Abstract
Integrins are a family of heterodimeric transmembrane receptors that mediate adhesion to the extracellular matrix (ECM). In addition to their role as adhesion receptors, integrins can act as “bidirectional signal transducers” that coordinate a large number of cellular activities in response to the extracellular environment and intracellular signaling events. This bidirectional signaling helps maintain tissue homeostasis. Dysregulated bidirectional signaling, however, could trigger the propagation of feedback loops that can lead to the establishment of a disease state such as glaucoma. Here we discuss the role of integrins and bidirectional signaling as they relate to the glaucomatous phenotype with special emphasis on the αvβ3 integrin. We present evidence that this particular integrin may have a significant impact on the pathogenesis of glaucoma.
Keywords: Integrins, Trabecular meshwork, Optic nerve head, Glaucoma, Extracellular matrix
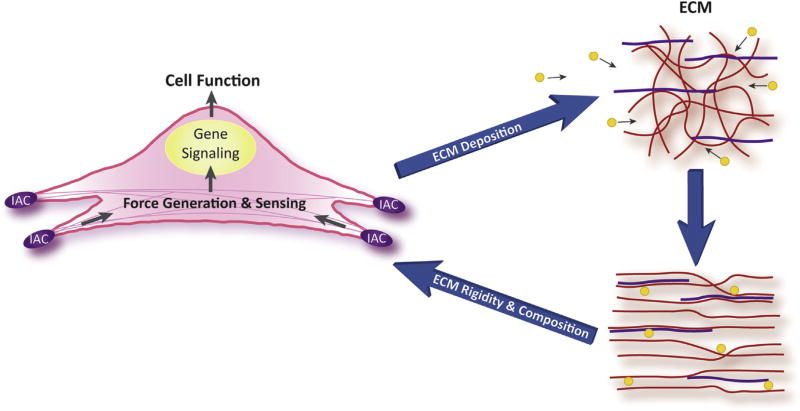
Glaucoma is a heterogeneous disease that is characterized by a number of changes in the trabecular meshwork and the optic nerve head (Morrison et al., 2005; Stamer and Acott, 2012; Braunger et al., 2015; Wallace et al., 2015; Downs, 2015; Schneider and Fuchshofer, 2016). The phenotypic changes most often associated with glaucoma involve the actomyosin-based contractile properties of the trabecular meshwork (TM), compliance of the extracellular matrix (ECM) and the types and amounts of proteins deposited in the ECM in both the TM and optic nerve head (ONH). In reality these changes are all interconnected and likely the result of bidirectional signaling between the ECM and cells. Bidirectional signaling involves the assessment of the tissue’s microenvironment by its resident cells to maintain tissue homeostasis (Acott et al., 2014). When changes in the compliance or composition of the ECM in the microenvironment occur, these changes are sensed by the resident cells (Fig. 1). The resident cells then respond by activating signaling pathways that may lead to other changes in cell function, the ECM or expression of cell receptors, for example.
Fig. 1.
Bidirectional signaling. The figure illustrates how changes made to the extracellular matrix (ECM) by the cell can in turn influence the signals perceived by the integrin adhesion complex (IAC) and alter the biological properties of the cell. Changes perceived by the IAC can be chemical in nature such as the expression of new ECM proteins (yellow circles) or physical due to a change in the 3-D architecture of the ECM or rigidity of the ECM. These physical changes can be triggered by contractile forces applied by cells through the IAC.
Bidirectional communication between cells and the extracellular environment is often directed by several classes of cell surface receptors. Perhaps the best characterized family of receptors involved in bidirectional communication is the integrins (Humphries et al., 2006; Barczyk et al., 2010; Gagen et al., 2014). How integrins respond to changes in the TM or the ONH is likely to contribute to the development of glaucoma and will be the subject of this review.
The review will emphasize the changes in integrin signaling induced by TGFβ2 and glucocorticoids. TGFβ2 levels are increased in most patients with primary open angle glaucoma (POAG) (Fuchshofer, 2011; Fuchshofer and Tamm, 2012; Wordinger et al., 2014) and treatments with glucocorticoids can lead to the development of ocular hypertension and an increased risk of developing POAG (Clark and Wordinger, 2009). Changes observed with glucocorticoid treatment also tend to mimic those observed in POAG patients and following TGFβ2 treatment. In addition, we will discuss the mechanosensitivity of integrins to mechanical forces that may be associated with glaucoma (Tamm, 2009; WuDunn, 2009; Tan et al., 2006).
1. Integrins
Integrins are a family of 24 transmembrane receptors (Humphries et al., 2006; Barczyk et al., 2010; Gagen et al., 2014). Each integrin is a heterodimer composed of an α- and a β-subunit and must be assembled as a heterodimer within the endoplasmic reticulum in order to be expressed on the cell surface (Bouvard et al., 2013). The individual α and β subunits are mixed and matched to form the 24members of the integrin family each having tissue-specific biological properties. Three of the α-subunits and four of the β-subunits can be expressed as alternatively spliced variants giving rise to integrins with different biological properties (Van der Flier and Sonnenberg, 2001). Almost all cells express one or more members of the integrin family, although the repertoire of integrin expression can vary during development, ageing, and in response to environmental conditions.
The major ligands for integrins are ECM proteins. It is generally thought that integrins bind ECM proteins via an arginine-glycine-aspartic acid (RGD) sequence within the ligands. However, only a third of integrins are known to bind this sequence in native ECM molecules. In ECM proteins like collagen and laminin, these RGD sequences are buried and are only available following degradation or denaturation of their fibrillar structure which limits these ECM-integrin interactions to specific circumstances (Barczyk et al., 2010). To bind collagens assembled into native fibrils, integrins use the consensus sequence GFOGER in the triple helical molecule. Accessibility to this binding sequence, however, is also limited and dependent on the fibrillar structure in vivo. This suggests that the collagen binding integrins may have a limited role in tissue homeostasis. The recognition sequence used by integrins to bind native, non-denatured laminin is unknown.
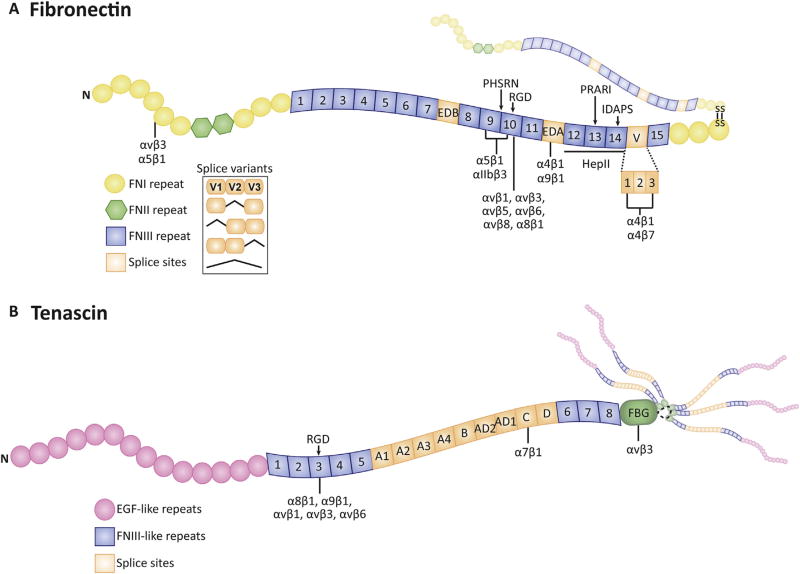
An additional level of complexity with integrin binding to its ECM ligand comes from the fact that some integrins also rely on auxiliary binding sites within the ECM ligand (Mould et al., 1997; Redick et al., 2000; Adair et al., 2005). The best characterized examples of this are the α5β1 and α4β1 fibronectin binding integrins. As shown in Fig. 2, the α5β1 integrin uses both the canonical RGD site and an auxiliary site called the synergy site (PHSRN) within a neighboring FN III module to bind fibronectin (Obara et al., 1988; Aota et al., 1994) while the α4β1 integrin uses the RGD homologue, IDAPS, and an auxiliary PRARI sequence in the 13th and 14th FN III modules to bind fibronectin (Sharma et al., 1999).
Fig. 2.
Integrin binding sites in fibronectin and tenascin-C. The figure illustrates the multiple integrin binding sites in fibronectin and tenascin-C and how alternative splicing would affect the availability of these sites. (A) Fibronectin is a dimeric protein disulfide bonded at its carboxyl terminus. It can be composed of two identical polypeptide chains or composed of two nonidentical chains due to alternative splicing of the EDA, EDB, and V region. It is composed of three types of repeating modules (FN-I, FN-II, and FN-III) and a variable region. Both chains can contain the integrin binding sites indicated which would essentially double the number of integrins bound to fibronectin. (B) Tenascin-C is an oligomeric protein consisting of six polypeptide chains. It consists of three repeating modules of heptad repeats, EGF-like repeats, FN-III repeats, and a C-terminal globular domain. Alternative splicing of the FN-III repeats results in a multitude of non-identical tenascin-C subunits differing in number and identity of FN-III repeats and integrin binding sites. Oligomerization of the hexamer is mediated by coiled-coil interactions and stabilized by disulfide bonds.
Another factor affecting integrin binding is that several ECM proteins undergo alternative splicing which generates or removes integrin binding sites from the ECM. The best-known example of this is fibronectin which can bind 12 different integrins (Fig. 2). Fibronectin is a modular glycoprotein found in the TM (Acott and Kelley, 2008) and in ONH cells in culture (Zode et al., 2009). It contains two alternatively spliced sites, generated by exon splicing, called the extra domains A (EDA) and B (EDB). There is also a third site called the variable (V) region which undoes alternative splicing to generate five isoforms of the V region in humans (Hynes, 1990). The fibronectin isoform containing the EDA domain provides an additional binding site for α4β1, α4β7 and α9β1 integrins (Leiss et al., 2008; White and Muro, 2011). Both the EDA and EDB iso-forms of fibronectin are detected in cultures of human TM cells (Medina-Ortiz et al., 2013) (Filla et al., manuscript in preparation). Both the EDA and EDB isoforms are upregulated in response to TGFβ2 (Medina-Ortiz et al., 2013) while dexamethasone upregulates the EDA isoform (Filla et al. manuscript in preparation). Alternative splicing of the V-region removes binding sites for α4β1 and α4β7 integrins (Leiss et al., 2008; White and Muro, 2011). In all these splice variants, the canonical RGD integrin binding site in fibronectin is still present, however, inclusion of the EDA and EDB domains have been reported to alter the cell-adhesive activity of α5β1 integrin to the RGD site. Presumably insertion of the EDA or EDB domains alters the spatial relationship between the RGD and synergy sites utilized by α5β1 integrin (Leahy et al., 1996; Manabe et al., 1997; Hashimoto-Uoshima et al., 1997).
Other ECM proteins can undergo alternative splicing as well. For example, tenascin C, collagen type XII, CD44 and versican expressed by TM cells are alternatively spliced in response to stretch (Keller et al., 2007). As shown in Fig. 2, alternative splicing of tenascin C would remove the α7β1 integrin binding site while leaving two other integrin binding sites capable of binding six separate integrins unaffected (Golledge et al., 2011; Yoshida et al., 2015). Alternative splicing of versican, on the other hand, would not affect its β1 integrin binding site (Wu et al., 2005), and to date no integrin binding sites have been reported in collagen type XII or CD44.
2. Integrins in the TM
At least eleven different integrins have been identified in the cells associated with the TM in the outflow pathway (Table 1). These integrins show a broad distribution and are found along the trabecular beams and in the juxtacanalicular tissue (JCT) demonstrating that multiple integrins are found on cells throughout the TM (Zhou et al., 1999a). Upon culturing, the integrin profile of TM cells appears to change slightly. For instance, the β4 subunit has been reported to be downregulated in cultured cells (Zhou et al., 1999a) and a similar finding has been observed with the β3 integrin. The β3 integrin is downregulated in an immortalized TM cell line even though it was found in normal TM cells in culture (Gagen et al., 2013).
Table 1.
Integrins Identified in Trabecular Meshwork and Optic Nerve Head in vitro and in situ (Zhou et al., 1995; Tervo et al., 1995; Diskin et al., 2009). The identity of these integrins is based onknown pairings between subunits (Humphries et al., 2006).
| Integrin | Location found |
Ligands | Consensus Motif |
Detection method(s) |
References |
|---|---|---|---|---|---|
| α1β1 | TM | Collagens (I, IV, IX), Laminin,Galectin-8 | GFOGER | IHC in situ and IP | (Zhou et al., 1999a) |
| α2β1 | TM and ONH | Collagens (I, III, IV, IX), Laminin, E-cadherin, TSP-1, Tenascin-C | GFOGER | IP and RT-PCR | (Zhou et al., 1999a; Morrison, 2006) |
| α3β1 | TM and ONH | Laminin, TSP-1, Galectin-8 | Not Identified | IHC/IF in situ and IP | (Tervo et al., 1995; Zhou et al., 1999a; Morrison, 2006) |
| α4β1 | TM and ONH | Fibronectin, VCAM-1, ICAM-4, TSP-1, Osteopontin, ADAM2 | LI/-D/E-V/S/T-P/S (LDV) | IHC in situ, IF in tissue culture, Flow, and RT-PCR | (Zhou et al., 1999a; Morrison, 2006; Schwinn et al., 2010; Faralli et al., 2011; Neumann et al., 2014) |
| α5β1 | TM and ONH | Fibronectin, Fibrillin, Osteopontin, TSP-1, Galectin-8, ADAM9,12,15,23 | RGD | IF in tissue culture, IP and RT-PCR | (Zhou et al., 1999a; Morrison, 2006; Junglass et al., 2009; Faralli et al., 2011; Neumann et al., 2014) |
| α6β1 | TM and ONH | Laminin, TSP-1, ADAM9,12,15,23 | Not Identified | IHC/IF in situ and IP | (Tervo et al., 1995; Zhou et al., 1999a; Morrison, 2006) |
| α6β4* | TM and ONH | Laminin | Not Identified | IHC/IF in situ, IP | (Tervo et al., 1995; Zhou et al., 1999a; Morrison, 2006; Perkumas and Stamer, 2012) |
| α9β1 | TM and ONH | Fibronectin | EDGIHEL | RT-PCR | (Neumann et al., 2014; Park et al., 2014) |
| αvβ1 | TM and ONH | Fibronectin, Vitronectin, Osteopontin, ADAM9,12,15,23, Galectin-8, LAP-TGF-β | RGD | IHC/IF in situ, RT-PCR, IP | (Zhou et al., 1999a; Morrison, 2006; Junglass et al., 2009) |
| αvβ3** | TM and ONH | Fibronectin, Vitronectin, TSP-1,2, tenascin, and Collagen IV, CTGF, MMP-2, PECAM-1, Fibrillin, LAP-TGF-β, Osteopontin | RGD | IHC/IF in situ, IF in tissue culture, Flow IP and PCR | (Tervo et al., 1995; Zhou et al., 1999a; Morrison, 2006; Filla et al., 2006; Filla et al., 2009; Filla et al., 2011; Gagen et al., 2013; Faralli et al., 2013; Pattabiraman and Rao, 2015) |
| αvβ5 | TM and ONH | Fibronectin, Vitronectin, LAP-TGF-β, Osteopontin, ADAM9,12,15,23 | RGD | IF in tissue culture and PCR | (Tervo et al., 1995; Zhou et al., 1999a; Morrison, 2006; Gagen et al., 2013; Neumann et al., 2014) |
β4 subunit has not been detectedinTM cell cultures.
The splice variants for β3A and β3B were detected in TM cell cultures by PCR (unpublished data). Splice variants for β1, β5, α3 and α6 integrin subunits have not been detected in TM or ONH.
Expression of the α2, α5, αv, and β3 integrin subunits is altered by dexamethasone treatment of TM cultures (Dickerson et al.,1998; Faralli et al., 2013), while the expression of the integrin subunits αv and β1 is altered by connective tissue growth factor (CTGF) in cultured TM cells (Junglass et al., 2009). As of yet, no difference in the expression patterns of any of these integrin subunits has been reported in normal versus glaucomatous derived TM cell lines (Dickerson et al., 1998).
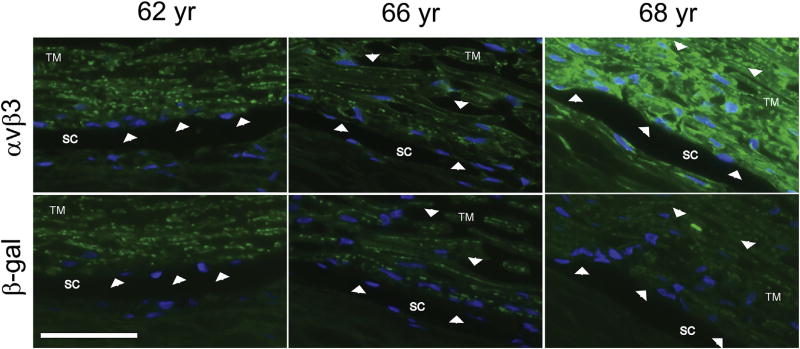
At least 5 integrin subunits have been identified in human Schlemm’s canal (SC) cells. Based on normal pairing patterns, this would suggest that SC cells express the collagen binding integrin α2β1, and the laminin binding integrins α3β1, α6β1, and α6β4 (Tervo et al., 1995; VanderWyst et al., 2011; Perkumas and Stamer, 2012). More recent studies, have shown that the α9 integrin subunit can be detected in mouse Schlemm’s canal (Park et al., 2014) and that both the αvβ3 and αvβ5 integrins are localized along the inner wall of Schlemm’s canal as well as within the JCT and TM beams in human anterior segments (Zhou et al., 1999b; Gagen et al., 2013). The expression of the αvβ3 integrin in Schlemm’s canal and within the TM, however, appears to vary (Fig. 3) between individuals with some individuals expressing high levels of αvβ3 integrin while others do not appear to express it at all. The finding that αvβ3 integrin is expressed by the endothelial cells of the inner wall of Schlemm’s Canal would be consistent with it being found predominantly in vascular endothelial cells and raises the question if it would have a similar role in SC cells. In the vasculature, αvβ3 integrin has been reported to have a mechanosensory role (Tzima et al., 2001) which could be important in mediating cellular responses of SC cells to shear stress (Ashpole et al., 2014).
Fig. 3.
Variable localization intensity of αvβ3 integrin in trabecular meshwork and Schlemm’s canal from 3 different donors. Anterior segment wedges from 3 donors were fixed in 4% p-formaldehyde prior to paraffin embedding. Sections were cut and then labeled with mAbs against either αvβ3 integrin (top) or β-galactosidase (bottom) as a negative control. Arrowheads indicate positive staining that is present in the αvβ3 integrin-labeled sections but absent in the β-galactosidase-labeled sections. Note the variation in labeling intensity observed in tissue from the different donors. Weak αvβ3 integrin labeling is present only along the inner wall of Schlemm’s Canal (SC) within the 62 year old donor tissue. In contrast, αvβ3 labeling is present within SC and along the trabecular meshwork (TM) beams in the other two donors’ tissue sections. However, the labeling is significantly stronger in the 68 year old donor than in the 66 year old donor. Specificity of the anti- αvβ3 integrin antibody (clone [BV3], Abcam, Cambridge, MA) was verified by FACs analysis (not shown) using TM-1 cell lines with known levels of αvβ3 integrin (Gagen et al., 2013). Scale bar = 50 µm.
3. Integrins in the ONH
In normal primate ONH, immunolabeling studies showed that α2β1, α3β1, α6β1, and α6β4 integrins have been localized to astrocytes along the margins of laminar beams and within glial columns. These integrins, as well as α5β1 and αvβ1 integrins, were found in vascular endothelial cells. In contrast, the α1 and β2 integrin sub-units were rarely expressed in the ONH and the β3 integrin subunit was found primarily in large blood vessels (Morrison, 2006). In human ONH astrocytes in culture, integrins were found that were not detected in situ. Both the αvβ3 and αvβ5 integrins were detected by immunolabeling studies in astrocytes in culture and by RT-PCR. Astrocytes were also found to express the α4, α5, α6, α9 and β1 integrin subunits by RT-PCR (Neumann et al., 2014).
4. Biological processes regulated by integrins
The intracellular signals generated by integrins, especially those mediated by members of the Rho GTPase family, regulate a large number of biological processes relevant to the function of the TM and ONH. In the TM and ONH, several functions have already been associated with specific integrins. For instance, αvβ5 integrins have been shown to regulate phagocytosis in TM cells in vitro (Gagen et al., 2013). This phagocytic pathway is not unique to TM cells and is shared by a number of other tissues in the eye where it is believed to play a protective and maintenance role. In retinal epithelium cells (RPE), the αvβ5 integrin-mediated phagocytic pathway is responsible for the phagocytosis of outer rod segments (Finnemann et al., 1997; Finnemann, 2003a, 2003b; Finnemann and Nandrot, 2006) and is believed to be responsible, in part, for the age-related blindness observed in mice null for the β5 integrin subunit (Nandrot et al., 2004, 2012). In lens epithelium, an αvβ5 integrin-mediated phagocytic pathway is responsible for the engulfment of apoptotic cells. Alterations in this pathway are associated with UV-induced cataract formation resulting from UV-induced degradation of αvβ5 integrin (Chauss et al., 2015).
In TM cells the αvβ5 integrin-mediated phagocytic process is similar to that observed in RPE cells and triggers a signaling pathway that utilizes focal adhesion kinase (FAK) to activate the GTPase Rac1 responsible for the engulfment of particles (Gagen et al., 2013) (Peotter et al., submitted for publication). Phagocytosis plays an important role in keeping the outflow pathway in the TM free of cellular debris, and decreases in the phagocytic activity of TM cells have been reported in both POAG and following steroid treatment (Grierson and Lee, 1973; Epstein et al., 1986; Johnson et al., 1989; Matsumoto and Johnson, 1997a, 1997b). Clearly, a reduction in αvβ5 integrin mediated phagocytosis in the TM could lead to an impairment in the outflow pathway thereby contributing to an elevation in intraocular pressure.
αvβ3 integrin participates in a number of activities in the TM. As discussed below, this integrin regulates assembly of extracellular matrices and organization of the actomyosin cytoskeleton in TM cells (Filla et al., 2006, 2009, 2011). Another function likely to be mediated by αvβ3 integrins in both the ONH and TM is growth factor signaling, especially CTGF and TGFβ1 signaling (Gagen et al., 2014). αvβ3 integrin has been reported to be a receptor for CTGF (Gao and Brigstock, 2004), and all members of the αv family of integrins participate in the activation of TGFβ1 (Sheppard et al., 1992).
The role of the α4 integrin subunit and its β subunit partner is less clear. Potentially, the α4 subunit could partner with either the β1 or β7 integrin subunit. However, there is no evidence that TM cells in culture or in situ express the β7 integrin subunit (Schwinn et al., 2010). Thus, the α4β1 integrin is the only α4-containing integrin that is likely to be expressed in the TM. This integrin is best known for its role in leukocyte and tumor cell migration (Holzmann et al., 1998; Rose et al., 2001, 2002) so it is interesting that it is also detected in the TM and ONH. In the TM, activation of the α4β1 integrin via the PRARI site in the Heparin II(HepII) binding domain of fibronectin appears to play a role in mediating the depolymerization of actin filaments and the disruption of adherens junctions (Gonzalez et al., 2006) which is consistent with its role in other migratory cell types especially leukocytes (Rose et al., 2001). Other studies have also suggested that activating α4β1 integrin with the Heparin II domain of fibronectin controls cellular contractility since the contraction of collagen gels by TM cells was reduced in the presence of the Heparin II binding domain (Schwinn et al., 2010). Presumably, this reduction in contractility was due to a decrease in RhoA activity since RhoA is known to control both actin polymerization and adherens junctions formation (Rao et al., 2008; Sumida and Stamer, 2010; Citi et al., 2011).
These results suggest that the α4β1 integrin interaction with one or more of its ECM ligands could be part of the normal homeostatic mechanisms proposed to regulate IOP (Acott et al., 2014). In support of this role, when human or monkey organ cultured anterior segments were perfused with the Heparin II binding domain or a PRARI peptide that activates α4β1 integrin signaling, outflow facility was increased (Santas et al., 2003; Gonzalez et al., 2009) and an expansion of the TM reminiscent of actin disrupting agents was observed (Gabelt et al., 2004, 2006; Schwinn et al., 2010). Interestingly, expression of the α4 integrin subunit is increased in the ONH in glaucomatous eyes (Morrison, 2006). It has been suggested that in the ONH, the α4 integrin subunit might play a role in astrocyte migration and microglia activation (Morrison, 2006) which would be consistent with its role in decreasing RhoA activity in other migratory cells.
Neurite outgrowth in the ONH would be another function that is potentially controlled by integrins, since a small molecule antagonist to the α4β1 integrin inhibited neurite outgrowth in cultures of retinal ganglion cells (RGCs) and amacrine cells in the chick retina (Leu et al., 2004). In this study, inhibition of the α4β1 integrin also triggered apoptosis in vivo causing a reduction in the number of RGCs in the developing chick retina, demonstrating a potentially important role for this integrin in the regeneration of RGCs in the ONH.
Finally, although different integrins often have overlapping functions that can be ascribed from tissue to tissue, it is important to keep in mind that tissue specific differences are observed. There are even some instances where distinct integrins can produce unique effects on cells by using different GTPase signaling pathways (Miao et al., 2002). The best characterized examples of this are the α5β1 and αvβ3 integrins. Both of these integrins bind the ECM protein fibronectin and activate Rho GTPases to promote migration. In mouse embryonic fibroblasts the α5β1 integrin appears to activate a RhoA-mediated pathway which promotes random migration whereas αvβ3 integrin supports a lamellipodium-driven directional migration that is believed to involve the GTPase Rac1 (Lin et al., 2013). Interestingly, the opposite effect is seen in Chinese hamster ovary cells indicating that differential regulation of Rho GTPases by integrins is cell type specific (Miao et al., 2002).
5. Integrin signaling
To understand the role of integrins in the pathogenesis of glaucoma, we need to understand how integrins control the function of cells in the TM and ONH. The major function originally ascribed to integrins was to mediate adhesion, however, it is now recognized that integrins also play critical signaling roles. They are known to transmit cues from the extracellular milieu across the plasma membrane into the cell via a process known as ‘outside-in’ signaling (Shattil et al., 2010; Bouvard et al., 2013; Iwamoto and Calderwood, 2015). These cues could be mechanical such as shear stress in Schlemm’s Canal or stretch in the TM or chemical in nature such as changes in the expression of matrix proteins in the TM and ONH.
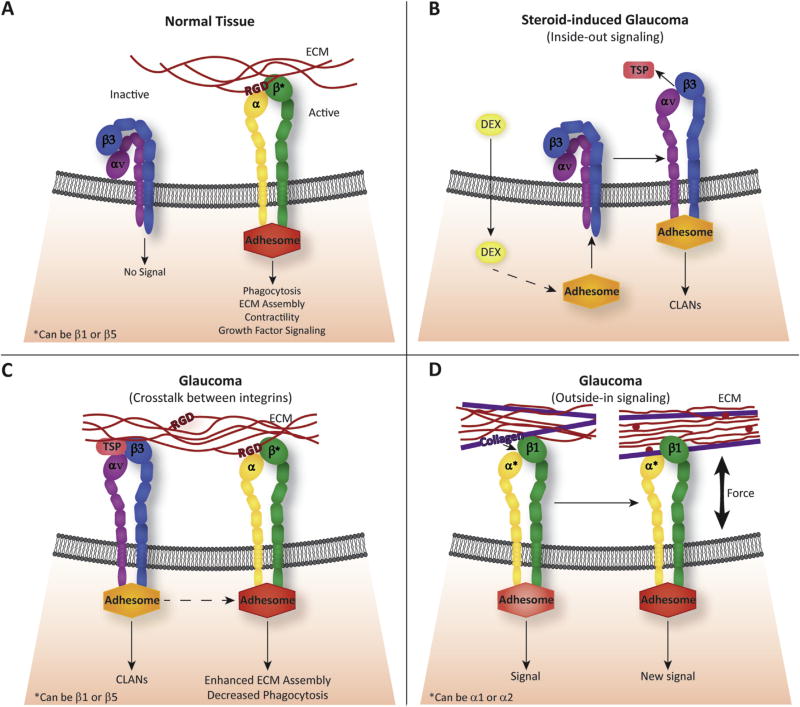
The conformation of the integrin plays a critical role in transmitting these signals (Askari et al., 2009). In the resting, or inactive, state, cell surface integrins exhibit a bent conformation of their extracellular domains and the cytoplasmic tails are closely bound together (Fig. 4A). In this conformation the integrin is unable to interact with the ECM or any cytoplasmic proteins. Thus, it is unlikely that a “resting” integrin on the cell surface would affect TM or ONH cell function. However, should the “resting” integrin become “active”, the extracellular domain would assume an upright conformation that can bind and transmit signals from ECM proteins.
Fig. 4.
Regulation of Integrin signaling. (A) Integrins can be found in both an inactive, bent conformation and an active state on the cell surface. In the active state, the integrin assumes a fully extended conformation and the subunits are separated to allow for interactions with their ECM ligands and a multi-protein intracellular complex called an adhesome. In this conformation, the ECM/integrin/adhesome complex can regulate a number of biological processes including phagocytosis, ECM assembly, contraction/relaxation of the trabecular meshwork and growth factor signaling. The consensus sequence used by many non-collagen binding integrins to bind the ECM is an Arg-Gly-Asp (RGD) motif. (B) Inactive αvβ3 integrins in the TM can be activated by dexamethasone-induced intracellular signaling pathway(s) that trigger the conformational change in the integrin to its active state. This process, called inside-out signaling, activates the integrin by promoting the binding of an adhesome complex to the cytoplasmic tail of the αvβ3 integrin. In turn, a conformational change is induced into the extracellular domain of the integrin that allows it to bind a ligand such as thrombospondin (TSP). Inside-out signaling has been shown to cause the formation of CLANs and hence may be involved in steroid-induced glaucoma. (C) Crosstalk between activated αvβ3 integrins and other integrins could affect other integrin-mediated signaling events in TM cells and induce a glaucomatous phenotype. In particular, activated αvβ3 integrin can cause a reduction in αvβ5 integrin-mediated phagocytosis, induce CLAN formation, and increase the amount of fibronectin assembled into the matrix. Adhesomes are represented as different colored shapes to indicate that each adhesome is unique to each integrin. (D) Newly synthesized proteins in the ECM can bind to integrins and initiate the formation of an adhesome and new signaling events. This is called outside-in signaling and could participate in the pathophysiology of glaucoma if constitutively active integrins were found on the cell surface of TM cells. Red circles represent newly synthesized ECM components.
Intracellular signals can also affect how integrins respond to the ECM. This process is called ‘inside-out’ signaling and results in the switch of the integrin from a resting, inactive conformation to a “high affinity or “activated state” that now enables the extracellular domain of the integrin to interact with its ECM ligands. Inside-out signaling is usually caused by the stimulation of secondary intracellular signaling pathways (Fig. 4B). For example in TM cells, stimulation of the glucocorticoid pathway by dexamethasone induced the active conformation of αvβ3 integrin and hence triggered an αvβ3 integrin-mediated pathway (Filla et al., 2011; Faralli et al., 2013).
Integrin signaling can also be controlled by the crosstalk between different integrin receptors. For example, cooperative signaling in TM cells between β1 and αvβ3 integrins alter intracellular signaling events that affect the organization of the acto-myosin network while signaling from an αvβ3 integrin inhibited αvβ5 integrin-mediated phagocytosis (Filla et al., 2006; Gagen et al., 2013) (Fig. 4C). Another example would be the cooperative signaling between α2β1 and α4β1 integrins which was shown to trigger the disassembly of actin stress fibers and reduced cell contractility in TM cells in culture (Schwinn et al., 2010).
Finally, changes in the composition and/or 3-D architecture of the ECM (Hytonen and Wehrle-Haller, 2015) can influence integrin signaling (Fig. 4D). In this instance, changes in the ECM can introduce new integrin binding sites via expression of a new ECM protein or unfolding of an existing ECM protein as discussed above which would activate a previously unactivated or resting integrin. Alternatively, these changes could propagate a change in intracellular tension (Hytonen and Wehrle-Haller, 2015) which could alter the composition of the signaling molecules associated with integrins as discussed below (Zaidel-Bar and Geiger, 2010; Ross et al., 2013; Horton et al., 2016) and hence the signal generated by the integrin.
6. Integrin activation in the TM and ONH
It is not known if all integrins in the TM or ONH are engaged with their ECM ligands and hence always in an active state or if some exist on the cell surface in an unoccupied, resting state. Hence, the development of glaucoma may arise from the activation of a resting integrin in the TM or ONH. Activation of the integrin could be induced by either mechanical forces such as stretch or shear stress as well as by some secondary signaling events. For instance, both stretch and shear force have been reported in the TM (Kelley et al., 2001; Keller et al., 2007; Ashpole et al., 2014). In smooth muscle cells and fibroblasts, stretch triggers the conversion of α5β1 integrin to the high affinity, activated state (Katsumi et al., 2005). Similarly in endothelial cells, increased shear stress similar to that reported to exist in Schlemm’s canal (Ashpole et al., 2014) led to the activation of αvβ3 integrin (Tzima et al., 2001).
As stated above, the αvβ3 integrin in cultured TM cells can be activated through an inside-out secondary signaling pathway induced by dexamethasone (Filla et al., 2011; Gagen et al., 2013; Faralli et al., 2013). These recent studies have shown that activation of this integrin can lead to several phenotypic changes associated with the pathogenesis of POAG including a decrease in phagocytosis (Gagen et al., 2013) and formation of crosslinked actin networks called CLANs (Filla et al., 2006, 2009). CLANs are actin geodesic dome-like structures that have been observed in both normal and glaucomatous TM cells in vivo (Clark et al., 1995, 2005; Hoare et al., 2009). They have also been observed in lamina cribrosa cells within ONH sections and in cultured lamina cribrosa cells that have either been isolated from glaucomatous donors or treated with glucocorticoids (Job et al., 2010). They appear with a greater frequency in glaucomatous TM where they are believed to alter the contractile properties of TM cells (Hoare et al., 2009).
Changes in the deposition of matrix proteins like fibronectin and collagen that have been reported to occur in glaucoma (Acott and Kelley, 2008; Tektas and Lutjen-Drecoll, 2009; Vranka et al., 2015) can also lead to integrin activation in the TM and ONH via an outside-in mechanism (Fig. 4D). One consequence of this last mechanism is that because different integrins bind a range of ECM molecules, each with its own signaling potential, the multiple changes in the ECM composition reported to occur in glaucoma are likely to activate and affect numerous integrin-mediated pathways in different ways (Chiquet et al., 2003; Orr et al., 2006). For example, in β3-null mouse embryonic fibroblasts, the α5β1 integrin was activated when the deposition of fibronectin into the ECM was increased (Lin et al., 2013). In contrast, the αvβ3 integrin was not activated in β1 null mouse embryonic fibroblasts when the levels of fibronectin were increased. Changes in the ECM could also affect how SC cells would respond to shear stress. For example, shear stress activates α2β1 integrin in endothelial cells plated on collagen, but not on fibronectin. Conversely, shear stress activates αvβ3 and α5β1 integrin in endothelial cells plated on fibronectin but not collagen.
7. Integrin adhesomes and glaucoma
Pivotal to the effect that an activated integrin has on cell behavior is the formation of a signaling complex, or adhesome, on the integrin cytoplasmic tails (Humphries et al., 2009; Zaidel-Bar and Geiger, 2010; Horton et al., 2016). When an integrin is in the “active” conformation the cytoplasmic tails of the subunits are separated. This separation exposes binding sites which allows a variety of intracellular proteins to bind and form the adhesome (Fig. 4). Since integrins do not possess any intrinsic catalytic activity, it is the composition of the adhesome which can be specific for each integrin that acts as the major signaling conduit that influences a wide variety of downstream signaling events. It also forms a bridge between the actomyosin network and the integrin which serves to convert the mechanical signals from the ECM into intracellular chemical signaling events.
The adhesome is a complex network of receptors, kinases, phosphatases, adaptor proteins and actin that is assembled onto integrin cytoplasmic tails upon engagement of their ECM ligands once the integrin is in an activated state (Zaidel-Bar and Geiger, 2010; Horton et al., 2015). It is now apparent that adhesomes are not static structures. Both fluorescence recovery experiments after photobleaching and quantitative time-lapse microscopy studies indicate that the receptors, adaptors and even actin in the adhesomes are in a constant state of flux (Hu et al., 2007; Worth and Parson, 2008). Assembly of proteins into the adhesome can be mediated by a series of auto- and trans-phosphorylation events or by mechanical forces that induce changes in actin binding proteins such as vinculin, filamin or talin which promote their binding to integrin cytoplasmic tails or other proteins within the adhesome (Horton et al., 2016). Thus, adhesomes are dynamic complexes with interactions that can be rapidly switched “on” or “off”. The dynamic nature of the adhesome makes it sensitive and responsive to external signaling cues and forces and is able to rapidly control gene expression in response to changes in the extracellular milieu.
Several adhesome components can also be observed to be shuttled between the nucleus and the adhesome. One such transient adhesome protein is Hic-5 (also called TGFB1I1) whose expression was recently identified to be upregulated by dexamethasome and TGFβ2 in TM cells (Clark et al., 2013; Pattabiraman and Rao, 2015). Hic-5 is an adaptor protein that divides its time between focal adhesions and the nucleus (Thomas et al.,1999; Yang et al., 2000; Chodankar et al., 2014). It plays a role in both cyto-skeletal organization and transcriptional regulation. In TM cells, it co-localized with αvβ3 integrins in focal adhesions and induced stress fiber formation and RhoA activity. Knockdown of its expression suppressed the dexamethasone-induced expression of myocilin, a protein known to be involved in POAG and TGFβ2-induced fibrogenic activity (Pattabiraman and Rao, 2015). Hic-5 shares homology with another adhesome protein called paxillin whose knockdown was also shown to block TGF-β2 responses in TM cells (Takahashi et al., 2013).
Zyxin is another transient adhesome protein whose expression may be altered by TGFβ2 (Bollinger et al., 2011). Zyxin is known to play a role in promoting actin polymerization at focal adhesions. Localization of zxyin to focal adhesions is force-dependent and requires highly contractile cells (Zaidel-Bar et al., 2003). In fact, it dissociates from the adhesome within seconds if contractile forces are reduced. In smooth muscle cells, zyxin moves to the nucleus in response to applied stretch (Cattaruzza et al., 2004). This suggests that zyxin, like Hic-5, may have a potentially important role in mediating transcriptional responses to force as well regulation of actin polymerization during normal homeostasis.
Recent proteomics studies onTM cells have also indicated that a number of actin-binding proteins associated with adhesome formation and stability are also altered upon treatment with dexamethasome or TGFβ2 suggesting that integrin-mediated signaling events may be altered in glaucoma (Bollinger et al., 2011, 2012; Clark et al., 2013). Filamin B for instance is upregulated following both dexamethasone and TGFβ2 treatment. Filamin A, another member of this actin-binding familyof proteins, is known tobind to the cytoplasmic tail of the integrin β-subunit and strengthen the adhesive interaction with the ECM (Schiller and Fassler, 2013). Fil-amin A is also recognized as an important mechanotransducer that regulates actin network stretching (Ohta et al., 2006; Ehrlicher et al., 2011) and its activity can be regulated by the tension generated by the actomyosin network (Ehrlicher et al., 2011; Nakamura et al., 2013). Whether filamin B has a similar function in TM cells is unknown. However, these properties could potentially make filamin B an important modulator of integrin signaling. Another actin-binding protein associated with integrins in adhesomes and reported to be altered by dexamethasone in TM cells is PDLIM1 (Clark et al., 2013). PDLIM1, along with filamin B, has been detected in CLANs. Filamin B was detected along the actin filaments and PDLIM1 was found in the vertices where the actin filaments interconnect. The increased expression of both filamin B and PDLIM1 would likely strengthen the actomyosin network connection to integrins in focal adhesions and stabilize the CLAN structure.
The dynamic nature of adhesome complexes coupled with their regulation by mechanical forces suggest that they play a role in regulating feedback loops that control tissue homeostasis (Acott et al., 2014) and theoretically would be involved in the response to changes in ECM composition and compliance in the TM and ONH. Thus, it is interesting that so many changes in the integrin adhesome are detected following TGFβ2 and dexamethasone treatment, since both these treatments are associated with the development of glaucoma. These alterations suggest that these integrin-mediated signaling complexes may be altered in glaucoma and/or could be responsible, in part, for the generation of the glaucomatous phenotype as studies with Hic-5 imply (Pattabiraman and Rao, 2015).
8. αvβ3 integrin and glaucoma
Of all the integrins studied to date, the αvβ3 integrin has the potential to be a key player in the pathophysiology of glaucoma. In contrast to other integrin subunits, the expression of the αv and β3 integrin subunits appears to be affected by several factors involved in the development of glaucoma. The αv integrin subunit inTM cells has been reported to be upregulated by CTGF (Junglass et al., 2009) and stretch (Rose, A.Y. et al., and Association for Research in Vision & Ophthalmology. Invest Ophthalmol Vis Sci, 2002; 43:E-Abstract 1045). Expression of the αv integrin subunit is also increased in the RGC layer and in the glial cells of the nerve head following optic nerve crush in mice (Wang et al., 2006). Finally, expression of the αv integrin subunit was downregulated by glucocorticoids in one study (Dickerson et al., 1998) while expression of the β3 integrin subunit was upregulated by glucocorticoid treatments in TM cells in vitro (Dickerson et al., 1998; Rozsa et al., 2006; Filla et al., 2011; Clark et al., 2013; Gagen et al., 2013; Faralli et al., 2013). The dexamethasone-induced increase in αvβ3 integrin was the result of a 4–6-fold increase in the mRNA level and the increased transcription of β3 integrin mRNA that occurs through a secondary glucocorticoid response mechanism similar to what has been shown for myocilin (Shepard et al., 2001; Joe et al., 2011; Faralli et al., 2013, 2015). This increase was dependent on calcineurin, a phosphatase that activates the NFAT family of transcription factors.
Interestingly, in the studies reporting the upregulation of these subunits only one or the other subunit appears to be upregulated indicating that the expression of the individual subunits may not be coordinated. Recent studies in TM cells support this idea and showed that knock down of the β5 integrin subunit did not affect expression of its αv integrin partner subunit (Gagen et al., 2013). In addition, expression of the αvβ3 integrin which shares the αv subunit was also unaffected (Faralli et al., 2013). Finally, it should be noted that increased cell surface expression of an integrin heterodimer does not indicate that it is in the activated state (Ginsberg et al., 1992; Arjonen et al., 2012; Bouvard et al., 2013; Faralli et al., 2013).
In addition to being upregulated by glucocorticoids, the αvβ3 integrin can be activated by a number of factors involved in glaucoma. In vascular endothelial cells, the αvβ3 integrin was activated by shear stress making it a potential mechanosensor (Tzima et al., 2001) and thus likely to be activated on SC cells when shear stress is increased (Ashpole et al., 2014). It is also activated by dexamethasone in TM cells in culture (Filla et al., 2011; Faralli et al., 2013; Gagen et al., 2013).
9. Surface expression and recycling of αvβ3 integrin
The dexamethasone-induced increase in αvβ3 integrin expression and cell surface activation persisted for over 2 weeks following the removal of dexamethasone (Faralli et al., 2013). This suggests that long term treatment with dexamethasone could result in a dysregulation of the cell surface expression of the αvβ3 integrin presumably due to changes in integrin recycling. This is worth noting because integrin recycling is important for controlling integrin-dependent signaling events, focal adhesion disassembly, matrix turnover, and the localized redistribution of integrins to new membrane sites. Recycling can remove both active and inactive integrins from the cell surface and it appears that the kinetics of recycling varies for each integrin depending on the α-subunit and its activation state (Caswell et al., 2009; Wickstrom and Fassler, 2011; De Franceschi et al., 2015). Thus, α5β1 integrin is rapidly and constitutively internalized whereas α4β1 or α3β1 integrin do not appear to be internalized at all. αvβ3 integrin, unlike other integrins, has been reported to be rapidly recycled back to the cell surface from early endosomes via a Rab4-dependent mechanism. Its recycling affects the recycling of other integrins, most notably α5β1 integrin which is a major factor in RhoA activation (White et al., 2007; De Franceschi et al., 2015). Hence the constitutive cell surface expression of αvβ3 integrin would likely trigger a dysregulation of other integrin signaling pathways.
A number of growth factors, kinases, and members of the Rab and ARF families are known to influence integrin recycling (Morgan et al., 2009; Jacquemet et al., 2013). For example, PDGF stimulates rapid recycling of internalized αvβ3 integrin from early endosomes. This process depends on the protein kinase D1 (PKD1) which binds directly to the β3-integrin cytoplasmic tail at endosomes and drives the rapid return of the heterodimer to the plasma membrane (White et al., 2007; di Blasio et al., 2010). PKD1 has been reported to be upregulated in dexamethasone-treated TM cells in culture (Clark et al., 2013) where we see prolonged expression of αvβ3 integrin on the cell surface even after the removal of dexamethasone. This supports the idea that the cell surface expression of the αvβ3 integrin may be impaired in dexamethasone treated TM cells (Faralli et al., 2013) and raises the question of whether the selective internalization of integrins (or lack of it) would play a critical role in controlling integrin-mediated pathways in TM and ONH.
10. Effects of αvβ3 integrin activation in TM cells
This prolonged expression and induction of αvβ3 integrin signaling pathways seen with dexamethasone could be significant. In adult tissues, αvβ3 integrin is mostly expressed on angiogenic endothelial cells found in tissues undergoing remodeling or in pathological states involving angiogenesis such as age-related macular degeneration and diabetic retinopathy (Brooks et al., 1995; Luna et al., 1996; Friedlander et al., 1996; Wilkinson-Berka et al., 2006; Lahdenranta et al., 2007) Thus, these studies suggest that upregulation of αvβ3 integrin may have adverse effects on TM cellular functions. Recent studies support this idea and have shown that expression and activation of the αvβ3 integrin can lead to several phenotypic changes associated with the pathogenesis of POAG. In particular, αvβ3 integrin activation leads to the inhibition of αvβ5 integrin-mediated phagocytosis in TM cells (Gagen et al., 2013) as well as alterations in the cytoskeleton associated with the development of CLANs in TM cells (Filla et al., 2006, 2009, 2011) which are more frequent in glaucomatous TM cells and steroid treated cells and organ cultures (Hoare et al., 2009; Filla et al., 2011).
CLANs are generally thought to play a role in the progression of glaucoma by restricting the contractile properties of the TM (Clark et al., 1994, 2005), although this concept in TM cells has never been tested. Recent studies in mouse embryonic fibroblasts, however, would support this idea (Schiller and Fassler, 2013; Lin et al., 2013) and suggest a signaling role for CLANs as well. These studies showed that the application of cyclic force through the αvβ3 integrin resulted in a stiffening of the cytoskeleton (Roca-Cusachs et al., 2009; Schiller et al., 2013) which was not seen when the same force was applied to the α5β1 integrin. This would suggest that the application of cyclic force in the TM as a result of changes in IOP would more likely to be perceived by the αvβ3 integrin and not the α5β1 integrin which is also found in the TM. This could explain why CLANs form in response to αvβ3 integrin signaling. αvβ3 integrin-mediated CLANs may be the structural adaptions to forces in the TM responsible for the stiffening of the tissue during the progression of POAG (Braunger et al., 2015).
How αvβ3 integrin is activated within the TM is unknown. Following dexamethasone treatment, three of the major ligands for αvβ3 integrin, fibronectin and thrombospondins-1 and −2 are upregulated (Steely et al., 1992; Zhou et al., 1998; Flugel-Koch et al., 2004). Thus, it is possible that unoccupied αvβ3 integrins on the TM cell surface could become engaged by one of these ligands thereby initiating an outside-in signaling pathway. Interestingly, thrombospondin-1 levels are increased in 1/3 of patients with POAG and thrombospondin-1 and −2 null mice have lower intraocular pressures (Haddadin et al., 2012). Thrombospondin levels are also increased in TGFβ-treated TM cultures where an increase in CLAN formation has also been observed (O’Reilly et al., 2011). Studies by Filla et al. (Filla et al., 2009) showed that the 4N1K peptide (RFYVVMWK) from thrombospondin-1, which is known to activate αvβ3 integrin signaling via its co-receptor CD47 caused an increase in the formation of CLANs. An earlier study by Filla et al. (Filla et al., 2006) also showed that the substrate the cell was plated on influenced the degree to which CLANs were formed. Thus, alterations in the actin cytoskeleton associated with POAG may be due to changes in the composition of the ECM altering integrin signaling pathways and/or possibly activating a previous “resting” αvβ3 integrin on the cell surface (Fig. 4B).
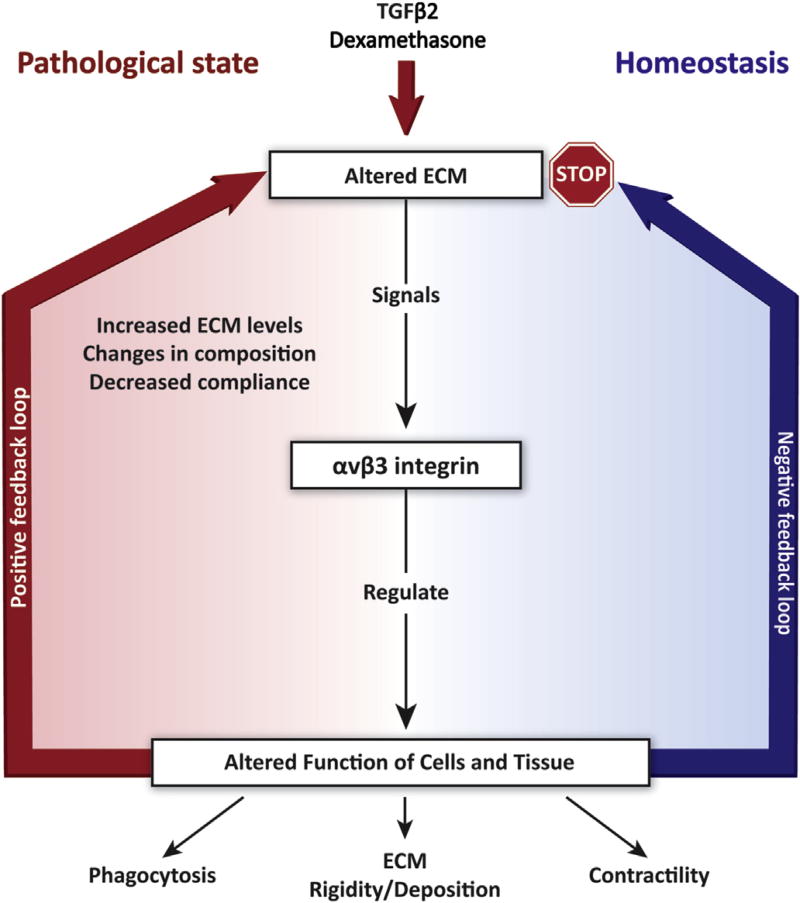
11. Activated αvβ3 integrin and the ECM: generation of a positive feedback loop
It was recognized over 30 years ago that the bidirectional signaling processes between cells and the ECM remains a driving force in understanding the pathogenesis of diseases (Humphrey et al., 2014). When either the physiological functions of cells or the ECM composition changes, a feedback loop is created (Fig. 5). In normal, healthy tissue, it has been postulated that cells would use negative feedback mechanisms to restore the tissue back to normal. If there has been either a loss of the negative feedback pathway and/or a switch to a positive feedback mechanism, then a diseased state is created (Humphrey et al., 2014).
Fig. 5.
Role of αvβ3 integrin signaling in the pathophysiology of glaucoma. Changes introduced into the ECM by TGFβ2-or dexamethasone-mediated signaling pathways are perceived by the αvβ3 integrin which then alters biological processes such as contractility, matrix deposition and phagocytosis. Homeostatic mechanisms (negative feedback loop) would downregulate the induced changes and turn off αvβ3 integrin signaling. However, prolonged expression of αvβ3 integrin signaling could establish a positive feedback loop that propagates the effects induced by TGFβ2 and dexameth-asone. The precise mechanism responsible for these negative and positive feedback loops are still unknown.
The αvβ3 integrin may be a key player in the generation of such a feedback loop in glaucoma. Recent studies have shown that in addition to causing a decrease in phagocytosis and an increase in CLAN formation in TM cells, activation of αvβ3 integrin contributes to the excess deposition of a fibronectin matrix (Filla et al., manuscript in preparation). The assembly of the ECM, especially a fibronectin matrix, is an integrin-dependent process (Schwarzbauer and DeSimone, 2011). A critical feature of the integrin-mediated assembly of fibronectin fibrils is the cytoskeletal connections with the integrins’ cytoplasmic tails. These integrin-cytoskeletal connections provide the necessary contractile forces that induce the conformational change in fibronectin necessary for fibril formation (Schwarzbauer and DeSimone, 2011). Using immortalized TM cells overexpressing either the wildtype or a constitutively active αvβ3 integrin, it was found that increased matrix deposition was only observed in cells expressing the constitutively active form of αvβ3 integrin. Thus, activation of this single integrin may be responsible for several of the phenotypic changes observed in glaucomatous cells.
Since this integrin can be activated by dexamethasone treatment or changes in the TM ECM, it would suggest that αvβ3 integrin signaling mediates the positive feedback loop that propagates the formation of these glaucomatous phenotypes and contributes to a “diseased” microenvironment (Fig. 5). In this scenario, the increased deposition of fibronectin into the ECM that is frequently observed following TGFβ signaling in POAG or steroid treatment in steroid-induced glaucoma could promote αvβ3 integrin activation. In turn, activation of αvβ3 integrin could change the contractile properties of the TM which are needed for matrix production (Zhang et al., 1997).
Exactly how αvβ3 integrin is able to mediate these phenotypic changes is still under investigation. The Rho family of small GTPases is a critical downstream target of integrin signaling. Integrins are responsible for the translocation and activation of Rho GTPases which occurs at membrane sites proximal to the integrin cytoplasmic tails (Lawson and Burridge, 2014). Like many Rho GTPase-mediated functions, it is the localized activity of the different GTPases at these proximal membrane sites and subtle differences in the integrin adhesome composition which control activities like phagocytosis or cellular contractility. For example, a switch from an αvβ3 integrin rich adhesome to an α5β1 integrin rich adhesome causes a switch from a Rac1-dominated to a RhoA-dominated GTPase signaling pathway that in turn causes an increase in cytoskeletal tension and adhesion complex maturation (Morgan et al., 2009).
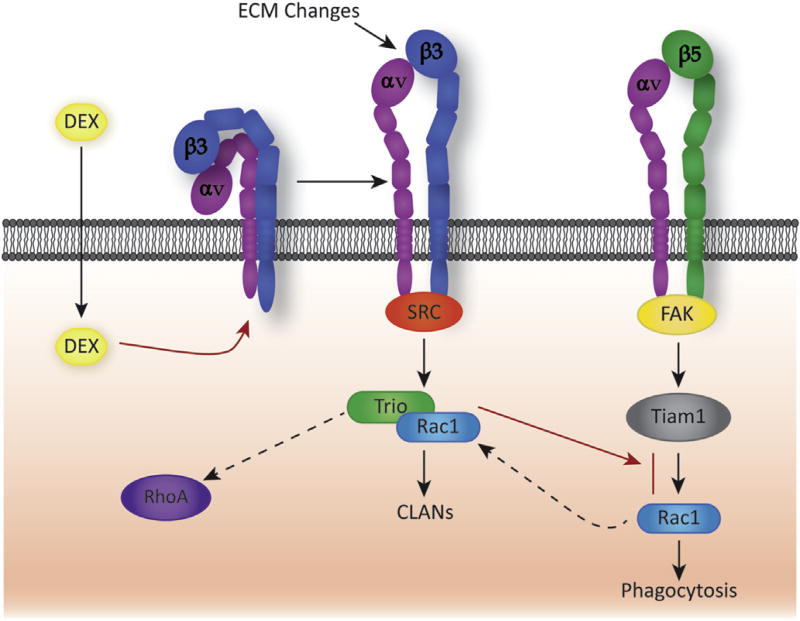
Recent studies with TM cells suggest that αvβ3 integrins may be affecting the phenotype of TM cells by altering how the Rho GTPase Rac1 is activated. In TM cells in vitro, Rac1 is required for αvβ5 integrin-mediated phagocytosis (Peotter et al., submitted for publication). However Rac1 activity is also needed for αvβ3 integrin-mediated CLAN formation (Filla et al., 2009). So it is interesting that constitutive activation of αvβ3 integrin inhibits phagocytosis. Recent studies suggest that it may be the differential use of kinases and specific guanine nucleotide exchange factors (GEFs) by integrins which regulate phagocytosis and CLAN formation (Fig. 6). Activated αvβ3 integrin uses a Src kinase and the GEF Trio to activate Rac1 and form CLANs whereas αvβ5 integrins appear to use FAK and the GEF Tiam1 to activate Rac1 and mediate phagocytosis. The glaucomatous phenotype therefore appears to involve a dysregulation in the spatiotemporal regulation of Rac1 activity triggered by αvβ3 integrin which, in a sense, enables the activated αvβ3 integrin to “steal” Rac1 away from the αvβ5 integrin-mediated phagocytic pathway. This would be consistent with previous reports that there is a differential and localized regulation of Rho GTPases by integrins (Miao et al., 2002).
Fig. 6.
Schematic showing differential use of GEFs by αvβ3 and αvβ5 integrins to activate Rac1. In normal human TM cells, αvβ5 integrin uses a Rac1-mediated pathway to induce phagocytosis. The pathway involves focal adhesion kinase (FAK) (Gagen et al., 2013) and the Rac1 specific GEF called Tiam1 (Peotter et al., submitted for publication). Following treatment with dexamethasone, inactive αvβ3 integrins on the cell surface become activated (Filla et al., 2011; Faralli et al., 2013; Gagen et al., 2013) which results in the activation of a Rac1-mediated pathway via the GEF called Trio that promotes the formation of CLANs (Filla et al., 2009; Filla et al., 2011). Utilization of Rac1 by this pathway may inhibit phagocytosis by limiting the availability of Rac1 GEFs as previously reported (Toth et al., 2009; Liu et al., 2013). Interestingly, Trio can act as a GEF for both Rac1 and RhoA (van Rijssel and Van Buul, 2012) which could explain why activation of αvβ3 integrin has recently been reported to activate RhoA (Pattabiraman and Rao, 2015). Use of Trio in this case may represent a way to temporally and spatially coordinate the activity of both Rac1 and RhoA as suggested in studies when both are at activated at the leading edge of migrating cells (Kraynov et al., 2000; Pertz et al., 2006).
12. Future directions
One area yet to be explored in the TM and ONH is the interaction of αvβ3 integrin with other intracellular pathways, especially growth factor signaling (Gagen et al., 2014). In most of these instances, studies show that αvβ3 integrin interacts with and controls growth factor receptors. αvβ3 integrin has also been shown to regulate TGFβ1 activity (Sheppard et al., 1992) and the expression and recycling of the VEGF receptor-2 (Reynolds et al., 2004, 2009). The αvβ3 integrin can also associate with Neuropilin-1, a VEGF coreceptor (Soker et al., 1998) that mediates signaling through VEGF receptor-2 (Zachary and Gliki, 2001) and is found on SC cells (Perkumas and Stamer, 2012). Finally, activation of αvβ3 integrin signaling via osteopontin has been shown to control the eNOS/NO pathway (Wang et al., 2011) which has recently been shown to play a role in outflow facility (Chang et al., 2015).
Clearly this integrin has the potential to play a critical role in mediating the pathophysiology of glaucoma and developing ways to control its activity could provide new therapeutic avenues for glaucoma. One such approach may be using peptide antagonists and/or antibodies. αvβ3 peptide antagonists are currently being used to treat wet age-related macular degeneration and diabetic retinopathy (Mousa, 2003) and anti-αvβ3 integrin monoclonal antibody therapies are used to treat cancer (Gutheil et al., 2000). Both of these clinically tested approaches may prove useful for the treatment of glaucoma (Gagen et al., 2014). Finally, since different integrins appear to use specific signaling molecules to activate GTPases, understanding which kinases and GEFs are used by the integrins to control the various GTPase-mediated activities of the TM may provide additional targets that would enable us to tailor treatments towards one specific signaling event.
Acknowledgments
This work was supported by NEI grants EY017006, EY026009 (D.M.P.), a Core grant to the Department of Ophthalmology and Visual Sciences (P30 EY016665).
References
- Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp. Eye Res. 2008;86:543–561. doi: 10.1016/j.exer.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acott TS, Kelley MJ, Keller KE, Vranka JA, Abu-Hassan DW, Li X, Aga M, Bradley JM. Intraocular pressure homeostasis: maintaining balance in a high-pressure environment. J. Ocul. Pharmacol. Ther. 2014;30:94–101. doi: 10.1089/jop.2013.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adair BD, Xiong JP, Maddock C, Goodman SL, Arnaout MA, Yeager M. Three-dimensional EM structure of the ectodomain of integrin {alpha}V{beta}3 in a complex with fibronectin. J. Cell Biol. 2005;168:1109–1118. doi: 10.1083/jcb.200410068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aota S, Nomizu M, Yamada KM. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J. Biol. Chem. 1994;269:24756–24761. [PubMed] [Google Scholar]
- Arjonen A, Akano J, Veltel S, Ivaska J. Distinct recycling of active and inactive β1 integrins. Traffic. 2012;13:610–625. doi: 10.1111/j.1600-0854.2012.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole NE, Overby DR, Ethier CR, Stamer WD. Shear stress-triggered nitric oxide release from Schlemm’s canal cells. Investig. Ophthal. Vis. Sci. 2014;55:8067–8076. doi: 10.1167/iovs.14-14722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askari JA, Buckley PA, Mould AP, Humphries MJ. Linking integrin conformation to function. J. Cell Sci. 2009;122:165–170. doi: 10.1242/jcs.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tiss. Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger KE, Crabb JS, Yuan X, Putliwala T, Clark AF, Crabb JW. Quantitative proteomics: TGFβ2 signaling in trabecular meshwork cells. Investig. Ophthal. Vis. Sci. 2011;52:8287–8294. doi: 10.1167/iovs.11-8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger KE, Crabb JS, Yuan X, Putliwala T, Clark AF, Crabb JW. Proteomic similarities in steroid responsiveness in normal and glaucomatous trabecular meshwork cells. Mol. Vis. 2012;18:2001–2011. [PMC free article] [PubMed] [Google Scholar]
- Bouvard D, Pouwels J, De Franceschi N, Ivaska J. Integrin inactivators: balancing cellular functions in vitro and in vivo. Nature. 2013;14 doi: 10.1038/nrm3599. [DOI] [PubMed] [Google Scholar]
- Braunger BM, Fuchshofer R, Tamm ER. The aqueous humor outflow pathways in glaucoma: a unifying concept of disease mechanisms and causative treatment. Eur. J. Pharm. Biopharm. 2015;95(Part B):173–181. doi: 10.1016/j.ejpb.2015.04.029. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA. Anti-integrin αvβ3 blocks human breast cancer growth and angiogenesis in human skin. J. Clin. Investig. 1995;96:1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat. Rev. Mol. Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- Cattaruzza M, Lattrich C, Hecker M. Focal adhesion protein zyxin is a mechanosensitive modulator of gene expression in vascular smooth muscle cells. Hypertension. 2004;43:726–730. doi: 10.1161/01.HYP.0000119189.82659.52. [DOI] [PubMed] [Google Scholar]
- Chang JY, Stamer WD, Bertrand J, Read AT, Marando CM, Ethier CR, Overby DR. Role of nitric oxide in murine conventional outflow physiology. Am. J. Physiol. Cell Physiol. 2015;309:C205–C214. doi: 10.1152/ajpcell.00347.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauss D, Brennan L, Bakina O, Kantorow M. Integrin αVβ5-mediated removal of apoptotic cell debris by the eye lens and its inhibition by UV light exposure. J. Biol. Chem. 2015;290:30253–30266. doi: 10.1074/jbc.M115.688390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet M, Renedo A, Huber F, Fluck M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol. 2003;22:73–80. doi: 10.1016/s0945-053x(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Chodankar R, Wu DY, Schiller BJ, Yamamoto KR, Stallcup MR. Hic-5 is a transcription coregulator that acts before and/or after glucocorticoid receptor genome occupancy in a gene-selective manner. Proc. Natl. Acad. Sci. U. S. A. 2014;111:4007–4012. doi: 10.1073/pnas.1400522111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S, Spadaro D, Schneider Y, Stutz J, Pulimeno P. Regulation of small GTPases at epithelial cell-cell junctions. Mol. Membr. Biol. 2011;28:427–444. doi: 10.3109/09687688.2011.603101. [DOI] [PubMed] [Google Scholar]
- Clark A, Wordinger RJ. The role of steroids in outflow resistance. Exp. Eye Res. 2009;88:752–759. doi: 10.1016/j.exer.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Clark AF, Brotchie D, Read AT, Hellberg P, English-Wright S, Pang IH, Ethier CR, Grierson I. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil. Cytoskelet. 2005;60:83–95. doi: 10.1002/cm.20049. [DOI] [PubMed] [Google Scholar]
- Clark AF, Miggans ST, Wilson K, Browder S, McCartney MD. Cytoskeletal changes in cultured human glaucoma trabecular meshwork cells. J. Glauc. 1995;4:183–188. [PubMed] [Google Scholar]
- Clark AF, Wilson K, McCartney MD, Miggans ST, Kunkle M, Howe W. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Investig. Ophthal. Vis. Sci. 1994;35:281–294. [PubMed] [Google Scholar]
- Clark R, Nosie A, Walker TM, Faralli JA, Filla MS, Barrett-Wilt G, Peters DM. Comparative genomic and proteomic analysis of cytoskeletal changes in dexamethasonetreated trabecular meshwork cells. Mol. Cell Proteomics. 2013;12:194–206. doi: 10.1074/mcp.M112.019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Franceschi N, Hamidi H, Alanko J, Sahgal P, Ivaska J. Integrin traffic -the update. J. Cell Sci. 2015;128:839–852. doi: 10.1242/jcs.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Blasio L, Droetto S, Norman J, Bussolino F, Primo L. Protein kinase D1 regulates VEGF-A-induced αvβ3 integrin trafficking and endothelial cell migration. Traffic. 2010;11:1107–1108. doi: 10.1111/j.1600-0854.2010.01077.x. [DOI] [PubMed] [Google Scholar]
- Dickerson JE, Jr, Steely HT, Jr, English-Wright SL, Clark AF. The effect of dexamethasone on integrin and laminin expression in cultured human trabecular meshwork cells. Exp. Eye Res. 1998;66:731–738. doi: 10.1006/exer.1997.0470. [DOI] [PubMed] [Google Scholar]
- Diskin S, Cao Z, Leffler H, Panjwani N. The role of integrin glycosylation in galectin-8-mediated trabecular meshwork cell adhesion and spreading. Glycobiology. 2009;19:29–37. doi: 10.1093/glycob/cwn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JC. Optic nerve head biomechanics in aging and disease. Exp. Eye Res. 2015;133:19–29. doi: 10.1016/j.exer.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlicher AJ, Nakamura F, Hartwig JH, Weitz DA, Stossel TP. Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature. 2011;478:260–263. doi: 10.1038/nature10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DL, Freddo TF, Anderson PJ, Patterson MM, Bassett-Chu S. Experimental obstruction to aqueous outflow by pigment particles in living monkeys. Investig. Ophthal. Vis. Sci. 1986;27:387–395. [PubMed] [Google Scholar]
- Faralli JA, Clark RW, Filla MS, Peters DM. NFATc1 activity regulates the expression of myocilin induced by dexamethasone. Exp. Eye Res. 2015;130:9–16. doi: 10.1016/j.exer.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faralli JA, Gagen D, Filla MS, Crotti TN, Peters DM. Dexamethasone increases αvβ3 integrin expression and affinity through a calcineurin/NFAT pathway. BBA- Mol. Cell Res. 2013;1833:3306–3313. doi: 10.1016/j.bbamcr.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faralli JA, Newman JR, Sheibani N, Dedhar S, Peters DM. Integrin linked kinase regulates integrin signaling in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2011;52:1684–1692. doi: 10.1167/iovs.10-6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filla M, Schwinn MK, Nosie AK, Clark RW, Peters DM. Dexamethasone-associated cross-linked actin network (CLAN) formation in human trabecular meshwork (HTM) cells involves β3 integrin signaling. Investig. Ophthal. Vis. Sci. 2011;52:2952–2959. doi: 10.1167/iovs.10-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filla M, Schwinn MK, Sheibani N, Kaufman PL, Peters DM. Regulation of cross-linked actin network (CLAN) formation in human trabecular meshwork (HTM) cells by convergence of distinct β 1 and β3 integrin pathways. Investig. Ophthal. Vis. Sci. 2009;50:5723–5731. doi: 10.1167/iovs.08-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filla M, Woods A, Kaufman PL, Peters DMP. β1 and β3 integrins cooperate to induce syndecan-4 containing cross-linked actin networks (CLANs) in human trabecular meshwork (HTM) cells. Investig. Ophthal. Vis. Sci. 2006;47:1956–1967. doi: 10.1167/iovs.05-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003a;22:4143–4154. doi: 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC. Role of alphavbeta5 integrin in regulating phagocytosis by the retinal pigment epithelium. Adv. Exp. Med. Biol. 2003b;533:337–342. doi: 10.1007/978-1-4615-0067-4_42. [DOI] [PubMed] [Google Scholar]
- Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc. Natl. Acad. Sci. U. S. A. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Nandrot EF. MerTK activation during RPE phagocytosis in vivo requires alphaVbeta5 integrin. Adv. Exp. Med. Biol. 2006;572:499–503. doi: 10.1007/0-387-32442-9_69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flugel-Koch C, Ohlmann A, Fuchshofer R, Welge-Lussen U, Tamm ER. Thrombospondin-1 in the trabecular meshwork: localization in normal and glaucomatous eyes, and induction by TGF-beta1 and dexamethasone. Exp. Eye Res. 2004;79:649–663. doi: 10.1016/j.exer.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Friedlander M, Theesfeld CL, Sugita M, Fruttiger M, Thomas MA, Chang S, Cheresh DA. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchshofer R. The pathogenic role of transforming growth factor- β2 in glaucomatous damage to the optic nerve head. Exp. Eye Res. 2011;93:165–169. doi: 10.1016/j.exer.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Tamm ER. The role of TGF-β in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012;347:279–290. doi: 10.1007/s00441-011-1274-7. [DOI] [PubMed] [Google Scholar]
- Gabelt BT, Hennes EA, Seeman JL, Tian B, Kaufman PL. H-7 effect on outflow facility after trabecular obstruction following long-term echothiophate treatment in monkeys. Investig. Ophthal. Vis. Sci. 2004;45:2732–2736. doi: 10.1167/iovs.04-0083. [DOI] [PubMed] [Google Scholar]
- Gabelt BT, Hu Y, Vittitow JL, Rasmussen CA, Grosheva I, Bershadsky AD, Geiger B, Borras T, Kaufman PL. Caldesmon transgene expression disrupts focal adhesions in HTM cells and increases outflow facility in organ-cultured human and monkey anterior segments. Exp. Eye Res. 2006;82:935–944. doi: 10.1016/j.exer.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Gagen D, Faralli JA, Filla M, Peters DM. The role of integrins in the trabecular meshwork. J. Ocul. Pharmacol. Ther. 2014;30:110–120. doi: 10.1089/jop.2013.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagen D, Filla M, Clark R, Litton P, Peters DM. Activated αvβ3 integrin regulates αvβ5 integrin-mediated phagocytosis in trabecular meshwork cells. Investig. Ophthal. Vis. Sci. 2013;54:5000–5011. doi: 10.1167/iovs.13-12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Brigstock DR. Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C-terminal domain to integrin alpha(v)beta(3) and heparan sulfate proteoglycan. J. Biol. Chem. 2004;279:8848–8855. doi: 10.1074/jbc.M313204200. [DOI] [PubMed] [Google Scholar]
- Ginsberg MH, Du X, Plow EF. Inside-out integrin signalling. Curr. Opin. Cell Biol. 1992;4:766–771. doi: 10.1016/0955-0674(92)90099-x. [DOI] [PubMed] [Google Scholar]
- Golledge J, Clancy P, Maguire J, Lincz L, Koblar S. The role of tenascin C in cardiovascular disease. J. Cardiovasc. Res. 2011;92:19–28. doi: 10.1093/cvr/cvr183. [DOI] [PubMed] [Google Scholar]
- Gonzalez JMJ, Hu Y, Gabelt B, Kaufman PL, Peters DM. Identification of the active site in the Heparin II domain of fibronectin that increases outflow facility in cultured monkey anterior segments. Investig. Ophthal. Vis. Sci. 2009;50:235–241. doi: 10.1167/iovs.08-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JMJ, Peterson JA, Peters JM, Newman J, Peters DM. Effect of heparin II domain of fibronectin on actin cytoskeleton and adherens junctions in human trabecular meshwork cultures. Investig. Ophthal. Vis. Sci. 2006;47:2924–2931. doi: 10.1167/iovs.06-0038. [DOI] [PubMed] [Google Scholar]
- Grierson I, Lee WR. Erythrocyte phagocytosis in the human trabecular meshwork. Br. J. Ophthalmol. 1973;57:400–415. doi: 10.1136/bjo.57.6.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutheil JC, Campbell TN, Pierce PR, Watkins JD, Huse WD, Bodkin DJ, Cheresh DA. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin αvβ3. Clin. Cancer Res. 2000;6:3056–3061. [PubMed] [Google Scholar]
- Haddadin R, Oh D, Kang M, Villarreal GJ, Kang J, Jin R, Gong H, Rhee D. Thrombospondin-1 (TSP1)-null and TSP2-null mice exhibit lower intraocular pressures. Investig. Ophthal. Vis. Sci. 2012;53:6708–6717. doi: 10.1167/iovs.11-9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Uoshima M, Yan YZ, Schneider G, Aukhil I. The alternatively spliced domains EIIIB and EIIIA of human fibronectin affect cell adhesion and spreading. J. Cell Sci. 1997;110:2271–2280. doi: 10.1242/jcs.110.18.2271. [DOI] [PubMed] [Google Scholar]
- Hoare MJ, Grierson I, Brotchie D, Pollock N, Cracknell K, Clark AF. Cross-linked actin networks (CLANs) in the trabecular meshwork of the normal and glaucomatous human eye in situ. Investig. Ophthal. Vis. Sci. 2009;50:1255–1263. doi: 10.1167/iovs.08-2706. [DOI] [PubMed] [Google Scholar]
- Holzmann B, Gosslar U, Bittner M. Alpha 4 integrins and tumor metastasis. Curr. Top. Microbiol. Immunol. 1998;231:125–141. doi: 10.1007/978-3-642-71987-5_8. [DOI] [PubMed] [Google Scholar]
- Horton ER, Astudillo P, Humphries MJ, Humphries JD. Mechanosensitivity of integrin adhesion complexes: role of the consensus adhesome. Exp. Cell Res. 2016;343:7–13. doi: 10.1016/j.yexcr.2015.10.025. [DOI] [PubMed] [Google Scholar]
- Horton ER, Byron A, Askari JA, Ng DH, Millon-Fremillon A, Robertson J, Koper EJ, Paul NR, Warwood S, Knight D, Humphries JD, Humphries MJ. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat. Cell Biol. 2015;17:1577–1587. doi: 10.1038/ncb3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science. 2007;315:111–115. doi: 10.1126/science.1135085. [DOI] [PubMed] [Google Scholar]
- Humphrey DJ, Dufresne ER, Schwartz MA. Mechanotransduction and extraceullar matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Bass MD, Craig SE, Pinney JW, Knight D, Humphries MJ. Proteomic analysis of integrin-associated complexes indentifies RCC2 as a dual regulator of Rac1 and Arf6. Sci. Signal. 2009;2:1–9. doi: 10.1126/scisignal.2000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J. Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Fibronectins. Springer-Verlag; New York: 1990. [Google Scholar]
- Hytönen VP, Wehrle-Haller B. Mechanosensing in cell-matrix adhesions -Converting tension into chemical signals. Exp. Cell Res. 2016;343:35–41. doi: 10.1016/j.yexcr.2015.10.027. [DOI] [PubMed] [Google Scholar]
- Iwamoto DV, Calderwood DA. Regulation of integrin-mediated adhesions. Curr. Opin. Cell Biol. 2015;36:41–47. doi: 10.1016/j.ceb.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemet J, Humphries MJ, Caswell PT. Role of adhesion receptor in trafficking in 3D cell migration. Curr. Opin. Cell Biol. 2013;25:627–632. doi: 10.1016/j.ceb.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job R, Raja V, Grierson I, Currie L, O’reilly S, Pollock N, Knight E, Clark AF. Cross-linked actin networks (CLANs) are present in lamina cribrosa cells. Br. J. Ophthalmol. 2010;94:1388–1392. doi: 10.1136/bjo.2009.176032. [DOI] [PubMed] [Google Scholar]
- Joe MK, Sohn S, Kim TE, Im J, Choi YR, Kee C. Analysis of glucocorticoid-induced MYOC expression in human trabecular meshwork cells. Vis. Res. 2011;51:1033–1038. doi: 10.1016/j.visres.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Johnson DH, Richardson TM, Epstein DL. Trabecular meshwork recovery after phagocytic challenge. Curr. Eye Res. 1989;8:1121–1130. doi: 10.3109/02713688909000037. [DOI] [PubMed] [Google Scholar]
- Junglass B, Yu AHL, Welge-Lussen U, Tamm E, Fuchshofer R. Connective tissue growth factor induces extracellular matrix deposition in human trabecular meshwork cells. Exp. Eye Res. 2009;88:1065–1075. doi: 10.1016/j.exer.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Katsumi A, Naoe T, Matsushita T, Kaibuchi K, Schwartz MA. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J. Biol. Chem. 2005;280:16546–16549. doi: 10.1074/jbc.C400455200. [DOI] [PubMed] [Google Scholar]
- Keller KE, Kelley MJ, Acott TS. Extracellular matrix gene alternative splicing by trabecular meshwork cells in response to mechanical stretching. Investig. Ophthal. Vis. Sci. 2007;48:1164–1172. doi: 10.1167/iovs.06-0875. [DOI] [PubMed] [Google Scholar]
- Kelley MJ, Rose A, Radecki M, Hernandez NI, Acott TS. Phosphorylation increases with mechanical stretch in trabecular meshwork cells. 11th International Conference on Second Messengers and Phosphoproteins. 2001:229. [Google Scholar]
- Kraynov VS, Chamberlain C, Bokoch GM, Schwartz MA, Slabaugh S, Hahn KM. Localized Rac activation dynamics visualized in living cells. Science. 2000;290:333–337. doi: 10.1126/science.290.5490.333. [DOI] [PubMed] [Google Scholar]
- Lahdenranta J, Sidman RL, Pasqualini R, Arap W. Treatment of hypoxia- induced retinopathy with targeted proapoptotic peptidomimetic in a mouse model of disease. FASEB J. 2007;2007:3272–3278. doi: 10.1096/fj.07-8273com. [DOI] [PubMed] [Google Scholar]
- Lawson CD, Burridge K. The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration. Small GTPases. 2014 doi: 10.4161/sgtp.27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy DJ, Aukhil I, Erickson HP. 2.0 A crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84:155–164. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- Leiss M, Beckmann K, Giros A, Costell M, Fassler R. The role of integrin binding sites in fibronectin matrix assembly in vivo. Curr. Opin. Cell Biol. 2008;20:502–507. doi: 10.1016/j.ceb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Leu ST, Jacques SAL, Wingerd KL, Hikita ST, Tolhurst EC, Pring JL, Wiswell D, Kinney L, Goodman NL, Jackson DY, Clegg DO. Integrin α4β1 function is required for cell survival in developing retina. Dev. Biol. 2004;276:416–430. doi: 10.1016/j.ydbio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Lin GL, Cohen DM, Desai RA, Breckenridge MY, Gao L, Humphries MJ, Chen CS. Activation of beta 1 but not beta 3 integrin increases cell traction forces. FEBS Lett. 2013;587:763–769. doi: 10.1016/j.febslet.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Collins C, Kiosses WB, Murray AM, Joshi M, Shepherd TR, Fuentes EJ, Tzima E. A novel pathway spatiotemporally activates Rac1 and redox signaling in response to fluid shear stress. J. Cell Biol. 2013;201:863–873. doi: 10.1083/jcb.201207115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna J, Tobe T, Mousa SA, Reilly TM, Campochiaro PA. Antagonists of integrin alpha v beta 3 inhibit retinal neovascularization in a murine model. Lab. Investig. 1996;75:563–573. [PubMed] [Google Scholar]
- Manabe R, Oh-e N, Maeda T, Fukuda T, Sekiguchi K. Modulation of cell-adhesive activity of fibronectin by the alternatively spliced EDA segment. J. Cell Biol. 1997;139:295–307. doi: 10.1083/jcb.139.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Johnson DH. Dexamethasone decreases phagocytosis by human trabecular meshwork cells in situ. Investig. Ophthal. Vis. Sci. 1997a;38:1902–1907. [PubMed] [Google Scholar]
- Matsumoto Y, Johnson DH. Trabecular meshwork phagocytosis in glaucomatous eyes. Ophthalmologica. 1997b;211:147–152. doi: 10.1159/000310782. [DOI] [PubMed] [Google Scholar]
- Medina-Ortiz WE, Belmares R, Neubauer S, Wordinger RJ, Clark AF. Cellular fibronectin expression in human trabecular meshwork and induction by transforming growth factor-ß2. Investig. Ophthal. Vis. Sci. 2013;54:6779–6788. doi: 10.1167/iovs.13-12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Li S, Hu YL, Yuan S, Zhao Y, Chen BP, Puzon-McLaughlin W, Tarui T, Shyy JY, Takada Y, Usami S, Chien S. Differential regulation of Rho GTPases by beta1 and beta3 integrins: the role of an extracellular domain of integrin in intracellular signaling. J. Cell Sci. 2002;115:2199–2206. doi: 10.1242/jcs.115.10.2199. [DOI] [PubMed] [Google Scholar]
- Morgan MR, Byron A, Humphries MJ, Bass MD. Giving off mixed signals-distinct functions of alpha5beta1 and alphavbeta3 integrins in regulating cell behaviour. IUBMB Life. 2009;61:731–731. doi: 10.1002/iub.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JC. Integrins in the optic nerve head: potential roles in glau-comatous optic neuropathy. Trans. Am. Ophthalmol. Soc. 2006;104:453–477. [PMC free article] [PubMed] [Google Scholar]
- Morrison JC, Johnson EC, Cepurna W, Jia L. Understanding mechanisms of pressure-induced optic nerve damage. Prog. Retin Eye Res. 2005;24:217–240. doi: 10.1016/j.preteyeres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Mould AP, Askari JA, Aota S, Yamada KM, Irie A, Takada Y, Mardon HJ, Humphries MJ. Defining the topology of integrin alpha5beta1-fibronectin interactions using inhibitory anti-alpha5 and anti-beta1 monoclonal antibodies. Evidence that the synergy sequence of fibronectin is recognized by the amino-terminal repeats of the alpha5 subunit. J. Biol. Chem. 1997;272:17283–17292. doi: 10.1074/jbc.272.28.17283. [DOI] [PubMed] [Google Scholar]
- Mousa SA. αv Vitronectin receptors in vascular-mediated disorders. Med. Res. Rev. 2003;23:190–199. doi: 10.1002/med.10031. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Stossel TP, Hartwig JH. The filamins. organizers of cell structure and function. Cell Adhes. Migr. 2013;5:160–169. doi: 10.4161/cam.5.2.14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking αvβ5 integrin. J. Exp. Med. 2004;200:1539–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Silva KE, Scelfo C, Finnemann SC. Retinal pigment epithelial cells use a MerTK-dependent mechanism to limit the phagocytic particle binding activity of αvβ5 integrin. Biol. Cell. 2012;104:326–341. doi: 10.1111/boc.201100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann C, Garreis F, Paulsen F, Hammer CM, Birke MT, Scholz M. Osteopontin is induced by TGF-β2 and regulates metabolic cell activity in cultured human optic nerve head astrocytes. PLoS One. 2014;9:e92762. doi: 10.1371/journal.pone.0092762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly S, Pollock N, Currie L, Paraoan L, Clark AF, Grierson I. Inducers of cross-linked actin networks in trabecular meshwork cells. Investig. Ophthal. Vis. Sci. 2011;52:7316–7324. doi: 10.1167/iovs.10-6692. [DOI] [PubMed] [Google Scholar]
- Obara M, Kang MS, Yamada KM. Site-directed mutagenesis of the cell-binding domain of human fibronectin: separable, synergistic sites mediate adhesive function. Cell. 1988;53:649–657. doi: 10.1016/0092-8674(88)90580-6. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Hartwig JH, Stossel TP. Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat. Cell Biol. 2006;8:803–814. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- Orr WA, Ginsberg MH, Shattil SJ, Deckmyn H, Schwartz MA. Matrix-Specific suppression of integrin activation shear stress signaling. Mol. Biol. Cell. 2006;17:4686–4697. doi: 10.1091/mbc.E06-04-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D-Y, Lee J, Park I, Choi D, Lee S, Song S, Hwang Y, Hong KY, Nakaoka Y, Makinen T, Kim P, Alitalo K, Hong Y-K, Koh GY. Lymphatic regulator PROX1 determines Schlemm’s canal integrity and identity. J. Clin. Investig. 2014;124:3960–3974. doi: 10.1172/JCI75392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattabiraman P, Rao P. Hic-5 regulates actin cytoskeletal reorganization and expression of fibrogenic markers and myocilin in trabecular meshwork cells. Investig. Ophthal. Vis. Sci. 2015;56:5656–5669. doi: 10.1167/iovs.15-17204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkumas KM, Stamer WD. Protein markers and differentiation in culture for Schlemm’s canal endothelial cells. Exp. Eye Res. 2012;96:82–87. doi: 10.1016/j.exer.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- Rao PV, Peterson YK, Inoue T, Casey PJ. Effects of pharmacologic inhibition of protein geranylgeranyltransferase type I on aqueous humor outflow through the trabecular meshwork. Investig. Ophthal. Vis. Sci. 2008;49:2464–2471. doi: 10.1167/iovs.07-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redick SD, Settles DL, Briscoe G, Erickson HP. Defining fibronectin’s cell adhesion synergy site by site directed mutagenesis. J. Cell Biol. 2000;149:521–527. doi: 10.1083/jcb.149.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, Da Violante G, Gourlaouen M, Salih M, Jones MC, Jones DT, Saunders G, Kostourou V, Perron-Sierra F, Norman JC, Tucker GC, Hodivala-Dilke KM. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat. Med. 2009;15:392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- Reynolds AR, Reynolds LE, Nagel TE, Lively JC, Robinson SD, Hicklin DJ, Bodary SC, Hodivala-Dilke KM. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta3-integrin-deficient mice. Cancer Res. 2004;64:8643–8650. doi: 10.1158/0008-5472.CAN-04-2760. [DOI] [PubMed] [Google Scholar]
- Roca-Cusachs P, Gauthier NC, del Rio A, Sheetz MP. Clustering of α5β1 integrins determines adhesion strength whereas αvβ3 and talin enable mechanotransduction. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16250–16425. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose DM, Grabovsky V, Alon R, Ginsberg MH. The affinity of integrin α4β1 governs lymphocyte migration. J. Immunol. 2001;167:2824–2830. doi: 10.4049/jimmunol.167.5.2824. [DOI] [PubMed] [Google Scholar]
- Rose DM, Han J, Ginsberg MH. α4 integrins and the immune response. Immunol. Rev. 2002;186:118–124. doi: 10.1034/j.1600-065x.2002.18611.x. [DOI] [PubMed] [Google Scholar]
- Ross TD, Coon BG, Yunm S, Baeyens N, Tanaka K, Ouyang M, Schwartz MA. Integrins in mechanotransduction. Curr. Opin. Cell Biol. 2013;25:613–618. doi: 10.1016/j.ceb.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozsa FW, Reed DM, Scott KM, Pawar H, Moroi SE, Kijek TG, Krafchak CM, Othman MI, Vollrath D, Elner VM, Richards JE. Gene expression profile of human trabecular meshwork cells in response to long-term dexa-methasone exposure. Mol. Vis. 2006;12:125–141. [PubMed] [Google Scholar]
- Santas AJ, Bahler C, Peterson JA, Filla MS, Kaufman PL, Tamm ER, Johnson D, Peters DP. Effect of Heparin II domain of fibronectin on aqueous outflow in cultured anterior segments of human eyes. Investig. Ophthal. Vis. Sci. 2003;44:4796–4801. doi: 10.1167/iovs.02-1083. [DOI] [PubMed] [Google Scholar]
- Schiller HB, Fassler R. Mechanosensitivity and compositional dynamics of cell-matrix adhesions. EMBO Rep. 2013;14:509–519. doi: 10.1038/embor.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller HB, Hermann M, Polleux J, Vignaud T, Zanivan S, Friedel CC, Sun Z, Raducanu A, Gottschalk K, Thery M, Mann M, Fassler R. β 1- and αv-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat. Cell Biol. 2013;15:625–636. doi: 10.1038/ncb2747. [DOI] [PubMed] [Google Scholar]
- Schneider M, Fuchshofer R. The role of astrocytes in optic nerve head fibrosis in glaucoma. Exp. Eye Res. 2016;142:49–55. doi: 10.1016/j.exer.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J, DeSimone DW. Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb. Perspect. Biol. 2011;3:a005041. doi: 10.1101/cshperspect.a005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwinn MK, Gonzalez JMJ, Gabelt B, Sheibani N, Kaufman P, Peters DM. Heparin II domain of fibronectin mediates contractility through an α4β1 integrin co-signaling pathway. Exp. Eye Res. 2010;316:1500–1512. doi: 10.1016/j.yexcr.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Askari JA, Humphries MJ, Jones EY, Stuart DI. Crystal structure of a heparin- and integrin-binding segment of human fibronectin. EMBO J. 1999;18:1468–1479. doi: 10.1093/emboj/18.6.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard AR, Jacobson N, Fingert JH, Stone EM, Sheffield VC, Clark AF. Delayed secondary glucocorticoid responsiveness of MYCO in human trabecular eshwork cells. Investig. Ophthal. Vis. Sci. 2001;42:3173–3181. [PubMed] [Google Scholar]
- Sheppard D, Cohen DS, Wang A, Busk M. Transforming growth factor β differentially regulates expression of integrin subunits in guinea pig airway epithelial cells. J. Biol. Chem. 1992;267:17409–17414. [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- Stamer WD, Acott TS. Current understanding of conventional outflow dysfunction in glaucoma. Curr. Opin. Ophthalmol. 2012;23:135–143. doi: 10.1097/ICU.0b013e32834ff23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steely HT, Browder SL, Julian MB, Miggans ST, Wilson KL, Clark AF. The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Investig. Ophthal. Vis. Sci. 1992;33:2242–2250. [PubMed] [Google Scholar]
- Sumida GM, Stamer WD. Sphingosine-1-phosphate enhancement of cortical actomyosin organization in cultured human Schlemm’s canal endothelial cell monolayers. Investig. Ophthal. Vis. Sci. 2010;51:6633–6638. doi: 10.1167/iovs.10-5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E, Inoue T, Fujimoto T, Kojima S, Tanihara H. Epithelial mesenchymal transition-like phenomenon in trabecular meshwork cells. Exp. Eye Res. 2013;118:72–79. doi: 10.1016/j.exer.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Tamm ER. The trabecular meshwork outflow pathways: structural and functional aspects. Exp. Eye Res. 2009;88:648–655. doi: 10.1016/j.exer.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Tan JC, Kalapesi FB, Coroneo MT. Mechanosensitivity and the eye: cells coping with the pressure. Br. J. Ophthalmol. 2006;90:383–388. doi: 10.1136/bjo.2005.079905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tektas OY, Lutjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp. Eye Res. 2009;88:769–775. doi: 10.1016/j.exer.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Tervo K, Paallysaho T, Virtanen I, Tervo T. Integrins in human anterior chamber angle. Graefes Arch. Clin. Exp. Ophthalmol. 1995;233:291–295. doi: 10.1007/BF00177651. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Hagel M, Turner CE. Characterization of a focal adhesion protein, Hic-5, that shares extensive homology with paxillin. J. Cell Sci. 1999;112(pt2):181–190. doi: 10.1242/jcs.112.2.181. [DOI] [PubMed] [Google Scholar]
- Toth B, Sarang Z, Vereb G, Zhang A, Tanaka S, Melino G, Fesus L, Szondy Z. Over-expression on integrin β3 can partially overcome the defect of integrin β3 signaling in transglutaminase 2 null macrophages. Immunol. Lett. 2009;126:22–28. doi: 10.1016/j.imlet.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Tzima E, Angel del Pozo M, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- van Rijssel J, Van Buul JD. The many faces of the guanine-nucleotide exchange factor trio. Cell Adhes. Migr. 2012;6:482–487. doi: 10.4161/cam.21418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWyst SS, Perkumas KM, Read AT, Overby DR, Stamer WD. Structural basement membrane components and corresponding integrins in Schlemm’s canal endothelia. Mol. Vis. 2011;17:199–209. [PMC free article] [PubMed] [Google Scholar]
- Vranka JA, Bradley JM, Yang YF, Keller KE, Acott TS. Mapping molecular differences and extracellular matrix gene expression in segmental outflow pathways of the human ocular trabecular meshwork. PLoS One. 2015;10(3):e0122483. doi: 10.1371/journal.pone.0122483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DM, Pokrovskaya O, O’Brien CJ. The function of matricellular proteins in the lamina cribosa and trabecular meshwork in glaucoma. J. Ocul. Pharmacol. Ther. 2015;31:1–10. doi: 10.1089/jop.2014.0163. [DOI] [PubMed] [Google Scholar]
- Wang A, Yen MY, Hsu WM, Fann MJ. Induction of vitronectin and integrin alphav in the retina after optic nerve injury. Mol. Vis. 2006;12:76–84. [PubMed] [Google Scholar]
- Wang Y, Yan W, Lu X, Qian C, Zhang J, Li P, Shi L, Zhao P, Fu Z, Pu P, Kang C, Jiang T, Liu N, You Y. Overexpression of osteopontin induces angiogenesis of endothelial progenitor cells via the αvβ3/PI3K/AKT/eNOS/NO signaling pathway in glioma cells. Euro J. Cell Biol. 2011;90:642–648. doi: 10.1016/j.ejcb.2011.03.005. [DOI] [PubMed] [Google Scholar]
- White DP, Caswell PT, Norman JC. Alpha v beta3 and alpha5beta1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J. Cell Biol. 2007:515–525. doi: 10.1083/jcb.200609004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White ES, Muro AF. Fibronectin splice variants: understanding their multiple roles in health and disease using engineered mouse models. IUBMB Life. 2011;63:538–546. doi: 10.1002/iub.493. [DOI] [PubMed] [Google Scholar]
- Wickstrom SA, Fassler R. Regulation of membrane traffic by interin signaling. Trends Cell Biol. 2011;21:266–274. doi: 10.1016/j.tcb.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Wilkinson-Berka JL, Jones D, Taylor G, Jaworski K, Kelly DJ, Ludbrook SB, Willette RN, Kumar S, Gilbert RE. SB-267268, a nonpeptidic antagonist of alpha(v)beta3 and alpha(v)beta5 integrins, reduces angiogenesis and VEGF expression in a mouse model of retinopathy of prematurity. Investig. Ophthalmol. Vis. Sci. 2006;47:1600–1605. doi: 10.1167/iovs.05-1314. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Sharma T, Clark AF. The role of TGF-β2 and bone morphogenetic proteins in the trabecular meshwork and glaucoma. J. Ocul. Pharmacol. Ther. 2014;30:154–162. doi: 10.1089/jop.2013.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worth DC, Parson M. Adhesion dynamics: mechanisms and measurements. Int. J. Biochem. Cell Biol. 2008;40:2397–2409. doi: 10.1016/j.biocel.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Wu YJ, La Pierre DP, Wu AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- WuDunn D. Mechanobiology of trabecular meshwork cells. Exp. Eye Res. 2009;88:718–723. doi: 10.1016/j.exer.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Yang L, Guerrero J, Hong H, DeFranco DB, Stallcup MR. Interaction of the tau2 transcriptional activation domain of glucocorticoid receptor with a novel steroid receptor coactivator, Hic-5, which localizes to both focal adhesions and the nuclear matrix. Mol. Cell Biol. 2000;11:2007–2018. doi: 10.1091/mbc.11.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Akatsuka T, Imanaka-Yoshida K. Tenascin-C and integrins in cancer. Cell Adhes. Migr. 2015;9:96–104. doi: 10.1080/19336918.2015.1008332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49:568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 2003;116:4013–4605. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Geiger B. The switchable integrin adhesome. J. Cell Sci. 2010;123:1385–1388. doi: 10.1242/jcs.066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Magnusson MK, Mosher DF. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol. Biol. Cell. 1997;8:1415–1425. doi: 10.1091/mbc.8.8.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Li Y, Yue BY. Glucocorticords effects on extracellular matrix proteins and integrins in bovine trabecular meshwork cells in relation to glaucoma. Int. J. Mol. Med. 1998;1:339–346. [PubMed] [Google Scholar]
- Zhou L, Maruyama I, Li Y, Cheng EL, Yue BY. Expression of integrin receptors in the human trabecular meshwork. Curr. Eye Res. 1999a;19:395–402. doi: 10.1076/ceyr.19.5.395.5297. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhang SR, Yue B. Expression of integrin receptors in the human trabecular meshwork. Investig. Ophthal. Vis. Sci. 1995;36:S726. [Google Scholar]
- Zhou LL, Maruyama I, Li YH, Cheng EL, Yue B. Expression of integrin receptors in the human trabecular meshwork. Curr. Eye Res. 1999b;19:395–402. doi: 10.1076/ceyr.19.5.395.5297. [DOI] [PubMed] [Google Scholar]
- Zode GS, Clark AF, Wordinger RJ. Bone morphogenetic protein 4 inhibits TGF-β2 stimulation of extracellular matrix proteins in optic nerve head cells: role of gremlin in ECM modulation. GLIA. 2009;57:755–766. doi: 10.1002/glia.20803. [DOI] [PubMed] [Google Scholar]