Abstract
It is now widely recognized that social bonds are critical to human health and well-being. One of the most important social bonds is the attachment relationship between two adults, known as the pair bond. The pair bond involves many characteristics that are inextricably linked to quality of health, including providing a secure psychological base and acting as a social buffer against stress. The majority of our knowledge about the neurobiology of pair bonding comes from studies of a socially monogamous rodent, the prairie vole (Microtus ochrogaster), and from human imaging studies, which inherently lack control. Here, we first review what is known of the neurobiology of pair bonding from humans and prairie voles. We then present a summary of the studies we have conducted in titi monkeys (Callicebus cupreus)—a species of socially monogamous New World primates. Finally, we construct a neural model based on the location of neuropeptide receptors in the titi monkey brain, as well as the location of neural changes in our imaging studies, with some basic assumptions based on the prairie vole model. In this model, we emphasize the role of visual mating stimuli as well as contributions of the dopaminergic reward system and a strong role for the lateral septum. This model represents an important step in understanding the neurobiology of social bonds in non-human primates, which will in turn facilitate a better understanding of these mechanisms in humans.
Keywords: pair bond, social monogamy, oxytocin, vasopressin, opioids, dopamine, prairie vole, relationships
Social Bonds are Fundamental to Mammalian Well-being
Social bonding lays the foundation for a range of adaptive processes within a species. Bonding facilitates group-level cooperation [1,2], fortifies the ability to cope with both internal and external stressors, and enhances physical health and psychological well-being [3-5]. Among the social bonds exhibited within species, research spanning multiple disciplines has converged on the conclusion that the pair bond may be particularly central to adaptive functioning [4,6]. Indeed, the quality of a pair bond is robustly associated with crucial survival outcomes such as faster recovery from injury [7], decreased risk of infection [8], and lower mortality rates [9,10]. Yet, for all that is known about the influence pair bonds exert on psychophysiological processes, comparatively little is known about the psychophysiological processes that give rise to, and maintain, those pair bonds.
The study of pair bonds is inevitably comparative [11], in part because the cross-section of research concerning social bonds and physiology is at an interdisciplinary impasse: on one hand, the vast majority of work investigating the psychological features of the pair bond has been conducted with human subjects (e.g., investment, intimacy, attachment [12]); on the other hand, the most rigorous attempts to identify the biological substrates of the pair bond have been undertaken with non-human animals whose neurobiology may vary widely from that of humans (e.g., prairie voles, Microtus ochrogaster [13]). The present review, therefore, aims to advance the study of the pair bond by centering our investigation on a species that exhibits comparatively more neurological and social similarities to humans: the titi monkey (Callicebus cupreus). In doing so, we expect that the proposed neurobiological model of pair bonding presented here will be more readily, and appropriately, generalized to humans (Figure 1).
Figure 1.
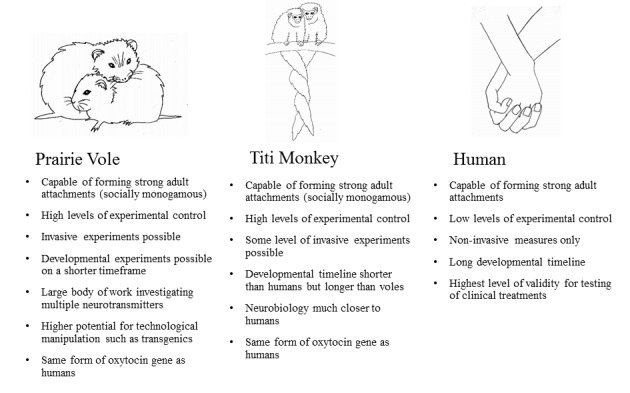
Summary of advantages and disadvantages of studying the neurobiology of pair bonding in prairie voles, titi monkeys, and humans.
A pair bond consists of a constellation of behavioral, biological, cognitive, and affective features. Beginning with the features that are most consistent across species, pair bonds are typically dyadic and formed between two adult conspecifics. Pair bonds may also be sexually monogamous, but the present work posits that a more consistent feature is the tendency toward, but not strict adherence to, sexual exclusivity. That is, whether referring to prairie voles, titi monkeys, or humans, those in a pair bond are almost always one another’s primary sexual partner, but they may also have secondary or tertiary sexual relationships. As such, it is the long-term, enduring nature of both social and sexual contact that distinguishes a pair bond from other relationships [12,14].
Pair bonds developed during adulthood are thought to stem from the attachment system established early in life [15,16]. Bowlby’s attachment theory [17] proposes that this biobehavioral attachment system supports proximity seeking between infants and their primary caregivers; this is thought to promote caregiver investment and offspring survival. Similarly, pair bonded partners across a range of species demonstrate a degree of psychological attachment to one another [18]. Accordingly, pair bonded partners usually develop a preference for their partner compared to alternative mates [19,20], seek and maintain proximity to one another [15], experience distress upon separation from one another [16], serve as a safe base for exploration [21], and act as a buffer against stressful situations [22]. Additionally, many species have evolved a repertoire of behaviors geared toward maintaining the stability of the pair bond, such as mate guarding, cohabitation, merging resources and resource provision, and social support.
Prairie Voles are the Main Rodent Model of Pair Bonding
Animal models are crucial to the study of pair bonds, as they enable researchers to experimentally manipulate and test specific neurobiological pathways and mechanisms using more invasive techniques than are allowable in humans. The premier model for research on mammalian pair bonding is the prairie vole (Microtus ochrogaster). Prairie voles are socially monogamous rodents which form stable female-male pair bonds in the field and in the laboratory [23-25], as well as in semi-free-ranging conditions [26]. This behavior, combined with their quick maturation and easy habituation to breeding in the laboratory, makes them an ideal model for basic research.
The timeline for the formation of pair bonds has been well studied in voles. In typical laboratory conditions, female prairie voles will reliably develop a selective preference for their partner over an unfamiliar potential mate after six hours of cohabitation, while males will form a preference for their partner after 24 hours of cohabitation. This 'partner preference’ is used as a behavioral indicator of the formation of a pair bond in this species [27-29]. Even in the absence of gonadal hormones [27,29,30], both sexes are capable of forming partner preferences through cohabitation alone, but mating further facilitates pair bond formation in both sexes [13,27]. For example, mating and pregnancy are necessary to elicit a marked increase in selective aggression in males, but not females [31], after two weeks of pairing [32,33]. Once formed, a pair bond often persists for the duration of the vole’s lifetime. It is rare for individuals of either sex to form a new bond following the death of a partner in the field [13]. Given the detailed body of work on pair bond formation in prairie voles, this model has largely informed our consideration of the potential neuroendocrine mechanisms that contribute to formation and maintenance of the pair bond in other socially monogamous mammals, including titi monkeys and humans.
Oxytocin and Vasopressin Play Key Roles in Prairie Vole and Human Pair Bonding
The nonapeptides oxytocin (OT) and arginine vasopressin (AVP), which are implicated in both parent-infant bonds and adult pair bonds across a number of species, appear to be key molecular mediators of mammalian pair bonding [34]. OT has a single receptor type (OTR) [35]; AVP has several receptor types, but almost certainly influences social behaviors via the AVP V1a receptor (AVPR1a) subclass [36]. These receptors are encoded by the OT receptor gene (OXTR) and AVP V1a gene (AVPR1A).
The importance of OT and AVP in pair bonding has been well established in prairie voles [37]. In the past, it was thought that OT was responsible for female pair bonding and AVP for male pair bonding [28], however, our current understanding is more nuanced. Pharmacological blockade of either neuropeptide in either sex results in loss of the pair bond [30]. In females, the enhancement of OTRs in the NAc increases the rate of pair bond formation [38], whereas the reduction of OTRs in the NAc via interference RNA disrupts pair bond formation [39]. In males, OTRs in the NAc coordinate sensory and reward processing during the establishment of the pair bond [40]. However, an increase in AVPR1a in the ventral pallidum (VP) via viral vector is sufficient to facilitate the formation of a pair bond in otherwise socially promiscuous meadow vole males. Similar studies of AVPR1a in female voles have yet to be conducted [41].
An emerging literature using peripheral hormone measures, genotyping, brain imaging, and intranasal administration in humans also supports the involvement of OT and AVP in several facets of pair bonds, including the formation and maintenance of romantic relationships [42]. OT and AVP play documented roles in human sexual behavior, but they are also integral to a number of nonsexual features that distinguish pair bonds from other sorts of sexual relationships. For instance, studies have shown significantly higher plasma OT levels in newly formed couples than in single controls [43-45], with couples expressing higher OT more likely to remain paired six months later [44]. New couples with higher plasma OT also had higher interactive reciprocity (including social focus, positive affect, affectionate touch, and synchronized dyadic states) [44]. Moreover, women who reported frequent hugs and massages from their partners had higher baseline levels of plasma OT [46], and married couples who participated in a four-month relationship intervention involving warm touch showed increased levels of salivary OT [47]. These findings support the notion that the integration of physiological processes, including the OT system, with social behaviors such as eye-gaze, vocalization, and touch, support dyad-specific affiliations [48].
AVP is also associated with differing qualities of the pair bond. Specifically, among men in long-term relationships, polymorphic variants of the AVPR1A gene were associated with differences in self-reported relationship bonding and the frequency of relationship crises [49]. Furthermore, among married couples, higher plasma AVP levels were associated with less negative communication during an observed task [50] and greater self-reported attachment security [51].
Changes in the OT and AVP systems may further facilitate pair bond maintenance in humans and other species. For instance, when OT was nasally administered to men before viewing pictures of their romantic partner it enhanced the attractiveness of their partner, which in turn corresponded with stronger responses in brain regions associated with reward [52]. Intranasal OT administration also promoted greater distancing between romantically involved men and an attractive female experimenter [53]. Collectively, these findings suggest that OT and AVP may interact with reward regions in the brain to enhance the reward response associated with the romantic partner, while reducing the motivation to interact with other potential mates.
Neuroimaging Studies of Romantic Love in Humans Implicate Social and Reward Systems
There are relatively few published imaging studies that have investigated the neural correlates of the romantic love associated with pair bonding in humans [54,55], and as most were carried out on established couples, they focus on pair bond maintenance rather than formation. Despite this limitation, evidence points toward specific patterns of neural activation that accompany strong, romantic bonds. Specifically, the experience of romantic love is associated with heightened activity in dopaminergic-related brain areas often associated with reward and motivation systems, such as the ventral tegmental area (VTA), medial insula, anterior cingulate cortex (ACC), hippocampus (Hipp), striatum, nucleus accumbens (NAc), and hypothalamus (Hyp) [56-62]. Romantic love is also associated with increased functional connectivity between these subcortical regions (i.e., caudate nucleus, NAc, amygdala (Amyg), insula; [63]). These findings are consistent with the hypothesis that the mesolimbic dopaminergic system functions in conjunction with systems mediating emotion and memory to facilitate the mechanisms that enable humans and other mammals to enact behaviors that maintain pair bonds [13,32,64].
Akin to the pattern of activation seen in romantic love, there is also a corresponding pattern of deactivation in several brain areas, including the posterior cingulate cortex (PCC) and Amyg [57,58,62,65]. Activation of the PCC is associated with the experience of grief [66], and the region is thought to play a central role in the interaction between emotion and memory [67]. Similarly, activation of the Amyg is tied to processing a variety of emotions, particularly fear and anxiety [68]. Thus, being in the presence of, or thinking about, a romantic partner may reduce experiences of fear and other negative emotions in pair bonded individuals.
A study comparing patterns of brain activity showed significant overlap in the brain regions activated or deactivated by maternal and romantic love [58]. However, activation of the periaqueductal gray was specific to maternal love, which is consistent with rodent studies showing that lesions in this area severely reduce maternal behavior [69], and romantic love differentially activated the dentate gyrus, hippocampus (Hipp), and hypothalamus (Hyp). The greater activation observed in hypothalamic regions may reflect the sexually arousing nature of romantic love [70]. Together these findings support the proposition that infant-caregiver bonds and pair bonds share similar biological substrates—presumably because adult attachment evolved from the parent-child attachment system [71,72]—yet, these biological substrates also include divergent features that support the unique characteristics of infant-caregiver or pair bonds.
The Dopamine System Plays a Key Role in Prairie Vole and Human Pair Bonding
The mesolimbic dopamine system is an evolutionarily well-conserved system that is comprised of connections between the VTA, prefrontal cortex (PFC), NAc, VP, Amyg, Hipp, Hyp, and lateral septum (LS). A primary function of this system is to generate the motivation to seek rewards and to avoid aversive stimuli; this has influences on many different behavioral systems, including pair bonding. Although the main neurotransmitter connections of the mesolimbic dopamine system are GABAergic, glutamatergic, oxytocinergic, and dopaminergic [73], it is dopamine that is critical for reward processing within this system, and in regard to pair bonding. As mentioned previously, neural imaging evidence in humans has repeatedly shown heightened activation of these dopaminergic-related brain areas when romantic love is experienced.
Dopamine signaling in the NAc is important for the facilitation and maintenance of pair bonds in prairie voles [33,74]. For instance, dopamine is released in the NAc in response to mating, and this increase is necessary for the formation of a pair bond [74]. Studies show that using antagonists to block the activity of D2—but not D1—receptors is enough to prevent the formation of pair bonds in prairie voles [75], indicating the importance of D2 receptors in the establishment of pair bonds. D1 receptors on the other hand are implicated in the maintenance of pair bonds. D1 receptors are upregulated in the NAc following the formation of a pair bond, and the activity of these receptors contributes to maintenance by inducing aggressive behaviors in mated prairie voles towards opposite-sex strangers [74].
The Opioid System Plays a Key Role in Prairie Vole and Human Pair Bonding
Literature on mammalian social attachments supports the importance of opioids for the formation and maintenance of pair bonds. For example, removal of an attachment figure (usually the mother) has been found to decrease endogenous opioid levels and activation in several mammalian species, including dogs, guinea pigs, talapoins, and rhesus monkeys [76]. Thus, activation of μ and κ opioid receptors may facilitate different aspects of monogamous pair bonds due to their roles in affective responses.
Resendez and Aragona [77] suggest that μ opioid receptors are important for pair bond formation in prairie voles due to their regulation of pleasant affective responses. Affiliative behaviors, such as grooming, naturally release β-endorphins [78], and over-activation of μ opioid receptors reduces the need for additional grooming in primates [78] and side-to-side contact in prairie voles [79]. Conversely, pair bond maintenance may be facilitated by activation of κ opioid receptors due to their role in producing unpleasant affective responses to stressors; in other words, κ opioid receptors may mediate the dysphoric effects of separation from the pair mate [33]. In prairie voles, pair bond formation is prevented by either systemic administration of naltrexone (a non-specific opioid antagonist) or local administration of CTAP (a selective μ-opioid receptor antagonist) into the dorsal striatum, confirming the importance of opioids in pair bond formation [80]. In monogamous voles, contact is not increased by blockade of μ-opioid receptors [79]; however, in non-monogamous primates, physical contact does increase following opioid blockade [81]. It will be important to determine whether this difference in behavioral response to opioid antagonists is due to phylogenetic differences or differences in social structure.
The κ and μ opioid receptors located in the limbic regions are likely essential for various aspects of monogamous pair bonding. In particular, the cingulate gyrus, striatum, and mediodorsal thalamus may play different roles in attenuating emotional responses during pair bond formation. The cingulate gyrus is important for emotion regulation, and the presence of both μ and κ opioid receptors in this brain region may help to regulate differing responses to a pair mate versus an unfamiliar conspecific. For instance, activity in the cingulate gyrus is down regulated in humans viewing pictures of loved ones [82], whereas humans experiencing social rejection exhibit increased activity in the dorsal cingulate gyrus [83]. Meanwhile, μ opioid receptors in the striatum appear to be particularly associated with positive affect [84]. In sum, these opioid receptors may serve to regulate both hedonic and dysphoric aspects of pair bonding.
Pair Bond Challenges Activate Several Neuroendocrine Systems in Prairie Voles and Humans
While involvement in a pair bond can be very rewarding, it can involve challenges, such as separation or jealousy, that can lead to mental and physical distress [85]. For instance, in male prairie voles, partner separation leads to greater anxiety and depressive-like behaviors during the elevated plus maze and forced swim test respectively, as well as an increase in the density of OT, AVP, and corticotropin-releasing hormone immunoreactive cells in the PVN and the supraoptic nucleus (SON) of the Hyp [85]. Prairie voles also show elevated plasma corticosterone levels for up to four weeks after the loss of a partner, indicating the importance of the corticotropin-releasing-factor system in modulating stress and coping after the loss of the partner [85-87]. Although no changes in plasma OT or AVP are observed in response to separation in voles, humans exhibit sex-specific changes in OT and AVP in response to relationship distress [85,88]. For example, reporting negative aspects of their relationship results in elevated plasma AVP concentrations in men, but elevated plasma OT levels in women [89].
Much remains unknown about the neurobiology of partner loss. Human studies focusing on the neurobiology of grief suggest roles for a number of different regions in mediating this response. For example, cingulate cortex and cerebellum were activated in bereaved women in response to words associated with the death of their deceased husband, as well as in response to pictures of the deceased (in comparison to pictures of a stranger) [66]. Another study focusing on complicated grief (e.g. prolonged, unabated grief), in humans showed activation of the NAc following presentation of stimuli related to the death of the loved one, indicating that after loss, memories of the attachment may still be rewarding and thus may make it more difficult to move on [90]. Avoidance of negative experiences such as separation may help to maintain the pair bond; thus, they are important aspects of the pair bond to study in both humans and animal models.
Titi monkeys are a Novel, Non-human Primate Model for the Neurobiology of Pair Bonding
Titi monkeys are small, arboreal, New World monkeys (Figure 2) [91]. While they are not a common laboratory model, a colony of titi monkeys was established at the California National Primate Research Center in 1971 and exists as of 2017 [92]; for management procedures, see [93]. Although titi monkeys are not the only potential primate model for pair bonding, the alternatives possess a number of disadvantages. For example, the Callitrichidae (marmosets and tamarins), another group of small New World primates common in laboratories, has been characterized variously as monogamous, polyandrous, and flexible [94]. Evidence for a pair bond is also equivocal, in that stress can be buffered by a familiar non-pair mate, the brother [95]. Another potential laboratory model, the owl monkey (Aotus azarae), has a number of visual system adaptations associated with nocturnality, which differ significantly from humans [96]. Finally, New World monkeys show a large number of amino acid substitutions in the 9-amino acid structure of OT. Out of the available New World monkey models, only titi monkeys share the conserved form of OT with humans (Leu8-OT) [97]. All other potential socially monogamous primate models (ex. some lemurs, gibbons, and siamangs) are not held in laboratories, and many of these species are endangered.
Figure 2.

Titi monkey family; pair bonded male and female with infant. © Kathy West, California National Primate Research Center.
Like prairie voles, titi monkeys display a preference for their partner over other potential mates, which is a key behavioral indicator that the pair bond has been established [98,99]. Titi monkey pair bonds are characterized by coordinated behavior, allowing them to maintain proximity during feeding and travel, and to spend time in contact during resting periods—sitting side by side with their tails entwined [100]. Titi pairs also display territorial behavior, aggression towards unfamiliar animals who might represent a threat to the bond [101], behavioral and physiological distress upon involuntary separation from the pair mate [102], social buffering [103], and biparental care [104]. The exact timing of the emergence of these behaviors is still being studied in titi monkeys, and there is a great variety of responses when two unfamiliar titis (female and male) are paired in the laboratory [105]. However, these behaviors appear to be similar in the field and in the laboratory. [100,106]. Both wild and captive pairs display a close affiliative relationship [104,106], with most of the sexual interactions occurring within the bonded pair [106], and extra pair sexual encounters being brief [100]. In the wild, titi monkey groups are also composed of a mated pair and their offspring. Titi monkey pairs duet both in the field [100] and in the laboratory [107]. Males are the primary carriers of offspring both in the field [108] and in the laboratory [104], and the birth of an offspring results in less time in contact between pair mates in both contexts.
Imaging Studies in Titi Monkeys
Positron emission tomography (PET) scans co-registered with structural magnetic resonance imaging have been used to experimentally examine changes in regional and global cerebral glucose metabolism during the formation and maintenance of pair bonds in male titi monkeys. Forty-eight hours after pairing, male titi monkeys showed changes in cerebral glucose metabolism in the right NAc and VP—two regions predicted to be involved in pair bonding based on their dopaminergic innervation [105]. After being paired for one week, they showed global increases in cerebral glucose metabolism [109], and these gains were maintained or strengthened four months after pairing [109]. These global increases in cerebral glucose metabolism were driven by areas involved in motivation (NAc, caudate, putamen, VP) and by areas involved in emotion, reproduction, and social memory (Amyg, LS, PCC, medial preoptic area) [109].
OT and AVP May Play Key Roles in Titi Monkey Pair Bonding
The distributions of OTR and AVPR1a have been well described in the prairie vole brain [110,111]; however, studies investigating these receptors in primates have only recently been published [112,113]. In all non-human primates studied to date (rhesus macaques, titi monkeys, and common marmosets), OTR binding was far more restricted than in rodents [114].
Across these three primate species, OTR and AVPR1a binding were observed in brain areas that modulate visual and sensory stimuli, unlike many rodent species that have high densities in areas that process olfactory stimuli. This is consistent with the understanding that primates rely more heavily on visual information, whereas rodents depend primarily on olfactory cues to navigate their social environments. In titi monkeys, AVPR1a binding was present in the lateral geniculate nucleus of the thalamus (LGN), which transduces visual stimuli to downstream regions such as the superior colliculus (SC) [115], a region in which contains both OTR and AVPR1a in the titi monkey. Moreover, both receptor types were detected in the primary visual cortex (V1), an area that has bi-directional neural projections with the SC [115]. OTR and AVPR1a were also observed in the pulvinar nucleus (PUL) of the thalamus, a region that transmits retinal and SC information downstream to the Amyg [116]. These binding patterns are similar binding to those observed in the rhesus macaque [112], including AVPR1a binding in the primary and secondary visual cortices, which reaffirms the importance of processing social stimuli via visual pathways across primate species. Furthermore, all primates studied to date (including humans) show OTR expression in the nucleus basalis of Meynert, a cholinergic region related to attentional processes [114,117]. These findings point to an important role for visual cues in the development and maintenance of pair bonds.
While there are similarities, most areas that express OTR in titi monkeys differ from those observed in rhesus macaques, a primate species that does not form pair bonds. In titi monkeys, OTR expression was observed in brain regions that are important for learning and memory, including the presubiculum and two sub-regions of the hippocampus—CA1 and dentate gyrus [118]. OTR and AVPR1a binding were also found in other areas of the titi monkey limbic system; AVPR1a binding was observed in the central amygdala (CeA), and OTR in the LS. While the CeA is critical to fear conditioning [119], and its role in anxiety is modulated by OT and AVP [120,121], it is also a crucial region for social recognition [122], as is the LS [123].
As in rhesus monkeys and marmosets [124,125], AVPR1a expression was found to be more prevalent than OTR in the central nervous system of titi monkeys, suggesting that AVP may play a more widespread role in the social behavior of primates than might be expected from work in rodents. For example, AVPR1a binding was present in areas that are important for reward and reinforcement, whereas OTR binding was not. Specifically, dense AVPR1a binding was observed in the striatum, and in the substantia nigra, a dopaminergic area that projects into the striatum.
A pharmacological study in male titi monkeys administered intranasal AVP [126] at two doses previously shown increase AVP concentration in cerebrospinal fluid (CSF) when given intranasally in humans [127]. Males were then exposed to their partner, a strange female, or an empty cage in a sequential (non-simultaneous) fashion. Males treated with either dose demonstrated decreased latencies to approach both females, but the high dose of AVP increased the frequency with which they touched their partner’s cage over the stranger’s cage [126].
The Opioid and Dopamine Systems Play Key Roles in Titi Monkey Pair Bonding
Recent studies have explored the role of opioids in titi monkey pair bonding, and suggest that morphine, a μ opioid receptor agonist, may have an anxiolytic effect in titi monkeys, whereas naloxone, an opioid antagonist, may have an anxiogenic effect [78]. While separated from their mate, titi monkeys often display increased locomotion, cortisol, and isolation peeps [102]. Using a mate-separation paradigm, Ragen and colleagues found that cortisol increases induced by partner separation were highest following naloxone treatment, but that medium and high doses of morphine attenuated cortisol responses [78]. Similarly, AVP concentrations rose significantly following naloxone administration when males were separated from their mates, but not when they remained with their partners [128]. Pair mates likely act as a buffer to the effects of stressors, which may explain why naloxone treatment increased locomotion and AVP when males were alone but not when they were with their partners [129]. As opioid antagonists did not increase affiliation, opioid receptor function in titi monkeys appears to be more similar to that of other monogamous species (like prairie voles) than that of non-monogamous primates [78].
In addition to μ opioid receptor manipulation, a follow-up study by Ragen and colleagues [128] examined the effects of a κ opioid agonist (U50, 488) and antagonist (GNTI) on titi monkey behavior and physiology. Given that rats show increased isolation calls following activation of κ opioid receptors, primates might also be expected to demonstrate separation behaviors when given κ opioid receptor agonists; however, agonist treatment failed to sustain separation behaviors (e.g. isolation peeps). This indicates further differences between non-human primates and rodents. GNTI decreased male locomotion during separation, but it did not attenuate isolation peeps. The authors did not find effects of any treatment on plasma AVP, OT, or cortisol. These findings suggest that κ opioid receptors may be important for regulating certain behavioral stress responses, like increased locomotion, but not the physiological components of distress in titi monkeys. As such, the blockade of κ opioid receptors may attenuate the negative affective experience of separation.
Little is known about dopaminergic processing in titi monkeys, though recent work suggests that neuroplasticity of the dopamine systems in the LS, as opposed to the NAc, may be important for pair bonding in this species [130]. For instance, PET scans of male titi monkeys taken four to nine weeks post-pairing revealed increases in dopamine-D1 binding in the LS, while there were no changes in the NAc.
Neural Results of Challenges to Titi Monkey Pair Bonds
Bales and colleagues examined neural and hormonal changes of adult male titi monkeys in response to acute and chronic separation from the pair mate [131]. An acute separation of 48 hours resulted in elevated levels of cortisol, CSF OT, and insulin compared to baseline levels (when the pair mate was present). The authors think these changes reflect an increase in motivation to find social proximity, and preparation to cope with the stress of separation [132,133]. In addition, a decrease in glucose uptake was observed in many regions which contain OTR and AVPR1a [113], including the VP, LS, PVN, periaqueductal gray, and cerebellum. In contrast, a long term chronic separation of two weeks resulted in reduced whole brain glucose uptake—the converse of what is found during the initiation of a pair bond [105,109,131] as well as increased levels of CSF OT, and insulin. Chronic separation also resulted in a decrease in glucose uptake in the central Amyg, a region known to contain dense AVPR1a binding in titi monkeys, which has been previously linked with mediation of stress responses in other species [113,134].
An Integrated Neural Model for Titi Monkey Pair Bonding
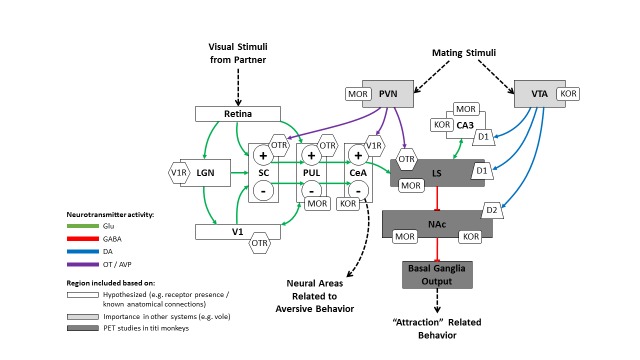
Our model for the neural regulation of pair bonding in titi monkeys (Figure 3) is based on several principles: 1) We incorporated data from our imaging studies with titi monkeys, which indicated which brain areas experienced changes in glucose uptake according to which social conditions; 2) We assumed special roles for areas that contained OTR and AVPR1a, particularly when those areas overlapped with dopaminergic and/or opioidergic innervation or receptors; and 3) We used the prairie vole model [135] for general guidance.
Figure 3.
Proposed model for the neurobiology of pair bond formation in male titi monkeys. Arrow heads indicate excitatory effects on the subsequent neural region while flat heads indicate inhibitory effects. Dashed lines represent either prior or later neural processing not detailed in the model. The + and – signs correspond to positively and negatively valenced neuronal populations respectively. Not all connections between areas are shown, in order to reduce the complexity of the figure. Region shading corresponds to the justification for inclusion in the model; white regions were hypothesized to be important for pair bond behavior based on a variety of factors (e.g. receptor presence, anatomical connections), light gray regions were included based on importance in other models (e.g. vole), and dark gray regions were included based on the results from PET studies we have previously conducted on titi monkeys. Abbreviations: CA3=hippocampus; CeA=central amygdala; LGN=lateral geniculate nucleus; LS=lateral septum; NAc=nucleus accumbens; PUL=pulvinar; PVN=paraventricular nucleus of the hypothalamus; SC=superior colliculus; VTA=ventral tegmental area; V1=primary visual cortex.
First, we posited that as primates, titi monkeys would primarily use visual signals for courtship and mating. This was supported by the distribution of OTR and AVPR1a in the titi monkey brain; OTR binding is present in the SC, PUL, and V1 [113]; AVPR1a binding is present in the LGN, SC, PUL, and V1 [113]. OT release within visual areas likely originates within the PVN. Given that rhesus monkeys and humans both demonstrate OTR binding and OT protein in the retina [136], OT peptide expression within the retina could potentially provide another source for OT release onto visual areas, though this has not yet been established in titi monkeys. Over time, OT release within visual areas might act to strengthen synapses and allow certain neurons to respond preferentially to visual stimuli from the partner. Given that the PUL extends to the CeA in primates [137], it may bridge visual processing (e.g. identification) and limbic activation in primates.
Several studies have demonstrated a role for the CeA in social recognition [121,122,138]. The CeA projects to the LS [139], another area crucial to social recognition in many species [139,140], which receives dopaminergic input from the VTA and regulates activity of dopaminergic areas in the forebrain [139,141]. In contrast to the rhesus monkey, a polygynous species that does not form pair bonds, titi monkeys have OTR in the LS [112-114]. The LS also appears to be implicated in pair bonding as demonstrated by differences in glucose uptake between paired and unpaired males [105], changes in glucose uptake across the course of pair bonding [105,109], a reduction in glucose uptake during short separations from the pair mate [131], and an up-regulation of dopamine D1 receptors four to nine weeks following pairing [130]. The LS also has strong reciprocal connections to the Hipp [139], which is involved in memory consolidation [142]. LS activation exerts inhibitory feedback on the NAc, which exerts inhibitory feedback on the basal ganglia [141]. Thus, the inhibition of the NAc would increase basal ganglia output, enabling the motor aspects of pair bonding.
The proposed model is detailed in Figure 3. Visual stimuli from a potential mate are received by the retina and propagated throughout visual processing regions. Subsequent copulation should stimulate PVN activity, releasing OT and/or AVP into visual areas. The synergistic activation of visual processing centers by the retina and the PVN may mediate individual recognition, conditioning certain neurons to respond preferentially to the associated visual stimuli henceforward. Consequently, these positively valenced neurons within the visual system then activate the CeA, which also might be sensitive to co-incidental OT/AVP conditioning from the PVN. Activation of the CeA should stimulate the LS, inhibiting the NAc. By decreasing output from the NAc, the basal ganglia system subsequently increases its output, ultimately enabling the motor aspects of pair bonding. Stimuli from mating episodes should also activate the VTA, mediating the rewarding aspects of mating behavior. Initially, VTA activation would be sufficient to activate affiliative motor behavior through LS activation via D1 receptors and NAc deactivation via D2 receptors.
Repeated mating episodes with paired visual stimuli from the same mate should enable visual stimuli alone to strongly activate the affiliative motor circuits associated with pair bonding. After OT/AVP conditioning within the visual system, visual stimuli from strangers should fail to activate the positively valenced neurons. Instead, these visual stimuli would activate negatively valenced neurons which project to neural areas that increase aversive/avoidance related behavior. The localization of opioid receptors across the circuit provides inferential evidence for the ability of the opioid system to modulate pair bonding behavior. For example, opioid receptor binding within the NAc should cause deactivation, increasing basal ganglia derived affiliative motor behavior.
Perspectives on the Titi Monkey Model, Implications for Future Research, and Conclusions
Our model for the neurobiology of titi pair bonding is intended to provide testable predictions for future research. Our assumption in creating this model was that the basic systems underlying pair bonding, including those underlying reward and emotion/social memory, are somewhat similar between all three species (prairie vole, titi monkey, and human). The findings presented in this paper support overlap in the neurobiological mechanisms underlying pair bonding across these three species (Table 1). For instance, we saw involvement of the reward system and limbic areas in multiple paradigms including pair bond formation, separation, and reunion.
Table 1. Summary of the current state of data on pair bonding from prairie voles, titi monkeys, and humans.
| Prairie voles | Titi monkeys | Humans | |
| Oxytocin | OTRa in the NAcb are crucial to pair bonding based on multiple methods: pharmacology [30], viral vectors [38], RNAic technology [39] | OTR present in some dopaminergic areas, like the LSd [113]; these areas are implicated in imaging studies of pair bonding [105,109]; however, no OTR present in the NAc as in voles | Peripheral OTe responsive to relationship cues [44-46]; intranasal OT enhances partner attractiveness, distance from strangers [52,53]; genotype associations [43]; OTR distribution known only for brainstem [117] |
| Vasopressin | AVPR1af in VPg are crucial to pair bonding based on multiple methods: pharmacology [30], viral vectors [41], RNAi technology | Intranasal AVPh causes changes in partner contact [126]; AVPR1a present in NAc rather than VP [113]; these areas implicated in imaging studies of pair bonding [105,109] | Peripheral AVP associated with relationship quality [50,51]; genotype associations [49]; AVPR1a distribution known only for brainstem [117] |
| Dopamine | Dopamine type D2 receptors involved in formation [64]; type D1 receptors involved in maintenance via up-regulation of aggression [33] | D1 receptors in LS up-regulated in males after pairing [130]; AVPR1a present in NAc [113] | Dopaminergic areas implicated in imaging studies [62] |
| Opioids | µ receptors regulate formation and ĸ receptors regulate maintenance [33,77]; pharmacology, gene expression in striatum | µ and ĸ receptors involved in maintenance, grooming [78] and responses to separation [128] | µ agonism promotes visual attention to faces and eyes [148]; touch from partner alters µ availability [149]; kappa system not studied |
| Visual system | Not studied, expected NOT to be heavily involved | Contains OTR and AVPR1a in titis [113] | Not studied directly, involvement expected (but see above) |
aOTR = oxytocin receptor, bNAc = nucleus accumbens, cRNAi = RNA interference, dLS = lateral septum, eOT = oxytocin, fAVPR1a = arginine vasopressin receptor type 1a, gVP = ventral pallidum, hAVP = arginine vasopressin
Titi monkey imaging and receptor distribution studies have provided some interesting contrasts with prairie vole neurobiology. One example is the involvement of the LS in titi monkey pair bonding, detailed in the previous section. The LS has been studied in relation to pair bonding in voles [143]; however, recent work has emphasized other dopaminergic forebrain areas such as the NAc and VP. While this may be a matter of differing methodology or focus, it is also possible that it is a taxonomic or species difference. For example, following pair bonding D1 receptors are upregulated in the NAc in voles and the LS in titi monkeys [130]. In addition, no OTR or AVPR1a are present in the VP of titi monkeys, while in male voles AVPR1a in the VP are considered necessary for pair bonding [144].
One caveat to the model above is that all of our information is currently derived from males. Sex differences are common in prairie vole pair bonding [28], and the same is likely to be true for titi monkeys and humans. This limitation highlights the need for additional studies focusing on pair bonding in female titi monkeys. It is also worth noting that some findings (like changes in D1 receptors with pairing) currently rest on only one study, while others (such as changes in global cerebral glucose metabolism with pairing and separation) have been replicated. This should be considered when weighting the significance of each finding.
A titi monkey model presents exciting new opportunities to explore the role of social bonds, and their neural bases, in processes best studied in primates rather than rodents. For instance, the use of visual tracking of eye gaze in non-human primates was recently identified as a priority area for development in animal studies of social cognition [145]. Along these lines, titi monkeys offer a novel model for studying the effects of treatments for social deficits on facial recognition and eye-tracking, techniques which capitalize on differences between primates and rodents in the use of the visual system for social behavior. Importantly, the extended period of development in primates presents opportunities to realistically model many aspects of maturation; in titi monkeys as in humans, offspring often stay in the natal group well past sexual maturity [146]. Reproductive processes also differ considerably between primates and non-primates. Thus, when treatments for social deficits might also affect reproductive hormones or maturation, a titi monkey model presents the possibility of assessing effects of treatments on social behavior and reproduction simultaneously [147]. In summary, the titi monkey model may serve as a novel, generalizable model for many aspects of human health.
Acknowledgments
The titi monkey research described in this article was funded by NICHD HD053555 to KLB, NIH OD011107 to the CNPRC, and the Good Nature Institute to KLB. During the writing of this paper, KLB was funded by NICHD HD071998, NIMH MH108319, NIMH MH110014, NIH OD011107 and the Good Nature Institute.
Glossary
- Amyg
Amygdala
- ACC
Anterior cingulate cortex
- AVP
Arginine vasopressin
- AVPR1a
AVP receptor
- AVPR1A
AVP V1a gene
- CA1
CA1 subregion of the hippocampus
- CA3
CA3 subregion of the hippocampus
- CeA
central amygdala
- D1
Dopamine receptors, type D1
- D2
Dopamine receptors, type D2
- Hipp
Hippocampus
- Hyp
Hypothalamus
- LGN
lateral geniculate nucleus of the thalamus
- LS
Lateral septum
- NAc
Nucleus accumbens
- OXTR
OTR gene
- OT
Oxytocin
- OTR
Oxytocin receptor
- PVN
Paraventricular nucleus
- PET
positron emission tomography
- PCC
Posterior cingulate cortex
- PFC
Prefrontal cortex
- PUL
pulvinar nucleus of the thalamus
- SC
superior colliculus
- SON
supraoptic nucleus
- VP
Ventral pallidum
- VTA
Ventral tegmental area
Author Contributions
All authors contributed to the conceptualization, writing, and editing of this review paper.
References
- Coan JA, Sbarra DA. Social baseline theory: The social regulation of risk and effort. Curr Opin Psychol. 2015;1:87–91. doi: 10.1016/j.copsyc.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd R, Richerson PJ, Henrich J. The cultural niche: Why social learning is essential for human adaptation. Proc Natl Acad Sci. 2011;1:87–91. doi: 10.1073/pnas.1100290108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA. Early social relationships: A psychobiologist's view. Child Develop. 1984;58:633–647. [PubMed] [Google Scholar]
- Berscheid E. The human's greatest strength: Other humans. In: Staudinger UM, editor. A Psychology of Human Strengths: Fundamental Questions and Future Directions for a Positive Psychology. Washington, D.C.: American Psychological Association; 2003. pp. 37–47. [Google Scholar]
- Loving TJ, Slatcher R. Romantic relationships and health. In: Simpson J, Campbell L, editors. The Oxford Handbook of Close Relationships. Oxford: Oxford University Press; 2013. pp. 617–637. [Google Scholar]
- Wardecker BM, Smith LK, Edelstein RS. et al. Intimate relationships then and now: How old hormonal processes are influenced by our modern psychology. Adapt Hum Behav Physiol. 2015;1:150–176. [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR. et al. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Robles TF, Kiecolt-Glaser JK. The physiology of marriage: Pathways to health. Physiol Behav. 2003;79:409–416. doi: 10.1016/s0031-9384(03)00160-4. [DOI] [PubMed] [Google Scholar]
- Coyne JC, Rohrbaugh MJ, Shoham VS. et al. Prognostic importance of marital quality for survival of congestive heart failure. Am J Cardiol. 2001;88:526–529. doi: 10.1016/s0002-9149(01)01731-3. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Bane C, Glaser R. et al. Love, marriage, and divorce: Newlyweds' stress hormones foreshadow relationship changes. J Consul Clin Psychol. 2003;71:176–188. doi: 10.1037//0022-006x.71.1.176. [DOI] [PubMed] [Google Scholar]
- Eastwick PW. Beyond the Pleistocene: Using phylogeny and constraint to inform the evolutionary psychology of human mating. Psychol Bull. 2009;135:794–821. doi: 10.1037/a0016845. [DOI] [PubMed] [Google Scholar]
- Eastwick PW. The psychology of the pair-bond: Past and future contributions of close relationships research to evolutionary psychology. Psychol Inq. 2013;24:183–191. [Google Scholar]
- Carter CS, DeVries AC, Taymans SE. et al. Peptides, steroids, and pair bonding. Ann NY Acad Sci. 1997;807:260–272. doi: 10.1111/j.1749-6632.1997.tb51925.x. [DOI] [PubMed] [Google Scholar]
- Gangestad SW, Simpson JA. The evolution of human mating: Trade-offs and strategic pluralism. Behav Brain Sci. 2000;23:573–644. doi: 10.1017/s0140525x0000337x. [DOI] [PubMed] [Google Scholar]
- Dewitte M, Houwer J, Buysse A. et al. Proximity seeking in adult attachment: Examining the role of automatic approach-avoidance tendencies. Brit J Soc Psychol. 2008;47:557–573. doi: 10.1348/014466607X265148. [DOI] [PubMed] [Google Scholar]
- Fraley RC, Shaver PR. Airport separations: A naturalistic study of adult attachment dynamics in separating couples. J Pers Soc Psychol. 1998;75:1198–1212. [Google Scholar]
- Bowlby J. Attachment and Loss. New York: Basic Books, Inc.; 1969. [Google Scholar]
- Zeifman D, Hazan C. Attachment: The bond in pair-bonds. In: Simpson JA, Kenrick DT, editors. Evolutionary Social Psychology. Hillsdale, N.J.: Lawrence Erlbaum Associates, Inc.; 1997. pp. 237–263. [Google Scholar]
- Holmes BM, Johnson KR. Adult attachment and romantic partner preference: A review. J Soc Pers Relat. 2009;26:833–852. [Google Scholar]
- Eastwick PW, Finkel EJ. The evolutionary armistice: Attachment bonds mdoerate the function of ovulatory cycle adaptations. Pers Soc Psychol Bull. 2012;38:174–184. doi: 10.1177/0146167211422366. [DOI] [PubMed] [Google Scholar]
- Feeney BC. A secure base: Responsive support of goal strivings and exploration in adult intimate relationships. J Pers Soc Psychol. 2004;87:631–648. doi: 10.1037/0022-3514.87.5.631. [DOI] [PubMed] [Google Scholar]
- Feeney BC, Kirkpatrick LA. Effects of adult attachment and presence of romantic partners on physiological responses to stress. J Pers Soc Psychol. 1996;70:255–270. doi: 10.1037//0022-3514.70.2.255. [DOI] [PubMed] [Google Scholar]
- Getz LL. Speculation on social structure and population cycles of microtine rodents. The Biologist. 1978;60:134–147. [Google Scholar]
- Getz LL, Carter CS. Social organization in Microtus ochrogaster populations. The Biologist. 1980;(62):56–69. [Google Scholar]
- Getz LL, Carter CS. Prairie vole partnerships. Am Sci. 1996;84:56–62. [Google Scholar]
- Ophir AG, Phelps SM, Sorin AB. et al. Morphological, genetic, and behavioral comparisons of two prairie vole populations in the field and laboratory. J Mammal. 2007;88:989–999. [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): The role of social and sexual experience. Horm Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding - oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109(4):782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Carter CS. Sex differences in temporal parameters of partner preference in prairie voles (Microtus ochrogaster). Can J Zool. 1999;77(6):885–889. [Google Scholar]
- Cho MM, DeVries AC, Williams JR. et al. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav Neurosci. 1999;113(5):1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Bowler CM, Cushing BS, Carter CS. Social factors regulate female-female aggression and affiliation in prairie voles. Physiol Behav. 2002;76(4-5):559–566. doi: 10.1016/s0031-9384(02)00755-2. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS. et al. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365(6446):545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Keyes PC, Day JJ. et al. Dopamine and opioid systems interact within the nucleus accumbens to maintain monogamous pair bonds. eLife. 2016;5:e15325. doi: 10.7554/eLife.15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J. From affiliative behaviors to romantic feelings: A role of nanopeptides. FEBS Lett. 2007;581:2580–2586. doi: 10.1016/j.febslet.2007.03.095. [DOI] [PubMed] [Google Scholar]
- Zingg HH, Laporte SA. The oxytocin receptor. Trends Endocrinol Metab. 2003;14:222–227. doi: 10.1016/s1043-2760(03)00080-8. [DOI] [PubMed] [Google Scholar]
- Maybauer MO, Maybauer DM, Enkhbaatar P. et al. Physiology of the vasopressin receptors. Best Pract Res Clin Anaesthesiol. 2008;22:253–263. doi: 10.1016/j.bpa.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, Young LJ. Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm Behav. 2011;60:498–504. doi: 10.1016/j.yhbeh.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, Barrett CE, Laprairie JL. et al. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc Neurosci. 2015;10:561–570. doi: 10.1080/17470919.2015.1040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Jamal YA. et al. Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Horm Behav. 2016;79:8–17. doi: 10.1016/j.yhbeh.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE. et al. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429(6993):754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Murphy MR, Seckl JR, Burton S. et al. Changes in oxytocin and vasopressin secretion during sexual activity in men. J Clin Endocrinol Metab. 1987;65:738–741. doi: 10.1210/jcem-65-4-738. [DOI] [PubMed] [Google Scholar]
- Schneiderman I, Kanat-Maymon Y, Ebstein RP. et al. Cumulative risk on the oxytocin receptor gene (OXTR) underpins empathic communication difficulties at the first stages of romantic love. Soc Cogn Affect Neurosci. 2014;9:1524–1529. doi: 10.1093/scan/nst142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman I, Zagoory-Sharon O, Leckman JF. et al. Oxytocin during the initial stages of romantic attachment: Relation to couples' interactive reciprocity. Psychoneuroendocrinology. 2012;37:1277–1285. doi: 10.1016/j.psyneuen.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer-Yaniv A, Avitsur R, Kanat-Maymon Y. et al. Affiliation, reward, and immune biomarkers coalescle to support social synchrony during periods of bond formation in humans. Brain Behav Immun. 2016;56:130–139. doi: 10.1016/j.bbi.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA. More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biol Psychol. 2005;69:5–21. doi: 10.1016/j.biopsycho.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Birmingham WA, Light KC. Influence of a "warm touch" support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosom Med. 2008;70:976–985. doi: 10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- Feldman R. Oxytocin and social affiliation in humans. Horm Behav. 2012;61:380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S. et al. Genetic variation in the vasopressin receptor 1a gene (AVPR1a) associates with pair-bonding behavior in humans. Proc Natl Acad Sci. 2008;105:14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin JP, Carter CS, Pournajafi-Nazarloo H. et al. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology. 2010;35:1082–1090. doi: 10.1016/j.psyneuen.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin JP, Carter CS, Pournajafi-Nazarloo H. et al. Plasma vasopressin and interpersonal functioning. Biol Psychol. 2012;91:270–274. doi: 10.1016/j.biopsycho.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM. et al. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci. 2013;110:20308–20313. doi: 10.1073/pnas.1314190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Striepens N, Gunturkun O. et al. Oxytocin modulates social distance between males and females. J Neurosci. 2012;32:16074–16079. doi: 10.1523/JNEUROSCI.2755-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer A, Van Buel EM, ter Horst GJ. et al. Love is more than just a kiss: a neurobiological perspective on love and affection. Neuroscience. 2012;201:114–124. doi: 10.1016/j.neuroscience.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Ortigue S, Bianchi-Demicheli F, Patel N. et al. Neuroimaging of love: fMRI meta-analysis evidence toward new perspectives in sexual medicine. J Sex Med. 2010;7:3541–3552. doi: 10.1111/j.1743-6109.2010.01999.x. [DOI] [PubMed] [Google Scholar]
- Zeki S. The neurobiology of love. FEBS Lett. 2007;581:2575–2579. doi: 10.1016/j.febslet.2007.03.094. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000;11:3829–3834. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Ortigue S, Bianchi-Demicheli F, Hamilton AFdC. et al. The neural basis of love as a subliminal prime: an event-related functional magnetic resonance imaging study. J Cogn Neurosci. 2007;19:1218–1230. doi: 10.1162/jocn.2007.19.7.1218. [DOI] [PubMed] [Google Scholar]
- Fisher HE, Brown LL, Aron A. et al. Reward, addiction, and emotion regulation systems associated with rejection in love. J Neurophysiol. 2010;104:51–60. doi: 10.1152/jn.00784.2009. [DOI] [PubMed] [Google Scholar]
- Acevedo BP, Aron A. Does a long-term relationship kill romantic love? Rev Gen Psychol. 2009;13:59. [Google Scholar]
- Xu S, Aron A, Brown LL. et al. Reward and motivation systems: a brain mapping study of early-stage intense romantic love in Chinese participants. Hum Brain Mapp. 2011;32:249–257. doi: 10.1002/hbm.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Zou Z, Kou J. et al. Love-related changes in the brain: a resting-state functional magnetic resonance imaging study. Front Hum Neurosci. 2015;9:71. doi: 10.3389/fnhum.2015.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis T. et al. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci. 2003;23(8):3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher H, Aron A, Brown LL. Romantic love: an fMRI study of a neural mechanism for mate choice. J Comp Neurol. 2005;493:58–62. doi: 10.1002/cne.20772. [DOI] [PubMed] [Google Scholar]
- Gundel H, O'Connor MF, Littrell L. et al. Functional neuroanatomy of grief: an FMRI study. Am J Psychiatry. 2003;160:1946–1953. doi: 10.1176/appi.ajp.160.11.1946. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- Dalgliesh T. The emotional brain. Nat Rev Neurosci. 2004;5:583–589. doi: 10.1038/nrn1432. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Site and behavioral specificity of periaqueductal gray lesions on postpartum sexual, maternal, and aggressive behaviors in rats. Brain Res. 1998;804(1):21–35. doi: 10.1016/s0006-8993(98)00642-8. [DOI] [PubMed] [Google Scholar]
- Arnow BA, Desmond JE, Banner LL. et al. Brain activation and sexual arousal in healthy, heterosexual males. Brain. 2002;125:1014–1023. doi: 10.1093/brain/awf108. [DOI] [PubMed] [Google Scholar]
- Fisher H. Lust, attraction, attachment: Biology and evolution of the three primary emotion systems for mating, reproduction, and parenting. J Sex Educ Ther. 2000;25:96–104. [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23(8):779–818. [DOI] [PubMed] [Google Scholar]
- Love TM. Oxytocin, motivation, and the role of dopamine. Pharmacol Biochem Behav. 2014;119:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. [DOI] [PubMed] [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang ZX, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav Neurosci. 2000;114(1):173–183. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22:437–452. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Dome M, Gormley G, Franco D, Nevarez N, Hamid AA, et al. µ-Opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms. J Neurosci. 2013;33:9140–9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragen BJ, Maninger N, Mendoza SP, Jarcho MR, Bales KL. Presence of a pair-mate regulates the behavioral and physiological effects of opioid manipulation in the monogamous titi monkey (Callicebus cupreus). Psychoneuroendocrinology. 2013;38:2448–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LE, Meyer ME, Dewsbury DA. Affiliative behavior in voles: effects of morphine, naloxone, and cross-fostering. Physiol Behav. 1989;46:719–723. [DOI] [PubMed] [Google Scholar]
- Burkett JP, Spiegel LL, Inoue K, Murphy AZ, Young LJ. Activation of u-opioid receptors in the dorsal striatum is necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology. 2011;36:2200–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre-Nys C, Meller RE, Keverne EB. Opiate antagonists stimulate affiliative behaviour in monkeys. Pharmacol Biochem Behav. 1982;16:653–659. [DOI] [PubMed] [Google Scholar]
- Diamond LM. Emerging perspectives on distinctions between romantic love and sexual desire. Curr Dir Psychol Sci. 2004;13:116–119. [Google Scholar]
- Way BM, Taylor SE, Eisenberger NI. Variation in the µ-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc Natl Acad Sci USA. 2009;106:15079–15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragen BJ, Freeman SM, Laredo SA, Mendoza SP, Bales KL. µ and ĸ opioid receptor distribution in the monogamous titi monkey (Callicebus cupreus): implications for social behavior and endocrine functioning. Neuroscience. 2015;290:421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Smith AS, Lei K, Liu Y, Wang Z. Breaking bonds in male prairie voles: long-term effects on emotional and social behavior, physiology, and neurochemistry. Behav Brain Res. 2014;265:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34:1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal N, Scotti M-AL, Wardwell J, Chandler DL, Bates SL, LaRocca M, et al. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Auton Neurosci. 2014;180:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P, Morin R, Kochkar R, Parker K, Cohn D, Lopez MH, et al. A balance sheet at 30 months: how the great recession has changed life in America. 2010. Pew Research Center, Social and Demographic Trends Project. Retrieved from: http://pewsocialtrends.org/assets/pdf/759-recession.pdf.
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychol Sci. 2010;21:3–7. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Wellisch DK, Stanton AL, Eisenberger NI, Irwin MR, Lieberman MD. Craving love? Enduring grief activates brain’s reward center. Neuroimage. 2008;42:969–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershkovitz P. Living New World Monkeys. Vol. 1 Chicago: University of Chicago Press; 1977. [Google Scholar]
- Mendoza A, Ng J, Bales KL, Mendoza SP, George DA, Smith DG, et al. Population genetics of the California National Primate Research Center’s (CNPRC) captive Callicebus cupreus colony. Primates. 2014;56:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif S, Bales K, Williams L, Moeller E, Abbott D, Schultz-Darken N, et al. Preparing New World monkeys for laboratory research. ILAR J. 2006;47:307–315. [DOI] [PubMed] [Google Scholar]
- Garber PA, Porter LM, Spross J, Fiore AD. Tamarins: insights into monogamous and non-monogamous single female social and breeding systems. Am J Primatol. 2016;78:298–314. [DOI] [PubMed] [Google Scholar]
- Galvao-Coelho NL, Silva HPA, De Sousa MBC. The influence of sex and relatedness on stress response in common marmosets (Callithrix jacchus). Am J Primatol. 2012;74:819–827. [DOI] [PubMed] [Google Scholar]
- Mundy NI, Morningstar NC, Baden AL, Fernandez-Duque E, Davalos VM, Bradley BJ. Can colour vision re-evolve? Variation in the X-linked opsin locus of cathemeral Azara’s owl monkeys (Aotus azarae azarae). Front Zool. 2016;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Taylor JH, Mustoe AC, Cavanaugh J. Neuropeptide diversity and the regulation of social behavior in New World primates. Front Neuroendocrinol. 2016;42:18–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA, Mendoza SP. Generic aspects of primate attachments: Parents, offspring and mates. Psychoneuroendocrinology. 1998;23(8):765–778. [DOI] [PubMed] [Google Scholar]
- Carp SB, Rothwell ES, Bourdon A, Freeman SM, Ferrer E, Bales KL. Development of a partner preference test that differentiates between established pair bonds and other relationships in socially monogamous titi monkeys (Callicebus cupreus). Am J Primatol. 2016;78:326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA. Social organization of the South American monkey, Callicebus moloch: a preliminary report. Tulane Stud Zool. 1966;13:23–28. [Google Scholar]
- Cubiciotti DDI, Mason WA. Comparative studies of social behavior in Callicebus and Saimiri: heterosexual jealousy behavior. Behav Ecol Sociobiol. 1978;3:311–322. [Google Scholar]
- Mendoza SP, Mason WA. Contrasting responses to intruders and to involuntary separation by monogamous and polygynous New World monkeys. Physiol Behav. 1986;38:795–801. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Capitanio JP, Mason WA. Chronic social stress: studies in non-human primates In: Moberg GP, Mench JA. Biology of Animal Stress: Basic Principles and Implications for Animal Welfare. New York: CABI Publishing; 2000. p. 227–247. [Google Scholar]
- Mendoza SP, Mason WA. Parental division of labour and differentiation of attachments in a monogamous primate (Callicebus cupreus). Anim Behav. 1986;34:1336–1347. [Google Scholar]
- Bales KL, Mason WA, Catana C, Cherry SR, Mendoza SP. Neural correlates of pair-bonding in a monogamous primate. Brain Res. 2007;1184:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Belle S, Fernandez-Duque E, Di Fiore A. Demography and life history of wild red titi monkeys (Callicebus discolor) and equatorial sakis (Pithecia aequatorialis) in Amazonian Ecuador: a 12-year study. Am J Primatol. 2016;78:204–215. [DOI] [PubMed] [Google Scholar]
- Muller AE, Anzenberger G. Duetting in the titi monkey Callicebus cupreus: structure, pair specificity and development of duets. Folia Primatol (Basel). 2002;73:104–115. [DOI] [PubMed] [Google Scholar]
- Spence-Aizenberg A, Di Fiore A, Fernandez-Duque E. Social monogamy, male-female relationships, and biparental care in wild titi monkeys (Callicebus discolor). Primates. 2016;57:103–112. [DOI] [PubMed] [Google Scholar]
- Maninger N, Hinde K, Mendoza SP, Mason WA, Larke RH, Ragen BJ, et al. Pair bond formation leads to a sustained increase in global cerebral glucose metabolism in monogamous male titi monkeys (Callicebus cupreus). Neuroscience. 2017;348:302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci USA. 1992;89(13):5981–5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci. 1994;14(9):5381–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta). Psychoneuroendocrinology. 2014;45:128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, et al. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus). Neuroscience. 2014;273:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Young LJ. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: translational implications. J Neuroendocrinol. 2016:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH. Eye, Brain, and Vision. W.H. Freeman; 1995. [Google Scholar]
- Ohman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30:953–958. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Smith AL, Goodman MM, Bales KL. Selective localization of oxytocin receptors and vasopressin 1a receptors in the human brainstem. Soc Neurosci. 2016;20:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Consiglio A, Lie DC, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R, Hegoburu Cvd B. E. New opportunities in vasopressin and oxytocin research: a perspective from the amygdala. Annu Rev Neurosci. 2015;38:369–388. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci. 2002;14:1264–1274. [DOI] [PubMed] [Google Scholar]
- Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav Neurosci. 2012;126:97–109. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Dupre A, Tribollet E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neurosci Lett. 2009;461:217–222. [DOI] [PubMed] [Google Scholar]
- Young LJ, Toloczko D, Insel TR. Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain. J Neuroendocrinol. 1999;11:291–297. [DOI] [PubMed] [Google Scholar]
- Jarcho MR, Mendoza SP, Mason WA, Yang X, Bales KL. Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes Brain Behav. 2011;10:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. [DOI] [PubMed] [Google Scholar]
- Ragen BJ, Maninger N, Mendoza SP, Bales KL. The effects of morphine, naloxone, and ĸ opioid manipulation on endocrine functioning and social behavior in monogamous titi monkeys (Callicebus cupreus). Neuroscience. 2015;287:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. WIlls TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- Hostetler CM, Hinde K, Maninger N, Mendoza SP, Mason WA, Rowland DJ, et al. Effects of pair bonding on dopamine D1 receptors in monogamous male titi monkeys (Callicebus cupreus). Am J Primatol. 2017;79:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde K, Muth C, Maninger N, Ragen BJ, Larke RH, Jarcho MR, et al. Challenges to the pair bond: neural and hormonal effects of separation and reunion in a monogamous primate. Front Behav Neurosci. 2016;10:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PW. The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry. 2015;20:32–47. [DOI] [PubMed] [Google Scholar]
- Mendoza SP. Social stress: concepts, assumptions, and animal models. 3rd ed. Hormones, Brain, and Behavior; 2017. [Google Scholar]
- Ebner K, Bosch OJ, Kromer SA, Singewald N, Neumann ID. Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology. 2005;30:223–230. [DOI] [PubMed] [Google Scholar]
- Numan M, Young LJ. Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Horm Behav. 2016;77:98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbach P, Pillers DA, York N, Asuma MP, Chiu MA, Luo W, et al. Oxytocin expression and function in the posterior retina: a novel signaling pathway. Invest Ophthalmol Vis Sci. 2015;56:751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve KL, Acuna C, Cudeiro J. The primate pulvinar nuclei: vision and action. Trends Neurosci. 2000;23:35–39. [DOI] [PubMed] [Google Scholar]
- Stoop R, Hegoburu C, van den Burg E. New opportunities in vasopressin and oxytocin research: a perspective from the amygdala. Annu Rev Neurosci. 2015;38:369–388. [DOI] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Brain Res Rev. 2004;46:71–117. [DOI] [PubMed] [Google Scholar]
- Everts HGJ, Koolhaas JM. Differential modulation of lateral septal vasopressin receptor blockade in spatial learning, social recognition, and anxiety-related behaviors in rats. Behav Brain Res. 1999;99(1):7–16. [DOI] [PubMed] [Google Scholar]
- Salgado S, Kaplitt MG. The nucleus accumbens: A comprehensive review. Stereotact Funct Neurosurg. 2015;93:75–93. [DOI] [PubMed] [Google Scholar]
- Squire LR, Genzel L, Wixted JT, Morris RG. Memory consolidation. Cold Spring Harb Perspect Biol. 2015;7:a021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang ZX. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster). Behav Neurosci. 2001;115(4):910–919. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125(1):35–45. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Bales KL. Towards improved animal models for evaluating social cognition and its disruption in schizophrenia: the CNTRICS initiative. Neurosci Biobehav Rev. 2013;37:2166–2180. [DOI] [PubMed] [Google Scholar]
- Mayeaux DJ, Mason WA, Mendoza SP. Developmental changes in responsiveness to parents and unfamiliar adults in a monogamous monkey (Callicebus moloch). Am J Primatol. 2002;58:71–89. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, ’t Hart BA, et al. Why primate models matter. Am J Primatol. 2014;76:801–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelnokova O, Laeng B, Loseth G, Eikemo M, Willoch F, Leknes S. The mu-opioid system promotes visual attention to faces and eyes. Soc Cogn Affect Neurosci. 2016;11:1902–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Tuominen L, Dunbar RIM, Hirvonen J, Manninen S, Arponen E, et al. Social touch modulates endogenous mu-opioid system activity in humans. Neuroimage. 2016;138:242–247. [DOI] [PubMed] [Google Scholar]