Abstract
Retinoic acid (RA) is a metabolite of vitamin A and has important roles in development, differentiation, and reproduction. Activin has been shown to regulate the RA pathway and affect granulosa cell (GC) proliferation, suggesting that RA is important for early follicle development. However, little is known about the effects of RA on GC functions, particularly steroidogenesis, during the early follicle stage. The aim of this study was to investigate the effects of all-trans-RA (atRA) on progesterone production in immature rat GCs cultured without gonadotropin. Our results demonstrated that atRA enhanced progesterone production by upregulating the levels of steroidogenic acute regulatory protein (StAR) and cytochrome P450scc (Cyp11a1) mRNAs, but not 3β-hydroxysteroid dehydrogenase mRNA in immature rat GCs. Additionally, analysis of the mechanisms through which atRA upregulated StAR and Cyp11a1 mRNAs revealed that atRA enhanced intracellular cAMP accumulation and phosphorylation of cAMP response-element binding protein (CREB). In addition, H-89, an inhibitor of protein kinase A (PKA), abolished the stimulatory effects of atRA, indicating that atRA enhanced progesterone synthesis through cAMP/PKA signaling. In conclusion, our data demonstrated that atRA has a crucial role in progesterone synthesis in rat GCs during the early follicle stage.
Keywords: Retinoic acid, Granulosa cell, Progesterone, Steroidogenic acute regulatory protein, cAMP, Protein kinase A
Highlights
-
•
atRA upregulated StAR and Cyp11a1 and enhanced progesterone production.
-
•
atRA enhanced intracellular cAMP accumulation and phosphorylation of CREB.
-
•
Inhibition of PKA abolished the stimulatory effects of atRA.
-
•
atRA mediated progesterone synthesis in rat GCs during the early follicle stage.
1. Introduction
Ovarian functions are regulated by many factors, including gonadotropin, transforming growth factor β (TGFβ), various cytokines, and retinoic acid (RA). RA is the active metabolite of vitamin A and is synthesized from retinol by retinol dehydrogenase (RDH) and retinaldehyde dehydrogenase (RALDH) [1]. RA exerts its activity by acting as a ligand for RA receptors (RARs) and retinoid X receptors (RXRs). RA mediates many physiological functions, including embryogenesis [2] and reproduction. In female rats, severe vitamin A deficiency prior to mating leads to reproductive failure prior to implantation [3]. Maternal vitamin A also plays a role in placental development and maintenance [4]. In addition, several studies have indicated that retinoids have important effects on oocyte maturation and function [5], [6].
Previous studies have reported that RA enhances steroid production in several different cell types. For example, in MA-10 mouse Leydig cells, RA increases progesterone production by upregulation of steroidogenic acute regulatory protein (StAR) expression [7], and in rat hippocampal slice cultures, RA increases 17β-estradiol and testosterone levels through upregulation of cytochrome P45017α expression [8]. In the ovary, Bagavandoss et al. reported that retinoids increase luteinized granulosa cell (GC) progesterone accumulation in gonadotropin-primed rat ovaries [9]. In a recent study, Kipp et al. reported that activin regulates the RA pathway to modulate GC proliferation and ovarian functions [10]. Because activin plays a key role in early follicle development [11], it has been hypothesized that RA may affect GC function, including steroidogenesis, during the early follicle stage. However, the effects of RA on steroidogenesis in GCs during the early follicle stage are still unclear.
In the present study, we investigated the effects of all-trans-RA (atRA) on progesterone production in immature rat GCs cultured without gonadotropin. Our results indicated that atRA enhanced progesterone synthesis through cAMP/protein kinase A (PKA)/cAMP response element-binding protein (CREB) signaling in GCs during the early follicle stage. Thus, these results provide important insights into the mechanisms of RA signaling in GCs.
2. Materials and methods
2.1. Hormones and reagents
DMEM/Ham's nutrient mixture F-12, diethylstilbestrol (DES), atRA, and H-89 dihydrochloride hydrate were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). Gentamicin sulfate and fungizone were purchased from Invitrogen Corp. (Carlsbad, CA, USA). The RNA labeling kit and nucleic acid detection kit were purchased from Roche Diagnostics (Indianapolis, IN, USA).
2.2. Animals
Immature female Wistar rats (Japan SLC, Inc.) were maintained at all times according to the NIH Guide for the Care and Use of Laboratory Animals and the policies of the Gunma University Animal Care and Use Committee. Animals were housed in a temperature- and light-controlled room (12-h light, 12-h dark cycle; lights on at 6:00 AM) with food and water provided ad libitum. All experiments were approved by the Gunma University Animal Care and Use Committee.
2.3. GC culture
GCs were obtained from immature female Wistar rats injected daily for 4 days with 2 mg DES in 0.2 mL of sesame oil. The ovaries were then excised, and the GCs were released by puncturing the follicles with 26-gauge needles. GCs were washed and collected by brief centrifugation, and cell viability was determined by trypan blue exclusion. The GCs were then cultured in DMEM/Ham's nutrient mixture F-12 supplemented with 20 mg/L gentamicin sulfate, 500 μg/L fungizone, and 1 g/L BSA on collagen-coated plates in a humidified atmosphere under 5% CO2 at 37 °C.
2.4. RNA isolation and reverse transcription
GCs were cultured in 35-mm dishes containing 2.5×106 viable cells in 2.5 mL of medium, and the test substances were added to the medium after 24 h of culture. GCs were further incubated, and the cultures were stopped at selected times using Isogen (Nippon Gene, Toyama, Japan). The final RNA pellet was dissolved in diethylpyrocarbonate-treated H2O. Total RNA was quantified by measuring the absorbance of samples at 260 nm. Isolated RNAs (2 μg of each sample) from the GC cultures were treated with deoxyribonuclease I (Invitrogen) to eliminate residual genomic DNA. These RNAs were reverse transcribed with random primers, 10 mM deoxynucleoside triphosphate mix, and SuperScript III reverse transcriptase (Invitrogen) according to the manufacturer's protocol. The reactions were incubated for 5 min at 25 °C, 60 min at 50 °C, and 15 min at 70 °C in a thermal cycler. To remove cRNA, ribonuclease H was added to the cDNAs and incubated for 20 min at 37 °C.
2.5. Reverse transcription polymerase chain reaction (RT-PCR)
To amplify rat Rar, Rxr, Raldh, and ribosomal protein L19 (Rpl19) cDNAs, RT-PCR was employed according to the manufacturer's instructions using an Advantage2 PCR Kit (TaKaRa Bio, Inc., Shiga, Japan). The PCR protocol was as follows: 95 °C for 1 min, 33 cycles of 95 °C for 30 s and 68 °C for 1 min, and a final extension at 68 °C for 1 min. The primer pairs use in this study are shown in Table 1. Amplification products were resolved on 2% agarose gels and stained with 0.25 μg/mL ethidium bromide. The gel image was photographed, and band intensities were analyzed using NIH ImageJ (version 1.60). The bands were excised from the gel, purified, and characterized by DNA sequencing.
Table 1.
RT-PCR primer pairs.
| Gene | Primer |
|---|---|
| Rarα | |
| Forward | 5′-GTGTCACCGGGACAAGAACT-3′ |
| Reverse | 5′-GGGCTTGGGTGTTTCTTTCT-3′ |
| Rarβ | |
| Forward | 5′-GCTTCGGTCCTCTGACTGAC-3′ |
| Reverse | 5′-GGCGGTCTCCACAGATTAAG-3′ |
| Rarγ | |
| Forward | 5′-CAGCATCCAGAAAAACATGG-3′ |
| Reverse | 5′-TTCCGGTCATTCCTTACAGC-3′ |
| Rxrα | |
| Forward | 5′-GTCAAGCAGCAGACAAGCAG-3′ |
| Reverse | 5′-GAGAAGGAGGCAATCAGCAG-3′ |
| Rxrβ | |
| Forward | 5′-AGACTGCACAGTGGACAAGC-3′ |
| Reverse | 5′-GTTGTCGCTCCTCCTGTACC-3′ |
| Rxrγ | |
| Forward | 5′-TTGCTGATTGCCTCCTTCTC-3′ |
| Reverse | 5′-CTTGGACACCAGCTCTGTGA-3′ |
| Raldh1 | |
| Forward | 5′-GGGCCATCACTGTGTCTTCT-3′ |
| Reverse | 5′-CATCTTGAATCCACCGAAGG-3′ |
| Raldh2 | |
| Forward | 5′-TGAGTTTGGCTTACGGGAGT-3′ |
| Reverse | 5′-AAGGAGGCCTGGTGATAGGT-3′ |
| Raldh3 | |
| Forward | 5′-ATCAACAATGACTGGCACGA-3′ |
| Reverse | 5′-CTTGTCCACATCGGGCTTAT-3′ |
| Rpl19 | |
| Forward | 5′-AGCCTGTGACTGTCCATTCC-3′ |
| Reverse | 5′-GGCAGTACCCTTCCTCTTCC-3′ |
2.6. Quantitative RT-PCR
Quantitative RT-PCR was performed using the EagleTaq Universal Master Mix with ROX (Roche Diagnostics) and an ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA) according to manufacturers’ instructions. The quantitative PCR conditions were as follows: initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The primers and probes for each gene were purchased from Applied Biosystems (steroidogenic acute regulatory protein: Rn00580695_m1; cytochrome P450, family 11, subfamily a, polypeptide 1: Rn00568733_m1; 3 beta-hydroxysteroid dehydrogenase/delta-5-delta-4 isomerase type II: Rn01789220_m1; and eukaryotic 18S rRNA: Hs99999901_s1 as an internal control). Relative quantification of mRNA was carried out using the comparative threshold cycle (CT) method.
2.7. Intracellular cAMP assay
Intracellular accumulation of cAMP was measured using a Cyclic AMP EIA Kit (Cayman Chemical Co., Ann Arbor, MI, USA). GCs were cultured in 24-well culture plates containing 5×105 viable cells per well in 0.5 mL of medium. After 24 h, the cells were incubated for 30 min in fresh medium in the presence of 0.5 mM 3-isobutyl-1-methylxanthine. Various concentrations of RA were then added to the wells, and the cells were incubated for an additional 30 min. After incubation, the cells were lysed, and intracellular cAMP levels were measured according to the manufacturer's instructions.
2.8. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and western blot analysis
GCs were cultured in 35-mm dishes containing 2.5×106 viable cells in 2.5 mL medium. After 24 h, the cells were incubated with or without H89 for 30 min at 37 °C, and RA was then added to the medium. After incubation, the cells were washed twice with 0.5 mL of cold buffer (20 mM HEPES, 0.15 M NaCl, pH 7.4). Cells were then lysed in RIPA buffer (150 mM NaCl, 50 mM Tris, 1 mM EDTA, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and protease complete inhibitor, pH 7.4) supplemented with 200 mM NaF and 200 mM sodium orthovanadate for 40 min with rotation at 4 °C. Lysates were clarified by centrifugation at 20,000×g for 10 min at 4 °C and stored at −80 °C. Protein lysates (25 μg) were resolved on 12% SDS gels and electrophoretically transferred to polyvinylidene difluoride membranes. After blocking, membranes were incubated overnight at 4 °C with rabbit anti-phospho-CREB (1:2000) or rabbit anti-CREB (1:2000) antibodies (Cell Signaling Technology, Beverly, MA, USA). After washing in Tris-buffered saline with Tween 20, the membranes were incubated at room temperature for 60 min with horseradish peroxidase-conjugated goat anti-rabbit IgG antibodies (no. 170-6515, 1:40,000 dilution; Bio-Rad Laboratories, Hercules, CA, USA). The proteins were visualized using enhanced chemiluminescence (Immobilon Western; Millipore). Luminescence was quantified by scanning the films with a CCD camera and digitizing the data using NIH ImageJ version 1.60.
2.9. Progesterone analysis by enzyme-linked immunosorbent assay (ELISA)
Progesterone accumulation was measured using a Progesterone EIA Kit (Cayman Chemical Co.). GCs were cultured in 24-well culture plates containing 5×105 viable cells per well in 0.5 mL of medium, and the reagents were added to the medium after 24 h of culture. After incubation, the culture medium was collected, and the concentration of progesterone was measured according to the manufacturer's instructions.
2.10. Data analysis
The data represent the means±standard errors of the means (SEMs) from at least three independent experiments. Comparisons between groups were performed by one-way analysis of variance (ANOVA). The significance of the differences between the mean values of the control group and each treated group was determined using Scheffé's multiple-comparison test. Differences with P values of 0.05 were considered significant.
3. Results
3.1. Expression of Rar, Rxr, and Raldh in GCs
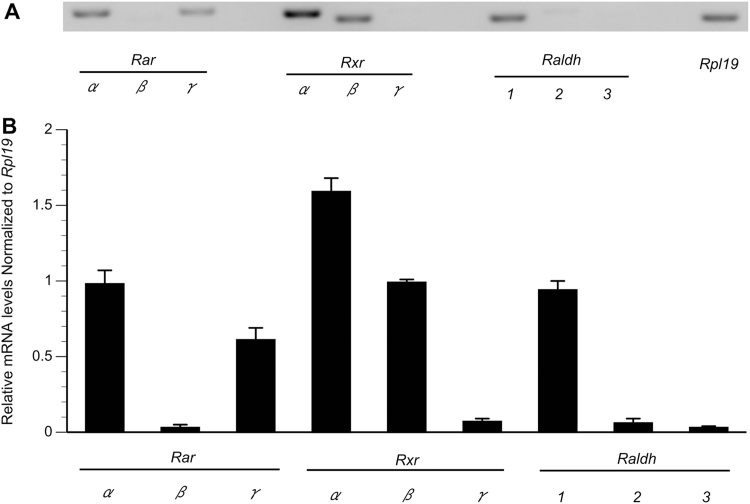
First, we investigated the expression of Rar, Rxr, and Raldh mRNA in primary GC cultures using RT-PCR (Fig. 1). The results revealed that Rarα, Rarγ, Rxrα, Rxrβ, and Raldh1 mRNAs were expressed at constant levels, while low levels of Rarβ, Rxrγ, Raldh2, and Raldh3 mRNAs were found. cDNAs from rat brain tissues and liver tissues were used as positive controls, and PCR products were detected as DNA bands (data not shown).
Fig. 1.
Expression of Rar, Rxr, and Raldh isoforms in rat GCs. GCs from DES-primed immature rats were cultured for 24 h. Levels of Rarα, Rarβ, Rarγ, Rxrα, Rxrβ, Rxrγ, Raldh1, Raldh2, and Raldh3 mRNAs (in triplicate) were determined by RT-PCR analysis. (A) Representative autoradiograms illustrating the expression of Rar, Rxr, and Raldh mRNAs using 2 μg of total RNA. (B) Results show the corresponding intensities of Rar, Rxr, and Raldh bands normalized with the corresponding Rpl19 bands. Data in the bar graphs represent the means±SEMs from three independent experiments.
3.2. atRA enhanced progesterone synthesis via upregulation of StAR and cytochrome P450scc (Cyp11a1) mRNAs
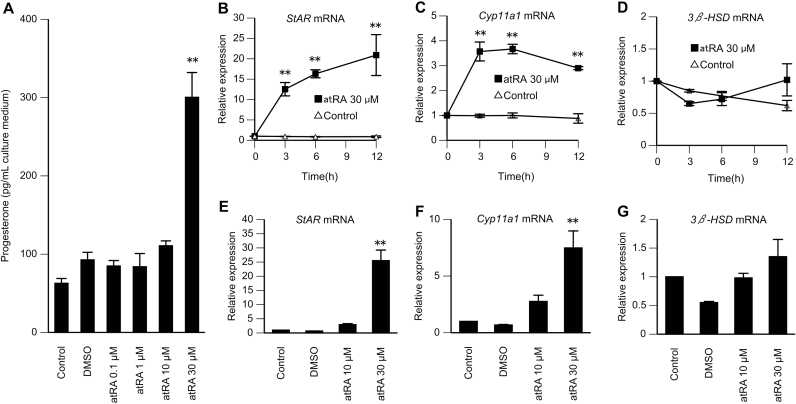
Next, we examined the effects of atRA on progesterone synthesis by analysis of progesterone accumulation in primary rat GC cultures incubated with atRA (0.1–30 μM) for 9 h (Fig. 2A). Progesterone synthesis was enhanced by 4.88±0.98 fold following treatment with 30 μM atRA compared with that at baseline. In contrast, progesterone accumulation after treatment with 0.1–10 μM atRA was not significantly enhanced. We also investigated the effects of atRA on the expression levels of StAR, Cyp11a1, and 3β-hydroxysteroid dehydrogenase (3β-HSD) mRNAs. GCs were cultured for 3–12 h with or without atRA (30 μM; Fig. 2B–D). Treatment with atRA resulted in significant augmentation of StAR and Cyp11a1 mRNA levels at all times. In contrast, 3β-HSD mRNA was unaltered. GCs were also cultured with different concentrations of atRA (10 or 30 μM) for 6 h (Fig. 2E–G). After treatment with 30 μM atRA, StAR and Cyp11a1 mRNA levels were increased by 25.5±3.73 and 7.48±1.50 fold compared with their respective basal values. By contrast, StAR and Cyp11a1 mRNA levels were higher after treatment with 10 μM atRA than that at baseline; however, these differences were not statistically significant.
Fig. 2.
Effects of atRA on progesterone synthesis and the mRNA levels of StAR, Cyp11a1, and 3β-HSD in rat GCs. (A) GCs from DES-primed immature rats were cultured for 24 h. The cells were then further incubated with atRA for 9 h. Progesterone accumulation was then measured in the culture medium using ELISA. As an additional control, the cells were also treated with DMSO in the absence of atRA. Data in the bar graphs represent the means±SEMs from three independent experiments. **P<0.01 compared with untreated cells. (B–D) GCs from DES-primed immature rats were cultured for 24 h. The cells were then further incubated with or without atRA (30 μM) for the indicated times. StAR, Cyp11a1, and 3β-HSD mRNA levels were determined relative to that of 18S rRNA by quantitative RT-PCR. The amount at 0 h for each target was set as 1. Data in the line graphs represent the means±SEMs from three independent experiments. **P<0.01 compared with the control (no treatment) at the same time. (E–G) GCs from DES-primed immature rats were cultured for 24 h. The cells were then further incubated with 10 or 30 μM atRA for 6 h. StAR, Cyp11a1, and 3β-HSD mRNA levels were determined relative to that of 18S rRNA by quantitative RT-PCR. As an additional control, the cells were also treated with DMSO in the absence of atRA. The basal amount of each mRNA was set as 1. Data in the bar graphs represent the means ± SEMs from three independent experiments. **P<0.01 compared with the basal level.
3.3. Effects of atRA on cAMP/PKA/CREB signaling
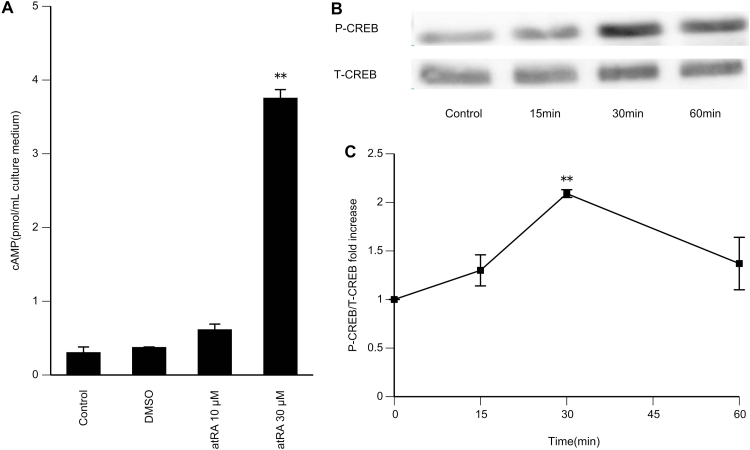
We then evaluated changes in intracellular cAMP concentrations following the addition of atRA (10 or 30 μM, 30 min) in GCs pre-incubated with 0.5 mM 3-isobutyl-1-methylxanthine for 30 min (Fig. 3A). Intracellular cAMP accumulation was enhanced by atRA. The response observed at 30 μM was 12.5±4.02 fold higher than the basal value. Because cAMP has been known to activate the PKA pathway which, in turn, induces CREB phosphorylation, we next investigated the effects of atRA on the phosphorylation of CREB. GCs were cultured for 15–60 min with atRA (30 μM), and western blot analysis was performed to assess the phosphorylation of CREB (Fig. 3B and C). The data showed that phosphorylation of CREB was increased by 2.09±0.04 fold following 30 min of atRA treatment.
Fig. 3.
Effects of atRA on intracellular cAMP accumulation and phosphorylation of CREB. (A) GCs from DES-primed immature rats were cultured for 24 h. The cells were then incubated for 30 min in fresh medium in the presence of 0.5 mM 3-isobutyl-1-methylxanthine. Then, 10 or 30 μM atRA was added, and cells were incubated for an additional 30 min. The cells were then lysed, and intracellular cAMP levels were determined by ELISA. As an additional control, the cells were also treated with DMSO in the absence of atRA. Data in the bar graphs represent the means±SEMs from three independent experiments. **P<0.01 compared with the basal level. (B, C) GCs from DES-primed immature rats were cultured for 24 h. The cells were then incubated with atRA (30 μM) for the indicated times. Whole cell lysates were used for western blot analysis. (B) Levels of phosphorylated CREB (P-CREB) and total CREB (T-CREB). (C) Relative ratios of P-CREB to T-CREB. The basal ratio was set as 1. Data in the line graphs represent the means±SEMs from three independent experiments. **P<0.01 compared with the basal level.
3.4. The effects of atRA on the upregulation of progesterone synthesis were mediated by the PKA pathway
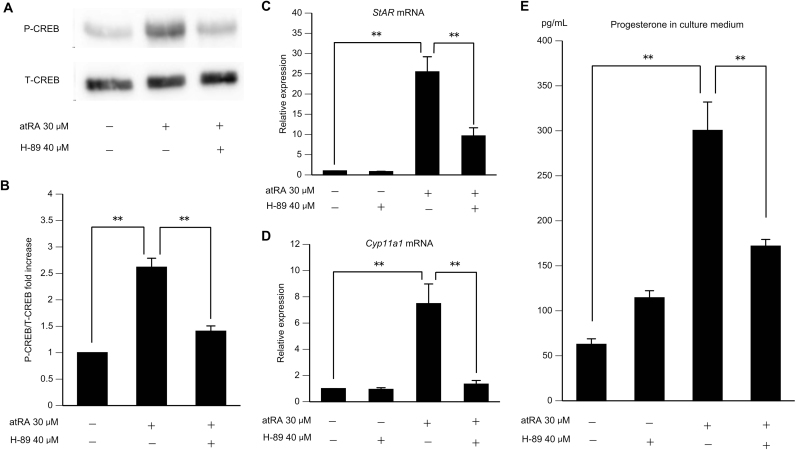
To investigate whether activation of cAMP/PKA/CREB signaling by atRA was involved in the enhancement of progesterone synthesis, we next examined StAR mRNA expression, Cyp11a1 mRNA expression, and progesterone accumulation during treatment with H-89 (a PKA inhibitor). We found that concurrent treatment with 40 μM H-89 blocked the effects of 30 μM atRA on CREB phosphorylation (Fig. 4A and B). Moreover, pretreatment for 30 min with 40 μM H-89, followed by 6 h of treatment with 30 μM atRA significantly blocked the atRA-dependent upregulation of StAR and Cyp11a1 mRNA expression (Fig. 4C and D). Notably, H-89 alone did not affect StAR and Cyp11a1 mRNA expression compared with that in untreated cells. We next examined the effects of H-89 on progesterone synthesis in GCs. GCs were pretreated for 1 h with or without 40 μM H-89 and then cultured for 9 h in the absence or presence of 30 μM atRA (Fig. 4E). H-89 significantly reduced progesterone accumulation induced by atRA, whereas H-89 alone did not affect progesterone accumulation compared with that in untreated cells. These results demonstrated that the cAMP/PKA/CREB pathway was important for mediating the effects of atRA on progesterone synthesis.
Fig. 4.
(A, B) Effects of pathway blockade in atRA-induced phosphorylation of CREB in rat GCs. GCs from DES-primed immature rats were cultured for 24 h, pretreated with H-89 for 30 min, and then treated with atRA for 30 min. Whole cell lysates were used for western blot. (A) Representative autoradiograms show levels of P-CREB and T-CREB. (B) Relative ratios of P-CREB to T-CREB. The basal ratio was set as 1. Data in the bar graphs represent the means±SEMs from three independent experiments. **P<0.01. (C, D) Effects of PKA inhibition on StAR and Cyp11a1 mRNA expression in rat GCs. GCs from DES-primed immature rats were cultured for 24 h. These cells were pretreated with H-89 for 30 min and then treated with atRA for 6 h. StAR and Cyp11a1 mRNA levels were determined relative to that of 18S rRNA by quantitative RT-PCR. The basal amount of each mRNA was set as 1. Data in the bar graphs represent the means±SEMs from three independent experiments. **P<0.01. (E) Effects of PKA inhibition on progesterone synthesis in rat GCs. GCs from DES-primed immature rats were cultured for 24 h, pretreated with H-89 for 1 h, and then treated with atRA for 9 h. Progesterone accumulation in the culture medium was then measured by ELISA. Data in the bar graphs represent the means±SEMs from three independent experiments. **P<0.01.
4. Discussion
In the present study, we aimed to elucidate the effects of atRA in GCs. Our data showed that atRA upregulated StAR and Cyp11a1 mRNAs via the cAMP/PKA pathway and enhanced progesterone production in immature rat GCs.
RA is a metabolite of vitamin A, and RA synthesis from the dietary precursor retinol occurs via two sequential enzymatic reactions. The first reaction, from retinol to retinaldehyde, is facilitated by enzymes with RDH activity, whereas the second reaction, from retinaldehyde to RA, is carried out by enzymes with RALDH activity [1]. The critical step in RA synthesis is the irreversible conversion of retinaldehyde into RA by RADHLs [12]. RA exerts its activity by acting as a ligand for two families of ligand-activated nuclear receptors, RARs and RXRs, each of which has three subtypes (α, β, and γ) [13], [14]. RARs and RXRs form a heterodimeric complex, which binds RA response elements [15]. In this study, we found that immature GCs expressed Rarα, Rarγ, Rxrα, Rxrβ, and Raldh1 mRNAs. These results suggested that RA was involved in mediating the functions of immature GCs.
Progesterone synthesis is regulated by StAR, Cyp11a1, and 3β-HSD. StAR transports cholesterol from cellular stores to the inner mitochondrial membrane which serves as a key regulatory step in steroidogenesis [16]. Cyp11a1 converts cholesterol to pregnenolone, whereas 3β-HSD converts pregnenolone to progesterone. Our results show that atRA enhances progesterone production through upregulation of StAR and Cyp11a1 mRNAs, without affecting 3β-HSD mRNA levels. In contrast with these findings, Manna et al. reported that RA enhances StAR expression but not Cyp11a1 or 3β-HSD expressions in MA-10 mouse Leydig cells [7]. Therefore, these data suggest that RA enhances progesterone production in both GCs and Leydig cells by activating StAR. Further studies are needed to clarify the mechanisms regulating these enzymes, including analysis of the promoter functions of these enzymes in different cell types.
Steroidogenesis is regulated by both cAMP/PKA-dependent and cAMP/PKA-independent signaling pathways [17]. Several studies have shown that RA stimulates intracellular cAMP production in various cellular models [18], [19]. Therefore, we hypothesized that atRA would enhance progesterone production by activating the cAMP/PKA signaling pathway. The results of the present study support this notion since atRA enhanced intracellular cAMP accumulation and phosphorylation of CREB, and treatment with a PKA inhibitor abolished the stimulatory effects of atRA. A previous study reported that adenylate cyclase activation could be responsible for the RA-triggered activation of cAMP signaling in acute promyelocytic leukemia cells [18]. In addition, another previous study reported that retinoids increase cAMP production via binding to or interacting with RXR/LXR/RAR in MA-10 cells [7]. Therefore, we speculate that atRA may bind to RAR/RXR and cause activation of adenylate cyclase. Further studies are needed to investigate the detail mechanisms underlying the signaling relationships between nuclear and cell-surface receptors.
Progesterone is an important substrate for other steroids, including estrogens, and several studies have suggested that estrogens have a positive role in follicle development [20]. In addition, activin has been shown to regulate the RA pathway to modulate GC proliferation [10]. Therefore, progesterone induced by RA in early follicles may be used as a substrate for estrogens in order to promote follicle development. Consistent with this notion, we have observed that atRA enhances aromatase activity in cultured GCs (data not presented). However, further studies are needed to confirm this hypothesis because cytochrome P45017α, which converts progesterone to androstenedione, is expressed at very low levels in GCs.
In conclusion, we have demonstrated that atRA enhanced progesterone production via the cAMP/PKA signaling pathway in immature rat GCs. Our results suggest that atRA may have an important role in GCs during the early stages of follicle growth.
Acknowledgments
We thank Hiroko Matsuda and Junko Sakurai for their excellent technical assistance and Rika Kuroda for her excellent secretarial assistance. This work was supported by a Grant-in-Aid for Scientific Research (B) (21390448) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2016.08.013.
Contributor Information
Hiroto Suwa, Email: m13702016@gunma-u.ac.jp.
Hiroshi Kishi, Email: hrskishi@gmail.com.
Fumiharu Imai, Email: fumihimai@gmail.com.
Kohshiro Nakao, Email: m12702010@gunma-u.ac.jp.
Takashi Hirakawa, Email: tahirakawa@gmail.com.
Takashi Minegishi, Email: tminegis@gunma-u.ac.jp.
Appendix A. Transparency document.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- 1.Duester G. Involvement of alcohol dehydrogenase, short-chain dehydrogenase/reductase, aldehyde dehydrogenase, and cytochrome P450 in the control of retinoid signaling by activation of retinoic acid synthesis. Biochemistry. 1996;35:12221–12227. doi: 10.1021/bi961176+. [DOI] [PubMed] [Google Scholar]
- 2.Thompson J.N. Vitamin A in development of the embryo. Am. J. Clin. Nutr. 1969;22:1063–1069. doi: 10.1093/ajcn/22.8.1063. [DOI] [PubMed] [Google Scholar]
- 3.Evans H.M. The effects of inadequate vitamin A on the sexual physiology of the female. J. Biol. Chem. 1928;77:651–654. [Google Scholar]
- 4.Howell J.M., Thompson J.N., Pitt G.A. Histology of the lesions produced in the reproductive tract of animals fed a diet deficient in vitamin A alcohol but containing vitamin A acid. II. The female rat. J. Reprod. Fertil. 1964;7:251–258. doi: 10.1530/jrf.0.0070251. [DOI] [PubMed] [Google Scholar]
- 5.Duque P., Díez C., Royo L., Lorenzo P.L., Carneiro G., Hidalgo C.O., Facal N., Gómez E. Enhancement of developmental capacity of meiotically inhibited bovine oocytes by retinoic acid. Hum. Reprod. 2002;17:2706–2714. doi: 10.1093/humrep/17.10.2706. [DOI] [PubMed] [Google Scholar]
- 6.Almiñana C., Gil M.A., Cuello C., Caballero I., Roca J., Vazquez J.M., Gomez E., Martinez E.A. In vitro maturation of porcine oocytes with retinoids improves embryonic development. Reprod. Fertil. Dev. 2008;20:483–489. doi: 10.1071/rd07175. [DOI] [PubMed] [Google Scholar]
- 7.Manna P.R., Slominski A.T., King S.R., Stetson C.L., Stocco D.M. Synergistic activation of steroidogenic acute regulatory protein expression and steroid biosynthesis by retinoids: involvement of cAMP/PKA signaling. Endocrinology. 2014;155:576–591. doi: 10.1210/en.2013-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munetsuna E., Hojo Y., Hattori M., Ishii H., Kawato S., Ishida A., Kominami S.A., Yamazaki T. Retinoic acid stimulates 17beta-estradiol and testosterone synthesis in rat hippocampal slice cultures. Endocrinology. 2009;150:4260–4269. doi: 10.1210/en.2008-1644. [DOI] [PubMed] [Google Scholar]
- 9.Bagavandoss P., Midgley A.R. Lack of difference between retinoic acid and retinol in stimulating progesterone production by luteinizing granulosa cells in vitro. Endocrinology. 1987;121:420–428. doi: 10.1210/endo-121-1-420. [DOI] [PubMed] [Google Scholar]
- 10.Kipp J.L., Golebiowski A., Rodriguez G., Demczuk M., Kilen S.M., Mayo K.E. Gene expression profiling reveals Cyp26b1 to be an activin regulated gene involved in ovarian granulosa cell proliferation. Endocrinology. 2011;152:303–312. doi: 10.1210/en.2010-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li R., Phillips D.M., Mather J.P. Activin promotes ovarian follicle development in vitro. Endocrinology. 1995;136:849–856. doi: 10.1210/endo.136.3.7867593. [DOI] [PubMed] [Google Scholar]
- 12.Simões-Costa M.S., Azambuja A.P., Xavier-Neto J. The search for non-chordate retinoic acid signaling: lessons from chordates. J. Exp. Zool. B: Mol. Dev. Evol. 2008;310:54–72. doi: 10.1002/jez.b.21139. [DOI] [PubMed] [Google Scholar]
- 13.Germain P., Chambon P., Eichele G., Evans R.M., Lazar M.A., Leid M., De Lera A.R., Lotan R., Mangelsdorf D.J., Gronemeyer H. International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol. Rev. 2006;58:712–725. doi: 10.1124/pr.58.4.4. [DOI] [PubMed] [Google Scholar]
- 14.Germain P., Chambon P., Eichele G., Evans R.M., Lazar M.A., Leid M., De Lera A.R., Lotan R., Mangelsdorf D.J., Gronemeyer H. International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol. Rev. 2006;58:760–772. doi: 10.1124/pr.58.4.7. [DOI] [PubMed] [Google Scholar]
- 15.McKenna N.J. EMBO retinoids 2011: mechanisms, biology and pathology of signaling by retinoic acid and retinoic acid receptors. Nucl. Recept. Signal. 2012;10:e003. doi: 10.1621/nrs.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin D., Sugawara T., Strauss J.F., Clark B.J., Stocco D.M., Saenger P., Rogol A., Miller W.L. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267:1828–1831. doi: 10.1126/science.7892608. [DOI] [PubMed] [Google Scholar]
- 17.Light A., Hammes S.R. Membrane receptor cross talk in steroidogenesis: recent insights and clinical implications. Steroids. 2013;78:633–638. doi: 10.1016/j.steroids.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Q., Tao J., Zhu Q., Jia P.M., Dou A.X., Li X., Cheng F., Waxman S., Chen G.Q., Chen S.J., Lanotte M., Chen Z., Tong J.H. Rapid induction of cAMP/PKA pathway during retinoic acid-induced acute promyelocytic leukemia cell differentiation. Leukemia. 2004;18:285–292. doi: 10.1038/sj.leu.2403226. [DOI] [PubMed] [Google Scholar]
- 19.He J.C., Lu T.C., Fleet M., Sunamoto M., Husain M., Fang W., Neves S., Chen Y., Shankland S., Iyengar R., Klotman P.E. Retinoic acid inhibits HIV-1-induced podocyte proliferation through the cAMP pathway. J. Am. Soc. Nephrol. 2007;18:93–102. doi: 10.1681/ASN.2006070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ting A.Y., Xu J., Stouffer R.L. Differential effects of estrogen and progesterone on development of primate secondary follicles in a steroid-depleted milieu in vitro. Hum. Reprod. 2015;30:1907–1917. doi: 10.1093/humrep/dev119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material