Graphical abstract
Keywords: Dermanyssus gallinae, Vaccine, Field trial, Serpin, Vitellogenin, Soluble mite extract (SME), Poultry red mite
Highlights
-
•
Field trial testing of a native and recombinant poultry red mite vaccines.
-
•
Vaccination with a soluble mite extract (SME) resulted in a 78% reduction in mite numbers.
-
•
Poor antibody persistence may relate to lack of effect of a recombinant cocktail vaccine.
-
•
A semi-protective naturally acquired immunity may develop.
Abstract
Vaccination is a desirable emerging strategy to combat poultry red mite (PRM), Dermanyssus gallinae. We performed trials, in laying hens in a commercial-style cage facility, to test the vaccine efficacy of a native preparation of soluble mite extract (SME) and of a recombinant antigen cocktail vaccine containing bacterially-expressed versions of the immunogenic SME proteins Deg-SRP-1, Deg-VIT-1 and Deg-PUF-1. Hens (n = 384 per group) were injected with either vaccine or adjuvant only (control group) at 12 and 17 weeks of age and then challenged with PRM 10 days later. PRM counts were monitored and, at the termination of the challenge period (17 weeks post challenge), average PRM counts in cages containing birds vaccinated with SME were reduced by 78% (p < 0.001), compared with those in the adjuvant-only control group. When the trial was repeated using the recombinant antigen cocktail vaccine, no statistically significant differences in mean PRM numbers were observed in cages containing vaccinated or adjuvant-only immunised birds. The roles of antigen-specific antibody levels and duration in providing vaccine-induced and exposure-related protective immunity are discussed.
1. Introduction
One of the biggest challenges facing the global commercial egg industry is the control of the blooding-feeding poultry red mite (PRM), Dermanyssus gallinae (De Geer, 1778). The prevalence of PRM in commercial laying facilities is estimated to range from 4 to 100% in several European countries (Sparagano et al., 2014) but is also high outwith Europe, for example, 64% in China (Wang et al., 2010) and 85% in Japan (Sparagano et al., 2014). PRM live mostly off-host in the structure and furniture of poultry facilities and emerge in darkness to feed on the blood of hens for short periods of approximately 30–60 min (Maurer et al., 1988). A moderate level of infestation in commercial facilities is considered to be approximately 50,000 mites per bird and this can rise to 500,000 in severe infestations (Kilpinen et al., 2005). A rise in host somatic stress indicators (Kowalski and Sokol, 2009) and psychogenic behaviours such as restlessness, irritation, feather pecking and cannibalism have been observed in response to PRM infestation (Kilpinen et al., 2005). In addition, PRM infestation can result in an increase in anaemia and hen mortality (Wojcik et al., 2000, Cosoroaba, 2001, Kilpinen et al., 2005, Arkle et al., 2006) and the PRM may also act as vectors of important avian diseases (Valiente-Moro et al., 2007; Sommer et al., 2016). Together these effects may result in reduced egg production, a lower feed conversion and reduced egg quality (Cosoroaba, 2001; Arkel et al., 2006; Mul et al., 2009). PRM is therefore a highly economically-important disease and, in 2005, was estimated to cost the EU egg industry €130 million in production loss and control costs annually (Van Emous, 2005).
Traditionally the egg industry has relied upon synthetic acaricides for PRM control (Sparagano et al., 2014). However prolonged use of these compounds has resulted in the development of acaricide resistance in many countries (Abbas et al., 2014) and legislation has seen with withdrawal of many compounds, particularly in the EU (Sparagano et al., 2014). Novel and effective methods of PRM control are required and vaccination as a control strategy offers several advantages over existing treatments; vaccines are acceptable to industry, they are not environmental toxins, resistance is unlikely to develop and they have the potential to be cost-effective and long lasting (Wright et al., 2016). Preliminary immunisation studies using different crude fractions of PRM extracts as vaccines have been performed previously (Arkle et al., 2008, Wright et al., 2009, Harrington et al., 2009, Bartley et al., 2015, Makert et al., 2016). PRM fed on heparinised blood which was enriched with IgY from hens vaccinated with PBS-soluble PRM proteins (soluble mite extract, SME) had a statistically significant higher rate of mortality than those fed on blood enriched with IgY from control hens (Wright et al., 2009, Bartley et al., 2015). Following on from the demonstration of protection using these native extracts, the challenge has been to simplify these protective PRM protein fractions, identify the key protein components responsible for protection and produce effective recombinant versions of the vaccine candidate proteins. The search for vaccine candidates was accelerated when PRM transcriptomic data became available (Bartley et al., 2009, Bartley et al., 2012, Schicht et al., 2013, Schicht et al., 2014, Makert et al., 2016) and, since then, a pragmatic approach of combined immuno-screening, proteomic analysis and MASCOT searching of transcriptomic datasets has been successful in identifying several such vaccine candidates (Bartley et al., 2015, Merkert et al., 2016).
To date, the testing of recombinant versions of PRM vaccine candidate antigens on PRM survivability following a blood meal from vaccinated hens has been carried out using an in vitro feeding mite device based on the McDevitt et al. (2006) design (Bartley et al., 2009, Bartley et al., 2012, Bartley et al., 2015; Wright et al., 2016). This process was used to identify the three most effective antigens in vitro: D. gallinae Vitellogenin-1 (Deg-VIT-1), Serpin-1 (Deg-SRP-1) and a Protein of Unknown Function-1 (Deg-PUF-1) (Bartley et al., 2015).
There are several advantages to the in vitro feeding strategy in determining vaccine efficacy, for example, antigen-specific IgY can be fed to PRMs in a controlled environment and mite mortality, oviposition and development of individual PRMs can be accurately monitored with ease. However, the in vitro feeding system lacks natural feeding cues and feeding rates can be variable (Wright et al., 2009). In addition, the blood is heparinised which has a residual toxicity (McDevitt et al., 2006) to PRMs and to induce feeding, the PRMs are pre-starved and exposed to high temperature, which may elevate background mortality (Kirkwood, 1971, Bruneau et al., 2001, McDevitt et al., 2006, Wright et al., 2009, Harrington et al., 2009). Attempts to maintain a laboratory isolate of PRM ex vivo using in vitro feeding devices have been partially successful (Bruneau et al., 2001), however PRM survival was severely diminished following the first feed. Therefore, to fully evaluate the effects of repeated feeding on vaccinated blood and secondary vaccine effects such as reduced oviposition and impaired development of successive generations, a field trial that permits natural feeding behaviour of PRM on live hens, coupled with PRM population monitoring over many generations is required.
The purpose of the experiments described here was, therefore, to test the efficacy of the SME and a cocktail vaccine containing recombinant versions of the SME-derived proteins Deg-VIT-1, Deg-SRP-1 and Deg-PUF-1 as prototype vaccines under field conditions akin to a commercial layer farm.
2. Materials and methods
2.1. Field trial design
Two field trials (trial 1 and trial 2) were performed at the same poultry facility in East Lothian, Scotland which was free from PRM before the commencement of each study. Trial 1 ran from 6th July 2010 for 36 weeks and trial 2 from 17th December 2014 for 38 weeks.
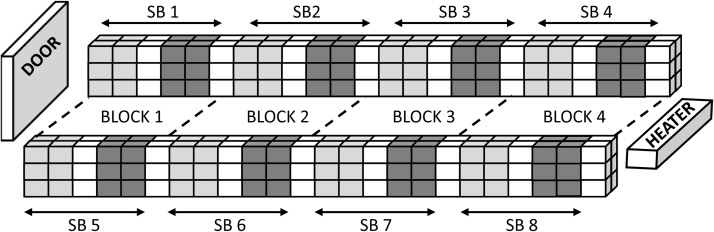
In both trials, 768 Lohmann Brown hens were randomly assigned to one of 2 groups (Placebo and Vaccine groups, 384 hens per group). The hens were initially group-housed from hatching (=week 0) in a loose litter floor pens, with layer mash and water provided ad libitum until week 17. In keeping with commercial laying hen rearing practices, all hens received several routine vaccinations and coccidiostat treatments during the first 17 weeks of life. In addition, the Vaccine groups received two 0.5 ml doses of prototype PRM vaccine (SME in trial 1; recombinant antigen cocktail in trial 2) formulated in adjuvant (Section 2.2), given intra-muscularly into the thigh, at 12 and 17 weeks old. The Placebo group hens received two 0.5 ml doses of adjuvant in diluent, also at weeks 12 and 17 and into the thigh. Following vaccination with the PRM vaccines or placebo, hens were moved to facilities equipped with a conventional battery cage system (Big Dutchman, Germany) arranged in a format of 4 lines of 24 cages set in a back to back format and 4 tiers high (Fig. 1). The top layer of cages remained empty. To account for the spatial effect due to placement and its proximity of cages to variable environmental factors, hens were placed 4 per cage into the alternating group format depicted in Fig. 1.
Fig. 1.
Schematic representation of a cage facility layout used in the field evaluation of prototype vaccines to control poultry red mite.
Four lines of 24 cages, 4 tiers high were set in a back to back format. The top tiers of cages are not shown and were empty. Hens were injected with either the red mite prototype vaccine (Vaccine group) or adjuvant only (Placebo group) and placed 4 hens to a cage in replicate 8 sub-blocks (SB) comprising of 12 cages of Vaccine and 12 cages of Placebo group hens. The Vaccinated group were placed in the cages shaded light grey (trial 1) and dark grey (trial 2) and the Placebo group in the cages shaded dark grey (trial 1) and light grey (trial 2). The un-shaded cages remained empty and served as a buffer zone to reduce mite migration between adjacent sub-blocks and groups. Barrier glue (Agralan Ltd., UK) was applied in 2 continuous parallel lines around each empty cage in the buffer zone, around the supporting legs of the housing structure and pipes and ducting.
Neighbouring groups were separated from each other by a single column of empty cages treated with a double line of adhesive insect barrier glue (Agralan Ltd., UK), which has since been shown to be effective in limiting PRM movement (Pritchard et al., 2016). The glue barriers were applied in continuous lines to the floor, ceiling, rear and front of the cages and extended around the feeding trough, egg collection tray, water pipes and ducting to ensure a complete barrier was in place to reduce PRM migration between adjacent blocks and groups of cages. Consistent with industry practices, food and water were provided ad libitum throughout the studies and manure removed daily using the manure belts located below each tier.
Ten days after the second immunisation and placement of the hens in the cage system, a PRM challenge infestation was released into the shed (Section 2.3) and the PRM population was subsequently monitored (Section 2.4) for the remainder of the challenge period.
Both trials were performed under the terms of UK Home Office licences (PPL 60/4111 and PPL 60/4324) and the experimental design was ratified by the ethics committees of the Moredun Research Institute and Roslin Nutrition Ltd.
2.2. Preparation of experimental vaccines
2.2.1. Trial 1 vaccines
SME was prepared from 1 g PRM which had been snap-frozen in liquid nitrogen within 24 h of collection from a commercial layer facility (Peeblesshire, Scotland) according to the protocol given in Wright et al. (2009). The SME was filtered through a 0.22 μm cellulose acetate low-binding filter unit (Corning Inc., USA) prior to estimating the concentration of proteins using a Pierce™ bicinchoninic acid (BCA) assay (Thermo Fisher Scientific, USA), following the manufacturer’s protocol. The experimental vaccine was prepared by a commercial vaccine company (Ridgeway Biologicals Ltd., UK) as follows: SME proteins were combined, in an emulsion, with the Montanide ISA207VG water in oil in water adjuvant (SEPPIC, France) in an equal ratio resulting in 285 μg SME present in a single 0.5 ml dose. In trial 1, a placebo emulsion was also prepared from sterile PBS and Montanide ISA207VG mixed in the same ratio.
2.2.2. Trial 2 vaccines
Recombinant poly-histidine tagged versions of Deg-SRP-1, Deg-PUF-1 and the N-terminal portion Deg-VIT-1 encoded by the nucleotide bases 43–3157, were expressed in Escherichia coli BL21(DE3)-RIPL competent cells (Agilent Technologies, USA) and affinity-purified using His-Trap™ HP columns (GE Healthcare, UK) as described previously (Bartley et al., 2015). Following purification, imdazole was removed from the soluble Deg-SRP-1 and Deg-PUF-1 proteins by dialysis against 10 mM Tris-HCl; 0.5 M NaCl, pH 7.4 for 16 h at 4 °C. The Deg-VIT-1 was purified from the insoluble inclusion bodies. The urea and imidazole were removed and the Deg-VIT-1 protein refolded by sequential dialysis against refolding buffer (100 mM Tris–HCl, pH 8.0; 0.4 M l-Arginine; 2 mM EDTA; 0.5 mM oxidative glutathione; 5 mM reduced glutathione) with decreasing concentrations of urea (6, 4, 2 and 1 M urea) and finally against 10 mM Tris–HCl; 0.5 M NaCl, pH 7.4 for 16 h at 4 °C. Following filter sterilisation and protein quantification (BCA assay) equal quantities of the 3 purified recombinant proteins were combined and formulated (by Ridgeway Biologicals Ltd., UK) with Montanide ISA70VG (SEPPIC, France) in a 3:7 ratio of protein to adjuvant. Each 0.5 ml dose contained 25 μg of each recombinant protein. A trial 2 Placebo emulsion was prepared by mixing 10 mM Tris–HCl; 0.5 M NaCl, pH 7.4 in a 3:7 ratio with Montanide ISA70VG.
2.3. Preparation and release of the poultry red mite challenge into the poultry house
Mixed developmental-stage and gender PRM were collected into 75 cm2 vented tissue culture flasks (Corning Inc.) at a commercial egg production unit in Scotland, UK, which had been free from pesticide treatment for at least 1 month. The mites were collected within a 10 day period prior to release into the trial shed. The identity of the mites was confirmed as PRM by PCR-amplification of a 737 bp fragment of the Cytochrome C oxidase -1 subunit (COX-1), DNA sequencing and phylogenetic analysis following the method of Oines and Brannstrom (2011). The challenge isolate partitioned with the ‘haplotype A group’, closely related to PRM isolates previously characterised from Finland and Scotland (results not shown).
PRMs were maintained at room temperature (RT) for 1–5 days and 192 individual doses (each containing ∼20,000 PRMs in trial 1; ∼10,000 PRMs in trial 2) of PRMs were prepared in universal tubes (Sterilin) fitted with a vented lid (Corning Inc.). The prepared live PRM challenge doses were then maintained at 7 °C at 75% humidity for a maximum of 4 days until required. Each universal tube of PRM was attached to the egg collection rack located at the front of each stocked cage and out of reach of the hens. The PRMs were released from the universal tube by piercing the filter in the vented cap several times. The universal tube was left in place until the end of the study to serve as a refuge for PRMs. At the same time as the infestation was initiated, a mite monitoring device (Elanco, USA) (see Section 2.4) was attached to the egg collection rack adjacent to the mite challenge tube to also serve a refuge for the emerging mites. The refuge traps remained in place for the duration of the trials.
2.4. Monitoring poultry red mite populations
PRM populations were monitored in both trials using ADAS-type MiteMonitor trap devices (Elanco). Mite trap counting was performed at 7 time points in trial 1 (at weeks 20.5, 22.5, 27, 29.5, 32, 34 and 36 post hen hatching) and 9 time points in trial 2 (weeks 21, 23, 25, 27, 29, 31, 33, 35 and 37 post hen hatching). Trap holders were attached to the egg collection tray, adjacent to the refuge trap. The corrugated plastic trap insert were placed into each holder for 24 h, then removed and immediately placed into 50 ml Sterilin falcon tubes (Thermo Fisher Scientific, USA) containing 10 ml 70% (v/v) ethanol to kill the PRMs. The dead PRMs were flushed from the corrugated traps using 70% (v/v) ethanol, transferred to a Petri dish and then manually counted with the aid of a dissecting microscope. In addition to counting the total numbers of mites per trap, the population composition was estimated in trial 2 by counting the numbers of adults and juveniles PRMs present in a subset of 32 traps (2 from each replicate block). The proportions of the adult and juvenile populations were expressed as a percentage of the total number of mites per trap
2.5. Evaluation of the immune response to vaccination and challenge
The generation and persistence of IgY responses were quantified by ELISA and Western blotting. In trial 1, 8 eggs were collected from each group (1 egg from each replicate sub-block) at weeks 20, 27, 29 and 36 and equal volumes of yolk from each egg were pooled according to group and time. In trial 2 serum IgY levels were measured at weeks 12 (pre-vaccination), 17, 19 and thereafter at weeks 23, 27, 31, 35 and 38. Yolk-IgY levels were also measured from eggs collected at these time points from week 19 onwards. Blood was withdrawn by venesection of the brachial wing vein of 1 bird randomly selected from each replicate sub-block group. Serum was recovered following clotting at 4 °C for 24 h and centrifugation at 3000 × g and stored at −20 °C. In trial 2 the serum and yolk samples were evaluated individually to allow the variation in individual hen responses to be assessed.
For ELISA, the wells of Microlon® high-binding 96-well microplates (Greiner Bio-one, UK) were coated overnight at 4 °C with 50 μl of antigen (SME, integral membrane mite extract (IMME, prepared as described in Wright et al., 2009) or each of the recombinant antigens) diluted at 10 μg/ml in coating buffer (50 mM sodium bicarbonate, pH 9.6). Plates were washed six times with 200 μl PBST (PBS containing 0.05% v/v Tween-20) to remove unbound antigen and non-specific binding was blocked with 200 μl/well of blocking buffer (10% w/v Infasoy (Cow & Gate, UK) in tris-buffered saline/Tween 20 (TBST) buffer (50 mM Tris, 150 mM NaCl, 0.05% v/v Tween20)) for 2 h at RT with agitation. Following washing with PBST buffer, 50 μl of sera or egg yolks diluted to an appropriate concentration (1/4000 for detection of recombinant antigens and 1/400 for native protein extracts) in TBST were placed into appropriate wells and incubated at RT for 1 h with agitation. Plates were washed as before and the bound IgY detected by incubation for 1 h with agitation at RT with 50 μl/well of rabbit anti-IgY-peroxidase conjugate (Sigma, UK), diluted 1/30,000 in TBST. Following washing as before, bound secondary antibody was detected with 50 μl/well of the colorimetric substrate o-Phenylenediamine dihydrochloride (SIGMAFAST™ OPD, Sigma, UK). The colorimetric reaction was stopped after 20 mins by the addition of 25 μl of 2.5 mM H2SO4 to each well and the A490nm of each well measured using an ELX808IU Ultra Microplate Reader (Bio-Tek Instruments, UK).
For Western blotting, 4 μg of SME prepared from engorged mites or 100 ng of recombinant antigen per lane were separated by electrophoresis on 12% Bis–Tris Novex gels in NuPAGE® MES SDS Running Buffer (GE Healthcare, UK). Proteins were transferred to nitrocellulose membrane using an Xcell II blot module (GE Healthcare, UK), following the manufacturer’s procedures. Western blot screening and development were carried out as previously described (Bartley et al., 2009). Briefly, individual lanes of the membrane were excised and blocked by incubation in 5% (w/v) Marvel skimmed milk in PBST at 4 °C for 12 h, washed in PBST, then probed with yolk or serum diluted 1/250 in PBS for 2 h at RT. Unbound IgY was washed off with PBST. Bound IgY was detected by incubation in rabbit anti-IgY-peroxidase conjugate (Sigma, UK), diluted 1/30,000 in PBS for 1 h at RT, followed by washing in PBST and colorimetric development with SIGMAFAST™ 3,3′-Diaminobenzidine substrate (Sigma,UK).
2.6. Statistical analyses
For the purpose of statistical analyses of the PRM count data and to account for spatial effects due to placement and proximity of cages to variable environmental factors, the house was partitioned into four blocks (Blocks 1-4) with each block comprising of two sub-blocks (SB 1-8). For each trial separately, a Poisson generalized additive mixed model (GAMM) with a logarithmic link function was fitted by penalised maximum-likelihood (PQL) estimation to test for differences in mean PRM counts between Vaccinated and Placebo groups over time. The model included the treatment group as fixed effect and spline-based smooth terms (one per treatment group) to account for the non-linear relationship of the response variable with time. The effect of sub-block within block was regarded as a random effect. A dispersion parameter was estimated to take into account excessive variability in the data. Trial 1 showed a delayed increase in PRM numbers and generated negligible number of counts in the first two time points. Therefore only data from week 27 on were considered in trial 1 for GAMM fitting. Differences in mean PRM trap counts at the end of the experiment (week 36 for trial 1 and week 38 from trial 2) were statistically tested by fitting a Poisson generalized linear mixed model (GLMM). In trial 2 the same model was fitted to also test for differences at the point of peak response (week 33), and the corresponding p-values were adjusted for multiplicity by controlling for false discovery rate (FDR). The Poisson GLMMs included group as fixed effect and a random intercept for each sub-block within block. An observation-level random effect was specified to account for over-dispersion. Differences in mean proportion of juvenile PRMs between Vaccinated and Placebo groups measured over 4 weeks in trial 2 (based on 16 mite traps from each group) were statistically tested by fitting a binomial GLMM. Statistical significance was assessed at the 5% significance level. All statistical analyses were carried out using R software version 3.2.4 (R Core Team, 2016).
3. Results
3.1. The poultry red mite populations
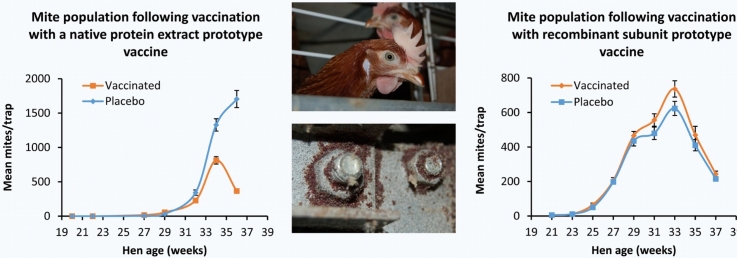
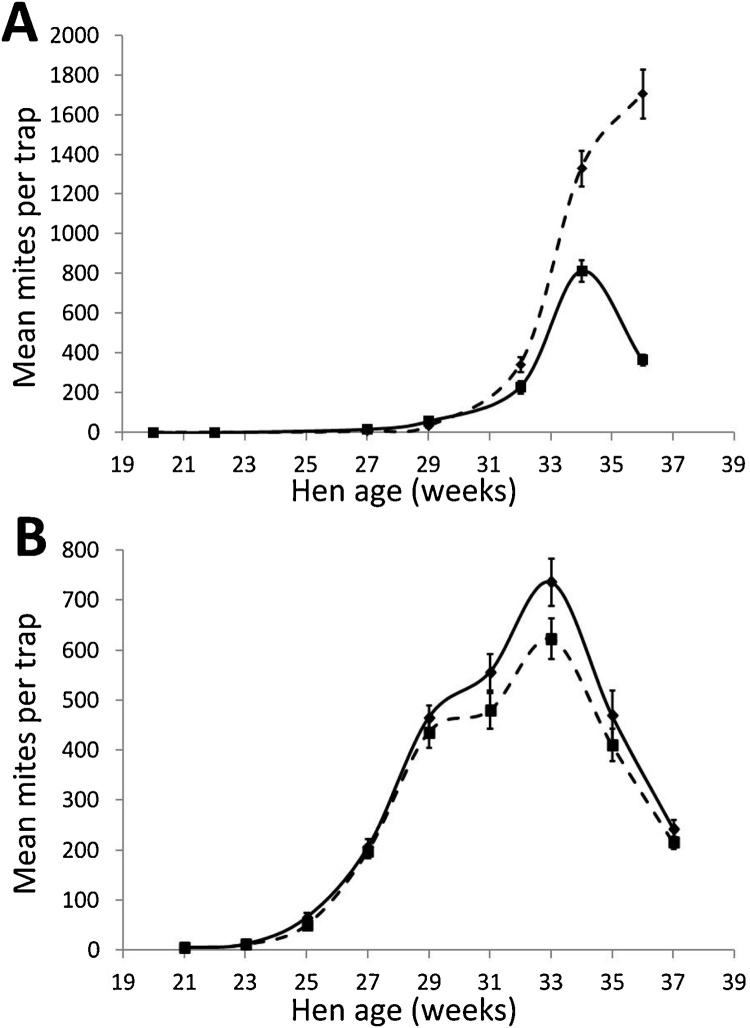
The mean numbers of PRM per trap for the Vaccine and Placebo groups over the course of both trial 1 (SME vaccine trial) and trial 2 (recombinant antigen cocktail vaccine trial) are shown in Fig. 2. Individual trap data for both trials are available in Supplementary Data File 1.
Fig. 2.
The changes in poultry red mite populations over time during field evaluation of prototype poultry red mite vaccines.
Hens in trial 1 (panel A) were immunised with a prototype vaccine containing a soluble mite protein extract (Vaccine group) or adjuvant only (Placebo group). Hens in trial 2 (panel B), received a recombinant antigen cocktail vaccine containing Deg-SRP-1, Deg-VIT-1 and Deg-PUF-1 (Vaccine group) or adjuvant only (Placebo group). Live mites were released into the cage system at week 18.5. Approximately every 2 weeks thereafter, a mite trap was placed into each cage (n = 96 per group) for a 24 h period and the mites in each trap were manually counted with the aid of a dissecting microscope. The mean number of mites per trap is plotted for both the Vaccine groups (solid black line) and Placebo groups (broken black line) for each time point. The error bars represent the standard error of the mean (S.E.M.).
In trial 1, the increase in PRM numbers in both Placebo and Vaccine groups was initially slow, characterised by a 10.5 week lag period in population expansion following challenge. After week 29, an expansion in PRM populations was observed in both groups (Fig. 2A). The PRM trap numbers peaked in the Vaccine group at week 34 and then declined, whereas PRM numbers in the Placebo group continued to rise until the end of the study. The difference between mean PRM counts in the Vaccine and Placebo groups over time, were analysed using a GAMM. Statistically significant differences in mean PRM counts were observed between the Vaccine and Placebo groups (p < 0.001), over the time period extending from 27 to 36 weeks. Analysis of group differences at the challenge endpoint individual time points was performed using GLMM. Statistically significant differences were observed between the mean PRM counts of the Vaccine and Placebo groups at the challenge endpoint (week 36) of the trial (p < 0.001), with mean PRM numbers in the Vaccine group (366.24 ± 27.44) being 78% less than those in the Placebo group (1671.34 ± 123.60).
In contrast to the slow PRM population growth following challenge in trial 1, a shorter lag period of 4.5 weeks was observed in trial 2 (Fig. 2B). After week 23, a rapid expansion in PRM populations was observed in both Vaccine and Placebo groups in trial 2. Numbers peaked in both groups at week 33 and the mean peak PRM counts numbers were not statistically significantly different (p = 0.058) between the Vaccine group (736.56 ± 46.03) and the Placebo group (623.55 ± 39.70). After the week 33 peak, PRM numbers declined in both groups following a similar pattern. No statistically significant differences in mean PRM counts over the course of trial 2 were observed between the Vaccine and Placebo groups (p = 0.338). Furthermore, at the end of the trial (week 37), there was no statistically significant difference (p = 0.232) between the mean trap counts for the Vaccine (243.45 ± 16.70) and Placebo (215.84 ± 12.42) groups.
To investigate any effects of the recombinant vaccine on PRM population structure in trial 2, the numbers of juvenile and adult PRMs were counted in 16 mite traps from each group at 4 trap points (weeks 25, 29, 33 and 37). The mean proportions of juvenile PRMs (overall mean of the 4 trapping points in Vaccine group was equal to 74.23 ± 5.51% and 72.15 ± 5.60% in the Placebo group) were not statistically significantly different whether the traps came from Placebo or Vaccine group hens (p = 0.397).
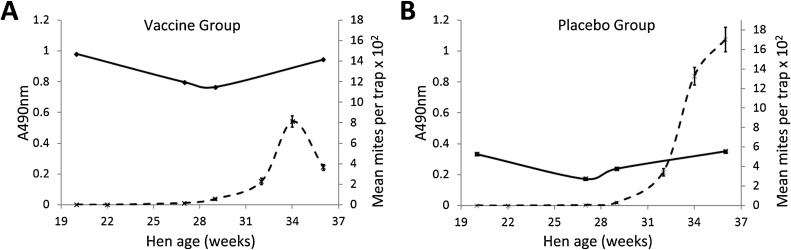
3.2. IgY levels in response to immunisation and to challenge with PRM
In trial 1, pools of yolks randomly sampled from 8 hens from each of the Vaccine and Placebo groups at weeks 20, 27, 29 and 36, were tested for IgY specific for the SME (Fig. 3). A high ELISA O.D. value (A490nm range 0.76–0.98) of the Vaccine group yolk pool was observed following vaccination and challenge and was maintained throughout the trial. In contrast, the Placebo group yolk pool ELISA A490nm remained consistently low (range 0.17–0.35) throughout the trial. The A490nm in both groups followed a similar trend: as PRM numbers increased, so did the A490nm. A similar trend in A490nm was observed in the IMME antigen ELISAs (Supplementary Fig. S1).
Fig. 3.
The yolk IgY levels during field trial evaluation of a prototype poultry red mite vaccine containing soluble mite protein extract (SME).
The IgY levels in pooled yolk sampled from hens immunised with SME (Vaccine group, panel A), or adjuvant only (Placebo group, panel B), at weeks 20, 27, 29 and 36 of trial 1, were quantified using an ELISA with SME as coating antigen. The anti-SME IgY levels (represented by A490nm) in the pooled yolk samples are plotted (solid black line) and the PRM populations data from the Vaccine and Placebo groups are overlaid (broken black lines), with the error bars giving the S.E.M.
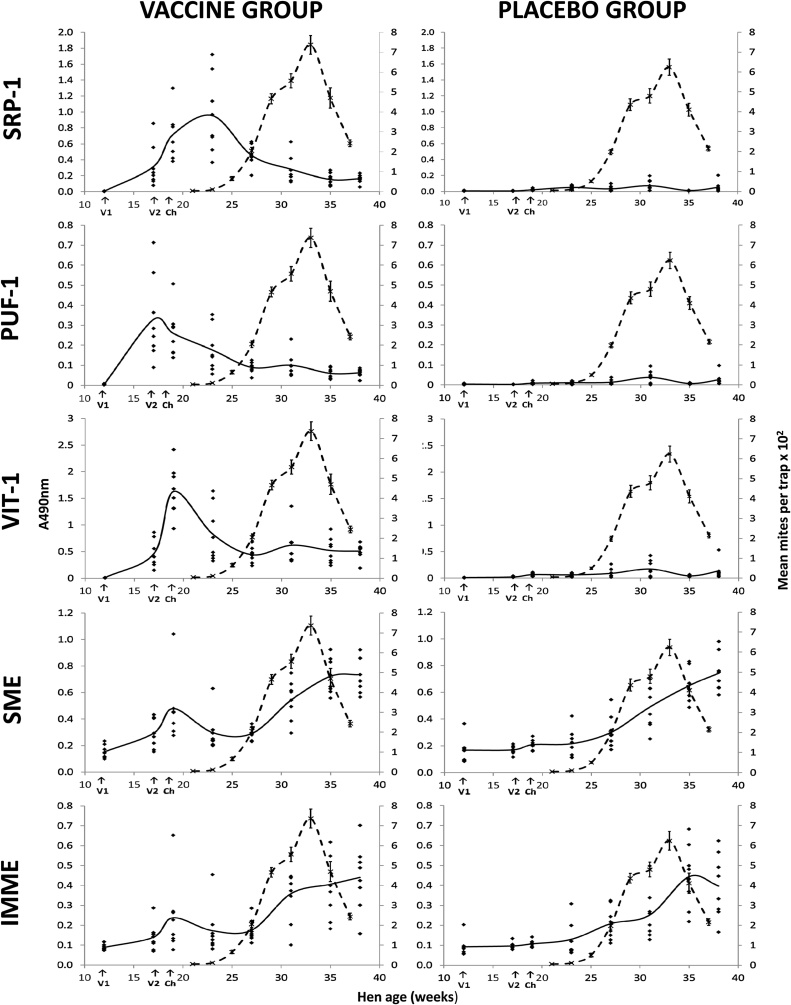
In trial 2, the longevity and magnitude of individual hen’s responses to each of the recombinant antigens were evaluated using serum IgY ELISAs. The individual hen and the mean Vaccine and Placebo group responses to Deg-SRP-1, Deg-PUF-1 and Deg-VIT-1 antigens at 8 time points are presented in Fig. 4 along with the PRM population data for comparison. Most hens in the Vaccine group produced high levels of antigen-specific IgY to Deg-SRP-1, Deg-VIT-1 and Deg-PUF-1 following primary immunisation and secondary boosting at 12 and 17 weeks old respectively. Maximum antigen-specific IgY levels were reached at weeks 17, 19 and 23 for Deg-PUF-1, VIT-1 and SRP-1 respectively; after which the antigen-specific IgY levels diminished rapidly. Peak IgY levels to Deg-PUF-1 and Deg-VIT-1 occurred before the PRM population started to expand (week 23) and the IgY levels to Deg-SRP-1 peaked at week 23. In contrast, the Placebo group hens maintained a low vaccine antigen-specific IgY level against all 3 recombinant vaccine antigens throughout the course of trial 2.
Fig. 4.
The generation and longevity of serum IgY levels specific for the individual components of a prototype poultry red mite recombinant antigen cocktail vaccine and native protein preparations during field trial evaluation.
The specific IgY levels of individual hens from the Vaccine (left panels) and Placebo (right panels) groups in trial 2 to the individual recombinant vaccine antigens: Deg-SRP-1, Deg-PUF-1, Deg-VIT-1 and the native protein mite extracts: soluble mite extract (SME) and integral membrane mite extract (IMME) were quantified using ELISAs. The antigen-specific IgY levels (represented by A490nm) of 8 individual hens randomly sampled from each group at 9 time points, are indicated with black diamonds (♦) and the mean response with the solid black line. The timings of the primary and secondary vaccinations (V1 and V2) and of the mite challenge (Ch) are indicated with the arrows on x-axis. The mite population data of Vaccine and Placebo groups are overlaid (broken black lines), with the error bars giving the S.E.M.
Yolk-IgY ELISAs were performed in parallel with serum-IgY ELISA (Supplementary Fig. S2) and largely reflected the patterns observed for the serum IgY ELISAs.
3.3. Hen IgY levels against native antigens following poultry red mite exposure
In order to investigate the generation of an IgY response to PRM infestation over the course of trial 2, the IgY responses against extracts of PRM antigens (SME and IMME) were monitored in Vaccine and Placebo group hens using serum-IgY ELISAs (Fig. 4) and yolk-IgY ELISAs (Supplementary Fig. S2, panels D and E).
The generation of a PRM-specific serum-IgY during infestation was observed in both Vaccinated and Placebo hens and was directed towards antigens present in both SME and IMME. Substantial increases in the levels of serum- and yolk-SME and IMME-specific IgY were observed in both Vaccine and Placebo groups from week 25 onwards. The rise in PRM antigen-specific serum IgY levels was comparable in both treatment groups, and was therefore not related to PRM-vaccination status of the hen. The rise in PRM antigen-specific serum IgY levels in both groups coincided with the onset of the rapid increase in PRM populations. Following a reduction in PRM numbers towards the end of the trial, the mean PRM antigen specific serum IgY levels of the Vaccine and Placebo groups against the SME and IMME antigens became static or reduced. A peak in native PRM antigen-specific serum and yolk IgY levels at week 19 following vaccine boosting was observed in the Vaccine group, but not the Placebo group (Fig. 4, Supplementary Fig. S2), suggesting that one of more of the recombinant vaccine antigens was represented in native form in the SME and IMME preparations.
3.4. The immuno-reactive profiles of hens in response to vaccination and poultry red mite infestation
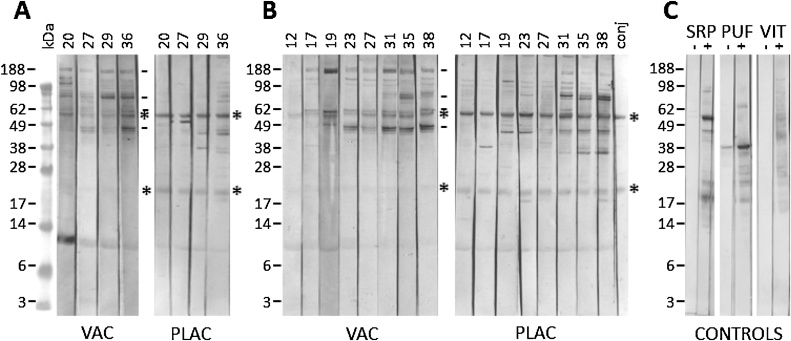
In trial 1, the yolk-IgY from the Vaccine group, collected 3 weeks after secondary immunisation, reacted strongly with multiple protein bands present in the SME (Fig. 5, panel A), indicating a vigorous and multi-antigen response to immunisation with SME. Reactivity was predominately seen in the mid (35 kDa) to the high (>188 kDa) mol. wt range. In contrast, only 2 bands were obvious in the immunoblots probed with Placebo group yolk IgY at the same time point. These 2 bands were also detected in the anti-IgY-HRP control lane (Fig. 5, panel C) and are IgY fragments derived from the ingested blood meal present in the digestive tract of the PRMs used in the preparation of SME. Band intensity in the immunoblots probed with yolk IgY from Vaccine group hens decreased at mid points in the trial (weeks 27 and 29), which coincided with low PRM numbers and the lower A490nmobtained at these time points with the yolk-IgY SME ELISA (Fig. 3). Thereafter, increased band intensity and complexity were observed at week 36 that differs in profile to that detected with Placebo group yolk-IgY from the same time point.
Fig. 5.
The changes in immuno-reactivity over time during the field trial evaluation of prototype poultry red mite vaccines.
Individual lanes of a western blot of soluble mite extract (SME) protein were probed with pooled egg yolks from hens from trial 1 assessing a SME prototype vaccine (panel A) or with pooled serum from trial 2 testing a recombinant antigen cocktail prototype vaccine (panel B). Pooled yolk or serum from Vaccinated hens (VAC) and the Placebo hens (PLAC) obtained at several time points throughout the trials were tested (trial 1: weeks 20, 27, 29 and 36; trial 2: weeks 12,17,19,23,27,31,35 and 38). Bound IgY was detected with rabbit anti-IgY-peroxidase conjugated antibody (Sigma, UK). PBS was substituted for the primary IgY and served as a conjugate control (conj, panel B). Additional controls were performed for trial 2 (panel C): individual western blot strips of the purified recombinant antigens: Deg-Serpin-1 (SRP), Deg-protein of unknown function-1 (PUF) and Deg-Vitellogenin (VIT) were probed with trial 2 serum pool from the Placebo group (−) and Vaccine group (+) obtained following vaccination (week 17). The bands marked with asterisk (*) indicate the detection of host IgY fragments present in SME. Bands marked with the lines (−) indicate immuno-reactive bands common to both in trial 1 and 2 Vaccine groups that developed following prolonged infestation with poultry red mite. The approximate mol. wt (kDa) of detected proteins were estimated by comparison to SeeBlue® Plus2 pre-stained standards (GE Healthcare, UK).
In trial 2, the only immuno-reactive bands detected in immunoblots of SME probed with serum from the Vaccine and Placebo groups at week 12 (pre-immunisation) corresponded to the IgY fragments derived from the ingested host blood meal contained in the PRM extract (Fig. 5, panel B). Following immunisation of hens with the 3 recombinant antigens, additional immuno-reactive bands on immunoblots of both SME (Fig. 5, panel B) and purified recombinant proteins (Fig. 5, panel C) probed with serum from the Vaccine group hens were visible at weeks 17, indicating a specific antibody response to vaccination with the recombinant PRM antigens and also the ability of IgY raised against the recombinant proteins to bind native versions of some of these proteins in SME. Thus, high mol. wt bands (>188 kDa) corresponding to the full length native Deg-VIT-1 (210 kDa) and some lower mol. wt bands that may correspond to native forms of processed Deg-VIT-1 were detected in immunoblots of SME probed with sera from Vaccine group hens by weeks 17 and 19 (Fig. 5, panel B). Bands corresponding with the predicted mol wt. of native forms of Deg-SRP-1 (42 kDa) and Deg-PUF-1 (20 kDa) were not clearly visible in immunoblots of SME probed with sera from Vaccine group hens (Fig. 5, panel B) suggesting that these proteins are of low abundance in SME and below the detectable level by Western blotting.
Following challenge, as the PRM populations increased in both the Vaccine and Placebo groups in trial 2, a diverse immuno-reactive profile emerged in immunoblots of SME probed with sera from both Vaccine and Placebo group hens over time. The profiles for the Vaccine and Placebo groups at week 38 were similar to each other, with notable differences in the band intensities in a high mol. wt. band (>188 kDa) and a lower mol. wt band at approximately 38 kDa. In addition, the profiles that emerged in both groups of hens at weeks 35 and 38 in trial 2, were comparable to those seen in the trial 1 Vaccine group (see bands marked with a line on Fig. 5 panels A and B).
4. Discussion
Here we have demonstrated, for the first time, the efficacy of a prototype PRM vaccine containing native proteins in a soluble mite extract (SME) in a commercial-style laying hen facility. The SME-based vaccine reduced PRM population size by up to 78% (trial 1). Previous studies demonstrated the feasibility of using vaccination with similar native PRM protein extracts to generate specific IgY immunoglobulins that induce PRM mortality when ingested in a blood meal (Wright et al., 2009, Harrington et al., 2009, Bartley et al., 2015). However, these studies were performed on a small scale, in a laboratory environment and the measurable vaccine effects were limited to mite mortality after a single heparinised blood meal delivered in vitro using an artificial mite feeding apparatus. In contrast, the reduction in PRM population numbers attributable to vaccination in the field-type trial described here incorporates several vaccine effects, such as mortality, morbidity and fecundity.
Previous immuno-proteomic analysis of SME identified several promising individual vaccine candidate antigens which induced statistically-significant increases in PRM mortality when blood from hens immunised with recombinant forms of each of the antigens was fed to PRM on a small-scale using the in vitro artificial feeding device (Bartley et al., 2015). In this current study (in trial 2), we selected three of the most promising of these vaccine candidates, Deg-SRP-1, Deg-PUF-1 and Deg-VIT-1, and performed the first large-scale PRM vaccine field trial using recombinant vaccine candidates. In spite of their efficacy individually in the in vitro feeding trials, the combined recombinant cocktail vaccine did not confer protective immunity in immunised hens in the field trial. The reasons for the inability of the recombinant cocktail vaccine to control PRM numbers in the field trial may include inappropriate antigen selection, and/or an inadequate protective response in terms of magnitude and longevity.
As all 3 recombinant antigens performed strongly in previous in vitro mite feeding assays (Bartley et al., 2015) and the antigen choice was based on their apparent potential. PRMs fed in vitro on blood from hens immunised with each of these antigens individually were more than twice as likely to die than those fed on blood from control hens (Bartley et al., 2015). Combining recombinant antigens into a cocktail vaccine has also been a useful method to enhance vaccine efficacy against parasites (cf. Nisbet et al., 2013, Burgess et al., 2016). In addition, Deg-VIT-1 is structurally similar to a second high performing antigen from the in vitro feeding screen, hemelipoglycoprotein (Deg-HGP-1), which induced significant PRM mortality in the previous studies (Bartley et al., 2015). These two proteins share many functional domains and were shown, by Western blotting, to be immunological cross-reactive (Bartley et al., unpublished data). Therefore vaccination with one protein may have the additional benefit of conferring cross-protection immunological cross-reactivity against the other. Likewise, Deg-SRP-1 also had a rational basis for its inclusion into the recombinant antigen cocktail vaccine: in addition to the induced mortality in PRM feeding on sera from hens immunised with recombinant Deg-SRP-1 (Bartley et al., 2015), previous vaccination studies on several tick species have shown that serpin-based vaccines induce a variety of effects including: increased parasite mortality, reduced reproductive parameters and interference with feeding (Sugino et al., 2003, Imamura et al., 2005, Imamura et al., 2006, Prevot et al., 2007, Jittapalapong et al., 2010, Kim et al., 2016). In contrast, the final protein included into the recombinant antigen cocktail, Deg-PUF-1, has no ascribed putative function. However, as Deg-PUF-1 produced the highest rates of mortality seen in the 10 antigens tested using the in vitro feeding studies (Bartley et al., 2015), it was selected for inclusion in the recombinant antigen cocktail vaccine.
Following immunisation with the recombinant protein cocktail, wide variation was observed in the magnitude of the initial antigen-specific IgY levels of individual hens to the vaccine components (Fig. 4). The mean recombinant antigen-specific IgY levels peaked at between 17 to 23 weeks, and then rapidly diminished before any substantial rise in PRM numbers occurred. It is therefore likely that the poor longevity of the vaccine-induced antibody response is largely responsible for the inability of the vaccine to control the rise in PRM numbers. In addition, the recombinant-antigen specific IgY levels were not boosted by exposure to high numbers of feeding PRMs. Therefore the choice of adjuvant is likely to be crucial for vaccination with these recombinant antigens to produce a sustained, protective response. The adjuvant used in trial 2, Montanide™ ISA70VG, synthetic oil in water in oil preparation, was chosen for its demonstrated safety and efficacy in other poultry vaccines (e.g. Dupuis et al., 2006, Jawale and Lee, 2016) but, on this occasion, sustained recombinant antigen-specific IgY levels were not observed. There was also little evidence for a substantial anamnestic response to recombinant antigens in the Vaccine group hens following PRM exposure. This suggests that either the antigens need to be administered in a suitable adjuvant as described above or that vaccine boosting would need to be performed during the lay period for sustained protection. Repeated PRM vaccination using an injection route is unlikely to be acceptable to industry, given the practical and economic implications of mass vaccination of commercial flocks, which often exceed 50,000 hens. To overcome the challenges of mass vaccination, many of the current commercial poultry vaccines are administered in drinking water or by aerosol spraying during the rearing period (e.g. vaccines which protect against Salmonella: SALMUNE® (CEVA, France) and Gallivac Se (Merial, France); Infectious Bronchitis and variants: CEVAC® IBird (CEVA), Infectious Bursal Disease: CEVAC®GUMBO L (CEVA) and Gallivac IBD (Merial); Newcastle Disease: CEVAC® VITAPEST L (CEVA)) and these may be coupled with an initial injectable vaccine either to prime an initial response or to boost responses just before the lay period (Knott et al., 2008). Alternative oral delivery routes for avian vaccine antigens include delivery in food (e.g. Zhou et al., 2004; Jacob et al., 2013, Ito et al., 2013, Kolotilin et al., 2014) and a similar regime may be beneficial in maintaining a strong response to a PRM vaccine if the delivery method was to result in antigen-specific circulating serum antibodies.
An alternative method to produce a sustained protective immune response would be to select a novel set of vaccine antigens from those proteins which generate a powerful immune response during natural parasite exposure. By their nature these “exposed antigens” should generate vaccine-induced antibody levels following immunisation which would be further boosted during natural infestation (Willadsen and Kemp, 1988, Munn, 1993, Nuttall et al., 2006). We have shown here, for the first time, that serum IgY levels against exposed PRM antigens increase with the duration and level of PRM infestation and that, after these IgY levels reach a peak, PRM numbers started to reduce (Fig. 4). This is in contrast to results obtained by Arkle et al. (2006), where a low PRM-specific serum IgY level was measured throughout the lay period of commercial hens and no significant relationship between antibody level and PRM numbers was observed. However, the antigen used in the ELISA in the Arkle et al. (2006) study was prepared from membrane extracts from starved PRMs which would probably lack many exposed antigens and many of the PRM proteins that are upregulated and secreted/excreted by feeding mites.
The shift in the specificity of the IgY response in hens vaccinated with SME (trial 1), from a diffuse response against a multitude of SME antigens at the start of infestation, to a focused response against fewer antigens later in the trial, coupled with reduction in PRM numbers, lends support for using a targeted approach using exposed antigens. It is clear from the problems caused by huge numbers of PRM in laying sheds (Van Emous, 2005) that the hens’ immune response to natural infestations is “too little, too late” to prevent PRM population expansion but, if the hens were already primed with a vaccine containing these exposed antigens (as in trial 1, here) the PRM populations may be controlled. Very little is currently known about the underlying mechanism(s) of adaptive protective immune response in hens to PRM but it may prove to be as complex as in other blood-feeding ectoparasite species. For example, in Rhipicephalus microplus infestation, immune-mediated resistance is associated with several factors including high levels of specific immunoglobulins, strong T-helper-1 (Th1) type response, particularly hypersensitivity with associated eosinophil and mast cell degranulation and increases in gamma-delta T-cells and CD25+ cells (reviewed Jonsson et al., 2014). Studies examining cytokine and chemokine gene expression levels and patterns in hens during the first 5 and 22 days of exposure to PRM did not identify any statistically significant differences in expression of selected Th1 and T-helper-2 (Th2) molecules (Harrington et al., 2010a; 2010b). There was however, a possible trend towards a Th1 response in the first 24 h post-infestation, followed by down regulation of Th1 responses at 48 h post infestation (Harrington et al., 2010a). After the removal of the PRMs on day 5, increases in IFNγ and IL-6 were observed; leading to the suggestion that PRM-mediated immuno-modulation of the host response may also be important for successful PRM feeding. Despite the lack of a clearly defined Th1/Th2 paradigm response, statistically significant increases in IgM and IgY levels, specific for PRM protein extracts, were observed over 22 days of infestation (Harrington et al., 2010b). This increase in IgY and IgM levels occurred concurrently with a decrease in the PRM oviposition rates, indicating a potential immunoglobulin mediated effect on PRM fecundity. As supplementation of blood meals with purified anti-PRM-IgYs induced mortality in feeding PRMs (Wright et al., 2009) and there is a statistically significant relationship between IgY level and mortality in this system (Harrington et al., 2009); it is clear that vaccine-induced humoral responses, at least, are implicated in vaccine efficacy. Similarly, the generation of a strong serum antibody response against the blood-feeding avian ectoparasite mite Ornithonyssus sylviarum during heavy infestations (DeVaney and Ziprin, 1980) is associated with a reduction in parasite burden (DeVaney and Augustine, 1988, Wikel et al., 1989). However, factors other than host antibody response are also important in natural acquired immunity to O. sylviarum, such as MHC-influenced skin inflammatory responses which are able to restrict the ability of the mite to reach the host capillaries and obtain a blood meal (Owen et al., 2008, Owen et al., 2009). Similar histopathological changes (e.g. epidermal hyperplasia, hyperkeratosis and parakeratosis) are also characteristic of PRM infestation of hens (Sokól and Rotkiewicz, 2010; Hobbenaghi et al., 2012), although the implications of the skin changes in the resistance of hens to PRM are unknown.
The dynamics of PRM population growth were different in trial 1 and 2, with growth in PRM populations being much slower in trial 1 Vaccine and Placebo groups, possibly as a consequence of seasonality and a lower external temperature. The development of PRM exposed antigen-specific IgY in vaccinated hens in trial 1 developed after just 5 weeks of rapidly rising PRM numbers (between weeks 29 to 34), whereas in trial 2 a similar response, in terms of both antigen profile and immunoreactivity, was observed after a longer period of 11 weeks of rapid PRM population growth. This suggests that the IgY response to exposed antigens during infestation develops more quickly in SME-immunised birds in response to increasing PRM numbers, possibly as a consequence of prior exposure to these antigens in the SME vaccine.
In conclusion, we have demonstrated that vaccine-induced immunity is able to reduce PRM population numbers in commercial style laying facilities. In addition, prolonged exposure to high numbers of PRMs may result in some degree of mite control through the development of natural acquired immunity. The vaccination trial using a recombinant vaccine cocktail has demonstrated the difficulties in maintaining a sustained IgY level throughout the laying period. The challenge is now to engineer a recombinant antigen-based vaccine capable of producing a response of sufficient magnitude and longevity that does not rely upon high numbers of PRM to continually boost the protective response. It is likely that such a vaccine would have an improved protective capacity during the early phase of the lay cycle, when PRM numbers are low and thus prevent PRM numbers reaching a level where they impact on the health, welfare and production of laying hens.
Acknowledgements
The authors gratefully acknowledge funding for this project from Scottish Government and BBRSC (grant reference BB/J01513X/1), Zoetis and Akita Co. Ltd., The British Egg Marketing Board Trust and Genomia. We would also like to thank all the staff at Roslin Nutrition Ltd., Helen Groves and Tim Wallis of Ridgeway Biologicals Ltd., and Dr Barry Thorp (St David’s Poultry Vets) for their assistance with the field trials and Sir. John Campbell O.B.E. and Mrs Karen Campbell, Glenrath Farms Ltd., for their continued support throughout this project.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vetpar.2017.06.020.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Abbas R.Z., Colwell D.D., Iqbal Z., Khan A. Acaricidal drug resistance in poultry red mite (Dermanyssus gallinae) and approaches to its management. World's Poultry Sci. J. 2014;70:113–124. [Google Scholar]
- Arkle S., Guy J.H., Sparagano O. Immunological effects and productivity variation of red mite (Dermanyssus gallinae) on laying hens-implications for egg production and quality. World’s Poultry Sci. J. 2006;62:249–257. [Google Scholar]
- Arkle S., Harrington D., Kaiser P., Rothwell L., De Luna C., George D., Guy J., Sparagano O.A. Immunological control of the poultry red mite. Ann. NY Acad. Sci. 2008;1149:36–40. doi: 10.1196/annals.1428.057. [DOI] [PubMed] [Google Scholar]
- Bartley K., Nisbet A.J., Offer J.E., Sparks N.H., Wright H.W., Huntley J.F. Histamine release factor from Dermanyssus gallinae (De Geer): characterization and in vitro assessment as a protective antigen. Int. J. Parasitol. 2009;39:447–456. doi: 10.1016/j.ijpara.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Bartley K., Huntley J.F., Wright H.W., Nath M., Nisbet A.J. Assessment of cathepsin D and L-like proteinases of poultry red mite, Dermanyssus gallinae (De Geer), as potential vaccine antigens. Parasitology. 2012;139:755–765. doi: 10.1017/S0031182011002356. [DOI] [PubMed] [Google Scholar]
- Bartley K., Wright H.W., Huntley J.F., Manson E.D., Inglis N.F., McLean K., Nath M., Bartley Y., Nisbet A.J. Identification and evaluation of vaccine candidate antigens from the poultry red mite (Dermanyssus gallinae) Int. J. Parasitol. 2015;45:819–830. doi: 10.1016/j.ijpara.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau A., Dernburg A., Chauve C., Zenner L. First in vitro cycle of the chicken mite Dermanyssus gallinae (DeGeer 1778), utilizing an artificial feeding device. Parasitology. 2001;123:583–589. doi: 10.1017/s0031182001008836. [DOI] [PubMed] [Google Scholar]
- Burgess S.T., Nunn F., Nath M., Frew D., Wells B., Marr E.J., Huntley J.F., McNeilly T.N., Nisbet A.J. A recombinant subunit vaccine for the control of ovine psoroptic mange (sheep scab) Vet. Res. 2016;47:26. doi: 10.1186/s13567-016-0315-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosoroaba I. Massive Dermanyssus gallinae invasion in battery-husbandry raised fowls. Rev. Med. Vet.-Toulouse. 2001;152:89–96. [Google Scholar]
- DeVaney J.A., Augustine P.C. Correlation of estimated and actual northern fowl mite populations with the evolution of specific antibody to a low molecular weight polypeptide in the sera of infested hens. Poultry Sci. 1988;67:549–556. doi: 10.3382/ps.0670549. [DOI] [PubMed] [Google Scholar]
- DeVaney J.A., Ziprin R.L. Acquired immune response of white leghorn hens to populations of northern fowl mite, Ornithonyssus sylviarum (Canestrini and Fanzago) Poultry Sci. 1980;59:1742–1744. doi: 10.3382/ps.0591742. [DOI] [PubMed] [Google Scholar]
- Dupuis L., Ascarateil S., Aucouturier J., Ganne V. SEPPIC vaccine adjuvants for poultry. Ann. NY Acad. Sci. 2006;1081:202–205. doi: 10.1196/annals.1373.024. [DOI] [PubMed] [Google Scholar]
- Harrington D., Din H.M., Guy J., Robinson K., Sparagano O. Characterization of the immune response of domestic fowl following immunization with proteins extracted from Dermanyssus gallinae. Vet. Parasitol. 2009;160:285–294. doi: 10.1016/j.vetpar.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Harrington D., Robinson K., Guy J., Sparagano O. Characterization of the immunological response to Dermanyssus gallinae infestation in domestic fowl. Transbound. Emerg. Dis. 2010;57:107–110. doi: 10.1111/j.1865-1682.2010.01109.x. [DOI] [PubMed] [Google Scholar]
- Harrington D.W., Robinson K., Sparagano O.A. Immune responses of the domestic fowl to Dermanyssus gallinae under laboratory conditions. Parasitol. Res. 2010;106:1425–1434. doi: 10.1007/s00436-010-1821-2. [DOI] [PubMed] [Google Scholar]
- Hobbenaghi R., Tavassoli M., Alimehr M., Shokrpoor S., Ghorbanzadeghan M. Histopathological study of the mite biting (Dermanyssus gallinae) in poultry skin. Vet. Res. Forum. 2012;3:205–208. [PMC free article] [PubMed] [Google Scholar]
- Imamura S., da Silva V.J.I., Sugino M., Ohashi K., Onuma M. A serine protease inhibitor (serpin) from Haemaphysalis longicornis as an anti-tick vaccine. Vaccine. 2005;23:1301–1311. doi: 10.1016/j.vaccine.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Imamura S., Namangala B., Tajima T., Tembo M.E., Yasuda J., Ohashi K., Onuma M. Two serine protease inhibitors (serpins) that induce a bovine protective immune response against Rhipicephalus appendiculatus ticks. Vaccine. 2006;24:2230–2237. doi: 10.1016/j.vaccine.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Ito A., Gotanda T., Himeno N., Itchoda N., Tabayashi N., Ike K., Sugimoto C., Matsumura T. Booster responses by oral vaccination with transgenic plants against chicken leucocytozoonosis. Avian Pathol. 2013;42:215–220. doi: 10.1080/03079457.2013.779635. [DOI] [PubMed] [Google Scholar]
- Jacob S.S., Cherian S., Sumithra T.G., Raina O.K., Sankar M. Edible vaccines against veterinary parasitic diseases–current status and future prospects. Vaccine. 2013;31:1879–1885. doi: 10.1016/j.vaccine.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Jawale C.V., Lee J.H. 2016. Evaluation of immunogenicity and protective efficacy of adjuvanted Salmonella typhimurium ghost vaccine against salmonellosis in chickens. Vet. Q. 2016:1–7. doi: 10.1080/01652176.2016.1138248. [DOI] [PubMed] [Google Scholar]
- Jittapalapong S., Kaewhom P., Pumhom P., Canales M., de la Fuente J., Stich R.W. Immunization of rabbits with recombinant serine protease inhibitor reduces the performance of adult female Rhipicephalus microplus. Transbound Emerg. Dis. 2010;57:103–106. doi: 10.1111/j.1865-1682.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- Jonsson N.N., Piper E.K., Constantinoiu C.C. Host resistance in cattle to infestation with the cattle tick Rhipicephalus microplus. Parasite Immunol. 2014;36:553–559. doi: 10.1111/pim.12140. [DOI] [PubMed] [Google Scholar]
- Kilpinen O., Roepstorff A., Permin A., Norgaard-Nielsen G., Lawson L.G., Simonsen H.B. Influence of Dermanyssus gallinae and Ascaridia galli infections on behaviour and health of laying hens (Gallus gallus domesticus) Br. Poult. Sci. 2005;46:26–34. doi: 10.1080/00071660400023839. [DOI] [PubMed] [Google Scholar]
- Kim T.K., Radulovic Z., Mulenga A. Target validation of highly conserved Amblyomma americanum tick saliva serine protease inhibitor 19. Ticks Tick Borne Dis. 2016;3:405–414. doi: 10.1016/j.ttbdis.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A.C. In vitro feeding of Dermanyssus gallinae. Exp. Parasitol. 1971;29:1–6. doi: 10.1016/0014-4894(71)90002-6. [DOI] [PubMed] [Google Scholar]
- Knott C.I.F., Lister S.A., Hammond P.P. Vaccination of poultry–all you ever wanted to know. Ranger. 2008;(February) [Google Scholar]
- Kolotilin I., Topp E., Cox E., Devriendt B., Conrad U., Joensuu J., Stoger E., Warzecha H., McAllister T., Potter A., McLean M.D., Hall J.C., Menassa R. Plant-based solutions for veterinary immunotherapeutics and prophylactics. Vet. Res. 2014;45:117. doi: 10.1186/s13567-014-0117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski A., Sokol R. Influence of Dermanyssus gallinae (poultry red mite) invasion on the plasma levels of corticosterone, catecholamines and proteins in layer hens. Pol. J. Vet. Sci. 2009;12:231–235. [PubMed] [Google Scholar]
- Makert G.R., Vorbruggen S., Krautwald-Junghanns M.E., Voss M., Sohn K., Buschmann T., Ulbert S. A method to identify protein antigens of Dermanyssus gallinae for the protection of birds from poultry mites. Parasitol. Res. 2016;115:2705–2713. doi: 10.1007/s00436-016-5017-2. [DOI] [PubMed] [Google Scholar]
- Maurer V., Bieri M., Fölsch D.W. Das suchverhalten von Dermanyssus gallinae in Hühnerställen. Arch. Geflügelk. 1988;52:209–215. [Google Scholar]
- McDevitt R., Nisbet A.J., Huntley J.F. Ability of a proteinase inhibitor mixture to kill poultry red mite, Dermanyssus gallinae in an in vitro feeding system. Vet. Parasitol. 2006;141:380–385. doi: 10.1016/j.vetpar.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Mul M., Van Niekerk T., Chirico J., Maurer V., Kilpinen O., Sparagano O., Thind B., Zoons J., Moore D., Bell B., Gjevre A.G., Chauve C. Control methods for Dermanyssus gallinae in systems for laying hens: results of an international seminar. World’s Poultry Sci. J. 2009;65:589–600. [Google Scholar]
- Munn E.A. Development of a vaccine against Haemonchus contortus. Parasitol. Today. 1993;9:338–339. doi: 10.1016/0169-4758(93)90237-a. [DOI] [PubMed] [Google Scholar]
- Nisbet A.J., McNeilly T.N., Wildblood L.A., Morrison A.A., Bartley D.J., Bartley Y., Longhi C., McKendrick I.J., Palarea-Albaladejo J., Matthews J.B. Successful immunization against a parasitic nematode by vaccination with recombinant proteins. Vaccine. 2013;37:4017–4023. doi: 10.1016/j.vaccine.2013.05.026. [DOI] [PubMed] [Google Scholar]
- Nuttall P.A., Trimnell A.R., Kazimirova M., Labuda M. Exposed and concealed antigens as vaccine targets for controlling ticks and tick-borne diseases. Parasite Immunol. 2006;28:155–163. doi: 10.1111/j.1365-3024.2006.00806.x. [DOI] [PubMed] [Google Scholar]
- Oines O., Brannstrom S. Molecular investigations of cytochrome c oxidase subunit I (COI) and the internal transcribed spacer (ITS) in the poultry red mite Dermanyssus gallinae, in northern Europe and implications for its transmission between laying poultry farms. Med. Vet. Entomol. 2011;25:402–412. doi: 10.1111/j.1365-2915.2011.00958.x. [DOI] [PubMed] [Google Scholar]
- Owen J.P., Delany M.E., Mullens B.A. MHC haplotype involvement in avian resistance to an ectoparasite. Immunogenetics. 2008;60:621–631. doi: 10.1007/s00251-008-0314-2. [DOI] [PubMed] [Google Scholar]
- Owen J.P., Delany M.E., Cardona C.J., Bickford A.A., Mullens B.A. Host inflammatory response governs fitness in an avian ectoparasite, the northern fowl mite (Ornithonyssus sylviarum) Int. J. Parasitol. 2009;39:789–799. doi: 10.1016/j.ijpara.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Prevot P.P., Couvreur B., Denis V., Brossard M., Vanhamme L., Godfroid E. Protective immunity against Ixodes ricinus induced by a salivary serpin. Vaccine. 2007;25:3284–3292. doi: 10.1016/j.vaccine.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Pritchard J., Küster T., George D., Sparagano O., Tomley F. Impeding movement of the poultry red mite, Dermanyssus gallinae. Vet. Parasitol. 2016;225:104–107. doi: 10.1016/j.vetpar.2016.06.006. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2016. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- Schicht S., Qi W., Poveda L., Strube C. The predicted secretome and transmembranome of the poultry red mite Dermanyssus gallinae. Parasit. Vectors. 2013;6:259. doi: 10.1186/1756-3305-6-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schicht S., Qi W., Poveda L., Strube C. Whole transcriptome analysis of the poultry red mite Dermanyssus gallinae (De Geer, 1778) Parasitology. 2014;141:336–346. doi: 10.1017/S0031182013001467. [DOI] [PubMed] [Google Scholar]
- Sommer D., Heffels-Redmann U., Kohler K., Lierz M., Kaleta E.F. Role of the poultry red mite (Dermanyssus gallinae) in the transmission of avian influenza A virus. Tierarztl. Prax. G. N. 2016;44:26–33. doi: 10.15653/TPG-150413. [DOI] [PubMed] [Google Scholar]
- Sparagano O.A., George D.R., Harrington D.W., Giangaspero A. Significance and control of the poultry red mite, Dermanyssus gallinae. Annu. Rev. Entomol. 2014;59:447–466. doi: 10.1146/annurev-ento-011613-162101. [DOI] [PubMed] [Google Scholar]
- Sugino M., Imamura S., Mulenga A., Nakajima M., Tsuda A., Ohashi K., Onuma M. A serine proteinase inhibitor (serpin) from ixodid tick Haemaphysalis longicornis; cloning and preliminary assessment of its suitability as a candidate for a tick vaccine. Vaccine. 2003;21:2844–2851. doi: 10.1016/s0264-410x(03)00167-1. [DOI] [PubMed] [Google Scholar]
- Valiente-Moro C., Chauve C., Zenner L. Experimental infection of Salmonella enteritidis by the poultry red mite, Dermanyssus gallinae. Vet. Parasitol. 2007;146:329–336. doi: 10.1016/j.vetpar.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Van Emous R. Wage war against the red mite! Poultry Int. 2005;44:26–33. [Google Scholar]
- Wang F.F., Wang M., Xu F.R., Liang D.M., Pan B.L. Survey of prevalence and control of ectoparasites in caged poultry in China. Vet. Rec. 2010;167:934–937. doi: 10.1136/vr.c6212. [DOI] [PubMed] [Google Scholar]
- Wikel S.K., DeVaney J.A., Augustine P.C. Host immune response to northern fowl mite: immunoblot and lectin blot identification of mite antigens. Avian Dis. 1989;33:668–675. [PubMed] [Google Scholar]
- Willadsen P., Kemp D.H. Vaccination with ‘concealed’ antigens for tick control. Parasitol. Today. 1988;4:196–198. doi: 10.1016/0169-4758(88)90084-1. [DOI] [PubMed] [Google Scholar]
- Wojcik A.R., Grygon-Franckiewicz B., Zbikowska E., Wasielewski L. Invasion of Dermanyssus gallinae (De Geer, 1778) in poultry farms in the Torun region. Wiad. Parazytol. 2000;46:511–515. [PubMed] [Google Scholar]
- Wright H.W., Bartley K., Nisbet A.J., McDevitt R.M., Sparks N.H., Brocklehurst S., Huntley J.F. The testing of antibodies raised against poultry red mite antigens in an in vitro feeding assay; preliminary screen for vaccine candidates. Exp. Appl. Acarol. 2009;48:81–91. doi: 10.1007/s10493-009-9243-5. [DOI] [PubMed] [Google Scholar]
- Wright H.W., Bartley K., Huntley J.F., Nisbet A.J. Characterisation of tropomyosin and paramyosin as vaccine candidate molecules for the poultry red mite, Dermanyssus gallinae. Parasit. Vectors. 2016;12:544. doi: 10.1186/s13071-016-1831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.Y., Cheng L.Q., Zheng X.J., Wu J.X., Shang S.B., Wang J.Y., Chen J.G. Generation of the transgenic potato expressing full-length spike protein of infectious bronchitis virus. J. Biotechnol. 2004;111:121–130. doi: 10.1016/j.jbiotec.2004.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.