Abstract
IMPORTANCE
Five medications have been approved for the management of obesity, but data on comparative effectiveness are limited.
OBJECTIVE
To compare weight loss and adverse events among drug treatments for obesity using a systematic review and network meta-analysis.
DATA SOURCES
MEDLINE, EMBASE, Web of Science, Scopus, and Cochrane Central from inception to March 23, 2016; clinical trial registries.
STUDY SELECTION
Randomized clinical trials conducted among overweight and obese adults treated with US Food and Drug Administration–approved long-term weight loss agents (orlistat, lorcaserin, naltrexone-bupropion, phentermine-topiramate, or liraglutide) for at least 1 year compared with another active agent or placebo.
DATA EXTRACTION AND SYNTHESIS
Two investigators identified studies and independently abstracted data using a predefined protocol. A Bayesian network meta-analysis was performed and relative ranking of agents was assessed using surface under the cumulative ranking (SUCRA) probabilities. Quality of evidence was assessed using GRADE criteria.
MAIN OUTCOMES AND MEASURES
Proportions of patients with at least 5%weight loss and at least 10% weight loss, magnitude of decrease in weight, and discontinuation of therapy because of adverse events at 1 year.
RESULTS
Twenty-eight randomized clinical trials with 29018 patients (median age, 46 years; 74%women; median baseline body weight, 100.5 kg; median baseline body mass index, 36.1) were included. A median 23%of placebo participants had at least 5%weight loss vs 75%of participants taking phentermine-topiramate (odds ratio [OR], 9.22; 95%credible interval [CrI], 6.63–12.85; SUCRA, 0.95), 63%of participants taking liraglutide (OR, 5.54; 95%CrI, 4.16–7.78; SUCRA, 0.83), 55%taking naltrexone-bupropion (OR, 3.96; 95%CrI, 3.03–5.11; SUCRA, 0.60), 49%taking lorcaserin (OR, 3.10; 95%CrI, 2.38–4.05; SUCRA, 0.39), and 44%taking orlistat (OR, 2.70; 95%CrI, 2.34–3.09; SUCRA, 0.22). All active agents were associated with significant excess weight loss compared with placebo at 1 year—phentermine-topiramate, 8.8 kg (95%CrI, −10.20 to −7.42 kg); liraglutide, 5.3 kg (95%CrI, −6.06 to −4.52 kg); naltrexone-bupropion, 5.0 kg (95%CrI, −5.94 to −3.96 kg); lorcaserin, 3.2 kg (95%CrI, −3.97 to −2.46 kg); and orlistat, 2.6 kg (95%CrI, −3.04 to −2.16 kg). Compared with placebo, liraglutide (OR, 2.95; 95%CrI, 2.11–4.23) and naltrexone-bupropion (OR, 2.64; 95%CrI, 2.10–3.35) were associated with the highest odds of adverse event–related treatment discontinuation. High attrition rates (30%–45%in all trials) were associated with lower confidence in estimates.
CONCLUSIONS AND RELEVANCE
Among overweight or obese adults, orlistat, lorcaserin, naltrexone-bupropion, phentermine-topiramate, and liraglutide, compared with placebo, were each associated with achieving at least 5%weight loss at 52 weeks. Phentermine-topiramate and liraglutide were associated with the highest odds of achieving at least 5%weight loss.
Approximately 1.9 billion adults are overweight and 600 million are obeseworldwide.1 Identifying effective long-term treatment strategies for overweight and obesity is of paramount importance. The US Food and Drug Administration (FDA) has approved 5 weight loss drugs (orlistat, lorcaserin, naltrexone-bupropion, phentermine-topiramate, and liraglutide) for long-term use in obese (body mass index [BMI] ≥30) or overweight (BMI≥27)individuals with at least 1 weight-associated co-morbidity (type 2 diabetes, hypertension, hyperlipidemia).2–4 (Body mass index is calculated as weight in kilograms divided by height in meters squared.) However, there is a paucity of randomized clinical trial (RCT) evidence comparing different pharmacological interventions with each other. Data regarding relative efficacy and adverse effects of each drug can inform patients, health care practitioners, and policymakers regarding optimal medication prescription to treat obesity and overweight. In this systematic review, associations of each drug with weight loss and adverse effects were compared using a direct meta-analysis and Bayesian network meta-analysis.
Methods
This systematic review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement extension for network meta-analysis5 and was conducted following an a priori-established protocol registered with PROSPERO (CRD42015026114).6 Good research practices outlined in the International Society for Pharmacoeconomics and Outcomes Research report on interpreting indirect treatment comparisons and network meta-analysis for health care decision making were followed.7 Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria for network meta-analysis were used to appraise quality of evidence.8
Selection Criteria
Randomized clinical trials were included in this meta-analysis if they studied any of the 5FDA-approvedweight loss drugs administered at the most effective recommended doses for at least 1 year compared with either placebo or each other in obese (BMI ≥30) or overweight (BMI ≥27) adults (aged ≥18 years), with or without weight-associated comorbidities, and reported either proportion of patients achieving at least 5%weight loss or differences in mean weight loss between different study groups.
Observational studies, trials of short-term or nonapproved pharmacological agents (eg, rimonabant, sibutramine), trials comparing individual components of the approved fixed-dose combination medications (eg, naltrexone-bupropion, phentermine-topiramate), studies in special populations (patients with nonalcoholic fatty liver disease or polycystic ovary syndrome), and studies comparing an active agent with another nonapproved weight loss therapy (eg, metformin, statins) were excluded.
Search Strategy
The search strategy was designed and conducted by an experienced medical librarian with input from study investigators using various databases from inception to March 23, 2016. The databases included Ovid MEDLINE, EMBASE, Scopus, Web of Science, and Cochrane Central Register of Controlled Trials. Clinical trial registries (http://www.clinicaltrials.gov and http://www.clinicaltrialsregister.eu), conference proceedings, and published systematic reviews were screened for additional studies. Details of the search strategy and study selection procedures are shown in the eAppendix in the Supplement.
Data Abstraction and Quality Assessment
Data on study-, patient- and treatment-related characteristics were abstracted onto a standardized form by 2 authors (R.K. and A.K.C.) independently and discrepancies were resolved by consensus in consultation with a third reviewer (S.S.).Details of the data abstraction are reported in the eAppendix in the Supplement. When trials randomized patients to different dosages of the active intervention, only data for the most effective FDA-approved dosage of the medication (orlistat, 120mg 3 times daily; lorcaserin, 10 mg twice daily; naltrexone-bupropion, 32 mg/360 mg twice daily; phentermine-topiramate, 15mg/92mg once daily; and liraglutide, 3-mg subcutaneous injection daily) were used.2–4 The risk of bias of individual studies was assessed in the context of the primary outcome using the Cochrane Risk of Bias assessment tool.9
Outcomes
All outcomes were assessed at 1 year of follow-up (52 [±4] weeks). The primary outcome was the proportion of patients achieving at least 5% weight loss from baseline, since this is the primary efficacy outcome mandated by the FDA in trials evaluating weight loss drugs and associated with clinically significant improvement in metabolic risk profile.10,11 Secondary weight loss outcomes were the proportion of individuals with at least 10%weight loss and change in weight from baseline. The primary adverse event outcome was rate of discontinuation of treatment due to adverse events. Serious adverse events were not consistently defined or reported.
All data were abstracted using study-reported modified intention-to-treat analysis(ie, patients who received at least 1 dose of the drug and had 1 post randomization weight assessment);imputation of missing values was performed in all studies using last observation carried forward (LOCF) in accordance with FDA guidelines regarding trials of weight loss agents.10
Quality of Evidence
The GRADE approach was used to rate the quality of evidence of estimates derived from network meta-analysis.8 In this approach, direct evidence from RCTs starts at high quality and can be downgraded based on risk of bias, indirectness, imprecision, inconsistency (or heterogeneity), and/or publication bias to levels of moderate, low, and very low quality. The rating of indirect estimates starts at the lowest rating of the 2 pair wise estimates that contribute as first-order loops to the indirect estimate but can be downgraded further for imprecision or intransitivity (dissimilarity between studies in clinical or methodological characteristics). If direct and indirect estimates were similar (ie, coherent), then the higher of their ratings was assigned to the network meta-analysis estimates.
Statistical Analysis
Direct meta-analysis was performed using DerSimonian and Laird random-effects model to estimate pooled odds ratios (ORs) and 95%confidence intervals incorporating within- and between-study heterogeneity.12 Statistical heterogeneity was assessed using the I2 statistic, with values higher than 50% indicating substantial heterogeneity.13 In post hoc sensitivity analyses, summary estimates were also derived using the Hartung-Knapp method to address possible type I error with the conventional DerSimonian and Laird approach.14 Publication bias was assessed by examining funnel-plot symmetry and using the Egger regression test, with P < .05 suggesting publication bias.15,16
To incorporate indirect comparisons with direct comparisons, random-effects Bayesian network meta-analyses were conducted using Markov chain Monte Carlo methods in WinBUGS version 1.4.3 (MRC Biostatistics Unit) and methods described by Lu and Ades.17,18 The relative ranking of agents on weight loss and adverse events outcomes was presented as their surface under the cumulative ranking (SUCRA) probabilities, which represent their likelihood of being ranked best.19 In this study, higher SUCRA scores reflect higher associated weight loss and a lower rate of adverse events. Furthermore, using ORs derived from the network meta-analysis for placebo comparisons and median placebo response rate as the assumed control risk, absolute event rates for each intervention were estimated.20 Details of the statistical analysis and the WinBUGS code are reported in the eAppendix in the Supplement. The level of statistical significance was set at P < .05 and all statistical tests were 2-sided.
Multiple sensitivity analyses were performed to assess the robustness of the findings. These were based on (1) use of an alternative statistical approach (random-effects frequentist model)21; (2) restricting only to studies in adults without diabetes (because antidiabetic medications may have independent weight-modifying effects); and (3) replacing trials of high-dose phentermine-topiramate with standard-dose phentermine-topiramate (7.5 mg/46 mg once daily). Additional post hoc sensitivity analyses were performed given potential bias associated with LOCF imputation using (1) worst-case scenario analysis, wherein all patients who were randomized but did not undergo assessment of outcomes at the end of the study were considered treatment failures and (2) complete-case analysis, which limited analysis to patients who completed the entire study and underwent an assessment at the end of the trial.
Results
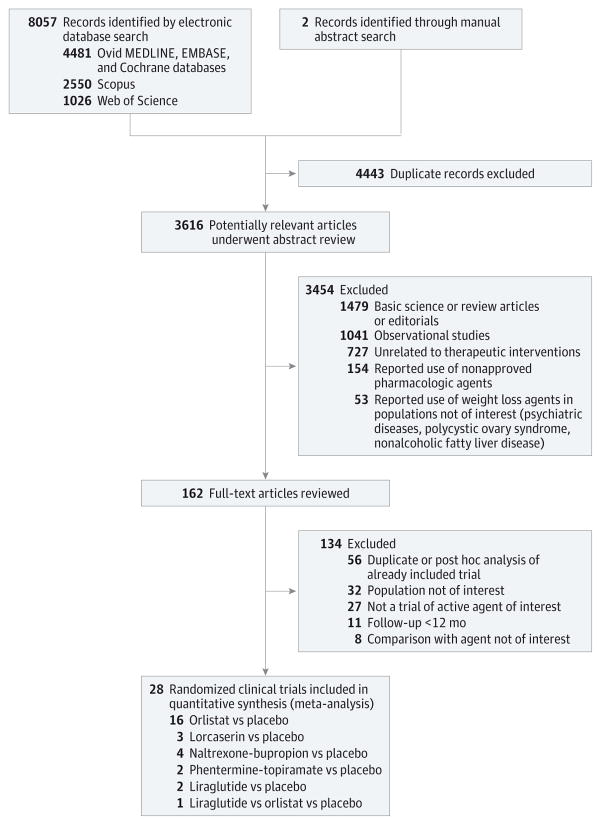
From a total of 3616 unique studies identified using the search strategy, 28 RCTs were included in this network meta-analysis. These included 27 two-group trials comparing active intervention to placebo (orlistat, 16 trials22–37; lorcaserin, 3 trials38–40; naltrexone-bupropion, 4 trials41–44; phentermine-topiramate, 2 trials45,46; liraglutide, 2 trials47,48) and 1 three group trial comparing liraglutide and orlistat against placebo.49 Study selection is shown in Figure 1. The available direct comparisons and network of trials are shown in Figure 2 and eFigure 1 in the Supplement.
Figure 1.
Study Identification and Selection
Figure 2. Network of Included Studies With Available Direct Comparisons for Primary Efficacy Outcome (≥5%Weight Loss).
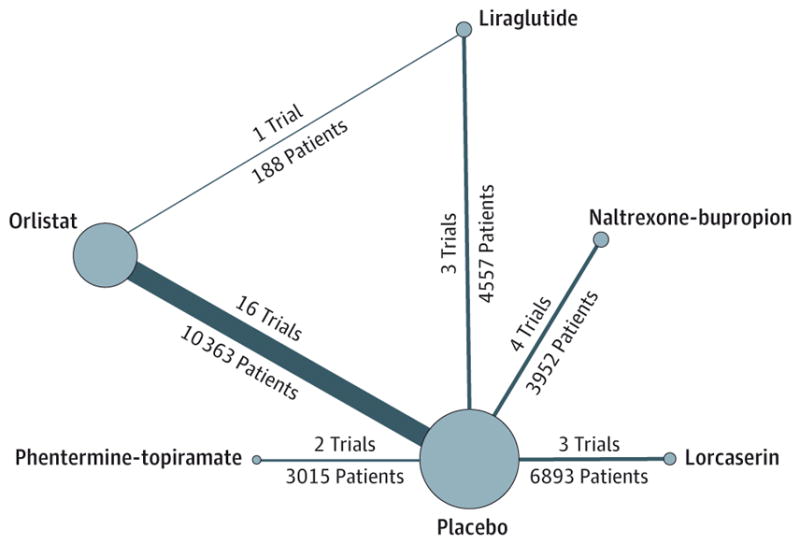
The size of the nodes and the thickness of the edges are weighted according to the number of studies evaluating each treatment and direct comparison, respectively. The study by Swinburn et al37 reported only continuous weight loss outcomes and is not included in this network. Network of included studies for all other outcomes is shown in eFigure 1 in the Supplement.
Characteristics and Quality of Included Studies
The RCTs included in the network meta-analysis are summarized in Table 1 and Table 2. Overall, these 28 trials were reported between 1998 and 2015 and included 29 018 participants (the range of size of trials was 220 to 3731 participants). The primary outcome (proportion of patients achieving at least 5%weight loss at 1 year) was reported in all studies except one, which reported only weight loss on a continuous scale.36
Table 1.
Characteristics of Included Randomized Clinical Trials Comparing Orlistat vs Placebo for Weight Lossa
| Source/Period | Total Enrolled/mITT/Completers | Cointerventions | Outcomes of Interest Reported | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Intervention | Control | Diet | Exercise | Behavior | Mean Weight Change | Proportion With ≥5% Weight Loss | Proportion With ≥10% Weight Loss | Treatment Withdrawals Due to Adverse Events | |
| Astrup et al,49 2012 (US)/2007–2009 | 95/67/45 | 98/67/47 | 500-kcal/d deficitb | Brisk walking ≥150 min/wk | Yes | ✓ | ✓ | ✓ | ✓ |
|
| |||||||||
| Swinburn et al,36 2005 (Australia and New Zealand) | 70/170/132 | 169/169/137 | Reduce fat intake (40 g/d) | Moderate, 30 min/d | Yes | ✓ | ✓ | ||
|
| |||||||||
| Berne,23 2005 (Sweden)/1999–2002 | 111/111/96 | 109/109/94 | 600-kcal/d deficit | Moderate, 30 min/d | Yes | ✓ | ✓ | ✓ | |
|
| |||||||||
| Torgerson et al,37 2004 (Sweden)/1997–2002 | 1650/1640/850 | 1655/1637/564 | 800-kcal/d deficit | Moderate, walk 1 extra km/d | Yes | ✓ | ✓ | ✓ | |
|
| |||||||||
| Krempf et al,31 2003 (France) | 346/346/224 | 350/350/201 | 20% energy reduction | No | ✓ | ✓ | ✓ | ✓ | |
|
| |||||||||
| Miles et al,33 2002 (US and Canada) | 255/250/165 | 261/254/146 | 600-kcal/d deficit | Nonspecific increase | Yes | ✓ | ✓ | ✓ | ✓ |
|
| |||||||||
| Hanefeld and Sachse,27 2002 (Germany) | 195/189/133 | 188/180/131 | 600-kcal/d deficit | No | ✓ | ✓ | ✓ | ||
|
| |||||||||
| Broom et al,24 2002 (UK) | 265/265/186 | 266/266/161 | 600-kcal/d deficit | No | ✓ | ✓ | ✓ | ✓ | |
|
| |||||||||
| Bakris et al,22 2002 (US) | 278/267/160 | 276/265/106 | 600-kcal/d deficit | Nonspecific increase | No | ✓ | ✓ | ✓ | |
|
| |||||||||
| Kelley et al,30 2002 (US) | 274/266/129 | 276/269/141 | 600-kcal/d deficit | Nonspecific increase | Yes | ✓ | ✓ | ✓ | ✓ |
|
| |||||||||
| Rossner et al,34 2000 (Europe) | 244/242/181 | 243/237/158 | 600-kcal/d deficit | No | ✓ | ✓ | ✓ | ✓ | |
|
| |||||||||
| Lindgarde,32 2000 (Sweden) | 190/190/159 | 186/186/164 | 600-kcal/d deficit | Moderate, 30 min/d | Yes | ✓ | ✓ | ✓ | ✓ |
|
| |||||||||
| Hauptman et al,28 2000 (US) | 210/210/151 | 212/212/122 | 600-kcal/d deficit | Moderate, 20–30 min/d (3–5 times/wk) | Yes | ✓ | ✓ | ✓ | ✓ |
|
| |||||||||
| Finer et al,26 2000 (UK) | 114/110/66 | 114/108/73 | 600-kcal/d deficit (reduced by further 300 kcal at 24 wk) | No | ✓ | ✓ | ✓ | ✓ | |
|
| |||||||||
| Davidson et al,25 1999 (US)/1992–1995 | 668/657/458 | 224/223/133 | Energy-deficient diet (30% energy from fat) | Brisk walking, 20–30 min/d (3–5 times/wk) | Yes | ✓ | ✓ | ✓ | ✓ |
|
| |||||||||
| Sjostrom et al,35 1998 (Europe) | 345/343/284 | 343/340/260 | 600-kcal/d deficit (reduced by further 300 kcal at 24 wk) | No | ✓ | ✓ | ✓ | ||
|
| |||||||||
| Hollander et al,29 1998 (US) | 162/162/139 | 159/159/115 | Energy-deficient diet (30% energy from fat) | No | ✓ | ✓ | ✓ | ✓ | |
Abbreviation: mITT, modified intention to treat (last-observation-carried-forward analysis).
All trials were randomized, double-blind, placebo-controlled, multicenter studies except Broom et al,25 a single-center study. All trials had follow-up through 52 weeks.
Baseline calculated using World Health Organization equations; 30% of energy from fat, 20% from protein, and 50% from carbohydrate.
Table 2.
Characteristics of Randomized Clinical Trials Comparing Lorcaserin, Naltrexone-Bupropion, Phentermine-Topiramate, and Liraglutide vs Placebo for Weight Lossa
| Source/Period | Total Enrolled/mITT/Completers | Cointerventions | Outcomes of Interest Reported | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Intervention | Control | Diet | Exercise | Behavior | Mean Weight Change | Proportion With ≥5% Weight Loss | Proportion With ≥10% Weight Loss | Treatment Withdrawals Due to Adverse Events | |
| Lorcaserin vs Placebo | |||||||||
|
| |||||||||
| O’Neil et al,39 2012 (US)/2007–2010 | 256/251/169 | 252/248/157 | 600-kcal/d deficitb | Moderate, 30 min/d | Yes | ✓ | ✓ | ✓ | ✓ |
|
| |||||||||
| Fidler et al,38 2011 (US)/2007–2009 | 1602/1561/917 | 1601/1541/834 | 600-kcal/d deficitb | Moderate, 30 min/d | Yes | ✓ | ✓ | ✓ | ✓ |
|
| |||||||||
| Smith et al,40 2010 (US)/2006–2009 | 1595/1538/883 | 1587/1499/676 | 600-kcal/d deficitb | Moderate, 30 min/d | Yes | ✓ | ✓ | ✓ | ✓ |
|
| |||||||||
| Naltrexone-Bupropion vs Placebo | |||||||||
|
| |||||||||
| Apovian et al,41 2013 (US)/2007–2009 | 1001/826/538 | 495/456/267 | 500-kcal/d deficit | Nonspecific increase | Yes | ✓ | ✓ | ✓ | ✓ |
|
| |||||||||
| Hollander et al,43 2013 (US)/2007–2009 | 333/265/175 | 169/159/100 | 500-kcal/d deficit | Brisk walking, 30 min/d | Yes | ✓ | ✓ | ✓ | |
|
| |||||||||
| Wadden et al,44 2011 (US)/2007–2009 | 591/482/301 | 202/193/106 | Graded energy balancec | Moderate, 180 min/wk, increasing to 360 min/wk | Yes | ✓ | ✓ | ✓ | |
|
| |||||||||
| Greenway et al,42 2010 (US)/2007–2009 | 583/471/296 | 581/511/290 | 500-kcal/d deficitb | Nonspecific increase | Yes | ✓ | ✓ | ✓ | ✓ |
|
| |||||||||
| Phentermine-Topiramate vs Placebo | |||||||||
|
| |||||||||
| Allison et al,45 2012 (US)/2007–2009 | 512/498/301 | 514/498/241 | 500-kcal/d deficitb | Nonspecific increase | Yes | ✓ | ✓ | ✓ | |
|
| |||||||||
| Gadde et al,46 2011 (US)/2007–2009 | 995/981/733 | 994/979/616 | 500-kcal/d deficitb | Yes | ✓ | ✓ | ✓ | ✓ | |
|
| |||||||||
| Liraglutide vs Placebo | |||||||||
|
| |||||||||
| Davies et al,47 2015/2011–2013d | 423/412/324 | 212/211/140 | 500-kcal/d deficitb,e | Brisk walking ≥150 min/wk | Yes | ✓ | ✓ | ✓ | ✓ |
|
| |||||||||
| Pi-Sunyer et al,48 2015/2011–2013d | 2487/2437/1789 | 1244/1225/801 | 500-kcal/d deficitb,e | Brisk walking ≥150 min/wk | Yes | ✓ | ✓ | ✓ | ✓ |
|
| |||||||||
| Astrup et al,49 2012/2007–2009d | 93/72/47 | 98/67/47 | 500-kcal/d deficitb,e | Brisk walking ≥150 min/wk | Yes | ✓ | ✓ | ✓ | ✓ |
|
| |||||||||
| Liraglutide vs Orlistat Johnston et al,55 2014 (US)/2007–2009/52 | 93/72/47 | 95/67/45 | 500-kcal/d deficitb,e | Brisk walking ≥150 min/wk | Yes | ✓ | ✓ | ✓ | ✓ |
Abbreviation: mITT, modified intention to treat (last-observation-carried-forward analysis).
All trials were randomized, double-blind, placebo-controlled, multicenter studies. Trials had follow-up through 52 weeks with the exception of the following, which went to 56 weeks: Apovian et al, Hollander et al, Wadden et al, and Greenway et al (all naltrexone-bupropion vs placebo); and Davies et al (liraglutide vs placebo).
Baseline calculated using World Health Organization equations.
Participants: weight ≤249 lb, 1200 kcal/d; weight 250–299 lb, 1500 kcal/d; weight 300–349 lb, 1800 kcal/d; weight ≥350 lb, 2000 kcal/d. Balanced deficit diet: approximately 15%–20% of energy from protein, ≤30% energy from fat, and the remainder from carbohydrate.
International trial.
Thirty percent of energy from fat, 20% from protein, and 50% from carbohydrate.
The baseline characteristics of patients included in these trials are described in eTable 1 in the Supplement. The median of average age of study participants was 45.9 years (range of average age, 40.0–59.8 years)and 74% of participants were women (range, 45%–92%). The median of average BMI of patients was 36.1 (range, 32.6–42.0)and the median of average base line weight was 100.5kg (range, 95.3–115.8kg). Sixteen trials were performed exclusively in patients without diabetes (or diet-controlled diabetes), whereas 8 trials were conducted in patients with diabetes treated with pharmacological therapy. Baseline patient characteristics and prognostic factors were comparably distributed in the active and comparator groups and across different trials. In all trials, participants received standard dietary and lifestyle counseling without a structured intervention; in 1 trial, all participants received intensive behavioral modification.44
Overall, studies were considered to be at high risk of bias, with attrition rates of 30% to 45% in all trials. Overall and study-level quality assessments are summarized in eFigure 2 in the Supplement.
Direct Meta-analysis
Results of direct pairwise meta-analysis are summarized in Table 3 and eFigure 3 in the Supplement. All agents were associated with higher proportions of patients achieving at least 5% and at least 10% weight loss compared with placebo. Overall, the excess weight loss compared with placebo (ie, weighted mean difference for the drug-to-placebo comparison for the respective drug) was 2.6 kg (95%CI, 2.3–2.9 kg)with orlistat, 3.2kg (95%CI, 3.0–3.6 kg) with lorcaserin, 5.0 kg (95% CI, 4.4–5.5 kg) with naltrexone-bupropion, 8.8 kg (95% CI, 8.0–9.6 kg) with phentermine-topiramate, and 5.2kg (95% CI, 4.9–5.6 kg)with liraglutide. All agents were more frequently discontinued because of adverse events than placebo (Table 3). Significant heterogeneity was observed for most comparisons, but the difference was primarily in the magnitude of effect size, not in the direction. In the only head-to-head comparison, liraglutide resulted in greater weight loss compared with orlistat, with no difference in adverse events.49 In post hoc sensitivity analysis using the Hartung-Knapp method, all results were consistent (eTable 2 in the Supplement).
Table 3.
Summary of Direct Meta-analysis for All Weight Loss and Adverse Event Outcomes
| Pharmacological Intervention | No. of Studies | Active Intervention | Control (Placebo Unless Otherwise Noted) | OR or Weighted Mean Difference, kg (95% CI) | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| No. With Event | Total No. | No. With Event | Total No. | |||
| ≥5% Weight Loss | ||||||
|
| ||||||
| Orlistat | 16 | 3140 | 5315 | 1694 | 4694 | 2.69 (2.36 to 3.07) |
|
| ||||||
| Lorcaserin | 3 | 1562 | 3350 | 729 | 3288 | 3.09 (2.49 to 3.83) |
|
| ||||||
| Naltrexone-bupropion | 4 | 1081 | 2044 | 274 | 1319 | 3.90 (2.91 to 5.22) |
|
| ||||||
| Phentermine-topiramate | 2 | 1019 | 1479 | 290 | 1477 | 9.10 (7.68 to 10.78) |
|
| ||||||
| Liraglutide | 3 | vs Placebo: 1798 | 2921 | Placebo: 380 | 1503 | 5.09 (4.07 to 6.37) |
| vs Orlistat: 53 | 72 | Orlistat: 29 | 67 | 3.66 (1.79 to 7.46) | ||
|
| ||||||
| ≥10% Weight Loss | ||||||
|
| ||||||
| Orlistat | 14 | 1520 | 4859 | 684 | 4249 | 2.41 (2.08 to 2.78) |
|
| ||||||
| Lorcaserin | 3 | 742 | 3350 | 276 | 3288 | 3.17 (2.53 to 3.97) |
|
| ||||||
| Naltrexone-bupropion | 4 | 599 | 2044 | 112 | 1319 | 4.11 (2.80 to 6.05) |
|
| ||||||
| Phentermine-topiramate | 2 | 702 | 1479 | 109 | 1477 | 11.34 (9.10 to 14.13) |
|
| ||||||
| Liraglutide | 3 | vs Placebo: 930 | 2921 | Placebo: 146 | 1503 | 4.36 (3.61 to 5.26) |
| vs Orlistat: 27 | 72 | Orlistat: 9 | 67 | 3.87 (1.65 to 9.04) | ||
|
| ||||||
| Mean Weight Loss in Excess of Placeboa | ||||||
|
| ||||||
| Orlistat | 14 | 3391 | 2777 | −2.63 (−2.94 to −2.32)b | ||
|
| ||||||
| Lorcaserin | 3 | 3350 | 3288 | −3.25 (−3.55 to −2.95)b | ||
|
| ||||||
| Naltrexone-bupropion | 2 | 1297 | 967 | −4.95 (−5.54 to −4.36)b | ||
|
| ||||||
| Phentermine-topiramate | 1 | 981 | 979 | −8.80 (−9.62 to −7.98)b | ||
|
| ||||||
| Liraglutide | 3 | 2921 | 1503 | −5.24 (−5.60 to −4.87)b | ||
| 72 | 67 | −3.90 (−5.18 to −2.62)b | ||||
|
| ||||||
| Discontinuation of Therapy Due to Adverse Events | ||||||
|
| ||||||
| Orlistat | 16 | 439 | 5323 | 224 | 4704 | 1.84 (1.55 to 2.18) |
|
| ||||||
| Lorcaserin | 3 | 250 | 3350 | 190 | 3288 | 1.40 (0.96 to 2.03) |
|
| ||||||
| Naltrexone-bupropion | 4 | 501 | 2044 | 175 | 1319 | 2.60 (2.15 to 3.14) |
|
| ||||||
| Phentermine-topiramate | 2 | 274 | 1479 | 132 | 1477 | 2.32 (1.86 to 2.89) |
|
| ||||||
| Liraglutide | 3 | vs Placebo: 292 | 2921 | Placebo: 57 | 1503 | 2.82 (2.10 to 3.77) |
| vs Orlistat: 7 | 72 | Orlistat: 2 | 67 | 3.50 (0.70 to 17.49) | ||
Abbreviation: OR, odds ratio.
Continuous outcome; event rate not applicable.
Weighted mean difference (or excess weight loss vs placebo).
Network Meta-analysis—Weight Loss Outcomes
Proportion of Patients With at Least 5%and at Least 10%Weight Loss
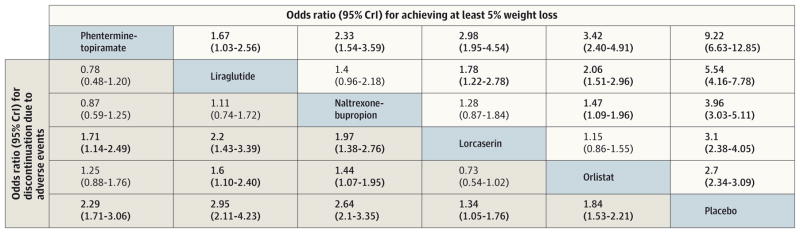
In network meta-analysis, compared with placebo, or list at was associated with an OR of 2.70(95%credible interval [CrI], 2.34–3.09), lorcaserin with an OR of 3.10 (95% CrI, 2.38–4.05), naltrexone-bupropion with an OR of 3.96 (95% CrI, 3.03–5.11), phentermine-topiramate an OR of 9.22 (95% CrI, 6.63–12.85), and liraglutide with an OR of 5.54 (95%CrI, 4.16–7.78) for achieving at least 5%weight loss (Figure 3). All agents were also associated with higher odds of at least 10% weight loss from baseline compared with placebo (eTable 3 in the Supplement). Placebo was associated with a 23% median rate of achieving at least 5% weight loss while phentermine-topiramate was associated with achieving at least 5% weight loss in an estimated 75% of participants, liraglutide in an estimated 63%, naltrexone-bupropion in an estimated 55%, lorcaserin in an estimated 49%, and orlistat in an estimated 44% (eTable 4 in the Supplement). Similarly, with a 9%median rate of achieving at least 10% weight loss in placebo-treated patients, phentermine-topiramate was associated with achieving at least 10% weight loss in an estimated 54% of participants, liraglutide in an estimated 34%, naltrexone-bupropion in an estimated 30%, lorcaserin in an estimated 25%, and orlistat in an estimated 20%.
Figure 3. Comparison of Weight Loss and Adverse Events With Pharmacological Weight Loss Agents in Network Meta-analysis.
Summary estimate represents odds ratio of achieving at least 5%weight loss (light gray background) and discontinuation due to adverse events (light blue background). Agents are ordered by rankings for the 5%weight loss outcome. Odds ratio for comparisons are in the cell in common between the column-defining and row-defining treatment. For weight loss outcome, row treatment is compared with column treatment (ie, column treatment is reference). For adverse event outcome, column treatment is compared with row treatment (ie, row treatment is reference). Numbers in parentheses indicate 95%credible intervals (95%CrIs). Numbers in bold represent statistically significant results.
Network meta-analysis suggested that phentermine-topiramate, 15 mg/92 mg once daily, was associated with the highest probability of achieving at least 5%weight loss (SUCRA, 0.95), followed by liraglutide (SUCRA, 0.83), naltrexone-bupropion (SUCRA, 0.60), lorcaserin (SUCRA, 0.39), and orlistat (SUCRA, 0.22) (Figure 4). Similarly, phentermine-topiramate was associated with the highest probability of achieving at least 10% weight loss (SUCRA, 0.99), followed by liraglutide (SUCRA, 0.71), naltrexone-bupropion (SUCRA, 0.64), lorcaserin (SUCRA, 0.44), and orlistat (SUCRA, 0.16).
Figure 4. SUCRAs for Weight Loss and Adverse Event Outcomes.
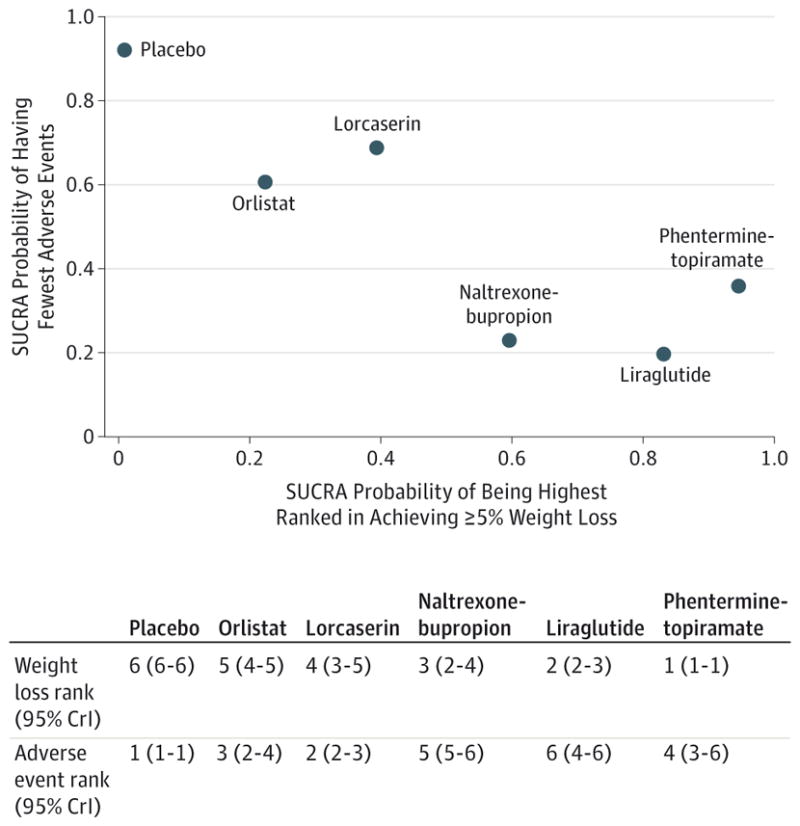
Surface under the cumulative rankings (SUCRAs) between 0 and 1 represent the probability of being ranked highest. For the weight loss outcomes, higher score corresponds to higher proportion achieving at least 5%weight loss with a particular therapy. For the adverse event outcome, higher scores reflect lower probability of discontinuation due to adverse events. The median ranks on both weight loss and adverse event rates (rank 1 through 6 on each scale) are tabulated along with their corresponding 95%credible intervals (95%CrIs).
Weight Loss in Excess of Placebo
In network meta-analysis, all active agents were associated with significant excess weight loss vs placebo at 1 year–orlistat, 2.6 kg (95%CrI, −3.04 to −2.16 kg); lorcaserin, 3.2 kg (95%CrI, −3.97 to −2.46 kg); naltrexone-bupropion, 5.0 kg (95% Cr I, −5.94 to −3.96 kg); phentermine-topiramate, 8.8 kg (95%CrI, −10.20 to−7.42 kg); and liraglutide, 5.3 kg (95% Cr I, −6.06 to −4.52 kg). Network meta-analysis also suggested that phentermine-topiramate, 15mg/92mg once daily, was associated with significant excess weight loss compared with all active agents (change vs orlistat,6.2kg; vs lorcaserin, 5.6kg; vs naltrexone-bupropion, 3.9 kg; and vs liraglutide, 3.5 kg) (eTable 3 in the Supplement).
Sensitivity Analysis
Results from multiple sensitivity analyses are reported in eTables 5–8 in the Supplement. Overall, the results were similar to the main analysis for the primary outcome in sensitivity analyses based on (1) alternative statistical model (frequentist approach using a random-effects inconsistency model, worst-case scenario, complete-case analysis); (2) restricting to only studies in adults without diabetes; and (3) replacing trials of high-dose phentermine-topiramate with standard-dose phentermine-topiramate (7.5mg/46mg once daily).
Network Meta-analysis—Adverse Event Outcome
In network meta-analysis, compared with placebo, all active agents had 1.3 to 2.9 higher odds of being associated with discontinuation due to adverse events (Figure 3). Compared with placebo, lorcaserin was associated with the lowest odds of being discontinued because of adverse events (OR, 1.34; 95% CrI, 1.05–1.76; SUCRA, 0.61), whereas liraglutide (OR, 2.95; 95% CrI, 2.11–4.23; SUCRA, 0.20) and naltrexone-bupropion (OR, 2.64; 95% CrI, 2.10–3.35; SUCRA, 0.23) were associated with the highest odds of being discontinued because of adverse events (Figure 4 and eTable 4 in the Supplement). Details of the most commonly observed adverse events and reported reasons for discontinuation are shown in eTable 9 in the Supplement.
Publication Bias and Network Coherence
There was no evidence of publication bias, either qualitatively based on funnel-plot asymmetry (eFigure 4 in the Supplement) or quantitatively (Egger regression test, P > .05 for all comparisons), although the number of studies included in each comparison was very small. There were no significant differences between direct and indirect estimates in the only closed loop that allowed assessment of network coherence (placebo-orlistat-liraglutide). Visual inspection of trace plots and evaluation of the Monte Carlo error and the Brooks-Gelman-Rubin statistic suggested adequacy of burn-in and convergence.50 Values of the total residual deviance suggested good model fit.
Quality of Evidence
Given high attrition rates for all trials (30%–45%), evidence was downgraded for risk of bias. Although several comparisons had statistically significant heterogeneity, the difference was primarily in the magnitude of effect size, not in the direction of effect, and hence, evidence was not downgraded for inconsistency. On applying GRADE to findings from the network meta-analysis combining direct and indirect evidence, there was moderate-quality evidence for all agents being associated with higher odds of achieving at least 5% weight loss compared with placebo. In comparing different drugs against each other, there was moderate quality evidence for phentermine-topiramate being associated with higher odds of achieving weight loss compared with all other drugs. There was also moderate-quality evidence for liraglutide being associated with higher odds of achieving weight loss compared with orlistat and lorcaserin and low-quality evidence for liraglutide being associated with higher odds of achieving weight loss compared with naltrexone-bupropion (which was downgraded for imprecision and risk of bias) (eTable 10 in the Supplement).
Discussion
In this systematic review and network meta-analysis, direct and indirect evidence from 28 RCTs in 29 018 overweight and obese patients was combined to compare the association of each drug with relative weight loss and adverse events. The study has several key findings. First, with at least 1 year of treatment, or list at, lorcaserin, naltrexone-bupropion, phentermine-topiramate, and liraglutide are all associated with higher odds of achieving weight loss compared with placebo, with moderate confidence in estimates. Second, phentermine-topiramate was associated with higher odds of achieving weight loss of at least 5% and weight loss of at least 10% compared with all other active agents, with moderate confidence in estimates, and there was no difference in the odds of adverse event–related drug discontinuation among phentermine-topiramate, liraglutide, and naltrexone-bupropion. Third, liraglutide was associated with higher odds of weight loss of at least 5% and weight loss of at least 10% compared with orlistat, lorcaserin, and naltrexone-bupropion, with low to moderate confidence in estimates, but was associated with higher odds of discontinuation due to adverse events.
The US Preventive Services Task Force recommends referral of all obese adults to intensive, multi component-interventions including behavioral interventions, pharmacological therapies, and surgical weight loss procedures.51 The Endocrine Society also suggests the use of approved weight loss medications for long-term weight maintenance, to ameliorate comorbidities, and to enhance adherence to behavior changes.52 However, there are no current recommendations to guide clinicians regarding choice of individual drugs.
The present study found moderate-quality evidence for phentermine-topiramate being associated with higher odds of achieving predefined thresholds of clinically meaningful weight loss compared with other currently approved agents. The odds of discontinuation of therapy due to medication-related adverse events was not different for phentermine-topiramate, liraglutide, and naltrexone-bupropion. While lorcaserin and or-list at were associated with lower rates of adverse events, they were also associated with lower rates of achieving all weight loss outcomes. Besides weight loss, treatment decisions may also be driven by coexisting medical conditions, which may either favor or preclude the use of specific agents.2 For example, liraglutide may be a more appropriate agent in people with diabetes because of its glucose-lowering effects.47 Conversely, naltrexone-bupropion in patients with chronic opiate or alcohol dependence may be associated with neuropsychiatric complications.2 Ultimately, given the differences in safety, efficacy, and response to therapy, the ideal approach to weight loss should be highly individualized, identifying appropriate candidates for pharmacotherapy, behavioral interventions, and surgical interventions.53 Historically, concerns regarding the long-term safety profile of pharmacotherapy for weight loss have limited their clinical use, particularly among medications with significant adrenergic actions (eg, sibutramine) or central appetite-suppressing actions (eg, rimonabant).54 Short-term clinical trials may not provide comprehensive information on the long-term safety of these agents, and prospective postmarketing surveillance studies are warranted.
This study has limitations. First, there was a paucity of direct comparative studies. Four of the 5 studied agents received approval from the FDA within the last 3 years, and because there is no established standard weight loss agent against which a new agent needs to be compared for approval, there is a paucity of head-to-head trials. Second, the biggest threat to validity of the results of any meta-analysis is conceptual heterogeneity–ie, considerable differences among trials in patient characteristics, studied interventions, cointerventions/background therapy, outcome assessment, or study design–which can limit the comparability of trials. Strategies to limit the effect of conceptual heterogeneity included strict inclusion and exclusion criteria and the use of multiple sensitivity analyses to assess the robustness of the results. Cointerventions in the studies, including diet and exercise recommendations and behavioral modification, were similar, although rigor of implementation and adherence by trial participants was not routinely measured, and their association with the relative efficacy of active interventions is unclear. Third, ranking probabilities may be affected by unequal numbers of trials per comparison, sample size of individual studies, network configuration, and effect sizes among treatments and should be interpreted with caution. Finally, all included trials had a high rate of attrition. Although statistical tools allowed interpretation of these data (using an LOCF imputation as suggested by the FDA guidelines), there are un addressed concerns regarding the long term effect of weight loss agents in a clinical setting.
Conclusions
Among overweight or obese adults, orlistat, lorcaserin, naltrexone-bupropion, phentermine-topiramate, and liraglutide, compared with placebo, were each associated with achieving at least 5% weight loss at 52 weeks. Phentermine-topiramate and liraglutide were associated with the highest odds of achieving at least 5% weight loss.
Supplementary Material
Acknowledgments
Funding/Support: Dr Singh is supported by National Library of Medicine training grant T15LM011271. Dr Dulai is supported by National Institute of Diabetes and Digestive and Kidney Diseases training grant 5T32DK007202.
Footnotes
Role of the Funders/Sponsors: The sponsors were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Author Contributions: Dr Singh had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Khera, Murad, Dulai, Loomba, Camilleri, Singh.
Acquisition, analysis, or interpretation of data: Khera, Murad, Chandar, Dulai, Wang, Prokop, Loomba, Singh.
Drafting of the manuscript: Khera, Murad, Dulai, Prokop, Singh.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Khera, Murad, Chandar, Wang, Singh.
Administrative, technical, or material support: Khera, Chandar.
Study supervision: Murad, Loomba, Singh.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Loomba reports research funded by the National Institutes of Health, National Science Foundation, and AGA-RSA; funding from Gilead, Merck, Promedior, Kinemed, Adheron, Tobira, Immuron, Siemens, GE, NGM Bio, Bristol-Myers Squibb, Arisaph, and Daiichi-Sankyo; participation in advisory committees for Galmed, Nimbus, Gilead, Bristol-Myers Squibb, Arrowhead Research, Conatus, and Tobira; consulting for Gilead, Bristol-Myers Squibb, Merck, Pfizer, Fibrogen, NGM Bio, Alnylam, DeuteRx, Zafgen, RuiYi, Shire, Scholar Rock, Metacrine, Viking, Receptos, Isis, Enanta, Celgene, Zafgen, Boehringer Ingelheim, Eli Lilly, Conatus, and Janssen; and is a cofounder of Liponexus Inc. Dr Camilleri reports conducting research on liraglutide, supported in part by NIH grant 2R56DK067071-11 and by NovoNordisk; VIVUS and NovoNordisk provided medication for research studies conducted in Dr Camilleri’s laboratory at Mayo Clinic. No other disclosures are reported.
References
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311(1):74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration. [Accessed April 26, 2016];FDA-approved drug products: Contrave. 2014 http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200063s000lbl.pdf.
- 4.US Food and Drug Administration. [Accessed April 26, 2016];FDA-approved drug products: Saxenda. 2015 http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206321s001lbl.pdf.
- 5.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 6.Khera R, Singh S, Chandar A, et al. [Accessed October 10, 2015];Comparative effectiveness of weight loss medications: a systematic review and network meta-analysis. 2015 http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015026114.
- 7.Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health. 2011;14(4):429–437. doi: 10.1016/j.jval.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Puhan MA, Schünemann HJ, Murad MH, et al. GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Center for Drug Evaluation and Research. [Accessed December 15, 2015];Guidance for Industry Developing Products for Weight Management. 2007 Feb; http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm071612.pdf.
- 11.Jensen MD, Ryan DH, Apovian CM, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation. 2014;129(25 suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. 2001;20(24):3875–3889. doi: 10.1002/sim.1009. [DOI] [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10):e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 18.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607–617. doi: 10.1177/0272989X12458724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPTGS. [Accessed March 24, 2016];Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011 Mar; http://handbook.cochrane.org/chapter_12/12_5_4_3_computing_absolute_risk_reduction_or_nnt_from_an_odds.htm.
- 21.White IR. Multivariate random-effects meta-regression: updates tomvmeta. Stata J. 2011;11(2):255. [Google Scholar]
- 22.Bakris G, Calhoun D, Egan B, Hellmann C, Dolker M, Kingma I Orlistat and Resistant Hypertension Investigators. Orlistat improves blood pressure control in obese subjects with treated but inadequately controlled hypertension. J Hypertens. 2002;20(11):2257–2267. doi: 10.1097/00004872-200211000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Berne C Orlistat Swedish Type 2 diabetes Study Group. A randomized study of orlistat in combination with a weight management programme in obese patients with type 2 diabetes treated with metformin. Diabet Med. 2005;22(5):612–618. doi: 10.1111/j.1464-5491.2004.01474.x. [DOI] [PubMed] [Google Scholar]
- 24.Broom I, Wilding J, Stott P, Myers N UK Multimorbidity Study Group. Randomised trial of the effect of orlistat on body weight and cardiovascular disease risk profile in obese patients: UK Multimorbidity Study. Int J Clin Pract. 2002;56(7):494–499. [PubMed] [Google Scholar]
- 25.Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA. 1999;281(3):235–242. doi: 10.1001/jama.281.3.235. [DOI] [PubMed] [Google Scholar]
- 26.Finer N, James WP, Kopelman PG, Lean ME, Williams G. One-year treatment of obesity: a randomized, double-blind, placebo-controlled, multicentre study of orlistat, a gastrointestinal lipase inhibitor. Int J Obes Relat Metab Disord. 2000;24(3):306–313. doi: 10.1038/sj.ijo.0801128. [DOI] [PubMed] [Google Scholar]
- 27.Hanefeld M, Sachse G. The effects of orlistat on body weight and glycaemic control in overweight patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2002;4(6):415–423. doi: 10.1046/j.1463-1326.2002.00237.x. [DOI] [PubMed] [Google Scholar]
- 28.Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med. 2000;9(2):160–167. doi: 10.1001/archfami.9.2.160. [DOI] [PubMed] [Google Scholar]
- 29.Hollander PA, Elbein SC, Hirsch IB, et al. Role of orlistat in the treatment of obese patients with type 2 diabetes: a 1-year randomized double-blind study. Diabetes Care. 1998;21(8):1288–1294. doi: 10.2337/diacare.21.8.1288. [DOI] [PubMed] [Google Scholar]
- 30.Kelley DE, Bray GA, Pi-Sunyer FX, et al. Clinical efficacy of orlistat therapy in overweight and obese patients with insulin-treated type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2002;25(6):1033–1041. doi: 10.2337/diacare.25.6.1033. [DOI] [PubMed] [Google Scholar]
- 31.Krempf M, Louvet JP, Allanic H, Miloradovich T, Joubert JM, Attali JR. Weight reduction and long-term maintenance after 18 months treatment with orlistat for obesity. Int J Obes Relat Metab Disord. 2003;27(5):591–597. doi: 10.1038/sj.ijo.0802281. [DOI] [PubMed] [Google Scholar]
- 32.Lindgärde F. The effect of orlistat on body weight and coronary heart disease risk profile in obese patients: the Swedish Multimorbidity Study. J Intern Med. 2000;248(3):245–254. doi: 10.1046/j.1365-2796.2000.00720.x. [DOI] [PubMed] [Google Scholar]
- 33.Miles JM, Leiter L, Hollander P, et al. Effect of orlistat in overweight and obese patients with type 2 diabetes treated with metformin. Diabetes Care. 2002;25(7):1123–1128. doi: 10.2337/diacare.25.7.1123. [DOI] [PubMed] [Google Scholar]
- 34.Rössner S, Sjöström L, Noack R, Meinders AE, Noseda G European Orlistat Obesity Study Group. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. Obes Res. 2000;8(1):49–61. doi: 10.1038/oby.2000.8. [DOI] [PubMed] [Google Scholar]
- 35.Sjöström L, Rissanen A, Andersen T, et al. European Multicentre Orlistat Study Group. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. Lancet. 1998;352(9123):167–172. doi: 10.1016/s0140-6736(97)11509-4. [DOI] [PubMed] [Google Scholar]
- 36.Swinburn BA, Carey D, Hills AP, et al. Effect of orlistat on cardiovascular disease risk in obese adults. Diabetes Obes Metab. 2005;7(3):254–262. doi: 10.1111/j.1463-1326.2004.00467.x. [DOI] [PubMed] [Google Scholar]
- 37.Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. Xenical in the Prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 38.Fidler MC, Sanchez M, Raether B, et al. BLOSSOM Clinical Trial Group. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96(10):3067–3077. doi: 10.1210/jc.2011-1256. [DOI] [PubMed] [Google Scholar]
- 39.O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012;20(7):1426–1436. doi: 10.1038/oby.2012.66. [DOI] [PubMed] [Google Scholar]
- 40.Smith SR, Weissman NJ, Anderson CM, et al. Behavioral Modification and Lorcaserin for Overweight and Obesity Management Study Group. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363(3):245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 41.Apovian CM, Aronne L, Rubino D, et al. COR-II Study Group. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II) Obesity (Silver Spring) 2013;21(5):935–943. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenway FL, Fujioka K, Plodkowski RA, et al. COR-I Study Group. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 43.Hollander P, Gupta AK, Plodkowski R, et al. COR-Diabetes Study Group. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12):4022–4029. doi: 10.2337/dc13-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wadden TA, Volger S, Sarwer DB, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365(21):1969–1979. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity (Silver Spring) 2012;20(2):330–342. doi: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 47.Davies MJ, Bergenstal R, Bode B, et al. NN8022-1922 Study Group. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–699. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 48.Pi-Sunyer X, Astrup A, Fujioka K, et al. SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 49.Astrup A, Carraro R, Finer N, et al. NN8022-1807 Investigators. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 2012;36(6):843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Stat Sci. 1992;7(4):457–472. [Google Scholar]
- 51.US Preventive Services Task Force. Screening for obesity in adults: recommendations and rationale. Ann Intern Med. 2003;139(11):930–932. doi: 10.7326/0003-4819-139-11-200312020-00012. [DOI] [PubMed] [Google Scholar]
- 52.Apovian CM, Aronne LJ, Bessesen DH, et al. Endocrine Society. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342–362. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 53.Camilleri M, Acosta A. Gastrointestinal traits: individualizing therapy for obesity with drugs and devices. Gastrointest Endosc. 2016;83(1):48–56. doi: 10.1016/j.gie.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daubresse M, Alexander GC. The uphill battle facing antiobesity drugs. Int J Obes (Lond) 2015;39(3):377–378. doi: 10.1038/ijo.2014.169. [DOI] [PubMed] [Google Scholar]
- 55.Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: ameta-analysis. JAMA. 2014;312(9):923–933. doi: 10.1001/jama.2014.10397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.