Abstract
Recent pilot clinical studies have demonstrated that subjects with severe disorders of movement and communication can exert direct neural control over assistive devices using invasive Brain-Machine Interface (BMI) technology, also referred to as ‘cortical neuroprosthetics’. These important proof-of-principle studies have generated great interest among those with disability and clinicians who provide general medical, neurological and/or rehabilitative care. Taking into account the perspective of providers who may be unfamiliar with the field, we first review the clinical goals and fundamentals of invasive BMI technology, and then briefly summarize the vast body of basic science research demonstrating its feasibility. We emphasize recent translational progress in the target clinical populations and discuss translational challenges and future directions.
INTRODUCTION
Over the past decade, as the rate and sophistication of research involving invasive Brain-Machine Interface (BMIs) has grown, so has its visibility to clinicians, the disabled and the general public. While the term BMI may encompass a wide array of methods and approaches (Andersen et al., 2010; Ethier et al., 2015; Moxon and Foffani, 2015; Nicolelis and Lebedev, 2009; Schwartz, 2004; Silvoni et al., 2011), we will focus on “motor BMIs”, i.e. a neural interface that allows direct neural control of a prosthetic device or a communication interface(Bensmaia and Miller, 2014; Homer et al., 2013; Nicolelis and Lebedev, 2009; Schwartz, 2004; Shenoy and Carmena, 2014). Communication interfaces typically fall into two categories: (1) direct neural control of a cursor for navigation of a computer or use of an optimized keyboard for typing (i.e. sending messages and/or emails) and (2) control of a speech synthesizing prosthetic (Brumberg et al., 2011; Guenther et al., 2009; Hochberg et al., 2006; Kennedy et al., 2000; Mugler et al., 2014). A motor BMI operates by recording a neural signal, processing that signal into device commands, and sending commands to a device; the user typically receives natural visual feedback regarding the outcome (Figure 1). In this review we will specifically focus on invasive approaches using either multielectrode arrays (MEAs) (Bensmaia and Miller, 2014; Homer et al., 2013; Nicolelis and Lebedev, 2009; Schwartz, 2004) or electrocorticography (ECoG) (Figure 2) (Chang et al., 2010; Crone et al., 1998a; Crone et al., 1998b; Ganguly et al., 2009; Leuthardt et al., 2004; Schalk et al., 2008; Vansteensel et al., 2010); a main reason for this is that the most recent translational trials demonstrating complex control of prosthetic devices have relied on decoding using invasive approaches (Collinger et al., 2013b; Hochberg et al., 2012). Hereafter, we will broadly refer to invasive recordings using MEAs or ECoG with the term “cortical neuroprosthetics”. Importantly, we do wish to note, however, that there is very active research into the use of non-invasive EEG based recordings for cursor control and communication interfaces (Birbaumer et al., 1999; Millan Jdel and Carmena, 2010; Nijboer et al., 2008; Sellers et al., 2010; Wolpaw et al., 2002); as also noted at the end of this review, clinical studies will have to eventually weigh the risks and the comparative benefits of invasive versus non-invasive approaches.
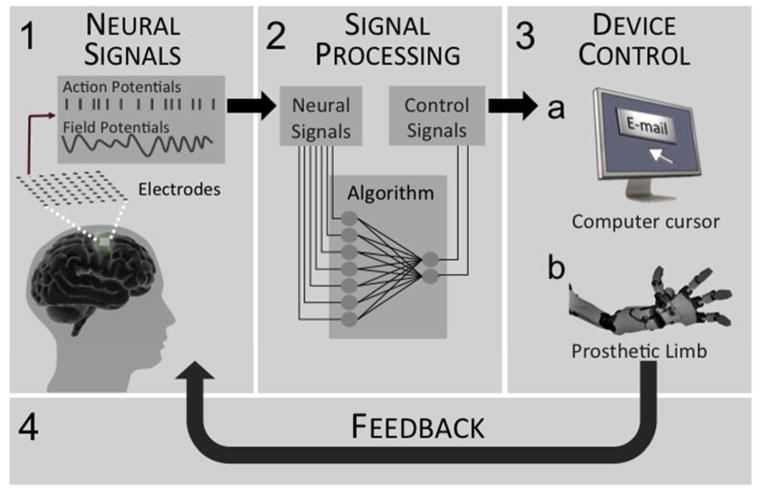
Figure 1.
Schematic of the basic components of a brain machine interface. 1) Sensors, composed of electrodes, connect the brain to a neural signal processor. 2) A signal processor uses mathematical algorithms to decode the neural signals into control signals. 3) An effector converts the control signal into a functional output, such as control of a cursor on a computer screen or a robotic limb. 4) Feedback (i.e. vision or artificial somatosensory) is incorporated to improve BMI neural control.
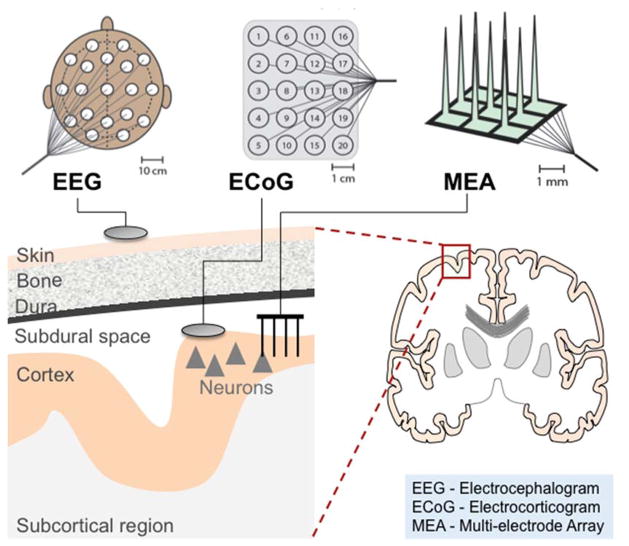
Figure 2.
Commonly used BMI sensors include electroencephalography (EEG), electrocorticography (ECoG), and multielectrode arrays (MEAs). EEG electrodes are noninvasively placed directly over the scalp to record synchronized electrical activity of thousands of cortical neurons. ECoG electrodes are placed directly on the cortical surface or overlying the dura to record aggregate local field potentials. MEAs are inserted directly into cortical tissue and can record aggregate local field potentials or action potentials (spikes) from single neurons or clusters of neurons. Please note that while the “discs” are used to generally depict EEG and ECoG, the MEA is specifically depicted to highlight the required penetration into cortical tissue.
Taking the perspective of clinical providers who care for individuals with impaired movement control and difficulties with communication, our primary aim is to review the clinical goals, current progress and barriers to translation of cortical neuroprosthetics. The following example of a patient under our care exemplifies the goals for the field and the need to educate clinical providers about current progress and the challenges. A sixty-eight year-old man first presented with six months of progressive fatigue, weakness and difficulty with walking. After a thorough evaluation and a series of diagnostic tests, he was diagnosed with amyotrophic lateral sclerosis (ALS). In the subsequent 2 years, his general strength continued to decline, resulting in difficulty typing on a keyboard, getting out of bed, bathing, and dressing. Evaluations of his breathing revealed reduced inspiratory capacity. As his speech deteriorated, he could no longer use his voice-activated software. Currently, he lives with his wife, who is his primary caretaker. He relies on a head tracking and a mouth joystick system as pointing devices. His long-term wish is for artificial ventilation and parenteral feedings. He has not experienced cognitive changes. Recently, he read an article on neuroprosthetics and asked about possible options. While he was generally interested in upper-limb and mobility improvements, his specific interest was in a more reliable communication interface. Taking the viewpoint of a clinical provider who is asked such a question, we aim to review current translational progress in the field of cortical neuroprosthetics.
CLINICAL GOALS FOR NEUROPROSTHETICS
The broad goals of cortical neuroprosthetics are to restore movement control and communication in a heterogeneous group of neurological disorders such as traumatic and non-traumatic spinal cord injury (SCI), ALS, severe neuropathies, myopathies and brainstem strokes; they can all result in variable degrees of weakness or paralysis of all four limbs and/or disorders of communication. As discussed below, research efforts have generally coalesced around the common restorative goals of improving upper-limb function as well as communication. The current research is primarily aimed towards those with the most severe disorders of communication and tetraparesis or tetraplegia, i.e. weakness or paralysis of all four limbs. While the adoption of broad goals are quite appropriate at this early stage of development, clinical differences among such conditions (e.g. progressive versus non-progressive, loss of sensation versus preserved sensation) may become important in the future and require individualized restorative goals tailored to the underlying disease process. Thus, goals for individuals with traumatic SCI, a typically non-progressive disorder with a fixed level of disability, would naturally differ from the goals of those with SCI from multiple sclerosis or someone with ALS, both progressive disorders where there is an increase in disability over time. Moreover, especially for ALS, compromise of respiratory function is a frequent cause of mortality; thus requiring a difficult decision to be placed on an artificial respirator(Malik et al., 2014). The patient’s future quality of life and continued ability to communicate will likely play a critical role in such decisions.
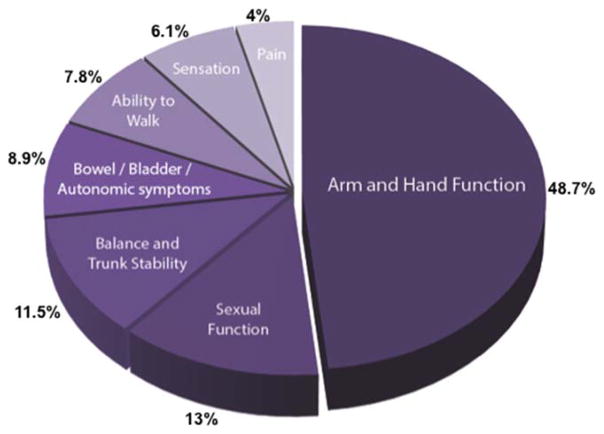
Restoring arm and hand function is a major clinical goal for cortical neuroprosthetics. A recent comprehensive assessment by the Reeve Foundation found that amongst people with paralysis, ~900,000 (16%) reported being “completely unable to move” (CRDF, 2009). Most of the neurological disorders listed above can result in severe weakness or paralysis of both upper extremities. Importantly, surveys indicate that arm and hand function are important restorative goals for those with severe paralysis. More specifically, a survey of subjects with tetraplegia resulting from SCI found that treatments to regain arm/hand function was a top priority (Figure 3) (Anderson, 2004). Those with ALS have also reported that neural control of a robotic arm would be of value (Huggins et al., 2011).
Figure 3.
Results from a survey of priorities in individuals with spinal cord injury. Tetraplegic patients were asked which disability, should it recover, would have the biggest impact on their lives. Data adapted from (Anderson, 2004).
In general, upper limb functions can be broadly broken down into arm movements (i.e. proximal muscles), wrist movements (e.g. orientating the hand), and hand movements; hand function, in turn, can be generally subdivided into grasping functions (i.e. a power grip), a pincer grip for finer manipulations, and individuated finger movements (Bullock and Dollar, 2011; Elliott and Connolly, 1984). The degrees of freedom (DOF), i.e. number of independent parameters that must be specified, increases with the complexity of control. In other words, the DOF needed to be controlled increases substantially when moving from gross reaching/grasping to reaching and grasping with wrist movements/finger movements. Importantly, current non-BMI approaches (e.g. using mechanical switches or joysticks) can have limitations when scaling up to control a prosthetic device with a large number of DOF (Resnik et al., 2014; Resnik et al., 2012). In contrast, direct neural control using intracortical approaches aims to allow intuitive and naturalistic control of the required DOF to achieve complex functional restoration. Current neuroprosthetic approaches primarily aim to restore basic reaching and grasping functions, which may eventually allow restoration of upper-limb functions such as self-feeding and object manipulation(Collinger et al., 2013b; Hochberg et al., 2012; Schwartz, 2004; Velliste et al., 2008). Importantly, such restoration will require integration of either an exoskeleton system (i.e. robotic device that can reanimate a paralyzed limb), a functional-electrical stimulation system (FES, i.e. stimulation of individual muscles to trigger movements) (Cornwell et al., 2012; Ethier et al., 2015; Ethier et al., 2012; Moritz et al., 2008) or the use of a separate prosthetic limb. While it currently remains unclear which approach is the best, there may be benefits based on the etiology of the weakness(Collinger et al., 2013a)... In individuals with ALS or incomplete SCI, for instance, an exoskeleton or an FES system may allow subjects to use residual natural tactile and proprioception.
Restoration of communication with a typing interface or perhaps a speech prosthesis is another important goal of BMIs. When the brainstem is affected, such as by basilar strokes or ALS, tetraplegia may be accompanied by paralysis of oral structures, leading to the loss of voluntary vocal communication. Individuals with severe traumatic brain injury or high cervical injuries who require mechanical ventilation may also have limited capacity to communicate (Kennedy, 1994; Kubler et al., 2001; Monti et al., 2010). Standard augmentative and alternative communication (AAC) devices are widely available (Selzer et al., 2014) but may not be suitable for individuals with severe or complete paralysis of the voluntary motor system. For example, the most commonly used devices are gaze trackers that allow computer cursor control through eye movements (Spataro et al., 2014). However, eye tracking may have a limited role in advanced cases of ALS and after brainstem strokes, where eye movements are frequently affected (Birbaumer et al., 1999; Kennedy and Bakay, 1998). They also require sustained visual attention and may create a high cognitive burden.
FUNDAMENTALS OF NEUROPROSTHETICS
Based on a strong foundation of basic and clinical research, the broader field of neural engineering has facilitated the translation of implantable neurotechnology to treat neurological diseases. For example, deep brain stimulation (DBS) has proven to be a highly successful implantable neuromodulatory device for Parkinson’s Disease and essential tremor (Awan et al., 2009; Perlmutter and Mink, 2006). Cochlear implants have also proven successful at restoring hearing through conversion of sounds into patterned electrical stimulation of the cochlear nerve (Clark et al., 2013; Kral and Sharma, 2012; Merzenich et al., 1980). More recently, the NeuroPace® RNS® stimulator, a neuromodulatory device using ECoG electrodes, has been approved to treat medically refractory epilepsy (Morrell, 2011).
In contrast to stimulation-based devices, motor BMI systems aim to interpret a user’s intentions directly from measurements of neural activity and to thereby allow control of external devices for movement and/or communication (Figure 1). Firstly, either MEA or ECoG arrays are implanted in order to monitor neural activity in real-time (Figure 2). Secondly, a signal processor uses mathematical algorithms to “decode” neural signals into control signals. These control signals can then drive an ‘effector’, such as a cursor or a robotic limb. Finally, sensory feedback is important for improved control and reliability (Bensmaia and Miller, 2014; Carmena et al., 2003; Serruya et al., 2002; Suminski et al., 2010; Taylor et al., 2002). While this is typically provided through natural visual inputs to the nervous system, there is ongoing research into the development of methods to generate artificial sensory percepts resembling proprioception and tactile sensation (Bensmaia and Miller, 2014; Dadarlat et al., 2015; O’Doherty et al., 2011).
Research into invasive cortical neuroprosthetics has primarily used MEA (Carmena et al., 2003; Hochberg et al., 2006; Kennedy and Bakay, 1998; Schwartz, 2004; Serruya et al., 2002; Taylor et al., 2002; Wessberg et al., 2000) or ECoG based recordings(Crone et al., 1998a; Ganguly et al., 2009; Leuthardt et al., 2004; Rouse et al., 2013; Schalk et al., 2008; Slutzky et al., 2011; Vansteensel et al., 2010; Yanagisawa et al., 2012). MEAs consist of an array of microelectrodes that are directly inserted into cortical tissue and can record action potentials (also referred to as ‘spikes’) from single neurons or clusters of neurons (Hochberg and Donoghue, 2006; Homer et al., 2013; Kennedy and Bakay, 1998; Schwartz, 2004; Wessberg et al., 2000). ECoG electrodes are placed on the cortical surface (Leuthardt et al., 2006) or overlying the dura (Slutzky et al., 2011) and can record field potentials which likely represent an aggregate of local cortical information. Although MEA techniques provide both high spatial and temporal resolution (Buzsaki, 2010; Schwartz, 2004), it is also perhaps the most invasive technique as it penetrates the cortical tissue. However, one commonly noted limitation of MEAs is the compromise of signal processing performance due to instability of recorded neural signals over time (Bensmaia and Miller, 2014; Chestek et al., 2009; Flint et al., 2013; Ganguly and Carmena, 2009a; Ganguly and Carmena, 2010; Suner et al., 2005). There are, however, emerging methods that might mitigate some of the effects of signal instability(Bishop et al., 2014; Shenoy and Carmena, 2014). In contrast, a growing body of research has suggested that ECoG signals may be stable over a period of months (Chao et al., 2010; Leuthardt et al., 2006; Morrell, 2011). Whereas ECoG recordings has shown promise in the ability to predict movement parameters (Chang et al., 2010; Crone et al., 1998a; Crone et al., 1998b; Ganguly et al., 2009; Leuthardt et al., 2004; Schalk et al., 2008), it remains an open question if the complexity of neuroprosthetic control will match that of MEA recordings.
The fundamental concept underlying motor BMIs, i.e. real-time decoding from neural activity and volitional control of cortical activity, can be traced back to basic research conducted decades ago using single electrode recordings of spikes (Elbert et al., 1980; Fetz, 1969; Fetz and Finocchio, 1972; Kennedy and Bakay, 1998; Schmidt, 1980) (Georgopoulos, 1991; Green and Kalaska, 2011; Lemon, 1988; Riehle and Vaadia, 2005; Schwartz, 2004). With the subsequent development of MEA technology and advances in electronics/computational techniques, neural interfaces were shown to be able to interpret neural activity in real-time (Carmena et al., 2003; Hatsopoulos et al., 1998; Serruya et al., 2002; Taylor et al., 2002; Wessberg et al., 2000). These efforts ultimately led to impressive demonstrations of rodents, non-human primates, and humans controlling devices in real-time through modulation of neural signals (Carmena et al., 2003; Chapin et al., 1999; Collinger et al., 2013b; Hochberg et al., 2012; Hochberg et al., 2006; Musallam et al., 2004; Santhanam et al., 2006; Schwartz, 2004; Serruya et al., 2002; Taylor et al., 2002; Velliste et al., 2008). While only a select few references are added above, we refer interested readers to more detailed reviews of the extensive basic research underlying the development of cortical neuroprosthetics (Bensmaia and Miller, 2014; Donoghue, 2008; Fetz, 2007; Homer et al., 2013; Nicolelis and Lebedev, 2009; Schwartz, 2004).
Studies have also demonstrated that cortical field potential recordings using ECoG can also be a viable alternative. ECoG studies in able-bodied subjects undergoing invasive monitoring to map seizure foci demonstrated that ECoG signals are also rich in information about neural processes (Crone et al., 1998a; Crone et al., 1998b; Leuthardt et al., 2006; Leuthardt et al., 2004; Schalk et al., 2008) and can be used to decode movement parameters (Anderson et al., 2012; Chao et al., 2010; Fifer et al., 2011; Flint et al., 2014; Ganguly et al., 2009; Miller et al., 2007; Miller et al., 2009; Rouse et al., 2013; Sanchez et al., 2006; Schalk et al., 2008). ECoG has also shown promise in the ability to control a computer cursor (Leuthardt et al., 2004; Schalk et al., 2008) and in the ability to decode human speech (Cogan et al., 2014; Mugler et al., 2014; Pasley et al., 2012; Pei et al., 2011); more specifically, features of speech heard by a subject could be decoded from sensory cortical areas (Pasley et al., 2012) and it was possible to classify the identity of 1 of 7 phonemes above chance levels (Cogan et al., 2014).
TRANSLATION OF CORTICAL NEURPROSTHETICS
Several pilot studies have provided important proof-of-principle for the potential clinical translation of neuroprosthetics for movement control. The first studies were conducted in a subject with ALS who had progressed to a ‘locked-in’ state and an individual with a brainstem stroke. Using an implanted small MEA electrode, participants demonstrated volitional control of neural signals between ON/OFF states (Kennedy and Bakay, 1998) and the control of a cursor (Kennedy et al., 2000). A subsequent important milestone was attained in a tetraplegic subject chronically implanted with a 100-channel MEA; spiking activity was shown to be capable of controlling a computer cursor with 2 degrees of freedom, i.e. control of the x- and y- position on the screen (Hochberg et al., 2006). The same group also demonstrated that tetraplegic participants could directly control a robotic arm to perform reaching and grasp movements (Hochberg et al., 2012). More recently, two MEA arrays implanted into motor areas allowed a paralyzed subject to control a complex robotic arm to complete tasks adopted from a validated clinical scale (i.e. a subset of the Action Research Arm Test) (Collinger et al., 2013b). More specifically, the subject reliably completed a range of reaching and grasping tasks, averaging 17 seconds to complete each task; the use of clinical standards represents an important step forward towards developing standard benchmarks and metrics for clinical translation. Investigators have also implanted an ECoG grid in a SCI subject with a C4 lesion for a 28-day period; the subject demonstrated direct control of a cursor in both two and three dimensions (Wang et al. 2013). It remains unclear if ECoG recordings in paralyzed subjects can be used to control a complex prosthetic device to allow reaching and grasping.
To a large extent, the work described above for movement control is likely directly applicable to communication interfaces. Communication interfaces typically fall into two categories, cursor control to allow typing on regular or language optimized keyboards, or actual speech prosthesis control. Intracortical recording systems have been implanted in subjects with locked-in-syndrome and ALS for the control of spelling devices and artificial speech synthesizers (Bacher et al., 2015; Brumberg et al., 2011; Guenther et al., 2009). A true speech prosthesis, consisting of a two-channel electrode with a wireless interface implanted into the speech motor cortex, was demonstrated in a 26 year-old study participant in a locked-in state after a brainstem stroke to produce vowels with some accuracy (Guenther et al., 2009), as well as phonemes at a level much greater than chance (Brumberg et al., 2011). More recently, a MEA-based BMI interface was used in a subject with incomplete locked-in syndrome to demonstrate the feasibility of communication with a ‘neural point-and-click’ system (Bacher et al., 2015). Interestingly, the authors demonstrated a significant increase in the rate of communication over a standard QWERTY keyboard using a more optimized keyboard layout.
CHALLENGES AND FUTURE DIRECTIONS
While there have been very impressive demonstrations of the proof-of-principle of BMI technology, cortical neuroprosthetics have not yet been used outside of the research environment. Multiple technological and clinical issues have to be addressed before cortical neuroprosthetic devices can be adopted as a viable clinical treatment (Table 1).
Table 1.
Summary of challenges to BMI translation.
| Summary of Translational Challenges |
|---|
| Technological Challenges |
| Improve robustness of BMI technology (i.e. longevity of recording technology and long-term stability of signal monitoring and decoding). |
| Need for a fully implantable wireless system. |
| Minimize complexity and footprint of systems for ease of use. |
| Successful incorporation of somatosensory feedback to improve control. |
| Clinical Challenges |
| Define practical clinical goals for each clinical population and rehabilitation goal. |
| Demonstrate clinical safety and benefits over current standard of care. |
| Move from pilot phase studies to later stage clinical trials in the coming decade. |
An important challenge for both basic and clinical research efforts is to improve the robustness and the versatility of the technology. Robustness applies to many aspects of the system including longevity of the recording technology, stability of “decoding schemes” (Ganguly and Carmena, 2009b; Gilja et al., 2012) to convert neural signals into control signals, and integration with the device being controlled. Improving robustness is critical to establishing a long-term clinically viable BMI device. Moreover, in order to transition from the laboratory to the home, the complexity of current setups involving sophisticated, unwieldy electrophysiological workstations, head-mounted wires anchoring the subject to those workstations, and complex software & actuator systems should be minimized. Current studies seeking to translate non-invasive EEG-based systems to the home provide valuable insights into this process and the need for iterative approaches for product optimization (Leeb et al., 2013; Sellers et al., 2010). Additionally, laboratory setups are built to accomplish a single task under heavily monitored, carefully controlled conditions. Moving this setup into the home will require an increased focus on handling a wide range of tasks in unexpected conditions and situations. Moreover, while a lack of somatosensory feedback may not be an absolute limit for the restoration of arm function (Collinger et al., 2013b; Hochberg et al., 2012), next-generation BMIs that incorporate hand function will likely attain more robust and versatile control if sensory modalities such as tactile and proprioceptive feedback are successfully incorporated (Bensmaia and Miller, 2014; Suminski et al., 2009; Weber et al., 2007).
Focusing on a specific indication for cortical neuroprosthetics and meeting the particular needs of a patient population maybe the most fruitful next step. More specifically, developing a system that can eventually obtain FDA approval for a specific clinical indication could help the broader translational potential for intracortical neuroprosthetics. For example, individuals with severe disorders of communication (e.g. advanced ALS and locked-in) are often unable to effectively use current assistive devices. It is reasonable to hypothesize that current BMI technology may consistently deliver clinically realistic benefits in those with such severe deficits (Bacher et al., 2015). Thus, focus on a robust communication interface that can be used in the home setting is a reasonable translation goal. Further tailoring of the system could be done using the current approaches of surveys to elucidate patient needs (Anderson, 2004; Collinger et al., 2013a; Huggins et al., 2015; Huggins et al., 2011). This framework would also allow direct comparison with the functional benefits of non-invasive approaches and ultimately allow the field to fully develop a clinically realistic algorithm to definitively assess the risks and benefits of an intracortical communication interface.
From a purely clinical perspective, the goal of cortical neuroprosthetics is to improve function and quality of life. If they can both objectively and subjectively improve function significantly beyond that of current standards of care (i.e. fulltime caregiver assistance, upper limb assistive devices, eye-gaze tracking) in a safe manner, it is quite likely they will be readily adopted. An emphasis on subjective improvement may ensure that complex assistive technology is ultimately used on a daily basis (Kyberd and Hill, 2011; Leeb et al., 2013; Schultz et al., 2007). Ideally, this would be demonstrated in a clinical trial with outcome and safety measures that reflect both subjective and objective metrics. Development of common platforms and outcome measures should ensure direct comparisons between approaches with common metrics. The ARAT is an example of a useful, validated clinical rehabilitation scale recently incorporated into BMI translational research (Collinger et al., 2013b). BMI research studies for communication applications can adopt common metrics such as bits/minute and words/minute. Through such a process, immediate ethical concerns regarding whether the possible benefits of an implanted BMI outweigh the associated risks would be greatly reduced (McGie et al., 2013).
Even in the face of the numerous challenges to widespread adoption of BMI devices, it is worth examining parallels with more established implantable technologies (i.e. DBS, cochlear implants). The wealth of evidence supporting the safety and efficacy of these treatments greatly simplifies the clinical and surgical decision-making. For example, after the introduction of DBS in the 1980’s (Olanow, 2002; Perlmutter and Mink, 2006), there was more widespread clinical use in the late 1990s (Perlmutter and Mink, 2006; Weaver et al., 2009). The first large multicenter clinical study in Parkinson’s patients was conducted in 2001(Deep-Brain Stimulation for Parkinson’s Disease Study, 2001), which led to FDA approval of DBS in 2002 for the treatment of advanced Parkinson’s disease. While current translational efforts for BMI technology are at an early pilot phase based either on FDA Investigational Device Exemptions (Collinger et al., 2013b; Guenther et al., 2009; Hochberg and Donoghue, 2006; Hochberg et al., 2006; Kennedy et al., 2000) or ‘off-label’ use of a device approved by a local Institutional Review Board (Wang et al., 2013), with continued advancements and dedicated clinical efforts, the hope is to advance to later stage clinical trials in the coming decade.
SUMMARY
Cortical neuroprosthetics have advanced significantly in the last decade. While there are numerous challenges remaining, a growing body of literature strongly supports the great potential for this technology to transform the lives of people with communication and motor disability. Major milestones to meet for the coming decade include the development of a robust, long-term implantable device, optimization of the BMI system to meet the prioritized needs of subjects with disability and caregivers, and demonstration of consistent safety and efficacy of the device through later stage clinical trials. Moreover, while broad restorative goals have helped drive the basic research forward, from a clinical and translational point-of-view, focus on a specific indication (e.g. communication interface for the most-disabled) may help overcome real-world challenges and lead to FDA approval of a “platform-device” that can further drive the field forward.
It is perhaps worth returning to our patient with ALS. His condition has proven to be relentlessly progressive and his greatest concern is the lack of a robust communication interface. In his case, even in the face of a progressive disorder, he will likely choose to get mechanical ventilation. Importantly, he has both the financial and social support to enable this option (Malik et al., 2014). Thus, the development of a robust communication interface will serve a great clinical need for him and others with severe disorders of communication. For now at least, a clinical benchmark for comparison is the eye-gaze tracking communication system. While this is perhaps adequate for now, his best future option, at least based on present advancements, is to seek enrollment in a clinical trial at a major academic center. This referral will hopefully also include evaluation of non-invasive (e.g. EEG-based) approaches in addition to intracortical approaches. Based on the rapid progress of this field, it is perhaps not overly optimistic to anticipate more widespread late-phase clinical trials for intracortical communication interfaces over the next decade.
Acknowledgments
This work was supported by the Burroughs Wellcome Fund, the Doris Duke Charitable Foundation and departmental funds from the UCSF Department of Neurology.
References
- Andersen RA, et al. Cognitive neural prosthetics. Annu Rev Psychol. 2010;61:169–90. C1–3. doi: 10.1146/annurev.psych.093008.100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–83. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Anderson NR, et al. Electrocorticographic (ECoG) correlates of human arm movements. Exp Brain Res. 2012;223:1–10. doi: 10.1007/s00221-012-3226-1. [DOI] [PubMed] [Google Scholar]
- Awan NR, et al. Deep brain stimulation: current and future perspectives. Neurosurgical Focus. 2009;27 doi: 10.3171/2009.4.FOCUS0982. [DOI] [PubMed] [Google Scholar]
- Bacher D, et al. Neural Point-and-Click Communication by a Person With Incomplete Locked-In Syndrome. Neurorehabil Neural Repair. 2015;29:462–71. doi: 10.1177/1545968314554624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensmaia SJ, Miller LE. Restoring sensorimotor function through intracortical interfaces: progress and looming challenges. Nat Rev Neurosci. 2014;15:313–25. doi: 10.1038/nrn3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N, et al. A spelling device for the paralysed. Nature. 1999;398:297–8. doi: 10.1038/18581. [DOI] [PubMed] [Google Scholar]
- Bishop W, et al. Self-recalibrating classifiers for intracortical brain-computer interfaces. J Neural Eng. 2014;11:026001. doi: 10.1088/1741-2560/11/2/026001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumberg JS, et al. Classification of intended phoneme production from chronic intracortical microelectrode recordings in speech-motor cortex. Front Neurosci. 2011;5:65. doi: 10.3389/fnins.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock IM, Dollar AM. Classifying human manipulation behavior. IEEE Int Conf Rehabil Robot. 2011;2011:5975408. doi: 10.1109/ICORR.2011.5975408. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68:362–85. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena JM, et al. Learning to control a brain-machine interface for reaching and grasping by primates. Plos Biology. 2003;1:193–208. doi: 10.1371/journal.pbio.0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, et al. Categorical speech representation in human superior temporal gyrus. Nat Neurosci. 2010;13:1428–32. doi: 10.1038/nn.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao ZC, et al. Long-term asynchronous decoding of arm motion using electrocorticographic signals in monkeys. Front Neuroengineering. 2010;3:3. doi: 10.3389/fneng.2010.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin JK, et al. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nature Neuroscience. 1999;2:664–70. doi: 10.1038/10223. [DOI] [PubMed] [Google Scholar]
- Chestek CA, et al. Neural prosthetic systems: Current problems and future directions. Conf Proc IEEE Eng Med Biol Soc. 2009;1:3369–75. doi: 10.1109/IEMBS.2009.5332822. [DOI] [PubMed] [Google Scholar]
- Clark GM, et al. The evolving science of cochlear implants. JAMA. 2013;310:1225–6. doi: 10.1001/jama.2013.278142. [DOI] [PubMed] [Google Scholar]
- Cogan GB, et al. Sensory-motor transformations for speech occur bilaterally. Nature. 2014;507:94–8. doi: 10.1038/nature12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinger JL, et al. Functional priorities, assistive technology, and brain-computer interfaces after spinal cord injury. J Rehabil Res Dev. 2013a;50:145–60. doi: 10.1682/jrrd.2011.11.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinger JL, et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2013b;381:557–64. doi: 10.1016/S0140-6736(12)61816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell AS, et al. Standard task set for evaluating rehabilitation interventions for individuals with arm paralysis. J Rehabil Res Dev. 2012;49:395–403. doi: 10.1682/jrrd.2011.03.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRDF. Paralysis and Spinal Cord Injury in the United States. 2009 http://www.christopherreeve.org.
- Crone NE, et al. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998a;121(Pt 12):2301–15. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- Crone NE, et al. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain. 1998b;121(Pt 12):2271–99. doi: 10.1093/brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- Dadarlat MC, et al. A learning-based approach to artificial sensory feedback leads to optimal integration. Nat Neurosci. 2015;18:138–44. doi: 10.1038/nn.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deep-Brain Stimulation for Parkinson’s Disease Study G. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345:956–63. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- Donoghue JP. Bridging the brain to the world: a perspective on neural interface systems. Neuron. 2008;60:511–21. doi: 10.1016/j.neuron.2008.10.037. [DOI] [PubMed] [Google Scholar]
- Elbert T, et al. Biofeedback of slow cortical potentials. I. Electroencephalogr Clin Neurophysiol. 1980;48:293–301. doi: 10.1016/0013-4694(80)90265-5. [DOI] [PubMed] [Google Scholar]
- Elliott JM, Connolly KJ. A classification of manipulative hand movements. Dev Med Child Neurol. 1984;26:283–96. doi: 10.1111/j.1469-8749.1984.tb04445.x. [DOI] [PubMed] [Google Scholar]
- Ethier C, et al. Brain-controlled neuromuscular stimulation to drive neural plasticity and functional recovery. Curr Opin Neurobiol. 2015;33:95–102. doi: 10.1016/j.conb.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier C, et al. Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature. 2012;485:368–71. doi: 10.1038/nature10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE. Operant conditioning of cortical unit activity. Science. 1969;163:955–8. doi: 10.1126/science.163.3870.955. [DOI] [PubMed] [Google Scholar]
- Fetz EE. Volitional control of neural activity: implications for brain-computer interfaces. J Physiol. 2007;579:571–9. doi: 10.1113/jphysiol.2006.127142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE, Finocchio DV. Operant conditioning of isolated activity in specific muscles and precentral cells. Brain Research. 1972;40:19–23. doi: 10.1016/0006-8993(72)90100-x. [DOI] [PubMed] [Google Scholar]
- Fifer MS, et al. Asynchronous decoding of grasp aperture from human ECoG during a reach-to-grasp task. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:4584–7. doi: 10.1109/IEMBS.2011.6091135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint RD, et al. Extracting kinetic information from human motor cortical signals. Neuroimage. 2014;101:695–703. doi: 10.1016/j.neuroimage.2014.07.049. [DOI] [PubMed] [Google Scholar]
- Flint RD, et al. Long term, stable brain machine interface performance using local field potentials and multiunit spikes. J Neural Eng. 2013;10:056005. doi: 10.1088/1741-2560/10/5/056005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Carmena JM. Emergence of a stable cortical map for neuroprosthetic control. Plos Biology. 2009a;7:e1000153. doi: 10.1371/journal.pbio.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Carmena JM. Emergence of a stable cortical map for neuroprosthetic control. PLoS Biol. 2009b;7:e1000153. doi: 10.1371/journal.pbio.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Carmena JM. Neural correlates of skill acquisition with a cortical brain-machine interface. J Mot Behav. 2010;42:355–60. doi: 10.1080/00222895.2010.526457. [DOI] [PubMed] [Google Scholar]
- Ganguly K, et al. Cortical representation of ipsilateral arm movements in monkey and man. Journal of Neuroscience. 2009;29:12948–56. doi: 10.1523/JNEUROSCI.2471-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP. Higher order motor control. Annu Rev Neurosci. 1991;14:361–77. doi: 10.1146/annurev.ne.14.030191.002045. [DOI] [PubMed] [Google Scholar]
- Gilja V, et al. A high-performance neural prosthesis enabled by control algorithm design. Nat Neurosci. 2012;15:1752–7. doi: 10.1038/nn.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AM, Kalaska JF. Learning to move machines with the mind. Trends Neurosci. 2011;34:61–75. doi: 10.1016/j.tins.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Guenther FH, et al. A Wireless Brain-Machine Interface for Real-Time Speech Synthesis. Plos One. 2009;4 doi: 10.1371/journal.pone.0008218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsopoulos NG, et al. Information about movement direction obtained from synchronous activity of motor cortical neurons. Proc Natl Acad Sci U S A. 1998;95:15706–11. doi: 10.1073/pnas.95.26.15706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg LR, et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485:372–5. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg LR, Donoghue JP. Sensors for brain-computer interfaces. IEEE Eng Med Biol Mag. 2006;25:32–8. doi: 10.1109/memb.2006.1705745. [DOI] [PubMed] [Google Scholar]
- Hochberg LR, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–71. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- Homer ML, et al. Sensors and decoding for intracortical brain computer interfaces. Annu Rev Biomed Eng. 2013;15:383–405. doi: 10.1146/annurev-bioeng-071910-124640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins JE, et al. What would brain-computer interface users want: opinions and priorities of potential users with spinal cord injury. Arch Phys Med Rehabil. 2015;96:S38–45. e1–5. doi: 10.1016/j.apmr.2014.05.028. [DOI] [PubMed] [Google Scholar]
- Huggins JE, et al. What would brain-computer interface users want? Opinions and priorities of potential users with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2011;12:318–24. doi: 10.3109/17482968.2011.572978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PR. ‘Locked-in’ patients. Neurology. 1994;44:366–7. doi: 10.1212/wnl.44.2.366-a. [DOI] [PubMed] [Google Scholar]
- Kennedy PR, Bakay RA. Restoration of neural output from a paralyzed patient by a direct brain connection. Neuroreport. 1998;9:1707–11. doi: 10.1097/00001756-199806010-00007. [DOI] [PubMed] [Google Scholar]
- Kennedy PR, et al. Direct control of a computer from the human central nervous system. IEEE Trans Rehabil Eng. 2000;8:198–202. doi: 10.1109/86.847815. [DOI] [PubMed] [Google Scholar]
- Kral A, Sharma A. Developmental neuroplasticity after cochlear implantation. Trends Neurosci. 2012;35:111–22. doi: 10.1016/j.tins.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler A, et al. Brain-computer communication: unlocking the locked in. Psychol Bull. 2001;127:358–75. doi: 10.1037/0033-2909.127.3.358. [DOI] [PubMed] [Google Scholar]
- Kyberd PJ, Hill W. Survey of upper limb prosthesis users in Sweden, the United Kingdom and Canada. Prosthet Orthot Int. 2011;35:234–41. doi: 10.1177/0309364611409099. [DOI] [PubMed] [Google Scholar]
- Leeb R, et al. Transferring brain-computer interfaces beyond the laboratory: successful application control for motor-disabled users. Artif Intell Med. 2013;59:121–32. doi: 10.1016/j.artmed.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Lemon R. The output map of the primate motor cortex. Trends Neurosci. 1988;11:501–6. doi: 10.1016/0166-2236(88)90012-4. [DOI] [PubMed] [Google Scholar]
- Leuthardt EC, et al. The emerging world of motor neuroprosthetics: A neurosurgical perspective. Neurosurgery. 2006;59:1–13. doi: 10.1227/01.NEU.0000221506.06947.AC. [DOI] [PubMed] [Google Scholar]
- Leuthardt EC, et al. A brain-computer interface using electrocorticographic signals in humans. Journal of Neural Engineering. 2004;1:63–71. doi: 10.1088/1741-2560/1/2/001. [DOI] [PubMed] [Google Scholar]
- Malik R, et al. Amyotrophic lateral sclerosis. Semin Neurol. 2014;34:534–41. doi: 10.1055/s-0034-1396007. [DOI] [PubMed] [Google Scholar]
- McGie S, et al. Clinical ethical concerns in the implantation of brain-machine interfaces: part I: overview, target populations, and alternatives. IEEE Pulse. 2013;4:28–32. doi: 10.1109/MPUL.2012.2228810. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, et al. Cochlear implant prostheses: strategies and progress. Ann Biomed Eng. 1980;8:361–8. doi: 10.1007/BF02363439. [DOI] [PubMed] [Google Scholar]
- del Millan JR, Carmena JM. Invasive or noninvasive: understanding brain-machine interface technology. IEEE Eng Med Biol Mag. 2010;29:16–22. doi: 10.1109/memb.2009.935475. [DOI] [PubMed] [Google Scholar]
- Miller KJ, et al. Spectral changes in cortical surface potentials during motor movement. Journal of Neuroscience. 2007;27:2424–32. doi: 10.1523/JNEUROSCI.3886-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, et al. Decoupling the cortical power spectrum reveals real-time representation of individual finger movements in humans. Journal of Neuroscience. 2009;29:3132–7. doi: 10.1523/JNEUROSCI.5506-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti MM, et al. Willful Modulation of Brain Activity in Disorders of Consciousness. New England Journal of Medicine. 2010;362:579–589. doi: 10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- Moritz CT, et al. Direct control of paralysed muscles by cortical neurons. Nature. 2008;456:639–42. doi: 10.1038/nature07418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- Moxon KA, Foffani G. Brain-machine interfaces beyond neuroprosthetics. Neuron. 2015;86:55–67. doi: 10.1016/j.neuron.2015.03.036. [DOI] [PubMed] [Google Scholar]
- Mugler EM, et al. Direct classification of all American English phonemes using signals from functional speech motor cortex. J Neural Eng. 2014;11:035015. doi: 10.1088/1741-2560/11/3/035015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musallam S, et al. Cognitive control signals for neural prosthetics. Science. 2004;305:258–62. doi: 10.1126/science.1097938. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Lebedev MA. Principles of neural ensemble physiology underlying the operation of brain-machine interfaces. Nat Rev Neurosci. 2009;10:530–40. doi: 10.1038/nrn2653. [DOI] [PubMed] [Google Scholar]
- Nijboer F, et al. A P300-based brain-computer interface for people with amyotrophic lateral sclerosis. Clinical Neurophysiology. 2008;119:1909–16. doi: 10.1016/j.clinph.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JE, et al. Active tactile exploration using a brain-machine-brain interface. Nature. 2011;479:228–31. doi: 10.1038/nature10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW. Surgical therapy for Parkinson’s disease. Eur J Neurol. 2002;9(Suppl 3):31–9. doi: 10.1046/j.1468-1331.9.s3.4.x. [DOI] [PubMed] [Google Scholar]
- Pasley BN, et al. Reconstructing speech from human auditory cortex. PLoS Biol. 2012;10:e1001251. doi: 10.1371/journal.pbio.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei X, et al. Decoding vowels and consonants in spoken and imagined words using electrocorticographic signals in humans. J Neural Eng. 2011;8:046028. doi: 10.1088/1741-2560/8/4/046028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229–57. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik L, et al. Controlling a multi-degree of freedom upper limb prosthesis using foot controls: user experience. Disabil Rehabil Assist Technol. 2014;9:318–29. doi: 10.3109/17483107.2013.822024. [DOI] [PubMed] [Google Scholar]
- Resnik L, et al. Advanced upper limb prosthetic devices: implications for upper limb prosthetic rehabilitation. Arch Phys Med Rehabil. 2012;93:710–7. doi: 10.1016/j.apmr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Riehle A, Vaadia E. Motor cortex in voluntary movements : a distributed system for distributed functions. CRC Press; Boca Raton: 2005. [Google Scholar]
- Rouse AG, et al. Cortical adaptation to a chronic micro-electrocorticographic brain computer interface. J Neurosci. 2013;33:1326–30. doi: 10.1523/JNEUROSCI.0271-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JC, et al. Analysis of amplitude modulated control features for ECoG neuroprosthetics. Conf Proc IEEE Eng Med Biol Soc. 2006;1:5468–71. doi: 10.1109/IEMBS.2006.259587. [DOI] [PubMed] [Google Scholar]
- Santhanam G, et al. A high-performance brain-computer interface. Nature. 2006;442:195–8. doi: 10.1038/nature04968. [DOI] [PubMed] [Google Scholar]
- Schalk G, et al. Two-dimensional movement control using electrocorticographic signals in humans. Journal of Neural Engineering. 2008;5:75–84. doi: 10.1088/1741-2560/5/1/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EM. Single neuron recording from motor cortex as a possible source of signals for control of external devices. Annals of Biomedical Engineering. 1980;8:339–49. doi: 10.1007/BF02363437. [DOI] [PubMed] [Google Scholar]
- Schultz AE, et al. Expert opinions on success factors for upper-limb prostheses. J Rehabil Res Dev. 2007;44:483–9. doi: 10.1682/jrrd.2006.08.0087. [DOI] [PubMed] [Google Scholar]
- Schwartz AB. Cortical neural prosthetics. Annual Review of Neuroscience. 2004;27:487–507. doi: 10.1146/annurev.neuro.27.070203.144233. [DOI] [PubMed] [Google Scholar]
- Sellers EW, et al. A brain-computer interface for long-term independent home use. Amyotroph Lateral Scler. 2010;11:449–55. doi: 10.3109/17482961003777470. [DOI] [PubMed] [Google Scholar]
- Selzer ME, et al. Textbook of neural repair and rehabilitation. Cambridge University Press; Cambridge: 2014. [Google Scholar]
- Serruya MD, et al. Instant neural control of a movement signal. Nature. 2002;416:141–2. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- Shenoy KV, Carmena JM. Combining decoder design and neural adaptation in brain-machine interfaces. Neuron. 2014;84:665–80. doi: 10.1016/j.neuron.2014.08.038. [DOI] [PubMed] [Google Scholar]
- Silvoni S, et al. Brain-computer interface in stroke: a review of progress. Clin EEG Neurosci. 2011;42:245–52. doi: 10.1177/155005941104200410. [DOI] [PubMed] [Google Scholar]
- Slutzky MW, et al. Decoding the rat forelimb movement direction from epidural and intracortical field potentials. J Neural Eng. 2011;8:036013. doi: 10.1088/1741-2560/8/3/036013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spataro R, et al. The eye-tracking computer device for communication in amyotrophic lateral sclerosis. Acta Neurol Scand. 2014;130:40–5. doi: 10.1111/ane.12214. [DOI] [PubMed] [Google Scholar]
- Suminski AJ, et al. Incorporating feedback from multiple sensory modalities enhances brain-machine interface control. J Neurosci. 2010;30:16777–87. doi: 10.1523/JNEUROSCI.3967-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suminski AJ, et al. Exploiting multiple sensory modalities in brain-machine interfaces. Neural Networks. 2009;22:1224–1234. doi: 10.1016/j.neunet.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suner S, et al. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. Ieee Transactions on Neural Systems and Rehabilitation Engineering. 2005;13:524–541. doi: 10.1109/TNSRE.2005.857687. [DOI] [PubMed] [Google Scholar]
- Taylor DM, et al. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296:1829–32. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- Vansteensel MJ, et al. Brain-computer interfacing based on cognitive control. Ann Neurol. 2010;67:809–16. doi: 10.1002/ana.21985. [DOI] [PubMed] [Google Scholar]
- Velliste M, et al. Cortical control of a prosthetic arm for self-feeding. Nature. 2008;453:1098–1101. doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- Wang W, et al. An electrocorticographic brain interface in an individual with tetraplegia. PLoS One. 2013;8:e55344. doi: 10.1371/journal.pone.0055344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver FM, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber DJ, et al. Limb-state feedback from ensembles of simultaneously recorded dorsal root ganglion neurons. J Neural Eng. 2007;4:S168–80. doi: 10.1088/1741-2560/4/3/S04. [DOI] [PubMed] [Google Scholar]
- Wessberg J, et al. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature. 2000;408:361–5. doi: 10.1038/35042582. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, et al. Brain-computer interfaces for communication and control. Clinical Neurophysiology. 2002;113:767–791. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]
- Yanagisawa T, et al. Electrocorticographic control of a prosthetic arm in paralyzed patients. Ann Neurol. 2012;71:353–61. doi: 10.1002/ana.22613. [DOI] [PubMed] [Google Scholar]