Abstract
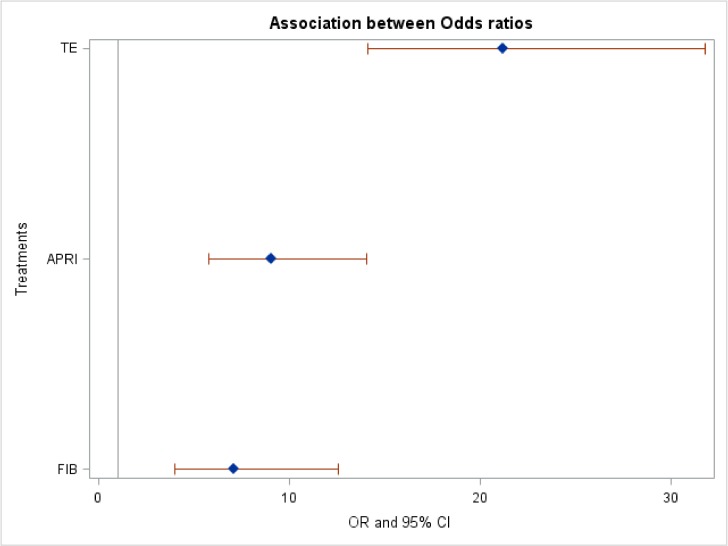
Recurrent fibrosis after liver transplantation (LT) impacts on long-term graft and patient survival. We performed a meta-analysis to compare the accuracy of non-invasive methods to diagnose significant recurrent fibrosis (stage F2-F4) following LT. Studies comparing serum fibrosis biomarkers, namely AST-to-platelet ratio index (APRI), fibrosis score 4 (FIB-4), or transient elastography (TE) with liver biopsy in LT recipients were systematically identified through electronic databases. In the meta-analysis, we calculated the weighted pooled odds ratio and used a fixed effect model, as there was no significant heterogeneity between studies. Eight studies were included for APRI, four for FIB-4, and twelve for TE. The mean prevalence of significant liver fibrosis was 37.4%. The summary odds ratio was significantly higher for TE (21.17, 95% CI confidence interval 14.10–31.77, p = 1X10-30) as compared to APRI (9.02, 95% CI 5.79–14.07; p = 1X10-30) and FIB-4 (7.08, 95% CI 4.00–12.55; p = 1.93X10-11). In conclusion, TE performs best to diagnose recurrent fibrosis in LT recipients. APRI and FIB-4 can be used as an estimate of significant fibrosis at centres where TE is not available. Longitudinal assessment of fibrosis by means of these non-invasive tests may reduce the need for liver biopsy.
Introduction
The development of hepatic fibrosis adversely affects the prognosis of any chronic liver disease, regardless of etiology. In the specific setting of liver transplantation (LT), recurrent liver fibrosis portends worse graft survival and need for re-transplantation, thus impacting on overall survival [1–3]. LT recipients may have multiple cofactors leading to liver fibrosis recurrence. Patients who undergo LT for hepatitis C virus (HCV) infection have universal recurrence, with development of cirrhosis in up to 30% by 5 years[4–6]. The landscape of antiviral therapy for hepatitis C has been dynamic, and has led to highly effective active, interferon-free regimens[7]. However, concerns about costs and availability in the post-transplant setting may limit the widespread use of interferon-free regimens, which highlights the need for prioritization of patients for therapy. The current AASLD/IDSA guidelines recommend that all patients with HCV be treated following LT[7]. Recurrent or de novo nonalcoholic fatty liver disease (NAFLD) also affects more than 20% of patients, given the high rate of metabolic syndrome triggered by rapid weight gain and immunosuppressive medications following LT[8, 9]. Various other factors, such as recipient age, donor age, type of immunosuppression, and cytomegalovirus (CMV) viremia increase the risk of recurrent fibrosis after LT[10].
Early identification of fibrosis recurrence in LT recipients is of paramount importance to permit risk stratification, ascertain prognosis and thereby provide targeted interventions and management readjustment. Liver biopsy has long been the gold standard to stage liver fibrosis. Annual protocol liver biopsies after LT were traditionally performed for hepatitis C[11], and there are certain conditions such as autoimmune hepatitis where they are considered. However, liver biopsy is impractical as a screening tool and for serial monitoring because of its invasiveness, cost and potential for sampling error[12, 13]. These are important considerations in the LT patient population, where longitudinal follow-up of recurrent fibrosis is required.
Serum biomarkers based on readily available parameters have been proposed to stage liver fibrosis in the pre-LT population, including the fibrosis score 4 (FIB-4) and aspartate aminotransferase (AST)-to-Platelet ratio index (APRI). Whereas the APRI is calculated on the basis of AST and platelet values, the FIB-4 score additionally includes age and alanine aminotransferase. An additional non-invasive tool is transient elastography (TE) (Fibroscan®), whose measurement of liver stiffness is proportional to the severity of fibrosis[3, 14, 15]. Although non-invasive methods do not enable distinction between single fibrosis stages(18), they have demonstrated good accuracy in detecting significant liver fibrosis, allowing for repeat assessment over time. Moreover, they also portend prognosis, by identifying patients at risk for liver-related complications such as death and need for LT[1, 2, 16]. As such, the routine incorporation of these non-invasive tests into the post-LT clinical setting could potentially facilitate screening and serial monitoring, thus identifying those patients in need of more immediate intervention.
APRI, FIB-4 and TE have been extensively validated in the pre-transplant clinical setting, where they have been recommended for clinical use by guidelines[17]. To our knowledge, there has been no meta-analysis of non-invasive diagnostic tests for liver fibrosis due to all etiologies of liver disease in the post-LT setting.
The objective of this study was to perform a meta-analysis of the diagnostic accuracy of simple serum biomarkers, namely APRI and FIB-4, and TE for the prediction of recurrent liver fibrosis in the post-LT setting. Although other non-invasive fibrosis measures have been studied in LT recipients, we focused on these tests due to their more extensive validation and widespread use.
Materials and methods
Literature search
A systematic literature search from 2003 to May 2017 was performed which included the following sources:
Electronic databases: Pubmed, MEDLINE, EMBASE and Cochrane library databases were systematically searched for original articles and abstracts.
Relevant websites and conference abstract books: American Association for the Study of the Liver, International Liver Transplant Society, European Association for the Study of the Liver, American Transplant Congress, Digestive Diseases Week, Asian Pacific Association for the Study of the Liver were searched for conference proceedings and abstracts.
Manual review of reference lists of relevant articles.
A combination of relevant text words and MeSH terms were applied, namely liver transplantation AND one of the following: fibrosis, non-invasive fibrosis markers, serum fibrosis markers, APRI, FIB-4, TE, and liver stiffness measurement. For management of the searched literature, Endnote version X7 (Thomson Reuters, New York, NY) was used. We chose these three non-invasive tests as they are widely used and available, as well as the literature search revealed more studies than other scores. For example, there has been limited evaluation of the Fibrotest in the detection of significant fibrosis after LT, with only 2 prospective studies comprising 82 patients, both of which reported a low diagnostic accuracy[18, 19]. Another patented non-invasive test, the Enhanced Liver Fibrosis (ELF) Score has been evaluated in two prospective studies comprising 113 patients with all etiologies of liver disease as indication for LT, with variable accuracy reported[19, 20].
Inclusion criteria
Studies were included if they met the following criteria: they evaluated APRI, FIB-4 or TE; they used liver biopsy as the reference standard; they employed comparable histologic staging systems: METAVIR, Ishak, Brunt, Ludwig, Knodell; they evaluated the diagnostic accuracy of the test expressed as area under the curve (AUC) for significant liver fibrosis (stage F2-4); and/or they evaluated sensitivity, specificity, positive predictive value (PPV) or negative predictive value (NPV) for the diagnosis of significant liver fibrosis based on cut-off values for APRI, FIB-4 or TE. Both adult and pediatric studies were included.
Exclusion criteria
Studies were excluded if they met the following criteria: they were not conducted in the post-LT setting; they did not use liver biopsy as the standard of reference; they did not report data on diagnostic accuracy (AUC), sensitivity, specificity, PPV or NPV for the diagnosis of significant liver fibrosis based on cut-off values for APRI, FIB-4 or TE; they were review articles, letters to the editor or editorials not reporting own original data; they were abstracts presenting data from the same study at different meetings.
Data extraction
One reviewer (M.B.) searched the various databases listed above, and identified pertinent studies (both articles and abstracts) for further assessment. Both reviewers (M.B. and G.S.) reviewed the abstracts and full text versions of the articles independently, evaluating study eligibility, grading study quality, and extracting data. We took note of study design, number of participants, non-invasive tool adopted among APRI, FIB-4 and TE. From the selected studies, we extracted the following data: study characteristics such as authors, year of publication, hospital or medical school and study design; demographic and clinical characteristics of participants; characteristics of liver biopsy; non-invasive method adopted among APRI, FIB-4 and TE; correlation with liver biopsy staging of fibrosis as data categories and relative cut-off value of the non-invasive test. In case of disagreement regarding the selected studies or data appropriate for extraction, studies were refereed by a third reviewer (M.T.) and resolved by consensus. Methodological quality was assessed using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) Score[21]. The primary outcome was the identification of significant fibrosis, defined as stage F2–4 according to the METAVIR staging system. This threshold was chosen because it indicates a progressive liver disease eventually leading to cirrhosis and requiring interventions, such as antiviral therapy (hepatitis C) and life style modifications (NAFLD)[7, 22]. In all the included studies, APRI and FIB-4 were computed as per original formulas: APRI as [100 x (AST/upper limit of normality)/platelet count (109 /L)[23]; FIB-4 as age (years) x AST /platelet count (109 /L)] x ALT1/2[24].
Data analysis
We estimated the diagnostic odds ratios and AUC with their corresponding 95% confidence intervals (95% CIs) for each study based on the diagnostic tests. In the meta-analysis we calculated the weighted pooled odds ratio. The heterogeneity statistics calculated the summary odds ratio under random effects model[25]. The summary receiving operating characteristics (ROC) curves (SROC) shows the overall diagnostic test including each study’s test and the sample size of each study.
Pooled measures for diagnostic performance, such as sensitivity, specificity, diagnostic odds ratios (DORs) with their corresponding 95% confidence intervals (95% CIs), and AUC were calculated. The DORs combine sensitivity and specificity into one measure for diagnostic performance. A DOR of 1 means that the test has no ability to discriminate between two outcomes. In the context of this study, the higher the DOR, the better the diagnostic accuracy of the non-invasive test for assessing significant liver fibrosis.
The forest plot was graphed for odds ratios, as a summary and for individual studies. A Chi-square-based test of homogeneity was performed, and the Cochran's Q inconsistency index (I2) statistics were determined. The heterogeneity between studies was calculated by I2 values. Publication bias was assessed by constructing funnel plots for DOR by Egger's test[26]. The absence of publication bias was indicated by the data points forming a symmetric funnel-shaped distribution and significance level p>0.05. We used the SAS statistical software 9.4 (SAS Institute, Cary, NC) to perform the statistical analysis at p<0.05 level of significant test and R program for SROC.
Sensitivity analyses
Sensitivity analyses were employed to examine the impact of the following factors on test performance for identifying significant liver fibrosis: study quality; prevalence of significant fibrosis; and quality of the reference standard for assessing fibrosis. The reference standard was deemed adequate if a study excluded liver biopsies <10 mm. If sufficient data were not reported in the manuscript, the reference standard was considered inadequate.
Results
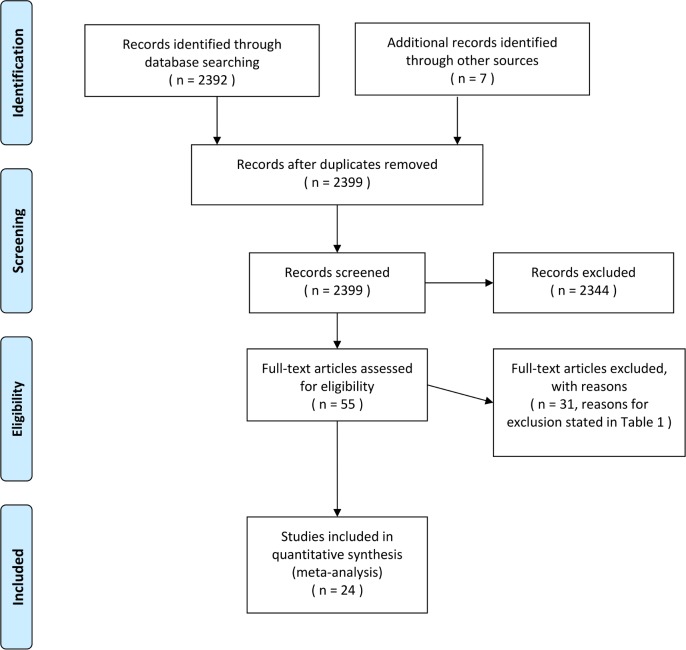
The literature search by the reviewers resulted in 2399 studies identified with our search terms (S1–S12 Files). Of these, 55 included original data. 48 were full-text manuscripts, and 7 studies were available in abstract form only. The systematic literature search and study selection are depicted in the flowchart of Fig 1. Ultimately, studies were excluded as they were not pertinent to non-invasive evaluation of fibrosis after LT, due to insufficient data and review articles/commentary. A total of 171 studies described TE, APRI or FIB-4, of which 33 included original data. 33 studies compared TE, APRI or FIB-4 versus liver biopsy in LT recipients. Finally, 24 studies were included in the meta-analysis. The studies excluded from the meta-analysis and reasons for exclusion are provided in Table 1. Our final data set for the meta-analysis included 8 studies assessing APRI, 4 assessing FIB-4 and 12 assessing TE (Table 2).
Fig 1. Flow diagram of the search strategy.
Table 1. Excluded studies and reasons for exclusion.
| Non-invasive test | Author, Year | Reason for exclusion |
|---|---|---|
| TE | Della Guardia, 2013 | Insufficient data |
| TE | Bellido-Munoz, 2012 | Insufficient data |
| TE | Adebajo et al, 2012 | Meta-analysis |
| TE | Vinciguerra et al, 2011 | Insufficient data |
| TE | Kamphues et al., 2010 | Insufficient data |
| TE | Masuzaki et al., 2009 | Insufficient data |
| TE | Harada et al., 2008 | Insufficient data |
| APRI, FIB-4 | Kitajima et al, 2016 | Insufficient data |
| APRI, FIB-4 | Sheen et al, 2016 | Insufficient data, and used F3-F4 as significant fibrosis |
| APRI | Carrion et al., 2010 | Insufficient data |
| APRI | Corradi et al., 2009 | Insufficient data |
Legend: TE: transient elastography, APRI: AST-to-platelet ratio index, FIB-4: Fibrosis score 4.
Table 2. Characteristics of the studies included in our meta-analysis of non-invasive tests to evaluate significant fibrosis after liver transplantation.
| Non-invasive test | Author/ Year | Study design | N | Time after LT (months) | Median/ mean age (male) | Etiology | Mean length biopsy, (interval biopsy & test) | Prevalence F2-4 (%) | QUADAS score |
|---|---|---|---|---|---|---|---|---|---|
| TE | Della-Guardia, 2017[36] | Prospective | 267 | 31.7 | 56 (67.7%) | 74.9% HCV, and others | At least 10 mm long or 10 portal tracts, mean not provided (same day) | 46.1 | 9 |
| TE | Crespo, 2016[37] | Retrospective | 64 | 12 | 59 (73.6%) | HCV | NA (same day) | 33 | 9 |
| TE | Mikołajczyk-Korniak, 2016[38] | Prospective | 36 | 29.2 | 54.6 | HCV | 20.9 mm (2.9 months) | 80.5 | 13 |
| TE | Lutz, 2016[39] | Prospective | 48 | 23±2 | 54.4 (68.8%) | 27.1% HCV, and others | NA (within 24 hours) | 27.1 | 12 |
| TE | Barrault, 2013 | Prospective | 43 | 55.8±4.9 | 58 yr (74.4%) | HCV, alcohol, other | 25.1 mm (<2 months) |
37.7 | 12 |
| TE | Goldsmith, 2013 | Prospective | 26 | 36 (1–176) |
5.6 yr (51.3%) | Biliary atresia, α1antitripsin, Wilson, other | NA (<6 months) |
NA | 12 |
| TE | Crespo, 2012 | Prospective | 87 | 43 | 60 yr (69%) | HCV, NAFLD, alcohol, cholestasis, other | 20 mm (<15 days) |
42 | 14 |
| TE | Sebagh, 2012 | Prospective | 91 | 240 | 37.3 yr (45%) |
HBV, HCV, alcohol, other | 28.2 ± 8.6 mm (same day) |
40.4 | 13 |
| TE | Beckebaum, 2010 | Prospective | 157 | 80.2±65.7 | 52.5 yr (61.1%) | HCV | At least 15 mm long (same day) | 58.6 | 13 |
| TE | Carrion, 2006 | Prospective | 124 | 24 (5–120) |
60 yr (66%) | HCV | NA (<2 weeks) |
43 | 13 |
| APRI, TE | Corradi, 2009 | Prospective | 56 | NA | 58 yr (83.9%) | HCV | 29 mm (<40 days) |
32 | 13 |
| TE | Rigamonti, 2008 | Prospective | 95 | 35 (6–156) |
54 yr (81%) | HCV | 32 mm (same day) |
NA | 14 |
| APRI, FIB-4 | Crespo, 2016[37] | Retrospective | 72 | 12 | 59 (73.6%) | HCV | NA (same day) | 33 | 9 |
| APRI | D’Souza, 2016 | Retrospective | 39 | 124.68 | 12.52 (51.2%) | Extrahepatic biliary cirrhosis, other |
NA (same day) | 38.5 | 9 |
| APRI | Pinto, 2014[40] | Prospective | 30 | 60 | 11 (63%) | Biliary atresia, metabolic disease, α1antitripsin, Wilson, other | NA (<4 months) |
20 | 9 |
| APRI, FIB-4, TE |
Kamphues, 2010[27] | Prospective | 94 | 80.6 | 51.7 (64.9%) | HCV | 1.5 cm (same day) |
68.1 | 13 |
| APRI, FIB-4 |
Pissaia, 2009[41] | Retrospective | 501 | 30.7 | 49.6 (62%) | All etiologies | NA (same day) | 28 | 9 |
| APRI | Harada, 2008[28] | Prospective | 56 | 25.6 | 63.1 (53.6%) | HCV | 15 mm (same day) |
37.5 | 12 |
| APRI | Toniutto, 2007[42] | Prospective | 512 | 24 | 56 (60.8%) | HCV | NA (same day) |
32.4 | 11 |
| FIB-4 | Segovia, 2008[43] | Retrospective | 219 | NA | 52.3 (68.95%) | All etiologies | NA (same day) | 29.9 | 10 |
Legend: TE: transient elastography, APRI: AST-to-platelet ratio index, FIB-4: Fibrosis score 4; NA, not available.
194 biopsies
2102 biopsies.
Characteristics of the included studies
We included eight studies investigating the ability of APRI to diagnose significant fibrosis (F2-4). These studies included a total of 448 LT recipients, of whom 30 were children. Five out of the eight studies focused on HCV patients, who comprised 329 out of the 448 patients. The overall prevalence of significant liver fibrosis was 36.2% (range 20–68.1%). Biopsy quality was considered acceptable in three of these studies[18, 27, 28]. None of the studies included patients with acute rejection.
A total of 435 patients were included in the four FIB-4 studies. The overall prevalence of significant liver fibrosis was 39.8% (range 28–68.1%). Two of these studies comprised only HCV-infected patients, while two included patients of mixed etiology of liver disease. Biopsy quality was considered acceptable in one of these studies[27].
In the twelve TE studies, a total of 1,196 LT recipients were included. The overall prevalence of significant liver fibrosis was 37.4% (range 27.1–68.1%). Six of these studies comprised only HCV-infected patients, while six included patients of mixed etiology of liver disease. Biopsy quality was considered acceptable in seven of these studies[18, 20, 27, 29, 30].
According to the QUADAS scale, the methodological quality of the included studies was good. Two studies met all 14 requirements of this scale, four studies met all but one of these requirements.
Diagnostic accuracy of APRI
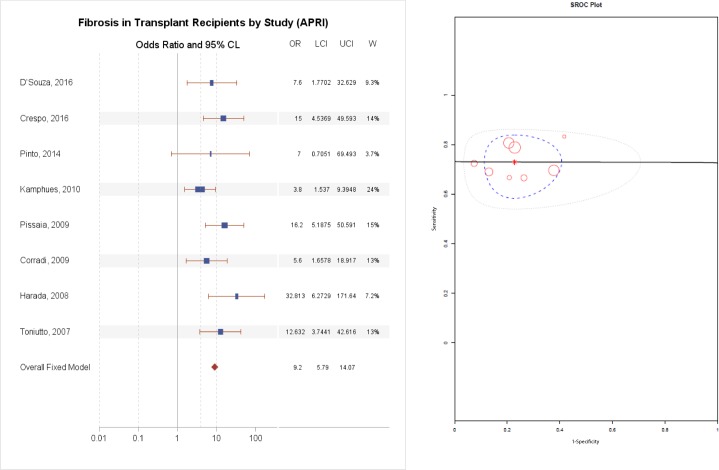
The AUC for significant fibrosis ranged from a low of 0.50 to a high of 0.83 for HCV, as reported in Table 3. The APRI test fared better in the studies of patients with all etiologies, with an AUC ranging from 0.74 to 0.87. As illustrated in Fig 2, the summary odds ratio for APRI was 9.20 (95% CI 5.79–14.07, p = 1X10-30).
Table 3. Diagnostic accuracy of non-invasive tests to predict fibrosis F2-4 in individual studies included in the meta-analysis.
| Author, Year | Test | Cut-off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC (95% CI) |
|---|---|---|---|---|---|---|---|
| Della-Guardia, 2017 | TE | 12.3 kPa |
43 | 91 | 72 | 76 | 0.78 (0.71–0.85) |
| Crespo, 2016 | TE | 6.8 kPa | 73 | 71 | 57 | 83 | 0.76 (0.64–0.88) |
| Mikołajczyk-Korniak, 2016[38] | TE | 4.7 kPa | 93 | 57 | 90 | 66 | 0.746 (0.53–0.95) |
| Lutz, 2016 | TE | 8.35 kPa | 84.6 | 91.4 | 0.89+/-0.057 | ||
| Barrault, 2013 | TE | 7 kPa | 88.2 | 67.9 | 62.5 | 90.5 | 0.83 (0.71–0.95) |
| Goldsmith, 2013 | TE | 9.3 kPa | 90 | 81 | 75 | 93 | 0.93 (NA) |
| Crespo, 2012 | TE | 8.4 kPa | 82 | 80 | 76 | 86 | 0.87 (NA) |
| Sebagh, 2012 | TE | 7.9 kPa | 62.5 | 66.7 | 45 | 80 | NA |
| Beckebaum, 2010 | TE | 7.3 kPa | 73 | 100 | 100 | 52 | 0.86 |
| Corradi, 2009 | TE | 10.1 kPa | 94 | 89 | 81 | 94 | 0.94 (0.85–0.99) |
| Harada, 2008 | TE | 9.9 kPa | 90 | 91 | 86 | 94 | 0.92 (NA) |
| Rigamonti, 2008 | TE | 7.9 kPa | 81 | 76 | 65 | 88 | 0.85 (0.76–0.92) |
| Carrion, 2006 | TE | 8.5 kPa | 90 | 81 | 79 | 92 | 0.90 (NA) |
| D’Souza, 2016 | APRI | 0.45 | 67 | 79 | 67 | 79 | 0.74 (0.57–0.91) |
| Crespo, 2016 | APRI | 1.36 | 69 | 87 | 75 | 83 | 0.83 (0.73–0.94) |
| Pinto, 2014 | APRI | 0.4 | 83 | 58 | 31 | 94 | 0.74 (0.54–0.95) |
| Kamphues, 2010 | APRI | 0.4845 | 70 | 63 | 80 | 80 | 0.68 (NA) |
| Carrion, 2010 | APRI | - | - | - | - | - | 0.50 |
| Pissaia, 2009 | APRI | 0.5 | 81 | 80 | 62 | 91 | 0.87 (NA) |
| Corradi, 2010 | APRI | 1.3 | 67 | 74 | 55 | 82 | 0.82 (0.64–0.99) |
| Harada, 2008 | APRI | 0.84 | 73 | 91 | 63 | 76 | 0.70 (NA) |
| Toniutto, 2007 | APRI | 1.4 | 76 | 77 | 46 | 93 | 0.80 (NA) |
| Crespo, 2016 | FIB-4 | 3.23 | 77 | 80 | 69 | 86 | 0.81 (0.70–0.92) |
| Kamphues, 2010 | FIB-4 | 2.8 | 44 | 87 | 88 | 42 | 0.66 (NA) |
| Pissaia, 2009 | FIB-4 | 3.25 | 31 | 94 | 67 | 77 | 0.78 (NA) |
| Segovia, 2008 | FIB-4 | 4.09 | 80 | 60 | 25 | 94 | NA |
Legend: TE: transient elastography, APRI: AST-to-platelet ratio index, FIB-4: Fibrosis score 4; NA, not available.
Fig 2.
(A) Diagnostic accuracy of APRI for the prediction of F2–4 fibrosis, (B) Summary Receiver Operating Curve.
Diagnostic accuracy of FIB-4
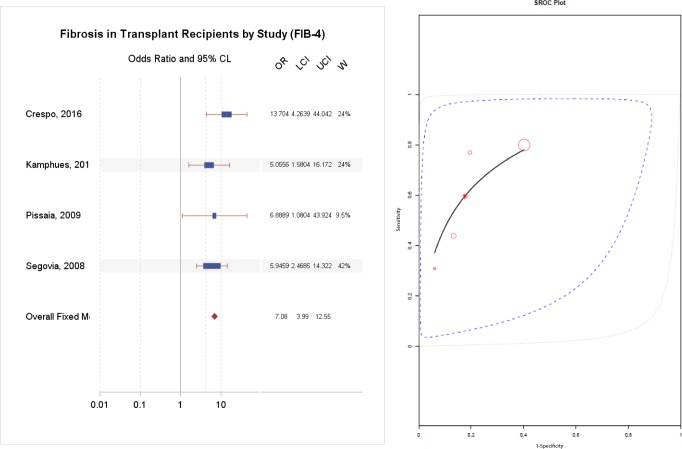
The AUC for FIB-4 ranged from 0.66 to 0.81 for HCV and an AUC of 0.78 for mixed etiologies, as reported in Table 3. As demonstrated in Fig 3, the summary odds ratio for FIB-4 was 7.08 (95% CI 4.00–12.55, p = 1.93X10-11).
Fig 3.
(A) Diagnostic accuracy of FIB-4 for the prediction of F2-4 fibrosis, (B) Summary Receiver Operating Curve.
Diagnostic accuracy of TE
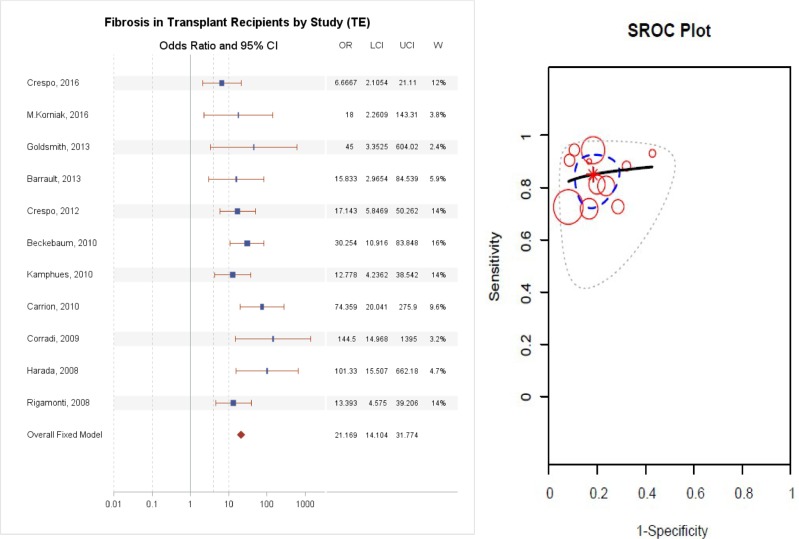
TE detected significant fibrosis with an AUC ranging from 0.75 to 0.96, as reported in Table 3. Two small prospective pediatric studies comprising 50 patients with various indications for LT revealed different results: the study of 24 patients negative for viral hepatitis had an AUC of 0.71, whereas a study of 26 patients with all etiologies had an excellent AUC of 0.93. As illustrated in Fig 4, the summary odds ratio for TE was the best, at 21.17 (95% CI 14.10–31.77, p = 1X10-30), having excluded one study due to publication bias as elucidated below. A comparison of diagnostic accuracy of the three tests is illustrated in Fig 5. There was no significant difference between APRI and FIB4 (p>0.05), but there was a significant difference between TE and FIB4 (p<0.05) and between TE and APRI (P<0.05).
Fig 4.
(A) Diagnostic accuracy of Fibroscan for the prediction of F2-4 fibrosis, (B) Summary Receiver Operating Curve.
Fig 5. Direct comparison of diagnostic accuracy of non-invasive tests.
There was no significant difference between APRI and FIB-4 (P>0.05), but there was a significant difference between TE and FIB-4 (P<0.05) and between TE and APRI (P<0.05).
Publication bias
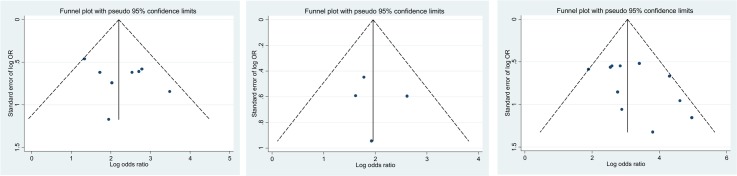
Funnel plots of diagnostic accuracy versus effective sample size for the prediction of significant liver fibrosis are illustrated in Fig 6. For APRI and FIB-4 (Fig 6A and 6B), the funnel plot did not detect a significant publication bias (p>0.05). For TE (Fig 6C), there was evidence of publication bias with a single study. The Egger test for the remaining 11 studies showed no evidence of publication bias (p>0.05). The sensitivity analysis including study quality, prevalence of significant fibrosis, and quality of the reference standard, did not explain this asymmetry.
Fig 6.
Funnel plots demonstrating the accuracy of the APRI (A), FIB-4 (B) and Fibroscan (C) versus the inverse of the square root of the effective sample size (ESS) for the prediction of F2–4 fibrosis. We derived the funnel plot and Egger test to the accuracy of meta-analysis inferences. Continuous lines represent the overall estimates of test accuracy. The Egger test did not detect a significant publication bias (p>0.05). For Fibroscan, the Egger test shows a borderline significant p-value (p = 0.0535) for Fibroscan data, which is driven by an outlier, the study by Carrion et al (2006).
Discussion
In this meta-analysis, we have evaluated the diagnostic accuracy of three non-invasive diagnostic tests to stage liver fibrosis in the post-LT setting. The identification of significant liver fibrosis is pivotal since it is a clear indication of recurrence of the primary disease (e.g. hepatitis C) or the occurrence of de novo liver disease (e.g. NAFLD) triggered by multiple risk factors related to immunosuppression or metabolic disorders which occur frequently after LT. Our data indicate that TE has good accuracy in detecting significant liver fibrosis in LT recipients and outperforms simple serum biomarkers, such as APRI and FIB-4. Our work has the strength of including all etiologies of liver disease and comparing TE with simple serum biomarkers. To our knowledge, there is only a single systematic review that included TE, APRI and FIB-4, however it was conducted only in HCV-positive recipients[31].
The development and validation of non-invasive diagnostic methods to diagnose liver fibrosis has gained much attention in recent years. These efforts have proceeded in parallel with increased evidence of the limitations and drawbacks of liver biopsy. Indeed, liver biopsy is an invasive and costly procedure, which could potentially lead to serious complications including bleeding and pneumothorax[17]. Moreover, there are intrinsic limitations in histological determination. Indeed, the quality and size of the specimen obtained through a percutaneous liver biopsy greatly affect the diagnostic reliability. Intra-observer and inter-observer variability have also been extensively studied. It has been suggested that liver biopsy is the best available standard of reference for fibrosis evaluation, although an imperfect gold standard[32].
Non-invasive methods for liver fibrosis have been extensively validated for the main etiologies of chronic liver diseases in the pre-transplant setting. TE and serum fibrosis biomarkers are the most validated tests and are recommended by recent guidelines. The recently endorsed guidelines of the European Association for the Study of the Liver (EASL) on the non-invasive diagnosis of liver fibrosis suggest that non-invasive methods could substitute liver biopsy when combined in the pre-transplant setting[17]. However, fewer data are available in LT recipients. After careful selection of available studies through a quality control check, we included 24 studies evaluating the performance of non-invasive diagnostic tools for liver fibrosis against liver histology. Although some of them included only patients with HCV, several included mixed etiologies of liver disease, such as alcoholic liver disease, NAFLD, cholestasis. There have been few studies employing other non-invasive tools, such as NAFLD fibrosis score, Magnetic Resonance Elastography (MRE) or Acoustic Radiation Force Impulse imaging (ARFI). However, we excluded those non-invasive tests either for poor quality of the studies or for the limited number of studies conducted, insufficient to perform a meta-analysis.
The primary outcome of our meta-analysis was the identification of significant liver fibrosis (stage F2-4), which indicates a progressive liver fibrosis that will eventually lead to liver cirrhosis if appropriate interventions are not implemented. In the specific setting of LT, significant liver fibrosis may mean both recurrence of primary liver disease (hepatitis C, NASH) or a de novo liver disease. In any case, it is a clear signal that further investigations are needed. Only a previous meta-analysis reported APRI’s performance in LT recipients and this was superior to other simple non-invasive diagnostic tests[31]. In our experience, there was no significant difference between the two simple fibrosis biomarkers APRI and FIB-4 in terms of diagnostic performance. Importantly, we did not find a significant publication bias in the studies including these two biomarkers, and this was confirmed by absence of statistically significant asymmetry on the funnel plots. APRI and FIB-4 have been employed serially in LT recipients not only for diagnostic purposes, but also for prognosis. The fact that both biomarkers and their longitudinal changes predict long-term outcomes in the recipient, including death and graft loss, confirms their pathophysiologic link with liver damage[33]. A major issue for both APRI and FIB-4 remains the wide range of cut-off values adopted in different studies to diagnose significant liver fibrosis, which may limit their applicability in clinical practice. Patented panels of fibrosis biomarkers, such as Fibrotest and ELF, have not been included in the present meta-analysis. Indeed, there are only a few studies which investigated the diagnostic accuracy of these tests in LT and they reported an unsatisfactory performance. This could be due to suboptimal adherence of the laboratory to pre-analytic recommendations to obtain reliable results[34] or on specific alterations of the individual components of the patented panels that could occur following LT due to chronic inflammation (elevation of alpha-2-macroglobulin, hyaluronan), hemolytic anemia (haptoglobin), multidrug induction (GGT) or cholestasis (bilirubin).
TE had the highest number of included studies among the three non-invasive methods we evaluated. In the pre-transplant setting, TE has been shown to outperform simple serum biomarkers to diagnose liver cirrhosis[17]. This gap in diagnostic accuracy is smaller for the detection of significant liver fibrosis. In the present study, we found that TE had higher diagnostic accuracy than APRI and FIB-4 to detect significant liver fibrosis in the post-transplant setting. Diagnostic accuracy was consistent in both HCV patients and other etiologies of liver disease. There was a tendency for a significant publication bias for TE, which was driven by only one study. However, we were not able to find any predictor of this marginally significant asymmetry with the sensitivity analysis. Interestingly, there have been several studies investigating the use of TE for serial assessment in LT recipients in order to identify liver fibrosis progression. On this topic, Rigamonti et al conducted two studies, one in patients transplanted for HCV and one in those transplanted for non-viral etiologies[30, 35]. In 95 patients transplanted for end-stage liver disease due to HCV and undergoing serial liver biopsies, the authors found an AUC of 0.85 for significant liver fibrosis, concluding that protocol liver biopsy could be avoided in patients with stable TE during follow-up. Along the same lines, in 69 LT recipients the same group found that TE allow accurate discrimination between presence or absence of liver graft damage, thus helping the selection of patients most in need of liver biopsy.
The EASL guidelines on non-invasive evaluation of liver fibrosis suggest combining TE with a serum biomarker in order to reduce the number of liver biopsies needed for correct clinical management[17]. The avoidance of a liver biopsy becomes a more complicated matter when dealing with the post-transplant setting. Indeed, in LT recipients, it is not only liver fibrosis stage that may cause graft damage, but also multiple liver pathologies that arise from the complex interaction between primary liver disease recurrence (hepatitis C, NAFLD), development of metabolic syndrome in the context of immunosuppression (calcineurin and mTOR inhibitors), and possible acute or chronic rejection. However, given the good accuracy we found in this meta-analysis, especially for TE, we feel that non-invasive methods for liver fibrosis could be used to reduce the number of liver biopsies in LT recipients. We acknowledge that non-invasive methods should always be used having carefully ascertained the clinical context and correlated with liver biochemistry and function tests.
Our study has certain limitations, including the higher proportion of HCV patients represented. We did also include pediatric studies, with various reasons for development of fibrosis post-transplant. Finally, the reported range of cut-off values for TE is wide (from 7.3 kPa to 12.3 kPa) in LT recipients, rendering potentially complicated the use in clinical practice for the individual patients. This finding is likely due to highly heterogeneous study populations due to etiology of liver disease, different post-LT time points for the assessment of liver fibrosis, study design and sample size and variable interval range between liver biopsy and the non-invasive assessment of liver fibrosis. We could not account for these different cut-offs for transient elastography for the various etiologies of CLD in our meta-analysis, as these were not provided in the studies. Nonetheless, the validity of our findings across this heterogeneous population suggests the usefulness of these non-invasive tools for fibrosis even in a complex context such as that of LT.
Conclusions
In conclusion, given non-invasiveness and feasibility for serial measurements, non-invasive tests for liver fibrosis could be used in the post-transplant clinical setting as an additive tool for suspected recurrent or de novo liver disease. The high accuracy we found in our meta-analysis, especially for TE, suggests that these tests have similar diagnostic value as in the pre-transplant setting. It should be underlined that liver biopsy remains a cornerstone for the clinical management of LT recipients, as non-invasive tests cannot differentiate between different liver pathologies that can coexist in this setting, such as acute or chronic rejection. Nonetheless, when the etiology of recurrent or de novo disease has been established, these non-invasive tests are helpful in following fibrosis progression longitudinally and implementing preventive therapies in a timely manner. Further studies aimed at defining optimal cut-off values for definition of significant liver fibrosis are warranted.
Supporting information
(DOC)
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
(DOCX)
Abbreviations
- LT
Liver transplantation
- APRI
AST-to-platelet ratio index
- FIB-4
Fibrosis score 4
- TE
Transient elastography
- AUC
Area under the curve
- CI
Confidence interval
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
GS holds a Chercheur-Boursier career award from the Fonds de recherche du Québec –Santé (FRQ-S). MB is the recipient of a Canadian Institutes of Health Research (CIHR) Fellowship for Health Professionals.
References
- 1.Vergniol J, Foucher J, Terrebonne E, Bernard PH, le Bail B, Merrouche W, et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology. 2011;140(7):1970–9, 9 e1-3. Epub 2011/03/08. doi: 10.1053/j.gastro.2011.02.058 . [DOI] [PubMed] [Google Scholar]
- 2.Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145(4):782–9 e4. Epub 2013/07/19. doi: 10.1053/j.gastro.2013.06.057 ; PubMed Central PMCID: PMC3931256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crespo G, Lens S, Gambato M, Carrion JA, Marino Z, Londono MC, et al. Liver stiffness 1 year after transplantation predicts clinical outcomes in patients with recurrent hepatitis C. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(2):375–83. Epub 2014/01/15. doi: 10.1111/ajt.12594 . [DOI] [PubMed] [Google Scholar]
- 4.Firpi RJ, Clark V, Soldevila-Pico C, Morelli G, Cabrera R, Levy C, et al. The natural history of hepatitis C cirrhosis after liver transplantation. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2009;15(9):1063–71. Epub 2009/09/01. doi: 10.1002/lt.21784 . [DOI] [PubMed] [Google Scholar]
- 5.Neumann UP, Berg T, Bahra M, Seehofer D, Langrehr JM, Neuhaus R, et al. Fibrosis progression after liver transplantation in patients with recurrent hepatitis C. Journal of hepatology. 2004;41(5):830–6. Epub 2004/11/03. doi: 10.1016/j.jhep.2004.06.029 . [DOI] [PubMed] [Google Scholar]
- 6.Charlton M, Seaberg E, Wiesner R, Everhart J, Zetterman R, Lake J, et al. Predictors of patient and graft survival following liver transplantation for hepatitis C. Hepatology. 1998;28(3):823–30. Epub 1998/09/10. doi: 10.1002/hep.510280333 . [DOI] [PubMed] [Google Scholar]
- 7.AASLD/IDSA/IAS–USA. Recommendations for testing, managing, and treating hepatitis C. [Accessed January 12th, 2015.]. Available from: http://www.hcvguidelines.org.
- 8.Seo S, Maganti K, Khehra M, Ramsamooj R, Tsodikov A, Bowlus C, et al. De novo nonalcoholic fatty liver disease after liver transplantation. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2007;13(6):844–7. Epub 2006/10/10. doi: 10.1002/lt.20932 . [DOI] [PubMed] [Google Scholar]
- 9.Marchetti P, Navalesi R. The metabolic effects of cyclosporin and tacrolimus. Journal of endocrinological investigation. 2000;23(7):482–90. Epub 2000/09/27. doi: 10.1007/BF03343761 . [DOI] [PubMed] [Google Scholar]
- 10.Bosch W, Heckman MG, Pungpapong S, Diehl NN, Shalev JA, Hellinger WC. Association of cytomegalovirus infection and disease with recurrent hepatitis C after liver transplantation. Transplantation. 2012;93(7):723–8. Epub 2012/03/13. doi: 10.1097/TP.0b013e3182472876 . [DOI] [PubMed] [Google Scholar]
- 11.Berenguer M, Rayon JM, Prieto M, Aguilera V, Nicolas D, Ortiz V, et al. Are posttransplantation protocol liver biopsies useful in the long term? Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2001;7(9):790–6. Epub 2001/09/12. doi: 10.1053/jlts.2001.23794 . [DOI] [PubMed] [Google Scholar]
- 12.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49(3):1017–44. Epub 2009/02/27. doi: 10.1002/hep.22742 . [DOI] [PubMed] [Google Scholar]
- 13.Sebastiani G. Non-invasive assessment of liver fibrosis in chronic liver diseases: implementation in clinical practice and decisional algorithms. World journal of gastroenterology: WJG. 2009;15(18):2190–203. Epub 2009/05/14. doi: 10.3748/wjg.15.2190 ; PubMed Central PMCID: PMC2682233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–6. Epub 2007/06/15. doi: 10.1002/hep.21669 . [DOI] [PubMed] [Google Scholar]
- 15.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–54. Epub 2007/03/30. doi: 10.1002/hep.21496 . [DOI] [PubMed] [Google Scholar]
- 16.Kim BK, Kim HS, Yoo EJ, Oh EJ, Park JY, Kim do Y, et al. Risk assessment of clinical outcomes in Asian patients with chronic hepatitis B using enhanced liver fibrosis test. Hepatology. 2014;60(6):1911–9. Epub 2014/08/22. 10.1002/hep.27389. 25142433. doi: 10.1002/hep.27389 [DOI] [PubMed] [Google Scholar]
- 17.EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. Journal of hepatology. 2015;63(1):237–64. Epub 2015/04/26. doi: 10.1016/j.jhep.2015.04.006 . [DOI] [PubMed] [Google Scholar]
- 18.Corradi F, Piscaglia F, Flori S, D'Errico-Grigioni A, Vasuri F, Tame MR, et al. Assessment of liver fibrosis in transplant recipients with recurrent HCV infection: usefulness of transient elastography. Dig Liver Dis. 2009;41(3):217–25. Epub 2008/08/02. 10.1016/j.dld.2008.06.009. 18672413. doi: 10.1016/j.dld.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 19.Goldschmidt I, Stieghorst H, Munteanu M, Poynard T, Schlue J, Streckenbach C, et al. The use of transient elastography and non-invasive serum markers of fibrosis in pediatric liver transplant recipients. Pediatric transplantation. 2013;17(6):525–34. Epub 2013/06/28. 10.1111/petr.12116. 23802661. doi: 10.1111/petr.12116 [DOI] [PubMed] [Google Scholar]
- 20.Crespo G, Fernandez-Varo G, Marino Z, Casals G, Miquel R, Martinez SM, et al. ARFI, FibroScan, ELF, and their combinations in the assessment of liver fibrosis: a prospective study. Journal of hepatology. 2012;57(2):281–7. Epub 2012/04/24. 10.1016/j.jhep.2012.03.016. 22521355. doi: 10.1016/j.jhep.2012.03.016 [DOI] [PubMed] [Google Scholar]
- 21.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of internal medicine. 2011;155(8):529–36. Epub 2011/10/19. 10.7326/0003-4819-155-8-201110180-00009. 22007046. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 22.Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline gastroenterology. 2014;5(3):211–8. Epub 2014/07/16. doi: 10.1136/flgastro-2013-100403 ; PubMed Central PMCID: PMC4078666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53(3):726–36. doi: 10.1002/hep.24105 . [DOI] [PubMed] [Google Scholar]
- 24.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–25. doi: 10.1002/hep.21178 . [DOI] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–88. Epub 1986/09/01. . [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. Epub 1997/10/06. ; PubMed Central PMCID: PMC2127453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamphues C, Lotz K, Rocken C, Berg T, Eurich D, Pratschke J, et al. Chances and limitations of non-invasive tests in the assessment of liver fibrosis in liver transplant patients. Clinical transplantation. 2010;24(5):652–9. Epub 2009/11/21. doi: 10.1111/j.1399-0012.2009.01152.x . [DOI] [PubMed] [Google Scholar]
- 28.Harada N, Soejima Y, Taketomi A, Yoshizumi T, Ikegami T, Yamashita Y, et al. Assessment of graft fibrosis by transient elastography in patients with recurrent hepatitis C after living donor liver transplantation. Transplantation. 2008;85(1):69–74. Epub 2008/01/15. doi: 10.1097/01.tp.0000297248.18483.16 . [DOI] [PubMed] [Google Scholar]
- 29.Barrault C, Roudot-Thoraval F, Tran Van Nhieu J, Atanasiu C, Kluger MD, Medkour F, et al. Non-invasive assessment of liver graft fibrosis by transient elastography after liver transplantation. Clin Res Hepatol Gastroenterol. 2013;37(4):347–52. Epub 2013/01/16. doi: 10.1016/j.clinre.2012.11.003 . [DOI] [PubMed] [Google Scholar]
- 30.Rigamonti C, Fraquelli M, Bastiampillai AJ, Caccamo L, Reggiani P, Rossi G, et al. Transient elastography identifies liver recipients with nonviral graft disease after transplantation: a guide for liver biopsy. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2012;18(5):566–76. Epub 2012/01/25. doi: 10.1002/lt.23391 . [DOI] [PubMed] [Google Scholar]
- 31.Cholongitas E, Tsochatzis E, Goulis J, Burroughs AK. Noninvasive tests for evaluation of fibrosis in HCV recurrence after liver transplantation: a systematic review. Transplant international: official journal of the European Society for Organ Transplantation. 2010;23(9):861–70. Epub 2010/08/14. doi: 10.1111/j.1432-2277.2010.01142.x . [DOI] [PubMed] [Google Scholar]
- 32.Mehta SH, Lau B, Afdhal NH, Thomas DL. Exceeding the limits of liver histology markers. Journal of hepatology. 2009;50(1):36–41. Epub 2008/11/18. doi: 10.1016/j.jhep.2008.07.039 ; PubMed Central PMCID: PMC2637134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhat M, Ghali P, Rollet-Kurhajec KC, Bhat A, Wong P, Deschenes M, et al. Serum fibrosis biomarkers predict death and graft loss in liver transplantation recipients. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2015;21(11):1383–94. Epub 2015/07/22. doi: 10.1002/lt.24217 . [DOI] [PubMed] [Google Scholar]
- 34.Imbert-Bismut F, Messous D, Thibault V, Myers RB, Piton A, Thabut D, et al. Intra-laboratory analytical variability of biochemical markers of fibrosis (Fibrotest) and activity (Actitest) and reference ranges in healthy blood donors. Clinical chemistry and laboratory medicine. 2004;42(3):323–33. doi: 10.1515/CCLM.2004.058 . [DOI] [PubMed] [Google Scholar]
- 35.Rigamonti C, Donato MF, Fraquelli M, Agnelli F, Ronchi G, Casazza G, et al. Transient elastography predicts fibrosis progression in patients with recurrent hepatitis C after liver transplantation. Gut. 2008;57(6):821–7. Epub 2008/01/26. doi: 10.1136/gut.2007.135046 . [DOI] [PubMed] [Google Scholar]
- 36.Della-Guardia B, Evangelista AS, Felga GE, Marins LV, Salvalaggio PR, Almeida MD. Diagnostic Accuracy of Transient Elastography for Detecting Liver Fibrosis After Liver Trannsplantation: A Specific Cut-Off Value Is Really Needed? Digestive diseases and sciences. 2017;62(1):264–72. Epub 2016/10/28. doi: 10.1007/s10620-016-4349-1 . [DOI] [PubMed] [Google Scholar]
- 37.Crespo G, Gambato M, Millan O, Casals G, Ruiz P, Londono MC, et al. Early non-invasive selection of patients at high risk of severe hepatitis C recurrence after liver transplantation. Transplant infectious disease: an official journal of the Transplantation Society. 2016;18(3):471–9. Epub 2016/03/19. doi: 10.1111/tid.12526 . [DOI] [PubMed] [Google Scholar]
- 38.Mikolajczyk-Korniak N, Tronina O, Slubowska K, Perkowska-Ptasinska A, Pacholczyk M, Baczkowska T, et al. Dynamic Elastography in Diagnostics of Liver Fibrosis in Patients After Liver Transplantation Due to Cirrhosis in the Course of Hepatitis C. Transplantation proceedings. 2016;48(5):1725–9. Epub 2016/08/09. doi: 10.1016/j.transproceed.2016.01.081 . [DOI] [PubMed] [Google Scholar]
- 39.Lutz HH, Schroeter B, Kroy DC, Neumann U, Trautwein C, Tischendorf JJ. Doppler Ultrasound and Transient Elastography in Liver Transplant Patients for Noninvasive Evaluation of Liver Fibrosis in Comparison with Histology: A Prospective Observational Study. Digestive diseases and sciences. 2015;60(9):2825–31. Epub 2015/05/15. doi: 10.1007/s10620-015-3682-0 . [DOI] [PubMed] [Google Scholar]
- 40.Pinto J, Matos H, Nobre S, Cipriano MA, Marques M, Pereira JM, et al. Comparison of acoustic radiation force impulse/serum noninvasive markers for fibrosis prediction in liver transplant. Journal of pediatric gastroenterology and nutrition. 2014;58(3):382–6. Epub 2013/10/30. doi: 10.1097/MPG.0000000000000226 . [DOI] [PubMed] [Google Scholar]
- 41.Pissaia A Jr., Borderie D, Bernard D, Scatton O, Calmus Y, Conti F. APRI and FIB-4 Scores Are Useful After Liver Transplantation Independently of Etiology. Transplantation proceedings. 2009;41(2):679–81. Epub 2009/03/31. doi: 10.1016/j.transproceed.2008.12.014 . [DOI] [PubMed] [Google Scholar]
- 42.Toniutto P, Fabris C, Bitetto D, Falleti E, Avellini C, Rossi E, et al. Role of AST to platelet ratio index in the detection of liver fibrosis in patients with recurrent hepatitis C after liver transplantation. Journal of gastroenterology and hepatology. 2007;22(11):1904–8. Epub 2007/10/05. doi: 10.1111/j.1440-1746.2006.04628.x . [DOI] [PubMed] [Google Scholar]
- 43.Segovia MC, Lyden ER, Bernard T, McCashland TM. Evaluation of Fib-4 as a Marker of Fibrosis in Hcv Infected Patients Who Underwent Liver Transplantation. Hepatology. 2008;48(4):576A–A. ISI:000259757400595. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.