Abstract
The Nuclear factor erythroid2-related factor2 (Nrf2), a master regulator of redox homoeostasis, is a key transcription factor regulating a wide array of genes for antioxidant and detoxification enzymes. It protects organs from various kinds of toxic insults. On the other hand, activation of Nrf2 is also correlated with cancer progression and chemoresistance. Downregulation of Nrf2 activity has attracted an increasing amount of attention as it may provide an alternative cancer therapy. In this review, we examine recent studies on roles of Nrf2 in several pathophysiological conditions emphasising cancer. We discuss elaborately the current knowledge on Nrf2 regulation including KEAP1-dependent and KEAP1-independent cascades. KEAP1/Nrf2 system is a master regulator of cellular response against a variety of environmental stresses. We also highlight several tightly controlled regulations of Nrf2 by numerous proteins, small molecules, toxic metals, etc. In addition, we evaluate the possible therapeutic approaches of increasing chemosensitivity via modulating Nrf2 signaling.
Keywords: Nrf2, Transcription factor, KEAP1, Oxidative stress, Cell proliferation, Carcinogenesis, Chemoprevention
1. Introduction
Exposure to xenobiotics first occurred since the introduction of heterotrophic nutrition in the ecological system comprising the fungi to modern human being, who were unable to synthesize organic compounds on their own [1], [2]. Xenobiotics consist of several organic compounds obtained from different plant and microbial sources, which were consumed by the heterotrophs as a source of energy to carryout different physiological functions [3]. Moreover, in the contemporary world, xenobiotics exposure can also occur due to the severe environmental pollution [4], [5]. Commonly, modern day human life style is encircled in an environment consisting of smoke, air pollution, ultraviolet radiations, γ radiation, several pharmaceutical drugs, etc [6] and all these cause the generation of reactive species inside the cellular compartments [7], [8]. These kinds of exogenous insults of toxic substances increase the intracellular level of reactive oxygen and nitrogen species (ROS and RNS) that irreversibly impair several cellular functions by damaging the cellular macromolecules such as nucleic acids, carbohydrates, lipids and proteins [9], [10], [11]. In this context, in order to make progress in human pathophysiological research, it is significant to apprehend several physiological responses to exposure to xenobiotics [12]. Under normal physiological conditions, low exposure to these xenobiotics or toxic substances, certain physiological processes get activated endogenously, and the cell can counteract the negative consequences of those toxic substances [13], [14]. However, excessive exposure to pollutants may lead to several diseases including diabetes, asthma, chronic obstructive pulmonary disorder (COPD), neurodegenerative disorders, cancer, etc [15], [16], [17], [18], [19]. Every type of mammalian cells held a diverse and synchronized defense mechanism to decontaminate the intracellular environment and retain normal physiological conditions [20], [21]. Cellular defense mechanism can be broadly classified in two layers. The first cellular defense layer consists of several anti-antioxidant molecules, which are not proteins by nature. Several such antioxidants like vitamin C, vitamin E, glutathione, bilirubin, β-carotene, etc. get oxidized by donating an electron to the reactive radical and generate a non-reactive species [12], [22], [23]. The second layer consists of assorted anti-oxidant proteins or a platter of enzymes such as superoxide dismutase, catalase, peroxidases, peroxiredoxins, etc [24], [25], [26]. These enzymes sequentially transform highly reactive superoxide radicals into water and oxygen [27]. Other than these enzymes there are certain xenobiotic transporters present to regulate the intracellular concentration of any toxic substance and thereby limiting their extent of toxicity by carrying them across the cell membrane [28], [29], [30], [31]. These two layers of cellular defense modulate the increasing oxidative load upon the cell by overexpression of these individual subcomponents [17], [21], [32], [33]. The level of these subcomponents inside the cell is regulated by the expression of several transcription factors which get activated under stressed conditions [34], [35], [36]. Researches round the globe suggest that in mammalian cells Nrf2 plays an important role to maintain normal cellular physiological conditions under exogenous oxidative insult by regulating the minimal and induced expression of several antioxidants molecules, enzymes and xenobiotic transporters [37], [38]. Nrf2 was first identified in different anticancer studies as an inducer of different antioxidant enzymes and found getting activated upon some drug candidates [39]. Comparable to the lower eukaryotic systems like yeast, the activation of Nrf2 is induced by the presence of electrophilic molecules and ROS [40]. Activation of Nrf2 leads to the expression of several other anti-oxidative genes [41], like superoxide dismutase-2 (SOD-2), hemoxygenase-1 (HO-1), etc. and attenuate the oxidative stress mediated cellular damage [42], [43], [44]. For a long period of time researches have considered Nrf2 as an anti-cancer molecule but recent interdisciplinary biological research reports revealed the role of this b-zip family transcription factor in cellular proliferation [45], [46]. Many studies involving the sequencing of the cancer genome unveiled the consequence of Nrf2 activation in the development and progression of cancer [47], [48]. Nrf2 activators are being used for the treatment of oxidative stress associated diseases and in the clinical trials for the prevention of cancer [49], [50], [51]. On the other hand constitutive activation of Nrf2 in many types of tumors has been reported to contribute to the survival/growth of cancer cells and resistance to anticancer therapy as well [52]. In this review, we provide a mechanistic overview of the Nrf2 signaling pathway and its role in progression and development of cancer. Additionally, we have also discussed the possible therapeutic application of regulation of the Nrf2 signaling pathway by nuclear receptors in cancer [53].
2. Nrf2 structure
Human Nrf2, a protein of 66.1 kDa molecular mass, consists of 589 amino acids. In 1994, Nrf2 was cloned within a 2.2 kB transcript as a protein that can activate transcription by binding to the Nuclear Factor Erythroid 2/Activator protein 1(NF-E2/AP-1) motif of the hypersensitive site-2 in the b-globin locus control region [39].
Nrf2 belongs to the Cap’n’collar (CNC) transcription factor family having a unique CNC domain which is linked to the N-terminal side of a highly conserved leucine zipper (bZIP) motif. These proteins function as transcription factors by pairing with other bZip proteins, including small MAF (sMAF) proteins which help in dimerization, DNA binding and nuclear import and export.
This family of proteins include p45 NF-E2, Nrf1, Nrf3, Broad complex, tramtrack and bric-a-brac (BTB) and CNC homology (Bach)1 and Bach2 in vertebrates [54]. Nrf1, Nrf2 and Nrf3 are ubiquitously expressed. In mammals, p45 NE-F2 is mainly expressed in hematopoietic progenitors essential for platelet formation and megakaryocyte maturation [55]. The NF-E2 p45 and Nrf2 transcription factors are soluble proteins, whereas upon translation Nrf1 and Nrf3 are initially anchored in the endoplasmic reticulum (ER) as glycosylated proteins. It influences uptake of cystine via the sodium-independent cystine-glutamate antiporter (Slc7a11) [56]. Nrf3 is expressed in a number of tissues, including placenta, B cells, and monocytes. Both Nrf1 and Nrf2 play important roles in the transcriptional up-regulation of cytoprotective genes [57].
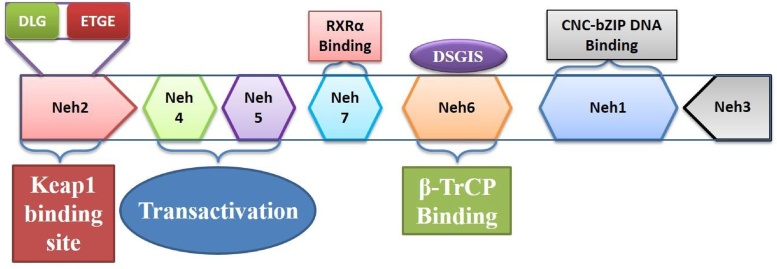
By structural and functional analysis of Nrf2 protein, seven highly conserved regions were identified known as (Nrf2-ECH homology) Neh1 to Neh7 [39]. Neh1, Neh3 and Neh6 are located in the C-terminal half of Nrf2 (Fig. 1). Neh1 contains a bZIP DNA-binding motif. Neh2 domain, which is located in the most N- terminal region, interacts with a cytoplasmic protein, Kelch-like ECH-associated protein 1(KEAP1) and acts as a negative regulatory domain. The Neh3 domain, located in the most C- terminal region is required for the activation of transcription, along with the Neh4 and Neh5 domains which enhance Nrf2-mediated reporter gene transcription in an ATP-dependent manner. Another transcriptional activator, BOI-related E3 ubiquitin-protein ligase 2 (BRG2) enhanced the activity. From a yeast two-hybrid screen experiment, it has been proven that Neh3 domain is importantfor interactions with a chromo-ATPase/helicase DNA-binding (CHD6) protein. In the N-terminal region, Neh4 and Neh5 domains (transactivation domains)are present that bind to the kinase-inducible domain interacting (KIX) and cysteine/histidine-rich domain 3 (CH3) domains of CBP (CREB (cAMP Responsive Element Binding protein)-binding protein). Neh6 contains a serine-rich conserved region which is involved in degradation of Nrf2.
Fig. 1.
The structure of Nrf2 containing seven domains.
There are two distinct binding sites (i.e., DLG and ETGE motifs) within the Neh2 domain of Nrf2. According to Fukutomi et al., the DLG motif is much more extended (DLGex motif (Met17–Gln51)) than those assumed by the classical DLG motif and DIDLID element [58]. The DLGex possesses triple helices: Helix-1 (Leu19–Arg25), Helix-2(Ile28–Leu30), and Helix-3(Arg34–Phe37). The DLGex structure shows huge differences from the β-hairpin structure of ETGE.
3. Regulations of Nrf2
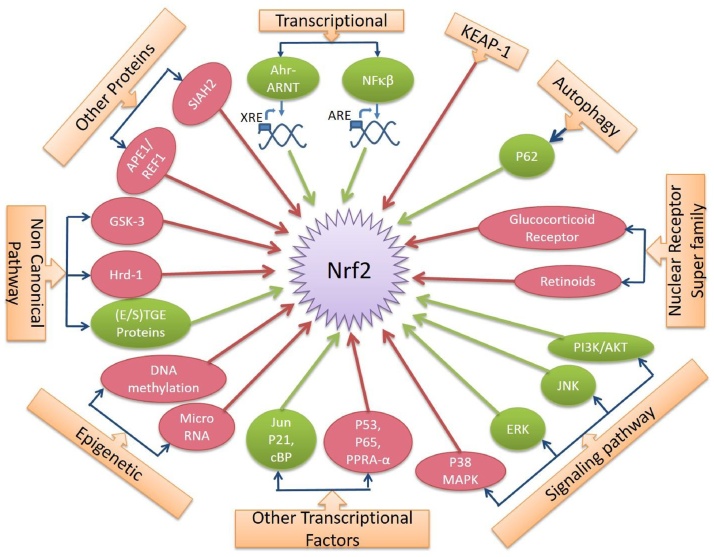
Expression and activities of Nrf2 are tightly controlled (Fig. 2). Several canonical and non canonical pathways are involved in regulating Nrf2 signaling pathways.
Fig. 2.
Regulations of Nrf2 by several factors. The green colour indicates upregulation of Nrf2 activity and the red colour indicates downregulation of Nrf2 activity.
3.1. KEAP1, the major regulator of Nrf2
From yeast two-hybrid screening it was identified that KEAP1 is the first major repressor of Nrf2. KEAP1, an adaptor subunit of Cullin3-based E3 ubiquitin ligase, acts as a sensor for oxidative and electrophilic stresses and regulates Nrf2. Site-directed mutagenesis study revealed the functional significance of cysteine residues of KEAP1 [59]. After mutation of Cys23, Cys273, and Cys288 residues, KEAP1 is unable to negatively regulate Nrf2, but Cys151 mutation represses Nrf2 constitutively [60].
There are two prevailing models for understanding the cytoprotective mechanism of Nrf2 regulation by KEAP1: (a) the hinge and latch model and (b) the Cul3-KEAP1 dissociation model.
In the first model, Nrf2 binds to a KEAP1 dimer first through a high affinity ETGE motif; which is called “open” conformation. At this stage, Nrf2 is not ubiquitinated. In the presence of inducers, the low affinity DLG motif dissociates from KEAP1. Then, the DLG motif of Nrf2 binds to the other part of the KEAP1 dimer which results in the “closed” conformation [61], [62]. Nrf2 is ubiquitinated by the Cul3-Rbx1-E3 ligase complex and degraded by the proteasome. The free KEAP1 dimer is now prepared to bind to Nrf2 for next cycle. When cells undergo oxidative stress, the oxidation or adduct formation of the cysteine residues of KEAP1 by ROS or electrophiles takes place which leads to the conformational changes of KEAP1. Although KEAP1 still binds to the DLG and ETGE motifs of Nrf2 by its kelch domain, it fails to align with the E2 ubiquitin-conjugating enzyme and thus prevents the ubiquitination. Thus, the cycle stops in the “closed” conformation of the KEAP1-Nrf2 complex and accumulation of newly translated Nrf2 occurs. Both motifs individually bind to a pocket within the DC (double glycine repeat and C-terminal) domain of KEAP1. Binding of the high-affinity ETGE motif and low-affinity DLG motif provides a hinge and latch. This phenomenon leads to optimal positioning of the lysine residues between the two motifs facilitating ubiquitin conjugation. The open and closed states of the KEAP1–Nrf2 complex were studied by fluorescence resonance energy transfer experiments [61]. In this model, the latch site becomes too tight, and thus Nrf2 is stabilized.
The mode of binding of DLGex and ETGE to KEAP1 are quite distinct from each other. The KEAP1–DLGex binding is both enthalpy and entropy driven while the ETGE–KEAP1 binding is purely enthalpy driven and contains a two-state reaction leading to a stable conformation. These observations explore DLGex motif as a converter transmitting environmental stress to Nrf2 induction as the latch site.
In the second model, the interaction between KEAP1 and Cul3 gets disrupted by inducers such as tert-butylhydroquinone (tBHQ) or eicosapentaenoic acid (EPA) and ubiquitination of Nrf2 is diminished [63]. Cysteine 151, localized far away from latch site was found to play an important role in this model in the BTB domain in KEAP1. DLG motif of Nrf2 binds to α12 KEAP1 dimer [61], [63].
After recovery of homeostatic balance, KEAP1 translocates into the nucleus and escorts Nrf2 out of the nucleus through a Karyopherin alpha6 (importin alpha7) (KPNA6), an adapter protein resulting in ubiquitination and degradation of Nrf2 in the cytoplasm.
The release of Nrf2 from KEAP1 requires phosphorylation of Ser40 in the Neh2 domain of Nrf2 by protein kinase C (PKC) [64]. Four reactive cysteine residues in a domain of KEAP1 are called the intervening regions (IVR), which serve as sensors for reactive oxygen/nitrogen species and electrophiles [40]. Chemical modification of these cysteine residues triggers a conformational change in KEAP1 which in turn allows the release of Nrf2.
There are five domains in KEAP1 an N-terminal domain (NTD, 1–60); a Broad complex, Tramtrack, and Bric-á-Brac (BTB, 61–178) domain; an intervening region (IVR, 179–321); a double glycine repeat (DGR, 322–608) and a Kelch domain; and the C-terminal region (CTR, 609–625) [63]. The DGR and CTR are also known as the DC domain, which mediates that association between KEAP1 and Nrf2. BTB domain facilitates homodimerization and then promotes interaction between the Neh2 domain of Nrf2 with its DC domain. The region flanked by the DLG and ETGE motifs of Nrf2 forms a-helix conformation containing seven lysine residues which are ubiquitination targets [65]. After this homodimerization, Nrf2 gets the proper conformation for ubiquitination. Single-particle electron microscopy demonstrated that KEAP1 has a forked-stem dimer structure, with two large spheres which enclose the intervening DC repeat and C-terminal domains [66].
Somatic KEAP1 and Nrf2 mutations in cancers antagonize one another as somatic mutations in tumor suppressor genes like TP53and oncogenes like MDM2. Oncogene activation transcriptionally upregulates Nrf2 which indicates Nrf2 as a member of oncogenic signaling [67]. Cancer genome sequencing revealed the non-overlapping nature of somatic KEAP1 and Nrf2 mutations. KEAP1 mutations tend to not have Nrf2 mutations [68]. Nrf2 activation confers a growth advantage in cancer cells and is therefore enriched in tumors.
3.2. Transcriptional regulation
KEAP1-independent mechanisms involve regulation at the transcriptional level by the binding of Aryl hydrocarbon receptor-arylhydrocarbon receptor nuclear translocator (AHr-ARNT) to xenobiotic response element (XRE) sequences and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), which then binds to the antioxidant-response-element (ARE) in the Nrf2 promotor region. NFE2L2 gets transcriptionally activated by polycyclic aromatic hydrocarbons (PAHs) [69], [70]. A feedback loop exists between the two xenobiotic-sensing transcription factors which are involved in the regulation of AhR [71]. Upstream of the transcription start site (TSS), the NFE2L2 promoter contains two ARE-like sequences that allow the transcription factor to increase modestly its own expression [72]. Downstream from the TSS, it contains a 12-O-tetradecanoylphorbol-13-acetate-response element that allows it to be transcriptionally activated by oncogenic V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KrasG12D) via c-Jun N-terminal kinases (c-Jun) and c-Fos [52], [73] and also a nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) binding site, which is involved in inflammatory stimuli [74]. A dynamic crosstalk exists between Nrf2 and peroxisome proliferator-activated receptor α (PPARα).
There are reports that transcription factors, including JDP2, Jun, CBP, BRG1, and p21 can promote Nrf2 activation. On the other hand, cFos, p53, p65, Fra1, Bach1, C/EB, ATF1, ATF3, estrogen receptors (ER), short-form estrogen-related receptor (SFERR), peroxisome proliferator-activated receptor α (PPAR-α), and RAR have been reported to negatively regulate Nrf2 transcription [75].
3.3. Epigenetic regulation
Expression of Nrf2 is controlled by epigenetic modification involving chromatin structural alterations like DNA methylation, histone modifications, and microRNAs. Transcription of Nrf2 is suppressed in prostate tumors of transgenic adenocarcinoma of the mouse prostate (TRAMP) mice due to the hypermethylation of select CpGs in the promoter of Nrf2 [76]. Repressive proteins, such as methyl-CpG-binding protein 2 (MBD2) and trimethyl-histone H3 (Lys9), are enriched in this region.
Hypermethylation of CpG sites in the KEAP1 promoter region leads to downregulation of KEAP1 expression in human lung cancer cell lines and tissues [77]. Muscarella et al. have shown that hypermethylation of KEAP1 is more frequent than KEAP1 gene mutations in this cancer [78]. Inactivation of the promoter region of the KEAP1 gene has been identified in lung, prostate [79], malignant glioma [78], colorectal [80] and breast cancer [81] suggesting epigenetic changes in KEAP1 which lead to constitutive activation of the Nrf2 pathway. This, in turn, promotes the survival of tumors and drug resistance in cancer cells.
Overexpression of miR-144, miR-153, miR-27a and miR-142-5, either individually or as a group, can suppress Nrf2 mRNA and protein levels [82]. MiR-144 and miR-28 mediate the degradation of Nrf2 mRNA by targeting the 3’-untranslated region (3’-UTR) [83], [84]. miR-34a negatively regulates Nrf2. Not only that, miR-200a mediates the degradation of KEAP1 mRNA in breast cancer cells which in turn results in activation of the Nrf2 pathway. Decrease in H3K27Me3 in the Nrf2 promoter elevated expression of Nrf2, NAD(P)H dehydrogenase quinone 1 (NQO1) and HO-1 in lung cancer tissues and cell lines [85]. Keap1-Nrf2 crosstalk with epigenetic modifications can be considered as a therapeutic approach in near future [86].
3.4. Autophagy
As discussed earlier that the Nrf2-KEAP1-ARE is a redox sensitive signaling cascadewhich gets activated in cellular pathophysiological conditions via the upregulation of different pro-survival and anti-oxidant genes. At the same time, to make sure a quick activation under stressed condition and its reversal, when the normal state is attained, the mammalian cellular system has evolved a well-regulated energy dependent signaling machinery as the constitutive expression of Nrf2 will increase the risk of carcinogenesis and extend multiple drug resistance to cancer cells [48], [52], [73], [87]. Autophagy adaptor protein, p62 plays an important role in the induced activity of Nrf2 modulating KEAP1, and it is quite obvious that dysfunctioning of the autophagic regulations will lead to a persistent activity of Nrf2 mediating p62 [88], [89]. Under normal autophagic dysfunction, p62 ceases the activity of KEAP1 and Nrf2 gets stabilized [90]. It results in prolonged expression of different ARE-reguated genes. Unlike Nrf2 authophagic pathways also play incongruous role in the regulation and development of cancer [91]. Autophagy is a critical physiological phenomenon which allows a cell to get rid of damaged organelles, old and misfolded proteins [92]. This self-clearing activity helps in maintaining the normal cellular homeostasis and to fight against endogenous or exogenous oxidative stress insult. Its downregulation will lead to constitutive activation of Nrf2 and other similar, or associated proteins and upregulation will facilitate the malignant cells to deal with the stressed intracellular environment, which is necessary for their survival [93], [94]. The understanding of the underlying signaling mechanism and interaction between the Nrf2-KEAP1-ARE axis and autophagy is a comprehensive display of the ongoing need for detailed depictions of normal physiological and diseased phenomena to aid the discovery of new therapeutic approach.
3.5. Electrophiles
Electrophiles trigger reactive cysteines in KEAP1. These residues have been shown to recognize cyclopentanone prostaglandins such as 15-deoxy-D12,14-prostaglandin J2 (by Cys-273) and alkenals such as acrolein and 4-hydroxynonenol (by Cys-288) [95], [96].
Within the BTB domain of KEAP1, Cys-151 is critical for the detection of electrophiles such as tBHQ, stratifin (SFN), and nitric oxide (NO) through the formation of a thiolate anion. Furthermore, replacement of Lys-131, Arg-135, and Lys-150 surrounding Cys-151 reduce the ability of electrophiles to induce ARE-driven gene expression. Within the DGR of KEAP1, Cys-434 acts as an electrophile sensor in KEAP1; it is triggered by 8-nitroguanosine-3′,5′-cyclic monophosphate (8-nitro-cGMP), which is generated by NO production leading to S-guanylation of KEAP1 and induction of Nrf2-target genes [97].
3.6. Reactive metals
Zn2+, Cd2+, Se4+and As3+ all interact non-covalently with KEAP1. Cys-226 and Cys-613 have been proven to be Zn2+sensor because zinc is the most likely endogenous trigger. They also sense ROS, upon exposure to H2O2 they can form a transient intramolecular disulfide bridge that is associated with brief stabilization of Nrf2 protein [97]. The loss in expression of selenoproteins elicits an increase in expression of cytoprotective proteins like thioredoxinreductase 1 (TXNRD1) [98].
3.7. Other signaling pathways regulating Nrf2
Nrf2 is phosphorylated by several signal transduction pathways which can alter the interactions between Nrf2 and KEAP1 [extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinases (JNK), mitogen-activated protein kinases (MAPK), p38], Nrf2 localization [protein kinases C(PKC), casein kinase 2 (CK2), Glycogen synthase kinase 3 (GSK3), Fyn], Nrf2 protein degradation [GSK3β, beta-transducin repeat containing protein (βTrCP)] and Nrf2-DNA binding [CREB-binding protein (CBP), musculoaponeurotic fibrosarcoma oncogene homolog (Maf)].
Phosphorylation of Nrf2 could lead to an increase in its stability and subsequent transactivation activity. It has been reported that the phosphorylation of PKC, MAPK, GSK3 and Phosphatidylinositol-3-kinase (PI3K) are strongly associated with the Nrf2 activity in a KEAP1-independent manner. Phosphorylation at Ser40, Ser568 and degron domain of Nrf2 by protein kinases triggers Nrf2 [99].
3.7.1. MAPK
Various cancer chemopreventive agents induce Nrf2- target genes and stimulate MAPK signaling, including ERKs, JNKs and p38 kinases. The use of kinase inhibitors and dominant-negative mutants suggest that ERK and JNK positively regulate Nrf2 activity. p38MAPK has been reported to both positive and negative regulation of Nrf2 [100].
3.7.2. PI3K
Salinas and colleagues showed that PI3K affected Nrf2 indirectly by nerve growth factor induced activation of PI3K [101]. Not only that, Nrf2 was required for HO-1 induction. GSK-3 provides the link between activation of the PI3K−PKB/Akt pathway and stimulation of Nrf2-mediated gene induction [102]. They have shown that activation of PKB/Akt resulted in inhibitory phosphorylation of GSK-3, this also resulted in failure by GSK-3 to phosphorylate Nrf2. Dietary phytochemicals can activate the Nrf2-ARE pathway through PI3K/Akt-dependent phosphorylation of Nrf2.
3.7.3. AMPK
Under conditions of glucose deprivation and low energy availability, 5′ adenosine monophosphate-activated protein kinase (AMPK) is activated by its upstream kinases, liver kinase B-1 (LKB-1) and Akt [103]. AMPK is a serine/threonine-specific kinase that respons to alterations in levels of adenylate in the cell; a low-ATP/high-AMP ratio, such as that observed during fasting, activates AMPK [104].
3.7.4. mTOR
Mechanistic target of rapamycin (mTOR) acts as a stress sensor. mTOR controls Nrf2 as it engages in signaling upstream of both KEAP1 and β-TrCP. The mTOR protein is a ubiquitously expressed serine–threonine protein kinase and a member of the PI3K-related kinase family which acts as a cellular energy sensor by integrating signals from hormones, energy levels, growth factors, and nutrients into the regulation of protein translation, autophagy, and lipid metabolism.
3.8. Nuclear receptor superfamily
Nuclear receptors which lack defined ligands are termed ‘orphan’ receptors. The Nrf2 signaling pathway can be regulated by nuclear receptors, which are highly-conserved, and selectively bind to small lipophilic/hydrophobic ligands that initiate the transactivation of specific cis-regulatory DNA sequences [105]. They bind with cofactors such as co-activators or co-repressors for the upregulation or downregulation of their downstream target genes.
The glucocorticoid receptor, activated by the steroid hormones regulate a number of genes through direct binding to glucocorticoid-responsive elements and play an important role in the regulation of immunity, intermediary metabolism, skeletal growth, cardiovascular function, reproduction, and cognition. Silencing mediator for retinoid or thyroid-hormone receptors (SMRT), a co-repressor of the steroid–GR complex, binds with the Neh4/5 domain of Nrf2 to mediate the repression of Nrf2-dependent gene expression [106].
Retinoids affect via interaction with two distinct classes of nuclear receptors: retinoic acid receptors (RARs) and retinoid X receptors (RXRs). Receptor alpha (RA) possesses the anticancer activity [107]. Wang et al. have shown that in a stable ARE-luciferase reporter cell line known as AREc32, the induction of ARE-driven luciferase activity is inhibited by all-trans retinoic acid (ATRA) which reduces the luciferase activity of ARE genes such as aldo–ketoreductase family 1member C1 (AKR1C1) and AKR1C2 at both them RNA and protein levels. ATRA is an endogenous Nrf2 inhibitor [108].
3.9. Other proteins
CR6-interacting factor 1 interacts with Nrf2 and promotes ubiquitinylation of Nrf2 by binding at both the C- and the N-terminal Neh3 and Neh2 domains of the transcription factor leading to its destruction. The protein apurinic/apyrimidinic endonuclease/redox factor-1 (APE1/REF-1) negatively regulates Nrf2 which in turn reduces the expression of selected target genes HO-1, Glutamate cysteine ligase catalytic subunit (GCLC), and Glutamate cysteine ligase modifier subunit (GCLM) in pancreatic cancer cell lines without causing the generation of ROS [109]. During hypoxia, Nrf2 levels are suppressed and seven in absentia homolog 2 (SIAH2) levels increase. SIAH2 suppresses Nrf2 through a KEAP1-independent manner. The SUMOylated Nrf2 gets ubiquitinated by RING finger protein 4 (RNF4) in the nucleus, leading to degradation of the transcription factor [110].
3.10. Non-canonical pathways
Under certain conditions, the non-canonical regulation of Nrf2 may act independent of the canonical KEAP1 pathway, playing a vital role in pathogenesis of diseases.
3.10.1. GSK-3
GSK3β mediated phosphorylation in the Neh6 domain of Nrf2 results in its recognition by the β-TrCP-S phase kinase association protein1 (Skp1)-cullin1 (Cul1) –RING-box protein 1 (Rbx1) E3ubiquitin ligase complex and its proteasomal degradation [111]. GSK-3 phosphorylates serine residues within the DSGIS motif [50], [112]. Epidermal growth factor, fibroblast growth factor, insulin, insulin-like growth factor, keratinocyte growth factor, nerve growth factor and platelet-derived growth factor causes inhibition of GSK-3α and GSK-3β by phosphorylation of their Ser21 and Ser9 residues, which in turn up-regulate Nrf2. GSK-3 suppression is responsible for an increase in the nuclear localization of Nrf2 and the levels of its target enzyme glutathione S-transferase, which in turn decreased oxidative stress.
Within the Neh6 domain of Nrf2, the β-TrCP substrate recognition motif, DSGϕXS, were identified (ϕ represents a hydrophobic residue, and X is any amino acid). β-TrCP is an F-box-containing protein, which binds to the Skp, Cullin, F-box containing complex (SCF) E3 ubiquitin ligase complex (SCFβ-TrCP) and an F-box substrate receptor protein [113].
3.10.2. Hrd1
HmgCoA reductase degradation1 (Hrd1) is an E3 ubiquitin ligase, a novel E3 ubiquitin ligase which mediates Nrf2 degradation. The most recently described ubiquitin-dependent system involved in Nrf2 degradation is the E3 ubiquitin ligase synoviolin, which resides in the endoplasmic reticulum [114].
3.10.3. Multiple (E/S)TGE-containing proteins
These proteins have been identified to disrupt the Nrf2-KEAP1 interaction through competing with Nrf2 for KEAP1 binding and stabilize Nrf2 [79].
4. Mode of action
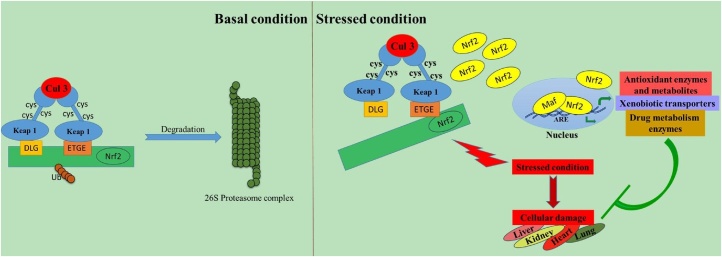
Chromatin immunoprecipitation followed by NGS (ChIP-Seq) revealed a set of Nrf2 target genes. The recognition sequence of CNC-sMAF heterodimers is called as Maf recognition element (MARE; TGCTGA G/C TCA GCA). sMAF heterodimers prefer to bind to an MARE-related sequence (CNC-sMAF binding element; GCTGA G/C TCA C/T) [115], [116]. The binding sequence containing a cis-regulatory element is called an ARE or electrophile responsive element (EpRE)(GCnnnG/C TCA). Genome wide collection of the cis-regulatory elements bound by Nrf2 is designated as the “Nrf2 cistrome”. The core ARE sequence has been described as 5′-A/GTGAC/GNNNGCA/G-3′, where “N” signifies apparently redundant residues [117]. After being relieved from the tight regulation of KEAP1, Nrf2 upregulates certain genes and performs a critical role in maintaining human health (Fig. 3).
Fig. 3.
Activation and mechanism of action of Nrf2. During normal condition, Nrf2 is sequestered in the cytoplasm by KEAP1. Any kind of stress leads to degradation of KEAP1 and Nrf2 mediated transcriptional upregulation.
5. Nrf2 function
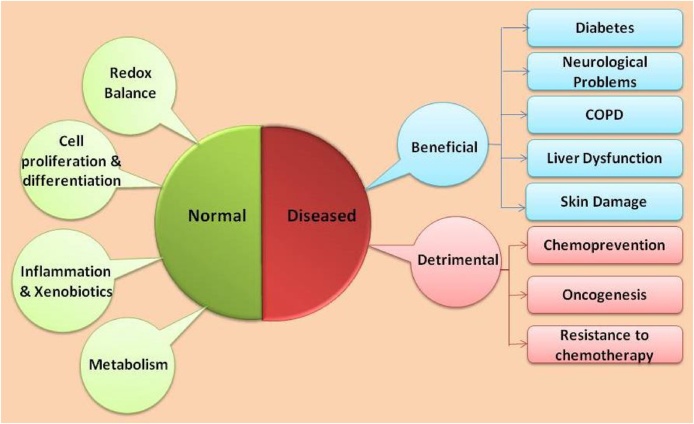
Nrf2 plays potential roles in normal physiological as well as diseased conditions (fig 4). It rescues cells from any kind of environmental and oxidative stress during normal physiological conditions as well as in several pathophysiological conditions. Not only that, it plays contradictory roles during cancer.
Fig. 4.
Functions of Nrf2. Nrf2 maintains homeostasis in the body. It plays a beneficial role in several diseased conditions, but during cancer, it plays a detrimental role in promoting cancer progression.
5.1. Role in normal physiological state
Nrf2 plays vital roles in normal physiological conditions and maintains homeostasis. It controls redox balance, metabolism, cell proliferation and protein folding. Imbalance of Nrf2 activity may hamper these phenomena.
5.1.1. Redox balance
It is known to be the master regulator of redox homeostasis. It can promote the expression of genes including superoxide dismutases (SODs), glutathione peroxidase (GPx), NQO1, HO-1, Gclc, Gclm, Prdx1 (also called MSP23), Slc7a11, Txn1, Txnrd1 and many other enzymes that are involved in glutathione production [118]. In addition, it also helps to decrease ROS by repressing the production of certain NOX enzymes in cell specific fashion [119].
5.1.2. Metabolism
Though Nrf2 is specially noted for its antioxidant related activity, its role in metabolic reprogramming is recently coming in light. It redirects glucose and glutamine metabolism and leads to increased production of ribose-5-phosphate and NADpH through anabolic pathways. Studies also revealed that it targets and activates metabolic genes like glucose-6-phosphate dehydrogenase (G6PD), phosphogluconate dehydrogenase (PGD), transketolase (TKT), transaldolase 1 (TALDO1), and malic enzyme 1 (ME-1) by binding their AREs. It has its role in efficiently synthesizing purine, nucleotides from glucose, in expression of phosphoribosyl pyrophosphate amidotransferase as well as the mitochondrial methylene tetrahydrofolate dehydrogenase 2. This upregulation drives in cell proliferation and thus tumor growth [120]. It also upregulates fatty acid oxidation and down regulates fatty acid synthesis [121]. Study reveals that the upregulation of Nrf2 decrease the expression of transcription factor PPARα which is responsible for controlling the expression of genes involved in lipid transport, β-oxidation of fatty acids, ketogenesis, lipogenesis, lipid mobilization and cholesterol metabolism and helps in adaptation in long-term fasting condition.
Activation of Nrf2 triggers the availability of substrates for respiration, increases mitochondrial membrane potential and enhances ATP production. Its deficiency may be detrimental in the case of neurons for the decrease in ATP caused by dysfunction of oxidative phosphorylation and reduced glycolysis. It also helps in mitochondrial activation of long chain and short chain fatty acids [122].
5.1.3. Inflammation and xenobiotics metabolism
Nrf2 exerts its effect in reduction of inflammation as well as oxidative stress. It may be due to the fact that it positively regulates PPARγ which has a strong inflammatory effect [121]. The diminished expression of antioxidant genes and higher production of interleukin (IL)-4 and IL-13 can be another cause. Additionally, there is a strong connection between Nrf2, NF-kB and inflammation. Study shows that Nrf2 may block lipopolysaccharide (LPS)-induced ROS generation of tumor necrosis factor alpha (TNF-α), IL-6 and chemokines (Mip2 and Mcp-1) and thus reducing inflammation.
Nrf2 is the controller of many phase II metabolizing enzymes of xenobiotics such as the GST family, the sulfotransferase 3A family, and the UDP-glucuronosyltransferase (UGT) family which often converts xenobiotics into more water-soluble and fewer toxic metabolites by reactions like glucuronidation, glutathione conjugation and sulfation. So, the absence of it increases the sensitivity of stressors including hyperoxia, acetaminophen, benzo[a]pyrene, etc. and DNA-damaging agents.
5.1.4. Cell proliferation and differentiation
Nrf2 affects cell proliferation and differentiation in various ways. Evidence show that during certain cell differentiation and proliferation, it is specifically down-regulated as ROS based signaling pathways play an important role there. Forced over expression of it in young neurons impair dendritic outgrowth and their development due to antagonism of JNK and Wnt pathways [123]. Its activation also inhibits differentiation of two major players involved in bone homeostasis, osteoclasts and osteoblasts by reducing the intracellular ROS and by suppressing the transcriptional activity of Runt-related transcription factor 2 (RUNX2) [124]. It regulates two genes, CCAAT/enhancer-binding protein β (CEBPβ) and peroxisome proliferator-activated receptor g (PPARγ) and its defect suppress adipogenesis in vitro [71]. Nrf2-null male mice exhibit decreased fertility due to accumulating ROS mediated damage to germ cells with increasing age concluding that its activity is closely related with spermatogenesis [125]. Its deficiency attenuates the reconstitution capacity of hematopoietic stem cells (HSCs) after the transplantation, indicating that it is required for the HSC maintenance. Moreover, liver regeneration is compromised in absence of Nrf2 as a result of blunted Notch 1 signaling caused by reduced expression of Notch1, p21, Hes1, Homocysteine-responsive endoplasmic reticulum-resident 1 (Herp1) and NOTCH-regulated ankyrin repeat protein (Nrarp) [126].
5.1.5. Role in protein folding
Evidence suggest that it have a potential role in folding proteins appropriately in their tertiary and quaternary structures thus reducing ER stress and unfolded protein response (UPR). So, it can be concluded that in Nrf2-null mice, the amount of Chop and X-box binding protein 1 (Xbp1) and the phosphorylation of Eukaryotic Initiation Factor 2α (eIF2α) are increased [126], [127]. The lower expression of Slc7a11 in Nrf2 null mice ultimately decreases the intracellular level of cysteine which is important for proper protein folding, thus leading to elevated ER stress [128]. Additionally, it plays a vital role in the availability of reduced glutathione (GSH) or in the proper reduction of oxidized glutathione (GSSH) which in turn helps in correct folding of membrane and secretary proteins [129].
5.2. Role in various pathophysiological conditions
Nrf2 reduces the drug-induced toxicity in different organs like brain, lung, skin, liver, gastrointestinal tract, kidney, etc. and also ameliorates several diseases [130], [131], [132].
The absence of Nrf2 in mice causes the neurological deficit and neuronal apoptosis which leads to traumatic brain injury, motor dysfunction, neuronal death and spinal cord injury [133], [134]. Intraperitoneal injection of sulforaphane protects from neuronal cell damage [135]. Nrf2 activity decreases in the lungs of patients suffering from COPD and emphysema. Nrf2−/− mice are more susceptible to cigarette smoke-induced emphysema [60]. Nrf2 is activated in the mouse liver after administration of non-toxic and toxic doses of hepatotoxin, acetaminophen [136]. It plays a role as a protective against ultraviolet A (UVA), and ultraviolet B (UVB)-induced inflammation and damage in the skin and represents itself as a promising therapeutic target for such disorders [137], [138].
Nrf2 rescues from diabetes induced nephropathy [139], retinopathy [140], and cardiomyopathy [141]. ROS plays both positive and negative roles in insulin signaling. Nrf2 has been documented to improve glucose homeostasis [118]. Improvement in glucose disposal occurs due to increased expression of fibroblast growth factor 21, which is repressed by Nrf2 [142]. Nrf2 appears to play an important role in the regulation of lipogenesis [143].
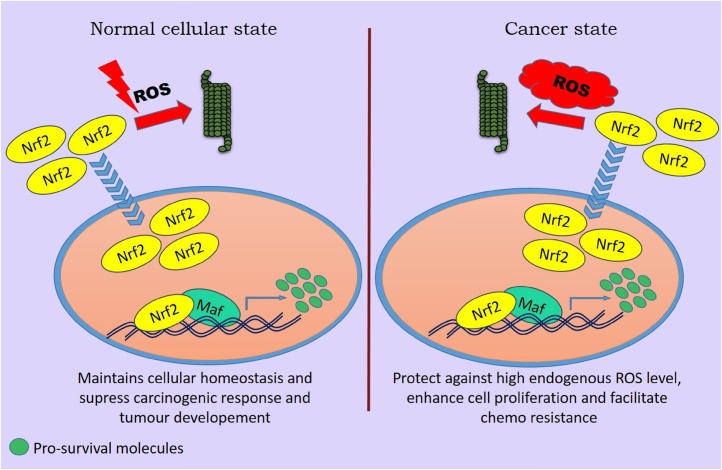
5.3. Role in cancer
Nrf2 plays not only crucial role in cancer chemoprevention and tumor suppression in normal cells but also is responsible in tumorigenesis and tumor protection. These two paradoxical roles have been concluded as the ‘dual role of Nrf2’ (Fig. 5).
Fig. 5.
Schematic representation of the dual role of Nrf2 in cancer progression and therapy.
5.3.1. Role of Nrf2 in chemoprevention
Nrf2 and HO-1 are upregulated in different types of tumors and correlate with tumor progression, aggressiveness and resistance to therapy. Hu et al. have identified that the mutations were in the purine bases of Nrf2. A point-mutation of glycine to cysteine was identified in the Kelch/DGR domain of KEAP1 which leads to a conformational change that reduces its affinity for Nrf2, resulting in activation of Nrf2 in cancer cells. Upregulation of Nrf2 is also related to angiogenesis which is promoted by hypoxia inducible factor-1(HIF-1α). Under hypoxic conditions, indeed, the O2-dependent regulator, prolyl-hydrolase domain (pH D), is catalytically inactive and increases the stability of a transcription factor, HIF-1α.
Suzuki et al. reported that single-nucleotide polymorphisms (SNP) in the human Nrf2 upstream promoter region (rs6721961) of minor A/A homozygotes affects the reduction of Nrf2 gene expression in lung cancer resulting in increased risk of lung cancer even in non-smokers [60]. SNP-homozygous (c.-617A/A) alleles in the Nrf2 gene are associated with lung adenocarcinoma [144]. Nrf2-null mice show elevated DNA adduct formation in lung tumors.
Overexpression of Nrf2 positively regulates the NQO1 gene in human hepatoblastoma. In Nrf2-deficient mice, burden of gastric neoplasia is increased after treatment with a carcinogen, such as benzo[a]pyrene, than wild type mice. In addition, Nrf2-knockout mice have lower hepatic and gastric activity of GST and NQO1 than wild-type. Constitutive activation of Nrf2 increases the sensitivity to chemotherapeutic drugs and radiation. Nrf2 mutated esophageal squamous cancer possesses malignant potential and resistance to chemo/radiotherapy [145]. Via rapamycin pathway, mutant Nrf2 induces proliferation, anchorage-independent growth and tumorigenicity in epithelial cells [146].
N-nitrosobutyl (4-hydroxybutyl)amine (BBN)-induced urinary bladder carcinoma and 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis are significantly higher in Nrf2 knockout than in wild-type.
5.3.2. Role of Nrf2 in oncogenesis
Elevated Nrf2 levels have been detected in various cancer tissues, including lung [147], [148], pancreas [52] and endometrium [149]. Missence mutations in KEAP1 suppress the DC domain of KEAP1 to bind Nrf2, and as a result, Nrf2 is constitutively stabilized under these conditions leading to cancer. Somatic KEAP1 gene mutations, insertions and deletions have been identified in the tumor tissue of lung [150], breast [151], [152], gallbladder [153], liver [153], ovarian [154], endometrial [155], papillary [156], head and neck, airway as well as skin cancer [157]. Disruption of Nrf2 signaling affects the cell cycle progression and proliferation of cancer cell lines in vitro [158], [159]. Nrf2 silencing in cancer cells causes attenuation of cell migration and tumor metastasis. Oncogenes K-Ras, B-Raf and Myc can stimulate Nrf2 transcription and activation in mouse fibroblasts leading to an overall reduction in the cellular burden of ROS [52]. The mechanism of Nrf2 activation is manifested in some hereditary cancer syndromes like hereditary leiomyomatosis and renal cell cancer (HLRCC) as well as hereditary tyrosinemia type 1 (HT1) [160]. These disorders are the results of inactivation of genes encoding metabolic enzymes, fumarate hydratase (FH) in HLRCC and fumaryl acetoacetate hydrolase (FAH) in HT1. They can modify key cysteine residues on KEAP1 which results in Nrf2 activation. The Cancer Genome Atlas has made a better understanding of several types of cancer and the regulation of Nrf-2 in its pathogenesis [161].
5.3.2.1. Lung carcinoma
The genomic and epigenomic analysis in lung squamous cell carcinomas has shown altered genes in KEAP1-NRF2 signaling pathway [67]. Mutually exclusive gain-of-function mutations and amplifications in NFE2L2 gene, as well as loss-of-function mutations in KEAP1 and CUL3, are mainly involved [162]. Lung adenocarcinoma (LUAD) leads to cancer-related deaths worldwide in never smokers, particularly women due to smoking. mRNA, miRNA and DNA profiling [67] indicated a high number of cytosine to adenine (C > A) nucleotide transversions in Keap1 [163].
5.3.2.2. Hepatocellular carcinoma (HCC)
HCC, the third most frequent cause of cancer-related deaths worldwide is strongly associated with hepatitis B virus infection and chronic inflammation in the liver and environmental exposure to toxicants such as alcohol [164]. In this case, genomic alterations were predominantly present in KEAP1. Liver cancer cell lines having the KEAP1 mutation and NRF2 overexpression were more sensitive to HSP90 inhibitor 17-N-allylamino-17-demethoxygeldanamycin (17-AAG), which is converted to its active form by an NRF2 target gene NAD(P)H quinone oxidoreductase-1 (NQO1) [165].
5.3.2.3. Renal and urinary bladder carcinoma
In urothelial bladder carcinoma there is mutations in thioredoxin interacting protein (TXNIP) gene mutually exclusive with NFE2L2 mutations which suggest a possible link with NRF2 and thioredoxin signaling [166] via gene amplification it has been shown that elevated p62 contribute to renal carcinogenesis via activation of NRF2 [167] in renal cell carcinoma. NRF2 activation is also characteristic of hereditary form of papillary renal cell carcinoma (PRCC), where fumarate is accumulated intracellularly and causes succinylation of KEAP1, leading to NRF2 activation [168], [169].
5.3.3. Role of Nrf2 in resistance to chemotherapy
Nrf2 is responsible for regulation of expression of efflux transporters especially from ATP-binding cassette (ABC) family which pumps out xenobiotics from the cell against a concentration gradient. Nrf2 binds to the ARE-like sequences in the promoters of multidrug resistance-associated proteins (MRP) genes like Mrp1, Mrp2, Mrp3, Mrp4, and Abcg2 and enhances their expressions, thus confers chemo resistance in cells. Nrf2 activation confers a growth advantage in cancer cells. Cancer cells show Warburg effect in which ATP generates through glycolysis providing a variety of glycolytic intermediates needed to synthesize nucleosides and amino acids that in turn leads to cell proliferation and division. Nrf2 plays key role in the development of drug resistance in patients undergoing chemotherapy. The activity of Nrf2 in cancer cells decreases their sensitivity to the common chemotherapeutic agents like doxorubicin, carboplatin, cisplatin, etc [159]. RNAi-mediated inhibition of Nrf2 signaling has been documented to reverse drug resistance [159]. Repression of Nrf2 promotes the cytotoxic effects of g-irradiation towards lung and pancreatic cancer cell lines. In vitro studies have revealed that Nrf2 signaling activity is downregulated in the doxorubicin-sensitive human ovarian carcinoma cell line, A2780 [161]. An elevated level of Cul3 in drug-sensitive breast cancer tissue samples and cell lines leads to constitutive repression of Nrf2. Knockdown of Cul3 upregulates expression of Nrf2 target genes [170]. A chemical compound K67 binds to the pocket of KEAP1 DC domain, which in turn disturbs the interaction between KEAP1 and p62 and maintains the interaction between KEAP1 and NRF2. Therefore, treatment with K67 enhances chemo-sensitivity.
5.3.4. Role of Nrf2 in immune system suppression
Nrf2 suppresses immune system against cancer by suppressing myeloid-derived suppressor cells (MDSC) which are a heterogeneous myeloid population containing macrophages, dendritic cells and neutrophils. ROS is an activator of immunosuppression by MDSC which promotes tumor development and metastasis by inhibiting innate and adaptive immunity [60]. As the proinflammatory cytokines play a critical role in sustaining the MDSC function, Nrf2 might suppress MDSC not only by ROS elimination but also by suppressing proinflammatory cytokines such as IL-6 and IL-1β.
6. Therapeutic role of Nrf2
Several natural products, small molecules, pharmacological compounds target Nrf2 signaling pathways, which may be used as therapeutic approaches against cancer and other diseased conditions.
6.1. Activation of Nrf2
Upregulation of Nrf2 activity is very useful in several pathophysiological conditions. Some herbal medicines [e.g., curcumin (turmeric), sulforaphane (broccoli), flavonoids like quercetin, fisetin, nordihydroguaiaretic acid (chapparal)] work via the alkylation/covalent modification mechanism [17], [171], [172], [173], [174], [175], [176]. There are multiple herbal supplements on the market based on curcumin (turmeric) in combination with other herbs such as protandim (Nrf2 activator), Nuley (Nrf2 optimizer), progressive nutracare (Nrf2 antiox), etc. Nrf2 activator, tecfidera (dimethylfumarate) is involved in the treatment of multiple sclerosis. Not only tecfidera but also bardoxolone is the most potent Nrf2 activator described to date, having a specific binding site in the KEAP1 IVR near Cys-298 and Cys-226 [177]. Upon hydrolysis monomethylfumarate (MMF) gives a natural metabolite – fumaric acid. It has a low alkylating potency and thus, low toxicity. It is used to treat chronic neurodegenerative disease such as PD. Antioxidant quercetin minimizes the cyto-lethal effect of UVA irradiated HaCaT cells. The Nrf2 activator, sulforaphane, protects against UVB-induced skin inflammation and sunburn in wild type, but not Nrf2−/− mice.
Several pharmacological products play a role as analogs of Nrf2. Combined treatment with 5-aza-29-deoxycytidine (a DNMT inhibitor) and trichostatin A (a histone deacetylase inhibitor) restores Nrf2 expression in TRAMP-C1 cells [178]. Nrf2-encoding lentivirus is effective in Alzheimer’s disease treatment [179]. CDDO represent a promising strategy for protection against Huntington’s disease and amyotrophiclateral sclerosis [180]. Pre-treatment of pharmacological activators of Nrf2 has been shown to be protective in animal models of numerous neurological conditions, such as Huntington’s disease, amyotrophic lateral sclerosis, PD, AD, and cerebral ischemia. Exposure of mice to 2-Cyano-3,12-dioxooleana-1,9-dien-28-imidazolide (CDDO-Im)reduces acetaminophen induced liver injury and concanavalin A induces T-cell-mediated acute inflammatory liver injury [181]. CDDO-Im pretreatment is beneficial against COPD, and it also enhances Nrf2-regulated cytoprotective responses in the kidney and protects against cisplatin toxicity [182].
6.2. Inhibition of Nrf2
Nrf2 downregulation is a potential therapeutic approach in cancer remedy. Inhibition of Nrf2 via RNAi knockdown can resensitize some cancer cells to anti-cancer agents [183]. Vitamins downregulate Nrf2 translocation into the nucleus leading to cancer cells death in leukemia [184]. Cryptotanshinone suppresses cell proliferation and increases apoptosis of cancer cells in acute lymphoblastic leukemia [185], human breast cancer [186] and colorectal cancer [187]. Some flavonoids like luteolin, apigenin, chrysin and 4-Methoxychalcone inhibit Nrf2 activity by promoting Nrf2 mRNA and protein degradation and sensitize cancer cells towards chemotherapeutic drugs [188], [189], [190]. Epigalocatechin3-gallate exerts an anticancer effect by downregulating Nrf2 –HO-1 axis [191].
The role of Nrf2 in cancer is very complex. Several studies have shown that Nrf2 plays an important role in preventing carcinogen-induced tumors. Numerous studies have shown that Nrf2 null mice are more susceptible to carcinogen-induced tumors than wild type. Satoh and co-workers found that Nrf2 does not promote initiation but enhances progression [192]. In HLRCC, Nrf2 activation is necessary to provide protection against intracellular fumarate accumulation [160]. Loss of either FH or FAH results in the intracellular accumulation of fumarate in the case of FH and fumarylacetoacetate in the case of FAH. Smoking associated lung cancer tends to have elevated expression of the Nrf2 target gene, aldose ketose reductase family 1 member B10 (AKR1B10) [3].
The chemopreventive effects of sulforaphane against the skin tumorigenesis and oltipraz against urinary bladder carcinogenesis are mediated through Nrf2 [193], [194]. The constitutive activation of Nrf2 in dermal fibroblasts derived from newborn KEAP1−/− mice causes huge decrease in the number of UVA irradiated apoptotic cells [138]. Exposure to the carcinogens 7,12-dimethylbenz(a)anthracene and 12-O-teradecanoylphorbol-13-acetate leads to a greater number of skin tumors [195]. Application of Nrf2 inducer, sulforaphane before carcinogen exposure provides protection against tumor development, in a Nrf2-dependent manner [196].
7. Concluding remarks
In this review, we have discussed both the beneficial and detrimental roles of Nrf2. In normal physiological condition, Nrf2 acts as a scavenger from stress and damage. But, this property of Nrf2 becomes destructive for cancer affected patients. Like any other normal cells, cancer cells also undergo apoptosis if intracellular ROS gets increased. Cancer cells harness the ability of Nrf2 to survive under stress conditions. Thus cancer cell undergoes proliferation easily by decreasing any kind of toxicity inside them. Mutational patterns of KEAP1 and Nrf2 are analogous to those of tumor suppressors and oncogenes. These represent new classes of genes which play important roles in carcinogenesis. There is a possibility to manage and treat these tumors with therapeutics. Several regulations are there to finely modulate Nrf2 activity. These modulations might be helpful for designing chemotherapeutic drugs in future.
References
- 1.Ariens E., Simonis A. General principles of nutritional toxicology. Nutr. Toxicol. 1982;1:17–80. [Google Scholar]
- 2.Godwill E.A., Jane I.C., Scholastica I.U., Marcellus U., Eugene A.L., Gloria O.A. Determination of some soft drink constituents and contamination by some heavy metals in Nigeria. Toxicol. Rep. 2015;2:384–390. doi: 10.1016/j.toxrep.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Praslicka B.J., Kerins M.J., Ooi A. The complex role of NRF2 in cancer: a genomic view. Curr. Opin. Toxicol. 2016;1:37–45. [Google Scholar]
- 4.Wittkopp S., Staimer N., Tjoa T., Stinchcombe T., Daher N., Schauer J.J., Shafer M.M., Sioutas C., Gillen D.L., Delfino R.J. Nrf2-related gene expression and exposure to traffic-related air pollution in elderly subjects with cardiovascular disease: an exploratory panel study. J. Expo. Sci. Environ. Epidemiol. 2016;26:141–149. doi: 10.1038/jes.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mossa A.-T.H., Swelam E.S., Mohafrash S.M. Sub-chronic exposure to fipronil induced oxidative stress. biochemical and histopathological changes in the liver and kidney of male albino rats. Toxicol. Rep. 2015;2:775–784. doi: 10.1016/j.toxrep.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nowak D.J., Hirabayashi S., Bodine A., Greenfield E. Tree and forest effects on air quality and human health in the United States. Environ. Pollut. 2014;193:119–129. doi: 10.1016/j.envpol.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Poljšak B., Fink R. The protective role of antioxidants in the defence against ROS/RNS-mediated environmental pollution. Oxid. Med. Cell. Longev. 2014;2014:671539. doi: 10.1155/2014/671539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogen K.T., Arnold L.L., Chowdhury A., Pennington K.L., Cohen S.M. Low-dose dose-response for reduced cell viability after exposure of human keratinocyte (HEK001) cells to arsenite. Toxicol. Rep. 2017;4:32–38. doi: 10.1016/j.toxrep.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das J., Sarkar A., Sil P.C. Hexavalent chromium induces apoptosis in human liver (HepG2) cells via redox imbalance. Toxicol. Rep. 2015;2:600–608. doi: 10.1016/j.toxrep.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Khishin I.A., El-fakharany Y.M.M., Hamid O.I.A. Role of garlic extract and silymarin compared to dimercaptosuccinic acid (DMSA) in treatment of lead induced nephropathy in adult male albino rats. Toxicol. Rep. 2015;2:824–832. doi: 10.1016/j.toxrep.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarkar K., Sil P.C. A 43 kDa protein from the herb Cajanus indicus L. protects thioacetamide induced cytotoxicity in hepatocytes. Toxicol. In Vitro. 2006;20:634–640. doi: 10.1016/j.tiv.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Sadhukhan P., Saha S., Sil P. Targeting oxidative stress: a novel approach in mitigating cancer. Biochem. Anal. Biochem. 2015;4 2161-1009.1000236. [Google Scholar]
- 14.Pari L., Karthikeyan A., Karthika P., Rathinam A. Protective effects of hesperidin on oxidative stress, dyslipidaemia and histological changes in iron-induced hepatic and renal toxicity in rats. Toxicol. Rep. 2015;2:46–55. doi: 10.1016/j.toxrep.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwal N., Sadhukhan P., Saha S., Sil Therapeutic P.C. Insights against oxidative stress induced diabetic nephropathy: a review. J. Autoimmune Disord. 2015;1:1. [Google Scholar]
- 17.Saha S., Sadhukhan P., Sinha K., Agarwal N., Sil P.C. Mangiferin attenuates oxidative stress induced renal cell damage through activation of PI3K induced Akt and Nrf-2 mediated signaling pathways. Biochem. Biophys. Rep. 2016;5:313–327. doi: 10.1016/j.bbrep.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saha S., Sadhukhan P., Sil P.C. Beneficial upshots of naturally occurring antioxidant compounds against neurological disorders, neuroprotective natural products. Clin. Asp. Mode Action. 2017:23–56. [Google Scholar]
- 19.Dutta S., Sadhukhan P., Saha S., Sil P.C. Regulation of oxidative stress by different naturally occurring polyphenolic compounds: an emerging anticancer therapeutic approach. React. Oxyg. Species. 2017;3:81–95. [Google Scholar]
- 20.Madrigal-Santillán E., Bautista M., Gayosso-De-Lucio J.A., Reyes-Rosales Y., Posadas-Mondragón A., Morales-González Á., Soriano-Ursúa M.A., García-Machorro J., Madrigal-Bujaidar E., Álvarez-González I. Hepatoprotective effect of Geranium schiedeanum against ethanol toxicity during liver regeneration. World J. Gastroenterol. 2015;21:7718. doi: 10.3748/wjg.v21.i25.7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pal S., Sarkar A., Pal P.B., Sil P.C. Protective effect of arjunolic acid against atorvastatin induced hepatic and renal pathophysiology via MAPK, mitochondria and ER dependent pathways. Biochimie. 2015;112:20–34. doi: 10.1016/j.biochi.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Sadhukhan P., Saha S., Sil P.C. Anti-oxidative effect of genistein and mangiferin on sodium fluoride induced oxidative insult of renal cells: a comparative study. Biomark. J. 2016;2:2. [Google Scholar]
- 23.Cao S.S., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rashid K., Sinha K., Sil P.C. An update on oxidative stress-mediated organ pathophysiology. Food Chem. Toxicol. 2013;62:584–600. doi: 10.1016/j.fct.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 25.Chowdhury S., Ghosh S., Rashid K., Sil P.C. Deciphering the role of ferulic acid against streptozotocin-induced cellular stress in the cardiac tissue of diabetic rats. Food Chem. Toxicol. 2016;97:187–198. doi: 10.1016/j.fct.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Bar-Or D., Bar-Or R., Rael L.T., Brody E.N. Oxidative stress in severe acute illness. Redox Biol. 2015;4:340–345. doi: 10.1016/j.redox.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal P.B., Sinha K., Sil P.C. Mangiferin attenuates diabetic nephropathy by inhibiting oxidative stress mediated signaling cascade, TNFα related and mitochondrial dependent apoptotic pathways in streptozotocin-induced diabetic rats. PLoS One. 2014;9:e107220. doi: 10.1371/journal.pone.0107220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki T., Motohashi H., Yamamoto M. Toward clinical application of the Keap1–Nrf2 pathway. Trends Pharmacol. Sci. 2013;34:340–346. doi: 10.1016/j.tips.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Chen L.-H., Tsai H.-C., Yu P.-L., Chung K.-R. A major facilitator superfamily transporter-Mediated resistance to oxidative stress and fungicides requires Yap1, Skn7, and MAP kinases in the citrus fungal pathogen alternaria alternata. PLoS One. 2017;12:e0169103. doi: 10.1371/journal.pone.0169103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh S., Bhattacharyya S., Rashid K., Sil P.C. Curcumin protects rat liver from streptozotocin-induced diabetic pathophysiology by counteracting reactive oxygen species and inhibiting the activation of p53 and MAPKs mediated stress response pathways. Toxicol. Rep. 2015;2:365–376. doi: 10.1016/j.toxrep.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onodera A., Nishiumi F., Kakiguchi K., Tanaka A., Tanabe N., Honma A., Yayama K., Yoshioka Y., Nakahira K., Yonemura S. Short-term changes in intracellular ROS localisation after the silver nanoparticles exposure depending on particle size. Toxicol. Rep. 2015;2:574–579. doi: 10.1016/j.toxrep.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhattacharyya S., Banerjee S., Guha C., Ghosh S., Sil P.C. A 35 kDa Phyllanthus niruri protein suppresses indomethacin mediated hepatic impairments: its role in Hsp70, HO-1, JNKs and Ca 2+ dependent inflammatory pathways. Food Chem. Toxicol. 2017;102:76–92. doi: 10.1016/j.fct.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh S., Sarkar A., Bhattacharyya S., Sil P.C. Silymarin protects mouse liver and kidney from thioacetamide induced toxicity by scavenging reactive oxygen species and activating PI3K-Akt pathway. Front. Pharmacol. 2016;7:481. doi: 10.3389/fphar.2016.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu L., Huang W., Wang J., Song H., Cen J., Ji B. Anthraquinone derivative exerted hormetic effect on the apoptosis in oxygen-glucose deprivation-induced PC12 cells via ERK and Akt activated Nrf2/HO-1 signaling pathway. Chem. Biol. Interact. 2017;262:1–11. doi: 10.1016/j.cbi.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Mastrantonio R., Cervelli M., Pietropaoli S., Mariottini P., Colasanti M., Persichini T. HIV-Tat induces the Nrf2/ARE pathway through NMDA receptor-elicited spermine oxidase activation in human neuroblastoma cells. PLoS One. 2016;11:e0149802. doi: 10.1371/journal.pone.0149802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aboonabi A., Singh I. Chemopreventive role of anthocyanins in atherosclerosis via activation of Nrf2–ARE as an indicator and modulator of redox. Biomed. Pharmacother. 2015;72:30–36. doi: 10.1016/j.biopha.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Niture S.K., Khatri R., Jaiswal A.K. Regulation of Nrf2—an update. Free Radic. Biol Med. 2014;66:36–44. doi: 10.1016/j.freeradbiomed.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizvi F., Shukla S., Kakkar P. Essential role of pH domain and leucine-rich repeat protein phosphatase 2 in Nrf2 suppression via modulation of Akt/GSK3β/Fyn kinase axis during oxidative hepatocellular toxicity. Cell Death Dis. 2014;5:e1153. doi: 10.1038/cddis.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaramillo M.C., Zhang D.D. The emerging role of the Nrf2–Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Mo C., Wang L., Zhang J., Numazawa S., Tang H., Tang X., Han X., Li J., Yang M., Wang Z. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. 2014;20:574–588. doi: 10.1089/ars.2012.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paredes‐Gonzalez X., Fuentes F., Jeffery S., Saw C.L.L., Shu L., Su Z.Y., Kong A.N.T. Induction of NRF2‐mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharm. Drug Dispos. 2015;36:440–451. doi: 10.1002/bdd.1956. [DOI] [PubMed] [Google Scholar]
- 43.Divya T., Dineshbabu V., Soumyakrishnan S., Sureshkumar A., Sudhandiran G. Celastrol enhances Nrf2 mediated antioxidant enzymes and exhibits anti-fibrotic effect through regulation of collagen production against bleomycin-induced pulmonary fibrosis. Chem. Biol. Interact. 2016;246:52–62. doi: 10.1016/j.cbi.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Lim J.L., Wilhelmus M.M., de Vries H.E., Drukarch B., Hoozemans J.J., van Horssen J. Antioxidative defense mechanisms controlled by Nrf2: state-of-the-art and clinical perspectives in neurodegenerative diseases. Arch. Toxicol. 2014;88:1773–1786. doi: 10.1007/s00204-014-1338-z. [DOI] [PubMed] [Google Scholar]
- 45.Fetoni A.R., Paciello F., Mezzogori D., Rolesi R., Eramo S.L.M., Paludetti G., Troiani D. Molecular targets for anticancer redox chemotherapy and cisplatin-induced ototoxicity: the role of curcumin on pSTAT3 and Nrf-2 signalling. Br. J. Cancer. 2015;10:1434–1444. doi: 10.1038/bjc.2015.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 47.Son Y.-O., Pratheeshkumar P., Roy R.V., Hitron J.A., Wang L., Divya S.P., Xu M., Luo J., Chen G., Zhang Z. Antioncogenic and oncogenic properties of Nrf2 in arsenic-induced carcinogenesis. J. Biol. Chem. 2015;290:27090–27100. doi: 10.1074/jbc.M115.675371. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Na H.-K., Surh Y.-J. Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1. Free Radic. Biol Med. 2014;67:353–365. doi: 10.1016/j.freeradbiomed.2013.10.819. [DOI] [PubMed] [Google Scholar]
- 49.Gardi C., Bauerova K., Stringa B., Kuncirova V., Slovak L., Ponist S., Drafi F., Bezakova L., Tedesco I., Acquaviva A. Quercetin reduced inflammation and increased antioxidant defense in rat adjuvant arthritis. Arch. Biochem. Biophys. 2015;583:150–157. doi: 10.1016/j.abb.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 50.Rojo A.I., Rada P., Mendiola M., Ortega-Molina A., Wojdyla K., Rogowska-Wrzesinska A., Hardisson D., Serrano M., Cuadrado A. The PTEN/NRF2 axis promotes human carcinogenesis. Antioxid. Redox Signal. 2014;21:2498–2514. doi: 10.1089/ars.2014.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Furfaro A., Traverso N., Domenicotti C., Piras S., Moretta L., Marinari U., Pronzato M., Nitti The M. Nrf2/HO-1 axis in cancer cell growth and chemoresistance. Oxid. Med. Cell. Longev. 2016;2016:1958174. doi: 10.1155/2016/1958174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeNicola G.M., Karreth F.A., Humpton T.J., Gopinathan A., Wei C., Frese K., Mangal D., Kenneth H.Y., Yeo C.J., Calhoun E.S. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sporn M.B., Liby K.T. NRF2 and cancer: the good, the bad and the importance of context. Nat. Rev. Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C.-Y., Wang Z.-Y., Xie J.-W., Cai J.-H., Wang T., Xu Y., Wang X., An L. CD36 upregulation mediated by intranasal LV-NRF2 treatment mitigates hypoxia-induced progression of Alzheimer’s-like pathogenesis. Antioxid. Redox Signal. 2014;21:2208–2230. doi: 10.1089/ars.2014.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gasiorek J.J., Blank V. Regulation and function of the NFE2 transcription factor in hematopoietic and non-hematopoietic cells. Cell Mol. Life Sci. 2015;72:2323–2335. doi: 10.1007/s00018-015-1866-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsujita T., Peirce V., Baird L., Matsuyama Y., Takaku M., Walsh S.V., Griffin J.L., Uruno A., Yamamoto M., Hayes J.D. Transcription factor Nrf1 negatively regulates the cystine/glutamate transporter and lipid-metabolizing enzymes. Mol. Cell Biol. 2014;34:3800–3816. doi: 10.1128/MCB.00110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biswas M., Chan J.Y. Role of Nrf1 in antioxidant response element-mediated gene expression and beyond. Toxicol. Appl. Pharmacol. 2010;244:16–20. doi: 10.1016/j.taap.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fukutomi T., Takagi K., Mizushima T., Ohuchi N., Yamamoto M. Kinetic, thermodynamic, and structural characterizations of the association between Nrf2-DLGex degron and Keap1. Mol. Cell Biol. 2014;34:832–846. doi: 10.1128/MCB.01191-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takaya K., Suzuki T., Motohashi H., Onodera K., Satomi S., Kensler T.W., Yamamoto M. Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radic. Biol Med. 2012;53:817–827. doi: 10.1016/j.freeradbiomed.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki M., Otsuki A., Keleku-Lukwete N., Yamamoto M. Overview of redox regulation by Keap1–Nrf2 system in toxicology and cancer. Curr. Opin. Toxicol. 2016;1:29–36. [Google Scholar]
- 61.Baird L., Llères D., Swift S., Dinkova-Kostova A.T. Regulatory flexibility in the Nrf2-mediated stress response is conferred by conformational cycling of the Keap1-Nrf2 protein complex. Proc. Natl. Acad. Sci. 2013;110:15259–15264. doi: 10.1073/pnas.1305687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dinkova-Kostova A.T., Abramov A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol Med. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki T., Yamamoto M. Molecular basis of the Keap1–Nrf2 system. Free Radic. Biol Med. 2015;88:93–100. doi: 10.1016/j.freeradbiomed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 64.Huang H.-C., Nguyen T., Pickett C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 65.Tong K.I., Katoh Y., Kusunoki H., Itoh K., Tanaka T., Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol. Cell Biol. 2006;26:2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ogura T., Tong K.I., Mio K., Maruyama Y., Kurokawa H., Sato C., Yamamoto M. Keap1 is a forked-stem dimer structure with two large spheres enclosing the intervening, double glycine repeat, and C-terminal domains. Proc. Natl. Acad. Sci. 2010;107:2842–2847. doi: 10.1073/pnas.0914036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cancer Genome Atlas Research Network Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miao W., Hu L., Scrivens P.J., Batist G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and Ii drug-metabolizing enzymes. J. Biol. Chem. 2005;280:20340–20348. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- 69.Cancer Genome Atlas Research Network Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qiang M., Kinneer K., Yongyi B. Induction of murine NAD (P) H: quinone oxidoreductase by 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin requires the CNC (cap n collar) basic leucine zipper transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2): cross-interaction between AhR (aryl hydrocarbon receptor) and Nrf2 signal transduction. Biochem. J. 2004;377:205–213. doi: 10.1042/BJ20031123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shin S., Wakabayashi N., Misra V., Biswal S., Lee G.H., Agoston E.S., Yamamoto M., Kensler T.W. NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol. Cell Biol. 2007;27:7188–7197. doi: 10.1128/MCB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwak M.-K., Itoh K., Yamamoto M., Kensler T.W. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol. Cell Biol. 2002;22:2883–2892. doi: 10.1128/MCB.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tao S., Wang S., Moghaddam S.J., Ooi A., Chapman E., Wong P.K., Zhang D.D. Oncogenic KRAS confers chemoresistance by upregulating NRF2. Cancer Res. 2014;74:7430–7441. doi: 10.1158/0008-5472.CAN-14-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rushworth S.A., Zaitseva L., Murray M.Y., Shah N.M., Bowles K.M., MacEwan D.J. The high Nrf2 expression in human acute myeloid leukemia is driven by NF-κB and underlies its chemo-resistance. Blood. 2012;120:5188–5198. doi: 10.1182/blood-2012-04-422121. [DOI] [PubMed] [Google Scholar]
- 75.Sanderson L.M., Boekschoten M.V., Desvergne B., Müller M., Kersten S. Transcriptional profiling reveals divergent roles of PPARα and PPARβ/δ in regulation of gene expression in mouse liver. Physiol. Genomics. 2010;41:42–52. doi: 10.1152/physiolgenomics.00127.2009. [DOI] [PubMed] [Google Scholar]
- 76.Guo Y., Yu S., Zhang C., Kong A.-N.T. Epigenetic regulation of Keap1-Nrf2 signaling. Free Radic. Biol Med. 2015;88:337–349. doi: 10.1016/j.freeradbiomed.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang R., An J., Ji F., Jiao H., Sun H., Zhou D. Hypermethylation of the Keap1 gene in human lung cancer cell lines and lung cancer tissues. Biochem. Biophys. Res. Commun. 2008;373:151–154. doi: 10.1016/j.bbrc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 78.Muscarella L.A., Barbano R., D’Angelo V., Copetti M., Coco M., Balsamo T., la Torre A., Notarangelo A., Troiano M., Parisi S. Regulation of KEAP1 expression by promoter methylation in malignant gliomas and association with patient’s outcome. Epigenetics. 2011;6:317–325. doi: 10.4161/epi.6.3.14408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang P., Singh A., Yegnasubramanian S., Esopi D., Kombairaju P., Bodas M., Wu H., Bova S.G., Biswal S. Loss of Kelch-like ECH-associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growth. Mol. Cancer Ther. 2010;9:336–346. doi: 10.1158/1535-7163.MCT-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanada N., Takahata T., Zhou Q., Ye X., Sun R., Itoh J., Ishiguro A., Kijima H., Mimura J., Itoh K. Methylation of the KEAP1 gene promoter region in human colorectal cancer. BMC Cancer. 2012;12:66. doi: 10.1186/1471-2407-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barbano R., Muscarella L.A., Pasculli B., Valori V.M., Fontana A., Coco M., La Torre A., Balsamo T., Poeta M.L., Marangi G.F. Aberrant Keap1 methylation in breast cancer and association with clinicopathological features. Epigenetics. 2013;8:105–112. doi: 10.4161/epi.23319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Narasimhan M., Patel D., Vedpathak D., Rathinam M., Henderson G., Mahimainathan L. Identification of novel microRNAs in post-transcriptional control of Nrf2 expression and redox homeostasis in neuronal, SH-SY5Y cells. PLoS One. 2012;7:e51111. doi: 10.1371/journal.pone.0051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sangokoya C., Telen M.J., Chi J.-T. microRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood. 2010;116:4338–4348. doi: 10.1182/blood-2009-04-214817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang M., Yao Y., Eades G., Zhang Y., Zhou Q. MiR-28 regulates Nrf2 expression through a Keap1-independent mechanism. Breast Cancer Res. Treat. 2011;129:983–991. doi: 10.1007/s10549-011-1604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsai C.-Y., Wang C.-C., Lai T.-Y., Tsu H.-N., Wang C.-H., Liang H.-Y., Kuo W.-W. Antioxidant effects of diallyl trisulfide on high glucose-induced apoptosis are mediated by the PI3K/Akt-dependent activation of Nrf2 in cardiomyocytes. Int. J. Cardiol. 2013;168:1286–1297. doi: 10.1016/j.ijcard.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 86.Cheng D., Wu R., Guo Y., Kong A.N. Regulation of Keap1–Nrf2 signaling: the role of epigenetics. Curr. Opin. Toxicol. 2016;1:134–138. doi: 10.1016/j.cotox.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gañán-Gómez I., Wei Y., Yang H., Boyano-Adánez M.C., García-Manero G. Oncogenic functions of the transcription factor Nrf2. Free Radic. Biol Med. 2013;65:750–764. doi: 10.1016/j.freeradbiomed.2013.06.041. [DOI] [PubMed] [Google Scholar]
- 88.Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y., Sou Y.-S., Ueno I., Sakamoto A., Tong K.I. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 89.Ichimura Y., Waguri S., Sou Y.-s., Kageyama S., Hasegawa J., Ishimura R., Saito T., Yang Y., Kouno T., Fukutomi T. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol. Cell. 2013;51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 90.Fan W., Tang Z., Chen D., Moughon D., Ding X., Chen S., Zhu M., Zhong Q. Keap1 facilitates p62-mediated ubiquitin aggregate clearance via autophagy. Autophagy. 2010;6:614–621. doi: 10.4161/auto.6.5.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Puissant A., Fenouille N., Auberger P. When autophagy meets cancer through p62/SQSTM1. Am. J. Cancer Res. 2012;2:397–413. [PMC free article] [PubMed] [Google Scholar]
- 92.Komatsu M., Ichimura Y. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett. 2010;584:1374–1378. doi: 10.1016/j.febslet.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 93.Filomeni G., De Zio D., Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22:377–388. doi: 10.1038/cdd.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mei Y., Thompson M.D., Cohen R.A., Tong X. Autophagy and oxidative stress in cardiovascular diseases. Biochim. Biophys. Acta. 2015;1852:243–251. doi: 10.1016/j.bbadis.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kobayashi M., Li L., Iwamoto N., Nakajima-Takagi Y., Kaneko H., Nakayama Y., Eguchi M., Wada Y., Kumagai Y., Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McMahon M., Lamont D.J., Beattie K.A., Hayes J.D. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc. Natl. Acad. Sci. 2010;107:18838–18843. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fujii S., Sawa T., Ihara H., Tong K.I., Ida T., Okamoto T., Ahtesham A.K., Ishima Y., Motohashi H., Yamamoto M. The critical role of nitric oxide signaling via protein S-guanylation and nitrated cyclic GMP, in the antioxidant adaptive response. J. Biol. Chem. 2010;285:23970–23984. doi: 10.1074/jbc.M110.145441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burk R.F., Hill K.E., Nakayama A., Mostert V., Levander X.A., Motley A.K., Johnson D.A., Johnson J.A., Freeman M.L., Austin L.M. Selenium deficiency activates mouse liver Nrf2–ARE but vitamin E deficiency does not. Free Radic. Biol Med. 2008;44:1617–1623. doi: 10.1016/j.freeradbiomed.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bryan H.K., Olayanju A., Goldring C.E., Park B.K. The Nrf2 cell defence pathway: Keap1-dependent and-independent mechanisms of regulation. Biochem. Pharmacol. 2013;85:705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 100.Yu R., Mandlekar S., Lei W., Fahl W.E., Tan T.-H., Kong A.-N.T. p38 mitogen-activated protein kinase negatively regulates the induction of phase II drug-metabolizing enzymes that detoxify carcinogens. J. Biol. Chem. 2000;275:2322–2327. doi: 10.1074/jbc.275.4.2322. [DOI] [PubMed] [Google Scholar]
- 101.Salinas M., Diaz R., Abraham N.G., de Galarreta C.M.R., Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J. Biol. Chem. 2003;278:13898–13904. doi: 10.1074/jbc.M209164200. [DOI] [PubMed] [Google Scholar]
- 102.Salazar M., Rojo A.I., Velasco D., de Sagarra R.M., Cuadrado A. Glycogen synthase kinase-3β inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J. Biol. Chem. 2006;281:14841–14851. doi: 10.1074/jbc.M513737200. [DOI] [PubMed] [Google Scholar]
- 103.Han D., Li S.-J., Zhu Y.-T., Liu L., Li M.-X. LKB1/AMPK/mTOR signaling pathway in non-small-cell lung cancer. Asian Pac. J. Cancer Prev. 2013;14:4033–4039. doi: 10.7314/apjcp.2013.14.7.4033. [DOI] [PubMed] [Google Scholar]
- 104.Hardie D.G. AMP-activated protein kinase: maintaining energy homeostasis at the cellular and whole-body levels. Annu. Rev. Nutr. 2014;34:31–55. doi: 10.1146/annurev-nutr-071812-161148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sladek F.M. What are nuclear receptor ligands? Mol. Cell. Endocrinol. 2011;334:3–13. doi: 10.1016/j.mce.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oakley R.H., Cidlowski J.A. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013;132:1033–1044. doi: 10.1016/j.jaci.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bushue N., Wan Y.-J.Y. Retinoid pathway and cancer therapeutics. Adv. Drug Deliv. Rev. 2010;62:1285–1298. doi: 10.1016/j.addr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang X.J., Hayes J.D., Henderson C.J., Wolf C.R. Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. Proc. Natl. Acad. Sci. 2007;104:19589–19594. doi: 10.1073/pnas.0709483104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fishel M.L., Wu X., Devlin C.M., Logsdon D.P., Jiang Y., Luo M., He Y., Yu Z., Tong Y., Lipking K.P. Apurinic/apyrimidinic endonuclease/redox factor-1 (APE1/Ref-1) redox function negatively regulates NRF2. J. Biol. Chem. 2015;290:3057–3068. doi: 10.1074/jbc.M114.621995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Malloy M.T., McIntosh D.J., Walters T.S., Flores A., Goodwin J.S., Arinze I.J. Trafficking of the transcription factor Nrf2 to promyelocytic leukemia-nuclear bodies: implications for degradation of Nrf2 In the nucleus. J. Biol. Chem. 2013;288:14569–14583. doi: 10.1074/jbc.M112.437392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Singh A., Bodas M., Wakabayashi N., Bunz F., Biswal S. Gain of Nrf2 function in non-small-cell lung cancer cells confers radioresistance. Antioxid. Redox Signal. 2010;13:1627–1637. doi: 10.1089/ars.2010.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rada P., Rojo A.I., Evrard-Todeschi N., Innamorato N.G., Cotte A., Jaworski T., Tobón-Velasco J.C., Devijver H., García-Mayoral M.F., Van Leuven F. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/β-TrCP axis. Mol. Cell Biol. 2012;32:3486–3499. doi: 10.1128/MCB.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chowdhry S., Zhang Y., McMahon M., Sutherland C., Cuadrado A., Hayes J.D. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu T., Zhao F., Gao B., Tan C., Yagishita N., Nakajima T., Wong P.K., Chapman E., Fang D., Zhang D.D. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes. Dev. 2014;28:708–722. doi: 10.1101/gad.238246.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hirotsu Y., Katsuoka F., Funayama R., Nagashima T., Nishida Y., Nakayama K., Engel J.D., Yamamoto M. Nrf2–MafG heterodimers contribute globally to antioxidant and metabolic networks. Nucl. Acids Res. 2012;40:10228–10239. doi: 10.1093/nar/gks827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fujita R., Takayama-Tsujimoto M., Satoh H., Gutiérrez L., Aburatani H., Fujii S., Sarai A., Bresnick E.H., Yamamoto M., Motohashi H. NF-E2 p45 is important for establishing normal function of platelets. Mol. Cell Biol. 2013;33:2659–2670. doi: 10.1128/MCB.01274-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hayes J.D., McMahon M., Chowdhry S., Dinkova-Kostova A.T. Cancer chemoprevention mechanisms mediated through the Keap1–Nrf2 pathway. Antioxid. Redox Signal. 2010;13:1713–1748. doi: 10.1089/ars.2010.3221. [DOI] [PubMed] [Google Scholar]
- 118.Cullinan S.B., Diehl J.A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 119.Jaquet V., Scapozza L., Clark R.A., Krause K.-H., Lambeth J.D. Small-molecule NOX inhibitors: ROS-generating NADpH oxidases as therapeutic targets. Antioxid. Redox Signal. 2009;11:2535–2552. doi: 10.1089/ars.2009.2585. [DOI] [PubMed] [Google Scholar]
- 120.Ludtmann M.H., Angelova P.R., Zhang Y., Abramov A.Y., Dinkova-Kostova A.T. Nrf2 affects the efficiency of mitochondrial fatty acid oxidation. Biochem. J. 2014;457:415–424. doi: 10.1042/BJ20130863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bastin J., Lopes-Costa A., Djouadi F. Exposure to resveratrol triggers pharmacological correction of fatty acid utilization in human fatty acid oxidation-deficient fibroblasts. Hum. Mol. Genet. 2011;20:2048–2057. doi: 10.1093/hmg/ddr089. [DOI] [PubMed] [Google Scholar]
- 122.Chorley B.N., Campbell M.R., Wang X., Karaca M., Sambandan D., Bangura F., Xue P., Pi J., Kleeberger S.R., Bell D.A. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucl. Acids Res. 2012;40:7416–7429. doi: 10.1093/nar/gks409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hochmuth C.E., Biteau B., Bohmann D., Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell. 2011;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ferrando M., Wan X., Meiss R., Yang J., De Siervi A., Navone N., Vazquez E. Heme oxygenase-1 (HO-1) expression in prostate cancer cells modulates the oxidative response in bone cells. PLoS One. 2013;8:e80315. doi: 10.1371/journal.pone.0080315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nakamura B.N., Lawson G., Chan J.Y., Banuelos J., Cortés M.M., Hoang Y.D., Ortiz L., Rau B.A., Luderer U. Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age-dependent manner. Free Radic. Biol. Med. 2010;49:1368–1379. doi: 10.1016/j.freeradbiomed.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wakabayashi N., Skoko J.J., Chartoumpekis D.V., Kimura S., Slocum S.L., Noda K., Palliyaguru D.L., Fujimuro M., Boley P.A., Tanaka Y. Notch-Nrf2 axis: regulation of Nrf2 gene expression and cytoprotection by notch signaling. Mol. Cell Biol. 2014;34:653–663. doi: 10.1128/MCB.01408-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Meakin P.J., Chowdhry S., Sharma R.S., Ashford F.B., Walsh S.V., McCrimmon R.J., Dinkova-Kostova A.T., Dillon J.F., Hayes J.D., Ashford M.L. Susceptibility of Nrf2-null mice to steatohepatitis and cirrhosis upon consumption of a high-fat diet is associated with oxidative stress, perturbation of the unfolded protein response, and disturbance in the expression of metabolic enzymes but not with insulin resistance. Mol. Cell Biol. 2014;34:3305–3320. doi: 10.1128/MCB.00677-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sasaki H., Sato H., Kuriyama-Matsumura K., Sato K., Maebara K., Wang H., Tamba M., Itoh K., Yamamoto M., Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J. Biol. Chem. 2002;277:44765–44771. doi: 10.1074/jbc.M208704200. [DOI] [PubMed] [Google Scholar]
- 129.Kojer K., Riemer J. Balancing oxidative protein folding: the influences of reducing pathways on disulfide bond formation. Biochim. Biophys.Acta. 2014;1844:1383–1390. doi: 10.1016/j.bbapap.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 130.Sinha K., Pal P.B., Sil Cadmium P.C. (Cd 2+) exposure differentially elicits both cell proliferation and cell death related responses in SK-RC-45. Toxicol. In Vitro. 2014;28:307–318. doi: 10.1016/j.tiv.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 131.Das J., Sil P.C. Taurine ameliorates alloxan-induced diabetic renal injury, oxidative stress-related signaling pathways and apoptosis in rats. Amino Acids. 2012;43:1509–1523. doi: 10.1007/s00726-012-1225-y. [DOI] [PubMed] [Google Scholar]
- 132.Rashid K., Sil P.C. Curcumin enhances recovery of pancreatic islets from cellular stress induced inflammation and apoptosis in diabetic rats. Toxicol. Appl. Pharmacol. 2015;282:297–310. doi: 10.1016/j.taap.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 133.Mao L., Wang H., Wang X., Liao H., Zhao X. Transcription factor Nrf2 protects the spinal cord from inflammation produced by spinal cord injury. J. Surg. Res. 2011;170:e105–e115. doi: 10.1016/j.jss.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 134.Mao L., Wang H.-D., Wang X.-L., Tian L., Xu J.-Y. Disruption of Nrf2 exacerbated the damage after spinal cord injury in mice. J. Trauma Acute Care Surg. 2012;72:189–198. doi: 10.1097/TA.0b013e31821bf541. [DOI] [PubMed] [Google Scholar]
- 135.Tu J., Zhang X., Zhu Y., Dai Y., Li N., Yang F., Zhang Q., Brann D.W., Wang R. Cell-permeable peptide targeting the Nrf2–Keap1 interaction: a potential novel therapy for global cerebral ischemia. J. Neurosci. 2015;35:14727–14739. doi: 10.1523/JNEUROSCI.1304-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Osburn W.O., Yates M.S., Dolan P.D., Chen S., Liby K.T., Sporn M.B., Taguchi K., Yamamoto M., Kensler T.W. Genetic or pharmacologic amplification of nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol. Sci. 2008;104:218–227. doi: 10.1093/toxsci/kfn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Saw C.L., Huang M.T., Liu Y., Khor T.O., Conney A.H., Kong A.N. Impact of Nrf2 on UVB‐induced skin inflammation/photoprotection and photoprotective effect of sulforaphane. Mol. Carcinog. 2011;50:479–486. doi: 10.1002/mc.20725. [DOI] [PubMed] [Google Scholar]
- 138.Hirota A., Kawachi Y., Itoh K., Nakamura Y., Xu X., Banno T., Takahashi T., Yamamoto M., Otsuka Ultraviolet F. A irradiation induces NF-E2-related factor 2 activation in dermal fibroblasts: protective role in UVA-induced apoptosis. J. Invest. Dermatol. 2005;124:825–832. doi: 10.1111/j.0022-202X.2005.23670.x. [DOI] [PubMed] [Google Scholar]
- 139.Zheng H., Whitman S.A., Wu W., Wondrak G.T., Wong P.K., Fang D., Zhang D.D. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011;60:3055–3066. doi: 10.2337/db11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nabavi S.F., Barber A.J., Spagnuolo C., Russo G.L., Daglia M., Nabavi S.M., Sobarzo-Sánchez E. Nrf2 as molecular target for polyphenols: a novel therapeutic strategy in diabetic retinopathy. Crit. Rev. Clin. Lab. Sci. 2016;53:293–312. doi: 10.3109/10408363.2015.1129530. [DOI] [PubMed] [Google Scholar]
- 141.Kannan S., Muthusamy V.R., Whitehead K.J., Wang L., Gomes A.V., Litwin S.E., Kensler T.W., Abel E.D., Hoidal J.R., Rajasekaran N.S. Nrf2 deficiency prevents reductive stress-induced hypertrophic cardiomyopathy. Cardiovascular research. 2013:cvt150. doi: 10.1093/cvr/cvt150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Furusawa Y., Uruno A., Yagishita Y., Higashi C., Yamamoto M. Nrf2 induces fibroblast growth factor 21 in diabetic mice. Genes Cells. 2014;19:864–878. doi: 10.1111/gtc.12186. [DOI] [PubMed] [Google Scholar]
- 143.Huang J., Tabbi-Anneni I., Gunda V., Wang L. Transcription factor Nrf2 regulates SHP and lipogenic gene expression in hepatic lipid metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G1211–G1221. doi: 10.1152/ajpgi.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Okano Y., Nezu U., Enokida Y., Lee M.T.M., Kinoshita H., Lezhava A., Hayashizaki Y., Morita S., Taguri M., Ichikawa Y. SNP. (−617C > A) in ARE-like loci of the NRF2 gene: a new biomarker for prognosis of lung adenocarcinoma in Japanese non-smoking women. PLoS One. 2013;8:e73794. doi: 10.1371/journal.pone.0073794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shibata T., Kokubu A., Saito S., Narisawa-Saito M., Sasaki H., Aoyagi K., Yoshimatsu Y., Tachimori Y., Kushima R., Kiyono T. NRF2 mutation confers malignant potential and resistance to chemoradiation therapy in advanced esophageal squamous cancer. Neoplasia. 2011;13:864–873. doi: 10.1593/neo.11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shibata T., Saito S., Kokubu A., Suzuki T., Yamamoto M., Hirohashi S. Global downstream pathway analysis reveals a dependence of oncogenic NF-E2–related factor 2 mutation on the mTOR growth signaling pathway. Cancer Res. 2010;70:9095–9105. doi: 10.1158/0008-5472.CAN-10-0384. [DOI] [PubMed] [Google Scholar]
- 147.Padmanabhan B., Tong K.I., Ohta T., Nakamura Y., Scharlock M., Ohtsuji M., Kang M.-I., Kobayashi A., Yokoyama S., Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol. Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 148.Takahashi T., Sonobe M., Menju T., Nakayama E., Mino N., Iwakiri S., Nagai S., Sato K., Miyahara R., Okubo K. Mutations in Keap1 are a potential prognostic factor in resected non‐small cell lung cancer. J. Surg. Oncol. 2010;101:500–506. doi: 10.1002/jso.21520. [DOI] [PubMed] [Google Scholar]
- 149.Jiang T., Huang Z., Lin Y., Zhang Z., Fang D., Zhang D.D. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59:850–860. doi: 10.2337/db09-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ohta T., Iijima K., Miyamoto M., Nakahara I., Tanaka H., Ohtsuji M., Suzuki T., Kobayashi A., Yokota J., Sakiyama T. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 151.Sjöblom T., Jones S., Wood L.D., Parsons D.W., Lin J., Barber T.D., Mandelker D., Leary R.J., Ptak J., Silliman N. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 152.Nioi P., Nguyen T. A mutation of Keap1 found in breast cancer impairs its ability to repress Nrf2 activity. Biochem. Biophys. Res. Commun. 2007;362:816–821. doi: 10.1016/j.bbrc.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 153.Shibata T., Kokubu A., Gotoh M., Ojima H., Ohta T., Yamamoto M., Hirohashi S. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology. 2008;135:1358–1368. doi: 10.1053/j.gastro.2008.06.082. e1354. [DOI] [PubMed] [Google Scholar]
- 154.Konstantinopoulos P.A., Spentzos D., Fountzilas E., Francoeur N., Sanisetty S., Grammatikos A.P., Hecht J.L., Cannistra S.A. Keap1 mutations and Nrf2 pathway activation in epithelial ovarian cancer. Cancer Res. 2011;71:5081–5089. doi: 10.1158/0008-5472.CAN-10-4668. [DOI] [PubMed] [Google Scholar]
- 155.Wong T.F., Yoshinaga K., Monma Y., Ito K., Niikura H., Nagase S., Yamamoto M., Yaegashi N. Association of keap1 and nrf2 genetic mutations and polymorphisms with endometrioid endometrial adenocarcinoma survival. Int. J. Gynecol. Cancer. 2011;21:1428–1435. doi: 10.1097/IGC.0b013e31822d0eb2. [DOI] [PubMed] [Google Scholar]
- 156.Li Q.K., Singh A., Biswal S., Askin F., Gabrielson E. KEAP1 gene mutations and NRF2 activation are common in pulmonary papillary adenocarcinoma. J. Hum. Genet. 2011;56:230–234. doi: 10.1038/jhg.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim Y.R., Oh J.E., Kim M.S., Kang M.R., Park S.W., Han J.Y., Eom H.S., Yoo N.J., Lee S.H. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J. Pathol. 2010;220:446–451. doi: 10.1002/path.2653. [DOI] [PubMed] [Google Scholar]
- 158.Ma X., Zhang J., Liu S., Huang Y., Chen B., Wang D. Nrf2 knockdown by shRNA inhibits tumor growth and increases efficacy of chemotherapy in cervical cancer. Cancer Chemother. Pharmacol. 2012;69:485–494. doi: 10.1007/s00280-011-1722-9. [DOI] [PubMed] [Google Scholar]
- 159.Lister A., Nedjadi T., Kitteringham N.R., Campbell F., Costello E., Lloyd B., Copple I.M., Williams S., Owen A., Neoptolemos J.P. Nrf2 is overexpressed in pancreatic cancer: implications for cell proliferation and therapy. Mol. Cancer. 2011;10:37. doi: 10.1186/1476-4598-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sandhu I.S., Maksim N.J., Amouzougan E.A., Gallion B.W., Raviele A.L., Ooi Sustained A. NRF2 activation in hereditary leiomyomatosis and renal cell cancer (HLRCC) and in hereditary tyrosinemia type 1 (HT1) Biochem. Soc. Trans. 2015;43:650–656. doi: 10.1042/BST20150041. [DOI] [PubMed] [Google Scholar]
- 161.Pölönen P., Levonen A.L. Insights into the role of NRF2 in cancer provided by cancer genomics. Curr. Opin. Toxicol. 2016;1:111–117. [Google Scholar]
- 162.Genschik P., Sumara I., Lechner E. The emerging family of CULLIN3‐RING ubiquitin ligases (CRL3s): cellular functions and disease implications. EMBO J. 2013;32:2307–2320. doi: 10.1038/emboj.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Govindan R., Ding L., Griffith M., Subramanian J., Dees N.D., Kanchi K.L., Maher C.A., Fulton R., Fulton L., Wallis J., Chen K. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150:1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Forner A., Llovet J.M., Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 165.Schulze K., Imbeaud S., Letouzé E., Alexandrov L.B., Calderaro J., Rebouissou S., Couchy G., Meiller C., Shinde J., Soysouvanh F., Calatayud A.L. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 2015;47:505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cancer Genome Atlas Research Network Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li L., Shen C., Nakamura E., Ando K., Signoretti S., Beroukhim R., Cowley G.S., Lizotte P., Liberzon E., Bair S., Root D.E. SQSTM1 is a pathogenic target of 5q copy number gains in kidney cancer. Cancer Cell. 2013;24:738–750. doi: 10.1016/j.ccr.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ooi A., Wong J.C., Petillo D., Roossien D., Perrier-Trudova V., Whitten D., Min B.W., Tan M.H., Zhang Z., Yang X.J., Zhou M. An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer Cell. 2011;20:511–523. doi: 10.1016/j.ccr.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 169.Adam J., Hatipoglu E., O’Flaherty L., Ternette N., Sahgal N., Lockstone H., Baban D., Nye E., Stamp G.W., Wolhuter K., Stevens M. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell. 2011;20:524–537. doi: 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shim G.-s., Manandhar S., Shin D.-h., Kim T.-H., Kwak M.-K. Acquisition of doxorubicin resistance in ovarian carcinoma cells accompanies activation of the NRF2 pathway. Free Radic. Biol Med. 2009;47:1619–1631. doi: 10.1016/j.freeradbiomed.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 171.Loignon M., Miao W., Hu L., Bier A., Bismar T.A., Scrivens P.J., Mann K., Basik M., Bouchard A., Fiset P.O. Cul3 overexpression depletes Nrf2 in breast cancer and is associated with sensitivity to carcinogens, to oxidative stress, and to chemotherapy. Mol. Cancer Ther. 2009;8:2432–2440. doi: 10.1158/1535-7163.MCT-08-1186. [DOI] [PubMed] [Google Scholar]
- 172.Saha S., Sadhukhan P., Sil Mangiferin P.C. A xanthonoid with multipotent anti‐inflammatory potential. Biofactors. 2016;42:459–474. doi: 10.1002/biof.1292. [DOI] [PubMed] [Google Scholar]
- 173.Ghosh S., Basak P., Dutta S., Chowdhury S., Sil P.C. New insights into the ameliorative effects of ferulic acid in pathophysiological conditions. Food Chem. Toxicol. 2017;103:41–55. doi: 10.1016/j.fct.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 174.Sarkar A., Sil P.C. Iron oxide nanoparticles mediated cytotoxicity via PI3K/AKT pathway: role of quercetin. Food Chem. Toxicol. 2014;71:106–115. doi: 10.1016/j.fct.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 175.Pal S., Pal P.B., Das J., Sil P.C. Involvement of both intrinsic and extrinsic pathways in hepatoprotection of arjunolic acid against cadmium induced acute damage in vitro. Toxicology. 2011;283:129–139. doi: 10.1016/j.tox.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 176.Bhattacharya S., Gachhui R., Sil P.C. Hepatoprotective properties of kombucha tea against TBHP-induced oxidative stress via suppression of mitochondria dependent apoptosis. Pathophysiology. 2011;18:221–234. doi: 10.1016/j.pathophys.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 177.Kaidery N.A., Banerjee R., Yang L., Smirnova N.A., Hushpulian D.M., Liby K.T., Williams C.R., Yamamoto M., Kensler T.W., Ratan R.R. Targeting Nrf2-mediated gene transcription by extremely potent synthetic triterpenoids attenuate dopaminergic neurotoxicity in the MPTP mouse model of Parkinson’s disease. Antioxid. Redox Signal. 2013;18:139–157. doi: 10.1089/ars.2011.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang C., Su Z.-Y., Khor T.O., Shu L., Kong A.-N.T. Sulforaphane enhances Nrf2 expression in prostate cancer TRAMP C1 cells through epigenetic regulation. Biochem. Pharmacol. 2013;85:1398–1404. doi: 10.1016/j.bcp.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kanninen K., Heikkinen R., Malm T., Rolova T., Kuhmonen S., Leinonen H., Ylä-Herttuala S., Tanila H., Levonen A.-L., Koistinaho M. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. 2009;106:16505–16510. doi: 10.1073/pnas.0908397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stack C., Ho D., Wille E., Calingasan N.Y., Williams C., Liby K., Sporn M., Dumont M., Beal M.F. Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington's disease. Free Radic. Biol Med. 2010;49:147–158. doi: 10.1016/j.freeradbiomed.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Samudio I., Konopleva M., Hail N., Shi Y.-X., McQueen T., Hsu T., Evans R., Honda T., Gribble G.W., Sporn M. 2-Cyano-3, 12-dioxooleana-1, 9-dien-28-imidazolide (CDDO-Im) directly targets mitochondrial glutathione to induce apoptosis in pancreatic cancer. J. Biol. Chem. 2005;280:36273–36282. doi: 10.1074/jbc.M507518200. [DOI] [PubMed] [Google Scholar]
- 182.Hassan M., Watari H., AbuAlmaaty A., Ohba Y., Sakuragi N. Apoptosis and molecular targeting therapy in cancer. BioMed Res. Int. 2014;2014:150845. doi: 10.1155/2014/150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 183.Clarke J.L., Murray J.B., Park B.K., Copple I.M. Roles of Nrf2 in drug and chemical toxicity. Curr. Opin. Toxicol. 2016;1:104–110. [Google Scholar]
- 184.Bobilev I., Novik V., Levi I., Shpilberg O., Levy J., Sharoni Y., Studzinski G.P., Danilenko M. The Nrf2 transcription factor is a positive regulator of myeloid differentiation of acute myeloid leukemia cells. Cancer Biol. Ther. 2011;11:317–329. doi: 10.4161/cbt.11.3.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Knight T., Irving J.A.E. Ras/Raf/MEK/ERK pathway activation in childhood acute lymphoblastic leukemia and its therapeutic targeting. Front. Oncol. 2014;4:160. doi: 10.3389/fonc.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shin D.-S., Kim H.-N., Shin K.D., Yoon Y.J., Kim S.-J., Han D.C., Kwon B.-M. Cryptotanshinone inhibits constitutive signal transducer and activator of transcription 3 function through blocking the dimerization in DU145 prostate cancer cells. Cancer Res. 2009;69:193–202. doi: 10.1158/0008-5472.CAN-08-2575. [DOI] [PubMed] [Google Scholar]
- 187.Li W., Saud S.M., Young M.R., Colburn N.H., Hua B. Cryptotanshinone a Stat3 inhibitor, suppresses colorectal cancer proliferation and growth in vitro. Mol. Cell. Biochem. 2015;406:63–73. doi: 10.1007/s11010-015-2424-0. [DOI] [PubMed] [Google Scholar]
- 188.Tang X., Wang H., Fan L., Wu X., Xin A., Ren H., Wang X.J. Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic. Biol. Med. 2011;50:1599–1609. doi: 10.1016/j.freeradbiomed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 189.Gao A.-M., Ke Z.-P., Shi F., Sun G.-C., Chen H. Chrysin enhances sensitivity of BEL-7402/ADM cells to doxorubicin by suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathway. Chem. Biol. Interact. 2013;206:100–108. doi: 10.1016/j.cbi.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 190.Gao A.-M., Ke Z.-P., Wang J.-N., Yang J.-Y., Chen S.-Y., Chen H. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis. 2013;34:1806–1814. doi: 10.1093/carcin/bgt108. [DOI] [PubMed] [Google Scholar]
- 191.Tsai P.-Y., Ka S.-M., Chang J.-M., Chen H.-C., Shui H.-A., Li C.-Y., Hua K.-F., Chang W.-L., Huang J.-J., Yang S.-S. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic. Biol Med. 2011;51:744–754. doi: 10.1016/j.freeradbiomed.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 192.Satoh H., Moriguchi T., Takai J., Ebina M., Yamamoto M. Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res. 2013;73:4158–4168. doi: 10.1158/0008-5472.CAN-12-4499. [DOI] [PubMed] [Google Scholar]
- 193.Xu C., Huang M.-T., Shen G., Yuan X., Lin W., Khor T.O., Conney A.H., Kong A.-N.T. Inhibition of 7, 12-dimethylbenz (a) anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2–related factor 2. Cancer Res. 2006;66:8293–8296. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- 194.Iida K., Itoh K., Kumagai Y., Oyasu R., Hattori K., Kawai K., Shimazui T., Akaza H., Yamamoto M. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64:6424–6431. doi: 10.1158/0008-5472.CAN-04-1906. [DOI] [PubMed] [Google Scholar]
- 195.Tsai M.-L., Lai C.-S., Chang Y.-H., Chen W.-J., Ho C.-T., Pan M.-H. Pterostilbene, a natural analogue of resveratrol, potently inhibits 7, 12-dimethylbenz [a] anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse skin carcinogenesis. Food Funct. 2012;3:1185–1194. doi: 10.1039/c2fo30105a. [DOI] [PubMed] [Google Scholar]