Abstract
Polarized epithelia develop distinct cell surface domains, with the apical membrane acquiring characteristic morphological features such as microvilli. Cell polarization is driven by polarity determinants including the evolutionarily conserved partitioning defective (PAR) proteins that are separated into distinct cortical domains. PAR protein segregation is thought to be a consequence of asymmetric actomyosin contractions. The mechanism of activation of apically polarized actomyosin contractility is unknown. Here we show that the Cdc42 effector MRCK activates Myosin-II at the apical pole to segregate aPKC-Par6 from junctional Par3, defining the apical domain. Apically polarized MRCK-activated actomyosin contractility is reinforced by cooperation with aPKC-Par6 downregulating antagonistic RhoA-driven junctional actomyosin contractility, and drives polarization of cytosolic brush border determinants and apical morphogenesis. MRCK-activated polarized actomyosin contractility is required for apical differentiation and morphogenesis in vertebrate epithelia and Drosophila photoreceptors. Our results identify an apical origin of actomyosin-driven morphogenesis that couples cytoskeletal reorganization to PAR polarity signalling.
Epithelial cells polarize and form distinct cell surface domains that have different biochemical compositions, reflecting their different functions1. The apical domain often undergoes a morphogenetic process leading to the development of actin-rich structures that support specific apical functions, such as the brush border membrane of absorptive epithelia or the light-harvesting domain of Drosophila photoreceptors. Formation of such apical specializations relies on the recruitment of specific cytosolic factors that determine apical morphogenesis and, hence, requires asymmetric distribution of cytosolic components2. Epithelial polarization is regulated by apical and basolateral polarity determinants3. Among which are the evolutionarily conserved PAR proteins that segregate into two distinct cortical domains4, 5. In epithelia, the boundary between the two domains, the apical/lateral border (tight junctions in vertebrates, adherens junctions in flies), is marked by Par3, which is recruited to the cell surface bound to the Par6-aPKC complex. In response to apical Cdc42 activation, Par3 dissociates, demarking the apical/lateral border, and the Par6-aPKC complex segregates into the differentiating apical domain6, 7. Studies in C. elegans one-cell stage embryos suggest that PAR protein segregation relies on asymmetric actomyosin activity, generating movement of anterior PAR complexes to the anterior pole, which results in the formation of two cortical domains that harbour distinct PAR proteins8–13. Anterior PAR proteins correspond to apical PARs in epithelia. The functional importance of actomyosin and, if relevant, how and where asymmetric Myosin-II activity is generated to drive apical accumulation of PAR proteins in epithelia is not clear. Identifying such mechanisms, however, is essential to understand how the interplay between mechanical forces generated by actomyosin contractility and biochemical signalling guide epithelial polarization and morphogenesis.
In epithelia, RhoA is known to generate contractile forces driving junction formation and remodelling, a mechanism important during apical constriction and developmental processes requiring epithelial sheet movement and elongation14–16. In contrast, apical Cdc42 activation not only drives apical differentiation but also promotes apical expansion at the cost of the basolateral domain, counteracting junctional actomyosin-generated apical constriction17. In analogy to the C. elegans embryo model, one would expect a mechanism of Myosin-II activation at the apical pole to create an actomyosin activity gradient that favours apical polarization if apical segregation of Par6-aPKC is indeed driven by actomyosin contractions. Therefore, we asked if and how apical Cdc42 signalling activates asymmetric actomyosin contractility to stimulate apical polarization and plasma membrane morphogenesis, and how such a mechanism interacts with counteracting junctional RhoA signalling. Here, we show that the Cdc42 effector MRCK activates apical actomyosin contractility, initiating a pathway regulating apical morphogenesis, and cooperates with the aPKC-Par6 complex, which downregulates RhoA-driven junctional actomyosin contractility, to drive apical polarization.
Results
MRCK-activated Myosin-II drives apical morphogenesis
As epithelial cells polarize and develop a specialized apical membrane domain, Myosin-II polarizes apically at distinct sites along the apical membrane domain including the junctional circumferential actomyosin belt18, 19. In cultured canine kidney epithelial MDCK cells that spontaneously differentiate, we found that phosphorylated MLC (myosin regulatory light chain), demarking active Myosin-II, is localised basolaterally in non-polarized cells and becomes increasingly enriched along the apical membrane domain, forming ‘caps’ that define the apical cellular cortex, as epithelial cells polarize and differentiate over a period of a few days (Fig.1a). Since apical polarization of PAR proteins and morphogenesis depends on apically polarized Cdc42 activation3, 20, we asked whether a Cdc42-dependent mechanism driving polarized Myosin-II activation is at the origin of apical polarization and morphogenesis.
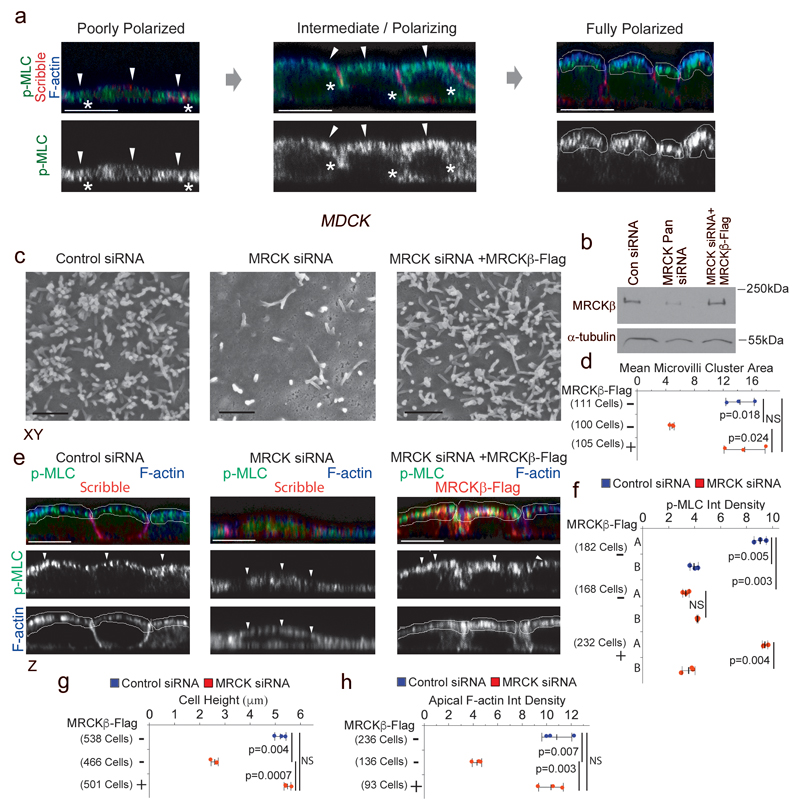
Figure 1. MRCK activates apical actomyosin contractility that controls apical morphogenesis.
(a) Spontaneous polarization of MDCK cells leads to the formation of apical actomyosin caps positive for p-MLC and F-actin. (b) Expression of MRCKβ as analysed by immunoblotting of total cell extracts. (c,d) Scanning electron microscopy of apical domains reveals levels of microvilli induction by MDCK cells upon depletion of MRCK without or with complementation with exogenously expressed MRCKβ-flag. (e,f) Measurement of active Myosin at cortical caps (A) and basal membrane (B) during polarization and differentiation of cells depleted of or rescued for MRCK expression by confocal immunofluorescence microscopy. (g,h) Measured cell height and F-actin levels in polarizing cells with or without MRCK function. For all quantifications, n=3 independent experiments and shown are the data points, means ± 1 SD (in black), the total number of cells analysed for each type of sample across all experiments, and p-values derived from t-tests. Arrowheads point to the apical cortex demarked by F-actin. Unprocessed original scans of blots are shown in Supplementary Figure 8. Scale bars: electron micrographs, 1μm; confocal immunofluorescence images, 10μm.
MRCKs are Cdc42 effector kinases that activate Myosin-II by phosphorylating MLC21. Using spontaneously differentiating MDCK cells, we asked whether siRNA-mediated MRCK knockdown affected formation of apical actomyosin caps. Indeed, MRCK depleted cells failed to form differentiated apical membranes as microvilli did not assemble, apical accumulation of F-actin and active Myosin-II did not occur, and had a reduced cell height (Fig. 1b-h, Supplementary Fig. 1a-c). All of these MRCK siRNA-induced effects were rescued by expression of siRNA resistant MRCKβ-flag, supporting specificity. MRCK was also required for the differentiation of human intestinal epithelial Caco-2 cells that also spontaneously polarize and differentiate (Supplementary Fig. 1c,d). Hence, MRCK is essential for the differentiation of vertebrate epithelial cells from different tissues.
To determine whether MRCK-generated apical Myosin-II activation is a conserved mechanism, we turned to D. melanogaster: their differentiating photoreceptors represent a well-characterized invertebrate model system to study epithelial polarity, PAR polarity signalling, and apical differentiation5, 7, 22–24. Drosophila photoreceptors undergo epithelial polarity remodelling during retinal development and evolve a characteristic, F-actin-rich apical membrane domain (Fig. 2a). Strikingly, differentiating photoreceptors displayed active Myosin caps at the apical cortex, which were not formed in cells mutant for gek/MRCK25 (Fig. 2b). Loss of gek function also resulted in attenuation of F-actin signal at the apical membrane domain and gross defects in the morphology of the F-actin rich apical organelle known as the rhabdomere (Fig. 2c-f), which consists of a stack of approximately 60.000 microvilli, indicative of defective actomyosin contractility22. Introduction of a phosphomimetic MLC transgene, spaghetti squash-EE (sqhEE), rescued the phenotype of gek mutant cells, demonstrating Myosin-II activation acts downstream of gek/MRCK (Fig. 2g,h). MRCK is thus a conserved driver of apical Myosin activation and morphogenesis in vertebrate epithelia and Drosophila photoreceptors.
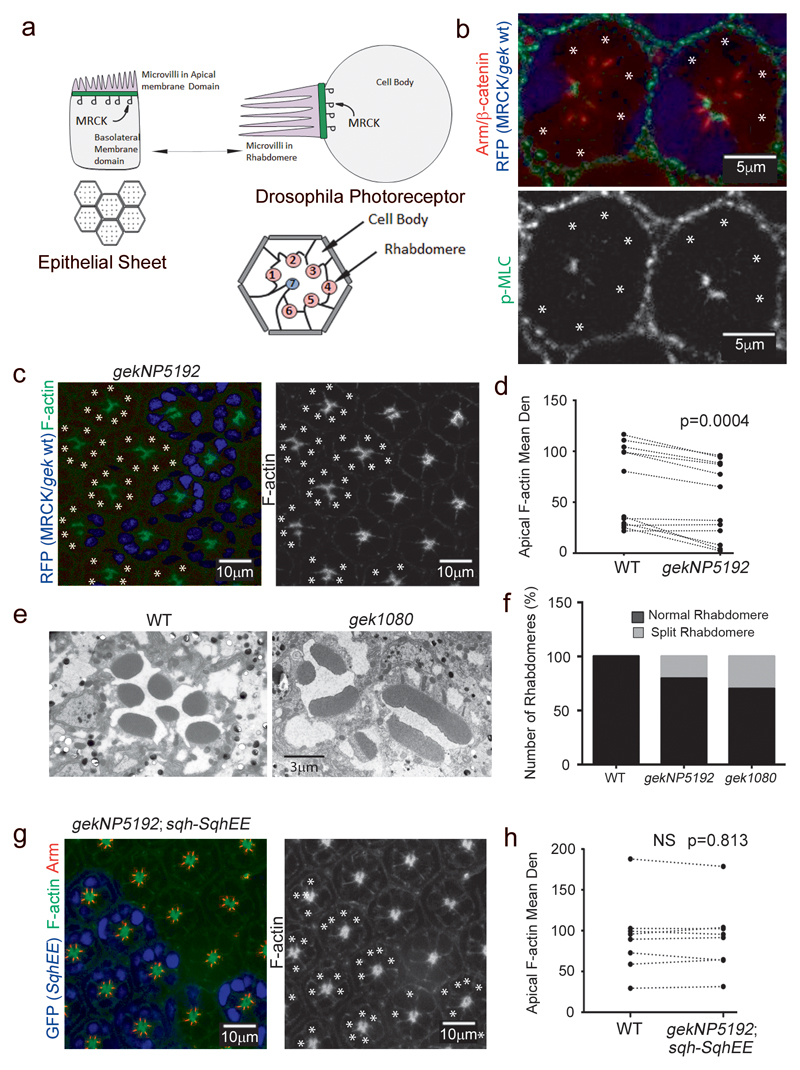
Figure 2. MRCK/gek regulates apical Myosin activation and morphogenesis in differentiating Drosophila photoreceptors.
(a) Scheme illustrating the similarities and corresponding plasma membrane domains of vertebrate epithelial cells and Drosophila photoreceptors. Indicated are apical microvilli and the apical zone enriched in active Myosin-II. (b-d) Confocal sections of Drosophila pupal mosaic retinas showing wild type cells (blue nuclei) and cells mutant for gek were stained for F-actin (c) or p-MLC (b). Mutant cells are labelled with asterisks. Quantification of apical actin enrichment shows analysis of wild type and mutant cells (paired within sections; n represents 11 animals, a t-test was used to calculate the p value). (e,f) Analysis of rhabdomere integrity in wild type and gek mutant cells using 2 different gek alleles (gekNP5192 or gek1080) demonstrates severe defects including splitting. (g,h) Confocal sections of Drosophila eyes showing wild type cells and cells mutant for gekNP5192 but expressing sqh-SqhEE (blue nuclei; labelled with asterisks; the quantification represents pairs within sections and is based on n = 8 animals, a t-test was used to calculate the p value).
Apical determinant Dbl3, a Cdc42 guanine nucleotide exchange factor, controls MRCK activity
We have previously shown that the guanine nucleotide exchange factor Dbl3 activates Cdc42 at the apical pole to drive epithelial polarization and morphogenesis17. To determine whether Dbl3 signalling stimulates MRCK-mediated Myosin activation, we conditionally expressed Dbl3-myc in MDCK cells. This resulted in increased apical phosphorylation of MLC along with enhanced apical F-actin accumulation and microvilli induction; both inhibited by knockdown of MRCK (Fig. 3a-e). Inhibition of ROCKI/II in polarizing MDCK cells did not prevent apical actomyosin activation and morphogenesis, indicating that apical Myosin activation is not Rho dependent (Supplementary Fig. 2). Moreover, our results indicate that Dbl3 is a major activator of MRCK in mammalian cells since constitutive overexpression of MRCKβ-Flag could not rescue Db3 knockdown effects (Supplementary Fig. 3a-d). Only catalytically active, but not inactive Dbl3 that is unable to activate Cdc4217, activated MLC phosphorylation (Fig. 3f). Thus, the activation of apical Myosin caps requires Cdc42 and MRCK activation.
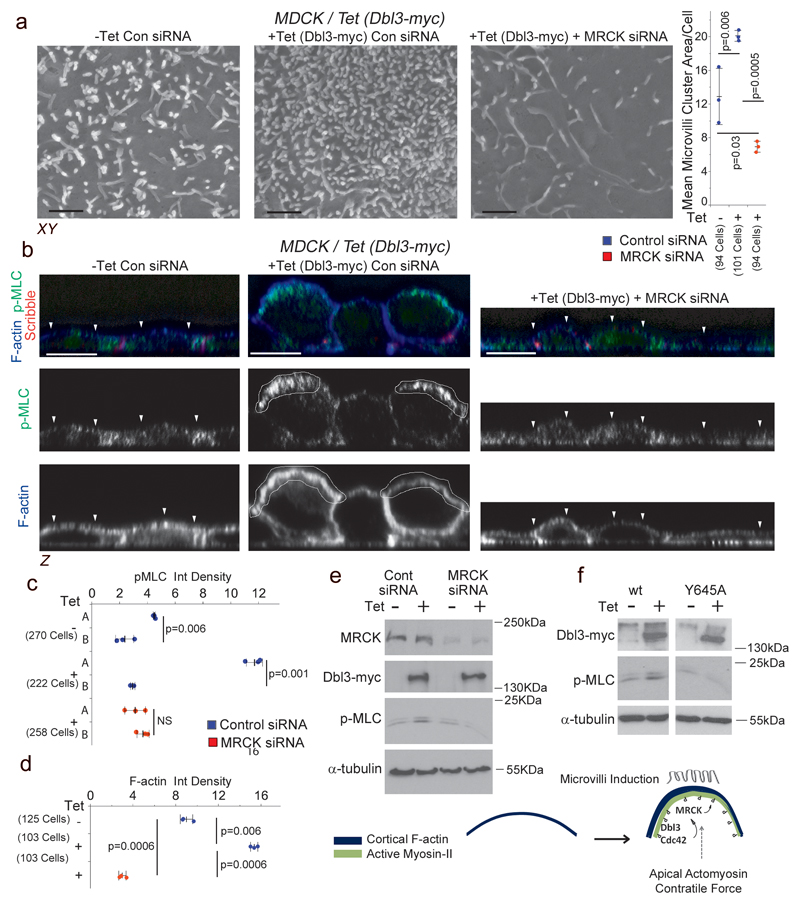
Figure 3. MRCK functions as an effector of Dbl3-Cdc42 signalling.
(a) Scanning electron microscopy analysis of microvilli induction by MDCK cells with tetracycline-inducible Dbl3-myc expression transfected with MRCK siRNA determined by measuring areas of microvilli clusters from SEM scans. (b-d) Induction of active Myosin at cortical caps (A) or basal membrane (B), and enrichment of F-actin at the apical cortex during polarization and differentiation, stimulated by conditional expression of Dbl3-myc with or without MRCK siRNA knockdown. White arrowheads highlight the apical membrane cortex labelled for F-actin. (e) Immunoblot analysis of total active Myosin levels following conditional expression of Dbl3-myc and siRNA knockdown of MRCK. (f) Immunoblot analysis of total active Myosin-II levels following conditional expression of Dbl3-myc or GEF inactive Dbl3Y645A-myc. The schematic illustrates the formation of apical caps formed by activated Myosin-II and F-actin. Unprocessed original scans of blots are shown in Supplementary Figure 8. For all quantifications, n=3 independent experiments and shown are the data points, means ± 1 SD (in black), the total number of cells analysed for each type of sample across all experiments, and p-values derived from t-tests. Scale bars: a, 1μm; b, 10μm.
To determine the temporal nature of apical MLC activation, we generated a conditional Dbl3-Myc MDCK-cell-line that constitutively expresses EGFP-MLC; the conditional Dbl3-myc transgene was then induced by the addition of tetracycline. Time-lapse fluorescence microscopy revealed that concurrent to induction of Dbl3-myc protein, increased apical EGFP-MLC activity was detected from 2.5h of tetracycline treatment (Supplementary video 1; Supplementary Fig. 3e). Analysis in fixed cells revealed that conditional expression of Dbl3-myc resulted in active Myosin cap formation 2.5 hours from the onset of tetracycline addition along with apical accumulation of MRCKβ increasing further at 6 hours, along the expanding apical membrane domain (Fig. 4a; Supplementary Fig. 3f,g). Thus, apical MRCK recruitment and MLC phosphorylation follow the Dbl3-myc protein expression profile. Apical actomyosin cap formation correlated with apical polarization of Par6-aPKC, the brush border component Ezrin, and microvilli induction (Fig. 4a-c, Supplementary Fig. 3g). Constitutive expression of MRCKβ-flag, which is autoinhibited until bound to Cdc42-GTP, in conditional Dbl3-expressing cells accelerated the formation of apically activated Myosin caps, which became enriched with MRCKβ-flag, polarization and brush border induction (Fig. 4b,c; Supplementary Fig. 3f,g). MRCK is thus recruited to the apical membrane by Dbl3-mediated Cdc42 activation to drive apical polarization and differentiation of epithelial cells. Apical localization of MRCKβ was also observed in vivo in mouse renal tubules and intestine (Fig. 4d), and Gek was found at the apical photoreceptor cortex (Fig. 4e). Hence, apical MRCK polarization occurs in vitro and in vivo, and in vertebrates and invertebrates.
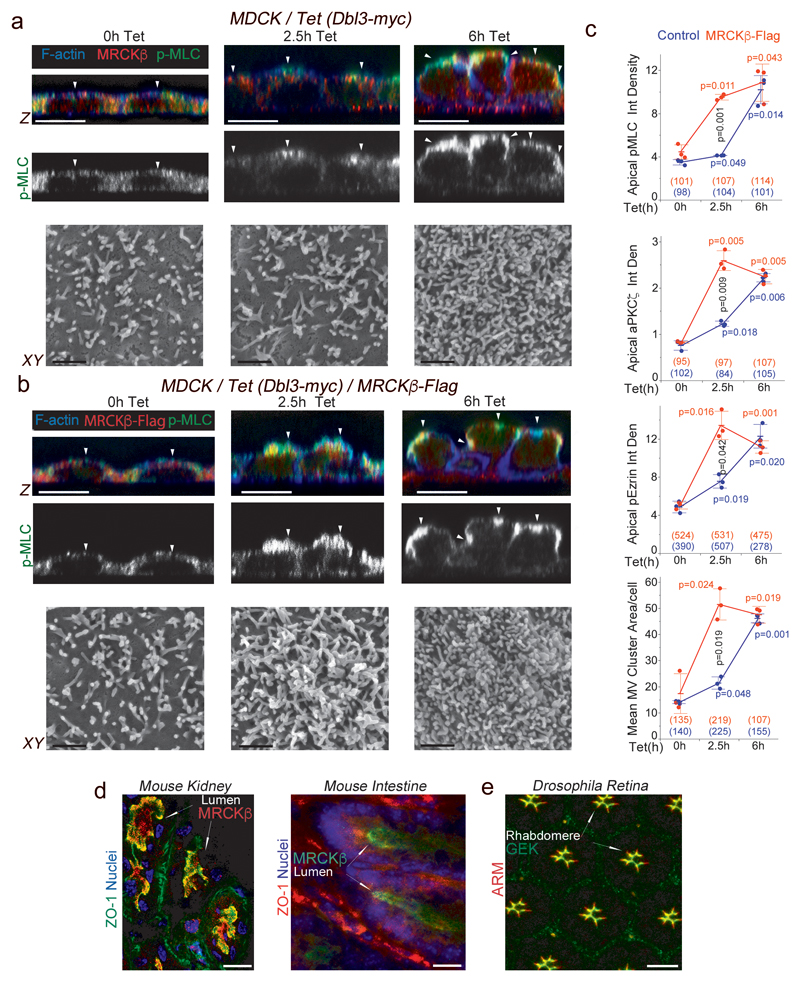
Figure 4. Cdc42 activates MRCK apically, stimulating apical Myosin-II activation and differentiation.
(a-c) Analysis and quantification of apical pMLC, aPKCζ and pEzrinT567 at the apical membrane domain using confocal z-section analysis and of brush border induction using scanning electron microscopy of MDCK cells with tetracycline-inducible Dbl3-myc expression with or without constitutive expression of MRCKβ-flag. White arrowheads highlight the apical membrane cortex labelled for F-actin. (d) Localization of MRCKβ in mouse kidney and small intestine. (e) Localization of Gek in polarizing pupal Drosophila photoreceptors. For all quantifications, n=3 independent experiments and shown are the data points, means ± 1 SD, the total number of cells analysed for each type of sample across all experiments, and p-values derived from t-tests (red/blue values refer to comparisons within categories and black value to comparisons between categories after 2.5h). In panels d and e, arrows point to apical domains positive for MRCK/Gek. Scale bars: electron micrographs, 1μm; confocal immunofluorescence images, 10 μm.
Apical Cdc42 activates a dual effector mechanism that antagonises junctional Myosin activity
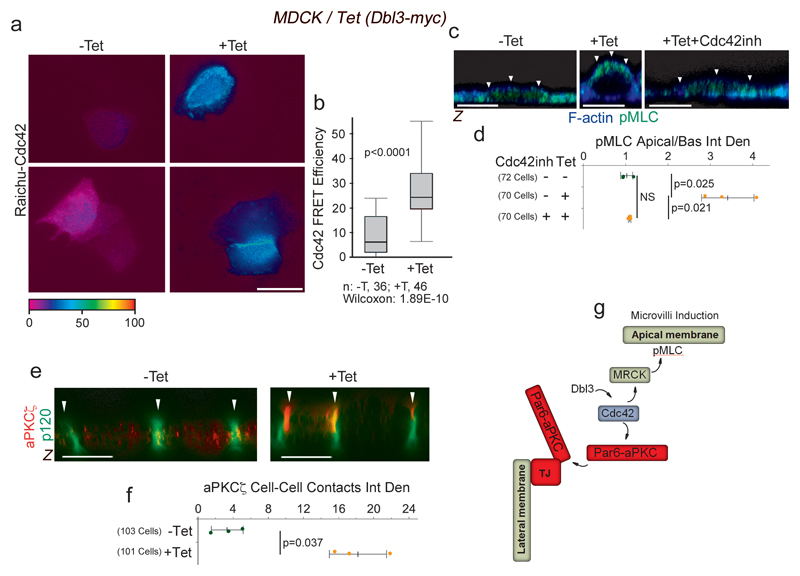
RhoA and ROCK regulate actomyosin activity along cell-cell junctions, generating a circumferential actomyosin belt that is required for junction assembly and remodelling, and apical constriction15, 18, 19. Activation of apical Cdc42 by Dbl3 promotes apical expansion17, a process opposing apical constriction. Apical Cdc42 signalling may thus not only promote apical MRCK-driven Myosin activation but also downregulate junctional actomyosin contractility to create an actomyosin activity gradient that favours apical expansion and apical PAR protein segregation. In agreement with apical activation of Myosin-II by MRCK, conditional expression of Dbl3-myc stimulated apical Cdc42 activity, as revealed by a FRET biosensor, confirming previously published loss of function experiments (Fig. 5a,b)17. Addition of a Cdc42 inhibitor during Dbl3 induction26, blocked the formation of apical active Myosin caps (Fig. 5c,d). Cdc42 activation is thus required for Dbl3 to induce MRCK-dependent, apically polarized actomyosin caps. Dbl3 stimulates apical Cdc42-dependent aPKC-PAR6 effector complex activation and, hence, enrichment of aPKC at the cell cortex (Fig. 5e,f; Supplementary Fig. 4a)17. As aPKC is an inhibitor of the RhoA activator LULU-216, we asked whether Cdc42 activates a dual effector mechanism that downregulates junctional Myosin-II activity via aPKC whilst activating apical actomyosin contractility via MRCK (Fig. 5g).
Figure 5. Dbl3 and Cdc42 drive apical actomyosin activation and aPKC recruitment.
(a,b) FRET analysis of Cdc42 activity at the apical aspect of MDCK cells conditionally expressing Dbl3-myc. (c,d) Confocal microscopy of pMLC and F-actin at the apical membrane domain of MDCK cells conditionally expressing Dbl3-myc induced with tetracycline in the absence or presence of Cdc42 inhibitor. White arrowheads highlight the apical membrane cortex labelled for F-actin. (e,f) Analysis of aPKCζ localization in MDCK cells with tetracycline-inducible Dbl3-myc expression. White arrowheads point to the lateral junctional complex. (g) Schematic diagram illustrating activation of two effector mechanisms, MRCK/Myosin-II and Par6-aPKC by Dbl3 stimulated apical Cd42-activation. Quantifications show shown are (b) box blots (25th to 75th percentiles, with a line at the median; whiskers extend to the max/min data points; n=36 cells -Tet and n=46 cells +Tet; p value was calculated with a Wilcoxon test), or (d, f) based on n=3 independent experiments and showing the data points, means ± 1 SD (in black), the total number of cells analysed for each type of sample across all experiments, and p-values derived from t-tests. Scale bars: 10 μm.
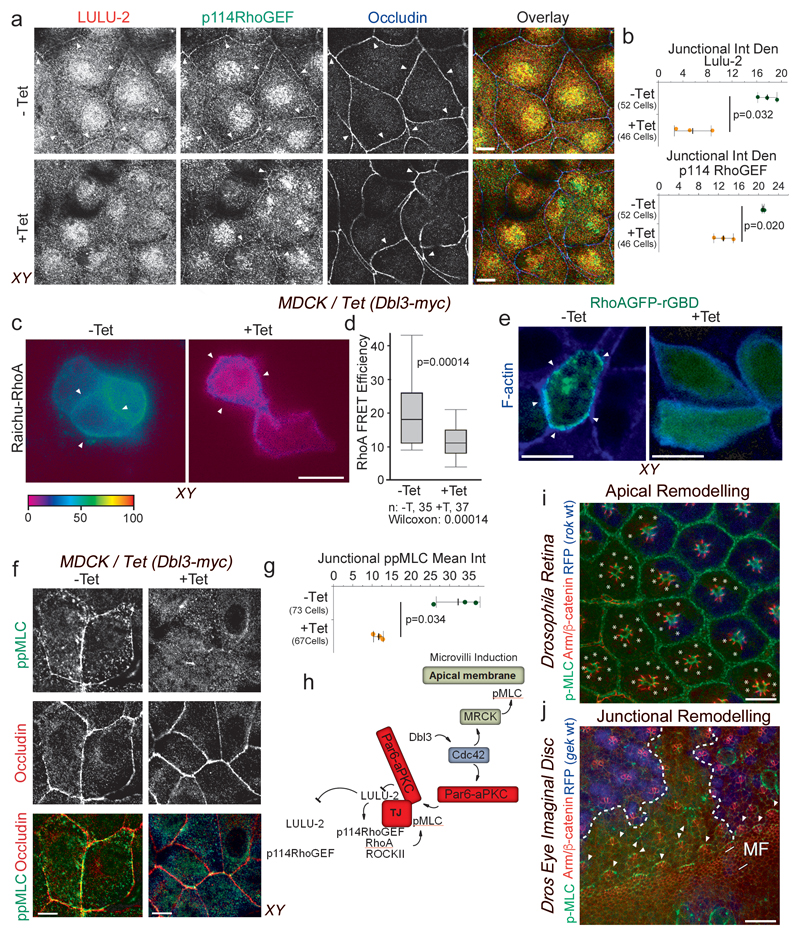
To determine whether Dbl3-induced Cdc42 activation is indeed an inhibitor of RhoA at junctions, we first monitored junctional localization of LULU-2 and p114RhoGEF. The latter protein is a major junctional guanine nucleotide exchange factor for RhoA that is activated by binding to LULU-2, a FERM (4.1 protein, ezrin, radixin, moesin) domain-containing protein homologous to Drosophila Yurt15, 16, 27. Concomitant with increased aPKCζ recruitment, conditional expression of Dbl3-myc promoted LULU-2 dissociation from junctions (Fig. 6a,b; Supplementary Fig. 4d). Junctional p114RhoGEF levels also decreased upon Dbl3 induction (Fig. 6a,b; Supplementary Fig. 4d). Dbl3 induction did not affect expression levels of LULU-2 and p114RhoGEF but resulted in enhanced Ezrin phosphorylation, a marker of apical differentiation (Supplementary Fig. 4b,c). Dbl3-myc induction thus leads to reduced junctional association of proteins that activate RhoA signalling. To confirm that junctional recruitment of LULU-2 is regulated by Dbl3 activated aPKC, we knocked down Dbl3 or inhibited aPKCζ activity, which resulted in an increase in LULU-2 localization at cell junctions (Supplementary Fig. 4e-h). These results indicate that apical activation of Cdc42 antagonizes the molecular machinery that activates RhoA at cell junctions.
Figure 6. Apical activation of Cdc42 by Dbl3 antagonizes junctional RhoA-stimulated Myosin-II activation.
(a,b) Conditional tetracycline-induced expression of Dbl3 in MDCK cells stimulates loss of junctional RhoA signalling components p114RhoGEF and LULU-2. Shown are confocal xy and z-sections. Junctions are indicated by white arrowheads. (c,d) FRET analysis of RhoA activity in MDCK cells conditionally expressing Dbl3-myc (white arrowheads indicate cell-cell contacts) (e) Confocal analysis of MDCK cells transiently expressing the active RhoA binding domain of rhotekin fused to GFP (GFP-rGBD), conditionally expressing Dbl3-myc (white arrowheads indicate cell-cell junctions). (f,g) Confocal analysis of junctional pMLC activity in MDCK cells conditionally expressing Dbl3-myc. (i) Confocal sections of a Drosophila retina showing wild type cells (blue) and cells mutant for the RhoA effector null allele rok2 (white asterisks) that were stained for pMLC and Armadillo (Arm), the orthologue of mammalian β-catenin. White asterisks indicate rok2-mtant cells. (j) Maximum intensity projection of confocal sections of a mosaic Drosophila eye imaginal disc showing wild type cells (blue nuclei) and cells mutant for gekNP5192 and stained for Armadillo (Arm) and pMLC (MF, morphogenetic furrow; the dashed white lines indicate the borders between wild type and mutant cells; arrowheads point to Myosin-II-rich cell junctions). The schematic diagram illustrates the generation of apically polarized actomyosin activation by apical activation of MRCK and inactivation of junctional RhoA signalling by aPKC. Quantifications show shown are (d) box blots (25th to 75th percentiles, with a line at the median; whiskers extend to the max/min data points; n=35 cells -Tet and n=37 cells +Tet; p value was calculated with a Wilcoxon test), or (b, d, g) based on n=3 independent experiments and showing the data points, means ± 1 SD (in black), the total number of cells analysed for each type of sample across all experiments, and p-values derived from t-tests. Scale bars: 10 μm.
We next asked if RhoA activity and junctional actomyosin activation is indeed affected by Dbl3 signalling. Analysis of RhoA activity at the apical domain using a FRET biosensor revealed enriched RhoA activity at cell-cell contacts as previously reported15. Induction of Dbl3 expression resulted in a 50% decrease in RhoA activity at cell-cell contacts (Fig. 6c,d). Similarly, a GFP fusion protein containing the RhoA binding domain of rhotekin, another sensor to monitor the cellular distribution of active RhoA28, was enriched at cell-cell contacts prior to Dbl3 induction but diffusely distributed when expression of the exchange factor had been induced, supporting the conclusion that enhanced Dbl3 signalling inhibits junctional RhoA activity (Fig. 6e). Active p114RhoGEF forms a stable complex with ROCKII and Myosin-II, stimulating junctional double phosphorylation of MLC (ppMLC)15. Conditional expression of Dbl3 strongly reduced junctional ppMLC staining (Fig. 6f,g), further supporting an inactivation of the p114RhoGEF/RhoA pathway that promotes junctional Myosin-II activation. Thus, Dbl3 activates apical Cdc42 and thereby stimulates two effector mechanisms: induction of apical MLC phosphorylation by MRCK and aPKC-stimulated downregulation of junctional RhoA-dependent actomyosin contractility. Dbl3 signalling hence leads to a shift in the actomyosin activity gradient to stimulate apical polarization (Fig. 6h). These results further indicate distinct roles for RhoA-ROCK and Cdc42-MRCK signalling in epithelia. Indeed, loss of the RhoA effector ROCK using the null allele rok2 did not affect apical Myosin-II activation in developing pupal Drosophila photoreceptors (Fig. 6i), which is MRCK dependent (Fig. 2b). In contrast, adherens junction remodelling in eye imaginal discs, a rok-dependent process requiring junctional actomyosin activity29, was not affected in cells mutant for gek/MRCK (Fig. 6k). MRCK and ROCK thus regulate distinct morphogenetic processes.
MRCK-activated Myosin-II motor activity promotes PAR protein dissociation and separation
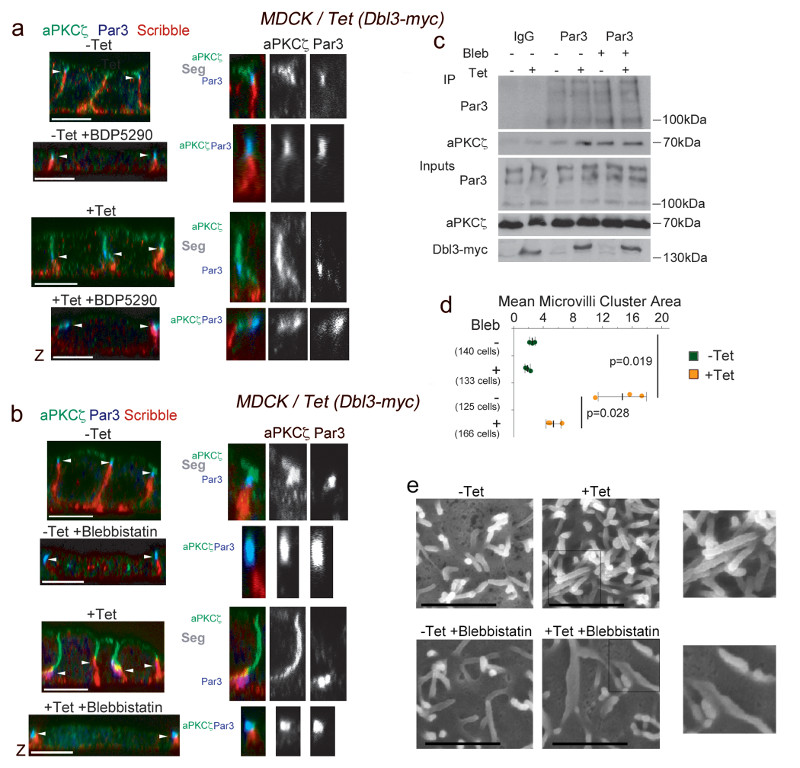
Cortical polarization of PAR proteins is thought to rely on both biochemical signals and mechanical forces generated by actomyosin contractions6–13. Cdc42 activation promotes apical polarization by stimulating activation of Par6-aPKC, leading to recruitment to junctions, Par3 phosphorylation and biochemical destabilisation of the initial Par6-aPKC/Par3 complex6, 7. Since activation of apical actomyosin contractility by MRCK stimulates apical differentiation, we asked whether MRCK-activated Myosin-II mediates aPKC-Par6 separation from Par3 at junctions. To determine first whether MRCK activity itself is required, we used an inhibitor that blocks the catalytic activity of MRCK30. Induction of Dbl3-myc expression led to shift of the position of tight junctions, demarked by Par3, from the apical end of the lateral membrane towards the basal membrane, and an extended apical domain, labelled by aPKC, and a reduced basolateral domain, stained with anti-scribble antibody, as previously described (Fig. 7a)17. Inhibition of MRCK blocked separation of Par3 from aPKC as well as the junctional shift in control and Dbl3 overexpressing cells, indicating that MRCK activity was required even when apical Cdc42 activation and, hence, aPKC signalling17 was increased by enhanced expression of the guanine nucleotide exchange factor. If the same experiments were repeated with blebbistatin, an inhibitor of Myosin-II motor activity that blocks actomyosin contractions31, separation of aPKC from Par3 and junctional shifting was also inhibited, and led to increased stable complex assembly (Fig. 7b,c). Blebbistatin also strongly attenuated brush border induction (Fig. 7d,e) comparable to what we had observed in response to depletion of MRCK (Fig. 3a). Thus, these results indicate that MRCK-activated Myosin-II motor activity is required for separation of Par6-aPKC from Par3 upon apical Cdc42 activation but not for the initial recruitment of Par6-aPKC to form the tight junction-associated complex with Par3. This is in agreement with the reported diffusive properties of PAR proteins in C. elegans, where PAR protein segregation, but not cortical recruitment, is actomyosin-dependent32. Since Dbl3-mediated Cdc42 activation also stimulates aPKC to phosphorylate Par317 and to down-regulate junctional RhoA signalling (Fig. 6), our results demonstrate that both effector mechanisms stimulated by active apical Cdc42 are required for apical PAR protein separation and segregation: The biochemical activity of aPKC-Par6 that destabilizes the PAR complex and downregulates junctional Myosin contractility, and the activation of apical Myosin contractility by MRCK that drives apical PAR protein separation and domain morphogenesis.
Figure 7. MRCK-stimulated Myosin-II activation drives PAR separation at tight junctions.
(a,b) Confocal analysis of aPKCζ localization at tights junctions in medium/advanced polarizing/differentiating MDCK cells with tetracycline-inducible Dbl3-myc expression without or with (a) BDP5290, a MRCK inhibitor, or (b) blebbistatin, a Myosin-II inhibitor. The position of tight junctions, demarked by Par3, is indicated by white arrowheads. Note, tight junctions move towards the basal membrane in response to apical expansion17. The larger magnifications show examples of cells in which aPKC segregates from Par3 (all controls and enhanced by Dbl3-myc expression) or in which aPKC and Par3 co-localise (cells treated with MRCK or Myosin-II inhibitor). aPKCζ segregation from Par3 at tight junctions is highlighted by ‘Seg’. (c) Immunoprecipitation analysis of aPKCζ-Par3 complex formation in cells conditionally expressing Dbl3-myc with or without blebbistatin. Note, increased Par3-aPKC complex formation in Dbl3-myc-induced cells without blebbistatin is due to increased transient interactions that promote apical expansion17. (d,e) Quantification of microvilli induction in MDCK cells conditionally expressing Dbl3-myc -/+ Myosin-II inhibition. The quantification in panel d is based on n=3 independent experiments and shown are the data points, means ± 1 SD (in black), the total number of cells analysed for each type of sample across all experiments, and p-values derived from t-tests. Unprocessed original scans of blots are shown in Supplementary Figure 8. Scale bars: electron micrographs, 1μm; confocal immunofluorescence images, 10 μm.
Dbl3/MRCK-stimulated actomyosin contractility drives apical segregation of PAR proteins and polarization of cytosolic brush border determinants
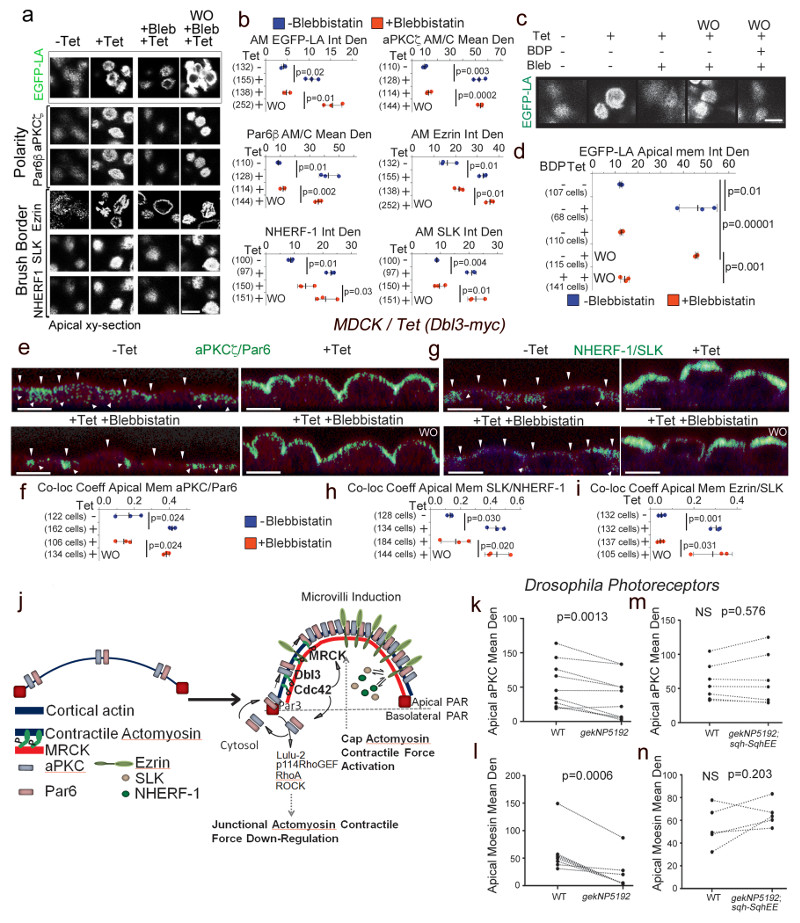
Cdc42-activated aPKC-Par6 recruitment to junctions is a transient process and the complex then proceeds to expand along the apical membrane, segregating from Par3 and tight junctions, to define the apical cortex33. Hence, we next asked whether expansion along the apical membrane is powered by MRCK-dependent and Myosin-II motor activity. Polarization of aPKC-Par6 along the apical cell cortex, which formed a stable complex, was inhibited in conditional Dbl3-myc expressing cells treated with blebbistatin and rapidly reversed following wash-out (Fig. 8a,b; Supplementary Fig. 5). The reversal of Myosin motor inhibition required MRCK kinase activity, confirming the requirement of MRCK-dependent Myosin-II activation for apical polarization of the aPKC-Par6 complex (Fig. 8c,d). It is thought that a rapid apical phosphorylation/dephosphorylation cycle of Ezrin is required to confine Ezrin and microvilli to the apical membrane domain in polarized epithelial cells, which is facilitated by apical localization of cytosolic factors such as the kinase SLK34,35. Similarly, NHERF-1/EBP-50 engages in a transient association with Ezrin to regulate microvillar structure34. Myosin motor-dependant cortical polarization was concomitant with an apical enrichment of such cytosolic regulators of brush border formation and was required for their co-localization with Ezrin at the apical pole, indicating that MRCK-induced apical domain formation included polarization of cytosolic factors required for plasma membrane specialization and morphogenesis (Fig. 8e-i and Supplementary Fig. 6). Our data thus support a model in which epithelial cortical polarization and cytosolic polarization are linked32. Hence, MRCK signalling unifies the mechanisms for asymmetric segregation of cytosolic components to the vicinity of the apical membrane with cortical polarization (Fig. 8j). Apical membrane staining of aPKC and Moesin in Drosophila gek mutant photoreceptors was also attenuated (Fig. 8k,l; Supplementary Fig. 7). These effects were rescued by co-expression of sqhEE, a phosphomimetic MLC construct, in mutant gek/MRCK cells (Fig. 8m,n), demonstrating the importance of gek/MRCK activation of Myosin-II for apical differentiation of Drosophila photoreceptors.
Figure 8. MRCK-activated actomyosin contractility drives apical PAR segregation and polarization of cytosolic factors.
(a,b) Levels of EGFP-Lifeact (EGFP-LA), Par polarity and brush border proteins localized at the apical membrane domain in MDCK cells conditionally expressing Dbl3-myc, treated with blebbistatin and followed by washout (WO). (c,d) Levels of EGFP-Lifeact (EGFP-LA) enrichment at the apical membrane domain of MDCK cells conditionally expressing Dbl3-myc following blebbistatin treatment and washout in the absence or presence of BDP5290 to inhibit catalytic activity of MRCK. (e-i) Measurements of co-localization coefficients of PAR polarity and brush border proteins at the apical membrane domain in MDCK cells conditionally expressing Dbl3-myc transiently inhibited with blebbistatin followed by wash out for 2 hours (green indicates co-localisation, see also Supplementary figures 5 and 6; arrowheads point to apical membrane). (j) Schematic model of polarity induced by apical stimulation of Cdc42, activating a dual effector mechanism to generate asymmetric actomyosin contractions, apical PAR domain formation and membrane morphogenesis. Bright green represents co-localization of Par and brush border proteins. (k-n) Quantification of confocal sections of pupal retinas stained for the apical markers aPKC and Moesin comparing wild type cells and gek mutant cells (k, l), or wild type cells and cells mutant for gek expressing SqhEE (m, n). Quantifications in panels b, d, f, h, and i are based on n=3 independent experiments and shown are the data points, means ± 1 SD (in black), the total number of cells analysed for each type of sample across all experiments, and p-values derived from t-tests. Quantifications in panels k-n of protein expression levels compare measurements from wild type and neighbouring mutant cells (paired within sections; see Supplementary Fig. 7 for representative images); k, n=10 animals; l, n=8 animals; m and n, n=7 animals; p values were calculated with t-tests). Scale bars: 10 μm.
Discussion
We have identified a distinct, evolutionarily conserved actomyosin-powered morphogenetic pathway at the apical domain that is activated by Cdc42/MRCK and drives epithelial morphogenesis and the development of epithelial polarity. Cdc42/MRCK signalling serves to coordinate biochemical signalling and actomyosin-generated mechanical forces. Unlike the mechanism of action of Cdc42, the spatial activation of Cdc42 is likely to vary across species since far less GEF diversity exists in Drosophila, and the closest orthologue of Dbl3 in insects lacks a Sec14 domain, which is crucial for Dbl3 function in vertebrates17.
Cell polarization is thought to be induced by mechanical forces generated by an actomyosin activity gradient11–13, 32. Our results indicate that apical Cdc42/MRCK signalling functions in the culminating stages of epithelial polarization leading to the accumulation of Par6 and aPKC at the apical domain by promoting the actomyosin-driven segregation of the Par6-aPKC complex from the junctional complex along the differentiating apical domain and thus promoting apical identity and morphogenesis. Moreover, cytosolic factors that regulate apical membrane specialization such as SLK and NHERF-1/EBP-50, which mediate localised Ezrin activation and brush border assembly, are concentrated at the apical pole, linking cortical and cytosolic polarization. As active Ezrin also promotes apical Dbl3 recruitment and Cdc42 activation17, this process represents a positive feedback mechanism that enhances apical differentiation and reinforces the polarized epithelial phenotype.
Apical Cdc42 activation stimulates two cooperating effector pathways that induce the formation of apically polarized actomyosin contractility to drive PAR protein polarization: MRCK-mediated apical Myosin-II activation and Par6-aPKC-mediated inhibition of junctional RhoA activation via negative regulation of LULU-2 and p114RhoGEF and, hence, reduced junctional actomyosin contractility. The biochemical activity of aPKC-Par6 also supports apical PAR segregation by phosphorylating Par3 and, thereby, destabilising its interaction with the aPKC-Par6 complex6, 7, 17. Hence, activation of the two Cdc42 effector pathways integrates biochemical and mechanical force-generating pathways stimulating apical segregation of PAR proteins, polarization and domain morphogenesis. The downregulation of junctional RhoA signalling by apical Cdc42 activation further suggests a sequential model for the function of ROCK and MRCK in Myosin-II activation: RhoA/ROCK signalling drives early steps of junction formation15 and remodelling such as in the early eye disc29, 36, 37, subsequent Cdc42/MRCK activation then leads to a reorientation of the actomyosin contractility gradient and, hence, apical polarization and differentiation. The antagonism between junctional RhoA and apical Cdc42 signalling may be bidirectional. In vertebrates, junctional RhoA activity is generated by p114RhoGEF, a pathway that is stimulated in cells that undergo apical constriction due to increased levels of LULU-215, 16. The fly homologue of LULU-2, Yurt, regulates apical domain size negatively by inhibiting the activity of Crumbs, which is positively regulated by aPKC27, suggesting that LULU-2/Yurt signalling may indeed antagonize apical Cdc42-driven mechanisms. Hence, mechanisms of mutual antagonism between Cdc42/MRCK and RhoA/ROCK driven actomyosin contractility may exist that create a cellular code for dynamic polarized morphogenetic processes underlying the generation of tissue- and organ-specific cell surface fates during differentiation of various cell types.
Methods
Cell culture and generation of mammalian expression vectors and cell Lines
MDCK and Caco-2 cells were grown in DMEM supplemented with 10% or 20%, respectively, foetal bovine serum (original cell stocks were obtained from Ira Mellman (MDCK cells) and Hans-Peter Hauri (Caco-2 cells) 17, 38, 39. Fresh batches of cells from a contamination-free stock that had been tested for mycoplasma (MycoAlert; Promega Inc.) were used to replace fresh cultures every 6 to 8 weeks. Cells were then weekly stained with Hoechst dye to reveal nuclei and DNA of contaminants such as mycoplasma. MRCK inhibitor BDP5290 was synthesized by the Cancer Research UK Beatson Institute Drug Discovery Group and used at a concentration of 10μM. Blebbistatin (10μM final concentration), the Cdc42 inhibitor ML141 (10μM), and the ROCKI/II inhibitor GSK 269962 (10nM) were purchased from Tocris Bioscience. The aPKCζ-myristoylated inhibitor was purchased from Merck Millipore and used at a concentration of 40μM. For conditional expression of Dbl3-myc, Dbl3 pcDNATO-myc expression vector system was constructed as described in Zihni et al., 201417. MDCK cells were transfected with a plasmid encoding the tetracycline repressor (pcDNA6-TR) using the calcium phosphate method17, cultured in DMEM with 10% foetal bovine serum and selected with Blasticidin (0.5μg/ml). Selected clones were then transfected with pCDNA4TO-Dbl3-myc and selected in DMEM with 10% foetal bovine serum with Blasticidin (0.5μg/ml; PAA Laboratories Inc.) and Zeocin (200μg/ml; Thermo Fisher Scientific). The MDCK pcDNA4TO-Dbl3-myc/MRCKβ-flag cell-line was generated by transfecting rat MRCKβ-flag into the MDCK pcDNATO-Dbl3-myc TET-R cell-line and selecting in DMEM with 10% foetal bovine serum with Blastocidin (0.5μg/ml), Zeocin (200μg/ml) and G418/Geneticin (600μg/ml; Thermo Fisher Scientific). MDCK and Caco-2 cells constitutively expressing MRCKβ-flag were transfected analogously and cultured in DMEM with either 10% or 20% foetal bovine serum and G418 (600μg/ml G418). To generate EGFP-LA or EGFP-MLC pcDNA4TO-Dbl3-myc MDCK TET-R cell-lines retroviral transduction constructs EGFP-Lifeact were cloned at EcoRI and XhoI sites using the following primers: 5’ GAA TTC ATG GGT GTC GCA GAT TTG ATC AAG AAA TTC GAA AGC ATC TCA AAG GAA GAA CTC GAG 3’ and 5’ CTC GAG TTC TTC CTT TGA GAT GCT TTC GAA TTT CTT GAT CAA ATC TGC GAC ACC CAT GAA TTC 3’ and MLC by PCR amplification using the following primers 5’ CAA TAA GAA TTC ATG TCC AGC AAG CGG GCC AAA GC 3’ and 5’ GAC TTC CTC GAG CTA GTC GTC TTT ATC CTT GGC G 3’ into retroviral packing vector pMSC28 EGFP IRES Puro, a MLV-based retroviral system as described previously40. Constructs were co-transfected with pVSVG into GP2-293 cells (Clontech). Supernatants were collected, clarified by centrifugation (200XG, 5 mins) filtered (0.45μm) and used to infect MDCK Dbl3 Tet-R cells in the presence of 8μg/ml polybrene (Millipore) at (MOI) <1. Cell lines were selected for with either Puromycin or G418 (Thermo Fisher Scientific) at final concentration of 2.0μg/ml or 800μg/ml respectively. Selected clonal cell lines were grown on maintenance concentration of 0.5μg/ml Puromycin or 400μg/ml G418.
Transfection
Cells were cultured and transfected using Interferin transfection reagent (Polyplus Transfection) using the method described in Zihni et al.17 using siRNAs targeting the following sequences: human MRCKpan 5’-CGAGAAGACTTTGAAATAA-3’; canine MRCKpan 5’-AGAGAAGACTTTGAGATAT-3’; canine Dbl3 5’-AAGAUAUCGCCUUCUUGUC-3’. The MRCK siRNA sequences are not conserved in the rat mRNA, enabling rescue experiments with the rat cDNA encoding MRCKβ.
Mammalian antibodies and immunological methods
Fixation and processing cells and mouse tissue sections was as previously described19. The following antibodies were used: EHM2/Lulu-2, goat polyclonal (abcam ab77484) 1/50 for immunofluorescence and 1/250 for immunoblotting16; Par3, rabbit polyclonal (Upstate, Millipore 07-330) 1/500 for immunofluorescence and 1/2000 for immunoblotting; PAR6β, rabbit polyclonal H-64 (Santa Cruz Biotechnology sc-67392) 1/200 for immunofluorescence and 1/1000 for immunoblotting; aPKCζ, mouse monoclonal (Santa Cruz Biotechnology sc-17781) 1/200 for immunofluorescence and 1/1000 for immunoblotting; occludin, mouse monoclonal (Invitrogen, ThermoFisher Scientific 33-150) 1/1000 for immunofluorescence; Scribble, goat polyclonal (Santa Cruz Biotechnology sc-11049) immunofluorescence 1/200; MRCKβ, rabbit polyclonal (Santa Cruz Biotechnology sc-48834) immunofluorescence 1/200 and immunoblotting 1/500, Ezrin Mouse monoclonal 3C12 (Santa Cruz Biotechnology sc-58758) immunofluorescence 1/500 and immunoblotting 1/1000; phospho- EzrinT567, rabbit polyclonal (abcam ab47293) immunofluorescence 1/500 and immunoblotting 1/2000; p-MLC S19, mouse monoclonal and pp-MLC Thr18, S19 rabbit polyclonal (Cell Signaling Technology 3675 and 3674) immunofluorescence 1/100 and 1/200, respectively, and immunoblotting 1/1000; SLK, goat polyclonal (Santa Cruz Biotechnology sc-79068) immunofluorescence 1/100 and immunoblotting 1/1000; NHERF1/EBP50, rabbit polyclonal (Santa Cruz Biotechnology sc-134485) immunofluorescence 1/100 and immunoblotting 1/1000; p114RhoGEF, mouse monoclonal (GeneTex GTX629806) immunoblotting 1/1000; p114RhoGEF, rabbit polyclonal (GeneTex GTX102223) immunofluorescence 1/50. An affinity-purified rabbit polyclonal was used for ZO-1, immunofluorescence 1/400 (see Supplementary Table 1 for additional details on antibodies) 41–43. Phalloidin-Atto 647 reagent was obtained from Sigma Aldrich and diluted 1/1000 (65906). Affinity-purified and cross-adsorbed Alexa488-, Cy3- and Cy5-labelled donkey anti-mouse, rabbit, and goat secondary antibodies were from Jackson ImmunoResearch Laboratories (1/300 diluted from 50% glycerol stocks). Affinity-purified HRP-conjugated goat anti mouse and rabbit, and donkey anti goat secondary antibodies 1/5000 were also from Jackson ImmunoResearch Laboratories (1/5000 diluted from 50% glycerol stocks). For immunofluorescence analysis cells and tissues were mounted using Prolong Gold antifade reagent (Life technologies P36930) and imaging was performed using Zeiss 700 and 710 confocal microscopes and a 64x oil lens/NA1.4. Images were processed using Zeiss Zen 2009 and Adobe Photoshop CS5 and 10 software. Co-immunoprecipitation and immunoblotting was carried out using methods previously described and were repeated at least three times19.
Rho GTPase activity assays
For FRET experiments, cells were plated into ibid multi-well chamber slides and then transfected with pRaichu-RhoA or pRaichu-Cdc42 (kindly provided by M. Matsuda, Osaka University, Japan)44. The FRET analysis was performed at 37°C with a Nikon Eclipse Ti-E inverted microscope equipped with excitation and emission CFP and YFP filters in external filter wheels and using a CFI Apochromat Nano-Crystal 60x oil lens (N.A., 1.2). Crossover between CFP and YFP filters was calibrated by imaging CFP and YFP expressed alone using all four emission/excitation filter combinations. FRET efficiency maps were then produced with the Nikon software and quantified with ImageJ. The plasmid encoding the GFP-RhoA-rGBD construct was obtained from addgene and was described previously28.
Mammalian electron microscopy
Cells were fixed and cut and mounted for scanning electron microscopy as previously described19. Samples were analysed in a Sigma Field Emission scanning electron microscope (Carl Zeiss) operating at 5kV. Digital images were recorded using Carl Zeiss (SmartSEM) software.
Drosophila strains and genetics
Flies were maintained at 25°C on standard food. To generate whole mutant eyes, the EGUF/GMRhid system was used45 in combination with the gekNP5192 and gekomb1080alleles 25. Canton S flies were used as control. Mosaic gek retinas where generated using the following genotype: eyflp ; FRT42D Ubi-myrRFP / FRT42D gek. Rescue experiments were performed using the the MARCM technique46 combined to the sqh-SqhE20,21 transgene 47. The following genotype was examined: hsFLP,Tubulin-Gal4, nuGFP/+ ; FRT42D, TubGal80/ FRT42, gekNP5192 ; sqh-SqhE20E21/+. Pupal retinas where staged and stained according as described48.
Drosophila antibodies and immunological methods
Whole mount retinas were prepared as described in48. The following antibodies were used: rabbit anti-p-MLC Ser19, 1/40 (Cell Signaling Technology 3671); rabbit anti-PKCζ 1/200 (Sigma Aldrich SAB4502380), mouse anti-Arm 1/200 (N27-A1, Developmental Studies Hybridoma Bank), rabbit anti-Gek 1/25 (gift from TR Clandinin25, rabbit anti-P-Moesin 1/50049, phalloidin-Texas Red (Sigma Aldrich, 300nM) with the appropriate combination of secondary antibodies conjugated to Dy405, Alexa488, Cy3 or Cy5 as appropriate at 1/200 each (Jackson ImmunoResearch). Retinas were mounted in VectaShield and imaging was performed using a Leica SP5 confocal. Images were processed using ImageJ and Adobe Photoshop 7.0.
Drosophila electron microscopy
Electron microscopy was performed as in50 using a Tecnai G2 Spirit transmission electron microscope (FEI, The Netherlands) equipped with a Morada CCD camera (Olympus Soft Imaging Systems). Image quantification was performed using iTEM software.
Image analysis
Mammalian epithelial cells
Microvilli induction was measured as cluster areas since clusters represent advanced microvilli induction during differentiation that proceeds initial bud formation at the early stage of differentiation51. Cluster areas were measured in scanning electron micrographs using Nikon Imaging software (NIS) (Supplementary Figure 1) by determining threshold intensity. 2-3 areas of each cell at a magnification of 40,000x were measured to accommodate cluster variation in each cell. Cell height was measured in Zeiss 700 confocal Z-sections using Zeiss Zen2000 imaging software. Co-localisation coefficients were calculated with the Zeiss 700 microscope software. Cell height was measured using basal and apical markers and at 3μM intervals to accommodate for variation of height along individual cells, and values were then averaged. p-MLC (ser19), F-actin, EFP-LA, polarity and brush border protein pixel intensity was measured using Image-J software. For each cell background was measured and subtracted from the sample value. Co-localization coefficients were measured in Zeiss Confocal XY or Z-scans using Zeiss Zen 2000 co-localization software that applies the principal of the Pearson’s coefficient, using cross-hairs facility to subtract background from each channel.
Drosophila
Pixel intensity/membrane length measurements of apical membrane and Arm domains were determined by analysing confocal images of gek mosaic retinas at 40% APF using ImageJ.
Statistics and reproducibility
For the quantifications shown, the provided n values refer to independent experiments and the numbers in the graphs refer to the total of analysed cells per type of sample across all experiments. Statistical significance was tested in most experiments using two-tailed Student’s t-tests, pairing values derived from the same experiment. The FRET experiments are shown as standard box plots (25th to 75th percentiles, with a line at the median; whiskers extend to the max/min data points) and were analysed with a nonparametric Mann-Whitney-Wilcoxon test as the data were not normally distributed. The experiments that were not quantified in figures1b, 3e and f, and 7c, as well as supplementary figures 3e, 5b, 6e and 7c are representative for 3 experiments. Localization of MRCK in mouse tissue was analysed in sections derived from 2 different animals (figure 4d). Graphs and statistical calculations were generated using MS Excel (V15) and JMP (V12). For the Drosophila experiments, a minimum of four independent retinas were used for each genotype. Parametric samples were tested for statistical significance using paired two-tailed Student’s t-test. The numbers of animals analysed: figure 2b-d, n=11; figure 2e,f, n=4 for each genotype; figure 2g,h, n=8; figure 4e, n=8; figure 6, n=4 (i); n=6 (j); figure 8, n=10 (k), n=8 (l), n=7 (m), n=7 (n); and Supplementary figure 9 n=9 (a), n=7 (b), and n=7 (c,d), n=7.
Data availability
All data supporting the conclusions here are available from the authors on reasonable request.
Supplementary Material
Acknowledgements
This work was supported by the BBSRC (BB/L007584/1 and BB/N014855/1) and the Wellcome Trust (099173/Z/12/Z). Work in FP lab, including support to EV, was funded by an MRC grant (MC_UU_12018/3). The N2 A71 monoclonal antibodies, developed by Eric Wieschaus was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study.
Footnotes
Author Contributions
CZ performed most of the vertebrate and EV the Drosophila experiments. All other authors performed particular subsets of experiments. CZ, MSB and KM designed the project and drafted the manuscript. All authors read and contributed to the final version of the manuscript.
Competing Interests
The authors declare that they have no competing financial interests.
References
- 1.Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol. 2008;9:833–845. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sauvanet C, Wayt J, Pelaseyed T, Bretscher A. Structure, regulation, and functional diversity of microvilli on the apical domain of epithelial cells. Annu Rev Cell Dev Biol. 2015;31:593–621. doi: 10.1146/annurev-cellbio-100814-125234. [DOI] [PubMed] [Google Scholar]
- 3.Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17:564–580. doi: 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]
- 4.Cowan CR, Hyman AA. Acto-myosin reorganization and PAR polarity in C. elegans. Development. 2007;134:1035–1043. doi: 10.1242/dev.000513. [DOI] [PubMed] [Google Scholar]
- 5.St Johnston D, Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 2010;141:757–774. doi: 10.1016/j.cell.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Morais-de-Sa E, Mirouse V, St Johnston D. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell. 2010;141:509–523. doi: 10.1016/j.cell.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walther RF, Pichaud F. Crumbs/DaPKC-dependent apical exclusion of Bazooka promotes photoreceptor polarity remodeling. Curr Biol. 2010;20:1065–1074. doi: 10.1016/j.cub.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 8.Hird SN, White JG. Cortical and cytoplasmic flow polarity in early embryonic cells of Caenorhabditis elegans. J Cell Biol. 1993;121:1343–1355. doi: 10.1083/jcb.121.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheeks RJ, et al. C. elegans PAR proteins function by mobilizing and stabilizing asymmetrically localized protein complexes. Curr Biol. 2004;14:851–862. doi: 10.1016/j.cub.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Munro E, Nance J, Priess JR. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev Cell. 2004;7:413–424. doi: 10.1016/j.devcel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Mayer M, Depken M, Bois JS, Julicher F, Grill SW. Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows. Nature. 2010;467:617–621. doi: 10.1038/nature09376. [DOI] [PubMed] [Google Scholar]
- 12.Bois JS, Julicher F, Grill SW. Pattern formation in active fluids. Phys Rev Lett. 2011;106:028103. doi: 10.1103/PhysRevLett.106.028103. [DOI] [PubMed] [Google Scholar]
- 13.Goehring NW, et al. Polarization of PAR proteins by advective triggering of a pattern-forming system. Science. 2011;334:1137–1141. doi: 10.1126/science.1208619. [DOI] [PubMed] [Google Scholar]
- 14.Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 15.Terry SJ, et al. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol. 2011;13:159–166. doi: 10.1038/ncb2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakajima H, Tanoue T. Lulu2 regulates the circumferential actomyosin tensile system in epithelial cells through p114RhoGEF. J Cell Biol. 2011;195:245–261. doi: 10.1083/jcb.201104118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zihni C, et al. Dbl3 drives Cdc42 signaling at the apical margin to regulate junction position and apical differentiation. J Cell Biol. 2014;204:111–127. doi: 10.1083/jcb.201304064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mooseker MS. Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu Rev Cell Biol. 1985;1:209–241. doi: 10.1146/annurev.cb.01.110185.001233. [DOI] [PubMed] [Google Scholar]
- 19.Castillo AM, Lagunes R, Urban M, Frixione E, Meza I. Myosin II-actin interaction in MDCK cells: role in cell shape changes in response to Ca2+ variations. J Muscle Res Cell Motil. 1998;19:557–574. doi: 10.1023/a:1005316711538. [DOI] [PubMed] [Google Scholar]
- 20.St Johnston D, Sanson B. Epithelial polarity and morphogenesis. Curr Opin Cell Biol. 2011;23:540–546. doi: 10.1016/j.ceb.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Unbekandt M, Olson MF. The actin-myosin regulatory MRCK kinases: regulation, biological functions and associations with human cancer. J Mol Med (Berl) 2014;92:217–225. doi: 10.1007/s00109-014-1133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reifegerste R, Moses K. Genetics of epithelial polarity and pattern in the Drosophila retina. Bioessays. 1999;21:275–285. doi: 10.1002/(SICI)1521-1878(199904)21:4<275::AID-BIES3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Pocha SM, Knust E. Complexities of Crumbs function and regulation in tissue morphogenesis. Curr Biol. 2013;23:R289–293. doi: 10.1016/j.cub.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Walther RF, Nunes de Almeida F, Vlassaks E, Burden JJ, Pichaud F. Pak4 Is Required during Epithelial Polarity Remodeling through Regulating AJ Stability and Bazooka Retention at the ZA. Cell Rep. 2016;15:45–53. doi: 10.1016/j.celrep.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gontang AC, Hwa JJ, Mast JD, Schwabe T, Clandinin TR. The cytoskeletal regulator Genghis khan is required for columnar target specificity in the Drosophila visual system. Development. 2011;138:4899–4909. doi: 10.1242/dev.069930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong L, et al. Characterization of a Cdc42 protein inhibitor and its use as a molecular probe. J Biol Chem. 2013;288:8531–8543. doi: 10.1074/jbc.M112.435941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laprise P, et al. Yurt, Coracle, Neurexin IV and the Na(+),K(+)-ATPase form a novel group of epithelial polarity proteins. Nature. 2009;459:1141–1145. doi: 10.1038/nature08067. [DOI] [PubMed] [Google Scholar]
- 28.Benink HA, Bement WM. Concentric zones of active RhoA and Cdc42 around single cell wounds. J Cell Biol. 2005;168:429–439. doi: 10.1083/jcb.200411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson F, Pinal N, Fichelson P, Pichaud F. Atonal and EGFR signalling orchestrate rok- and Drak-dependent adherens junction remodelling during ommatidia morphogenesis. Development. 2012;139:3432–3441. doi: 10.1242/dev.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unbekandt M, et al. A novel small-molecule MRCK inhibitor blocks cancer cell invasion. Cell Commun Signal. 2014;12:54. doi: 10.1186/s12964-014-0054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bond LM, Tumbarello DA, Kendrick-Jones J, Buss F. Small-molecule inhibitors of myosin proteins. Future Med Chem. 2013;5:41–52. doi: 10.4155/fmc.12.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoege C, Hyman AA. Principles of PAR polarity in Caenorhabditis elegans embryos. Nat Rev Mol Cell Biol. 2013;14:315–322. doi: 10.1038/nrm3558. [DOI] [PubMed] [Google Scholar]
- 33.Zihni C, Balda MS, Matter K. Signalling at tight junctions during epithelial differentiation and microbial pathogenesis. J Cell Sci. 2014;127:3401–3413. doi: 10.1242/jcs.145029. [DOI] [PubMed] [Google Scholar]
- 34.Viswanatha R, Bretscher A, Garbett D. Dynamics of ezrin and EBP50 in regulating microvilli on the apical aspect of epithelial cells. Biochem Soc Trans. 2014;42:189–194. doi: 10.1042/BST20130263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viswanatha R, Ohouo PY, Smolka MB, Bretscher A. Local phosphocycling mediated by LOK/SLK restricts ezrin function to the apical aspect of epithelial cells. J Cell Biol. 2012;199:969–984. doi: 10.1083/jcb.201207047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escudero LM, Bischoff M, Freeman M. Myosin II regulates complex cellular arrangement and epithelial architecture in Drosophila. Dev Cell. 2007;13:717–729. doi: 10.1016/j.devcel.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Corrigall D, Walther RF, Rodriguez L, Fichelson P, Pichaud F. Hedgehog signaling is a principal inducer of Myosin-II-driven cell ingression in Drosophila epithelia. Dev Cell. 2007;13:730–742. doi: 10.1016/j.devcel.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Matter K, Brauchbar M, Bucher K, Hauri HP. Sorting of endogenous plasma membrane proteins occurs from two sites in cultured human intestinal epithelial cells (Caco-2) Cell. 1990;60:429–437. doi: 10.1016/0092-8674(90)90594-5. [DOI] [PubMed] [Google Scholar]
- 39.Matter K, Hunziker W, Mellman I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992;71:741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- 40.Carlton JG, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 2007;316:1908–1912. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- 41.Benais-Pont G, et al. Identification of a tight junction-associated guanine nucleotide exchange factor that activates Rho and regulates paracellular permeability. J Cell Biol. 2003;160:729–740. doi: 10.1083/jcb.200211047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol. 2003;160:423–432. doi: 10.1083/jcb.200210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sourisseau T, et al. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol Cell Biol. 2006;26:2387–2398. doi: 10.1128/MCB.26.6.2387-2398.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshizaki H, et al. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J Cell Biol. 2003;162:223–232. doi: 10.1083/jcb.200212049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stowers RS, Schwarz TL. A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics. 1999;152:1631–1639. doi: 10.1093/genetics/152.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 47.Winter CG, et al. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 48.Walther RF, Pichaud F. Immunofluorescent staining and imaging of the pupal and adult Drosophila visual system. Nat Protoc. 2006;1:2635–2642. doi: 10.1038/nprot.2006.379. [DOI] [PubMed] [Google Scholar]
- 49.Karagiosis SA, Ready DF. Moesin contributes an essential structural role in Drosophila photoreceptor morphogenesis. Development. 2004;131:725–732. doi: 10.1242/dev.00976. [DOI] [PubMed] [Google Scholar]
- 50.Pinal N, et al. Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Curr Biol. 2006;16:140–149. doi: 10.1016/j.cub.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 51.Crawley SW, Mooseker MS, Tyska MJ. Shaping the intestinal brush border. J Cell Biol. 2014;207:441–451. doi: 10.1083/jcb.201407015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the conclusions here are available from the authors on reasonable request.