Abstract
Background: Epidemiologic evidence regarding niacin, folate, vitamin B-6, and vitamin B-12 intake in relation to cognitive function is limited, especially in midlife.
Objective: We hypothesize that higher intake of these B vitamins in young adulthood is associated with better cognition later in life.
Design: This study comprised a community-based multicenter cohort of black and white men and women aged 18–30 y in 1985–1986 (year 0, i.e., baseline) from the Coronary Artery Risk Development in Young Adults (CARDIA) study (n = 3136). We examined participants’ CARDIA diet history at years 0, 7, and 20 to assess nutrient intake, including dietary and supplemental B vitamins. We measured cognitive function at year 25 (mean ± SD age: 50 ± 4 y) through the use of the Rey Auditory Verbal Learning Test (RAVLT) for verbal memory, the Digit Symbol Substitution Test (DSST) for psychomotor speed, and a modified Stroop interference test for executive function. Higher RAVLT and DSST scores and a lower Stroop score indicated better cognitive function. We used multivariable-adjusted linear regressions to estimate mean differences in cognitive scores and 95% CIs.
Results: Comparing the highest quintile with the lowest (quintile 5 compared with quintile 1), cumulative total intake of niacin was significantly associated with 3.92 more digits on the DSST (95% CI: 2.28, 5.55; P-trend < 0.01) and 1.89 points lower interference score on the Stroop test (95% CI: −3.10, −0.68; P-trend = 0.05). Total folate was associated with 2.56 more digits on the DSST (95% CI: 0.82, 4.31; P-trend = 0.01). We also found that higher intakes of vitamin B-6 (quartile 5 compared with quartile 1: 2.62; 95% CI: 0.97, 4.28; P-trend = 0.02) and vitamin B-12 (quartile 5 compared with quartile 1: 2.08; 95% CI: 0.52, 3.65; P-trend = 0.02) resulted in better psychomotor speed measured by DSST scores.
Conclusion: Higher intake of B vitamins throughout young adulthood was associated with better cognitive function in midlife.
Keywords: epidemiology, cognitive function, niacin, folate, vitamin B-6, vitamin B-12, middle age
INTRODUCTION
Maintaining a healthy lifestyle and diet may be an important strategy for reducing the global burden of cognitive impairment and dementia. A modest 1-y delay in the onset and progression of Alzheimer disease (AD), the most common form of dementia, is estimated to reduce the number of cases by 9.2 million throughout the world (1). In particular, B-vitamin intake has been thought to reduce the risk of later adulthood cognitive dysfunction. Multiple mechanisms linking B vitamins to cognitive function have been observed, chiefly through their influence on homocysteine metabolism (2, 3).
To the best of our knowledge, 3 studies have examined niacin intake and cognitive outcome in older adults. These studies found that higher intake of niacin was correlated with better performance on cognitive tests (4, 5) or was associated with lower risk of AD (6). Several systematic reviews and meta-analyses of cohort studies reported that lower intakes of folate and vitamin B-6 in mid- and late life were associated with poorer cognitive outcomes in older adults (7, 8). Vitamin B-12 status, measured by sensitive markers holotranscobalamin and methylmalonic acid, was inversely associated with impaired cognition or risk of dementia (9). Randomized controlled trials (RCTs) revealed an inconsistent picture of B-vitamin supplementation and cognitive function (10–12), but RCTs carried out on participants with a low dietary intake of B vitamins or with early signs of cognitive impairment found a favorable effect of B-vitamin supplementation on cognitive function (13–15).
Evidence suggests that cognitive decline is already evident by middle age (16), which indicates a possibility that the critical period to prevent the onset of dementia may be earlier than has been predicted. However, few studies, if any, have evaluated whether the intake of B vitamins in young adulthood is associated with cognitive function in midlife. Thus, we aimed to investigate the association between the intakes of niacin, folate, vitamin B-6, and vitamin B-12 through young adulthood and cognitive function in midlife. The CARDIA (Coronary Artery Risk Development in Young Adults) study, which followed participants for 25 y starting from a young age, offers a unique opportunity to examine these relations.
METHODS
Study population
The CARDIA study was a community-based, multicenter, longitudinal study of black and white men and women initially aged 18–30 y in 1985–1986 (year 0, i.e., baseline) (17). A total of 5115 participants were recruited from 4 sites in the United States (Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California). They were re-examined during 7 follow-ups at years 2, 5, 7, 10, 15, 20, and 25, with retention rates of 91%, 86%, 81%, 79%, 74%, 72%, and 72%, respectively. All the participants provided written informed consent, and the institutional review boards at all centers approved the study.
The present analyses included participants who had undergone cognitive measurements at year 25 and ≥1 measurement of B-vitamin intake at baseline, year 7, or year 20. Dietary data for participants who were pregnant or reported an extreme energy intake (<600 or >6000 kcal/d for women; <800 or >8000 kcal/d for men) were set as missing. Among 3499 participants at year 25, 3322 had undergone cognitive testing (the Rey Auditory Verbal Learning Test, the Digit Symbol Substitution Test, and the Stroop Test). After excluding 96 individuals who did not participate in year 7 and year 20 surveys, 8 participants who were pregnant, 34 who reported an extreme energy intake, and 48 who had missing cumulative dietary information, 3136 participants remained in the analyses (Supplemental Figure 1).
Dietary assessment
We collected dietary intake information at baseline, year 7, and year 20 through the use of an interviewer-administered CARDIA Diet History (18). In brief, participants were questioned about their eating habits in the previous month, including portion sizes and common additions (e.g., toppings, condiments). We calculated nutrient intake based on information from the food and nutrient database Nutrition Data System for Research (version 10, 20, and 36 at baseline, year 7, and year 20, respectively; Nutrition Coordinating Center at the University of Minnesota). We also recorded participants’ supplemental intake of B vitamins including intake from multivitamin sources. Total intake of each B vitamin includes that from both dietary and supplemental sources. A validation study of a sample of 128 CARDIA participants has been described elsewhere (19). The correlation coefficients for logarithmically transformed nutrient values and energy-adjusted nutrient values from 2 dietary histories ranged from 0.30 to 0.80.
To represent long-term intake and to minimize sample loss due to missing data, the cumulative total intake was calculated based on the mean total intake or 1 instance of total intake across baseline, year 7, or year 20 among participants who had completed the diet history ≥1 time (72% had all 3 measures). Total intake of vitamin B-6 and vitamin B-12 was based on year 7 and year 20 mean values because intake of those vitamins was not assessed at baseline. We reported the median value of individual B vitamins at each wave and by the number of dietary assessments (e.g., all 3 waves compared with 1–2 waves) to facilitate the understanding of trends in intake over time and in nutrient intake by participation status, respectively.
Cognitive function assessment
Trained and certified CARDIA technicians measured cognitive function at year 25 with 3 standardized tests. The Rey Auditory Verbal Learning Test (RAVLT) assessed verbal memory. Results from a 10-min delayed free recall were used, with possible scores ranging from 0 to 15. More words recalled indicated better performance. The Digit Symbol Substitution Test (DSST) from the Wechsler Adult Intelligence Scale–III assessed psychomotor speed, sustained attention, and working memory (20). The possible scores ranged from 0 to 133, with a higher score indicating better function. The Stroop interference test evaluated executive function through viewing multiple visual stimuli, which assessed the ability to respond to one stimulus dimension while suppressing the response to another (21). In the first and second congruent trials of the Stroop task, participants read a list of names of colors and identified the colors of 40 rectangular boxes, respectively. In the final incongruent trial, participants named the color of words printed in a color different from the written name of the color. Each trial was scored by adding the number of errors and the amount of time (s) required to complete the trial. The Stroop interference score was calculated by subtracting the score on the final incongruent trial from the second congruent trial (22). Higher scores indicated worse cognitive performance.
Covariates
We assessed all covariates with standardized questionnaires or clinical examinations at baseline and follow-up. Education through year 25 was measured in years and grouped by level of education achieved: high school or below, college, or graduate school and beyond in stratified analysis. Participants were classified as never or ever smokers. Alcohol intake, assessed by a self-administered questionnaire, was converted to milliliters per day (23). Physical activity (exercise units) was measured by the validated, interviewer-administered CARDIA Physical Activity History questionnaire (24). BMI was calculated by measuring participants’ weight and height, which were taken in duplicate and averaged. Depressive symptoms were assessed on the Center for Epidemiologic Studies Depression (CES-D) scale, which has been validated and extensively used (25). The possible score ranged from 0 to 60. A higher score indicated more depressive symptoms. Blood pressure was measured from the right arm of seated participants at 1-min intervals after a 5-min rest. The average of the second and the third measurements was used. The presence of diabetes was detected through fasting blood glucose or glucose tolerance tests (≥126 or ≥200 mg/dL respectively), glycated hemoglobin assay (≥6.5%), or reported use of antidiabetic medications (26). Except for smoking and diabetes, which were represented as binary status through year 25, all other nondietary lifestyle factors or medical factors were averaged across repeated measurements from baseline to year 25. Dietary covariates were averaged across measures at baseline, year 7, and year 20. For participants who had a missing education (n = 7) or smoking (n = 13) or diabetes status (n = 10) at year 25, their last observation at year 20 was carried forward.
Statistical analysis
We examined characteristics of participants by quintiles of total niacin, folate, vitamin B-6, and vitamin B-12 intake in nutrient density form through the use of ANOVA, chi-square test, or Kruskal-Wallis equality-of-populations rank test as appropriate. Characteristics were described as mean or percentage. The median value was provided for variables (e.g., alcohol consumption) with skewed distribution. We also compared the correlations between the individual B vitamins under study through the use of Spearman’s rank correlation coefficients.
We used multivariable-adjusted linear regression to model the association between the intake of B vitamins and the outcome of interest. The first model adjusted for age, sex, race, center, and educational attainment. The second model further adjusted for smoking status, alcohol consumption, physical activity, BMI, CES-D scores, systolic blood pressure, diabetes, total energy intake, saturated fat and cholesterol intake, and additional covariates that changed the effect estimate over 10% for corresponding models. For niacin and folate, the second model additionally adjusted for intakes of vitamin C and polyunsaturated fat. For vitamin B-6 and vitamin B-12, the model further adjusted for vitamin C intake, or intakes of vitamin E and polyunsaturated fat, respectively. The parsimonious set of nutrient variables in each model was based on a priori potential confounders and was aided by backward stepwise elimination. Linear trends were tested by creating a continuous variable for the exposure of interest by using the median value of each quintile. We did not adjust for multiple comparisons because the associations examined were from a priori hypotheses based on the findings of previous literature on niacin, folate, vitamin B-6, and vitamin B-12 with cognitive outcomes (6–9). Considering the challenge of highly correlated nature of B vitamins in model adjustment and interpretation, we presented the results adjusting for other B vitamins as continuous variables in a sensitivity analysis. We performed the joint F test for a global hypothesis, testing that among the highly correlated B vitamins, the association with each cognitive outcome was not null.
Based on the findings of previous literature on B vitamins and cognitive function or other health outcomes, and the role of education in cognitive resilience (27–30), we developed a priori hypotheses to examine whether the associations were modified by race, educational level, CES-D score, and supplementation via likelihood ratio test with and without the product term between the B vitamins under study (quintiles) and the stratification term (categories). We repeated the analysis by excluding participants who experienced a stroke event through year 25 (n = 40). We also repeated the analysis by limiting it to those who had diet information in all 3 waves (n = 2268). Additionally to the nutrient density method, we also applied the standard multivariate method which used the absolute nutrient intake with adjustment for the total energy intake. A 2-sided P value ≤0.05 was defined as statistical significance. All statistical analyses were performed with Stata (version 14.1; StataCorp).
RESULTS
The current study included 1398 blacks (45%) and 1738 whites (55%) with 56% female and mean ± SD age of 25.1 ± 3.6 y at baseline. Participants with a higher intake of niacin, folate, vitamin B-6, or vitamin B-12 were older, were more likely to be female and white, exercised more, and had a higher education level, fewer depression symptoms as indicated by lower CES-D scores, lower BMI and systolic blood pressure, and a lower prevalence of diabetes (for niacin and folate only) (Table 1). A higher B-vitamin intake was related to lower intakes of total energy, saturated fat, and cholesterol. B-vitamin intake was positively correlated to the intakes of vitamins C and E. The Spearman rank correlation coefficients between these B vitamins ranged from 0.65 to 0.82 (Supplemental Table 1) as expected, because fortified breakfast cereals and multivitamins are their shared major sources (31). The median intake of niacin, vitamin B-6, and vitamin B-12 slightly increased over time (Supplemental Table 2), while folate intake had appreciably increased at year 20 after the Food and Drug Association–mandated folic acid fortification of grain products in the United States went into effect in 1998. Participants who had all 3 waves of dietary assessment tended to have higher intakes of B vitamins at each wave.
TABLE 1.
Characteristics of study population by quintiles of cumulative daily total intake of niacin, folate, vitamin B-6, and vitamin B-12 through year 25 of the CARDIA study (n = 3136)1
| Niacin, mg/1000 kcal |
Folate, μg/1000 kcal |
Vitamin B-6, mg/1000 kcal |
Vitamin B-12, μg/1000 kcal |
|||||||||||||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | Q1 | Q2 | Q3 | Q4 | Q5 | Q1 | Q2 | Q3 | Q4 | Q5 | Q1 | Q2 | Q3 | Q4 | Q5 | |
| n | 630 | 631 | 623 | 625 | 627 | 629 | 626 | 627 | 628 | 626 | 632 | 635 | 617 | 629 | 623 | 634 | 622 | 626 | 627 | 627 |
| Median daily intake | 8.6 | 10.2 | 11.9 | 14.8 | 24.7 | 113.1 | 151.6 | 190.6 | 250.9 | 384.0 | 0.7 | 0.9 | 1.1 | 1.5 | 3.0 | 1.5 | 2.2 | 3.0 | 4.4 | 8.7 |
| Socioeconomic factors | ||||||||||||||||||||
| Age, y | 49.3 | 49.8 | 50.3 | 50.3 | 50.9 | 49.2 | 50.0 | 50.2 | 50.4 | 50.8 | 49.4 | 50.0 | 50.2 | 50.3 | 50.7 | 49.8 | 49.8 | 50.1 | 50.3 | 50.6 |
| Female, % | 55.4 | 49.9 | 54.9 | 55.8 | 65.9 | 46.4 | 46.5 | 54.2 | 57.6 | 77.2 | 47.6 | 50.7 | 56.4 | 60.1 | 67.3 | 54.9 | 49.7 | 55.1 | 60.5 | 61.7 |
| Black, % | 63.8 | 52.1 | 40.1 | 37.8 | 28.9 | 68.4 | 49.5 | 40.8 | 34.9 | 29.2 | 63.5 | 46.0 | 41.2 | 35.9 | 36.1 | 56.5 | 47.1 | 42.0 | 40.7 | 36.5 |
| Education attained through year 25, y | 14.5 | 14.8 | 15.2 | 15.6 | 15.8 | 14.0 | 14.9 | 15.3 | 15.7 | 16.0 | 14.2 | 15.0 | 15.4 | 15.6 | 15.7 | 14.8 | 14.9 | 15.2 | 15.3 | 15.6 |
| Lifestyle factors and medical history | ||||||||||||||||||||
| Never smoker, % | 51.3 | 52.0 | 49.9 | 57.3 | 52.02 | 45.3 | 53.8 | 52.3 | 54.3 | 56.7 | 45.3 | 52.6 | 55.6 | 56.4 | 52.7 | 52.4 | 50.5 | 53.8 | 51.7 | 54.12 |
| Alcohol intake,3 mL/d | 4.1 | 6.1 | 6.4 | 4.3 | 5.7 | 5.6 | 6.5 | 5.6 | 4.9 | 4.0 | 6.1 | 5.7 | 5.2 | 4.9 | 4.22 | 4.8 | 5.7 | 5.7 | 4.9 | 4.92 |
| Physical activity,3 exercise units | 292.5 | 304.9 | 310.5 | 339.4 | 369.2 | 267.3 | 319.4 | 323.2 | 348.4 | 348.3 | 273.0 | 314.0 | 335.0 | 340.7 | 360.0 | 301.8 | 316.9 | 324.1 | 311.4 | 368.3 |
| BMI, kg/m2 | 27.6 | 27.6 | 27.5 | 27.2 | 26.3 | 28.3 | 27.7 | 27.0 | 26.9 | 26.3 | 27.7 | 27.8 | 27.2 | 26.8 | 26.6 | 27.5 | 27.6 | 27.2 | 27.3 | 26.6 |
| CES-D score | 10.6 | 10.0 | 9.3 | 9.1 | 9.0 | 10.9 | 9.5 | 9.7 | 9.1 | 8.8 | 10.9 | 9.5 | 9.6 | 9.1 | 8.9 | 10.1 | 9.9 | 9.3 | 9.7 | 9.0 |
| Systolic blood pressure, mm Hg | 112.4 | 112.4 | 111.6 | 110.7 | 109.6 | 113.8 | 112.9 | 110.9 | 110.1 | 108.9 | 113.2 | 112.7 | 110.7 | 110.2 | 109.8 | 111.7 | 112.4 | 111.3 | 111.0 | 110.2 |
| Diabetes, % | 12.7 | 17.1 | 12.7 | 13.9 | 10.1 | 16.2 | 13.9 | 12.9 | 13.1 | 10.4 | 13.9 | 16.1 | 13.0 | 10.3 | 13.22 | 12.6 | 14.2 | 13.4 | 13.9 | 12.42 |
| Stroke, % | 1.6 | 1.4 | 1.0 | 1.1 | 1.32 | 1.9 | 1.0 | 0.8 | 1.4 | 1.32 | 2.2 | 0.8 | 1.0 | 0.6 | 1.82 | 1.0 | 1.8 | 0.5 | 1.8 | 1.42 |
| Daily nutrient intake | ||||||||||||||||||||
| Total energy, kcal | 2852.5 | 2840.1 | 2613.5 | 2513.9 | 2293.5 | 2961.7 | 2822.7 | 2657.4 | 2529.4 | 2143.8 | 2953.3 | 2720.5 | 2654.8 | 2502.0 | 2280.6 | 2765.4 | 2756.2 | 2712.5 | 2527.5 | 2354.3 |
| Total vitamin C,3 mg/1000 kcal | 54.4 | 58.5 | 67.0 | 88.9 | 143.1 | 44.5 | 59.0 | 73.6 | 94.8 | 136.7 | 46.6 | 61.7 | 73.7 | 93.5 | 134.4 | 59.1 | 60.6 | 68.6 | 89.8 | 122.9 |
| Total vitamin E,3 mg/1000 kcal | 3.4 | 3.9 | 4.9 | 7.3 | 11.9 | 3.3 | 3.8 | 5.2 | 7.0 | 11.5 | 3.3 | 3.9 | 5.0 | 7.2 | 11.9 | 3.8 | 3.9 | 5.0 | 7.0 | 11.2 |
| Saturated fat, g/1000 kcal | 15.0 | 14.6 | 14.2 | 13.6 | 13.2 | 15.2 | 14.7 | 14.2 | 13.7 | 12.9 | 15.2 | 14.5 | 14.0 | 13.6 | 13.2 | 14.2 | 14.6 | 14.4 | 13.9 | 13.4 |
| Cholesterol, mg/1000 kcal | 148.1 | 148.7 | 145.1 | 140.4 | 138.3 | 152.3 | 151.4 | 144.3 | 139.3 | 133.3 | 151.2 | 147.5 | 143.5 | 139.3 | 139.0 | 138.3 | 146.2 | 147.8 | 145.1 | 143.4 |
| Polyunsaturated fat, g/1000 kcal | 8.1 | 8.4 | 8.2 | 8.2 | 8.22 | 8.3 | 8.3 | 8.3 | 8.1 | 8.0 | 8.3 | 8.2 | 8.2 | 8.0 | 8.12 | 8.5 | 8.2 | 8.0 | 8.1 | 8.0 |
Values are means or percentages as appropriate. Except for vitamin B-6 and vitamin B-12, which were based on the average of year 7 and year 20 values, other mean values of nutrient intake were based on the averages of year 0, 7, and 20 values. CARDIA, Coronary Artery Risk Development in Young Adults; CES-D, Center for Epidemiologic Studies Depression Scale; Q, quintile.
P > 0.05. All others were significant at P ≤ 0.05. ANOVA, chi-square test, or Kruskal-Wallis equality-of-populations rank test were used as appropriate.
Median values were provided for skewed distributed variables.
The mean ± SD scores of RAVLT, DSST, and the Stroop test were 8.4 ± 3.2, 70.5 ± 15.9, and 22.5 ± 10.7, respectively. Higher niacin intake was significantly associated with a better performance on 2 of the 3 cognitive tests (Table 2). Compared with participants in the lowest quintile (quintile 1) of niacin intake, those in the highest quintile (quintile 5) had 3.92 more digits in DSST (95% CI: 2.28, 5.55; P-trend < 0.01), and 1.89 points lower interference score on the Stroop test (95% CI: −3.10, −0.68; P-trend = 0.05), which are equivalent to results observed in participants 6.1 and 5.9 y younger in age, respectively. No significant association was observed between niacin intake and RAVLT. The highest quintile of folate intake was associated with 2.56 more digits in DSST (95% CI: 0.82, 4.31; P-trend = 0.01), equivalent to 4.1 y younger in age. The positive association between folate intake and RAVLT was marginally significant (quintile 5 compared with quintile 1: 0.30; 95% CI: −0.07, 0.68; P-trend = 0.09). The association with Stroop test scores was attenuated and not significant after multivariable adjustment. For vitamin B-6, higher intake was associated with a better performance in DSST (quintile 5 compared with quintile 1: 2.62; 95% CI: 0.97, 4.28; P-trend = 0.02), but not in RAVLT or Stroop test. Except for the highest quintile of vitamin B-12 intake, which was associated with 2.08 more digits in DSST (95% CI: 0.52, 3.65; P-trend = 0.02), no significant association was found between vitamin B-12 and other cognitive tests. Results remained materially unchanged after further adjusting for other B vitamins under study despite the increased standard errors of the estimates (Supplemental Table 3). As suggested by the conditional F test, the estimates of B vitamins under study were jointly significant for each cognitive outcome (P ≤ 0.01).
TABLE 2.
Multivariable-adjusted mean differences (95% CIs) in cognitive test scores according to quintile of cumulative daily total intake of niacin, folate, vitamin B-6, and vitamin B-12 in the CARDIA study (n = 3136)1
| RAVLT words(mean ± SD: 8.4 ± 3.2) |
DSST symbols(mean ± SD: 70.5 ± 15.9) |
Stroop test points(mean ± SD: 22.5 ± 10.7) |
||||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |||
| Niacin (mg/1000 kcal) | ||||||||
| Q1 (≤9.43) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | ||
| Q2 (9.44–10.98) | 0.06 (−0.25, 0.38) | 0.08 (−0.23, 0.40) | 1.85 (0.35, 3.35) | 1.80 (0.33, 3.27) | −1.67 (−2.76, −0.58) | −1.68 (−2.76, −0.59) | ||
| Q3 (10.99–13.00) | 0.19 (−0.13, 0.51) | 0.19 (−0.13, 0.52) | 2.10 (0.57, 3.62) | 1.99 (0.49, 3.49) | −1.60 (−2.71, −0.49) | −1.59 (−2.70, −0.48) | ||
| Q4 (13.01–17.52) | 0.22 (−0.11, 0.54) | 0.23 (−0.10, 0.56) | 2.48 (0.94, 4.01) | 2.26 (0.73, 3.79) | −1.76 (−2.88, −0.65) | −1.74 (−2.87, −0.61) | ||
| Q5 (≥17.53) | 0.13 (−0.20, 0.46) | 0.17 (−0.18, 0.52) | 3.76 (2.20, 5.32) | 3.92 (2.28, 5.55) | −1.85 (−2.98, −0.71) | −1.89 (−3.10, −0.68) | ||
| P-trend | 0.58 | 0.49 | <0.01 | <0.01 | 0.04 | 0.048 | ||
| Folate (μg/1000 kcal) | ||||||||
| Q1 (≤133.2) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | ||
| Q2 (133.3–169.7) | 0.17 (−0.15, 0.49) | 0.17 (−0.15, 0.48) | 1.75 (0.23, 3.26) | 1.32 (−0.17, 2.81) | −1.29 (−2.39, −0.19) | −1.16 (−2.26, −0.06) | ||
| Q3 (169.8–218.1) | −0.03 (−0.36, 0.29) | −0.00 (−0.33, 0.32) | 1.86 (0.32, 3.40) | 1.53 (0.00, 3.05) | −1.40 (−2.51, −0.28) | −1.28 (−2.41, −0.15) | ||
| Q4 (218.2–294.4) | 0.30 (−0.03, 0.63) | 0.33 (−0.01, 0.67) | 1.89 (0.32, 3.45) | 1.36 (−0.22, 2.95) | −1.21 (−2.35, −0.07) | −1.04 (−2.22, 0.13) | ||
| Q5 (≥294.5) | 0.25 (−0.09, 0.60) | 0.30 (−0.07, 0.68) | 3.11 (1.48, 4.73) | 2.56 (0.82, 4.31) | −1.36 (−2.53, −0.18) | −1.13 (−2.42, 0.16) | ||
| P-trend | 0.12 | 0.09 | <0.01 | 0.01 | 0.15 | 0.33 | ||
| Vitamin B-6 (mg/1000 kcal) | ||||||||
| Q1 (≤0.77) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | ||
| Q2 (0.78–0.95) | 0.06 (−0.26, 0.37) | 0.04 (−0.28, 0.36) | 2.01 (0.51, 3.52) | 1.62 (0.13, 3.10) | −0.71 (−1.80, 0.39) | −0.62 (−1.72, 0.48) | ||
| Q3 (0.96–1.23) | 0.15 (−0.18, 0.47) | 0.15 (−0.18, 0.48) | 1.97 (0.44, 3.51) | 1.52 (−0.00, 3.05) | −1.03 (−2.14, 0.09) | −0.92 (−2.05, 0.21) | ||
| Q4 (1.24–1.93) | 0.13 (−0.20, 0.45) | 0.13 (−0.21, 0.46) | 2.87 (1.33, 4.41) | 2.24 (0.68, 3.80) | −1.11 (−2.23, 0.01) | −0.94 (−2.09, 0.21) | ||
| Q5 (≥1.94) | 0.19 (−0.14, 0.52) | 0.20 (−0.15, 0.56) | 3.02 (1.46, 4.58) | 2.62 (0.97, 4.28) | −1.29 (−2.42, −0.15) | −1.17 (−2.39, 0.06) | ||
| P-trend | 0.33 | 0.31 | <0.01 | 0.02 | 0.09 | 0.16 | ||
| Vitamin B-12 (μg/1000 kcal) | ||||||||
| Q1 (≤1.86) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) | ||
| Q2 (1.87–2.54) | −0.07 (−0.39, 0.24) | −0.04 (−0.36, 0.27) | 0.60 (−0.91, 2.10) | 1.05 (−0.42, 2.52) | −0.27 (−1.36, 0.82) | −0.47 (−1.55, 0.62) | ||
| Q3 (2.55–3.53) | 0.08 (−0.23, 0.40) | 0.10 (−0.21, 0.42) | 0.24 (−1.27, 1.74) | 0.53 (−0.94, 2.01) | −0.45 (−1.54, 0.64) | −0.56 (−1.65, 0.53) | ||
| Q4 (3.54–5.74) | −0.15 (−0.47, 0.17) | −0.12 (−0.44, 0.20) | 0.22 (−1.29, 1.73) | 0.70 (−0.78, 2.19) | −0.17 (−1.27, 0.92) | −0.36 (−1.45, 0.74) | ||
| Q5 (≥5.75) | −0.12 (−0.44, 0.20) | −0.11 (−0.45, 0.22) | 1.80 (0.28, 3.32) | 2.08 (0.52, 3.65) | −0.92 (−2.02, 0.19) | −0.99 (−2.14, 0.17) | ||
| P-trend | 0.38 | 0.39 | 0.02 | 0.02 | 0.13 | 0.15 | ||
Model 1 was adjusted for age, sex, race (white or black), center (Alabama, Illinoi, Minnesota, or California), and educational attainment through year 25. Model 2 was adjusted for model 1 covariates plus for ever smoking (yes or no), alcohol intake, physical activity, BMI, Center for Epidemiologic Studies Depression Scale scores, systolic blood pressure, diabetes (yes or no), total energy intake, saturated fat intake, and cholesterol intake. For niacin and folate, models were further adjusted for vitamin C and polyunsaturated fat intake; for vitamin B-6, models were further adjusted for vitamin C intake; and for vitamin B-12, models were further adjusted for vitamin E intake and polyunsaturated fat intake. Because vitamin B-6 and vitamin B-12 values were not available in year 0, the average intake was calculated with year 7 and 20 values. CARDIA, Coronary Artery Risk Development in Young Adults; DSST, Digit Symbol Substitution Test; Q, quintile; RAVLT, Rey Auditory Verbal Learning Test; ref, reference.
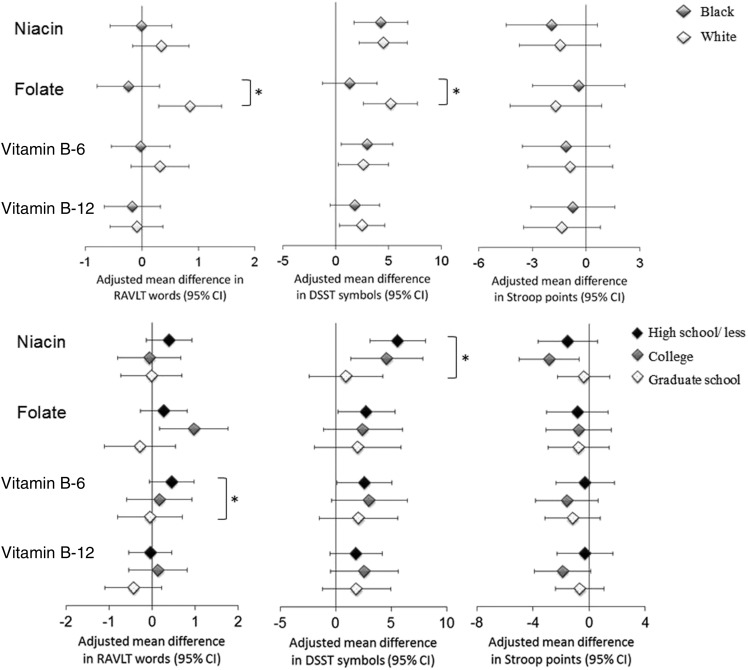
We stratified data according to a few prespecified factors, including race and educational level, to examine the potential modifications (Figure 1). We found that the significant associations between folate intake and RAVLT or DSST scores persisted among whites, but not blacks (P-interaction = 0.001 and 0.03, respectively). In addition, the positive associations between niacin intake and DSST scores and between vitamin B-6 and RAVLT scores were diluted in participants with higher levels of education (P-interaction = 0.03 and 0.01, respectively). The effect estimates between vitamin B-12 intake and Stroop interference scores were more pronounced in participants with a higher depression score (below the median, i.e., ≤8.4 CES-D scores—quintile 5 compared with quintile 1: −0.58; 95% CI: −1.99, 0.82; above the median: −1.30; 95% CI: −3.18, 0.57), with a borderline significant interaction (P-interaction = 0.07). Approximately 50% of participants had ever taken supplements containing B vitamins, but we did not find a significant interaction by supplement use. Moreover, excluding participants who had ever experienced a stroke or those who had missing waves of dietary assessment did not materially alter the results (data not shown). Using the absolute nutrient intake with adjustment for total energy intake had essentially no impact on results (Supplemental Table 4).
FIGURE 1.
Multivariable-adjusted mean differences (95% CIs) in cognitive test scores by race (upper panel) and education (lower panel) comparing quintile 5 with quintile 1 of cumulative daily total intake of B vitamins. Model adjusted for covariates listed in model 2 (see Table 2, footnote 1). *P-interaction < 0.05. DSST, Digit Symbol Substitution Test; RAVLT, Rey Auditory Verbal Learning Test.
DISCUSSION
The present study is among the first to evaluate the intake of B vitamins through young adulthood in relation to cognitive performance in middle age. We found that higher intakes of these B vitamins from both food and supplement sources were generally associated with better psychomotor speed. The observed associations were more pronounced in participants with a low educational level and in white individuals.
A number of studies have examined the relation between the intake of B vitamins and cognitive function or the risk of dementia in older adults, focusing on folate, vitamin B-6, and vitamin B-12, but the results were inconsistent. The null results from most of the RCTs are difficult to interpret because of the small sample sizes and short periods of follow-up (32). Among several relatively large RCTs that followed participants over several years, 2 studies reported beneficial effects of B-vitamin supplementation on cognitive function in participants with low dietary intake of B vitamins (13, 14), which generally support our findings.
B vitamins are involved in the metabolism of homocysteine and are therefore used either separately or jointly in homocysteine-lowering treatment. Elevated homocysteine concentrations have been hypothesized to increase the risk of dementia, supported by several lines of evidence (3, 10). Homocysteine concentration was found to be positively associated with amyloid neurotoxicity (33, 34), regional brain atrophy (35–39), cognitive impairment (40–42), or dementia (43). If the relations were causal, it was estimated that there would be an ∼20% risk reduction in dementia from homocysteine-lowering treatment involving B vitamins (43). Elevated midlife homocysteine was suggested as a risk factor for dementia (44), which was in line with evidence indicating that the pathologic process in AD may begin early in life (45). Our findings were concordant with the potential beneficial effects of B-vitamin intake on cognitive function, suggesting that B vitamins may be an early preventive measure.
Of the 4 B vitamins examined in the present study, we found that all were positively associated with psychomotor speed. It is possible, as observed in previous studies (46–48), that the cognitive domain of visual motor speed is more likely to be influenced by B-vitamin intake or homocysteine concentration. These B vitamins were found to be associated with multiple vascular risk factors (31, 49–51), whose relations with cognitive performance were most pronounced in psychomotor speed (52–54). However, the largely null findings on verbal memory performance do not necessarily indicate that such a benefit of B vitamins does not exist. The delayed word-recall task may be less challenging to perform than the DSST and Stroop tests. This may have diminished our ability to detect any possible association at a relatively young age.
We observed no relation between niacin or vitamin B-6 intake and cognitive performance among participants who attended graduate school, but the association was pronounced among participants at the high school educational level and below. It is possible that education or education-related variables such as cognitive activities may increase the ability to tolerate the accumulation of cognitive dysfunction induced by low intake of B vitamins (27). In our study, we also found that greater intake of folate was associated with better cognitive performance only among whites. This observation is in line with many previous studies, which found folate-race interactions in other health outcomes such as hypertension and cancer risk (28, 29). Presumably, blacks tend to have lower folate status response than whites (55), although the response mechanisms are not well understood.
A few limitations of the present study should be considered. First, cognitive function was assessed only once, which did not allow us to evaluate the association with changes in cognitive function. Second, it is challenging to estimate the independent association of individual B vitamins because of the high correlation between them. Although we adjusted for the other B vitamins under study in a sensitivity analysis and found that the results were materially unchanged, the concern still remains. Third, because there are multiple sources of B vitamins (e.g., vitamin-rich foods like legumes, meat, and fish; fortified foods, especially breakfast cereals; and dietary supplements), the association we observed may be different for each source of B vitamins, but we did not evaluate that in the current study. The biochemical markers of B-vitamin status were not examined, and the intake amounts of these B vitamins may not reflect the amount available to the body. For example, vitamin B-12 bioavailability decreases substantially in doses >1.5–2.0 μg/meal (56). Because we observed an increasing trend of intake over time for some B vitamins, especially folate, confounding cannot be ruled out if there was any unobserved factor that influenced the participation in dietary assessment and the performance of cognitive function. However, the consistent results among participants with all 3 waves of dietary assessments provide reassurance about the validity of our findings. Residual confounding may not be ruled out, as it applies to all observational studies. Although we adjusted for a wide array of dietary and nondietary confounders, confounding should not substantially skew our results. Furthermore, although we did not examine the potential influence of genetic risk factors in the current study, it would be interesting in future studies to examine the potential diet-gene interaction between vitamin B-12 and the presence of the apolipoprotein E ε4 allele on cognitive performance (30).
One major strength of this study is that participants who were aged 18–30 y at baseline were reassessed for their cognitive performance 25 y later, which enabled us to study B-vitamin intake in young adulthood in relation to cognitive function in midlife. Another strength is the use of a community-based cohort relatively balanced across race, sex, and educational level, which enabled more efficient adjustment in the analysis and identification of potential modifiers. The rigorous quality control in the CARDIA study gives us reassurance about the validity of our findings.
In conclusion, the present study suggests that greater intake of B vitamins throughout young adulthood may be associated with better cognitive performance in middle age, especially in the test for psychomotor speed. Considering the rapidly growing population at risk for dementia, increasing B-vitamin intake in early life may have important implications for cognitive performance later in life.
Acknowledgments
The authors’ responsibilities were as follows—KH: designed the study; SS: acquired the data; BQ and PX: analyzed the data; BQ: drafted the manuscript and took primary responsibility for the final content; and all authors: critically revised the manuscript and read and approved the final version of the manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: AD, Alzheimer disease; CARDIA, Coronary Artery Risk Development in Young Adults; CES-D, Center for Epidemiologic Studies Depression; DSST, Digit Symbol Substitution Test; RAVLT, Rey Auditory Verbal Learning Test; RCT, randomized controlled trial.
REFERENCES
- 1.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement 2007;3:186–91. [DOI] [PubMed] [Google Scholar]
- 2.McCaddon A. Homocysteine and cognition—a historical perspective. J Alzheimers Dis 2006;9:361–80. [DOI] [PubMed] [Google Scholar]
- 3.Smith AD, Refsum H. Homocysteine, B vitamins, and cognitive impairment. Annu Rev Nutr 2016;36:211–39. [DOI] [PubMed] [Google Scholar]
- 4.La Rue A, Koehler KM, Wayne SJ, Chiulli SJ, Haaland KY, Garry PJ. Nutritional status and cognitive functioning in a normally aging sample: a 6-y reassessment. Am J Clin Nutr 1997;65:20–9. [DOI] [PubMed] [Google Scholar]
- 5.Lee L, Kang SA, Lee HO, Lee BH, Park JS, Kim JH, Jung IK, Park YJ, Lee JE. Relationships between dietary intake and cognitive function level in Korean elderly people. Public Health 2001;115:133–8. [DOI] [PubMed] [Google Scholar]
- 6.Morris MC, Evans DA, Bienias JL, Scherr PA, Tangney CC, Hebert LE, Bennett DA, Wilson RS, Aggarwal N. Dietary niacin and the risk of incident Alzheimer’s disease and of cognitive decline. J Neurol Neurosurg Psychiatry 2004;75:1093–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health 2014;14:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dangour AD, Whitehouse PJ, Rafferty K, Mitchell SA, Smith L, Hawkesworth S, Vellas B. B-vitamins and fatty acids in the prevention and treatment of Alzheimer’s disease and dementia: a systematic review. J Alzheimers Dis 2010;22:205–24. [DOI] [PubMed] [Google Scholar]
- 9.Doets EL, van Wijngaarden JP, Szczecińska A, Dullemeijer C, Souverein OW, Dhonukshe-Rutten RA, Cavelaars AE, van ’t Veer P, Brzozowska A, de Groot LC. Vitamin B12 intake and status and cognitive function in elderly people. Epidemiol Rev 2013;35:2–21. [DOI] [PubMed] [Google Scholar]
- 10.McCaddon A, Miller JW. Assessing the association between homocysteine and cognition: reflections on Bradford Hill, meta-analyses, and causality. Nutr Rev 2015;73:723–35. [DOI] [PubMed] [Google Scholar]
- 11.Clarke R, Bennett D, Parish S, Lewington S, Skeaff M, Eussen SJ, Lewerin C, Stott DJ, Armitage J, Hankey GJ, et al. Effects of homocysteine lowering with B vitamins on cognitive aging: meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am J Clin Nutr 2014;100:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balk EM, Raman G, Tatsioni A, Chung M, Lau J, Rosenberg IH. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med 2007;167:21–30. [DOI] [PubMed] [Google Scholar]
- 13.Durga J, Van Boxtel MP, Schouten EG, Kok FJ, Jolles J, Katan MB, Verhoef P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet 2007;369:208–16. [DOI] [PubMed] [Google Scholar]
- 14.Kang JH, Cook N, Manson J, Buring JE, Albert CM, Grodstein F. A trial of B vitamins and cognitive function among women at high risk of cardiovascular disease. Am J Clin Nutr 2008;88:1602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jager CA, Oulhaj A, Jacoby R, Refsum H, Smith AD. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. Int J Geriatr Psychiatry 2012;27:592–600. [DOI] [PubMed] [Google Scholar]
- 16.Singh-Manoux A, Kivimaki M, Glymour MM, Elbaz A, Berr C, Ebmeier KP, Ferrie JE, Dugravot A. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ 2012;344:d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes GH, Cutter G, Donahue R, Friedman GD, Hulley S, Hunkeler E, Jacobs DR Jr., Liu K, Orden S, Pirie P, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (Cardia) Study. Control Clin Trials 1987;8(4 Suppl):68S–73S. [DOI] [PubMed] [Google Scholar]
- 18.McDonald A, Van Horn L, Slattery M, Hilner J, Bragg C, Caan B, Jacobs D Jr., Liu K, Hubert H, Gernhofer N, et al. The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc 1991;91:1104–12. [PubMed] [Google Scholar]
- 19.Liu K, Slattery M, Jacobs D Jr, Cutter G, McDonald A, Van Horn L, Hilner JE, Caan B, Bragg C, Dyer A, et al. A study of the reliability and comparative validity of the cardia dietary history. Ethn Dis 1994;4:15–27. [PubMed] [Google Scholar]
- 20.Wechsler D. WAIS-III: administration and scoring manual: Wechsler adult intelligence scale. San Antonio (TX): Psychological Corporation; 1997. [Google Scholar]
- 21.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol 1935;18:643–62. [Google Scholar]
- 22.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull 1991;109:163–203. [DOI] [PubMed] [Google Scholar]
- 23.Dyer AR, Cutter GR, Liu KQ, Armstrong MA, Friedman GD, Hughes GH, Dolce JJ, Raczynski J, Burke G, Manolio T. Alcohol intake and blood pressure in young adults: the CARDIA study. J Clin Epidemiol 1990;43:1–13. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs DR Jr., Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil 1989;9:448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol 1977;106:203–14. [DOI] [PubMed] [Google Scholar]
- 26.He K, Xun P, Liu K, Morris S, Reis J, Guallar E. Mercury exposure in young adulthood and incidence of diabetes later in life: the CARDIA Trace Element Study. Diabetes Care 2013;36:1584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, Barnes LL, Bienias JL. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 2003;60:1909–15. [DOI] [PubMed] [Google Scholar]
- 28.Xun P, Liu K, Loria CM, Bujnowski D, Shikany JM, Schreiner PJ, Sidney S, He K. Folate intake and incidence of hypertension among American young adults: a 20-y follow-up study. Am J Clin Nutr 2012;95:1023–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong Z, Ambrosone CB, McCann SE, Zirpoli G, Chandran U, Hong CC, Bovbjerg DH, Jandorf L, Ciupak G, Pawlish K, et al. Associations of dietary folate, Vitamins B6 and B12 and methionine intake with risk of breast cancer among African American and European American women. Int J Cancer 2014;134:1422–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogiatzoglou A, Smith AD, Nurk E, Drevon CA, Ueland PM, Vollset SE, Nygaard HA, Engedal K, Tell GS, Refsum H. Cognitive function in an elderly population: interaction between vitamin B12 status, depression, and apolipoprotein E ϵ4: the Hordaland Homocysteine Study. Psychosom Med 2013;75:20–9. [DOI] [PubMed] [Google Scholar]
- 31.Rimm EB, Willett WC, Hu FB, Sampson L, Colditz GA, Manson JE, Hennekens C, Stampfer MJ. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA 1998;279:359–64. [DOI] [PubMed] [Google Scholar]
- 32.Morris MC. Nutritional determinants of cognitive aging and dementia. Proc Nutr Soc 2012;71:1–13. [DOI] [PubMed] [Google Scholar]
- 33.Ho PI, Collins SC, Dhitavat S, Ortiz D, Ashline D, Rogers E, Shea TB. Homocysteine potentiates beta-amyloid neurotoxicity: role of oxidative stress. J Neurochem 2001;78:249–53. [DOI] [PubMed] [Google Scholar]
- 34.Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, Haughey N, Lee J, Evans M, Mattson MP. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci 2002;22:1752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sachdev PS, Valenzuela M, Wang XL, Looi JC, Brodaty H. Relationship between plasma homocysteine levels and brain atrophy in healthy elderly individuals. Neurology 2002;58:1539–41. [DOI] [PubMed] [Google Scholar]
- 36.den Heijer T, Vermeer SE, Clarke R, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MM. Homocysteine and brain atrophy on MRI of non‐demented elderly. Brain 2003;126:170–5. [DOI] [PubMed] [Google Scholar]
- 37.Seshadri S, Wolf PA, Beiser AS, Selhub J, Au R, Jacques PF, Yoshita M, Rosenberg IH, D’Agostino RB, DeCarli C. Association of plasma total homocysteine levels with subclinical brain injury: cerebral volumes, white matter hyperintensity, and silent brain infarcts at volumetric magnetic resonance imaging in the Framingham Offspring Study. Arch Neurol 2008;65:642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith AD, Smith SM, de Jager CA, Whitbread P, Johnston C, Agacinski G, Oulhaj A, Bradley KM, Jacoby R, Refsum H. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One 2010;5:e12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douaud G, Refsum H, de Jager CA, Jacoby R, Nichols TE, Smith SM, Smith AD. Preventing Alzheimer’s disease–related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci USA 2013;110:9523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quadri P, Fragiacomo C, Pezzati R, Zanda E, Forloni G, Tettamanti M, Lucca U. Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am J Clin Nutr 2004;80:114–22. [DOI] [PubMed] [Google Scholar]
- 41.Haan MN, Miller JW, Aiello AE, Whitmer RA, Jagust WJ, Mungas DM, Allen LH, Green R. Homocysteine, B vitamins, and the incidence of dementia and cognitive impairment: results from the Sacramento Area Latino Study on Aging. Am J Clin Nutr 2007;85:511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehmann M, Gottfries CG, Regland B. Identification of cognitive impairment in the elderly: homocysteine is an early marker. Dement Geriatr Cogn Disord 1999;10:12–20. [DOI] [PubMed] [Google Scholar]
- 43.Wald DS, Kasturiratne A, Simmonds M. Serum homocysteine and dementia: meta-analysis of eight cohort studies including 8669 participants. Alzheimers Dement 2011;7:412–7. [DOI] [PubMed] [Google Scholar]
- 44.Zylberstein DE, Lissner L, Björkelund C, Mehlig K, Thelle DS, Gustafson D, Ostling S, Waern M, Guo X, Skoog I. Midlife homocysteine and late-life dementia in women. A prospective population study. Neurobiol Aging 2011;32:380–6. [DOI] [PubMed] [Google Scholar]
- 45.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 2011;70:960–9. [DOI] [PubMed] [Google Scholar]
- 46.Prins ND, Den Heijer T, Hofman A, Koudstaal PJ, Jolles J, Clarke R, Breteler MM; Rotterdam Scan Study. Homocysteine and cognitive function in the elderly: the Rotterdam Scan Study. Neurology 2002;59:1375–80. [DOI] [PubMed] [Google Scholar]
- 47.Hooshmand B, Solomon A, Kåreholt I, Rusanen M, Hänninen T, Leiviskä J, Winblad B, Laatikainen T, Soininen H, Kivipelto M. Associations between serum homocysteine, holotranscobalamin, folate and cognition in the elderly: a longitudinal study. J Intern Med 2012;271:204–12. [DOI] [PubMed] [Google Scholar]
- 48.Schafer JH, Glass TA, Bolla KI, Mintz M, Jedlicka AE, Schwartz BS. Homocysteine and cognitive function in a population-based study of older adults. J Am Geriatr Soc 2005;53:381–8. [DOI] [PubMed] [Google Scholar]
- 49.Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol 2013;61:440–6. [DOI] [PubMed] [Google Scholar]
- 50.Hackam DG, Anand SS. Emerging risk factors for atherosclerotic vascular disease: a critical review of the evidence. JAMA 2003;290:932–40. [DOI] [PubMed] [Google Scholar]
- 51.Robinson K, Arheart K, Refsum H, Brattström L, Boers G, Ueland P, Rubba P, Palma-Reis R, Meleady R, Daly L, et al. Low circulating folate and vitamin B6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. European Comac Group. Circulation 1998;97:437–43. [DOI] [PubMed] [Google Scholar]
- 52.Reis JP, Loria CM, Launer LJ, Sidney S, Liu K, Jacobs DR Jr, Zhu N, Lloyd‐Jones DM, He K, Yaffe K. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol 2013;73:170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, McGovern P, Folsom AR; Atherosclerosis Risk in Communities (ARIC) Study Investigators. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001;56:42–8. [DOI] [PubMed] [Google Scholar]
- 54.Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 2004;292:2237–42. [DOI] [PubMed] [Google Scholar]
- 55.Perry CA, Renna SA, Khitun E, Ortiz M, Moriarty DJ, Caudill MA. Ethnicity and race influence the folate status response to controlled folate intakes in young women. J Nutr 2004;134:1786–92. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe F. Vitamin B12 sources and bioavailability. Exp Biol Med (Maywood) 2007;232:1266–74. [DOI] [PubMed] [Google Scholar]