SUMMARY
Brain development requires correct targeting of multiple thousand synaptic terminals onto staggeringly complex dendritic arbors. The mechanisms by which input synapse numbers are matched to dendrite size, and by which synaptic inputs from different transmitter systems are correctly partitioned onto a postsynaptic arbor, are incompletely understood. By combining quantitative neuroanatomy with targeted genetic manipulation of synaptic input to an identified Drosophila neuron, we show that synaptic inputs of two different transmitter classes locally direct dendrite growth in a competitive manner. During development, the relative amounts of GABAergic and cholinergic synaptic drive shift dendrites between different input domains of one postsynaptic neuron without affecting total arbor size. Therefore, synaptic input locally directs dendrite growth, but intra-neuronal dendrite redistributions limit morphological variability, a phenomenon also described for cortical neurons. Mechanistically, this requires local dendritic Ca2+ influx through Dα7nAChRs or through LVA channels following GABAAR-mediated depolarizations.
In Brief
Ryglewski et al. report that competitive mechanisms are required to correctly partition synaptic inputs from different transmitter systems on the dendritic arbor of a Drosophila motoneuron. Perturbations cause dendrite shifts and result in defects of adaptive motor control.
INTRODUCTION
Dendrites are the highly branched processes of a neuron specialized for receiving synaptic input, and thus, dendritic architecture forms the blueprint for brain wiring (Fiala et al., 2008). During development, overall dendritic gestalt is determined by innate genetic factors (Montague and Friedlander, 1989; Grueber et al., 2003; Gao and Bogert, 2003; Puram and Bonni, 2013), and external molecular cues (McAllister, 2002; Polleux and Ghosh, 2008; Valnegri et al., 2015) guide growth into the correct neuropil regions. In addition, both overall dendritic growth rates and the fine tuning of high order branching often require activity-dependent control (Cline, 2001). However, the mechanisms by which the size of a postsynaptic dendrite is matched to the number of synaptic inputs, and by which synaptic inputs of different neurotransmitter classes are correctly partitioned onto a postsynaptic dendritic arbor, are incompletely understood.
One possible mechanism to match synapse number to postsynaptic dendrite length is to selectively stabilize branches that house functional synapses but retract other branches during dendritic arbor development. Local dendritic branch stabilization at sites of new synapses has been demonstrated in Xenopus tectal neurons (Niell et al., 2004) and in chicken retina (Lohmann et al., 2002). However, it is not clear to what extent local “synaptotropic” control shapes the architecture of a dendritic arbor in its entirety, how it operates in conjunction with global growth control, or whether synaptotropic growth control of postsynaptic dendrites is exerted in parallel by multiple different types of presynaptic partners.
We hypothesize that synaptic mechanisms shape dendritic fine branching during the partitioning of synaptic inputs of different transmitter classes onto one postsynaptic arbor. We test this hypothesis by combining genetic manipulation with quantitative three-dimensional dendritic architecture analysis of an identified Drosophila flight motoneuron, named MN5. MN5 shows a stereotypical overall dendritic morphology, but fine branching structure differs between animals (Vonhoff and Duch, 2010). The complex MN5 dendritic arbor with more than 6mmtotal length and more than 4,000 branches covers a diffuse motor neuropil with intermingled terminals of GABAergic and cholinergic synaptic terminals. We have previously shown that cholinergic inputs to the Dα7nAChR are targeted preferentially to the proximal dendritic domain of MN5, whereas GABAergic inputs to the Rdl GABAAR are targeted preferentially to the distal dendritic domain (Kuehn and Duch, 2013) (Figure 1).
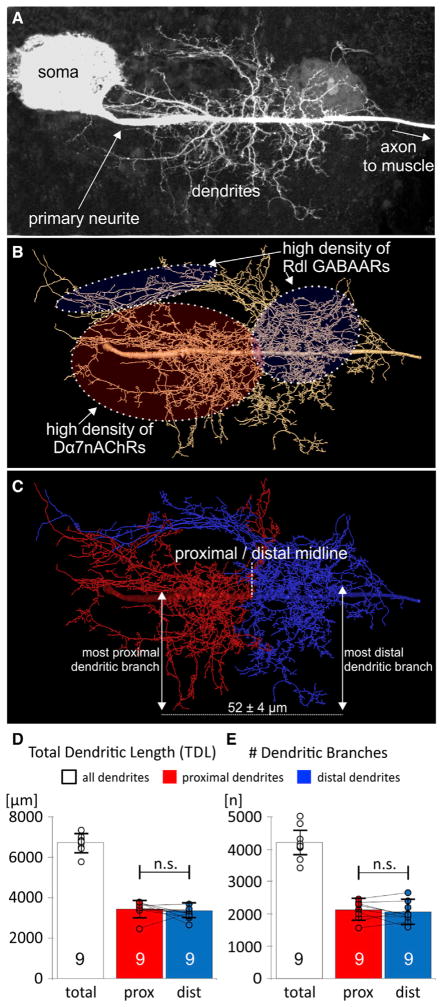
Figure 1. Equal Dendrite Distribution to the Proximal Cholinergic and Distal GABAergic Domain of the Identified Motoneuron 5.
(A) MN5 dendrite structure as maximum intensity projection view from a representative confocal image stack. MN5 is a unipolar neuron with a primary neurite arising from the soma. All dendritic sub-trees and the axon emerge from the primary neurite.
(B) Geometric dendrite reconstruction from the image stack shown in (A). Soma is omitted. Predominantly cholinergic (>75% of all inputs to the Dα7nAChR) and GABAergic (>75% of all inputs to the Rdl GABAAR) input domains are color coded and encircled by dotted lines. All branches with high densities of Dα7nAChRs (cholinergic input domain, red) arise from the proximal part of the primary neurite. All dendrites with high densities of GABAergic input synapses to the Rdl receptor (GABAergic input domain, blue) arise from the distal part of the primary neurite (Kuehn and Duch, 2013). Therefore, dendrites that originate from the proximal part of the primary neurite receive mostly cholinergic input whereas dendrites originating from the distal part of the primary neurite receive mostly GABAergic input.
(C) The distance between the most proximal cholinergic and the most distal GABAergic dendritic sub-tree is 52 ± 4 μm (mean and SD from 30 MN5 reconstructions). We defined the exact midpoint between these two dendritic sub-trees as the midline between the proximal cholinergic (red) and the distal GABAergic input domain (blue). This reproducibly divided all reconstructions into distal and proximal parts by the identical method without introducing bias. In all experiments, the midpoints were determined blindly without any knowledge of the experimental group.
(D and E) Quantification revealed equal dendritic length (D) and branch numbers (E) in the cholinergic (red) and in the GABAergic (blue) input domains of control MN5 (means and SD; p > 0.8; paired two-sided t test; circles show individual data points, lines connect data points from both input domains for each individual animal).
Our data indicate that dendrites are redistributed in a synaptic activity-dependent and competitive manner between cholinergic and GABAergic input domains while conserving total dendritic length. GABAergic and cholinergic inputs both cause local dendritic Ca2+ influx, which is required to successfully compete for a given amount of dendritic building material that is defined by global cues.
RESULTS
To quantitatively test whether inputs of multiple transmitter classes can direct postsynaptic dendrite arbor growth, we needed an identified central neuron that displays a stereotyped dendritic morphology between animals, contains different segregated synaptic input domains from presynaptic partners of different transmitter classes, and allows measuring dendritic length and branch numbers in control animals as well as following selective manipulation of synaptic input to different parts of its developing arbor. The identified adult flight motoneuron 5 (MN5) (Consoulas et al., 2002) of Drosophila melanogaster fulfills these requirements. First, MN5 displays a stereotyped dendritic morphology (Figure 1A) with metric and topological features that are sufficiently constant across animals to allow statistical comparison of measurements between control and genetic manipulation. MN5 contains a fixed number of 23 dendritic subtrees that all arise from the primary neurite, tile the input space, and, on average, comprise 4,000 ± 577 dendritic branches with a total length of 6,716 ± 731 μm (means ± SD) (Vonhoff and Duch, 2010). Second, MN5 receives synaptic input of different transmitter classes. The main excitatory input is cholinergic through the Dα7 nicotinic acetylcholine receptor (Dα7nAChR) (Fayyazuddin et al., 2006), whereas the main inhibitory input is GABAergic to the Rdl GABAA receptor. We have previously shown (Kuehn and Duch, 2013) that more than 75% of all cholinergic inputs to the Dα7nAChR are targeted to the proximal dendritic domain of MN5 (Figure 1B, dotted red oval), whereas more than 75% of all GABAergic inputs to the Rdl GABAAR are targeted to dendrites originating from the distal part of the primary neurite (distal domain; Figure 1B, dotted blue ovals). Third, genetic tools allow selective manipulation of synaptic input to the GABAergic or to the cholinergic domain of MN5.
Equal Distribution of Dendrites to the Cholinergic and GABAergic Input Domains
We used 30 geometric MN5 dendrite reconstructions (Schmitt et al., 2004; Evers et al., 2005) to quantify dendrites in the distal GABAergic and in the proximal cholinergic input domains. The average distance between the most proximal (in the cholinergic input domain) and the most distal (in the GABAergic input domain) dendritic sub-tree branching off the primary neurite is 52 ± 4 μm (mean ± SD, n = 30), thus highly stereotyped between animals. We used the midpoint of this distance as landmark to divide each geometric dendrite reconstruction into a proximal (Figure 1C, red, mainly cholinergic inputs) and a distal half (Figure 1C, blue, mainly GABAergic inputs). Quantification of MN5 dendrite reconstructions from nine different control animals demonstrated that, on average, the total dendritic length of 6,416 ± 423 μm (Figure 1D, white bar) was distributed to equal amounts to proximal (Figure 1D, red bar) and distal dendrites (Figure 1D, blue bar). Similarly, in controls half of the 3,998 ± 512 branches were proximal, the other half were distal dendrites (Figure 1E). Therefore, during normal development dendritic building material is assigned in equal proportions to the excitatory cholinergic (Figure 1C, red) and to the inhibitory GABAergic domain (Figure 1C, blue). However, although mean branch numbers and dendrite length were statistically identical in both input domains, in individual animals the relative sizes of both domains to each other showed some variation (Figures 1D and 1E, individual data points are depicted as circles, the lines connect proximal and distal length values of the respective neurons). This may indicate developmental plasticity in assigning dendrites to both input domains, but there were no hints for systematic size increases of one over the other input domain in controls. We next tested whether synaptic input to either dendritic domain could locally shape dendrite growth.
GABAergic and Cholinergic Synapses Compete for Dendritic Building Material
All MN5 dendrites (Ryglewski et al., 2014a) as well as cholinergic and GABAergic inputs form during pupal life (Kuehn and Duch, 2013). We employed targeted genetic manipulation to selectively alter cholinergic or GABAergic synaptic activity in different parts of the developing MN5 arbor. In order to increase cholinergic synaptic drive to the proximal dendritic domain, we overexpressed Dα7nAChRs as UAS transgene postsynaptically in MN5 under the control of P103.3-GAL4. Correct localization of UAS-Dα7nAChR has previously been confirmed by comparison with immunolabeling of native Dα7nAChRs (Kuehn and Duch, 2013). Therefore, Dα7nAChR overexpression increases receptor number but does not change localization predominantly to the proximal dendritic domain of MN5. Although we did not precisely quantify the amount of Dα7nAChR overexpression by immuno EM following expression under the control of P103.3-GAL4, we estimated an increase of 23% ± 11% of Dα7nAChR immuno-positive puncta in flight motoneuron dendrites on the light microscopy level (Figures S1A, S1B, S1D, and S1E). Co-labeling of native and GFP-tagged receptors revealed that expression of Dα7nAChR-GFP under the control of P103.3-GAL4 resulted in ~40% GFP-positive and 60% native receptors in flight motoneuron dendrites (Figures S1F and S1G).
The addition of ~20% of postsynaptic Dα7nAChRs caused a significant increase in the length of proximal dendrites (Figures 2A and 2D), i.e., the cholinergic dendritic domain. Importantly, total dendritic length was not affected by this manipulation. The reason for constant total dendrite length was that a 20% increase of proximal dendrites was accompanied by a 20% reduction of distal dendrites with mainly GABAergic inputs (Figure 2D). Consequently, the proximal cholinergic domain was on average 40% larger than the distal GABAergic domain (Figures 2D and 2F). The same was measured for the number of dendritic branches (Figure 2E). Importantly, all animals with increased Dα7nAChR expression showed increased branch numbers and dendrite length in the proximal cholinergic domain (Figures 2D and 2E). By contrast, controls showed no preferences for size increases of one over the other domain, despite some variation in the relative sizes of both dendritic domains (see Figures 1D and 1E). Therefore, increasing receptor availability for cholinergic input caused a significant redistribution of dendrites from the GABAergic to the cholinergic input domain. Branch order analysis revealed that this intra-neuronal dendrite shift affected branch orders higher than ten (see Figure S2).
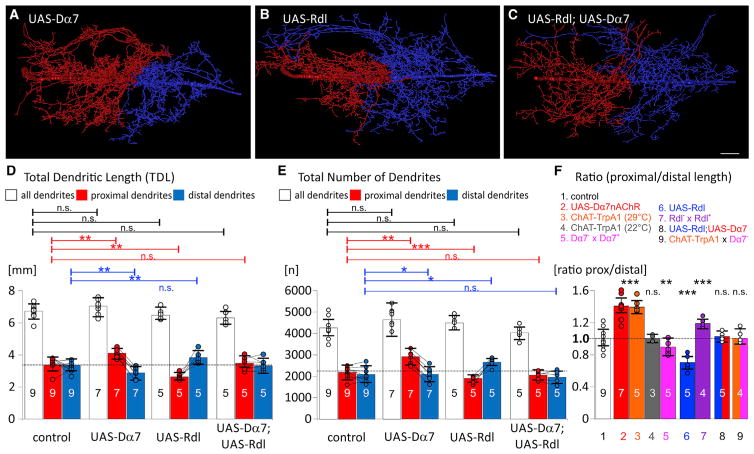
Figure 2. Competition for Dendrites by Cholinergic and GABAergic Synaptic Drive.
(A–C) Representative reconstructions color coded for proximally originating dendritic sub-trees with high densities of Dα7nAChRs (red) and distally originating dendrites with high densities of Rdl GABAARs (blue). The separation point for proximal and distal dendrites is defined as the midpoint between the origins of the most proximal cholinergic and the most distal GABAergic sub-tree (see Figure 1C). Increasing the availability of nAChRs by targeted overexpression of UAS-Dα7 (A) increased the quantity of proximal (red) in relation to (blue) distal dendrites. Vice versa, increasing the availability of GABAARs by targeted overexpression of UAS-Rdl (B) increased the quantity of distal (blue) in relation to proximal (red) dendrites. By contrast increasing the availability of both receptors (C; UAS-Rdl; UAS-Dα7) had no effect. Scale is 10 μm.
(D and E) Quantification of dendritic length (D) and the number of branches (E) revealed significant redistribution of dendrites from the distal GABAergic domain to the proximal cholinergic domain following increased availability of Dα7nAChRs, but total arbor length and branch numbers remained unchanged. Vice versa, significant redistribution of dendrites from the proximal cholinergic to the distal GABAergic domain were observed following increased availability of GABAARs, again total arbor size remained unchanged. No significant dendrite redistribution occurred upon increasing GABAARs and Dα7nAChRs.
(F) The ratio of dendrite length in the proximal cholinergic relative to the distal GABAergic domain was 1.02 ± 0.11 in controls (bar 1, white). Intra-neuronal dendrite redistribution from the distal GABAergic to the proximal cholinergic domain are depicted by a ratio significantly larger than 1.0 and were caused by overexpression of Dα7nAChRs (bar 2, red), increasing presynaptic cholinergic neuron activity by activation of TrpA1 channels (ChAT-TrpA1 at 29°C, bar 3, orange), and in heterozygous Rdl mutants (Rdl− × Rdl+, bar 7, purple). Redistribution from the proximal cholinergic to the distal GABAergic domain resulted in a ratio smaller than 1.0 and was caused by overexpression of Rdl receptors (UAS-Rdl, bar 6, blue), and in heterozygous Dα7nAChR mutants (Dα7− × Dα7+, bar 5, pink). No significant dendrite redistribution occurred in controls with TrpA1 channel activation (ChAT-TrpA1 at 22°C, bar 4, gray), following co-expression of Rdl and Dα7nAChRs (UAS-Rdl; UAS− Dα7, bar 8, blue/red), and with activation of presynaptic cholinergic neurons in heterozygous Dα7nAChR mutants (ChAT-TrpA1 ×Dα7−, bar 9, orange/pink). (D)–(F) show means and SDs. Circles depict individual data points, lines connect data points for cholinergic and GABAergic domains from the same animals. *p < 0.05; **p < 0.01; ***p < 0.001; n.s. p > 0.1, ANOVA with Newman-Keuls post-hoc test.
See also Figure S1 for an estimate of the amount of Dα7nAChR overexpression, Figure S2 for quantitative branch order analysis in controls and following Dα7 overexpression, and Figure S3 for increasing the magnitude of intra-neuronal dendrite shift by further increasing Dα7 receptor overexpression.
Vice versa, overexpression of Rdl GABAARs in MN5 caused the exact opposite effect: an increase in dendritic length and branch numbers in the distal GABAergic domain that came at the cost of a reduced arbor in the proximal cholinergic domain. All individuals tested showed increased dendritic length (Figures 2B and 2D) and branch numbers (Figure 2E) in the distal GABAergic domain. Again, neither total dendritic length nor the total number of branches were changed (Figures 2D and 2E). With respect to the amount of Rdl overexpression, we yielded similar estimates for increased receptor density as described above for overexpression of Dα7nAChR (data not shown). Therefore, an increase of either Rdl GABAA or Dα7nAChR density by ~20% caused an intra-neuronal shift of ~20% of dendrites away from the input domain with only native receptors.
Additional genetic manipulations further supported these findings. First, reducing the amount of available Dα7nAChRs in heterozygous Dα7 mutants caused a redistribution of dendrites to the GABAergic domain (Figure 2F). We did not use Dα7 homozygous null mutants, because MN5 total dendritic length is significantly reduced in these animals (Vonhoff et al., 2013). Second, a reduction of available GABAA receptors in heterozygous Rdl mutants caused a shift of dendrites to the cholinergic domain (Figure 2F). Third, redistribution of dendrites to the cholinergic input domain was induced not only by overexpression of receptors but also by activating presynaptic cholinergic neurons during pupal life by temperature shifts to 29°C following expression of TrpA1 channels under the control of ChAT-GAL4 (Figure 2F). And finally, no dendrite redistribution was observed following TrpA1-activation of presynaptic cholinergic neurons in a Dα7 heterozygous mutant background (Figure 2F). Therefore, intra-neuronal dendrite redistribution could either be induced by manipulation of presynaptic activity or by changing postsynaptic receptor availability. These manipulations altered neither total dendritic length nor the total number of branches through the dendritic tree. Furthermore, our data indicate that GABAergic and cholinergic inputs locally direct dendrite growth in a competitive manner, because overexpression of both Dα7nAChRs and Rdl GABAARs had no effect (Figures 2C–2F).
Upon supplying an estimated 20% of extra postsynaptic neurotransmitter receptors, we observed an average 20% intra-neuronal dendrite shift to the domain with increased receptor density. At least for Dα7nAChRs, we have indications that this effect can be enhanced by further increasing receptor density. Expression of UAS-Dα7Rs under the control of the stronger motoneuron driver, D42-GAL4, yielded an estimated 40% increase over control of receptor density in flight motoneuron dendrites (Figures S1C–S1E). Expression was restricted mainly to flight motoneurons by inclusion of ChAT-GAL80 to inhibit GAL4 in all cholinergic interneurons (Boerner and Duch, 2010). Increasing Dα7nAChR density in the cholinergic input domain by ~40% caused a significantly stronger dendrite shift to that domain as compared to an estimated 20% of Dα7nAChR density (Figure S3). In controls, the proximal to distal size ratio is on average 1.02 ± 0.11. Increasing Dα7nAChR density by ~20% yields an average proximal to distal domain size ratio of 1.39 ± 0.10, and increasing Dα7nAChR density by ~40% yields a ratio of 1.81 ± 0.19 (Figure S3D). This indicated that cholinergic synaptic input might locally direct dendrite growth in a dose-dependent manner. Although both drivers express from pupal stage P5 to adulthood, expression in cholinergic interneurons was inhibited, and expression was restricted mainly to motoneurons, we cannot exclude the possibility that some indirect effects may have been caused by spatial or temporal differences in Dα7nAChR expression in both sets of experiments. Although dendrites may contain output synapses, we judge indirect effects via feedback into the flight circuit upon receptor expression in motoneurons unlikely. In general, central output synapses are rare in insect motoneurons, and we have not found presynaptic specializations in Drosophila wing motoneuron dendrites by expression of fluorescently tagged proteins, which reliably label presynaptic specializations in the axon terminals (not shown). However, without a rigorous electron microscopic study we cannot completely exclude the possibility of some output synapses from these dendrites.
We next addressed part of the mechanism by which cholinergic and GABAergic synaptic inputs compete for postsynaptic dendrites, to then test functional consequences for motoneuron firing patterns during behavior.
Cholinergic Synaptic Transmission Induces Locally Restricted Dendritic Ca2+ Signals
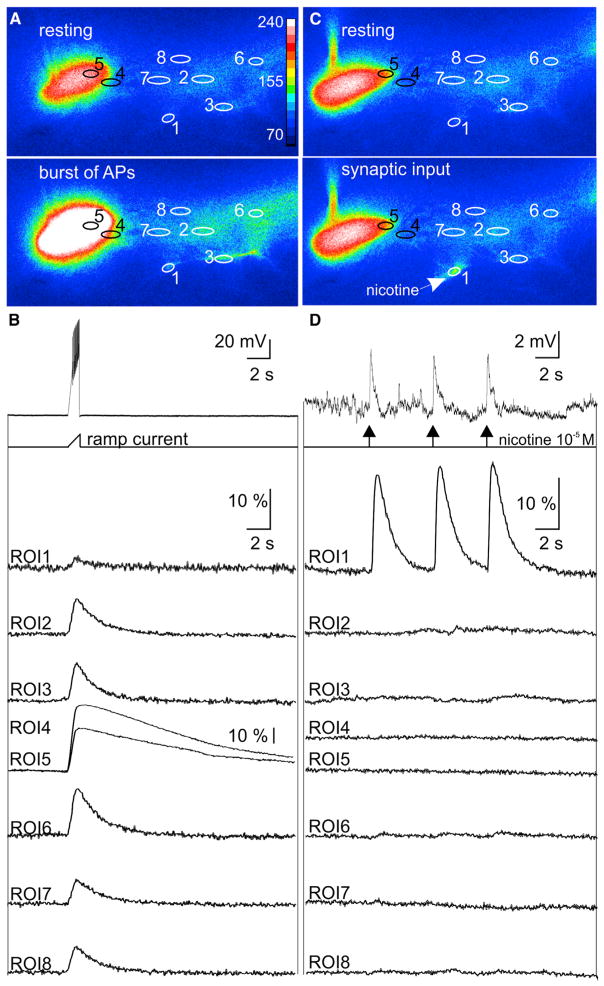
Since we have previously reported that increases in MN5 firing activity enhance dendrite growth via transcriptional mechanisms (Duch et al., 2008; Vonhoff et al., 2013), at first it seemed counterintuitive that excitatory cholinergic synaptic transmission caused dendrite redistribution without affecting total dendrite length. One possible explanation could be that cholinergic synaptic transmission as occurring during normal pupal life causes dendrite shifts via locally restricted intracellular signaling without affecting transcriptional mechanisms. In developing chicken retinal ganglion cells, cholinergic synaptic transmission can evoke local Ca2+ signals that are restricted to individual dendritic segments and are required for new dendritic branch stabilization. By contrast, retinal ganglion cell firing causes global Ca2+ signals through all arbors and these have no effect on local dendrite growth and retraction (Lohmann et al., 2002). To test whether similar local and global Ca2+-dependent mechanisms also existed in Drosophila motoneurons, we conducted simultaneous somatic current clamp and Ca2+ imaging recordings from MN5 (Figure 3). Bursts of action potentials as caused by somatic ramp current injections evoked Ca2+ influx into the soma and all dendritic arbors (Figures 3A and 3B, n = 16; Movie S1). By contrast, if brief pulses (5 ms) of local pressure application of the cholinergic agonist nicotine (10−5 M) were focally targeted to individual dendritic segments, they caused locally restricted Ca2+ signals in that dendritic branch along with depolarizing postsynaptic potentials of large enough amplitude to be reliably monitored by somatic recordings (Figures 3C and 3D; Movie S2). All 37 different regions within the cholinergic input domain that were tested in 25 different animals displayed local dendritic Ca2+ signals upon focal nicotine application. Focal nicotine application to two different dendritic branches within the cholinergic input domain further supported the finding that cholinergic synaptic transmission caused local dendritic Ca2+ responses that are independent of each other and do not affect somatic Ca2+ signaling (Figure S4; Movies S3 and S4). Therefore, as in chicken retinal ganglion cells, focally restricted cholinergic synaptic inputs can cause local dendritic Ca2+ signals that are distinct from global spiking-dependent Ca2+ elevations and Ca2+-dependent transcriptional mechanisms in the soma.
Figure 3. Global versus Local Dendritic Ca2+ Signals.
(A) In situ Ca2+ imaging of MN5 with targeted expression of GCaMP6s reveals global Ca2+ signals during a train of action potentials as evoked by somatic ramp current injection. Upper image is at rest and lower image during a train of action potentials as induced by somatic ramp current injection (original images overlaid as false color code for gray values from 70 to 240). Eight regions of interest (ROIs) for ΔF/F measurement are depicted by numbered ovals.
(B) Simultaneous somatic current clamp (upper trace) and Ca2+ imaging recordings from all eight ROIs before, during, and after a train of action potentials reveals global increases in ΔF/F. Note different scale for ROIs 4 and 5 (soma and primary neurite), which displayed the largest amplitude signals upon firing.
(C) In situ Ca2+ imaging of the same MN5 with identical ROIs as in (A) at rest (upper image) and during a brief (5 ms) puff of nicotine (10−5 M) onto a distal dendrite (lower image).
(D) Simultaneous somatic current clamp (upper trace) and Ca2+ imaging recordings from all eight ROIs before, during, and after three consecutive nicotine puffs to ROI1. Nicotinic AChR activation in ROI1 caused locally restricted dendritic Ca2+ signals.
See also original data movies for global (Movie S1) and local (Movie S2) dendritic signals as well as Figure S4 and Movies S3 and S4 for local Ca2+ signals at different dendritic sites.
If synaptically evoked dendritic Ca2+ signals were mechanistically required to locally direct dendrite growth during development, synaptic transmission needed to occur at early pupal stages during motoneuron dendrite growth. We have previously shown that Dα7nAChRs are expressed from early stages of MN5 dendritic growth in Vonhoff et al. (2013). In the present study, we now provide in situ evidence that cholinergic inputs to the Dα7nAChR are functional already at the onset of MN5 dendrite growth (Figures S5A–S5C). Therefore, moderate network activity during pupal life may cause local dendritic Ca2+ signals. In contrast to the work in retinal ganglion cells (Lohmann et al., 2002), we did not directly test by live imaging during dendrite growth whether such locally restricted Ca2+ signals were required for local dendritic branch stabilization. However, we provide indirect evidence for a critical requirement of local dendritic Ca2+ signals in intra-neuronal dendrite shifts (see below).
GABAergic Synaptic Transmission Causes Locally Restricted Dendritic Ca2+ Signals
GABAergic input synapses to the Rdl GABAAR are formed simultaneously with cholinergic synapses to the Dα7nAChRs during early pupal stages of MN5 dendrite growth (Kuehn, 2012) (Figures S5A–S5C). If GABAergic and cholinergic synaptic inputs competed for a given amount of available dendrites via locally restricted Ca2+-dependent mechanisms, one has to postulate that activation of the Rdl GABAARs also causes local dendritic Ca2+ influx. Paradoxically, in insect neurons, including adult Drosophila (Ryglewski et al., 2014b), chloride influx through GABAARs is inhibitory and hyperpolarizing relative to resting. Therefore, at first, local dendritic Ca2+ influx did not seem reconcilable with Rdl receptor activation. By contrast, during vertebrate embryonic development GABAARs are excitatory during early developmental stages because of elevated intracellular chloride levels, and GABA becomes inhibitory during later stages when a chloride exporter becomes expressed (Ben-Ari, 2002). If a similar developmental shift in chloride reversal potential occurred during Drosophila pupal life, Rdl receptor activation could also cause local dendritic Ca2+ influx through voltage-gated Ca2+ channels, but chloride shifts have not been reported during invertebrate CNS development.
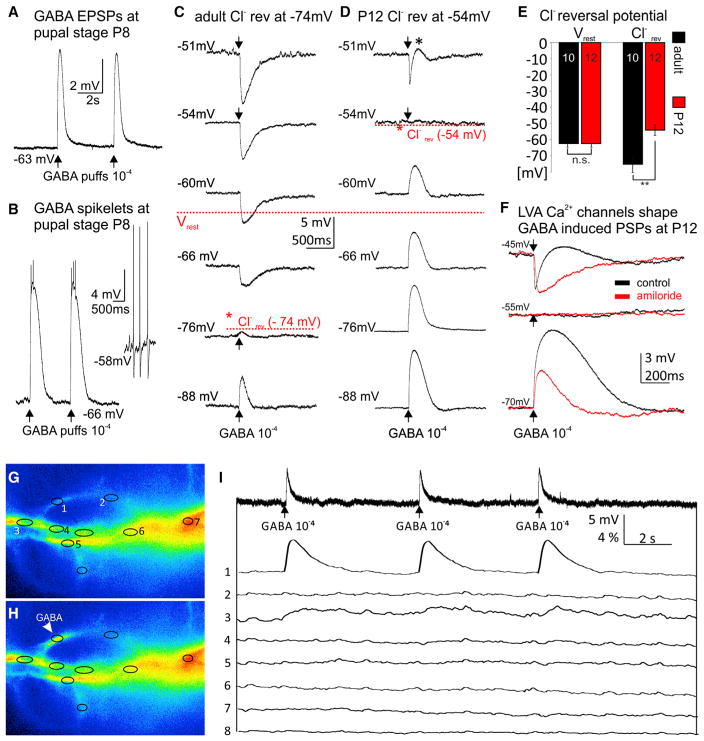
To test this possibility, we paired sharp electrode recordings, thus not directly interfering with the intracellular chloride concentration (see the STAR Methods), with focal pressure application of GABA to individual dendritic segments with a second pipette. Indeed, during pupal life (stages P6–P13, n = 32), Rdl receptor activation caused depolarizations relative to resting (Figure 4A). In some instances, and depending on the dendritic location, GABA puffs even induced spikelets riding on top of large amplitude GABA-induced depolarizations (Figure 4B) and were different from spontaneously occurring action potentials as recorded from the soma (Figure 4B, inset). GABA-induced depolarizations were likely depending on chloride efflux because they were fully blocked by bath application of the chloride channel blocker picrotoxin (10−4 M; Figures S5D–S5F). This was consistent with a developmental shift in chloride reversal potential. In adult motoneurons, GABA was inhibitory because chloride reversal was at −74 mV (Figures 4C and 4E), whereas during pupal life, chloride reversal was significantly more depolarized at −54 mV (Figures 4D and 4E). By contrast, resting membrane potential was not altered during development (Figures 4C–4E). Consequently, GABA caused depolarizations relative to resting during pupal life.
Figure 4. GABA Induces Depolarization, LVA Ca2+ Channel Activation, and Dendritic Ca2+ Signals during Immature Stages.
(A) During pupal life somatic current clamp recordings revealed depolarizations upon brief (5 ms) puffs of GABA (10−4 M) onto dendrites.
(B) For some high order branches within the GABAergic domain GABA puffs caused large amplitude depolarizations with spikelets. GABA-induced spikelets displayed distinctly different amplitudes and shapes as compared to spontaneously occurring action potentials as recorded from the soma (see inset).
(C and D) Sharp electrode recordings were used to determine the chloride reversal potential in adult (C) and pupal (D) MN5. (C) Representative responses to GABA puffs at differentmembranepotentials revealeda chloride reversal potential of −74mV for the adult flight MN5, more than 10m V more hyperpolarized than the average resting membrane potential (−63 mV). (D) By contrast, at P12 the chloride reversal was at −54 mV, about 10 mV more depolarized than resting membrane potential.
(E) Quantification revealed that resting membrane potential remained unchanged through development (−63 ± 2 mV), but chloride reversal was significantly shifted from values more positive than resting during pupal life (−54 ± 3 mV) to values more negative than resting in mature stages (−74 ± 4 mV).
(F) GABA-induced depolarizations are amplified by Ca2+ influx through LVA channels. Sharp electrode recording from a representative pupal stage P12 MN5 held at −70 mV displayed a large depolarizing response to dendritic GABA puffs (lower traces, black). Pharmacological blockade of LVA DmαG Ca2+ channels by amiloride (10−3 M) significantly reduced amplitude and duration of this GABA response (lower traces, red). No response to GABA puff was observed when holding the cell at the chloride reversal potential (middle traces). A biphasic response, hyperpolarization followed by depolarization, was observed when holding membrane potential at −45 mV (upper traces, black). Blockade of LVA channels isolated the GABA-induced hyperpolarizing response (upper traces, red).
(G–I) During pupal life GABA puffs (10−4 M) cause somatic depolarizations but local dendritic Ca2+ signals at the site of GABAAR activation. Ca2+ imaging of parts of MN5 dendrites before (G) and after (H) GABA puff to ROI 1. (I) Simultaneous somatic current clamp recording (upper trace) and Ca2+ imaging from ROIs 1–8 as defined in (G) and (H) in response to three GABA puffs to ROI 1 (for signal spread, see Movie S5).
See also Figures S5D–S5F for pharmacological evidence that GABA-mediated depolarizations required chloride channels. Local dendritic Ca2+ responses to GABA puffs at different dendritic sites are shown in Figure S6 and Movie S6.
Note that at pupal stages the time course for GABA-mediated depolarizations (Figure 4D, lower four traces) differed markedly from GABA-induced hyperpolarizations at membrane potentials more depolarized than the GABA reversal potential (Figure 4D, top trace). GABA-induced hyperpolarizations at depolarized membrane potentials appeared much sharper than GABA-induced depolarizations at normal or hyperpolarized membrane potentials (Figure 4D). A possible explanation for different time courses in the responses to GABA could be activation and de-inactivation of low voltage activated transient (LVA) Ca2+ currents, which we have previously described in this neuron (Ryglewski et al., 2012). At membrane potentials more depolarized than chloride reversal (e.g., −51 mV; Figure 4D, top trace), LVA Ca2+ currents are voltage inactivated. Therefore, GABAAR activation caused a hyperpolarization that may have de-inactivated LVA channels, and thus, caused an inward Ca2+ current, which in turn, opposed the GABA-induced hyperpolarization and sharpened the time course of the inhibitory postsynaptic potential (IPSP). Depending on the dendritic location, LVA channel de-inactivation outlasted GABAAR opening, which gave rise to a biphasic response, a sharp GABA-induced hyperpolarization followed by a transient depolarization (Figure 4D, top trace, asterisk). By contrast, at −70 mV and at more hyperpolarized membrane potentials, LVA channels were closed but ready for activation. Therefore, it seems likely that at negative membrane potentials, LVA channel activation added to GABA-induced depolarization and prolonged it.
Pharmacological blockade of LVA channels provide additional support for this interpretation (Figure 4F).DmαGchannels underlie pupal LVA Ca2+ currents in MN5 and can be blocked with amiloride (10−3 M) (Ryglewski et al., 2012). Blockade of LVA Ca2+ channels abolished the biphasic responses to GABAAR activation at pupal stages and isolated the hyperpolarization as caused by chloride flux through Rdl receptors (Figure 4F, top traces). Furthermore, pharmacological block of LVA channels resulted in similar time courses for depolarizing and hyperpolarizing PSPs as induced by GABA at positive and negative potentials (Figure 4F). Note that depolarizations as induced by GABA puffs to pupal MN5 dendrites were significantly reduced in amplitude and duration by bath application of amiloride (Figure 4F, lower traces). Therefore, the most likely explanation for the different time courses of GABA-induced depolarizing and hyperpolarizing PSPs in pupal motoneurons is that depolarizations as induced by chloride efflux through Rdl receptors caused opening of DmαG channels, and thus dendritic Ca2+ influx. However, possible additional roles of other GABARs cannot be excluded.
Taken together, our data indicate that a developmental regulation of the chloride reversal potential might not be a unique feature of vertebrates but evolutionarily more conserved than previously known. Consequently, GABAARs cause depolarizations during pupal life when MN5 dendrites form. These depolarizations are sufficient to open dendritic LVA T-type Ca2+ channels, which in turn, cause dendritic Ca2+ influx upon GABAergic synaptic transmission. Therefore, we hypothesized that, similar to focally restricted cholinergic synaptic inputs (see Figure 3), GABAergic inputs may also cause local dendritic Ca2+ signals that are distinct from global spiking-dependent Ca2+ elevations and Ca2+-dependent transcriptional mechanisms in the soma.
Paired somatic electrode and dendritic Ca2+ imaging recordings revealed that depolarizations as induced by focal dendritic GABA application also caused locally restricted dendritic Ca2+ elevations (Figures 4G–4I, compare regions of interest [ROIs] 1–8; Movie S5). Local postsynaptic Ca2+ signals remained in TTX, thus indirect effects via de-inhibition were unlikely. In contrast to local cholinergic Ca2+ signals, local GABA-induced signals were not observed for all dendritic locations tested, they were less reliable upon repeated GABA application, and the spread of GABA-induced Ca2+ signals to neighboring dendritic branches was generally broader than following nAChR activation. This was in agreement with a requirement for co-localization of dendritic Rdl receptors with transient LVA Ca2+ channels (Figure 4F). However, GABA-induced dendritic Ca2+ signals were local in the sense that they never spread through all dendrites, along the primary neurite, or to the soma. To further support this, we simultaneously puffed GABA to two different dendritic locations within the GABAergic input domain. This showed that GABA-induced dendritic Ca2+ signals at different locations remained entirely separated (Figure S6; Movie S6).
GABA-Induced Intra-neuronal Dendritic Wire Shifts Require T-type Channels
Local dendritic Ca2+ signals have been reported to mediate dendritic branch stabilization upon cholinergic synaptic transmission to developing retinal ganglion cells (Lohmann et al., 2002). We observed local dendritic Ca2+ signals upon cholinergic (Figure 3) and upon GABAergic synaptic input to developing Drosophila motoneuron dendrites (Figure 4). Therefore, cholinergic and GABAergic synapses could potentially compete for postsynaptic partner dendrites via local Ca2+ influx and selected branch stabilization. Because GABA-mediated Ca2+ signals required DmαG LVA channels (Figure 4F), we hypothesized that no dendrite redistribution to the GABAergic domain would occur in DmαG mutants. We have previously generated DmαG excision mutants that are homozygous-viable (Ryglewski et al., 2012). Indeed, overexpression of Rdl receptors in MN5 caused a dendrite shift toward the distal GABAergic domain in the presence of DmαG LVA channels (Figures 2, 5A, and 5C), but not in DmαG mutants (Figures 5B and 5C). Moreover, in the absence of DmαG, there was always a small but significant dendrite shift to the cholinergic domain (Figure 5C). A possible explanation is that local dendritic Ca2+ signals were strongly reduced in the distal domain with predominantly GABAergic inputs, but not from the predominantly proximal cholinergic domain, because the Dα7nAChR itself conducts Ca2+ upon opening (Séguéla et al., 1993).
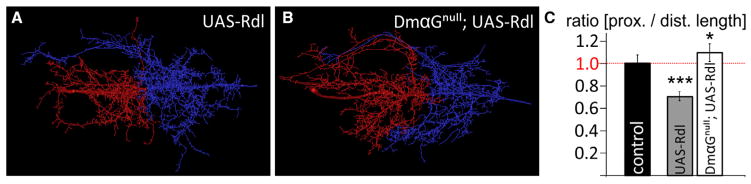
Figure 5. GABA-Induced LVA Channel Activation Is Required for Dendrite Redistribution.
(A and B) Representative MN5 dendrite reconstructions with color-coded proximal cholinergic (red) and distal GABAergic dendritic domain (blue) following overexpression of Rdl GABAARs (UAS-Rdl) in a control background (A) and in a DmαG LVA Ca2+ channel null mutant background (B, DmαGnull;UAS-Rdl).
(C) Quantification revealed a statistically significant dendrite shift from the proximal to the distal domain upon overexpression of Rdl GABAARs in a control background. No dendrite shift to the distal domain was observed upon overexpression of Rdl GABAARs in a DmαG null mutant background. By contrast, lack of LVA Ca2+ channels caused a slight but significant shift to the proximal cholinergic domain.
Functional Consequences
Overexpression of Rdl GABAARs or Dα7nAChRs and dendrite shift did not cause any obvious motor defects in tethered flight (Figure 6A). To directly address flight motoneuron and wing power output, we recorded MN5 extracellularly with sharpened tungsten electrodes during flight and simultaneously measured wing beat frequencies (Figures 6A and 6B). Neither mean motoneuron firing rates nor wingbeat frequencies were affected (Figures 6C and 6D), and animals showed normal flight durations in response to wind stimuli to the head (Figure 6E). Therefore, no significant consequences were observed with regard to basic motor performance. By contrast, the relation of motoneuron firing and wing beat frequencies was affected (Figure 6F). Linear correlations between motoneuron firing rates, wing beat frequencies, and wing power output have previously been shown in Drosophila asynchronous wing depressor muscle (Gordon and Dickinson, 2006). We confirmed a significant positive correlation of wing beat and motoneuron firing rates in controls and following overexpression of both receptors (Figure 6F), but intra-neuronal dendrite shifts as induced by overexpression of either GABAARs or nAChRs impaired this correlation (Figure 6F). Based on this, we hypothesized impairments in the fine control of motoneuron firing rates and measured responses to visual input during flight.
Figure 6. Intra-neuronal Dendrite Shifts Impair Adaptive Adjustments of Firing Rates.
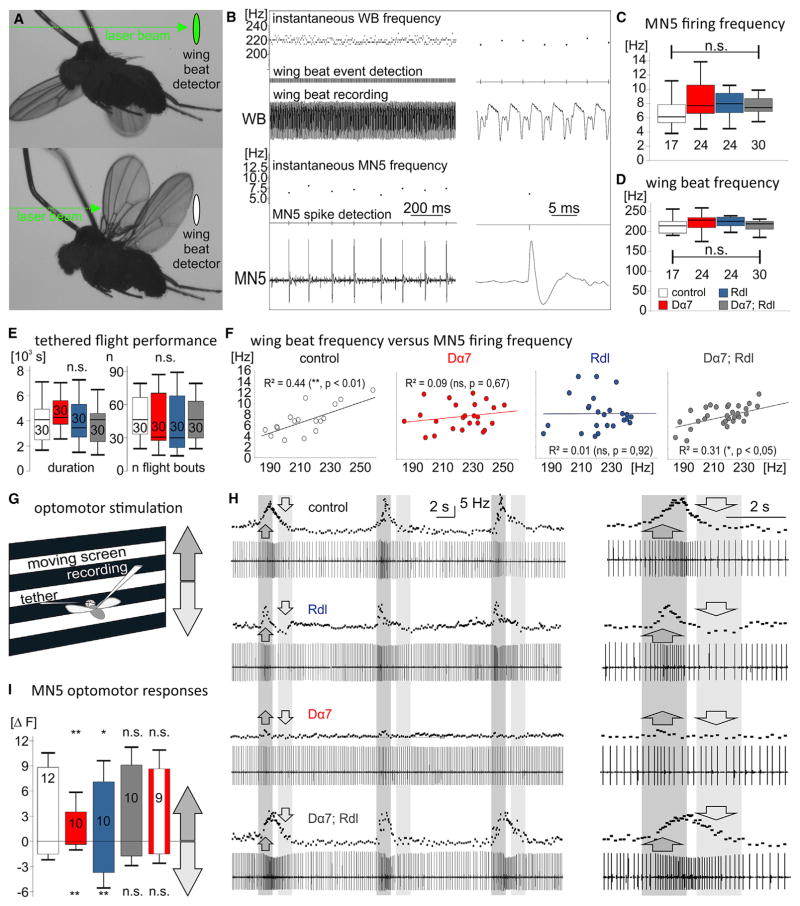
(A) Wing beat detection by laser beam during tethered flight.
(B) Simultaneous recording of MN5 from its target flight muscle fiber (lower trace) and wing beat (WB) recording during tethered flight. Threshold-based event detection and instantaneous frequencies are depicted above the original MN5 and WB recordings.
(C and D) No statistical differences were detected in mean MN5 firing frequencies (C) or mean wing beat frequencies (D) during tethered flight in controls (white bars), following overexpression of Dα7nAChRs (red bars), Rdl GABAARs (blue bars), or both (gray bars). (legend continued on next page)
(E) Tethered flight durations were not statistically different between receptor overexpression with intra-neuronal dendrite shift and controls. Boxplots in (C)–(E) depict medians, quartiles, and 10 and 90 percentiles (n.s., p > 0.1, Kruskal-Wallis ANOVA).
(F) In controls (left) and following overexpression of both Dα7nAChRs and Rdl GABAARs (right) wing beat frequencies correlated significantly with motoneuron firing rates (R2, Spearman’s rank correlation coefficient). No correlation was detected following overexpression of either Rdl GABAARs or Dα7nAChRs alone (middle).
(G) Optomotor modulation of MN5 firing rates were elicited by using a visual grating pattern that moved either up or down.
(H) Upward and downward movement simulated decreasing or increasing flight altitude and resulted in compensatory adjustments of MN5 firing frequencies in controls (upper trace) and following overexpression of both Rdl GABAARs and Dα7nAChRs (lower trace). Following overexpression of Rdl GABAARs or Dα7nAChRs and intra-neuronal dendrite shift to the respective dendritic domain, adjustments of frequencies in response to visual stimulation were significantly altered (second and third trace from top).
(I) Quantification showed that in controls (white bar), following overexpression of both receptors (gray bar), and with conditional expression of Dα7nAChRs in the adult stage only (red and white bar), upward movement caused firing rate increases by ~9 Hz (upward bars, means ± SD) whereas downward movement caused firing rate decreases by ~2 Hz (downward bars, means ± SD). Following increased Rdl GABAARs and dendrite shifts to the GABAergic domain, upregulation of firing rate was slightly but significantly smaller (blue bar), and downregulation of firing rate was significantly increased (blue bar). Following increased Dα7nAChRs and dendrite shifts to the cholinergic domain upregulation of firing rates was significantly reduced by ~65% and downregulation was significantly impaired (red bar, ANOVA, Newman-Keuls post hoc test, p ≤ 0.05).
During tethered flight in front of a screen with horizontal stripe patterns (Figure 6G), animals respond with modulation of flight motoneuron firing rates to up- and downward movement of the patterns. Upward movement causes the visual impression of losing flight altitude, and firing frequencies are increased (Figures 6H and 6I). Downward movement causes the impression of increasing flight altitude, and firing rates are decreased (Figures 6G–6I). Following overexpression of Rdl GABAARs and dendrite shifts to the distal dendritic domain, decreases of motoneuron firing in response to the respective optomotor input were significantly stronger (Figures 6H and 6I). Vice versa, increasing motoneuron firing and power output in response to the opposite optomotor stimulus was slightly, but statistically significantly, decreased. Note that with increased GABAergic input, optomotor adjustments of firing rates were still possible at the normal net range, but increases in firing rates were significantly smaller whereas decreases were significantly larger than in controls (Figure 6I). By contrast, upon overexpression of Da7nAChRs and dendrite shift to the proximal cholinergic domain, increases in motoneuron firing rates were approximately three times smaller, and decreases in power output in response to the respective optomotor stimulus were diminished (Figures 6H and 6I).
In an attempt to distinguish between the functional consequences of receptor overexpression and intra-neuronal dendrite shifts, we also overexpressed receptors conditionally only in the adult stage after inhibition of expression through pupal development with the temperature-sensitive GAL4 inhibitor GAL80. Conditional receptor expression in adult flight motoneurons was confirmed by immunocytochemistry, caused no dendrite shifts, and had no effects on optomotor adjustments of motoneuron firing frequencies in our paradigm (Figure 6I). Therefore, functional impairments of adaptive adjustments of motoneuron firing rates in response to visual input were a consequence of receptor overexpression and subsequent dendrite shift, but likely not of receptor overexpression alone (see the Discussion).
DISCUSSION
Local Competition for Globally Controlled Total Amounts of Dendrite
Our results are consistent with competition of GABAergic and cholinergic inputs for a given amount of dendrites. We propose that total dendrite size is determined by global cues, such as the transcriptional identity of the neuron, hormone signals, and overall firing activity, all of which regulate dendritic length and branch numbers in many types of neurons (Wong and Ghosh, 2002; Redmond et al., 2002; Libersat and Duch, 2004; Redmond, 2008; Puram and Bonni, 2013). Manipulations that increase MN5 firing activity cause dendrite overgrowth via activation of CaMKII− and AP1-dependent transcription (Vonhoff et al., 2013). MN5 firing causes global Ca2+ influx into the soma and the dendrites (Figure 3A). Conversely, knockdown of the Drosophila Cav2 homolog cacophony reduces somatic and dendritic Ca2+ influx upon firing and decreases dendrite arbor size (Ryglewski et al., 2014a). Therefore, overall MN5 firing activity and global Ca2+ influx regulate total dendrite length, likely together with steroid hormones (Weeks and Levine, 1995). We now show that the total amount of available dendrite becomes locally redistributed within the arbor by competitive synaptic mechanisms. A mechanistic separation between global overall growth and local fine branching control might enable the formation and maintenance of dendritic branches at sites of newly formed input synapses while preventing dendrite overgrowth, thus enabling opportunistic functional connections with minimal dendrites.
GABAergic and Cholinergic Input Synapses Simultaneously Direct Fine Branching of the Same Postsynaptic Arbor
Competitive interactions between GABAergic and cholinergic inputs may optimize dendritic gestalt to partition synaptic inputs from different transmitter systems to segregated postsynaptic input domains. Whenever synaptic input to the GABAergic or to the cholinergic domain is increased, dendrites shift toward this domain and away from the other one. Vice versa decreased input to one domain causes dendrite shift to the other domain. Increasing input to both domains has no effect, thus indicating synaptic activity-dependent competition. Although regulation of dendrite growth by synaptic activity has previously been reported for excitatory cholinergic (Lohmann et al., 2002) and glutamatergic (Niell et al., 2004), as well as excitatory (Cancedda et al., 2007; Chen and Kriegstein, 2015) and inhibitory GABAergic (Shen et al., 2009) synapses, intra-neuronal dendrite shifts that reshape the entire tree have not been demonstrated.
However, it must be noted that not all neurons contain distinctly different input domains, but some receive intermingled inputs of different transmitter classes. In addition, our analysis focused on the most abundant excitatory and inhibitory inputs to MN5, which segregate to different input domains (Kuehn and Duch, 2013). MN5 likely receives also glutamatergic inputs (S.R., unpublished data) and potentially also aminergic modulatory inputs. It remains unclear whether such input classes are also targeted to specific input domains, or whether these also compete for dendrites. Nonetheless, our findings are likely of general interest, because segregated input domains for different transmitter classes also exist in mammalian pyramidal neurons (Ishizuka et al., 1990; Di Cristo et al., 2004). Although the underlying mechanisms have not been addressed in the mammalian brain, size reductions in pyramidal neuron apical dendrites are accompanied by size increases of the basal dendrites and vice versa. Moreover, the relative sizes of pyramidal neuron dendritic sub-trees are counterbalanced and stabilize total dendritic length (Samsonovich and Ascoli, 2006). Similarly, for the Drosophila MN5, we have previously reported high size variability of individual dendritic sub-trees while counterbalance between sub-tree sizes keeps total dendrite size variability low (Vonhoff and Duch, 2010). In this study, we provide evidence that competitive interactions between different synaptic inputs locally direct arbor growth in different input domains. This may be a general means to fine tune the gestalt of neurons with segregated input domains. Our data on an identified Drosophila motoneuron may provide an entry point into understanding the mechanism by which presynaptic partners may instruct the redistribution of postsynaptic dendrites between different input domains in a “synaptotropic” manner (see below).
Local Dendritic Ca2+ Signals Are Required for Intra-neuronal Dendrite Shift
Although the mechanisms by which competition between GABAergic and cholinergic inputs locally directs dendrite growth require further studies, our data indicate that both competitors employ the same intracellular signaling mechanism. In retinal ganglion cells, newly formed dendritic branches become selectively stabilized at sites of cholinergic input synapses from amacrine cells, and strictly local dendritic Ca2+ signals are required for this process (Lohmann et al., 2002). We also find local dendritic Ca2+ signals at sites of cholinergic and at sites of GABAergic input, although we have no direct live imaging evidence of local branch stabilization by these signals. However, any manipulation, be it presynaptic or postsynaptic, that affects synaptic input to either the cholinergic or the GABAergic domain changes arbor size in that domain. Local dendritic Ca2+ signals at cholinergic synapses are expected, because the Dα7nAChR conducts Ca2+ (Séguéla et al., 1993). By contrast, the Rdl GABAAR is a chloride channel (Grolleau and Sattelle, 2000). During vertebrate embryonic development, GABAAR activation is initially excitatory because of elevated intracellular chloride concentrations (Ben-Ari, 2002). We find that such a developmental shift of the chloride reversal potential to more negative values at mature stages likely also exists in insect neurons and thus, may be more conserved than previously known.
As a result, GABAAR activation during Drosophila pupal stages causes depolarization, which in turn, induces local dendritic Ca2+ influx through DmαG LVA Ca2+ channels. LVA channels are required for GABA-mediated dendrite redistribution, because GABAergic input fails to direct dendrites to the GABAergic domain in DmαG null mutants. Our interpretation is consistent with a recent study demonstrating that depolarizing GABAergic input selectively promotes apical dendrite growth via Ca2+ influx during neonatal cortical neuron development (Chen and Kriegstein, 2015). Therefore, directing dendrite arbor growth via GABA-mediated Ca2+ signals takes place in flies and mammals.
Functional Consequences of Intra-neuronal Dendrite Shifts
In agreement with findings that basic flight motoneuron function is retained even with a minimal set of dendrites (Ryglewski et al., 2014b), intra-neuronal dendrite shifts as caused by either Rdl or nAChR overexpression did not alter firing rates or wingbeat frequencies during tethered flight. By contrast, adaptive adjustments of motoneuron firing frequencies in response to visual input during flight behavior were impaired. The defect was more severe with increased excitation and dendrite shifts to the cholinergic domain than with increased inhibition and dendrite shifts to the GABAergic domain. By contrast, motoneuron firing frequency adjustments were not affected following conditional receptor expression in mature neurons that caused no dendrite shifts. This suggested that the impairment was at least in part caused by dendrite redistribution, although this interpretation requires caution. First, we cannot completely exclude possible indirect effects on network properties to result from receptor overexpression in MN5 through development. Second, although we estimated the amount of receptor overexpression, light microscopy analysis cannot rule out potential differences between conditional and chronic receptor expression. However, our data indicate that the balance between excitatory cholinergic input to the proximal dendritic domain and inhibitory GABAergic input to the distal domain may play a critical role for adaptive adjustments of motoneuron firing rates during behavior.
Dendritic Conservation versus Structural Homeostasis
Directing more dendrites to the input domain of increased GABAergic synaptic input and away from the domain of decreased cholinergic input, or vice versa, seems to contradict the concept of homeostasis. Why to shift more dendrites to sites with many synaptic partners, but away from sites with lower synapse densities? First, a mechanism that locally matches the amount of available dendritic surface to the amount of synapses is in accordance to minimizing cost. Maximum synapse numbers on minimal dendritic length has been proposed as a general principle in cortex (Chklovskii, 2004). Second, redistribution of dendrites to domains with high synapse density may prevent superfluous increases of total arbor length. We suggest naming dendrite redistribution while maintaining total length “dendritic conservation.” Can dendritic conservation function in parallel to homeostatic control of excitability? Tight regulation of excitation-inhibition ratios is essential for normal circuit function (Nelson and Valakh, 2015). Providing more dendrites to inhibitory or to excitatory input domains, depending on GABAergic or cholinergic synaptic activity during arbor growth does not preclude compensatory adjustments of neuronal excitability on the levels of synapse strength or ion channel expression, both of which have been reported conserved mechanisms from invertebrates to mammals (Turrigiano and Nelson, 2004; Turrigiano, 2008; Marder and Goaillard, 2006). In fact, even with forced receptor overexpression or massive thermogenetic stimulation of synaptic inputs through development, both of which caused dendrite shifts, multiple aspects of basic flight motor performance were normal. Therefore, at least to a certain degree, intra-neuronal dendrite shifts may operate in concert with homeostatic adjustments to keep overall excitability in check.
STAR★METHODS
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| chicken polyclonal anti-GFP | Thermo Fisher Scientific | Cat# A10262; RRID: AB_2534023 |
| rat polyclonal anti-Drosophila nAChRα7 | H. Bellen, BCM Houston, Texas, USA; Fayyazuddin et al., 2006 | N/A |
| rabbit anti-Rdl | D. Naessel, Stockholm University, Stockholm, Sweden; Enell et al., 2007 | N/A |
| Anti-HA High Affinity; Rat monoclonal antibody (clone 3F10) | Roche | Cat# 11867423001; RRID: AB_10094468 |
| donkey α-rat IgG (H+L) Secondary Antibody, Alexa Fluor 647 conjugate | Jackson Immunoresearch | Cat# 712-605-150; RRID: AB_2340693 |
| Goat anti-Chicken IgY (H+L) Secondary Antibody, Alexa Fluor 488 conjugate | Thermo Fisher Scientific | Cat# A11039; RRID: AB_2534096 |
| Donkey anti-Rabbit IgG (H+L) Secondary Antibody, Alexa Fluor 568 conjugate | Thermo Fisher Scientific | Cat# A10042; RRID: AB_2534017 |
| Cy3-Streptavidin polyclonal antibody | Jackson ImmunoResearch | Cat# 016-160-084; RRID: AB_2337244 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Picrotoxin | Sigma Aldrich | P1675; CAS 124-87-8 |
| Nicotine | Sigma Aldrich | N3876; CAS 54-11-5 |
| GABA | Sigma Aldrich | A2129; CAS 56-12-2 |
| Amiloride | Sigma Aldrich | A7410; CAS 2016-88-8 (anhydrous) |
| Protease Type XIV from Streptomyces griseus | Sigma Aldrich | P5147; CAS 9036-06-0 |
| Tetrodotoxin | Roth Chemicals Germany | Cat# 6973.1; CAS 4368-28-9 |
| Neurobiotin | Vector Laboratories | Cat# SP-1120 |
| Tetramethylrhodaminedextran (TRITC-Dextran) 3000 lysine fixable | Thermo Fisher Scientific | Cat # D3308 |
| Methylsalicylate | Sigma Aldrich | M6752; CAS 119-36-8 |
| Sylgard 184 Dow Corning | Biesterfeld Spezialchemie Hamburg | Sylgard 184 |
| Experimental Models: Organisms/Strains | ||
| D. melanogaster: HA-tagged UAS-RDL on chromosome 3: w[*]; P{w[+mC] = UAS-Rdl.HA}C; MKRS/TM6B | Bloomington Drosophila Stock Center | RRID: BDSC_29038 |
| D. melanogaster: HA-tagged UAS-RDL on chromosome 1: w[*] P{w[+mC] = UAS-Rdl.HA}2A; sna[Sco]/CyO | Bloomington Drosophila Stock Center | RRID: BDSC_29035 |
| D. melanogaster: RDL mutant: Rdl[1]/TM3, Sb[1] | Bloomington Drosophila Stock Center | RRID: BDSC_1687 |
| D. melanogaster: GAL4 driver under the control of toll-6 (D42-GAL4) and suppression of expression in cholinergic neurons by inclusion of ChAT-GAL80 on the same chromosome: w[*]; P{w[+mW.hs] = GawB}D42 | Bloomington Drosophila Stock Center | RRID: BDSC_8816 |
| D. melanogaster: GAL4 driver under the control of an enhancer fragment of anachronism: w[1118]; P{y[+t7.7] w[+mC] = GMR23H06-GAL4}attP2 | Bloomington Drosophila Stock Center | RRID: BDSC_49050 |
| D. melanogaster: GAL4 driver under the control of choline acetyltransferase (ChAT): w[1118]; P{w[+mC] = ChAT-GAL4.7.4}19B/CyO, P{ry[+t7.2] = sevRas1.V12}FK1 | Bloomington Drosophila Stock Center | RRID: BDSC_6798 |
| D. melanogaster: membrane anchored UAS-GFP: w[*]; P{y[+t7.7] w[+mC] = 10XUAS-IVS-mCD8::GFP}attP40 | Bloomington Drosophila Stock Center | RRID: BDSC_32186 |
| D. melanogaster: genetically expressed calcium indicator GCaMP6s with slow decay kinetics: w[1118]; P{y[+t7.7] w[+mC] = 20XUAS-IVS-GCaMP6s}attP40 | Bloomington Drosophila Stock Center | RRID: BDSC_42746 |
| D. melanogaster: temperature-sensitive cation channel: UAS-TRPA1 on chromosome 2: w;UAS-TRPA1;+ | L. Griffith, Brandeis University, Boston, USA | N/A |
| D. melanogaster: GAL4 driver under the control of futsch on chromosome 1 and suppression of expression in cholinergic neurons by inclusion of ChAT-GAL80 on chromosome 3: C380-GAL4;;ChAT-GAL80 | S. Sanyal, Biogen Inc., Boston, USA | N/A |
| D. melanogaster: GAL4 driver that drives expression in DLM motoneurons and few other neurons on chromosome 2 and suppression of expression in cholinergic neurons by inclusion of ChAT-GAL80 on chromosome 3: P103.3-GAL4 | C. Consoulas, Medical School University of Athens, Athens, Greece | N/A |
| D. melanogaster: crossed together to be able to express UAS-transgenes conditionally: w[*];P{w[+mC] = tubP-GAL80[ts]}10; P{w[+mW.hs] = GawB}D42 | Bloomington Drosophila Stock Center | RRID: BDSC_7108 and RRID: BDSC_8816 |
| D. melanogaster: loss of function mutation of the Dα7 nAChR: nAChRalpha7[PDeltaEY6] | Bloomington Drosophila Stock Center | RRID: BDSC_24879 |
| Software and Algorithms | ||
| Amira 5.3.3 with custom plug-ins | FEI Hillsboro, Oregon, US; Schmitt et al., 2004; Evers et al., 2005 | Amira 3D analysis, RRID: SCR_014305 |
| Corel Draw X7 | Corel Corporation | N/A |
| SPSS Statistics 22 | IBM | SPSS, RRID: SCR_002865 |
| pClamp10.4 electrophysiology software | Molecular Devices | pClamp {SCR:011323} |
| HoKaWo imaging software, v 2.10, release pf01 | Hamamatsu | N/A |
| Other | ||
| Glass microelectrodes with filament, i.d. 0.86 mm, o.d. 1.0 mm | Sutter Instruments | Cat# BF 100-50-10 |
| Glass microelectrodes without filament, i.d. 1.0 mm, o.d. 1.5 mm | World Precision Instruments | Cat# PG52151-4 |
| Tungsten wire, diameter 100 μm, uncoated | Science Products | Cat# TW4-3 |
CONTACT FOR REAGENT AND RESOURCE SHARING
Please contact Carsten Duch (cduch@uni-mainz.de) for reagents, animals, and resources used in this study.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Fly Maintenance
Experiments were done in adult or pupal Drosophila melanogaster with different genetic backgrounds and/or transgenes (for fly strains, see the Key Resources Table). Flies were reared in plastic vials on 18°C, 25°C, or 29°C, depending on the experiment, with a 12hr light/dark cycle on a standard cornmeal/glucose/yeast/agar diet.
For flight experiments male flies between 2 and 5 days of age were used. For in situ patch clamp experiments and sharp electrode intracellular recordings pupal stages P6, P8, P12, and P13 pupae as well as 2 days old adult male and female flies were used. For intracellular dye fills 2 days old adult male and female flies were used.
Staging
Staging of pupae for electrophysiological and calcium imaging recordings was done by external criteria according to Bainbridge and Bownes (1981). Pupal stage P6: Malpighian tubules were green, and the yellow body appeared at the most anterior part of the abdomen but had not yet moved between the Malpighian tubules. Pupal stage P8: Eyes were light orange. Pupal stage P10: Eyes had just turned red, and tips of wings were not yet light gray. Pupal stage P12: Wings had turned dark gray but claws at the end of each leg had not yet appeared. Pupal stage P13: Claws are now visible at the end of each leg but meconium has not yet appeared.
Choice of GAL4-Driver lines
GAL4 driver lines to drive expression of UAS-transgenes in motoneurons were (1) w; P103.3-GAL4, UAS-mCD8-GFP;+, (2) C380− GAL4, UAS-mCD8-GFP;; ChAT-GAL80 and (3) w;UAS-mCD8-GFP; D42-GAL4, ChAT-GAL80 (see the Key Resources Table). All express in subsets of motoneurons including MN5 and some other non-identified neurons (Sanyal et al., 2003; Sanyal, 2009; Consoulas et al., 2002; Kuehn and Duch, 2013). To suppress expression in cholinergic interneurons and sensory neurons the ChAT-GAL80 transgene was included. Expression of UAS-nAChRDα7-GFP in MN5 was driven under the control of P103.3 (see the Key Resources Table). Previous work with co-labeling of native Dα7 receptors by immunohistochemistry has revealed correct localization of UASDα7− GFP to the cholinergic input domain of MN5 (Kuehn and Duch, 2013). Expression of UAS-Rdl-HA in MN5 was driven under the control of C380 (see the Key Resources Table). Previous work with co-labeling of native Rdl receptors by immunocytochemistry has revealed correct localization of UAS-Rdl-HA to the GABAergic input domain of MN5 (Kuehn and Duch, 2013). Co-expression of UASRdl and UAS-nAChRDα7 in MN5 was done with P103.3 (see the Key Resources Table). ChAT-GAL4 was used to drive expression of temperature sensitive TrpA1 channels (Pulver et al., 2009) in cholinergic neurons, either in a control background for Dα7Rs or in a Dα7 heterozygous mutant background (nAChRα7PΔEY6, see the Key Resources Table). UAS-mCD8::GFP was driven under the control of D42-GAL4 in a Dα7 heterozygous mutant background for identification of MN5 for intracellular dye filling (see the Key Resources Table). UAS-mCD8::GFP was driven under the control of C380-GAL4 in a Rdl1 (Ffrench-Constant et al., 1991) heterozygous mutant background for identification of MN5 for intracellular dye filling (see the Key Resources Table). D42-GAL4 was used to drive expression of UAS-Rdl in a null mutant background for DmαG (Ryglewski et al., 2012) low voltage activated calcium channels (see the Key Resources Table). GMR23H06-GAL4 (part of the anachronism enhancer, Jenett et al., 2012) was used to drive expression of either UAS-mCD8::GFP or 20xUAS-IVS-GCaMP6s in MN5 and few other neurons.
Conditional Expression of UAS-Transgenes
To test whether developmental or acute overexpression of Rdl or Dα7 receptors lead to behavioral changes, UAS-receptor transgenes were expressed either throughout development or acutely. For expression throughout development flies were reared at 25°C using different GAL4 drivers expressing also in the DLM motoneurons (D42-GAL4, P103.3-GAL4, C380-GAL4, 23H06-GAL4). For conditional expression, a tubulin 86C transgene with temperature sensitive GAL80 was used. In this case flies were reared at 18°C until 1 day old and then shifted to 29°C for 12, 24, 36, 48, 60, 72, and 100 hr to determine when maximum transgene expression was achieved. Expression levels were analyzed by immunohistochemistry and subsequent confocal microscopy (see Immunohistochemistry and Confocal Microscopy sections).
METHOD DETAILS
Animal Dissection and Treatment
Dissection for electrophysiology, dye fills, and immunohistochemistry
The dissection was done as previously described (Ryglewski and Duch, 2009). Wings and legs were cut off adult flies using fine iris scissors. For pupal stages the pupal case was removed with fine forceps. Before placing a steel insect needle at the tip of the abdomen with forceps to pin the respective animal dorsal side up into a sylgard coated petri dish (diameter 35 mm). In adult animals another insect needle was placed through the head, stretching the animal a little. For dissection at pupal stages the animals’ enclosure was opened between the thorax and the head using iris scissors before placing the needle through the head. After submerging the animal in saline and removal of air underneath the animal, a vertical incision was made close to the end of the abdomen to then cut the animal along its midline all the way up to the neck connectives. Then the two halves of the DLM were pinned to their respective sides with one insect needle each using fine forceps to then remove gut, esophagus, and other inner organs to expose the ventral nerve cord (VNC). Tracheae were removed as far as possible and the preparation rinsed thoroughly with saline.
For electrophysiology and dye fills the preparation was mounted onto an upright Zeiss Axio Examiner A1 Fluorescence microscope (Zeiss Germany) with a fixed stage and visualized with a 40× DIC VIS-IR water immersion lens, NA 1.0. Independent of the developmental stage both MN5 were located on the dorsal surface of the VNC in the anterior part of the mesothoracic neuromere and were readily identifiable from animal to animal, with or without UAS-mCD8::GFP or UAS-IVS-GCaMP6s-expression.
To access MN5 and its dendrites the ganglionic sheath was penetrated using a broken patch pipette loaded with 1% protease that was attached to the patch clamp electrode holder with tubing for application of pressure. By applying positive and negative pressure, the sheath was treated with enzyme and then removed focally (Ryglewski and Duch, 2012). Afterward the preparation was rinsed for 5 min with recording saline through a gravitation perfusion system.
For immunohistochemistry preparations were fixed at RT with 4% paraformaldehyde or 2% formalin/1% SMB immediately following dissection (see Immunohistochemistry).
Behavioral testing
Only male flies were used for behavioral testing to avoid weight differences due to egg load. Flies were tested double blind so that the genotype was unknown to the experimenter.
Tethered flight behavior
Tethered flight experiments were essentially done as described in Brembs et al. (2007). Flies were cold anaesthetized for 30 s and placed dorsal side up, head facing away, on a cold plate (at 4°C). Viewed through binoculars, small triangular metal hooks were glued between the head and the anterior part of the fly’s thorax in a way that wing beat was not disturbed. Metal hooks were secured using UV glass adhesive (Super Glue Corporation, US) that cured with a Coltolux 75 UV Curing Light (Peterson Dental Supplies, US) for 30 s. The fly was then immediately placed in a small glass jar with a filter paper soaked with 30 μl5% sucrose solution and left to rest for 1 hr before hanging it on self-closing forceps in front of a black and white horizontally striped paper. Flight was induced by a gentle air pulse to the head. The flight duration was recorded and the clock stopped each time the fly stopped flying. Flight was reinitiated by another air pulse. This was repeated until the fly would not resume flying upon three consecutive air stimuli. In that case the experiment was terminated, the total flight time recorded as well as the number and durations of the single flight bouts.
Extracellular recordings of wing depressor motoneurons during tethered flight
Extracellular recordings from identified wing depressor muscle fibers were conducted as previously described (Ryglewski et al., 2014b). Flies were glued to metal hooks and prepared for the experiment as described for tethered flight assays (see Tethered Flight Behavior). Two sharpened tungsten wires (100 μm diameter) were used for extracellular recordings. Sharpening was done via electrolysis using NaNO2 and KOH solution (see Solutions). One wire was placed in the abdomen as reference electrode. The second wire was placed in the dorsal-most DLM fiber to record MN5 action potentials extracellularly. MN firing is relayed to the muscle in a 1:1 fashion, so that every MN action potential is represented by one muscle spike. Muscle recordings were amplified 100-fold with a differential AC amplifier (A-M Systems Model 1700), digitized with a Digidata 1322 (Molecular Devices) and sampled at 20 kHz. Wing beat frequency was recorded by positioning the tethered fly in a laser beam that was detected with a photo diode. Every wing upstroke broke the laser beam once and was detected as altered light intensity with the photo diode. For optomotor stimulation, the fly was placed in front of a screen with moving horizontal stripes.
In Situ Whole Cell Patch Clamp and Intracellular Recordings
Electrodes
For sharp electrode recordings, intracellular dye fills, and pressure application of agonists sharp electrodes were pulled from thin walled borosilicate glass capillaries with filament (see the Key Resources Table) using a Sutter Flaming Brown P97 horizontal electrode puller. Electrode tips for intracellular recordings had ~100 MΩ tip resistance when filled with 2M potassium acetate as charge carrier and between 80 and 100MΩ when filled with tetramethylrhodamine (TRITC) dextran 3000 lysine fixable/neurobiotin in 2M potassium acetate (see the Key Resources Table and Dye Filling of Motoneuron Dendrites). For dye fills electrode tips were loaded with dye by putting them upside down in the dye solution, and subsequently the shaft was filled with 2M potassium acetate leaving an air bubble between the dye loaded tip and the shaft to avoid dilution.
Patch pipettes and cleaning pipettes were pulled from borosilicate glass capillaries without filament (see the Key Resources Table) using a Narishige PC-10 vertical pipette puller. Patch pipettes had a tip resistance of ~5MΩ with internal patch solution (composition, see Solutions). Tips of cleaning pipettes were broken with forceps.
Sharp Electrode Intracellular Recordings
Sharp electrode intracellular recordings were done with an Axoclamp 2B amplifier (Molecular Devices) in bridge mode with a HS2A × 0.1xLU headstage. Data were digitized with a Digidata 1440 (Molecular Devices), sampled at 50 kHz and acquired with pClamp10.4 software (Molecular Devices).
After removal of the ganglionic sheath (see under Dissection for Electrophysiology, Dye Fills, and Immunohistochemistry) the preparation was steadily perfused with normal saline (see Solutions) at a flow rate of 1ml/min through a gravitation perfusion system. The volume of the recording chamber was ~300 μl.
Before impalement offset was nulled. Under visualization with a 40× water immersion lens the MN5 soma was impaled with a sharp glass microelectrode with filament (for specifications see Electrodes and the Key Resources Table) filled with 2M potassium acetate. If GFP was used, the preparation was viewed using a respective filter cube and a 40× water dipping lens; mostly the neuron was recorded without GFP staining. To determine chloride reversal potentials, the membrane potential was changed by manually injecting current into the soma using the respective dial on the amplifier. Potential offset shifts during the course of the recording were checked for after removal of the recording electrode. If offset shift was detected the recording was discarded. Recordings were done at room temperature (24°C). Potassium acetate was used to avoid direct changes of the intracellular chloride concentration via diffusion of chloride from the pipette tip into the cell. However, we cannot exclude potential indirect shifts in chloride ion distribution upon hyperor depolarization with potassium acetate filled electrodes.
Whole cell patch clamp
Whole cell patch clamp was performed with an Axopatch 200B patch clamp amplifier (Molecular Devices), data were digitized with a Digidata 1440 (Molecular Devices) with a sampling rate of 50 kHz and filtered at 5 kHz through a low pass Bessel filter. Data acquisition was done with pClamp10.4 software (Molecular Devices). After removal of the ganglionic sheath (see Dissection for Electrophysiology, Dye Fills, and Immunohistochemistry) the preparation was steadily perfused with recording saline at a flow rate of 1ml/min through a gravitation perfusion system. The volume of the recording chamber was ~300 μl. After determination of the chloride reversal potential with sharp electrode recordings in the adult and in pupae (see Sharp Electrode Intracellular Recordings), the chloride concentration of the internal patch solution was adjusted to resemble the correct chloride reversal potential (see Solutions).
After formation of a giga seal, the holding potential was changed to −70 mV and the capacitive artifacts were canceled out before breaking into the cell to acquire whole cell mode. After determination of whole cell parameters and series resistance the recording was allowed 2 min to stabilize. MN5 responses to agonist pressure application were recorded in gap free mode within current clamp mode. Only recordings with series resistances below 10 MΩ and an input resistance above 100 MΩ were used for analysis. Recordings were done at room temperature (24°C).
Bath application of blockers
In some experiments tetrodotoxin (TTX, 10−7M, see the Key Resources Table) was used to block action potentials to avoid synaptic transmission onto MN5 dendrites from neighboring neurons. For application the saline flow was halted for two minutes while TTX was applied directly into the recording chamber. Picrotoxin (PTX, 10−4 M,see theKeyResources Table)was usedto block chloridechannels and was applied via perfusion system. Amiloride (10−3 M, see the Key Resources Table) was used to blockDmαG low voltage activated calciumchannels and was applied via perfusion system. Amiloridewas dissolved inDMSO(finalDMSOconcentration 0.1% which did not affect the recordings), added to the recording saline, and mixed well. Amiloride containing solution was prepared fresh every day.
Pressure application of agonists
Before agonists could be applied, the ganglionic sheath had to be removed to gain access to MN5 dendrites (see Dissection for Electrophysiology, Dye Fills, and Immunohistochemistry). Without removal of the sheath, no reliable responses upon pressure application were obtained. The agonists nicotine (10−5 M) and GABA (10−4 M, see the Key Resources Table) were applied using a pressure application setup with a Picospritzer II (General Valve Corporation). The pressure was set between 10 and 40 psi depending on the experiment. Pulse durations and frequencies were set with a pulse generator (Model 2100, A-M Systems). To switch between one electrode pressure application and simultaneous puffs with two separate electrodes the pressure was diverted to either one or both electrodes, thus resulting in half the pressure per electrode.
Calcium imaging
For calcium imaging experiments 20xUAS-GCaMP6s attP40 was expressed under the control of the GAL4 driver GMR23H06-GAL4 (see the Key Resources Table). The slow decay version of GCaMP6 (decay kinetics > 100 ms, Chen et al., 2013) was chosen to increase photon yield for better detection reliability of calcium signals in small dendrites. An Orca Flash 4.0 LT CMOS camera (C11440-42U; Hamamatsu Photonics K.K.) with HOKAWO 2.10 software was used for image acquisition. Exposure times were between 75 and 100ms. Image series were streamed. Raw data were exported to Excel 2010, and ΔF/F was calculated as previously described (Duch and Levine, 2002).
Action potentials were elicited during whole cell patch clamp in current clamp mode using ramp current injection protocols to depolarize the membrane above firing threshold.
Dye filling of motoneuron dendrites
Dye filling of MN5 with a 1:1 mixture of TRITC dextran 3000 lysine fixable/neurobiotin (see the Key Resources Table) in 2M potassium acetate with sharp electrodes and subsequent histology were done as previously described (Vonhoff and Duch, 2010). MN5 was impaled with the dye loaded sharp microelectrode. The positively charged dye was forced into the cell by application of positive current between 0.3 and 1.0 nA until entirely filled. After careful removal of the electrode, the preparation was fixed for 50 min with 4% paraformaldehyde and then washed 6×30 min with PBS. This was followed by 6×30 min washes with 0.5% PBS-Triton-X. Then the preparation was incubated over night with Cy3 coupled streptavidin (see the Key Resources Table) at a dilution of 1:500. Then preparations were washed with PBS 6×30 min followed by an ascending ethanol series (50%, 70%, 90%, 100%, 10 min each) and mounted in methylsalicylate (see the Key Resources Table) on metal slides. Metal slides were 200μm thick, had a round hole in the middle, and a glass coverslip glued underneath the hole. Specimen were put on top of the coverslip, submerged in methylsalicylate, a second coverslip was used to cover the top sealed with clear nail polish. In this way the preparation was not deformed by the weight of the coverslip and could be viewed from both sides.
Solutions
Normal saline [mM]: NaCl 128, KCl 2, MgCl2 4, CaCl2 1.8, HEPES 5, Sucrose ~35.5. pH was adjusted to 7.24 with 1N NaOH, osmolality was adjusted to 300 mOsM/kg with sucrose if needed.
Recording saline for calcium imaging experiments [mM]: NaCl 121.6, KCl 2, MgCl2 4, CaCl2 5, HEPES 5, Sucrose ~35.5. pH was adjusted to 7.24 with 1N NaOH, osmolality was adjusted to 300 mOsM/kg with sucrose if needed.
Internal patch solution with simultaneous calcium imaging (adult recordings, Cl rev: −74 mV) [mM]: KGluc 140, HEPES 10, phosphocreatine di tris 10, Mg-ATP 2, MgCl2 2, Na2-GTP 0.3. pH was adjusted to 7.24 with 1N KOH, osmolality was 310 mOsM/kg.
Internal patch solution with simultaneous calcium imaging (pupal recordings, Cl rev: −54 mV) [mM]: KGluc 127, KCl 13, HEPES 10, phosphocreatine di tris 10, Mg-ATP 2, MgCl2 2, Na2-GTP 0.3. pH was adjusted to 7.24 with 1N KOH, osmolality was 309 mOsM/kg.
2M potassium acetate solution for sharp electrode intracellular recordings without calcium imaging.
Electrolysis solution for sharpening tungsten wires for muscle recordings during restrained flight: NaNO2 10.3 M, KOH 6.05 M. First NaOH2 was dissolved in Millipore water, then KOH was added.
Immunohistochemistry
Immunohistochemistry for Dα7nAChRs and for Rdl GABAARs was conducted as previously described (Kuehn and Duch, 2013). The Dα7nAChR antibody was a kind gift of Dr. H Bellen (BCM, Houston Texas, see the Key Resources Table), and antibody specificity has previously been proven by absence of immunolabel in null mutants (Fayyazuddin et al., 2006). The anti-Rdl GABAA receptor antibody was a kind gift of Dr. Naessel (Stockholm, Sweden, see the Key Resources Table), and its specificity has been characterized by western blotting, immunohistochemistry, pre-absorption with immunization peptide, and by tests with pre-immune serum (Enell et al., 2007). Dα7-GFP was also detected with a primary chicken anti-GFP antibody and labeled with goat anti-chicken Alexa 488 secondary antibody, and Rdl-HA was detected with a primary rat anti-HA antibody and labeled with donkey anti-rat Alexa 647 secondary antibody. Native Rdl receptor was detected with rabbit anti-Rdl antibody and labeled with donkey anti-rabbit Alexa 568 secondary antibody. For antibodies see the Key Resources Table.
For anti-Dα7 staining, after dissection, animals were fixed with 4% paraformaldehyde (PFA) for 25min at RT, for anti-Rdl staining preparations were fixed with 2% formalin/1% SMB at RT for 30min, not shaking, to then be rinsed a few times with phosphate buffered saline 0.1M (PBS) and washed with PBS for 6×30 min atRT, shaking. This was followed by 6×30 min washes with PBS with 0.5% Triton-X (PBS-Tx 0.5%) at RT, shaking. The rat anti-Dα7 antibody was used at a dilution of 1:2000 in 0.3% PBS-Tx with 3% bovine serum albumin and incubated for 48 hr at 4°C, shaking. After 24 hr, chicken anti-GFP antibody was added at a concentration of 1:400. The rabbit anti-Rdl antibody was used at a concentration of 1:10,000 with 2% normal goat serum, the rat anti-HA antibody was used at a dilution of 1:100 in 0.5% PBS-Tx and incubated for 24 hr at 4°C, shaking. After antibody incubation preparations were washed for 6×30min with PBS at RT. Secondary antibodies (see above and theKeyResources Table) were applied overnight in the dark at 4°C, shaking, at a concentration of 1:500 in PBS. Next, preparations were washed 6×30 min atRT, kept in the dark, and then treated with an ascending ethanol series, 50%, 70%, 90, 100% for 10min each. Then preparations were mounted in methylsalicylate on metal slides as described above (see Dye Filling of Motoneuron Dendrites). Preparations were viewed and scanned with a Leica TCS SP8 confocal microscope (see Confocal Microscopy).
Confocal Microscopy
Dα7 and Rdl immunolabel and intracellular dye fills
Antibody staining was visualized and scanned with a Leica TCS SP8 confocal microscope (Leica Germany). Image resolution was 1024×1024 pixels. Images were acquired with a 40× oil immersion lens (Na = 1.25) with a 3.5 zoom factor. Voxel dimensions are 86 × 86 × 290 nm (x,y,z). For antibody specifics see the Key Resources Table. Alexa 488 was excited with an argon laser at 488 nm, and detected with a photomultiplier between 510 and 550 nm. Alexa 568 was excited with a DPSS laser at 561nm and emission was detected between 570 and 600 nm. Alexa 647 was excited with a helium-neon laser at 633 nm, and fluorescence was detected with a hybrid detector (HyD) between 650 and 700 nm. For multiple label within one preparation 488 and 633 were scanned simultaneously, but sequential scan mode was used for either one in conjunction with 561 excitation to avoid crosstalk.
Conditional expression of UAS-transgenes
For conditional overexpression of receptors a transgene with temperature sensitive GAL80 associated with tubulin 86C was expressed along with GAL4-drivers and UAS-transgenes for receptors. Flies were reared at 18°C until 1–2 days post eclosion and then shifted to 29°C to allow expression of GAL4 and subsequent receptor transgene expression. Preparations were evaluated for receptor transgene expression after 12, 24, 36, 48, 60, 72, and 100 hr by immunostaining and confocal imaging as described above (Dα7 and Rdl immunolabel). For all preparations carrying the same transgenes the same settings were used. Maximum transgene expression was observed after a 48 hr heat shock.
Movies
Calcium imaging time series as acquired with the HoKaWo imaging software were exported as TIFF image series to ImageJ. For some TIFF series a 16-bit false color code was applied, or the TIFF series were transformed to 8-bit gray scale. For presentation as movies TIFF image series were exported as .avi movies.
Quantification and Statistical Analysis
3D reconstruction of neuronal structure
Geometric reconstruction of dendritic architecture from confocal image stacks were conducted and quantified with custom made plug-ins for commercially available image analysis software (AMIRA 5.3.3, FEI, Hillsboro, Oregon, US) as described previously (Schmitt et al., 2004; Evers et al., 2005). Images were further processed with CorelDrawX7 (Corel Corporation).
Statistical analysis
All statistics were done with SPSS Statistics 22 software. Morphometric data of 3D dendrite reconstructions was normally distributed and displayed in Figures 1, 2, 5, S1, and S3 as means ± SD. Two sided paired Student’s t test was used for within neuron comparison of dendrite length and branch numbers in the cholinergic versus the GABAergic domain. Two sided non-paired Student’s t test was used for comparison of two groups, and ANOVA with Newman Keuls posthoc testing was used for comparison among multiple experimental groups. Physiological properties such as chloride reversal and resting membrane potential were treated similarly (Figure 4). Behavioral data from tethered flight experiments was not normally distributed and displayed as medians, quartiles, and 10 and 90 percentiles (Figure 6). Kruskal Wallis ANOVA was used for testing between multiple experimental groups (Figures 6C–6E). Spearman’s rank correlation with two sided testing for significance was used to analyze bivariate correlations between wing beat frequencies and motoneuron firing rates (Figure 6F). Changes in motoneuron firing rates in response to optomotor input were normally distributed, displayed as means ± SD, and tested for statistical differences with ANOVA with Newman Keuls posthoc testing. Significant statistical difference between groups was defined at p < 0.05 and indicated by one asterisks, highly significant difference was defined as p < 0.01 and indicated by two asterisks or by three asterisks at p > 0.001.
DATA AND SOFTWARE AVAILABILITY
For access to the custom made AMIRA plugins for semi-automated quantitative 3D neuron reconstruction from confocal image stacks, please contact the corresponding author.
Supplementary Material
Highlights.
Synaptic activity can direct postsynaptic arbor formation during dendritic growth
Competition of GABAergic and cholinergic synaptic inputs redistributes dendrites
Local dendritic calcium signaling is required for dendrite redistributions
Perturbations of correct input partitioning cause defects in fine motor control
Acknowledgments
Fly lines carrying UAS-Dα7nAChR and UAS-Rdl constructs were kindly provided by Drs. S. Sigrist (Berlin, Germany) and A. Prokop (Manchester, UK). The Dα7nAChR antibody was a kind gift of Dr. H.J. Bellen (Houston, USA). We thank C. Kuehn for help with some image segmentation work. We gratefully acknowledge support by the German Research Foundation (DFG) to S.R. (Ry 117/3-1) and C.D. (Du-331/6-1, Du-331/12-1, and INST 247/736-1).
Footnotes
Supplemental Information includes six figures and six movies and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2016.12.043.
A video abstract is available at http://dx.doi.org/10.1016/j.neuron.2016.12.043#mmc9.
AUTHOR CONTRIBUTIONS
C.D. and S.R. conceived and performed experiments, analyzed data, wrote the manuscript, and secured funding. F.V. conceived and performed experiments. All authors performed experiments. F.V., K.S., and C.D. conducted and formally analyzed neuroanatomical experiments. S.R. conducted and analyzed imaging and intracellular physiological experiments. S.R. and C.D. conducted and analyzed extracellular physiological and behavioral experiments.
References
- Bainbridge SP, Bownes M. Staging the metamorphosis of Drosophila melanogaster. J Embryol Exp Morphol. 1981;66:57–80. [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Boerner J, Duch C. Average shape standard atlas for the adult Drosophila ventral nerve cord. J Comp Neurol. 2010;518:2437–2455. doi: 10.1002/cne.22346. [DOI] [PubMed] [Google Scholar]
- Brembs B, Christiansen F, Pflüger HJ, Duch C. Flight initiation and maintenance deficits in flies with genetically altered biogenic amine levels. J Neurosci. 2007;27:11122–11131. doi: 10.1523/JNEUROSCI.2704-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda L, Fiumelli H, Chen K, Poo MM. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J Neurosci. 2007;27:5224–5235. doi: 10.1523/JNEUROSCI.5169-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kriegstein AR. A GABAergic projection from the zona incerta to cortex promotes cortical neuron development. Science. 2015;350:554–558. doi: 10.1126/science.aac6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chklovskii DB. Synaptic connectivity and neuronal morphology: two sides of the same coin. Neuron. 2004;43:609–617. doi: 10.1016/j.neuron.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Cline HT. Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol. 2001;11:118–126. doi: 10.1016/s0959-4388(00)00182-3. [DOI] [PubMed] [Google Scholar]
- Consoulas C, Restifo LL, Levine RB. Dendritic remodeling and growth of motoneurons during metamorphosis of Drosophila melanogaster. J Neurosci. 2002;22:4906–4917. doi: 10.1523/JNEUROSCI.22-12-04906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristo G, Wu C, Chattopadhyaya B, Ango F, Knott G, Welker E, Svoboda K, Huang ZJ. Subcellular domain-restricted GABAergic innervation in primary visual cortex in the absence of sensory and thalamic inputs. Nat Neurosci. 2004;7:1184–1186. doi: 10.1038/nn1334. [DOI] [PubMed] [Google Scholar]
- Duch C, Levine RB. Changes in calcium signaling during postembryonic dendritic growth in Manduca sexta. J Neurophysiol. 2002;87:1415–1425. doi: 10.1152/jn.00524.2001. [DOI] [PubMed] [Google Scholar]
- Duch C, Vonhoff F, Ryglewski S. Dendrite elongation and dendritic branching are affected separately by different forms of intrinsic motoneuron excitability. J Neurophysiol. 2008;100:2525–2536. doi: 10.1152/jn.90758.2008. [DOI] [PubMed] [Google Scholar]
- Enell L, Hamasaka Y, Kolodziejczyk A, Nässel DR. gamma-Aminobutyric acid (GABA) signaling components in Drosophila: immunocytochemical localization of GABA(B) receptors in relation to the GABA(A) receptor subunit RDL and a vesicular GABA transporter. J Comp Neurol. 2007;505:18–31. doi: 10.1002/cne.21472. [DOI] [PubMed] [Google Scholar]
- Evers JF, Schmitt S, Sibila M, Duch C. Progress in functional neuroanatomy: precise automatic geometric reconstruction of neuronal morphology from confocal image stacks. J Neurophysiol. 2005;93:2331–2342. doi: 10.1152/jn.00761.2004. [DOI] [PubMed] [Google Scholar]
- Fayyazuddin A, Zaheer MA, Hiesinger PR, Bellen HJ. The nicotinic acetylcholine receptor Dalpha7 is required for an escape behavior in Drosophila. PLoS Biol. 2006;4:e63. doi: 10.1371/journal.pbio.0040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffrench-Constant RH, Mortlock DP, Shaffer CD, MacIntyre RJ, Roush RT. Molecular cloning and transformation of cyclodiene resistance in Drosophila: an invertebrate gamma-aminobutyric acid subtype A receptor locus. Proc Natl Acad Sci USA. 1991;88:7209–7213. doi: 10.1073/pnas.88.16.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala CJ, Spacek J, Harris KM. Dendrite structure. In: Stuart G, Spruston N, Häusser M, editors. Dendrites. 2. Oxford University Press; 2008. pp. 1–11. [Google Scholar]
- Gao FB, Bogert BA. Genetic control of dendritic morphogenesis in Drosophila. Trends Neurosci. 2003;26:262–268. doi: 10.1016/S0166-2236(03)00078-X. [DOI] [PubMed] [Google Scholar]
- Gordon S, Dickinson MH. Role of calcium in the regulation of mechanical power in insect flight. Proc Natl Acad Sci USA. 2006;103:4311–4315. doi: 10.1073/pnas.0510109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grolleau F, Sattelle DB. Single channel analysis of the blocking actions of BIDN and fipronil on a Drosophila melanogaster GABA receptor (RDL) stably expressed in a Drosophila cell line. Br J Pharmacol. 2000;130:1833–1842. doi: 10.1038/sj.bjp.0703507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Different levels of the homeo-domain protein cut regulate distinct dendrite branching patterns of Drosophila multidendritic neurons. Cell. 2003;112:805–818. doi: 10.1016/s0092-8674(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jenett A, Rubin GM, Ngo TTB, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn C. Dissertation. Free University of Berlin; 2012. Localization of excitatory and inhibitory neurotransmitter receptors in an identified motoneuron of the Drosophila flight system. [Google Scholar]
- Kuehn C, Duch C. Putative excitatory and putative inhibitory inputs are localised in different dendritic domains in a Drosophila flight motoneuron. Eur J Neurosci. 2013;37:860–875. doi: 10.1111/ejn.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libersat F, Duch C. Mechanisms of dendritic maturation. Mol Neurobiol. 2004;29:303–320. doi: 10.1385/MN:29:3:303. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Myhr KL, Wong RO. Transmitter-evoked local calcium release stabilizes developing dendrites. Nature. 2002;418:177–181. doi: 10.1038/nature00850. [DOI] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- McAllister AK. Conserved cues for axon and dendrite growth in the developing cortex. Neuron. 2002;33:2–4. doi: 10.1016/s0896-6273(01)00577-3. [DOI] [PubMed] [Google Scholar]
- Montague PR, Friedlander MJ. Expression of an intrinsic growth strategy by mammalian retinal neurons. Proc Natl Acad Sci USA. 1989;86:7223–7227. doi: 10.1073/pnas.86.18.7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Valakh V. Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron. 2015;87:684–698. doi: 10.1016/j.neuron.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–260. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- Polleux F, Ghosh A. Molecular determinants of dendrite and spine development. In: Stuart G, Spruston N, Häusser M, editors. Dendrites. 2. Oxford University Press; 2008. pp. 96–108. [Google Scholar]
- Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol. 2009;101:3075–3088. doi: 10.1152/jn.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puram SV, Bonni A. Cell-intrinsic drivers of dendrite morphogenesis. Development. 2013;140:4657–4671. doi: 10.1242/dev.087676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond L. Translating neuronal activity into dendrite elaboration: signaling to the nucleus. Neurosignals. 2008;16:194–208. doi: 10.1159/000111563. [DOI] [PubMed] [Google Scholar]
- Redmond L, Kashani AH, Ghosh A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron. 2002;34:999–1010. doi: 10.1016/s0896-6273(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Ryglewski S, Duch C. Shaker and Shal mediate transient calcium-independent potassium current in a Drosophila flight motoneuron. J Neurophysiol. 2009;102:3673–3688. doi: 10.1152/jn.00693.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryglewski S, Duch C. Preparation of Drosophila central neurons for in situ patch clamping. J Vis Exp. 2012;68:4264. doi: 10.3791/4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryglewski S, Lance K, Levine RB, Duch C. Ca(v)2 channels mediate low and high voltage-activated calcium currents in Drosophila motoneurons. J Physiol. 2012;590:809–825. doi: 10.1113/jphysiol.2011.222836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryglewski S, Kilo L, Duch C. Sequential acquisition of cacophony calcium currents, sodium channels and voltage-dependent potassium currents affects spike shape and dendrite growth during postembryonic maturation of an identified Drosophila motoneuron. Eur J Neurosci. 2014a;39:1572–1585. doi: 10.1111/ejn.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryglewski S, Kadas D, Hutchinson K, Schuetzler N, Vonhoff F, Duch C. Dendrites are dispensable for basic motoneuron function but essential for fine tuning of behavior. Proc Natl Acad Sci USA. 2014b;111:18049–18054. doi: 10.1073/pnas.1416247111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsonovich AV, Ascoli GA. Morphological homeostasis in cortical dendrites. Proc Natl Acad Sci USA. 2006;103:1569–1574. doi: 10.1073/pnas.0510057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S. Genomic mapping and expression patterns of C380, OK6 and D42 enhancer trap lines in the larval nervous system of Drosophila. Gene Expr Patterns. 2009;9:371–380. doi: 10.1016/j.gep.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Narayanan R, Consoulas C, Ramaswami M. Evidence for cell autonomous AP1 function in regulation of Drosophila motor-neuron plasticity. BMC Neurosci. 2003;4:20. doi: 10.1186/1471-2202-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt S, Evers JF, Duch C, Scholz M, Obermayer K. New methods for the computer-assisted 3-D reconstruction of neurons from confocal image stacks. Neuroimage. 2004;23:1283–1298. doi: 10.1016/j.neuroimage.2004.06.047. [DOI] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Da Silva JS, He H, Cline HT. Type A GABA-receptor-dependent synaptic transmission sculpts dendritic arbor structure in Xenopus tadpoles in vivo. J Neurosci. 2009;29:5032–5043. doi: 10.1523/JNEUROSCI.5331-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Valnegri P, Puram SV, Bonni A. Regulation of dendrite morphogenesis by extrinsic cues. Trends Neurosci. 2015;38:439–447. doi: 10.1016/j.tins.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonhoff F, Duch C. Tiling among stereotyped dendritic branches in an identified Drosophila motoneuron. J Comp Neurol. 2010;518:2169–2185. doi: 10.1002/cne.22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonhoff F, Kuehn C, Blumenstock S, Sanyal S, Duch C. Temporal coherency between receptor expression, neural activity and AP-1-dependent transcription regulates Drosophila motoneuron dendrite development. Development. 2013;140:606–616. doi: 10.1242/dev.089235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JC, Levine RB. Steroid hormone effects on neurons sub-serving behavior. Curr Opin Neurobiol. 1995;5:809–815. doi: 10.1016/0959-4388(95)80110-3. [DOI] [PubMed] [Google Scholar]
- Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002;3:803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.