Abstract
The field of tissue engineering is advancing steadily, partly due to advancements in rapid prototyping technology. Even with increasing focus, successful complex tissue regeneration of vascularized bone, cartilage and the osteochondral interface remains largely illusive. This review examines current three-dimensional printing techniques and their application towards bone, cartilage and osteochondral regeneration. The importance of, and benefit to, nanomaterial integration is also highlighted with recent published examples. Early-stage successes and challenges of recent studies are discussed, with an outlook to future research in the related areas.
Keywords: 3D bioprinting, tissue engineering, osteochondral tissue
Introduction
Osteoarthritis (OA) is the gradual degeneration of cartilage in articulating joints causing joint pain and dysfunction for the patient [1]. In 2005, 47.8 million Americans were diagnosed with symptomatic OA; this number is expected to grow to 67 million by 2030. It is estimated that 33% of this group will be workforce contributors aged between 45 and 64 years [2] where adverse effects, including decreased patient quality of life, directly and indirectly lead to a large global economic impact through decreased productivity, reduced contribution to the work force, and increased disability compensation. This impact is likely to grow as the United States population ages [1]. Another potential contributing factor not currently included in the above 2030 prediction is an increasing national obesity epidemic [2]. Increased body weight leads to increased joint loading, leading to and further exacerbating existing joint injury and degeneration.
Joint degeneration is characterized by chronic or acute damage to cartilaginous tissue and bone at the joint surfaces. Bone tissue has effective self-healing properties and has proven a favorable candidate for tissue engineered scaffolds and grafts though some challenges remain, especially for large-scale defects. Cartilage, on the other hand, is largely avascular and has low regenerative capacity; once injured it is very difficult for this tissue to self-heal [3]. Available surgical methods are predicated on the severity and size of the defect as shown in figure 1 ranging from focal sized (<2 cm2 diameter) defects where no efficient treatment options exist to complete joint degeneration requiring highly invasive total joint arthroplasty [4]. Less invasive clinical treatment methods to modulate disease progression are available, but are ineffective in regenerating the native extracellular matrix niche. This mismatch leads to increased defect severity and, in most severe cases, to total joint replacement. Large defects involving both joint cartilage and bone are even more difficult to manage. Physical and mechanical property requirements of the osteochondral transitional tissue region are difficult to replicate. Currently, large defect tissue repair is conducted through the use of autograft or allograft when available and feasible. If the damage is too severe, and/or the supply of auto- or allograft is limited, total joint replacement is necessary to mobilize the patient.
Figure 1.
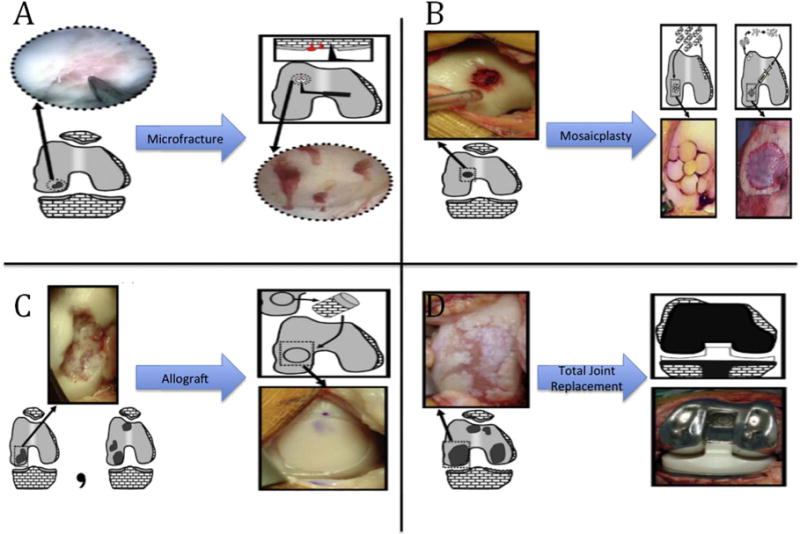
Current clinical treatment options for articular joint damage. Treatment options depend on defect size and severity: (A) defects < 2 cm2; (B) 2 cm2 < defects < 3 cm2; (C) defect ≈ 4 cm2 or multiple defects; (D) end-stage degeneration. Reprinted from [4], Copyright (2010), with permission from Elsevier.
Tissue engineering (TE) holds great promise in developing methods of repairing tissue damage and prolonging the need for more invasive orthopedic procedures. TE applies the principles of engineering and life sciences towards the development of biological substitutes which restore, maintain, or improve tissue function [5]. For focal defects, autologous stem cells can be harvested from the patient, seeded and expanded in vitro upon a three-dimensional (3D) scaffold then transplanted back into the patient after successful cell differentiation. Although traditional scaffold fabrication techniques such as particle leaching [6–8] and gas foaming [9–12] have been used to manufacture porous scaffolding for tissue regeneration, limitations exist with regards to insufficient pore interconnectivity leading to poor tissue integration and inefficient nutrient delivery and waste removal.
3D printing is a technology that has garnered greater attention for the fabrication of highly ordered tissue engineered scaffolds. Multiple 3D printing platforms exist and provide a breadth of material options for scaffold fabrication. 3D printing technology is also advancing in capabilities for direct cell printing, further expanding scaffold design potential. Many 3D printing platforms and products facilitate the integration of nano/micro-particles and growth factors, which enhance cytocompatibility and overall scaffold performance. Nanomaterials provide physiochemical cues that help guide desired cellular differentiation of stem cells used in culture and often improve the biocompatibility of inert scaffold materials [13].
Material selection is critical, both for the bulk scaffold material and for any additional supplementation. It is important to consider both material and mechanical properties in the selection process since both provide cues to the surrounding cells guiding acceptance and function [14, 15]. Scaffolds must present favorable hyrophilicity, roughness, and surface topography, at the micron and sub-micron level, to replicate the natural environment of native tissue. Nanoscale features on the surface topography of a scaffold increase surface area, surface-to-volume ration, and surface roughness enhancing cellular adhesion and promoting favorable biocompatibility [16]. These nanoscale features are often created through integration of nanoscale materials within or on the surface of the bulk scaffold material. Toxicity of nanomaterials is a topic of great debate, both in their fabrication and in their use in the human body. The nanomaterials discussed in this article, and the majority of nanomaterials used for musculoskeletal regeneration, are biological markers that already exist in the human body so toxicity will not be covered in this review. The current review will present various 3D printing modalities and their application towards cartilage, bone, and osteochondral tissue regeneration. Discussions on the current challenges inhibiting efficient and long-term efficacy of scaffolds for articulating joint lesion treatment, along with the benefits of nanomaterial integration during scaffold development, are included.
3D printing
Pham and Gault define rapid prototyping (RP) as an enabling technology whose purpose is to reduce product development and production times, as well as cost, resulting in an increase in market competitiveness [17]. Though this definition, and their article, focuses on RP in manufacturing, the fundamental ideas presented hold true for TE. RP technologies via additive manufacturing 3D printing can readily enable physicians to efficiently engineer personalized scaffolds for patient-specific treatment. Ultimately, patients will not have to wait for a viable donor, or surrender to total joint replacement at an early age due to disease progression. They will instead be treated with cell-laden or bioactive 3D scaffolds prolonging or eliminating the need for more invasive/destructive procedures. Advances in biomaterials research is a critical component to the realization of functional and efficient scaffolding where processability and biocompatibility must be taken into consideration. Several RP technologies for 3D bioactive scaffold fabrication exist, including: bioplotting, inkjet bioprinting, selective laser sintering (SLS), stereo-lithography (SL), and fused deposition modeling (FDM). This review will discuss advantages and disadvantages, as well as potential future applications, of these technologies as they relate to bone, cartilage, and/or osteochondral scaffold fabrication. It will also touch on the integration of bioactive nanoparticles, during or after printing, to enhance scaffold performance. Table 1 summarizes musculoskeletal applications for presented 3D printing techniques along with common materials available for use on the various platforms [18–26].
Table 1.
A summary of musculoskeletal applications for various 3D printing techniques with some common materials used on respective platforms.
| Targeted musculoskeletal tissue region | Applicable printing platforms | Common scaffold materials | Common nanomaterial supplementation |
|---|---|---|---|
| Bone | SLS | TCP, CaP, Hydroxyapatite, Ti, silica, CoCr |
nHA BMP |
| FDM | PCL, PLA, PLGA, PLLA, PEGa, TCP |
||
| Cartilage | SL | PPF/DEF—HA, PDLLA/HA | TGF-β IGF |
| FDM | PCL, PLA, PLGA, PLLA, PEGa | ||
| Bioplotting | GELMA, PEGDMA, Collagen, Alginate, Chitosan, HAMA, NFC | ||
| Inkjet bioprinting | |||
| Osteochondral | FDM | PCL, PLA, PLGA, PLLA, PEGa | nHA BMP |
| GELMA, PEGDMA, Collagen, Alginate, Chitosan, HAMA, NFC | TGF-β | ||
| Bioplotting | IGF | ||
| Inkjet bioprinting |
Note. TCP, tricalcium phosphate: CaP, calcium phosphate: Ti, titanium: CoCr, cobalt-chromium: PCL, polycaprolactone: PLA, poly(lactic acid): PLGA, poly (lactic-co-glycolic)acid: PLLA, poly(L-lactic acid): PEG, polyethylene glycol: PPF, poly(propylene fumerate): DEF, diethyl fumerate: HA, hyaluronin: PDLLA, poly(D, L-lactic acid): GELMA, gelatin methacrylate: PEGDMA, polyethylene glycol dimethylactrilate, HAMA, methacrylated hyaluronan: NFC, nanofibrillated cellulose: nHA, nanohydroxyapatite: TGF, transforming growth factor: IGF, insulin-like growth factor: BMP, bone morphogenic protein.
This material is used for investment casting on the specified platform.
Bioplotting
Bioplotting is an additive manufacturing technique for the fabrication of 3D TE scaffolds where cell-laden and/or non-cell-laden materials are extruded drop-wise as a viscous fluid through a syringe into tubular or spherical shapes and cured using ultraviolet radiation, chemical crosslinking agents, or time dependent solidification [27]. Figure 2 displays basic schematic procedures of the 3D bioplotting process with representative scaffolds. Bioplotters allow for the use of one or multiple syringes leading to the formation of compositionally complex constructs. This capability allows for the integration of cells and biomaterials to replicate physiologically relevant spatial orientations and geometries [28]. The main challenge with this technology is the necessity for high viscosity bioprinting materials to provide support while curing [29].
Figure 2.
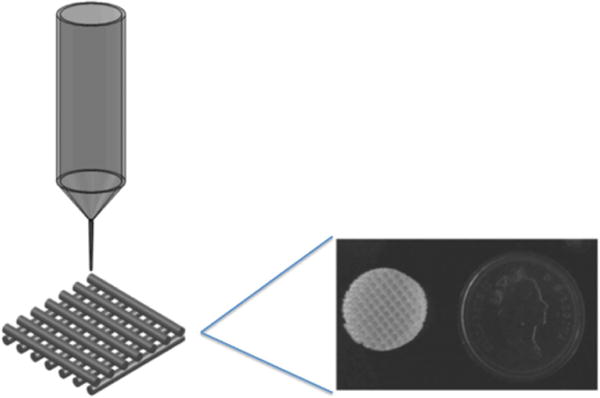
Schematic image of a bioplotting system with photo image of potential resulting scaffold. Reproduced from [30]. © IOP Publishing Ltd. All rights reserved.
The ability to directly impregnate biomaterials with cells while bioprinting is certainly an advantage of these systems thus reducing material processing. However, bioplotted scaffolds commonly exhibit a very smooth surface, which is attributed to the extrusion and curing process. Since surface characteristics have been extensively linked to cell adhesion and proliferation, additional scaffold surface modification is often necessary to create surface roughness and improve the cellular response [31–33]. Furthermore, the materials currently available for syringe-based extrusion often do not possess mechanical properties suitable for human implantation [27]. Although bioplotters show promise for the rapid fabrication of cell impregnated biomaterials, more focused research is necessary for the development of extrudable, biocompatible materials possessing favorable mechanical and physical properties.
Inkjet bioprinting
Inkjet bioprinting is a method of depositing bio-inks containing cells and/or biological materials via commercial inkjet printers. Much like the preceding technologies, inkjet bioprinting is a bottom-up scaffold fabrication technique in which cells and hydrogels can be directly and precisely printed into 3D tissue engineered scaffolds as illustrated with representative scaffold in figure 3 [34]. Additionally, inkjet bioprinters containing multiple print cartridges have the capability of allowing simultaneous printing of multiple types of cells [35]. Owing to the relative accessibility and availability of commercial printers, very precise non-contact deposition of cells can be directly applied upon pre-designed scaffolds.
Figure 3.
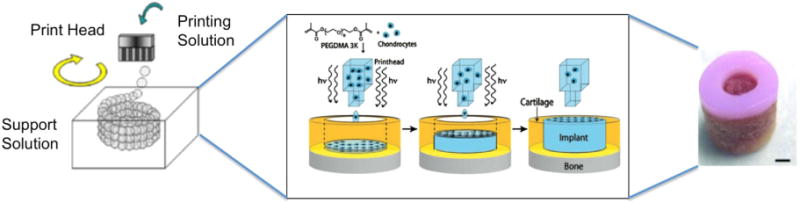
Schematic of inkjet bioprinting system, scaffold production schematic, and image of final scaffold created using inkjet bioprinting. Reproduced from [36]. CC BY 3.0.; process schematic and scaffold, scale bar = 2 mm, reproduced with permission from [37]. [The publisher for this copyrighted material is Mary Ann Liebert, Inc. publishers.].
The biggest challenge with inkjet bioprinting devices is cell settling and aggregation at the printer nozzle and within the printer cartridge, which can lead to obstruction and nonuniform distribution during printing. Some solutions have been evaluated in an attempt to resolve this problem, such as agitation of medium contained in the cartridge, which directly impacts cell viability [38]. More research is needed to find stand-alone materials capable of acting as the cell-carrying medium within the cartridges, and solidifying to a supportable, biocompatible scaffold upon deposition.
Most of the current research has focused on hybrid printing techniques using multiple printing materials wherein one contains a cell suspension and the other serves to deposit the printing substrate or ‘biopaper’. One source acts as the cross-linking agent after contact in an effort to drive material solidification. This technique has shown positive results in vitro and in vivo illustrating adequate proliferation rates and good cell viability. The deposited cells survived and matured into functional tissue showing adequate vascularization [35]. This type of success is driving more advanced research and in vivo studies using hybrid devices, moving focus away from directly printing cell-laden materials. Further research in bio-ink formulations, especially for direct printing, could lead to continued advancement in cell printing for TE [38].
Selective laser sintering
For the manufacture of hard tissue and rigid support structures for tissue regeneration applications, SLS is often a suitable technique for scaffold fabrication. This process uses a laser beam to fuse nano/micro particles together into a solid component as illustrated in figure 4. This technique can also be used with slurries, combinations of powder particles, and gels. Both techniques involve the spreading of a thin layer of powder/slurry, treating the layer with the laser to fuse/solidify, and repeating as necessary to obtain the pre-designed 3D structure [39]. The laser evaporates the liquid phase, solidifying the slurry and allowing the solid phases to fuse. The process of bonding/fusing creates surfaces with favorable roughness at scales desired for biocompatibility.
Figure 4.
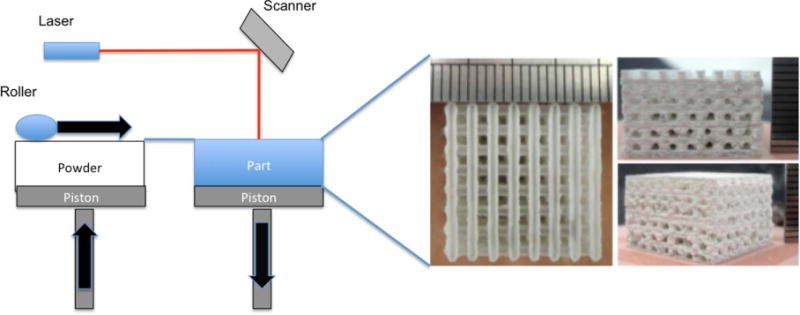
Schematic of selective laser sintering system and images of scaffolds created using selective laser sintering. Scaffold images reproduced from [40]. CC BY 3.0.
Benefits of this technique include the ability to integrate materials already approved for orthopedic implantation. Research exists on SLS of pure and alloyed titanium (Ti) powder with silica sol, cobalt chrome (CoCr), and hydroxyapatite (HA) with silica sol [39, 41, 42]. These studies illustrate successful fabrication and evaluation of SLS-based components constructed with materials previously approved for implantation. Further evolution of this technique can lead to efficient and viable scaffolds for bone and other hard tissues by facilitating clinical approval and increasing integration based on the extensive use of FDA approved materials. This is a huge benefit not shared by most other bioprinting techniques at the present time.
Some drawbacks of SLS printing are largely predicated on equipment cost; SLS-based printers are considerably more expensive than other 3D printing technologies. Also, printing times are longer than other techniques. Proper manufacturing of components is a careful balance between laser strength, speed, and spacing to achieve desired mechanical strengths [39]. Similar to other 3D printing techniques, SLS processing parameters and material properties of powders have significant implications on the overall performance of the sintered construct [43].
Stereolithography
As one of the oldest RP technologies, SL can be readily employed to manufacture stratified, multilayered scaffolds through UV photopolymerization of photocureable resins; examples of SL setup and printed products can be seen in figure 5. The geometry and architecture of the cured resin can be readily controlled by a focused laser beam [44–49] or digital mask projected from computer aided design interfaces [50]. This technology remains one of the most powerful and versatile, exhibiting the highest fabrication accuracy. It is largely a bottom-up process although research does exist on top-down methods for fabrication.
Figure 5.
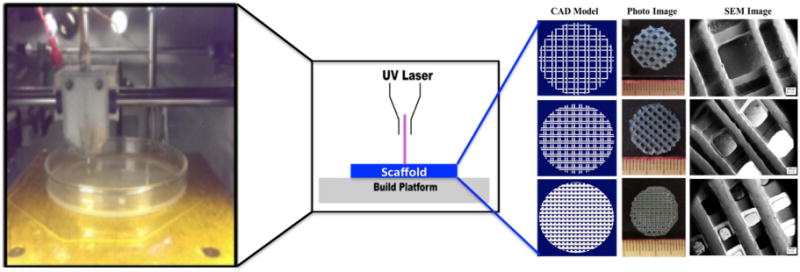
Images of stereolithography printer and resulting scaffolds as well as a system schematic of the process in the center. Reproduced from [51] with permission of The Royal Society of Chemistry.
Some challenges associated with SL include available biomaterials, material toxicity due to incorporated photoinitiators, and inadequate cell adhesion. Suitable materials for SL are limited to liquid resins that solidify when exposed to UV light. The breadth of available materials is steadily increasing, subsequently delivering a broader spectrum of resulting mechanical properties. Additionally, for implantation, a compounding complication is the presence of photoinitiators, which are added to the liquid resin in order to expedite cross-linking [52]. These materials must be carefully controlled and monitored to ensure that in vivo degradation is not marred with excessive build-up leading to cytotoxicity, inadequate tissue integration and failure. Scaffolds fabricated by SL are largely based on poly(ethylene glycol) hydrogels which are inherently non-cell adherent. Often, micro/nano particles and growth factors must be added to improve adhesion and proliferation. In order for this technology to progress towards clinically relevant tissue engineered scaffolds, more research on biocompatible materials is needed. Photocrosslinkable natural polymers are an emerging research area in this field that can address some of these challenges [52].
Fused deposition modeling
One of the fastest and least expensive methods of 3D scaffold fabrication is FDM. FDM is the bottom-up, layer-by-layer extrusion of a thermoplastic polymer filament yielding more rigid structures than most other 3D biofabrication techniques [53]. The printer nozzle moves in the x- and y-axis while the printing platform lowers in the z-axis as illustrated in figure 6 with representative scaffold [54]. Even though this technology is rapid, limitations exist, most notably structural stability, especially in designs with horizontal channels (x- and y-axis) or overhangs, due to the bottom up approach and viscosity of the extruded filament. Soluble support material is one solution when overhangs or channels require external support until the extruded filament cools to a temperature where it is capable of self-support.
Figure 6.
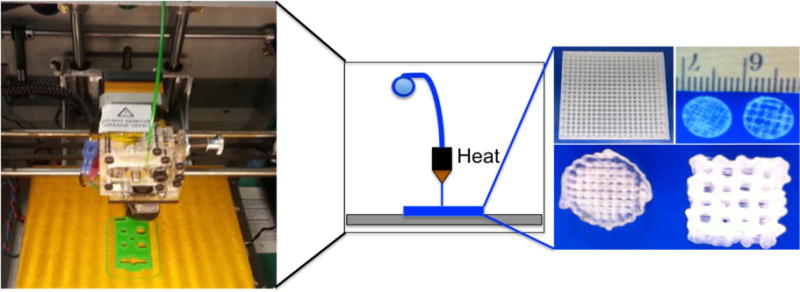
Photo of fused deposition modeling printer and resulting scaffolds with system schematic in the center.
The commercial availability of printable filaments is fairly broad, but availability of biomaterial filaments is still limited. Additionally, less robust polymers often require a difficult balance between temperature and extrusion speed to produce an optimal result. The limiting force when utilizing FDM is often the ramming force. This is a force generated by the drive wheel as the filament is fed into the heated nozzle. This force causes the filament to act as a piston for the distance between the drive wheel and nozzle; this is a difficult force to balance [54]. By violating the balance between ramming force and extrusion temperature the filament readily kinks and deforms on the forcing end or becomes too viscous at the nozzle tip. More research is needed to tune FDM-based printers to extend their capabilities to process more bio-compatible materials like polycaprolactone (PCL), especially given the proven positive in vivo response of this material.
Investment casting is a fabrication technique often implemented to take advantage of the robust dimensions realized with the FDM platforms without relying solely on available biofilaments. The FDM is used to fabricate a mold using any liquid/heat-soluble filament. Biocompatible resins/gels can be cast into the mold and solidified using various crosslinking techniques. After solidification, the mold is leached leaving a biocompatible polymer resin/gel scaffold. Challenges with this technique include viscosity of casting liquid, balancing solvent removal with polymer/gel swellability, and carefully controlling photoinitiators or other cross-linking agents to reduce cytotoxicity. Even with potential challenges, investment casting further diversifies FDM as a reliable platform for creating robust scaffolds with complex geometries.
Even though material limitations persist for FDM-based printers, it still remains one of the more reliable fabrication methods for TE scaffolds. Compared to other 3D printing methods, it produces scaffolds with favorable mechanical strength that are customizable, reproducible, and support cell growth [42]. It additionally illustrates great promise for patient-specific scaffolds when coupled with computed tomography (CT) or other similar medical imaging modalities.
3D printing and nanomaterials for vascularized bone, cartilage, and osteochondral regeneration
3D printing for TE is often augmented with nanomaterial integration during, or after, fabrication to improve component biocompatibility and direct cellular performance. Human mesenchymal stem cells are one of the most common cell lines used for bone and cartilage TE [55]. These stem cells must be directed to differentiate into the desired cell type required on the engineered scaffold. Physiochemical, as well as mechanical properties often determine the cell differentiation path. Certain nanomaterials can provide these necessary physiochemical cues and are often used as a method for guiding desired cellular differentiation of stem cells used in culture [13].
3D printing of vascularized bone
Bone tissue is composed of highly porous and vascularized cancellous tissue in inner bone, surrounded by dense and highly mineralized cortical tissue. The primary function of the cortical regions is load-bearing and support. Bone possesses natural self-healing properties, but its ability to effectively repair is limited by defect size. Larger defects often cannot effectively self-heal without external assistance [17]. Tissue-engineered scaffolds for large defect regions also experience challenges with oxygen and nutrient diffusion throughout the scaffold. Integrating appropriate porous geometries within tissue-engineered scaffolds helps mitigate this challenge [14].
Scaffold porosity is an essential design criterion for effective de novo bone formation. Open and interconnected pores allow for the movement of nutrients within the scaffold, promoting cell proliferation and differentiation, neovascularization, and waste removal [17, 56]. During scaffold design a strong interplay between pore size and pore distribution must be carefully balanced to produce a construct exhibiting a favorable cell microenvironment and appropriate mechanical strength. Increasing pore size and density improves cellular response and neovascularization while decreasing mechanical strength [17].
Presently, the gold standard for bone tissue replacement is autograft bone. This will likely remain as such until a more effective and reliable synthetic method is developed and clinically proven [57]. Autografts provide a suitable alternative but face challenges of their own; lack of usable tissue supply remains the greatest challenge for successful autograft implementation, especially for patients suffering large-scale trauma. Additionally, the most challenging area to treat is the craniomaxillofacial region where both a limited autologous tissue supply, combined with extremely personalized and complex geometries, are present and necessary [58]. It is not surprising scaffolds focused on defects of this region have seen the most rapid advancement and approval of engineered bone tissue scaffolds.
Human bone ECM contains collagen, osteopontin, fibronectin, HA, water, and other organic and inorganic compounds. One of the most prevalent inorganic compounds, nanohydroxyapatite (nHA), constitutes nearly 70% of the overall bone matrix [59]. nHA displays excellent cytocompatibility, osteoconductive and bioactive characteristics, and is easily fabricated in a lab setting with minimal equipment [60]. Integrating nHA into constructs is possible both during and after printing. If working with hydrogels, powders, or other fluid materials, nHA can be incorporated into the material prior to crosslinking or fusing. If working with solid polymers, in methods such as FDM, it is possible to incorporate nHA after printing as a surface modification. When culturing with stem cells, the presence of nHA can help direct the cells toward an osteogenic response necessary for scaffolds targeting bone. nHA is just one type of nanomaterial often used for osteogenesis; tricalcium phosphate, calcium phosphatase among other materials also enhance favorable osteogenic response. Cui, Temple, Gao, and Wang provide a few recent examples of 3D printing, and nanomaterial integration, of vascularized bone [58, 61–65].
Our lab recently created an innovative, nano/micro-featured, vascularized bone construct with self-modulating angiogenic and osteogenic growth factor delivery. This study implemented a concept of integrating a biologically inspired smart-release nano-coating strategy together with a 3D printed, fluid-perfused microstructure driving the realization of a biomimetic vascularized bone construct. Additionally, this research employed the use of a perfusion bioreactor to simulate in vivo shear stresses on cells created by nutrient flow over surfaces [61, 62]. Another recent study in our lab also investigates the effectiveness in combining multiple printing modalities to achieve a biomimetic vascularized bone construct. In this research, we successfully integrate FDM and SL to effectively recapitulate the intrinsic complexities of vascularized bone [63]. The results show great promise for both techniques moving forward to animal studies and clinical trials.
Recent studies by Temple et al examine 3D printing of large craniomaxillofacial bone defects and their ability to support human adipose-derived stem cell differentiation into vasculature and bone. This research investigates CT interaction with a homemade 3D printer to create patient-specific PCL scaffolds. The group also varied the internal porosity of the printed scaffolds to evaluate the effect of pores on scaffold success. Their results show great potential for patient-specific 3D TE scaffolds as vascularized bone grafts [58].
In addition to porosity within vascularized bone constructs, integration of bioactive materials also assists in the successful differentiation of multiple cell types in larger bone and vascularized bone scaffolds. Gao et al investigated the integration of nanoparticles of bioactive glass and HA embedded within poly(ethylene glycol) dimethacrylate scaffolds and their effect on human mesenchymal stem cell performance. They found that the biomimetic properties of HA significantly improved osteogenesis in the hydrogel bone scaffold and indicated the ability to achieve both hard and soft tissue engineered constructs using hydrogel matrices [64]. Wang et al recently investigated the potential for integrating virus-activated phage nanofibers displaying a high-density concentration of RGD peptide into 3D printed bioactive ceramic scaffolds for vascularized bone regeneration. The phage nanofibers did effectively induce both osteogenesis and angiogenesis within the group’s scaffolds [65].
3D printing cartilage
The successful application and effectiveness of 3D printed cartilage varies greatly depending on the site and extent of injury. Biological mechanisms for the regeneration of cartilage are still poorly understood. Unlike bone, cartilage lacks self-healing properties thus complicating regeneration and repair, especially in weight bearing regions. To date, the quality of repaired, load-bearing cartilage cannot be categorized as satisfactory functional restoration [66]. Researchers continue to struggle with the balance of biocompatibility, bioactivity, and acceptable mechanical properties of 3D printed implants. Currently, no effective scaffold has been fabricated to recapitulate the zonal organization, extracellular matrix composition, and mechanical properties of native load bearing cartilage, like that found within articulating joints [67]. Conversely, recent successes in non-load-bearing regions provide insight and promise of progress for this challenging tissue moving forward.
Nanomaterials can help induce stem cell chondrogenic differentiation, enhancing cartilage’s limited self-repair ability, as a way of improving cartilaginous tissue regeneration in 3D printed constructs. Two of the most common environmental stimulants for cartilage regeneration are insulin-like growth factor-1 (IGF-1) and transforming growth factor β (TGF-β) [37]. Strategies such as electrospraying are often used to encapsulate growth factors at the nanoscale into spheres for incorporation into the larger tissue engineered scaffold. For cartilage, IGF-1 is a key growth factor responsible for homeostasis as well as balancing matrix synthesis and degradation by chondrocytes [37]. It is used for numerous reasons, to include: maintaining viability of native cartilage [68], enhancing mechanical properties of engineered cartilage [69], and stimulating gene expression of aggrecan and type II collagen by chondrocytes [70, 71] leading to protein molecule biosynthesis [72]. TGF-β is a more complex growth factor belonging to a family of three isoforms :TGF-β1, TGF-β2, and TGF-β3. All three isoforms are associated with chondrocyte activity, inducing ECM deposition while inhibiting protease production by cells [37]. IGF-1 and TGF-β are just two examples of growth factors that, when incorporated into tissue engineered constructs as nanoparticles, benefit chondrogenesis and cartilage remodeling. Several examples are outlined in the remainder of this section.
Current research is also active in evaluating methods of stimulating cellular performance without merely increasing supplemental concentrations of growth factors and nutrients. Recent research by Aliabouzar et al investigated the impact of low intensity pulsed ultrasound (LIPUS), with and without lipid-coated microbubbles, on proliferation and chondrogenic differentiation of hMSCs in 3D printed scaffolds in vitro. The goal of this research was to find a way to enhance cellular growth and differentiation beyond the capacity of applied growth factors. Their results indicate that periodic mechanical stimulation of the culture environment through LIPUS improves chondrogenic performance of hMSCs on 3D printed cartilage scaffolds, and the introduction of microbubbles to the LIPUS environment further improves this chondrogenic response [73].
For non-load-bearing cartilaginous applications Chang et al successfully treated, in vivo, rabbit tracheal defects and Lee et al successfully replicated, in vitro, a human ear with satisfactory geometry and anatomical congruency [74, 75]. The fabricated tracheal implant displayed sufficient neo-cartilage formation upon the implanted graft with regenerated respiratory mucosa within the trachea. No respiratory distress was noted with minimal to no graft rejection [74]. Additionally, tracheal implants do undergo some mechanical stimulation internally via respiratory expansion and contraction. The regenerated cartilaginous tissue was able to withstand this type of loading. Though different than the frictional loading experienced at the surface of articulating joints, the ability to handle physiologically relevant mechanical loading is a promising outcome of this study. Lee’s study employed 3D printing to fabricate a bioactive anatomically correct scaffold for guided osteogenesis and adipogenesis of a human ear. To achieve appropriate geometries the group selected a dual extrusion printing system loaded with PCL as the primary print material and PEG as the sacrificial support material. Their setup demonstrated appropriate tissue formation derived from separately bioprinted chondrocytes and adipocytes in a geometry resembling that of a human ear [75]. The ear does not experience any necessary loading function but does present unique requirements for intricate and controlled design. The ability to control the print geometry in this manner and duplicate patient-specific structures using CT shows great promise for the modulation and control of cartilaginous tissue development, which may prove beneficial in other defect sites in the future.
Zopf et al investigated the non-load-bearing application of cartilage repair in the craniomaxillofacial region, specifically present in the auricular and nasal region. This region requires less emphasis on mechanical strength but greater focus on biocompatibility and controlled remodeling in order maintain the desired anatomical shape. CAD software was used to model the auricular or nasal features of each subject. The features were then printed using SLS of PCL. After printing, scaffolds were then seeded with a chondrocyte-enhanced type I collagen gel. In vivo testing of samples was conducted in porcine specimens. The PCL scaffolds maintained good shape upon implantation and showed cartilage growth. However, after two months in situ the scaffolds did not induce adequate de novo cartilage tissue [76]. Extended implantation time and analysis is required to validate the long-term performance of these scaffolds for human use, but initial results are promising. Successful biocompatibility in this procedure is worth noting and merits further investigation and development for extension towards load-bearing applications.
Another promising study for non-load-bearing advances by Mannoor et al effectively illustrated how 3D printing of TE constructs aims to improve patient quality of life in the future. This study merged biological and nanoelectronic functionalities to create functioning bionic ears. The group was able to print an ear with a cell-seeded hydrogel matrix containing an intertwined conducting polymer infused with silver nanoparticles. The group realized successful in vitro culturing of the cartilaginous tissue around the inductive coil. The ears were capable of sensing auditory signals and acting as complimentary left–right pairs for receiving stereo signals [77]. This groups success, among other similar successes, illustrates the vast potential for TE and the revolutionary improvement it will have on patient quality of life.
Load bearing cartilage repair requires a broad range of physical and mechanical properties not present in non-load-bearing cartilage applications. Cui et al investigated the effectiveness of hybrid scaffolds comprised of both natural and synthetic materials. The scaffold base was harvested using an 8 mm diameter stainless steel biopsy punch from bovine femoral condyles. The group then removed a 4 mm diameter plug from the center of the larger sample; depths of the induced defect ranged from 2–5 mm depending on thickness of cartilaginous layer as shown in figure 4. The group used inkjet bioprinting to deposit poly(ethylene glycol) dimethacrylate (PEGDMA) and human chondrocytes through a process of simultaneous polymerization into the center void in each scaffold. A schematic of this process is shown in figure 4. Findings of this study revealed increased levels of collagen type I and type II formation as well as increased glycosaminoglycan and DNA production when compared to samples without printed chondrocytes [67]. In vitro results show promise as a hybrid approach for repairing articular cartilage. More research is needed to validate the long-term benefits of such a procedure and the in vivo response upon implantation.
The difficulty with matching material properties, mechanical properties, architecture, and biocompatibility is perhaps most evident in cartilage regeneration. The above studies highlight the progress being made through the integration of 3D printed models on numerous printing platforms. However, much work is still needed to develop an ideal system for the fabrication of biodegradable, biomimetic, load bearing cartilage tissue replacement.
3D printing osteochondral tissue
Osteochondral repair is often closely linked with cartilage repair; research even suggests that cartilage and subchondral bone, as discussed in previous sections, should be considered interdependent when addressing osteochondral defects and repair [78]. Native osteochondral tissue, along with cartilaginous tissue, lacks significant vascularity. The lack of vascular networks hinders effective nutrient transport as well as the inherent compositional complexity of the tissue are the primary underlying reasons it is so difficult to effectively repair this region. Hyaline cartilage is smooth, connective, tissue covering the articular surface of joints to minimize friction. It is mainly composed of type II collagen is void of nerves, and blood cells, and has a limited capacity to regenerate and self-repair. Damaged hyaline cartilage is often replaced with fibrocartilage which possesses completely different physical and mechanical properties than native hyaline cartilage [79].
Recent research has focused on the fabrication of biphasic tissue engineered scaffolds that have shown promise in improving osteochondral repair. Zhang et al conducted a yearlong study of rabbit trochlea defects using biomimetic biphasic osteochondral composite scaffolds to address some of the challenges of cartilage in load-bearing regions. The scaffolds were two distinct regions. The lower region of the scaffold in contact with the subchondral bone was fabricated using gel casting of β-tricalciumphosphate (TCP). SL was then used to print layers of Poly(ethylene glycol) (PEG) on top of the β-TCP for the cartilage layer. This study yielded favorable results with evidence of hyaline-like cartilage beginning at week 24. The mechanical properties of both layers effectively simulated the mechanical properties of the native tissue [66].
According to Zhang, this study shows promise for effective healing of the complex interface between bone and cartilage [66]. The rabbits were not immobilized during healing, but were confined to their cages; therefore, load-bearing activities were minimal. This success is promising, but more research is required to further examine the long-term effects of such implants. The team also noted tidemark formation at week 52, which is a good indicator of successful osteochondral response. There is clear evidence in this study that calcified cartilage formed between the subchondral bone and calcium-void articular cartilage. The region of calcified cartilage beneath the tidemark is shown in figure 7, a qualitative illustration of effective osteochondral repair. In this study it was also noted that the repair occurred in a flow-like manner working from the surrounding bone inward, to the center of the defect, figure 8 [66].
Figure 7.
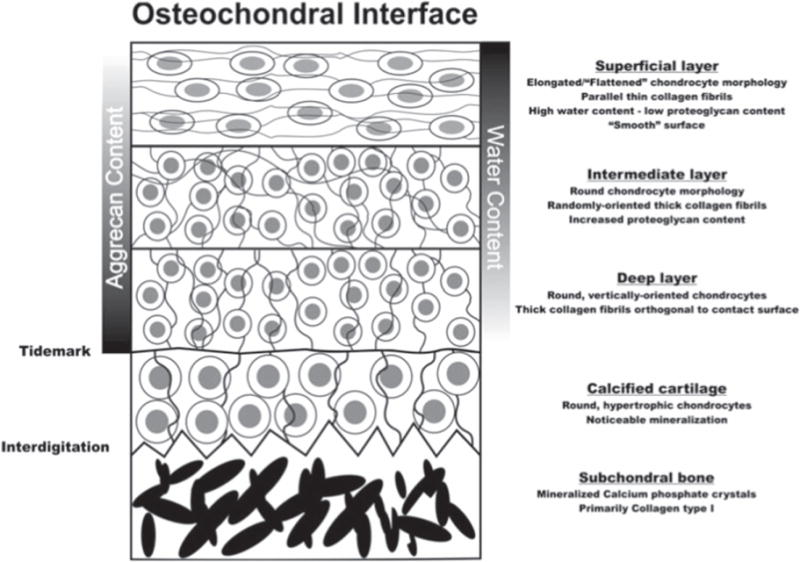
Schematic of the osteochondral interface. Images are from [80] (2012) (© Biomedical Engineering Society 2012). With permission of Springer.
Figure 8.
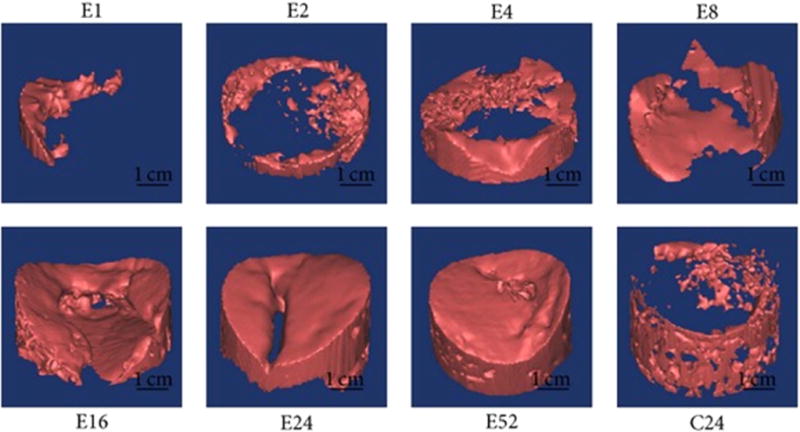
Computer imaging of a defect repairing itself from the outer bone contact inward to the defect center. Images labeled with E represent a defect with scaffold after respective number of days. Image with C24 represents the control defect, without scaffold, after 24 d. Reproduced from [67]. CC BY 3.0.
The above study demonstrates the importance of the interface between existing tissue and the implanted scaffold. Equally important is the interface between material types when using heterogeneous scaffolds. Heterogeneous scaffolds are one method used to address the disparate nature of the osteochondral region. Zhang et al investigated a biphasic scaffold design where each phase is separated by an interfacial structure with varying porosity. The study determined that structures with interfaces outperformed those without in every instance [81]. Sherwood et al also investigated a biphasic osteochondral scaffold with an interfacial region that gradually transitioned between disparate regions by varying materials and porosity in a gradient manner. The study achieved successful chondrogenic and osteogenic differentiation within the respective regions which helped prevent delamination while improving mechanical performance [82].
Structural stability of biphasic scaffolds is often compromised at the phase interfaces if not carefully managed. Recent research in our lab focuses on mitigating this risk by fabricating single-cast scaffolds capable of achieving biphasic effects. Initial research realized a positive osteogenic and chondrogenic response in single cast scaffolds augmented with nHA and chondrogenic media. This technique shows great promise for further exploring the ability to control multiphasic responses of structurally sound, single-cast, tissue engineered scaffolds [83].
Other studies analyze the effectiveness of direct printing specific cell types in an ordered, layer-by-layer manner to create the native cellular distribution found within the ostechondral region. Fedorovich et al developed a technique for 3D printing cell-laden hydrogels on fiber constructs which mimic the mechanical and physical properties found in the osteochondral region. The study suggests the technique was successful in creating centimeter-scaled constructs [84]. Though this technique seems promising it must be scaled down to at least micrometer scale to effectively replicate the intricacies of this region.
Like cartilage TE, replicating properties and complex architectures while ensuring biocompatibility in the osteochondral region is a significant challenge to full regeneration. The above studies indicate progress toward this end but more research is required on material availability, printability, and modeling of biomimetic architectures. Hybrid approaches currently show the greatest promise and ability toward matching the full complexity of this region.
Conclusions and future direction
3D printing of tissue-engineered scaffolds is rapidly advancing with diverse research occurring on numerous printing platforms at various universities and agencies. The largest challenge in this field remains the ability to balance all performance objectives for successful scaffold integration within areas of biological and physical complexity such as bone, cartilage, and the osteochondral interface. A large volume of current research has shown marked advancement in one direction, biocompatibility for example, with inadequate results in mechanical strength. It should be evident through the course of this discussion that material properties of printing medium and microscale geometry of printed scaffolds are critical components driving the overall biological success or failure of 3D printed scaffolds.
Materials that can closely mimic the properties of the natural tissue are essential. Candidate materials must be able to satisfy biocompatibility as well as mechanical loading requirements. More research is needed to better match biocompatible materials with the various printing platforms. In addition, the benefits of directly printing cells with novel bio-inks certainly warrant further investigation.
Research has also shown that neovascularization is essential in the healing cascade [58]. Scaffold pore size and distribution directly influence cell behavior and neovascularization. These pores, prior to cell confluence, also act as small defects that affect the mechanical performance of the scaffolds. More research is necessary to effectively integrate porosity into manufactured scaffolds to improve cell viability and promote healthy vasculature throughout the repaired tissue.
3D printing of TE scaffolds continues to hold great promise for improving patient quality of life in the future over traditional procedures currently in practice. Most of the leading advancements in this field are in non-load-bearing applications. As progress is made in this realm, it is necessary to investigate the cross-over potential for these materials to facilitate approval of load-bearing applications in the future.
Acknowledgments
This work is supported by NIH Director’s New Innovator Award 1DP2EB020549-01 and NSF BME program grant # 1510561.
References
- 1.Buckwalter JA, Martin JA. Osteoarthritis. Adv Drug Deliv Rev. 2006;58:150–67. doi: 10.1016/j.addr.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheumatol. 2006;54:226–9. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Hu J, Athanasiou KA. The role of tissue engineering in articular cartilage repair and regeneration. Crit Rev Biomed Eng. 2009;37:1–57. doi: 10.1615/critrevbiomedeng.v37.i1-2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams GM, et al. Shape, loading, and motion in the bioengineering design, fabrication, and testing of personalized synovial joints. J Biomech. 2010;43:156–65. doi: 10.1016/j.jbiomech.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 6.Guan L, Davies JE. Preparation and characterization of a highly macroporous biodegradable composite tissue engineering scaffold. J Biomed Mater Res A. 2004;71:480–7. doi: 10.1002/jbm.a.30173. [DOI] [PubMed] [Google Scholar]
- 7.Lebourg M, Sabater Serra R, Mas Estelles J, Hernandez Sanchez F, Gomez Ribelles JL, Suay Anton J. Biodegradable polycaprolactone scaffold with controlled porosity obtained by modified particle-leaching technique. J Mater Sci, Mater Med. 2008;19:2047–53. doi: 10.1007/s10856-007-3282-4. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Li B, Li YL, Jiang YZ, Ouyang HW, Gao CY. In vivo restoration of full-thickness cartilage defects by poly(lactide-co-glycolide) sponges filled with fibrin gel, bone marrow mesenchymal stem cells and DNA complexes. Biomaterials. 2010;31:5953–65. doi: 10.1016/j.biomaterials.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 9.Yoo HS, Lee EA, Yoon JJ, Park TG. Hyaluronic acid modified biodegradable scaffolds for cartilage tissue engineering. Biomaterials. 2005;26:1925–33. doi: 10.1016/j.biomaterials.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Mathieu LM, Mueller TL, Bourban PE, Pioletti DP, Muller R, Manson JAE. Architecture and properties of anisotropic polymer composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:905–16. doi: 10.1016/j.biomaterials.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Salerno A, Iannace S, Netti PA. Graded biomimetic osteochondral scaffold prepared via CO2 foaming and micronized NaCl leaching. Mater Lett. 2012;82:137–40. [Google Scholar]
- 12.Prieto EM, Page JM, Harmata AJ, Guelcher SA. Injectable foams for regenerative medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6:136–54. doi: 10.1002/wnan.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui H, Nowicki M, Fisher JP, Zhang LG. 3D bioprinting for organ regeneration. Adv Healthcare Mater. 2017;6:1601118. doi: 10.1002/adhm.201601118. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng B. 2013;19:485–502. doi: 10.1089/ten.teb.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Brien FJ. Biomaterials & scaffolds for tissue engineering. ScienceDirect. 2011;14:88–95. [Google Scholar]
- 16.Asa’ad F, Pagni G, Pilipchuk SP, Gianni AB, Giannobile WV, Rasperini G. 3D-printed scaffolds and biomaterials: review of alveolar bone augmentation and periodontal regeneration applications. Int J Dentistry. 2016;2016:1239842. doi: 10.1155/2016/1239842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pham DT, Gault RS. A comparison of rapid prototyping technologies. Int J Mach Tool Manuf. 1998;38:1257–87. [Google Scholar]
- 18.Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today. 2013;16:496–504. [Google Scholar]
- 19.Jia A, Joanne Ee Mei T, Ratima S, Chee Kai C. Design and 3D printing of scaffolds and tissues. Engineering. 2015;1:261–8. [Google Scholar]
- 20.Goddard JM, Hotchkiss JH. Polymer surface modification for the attachment of bioactive compounds. Prog Polym Sci. 2007;32:698–725. [Google Scholar]
- 21.Hoffman W, et al. Rapid prototyped porous nickel-titanium scaffolds as bone substitutes. J Tissue Eng. 2004;5:1–14. doi: 10.1177/2041731414540674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng K, Kisaalita WS. Exploring cellular adhesion and differentiation in a micr-/nano-hybrid polymer scaffold. Biotechnol Prog. 2010;26:838–46. doi: 10.1002/btpr.391. [DOI] [PubMed] [Google Scholar]
- 23.Webster TJ, Smith TA. Increased osteoblast function on PLGA composites containing nanophase titania. J Biomed Mater Res A. 2005;74:677–86. doi: 10.1002/jbm.a.30358. [DOI] [PubMed] [Google Scholar]
- 24.Murphy SV, Atala A. 3D bioprinitng of tissues and organs. Nat Biotechnol. 2014;32:773–85. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 25.Rosenweig DH, Carelli E, Steffen T, Jarzem P, Haglund L. 3D-printed ABS and PLA scaffolds for cartilage and nucleus pulposus tissue regeneration. Int J Mol Sci. 2015;16:15118–35. doi: 10.3390/ijms160715118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo T, Lembong J, Zhang LG, Fisher JP. Three-dimensioanl printing articular cartilage: recapitulating the complexity of native tissue. Tissue Eng B. 2017;23:225–36. doi: 10.1089/ten.TEB.2016.0316. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien CM, Holmes B, Faucett S, Zhang LG. Three-dimensional printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Eng B. 2014;21:103–14. doi: 10.1089/ten.teb.2014.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skardal A, Zhang J, Prestwich GD. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials. 2010;31:6173–81. doi: 10.1016/j.biomaterials.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 29.Pfister A, Landers R, Laib A, Hubner U, Schmelzeisen R, Mulhaupt R. Biofunctional rapid prototyping for tissue-engineering applications: 3D bioplotting versus 3D printing. J Polym Sci Pol Chem. 2004;42:624–38. [Google Scholar]
- 30.Daoud J, Asami K, Rosenberg L, Tabrizian M. Dielectric spectroscopy for non-invasive monitoring of epithelial cell differentiation within three-dimensional scaffolds. Phys Med Biol. 2012;57:5097–112. doi: 10.1088/0031-9155/57/16/5097. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Slamovich EB, Webster TJ. Increased osteoblast functions among nanophase titania/poly(lactide-co-glycolide) composites of the highest nanometer surface roughness. J Biomed Mater Res A. 2006;78:798–807. doi: 10.1002/jbm.a.30734. [DOI] [PubMed] [Google Scholar]
- 32.Marszalek JE, Simon CG, Jr, Thodeti C, Adapala RK, Murthy A, Karim A. 2.5D constructs for characterizing phase separated polymer blend surface morphology in tissue engineering scaffolds. J Biomed Mater Res A. 2013;101:1502–10. doi: 10.1002/jbm.a.34439. [DOI] [PubMed] [Google Scholar]
- 33.Peltola SM, Melchels FPW, Grijpma DW, Kellomaki M. A review of rapid prototyping techniques for tissue engineering purposes. Ann Med. 2008;40:268–80. doi: 10.1080/07853890701881788. [DOI] [PubMed] [Google Scholar]
- 34.Xu T, et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2013;5:015001. doi: 10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]
- 35.Xu T, Zhao WX, Zhu JM, Albanna MZ, Yoo JJ, Atala A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials. 2013;34:130–9. doi: 10.1016/j.biomaterials.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 36.Wust S, Muller R, Hofmann S. Controlled positioning of cells in biomaterials-approaches towards 3D tissue printing. J Funct Biomater. 2011;2:119–54. doi: 10.3390/jfb2030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui X, Breitenkamp K, Finn MG, Lotz M, D’Lima DD. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng A. 2012;18:1304–12. doi: 10.1089/ten.tea.2011.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferris CJ, Gilmore KJ, Beirne S, McCallum D, Wallace GG, Panhuis MIH. Bio-ink for on-demand printing of living cells. Biomater Sci. 2013;1:224–30. doi: 10.1039/c2bm00114d. [DOI] [PubMed] [Google Scholar]
- 39.Liu FH, Shen YK, Lee JL. Selective laser sintering of a hydroxyapatite-silica scaffold on cultured MG63 osteoblasts. in vitro Int J Precis Eng Manuf. 2012;13:439–44. [Google Scholar]
- 40.Gao CD, et al. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int J Mol Sci. 2014;15:4714–32. doi: 10.3390/ijms15034714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu FH, Lee RT, Lin WH, Liao YS. Selective laser sintering of bio-metal scaffold. First Cirp Conf on Biomanufacturing. 2013;5:83–7. [Google Scholar]
- 42.Lodererova M, et al. Biocompatibility of metal sintered materials in dependence on multi-material graded structure; 13th Int. Conf. on Biomedical Engineering; Berlin: Springer; 2009. pp. 1204–7. [Google Scholar]
- 43.Hao L, Savalani MM, Harris RA, Zhang Y, Tanner KE. Characterisation of HA/polymer bio-composite structure fabricated by selective laser sintering. In: da Silva Bartolo PJ, et al., editors. Virtual and Rapid Manufacturing. London: Taylor and Francis; 2008. pp. 121–7. [Google Scholar]
- 44.Fisher JP, et al. Soft and hard tissue response to photocrosslinked poly(propylene fumarate) scaffolds in a rabbit model. J Biomed Mater Res. 2002;59:547–56. doi: 10.1002/jbm.1268. [DOI] [PubMed] [Google Scholar]
- 45.Cooke MN, Fisher JP, Dean D, Rimnac C, Mikos AG. Use of stereolithography to manufacture critical-sized 3D biodegradable scaffolds for bone ingrowth. J Biomed Mater Res B. 2003;64B:65–9. doi: 10.1002/jbm.b.10485. [DOI] [PubMed] [Google Scholar]
- 46.Arcaute K, Mann BK, Wicker RB. Stereolithography of three-dimensional bioactive poly(ethylene glycol) constructs with encapsulated cells. Ann Biomed Eng. 2006;34:1429–41. doi: 10.1007/s10439-006-9156-y. [DOI] [PubMed] [Google Scholar]
- 47.Lee KW, Wang S, Fox BC, Ritman EL, Yaszemski MJ, Lu L. Poly(propylene fumarate) bone tissue engineering scaffold fabrication using stereolithography: effects of resin formulations and laser parameters. Biomacromolecules. 2007;8:1077–84. doi: 10.1021/bm060834v. [DOI] [PubMed] [Google Scholar]
- 48.Castro N, Goldstein P, Cooke MN. Synthesis and manufacture of photocrosslinkable poly(caprolactone)-based three-dimensional scaffolds for tissue engineering applications. Adv Biosci Biotechnol. 2011;2:167–73. [Google Scholar]
- 49.Beke S, et al. Towards excimer-laser-based stereolithography: a rapid process to fabricate rigid biodegradable photopolymer scaffolds. J R Soc Interface. 2012;9:3017–26. doi: 10.1098/rsif.2012.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu Y, Chen SC. Projection printing of 3-dimensional tissue scaffolds. Comput-Aided Tissue Eng. 2012;868:289–302. doi: 10.1007/978-1-61779-764-4_17. [DOI] [PubMed] [Google Scholar]
- 51.Castro NJ, O’Brien J, Zhang LG. Integrating biologically inspired nanomaterials and table-top stereolithography for 3D printed biomimetic osteochondral scaffolds. Nanoscale. 2015;9:14010–22. doi: 10.1039/c5nr03425f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melchels FPW, Feijen J, Grijpma DW. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31:6121–30. doi: 10.1016/j.biomaterials.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 53.Candiotti H, et al. Fused deposition modeling BioPrinter; 2013 39th Annual Northeast Bioengineering Conf. (Nebec 2013); 2013. pp. 177–8. [Google Scholar]
- 54.Korpela J, Kokkari A, Korhonen H, Malin M, Narhi T, Seppala J. Biodegradable and bioactive porous scaffold structures prepared using fused deposition modeling. J Biomed Mater Res B. 2013;101B:610–9. doi: 10.1002/jbm.b.32863. [DOI] [PubMed] [Google Scholar]
- 55.Zhao C, Tan A, Pastorin G, Ho HK. Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol Adv. 2013;31:654. doi: 10.1016/j.biotechadv.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Fedorovich NE, Kuipers E, Gawlitta D, Dhert WJA, Alblas J. Scaffold porosity and oxygenation of printed hydrogel constructs affect functionality of embedded osteogenic progenitors. Tissue Eng A. 2011;17:2473–86. doi: 10.1089/ten.TEA.2011.0001. [DOI] [PubMed] [Google Scholar]
- 57.Chen HP, Liu YY, Jiang ZL, Chen WH, Yu YZ, Hu QX. Cell-scaffold interaction within engineered tissue. Exp Cell Res. 2014;323:346–51. doi: 10.1016/j.yexcr.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 58.Calvert JW, Weiss LE, Sundine MJ. New frontiers in bone tissue engineering. Clin Plast Surg. 2003;30:641. doi: 10.1016/s0094-1298(03)00081-6. [DOI] [PubMed] [Google Scholar]
- 59.Temple JP, et al. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J Biomed Mater Res A. 2014;102:4317–25. doi: 10.1002/jbm.a.35107. [DOI] [PubMed] [Google Scholar]
- 60.Kaplan FS, Hayes WC, Keaveny TM, Boskey A, Einhorn TA, Iannotti JP. Form and function of bone. In: Sinmon SP, editor. Orthopedic Basic Science. Rosemont, IL: American Academy of Orthopedic Surgeons; 1994. p. 127. [Google Scholar]
- 61.Im O, Li J, Wang M, Zhang LG, Keidar M. Biomimetic three-dimensional nanocrystalline hydroxyapatitite and magnetically synthesized single-walled carbon nanotube chitosan nanocomposite for bone regeneration. Int J Nanomed. 2012;7:2087. doi: 10.2147/IJN.S29743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holmes B, Bulusu K, Plesniak M, Zhang LG. A synergistic approach to the design, fabrication and evluation of 3D printed micro and nano featured scaffolds for vascularized bone tissue repair. Nanotechnology. 2016;27:064001. doi: 10.1088/0957-4484/27/6/064001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui H, Zhu W, Holmes B, Zhang LG. Biologically inspired smart release system based on 3D bioprinted perfused scaffold for vascularized tissue regeneration. Adv Sci. 2016;3:1600058. doi: 10.1002/advs.201600058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cui H, Zhu W, Nowicki M, Zhang LG. Hierarchical fabrication of engineered vascularized bone biphasic constructs via dual 3D bioprinting: integrating regional bioactive factors into architectural design. Adv Mater. 2016;5:2174–81. doi: 10.1002/adhm.201600505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao GF, Schilling AF, Yonezawa T, Wang J, Dai GH, Cui XF. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol J. 2014;9:1304–11. doi: 10.1002/biot.201400305. [DOI] [PubMed] [Google Scholar]
- 66.Wang JL, Yang MY, Zhu Y, Wang L, Tomsia AP, Mao CB. Phage nanofibers induce vascularized osteogenesis in 3D printed bone scaffolds. Adv Mater. 2014;26:4961–6. doi: 10.1002/adma.201400154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang W, et al. Cartilage repair and subchondral bone migration using 3D printing osteochondral composites: a one-year-period study in rabbit trochlea. BioMed Res Int. 2014;2014:746138. doi: 10.1155/2014/746138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang LG, Khademhosseini A, Webster TJ. Tissue and Organ Regeneration: Advances in Micro- and Nanotechnology. Singapore: Pan Stanford Publishing Pte. Ltd; 2014. p. 423. [Google Scholar]
- 69.Sah RL, Trippel SB, Grodzinsky AJ. Differential effects of serum, insulin-like growth factor-1, and fibroblast growth factor-2 on the maintenance of cartilage physical properties during long-term culture. J Orthop Res. 1996;14:44–52. doi: 10.1002/jor.1100140109. [DOI] [PubMed] [Google Scholar]
- 70.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 71.Davies LC, Blain EJ, Gilbert SJ, Caterson B, Duance VC. The potential of insulin-like growth factor-1 and transforming growth factor-β 1 for promoting ‘adult’ articular cartilage repair; an in vitro study. Tissue Eng A. 2008;14:1251–61. doi: 10.1089/ten.tea.2007.0211. [DOI] [PubMed] [Google Scholar]
- 72.Yaeger PC, Masi TL, De Ortiz JLB, Binette F, Tubo R, Mcpherson JM. Synergistic action of transforming growth factor-β 1 and insulin-like growth factor-1 induces expression of type-II collagen and aggrecan genes in adult human articular chondrocytes. Exp Cell Res. 1997;237:318–25. doi: 10.1006/excr.1997.3781. [DOI] [PubMed] [Google Scholar]
- 73.Jin M, Emkey GR, Siparsky P, Trippel SB, Grodzinsky AJ. Combined effects of dynamic tissue shear deformation and insulin-like growth factor-1 on chondrocyte biosynthesis in cartilage explants. Arch Biochem Biophys. 2003;58:1731–40. doi: 10.1016/s0003-9861(03)00195-4. [DOI] [PubMed] [Google Scholar]
- 74.Aliabouzar M, Zhang LG, Sarkar K. Lipid coated microbubbles and low intebsity pulsed ultrasound enhance chondrogenesis of human mesenchymal stem cells in 3D printed scaffolds. Sci Rep. 2016;6:37728. doi: 10.1038/srep37728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang JW, et al. Tissue-engineered tracheal reconstruction using three-dimensionally printed artificial tracheal graft: preliminary report. Artif Organs. 2014;38:E95–105. doi: 10.1111/aor.12310. [DOI] [PubMed] [Google Scholar]
- 76.Lee JS, Hong JM, Jung JW, Shim JH, Oh JH, Cho DW. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication. 20146:024103. doi: 10.1088/1758-5082/6/2/024103. [DOI] [PubMed] [Google Scholar]
- 77.Zopf DA, Mitsak AG, Flanagan CL, Wheeler M, Green GE, Hollister SJ. Computer aided-designed, 3-dimensionally printed porous tissue bioscaffolds for craniofacial soft tissue reconstruction. Otolaryngology—Head Neck Surg. 2014;152:57–62. doi: 10.1177/0194599814552065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mannoor MS, et al. 3D printed bionic ears. Nano Lett. 2013;13:2634–9. doi: 10.1021/nl4007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y, Yuan X, Wang A, Peng J, Lu S. [Treatment strategy of osteochondral defects of knee joint]. Zhongguo xiu fu chong jian wai ke za zhi = Zhongguo xiufu chongjian waike zazhi = Chin J Reparative Reconstructive Surg. 2014;28:113–824693792. [PubMed] [Google Scholar]
- 80.Castro NJ, Hacking SA, Zhang LG. Recent progress in interfacial tissue engineering approaches for osteochondral defects. Ann Biomed Eng. 2012;40:1628–40. doi: 10.1007/s10439-012-0605-5. [DOI] [PubMed] [Google Scholar]
- 81.Ye K, Di Bella C, Myers DE, Choong PF. The osteochondral dilemma: review of current management and future trends. ANZ J Surg. 2014;84:211–7. doi: 10.1111/ans.12108. [DOI] [PubMed] [Google Scholar]
- 82.Zhang WJ, et al. The effect of interface microstructure on interfacial shear strength for osteochondral scaffolds based on biomimetic design and 3D printing. Mater Sci Eng C. 2015;46:10–5. doi: 10.1016/j.msec.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 83.Sherwood JK, et al. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials. 2002;23:4739–51. doi: 10.1016/s0142-9612(02)00223-5. [DOI] [PubMed] [Google Scholar]
- 84.Nowicki M, Castro N, Plesniak M, Zhang LG. 3D printing of novel osteochondral scaffolds with graded microstructure. Nanotechnology. 2016;27 doi: 10.1088/0957-4484/27/41/414001. [DOI] [PubMed] [Google Scholar]
- 85.Fedorovich NE, et al. Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Eng C. 2012;18:33–44. doi: 10.1089/ten.tec.2011.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]


