Abstract
Current evidence suggests lutein and its isomers play important roles in ocular development in utero and throughout the life span, in vision performance in young and later adulthood, and in lowering risk for the development of common age-related eye diseases in older age. These xanthophyll (oxygen-containing) carotenoids are found in a wide variety of vegetables and fruits, and they are present in especially high concentrations in leafy green vegetables. Additionally, egg yolks and human milk appear to be bioavailable sources. The prevalence of lutein, zeaxanthin, and meso-zeaxanthin in supplements is increasing. Setting optimal and safe ranges of intake requires additional research, particularly in pregnant and lactating women. Accumulating evidence about variable interindividual response to dietary intake of these carotenoids, based on genetic or metabolic influences, suggests that there may be subgroups that benefit from higher levels of intake and/or alternate strategies to improve lutein and zeaxanthin status.
Keywords: macula, carotenoids, macular degeneration, cataract, vision, retinopathy
Introduction
Certain xanthophyll (oxygen-containing) carotenoids are highly concentrated in the light-exposed structures in plants and in the human retina. These carotenoids include lutein (L), its structural isomer zeaxanthin (Z), and meso-zeaxanthin (meso-Z), a lutein metabolite (19, 30, 31) and zeaxanthin stereoisomer (31, 129). L and Z are widely distributed in nature and are common in plants. Animals do not synthesize carotenoids. In primates, dietary L and its isomers are selectively concentrated in the visual system (eye and brain) over other carotenoids in the blood, comprising 80% to 90% of carotenoids in human eyes and the majority of carotenoids in the brain (48, 185). They are the exclusive carotenoids in the neural retina and lens (19).
This article provides an overview of evidence accumulated over the past three decades and a description of these compounds' functions and roles in ocular health and vision performance [for details, see previous reviews (18, 65, 89, 92, 97, 121, 123, 161, 183)]. The focus of the present review is on incorporating recent advances and current controversies. In particular, this review discusses newly available information that describes genetic, dietary, and physiological influences on the variable levels of L and Z accumulation between individuals. In addition, new evidence that hints at their role in ocular development and protection in the neonatal period is summarized, and studies that demonstrate specific improvements in vision performance with supplementation are discussed. Finally, the review describes the levels and sources of L and Z in human diets and highlights current gaps in knowledge, offering suggestions for future research needed to establish guidelines for intake.
Distribution and Roles of Lutein and Zeaxanthin in The Eye
Distribution
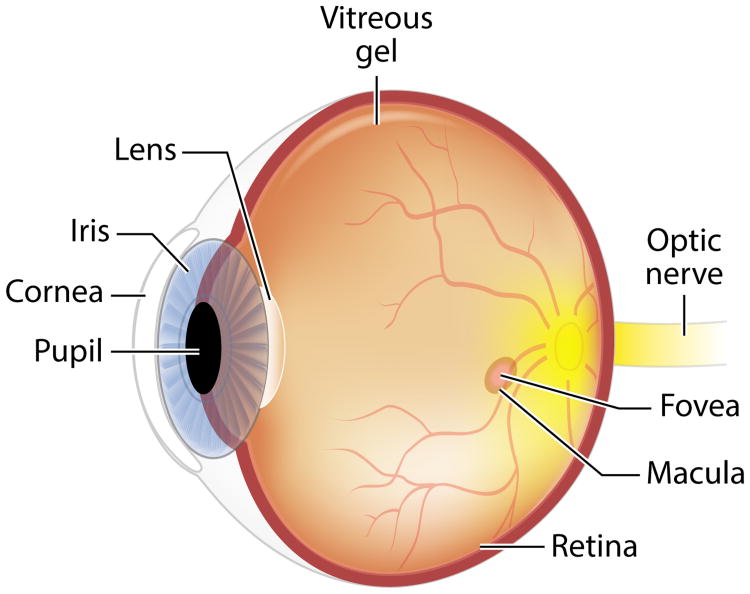
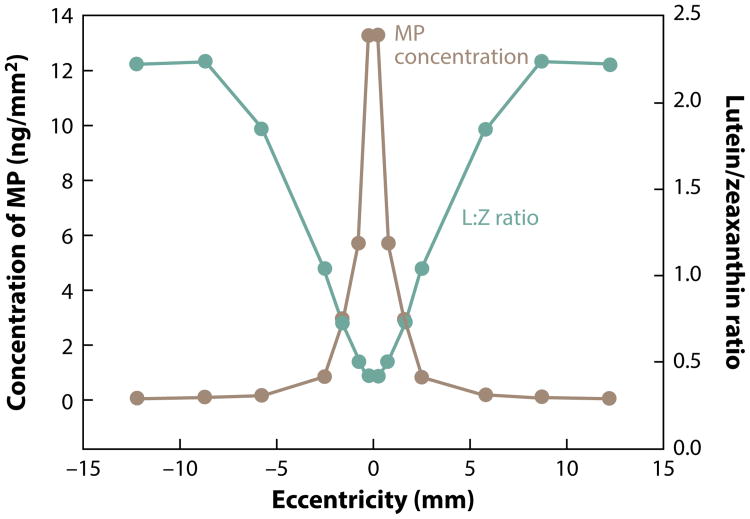
Lutein is the most abundant isomer in vision-related tissues (the eye and brain) (83). Nearly all human ocular structures (Figure 1) except the vitreous, cornea, and sclera contain L, Z, and metabolites (recently reviewed) (19). The highest concentration of L and Z in the eye is in the macula of the retina. Only humans (and nonhuman primates) have a macula with a central fovea, and concentrations of L and Z in the central fovea are 100-fold higher than elsewhere in the eye (Figure 2) (170). This cone-rich area confers the highest visual acuity and has the highest exposure to light in the retina. The selective distribution within the macula and throughout the retina and visual cortex of the brain suggest distinct properties and functions of the different isomers. In the macula of the eye, the ratio of Z to L declines and concentrations of Z (Figure 3) decrease with increasing distance away from the fovea. Concentrations of lutein metabolite meso-Z decrease with distance from the foveal center (31). The retina is a highly specialized tissue with ten discernible layers from the anterior to posterior poles (Figures 2 and 4); L and Z exist in varying amounts in specific layers, where they may play different predominant physiological functions.
Figure 1.
Image of the human eye.
Figure 2.
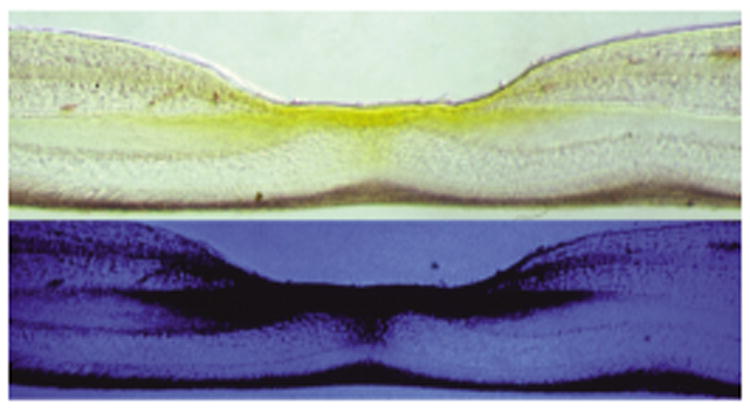
Cross-section of a primate retina, in the macula, photographed in either white or blue light, indicating macular pigment (composed of lutein, zeaxanthin, and meso-zeaxanthin) in retinal layers and its absorption of blue light from macular pigment. Figure adapted with permission from the American Journal of Clinical Nutrition and D. Max Snodderly (170).
Figure 3.
The concentration of macular pigment (MP) in human eyes and ratio of lutein (L) to zeaxanthin (Z) in relation to distance from the center of the fovea within the macula. Figure courtesy of John Landrum and Richard Bone, as published in Carotenoids and Retinal Disease, CRC Press (98). Adapted with permission.
Figure 4.
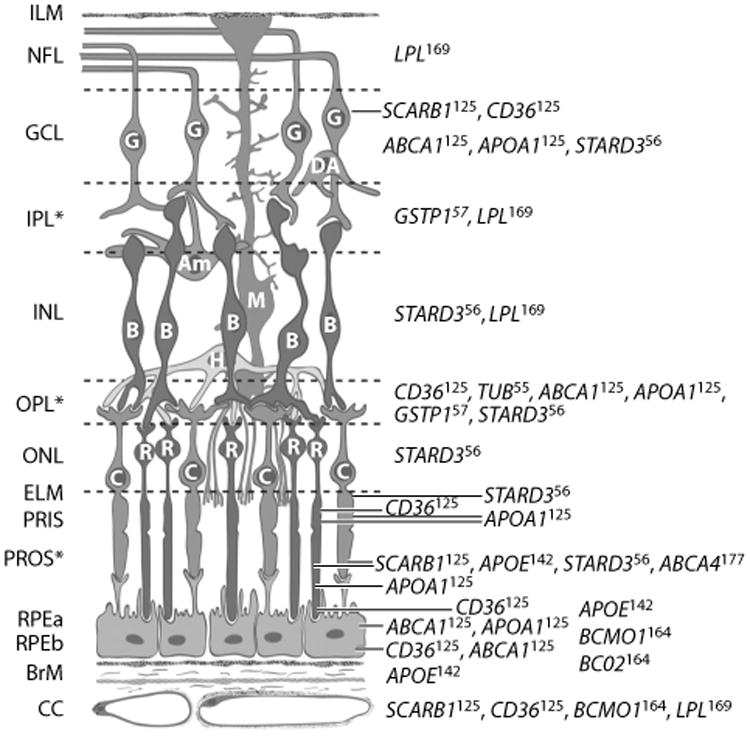
Distribution of proteins affecting or affected by macular xanthophylls in primate retina. Full names for genes are available from http://www.ncbi.nlm.nih.gov/gene. Superscripts on gene symbols refer to reference numbers of immunolocalization studies containing micrographs in Reference 161. Abbreviations: Am, amacrine cell; B, bipolar cell; BrM, Bruch's membrane; C, cone photoreceptors; CC, retinal choroid layer; DA, displaced amacrine cell; ELM, external limiting membrane; G, ganglion cell; GCL, ganglion cell layer; H, horizontal cell; ILM, inner limiting membrane; INL, inner nuclear layer; IPL, inner plexiform layer (interneurons); M, Muller cell; NFL, nerve fiber layer; ONL, outer nuclear layer; OPL, outer plexiform layer; PRIS, photoreceptor inner segment; PROS, photoreceptor outer segment; R, rod photoreceptor; RPEa, retinal pigment epithelium apical area; RPEb, retinal pigment epithelium basal area. ∗Retinal layers with macular xanthophyll concentrations. Schematic created by D. Fisher and adapted with permission from the American Journal of Clinical Nutrition (161) and John Paul SanGiovanni.
L and Z (193) and oxidized metabolites (19) are also the only carotenoids present in the lens of the eye (19). The most light-exposed and metabolically active lens tissue of the epithelial/cortical lens layers contains about 75% of the L/Z (193). The center (nuclear) region has lower levels. Approximately ten percent of the L and Z in the eye is contained in ciliary body, which is the metabolically active tissue responsible for aqueous humor formation; defects in aqueous humor flow contribute to the major form of glaucoma. Relationships of L and Z status to cataracts and glaucoma are discussed in the section titled Lutein and Zeaxanthin Status in Relation to Disease.
Biological Functions
Light absorption
Macular carotenoids are estimated to absorb 40% to 90% of incident blue light (depending on concentration) (95); this absorption protects the retina from light-related damage (10) and reduces light scatter. The highest density of macular carotenoids in the fovea is in the outer plexiform layer, a layer of neuronal synapses in the retina that is localized between rod and cone photoreceptors and their axons and other retinal neurons. This location is thought to be ideal to protect the outer retina (containing rod and cone photoreceptors) from photo-oxidative damage (171).
Protection against oxidative stress
L and Z, like all carotenoids, are potent antioxidants (for a review, see 91) and also reduce oxidative damage indirectly by light absorption (as described above). In the retinal pigment epithelium (RPE), lens, ciliary body, and iris, the presence of oxidized metabolites (19) suggests that L and Z protect against oxidative stress. Oxidative metabolism in the RPE is higher than anywhere else in the body; L and Z are two of many carotenoids contained in the RPE, and they are the predominant carotenoids in membranes (189). In the rod and cone photoreceptor outer segment membranes, they are most abundant in the lipid-rich bulk domain, which also contains the visual pigment rhodopsin, responsible for the first step of visual transduction (189). In this domain are also high concentrations of long-chain polyunsaturated lipids that are particularly vulnerable to oxidative damage (151, 189).
Protection against inflammation
Evidence indicates that L also protects against inflammation, a pathogenic mechanism in many ocular diseases, that can affect many regions of the eye. Possible mechanisms include preventing the increase in oxidation-induced cytokines and upregulating the expression of inflammation-related genes (24, 162). L may also indirectly influence ocular inflammation by reducing systemic inflammation via reducing factor D, a rate-limiting enzyme of the alternative complement activation pathway (reviewed in 178).
Other functions
Evidence suggests that carotenoids can play a role in cell-to-cell communication, through intercellular membrane structures known as gap junctions, which can play a role in homeostasis (108, 167). However, this role has not, to this author's knowledge, been specifically investigated in ocular tissues.
The presence of L and Z in membranes and their unique alignment decrease membrane fluidity, which could influence many membrane functions in photoreceptors and other parts of the neural retina and brain (discussed in 189). Outside the fovea, macular carotenoids are most dense in the inner plexiform layer (171), where lateral interneuronal processes transmit light to the nerve fiber layers (which also contain L and Z). Neurons from the fovea and parafovea transmit impulses from the axons of cone and rod photoreceptors to the brain, through the optic nerve. L is also the predominant carotenoid in the visual (occipital) cortex of human and nonhuman primates (48, 184, 185). Interestingly, levels in the visual cortex are highly correlated with levels in the retina (185). The presence of L and Z throughout the neural retina and brain supports the possibility that L might play a role in preserving long-chain polyunsaturated-rich neural tissue and ultimately enhance the transmission of visual impulses to the brain. Relationships between L and Z status and measurements of critical flicker frequency (thought to reflect visual processing speed) are supportive of this possibility (discussed in the section titled Visual Performance).
Measurement of Macular Pigment Levels
Several techniques exist to noninvasively assess levels of macular pigment (MP) (composed of L, Z, and meso-Z) by reliable psychophysical and optical means in adults (for a recent review, see 18). Efforts to adapt these methods for 7- to 10-year-old children, to date, have been demonstrated to be moderately reliable (125). Recently, new methods have been introduced to assess MP in infants (20, 26). Most of these measures use the unit of “optical density”; one optical density unit obtained from in vivo assessment of MP levels is equivalent to approximately 0.025 ng MP over a 1 mm2 area of retinal tissue (30), with a few percent variation, based on the nonrandom orientation of these molecules (R. Bone, personal communication).
Some evidence suggests that macular pigment optical density (MPOD) levels reflect carotenoid status throughout the eye and brain. Biochemically assessed levels of macular carotenoids are correlated with levels in more peripheral retinal areas (29) and in the brain (185). Autopsy specimens from donors who took L- and/or Z-containing carotenoid supplements had elevated xanthophyll carotenoid levels not only in the macula but also in the peripheral retina and lens (25). Thus, the sum of the current evidence suggests that levels in the macula are likely to be markers for levels in other areas of visual systems, enabling studies with broader ocular outcomes.
Influences on Accumulation in Retinal Tissues
Levels of L and Z in the serum and macula vary between individuals more than tenfold, whether assessed in autopsy tissues by high-performance liquid chromatography (19, 30, 31, 33) or by noninvasive assessment of MPOD. A substantial amount of evidence, summarized below, suggests numerous dietary, metabolic, and genetic influences on L and Z absorption, transport in the blood, and accumulation in the eye. Consistent with this idea, responses to dietary supplementation with L and Z are quite variable between individuals. Dietary supplementation with macular carotenoids for 6 to 24 months has been observed to increase MPOD in 36% (138) to 95% (135) of subjects, with many studies suggesting estimates between these two extremes (3, 4, 15, 28, 32, 52, 68, 71, 84, 88, 94, 116, 138, 156, 164, 179). The magnitude of individual response within studies is also quite variable, although the definition of macular “response” varies across studies.
The large interindividual variability in response to supplementation suggests there are many exogenous and endogenous influences on the uptake, transport, and retinal capture of L and Z. Several recent reviews detail the many dietary and host phenotypes and genotypes that influence the absorption of L and Z, their transport in the blood (35, 104, 166), and their uptake and stabilization in the retina (161). A summary is provided below, together with more recent evidence. Figure 5 provides an overview of phenotypes and genotypes associated with variation in MPOD levels in the Carotenoids in Age-Related Eye Disease Study (CAREDS) sample, the largest study sample in which a broad array of potential phenotype and genotype correlates of MPOD have been evaluated.
Figure 5.
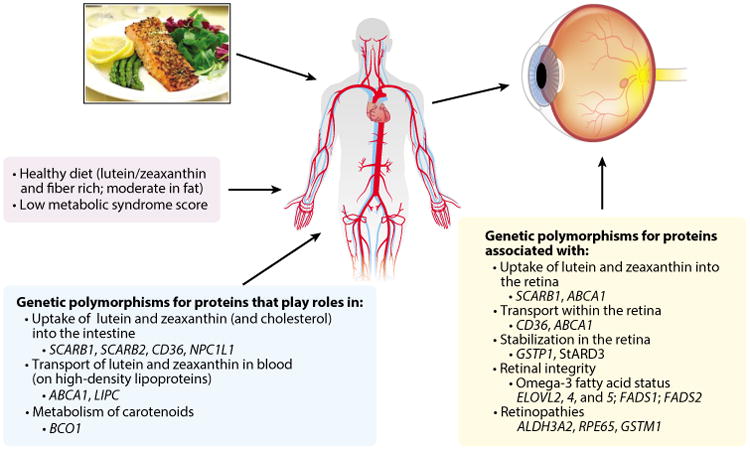
Phenotypes and genotypes associated with macular pigment optical density in the Carotenoids in Age-Related Eye Disease Study (120, 126, 128, 129). The evidence suggests that the indicated aspects of diet, metabolism, and genotype might influence the absorption and transport of lutein and zeaxanthin in the blood (left and center boxes) and that the indicated genotypes and activities of the proteins these genes encode (right box) might influence their uptake and/or stabilization in the retina.
Absorption
Some of the variability in MPOD levels appears to be the result of variable absorption. A large body of evidence indicates that the serum response to ingested L and/or Z is highly variable (similar to that of other carotenoids) (reviewed in 35, 60, 166). This variation may stem from differences in food preparation methods, bioavailability of L and/or Z in foods, genetic variations that influence the expression of proteins that regulate absorption, and competition with other nutrients. Grinding or cooking foods (which may release carotenoids from the food matrix) and consuming L or Z with a fat source increases blood response (38, 197). The context of food matters. For example, L and Z are more bioavailable in eggs than in spinach (45). Recent evidence suggests that this higher bioavailability may be the result of carotenoid presence within the lipid matrix of the egg and/or increased transfer to blood high-density lipoproteins (HDLs) (87, 132, 181, 182). In contrast to other carotenoids, at least half of these more polar carotenoids are carried on HDLs.
The bioavailability may also depend on polymorphisms in genes for proteins that influence cholesterol uptake into the intestinal lumen (73). Results of human cell studies and L feeding studies in humans suggest that L is transported (129) into the intestinal mucosal cells on cholesterol transporter proteins (for a recent review, see 35). In the intestinal lumen, these and other proteins on the apical side of the enterocyte can modulate the uptake of xanthophylls from micelles (35, 36). Common variants in genes (ABCG5, ABCG8, NPC1L1, SCARB1, and CD36) encoding these proteins were related to levels of serum L plus Z in the CAREDS sample (130).
Absorption of L and Z also appears to be related to intake and/or status of beta-carotene and the activity of the enzyme beta-carotene oxygenase 1 (BCO1), which catalyzes the cleavage of beta-carotene to yield retinoids. Within the intestinal cell, BCO1 and retinoid cleavage products were demonstrated to be part of a regulatory network that controls the absorption of L and Z, other carotenoids, and fat-soluble vitamins (188). A series of experiments in mouse knockout models (188) provided evidence of a negative feedback regulation of xanthophyll absorption catalyzed by BCO1 and indicated that both retinoids and homeobox transcription factor ISX suppress the expression of BCO1 in the presence of beta-carotene. Further evidence indicated that ISX signaling also targets the apical membrane protein SR-B1 receptor, reducing xanthophyll absorption. These results are consistent with the observation that ISX polymorphisms are among the genotypes selected in a model that best explains L absorption into chylomicrons in humans (36). These findings provide a mechanistic explanation for other recent evidence indicating that polymorphisms for the BCO1 gene are a predictor of L absorption (36) and of levels of L in MP (126) and serum (130, 194).
Jointly, these data would predict that beta-carotene availability in the diet, together with active cleavage by BCO1, reduces the absorption of L and Z. Consistent with this prediction, BCO1 polymorphisms associated with higher BCO1 activity are associated with higher levels of beta-carotene and lower levels of L and Z in serum (54). Interestingly, this might also explain a result of the recent second Age-Related Eye Disease Study (AREDS2) (1, 41). In this randomized trial, adding L and Z to a high-dose antioxidant that contained beta-carotene did not lower age-related macular degeneration (AMD) progression to late AMD, except among individuals who received a formulation lacking beta-carotene. In addition, after five years, serum levels of L and Z were higher in participants taking supplements containing L and Z without, compared to supplements with, beta-carotene, suggesting that absorption of L and Z was higher when beta-carotene was omitted.
Transport and tissue distribution
Carotenoid efflux from the enterocytes occurs via their secretion in chylomicrons and transport to the liver, where they are repackaged into lipoproteins and distributed throughout the body. Evidence implicates the involvement of several transport proteins that are related to chylomicron assembly and lipoprotein clearance in the efflux of L from the intestine and transport in the blood (for recent reviews, see 36, 104). Common variants in the ABCA1, ApoB, and LPL genes, which express different carrier proteins, were related to postprandial L response in chylomicrons (36). Variants in ABCA1 were also related to levels of serum L and Z in CAREDS (130) and to the response to L and Z supplements in a separate study (194).
Adipose tissue is a storage tissue for carotenoids. Current evidence suggests that the metabolic status of individuals might influence the distribution of carotenoids between adipose tissue and the eye. A large body of evidence indicates that obesity and diabetes are associated with lower levels of serum carotenoids and lower MPOD. Moreover, we recently observed a strong linear and inverse relationship between MPOD and metabolic syndrome risk scores composed of these and other phenotypes (e.g., serum triglycerides >3 mmol/L, use of cholesterol-lowering medications, history of hypertension) and of genotypes related to low HDL levels. Women with scores ≥5 had more than two-fold higher MPOD than women with scores of 0, even after limiting the sample to women with above-average L and Z intake (128).
At least three possible explanations exist for lower levels of L and Z in serum and MPOD in individuals with metabolic syndrome phenotypes. First, a large body of evidence indicates that metabolic syndrome phenotypes are associated with higher oxidative stress and inflammation, which could increase the turnover of carotenoids. Second, larger body fat compartments may shift the distribution of carotenoids away from the blood and retina and into adipose tissue. Third, interesting recent evidence suggests that carotenoid status and/or carotenoid cleavage enzymes directly influence adiposity (5, 74, 112).
Uptake, transport, and stability in the retina
An individual's MPOD level and spatial distribution have been observed to be relatively stable over at least a decade in one study (70) and are also stable despite short-term fluctuations in intake in other studies (96, 136). This is expected because an individual's retinal microstructure and lipid environment, and the activity of proteins that facilitate uptake into the retina and transport or stabilization within the retina, are all suspected of influencing the levels and spatial distribution of MP. Diminished levels or abnormal distributions of MP have been associated with the presence of a variety of retinal diseases, such as macular telangiectasia, albinism, and Sjörgen-Larsson syndrome (for a recent review, see 18). The relative stability of MP levels, selective accumulation of xanthophyll carotenoids in the retina, and specific localization of L and Z isomers within the primate retina all suggest the presence of transport and binding proteins with affinity for these carotenoids, which populate and stabilize them in specific retinal layers. Figure 4 describes the large number of retinal proteins that previous evidence suggests may be related to the distribution of L and Z isomers in the primate retina (161). Common genetic variants in genes for most of these proteins (except APOA1, LPL, and TUB polymorphisms, which were not studied) were observed to be related to levels of MPOD in CAREDS (Figure 5) (126). An exception is the gene for a carotenoid cleavage enzyme, beta-carotene 9,10-dioxygenase (BCO2).
The recent results of Li and colleagues' studies (102), but not those of Babino et al. (7), suggested that inactivity of BCO2 plays a role in the selective accumulation of lutein and zeaxanthin in the primate retina. This evidence, and other evidence from Babino et al. (for a recent review, see 143) suggests, instead, that BCO2 activity is localized to the inner mitochondrial membrane, where it plays a role in protecting against oxidative stress. Data from CAREDS support this second interpretation. Polymorphisms in BCO2 were unrelated to MPOD (126) but related to higher odds of AMD, which is known to be promoted by oxidative stress (130).
It has been argued that the selective localization of L, Z, and meso-Z in the retina might also be the result of their uniquely polar nature and shape (relative to other carotenoids), influencing their alignment into specific membrane domains that are rich in long-chain polyunsaturated fatty acids (189). Consistent with this idea, polymorphisms in genes for proteins involved in the metabolism and synthesis of long-chain fatty acids have been related to MPOD levels (Figure 5) (126). The unique chemical properties of macular carotenoids could influence their affinity for and interaction with membrane proteins. Genotypes for many proteins related to MPOD levels (126) are for proteins that traffic cholesterol and other lipids within the retina (and possibly elsewhere in the eye).
Accumulation of Lutein and Isomers Over the Neonatal Period
Considerably less is known about the levels of L in the eye in neonatal development and infancy. Two recent reviews have suggested that exposure to L and Z in fetal life and infancy might be important for early-life visual development and may have a lifelong influence on vision (64, 83). Also, a role for L and Z in ocular development in utero is suggested by the fact that L and Z appear transiently in the fetal vitreous in the second and third trimesters (79), which is consistent with the appearance of L and oxidized metabolites in arterial cord blood at levels that peak in the beginning of the third trimester and then decline later in gestation (146). L and Z have been found in human retinas as early as 20 weeks of gestation (30). At birth, all parts of the eye have been formed except the macula; differentiation of this region continues until age four (148), but the timing for the majority of L and Z accumulation is related to the maturation of the fovea of the retina, which permits the ability to see fine detail (20). During this time, there is also maturation of the RPE and its relation to photoreceptors (196).
L and Z levels in the macula appear to increase with age, particularly in the first year of life (30). Also, the proportion of L and Z isomers change over the first few years of life; the evidence for this in human and nonhuman primates has been described (97). Briefly, L is the dominant carotenoid through about age two (30). After that age the ratio changes, and Z becomes the dominant carotenoid in the fovea, as it is in adults. Meso-Z concentrations in macaques are below the limits of detection at birth and presumably are also absent in human retinas; concentrations increase in proportion to age in young humans (31) and macaques (97).
Significant direct relationships between L and Z concentrations in the macula and age from birth to seven years have been observed (20, 30). Premature human infants and monkeys born to mothers raised on L- and Z-free diets do not have MP (20, 134). Accumulation of macular L and Z is likely to depend on maternal L and Z status, and this hypothesis has been supported by evidence from a small sample that indicated direct relationships between maternal serum Z and infant MPOD levels (72). More studies are needed to confirm this finding. Recent evidence indicates that MPOD is higher in older women with early-life exposure to L and Z in breast milk, which contains higher concentrations than formulas made from cow's milk (111). (Cow's milk was the predominant source of formula for infants not breast fed in the 1920s to 1940s.) These early data suggest that levels later in life may be influenced by L and Z exposure in the first years.
Breast milk appears to selectively concentrate these carotenoids, particularly in early lactation. L and Z are proportionally more prevalent in breast milk than in maternal serum (105, 107). The molar ratio of carotenoids in human milk to maternal plasma was observed to be highest for L relative to other carotenoids (107). Currently, carotenoids are not routinely added to infant formulas, and they were not added to any formulas prior to about 2012.
L and Z were the most abundant carotenoids in samples of human milk in one multinational study (107), but not in five of nine countries in other multinational studies to date (39). Breast-milk carotenoids are related to maternal serum carotenoids (107), which are influenced not only by diet but also likely by conditions known to influence serum carotenoid levels, such as obesity, smoking, and alcohol use and markers of inflammation (63). L in breast milk appears to be approximately fourfold more bioavailable than L in infant formulas to date (22, 106). Given the presence and accumulation of L and Z in the eye in gestation and infancy, and the potential for influence on lifelong vision, additional research is critically needed to establish requirements for L and Z intake in infancy. Such requirements would help to determine guidelines for the intake of L and Z by pregnant and nursing mothers and infants who are not breast fed.
Roles in Visual Health
Visual Performance
For more than a century, it has been speculated that MP plays a role in enhancing visual performance; in the past two decades, observational studies have, indeed, suggested that MP influences performance on visual tasks (for a recent review and critical discussion, see 65, 173). New observational, experimental, and clinical evidence (summarized below and in Table 1) suggests that L, Z, and/or meso-Z could improve performance on tasks involving several different aspects of the visual system, from the eye to the brain, in both young, healthy people and people with ocular diseases.
Table 1. Summary of studies (2004–2015; including more than 50 subjects) investigating the relationships among macular pigment optical density, supplementation with lutein, zeaxanthin, and/or meso-zeaxanthin, and visual performance.
| Cross-sectional observational studies | ||||||
|---|---|---|---|---|---|---|
| Study author(s) and year (reference) | Sample | Measure of lutein/zeaxanthin status | Vision outcome | Result | ||
| Beirne 2013 (14) | 73 subjects (ages 20–71) | MPOD | Foveal acuity | MPOD was not significantly related to foveal acuity | ||
| Hammond et al. 2013 (66) | 150 healthy young subjects (mean age 22) | MPOD | GD, photostress recovery, chromatic contrast | Higher MPOD was significantly correlated with improved GD, photostress recovery, and chromatic contrast | ||
| Renzi & Hammond 2010 (152) | 50 healthy elderly subjects (mean age 72) 28 healthy young subjects (mean age 23) |
MPOD | Luminance contrast | Higher MPOD levels were significantly correlated with a greater luminance contrast | ||
| Renzi & Hammond 2010 (153) | 70 subjects, full-temporal function outcome (ages 15–84) 354 subjects for CFF outcome (ages 16–92) |
MPOD | CFF, TCSF | MPOD was positively correlated with TCSF in the center but not the parafovea. It also positively correlated with CFF | ||
| Randomized trials | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study author(s) and year (reference) | Sample | Intervention | Intervention influence on L/Z status | Duration | Vision outcome | Outcomes associated withsupplementation | ||
| Chous et al. 2016 (43) | 67 type 1 and type 2 diabetes patients (mean age 56) |
|
Increased mean MPOD | 6 months | CS, color discrimination | Better visual performance was observed for all measures in the supplemented group | ||
| Huang et al. 2015 (78) | 112 early-AMD patients (ages 61–78) |
|
Increased mean MPOD and serum L/Z | 2 years | VA, CS, photostress | CS significantly improved with supplementation; intervention 2 had the most improvement. No significant changes in photostress recovery or VA were observed | ||
| Akuffo et al. 2015 (2) | 47 early-AMD patients (mean age 66) |
|
Increased mean MPOD except in intervention 1 at the location of 1.75° from the foveal center | 3 years | VA, CSAMD progression | CS significantly improved at certain spatial frequencies in all interventions. No improvements in VA or AMD progression were observed | ||
| Hammond et al. 2014 (67) | 115 healthy college students (ages 18–40) |
|
Increased mean MPOD and serum L/Z | 1 year | Photostress recovery, chromatic contrast, GD | Photostress recovery and chromatic contrast were improved but GD was not | ||
| Bovier et al. 2014 (37) | 92 healdiy young subjects (ages 18–32) |
|
Increased MPOD | 4 months | CFF thresholds, visual motor reaction time, visual processing speed | Increased CFF thresholds, visual motor reaction times, and visual processing speeds were observed for groups that received any supplement | ||
| Sabour-Pickett et al. 2014 (159) | 52 early-AMD patients (mean age 66) |
|
Increased mean MPOD at all locations in interventions 2 and 3 but only at 1.75° in group 1 | 1 year | CS | CS improved at all spatial frequencies in intervention 3 and in low spatial frequencies in interventions 1 and 2 | ||
| Age-Related Eye Disease Study Investig. 2013 (1) | 4,203 participants (ages 50-85) |
|
ND | 5 years | VA more than 15 letters | No improvements were observed for VA of 15 letters or more | ||
| Yao et al. 2013 (192) | 120 drivers (ages 25–47) |
|
Increased mean MPOD and increased serum L | 1 year | VA, CS, GD | No significant improvements in VA were observed, but CS and GD in supplemented group increased significantly. Improved scores on the NEI-VFQ were also observed for the supplemented group | ||
| Ma et al. 2012 (116) | 108 AMD patients (ages 50-79) |
|
Increased mean MPOD | 48 weeks | BCVA, CS | Improvements in MPOD were positively correlated with improvements in VA and CS at 3, 6, and 12 cycles | ||
| Weigert et al. 2011 (187) | 126 patients (ages 50–90) |
|
Increased mean MPOD | 6 months | VA | Despite no significant improvement in VA, MPOD increases were related to improvements in VA | ||
| Piermarocchi et al. 2012 (147) | 145 patients (ages 55–80) |
|
ND | 2 years | VA, CS, NEI-VFQ | VA was stabilized, and improvements in CS and NEI-VFQ score were observed | ||
| Nolan etal. 2011 (135) | 121 healthy subjects (ages 18–41) |
|
Increased mean MPOD and increased serum L/Z | 1 year | VA, CS, GD, photostress | No significant improvement of visual performance despite increased MPOD levels | ||
| Dawczynski et al. 2013 (49) | 172 patients with early, intermediate, and advanced AMD (ages 50–95) |
|
Increased mean MPOD | 1 year | VA | VA was improved (mean 2 letters for dose 1 and 1.4 letters for dose 2) | ||
| Beatty et al. 2013 (12) | 433 patients with early AMD in both eyes or in one eye accompanied by advanced AMD in the fellow eye (ages 50–85) |
|
Increased serum L/Z | 3 years | VA, CS, AMD progression | VA improved in the supplemented group and progression along AMD severity scale was lower. No significant improvements in CS were observed | ||
| Richer et al. 2011 (156) | 60 patients with atrophic AMD (mean age 75) |
|
Increased mean MPOD at 1° | 1 year | VA, GD, CS | High-contrast VA improved in intervention 1. Low-contrast VA, CS, and GD improved in intervention 3 | ||
| Hu et al. 2011 (77) | 67 patients with nonproliferative diabetic retinopathy (ages 42–76) |
|
Increased serum L/Z | 3 months | VA, CS, macular edema (foveal thickness) | VA and CS improved, and macular edema was reduced in supplemented group | ||
Abbreviations: AMD, age-related macular degeneration; BCVA, best-corrected visual acuity; CFF, critical flicker frequency; CS, contrast sensitivity; GD, glare disability; L, lutein; MPOD, macular pigment optical density; MZ, meso-zeaxanthin; ND, MPOD or serum L/Z were not determined; NEI-VFQ, National Eye Institute Vision Function Questionnaire; TCSF, temporal contrast sensitivity function; VA, visual acuity; Z, zeaxanthin.
Visual acuity
Visual acuity reflects the ability to resolve objects that are in high contrast to their background, as measured by the ability to distinguish smaller and smaller letters at a given distance. Results of some but not all intervention studies (1, 11, 115, 133, 135, 187, 192) indicate improvements in visual acuity when L and/or Z are supplemented alone (77, 113, 139) or, more often, in conjunction with other antioxidants and/or omega-3 fatty acids (1, 12, 43, 49, 147, 155). Three of seven randomized trials in >50 individuals with early or advanced AMD (116, 156) or with diabetic retinopathy (77) indicate improvements in visual acuity. A recent meta-analysis of L and/or Z supplementation (six months to three years) in individuals with AMD indicates a significant protective effect (109) and a direct relationship between improvement in visual acuity and the change in MPOD level achieved. To date, the magnitude of effect in these short trials is modest.
In AREDS2 (1), adding L and Z to high-dose antioxidants did not significantly lower the loss of visual acuity by 15 letters or more over five years in people who already had intermediate or advanced AMD. Observing an impact of this magnitude might require longer periods of time and be limited to people who have a higher MPOD response and/or who receive a higher dose of supplements. In a study of middle-aged individuals, supplementation for six months with 20 mg L and/or Z resulted in significant improvement in visual acuity (113). This level (20 mg) is approximately five times the levels that are expected to be achieved if an individual follows dietary guidelines for fruit and vegetable intakes. Better L and Z status over a long period might also lead to better visual acuity in middle age and old age as a result of a lower risk for the common causes of visual impairment (see Lutein and Zeaxanthin Status in Relation to Disease section). Results of ongoing prospective studies are expected to provide an estimate of the magnitude of improvement of visual acuity that results from these combined effects.
Regardless of whether MP improves the most common measure of acuity in indoor tests, it could help us see farther. It is estimated that a person with 1.0 density unit of MP can see 26% farther through the atmosphere than someone with little or no MP (190). This improvement in vision, which might have conferred an advantage for previous humans in hunting, gathering, and remaining safe from predators, would, in today's world, be of greatest benefit to people for whom long-range acuity is important, such as pilots and sailors.
Contrast sensitivity
Impairments in contrast sensitivity occur before visual acuity is affected by aging or disease (for a recent review, see 140). Contrast sensitivity is the ability to detect contrasts in levels of lightness or darkness of an object, or of colors, relative to the objects background. Detecting contrasts can be especially difficult in dim lighting, as occurs at dawn and twilight. An example would be the ability to distinguish the edges of stairs; in this case, better contrast sensitivity could reduce the likelihood of falls and improve confidence in walking down steps. Contrast sensitivity is well correlated with various aspects of visual ability, such as orientation and mobility, reading speed, and driving (157), and with the satisfaction of individuals with their visual function and how their visual ability affects their quality of life (53). Supplements containing L and Z at various amounts and taken for three months to three years have improved contrast sensitivity in most previous studies, including in young, healthy subjects (37, 67, 94, 113, 115), in people with early (78, 116, 156) and/or advanced (12) AMD, and in individuals with diabetes (43, 77). Four of five randomized, placebo-controlled trials of solely L and/or Z isomers in >50 subjects observed improvements in spatial contrast sensitivity (77, 78, 116, 192); results of one other trial did not (135). The long-term influences of L and Z status on contrast sensitivity in later life would include not only these short-term effects but also protection against age- and disease-related changes. Long-term relationships between L and Z status and contrast sensitivity have not yet been described.
Photostress recovery and glare reduction
A considerable body of observational evidence suggests that higher levels of MP reduce the impact of bright lights by shortening the time needed to recover from bright lights (photostress recovery) and by enhancing the ability to see in conditions of glare (lower glare disability) (66, 174, 175), as might occur from oncoming headlights while driving at night. High MP levels may also enhance visual performance in moderate and bright lights by increasing the range over which vision-related tasks can be comfortably performed (173). For example, it has been estimated that increasing MPOD at a 1° diameter around the center of the fovea by 0.3 density units would roughly double the intensity of white light necessary to produce visual discomfort. One (116) in four randomized trials in more than 50 people indicated a benefit of L and/or Z supplements on glare disability; one (78) of three trials found beneficial effects on photostress recovery (see Table 1).
Visual processing speed
MP density levels appear to be a marker for L and Z status throughout the visual system. Better health of neurons in the eye and brain might speed transmission of visual impulses from the photoreceptors to the visual cortex in the brain and/or increase lateral neural connections on this path, improving the speed of resolving changes in visual stimuli (67, 69, 153, 201). Improved speed could be important in performing vision-related tasks safely as we age; however, improvements in visual processing speed might also be relevant at other ages. Improved critical flicker-frequency thresholds, visual motor reaction time, and visual processing speed have been observed in adults 18 to 32 years of age who received Z supplements, alone or with L and omega-3 fatty acids, for only four months (37). Increases in about 0.09 MPOD units through supplementation were estimated to significantly improve visual processing speed in these young, healthy adults. These findings, if replicated, suggest that high MP levels might improve vision function for people of all ages in tasks requiring quick responses, such as tasks in computer-related work.
Dark adaptation
It has been hypothesized (144) that MP could protect against impairments in rod-mediated dark adaptation, such as is required to regain visual sensitivity when moving from bright to dim lights. Impairments were recently observed in more than 20% of people ≥60 years of age without clinical signs of retinal disease (142). Impairment in dark adaptation rate is thought to be related to the accumulation of hydrophobic lipids in the RPE, which creates a diffusion barrier that slows the delivery of nutrients, such as vitamin A, to rods. In visual transduction, the delivery of vitamin A metabolite 11-cis-retinal is needed to combine with the protein opsin to form the visual pigment rhodopsin. Impaired delivery would slow the rate of rhodopsin regeneration and require longer time for dark adaptation. Longer time for dark adaptation in persons with low MPOD has been observed in two small observational studies (144, 173).
Future directions and challenges
The current body of observational evidence suggests that higher levels of MP, a marker for L and Z throughout the visual system, are associated with optical and physiological effects that improve several aspects of vision. In some cases, the degree to which tissue concentrations of MP are specifically responsible for these effects is not clear. Although MP is entirely composed of dietary xanthophylls and metabolites, higher MPOD levels are related to having healthy diets and phenotypes in general (122), which might also contribute to retinal integrity. Consistent with this idea, higher scores on the Healthy Eating Index–2005 were associated with higher MPOD, even after adjusting for L and Z intake (124).
Results of several short-term supplementation studies with one or more xanthophyll carotenoids suggest specific benefits of L and Z; greater increases in MPOD levels have been related to greater improvements in visual acuity and contrast sensitivity (see Table 1). The interpretation of these currently available data is complicated by the small sample sizes, especially given the variability among people in the ability to enhance MP with increasing L and Z intake over short periods of time. Additional data from well-powered intervention studies are needed to determine the levels and formulations that are required for optimal benefit in the short term and whether these are modified by phenotypes and genotypes that influence their accumulation in the eye and brain.
Long-term influences of better L and Z status, which include both direct optical effects and a reduction in risk for aging and age-related diseases, can be better captured in long-term prospective observational studies. Several are under way; eventual pooling of data from available cohorts will further enable well-powered investigations of the degree to which phenotypes and genotypes modify L and Z levels needed in diet or supplements for optimal benefit over a wide range of intake levels.
Lutein and Zeaxanthin Status in Relation to Disease
Age-related macular degeneration
Strong evidence indicates that L and Z protect against the development of AMD, the leading cause of blindness in persons over 40 years of age in the United States (47, 55). AMD is a progressive condition of vision loss that develops as a result of a complex interplay of multiple dietary, environmental, and genetic factors that influence oxidative stress, inflammation, and light damage (76). In early and intermediate stages, rod function declines, which reduces the ability to see contrasts in dim light and adapt to moving from well-lit to poorly lit areas (for a recent discussion, see 141). Advanced AMD is associated with the loss of sharp acuity or vision in the center of the visual field (such as that needed to view a person's face straight on). It is also associated with loss of the ability to perceive fine detail, making it difficult for individuals with advanced AMD to read newspapers and pill bottles. For individuals with neovascular/exudative (“wet”) AMD, new blood vessels grow and bleed and fluid accumulates, which can cause further limits in vision. At this time, treatment options for AMD are limited, not without risk (168), and costly (169).
The strong biological plausibility for a protective influence of L and Z on AMD development and progression has been recently reviewed (18, 183). In the fovea, MP is particularly concentrated in the photoreceptor axon layer (27, 171, 172), where it can absorb 40% to 90% of short-wavelength light (95) that could otherwise damage the retina (10, 24). Throughout the retina, L and Z might also protect against AMD by their demonstrated ability to lower oxidative stress (91, 103, 162) and inflammation (25, 80, 162). In addition to retinal effects, supplementation with L has been demonstrated to lower circulating levels of a rate-limiting enzyme of the alternative complement activation pathway that may play an important role in inflammatory response and the development of AMD (16, 178).
A large body of epidemiological evidence supports a protective influence of higher L and Z in the diet or blood on risk for advanced AMD (for a recent review, see 121; for newly published results, see 191). However, associations between L and Z in serum or diet and earlier stages of AMD are more challenging to study and are sometimes, but not always, observed. One reason for a failure to find consistent protective associations of L and Z intake to AMD could be variability in the proportions of people who readily accumulate MP in response to supplementation (discussed in section titled Influences on Accumulation in Retinal Tissues). Measuring MP levels directly, rather than relying on intake levels as the predictor, reduces the opportunity for masking associations between L and Z status and AMD. Previous evidence from cross-sectional studies supports the possibility that higher MPOD is related to lower risk for AMD (13, 21, 34), but only in younger subjects with stable diets in the CAREDS cohort (99) and not in some other samples (17, 51, 82, 137). In the only prospective study of the relation of MPOD to risk of AMD in 450 persons, a protective trend was observed (86). Larger prospective studies are needed to better assess the temporal relationship between MPOD and AMD. These are under way in several large study samples.
A second line of evidence in support of a protective role of L and Z status on early AMD risk includes evidence that many of the conditions that are associated with low MPOD are also risk factors for AMD (89). These include not only low levels of L and Z in the diet and serum, but also smoking, indicators of metabolic syndrome (i.e., obesity, diabetes, and hypertension), and more recently, common variants in genes related to MPOD and/or serum L and Z (126) that predicted the odds for having early/intermediate AMD (130). Of these, the genetic evidence is particularly supportive of a protective association, having the advantage of being unconfounded by lifestyle factors and reflective of carotenoid status over a person's lifetime rather than only a few years or decades. A protective role of L and Z may be limited to, or more pronounced, in people with high genetic risk for AMD. This is suggested by results of significant interactions between L and Z intake and genotype in AMD risk in previous prospective cohort studies [the Rotterdam Study (75) and a pooled analysis of the Rotterdam and Blue Mountain Eye studies (186)]. A similar trend for reduction in genetic risk for AMD among individuals with high L and Z intake was observed in the CAREDS cohort (127). The lowest rates of AMD have been reported in persons consuming 5 to 6 mg/day (118, 131, 165, 191). However, diets high in L and Z are also high in other carotenoids, which may contribute to risk lowering (191). The levels needed to lower AMD risk may be higher in people who have phenotypes and genotypes associated with low absorption, low HDL cholesterol levels, and/or low uptake or retention in the retina.
A small number of studies have evaluated the impact of supplementation with L and/or Z on the progression of AMD over three to five years. L and Z supplements alone did not influence AMD progression over three years (2), but L and Z supplements in combination with other antioxidant supplements did (12). Results of primary analyses in the AREDS2 (1) trial suggested that including 10 mg L and 2 mg Z in high-dose antioxidant supplements did not lower progression to advanced AMD, but secondary analyses (41) indicated a modestly lower five-year risk of progression in participants who began the study with low levels of these (and likely other) carotenoids in their diets or when the antioxidant supplements did not contain beta-carotene. Risk lowering was greater when restricting analyses to persons with earlier stages of the disease (eyes with bilateral large drusen at baseline), but risk lowering was not observed in relation to the five-year loss of photoreceptors and RPE (geographic atrophy) (41).
In summary, L and Z intakes of 5 to 6 mg/day are associated with lowering the risk of developing AMD, and supplemental intakes at 12 mg/day (in the absence of beta-carotene but in the presence of other antioxidants) slowed AMD progression in people who already had intermediate AMD. However, it is not clear whether intakes above these levels would have additional benefit and are without other adverse consequences over decades. It is also not known whether beneficial and safe levels might differ in certain segments of the population. The benefit of other carotenoids in slowing AMD, and the impact of all carotenoids on preventing early stages of AMD, is also unclear. Thus, a prudent approach until further data are available may be a public health strategy aimed at increasing the consumption of lutein-rich vegetables and fruits, moderately increasing egg consumption in people without AMD, and promoting the daily use of antioxidant supplements containing 12 mg L and Z in people with intermediate AMD.
Cataract
Cataract, the leading cause of blindness worldwide (154), results from opacification of the lens of the eye. This opacification diffuses and ultimately blocks light from entering the eye. The lens with a cataract can be replaced with a synthetic lens, but this surgery is not often available in developing countries. In developed countries, such as the United States, cataract surgery is common (47) and costly, although the cost per surgery has declined in the past decade. In addition, severe complications develop in 1% to 2% of surgeries. Therefore, preventing cataract could have a significant economic and health impact. It is well known that oxidative stress contributes to cataract development and that L and Z are the only two carotenoids, and two of the many exogenous antioxidants, that protect against the development of lens opacities and their severe form, cataract (for a recent review and discussion, see 119, 123, 183). Results of experiments in cultured cells (42, 56) and rats (6) demonstrate that these carotenoids reduce the oxidative stress and damage to the lens that lead to cataract development.
A large body of evidence from longitudinal observational studies (110, 114) supports a protective association of L and Z in diet and/or serum with the subsequent prevalence or incidence of cataract (except in well-nourished samples; 59, 81), particularly for opacities in the nuclear (central) region of the lens, where opacities develop slowly over a lifetime. However, the degree to which these associations reflect the specific effect of L and Z status in cataract development, independent from other components of fruit- and vegetable-rich diets, is difficult to isolate in observational studies. This is because it is difficult to adequately adjust for the intake of all antioxidants in foods that are consumed over the long period of time during which cataracts develop and that might confound the association of L and Z intake to cataract. In one large multisite clinical trial, adding L and Z to other antioxidant supplements did not slow progression to cataract surgery over five years, except in secondary analyses in the participants with the lowest levels of L and Z from foods (1). This result suggests the possibility that L and Z may indeed independently decrease risk for cataract, and if so, the levels of L and Z required for optimum protection might be easily achieved in well-nourished populations. Conducting additional clinical trials that are large enough to further evaluate the independent protective effect of L and Z would be costly, given the fact that cataracts develop over many years. However, adding cataract outcomes to existing intervention trials of L and Z and conducting pooled analyses across trials would provide an opportunity to gain additional insight. Results of pooled long-term prospective studies across strata of populations with varying levels of confounding influences might also contribute additional data to evaluate the degree to which L and Z intake or status contributes to cataract protection.
In summary, the body of evidence from different study types suggests that L and Z are likely to be two dietary components that protect against nuclear cataract, but if this is the case, the level needed to protect against lens opacity development is likely to be in the range of 0.5 to 1 mg/day, which is below the average intake in American adults (1.7 mg/day) (180). Assuring that all subgroups of the population achieve this minimal intake of L and Z might substantially lower the visual burden of cataracts and the risks and expense associated with cataract surgery, on a population level. It has been estimated that slowing cataract development by only 10 years would cut the need for cataract surgery in half (93).
Glaucoma and diabetic retinopathy
An early body of research suggests that L and Z might help prevent the development and progression of glaucoma and diabetic retinopathy, which are more common in people with diabetes. Diabetes is associated with lower MPOD (122, 163) and could increase the risk of low retinal response to dietary xanthophylls in individuals at risk for these conditions (as previously discussed).
Glaucoma is a group of conditions that cause optic neuropathy and the degeneration of the retinal ganglion cells and their axons, leading to reduced visual sensitivity, particularly in the peripheral field of vision. It is a long-term, chronic disease with no cure and is the leading cause of irreversible blindness in the world because many people continue to have progressive, severe vision loss despite treatment to lower eye pressure (149). Modifiable risk factors for glaucoma are less studied than those for cataract and AMD.
Current evidence in animals and humans suggests that pathogenic mechanisms underlying glaucoma include oxidative stress (for a review, see 177), which xanthophyll carotenoids are well known to protect against. Recently, L was observed to suppress damage to the retinal ganglion cells in rats (198). Damage to retinal ganglion cells occurs in glaucoma through hypoxia, oxidative stress, and inflammation. Also, L and Z present in the ciliary body (19), which produces aqueous humor, might protect against damage to the trabecular meshwork, lowering risk for high intraocular pressure (IOP), which is a well-known risk factor for glaucoma. This possibility has not been studied.
Higher dietary intake of fruits and vegetables rich in carotenoids (46, 58) has been associated with lower glaucoma occurrence in two observational studies, but two other studies did not find an association (85, 150). Interindividual variability in the absorption and retinal capture of L and Z is high and may explain the lack of associations with dietary intake. In particular, diabetes or other aspects of metabolic syndrome associated with low MPOD (see section titled Influences on Accumulation in Retinal Tissues) was more common in people with glaucoma in a large sample representative of the general US population (199).
A small body of evidence from experiments in rodents and human observational studies suggests a role for xanthophylls in protecting against diabetic retinopathy (for a recent review, see 61). Diabetic retinopathy is the most common acquired cause of blindness in individuals of working age (47). The growth in obesity rates and life span will increase the magnitude of the problem worldwide (200). Hyperglycemia can lead to damage of the retinal microvasculature, indicative of diabetic retinopathy, through a number of pathways that involve oxidative stress and inflammation (for a review, see 61).
In diabetic mice, dietary intake of Z (90) or a Z- and L-rich food (wolfberry) (176) mitigated retinal abnormalities, attenuated mitochondrial stress (195), and upregulated the expression of genes for proteins that play roles in the uptake of L and Z into the gut and retina (i.e., SCARB1), binding in the retina (i.e., GSTP1), and protection against mitochondrial damage as a result of oxidative stress (i.e., BCO2). Data from human epidemiological studies and clinical trials are limited and conflicting (123). In a small placebo-controlled, randomized trial, L was one of several antioxidants that prevented progression of diabetic retinopathy over five years (57).
In summary, clinical, epidemiological, and animal studies suggest the possibility that better L and Z intake could lower risk for glaucoma and diabetic retinopathy, but data are limited. Prospective epidemiological study results are needed to estimate the influence of L and Z status on the incidence and progression of diabetic retinopathy and glaucoma. Also, randomized controlled trials are needed to determine whether L and Z, specifically, lower the progression of these conditions. This research would be most efficiently accomplished by adding glaucoma and diabetic retinopathy outcomes to existing observational studies and clinical trials.
Retinopathy of prematurity
Xanthophyll carotenoids might prevent retinopathy of prematurity (ROP), a retinal neovascularization that leads to blindness in children born prematurely (for a recent review, see 61). In ROP, hypoxic damage to the retina occurs as a result of suppression of growth factors needed to complete the development of the retinal vasculature (which supplies the inner retina) and leads to an increased metabolic demand after birth, causing the growth of abnormal blood vessels in the vitreous. Recent small trials in human neonates indicate that carotenoid supplementation lowers oxidative stress (145) and inflammatory markers (158). A meta-analysis of four randomized controlled trials of L supplementation in premature infants found a 26% reduction in severe ROP (61). Another study (117) observed a lower risk of ROP among very-low-birth-weight infants who received human milk, which contains L and Z (but also many other components lacking in infant formulas). In summary, the biological plausibility that L lowers the risk of ROP in preterm infants is high, but additional larger trials need to examine this possibility with higher power and determine the dose of L needed for optimal effects and safety when human milk cannot be provided.
Retinitis pigmentosa
Retinitis pigmentosa (RP), a group of largely inherited genetic disorders of rod and cone photoreceptors, is the leading cause of inherited blindness in the developed world (23). A loss of night vision early in the disorder is followed by a loss of vision in the rod-dominated peripheral field of vision and eventually in the cone-rich center. The high level of nonresponse (50%) to L supplementation (4) might be secondary to loss of retinal tissue (160). L supplementation alone (10 mg/day for 12 weeks followed by 30 mg/day for 12 weeks) in 34 people improved the visual field in one randomized controlled crossover study (8), but 20 mg L supplementation did not improve central vision in another small study of patients with RP and Usher syndrome (4). In a larger and longer randomized controlled trial, L (12 mg/d) added to vitamin A supplements modestly slowed visual field loss over four years among nonsmoking adults with retinitis pigmentosa. It has been suggested that adding omega-3 fatty acids to L and vitamin A might additionally improve central vision (183).
Summary
Poor status for L and/or Z is associated with age-related cataract and a wide range of inherited and acquired diseases of the retina from early to late life. Supplementation with these xanthophylls often improves visual outcomes. However, results are inconsistent,, and levels that are necessary and safe over the long term, are poorly understood. Supplementation with other nutrients or antioxidants may improve visual outcomes, possibly by mechanisms that also increase the ability to retain retinal xanthophylls.
Levels in the Diet, Serum, and Supplements
Diet
The first national estimates of dietary L and Z levels in US adults came from food records reported in the 1986 Continuing Survey of Food Intake in Individuals. Results indicated that the average intake of L and Z in adults 19 to 50 years of age was 1.3 mg/day. Subsequently, mean intake levels in adults reported in the National Health and Nutrition Examination Survey (NHANES) have been similar, whether estimated from responses to food frequency questionnaires (118) or, more recently, one-day diet recall data. Mean intake levels estimated in the 2011–2012 NHANES were 1.7 mg/day in adults age 20 and older and 0.8 mg/day in children ages 2 to 19 (180). Levels of L and Z intake in some South Pacific study samples are higher (101) and reached 25 mg/day in one study in Fiji (100).
The major food contributors to intake in the United States have changed somewhat over the past 25 years. The size and composition of populations sampled, increased completeness of food composition databases, and trends of intake over this time likely all contribute to these changes. In 1988, the major contributors were spinach; collard, mustard, or turnip greens; and broccoli (44). These major contributors were followed by corn, green beans, and peas. These foods accounted for about half of L and Z intake, and more minor amounts came mostly from other vegetables. In comparison, in 2003–2004, when improved food composition databases for L and Z separately were available, the major contributors (>50%) of L were leafy green vegetables, corn tortillas, eggs, orange juice, and broccoli, and major contributors of Z were corn tortillas, eggs, and orange juice.
Serum
Serum L/Z concentrations averaged 13 μg/dl in Americans over age 6 in two separate NHANES cycles (2001–2002 and 2005–2006) (40), ranging from 6 to 30/31 μg/dl in the fifth and ninety-fifth percentiles, respectively. Population groups at risk for lower serum L/Z status included teens, whites, females, smokers, people who are abdominally obese, and those who are less physically active.
Supplements
L and Z supplementation for eye health has become increasingly common since supplements containing them entered the market in the late 1990s. The use of supplements for eye health in general is increasing (50). Data from adults 20 years or older from the 2007–2010 NHANES indicated 4% used supplements for eye health, but for Americans over age 60, the prevalence was more than twice that level (9). Two recent trends are likely to increase intakes of L and Z supplements. First, the American Academy of Ophthalmology recently recommended that persons with intermediate or advanced AMD be treated with a high-dose antioxidant supplement that contains L and Z; this recommendation was made on the basis of the previously discussed AREDS2 results (41). Moreover, individuals who have a family history of AMD might choose to supplement with similar formulations (even though there is currently no evidence that L and Z supplementation has prevented AMD). Second, techniques to assess the density of retinal carotenoids are being commercialized for use in optometric and ophthalmological practices, which may lead to identification of people in middle age with low MP levels and increased propensity of individuals with low levels to take supplements. In 2014, L and Z were ingredients in most eye supplements; L and Z levels in multivitamins were 0.25 mg, whereas doses in other supplements ranged from approximately 5 mg/day to 50 mg L/day and 10 mg Z/day (62).
Levels associated with benefit and risks
At this time, there is no consensus or recommendation for levels of L and Z intake that are safe and beneficial. However, intake for individuals who follow the fruit and vegetable intake recommendations of the Dietary Guidelines for Americans would be approximately 5 mg L and Z per day. Whereas the lowest AMD risk has been demonstrated in persons with L and Z intakes of 5 to 6 mg per day, the levels needed to slow cataracts (if future results confirm suspected protective effects) may be much lower. Results of short-term clinical trials of L and Z supplements in relation to specific visual functions are inconsistent, and evidence is insufficient to determine the level of intake required to attain maximal visual performance. Also, there is a need to develop consensus about which visual functions are optimal for persons of specific ages and for daily activities requiring vision. Recommendations might also be established based on levels of L and Z needed to increase MP levels in trials. However, intake levels needed to increase MP in short-term studies may be higher than intake levels that increase MP over a longer time period. Current evidence suggests that levels of intake needed by individuals might differ, depending on their ability to absorb L and Z, deliver them to the eye, and stabilize them within the eye; these are influenced by both genetic and metabolic factors. Strategies to increase ocular levels of MP might include more than increasing intake; they might include getting more physical activity, eating diets rich in fruits and vegetables, and not smoking. These lifestyle changes might enhance lutein and zeaxanthin status in the blood and retina by modifying metabolic phenotype. This remains to be tested.
Data are insufficient to evaluate whether there are long-term risks of high L and/or Z intake from supplements because L and Z supplement use prior to the past five years has been uncommon. Clinical trials are not powered to evaluate adverse events and high L and Z supplement use. Continued monitoring in observational studies is needed.
Summary Points.
L and Z appear to play specific roles in optimal visual performance in health and disease and in lowering risk for common causes of visual impairment.
Well-powered clinical trials and long-term prospective studies are needed to determine optimal levels associated with aspects of vision that impact daily function. In addition, supplementation levels should be evaluated for adverse events, if any, related to the long-term intake of these carotenoids in supplements.
Individuals who have lower blood and retinal responses to the intake of these carotenoids might require higher intakes of lutein and zeaxanthin or longer periods of time to accumulate optimal levels of macular pigment.
A critical need exists for data to establish guidelines for the safe and adequate intake of L and Z in pregnant and lactating women and infants.
Acknowledgments
I am grateful to Ryleigh White for literature research and to Courtney Blomme for critical editing that contributed to this review. This work was supported by National Eye Institute grants EY013018, EY016886, and EY025292 and by an unrestricted grant to the University of Wisconsin Department of Ophthalmology and Visual Sciences from the Research to Prevent Blindness.
Footnotes
Disclosure Statement: The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Literature Cited
- 1.Age-Related Eye Disease Study Investig. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–15. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 2.Akuffo KO, Nolan JM, Howard AN, Moran R, Stack J, et al. Sustained supplementation and monitored response with differing carotenoid formulations in early age-related macular degeneration. Eye (Lond) 2015;29:902–12. doi: 10.1038/eye.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aleman TS, Cideciyan AV, Windsor EA, Schwartz SB, Swider M, et al. Macular pigment and lutein supplementation in ABCA4-associated retinal degenerations. Investig Ophthalmol Vis Sci. 2007;48:1319–29. doi: 10.1167/iovs.06-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aleman TS, Duncan JL, Bieber ML, de Castro E, Marks DA, et al. Macular pigment and lutein supplementation in retinitis pigmentosa and Usher syndrome. Investig Ophthalmol Vis Sci. 2001;42:1873–81. [PubMed] [Google Scholar]
- 5.Amengual J, Lobo GP, Golczak M, Li HN, Klimova T, et al. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2011;25:948–59. doi: 10.1096/fj.10-173906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnal E, Miranda M, Almansa I, Muriach M, Barcia JM, et al. Lutein prevents cataract development and progression in diabetic rats. Graefes Arch Clin Exp Ophthalmol. 2009;247:115–20. doi: 10.1007/s00417-008-0935-z. [DOI] [PubMed] [Google Scholar]
- 7.Babino D, Palczewski G, Widjaja-Adhi MA, Kiser PD, Golczak M, von Lintig J. Characterization of the role of beta-carotene 9,10-dioxygenase in macular pigment metabolism. J Biol Chem. 2015;290:24844–57. doi: 10.1074/jbc.M115.668822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahrami H, Melia M, Dagnelie G. Lutein supplementation in retinitis pigmentosa: PC-based vision assessment in a randomized double-masked placebo-controlled clinical trial [ NCT00029289] BMC Ophthalmol. 2006;6:23. doi: 10.1186/1471-2415-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med. 2013;173:355–61. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- 10.Barker FM, 2nd, Snodderly DM, Johnson EJ, Schalch W, Koepcke W, et al. Nutritional manipulation of primate retinas, V: effects of lutein, zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light-induced damage. Investig Ophthalmol Vis Sci. 2011;52:3934–42. doi: 10.1167/iovs.10-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartlett HE, Eperjesi F. A randomised controlled trial investigating the effect of lutein and antioxidant dietary supplementation on visual function in healthy eyes. Clin Nutr. 2008;27:218–27. doi: 10.1016/j.clnu.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Beatty S, Chakravarthy U, Nolan JM, Muldrew KA, Woodside JV, et al. Secondary outcomes in a clinical trial of carotenoids with coantioxidants versus placebo in early age-related macular degeneration. Ophthalmology. 2013;120:600–6. doi: 10.1016/j.ophtha.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 13.Beatty S, Murray IJ, Henson DB, Carden D, Koh H, Boulton ME. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Investig Ophthalmol Vis Sci. 2001;42:439–46. [PubMed] [Google Scholar]
- 14.Beirne RO. The macular pigment optical density spatial profile and increasing age. Graefes Arch Clin Exp Ophthalmol. 2013;252:383–88. doi: 10.1007/s00417-013-2471-8. [DOI] [PubMed] [Google Scholar]
- 15.Berendschot TT, Goldbohm RA, Klopping WA, Van de Kraats J, Van Norel J, Van Norren D. Influence of lutein supplementation on macular pigment, assessed with two objective techniques. Investig Ophthalmol Vis Sci. 2000;41:3322–26. [PubMed] [Google Scholar]
- 16.Berendschot TTJM, Tian Y, Murray I, Makridaki M. Lutein supplementation leads to a decreased level of circulating complement factors. Investig Ophthalmol Vis Sci. 2013;54:4124. Abstr. [Google Scholar]
- 17.Berendschot TT, Willemse-Assink JJ, Bastiaanse M, de Jong PT, van Norren D. Macular pigment and melaninin age-related maculopathy in a general population. Investig Ophthalmol Vis Sci. 2002;43:1928–32. [PubMed] [Google Scholar]
- 18.Bernstein P, Li B, Vachali P, Goruspudi A, Shyam R, et al. Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res. 2016;50:34–66. doi: 10.1016/j.preteyeres.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res. 2001;72:215–23. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein PS, Sharifzadeh M, Liu A, Ermakov I, Nelson K, et al. Blue-light reflectance imaging of macular pigment in infants and children. Investig Ophthalmol Vis Sci. 2013;54:4034–40. doi: 10.1167/iovs.13-11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernstein PS, Zhao DY, Wintch SW, Ermakov IV, McClane RW, Gellermann W. Resonance Raman measurement of macular carotenoids in normal subjects and in age-related macular degeneration patients. Ophthalmology. 2002;109:1780–87. doi: 10.1016/s0161-6420(02)01173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bettler J, Zimmer JP, Neuringer M, DeRusso PA. Serum lutein concentrations in healthy term infants fed human milk or infant formula with lutein. Eur J Nutr. 2010;49:45–51. doi: 10.1007/s00394-009-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatti MT. Retinitis pigmentosa, pigmentary retinopathies, and neurologic diseases. Curr Neurol Neurosci Rep. 2006;6:403–13. doi: 10.1007/s11910-996-0021-z. [DOI] [PubMed] [Google Scholar]
- 24.Bian Q, Gao S, Zhou J, Qin J, Taylor A, et al. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radic Biol Med. 2012;53:1298–307. doi: 10.1016/j.freeradbiomed.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bian Q, Qin T, Ren Z, Wu D, Shang F. Lutein or zeaxanthin supplementation suppresses inflammatory responses in retinal pigment epithelial cells and macrophages. Adv Exp Med Biol. 2012;723:43–50. doi: 10.1007/978-1-4614-0631-0_7. [DOI] [PubMed] [Google Scholar]
- 26.Bone RA, Brener B, Gibert JC. Macular pigment, photopigments, and melanin: distributions in young subjects determined by four-wavelength reflectometry. Vis Res. 2007;47:3259–68. doi: 10.1016/j.visres.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bone RA, Landrum JT. Macular pigment in Henle fiber membranes: a model for Haidinger's brushes. Vis Res. 1984;24:103–8. doi: 10.1016/0042-6989(84)90094-4. [DOI] [PubMed] [Google Scholar]
- 28.Bone RA, Landrum JT, Cao Y, Howard AN, Alvarez-Calderon F. Macular pigment response to a supplement containing meso-zeaxanthin, lutein and zeaxanthin. Nutr Metab (Lond) 2007;4:12. doi: 10.1186/1743-7075-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bone RA, Landrum JT, Dixon Z, Chen Y, Llerena CM. Lutein and zeaxanthin in the eyes, serum and diet of human subjects. Exp Eye Res. 2000;71:239–45. doi: 10.1006/exer.2000.0870. [DOI] [PubMed] [Google Scholar]
- 30.Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Investig Ophthalmol Vis Sci. 1988;29:843–49. [PubMed] [Google Scholar]
- 31.Bone RA, Landrum JT, Friedes LM, Gomez CM, Kilburn MD, et al. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res. 1997;64:211–18. doi: 10.1006/exer.1996.0210. [DOI] [PubMed] [Google Scholar]
- 32.Bone RA, Landrum JT, Guerra LH, Ruiz CA. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J Nutr. 2003;133:992–98. doi: 10.1093/jn/133.4.992. [DOI] [PubMed] [Google Scholar]
- 33.Bone RA, Landrum JT, Hime GW, Cains A, Zamor J. Stereochemistry of the human macular carotenoids. Investig Ophthalmol Vis Sci. 1993;34:2033–40. [PubMed] [Google Scholar]
- 34.Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE. Macular pigment in donor eyes with and without AMD: a case-control study. Investig Ophthalmol Vis Sci. 2001;42:235–40. [PubMed] [Google Scholar]
- 35.Borel P. Genetic variations involved in interindividual variability in carotenoid status. Mol Nutr Food Res. 2012;56:228–40. doi: 10.1002/mnfr.201100322. [DOI] [PubMed] [Google Scholar]
- 36.Borel P, Desmarchelier C, Nowicki M, Bott R, Morange S, Lesavre N. Interindividual variability of lutein bioavailability in healthy men: characterization, genetic variants involved, and relation with fasting plasma lutein concentration. Am J Clin Nutr. 2014;100:168–75. doi: 10.3945/ajcn.114.085720. [DOI] [PubMed] [Google Scholar]
- 37.Bovier ER, Renzi LM, Hammond BR. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on neural processing speed and efficiency. PLOS ONE. 2014;9:e108178. doi: 10.1371/journal.pone.0108178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, et al. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am J Clin Nutr. 2004;80:396–403. doi: 10.1093/ajcn/80.2.396. [DOI] [PubMed] [Google Scholar]
- 39.Canfield LM, Clandinin MT, Davies DP, Fernandez MC, Jackson J, et al. Multinational study of major breast milk carotenoids of healthy mothers. Eur J Nutr. 2003;42:133–41. doi: 10.1007/s00394-003-0403-9. [DOI] [PubMed] [Google Scholar]
- 40.Cent. Dis. Control Prev. Second national report on biochemical indicators of diet and nutrition in the US population 2012. Atlanta, GA: Natl. Cent. Environ. Health; 2012. http://www.cdc.gov/nutritionreport. [Google Scholar]
- 41.Chew EY, Clemons TE, Sangiovanni JP, Danis RP, Ferris FL, 3rd, et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 Report No. 3. JAMA Ophthalmol. 2013;132:142–49. doi: 10.1001/jamaophthalmol.2013.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chitchumroonchokchai C, Bomser JA, Glamm JE, Failla ML. Xanthophylls and alpha-tocopherol decrease UVB-induced lipid peroxidation and stress signaling in human lens epithelial cells. J Nutr. 2004;134:3225–32. doi: 10.1093/jn/134.12.3225. [DOI] [PubMed] [Google Scholar]
- 43.Chous AP, Richer SP, Gerson JD, Kowluru RA. The Diabetes Visual Function Supplement Study (DiVFuSS) Br J Ophthalmol. 2016;100:227–34. doi: 10.1136/bjophthalmol-2014-306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chug-Ahuja JK, Holden JM, Forman MR, Mangels AR, Beecher GR, Lanza E. The development and application of a carotenoid database for fruits, vegetables, and selected multicomponent foods. J Am Diet Assoc. 1993;93:318–23. doi: 10.1016/0002-8223(93)91559-9. [DOI] [PubMed] [Google Scholar]
- 45.Chung HY, Rasmussen HM, Johnson EJ. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J Nutr. 2004;134:1887–93. doi: 10.1093/jn/134.8.1887. [DOI] [PubMed] [Google Scholar]
- 46.Coleman AL, Stone KL, Kodjebacheva G, Yu F, Pedula KL, et al. Glaucoma risk and the consumption of fruits and vegetables among older women in the study of osteoporotic fractures. Am J Ophthalmol. 2008;145:1081–89. doi: 10.1016/j.ajo.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 47.Congdon N, O'Colmain B, Klaver CC, Klein R, Munoz B, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 48.Craft NE, Haitema TB, Garnett KM, Fitch KA, Dorey CK. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J Nutr Health Aging. 2004;8:156–62. [PubMed] [Google Scholar]
- 49.Dawczynski J, Jentsch S, Schweitzer D, Hammer M, Lang GE, Strobel J. Long term effects of lutein, zeaxanthin and omega-3-LCPUFAs supplementation on optical density of macular pigment in AMD patients: the LUTEGA study. Graefes Arch Clin Exp Ophthalmol. 2013;251:2711–23. doi: 10.1007/s00417-013-2376-6. [DOI] [PubMed] [Google Scholar]
- 50.Dickinson A, Blatman J, El-Dash N, Franco JC. Consumer usage and reasons for using dietary supplements: report of a series of surveys. J Am Coll Nutr. 2014;33:176–82. doi: 10.1080/07315724.2013.875423. [DOI] [PubMed] [Google Scholar]
- 51.Dietzel M, Zeimer M, Heimes B, Claes B, Pauleikhoff D, Hense HW. Determinants of macular pigment optical density and its relation to age-related maculopathy: results from the Muenster Aging and Retina Study (MARS) Investig Ophthalmol Vis Sci. 2011;52:3452–57. doi: 10.1167/iovs.10-6713. [DOI] [PubMed] [Google Scholar]
- 52.Duncan DD, Munoz B, West SK. Assessment of ocular exposure to visible light for population studies. Dev Ophthalmol. 2002;35:76–92. doi: 10.1159/000060812. [DOI] [PubMed] [Google Scholar]
- 53.Ekici F, Loh R, Waisbourd M, Sun Y, Martinez P, et al. Relationships between measures of the ability to perform vision-related activities, vision-related quality of life, and clinical findings in patients with glaucoma. JAMA Ophthalmol. 2015;133:1377–85. doi: 10.1001/jamaophthalmol.2015.3426. [DOI] [PubMed] [Google Scholar]
- 54.Ferrucci L, Perry JR, Matteini A, Perola M, Tanaka T, et al. Common variation in the beta-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet. 2009;84:123–33. doi: 10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 56.Gao S, Qin T, Liu Z, Caceres MA, Ronchi CF, et al. Lutein and zeaxanthin supplementation reduces H2O2-induced oxidative damage in human lens epithelial cells. Mol Vis. 2011;17:3180–90. [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia-Medina JJ, Pinazo-Duran MD, Garcia-Medina M, Zanon-Moreno V, Pons-Vazquez S. A 5-year follow-up of antioxidant supplementation in type 2 diabetic retinopathy. Eur J Ophthalmol. 2011;21:637–43. doi: 10.5301/EJO.2010.6212. [DOI] [PubMed] [Google Scholar]
- 58.Giaconi JA, Yu F, Stone KL, Pedula KL, Ensrud KE, et al. The association of consumption of fruits/vegetables with decreased risk of glaucoma among older African-American women in the study of osteoporotic fractures. Am J Ophthalmol. 2012;154:635–44. doi: 10.1016/j.ajo.2012.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glaser TS, Doss LE, Shih G, Nigam D, Sperduto RD, et al. The association of dietary lutein plus zeaxanthin and B vitamins with cataracts in the Age-Related Eye Disease Study: AREDS Report No. 37. Ophthalmology. 2015;122:1471–79. doi: 10.1016/j.ophtha.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goltz SR, Ferruzzi MG. Carotenoid bioavailability: influence of dietary lipid and fiber. In: Tanumihardjo S, editor. Carotenoids and Human Health. New York: Humana; 2013. pp. 111–28. [Google Scholar]
- 61.Gong X, Rubin LP. Role of macular xanthophylls in prevention of common neovascular retinopathies: retinopathy of prematurity and diabetic retinopathy. Arch Biochem Biophys. 2015;572:40–48. doi: 10.1016/j.abb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Grover AK, Samson SE. Antioxidants and vision health: facts and fiction. Mol Cell Biochem. 2014;388:173–83. doi: 10.1007/s11010-013-1908-z. [DOI] [PubMed] [Google Scholar]
- 63.Gruber M, Chappell R, Millen A, LaRowe T, Moeller SM, et al. Correlates of serum lutein + zeaxanthin: findings from the Third National Health and Nutrition Examination Survey. J Nutr. 2004;134:2387–94. doi: 10.1093/jn/134.9.2387. [DOI] [PubMed] [Google Scholar]
- 64.Hammond BR., Jr Possible role for dietary lutein and zeaxanthin in visual development. Nutr Rev. 2008;66:695–702. doi: 10.1111/j.1753-4887.2008.00121.x. [DOI] [PubMed] [Google Scholar]
- 65.Hammond BR, Jr, Elliott J. Multiple influences of xanthophylls within the visual system. 2013:147–70. See Ref, 98. [Google Scholar]
- 66.Hammond BR, Jr, Fletcher LM, Elliott JG. Glare disability, photostress recovery, and chromatic contrast: relation to macular pigment and serum lutein and zeaxanthin. Investig Ophthalmol Vis Sci. 2013;54:476–81. doi: 10.1167/iovs.12-10411. [DOI] [PubMed] [Google Scholar]
- 67.Hammond BR, Jr, Fletcher LM, Roos F, Wittwer J, Schalch W. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on photostress recovery, glare disability, and chromatic contrast. Investig Ophthalmol Vis Sci. 2014;55:8583–89. doi: 10.1167/iovs.14-15573. [DOI] [PubMed] [Google Scholar]
- 68.Hammond BR, Jr, Johnson EJ, Russell RM, Krinsky NI, Yeum KJ, et al. Dietary modification of human macular pigment density. Investig Ophthalmol Vis Sci. 1997;38:1795–801. [PubMed] [Google Scholar]
- 69.Hammond BR, Jr, Wooten BR. CFF thresholds: relation to macular pigment optical density. Ophthalmic Physiol Opt. 2005;25:315–19. doi: 10.1111/j.1475-1313.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 70.Hammond BR, Jr, Wooten BR, Snodderly DM. Individual variations in the spatial profile of human macular pigment. J Opt Soc Am A Opt Image Sci Vis. 1997;14:1187–96. doi: 10.1364/josaa.14.001187. [DOI] [PubMed] [Google Scholar]
- 71.Hammond CJ, Liew SM, Van Kuijk FJ, Beatty S, Nolan JM, et al. The heritability of macular response to supplemental lutein and zeaxanthin: a classical twin study. Investig Ophthalmol Vis Sci. 2012;53:4963–68. doi: 10.1167/iovs.12-9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Henriksen BS, Chan G, Hoffman RO, Sharifzadeh M, Ermakov IV, et al. Interrelationships between maternal carotenoid status and newborn infant macular pigment optical density and carotenoid status. Investig Ophthalmol Vis Sci. 2013;54:5568–78. doi: 10.1167/iovs.13-12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herron KL, McGrane MM, Waters D, Lofgren IE, Clark RM, et al. The ABCG5 polymorphism contributes to individual responses to dietary cholesterol and carotenoids in eggs. J Nutr. 2006;136:1161–65. doi: 10.1093/jn/136.5.1161. [DOI] [PubMed] [Google Scholar]
- 74.Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, et al. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem. 2007;282:33553–61. doi: 10.1074/jbc.M706763200. [DOI] [PubMed] [Google Scholar]
- 75.Ho L, van Leeuwen R, Witteman JC, Van Duijn CM, Uitterlinden AG, et al. Reducing the genetic risk of age-related macular degeneration with dietary antioxidants, zinc, and omega-3 fatty acids: the Rotterdam Study. Arch Ophthalmol. 2011;129:758–66. doi: 10.1001/archophthalmol.2011.141. [DOI] [PubMed] [Google Scholar]
- 76.Hollyfield JG. Age-related macular degeneration: the molecular link between oxidative damage, tissue-specific inflammation and outer retinal disease: the Proctor Lecture. Investig Ophthalmol Vis Sci. 2010;51:1275–81. doi: 10.1167/iovs.09-4478. [DOI] [PubMed] [Google Scholar]
- 77.Hu BJ, Hu YN, Lin S, Ma WJ, Li XR. Application of lutein and zeaxanthin in nonproliferative diabetic retinopathy. Int J Ophthalmol. 2011;4:303–6. doi: 10.3980/j.issn.2222-3959.2011.03.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang YM, Dou HL, Huang FF, Xu XR, Zou ZY, Lin XM. Effect of supplemental lutein and zeaxanthin on serum, macular pigmentation, and visual performance in patients with early age-related macular degeneration. BioMed Res Int. 2015;2015:564738. doi: 10.1155/2015/564738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iakovleva M, Panova I, Fel'dman T, Zak P, Tatikolov A, et al. Detection of carotenoids in the vitreous body of the human eye during prenatal development. Ontogenez. 2007;38:380–85. [PubMed] [Google Scholar]
- 80.Izumi-Nagai K, Nagai N, Ohgami K, Satofuka S, Ozawa Y, et al. Macular pigment lutein is antiinflammatory in preventing choroidal neovascularization. Arterioscler Thromb Vasc Biol. 2007;27:2555–62. doi: 10.1161/ATVBAHA.107.151431. [DOI] [PubMed] [Google Scholar]
- 81.Jacques PF, Chylack LT, Jr, Hankinson SE, Khu PM, Rogers G, et al. Long-term nutrient intake and early age-related nuclear lens opacities. Arch Ophthalmol. 2001;119:1009–19. doi: 10.1001/archopht.119.7.1009. [DOI] [PubMed] [Google Scholar]
- 82.Jahn C, Wustemeyer H, Brinkmann C, Trautmann S, Moosner A, Wolf S. Macular pigment density in age-related maculopathy. Graefe's Arch Clin Exp Ophthalmol. 2005;243:222–27. doi: 10.1007/s00417-004-0995-7. [DOI] [PubMed] [Google Scholar]
- 83.Johnson EJ. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr Rev. 2014;72:605–12. doi: 10.1111/nure.12133. [DOI] [PubMed] [Google Scholar]
- 84.Johnson EJ, Hammond BR, Yeum KJ, Qin J, Wang XD, et al. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am J Clin Nutr. 2000;71:1555–62. doi: 10.1093/ajcn/71.6.1555. [DOI] [PubMed] [Google Scholar]
- 85.Kang JH, Pasquale LR, Willett W, Rosner B, Egan KM, et al. Antioxidant intake and primary open-angle glaucoma: a prospective study. Am J Epidemiol. 2003;158:337–46. doi: 10.1093/aje/kwg167. [DOI] [PubMed] [Google Scholar]
- 86.Kanis MJ, Berendschot TT, Van Norren D. Influence of macular pigment and melanin on incident early AMD in a white population. Graefes Arch Clin Exp Ophthalmol. 2007;245:767–73. doi: 10.1007/s00417-006-0478-0. [DOI] [PubMed] [Google Scholar]
- 87.Kelly ER, Plat J, Haenen GR, Kijlstra A, Berendschot TT. The effect of modified eggs and an egg-yolk based beverage on serum lutein and zeaxanthin concentrations and macular pigment optical density: results from a randomized trial. PLOS ONE. 2014;9:e92659. doi: 10.1371/journal.pone.0092659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koh HH, Murray IJ, Nolan D, Carden D, Feather J, Beatty S. Plasma and macular responses to lutein supplement in subjects with and without age-related maculopathy: a pilot study. Exp Eye Res. 2004;79:21–27. doi: 10.1016/j.exer.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 89.Koo E, Neuringer M, SanGiovanni JP. Macular xanthophylls, lipoprotein-related genes, and age-related macular degeneration. Am J Clin Nutr. 2014;100(Suppl. 1):336–46S. doi: 10.3945/ajcn.113.071563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kowluru RA, Menon B, Gierhart DL. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Investig Ophthalmol Vis Sci. 2008;49:1645–51. doi: 10.1167/iovs.07-0764. [DOI] [PubMed] [Google Scholar]
- 91.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 92.Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- 93.Kupfer C. Bowman lecture. Trans Ophthalmol Soc. Part 1. Vol. 104. UK: 1985. The conquest of cataract: a global challenge; pp. 1–10. [PubMed] [Google Scholar]
- 94.Kvansakul J, Rodriguez-Carmona M, Edgar D, Barker F, Kopcke W, et al. Supplementation with the carotenoids lutein or zeaxanthin improves human visual performance. Ophthalmic Physiol Opt. 2006;26:362–71. doi: 10.1111/j.1475-1313.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 95.Landrum JT, Bone RA. Mechanistic evidence for eye diseases and carotenoids. In: Krinsky NI, Mayne ST, Sies H, editors. Carotenoids in Health and Disease. New York: Marcel Dekker; 2004. pp. 445–72. [Google Scholar]
- 96.Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Exp Eye Res. 1997;65:57–62. doi: 10.1006/exer.1997.0309. [DOI] [PubMed] [Google Scholar]
- 97.Landrum JT, Bone RA, Neuringer M, Cao Y. Macular pigment from discovery to function. 2013:1–22. See Ref. 98. [Google Scholar]
- 98.Landrum JT, Nolan JM, editors. Carotenoids and Retinal Disease. Boca Raton, FL: CRC; 2013. [Google Scholar]
- 99.LaRowe TL, Mares JA, Snodderly DM, Klein ML, Wooten BR, Chappell R. Macular pigment density and age-related maculopathy in the Carotenoids in Age-Related Eye Disease Study. An ancillary study of the Women's Health Initiative Ophthalmology. 2008;115:876–83.e1. doi: 10.1016/j.ophtha.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 100.Le Marchand L, Hankin JH, Bach F, Kolonel LN, Wilkens LR, et al. An ecological study of diet and lung cancer in the South Pacific. Int J Cancer. 1995;63:18–23. doi: 10.1002/ijc.2910630105. [DOI] [PubMed] [Google Scholar]
- 101.Lee HS, Cho YH, Park J, Shin HR, Sung MK. Dietary intake of phytonutrients in relation to fruit and vegetable consumption in Korea. J Acad Nutr Diet. 2013;113:1194–99. doi: 10.1016/j.jand.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 102.Li B, Vachali PP, Gorusupudi A, Shen Z, Sharifzadeh H, et al. Inactivity of human |3, |3-carotene-9/,10/-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment. PNAS. 2014;111:10173–78. doi: 10.1073/pnas.1402526111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li S, Lo A. Lutein protects RGC-5 cells against hypoxia and oxidative stress. Int J Mol Sci. 2010;11:2109–17. doi: 10.3390/ijms11052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lietz G. Host factors that affect carotenoid metabolism. In: Tanumihardjo SA, editor. Carotenoids and Human Health. New York: Springer; 2013. pp. 129–40. [Google Scholar]
- 105.Lietz G, Mulokozi G, Henry JC, Tomkins AM. Xanthophyll and hydrocarbon carotenoid patterns differ in plasma and breast milk of women supplemented with red palm oil during pregnancy and lactation. J Nutr. 2006;136:1821–27. doi: 10.1093/jn/136.7.1821. [DOI] [PubMed] [Google Scholar]
- 106.Lipkie TE, Banavara D, Shah B, Morrow AL, McMahon RJ, et al. Caco-2 accumulation of lutein is greater from human milk than from infant formula despite similar bioaccessibility. Mol Nutr Food Res. 2014;58:2014–22. doi: 10.1002/mnfr.201400126. [DOI] [PubMed] [Google Scholar]
- 107.Lipkie TE, Morrow AL, Jouni ZE, McMahon RJ, Ferruzzi MG. Longitudinal survey of carotenoids in human milk from urban cohorts in China, Mexico, and the USA. PLOS ONE. 2015;10:e0127729. doi: 10.1371/journal.pone.0127729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu CL, Huang YS, Hosokawa M, Miyashita K, Hu ML. Inhibition of proliferation of a hepatoma cell line by fucoxanthin in relation to cell cycle arrest and enhanced gap junctional intercellular communication. Chem Biol Interact. 2009;182:165–72. doi: 10.1016/j.cbi.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 109.Liu R, Wang T, Zhang B, Qin L, Wu C, et al. Lutein and zeaxanthin supplementation and association with visual function in age-related macular degeneration. Investig Ophthalmol Vis Sci. 2015;56:252–58. doi: 10.1167/iovs.14-15553. [DOI] [PubMed] [Google Scholar]
- 110.Liu XH, Yu RB, Liu R, Hao ZX, Han CC, et al. Association between lutein and zeaxanthin status and the risk of cataract: a meta-analysis. Nutrients. 2014;6:452–65. doi: 10.3390/nu6010452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Z, Meyers K, Johnson E, Snodderly D, Tinker L, et al. Exposure to lutein in infancy via breast milk and later life macular pigment optical density. Investig Ophthal Vis Sci. 2015;56:192. [Google Scholar]
- 112.Lobo GP, Amengual J, Li HN, Golczak M, Bonet ML, et al. β,β-carotene decreases peroxisome proliferator receptor gamma activity and reduces lipid storage capacity of adipocytes in a β,β-carotene oxygenase 1-dependent manner. J Biol Chem. 2010;285:27891–99. doi: 10.1074/jbc.M110.132571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Loughman J, Nolan JM, Howard AN, Connolly E, Meagher K, Beatty S. The impact of macular pigment augmentation on visual performance using different carotenoid formulations. Investig Ophthalmol Vis Sci. 2012;53:7871–80. doi: 10.1167/iovs.12-10690. [DOI] [PubMed] [Google Scholar]
- 114.Ma L, Hao ZX, Liu RR, Yu RB, Shi Q, Pan JP. A dose-response meta-analysis of dietary lutein and zeaxanthin intake in relation to risk of age-related cataract. Graefes Arch Clin Exp Ophthalmol. 2014;252:63–70. doi: 10.1007/s00417-013-2492-3. [DOI] [PubMed] [Google Scholar]
- 115.Ma L, Lin XM, Zou ZY, Xu XR, Li Y, Xu R. A 12-week lutein supplementation improves visual function in Chinese people with long-term computer display light exposure. Br J Nutr. 2009;102:186–90. doi: 10.1017/S0007114508163000. [DOI] [PubMed] [Google Scholar]
- 116.Ma L, Yan SF, Huang YM, Lu XR, Qian F, et al. Effect of lutein and zeaxanthin on macular pigment and visual function in patients with early age-related macular degeneration. Ophthalmology. 2012;119:2290–97. doi: 10.1016/j.ophtha.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 117.Manzoni P, Stolfi I, Pedicino R, Vagnarelli F, Mosca F, et al. Human milk feeding prevents retinopathy of prematurity (ROP) in preterm VLBW neonates. Early Hum Dev. 2013;89(Suppl. 1):S64–68. doi: 10.1016/S0378-3782(13)70019-7. [DOI] [PubMed] [Google Scholar]
- 118.Mares-Perlman JA, Fisher AI, Klein R, Palta M, Block G, et al. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third National Health and Nutrition Examination Survey. Am J Epidemiol. 2001;153:424–32. doi: 10.1093/aje/153.5.424. [DOI] [PubMed] [Google Scholar]
- 119.Mares J. Food antioxidants to prevent cataract. JAMA. 2015;313:1048–49. doi: 10.1001/jama.2014.15301. [DOI] [PubMed] [Google Scholar]
- 120.Mares J, Meyers K, Liu Z, Gehrs K, Iyengar S, et al. Joint phenotypic and genotypic predictors of macular pigment optical density. Investig Ophthal Vis Sci. 2015;56:1082. Abstr. [Google Scholar]
- 121.Mares JA. Relationships of lutein and zeaxanthin to age-related macular degeneration: epidemio-logical evidence. 2013:63–74. See Ref. 98. [Google Scholar]
- 122.Mares JA, LaRowe TL, Snodderly DM, Moeller SM, Gruber MJ, et al. Predictors of optical density of lutein and zeaxanthin in retinas of older women in the Carotenoids in Age-Related Eye Disease Study, an ancillary study of the Women's Health Initiative. Am J Clin Nutr. 2006;84:1107–22. doi: 10.1093/ajcn/84.5.1107. [DOI] [PubMed] [Google Scholar]
- 123.Mares JA, Millen AE, Meyers K. Diet and supplements and the prevention and treatment of eye diseases. In: Coulston AM, Boushey CJ, Ferruzzi M, editors. Nutrition in the Prevention and Treatment of Disease. San Diego, CA: Elsevier; 2012. pp. 341–72. [Google Scholar]
- 124.Mares JA, Voland RP, Sondel SA, Millen AE, Larowe T, et al. Healthy lifestyles related to subsequent prevalence of age-related macular degeneration. Arch Ophthalmol. 2011;129:470–80. doi: 10.1001/archophthalmol.2010.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McCorkle S, Raine L, Hammond B, Renzi-Hammond L, Hillman C, Khan N. Reliability of heterochromatic flicker photometry in measuring macular pigment optical density among preadolescent children. Foods. 2015;4:594–604. doi: 10.3390/foods4040594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meyers KJ, Johnson EJ, Bernstein PS, Iyengar SK, Engelman CD, et al. Genetic determinants of macular pigments in women of the Carotenoids in Age-Related Eye Disease Study. Investig Ophthalmol Vis Sci. 2013;54:2333–45. doi: 10.1167/iovs.12-10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Meyers KJ, Liu Z, Millen AE, Iyengar SK, Blodi BA, et al. Joint associations of diet, lifestyle, and genes with age-related macular degeneration. Ophthalmology. 2015;122:2286–94. doi: 10.1016/j.ophtha.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meyers KJ, Liu Z, Wallace R, Tinker L, Engelman C, et al. Correlates of metabolic syndrome are associated with low macular pigment despite moderate to high intakes of macular carotenoids. Investig Ophthalmol Vis Sci. 2014;55:6009. Abstr. [Google Scholar]
- 129.Meyers KJ, Liu Z, Wang S, Klein M, Wallace RT, et al. Phenotypic and genotypic predictors of low retinal response to diets high in lutein. Investig Ophthal Vis Sci. 2015;56:1083. Abstr. [Google Scholar]
- 130.Meyers KJ, Mares JA, Igo RP, Jr, Truitt B, Liu Z, et al. Genetic evidence for role of carotenoids in age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS) Investig Ophthalmol Vis Sci. 2014;55:587–99. doi: 10.1167/iovs.13-13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moeller SM, Parekh N, Tinker L, Ritenbaugh C, Blodi B, et al. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-Related Eye Disease Study (CAREDS): ancillary study of the Women's Health Initiative. Arch Ophthalmol. 2006;124:1151–62. doi: 10.1001/archopht.124.8.1151. [DOI] [PubMed] [Google Scholar]
- 132.Mukai M, Hayashi Y, Kitadate Y, Shigenobu S, Arita K, Kobayashi S. MAMO, a maternal BTB/POZ-Zn-finger protein enriched in germline progenitors, is required for the production of functional eggs in Drosophila. Mech Dev. 2007;124:570–83. doi: 10.1016/j.mod.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 133.Murray IJ, Makridaki M, van der Veen RL, Carden D, Parry NR, Berendschot TT. Lutein supplementation over a one-year period in early AMD might have a mild beneficial effect on visual acuity: the CLEAR study. Investig Ophthalmol Vis Sci. 2013;54:1781–88. doi: 10.1167/iovs.12-10715. [DOI] [PubMed] [Google Scholar]
- 134.Neuringer M, Sandstrom MM, Johnson EJ, Snodderly DM. Nutritional manipulation of primate retinas, I: effects of lutein or zeaxanthin supplements on serum and macular pigment in xanthophyll-free rhesus monkeys. Investig Ophthalmol Vis Sci. 2004;45:3234–43. doi: 10.1167/iovs.02-1243. [DOI] [PubMed] [Google Scholar]
- 135.Nolan JM, Loughman J, Akkali MC, Stack J, Scanlon G, et al. The impact of macular pigment augmentation on visual performance in normal subjects: COMPASS. Vis Res. 2011;51:459–69. doi: 10.1016/j.visres.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 136.Nolan JM, Stack J, Mellerio J, Godhinio M, O'Donovan O, et al. Monthly consistency of macular pigment optical density and serum concentrations of lutein and zeaxanthin. Curr Eye Res. 2006;31:199–213. doi: 10.1080/02713680500514677. [DOI] [PubMed] [Google Scholar]
- 137.Obana A, Hiramitsu T, Gohto Y, Ohira A, Mizuno S, et al. Macular carotenoid levels of normal subjects and age-related maculopathy patients in a Japanese population. Ophthalmology. 2008;115:147–57. doi: 10.1016/j.ophtha.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 138.Obana A, Tanito M, Gohto Y, Okazaki S, Gellermann W, Bernstein PS. Changes in macular pigment optical density and serum lutein concentration in Japanese subjects taking two different lutein supplements. PLOS ONE. 2015;10:e0139257. doi: 10.1371/journal.pone.0139257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Olmedilla B, Granado F, Blanco I, Vaquero M. Lutein, but not alpha-tocopherol, supplementation improves visual function in patients with age-related cataracts: a 2-y double-blind, placebo-controlled pilot study. Nutrition. 2003;19:21–24. doi: 10.1016/s0899-9007(02)00861-4. [DOI] [PubMed] [Google Scholar]
- 140.Owsley C. Aging and vision. Vis Res. 2011;51:1610–22. doi: 10.1016/j.visres.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Owsley C. Comparison of visual function in older eyes in the earliest stages of age-related macular degeneration to those in normal macular health. Curr Eye Res. 2016;41:266–72. doi: 10.3109/02713683.2015.1011282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Owsley C, Huisingh C, Jackson GR, Curcio CA, Szalai AJ, et al. Associations between abnormal rod-mediated dark adaptation and health and functioning in older adults with normal macular health. Investig Ophthalmol Vis Sci. 2014;55:4776–89. doi: 10.1167/iovs.14-14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Palczewski G, Amengual J, Hoppel CL, von Lintig J. Evidence for compartmentalization of mammalian carotenoid metabolism. FASEB J. 2014;28:4457–69. doi: 10.1096/fj.14-252411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Patryas L, Parry NR, Carden D, Aslam T, Murray IJ. The association between dark adaptation and macular pigment optical density in healthy subjects. Graefes Arch Clin Exp Ophthalmol. 2014;252:657–63. doi: 10.1007/s00417-014-2564-z. [DOI] [PubMed] [Google Scholar]
- 145.Perrone S, Longini M, Marzocchi B, Picardi A, Bellieni CV, et al. Effects of lutein on oxidative stress in the term newborn: a pilot study. Neonatology. 2010;97:36–40. doi: 10.1159/000227291. [DOI] [PubMed] [Google Scholar]
- 146.Picone S, Ritieni A, Fabiano A, Troise AD, Graziani G, et al. Arterial cord blood lutein levels in preterm and term healthy newborns are sex and gestational age dependent. Clin Biochem. 2012;45:1558–63. doi: 10.1016/j.clinbiochem.2012.07.109. [DOI] [PubMed] [Google Scholar]
- 147.Piermarocchi S, Saviano S, Parisi V, Tedeschi M, Panozzo G, et al. Carotenoids in Age-Related Maculopathy Italian Study (CARMIS): two-year results of a randomized study. Eur J Ophthalmol. 2012;22:216–25. doi: 10.5301/ejo.5000069. [DOI] [PubMed] [Google Scholar]
- 148.Provis JM, Penfold PL, Cornish EE, Sandercoe TM, Madigan MC. Anatomy and development of the macula: specialisation and the vulnerability to macular degeneration. Clin Exp Optom. 2005;88:269–81. doi: 10.1111/j.1444-0938.2005.tb06711.x. [DOI] [PubMed] [Google Scholar]
- 149.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–93. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ramdas WD, Wolfs RC, Kiefte-de Jong JC, Hofman A, de Jong PT, et al. Nutrient intake and risk of open-angle glaucoma: the Rotterdam Study. Eur J Epidemiol. 2012;27:385–93. doi: 10.1007/s10654-012-9672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rapp LM, Maple SS, Choi JH. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Investig Ophthalmol Vis Sci. 2000;41:1200–9. [PubMed] [Google Scholar]
- 152.Renzi LM, Hammond BR., Jr The effect of macular pigment on heterochromatic luminance contrast. Exp Eye Res. 2010;91:896–900. doi: 10.1016/j.exer.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 153.Renzi LM, Hammond BR., Jr The relation between the macular carotenoids, lutein and zeaxanthin, and temporal vision. Ophthalmic Physiol Opt. 2010;30:351–57. doi: 10.1111/j.1475-1313.2010.00720.x. [DOI] [PubMed] [Google Scholar]
- 154.Resnikoff S, Pascolini D, Etya'aale D, Kocur I, Pararajasegaram R, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51. [PMC free article] [PubMed] [Google Scholar]
- 155.Richer S, Stiles W, Statkute L, Pulido J, Frankowski J, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial) Optometry. 2004;75:216–30. doi: 10.1016/s1529-1839(04)70049-4. [DOI] [PubMed] [Google Scholar]
- 156.Richer SP, Stiles W, Graham-Hoffman K, Levin M, Ruskin D, et al. Randomized, double-blind, placebo-controlled study of zeaxanthin and visual function in patients with atrophic age-related macular degeneration: the Zeaxanthin and Visual Function study (ZVF) FDA IND #78, 973. Optometry. 2011;82:667–80.e6. doi: 10.1016/j.optm.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 157.Rubin GS, Roche KB, Prasada-Rao P, Fried LP. Visual impairment and disability in older adults. Optom Vis Sci. 1994;71:750–60. doi: 10.1097/00006324-199412000-00005. [DOI] [PubMed] [Google Scholar]
- 158.Rubin LP, Chan GM, Barrett-Reis BM, Fulton AB, Hansen RM, et al. Effect of carotenoid supplementation on plasma carotenoids, inflammation and visual development in preterm infants. J Perinatol. 2012;32:418–24. doi: 10.1038/jp.2011.87. [DOI] [PubMed] [Google Scholar]
- 159.Sabour-Pickett S, Beatty S, Connolly E, Loughman J, Stack J, et al. Supplementation with three different macular carotenoid formulations in patients with early age-related macular degeneration. Retina. 2014;34:1757–66. doi: 10.1097/IAE.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 160.Sandberg MA, Johnson EJ, Berson EL. The relationship of macular pigment optical density to serum lutein in retinitis pigmentosa. Investig Ophthalmol Vis Sci. 2010;51:1086–91. doi: 10.1167/iovs.09-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.SanGiovanni JP, Neuringer M. The putative role of lutein and zeaxanthin as protective agents against age-related macular degeneration: promise of molecular genetics for guiding mechanistic and translational research in the field. Am J Clin Nutr. 2012;96:1223–33s. doi: 10.3945/ajcn.112.038240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sasaki M, Ozawa Y, Kurihara T, Noda K, Imamura Y, et al. Neuroprotective effect of anantioxidant, lutein, during retinal inflammation. Investig Ophthalmol Vis Sci. 2009;50:1433–39. doi: 10.1167/iovs.08-2493. [DOI] [PubMed] [Google Scholar]
- 163.Scanlon G, Connell P, Ratzlaff M, Foerg B, McCartney D, et al. Macular pigment optical density is lower in type 2 diabetes, compared with type 1 diabetes and normal controls. Retina. 2015;35:1808–16. doi: 10.1097/IAE.0000000000000551. [DOI] [PubMed] [Google Scholar]
- 164.Schalch W, Cohn W, Barker FM, Kopcke W, Mellerio J, et al. Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin—the LUXEA (LUtein Xanthophyll Eye Accumulation) study. Arch Biochem Biophys. 2007;458:128–35. doi: 10.1016/j.abb.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 165.Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA. 1994;272:1413–20. [PubMed] [Google Scholar]
- 166.Shmarakov IO, Yuen JJ, Blaner WS. Carotenoid metabolism and enzymology. In: Tanumihardjo SA, editor. Carotenoids and Human Health. New York: Humana; 2013. pp. 29–56. [Google Scholar]
- 167.Sies H, Stahl W. Carotenoids and intercellular communication via gap junctions. Int J Vitam Nutr Res. 1997;67:364–67. [PubMed] [Google Scholar]
- 168.Singh RS, Kim JE. Ocular hypertension following intravitreal anti-vascular endothelial growth factor agents. Drugs Aging. 2012;29:949–56. doi: 10.1007/s40266-012-0031-2. [DOI] [PubMed] [Google Scholar]
- 169.Smiddy WE. Relative cost of a line of vision in age-related macular degeneration. Ophthalmology. 2007;114:847–54. doi: 10.1016/j.ophtha.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 170.Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr. 1995;62:1448–61S. doi: 10.1093/ajcn/62.6.1448S. [DOI] [PubMed] [Google Scholar]
- 171.Snodderly DM, Auran JD, Delori FC. The macular pigment. II Spatial distribution in primate retinas. Investig Ophthalmol Vis Sci. 1984;25:674–85. [PubMed] [Google Scholar]
- 172.Snodderly DM, Brown PK, Delori FC, Auran JD. The macular pigment. I Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Investig Ophthalmol Vis Sci. 1984;25:660–73. [PubMed] [Google Scholar]
- 173.Stringham JM, Garcia PV, Smith PA, Hiers PL, McLin LN, et al. Macular pigment and visual performance in low-light conditions. Investig Ophthalmol Vis Sci. 2015;56:2459–68. doi: 10.1167/iovs.14-15716. [DOI] [PubMed] [Google Scholar]
- 174.Stringham JM, Garcia PV, Smith PA, McLin LN, Foutch BK. Macular pigment and visual performance in glare: benefits for photostress recovery, disability glare, and visual discomfort. Investig Ophthalmol Vis Sci. 2011;52:7406–15. doi: 10.1167/iovs.10-6699. [DOI] [PubMed] [Google Scholar]
- 175.Stringham JM, Snodderly DM. Enhancing performance while avoiding damage: a contribution of macular pigment. Investig Ophthalmol Vis Sci. 2013;54:6298–306. doi: 10.1167/iovs.13-12365. [DOI] [PubMed] [Google Scholar]
- 176.Tang L, Zhang Y, Jiang Y, Willard L, Ortiz E, et al. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp Biol Med (Maywood) 2011;236:1051–63. doi: 10.1258/ebm.2011.010400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tian Y, Kijlstra A, Webers CA, Berendschot TT. Lutein and factor D: two intriguing players in the field of age-related macular degeneration. Arch Biochem Biophys. 2015;572:49–53. doi: 10.1016/j.abb.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 179.Trieschmann M, Beatty S, Nolan JM, Hense HW, Heimes B, et al. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: the LUNA study. Exp Eye Res. 2007;84:718–28. doi: 10.1016/j.exer.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 180.U.S. Dep. Agric., Agric. Res. Serv. What We Eat in America, NHANES 011–2012. Washington, DC: USDA ARS; 2014. http://www.ars.usda.gov/Services/docs.htm?docid=13793. [Google Scholar]
- 181.van der Made SM, Kelly ER, Berendschot TT, Kijlstra A, Lutjohann D, Plat J. Consuming a buttermilk drink containing lutein-enriched egg yolk daily for 1 year increased plasma lutein but did not affect serum lipid or lipoprotein concentrations in adults with early signs of age-related macular degeneration. J Nutr. 2014;144:1370–77. doi: 10.3945/jn.114.195503. [DOI] [PubMed] [Google Scholar]
- 182.Vishwanathan R, Goodrow-Kotyla EF, Wooten BR, Wilson TA, Nicolosi RJ. Consumption of 2 and 4 egg yolks/d for 5 wk increases macular pigment concentrations in older adults with low macular pigment taking cholesterol-lowering statins. Am J Clin Nutr. 2009;90:1272–79. doi: 10.3945/ajcn.2009.28013. [DOI] [PubMed] [Google Scholar]
- 183.Vishwanathan R, Johnson EJ. Lutein and zeaxanthin and eye disease. In: Tanumihardjo SA, editor. Carotenoids in Human Health. New York: Springer Sci; 2014. pp. 215–35. [Google Scholar]
- 184.Vishwanathan R, Neuringer M, Snodderly DM, Schalch W, Johnson EJ. Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutr Neurosci. 2013;16:21–29. doi: 10.1179/1476830512Y.0000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vishwanathan R, Schalch W, Johnson EJ. Macular pigment carotenoids in the retina and occipital cortex are related in humans. Nutr Neurosci. 2016;19:95–101. doi: 10.1179/1476830514Y.0000000141. [DOI] [PubMed] [Google Scholar]
- 186.Wang JJ, Buitendijk GH, Rochtchina E, Lee KE, Klein BE, et al. Genetic susceptibility, dietary antioxidants, and long-term incidence of age-related macular degeneration in two populations. Ophthalmology. 2014;121:667–75. doi: 10.1016/j.ophtha.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 187.Weigert G, Kaya S, Pemp B, Sacu S, Lasta M, et al. Effects of lutein supplementation on macular pigment optical density and visual acuity in patients with age-related macular degeneration. Investig Ophthalmol Vis Sci. 2011;52:8174–78. doi: 10.1167/iovs.11-7522. [DOI] [PubMed] [Google Scholar]
- 188.Widjaja-Adhi MA, Lobo GP, Golczak M, Von Lintig J. A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption. Hum Mol Genet. 2015;24:3206–19. doi: 10.1093/hmg/ddv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Widomska J, Subczynski WK. Why has nature chosen lutein and zeaxanthin to protect the retina? J Clin Exp Ophthalmol. 2014;5:326. doi: 10.4172/2155-9570.1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wooten B, Hammond B. Macular pigment: influences on visual acuity and visibility. Prog Retin Eye Res. 2002;21:225–40. doi: 10.1016/s1350-9462(02)00003-4. [DOI] [PubMed] [Google Scholar]
- 191.Wu J, Cho E, Willett WC, Sastry SM, Schaumberg DA. Intakes of lutein, zeaxanthin, and other carotenoids and age-related macular degeneration during 2 decades of prospective follow-up. JAMA Ophthalmol. 2015;133:1415–24. doi: 10.1001/jamaophthalmol.2015.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yao Y, Qiu QH, Wu XW, Cai ZY, Xu S, Liang XQ. Lutein supplementation improves visual performance in Chinese drivers: 1-year randomized, double-blind, placebo-controlled study. Nutrition. 2013;29:958–64. doi: 10.1016/j.nut.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 193.Yeum KJ, Shang FM, Schalch WM, Russell RM, Taylor A. Fat-soluble nutrient concentrations in different layers of human cataractous lens. Curr Eye Res. 1999;19:502–5. doi: 10.1076/ceyr.19.6.502.5282. [DOI] [PubMed] [Google Scholar]
- 194.Yonova-Doing E, Hysi PG, Venturini C, Williams KM, Nag A, et al. Candidate gene study of macular response to supplemental lutein and zeaxanthin. Exp Eye Res. 2013;115:172–77. doi: 10.1016/j.exer.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yu H, Wark L, Ji H, Willard L, Jaing Y, et al. Dietary wolfberry upregulates carotenoid metabolic genes and enhances mitochondrial biogenesis in the retina of db/db diabetic mice. Mol Nutr Food Res. 2013;57:1158–69. doi: 10.1002/mnfr.201200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yuodelis C, Hendrickson A. A qualitative and quantitative analysis of the human fovea during development. Vis Res. 1986;26:847–55. doi: 10.1016/0042-6989(86)90143-4. [DOI] [PubMed] [Google Scholar]
- 197.Zaripheh S, Erdman JW., Jr Factors that influence the bioavailability of xanthophylls. J Nutr. 2002;132:531–34S. doi: 10.1093/jn/132.3.531S. [DOI] [PubMed] [Google Scholar]
- 198.Zhang C, Wang Z, Zhao J, Li Q, Huang C, et al. Neuroprotective effect of lutein on nmda-induced retinal ganglion cell injury in rat retina. Cell Mol Neurobiol. 2015 doi: 10.1007/s10571-015-0231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhao D, Cho J, Kim MH, Friedman D, Guallar E. Diabetes, glucose metabolism, and glaucoma: the 2005–2008 National Health and Nutrition Examination Survey. PLOS ONE. 2014;9:e112460. doi: 10.1371/journal.pone.0112460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthal-mol. 2012;60:428–31. doi: 10.4103/0301-4738.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zimmer JP, Hammond BR., Jr Possible influences of lutein and zeaxanthin on the developing retina. Clin Ophthalmol. 2007;1:25–35. [PMC free article] [PubMed] [Google Scholar]