Abstract
As adults, we have structured conceptual representations of our emotions that help us to make sense of and regulate our ongoing affective experience. The ability to use emotion concepts is critical to make predictions about the world and choose appropriate action, such as ‘I am afraid, and going to run away’ or ‘I am hungry and going to eat’. Thus, emotion concepts have an important role in helping us maintain our ongoing physiological balance, or allostasis. We will suggest here that infants can learn emotion concepts for the purpose of allostasis regulation, and that conceptualization is key component in emotional development. Moreover, we will suggest that social dyads facilitate concept learning because of a robust evolutionary feature seen in newborns of social species: they cannot survive alone and depend on conspecifics for allostasis regulation. Such social dependency creates a robust driving force for social learning of emotion concepts, and makes the social dyad, which is designed to regulate the infant’s allostasis, an optimal medium for concept learning. In line with that, we will review evidence showing that the neural reference space for emotion overlaps with neural circuits that support allostasis (striatum, amygdala, hypothalamus) and conceptualization (medial prefrontal cortex, posterior cingulate cortex), and that their developmental trajectories are interrelated, and depend on synchronous social care.
Self-regulation in Adulthood Biologically Depends on Social Regulation in Early Life
Among social species, such as mammals and most birds, newborns cannot survive on their own, and completely rely on a dedicated caregiver to regulate their ongoing physiological balance, or allostasis **[1, 2]. Newborns need social assistance in regulating their energy expenditure, temperature and immune function **[3], and accordingly, early life care ensures the newborn’s survival and growth. Importantly, while mothers repeatedly regulate their offspring’s allostasis, they also foster the development of self-regulation *[4, 5]. Offspring brain development depends on the provisioning of adequate maternal care in early life, particularly in neural circuitry involved in allostasis, including the nucleus accumbens (NAcc), amygdala, and hypothalamus-pituitary-adrenal axis (HPA axis) **[4, 6, 7]. It was demonstrated in mice that insufficient regulation of pups’ allostasis, such as in cases of maternal separation [8] or low levels of maternal care [9], cause lasting modifications of allostasis regulation system, reducing its efficiency to accommodate stressful events **[6]. Specifically, insufficient maternal care causes increased sensitivity of neuroendocrine and behavioral stress response, including long term increase in HPA axis reactivity, changes in glucocorticoid receptor expression in the hippocampus, and increased basal levels of corticosterone [8], all key allostatic agents **[6]. The manifestations of such neuroendocrine changes relate to the animal’s behavioral phenotype of self-regulation, including decreased exploration and increased inhibition behaviors in pups *[10]. In addition to these HPA effects, early life social care has consequences for the developmental trajectory of limbic-motivational regions supporting allostasis, and shapes behavior. For example, offspring of low maternal behavior dams show a decreased density of benzodiazepine receptors in the amygdala, less expression of estrogen receptors in the hypothalamus, and altered dopaminergic release in reward regions, behaviorally manifested by increased indices of anxiety-like behavior and decreased maternal sensitivity later in life (for review see *[10]). Thus, the development of the neural infrastructure needed for allostatic self-regulation in adulthood depends on social regulation of allostasis in early life, which also “programs” the offspring’s long-term behavioral phenotype of self-regulation.
Synchrony is a Strategy for Social Regulation of Allostasis
One efficient strategy to regulate someone else’s allostasis is with bio-behavioral synchrony. Parent-infant bio-behavioral synchrony is the matching of behavior, affective states, and biological rhythms between parent and child, organized in an ongoing coherent pattern **[11]. In humans, parent-infant synchrony has been thoroughly studied and synchronous parenting behavior was found to be a reliable proxy for parent-infant attachment, and reflects parental sensitivity and attunement to the infant **[11]. Synchrony predicts optimal emotional development, including the ability to recognize emotion in others and to self-regulate emotion **[11]. Synchronous caregiving is often considered the source of infants’ affective regulation, which sets the ground for optimal emotional development **[11]. On the contrary, children raised without experiencing sufficient social synchrony will suffer from atypical emotional development **[11, 12]. However, while parent-infant synchrony is established as important for attachment and affective regulation, we propose here that synchrony regulates much more than the infant’s affect; synchrony is evolutionarily predisposed to keep infants alive. Starting from gestation, a mother controls her fetus’s allostasis via mother-fetus physiological synchronization [13]. After birth, mothers continue to regulate the infants’ allostasis [14–16] using the same synchrony strategy. Mothers regulate their infants’ temperature by holding them close so that their temperatures synchronize [15]. Mothers regulate their infants’ arousal with voice (by singing, or speaking loudly or softly) [17], synchronizing their heart rates [18]. Mothers regulate their infants’ immune function by breastfeeding, synchronizing their gut-macrobiota and antigen-specific antibodies [19]. Synchrony is an efficient strategy for social regulation of allostasis in multiple physiological systems. Critically, when a caretaker consistently cares for the infant via synchrony, in addition to ensuring survival, she/he implicitly creates an optimal environment for learning.
Synchronous Care Facilitates Learning of Abstract (Emotion) Concepts
Early social care determines the development of offspring self-regulation [20]. In contrast with other species, humans are special because they have the cognitive ability to link abstracti concepts to the regulation of allostasis. Infants learn to categorize information based on co-occurrence probability via statistical learning [23–25]. Detecting structure within the environment is a critical step in development, from a meaningless stream of unpredicted sensory information to populations of instances grouped into categories that can be mentally represented as concepts [25–27]. (For example, with experience the spatial statistical regularity in facial features perceived by the infant, will gradually be conceptualized as a ‘face’). Within social dyads, caretakers implicitly facilitate statistical learning, by providing temporal conditioning between concept learning and the reward gained from social interaction. This is consistent with the broader view that infants are “rational constructivists” who are actively sampling information in their environment [28]. Caretakers act as both the tutor (introducing new concepts, often with language) and the reinforcement (via the social regulation of allostasis). Since allostasis is regulated by a social agent, certain social concepts appear to be the first and most robust concepts to be learned: the conditioning between allostasis and human will result in a rapid and powerful learning of a fundamental social concept: mommy (i.e., the agent that repeatedly makes it all better) [29]. Similarly, this concept can be daddy, or other caretaker(s) who consistently meets the allostatic needs of the infant. Once fundamental social concepts for caregivers are learned, infants will gradually acquire other culturally relevant concepts, introduced by caretakers, and aid in the regulation of allostasis.
Emotion concepts are among the concepts that best predict an individuals’ ability to regulate their own allostasis throughout the lifespan [30]. Affect can be thought of as the interoceptive consequences of allostatic changes. As such, the properties of affect, namely valence and arousal, can be defined using the concept allostasis: valence represents the subjective experience of deviation from allostatic (negative valence) or regaining allostasis (positive affect), while arousal represents the amplitude of the change. Emotion concepts help to organize affective states within a given situation into meaningful events based on past experiences. These concepts convey and organize information about eliciting circumstances, actions and predicted outcomes. Critically, emotion concepts are not static representations, they are flexible predictions that are populated by a set of variable instances—they are grounded [31] by modality specific information tied to the situations in which they occur [32, 33]. Simply invoking an emotion concept can have a powerful impact on the experience or perception of affective (i.e., allostatically relevant) information [34]. As such, emotion concepts shape behavior and physiology [35], serving as tools to regulate allostasis.
Beginning in early development, emotion concepts are built in the context of social dyads. Infants learn new concepts by synchronizing their attention with others, during instances of joint attention [36] and by harnessing the power of language. Emotion categories are not made of homogenous instances of experience, and not all instances within an emotion category (for example ‘fear’) look alike, feel alike, or have the same biological signature [37]. We have proposed that language (including words for emotions) helps to overcome the abstract, varied and situated nature of emotional instances *[37, 38]. Words overcome variation by serving as an “essence placeholder” or “glue” to join the instances into a category [39]. Acquisition of emotion concepts appears to start broadly. Rudimentary concepts for affective states-- displeasure and pleasure reliably appear first **[40], and broadly indicate allostatic status (e.g., negative affect- deviation from allostasis versus positive affect- regaining allostasis). Detailed emotion concepts (e.g., anger or sadness) emerge more slowly over early childhood **[40]. In the social dyad, language use guides the complex statistical learning about emotions [41]. Caretaker use of emotion words (as well as other mental language, including words for thoughts and desires) predicts later offspring use of emotion terms [42]. Importantly, early caregiver use of emotion words is most frequently directed at labeling the infant’s state (as opposed to self-labeling in the adult) in the context of regulation [43], serving to directly tie these concepts to an infant’s allostasis. Moreover, prevalence of joint- attention in the social-dyad within the first year of life predicts the degree to which a child uses emotion language later on [44]. Thus, the social dyad is a vehicle that promotes the temporal conditioning between the use of emotion concepts and allostasis regulation. In synchronous dyads, this emotion concept learning is augmented by reinforcement from allostasis regulation, such that synchronous care facilitates the development of functional abstract (e.g., emotion) concepts.
Caregiver construction of emotions is a social strategy designed to acknowledge and eventually regulate the infant’s allostasis **[6]. The caretaker, who is attuned to their infant’s allostasis, constantly organizes their infant’s momentary subjective experience into constructed emotion categories. Humans can learn to categorize instances of their own affective experience into emotion categories by relying on these early experiences conferred by the social dyad [45]. Children gradually learn to share their own emotions with others, and later to detect and share the emotions of others [46, 47]. As such, emotion is inherently a social feature of experience. Learning to independently categorize subjective experience into emotion categories is a milestone in emotion development [48, 49], which depends on synchronous care (Figure 1). Correspondingly, emotion concepts also vary considerably cross-culturally [50] because the situations, actions, social perspectives, etc., that are highlighted by a shared concept vary based on the values and structure of a culture. Different cultures use different modes of regulating the nervous system with concepts. For example some cultures appear to conceptualize “affective” events with less mentalization but more action concepts *[51, 52], suggesting that there may be uncharted cultural variability in allostasis regulation, and the optimal trajectory for the social dyadic continuum (Figure 1).
Figure 1. A dyadic continuum of emotion development.
Infants’ developmental milestones are contingent on caregiver provisioning of allostasis regulation and language use. As caregivers regulate the infants’ allostasis, infants gain experience in the rudimentary social skill of synchrony. Thereafter, as attention develops [53], infants learn to share their attention with the caretaker [54], and to synchronize conceptual knowledge. Synchronous parenting fosters the child’s ability to use concepts *[55], and parental use of mental state language promote children to label their own emotions [42] and later to recognize and represent other people’s mental states [42, 43, 46]. Children rely on the social conditioning between emotion concepts and allostasis for the development of social cognition, as they learn to use emotion concepts for understanding and regulating other people’s allostasis. The color gradient represents the development of concepts; darker color indicates the infant’s growing ability to represent concepts and therefore engage in self-regulation. Synchronous caregivers carefully adjust their input per the infant’s developmental stage.
Caregiver Processes Are Critical for Conceptualization and Emotion Development
As illustrated in Figure 1a cascade of caregiver processes unfold across development, which promote conceptualization, and allow for emotion development in the infant to occur. Variability in the quality of caregiver processes can impact this developmental trajectory of emotion. In cases of insufficient maternal regulation, the optimal developmental trajectory shifts to place infants at risk of psychopathology [56]. It was recently demonstrated that mothers who suffer from post-partum depression are less synchronous with their infants, and such reduced social synchrony disrupts the infant’s emotion regulation *[57]. The authors suggested that compromised regulation of emotion is behaviorally transferred from the depressed mother to her infant. It was separately demonstrated that parent-infant synchrony promotes children’s use of concepts *[12], and reduced maternal responsiveness among post-partum depressed mothers impairs infants’ concept learning [58]. Mothers suffering from post-partum depression use less infant directed speech and show difficulty in establishing and maintaining events of joint attention *[55]. Accordingly, post-partum maternal depression adversely affects infants’ language development *[55]. Moreover, aberrant social experience among abused children is associated with children’s perceptions of emotion expressions, such as higher sensitivity to anger cues *[59]. Importantly, lack of early emotion socialization can be remediated in part by later caregiver use of emotion language (i.e., introduced to adopted children at 3 years of age) [60], suggesting some flexibility in the ideal developmental trajectory, and marking the importance of childhood experience in emotion development.
Building on this evidence that social experience and maternal care are crucial for both concept and emotion development, it is suggested here that synchrony, emotion and conceptualization are interrelated. We propose that synchronous care supports optimal emotion development because it fosters the conceptualization of emotional events, which is critical for self-regulation. The shift in emotion development seen in pathological dyads could be the result of impaired conceptualization due to insufficient maternal regulation. Thus, the mechanistic role of conceptualization in emotion development across childhood warrants future empirical investigations.
Common Neural Reference Space for Emotion, Allostasis, Conceptualization and Synchronous Maternal Care
Inspecting the neural circuits that support conceptualization of emotion corresponds with the theoretical and empirical links between emotion concepts and allostasis. The neural reference space for emotions, as assessed in neuroimaging meta-analysis **[61], can be decomposed to regions associated with conceptualization, including the medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC) [62], and regions associated with allostasis, particularly the striatum, hypothalamus and amygdala **[6]. These regions are consistently recruited in studies of any discrete emotion category, including fear, disgust, happiness, sadness and anger **[61]. Thus, these regions are not specific to one emotion category, but instead are involved when emotions are conceptualized as a category. This suggests that humans can associate neural features supporting allostasis and conceptualization when categorizing emotions.
The use of concepts for allostasis regulation is not suggested to be specific to emotion. Instead it is suggested to be a domain general mechanism for regulation and learning. Conceptualization supports social and self-regulation, while allostasis regulation reinforces and fosters concept learning. The same subcortical neural circuits associated with allostasis and cortical circuits associated with conceptualization (both consistently involved in emotion), are also involved in social processing [63], and specifically in synchronous mothering, as demonstrated in mothers’ brains *[64]. Synchronous mothers who are extremely attuned to their infants’ allostasis have increased neural connectivity between the cortical circuit supporting conceptualization (mPFC and PCC) and the subcortical circuit supporting allostasis (NAcc, hypothalamus and amygdala) *[64]. Moreover, highly synchronous mothers show stronger striatal dopaminergic responses to their infant *[64]. Striatal dopamine, which was linked to maternal synchrony *[64], is a key allostatic agent [65–67]. Thus, it seems that neural circuits that support allostasis regulation and those supporting conceptualization are domain general neural circuits, which interact to realize different kinds of experiences, including emotion (regulation) and social regulation (Figure 2).
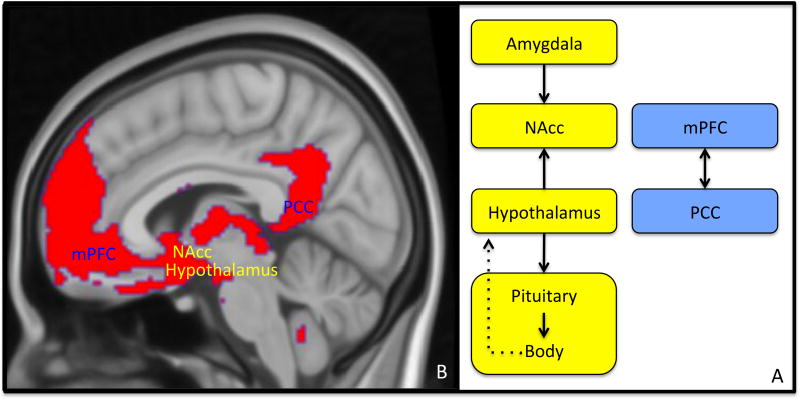
Figure 2. Neural circuits supporting allostasis and conceptualization associate in the human brain to support multiple mental experiences, including emotion.
A) A neural model for allostasis (yellow) and conceptualization (blue). The amygdala, nucleus accumbens (NAcc), hypothalamus and connecting pituitary secretory gland are involved in allostasis regulation [6]. The hypothalamus-pituitary endocrine system is a brain-body feedback pathway, which regulates the adrenal gland (via the HPA axis), but also gonadal and thyroid function, altogether controlling multiple allostatic processes including growth, reproduction, immunity, stress and metabolism [68]. The medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC) have been shown to be involved in humans’ mental ability to hold and use internal representations of concepts [62, 69]. B) In humans, these regions are intrinsically connected, forming a neural network [63] that associates limbic circuits (in yellow) with cortical circuits (in blue), potentially demonstrating a functional association between allostasis and conceptualization. Neural function in this circuitry has been associated with different experiences, including emotion **[70], social functioning [71] and synchronous bonding *[64].
Similar to the developmental trajectory of emotion, the neural circuitry that supports conceptualization takes years to develop [72]. The neural association between the PCC and mPFC, is not wired at birth **[73] and strengthens linearly throughout adolescence [74]. Interestingly, there is a temporal link between the maturation of this mPFC-PCC circuit and children’s ability to use concepts [75, 76], and to self-regulate their emotions **[77]. In addition to the cortical circuit potentially supporting conceptualization, the cortico-limbic association, which potentially links conceptualization to allostasis, also takes years to develop. The mesolimbic dopaminergic system, which has a role in detecting allostatic deviations [78], matures very early in life, whereas mesocortical system, involved in cortico-limbic integration, continue to develop into early adulthood [67]. It was recently documented that in the presence of mothers, children not only show improved affective regulation, they also show improved neural connectivity between the amygdala and mPFC **[79]. This suggests that caregivers support the infant’s self- regulation via consolidation of the infants’ cortico-limbic pathways **[79], which potentially links allostasis regulation to conceptualization.
Conclusions
Evidence suggests that while mothers synchronously care for their infants, using brain circuitry involved in conceptualization and allostasis *[64], infants gradually develop the equivalent neural circuitry [73], providing the infrastructure for cognitive and emotional skills. With maturation of this neural circuitry, children develop independence in using emotion concepts to regulate their own allostasis. Moreover, with synchronous care, the social dyad becomes a template: children are experientially trained to become social experts, eventually learning to use emotion concepts to regulate their own, and other people’s allostasis. This hypothesized mechanism for intergenerational transmission of emotion incorporates the important epigenetic and post-natal influences on emotion development, and points to future research that will assess emotion as an acquired scheme of culturally relevant concepts.
Highlights.
Newborns vitally depend on a caregiver for physiological regulation, or allostasis.
Parent-infant Bio-Behavioral Synchrony (e.g., matching behavior, affective states, and biological rhythms) supports the ongoing regulation of the infant’s allostasis.
Social interactions are rewarding because allostasis-regulation is a natural reinforcement.
Social interaction facilitates emotion development by reinforcing learning of emotion concepts with allostasis-mediated social reward.
Brain circuits involved in emotion overlap with the circuits for allostasis and conceptualization.
The role of social interaction in shaping emotion development highlights emotions as learned concepts.
Acknowledgments
Preparation of this manuscript was supported by a grant from National Institute of Child Health and Human Development (R21HD076164) awarded to SA and a National Institute of Mental Health NRSA fellowship (5F32MH105052) awarded to MG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Here we use the term abstract to refer to emotion concepts as relatively less physically or spatially constrained than other more “concrete” concepts. This does not imply that these concepts cannot also be ‘grounded’ in the sense that their representation relies on whole brain patterns of activity and impacts the periphery. Indeed, there is ample evidence that emotion concepts are grounded in different sensory and motor modalities 21. Fernandino L, Humphries CJ, Conant LL, Seidenberg MS, Binder JR: Heteromodal cortical areas encode sensory-motor features of word meaning. Journal of Neuroscience 2016, 36:9763-9769.(for review see 22. Niedenthal PM: Emotion concepts. Handbook of emotions 2008:587-600.).
References
- *1.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones and Behavior. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- *2.Sterling P. Allostasis: A model of predictive regulation. Physiology & Behavior. 2012;106:5–15. doi: 10.1016/j.physbeh.2011.06.004. [DOI] [PubMed] [Google Scholar]
- *3.Winberg J. Mother and newborn baby: mutual regulation of physiology and behavior—a selective review. Developmental psychobiology. 2005;47:217–229. doi: 10.1002/dev.20094. [DOI] [PubMed] [Google Scholar]
- *4.Sanchez MM, McCormack KM, Howell BR. Social buffering of stress responses in nonhuman primates: Maternal regulation of the development of emotional regulatory brain circuits. Soc Neurosci. 2015;10:512–526. doi: 10.1080/17470919.2015.1087426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *5.Meaney MJ. Mother nurture and the social definition of neurodevelopment. Proceedings of the National Academy of Sciences. 2016:201605859. doi: 10.1073/pnas.1605859113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **6.Ganzel BL, Morris PA, Wethington E. Allostasis and the human brain: Integrating models of stress from the social and life sciences. Psychol Rev. 2010;117:134–174. doi: 10.1037/a0017773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murgatroyd C, Spengler D. Epigenetics of early child development. Front Psychiatry. 2011;2:16. doi: 10.3389/fpsyt.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29:386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *10.Curley JP, Champagne FA. Influence of maternal care on the developing brain: Mechanisms, temporal dynamics and sensitive periods. Frontiers in neuroendocrinology. 2016;40:52–66. doi: 10.1016/j.yfrne.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **11.Feldman R. Parent-infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. J Child Psychol Psychiatry. 2007;48:329–354. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- *12.Feldman R. On the origins of background emotions: from affect synchrony to symbolic expression. Emotion. 2007;7:601–611. doi: 10.1037/1528-3542.7.3.601. [DOI] [PubMed] [Google Scholar]
- 13.Rao PNS, Shashidhar A, Ashok C. In utero fuel homeostasis: Lessons for a clinician. Indian Journal of Endocrinology and Metabolism. 2013;17:60–68. doi: 10.4103/2230-8210.107851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogt Merly C, Paeger L, Hess S, Steculorum Sophie M, Awazawa M, Hampel B, Neupert S, Nicholls Hayley T, Mauer J, Hausen AC, et al. Neonatal Insulin Action Impairs Hypothalamic Neurocircuit Formation in Response to Maternal High-Fat Feeding. Cell. 156:495–509. doi: 10.1016/j.cell.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin BE. Metabolic imprinting: critical impact of the perinatal environment on the regulation of energy homeostasis. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2006;361:1107–1121. doi: 10.1098/rstb.2006.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauman D. Regulation of nutrient partitioning during lactation: homeostasis and homeorhesis revisited. Ruminant physiology: digestion, metabolism, growth and reproduction. 2000:311–328. [Google Scholar]
- 17.Nakata T, Trehub SE. Infants’ responsiveness to maternal speech and singing. Infant Behavior and Development. 2004;27:455–464. [Google Scholar]
- 18.Feldman R, Magori-Cohen R, Galili G, Singer M, Louzoun Y. Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant behavior & development. 2011;34:569–577. doi: 10.1016/j.infbeh.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Front Immunol. 2014;5:427. doi: 10.3389/fimmu.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman R, Rosenthal Z, Eidelman AI. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biological Psychiatry. 2014;75:56–64. doi: 10.1016/j.biopsych.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Fernandino L, Humphries CJ, Conant LL, Seidenberg MS, Binder JR. Heteromodal cortical areas encode sensory-motor features of word meaning. Journal of Neuroscience. 2016;36:9763–9769. doi: 10.1523/JNEUROSCI.4095-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niedenthal PM. Emotion concepts. Handbook of emotions. 2008:587–600. [Google Scholar]
- 23.Siegelman N, Frost R. Statistical learning as an individual ability: Theoretical perspectives and empirical evidence. Journal of Memory and Language. 2015;81:105–120. doi: 10.1016/j.jml.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krogh L, Vlach HA, Johnson SP. Statistical learning across development: flexible yet constrained. Front Psychol. 2012;3:598. doi: 10.3389/fpsyg.2012.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saffran JR, Aslin RN, Newport EL. Statistical learning by 8-month-old infants. Science. 1996;274:1926–1928. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- 26.Tenenbaum JB, Kemp C, Griffiths TL, Goodman ND. How to grow a mind: statistics, structure, and abstraction. Science. 2011;331:1279–1285. doi: 10.1126/science.1192788. [DOI] [PubMed] [Google Scholar]
- 27.Aslin RN, Newport EL. Statistical learning: From acquiring specific items to forming general rules. Curr Dir Psychol Sci. 2012;21:170–176. doi: 10.1177/0963721412436806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu F, Kushnir T. Infants are rational constructivist learners. Current Directions in Psychological Science. 2013;22:28–32. [Google Scholar]
- 29.Welles-Nyström B, New R, Richman A. The ‘Good Mother’. Scandinavian Journal of Caring Sciences. 1994;8:81–86. doi: 10.1111/j.1471-6712.1994.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 30.Barrett LF. How emotions are made. Houghton Mifflin Harcourt; 2017. [Google Scholar]
- 31.Barsalou LW. Grounded cognition. Annual Review of Psychology. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- 32.Wilson-Mendenhall CD, Barrett LF, Barsalou LW. Situating emotional experience. Frontiers in Human Neuroscience. 2013;7:1–16. doi: 10.3389/fnhum.2013.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oosterwijk S, Mackey S, Wilson-Mendenhall C, Winkielman P, Paulus MP. Concepts in context: Processing mental state concepts with internal or external focus involves different neural systems. Social neuroscience. 2015;10:294–307. doi: 10.1080/17470919.2014.998840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindquist KA, MacCormack JK, Shablack H. The role of language in emotion: predictions from psychological constructionism. Front Psychol. 2015;6:444. doi: 10.3389/fpsyg.2015.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winkielman P, Niedenthal P, Wielgosz J, Eelen J, Kavanagh LC. Embodiment of cognition and emotion. APA handbook of personality and social psychology. 2015;1:151–175. [Google Scholar]
- 36.Tomasello M. Joint attention as social cognition. Joint attention: Its origins and role in development. 1995:103–130. [Google Scholar]
- *37.Barrett LF, Lindquist KA, Gendron M. Language as context for the perception of emotion. Trends in Cognitive Sciences. 2007;11:327–332. doi: 10.1016/j.tics.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *38.Lindquist KA, Satpute AB, Gendron M. Does language do more than communicate emotion? Curr Dir Psychol Sci. 2015;24:99–108. doi: 10.1177/0963721414553440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu F. The role of language in acquiring object kind concepts in infancy. Cognition. 2002;85:223–250. doi: 10.1016/s0010-0277(02)00109-9. [DOI] [PubMed] [Google Scholar]
- **40.Widen SC, Russell JA. Children acquire emotion categories gradually. Cognitive development. 2008;23:291–312. [Google Scholar]
- 41.Ruffman T, Taumoepeau M, Perkins C. Statistical learning as a basis for social understanding in children. British Journal of Developmental Psychology. 2012;30:87–104. doi: 10.1111/j.2044-835X.2011.02045.x. [DOI] [PubMed] [Google Scholar]
- 42.Taumoepeau M, Ruffman T. Stepping stones to others' minds: Maternal talk relates to child mental state language and emotion understanding at 15, 24, and 33 months. Child Development. 2008;79:284–302. doi: 10.1111/j.1467-8624.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- 43.Brown JR, Dunn J. 'You can, cry, mum': The social and developmental implications of talk about internal states. British Journal of Developmental Psychology. 1991;9:237–256. [Google Scholar]
- 44.Brooks R, Meltzoff AN. Connecting the dots from infancy to childhood: A longitudinal study connecting gaze following, language, and explicit theory of mind. Journal of Experimental Child Psychology. 2015;130:67–78. doi: 10.1016/j.jecp.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q. “Did you have fun?”: American and Chinese mother–child conversations about shared emotional experiences. Cognitive Development. 2001;16:693–715. [Google Scholar]
- 46.Rollo D, Sulla F. Maternal Talk in Cognitive Development: Relations between Psychological Lexicon, Semantic Development, Empathy, and Temperament. Frontiers in psychology. 2016;7 doi: 10.3389/fpsyg.2016.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adamson LB, Russell CL. Emotion regulation and the emergence of joint attention. Early social cognition: Understanding others in the first months of life. 1999:281–297. [Google Scholar]
- 48.Smiley P, Huttenlocher J. Young children’s acquisition of emotion concepts. Children’s understanding of emotion. 1989:27–49. [Google Scholar]
- 49.Balconi M, Amenta S, Ferrari C. Emotional decoding in facial expression, scripts and videos: A comparison between normal, autistic and Asperger children. Research in Autism Spectrum Disorders. 2012;6:193–203. [Google Scholar]
- 50.Russell JA. Culture and the categorization of emotions. Psychol Bull. 1991;110:426–450. doi: 10.1037/0033-2909.110.3.426. [DOI] [PubMed] [Google Scholar]
- *51.Gendron M, Roberson D, van der Vyver JM, Barrett LF. Perceptions of emotion from facial expressions are not culturally universal: Evidence from a remote culture. Emotion. 2014;14:251–262. doi: 10.1037/a0036052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *52.Gendron M, Roberson D, van der Vyver JM, Barrett LF. Cultural relativity in perceiving emotion from vocalizations. Psychological science. 2014;25:911–920. doi: 10.1177/0956797613517239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amso D, Scerif G. The attentive brain: insights from developmental cognitive neuroscience. Nat Rev Neurosci. 2015;16:606–619. doi: 10.1038/nrn4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Charman T, Swettenham J, Baron-Cohen S, Cox A, Baird G, Drew A. Infants with autism: an investigation of empathy, pretend play, joint attention, and imitation. Dev Psychol. 1997;33:781–789. doi: 10.1037//0012-1649.33.5.781. [DOI] [PubMed] [Google Scholar]
- *55.Sohr-Preston SL, Scaramella LV. Implications of timing of maternal depressive symptoms for early cognitive and language development. Clin Child Fam Psychol Rev. 2006;9:65–83. doi: 10.1007/s10567-006-0004-2. [DOI] [PubMed] [Google Scholar]
- 56.Leerkes EM, Su J, Calkins SD, O'Brien M, Supple AJ. Maternal physiological dysregulation while parenting poses risk for infant attachment disorganization and behavior problems. Dev Psychopathol. 2016:1–13. doi: 10.1017/S0954579416000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *57.Granat A, Gadassi R, Gilboa-Schechtman E, Feldman R. Maternal Depression and Anxiety, Social Synchrony, and Infant Regulation of Negative and Positive Emotions. 2016 doi: 10.1037/emo0000204. [DOI] [PubMed] [Google Scholar]
- 58.Milgrom J, Westley DT, Gemmill AW. The mediating role of maternal responsiveness in some longer term effects of postnatal depression on infant development. Infant Behavior and Development. 2004;27:443–454. [Google Scholar]
- *59.Pollak SD, Kistler DJ. Early experience is associated with the development of categorical representations for facial expressions of emotion. Proceedings of the National Academy of Sciences. 2002;99:9072–9076. doi: 10.1073/pnas.142165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tarullo AR, Youssef A, Frenn KA, Wiik K, Garvin MC, Gunnar MR. Emotion understanding, parent mental state language, and behavior problems in internationally adopted children. Development and Psychopathology. 2015;28:1–13. doi: 10.1017/S095457941500111X. [DOI] [PubMed] [Google Scholar]
- **61.Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. The Behavioral and brain sciences. 2012;35:121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 63.Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC. Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:14729–14741. doi: 10.1523/JNEUROSCI.1599-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *64.Atzil S, Touroutoglou A, Rudy T, Salcedo S, Feldman R, Hooker J, Dickerson BC, Catana C, Barrett LF. The Chemistry of Human Bonding: A Role for Dopamine. doi: 10.1073/pnas.1612233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.George O, Le Moal M, Koob GF. Allostasis and addiction: role of the dopamine and corticotropin-releasing factor systems. Physiology & behavior. 2012;106:58–64. doi: 10.1016/j.physbeh.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 67.Beauchaine TP, Neuhaus E, Zalewski M, Crowell SE, Potapova N. The effects of allostatic load on neural systems subserving motivation, mood regulation, and social affiliation. Development and Psychopathology. 2011;23:975–999. doi: 10.1017/S0954579411000459. [DOI] [PubMed] [Google Scholar]
- 68.Sower SA, Freamat M, Kavanaugh SI. The origins of the vertebrate hypothalamic–pituitary–gonadal (HPG) and hypothalamic–pituitary–thyroid (HPT) endocrine systems: new insights from lampreys. General and comparative endocrinology. 2009;161:20–29. doi: 10.1016/j.ygcen.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 69.Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- **70.Lindquist KA, Barrett LF. A functional architecture of the human brain: emerging insights from the science of emotion. Trends in cognitive sciences. 2012;16:533–540. doi: 10.1016/j.tics.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bickart KC, Dickerson BC, Barrett LF. The amygdala as a hub in brain networks that support social life. Neuropsychologia. 2014;63:235–248. doi: 10.1016/j.neuropsychologia.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. NeuroImage. 2010;52:290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **73.Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, Lin W. Evidence on the emergence of the brain's default network from 2-week-old to 2-year-old healthy pediatric subjects. Proceedings of the National Academy of Sciences. 2009;106:6790–6795. doi: 10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain's default network. Proceedings of the National Academy of Sciences. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fransson P, Skiöld B, Horsch S, Nordell A, Blennow M, Lagercrantz H, Åden U. Resting-state networks in the infant brain. Proceedings of the National Academy of Sciences. 2007;104:15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blakemore SJ, den Ouden H, Choudhury S, Frith C. Adolescent development of the neural circuitry for thinking about intentions. Soc Cogn Affect Neurosci. 2007;2:130–139. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **77.McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Gabrieli JD, Ochsner KN. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Social cognitive and affective neuroscience. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- **79.Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Flannery J, Lumian DS, Fareri DS, Caldera C, et al. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol Sci. 2014;25:2067–2078. doi: 10.1177/0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]