Abstract
Allergic conditions result in the increase of immunoglobulin (Ig)E-producing plasma cells (IgE-PCs); however, it is unclear how IgE production is qualitatively controlled. In this study, we found that IgE-PCs in spleen of immunized mice formed homotypic cell aggregates. By employing IgE-producing hybridomas (IgE-hybridomas) as a model of IgE-PCs, we showed that these cells formed aggregates in the presence of specific antigens (Ags). The formation of the Ag-induced cell aggregation involved secreted IgE and Fcγ receptor (FcγR)II/FcγRIII, but not FcεRs. Ag-induced cell aggregation plus lipopolysaccharide signaling resulted in an enhancement of IgE production in aggregated IgE-hybridomas. Furthermore, the administration of anti-FcγRII/FcγRIII antagonistic monoclonal antibody to immunized mice tended to reduce the splenic IgE-PC aggregation as well as the serum IgE levels. Taken together, our results suggested that Ag-IgE complexes induced IgE-PCs aggregation via FcγRII/FcγRIII, leading to the enhancement of IgE production. These findings suggest the presence of a novel mechanism for regulation of IgE production.
Abbreviations: PC, plasma cell; mAb, monoclonal antibody; TNP, 2,4,6-trinitrophenyl; SN, supernatant; AP, alkaline phosphatase; AI, aggregation index
Keywords: IgE, Plasma cell, Cell aggregation, Antibody production, Fc gamma receptor, Antigen
Highlights
-
•
IgE producing hybridomas aggregated in the presence of antigen and secreted IgE.
-
•
FcγRs but not FcεRs were required for the cell aggregation.
-
•
The cell aggregation with LPS stimulation enhanced IgE production.
-
•
IgE producing plasma cells formed the cell aggregates in spleen of immunized mice.
-
•
Inhibition of FcγRs reduced the IgE-producing plasma cell aggregation and serum IgE.
1. Introduction
Immunoglobulin (Ig)E antibody (Ab) is often increased in patients with allergic disorders and plays critical roles in the pathogenesis [1]. The elevation of IgE levels can be explained by quantitative and qualitative regulations. The quantitative regulation is the increase in the number of IgE-producing plasma cells (IgE-PCs) due to the allergen-induced T helper type 2 responses that promote the B cell differentiation into IgE-PCs [2], [3]. The qualitative regulation is the upregulation of IgE secretion by each IgE-PC. Qualitative control of Ab production by several stimuli does exist. In human IgM- or IgG-PCs, it has been reported that their Ab production is upregulated in response to several toll-like receptor (TLR) ligands such as peptidoglycan, polyinosinic-polycytidylic acid or lipopolysaccharide (LPS) [4]. Moreover, interleukin-6 signaling in IgG-PCs is suggested to control the Ab production of PCs [5]. However, little is known about the signaling controlling the IgE production by IgE-PCs.
We have analyzed spleen tissue sections of immunized mice and observed that some IgE strong positive cells (i.e. IgE-PCs) formed homotypic cell aggregates (Supplementary Fig. S1). Several reports have shown that IgM- and IgG1-PCs form cell clusters in the spleen of immunized mice, which may be due to the local cell division of plasmablasts [6], [7], [8]. However, the mechanism and the role(s) of IgE-PC aggregation remain unknown.
The expression of B cell antigen receptors (BCR) is normally downregulated during B cell differentiation into PCs [9]. Interestingly, recent studies have demonstrated that terminally-differentiated IgE-PCs still express the membrane-bound IgE as BCR [2], [10]. Naïve B cells are known to form adhesion molecule-mediated homotypic cell aggregates upon stimulation via BCR in vitro [11]. These findings led us to the idea that IgE-PCs may respond to and their functions may be regulated by their specific antigens (Ags).
In this study, we investigated the mechanism and the role of IgE-PC aggregation using IgE-producing hybridomas as a model of IgE-PCs. The IgE-hybridoma cell aggregation was induced by their specific Ags and secreted IgE via Fcγ receptors (FcγRs) rather than membrane-bound IgE. The IgE production of the aggregated IgE-hybridomas was upregulated when co-stimulated with LPS. In vivo IgE-PC aggregation and serum IgE level tended to be reduced by administration of an antagonistic monoclonal Ab (mAb) against FcγRs. Therefore, IgE-PC aggregation might play an important role in the qualitative control of IgE production.
2. Materials and methods
2.1. Mice and cell lines
BALB/c mice were purchased from Japan CLEA (Tokyo, Japan), and maintained under specific pathogen-free (SPF) conditions at Animal Science Research Center for Bioscience and Technology, Tottori University. All experiments were approved and performed in accordance with the guidelines of Institutional Animal Care and Use Committee of Tottori University (Permit Number: 15-Y-6).
As shown in Supplementary Table S1, three mouse IgE-hybridomas (IGEL b4, IGEL a2 and H1 DNP-ε-26) were maintained in RPMI-1640 (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS: Nichirei biosciences Inc., Tokyo, Japan) with 10−5M 2-mercaptoethanol (Wako Pure Chemical Industries, Osaka, Japan) and antibiotics (50 U/ml penicillin and 50 μg/ml streptomycin [Meiji Seika, Tokyo, Japan]) (RPMI/10% FBS).
2.2. Reagents and antibodies
The information of reagents and mAbs (including concentration) were shown in Supplementary Table S2 and S3, respectively.
2.3. Immunization
BALB/c mice were immunized intraperitoneally with 2,4,6-trinitrophenyl (TNP)18-conjugated ovalbumin (TNP18-OVA) (100 μg) in aluminum hydroxide gel (alum) (2 mg) (Sigma-Aldrich Co. LLC, St Louis, MO), and after more than 4 weeks, boosted with same Ags in alum. Each spleen was cut in half in the long axis direction and was used for the immunohistochemistry and the enzyme-linked immunosorbent spot (ELISPOT) assay, respectively.
2.4. Immunohistochemistry
The half of the spleen was frozen in liquid nitrogen using Tissue Tek OCT compound (Sakura Finetek USA Inc, Torrance, CA). Sections (6–7 µm in thick) were fixed in 4% paraformaldehyde (Wako), stained with a biotinylated anti-IgE mAb and with the ImmPRESS™ REAGENT KIT (Vector Laboratories, Inc., Burlingame, CA) or the Vectastain Elite ABC standard kit (Vector). The mAb was visualized using ImmPACT™ DAB Peroxidase Substrate (Vector). For counterstaining, Hematoxylin QS (H-3404, Vector) was used.
2.5. Aggregation assay
Hybridomas (0.5-2×104/well) were incubated with TNP-LPS (10 μg/ml) or LPS (10 μg/ml) in 96 well cell culture plates (Corning Costar, Corning, NY) for 2 days at 37 °C.
The short-term aggregation assay was performed in 96 well plates (IGEL b4 cells; 4×104/well) under constant agitation (600 rpm) using a MicroMixer E-36 (Taitec, Saitama, Japan) for 2 h at 37 or 4 °C, with TNP-LPS (10 μg/ml) and IGEL b4 cell culture supernatant (b4-SN). The b4-SN was collected after culturing IGEL b4 cells for 7 days and passed through 0.45 µm Sterile Syringe Filters (Corning Costar). Heat inactivation of b4-SN was done at 56 °C for 60 min. In the assays with blocking mAbs, IGEL b4 cells were pretreated with mAbs against FcεRI and FcεRII (on ice) or against FcγRII/FcγRIII (at 37 °C) for 30 min. Cells were diluted with medium (for mAbs against FcεRI and FcεRII) or washed once (for an anti-FcγRII/FcγRIII mAb), and then the short-term aggregation assay was performed.
After the plates were shaken at 1000 rpm with a MicroMixer E-36 (Taitec) for 10 s in order to equally distribute the cell aggregates across wells, the photomicrographs of three different places in each well were taken. The number of cell aggregates and cells in each aggregate in the randomly selected area (1.186 mm2) were counted.
The enrichment of aggregated cells was performed with 1-g sedimentation as described [12]. Briefly, IGEL b4 cells (1.48×104/well) were incubated with each regent in 48 well plates (Corning Costar) at 37 °C for 2 days. Cells were laid on 5 ml of RPMI/50% FBS in a tube and incubated for 20 min at RT. Cells in the lower layer were used as aggregated cells. In the case of culturing with PBS or LPS, all the cells (upper and lower layers) in a tube were collected and used for the subsequent experiments because IGEL b4 cells did not aggregate.
2.6. ELISPOT assay
Cells were incubated at 37 °C for 6–18 h in MultiScreen-HA 96 well plates (Merck Millipore Corporation., Darmstadt, Germany) coated with TNP26-conjugated bovine serum albumin (TNP26-BSA). After removing cells, secreted Abs bound to wells were detected by alkaline phosphatase (AP)-conjugated polyclonal Abs against IgE or IgG1 and NBT-BCIP substrate (Wako). The spot number and the area (pixels) were quantified using Image J software (National Institute of Health, Bethesda, MD).
2.7. Enzyme-linked immunosorbent assay (ELISA) assay
Samples were incubated in the plate for ELISA (Sumitomo Bakelite Co., Ltd., Tokyo, Japan) coated with a biotinylated anti-IgE mAb (for serum IgE) or TNP26-BSA (2.5 μg/ml) (for b4-SN). IgE concentration was quantified by staining with an AP-conjugated anti-IgE polyclonal Ab and SIGMAFAST™ p-Nitrophenyl phosphate Tablets (Sigma-Aldrich) and by measuring the absorbance at 405 nm.
2.8. Statistical analysis
All experiments were performed more than twice with similar results unless otherwise indicated. Data of aggregation assay represent the mean ±S.E. of triplicate cultures, and significance was determined by either one-way ANOVA with Tukey's post-hoc test for differences or the unpaired two-tailed Student's t-test. Statistical significance was established at p<0.05.
3. Results
3.1. IgE-PCs in the spleen of immunized mice formed homotypic cell aggregates
To investigate IgE-PCs in vivo, mice were immunized intraperitoneally twice with TNP-OVA in alum (Supplementary Fig. S1). Seven days after the last immunization, spleen tissue sections were stained with an anti-IgE mAb. The strongly cytoplasmic IgE positive cells were detected as IgE-PCs. We found that IgE-PCs formed homotypic cell aggregates in both naïve and immunized mice. The immunized mice had an increased number of IgE-PC aggregates and each IgE-PC aggregate contained three to five cells. Approximately 26.5±16.7% of the IgE-PCs participated in the cell aggregation. The majority of these cell aggregates localized in the white pulp.
3.2. An IgE-producing hybridoma, IGEL b4, formed cell aggregates in the presence of the specific Ag
To investigate the mechanism of the IgE-PC aggregation, we used three lines of anti-TNP IgE-hybridomas as a model of IgE-PCs. The use of hybridomas allowed us to overcome the difficulty in in vitro maintenance of the isolated PCs from mice [13] and to examine the response of a clonal population of IgE-PCs with a single specificity of their IgE.
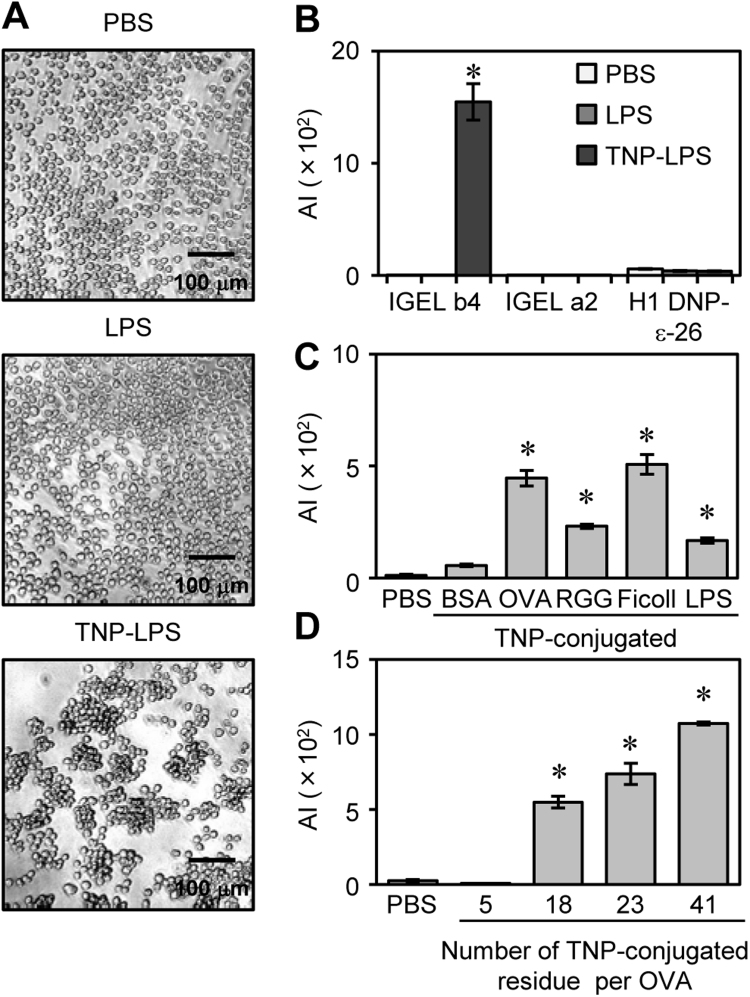
After the incubation of IgE-hybridomas with TNP-LPS for 2 days, we found that IGEL b4 cells, but not IGEL a2 or H1 DNP-ε-26 cells, formed the aggregates (Fig. 1A, Supplementary Fig. S2A). Incubation with PBS or LPS alone did not induce the cell aggregation. To quantify the cell aggregation, an ‘‘aggregation index (AI)’’ were defined (Supplementary Fig. S2B–S2D) and used for the subsequent analyses. Only IGEL b4 cells exhibited a high AI value in the presence of TNP-LPS (Fig. 1B). The cell growth rate and the viability of each IgE-hybridoma were comparable (Supplementary Fig. S2E, S2F), suggesting that the IGEL b4 cell aggregation was not the consequence of promotion of cell division or aggregation of cellular debris. The aggregation was also induced by TNP-conjugated other carriers (Fig. 1C). The magnitude of this cell aggregation correlated with the valence of TNP residues in an OVA protein (Fig. 1D). These results indicate that IGEL b4 cells can respond to their specific Ag and form homotypic cell aggregates like some IgE-PCs in vivo. Therefore, IGEL b4 cells were used in subsequent in vitro analyses to reveal the mechanism of cell aggregation.
Fig. 1.
An IgE-producing hybridoma line, IGEL b4, aggregated in the presence of specific Ags. (A, B) Three anti-TNP IgE-producing hybridoma cell lines (IGEL b4, IGEL a2 and H1 DNP-ε-26) were cultured with LPS or TNP-LPS (10 μg/ml) or the same volume of PBS (5% v/v) for 2 days. (A) Representative photomicrographs of IGEL b4 cells were shown. (B) The calculated AI values (see Supplementary Fig. S2) of each hybridoma were shown. (C, D) IGEL b4 cells were cultured with indicated Ags (1 μg/ml each) or the same volume of PBS (5% v/v) for 2 days. The AI values were shown. *p<0.05.
3.3. Ag-induced cell aggregation required secreted IgE in IGEL b4 cells
How did the Ag induce the IGEL b4 cell aggregation? The flow cytometric analysis demonstrated that three IgE-hybridomas had the IgE molecules on their surfaces and IGEL b4 had the highest level (Supplementary Fig. S3B). RT-PCR analysis showed that all these IgE-hybridomas expressed both secreted IgE and membrane-bound IgE mRNAs (Supplementary Fig. S3A).
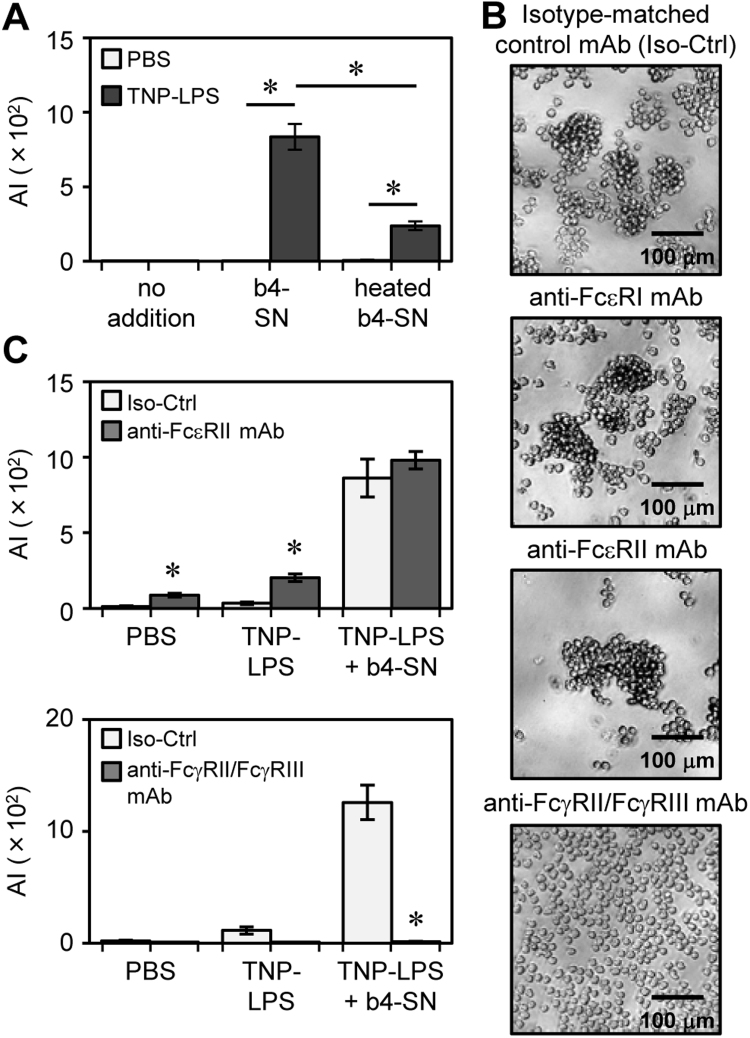
To investigate which forms of IgE were involved in cell aggregation, a short-term aggregation assay was conducted with constant agitation for 2 h (Fig. 2A), because the supernatant (SN) of IGEL b4 contained less than 0.02 μg/ml secreted IgE until 2 h after seeding (Supplementary Table S4). In this condition, IGEL b4 did not aggregate in the presence of TNP-LPS, suggesting that membrane-bound IgE may be dispensable for the induction of cell aggregation. In contrast, when a pooled b4-SN containing an abundant secreted IgE was added with the TNP-LPS, IGEL b4 cells aggregated. Moreover, cell aggregation was impaired by the heat-inactivation of b4-SN that ablated the conformation of the Fc portion of secreted IgE [14]. Instead of b4-SN, we added purified anti-TNP IgE, and detected the cell aggregation in the presence of TNP41-OVA (Supplementary Fig. S4). These data indicate that Ag-induced IGEL b4 cell aggregation involves secreted IgE rather than membrane-bound IgE.
Fig. 2.
Cell aggregation of IGEL b4 cells involved secreted IgE. (A) The AI values of IGEL b4 cells after the short-term aggregation assay with PBS (5% v/v) or TNP-LPS (10 μg/ml) in addition to b4-SN (80% v/v) or the same volume of medium. (B, C) IGEL b4 cells were subjected to the short-term aggregation assay with TNP-LPS and b4-SN in addition to antagonistic mAbs against each Fc receptor. (B) Representative photomicrographs were shown. (C) The AI values in each culture condition were shown. *p<0.05.
3.4. FcγRs, but not FcεRII, were required for the Ag-induced IGEL b4 cell aggregation
The above findings suggested that the Fc receptor(s) for IgE, FcεRs was involved in the induction of the aggregation. However, IGEL b4 cells expressed neither surface FcεRI nor FcεRII, although mRNAs of FcεRII were detected (Supplementary Fig. S3A, S3C). Addition of antagonistic mAbs against FcεRI or FcεRII did not inhibit the aggregation (Fig. 2B, 2C), suggesting that FcεRI and FcεRII did not mediate the cell aggregation.
Secreted IgE also binds to FcRs for IgGs such as FcγRII, FcγRIII and FcγRIV [15]. IGEL b4 cells expressed all of the above FcγRs at mRNA levels (Supplementary Fig. S3A). Flow cytometric analysis with a mAb specific to the common epitope of FcγRII and FcγRIII showed that all IgE-hybridomas expressed these receptors and IGEL b4 cells had the highest level (Supplementary Fig. S3D). Pre-treatment of IGEL b4 cells with an antagonistic mAb against FcγRII/FcγRIII (2.4G2) abolished the binding of secreted IgE-Ag complexes to IGEL b4 cells, indicating that FcγRII/FcγRIII on IGEL b4 can bind secreted IgE-Ag complexes (Supplementary Fig. S3E). The short-term aggregation assay demonstrated that blocking with 2.4G2 completely inhibited the IGEL b4 cell aggregation (Fig. 2B, 2C).
These results indicated that Ag-induced cell aggregation was induced by the binding of Ag-IgE complexes to FcγRII and/or FcγRIII, but not FcεRs. Moreover, cell aggregation required some signal transduction(s) leading to actin polymerization (Supplementary Fig. S5A–S5D), suggesting that the cell aggregation was not merely the cross-linking of adjacent cells via Ag-IgE complexes but was an actively controlled process.
3.5. Ag-induced cell aggregation in conjunction with LPS-stimulation resulted in the enhanced IgE production by IGEL b4 cells
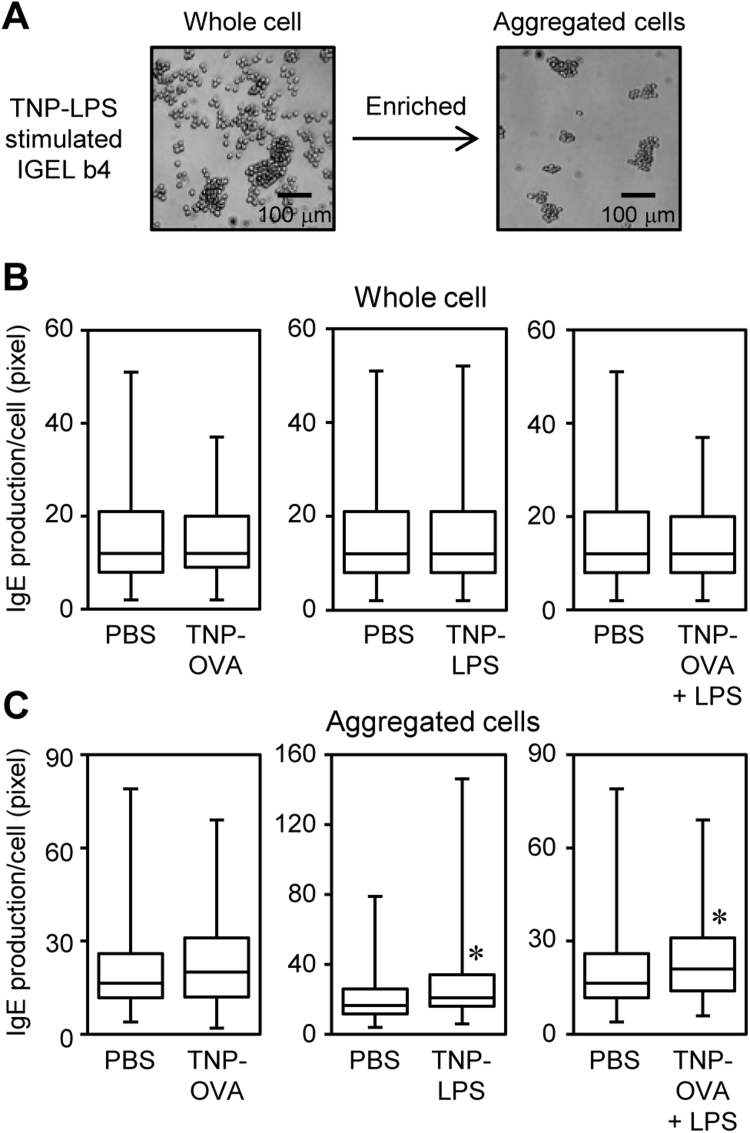
To investigate whether the aggregates affected IgE production, Ab production per cell was evaluated as the size of spot areas by ELISPOT assay (Supplementary Fig. S6A, Fig. 3A). The spot areas of IGEL b4 cells stimulated with each reagent for 2 days were comparable with those cultured with PBS alone (Fig. 3B). The spot area of IGEL b4 cells stimulated with LPS alone was unchanged in one experiment and slightly decreased in one experiment (Supplementary Fig. S6B). As not all the IGEL b4 cells in culture participated in cell aggregation, the aggregated cells were enriched by 1-g sedimentation [12] and their IgE production was assessed (Fig. 3A, 3C). The spot area was significantly increased in the aggregated IGEL b4 cells that were cultured with TNP-LPS. The IgE production of aggregated IGEL b4 cells by TNP-OVA was unchanged, while that of cultured with TNP-OVA plus LPS was enhanced. These results suggested that the Ag-induced cell aggregation triggered the acceleration of IgE production when combined with the LPS stimulation. The signaling via FcγRII/FcγRIII and TLR4 may cooperatively function in the upregulation of IgE production.
Fig. 3.
Ag-specific cell aggregation and LPS stimulation promoted IgE production in aggregated IGEL b4. (A–C) IGEL b4 cells were cultured for 2 days with PBS (5% v/v), LPS or TNP-LPS (10 μg/ml each) or TNP-OVA (1 μg/ml). (A) The representative photomicrograph of enriched aggregated cells was shown. (B, C) IgE production of (B) whole cells (not enriched) or (C) enriched aggregated cells were measured as each spot area (pixels) by ELISPOT assays. Approximately 300 spots were measured in each stimulation condition and the data were shown as the box-and-whisker plots. The same data of control condition (PBS) was used for each comparison of the data. Significant differences were determined by the Mann-Whitney U-test. *p<0.05.
3.6. Administration of an anti-FcγRII/FcγRIII mAb tended to reduce IgE-PC aggregation and the serum IgE level in vivo
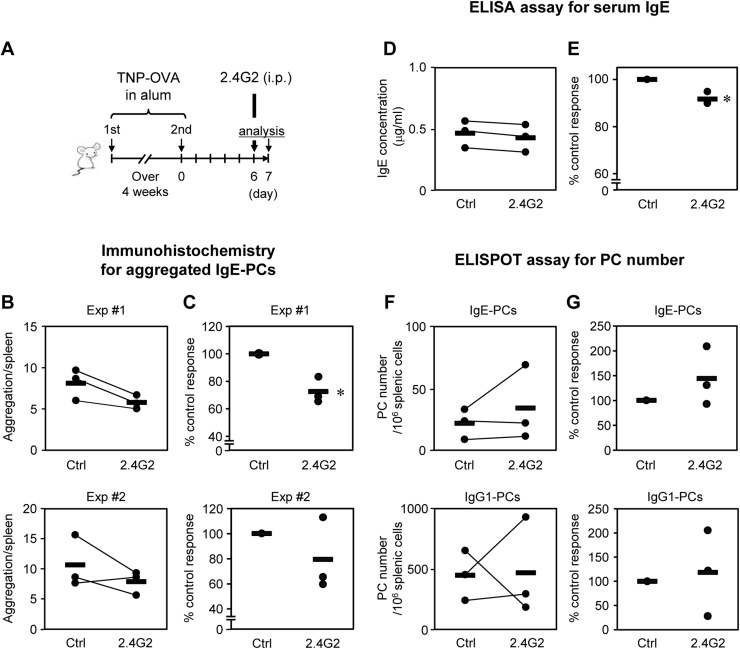
To investigate whether the in vivo IgE-PC aggregation was regulated by FcγRII/FcγRIII, we administered an antagonistic mAb against these receptors (2.4G2) to immunized mice one day before the analysis (Fig. 4A). The experiment was performed with three independent paired mice (one control mouse and one 2.4G2-injected mouse). The 2.4G2-treatment tended to reduce the aggregate numbers (Fig. 4B). We then calculated the ratio of aggregates of 2.4G2-treated mice compared to that of control mouse in each pair. In this calculation, the percentages of IgE-PC aggregates were significantly reduced by the injection of 2.4G2 (Fig. 4C, Experiment #1). In the secondary experiment, a significant difference was not observed while there was a reduction tendency (Fig. 4C, Experiment #2). We then measured the serum IgE level collected on the 7th day. The measured values of serum IgE made little difference between PBS- and 2.4G2- treated mice (Fig. 4D). However, as above, the calculation of the ratio of serum IgE levels in 2.4G2-treated mice against that of control mouse in each pair showed the significant reduction (Fig. 4E).
Fig. 4.
Administration of an anti-FcγRII/FcγRIII mAb tended to reduce splenic IgE-PC aggregation and serum IgE. (A) Experimental design. Immunized mice were injected intraperitoneally with an antagonistic mAb against FcγRII/FcγRIII (2.4G2, 200 μg/mouse) or the same volume of PBS (500 μl) one day before the analyses. Each experiment was performed with 3 pairs of a PBS- and a 2.4G2-injected mouse that were immunized and sampled in different days. (B, C) The numbers of IgE-PC aggregates (clusters consisting of more than three strongly IgE+ cells) in an entire spleen section were counted. The results of two independent experiments were shown. (D–G) The serum IgE levels (D, E) and the numbers of splenic IgE- and IgG1-PCs (F, G) in each pair of PBS- and 2.4G2-injected mice from total one experiment were shown. The data of each mouse showed a mean of triplicate measurements. The bars represent the mean of three mice. Significant differences were determined by the unpaired one-tailed Student's t-test. *p<0.05. (B, D, F) Actual measured values were shown. (C, E, G) The percentages of responses of 2.4G2-treated mice relative to their paired control mice were calculated.
The numbers of IgE- and IgG1-PCs in the spleen were comparable between PBS- and 2.4G2-injected immunized mice (Figs. 4F, 4G). These data suggested that their reduction tendencies may not be due to the inhibition of the quantitative regulation, that is, IgE-PCs’ increment.
These data suggested the possibility that the some IgE-PC aggregation in vivo may in part require FcγRII/FcγRIII and may be involved in the acceleration of IgE production, like IGEL b4 cells.
4. Discussion
In this study, we demonstrated that Ag-IgE complexes induced the homotypic cell aggregation of an IgE-hybridoma cell line via FcγRII/FcγRIII, leading to the acceleration of the Ab production in conjunction with LPS stimulation. The homotypic IgE-PC aggregation also occurred in spleens of immunized mice, and in vivo the injection of the anti-FcγRII/FcγRIII antagonistic mAb showed a tendency to reduce the splenic IgE-PC aggregation and the serum IgE levels. These results suggested the presence of the novel regulation of IgE production in IgE-PCs.
Cell aggregation was dependent on the cell metabolism but independent of de novo protein synthesis, suggesting that newly synthesized adhesion molecules after the induction of cell aggregation may not be required. The expression of several cell adhesion molecules, such as integrins and intercellular adhesion molecule-1 were not also upregulated in the aggregated IGEL b4 cells (Supplementary Fig. S7). It is possible that the conformational change of adhesion molecules such as integrins induced by FcγR signaling is involved in the IGEL b4 cell aggregation [16].
FcγRII and FcγRIII are known to be expressed on several cell types, and are involved in the promotion of differentiation into PCs [17], [18]. We injected 2.4G2 into immunized mice one day before the analysis and demonstrated the decrease tendency of serum IgE level and the aggregated IgE-PCs, even though the total numbers of IgE- and IgG1-PCs were not altered. Thus, our results suggest that in our experimental system, the inhibition of FcγRII/FcγRIII did not have an effect on the quantitative regulation, meaning that FcγRII and/or FcγRIII on IgE-PCs may function as the qualitative regulator of IgE production.
FcγRII/FcγRIII are classified as activating receptors (FcγRIII) and inhibitory receptors (FcγRII) by the presence of immunoreceptor tyrosine-based activation motif and immunoreceptor tyrosine-based inhibition motif, respectively, in their cytoplasmic domains [15]. IgM- and IgG-PCs are known to express FcγRIIb, but not FcγRIII, and its signaling has been shown to negatively regulate the number of PCs and the serum Ab level in vivo [19]. Therefore, the signaling via FcγRs in those PCs is generally considered to be a negative regulator of PC functions. In contrast, our data indicated the positive regulation of Ab production in IgE-PCs by FcγRII/FcγRIII, although it is still unclear which receptor is expressed on the IgE-PCs in vivo. Our results suggest that FcγRII/FcγRIII signaling may have the opposing function in the regulation of Ab production between IgM- and IgG-PCs (inhibitory) and IgE-PCs (promoting).
IGEL b4 expressed membrane-bound IgE mRNA, much like IgE-PCs in vivo, while membrane-bound IgE was not implicated in the Ag-induced IGEL b4 cell aggregation. Instead, Ag-IgE complexes bound to FcγRs were required for the cell aggregation. Addition of Ag-IgG1 complexes in culture also induced IGEL b4 cell aggregation in the short-term aggregation assay (Supplementary Fig. S8), suggesting that IgE-PC aggregation might not be restricted to Ag-specificity of IgE-PCs, but required for the activation of FcγR signaling, regardless of the isotype.
Both FcγRII and FcγRIII are known to bind to the Ag-Ab complexes composed of IgG1, IgG2a, IgG2b and IgE, but not to bind to monomeric Abs [20], [21]. Therefore, if there are monomeric IgGs with different specificities surrounding IgE-PCs in vivo, the binding of Ag-IgE or Ag-IgG complexes to FcγRs will preferentially occur and the cell aggregation will not be inhibited in the presence of monomeric IgGs with different specificity.
The amount of serum Ag-specific IgGs are larger than IgE [2], and the binding affinities of Ag-IgG complexes to FcγRs are higher than those of Ag-IgE complexes [22]. Our results suggested that Ag-IgG1 was also able to induce the IGEL b4 cell aggregation. Therefore, it is tempting to speculate that IgE-PC aggregation involves mainly Ag-IgG complexes. However, since local concentration of Ag-specific IgE surrounding the IgE-PCs should be high, the majority of Ag-Ig complexes inducing the cell aggregation might be the Ag-IgE complexes formed with the self-produced IgE.
Aggregation assays demonstrated that only IGEL b4 cells in the three tested IgE-hybridomas formed cell aggregates. Even in IGEL b4 cells, only a fraction of the cells participated in cell aggregation, resulting in the acceleration of Ab production in this cell fraction. Understanding this heterogeneity may give us clues as to why not all IgE-PCs formed cell aggregates.
IgE production via cell aggregation required additional LPS stimulation, suggesting that both signals via cell aggregation and pattern recognition receptors (PRR) may be required for in vivo qualitative regulation. We used alum instead of LPS as the adjuvant for in vivo immunization, but alum is known to active some PRRs [23]. Therefore, the enhancement of IgE production by IgE-PCs may also involve the signal activation via cell aggregation and PRRs.
Finally, patients with allergic diseases are known to have increased Ag-IgE and Ag-IgG1 complexes in their serum [24]. Moreover, endotoxin exposure is frequently associated with exacerbation of Th2 responses (i.e. upregulation of IgE) [25], [26]. Therefore, it will be important to elucidate the roles of FcγRs and the PRR on IgE-PCs in the qualitative control of IgE production.
Acknowledgments
We thank Dr. Hajime Karasuyama (Tokyo Medical and Dental University), Dr. Masaki Hikida (Akita University), Dr. Shinsuke Taki (Shinshu University), Dr. Sho Yamasaki (Kyushu University), Dr. Hitoshi Ohmori (Okayama University), Dr. Shiro Ono (Osaka Ohtani University), Dr. Kensuke Miyake (The University of Tokyo) and Dr. Katsuhiko Ishihara (Kawasaki Medical School) for materials; Dr. Kazuki Okuyama (Linkoping University, Sweden), Dr. Soichiro Yoshikawa (Tokyo Medical and Dental University) for the helpful discussion; and Ms. Toshie Shinohara (Tottori University) for the technical assistance. This work was supported by grants from JSPS KAKENHI [grant numbers 26460488 (SIH) and 15K19076 (AM)] and Tottori University (MY).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.04.007.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
Supplementary material
References
- 1.Gould H.J., Ramadani F. IgE responses in mouse and man and the persistence of IgE memory. Trends Immunol. 2015;36:40–48. doi: 10.1016/j.it.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Yang Z., Sullivan B.M., Allen C.D. Fluorescent in vivo detection reveals that IgE(+) B cells are restrained by an intrinsic cell fate predisposition. Immunity. 2012;36:857–872. doi: 10.1016/j.immuni.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Horst A., Hunzelmann N., Arce S. Detection and characterization of plasma cells in peripheral blood: correlation of IgE+ plasma cell frequency with IgE serum titre. Clin. Exp. Immunol. 2002;130:370–378. doi: 10.1046/j.1365-2249.2002.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorner M., Brandt S., Tinguely M. Plasma cell toll-like receptor (TLR) expression differs from that of B cells, and plasma cell TLR triggering enhances immunoglobulin production. Immunology. 2009;128:573–579. doi: 10.1111/j.1365-2567.2009.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rozanski C.H., Arens R., Carlson L.M. Sustained antibody responses depend on CD28 function in bone marrow-resident plasma cells. J. Exp. Med. 2011;208:1435–1446. doi: 10.1084/jem.20110040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angelin-Duclos C., Cattoretti G., Lin K.I. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J. Immunol. 2000;165:5462–5471. doi: 10.4049/jimmunol.165.10.5462. [DOI] [PubMed] [Google Scholar]
- 7.Vinuesa C. García De, Gulbranson-Judge A., Khan M. Dendritic cells associated with plasmablast survival. Eur. J. Immunol. 1999;29:3712–3721. doi: 10.1002/(SICI)1521-4141(199911)29:11<3712::AID-IMMU3712>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 8.Hasbold J., Corcoran L.M., Tarlinton D.M. Evidence from the generation of immunoglobulin G-secreting cells that stochastic mechanisms regulate lymphocyte differentiation. Nat. Immunol. 2004;5:55–63. doi: 10.1038/ni1016. [DOI] [PubMed] [Google Scholar]
- 9.Milcarek C., Albring M., Langer C. The eleven-nineteen lysine-rich leukemia gene (ELL2) influences the histone H3 protein modifications accompanying the shift to secretory immunoglobulin heavy chain mRNA production. J. Biol. Chem. 2011;286:33795–33803. doi: 10.1074/jbc.M111.272096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He J.S., Meyer-Hermann M., Xiangying D. The distinctive germinal center phase of IgE+ B lymphocytes limits their contribution to the classical memory response. J. Exp. Med. 2013;210:2755–2771. doi: 10.1084/jem.20131539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dang L.H., Rock K.L. Stimulation of B lymphocytes through surface Ig receptors induces LFA-1 and ICAM-1-dependent adhesion. J. Immunol. 1991;146:3273–3279. [PubMed] [Google Scholar]
- 12.Inaba K., Romani N., Steinman R.M. An antigen-independent contact mechanism as an early step in T cell-proliferative responses to dendritic cells. J. Exp. Med. 1989;170:527–542. doi: 10.1084/jem.170.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassese G., Arce S., Hauser A.E. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 2003;171:1684–1690. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 14.Ishizaka K., Ishizaka T., Menzel A.E. Physicochemical properties of reaginic antibody. VI. Effect of heat on gamma-E-, gamma-G- and gamma-A-antibodies in the sera of ragweed sensitive patients. J. Immunol. 1967;99:610–618. [PubMed] [Google Scholar]
- 15.Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119:5640–5649. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- 16.Botelho R.J., Harrison R.E., Stone J.C. Localized diacylglycerol-dependent stimulation of Ras and Rap1 during phagocytosis. J. Biol. Chem. 2009;284:28522–28532. doi: 10.1074/jbc.M109.009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohmori H., Hase N., Hikida M. Enhancement of antigen-induced interleukin 4 and IgE production by specific IgG1 in murine lymphocytes. Cell. Immunol. 1992;145:299–310. doi: 10.1016/0008-8749(92)90333-k. [DOI] [PubMed] [Google Scholar]
- 18.Qin D., Wu J., Vora K.A. Fc gamma receptor IIB on follicular dendritic cells regulates the B cell recall response. J. Immunol. 2000;164:6268–6275. doi: 10.4049/jimmunol.164.12.6268. [DOI] [PubMed] [Google Scholar]
- 19.Xiang Z., Cutler A.J., Brownlie R.J. FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nat. Immunol. 2007;8:419–429. doi: 10.1038/ni1440. [DOI] [PubMed] [Google Scholar]
- 20.Nimmerjahn F., Ravetch J.V. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 21.Mancardi D.A., Iannascoli B., Hoos S. FcgammaRIV is a mouse IgE receptor that resembles macrophage FcepsilonRI in humans and promotes IgE-induced lung inflammation. J. Clin. Invest. 2008;118:3738–3750. doi: 10.1172/JCI36452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jönsson F., Daëron M. Mast cells and company. Front. Immunol. 2012;3:16. doi: 10.3389/fimmu.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maisonneuve C., Bertholet S., Philpott D.J. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc. Natl. Acad. Sci. USA. 2014;111:12294–12299. doi: 10.1073/pnas.1400478111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens W.J., Bridts C.H. IgG-containing and IgE-containing circulating immune complexes in patients with asthma and rhinitis. J. Allergy Clin. Immunol. 1984;73:276–282. doi: 10.1016/s0091-6749(84)80020-2. [DOI] [PubMed] [Google Scholar]
- 25.Min K.B., Min J.Y. Exposure to household endotoxin and total and allergen-specific IgE in the US population. Environ. Pollut. 2015;199:148–154. doi: 10.1016/j.envpol.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Sahiner U.M., Semic-Jusufagic A., Curtin J.A. Polymorphisms of endotoxin pathway and endotoxin exposure: in vitro IgE synthesis and replication in a birth cohort. Allergy. 2014;69:1648–1658. doi: 10.1111/all.12504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material