24-2 SAP significantly increases the likelihood of glaucoma.24 In this study, we employed a similar definition (with or without a PSD < 5%) to define visual field damage. This is also the definition employed in the OHTS and EGPS to define early functional damage. Our findings therefore underscore that many patients often called suspects or pre-perimetric using the above criteria may in fact have established glaucomatous functional damage. In fact, of the 9,080 OHTS-defined normal and reliable visual field tests (on two out of three tests), 388 (4.2%) were identified by the visual field reading center as questionable because of the presence of suspicious clusters of points with abnormal sensitivity.25 Among OHTS participants who later developed abnormal 30-2 visual fields results, 15.6% had paracentral defects (however, if one includes other types of visual field patters that can extend to the central 10 degrees – such as arcuate, central, altitudinal, and widespread – this number exceeds 30%).26 Finally, upon retesting, OHTS-defined 30-2 abnormalities were not confirmed for 85% of originally abnormal and reliable visual fields.27
In addition, our findings have clinical implication because not only would these patients now be classified as having functional loss, but they would also be classified as having advanced glaucomatous damage based upon many classification systems, which consider the presence of central damage as a sign of severity20 due to the important role this region plays in daily activities and behavior.10–14 Remarkably, patients of African descent were more likely to have central visual field abnormalities, which underscores the role of race as a significant risk factor for the presence and severity of glaucoma.
One important concept is that the proportion of eyes classified as having abnormal visual fields depends upon how visual field abnormalities are defined. Here we used the ADAGES classification system (which is similar to the OHTS/EGPS systems) to define an abnormal 24-2 visual field result. This system, however, does not provide topographic information, that is, it does not show where the damage is. Hence, we applied criteria for visual field abnormality based upon clusters to be able to assess the location of damage. These two differing classification systems ought to be kept in mind when interpreting our results. For instance, this explains why some patients with established glaucoma (i.e., GON with abnormal 24-2 SAP based upon ADAGES criteria) did not have abnormalities based upon cluster criteria (26 of 364, 7%). Also, it explains why some glaucoma suspects (i.e.: GON with normal 24-2 SAP based upon ADAGES criteria) had abnormalities based upon cluster criteria (103 of 303, 34%). Our ancillary analysis revealed that, even when different criteria are employed, 24-2 tests still miss a meaningful proportion of eyes with central damage seen on 10-2 tests. Yet, the possibility of false-positive results should be kept in mind, and further confirmation with macular sdOCT is warranted.
Although we included patients whose tests were reliable and repeatable, it is still possible that some of the abnormalities detected on 10-2 tests, but not 24-2, could have been false-positive results. As we showed, about 5% of healthy eyes had false-positive results based upon our 10-2 cluster criteria. Moreover, when the presence of macular damage was defined using sdOCT and 10-2 tests simultaneously, 24-2 tests still missed a significant number of eyes with central damage (52%).16 The main reason for these findings is the sparse distribution of test locations in the 24-2 grid (6 degrees apart), as opposed to 10-2 (2 degrees). In fact, sdOCT studies have shown that the commonly seen arcuate-like damage of the retinal ganglion cell layer damage close to fixation falls within the central four points of the 24-2 test pattern.1
Our findings are consistent with recent studies from other groups investigating the prevalence of central visual field damage using 24-2 and 10-2 tests. In a one study, repeatable 10-2 visual field defects were present in 49% of subjects with established or suspected glaucoma.6 The authors reported that in eyes with no, mild, moderate, and advanced 24-2 visual field loss, 6%, 73%, 96%, and 100% had 10-2 visual field defects, respectively. In another study, 74.7% of eyes that underwent 24-2 (with MD better than −6 dB) and 10-2 had a paracentral scotoma detected on the 10-2 visual field test.5 The authors also investigated characteristics that predicted the presence of paracentral scotoma on the 10-2 visual field in early glaucoma patients and found that presence of abnormal 24-2 visual field points on the pattern deviation plot at P<0.5% was significantly associated with an abnormal 10-2 result (OR=18.00 and 3.89, for the 4 and 8 central-most points, respectively, both P<0.01).5 Our findings are consistent with those as the presence of central abnormalities on the 24-2, even if isolated and at P<5%, were significantly associated with an abnormal cluster on 10-2 tests (Table 6).
Many clinicians wait until significant visual field abnormalities are seen on 24-2 SAP (the reference standard used in clinical practice) to define whether a patient has glaucoma. Our findings suggest that many patients therefore may have had their treatment postponed due to the exclusive reliance on 24-2 results to tailor therapy. Of course in practice clinicians often complement their findings with clinical information, such as intraocular pressure, central corneal thickness, family history, and other risk factors, in addition to 24-2 test when making treatment decisions. Nonetheless, our results suggest that, had clinicians performed 10-2 tests in some of these patients and the results came out abnormal, the decision process would have been very different, with earlier and likely more aggressive treatment. Alternatively, the macular RGC thickness measured with sdOCT has excellent ability to detect glaucomatous damage 18 and has been shown to improve early diagnosis of glaucoma.19
Although we still do not have precise guidelines on when and how often 10-2 visual fields should be performed, some clinicians alternate 24-2 and 10-2 tests over time, while others add the 10-2 if there is any reason to be concerned about macular damage. As we described, the presence of abnormalities within the central 10 degrees of the 24-2, even if represented by isolated test points at probabilities often overlooked, can help decide when to perform 10-2 test as well. Also, the presence of a localized nerve fiber layer defect in the inferior-temporal region of the optic disc during assessment of disc photographs is likely to indicate significant macular damage.18 Alternatively, new developments in visual field testing algorithms (particularly the inclusion of more test locations within the central field) 28 and the combination of sdOCT and visual field tests results 29 may be helpful to overcome current challenges to perform multiple tests in daily practice. However, we suggest that clinicians should consider performing 10-2 tests not only in patients with established glaucoma, but also in glaucoma suspects and ocular hypertensives to prevent misdiagnosis and/or misclassification of disease severity. A normal 10-2 examination also provides and important baseline for future comparison, should a central field deficit develop or be suspected.
Acknowledgments
Supported by: National Eye Institute Grants U10EY14267, EY08208, EY11008, EY019869, EY13959, EY02115 (DCH), EY025253 (CGDM); Eyesight Foundation of Alabama; Alcon Laboratories Inc.; Allergan Inc.; Pfizer Inc.; Merck Inc.; Santen Inc.; unrestricted departmental grant from Research to Prevent Blindness, New York, NY (Department of Ophthalmology, Columbia University Medical Center and Department of Ophthalmology, University of California San Diego), Edith C. Blum Foundation, New York, NY.
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00221923.
Financial disclosures:
C.G.D.M.: Consultant– SENSIMED AG; Grants – Carl Zeiss Medictec, Inc.; Research Support: Heidelberg Engineering; Topcon Inc.
D.C.H.: Consultant: Carl Zeiss Meditec Inc; Research Support: Heidelberg Engineering; Topcon Inc.
A.T.: None.
C.A.G.: Research support – Heidelberg Engineering GmbH.
F.A.M.: Financial support – Alcon Laboratories Inc., Carl Zeiss Meditec Inc., Pfizer Inc.; Consultant – Alcon Laboratories Inc., Allergan Inc., Pfizer Inc.; Research support – Alcon Laboratories Inc., Allergan Inc., Carl Zeiss Meditec Inc., Pfizer Inc., Reicherts Inc.
R.N.W.: Financial support – Carl Zeiss Meditec Inc., Heidelberg Engineering GmbH, Optovue Inc., Topcon Medical Systems; Consultant – Aerie, Alcon Laboratories Inc., Allergan Inc, Bausch & Lomb, Unity; Grants – Quark, Genentech
L.M.Z.: Financial support – Carl Zeiss Meditec Inc., Heidelberg Engineering GmbH, Optovue Inc., Topcon Medical Systems Inc.; Research support – Heidelberg Engineering.
J.M.L.: Consultant – Alcon Laboratories Inc., Allergan Inc., Carl Zeiss Meditec Inc., Dyopsis Inc., Pfizer Inc., Topcon Medical Systems Inc.
The sponsors or funding organizations had no role in the design or conduct of this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hood DC, Raza AS, de Moraes CG, et al. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013;32:1–21. doi: 10.1016/j.preteyeres.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drance SM. The early field defects in glaucoma. Invest Ophthalmol. 1969;8:84–91. [PubMed] [Google Scholar]
- 3.Langerhorst CT, Carenini LL, Bakker D, De Bie-Raakman MAC. Measurements for description of very early glaucomatous field defects. In: Wall M, Heiji A, editors. Perimetry Update 1996/1997. New York, NY: Kugler Publications; 1997. [Google Scholar]
- 4.Traynis I, De Moraes CG, Raza AS, et al. Prevalence and nature of early glaucomatous defects in the central 10 degrees of the visual field. JAMA Ophthalmol. 2014;132:291–297. doi: 10.1001/jamaophthalmol.2013.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park HY, Hwang BE, Shin HY, Park CK. Clinical Clues to Predict the Presence of Parafoveal Scotoma on Humphrey 10-2 Visual Field Using a Humphrey 24-2 Visual Field. Am J Ophthalmol. 2016;161:150–159. doi: 10.1016/j.ajo.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan-Mee M, Karin Tran MT, Pensyl D, et al. Prevalence, Features, and Severity of Glaucomatous Visual Field Loss Measured With the 10-2 Achromatic Threshold Visual Field Test. Am J Ophthalmol. 2016;168:40–51. doi: 10.1016/j.ajo.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Schiefer U, Papageorgiou E, Sample PA, et al. Spatial pattern of glaucomatous visual field loss obtained with regionally condensed stimulus arrangements. Invest Ophthalmol Vis Sci. 2010;51:5685–5689. doi: 10.1167/iovs.09-5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300:5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]
- 9.McFadzean R, Brosnahan D, Hadley D, Mutlukan E. Representation of the visual field in the occipital striate cortex. Br J Ophthalmol. 1994;78:185–190. doi: 10.1136/bjo.78.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murata H, Hirasawa H, Aoyama Y, et al. Identifying areas of the visual field important for quality of life in patients with glaucoma. PLoS One. 2013;8:e58695. doi: 10.1371/journal.pone.0058695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramulu PY, West SK, Munoz B, et al. Driving cessation and driving limitation in glaucoma: the Salisbury Eye Evaluation Project. Ophthalmology. 2009;116:1846–1853. doi: 10.1016/j.ophtha.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baig S, Diniz-Filho A, Wu Z, et al. Association of Fast Visual Field Loss With Risk of Falling in Patients With Glaucoma. JAMA Ophthalmol. 2016 doi: 10.1001/jamaophthalmol.2016.1659. [DOI] [PubMed] [Google Scholar]
- 13.Ramulu PY, West SK, Munoz B, et al. Glaucoma and reading speed: the Salisbury Eye Evaluation project. Arch Ophthalmol. 2009;127:82–87. doi: 10.1001/archophthalmol.2008.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jampel HD, Frick KD, Janz NK, et al. Depression and mood indicators in newly diagnosed glaucoma patients. Am J Ophthalmol. 2007;144:238–244. doi: 10.1016/j.ajo.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 15.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. Jama. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grillo LM, Wang DL, Ramachandran R, et al. The 24-2 Visual Field Test Misses Central Macular Damage Confirmed by the 10-2 Visual Field Test and Optical Coherence Tomography. Transl Vis Sci Technol. 2016;5:15. doi: 10.1167/tvst.5.2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang DL, Raza AS, de Moraes CG, et al. Central Glaucomatous Damage of the Macula Can Be Overlooked by Conventional OCT Retinal Nerve Fiber Layer Thickness Analyses. Transl Vis Sci Technol. 2015;4:4. doi: 10.1167/tvst.4.6.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C, Tatham AJ, Abe RY, et al. Macular Ganglion Cell Inner Plexiform Layer Thickness in Glaucomatous Eyes with Localized Retinal Nerve Fiber Layer Defects. PLoS One. 2016;11:e0160549. doi: 10.1371/journal.pone.0160549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tatham AJ, Medeiros FA, Zangwill LM, Weinreb RN. Strategies to improve early diagnosis in glaucoma. Prog Brain Res. 2015;221:103–133. doi: 10.1016/bs.pbr.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Mills RP, Budenz DL, Lee PP, et al. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am J Ophthalmol. 2006;141:24–30. doi: 10.1016/j.ajo.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 21.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127:1136–1145. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feuer WJ, Parrish RK, 2nd, Schiffman JC, et al. The Ocular Hypertension Treatment Study: reproducibility of cup/disk ratio measurements over time at an optic disc reading center. Am J Ophthalmol. 2002;133:19–28. doi: 10.1016/s0002-9394(01)01338-1. [DOI] [PubMed] [Google Scholar]
- 23.Zeyen T, Miglior S, Pfeiffer N, et al. Reproducibility of evaluation of optic disc change for glaucoma with stereo optic disc photographs. Ophthalmology. 2003;110:340–344. doi: 10.1016/s0161-6420(02)01754-2. [DOI] [PubMed] [Google Scholar]
- 24.Weinreb RN, Liebmann JM, Medeiros FA. Diagnosis of Primary Open-Angle Glaucoma: Consensus. 2016. [Google Scholar]
- 25.Johnson CA, Keltner JL, Cello KE, et al. Baseline visual field characteristics in the ocular hypertension treatment study. Ophthalmology. 2002;109:432–437. doi: 10.1016/s0161-6420(01)00948-4. [DOI] [PubMed] [Google Scholar]
- 26.Keltner JL, Johnson CA, Cello KE, et al. Classification of visual field abnormalities in the ocular hypertension treatment study. Arch Ophthalmol. 2003;121:643–650. doi: 10.1001/archopht.121.5.643. [DOI] [PubMed] [Google Scholar]
- 27.Keltner JL, Johnson CA, Quigg JM, et al. Confirmation of visual field abnormalities in the Ocular Hypertension Treatment Study. Ocular Hypertension Treatment Study Group. Arch Ophthalmol. 2000;118:1187–1194. doi: 10.1001/archopht.118.9.1187. [DOI] [PubMed] [Google Scholar]
- 28.Ehrlich AC, Raza AS, Ritch R, Hood DC. Modifying the Conventional Visual Field Test Pattern to Improve the Detection of Early Glaucomatous Defects in the Central 10 degrees. Transl Vis Sci Technol. 2014;3:6. doi: 10.1167/tvst.3.6.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hood DC, Raza AS, De Moraes CG, et al. Evaluation of a One-Page Report to Aid in Detecting Glaucomatous Damage. Transl Vis Sci Technol. 2014;3:8. doi: 10.1167/tvst.3.6.8. [DOI] [PMC free article] [PubMed] [Google Scholar]