Abstract
Background
Out-of-hospital hypotension has been associated with increased mortality in traumatic brain injury (TBI). The association of TBI mortality with the depth or duration of out-of-hospital hypotension is unknown.
Methods
We evaluated adults and older children with moderate/severe TBI in the pre-implementation cohort of Arizona’s statewide Excellence in Prehospital Injury Care (EPIC) Study. We used logistic regression to determine the association between the depth-duration dose of hypotension [depth of systolic blood pressure <90 mmHg integrated over duration (minutes) of hypotension] and odds of in-hospital death, controlling for significant confounders.
Results
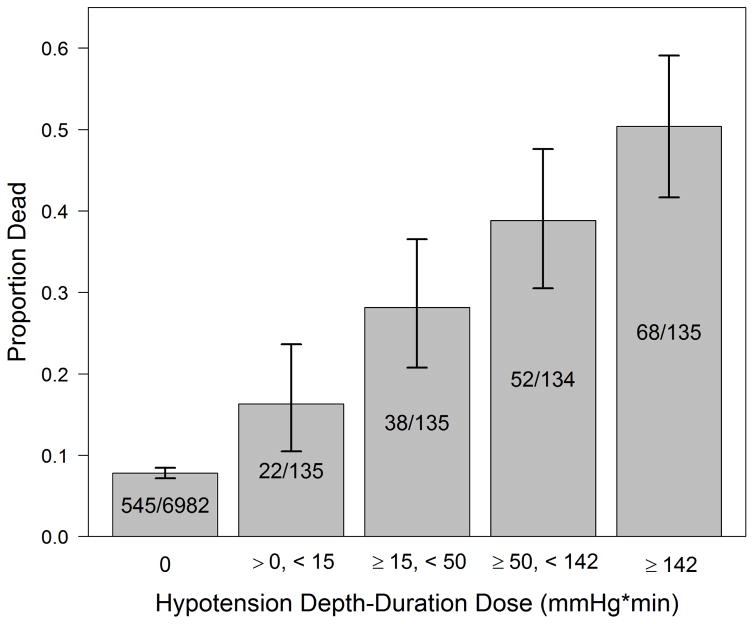
There were 7,521 TBI cases included [70.6% male, median age 40 (interquartile range 24, 58)]. Mortality was 7.8% (95%CI: 7.2–8.5%) among the 6982 cases without hypotension (SBP≥90 mmHg) and 33.4% (95%CI: 29.4–37.6%) among the 539 hypotensive cases (SBP<90 mmHg). Mortality was higher with increased hypotension dose: 0.01–14.99 mmHg*min, 16.3%; 15–49.99, 28.1%; 50–141.99, 38.8%; ≥142, 50.4%. Log2 (the logarithm in base 2) of hypotension dose was associated with TBI mortality [adjusted odds ratio 1.19 (95% CI: 1.14–1.25) per two-fold increase of dose].
Conclusion
In this study, the depth and duration of out-of-hospital hypotension were associated with increased TBI mortality. Assessments linking out-of-hospital blood pressure with TBI outcomes should consider both depth and duration of hypotension.
INTRODUCTION
Background
During the out-of-hospital and early in-hospital resuscitative care of Traumatic Brain Injury (TBI), hypotension is associated with increased mortality.1–31 The literature supporting this concept is based upon small series with only limited Emergency Medical Services (EMS) data that characterized hypotension on a dichotomous basis (</≥ 90 mmHg).3,16,21,28–31 Thus, very little is known about the impact of the depth of hypotension. Another limitation of these studies is the absence of repeated blood pressure measurements. Because of this, there are no descriptions of the depth and duration of out-of-hospital hypotension in TBI patients.
Importance
Hypotension is believed to reduce cerebral perfusion pressure to the injured brain.4,6,11,26,32 While not yet characterized, the extent of brain injury is likely linked to both the depth and duration of hypotensive episodes. Quantification of hypotension dose could offer an additional therapeutic target for refining out-of-hospital TBI care.
Goal of this Study
The objective of this study was to determine the association of out-of-hospital hypotension depth and duration with TBI mortality.
MATERIALS AND METHODS
Setting-The EPIC Study
Details of the Excellence in Prehospital Injury Care (EPIC) Study have been described previously.33–35 EPIC is evaluating the impact of implementing the EMS TBI guidelines36–39 in patients with major TBI throughout Arizona using a before-after, controlled, multisystem, observational design33 (ClinicalTrials.gov #NCT01339702). We obtained the necessary regulatory approvals for EPIC from the Arizona Department of Health Services (ADHS) and the State Attorney General. The University of Arizona Institutional Review Board and the ADHS Human Subjects Review Board have approved the project and have determined that, by virtue of being a public health initiative, neither the interventions nor their evaluation constitute human subjects research and have approved the publication of de-identified data.
Selection of Subjects
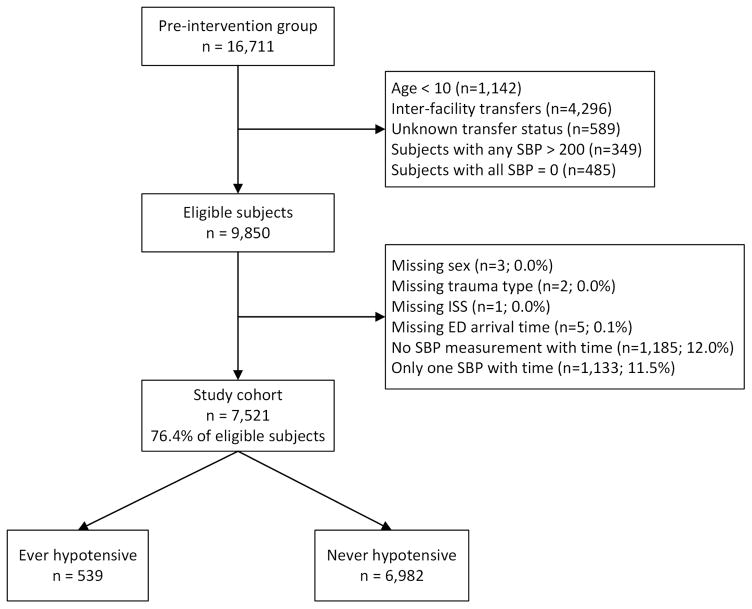
The patients in this evaluation are in the pre-implementation cohort of EPIC (treated by an EMS agency between 1/1/2007 and 3/31/2014 without receiving EPIC Study interventions). In this secondary analysis we included patients 10 years of age or older with physical trauma who have trauma center (TC) diagnosis(es) consistent with TBI (isolated or multisystem trauma) and meet at least one of the following definitions for moderate or severe (“major”) TBI: a) Centers for Disease Control (CDC) Barell Matrix-Type 1, b) International Classification of Diseases-Version 9 (ICD-9) head region severity score ≥3, c) Abbreviated Injury Scale (AIS)-head region score ≥3.33,34 We excluded cases with age <10 years, inter-facility transfer (or unknown), any SBP >200mmHg, SBP of 0 indicating traumatic arrest, missing important confounders/risk-adjusters, and zero or only one recorded out-of-hospital SBP with documented time between six hours before ED arrival to 10 minutes after ED arrival (excludes extreme or obviously-inaccurate time data). The patients with only one timed, recorded SBP measurement were excluded because at least two are needed to establish depth-duration dose.
Methods of Measurement
The EPIC Database contains the subset of patients from the Arizona State Trauma Registry (ASTR) meeting EPIC Study criteria for major TBI (defined above).33–35 The ASTR has detailed in-hospital data on all trauma patients taken to the state-designated level I TCs in Arizona. All cases from the ASTR that meet EPIC criteria are entered into the database. Each participating EMS agency receives the list of EPIC patients cared for in their system. The cases are matched by incident date, name, and other identifiers. Either scanned copies [paper-based patient care records (PCRs)] or electronic data files (e-PCRs) are sent to the EPIC Data Center. Database personnel then comprehensively abstract and enter the data yielding an extensive, linked dataset that includes both EMS and TC data. The processes of case identification, linkage, data entry, and data quality management have been reported in detail.33 We have enrolled over 20,000 cases into the EPIC study and the ASTR/EMS data linkage rate is well over 90%.
We included all SBP measurements with a recorded value and time. When multiple agencies cared for a given patient, we combined all available measurements. Patients that had at least two timed SBPs were included in this analysis. We excluded cases with only one recorded SBP since the duration of hypotension could not be accurately estimated in these.
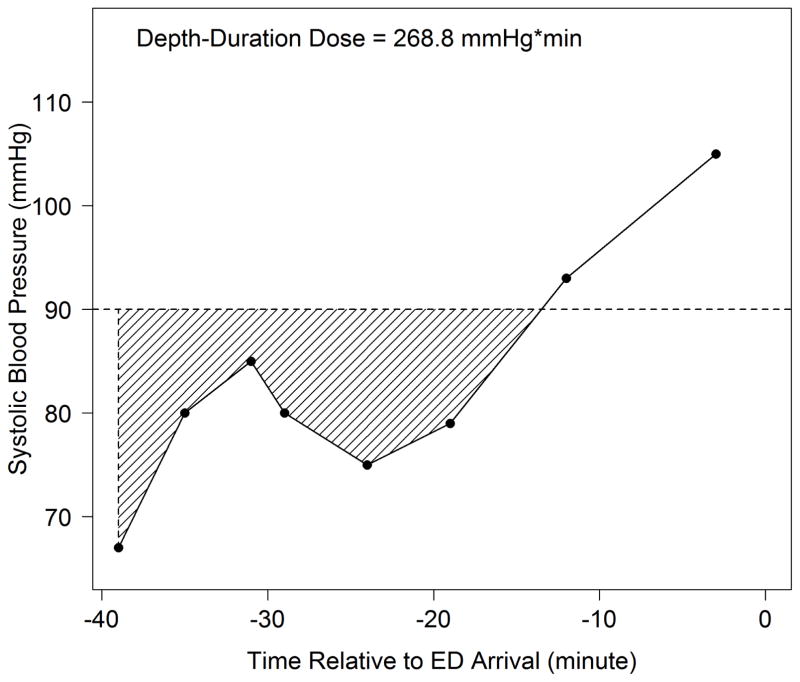
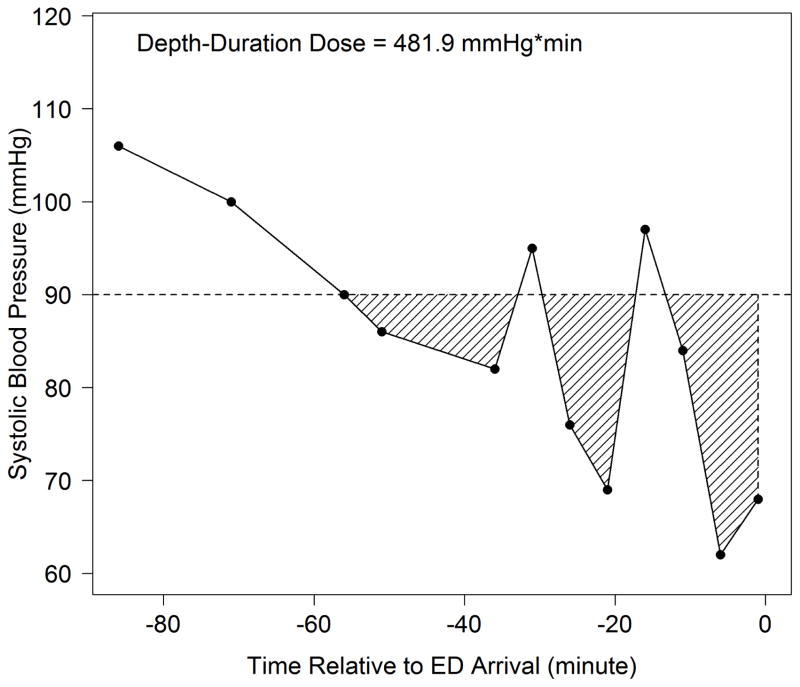
Our strategy for determining hypotension dosage was modeled after pharmacokinetic techniques.40 We defined hypotension depth-duration as the total amount of systolic hypotension (SBP<90mmHg) accumulated over a given time. Hypotensive “depth” referred to the difference between 90mmHg and the measured value. “Duration” referred to the total time during which SBP was <90mmHg. To calculate the depth-duration dose, we linked consecutive SBP measurements over time, calculating hypotension dose as the integrated “area under the curve” for values below 90mmHg. (Figure 1) In situations with multiple separate hypotensive episodes, we added the integrated values from all hypotensive segments. (Figure 2)
Figure 1. Depth-Duration Dose Plot from a Study Patient.
Depth-Duration Dose = Total area of shaded region under 90 mmHg
When calculating the dose, if either the first (as in this case) or last recorded SBP is a hypotensive value, the shaded region is closed by a vertical line passing through this point
Figure 2. Depth-Duration Dose Plot from a Study Patient with Multiple Hypotensive Episodes.
Depth-Duration Dose = Total area of shaded region under 90 mmHg
When calculating the dose, if either the first or last (as in this case) recorded SBP is a hypotensive value, the shaded region is closed by a vertical line passing through this point
This case shows a patient with three separate hypotensive episodes where the total dose is the sum of the AUC from all of the shaded regions
Outcome
The primary outcome was survival to hospital discharge.33 Deaths that occurred after hospital discharge were not included in the analysis.
Data Analysis
We determined TBI mortality for the cohort and the quartile of hypotension dose. We then examined the association between mortality and dose by logistic regression, adjusting for potential confounders. Age, sex, race, ethnicity, Injury Severity Score (ISS), and head region injury score (International Classification of Diseases-Version 9 matched to Abbreviated Injury Scale score)41–43 were included, a priori, in the model (since they have been used nearly universally in trauma risk adjustment). Trauma type (blunt versus penetrating), payment source, and treating TC were included because they have often been confounders in trauma outcome studies44,45 and were found to be significant covariates in previous EPIC reports.34,35
Because of the skewed distribution of hypotension dose, we log-transformed hypotension dose [log2(dose+1)]. This approach yields a value of 0 for patients without hypotension, and positive values for hypotensive cases. The effects of the log2 hypotension dose and age in the regression were fitted non-parametrically using penalized thin plate regression splines through the generalized additive model,46 with the smoothing parameter chosen to optimize the Akaike information criterion (AIC). Nested models were compared using an analysis of deviance table. We assessed the fitted model by deviance residual plots and the area under the receiver operational characteristic curve (AUC) with 95% CI obtained by the Delong method.47 We checked for collinearity using variance inflation factors for the parametric terms and concurvity for the nonparametric term. Mixed effect models were used to assess the impact of the correlation of subjects treated by the same TC.
We evaluated the predictive power of the hypotension dose by first fitting a logistic regression model for survival with demographic variables as predictors (Model 1), then adding the binary hypotensive indicator (</≥90 mmHg) as another predictor (Model 2), and then adding dose [log2(hypotension dose+1)] (Model 3). The AUC was estimated for each model. We further evaluated predictive power by comparing different models using the continuous Net Reclassification Improvement (NRI)48, with 95% CI estimated by the bootstrap method.
We used the software environment R for the analysis49 and the R package mgcv46,50 for the generalized additive model.
MAIN RESULTS
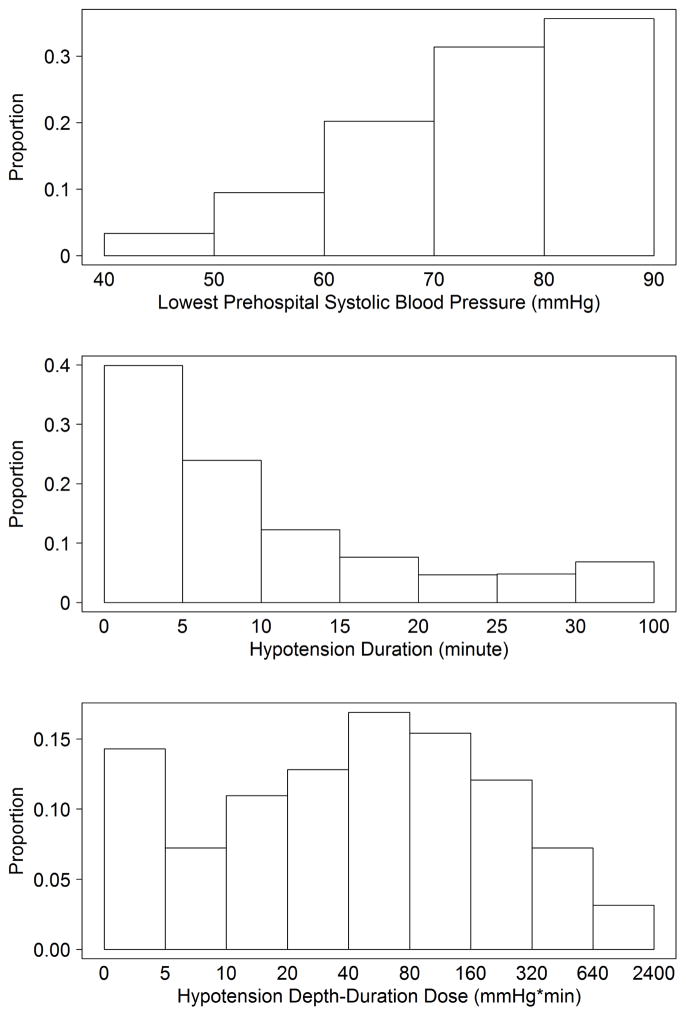
Among 16,711 TBI subjects, we included 7,521 in the analysis (Figure 3). Median age was 40 years (IQR: 24, 57), 70.6% were male, and overall mortality was 9.6% (95% CI: 9.0–10.3%). In the study group, 539 (7.2%) had hypotension. Among those with no hypotension, 7.8% died (95%CI: 7.2–8.5%) compared to 33.4% (95%CI: 29.4–37.6%) in the group with at least one hypotensive episode. Demographics and patient characteristics are shown in Table 1 (by hypotension status) and Appendix 1 (by survival status). Figure 4 shows the distribution of 1) depth, 2) duration, and 3) dose among the 539 hypotensive patients. All factors associated with hypotension status were also associated with risk of death (trauma type, head region injury score, ISS, out-of-hospital hypoxia), while age and payment source were associated with death but not hypotension status. As with previous reports, risk-adjusted outcomes varied among TCs.44,45 Thus, we adjusted for it in the model.
Figure 3. Case Inclusion/Exclusion Flow Chart.
SBP = systolic blood pressure
ISS = Injury Severity Score
Table 1.
Patient Characteristics by Hypotension Status
| Group | Never Hypotensive# @ | Ever Hypotensive# @ | |
|---|---|---|---|
| # of Subjects | N=6982 | N=539 | |
| Age (years) | 40 (24, 58) | 37 (23, 55) | |
| Male | No | 2047 (29.3%) | 161 (29.9%) |
| Yes | 4935 (70.7%) | 378 (70.1%) | |
| Race | Black | 234 (3.4%) | 10 (1.9%) |
| Asian | 68 (1%) | 8 (1.5%) | |
| American Indian/Alaska Nat. | 388 (5.6%) | 39 (7.2%) | |
| White | 5373 (77%) | 412 (76.4%) | |
| Other | 843 (12.1%) | 59 (10.9%) | |
| Unknown | 76 (1.1%) | 11 (2%) | |
| Hispanic | No | 5256 (75.3%) | 400 (74.2%) |
| Yes | 1512 (21.7%) | 114 (21.2%) | |
| Unknown | 214 (3.1%) | 25 (4.6%) | |
| Payer | Private | 2593 (37.1%) | 196 (36.4%) |
| AHCCCS+/Medicaid | 1805 (25.9%) | 154 (28.6%) | |
| Medicare | 1062 (15.2%) | 64 (11.9%) | |
| Self Pay | 1084 (15.5%) | 84 (15.6%) | |
| Other | 299 (4.3%) | 26 (4.8%) | |
| Unknown | 139 (2%) | 15 (2.8%) | |
| Trauma Type | Blunt | 6685 (95.7%) | 463 (85.9%) |
| Penetrating | 297 (4.3%) | 76 (14.1%) | |
| Head Injury Severity Score (ICD&) | 1–3 | 4043 (57.9%) | 207 (38.4%) |
| 4 | 1835 (26.3%) | 110 (20.4%) | |
| 5–6 | 1027 (14.7%) | 209 (38.8%) | |
| Unknown | 77 (1.1%) | 13 (2.4%) | |
| Injury Severity Score (ICD&) | 1–14 | 2954 (42.3%) | 81 (15%) |
| 16–24 | 2147 (30.8%) | 100 (18.6%) | |
| 25+ | 1881 (26.9%) | 358 (66.4%) | |
| Hypotension dose (mmHg*min) | 0 (0, 0) | 49 (15, 142.5) | |
| Prehospital hypoxia | No | 6205 (88.9%) | 348 (64.6%) |
| Yes | 480 (6.9%) | 147 (27.3%) | |
| Unknown | 297 (4.3%) | 44 (8.2%) | |
Median (IQR) for continuous variables and count (percentage) for categorical variables
Hypotension defined as systolic blood pressure (SBP) <90 mmHg
Arizona Health Care Cost Containment System
International Classification of Diseases-Version 9
Figure 4. Distribution of Hypotension Depth, Duration, and Dose Across the Hypotensive Cohort.
Histograms showing the proportions of hypotensive patients by depth, duration, and dose of hypotension
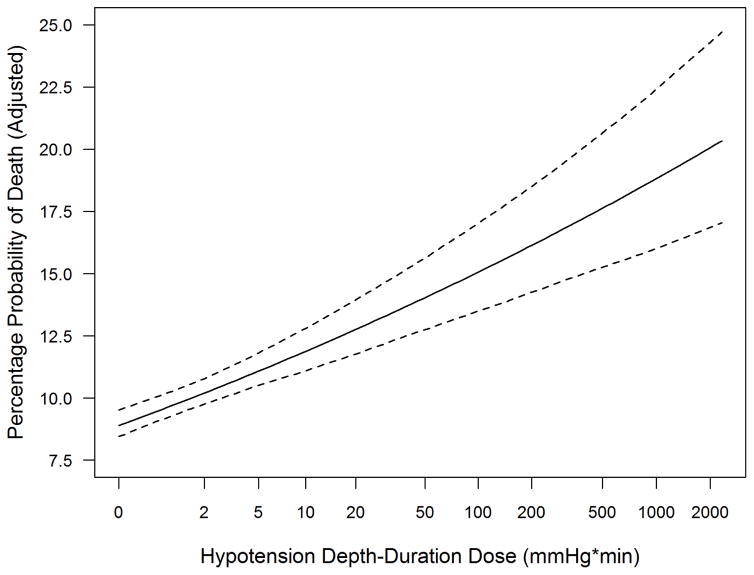
The unadjusted probability of death increased with higher hypotension dose (Figure 5). We used logistic regression to examine the association between log2 dose and the risk of death, controlling for potential confounders, with the effects of the continuous variables (log2 dose and age) modeled as nonparametric functions. We observed a monotonically increasing linear relationship between log2 dose and log odds of death [aOR=1.19 (95% CI: 1.14–1.25) per two-fold hypotension dose increase]. (Table 2, Figure 6)
Figure 5. Unadjusted Death Proportion by Hypotension Dose Categories.
Error bars represent 95% confidence intervals
Hypotension defined as systolic blood pressure <90 mmHg
Table 2.
Logistic Regression Model for Survival Status
| Variable* | Category | OR# | 95% CI |
|---|---|---|---|
| log2(SBP dose +1) | --- | 1.19 | (1.14, 1.25) |
| Male | No | --- | --- |
| Yes | 0.95 | (0.74, 1.21) | |
| Race | Black | --- | --- |
| Asian | 1.77 | (0.51, 6.15) | |
| American Indian/Alaska Nat. | 2.07 | (0.92, 4.65) | |
| White | 2.33 | (1.19, 4.57) | |
| Other | 2.38 | (1.08, 5.24) | |
| Unknown | 3.41 | (1.14, 10.23) | |
| Hispanic | No | --- | --- |
| Yes | 0.55 | (0.39, 0.79) | |
| Unknown | 1.43 | (0.78, 2.60) | |
| Payer | Private | --- | --- |
| AHCCCS+/Medicaid | 0.95 | (0.71, 1.29) | |
| Medicare | 1.16 | (0.78, 1.74) | |
| Self Pay | 3.27 | (2.31, 4.61) | |
| Other | 1.56 | (0.95, 2.57) | |
| Unknown | 2.86 | (1.44, 5.65) | |
| Trauma Type | Blunt | --- | --- |
| Penetrating | 4.96 | (3.54, 6.95) | |
| Head Region Severity Score (ICD&) | 1–3 | --- | --- |
| 4 | 1.17 | (0.77, 1.78) | |
| 5–6 | 14.21 | (9.64, 20.96) | |
| Unknown | 6.29 | (2.70, 14.64) | |
| Injury Severity Score (ICD&) | 1–14 | --- | --- |
| 16–24 | 4.92 | (2.18, 11.09) | |
| 25+ | 23.58 | (10.88, 51.11) | |
| Prehospital Hypoxia | No | --- | --- |
| Yes | 2.47 | (1.88, 3.24) | |
| Unknown | 2.91 | (1.96, 4.31) | |
| Age | Fitted non-parametrically | ||
Also adjusted for treating trauma centers (details not shown)
Odds ratio for death associated with 1 unit increase in continuous variable, or compared to the referent category for categorical variables
Arizona Health Care Cost Containment System
International Classification of Diseases-Version 9
Figure 6. Relationship of Hypotension Depth-Duration Dose to Adjusted Probability of Death.
Dotted lines represent pointwise 95% confidence band
Hypotension defined as systolic blood pressure <90 mmHg
x-axis is log2 scale
Deviance residual plots did not indicate any deviation from the model assumptions. The effect of dose (after transformation), when fitted as a nonparametric function, was not statistically different from a simple linear function. The AUC was estimated to be 0.952 (95% CI: 0.945–0.958), indicating a high discriminative ability of the model. No multicollinearity was detected in the covariates.
As a sensitivity analysis, random TC effects were included in the model (instead of fixed effects) to explore the potential correlation among subjects treated by the same TC. The differences were minimal with a change in the estimated OR for log2 dose of only 0.1% and in the standard error estimate for the corresponding regression coefficient of only 0.5%. Among the eight TCs, there was an average of 940 subjects per site and the intraclass correlation coefficient for the TC effect was 0.066. In a separate sensitivity analysis, instead of log2 hypotension dose we included the standardized hypotension dose [dose minus the sample mean, then divided by the standard deviation (SD)] in the logistic regression. The resulting inferences were similar (aOR 1.27 per SD increase in hypotension dose; 95% CI: 1.17–1.37). (Appendix 2).
In a model with only basic demographic variables as predictors, the AUC was 0.585 (95% CI: 0.563–0.607). Adding binary hypotension (SBP<90 vs. SBP≥90) improved AUC to 0.6635 (95% CI: 0.6409–0.6860) and the NRI was 39.1% (95% CI: 32.5%–45.5%). When hypotension dose [log2(dose + 1)] was added to the binary model, the AUC improved slightly to 0.6638 (95% CI: 0.6411–0.6865) the NRI was 8.1% (95% CI: −5.6%–21.8%) for the dose-based model over the binary model. When limiting the analysis to the 539 subjects with hypotension, the basic model had an AUC of 0.616 (95% CI: 0.566–0.666). Addition of hypotension dose improved AUC to 0.707 (95% CI: 0.659–0.754) and the NRI was 47.5% (95% CI: 27.5%–69.8%).
LIMITATIONS
This study has limitations. The design is observational and, thus, we cannot determine whether the treatment of hypotension is effective at reducing mortality (this hypothesis is part of the main study). However, this analysis does allow us, for the first time, to identify significant associations between the dose of hypotension and outcome.
There are missing data. While the missing rate for EMS SBP measurements is very low (<5%),51 the addition of the requirement for two timed SBPs for this analysis led to a rate of 23.6% (Figure 3). The database only contains measurements that were documented by EMS personnel and we cannot independently verify the accuracy of the measurements. However, the data are abstracted directly, consistently, and comprehensively from the PCRs. This level of data collection scrutiny is rare in EMS research.51
The hypotension dose estimate is impacted by how frequently BP was measured. Indeed, we found that a low measurement was more likely to be repeated quickly which would lead to a more accurate estimation of the dose. However, the fact that non-hypotensive values tended to lead to fewer repeat measurements is not likely to have significantly affected our findings since the dose in non-hypotensive patients is zero regardless of how many times BP was measured. Finally, we did not evaluate the effects of treatment. Future studies will assess the influence of TBI care on outcomes.
DISCUSSION
It is well established that out-of-hospital hypotension is associated with increased TBI mortality.3,16,21,28–31,34,38,52 However, the literature that has shaped this understanding has evaluated hypotension as a simple dichotomy (</≥90 mmHg).3,16,21,28–31,38 Currently there are no published reports with data evaluating the impact of either the depth or the duration of out-of-hospital hypotension. The paucity of knowledge related to these parameters in the field is reflected in the most recent EMS TBI treatment guidelines which state that a major area needing investigation is identifying “the critical values for duration and magnitude of hypotensive…episodes.”38,53 Our study offers one of the first assessments of the association between hypotension dose and TBI outcomes. These findings add to the growing evidence that close and frequent blood pressure monitoring and management may potentially contribute to improved TBI outcomes.4,7,8,15,23,30,32,33,36,38
The EPIC database contains all vital signs measurements and their associated times that are recorded on the EMS PCRs. The data entry system allows an unlimited number of data entries for vital signs.33,34 In fact, in this sub-study, there are patients with as many as 25 EMS BP measurements recorded in the database and the median number is four per patient. This feature allows the plotting of out-of-hospital blood pressure over time and, hence, an estimation of the depth and duration of hypotensive events (Figures 1, 2). These strengths allowed us to evaluate the hypotension dose as a novel measure.
Our study affirmed the presence of a dose-response association between hypotension dosage and mortality. The simple, unadjusted mortality rate increased significantly and consistently across the four quartiles (by dose) of hypotensive patients (Figure 5). Furthermore, a doubling of dose was associated with an aOR for death of 1.19 and this association held over a wide range of hypotension doses (Figure 6). Thus, with other factors being equal, in hypotensive TBI patients, a doubling of dose yielded a 19% increase in adjusted odds of death. For example, a case where SBP drops to 80 for 10 min (dose = 100mmHg*min) has 19% higher odds of dying than one with a dose of only 50mmHg-min (e.g. 85 for 10 min or 80 for 5 min).
Our findings not only provide evidence for the face validity of the dose-duration construct, but may also support the notion of minimizing both hypotension depth and duration during clinical care. Our findings did not show a marked improvement in model discrimination or NRI for the hypotensive dosage model compared with the binary hypotension model in the overall study group. However, we believe this was predictable since 92.8% of the subjects were non-hypotensive. Hence, this comparison is dominated by the non-hypotensive patients and only small improvement is expected when evaluating the entire study group no matter how well the dose model discriminates between hypotensive patients. On the other hand, in the assessment of the hypotensive cohort, the binary model becomes moot since all patients in this subgroup have the same value (positive for hypotension) and, unlike depth-duration dose, it has no discriminative value among hypotensive patients at all. The implementation phase of the larger EPIC study is applying the evidence-based guidelines for out-of-hospital TBI care. We plan to use the post-implementation cases not only to validate the current findings but also to identify alternate functional forms with clearer improvement of the dosage-based model over binary hypotension. For instance, since our previous work revealed a complete absence of an identifiable physiological threshold anywhere between an SBP of 40 and 120 mmHg,35 the discriminatory power of the model may improve when hypotension is defined as <100, <110, or <120 mmHg.53 Furthermore, when higher thresholds are evaluated, comparing the binary model versus the dose-based model in the overall study cohort will be pertinent since such a comparison will be much less likely to be dominated by the non-hypotensive subgroup. We will also be able to explore questions like whether it is better to be less hypotensive for longer or more hypotensive for a shorter time. The current study underscores the importance of hypotension dosage in TBI care and sets the stage for these future analyses.
Another important consideration is how to implement these findings into EMS practice. We hesitate to recommend specific measures until additional validation has identified the most accurate model. However, our results do identify the technical challenges at hand. Calculation of hypotension dosage requires real-time computer decision support. Current portable cardiac monitors are able to give real-time feedback such as CPR chest compression rate, depth, and fraction.54 Future efforts must consider the technological support required to implement the new measure in TBI patients.
In summary, this statewide, multisystem study of major TBI found that the depth and duration of out-of-hospital hypotension were strongly associated with increased mortality. Assessments linking out-of-hospital blood pressure with TBI outcomes should account for both the depth and duration of hypotension.
Supplementary Material
Acknowledgments
The authors express our deep gratitude to the exceptional, dedicated professionals in the EMS agencies and Level I Trauma Centers of Arizona.
Funding: Research reported in this publication was supported by the National Institute of Neurological Disorders And Stroke of the National Institutes of Health under Award Number R01NS071049. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Presented, in part, to the Resuscitation Symposium of the American Heart Association, New Orleans, LA, November 13, 2016
Registration: This is an observational, non-interventional analysis of a subset of the data in the EPIC study. The parent study, while not a randomized clinical trial, is registered at ClinicalTrials.gov: #NCT01339702
Disclosures: The University of Arizona receives funding from the NIH supporting the EPIC Study. This includes support for the following authors: DWS, BJB, VC, BB, JBG, KRD, PDA, CV, and DS. No COI to report: CH, SMK, TM and ADR.
Author Contributions: Study concept and design: DWS, BJB, JBG, KRD, PDA, CV, DS; Acquisition of the data: DWS, CH, BJB, VC, BB; Analysis and interpretation of the data: DWS, CH, BJB, VC, DS; Drafting of the manuscript: DWS, CH, BJB; Critical revision of the manuscript for important intellectual content: DWS, CH, BJB, VC, BB, JBG, KRD, SMK, PDA, CV, TM, ADR, DS; Statistical expertise: CH, DS, VC; Obtained funding: DWS, BJB, JBG, KRD, CV, DS; Administrative, technical, or material support: VC, BB, TM.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34(2):216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Jankowitz BT, Adelson PD. Pediatric traumatic brain injury: past, present and future. Dev Neurosci. 2006;28(4–5):264–275. doi: 10.1159/000094153. [DOI] [PubMed] [Google Scholar]
- 3.Fearnside MR, Cook RJ, McDougall P, McNeil RJ. The Westmead Head Injury Project outcome in severe head injury. A comparative analysis of pre-hospital, clinical and CT variables. Br J Neurosurg. 1993;7(3):267–279. doi: 10.3109/02688699309023809. [DOI] [PubMed] [Google Scholar]
- 4.Gentleman D. Causes and effects of systemic complications among severely head injured patients transferred to a neurosurgical unit. Int Surg. 1992;77(4):297–302. [PubMed] [Google Scholar]
- 5.Haddad S, Arabi Y, Al Shimemeri A. Initial management of traumatic brain injury. Middle East J Anesthesiol. 2005;18(1):45–68. [PubMed] [Google Scholar]
- 6.Shutter LA, Narayan RK. Blood Pressure Management in Traumatic Brain Injury. Annals of Emergency Medicine. 2008;51(3, Supplement 1):S37–S38. doi: 10.1016/j.annemergmed.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Pigula FA, Wald SL, Shackford SR, Vane DW. The effect of hypotension and hypoxia on children with severe head injuries. J Pediatr Surg. 1993;28(3):310–314. doi: 10.1016/0022-3468(93)90223-8. discussion 315–316. [DOI] [PubMed] [Google Scholar]
- 8.Kokoska ER, Smith GS, Pittman T, Weber TR. Early hypotension worsens neurological outcome in pediatric patients with moderately severe head trauma. J Pediatr Surg. 1998;33(2):333–338. doi: 10.1016/s0022-3468(98)90457-2. [DOI] [PubMed] [Google Scholar]
- 9.Miller JD, Becker DP. Secondary insults to the injured brain. J R Coll Surg Edinb. 1982;27(5):292–298. [PubMed] [Google Scholar]
- 10.Barton CW, Hemphill JC, Morabito D, Manley G. A novel method of evaluating the impact of secondary brain insults on functional outcomes in traumatic brain-injured patients. Acad Emerg Med. 2005;12(1):1–6. doi: 10.1197/j.aem.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 11.Manley G, Knudson MM, Morabito D, Damron S, Erickson V, Pitts L. Hypotension, hypoxia, and head injury: frequency, duration, and consequences. Arch Surg. 2001;136(10):1118–1123. doi: 10.1001/archsurg.136.10.1118. [DOI] [PubMed] [Google Scholar]
- 12.Johnson DL, Boal D, Baule R. Role of apnea in nonaccidental head injury. Pediatr Neurosurg. 1995;23(6):305–310. doi: 10.1159/000120976. [DOI] [PubMed] [Google Scholar]
- 13.Mayer TA, Walker ML. Pediatric head injury: the critical role of the emergency physician. Ann Emerg Med. 1985;14(12):1178–1184. doi: 10.1016/s0196-0644(85)81025-8. [DOI] [PubMed] [Google Scholar]
- 14.Ong L, Selladurai BM, Dhillon MK, Atan M, Lye MS. The prognostic value of the Glasgow Coma Scale, hypoxia and computerised tomography in outcome prediction of pediatric head injury. Pediatr Neurosurg. 1996;24(6):285–291. doi: 10.1159/000121057. [DOI] [PubMed] [Google Scholar]
- 15.Price DJ, Murray A. The influence of hypoxia and hypotension on recovery from head injury. Injury. 1972;3(4):218–224. doi: 10.1016/0020-1383(72)90104-0. [DOI] [PubMed] [Google Scholar]
- 16.Stocchetti N, Furlan A, Volta F. Hypoxemia and arterial hypotension at the accident scene in head injury. J Trauma. 1996;40(5):764–767. doi: 10.1097/00005373-199605000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Michaud LJ, Rivara FP, Grady MS, Reay DT. Predictors of survival and severity of disability after severe brain injury in children. Neurosurgery. 1992;31(2):254–264. doi: 10.1227/00006123-199208000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Levin HS, Aldrich EF, Saydjari C, et al. Severe head injury in children: experience of the Traumatic Coma Data Bank. Neurosurgery. 1992;31(3):435–443. doi: 10.1227/00006123-199209000-00008. discussion 443–434. [DOI] [PubMed] [Google Scholar]
- 19.Luerssen TG, Klauber MR, Marshall LF. Outcome from head injury related to patient’s age. A longitudinal prospective study of adult and pediatric head injury. J Neurosurg. 1988;68(3):409–416. doi: 10.3171/jns.1988.68.3.0409. [DOI] [PubMed] [Google Scholar]
- 20.Miller JD, Sweet RC, Narayan R, Becker DP. Early insults to the injured brain. Jama. 1978;240(5):439–442. [PubMed] [Google Scholar]
- 21.Carrel M, Moeschler O, Ravussin P, Favre JB, Boulard G. Prehospital air ambulance and systemic secondary cerebral damage in severe craniocerebral injuries. Ann Fr Anesth Reanim. 1994;13(3):326–335. doi: 10.1016/s0750-7658(94)80041-3. [DOI] [PubMed] [Google Scholar]
- 22.Jeffreys RV, Jones JJ. Avoidable factors contributing to the death of head injury patients in general hospitals in Mersey Region. Lancet. 1981;2(8244):459–461. doi: 10.1016/s0140-6736(81)90786-8. [DOI] [PubMed] [Google Scholar]
- 23.Kohi YM, Mendelow AD, Teasdale GM, Allardice GM. Extracranial insults and outcome in patients with acute head injury--relationship to the Glasgow Coma Scale. Injury. 1984;16(1):25–29. doi: 10.1016/0020-1383(84)90110-4. [DOI] [PubMed] [Google Scholar]
- 24.Rose J, Valtonen S, Jennett B. Avoidable factors contributing to death after head injury. Br Med J. 1977;2(6087):615–618. doi: 10.1136/bmj.2.6087.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seelig JM, Klauber MR, Toole BM, Marshall LF, Bowers SA. Increased ICP and systemic hypotension during the first 72 hours following severe head injury. In: Miller JD, Teasdale GM, Rowan JO, editors. Intracranial Pressure VI. Berlin: Springer-Verlag; 1986. pp. 675–679. [Google Scholar]
- 26.Chesnut RM, Ghajar J, Maas AIR, et al. Part 2: Early indicators of prognosis in severe traumatic brain injury. J Neurotrauma. 2000;17(6–7):555. [Google Scholar]
- 27.McHugh GS, Engel DC, Butcher I, et al. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):287–293. doi: 10.1089/neu.2006.0031. [DOI] [PubMed] [Google Scholar]
- 28.Hill DA, Abraham KJ, West RH. Factors affecting outcome in the resuscitation of severely injured patients. Aust N Z J Surg. 1993;63(8):604–609. doi: 10.1111/j.1445-2197.1993.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 29.Chi JH, Knudson MM, Vassar MJ, et al. Prehospital hypoxia affects outcome in patients with traumatic brain injury: a prospective multicenter study. J Trauma. 2006;61(5):1134–1141. doi: 10.1097/01.ta.0000196644.64653.d8. [DOI] [PubMed] [Google Scholar]
- 30.Stassen W, Welzel T. The prevalence of hypotension and hypoxaemia in blunt traumatic brain injury in the prehospital setting of Johannesburg, South Africa: A retrospective chart review. S Afr Med J. 2014;104(6):424–427. doi: 10.7196/samj.7494. [DOI] [PubMed] [Google Scholar]
- 31.Franschman G, Peerdeman SM, Andriessen TM, et al. Effect of secondary prehospital risk factors on outcome in severe traumatic brain injury in the context of fast access to trauma care. J Trauma. 2011;71(4):826–832. doi: 10.1097/TA.0b013e31820cebf0. [DOI] [PubMed] [Google Scholar]
- 32.Chesnut RM, Marshall SB, Piek J, Blunt BA, Klauber MR, Marshall LF. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir Suppl (Wien) 1993;59:121–125. doi: 10.1007/978-3-7091-9302-0_21. [DOI] [PubMed] [Google Scholar]
- 33.Spaite DW, Bobrow BJ, Stolz U, et al. Evaluation of the impact of implementing the emergency medical services traumatic brain injury guidelines in Arizona: the Excellence in Prehospital Injury Care (EPIC) study methodology. Acad Emerg Med. 2014;21(7):818–830. doi: 10.1111/acem.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spaite DW, Hu C, Bobrow BJ, et al. The Effect of Combined Out-of-Hospital Hypotension and Hypoxia on Mortality in Major Traumatic Brain Injury. Ann Emerg Med. 2016 doi: 10.1016/j.annemergmed.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spaite DW, Hu C, Bobrow BJ, et al. Mortality and Prehospital Blood Pressure in Patients With Major Traumatic Brain Injury: Implications for the Hypotension Threshold. JAMA Surg. 2016 doi: 10.1001/jamasurg.2016.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S1–106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- 37.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Pediatr Crit Care Med. 2003;3(S3):S2–S81. doi: 10.1097/01.CCM.0000066600.71233.01. [DOI] [PubMed] [Google Scholar]
- 38.Badjatia N, Carney N, Crocco TJ, et al. Guidelines for prehospital management of traumatic brain injury 2nd edition. Prehosp Emerg Care. 2008;12(Suppl 1):S1–52. doi: 10.1080/10903120701732052. [DOI] [PubMed] [Google Scholar]
- 39.Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatr Crit Care Med. 2012;13(Jan Suppl 1):S1–S82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 40.Yeh KC, Kwan KC. A comparison of numerical integrating algorithms by trapezoidal, Lagrange, and spline approximation. J Pharmacokinet Biopharm. 1978;6(1):79–98. doi: 10.1007/BF01066064. [DOI] [PubMed] [Google Scholar]
- 41.Hannan EL, Waller CH, Farrell LS, Cayten CG. A comparison among the abilities of various injury severity measures to predict mortality with and without accompanying physiologic information. J Trauma. 2005;58(2):244–251. doi: 10.1097/01.ta.0000141995.44721.44. [DOI] [PubMed] [Google Scholar]
- 42.MacKenzie EJ, Steinwachs DM, Shankar B. Classifying trauma severity based on hospital discharge diagnoses. Validation of an ICD-9CM to AIS-85 conversion table. Med Care. 1989;27(4):412–422. doi: 10.1097/00005650-198904000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Stewart KE, Cowan LD, Thompson DM. Changing to AIS 2005 and agreement of injury severity scores in a trauma registry with scores based on manual chart review. Injury. 2011;42(9):934–939. doi: 10.1016/j.injury.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 44.Newgard CD, Fildes JJ, Wu L, et al. Methodology and analytic rationale for the American College of Surgeons Trauma Quality Improvement Program. J Am Coll Surg. 2013;216(1):147–157. doi: 10.1016/j.jamcollsurg.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 45.Nathens AB, Jurkovich GJ, Maier RV, et al. Relationship between trauma center volume and outcomes. JAMA. 2001;285(9):1164–1171. doi: 10.1001/jama.285.9.1164. [DOI] [PubMed] [Google Scholar]
- 46.Wood SN. Generalized additive models : an introduction with R. Boca Raton, FL: Chapman & Hall/CRC; 2006. [Google Scholar]
- 47.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 48.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.R Core Team. [Accessed October 5, 2015];R: A language and environment for statistical computing. 2015 http://www.R-project.org/
- 50.Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc B. 2011;73:3–36. [Google Scholar]
- 51.Spaite DW, Valenzuela TD, Meislin HW. Barriers to EMS System Evaluation: Problems Associated with Field Data Collection. Prehospital Disaster Med. 1993;8(S1):S35–S40. doi: 10.1017/s1049023x00040541. [DOI] [PubMed] [Google Scholar]
- 52.Moppett IK. Traumatic brain injury: assessment, resuscitation and early management. Br J Anaesth. 2007;99(1):18–31. doi: 10.1093/bja/aem128. [DOI] [PubMed] [Google Scholar]
- 53.Brenner M, Stein DM, Hu PF, Aarabi B, Sheth K, Scalea TM. Traditional systolic blood pressure targets underestimate hypotension-induced secondary brain injury. The journal of trauma and acute care surgery. 2012;72(5):1135–1139. doi: 10.1097/TA.0b013e31824af90b. [DOI] [PubMed] [Google Scholar]
- 54.Bobrow BJ, Vadeboncoeur TF, Stolz U, et al. The influence of scenario-based training and real-time audiovisual feedback on out-of-hospital cardiopulmonary resuscitation quality and survival from out-of-hospital cardiac arrest. Ann Emerg Med. 2013;62(1):47–56. e41. doi: 10.1016/j.annemergmed.2012.12.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.