Abstract
Little information is available about the bioaccumulation and biomagnification of antibiotics in marine food webs. Here we investigate the levels and trophic transfer of 9 sulfonamide (SA), 5 fluoroquinolone (FQ), and 4 macrolide (ML) antibiotics, as well as trimethoprim in nine invertebrate and ten fish species collected from a marine food web in Laizhou Bay, North China in 2014 and 2015. All the antibiotics were detected in the marine organisms, with SAs and FQs being the most abundant antibiotics. Benthic fish accumulated more SAs than invertebrates and pelagic fish, while invertebrates exhibited higher FQ levels than fish. Generally, SAs and trimethoprim biomagnified in the food web, while the FQs and MLs were biodiluted. Trophic magnification factors (TMF) were 1.2 – 3.9 for SAs and trimethoprim, 0.3 – 1.0 for FQs and MLs. Limited biotransformation and relatively high assimilation efficiencies are the likely reasons for the biomagnification of SAs. The pH dependent distribution coefficients (logD) but not the lipophilicity (logKOW) of SAs and FQs had a significant correlation (r = 0.73; p < 0.05) with their TMFs. Although the calculated estimated daily intakes (EDI) for antibiotics suggest that consumption of seafood from Laizhou Bay is not associated with significant human health risks, this study provides important insights into the guidance of risk management of antibiotics.
Keywords: Antibiotics, Biomagnification, Health risk, Trophic dilution
Graphical abstract
INTRODUCTION
Antibiotics are largely used in human medicine, animal husbandry, agriculture and aquaculture. In 2013 alone, China, the largest producer and user of antibiotics in the world, used about 162,000 tons of antibiotics.1,2 Most antibiotics are only partially metabolized in humans or animals.3 Approximately 60% of antibiotics are ultimately released as the parent compounds into the environment.2 As a consequence, over 30 antibiotics, including sulfonamide (SA), fluoroquinolone (FQ), and macrolide (ML) antibiotics, have been detected with concentrations up to µg/L levels in surface waters and ng/g levels in sediments.4–7 For example, eleven antibiotics were found in seawater of Bohai Bay, North China with the total concentrations ranging from 2.3 to 6,800 ng/L.4 Seventeen antibiotics were detected in sediments from East Sea of China at concentrations ranging from not detected to 537.4 ng/g dry weight (dw).5 Continuous inputs from numerous pollution sources have led to antibiotics with the characteristic of “pseudo-persistence” (i.e., the inputs of antibiotics exceed the ability of the ecosystem to remove them completely from the natural environment8) in natural water environment, which increases the possibility of bioaccumulation and biomagnification of antibiotics in aquatic organisms. Furthermore, antibiotics can give rise to an increase in the antibiotic resistance of pathogenic bacteria, which is a particular concern.1 As a result, there is a need to investigate and assess the negative effects of antibiotic contamination on ecosystem and, ultimately, human health.
Antibiotics have been reported to have the potential to accumulate in aquatic biota by several field-monitoring data in aquatic organisms at various trophic levels, such as aquatic plants, mussels, and fish.7,9–11 For example, the concentrations of ΣSAs, ΣFQs and ΣMLs were as high as 15 ng/g dw, 6,500 ng/g dw, and 6 ng/g dw, respectively, in hydrophytes from Baiyangdian Lake, North China,7 and 77 ng/g dw, 1,600 ng/g dw, and 36 ng/g dw, respectively, in mollusks from Bohai Sea, China.9 Higher concentrations of ΣSAs and ΣMLs were found in wild fish from the Haihe River, North China and reached to 996 ng/g dw and 45 ng/g dw, respectively.10 Consistent with the occurrence of antibiotics in wild aquatic species, our earlier laboratory study revealed significant bioconcentration of sulfadiazine and sulfamethoxazole (BCF values: 1.7 – 170 L/kg) in common carp (Cyprinus carpio) at environmentally relevant concentrations.12 Hence, these antibiotics (i.e., SAs, FQs and MLs) may have a comparable trophic magnification potential and, thus, pose a risk for aquatic organisms, and even humans. Trophodynamics of these chemicals in aquatic food webs are an important criterion for assessing their ecological and health risks.13 However, there are only limited works about the trophic transfer of antibiotics in aquatic food webs.14,15 Boonsaner et al. found that oxytetracycline can transfer into seven-striped carp (Probarbus jullieni) from watermeal (Wolffia globosa).14 Roxithromycin was reported to have significant bioaccumulation through dietary uptake but no biomagnification potential in an artificial food chain including algae, water flea and fish.15 These studies only included one antibiotic, and the artificial food chains investigated consisted of two or three species. Overall, little comprehensive data are available about the trophodynamics of antibiotics to aquatic organisms, in particular with respect to marine food webs.
To explore the trophodynamics of antibiotics in aquatic ecosystems, this study analyzed 19 widely-used antibiotics (Table S1), including 9 SA, 5 FQ, and 4 ML antibiotics, as well as trimethoprim, in a marine food web (9 invertebrate and 10 fish species) from the coastal area of Laizhou Bay, North China. The trophic levels of all organisms were determined by using the stable carbon and nitrogen isotope method. The influence of physicochemical factors including octanol/water partition coefficient (KOW), fraction of neutral molecules (fn) and pH dependent distribution coefficient (logD) on trophodynamics of antibiotics was also investigated. In addition, antibiotic concentrations were used to assess human health risks associated with the consumption of seafood from Laizhou Bay. This study presents the first characterization of trophic transfer of antibiotics in the marine food web of Laizhou Bay, and provides important insights that can guide the risk assessment of antibiotics.
EXPERIMENTAL SECTION
Sampling
Samples of marine organism, including 9 invertebrate species (3 shellfish, 5 crustaceans, and 1 cephalopod) and 10 fish species (Table S2), were collected from Laizhou Bay with a bottom trawl from March 2014 to April 2015. The sampling months and sites for each species are described in detail in Table S2. The location of sampling sites is shown in Figure S1.
All the samples were sealed in polytetrafluoroethylene plastic bags and transported immediately on ice to the laboratory. The invertebrates and fish species were cleaned with deionized water, and their lengths and weights were measured. Because the reproducible homogenization of whole marine organisms is challenging, the following tissues were dissected: soft abdomen for crabs; soft body for other invertebrates; and muscle from the backbone for fish. The tissue samples from one to six similarly sized individuals for the same species were homogenized, lyophilized, wrapped in aluminum foil and stored at −20°C until analysis. Three different homogenized samples were analyzed for each species.
Sample Preparation and Instrumental Analysis
Sample extraction and clean-up were performed following a reported method with minor modifications.7 Briefly, target antibiotics were extracted from the biota samples with an accelerated solvent extraction system (ASE 350; Dionex, Sunnyvale, CA, USA) and purified using Oasis HLB cartridges (6 mL, 500 mg; Waters, Milford, MA, USA) as detailed in the Supporting Information (SI).
Chemical analysis was performed on an Agilent 1100 high performance liquid chromatograph-tandem 6410B quadrupole mass spectrometer (LC-MS/MS) equipped with an electrospray ionization (ESI) source using multiple reaction monitoring (MRM) positive mode. Ten µL of the final extract were injected into an XTerra® MS C18 column (2.1 mm × 100 mm, 3.5 µm; Waters, Milford, MA, USA) maintained at 40 °C. A gradient elution program was initiated with 95% A (0.1% v/v formic acid with 15.9 mM ammonium formate in water, pH = 2.4) and 5% B (methanol/acetonitrile, 1/1 in v/v), followed by a linear gradient from 5% to 88% B (0 – 30 min) at a flow rate of 0.3 mL/min. The MRM transitions and fragment voltage, collision energy, and retention times are listed in Table S3.
The carbon stable-isotope ratio (δ13C) and nitrogen stable-isotope ratio (δ15N) of the samples were determined using an Isoprime 100 isotope ratio mass spectrometer interfaced to a vario PYRO cube elemental analyzer (Elementar, Hanau, Germany) as described in the SI.
Quality Assurance and Quality Control
All the analytical standards had purities exceeding 98% and their sources are described in the SI. The method detection limits (MLOD) and the method quantification limits (MLOQ) are defined as three and ten times the standard deviation (SD) of the mean procedural blanks (n = 6), respectively. The MLODs and MLOQs ranged from 0.02 to 1.5 ng/g wet weight (ww) and 0.08 to 5.0 ng/g ww, respectively (Table S4). The recoveries (n = 6) of SAs, FQs, MLs and trimethoprim from spiked samples (i.e., farm raised fish) were 67% to 117%, 49% to 128%, 64% to 111% and 90%, respectively, and the relative standard deviations (RSD) were less than 19% (Table S4). All samples were spiked with surrogate standards prior to extraction to assess recoveries of target compounds in biotic samples (Table S4). The recoveries of the surrogate standards varied from 32% to 121%. The reported final concentrations of antibiotics were corrected for the recoveries of their respective surrogates.
The levels of all the target antibiotics in solvent blanks were below or near to their respective MLODs. Enrofloxacin, ofloxacin, ciprofloxacin, norfloxacin and enoxacin were detected in 58% to 71% of the procedural blanks. Concentrations of these five antibiotics in procedural blanks were subtracted from those in the corresponding samples for batches with blank contamination.
Data Analysis
To account for lipid effects on δ13C, all δ13C values were adjusted for the C/N ratios as follows:16
| (1) |
Trophic level of a consumer (TLconsumer) was calculated using the following equation:
| (2) |
where ΔN is the enrichment factor of 3.8‰ representing the increase in δ15N from one TL to the next;17,18 TLbaseline is the trophic level of the baseline indicator. In the present study, northern maoxia shrimp (Acetes chinensis) was selected as the baseline indicator and assigned to TL = 2 according to previous studies.19,20
The relative contribution of benthic vs. pelagic carbon sources in the diets of biota (i.e., relative carbon sources) was calculated using δ15N and δ13C values as detailed in the SI.21
The antibiotic concentration below the MLOQ was assigned a randomly-generated value between zero and the compound-specific MLOQ for statistical analysis. The random values obtained followed normal distribution. The data were log-transformed prior to analysis to obtain a greater homogeneity of variance. The differences in antibiotic concentrations between invertebrates and fish species and between benthic and pelagic fish were analyzed using the Student's t-test. The Mann-Whitney U test was used if the data were not normally distributed. All the data are presented as the arithmetic mean ± one standard deviation if they follow normal distribution otherwise the geometric mean (GM) values and 95% confidence intervals (CI) are given. A p-value of < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Antibiotic Concentrations and Profiles in the Biotas
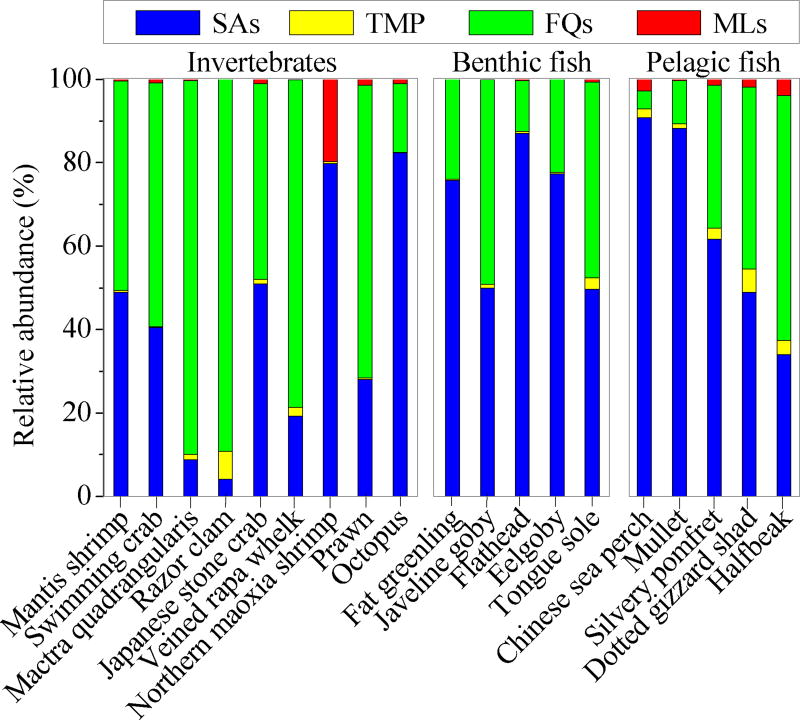
The detection frequencies of the 19 target antibiotics in all the samples are listed in Table S5. Sulfamethoxazole had the highest detection frequency (95%), followed by trimethoprim (91%). Ofloxacin (67%) and anhydroerythromycin (58%) occurred with the highest detection frequency among FQs and MLs, respectively. Among the marine species investigated, the swimming crab (Portunus trituberculatus) was the organism with the highest detection frequency (90%), while the fat greenling (Hexagrammos otakii) had the lowest detection frequency (21%) (Table S6). Overall, the high detection frequencies indicate widespread antibiotic contamination in marine organisms from Laizhou Bay.
The total concentrations of all the antibiotics ranged from 57 to 1,370 ng/g ww (GM (95% CI): 300 (250, 360) ng/g ww) in all the biota samples. The total antibiotic concentrations in invertebrates (range: 57 to 1,230 ng/g ww; 370 (270, 500) ng/g ww) were significantly higher (p < 0.05) than those in fish (98 to 610 ng/g ww; 240 (200, 300) ng/g ww). Benthic fish (i.e., flathead, eelgoby, javeline goby, fat greenling and tongue sole; 170 to 610 ng/g ww, mean ± SD: 360 ± 130 ng/g ww) tended to accumulate more antibiotics (p < 0.05) than pelagic fish (i.e., halfbeak, dotted gizzard shad, silvery pomfret, Chinese sea perch and mullet; 98 to 440 ng/g ww, 190 (150, 240) ng/g ww). The highest antibiotic residues were detected in mactra quadrangularis (Mactra veneriformis; 1,300 ± 90 ng/g ww), with FQs accounting for 90% of the total antibiotic concentrations. Similar observations have been reported for mollusks (Meretrix merehjgntrix; 0.7 to 1,580 ng/g dw) from the Bohai Sea, China.9 The lowest antibiotic concentrations were detected in northern maoxia shrimp (64 ± 10 ng/g ww), most likely due to their variable exposure to antibiotics associated with their strong floating habit.17
Concentrations of ΣSAs, ΣFQs, ΣMLs and trimethoprim in all the biota samples ranged from 19 to 451 ng/g ww, not detected (nd) to 1,200 ng/g ww, nd to 15 ng/g ww and nd to 50 ng/g ww, respectively. SAs and FQs were the most abundant antibiotic classes detected in most biota samples, accounting for 52% and 45% of total antibiotic concentrations, respectively (Figure 1). Trimethoprim (2%) and MLs (1%) made only minor contributions to the total antibiotic concentrations. The levels of ΣFQs in invertebrates (175 (92, 330) ng/g ww) were significantly higher (p < 0.05) than those in fish (56 (38, 81) ng/g ww). The opposite trend was found for ΣSAs (invertebrates: 100 (68, 140) ng/g ww; fish: 160 (120, 210) ng/g ww; p < 0.05). The average concentrations of ΣSAs in benthic fish (260 ± 130 ng/g ww) was significantly higher (p < 0.05) than those in pelagic fish (120 (86, 160) ng/g ww). No statistically significant difference in ΣFQ levels was observed for benthic vs. pelagic fish (benthic fish: 89 ± 33 ng/g ww; pelagic fish: 43 (24, 77) ng/g ww; p > 0.05). Overall, benthic fish accumulates more SAs than invertebrates and pelagic fish, whereas invertebrates exhibit higher FQ levels than fish. Several factors, including different habits and/or antibiotic partition between water and sediments, likely explain differences in levels of the antibiotic classes investigated. For example, the higher concentrations of antibiotics in invertebrates and benthic fish are likely a result of their intimate contact with antibiotic-contaminated sediments. It is well established that antibiotics, especially FQs, can be adsorbed to organic matter in sediments.22 As a consequence, some FQs (e.g., ofloxacin, ciprofloxacin, norfloxacin and enrofloxacin) accumulate in sediments in many polluted environments.7,23
Figure 1.
Abundance profiles of different antibiotic classes (percentage of the total measured concentrations) reveal significant differences among invertebrates, benthic fish and pelagic fish. SAs, TMP, FQs and MLs represent sulfonamides, trimethoprim, fluoroquinolones and macrolides, respectively.
Antibiotic concentrations normalized for dry weight (Table S6) exhibited the same trends observed for wet weight adjusted concentrations. Briefly, normalized ΣSA concentrations (dw) were significantly higher (p < 0.05) in fish than those in invertebrates, whereas no statistically significant difference was observed between benthic and pelagic fish (p > 0.05). Moreover, there were no significant differences (p > 0.05) in total antibiotic or ΣFQ concentrations (dw) between fish and invertebrates or between benthic and pelagic fish. The moisture content of all organisms ranged from 41% for fat greenling to 83% for octopus and significantly correlated (p < 0.05) with dry weight normalized concentrations of ΣMLs. No significant correlations (p > 0.05) were observed between moisture content and wet weight normalized antibiotic concentrations. Significant positive relationships (p < 0.05) were identified between sizes (lengths and weights) and wet weight concentrations of ΣSAs.
A comparison of antibiotic levels in the organisms collected from the Laizhou Bay with levels reported in earlier studies from China reveals several distinct differences (Table S7). The levels of ΣSAs in all the species from Laizhou Bay were much higher than those reported for aquatic organisms from coastal areas of Dalian6 and Hailing Island.24 Levels of ΣFQs in organisms from Laizhou Bay were also higher than those reported for Haihe River10 and coastal areas of Hailing Island.24 Levels of these three antibiotic classes in fish from Laizhou Bay were higher than those detected in fish from rivers of the Pearl River Delta.25 In contrast, levels of ΣMLs in this study were much lower than those reported in organisms from Baiyangdian Lake,7 Bohai Sea,9 Haihe River,10 and coastal areas of Hailing Island.24 Levels of ΣSAs and ΣFQs in biota samples from Laizhou Bay were similar to those from Baiyangdian Lake.7 The different levels for antibiotics in different parts of China are most likely related to regional differences in the use of antibiotics.2
Only limited information regarding levels of antibiotics in global biotas is currently available (Table S7). The levels of ΣFQs (nd to 0.73 ng/g ww) in shrimps and fish collected from Canada fish markets were much lower than those reported from China.26 In mussels from the coastal areas of Ireland, the concentrations of trimethoprim were up to 9.22 ng/g dw, which is similar to those observed in shellfish (2.0 to 9.7 ng/g dw) in the present study.29 Sulfamethoxazole and trimethoprim, the only two antibiotics analyzed, were not detected in any sample in national monitoring studies conducted in Germany and the United States.27,28
Food Web Structure
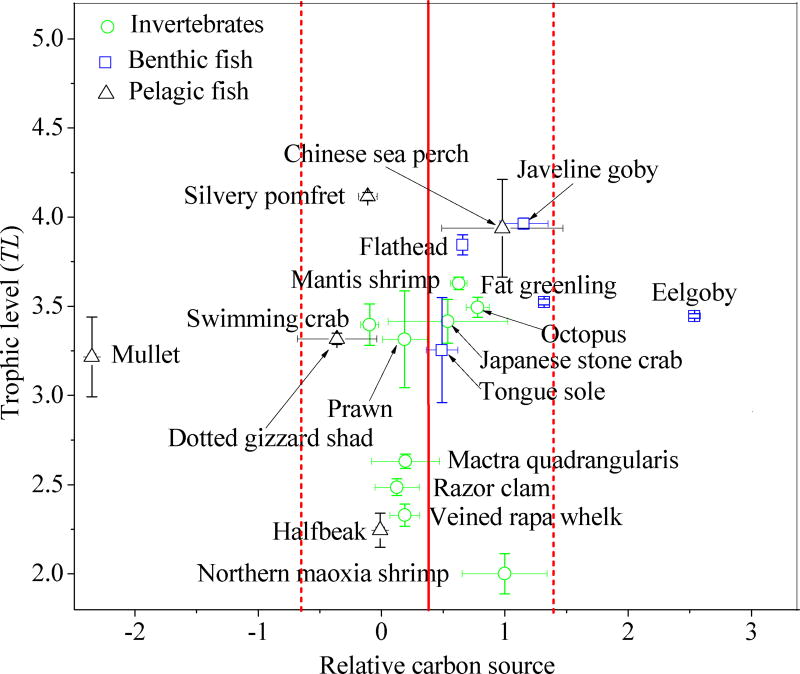
To characterize food web relationships of the aquatic organisms collected from Laizhou Bay and assess the trophodynamics of antibiotics, TLs and relative carbon sources were calculated using adjusted δ13C and δ15N values (Figure S2). Adjusted δ13C values ranged from −22.8 ± 0.1 for eelgoby to −13.2 ± 2.3 for mullet. The δ15N values ranged from 6.6 ± 0.4 for northern maoxia shrimp to 14.6 ± 0.1 for silvery pomfret. Estimated TLs ranged from 2.00 ± 0.10 for northern maoxia shrimp to 4.10 ± 0.02 for silvery pomfret (Figure 2). Apparent intra-species variability in TL values was observed in this study and, as has been suggested by previous studies, may be attributed to the variation in the body sizes of the collected aquatic organisms.18,30,31 No significant seasonal variation was found in the TL values of consumers (Table S8, p > 0.05).
Figure 2.
Relative carbon sources and trophic levels (TL) characterize the food web relationships of the species collected from Laizhou Bay. Green circles (
 ), blue diamonds (
), blue diamonds (
 ) and black triangles (△) represent invertebrates, benthic fish and pelagic fish, respectively. Data are presented as mean ± one standard deviation of relative carbon sources (n = 3) and TL values (n = 3). The red vertical solid line and dotted lines represent the mean value of relative carbon sources for all species and the boundaries of relative carbon sources for species in the same food web.32
) and black triangles (△) represent invertebrates, benthic fish and pelagic fish, respectively. Data are presented as mean ± one standard deviation of relative carbon sources (n = 3) and TL values (n = 3). The red vertical solid line and dotted lines represent the mean value of relative carbon sources for all species and the boundaries of relative carbon sources for species in the same food web.32
Relative carbon sources of these species were used to assess if different species belong to the same food web.21 Relative carbon source values close to 1 are thought to indicate more pelagic feeding, whereas values close to 0 indicate more benthic feeding.21 Values of relative carbon sources ranged from −0.40 ± 0.30 for dotted gizzard shad to 1.40 ± 0.01 for fat greenling (Figure 2). All species investigated were distributed close to the mean value of the relative carbon sources of 0.4 (vertical line in Figure 2). Eelgoby (Odontamblyopus rubicundus; 2.70 ± 0.05) and mullet (Liza haematocheilus; −2.40 ± 0.03) were an exception, which indicates that their diets might include sources which do not belong to the food web under investigation. Therefore, both species were not included in the calculation of trophic magnification factors (TMF) in this study.
Trophic Transfer of Antibiotics in the Marine Food Web
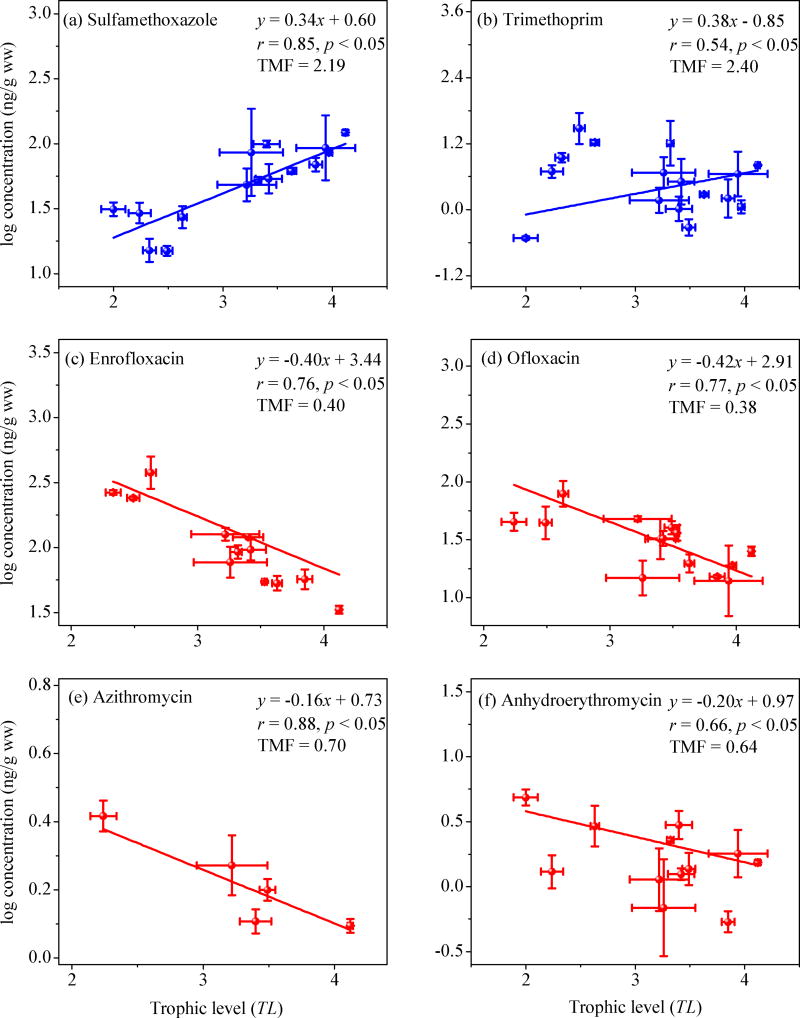
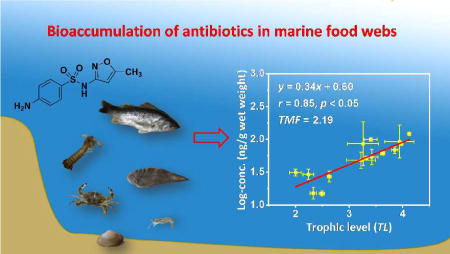
TMFs of individual antibiotics and the three antibiotic classes (i.e, SAs, FQs, and MLs) in the food web were determined from the slope of the regression between log-transformed antibiotic concentrations (ww) in biota and the TLs of the corresponding species (Figure 3; Table S9). Overall, the TMF values followed the order SAs ≥ trimethoprim > MLs > FQs. For SAs, concentrations (ww) of sulfadiazine, sulfamethoxazole, sulfamonomethoxine, sulfachlorpyridazine, sulfadimidine, and ΣSAs increased significantly with increasing TLs (p < 0.05). Similarly, a significant positive correlation (p < 0.05) was observed between the TL values and trimethoprim concentrations (ww). The TMF values were 1.2 – 3.9 for SAs and 2.4 for trimethoprim. These TMF values are greater than those reported previously for other pharmaceuticals, such as diphenhydramine (TMF = 0.38) and carbamazepine (TMF = 1.17) in an effluent stream from the North Bosque River, USA and propranolol (TMF = 0.31) and diclofenac (TMF = 1.06) in a freshwater food web from Taihu Lake, China.18,31 A similar trophic transfer for SAs was also observed in Baiyangdian Lake, North China, where fish (nd − 98.3 ng/g dw, mean: 20.8 ng/g dw) had the highest concentrations of SAs, followed by crustaceans (nd − 30.6 ng/g dw, 11.7 ng/g dw) and then aquatic plants (nd −15 ng/g dw, 1.67 ng/g dw).7 These results demonstrate that SAs and trimethoprim, like other pharmaceuticals, have the potential to biomagnify in a marine food web. Previous studies have shown that SAs such as sulfamethoxazole and trimethoprim are well absorbed in the intestinal tracts33–35 but not efficiently metabolized in aquatic organisms, such as crustaceans, mollusks, fish.35–38 Therefore, trophic magnification of SAs and trimethoprim in this marine food web is likely due to their low metabolic transformation and efficient assimilation in animals at higher trophic levels.
Figure 3.
Relationships between trophic levels (TL) and log-transformed wet weight normalized concentrations (ww) describe trophic magnification for (a) sulfamethoxazole, (b) trimethoprim and trophic dilution for (c) enrofloxacin, (d) ofloxacin, (e) azithromycin and (f) anhydroerythromycin in the marine food web from Laizhou Bay. Slopes, correlation coefficients (r), p-values and trophic magnification factors (TMF) from the regression analyses are shown (Table S9 for additional details). Data are presented as mean ± one standard deviation of TL values (n = 3) and log-transformed antibiotic concentrations which were detected in at least two out of three homogenized samples.
The TMF values ranged from 0.3 to 0.8 for FQs and from 0.5 to 1.0 for MLs, respectively. Significant negative relationships (p < 0.05) were observed for correlations between TL values and concentrations of ΣFQs and ΣMLs. Moreover, there were significant negative correlations (p < 0.05) between the TL values and concentrations of three FQs (i.e., enoxacin, enrofloxacin, and ofloxacin) and two MLs (i.e., azithromycin and anhydroerythromycin) in the species under investigation. These findings demonstrate that both antibiotic classes undergo trophic dilution in the marine food web of Laizhou Bay. Literature TMF values for both classes of antibiotics are limited and only TMF values for roxithromycin have been reported.15,18 In this study, the TMF values for azithromycin (0.7, 95% CI: 0.5 – 0.9) and anhydroerythromycin (0.6, 95% CI: 0.5 – 0.9) were higher than values reported for roxithromycin (0.21 – 0.29) in an artificial aquatic food chain but slight lower than values for roxithromycin (1.11) in a freshwater food web from Taihu Lake, China.15,18 Unfortunately, the detection frequency of roxithromycin (23%) was too low to calculate a TMF value in this study (Table S5). For the other seven antibiotics, no significant correlations (p > 0.05) were observed between TL values and their wet weight concentrations.
Influence of Physicochemical Factors on Trophic Magnification of Antibiotics
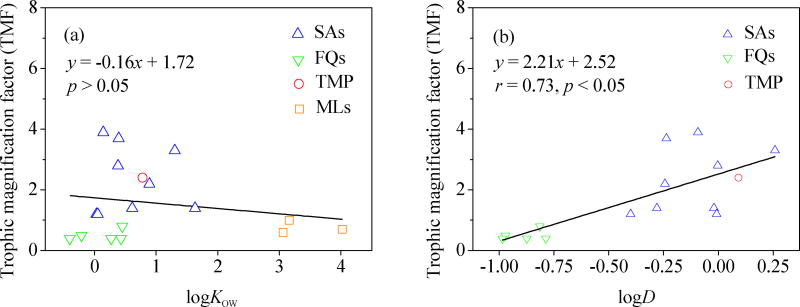
Octanol-water partition coefficients (Kow) are excellent predictors of the biomagnification of hydrophobic organic compounds in aquatic food webs.39 The TMF values of the antibiotics investigated in this study decreased with increasing logKOW; however, this correlation was not statistically significant (p > 0.05, Figure 4a). Negative relationships or no relationships between TMF and logKOW values are also observed for phthalate esters,40 and some hydrophobic compounds (e.g., dioxins, polychlorinated biphenyls).41–43 No significant correlation between TMFs of these antibiotics and their logKOW values is not entirely surprising because the antibiotics investigated are polar molecules and contain ionizable functional groups. Like other ionic compounds, an ionic form of an antibiotic cannot diffuse across cell membranes and is not readily absorbed in the intestinal tracts of marine organisms.29,44 Therefore, logKOW is not a good biomagnification predictor for these antibiotics. We also investigated if measures of the fraction of the non-ionic form of an antibiotic, such as the fraction of neutral molecules (fn) and the pH dependent distribution coefficient (logD),45 are better predictors of its biomagnification in a marine food web. Indeed, we found that the SAs and trimethoprim with higher TMF values displayed higher fn values (Table S1, p < 0.05) than the FQs and MLs (Figure S3). Furthermore, a significant positive correlation (r = 0.73; p < 0.05) was observed between TMFs of the antibiotics and their logD (pH = 7.5, Table S1) values, with the exception of the three ML antibiotics (Figure 4b). A similar correlation between BCF values of other pharmaceuticals and their pH-corrected liposome-water partition coefficients was observed in some invertebrates.46 The correlation suggests that logD of ionizable organic chemicals, such as antibiotics, is a better predictor of their biomagnification potential in marine food webs than logKOW.
Figure 4.
Relationships between TMFs of detected antibiotics and physicochemical factors (logKOW (a) and logD values (b)). The TMF values for SAs, trimethoprim, FQs and MLs are indicated by blue triangles (
 ), red circles (
), red circles (
 ), green inverted triangles (
), green inverted triangles (
 ) and orange diamonds (
) and orange diamonds (
 ), respectively. Equations, correlation coefficients (r) and p-values of the regression analyses are shown. LogKOW and logD values of antibiotics (Table S1) were collected from elsewhere.
), respectively. Equations, correlation coefficients (r) and p-values of the regression analyses are shown. LogKOW and logD values of antibiotics (Table S1) were collected from elsewhere.
Biotransformation processes within the food web are one possible explanation for the lack of a correlation between TMFs and logD values for the ML antibiotics. Although biotransformation was not directly assessed in this study, there is some literature evidence demonstrating that aquatic organisms have the capacity to metabolize ML antibiotics.47–49 For example, Liu et al. reported that erythromycin and roxithromycin are readily metabolized by crucian carp (Carassius auratus) and eliminated via the bile, most likely due to the induction of cytochrome P450 enzymes (measured as ethoxyresorufin-O-deethylase activity) following antibiotic exposure.47,48 Chen et al. observed faster hepatic clearance rates for tertiary amines (e.g., MLs) than other amines (e.g., SAs) in liver S9 fractions of rainbow trout (Oncorhynchus mykiss).49 Unfortunately, the biotransformation of antibiotics by aquatic organisms at different TLs is not fully understood and future studies are needed.
Assessment of Human Health Risks
All species investigated in this study are caught for human consumption.50–52 Estimated daily intakes (EDI, Table S10) for each antibiotic following consumption of seafood were calculated assuming a worst-case scenario for rural and urban residents in China.53 For urban residents, EDIs of antibiotics ranged from 0.5 ng/kg bw/d for roxithromycin to 144 ng/kg bw/d for enrofloxacin. EDIs for rural residents ranged from 0.3 ng/kg bw/d for roxithromycin to 78 ng/kg bw/d for enrofloxacin. The EDIs for urban residents were two times higher than those for rural residents due to the higher fish consumption of urban residents.54 All EDI values were two to five orders of magnitude lower than the respective acceptable daily intakes (ADI, Table S10). Based on the EDI values and the respective ADIs, hazard quotients of individual antibiotics (HQ; Table S10, Figure S4) were calculated for urban and rural residents and used to assess potential human health risks from dietary exposure to antibiotics.53 Vragovic et al. suggested that a HQ < 0.01 indicates a negligible risk whereas a HQ ≥ 0.01 indicates a considerable risk.55 A HQ > 0.05 is considered a distinct risk.55 The HQ of enrofloxacin for urban residents (HQ = 0.07, i.e., 7% of ADI) exceeded 0.05 (Figure S4). The HQs of ciprofloxacin (HQ = 0.01, i.e., 1% of ADI) and clarithromycin (HQ = 0.01, i.e., 1% of ADI) for urban residents and enrofloxacin for rural residents (HQ = 0.04, i.e., 4% of ADI) were ≥0.01. These results suggest that there is an appreciable human health risk associated with the exposure to antibiotics due to consumption of seafood from Laizhou Bay.
We also calculated hazard index values (HI = ΣHQi) assuming a similar toxicological mode of action (MOA) for substances belong to the same class to assess if consumption of seafood from Laizhou Bay represents a human health risk.53 The HI values of ΣMLs for urban residents (HI = 0.014) exceeded 0.01. The HI values of ΣFQs were 0.094 and 0.050 for urban and rural residents, respectively (Table S10). The high risk of FQs in seafood represents a potential concern because of the relatively low ADIs (1,600 to 11,400 ng/kg bw/d) and high EDIs (8.6 to 144 ng/kg bw/d). Compared to FQs and MLs, SAs exhibited relatively high biomagnification potential but represented a small ingestion risk (i.e., the HI values of SAs are < 0.01).
Exposure Implications
Due to intensive local aquaculture activities, ambient wastewater discharge and runoff from farming, large amounts of antibiotic residues have been discharged into the water bodies of Laizhou Bay,4,56,57 which increases the possibility of bioaccumulation and biomagnification of antibiotics in aquatic organisms. Our comprehensive investigation of the trophodynamics of antibiotics in a marine food web from this region demonstrates that several antibiotics undergo trophic transfer to top predators or low-trophic level invertebrates and accumulate in certain tissues, such as the liver and blood.25 In order to reduce the potential exposure risks, more strict maximal residue limits (MRL) of antibiotics in seafood need to be set for Chinese residents. While consumption of seafood alone is unlikely to represent a major human health risk in China, humans are exposed to antibiotics by a variety of other exposure pathways, such as other food intakes,58,59 drug abuse,60 tap water,61 and inhalation of contaminated dust.62 Moreover, this study did not investigate levels of antibiotic metabolites in the food web from Laizhou Bay. Emerging evidence suggests that the biotransformation products of antibiotics may be more toxic (e.g., protein-reactive) and/or bioaccumulative than the parent compounds.63–65 For example, sulfamethoxazole can be hydroxylated and further oxidized in humans and rats to nitroso-sulfamethoxazole, which can bind covalently to cysteine residues of cellular proteins and cause toxicity.63,64 Furthermore, decarboxylation of antibiotics, such as FQs and cephalosporins, may generate more fat-soluble metabolites that are more bioaccumulative than the parent antibiotics.65 In addition, ecotoxicological studies suggest that some antibiotics investigated (e.g., ciprofloxacin, erythromycin, sulfamethoxazole and trimethoprim) can induce phototoxicity to Daphnia magna, disrupt the immune system in mollusks, or impair the development of fish in their early life stages.66–68 Therefore, the environmental behavior of antibiotics in aquatic food webs and potential ecological and human health risks associated with exposure to antibiotics and their metabolites warrant further attention.
Supplementary Material
Acknowledgments
This study was supported by the National Basic Research Program of China (2013CB430403), the National Natural Science Foundation of China (21325729, 21277017) and the Basic Research Project of Key Laboratory of Liaoning Provincial Education Department (LZ2015023).
Footnotes
ASSOCIATED CONTENT
Supporting Information. Text, figures, and tables addressing (1) physicochemical properties of selected antibiotics in this study; (2) chemicals used in the analysis; (3) details about sample extraction and cleanup, and stable isotope analysis; (4) calculations of TMFs, relative carbon sources, estimated daily intakes (EDI) and hazard quotients (HQ); (5) sampling figure and tables about sample information; (6) table about MLODs, MLOQs, spiked recoveries and RSD of target antibiotics; (7) table about LC-MS/MS parameters and retention times of antibiotics; (8) tables about detection frequencies and concentrations in biota samples; (9) table about slopes, r and p-values of regression analyses between logarithm concentrations and TLs, and TMF values of antibiotics; (10) table about the comparison of antibiotic levels in aquatic organisms from Laizhou Bay with those reported in other locations; (11) table and figure about EDIs and HQs of antibiotics for rural and urban residents; (12) figure about relationship between TMFs and logKOW values. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Zhu YG, Johnson TA, Su JQ, Qiao M, Guo GX, Stedtfeld RD, Hashsham SA, Tiedje JM. Diverse and abundant antibiotic resistance genes in Chinese swine farms. P. Natl. Acad. Sci. USA. 2013;110(9):3435–3440. doi: 10.1073/pnas.1222743110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao JL. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modelling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015;49(11):6772–6782. doi: 10.1021/acs.est.5b00729. [DOI] [PubMed] [Google Scholar]
- 3.Bound JP, Voulvoulis N. Pharmaceuticals in the aquatic environment - a comparison of risk assessment strategies. Chemosphere. 2004;56(11):1143–1155. doi: 10.1016/j.chemosphere.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Zou SC, Xu WH, Zhang RJ, Tang JH, Chen YJ, Zhang G. Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: Impacts of river discharge and aquaculture activities. Environ. Pollut. 2011;159(10):2913–2920. doi: 10.1016/j.envpol.2011.04.037. [DOI] [PubMed] [Google Scholar]
- 5.Shi H, Yang Y, Liu M, Yan CX, Yue HY, Zhou JL. Occurrence and distribution of antibiotics in the surface sediments of the Yangtze Estuary and nearby coastal areas. Mar. Pollut. Bull. 2014;83(1):317–323. doi: 10.1016/j.marpolbul.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 6.Na GS, Fang XD, Cai YQ, Ge LK, Zong HM, Yuan XT, Yao ZW, Zhang ZF. Occurrence, distribution, and bioaccumulation of antibiotics in coastal environment of Dalian, China. Mar. Pollut. Bull. 2013;69(1–2):233–237. doi: 10.1016/j.marpolbul.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Li WH, Shi YL, Gao LH, Liu JM, Cai YQ. Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China. Chemosphere. 2012;89(11):1307–1315. doi: 10.1016/j.chemosphere.2012.05.079. [DOI] [PubMed] [Google Scholar]
- 8.Mackay D, Hughes DM, Romano ML, Bonnell M. The role of persistence in chemical evaluations. Integr. Environ. Assess. Manag. 2014;10(4):588–594. doi: 10.1002/ieam.1545. [DOI] [PubMed] [Google Scholar]
- 9.Li WH, Shi YL, Gao LH, Liu JM, Cai YQ. Investigation of antibiotics in mollusks from coastal waters in the Bohai Sea of China. Environ. Pollut. 2012;162:56–62. doi: 10.1016/j.envpol.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Gao LH, Shi YL, Li WH, Liu JM, Cai YQ. Occurrence, distribution and bioaccumulation of antibiotics in the Haihe River in China. J. Environ. Monitor. 2012;14(4):1248–1255. doi: 10.1039/C2em10916f. [DOI] [PubMed] [Google Scholar]
- 11.He XT, Wang ZH, Nie XP, Yang YF, Pan DB, Leung AOW, Cheng Z, Yang YT, Li KB, Chen KC. Residues of fluoroquinolones in marine aquaculture environment of the Pearl River Delta, South China. Environ. Geochem. Health. 2012;34(3):323–335. doi: 10.1007/s10653-011-9420-4. [DOI] [PubMed] [Google Scholar]
- 12.Zhao HX, Liu SS, Chen JW, Jiang JQ, Xie Q, Quan X. Biological uptake and depuration of sulfadiazine and sulfamethoxazole in common carp (Cyprinus carpio) Chemosphere. 2015;120:592–597. doi: 10.1016/j.chemosphere.2014.09.075. [DOI] [PubMed] [Google Scholar]
- 13.Zhang K, Wan Y, Jones PD, Wisemans S, Giesy JP, Hu JY. Occurrences and fates of hydroxylated polybrominated diphenyl ethers in marine sediments in relation to trophodynamics. Environ. Sci. Technol. 2012;46(4):2148–2155. doi: 10.1021/es203195s. [DOI] [PubMed] [Google Scholar]
- 14.Boonsaner M, Hawker DW. Evaluation of food chain transfer of the antibiotic oxytetracycline and human risk assessment. Chemosphere. 2013;93(6):1009–1014. doi: 10.1016/j.chemosphere.2013.05.070. [DOI] [PubMed] [Google Scholar]
- 15.Ding JN, Lu GH, Liu JC, Zhang ZH. Evaluation of the potential for trophic transfer of roxithromycin along an experimental food chain. Environ. Sci. Pollut. Res. 2015;22(14):10592–10600. doi: 10.1007/s11356-015-4265-5. [DOI] [PubMed] [Google Scholar]
- 16.Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montana CG. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia. 2007;152(1):179–189. doi: 10.1007/s00442-006-0630-x. [DOI] [PubMed] [Google Scholar]
- 17.Ma XD, Zhang HJ, Wang Z, Yao ZW, Chen JW, Chen JP. Bioaccumulation and trophic transfer of short chain chlorinated paraffins in a marine food web from Liaodong Bay, North China. Environ. Sci. Technol. 2014;48(10):5964–5971. doi: 10.1021/Es500940p. [DOI] [PubMed] [Google Scholar]
- 18.Xie ZX, Lu GH, Liu JC, Yan ZH, Ma BN, Zhang ZH, Chen W. Occurrence, bioaccumulation, and trophic magnification of pharmaceutically active compounds in Taihu Lake, China. Chemosphere. 2015;138:140–147. doi: 10.1016/j.chemosphere.2015.05.086. [DOI] [PubMed] [Google Scholar]
- 19.Cui Y, Wu Y, Xu ZL, Zhang J. Potential dietary influence on the stable isotopes and fatty acid composition of migratory anchovy (Coilia mystus) around the Changjiang Estuary. J. Mar. Biol. Assoc. UK. 2015;95(1):193–205. doi: 10.1017/S0025315414000873. [DOI] [Google Scholar]
- 20.Cabana G, Rasmussen JB. Comparison of aquatic food chains using nitrogen isotopes. P. Natl. Acad. Sci. USA. 1996;93(20):10844–10847. doi: 10.1073/pnas.93.20.10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKinney MA, McMeans BC, Tomy GT, Rosenberg B, Ferguson SH, Morris A, Muir DCG, Fisk AT. Trophic transfer of contaminants in a changing arctic marine food web: Cumberland Sound, Nunavut, Canada. Environ. Sci. Technol. 2012;46(18):9914–9922. doi: 10.1021/es302761p. [DOI] [PubMed] [Google Scholar]
- 22.Tolls J. Sorption of veterinary pharmaceuticals in soils: A review. Environ. Sci. Technol. 2001;35(17):3397–3406. doi: 10.1021/es0003021. [DOI] [PubMed] [Google Scholar]
- 23.Kim SC, Carlson K. Temporal and spatial trends in the occurrence of human and veterinary antibiotics in aqueous and river sediment matrices. Environ. Sci. Technol. 2007;41(1):50–57. doi: 10.1021/es060737+. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Liu S, Xu XR, Liu SS, Zhou GJ, Sun KY, Zhao JL, Ying GG. Antibiotics in typical marine aquaculture farms surrounding Hailing Island, South China: Occurrence, bioaccumulation and human dietary exposure. Mar. Pollut. Bull. 2015;90(1–2):181–187. doi: 10.1016/j.marpolbul.2014.10.053. [DOI] [PubMed] [Google Scholar]
- 25.Zhao JL, Liu YS, Liu WR, Jiang YX, Su HC, Zhang QQ, Chen XW, Yang YY, Chen J, Liu SS, Pan CG, Huang GY, Ying GG. Tissue-specific bioaccumulation of human and veterinary antibiotics in bile, plasma, liver and muscle tissues of wild fish from a highly urbanized region. Environ. Pollut. 2015;198:15–24. doi: 10.1016/j.envpol.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 26.Tittlemier SA, Van de Riet J, Burns G, Potter R, Murphy C, Rourke W, Pearce H, Dufresne G. Analysis of veterinary drug residues in fish and shrimp composites collected during the Canadian Total Diet Study, 1993–2004. Food Addit. Contam. 2007;24(1):14–20. doi: 10.1080/02652030600932937. [DOI] [PubMed] [Google Scholar]
- 27.Subedi B, Du BW, Chambliss CK, Koschorreck J, Rudel H, Quack M, Brooks BW, Usenko S. Occurrence of pharmaceuticals and personal care products in German fish tissue: A national study. Environ. Sci. Technol. 2012;46(16):9047–9054. doi: 10.1021/es301359t. [DOI] [PubMed] [Google Scholar]
- 28.Ramirez AJ, Brain RA, Usenko S, Mottaleb MA, O'Donnell JG, Stahl LL, Wathen JB, Snyder BD, Pitt JL, Perez-Hurtado P, Dobbins LL, Brooks BW, Chambliss CK. Occurrence of pharmaceuticals and personal care products in fish: Results of a national pilot study in the United States. Environ. Toxicol. Chem. 2009;28(12):2587–2597. doi: 10.1897/08-561.1. [DOI] [PubMed] [Google Scholar]
- 29.McEneff G, Barron L, Kelleher B, Paull B, Quinn B. A year-long study of the spatial occurrence and relative distribution of pharmaceutical residues in sewage effluent, receiving marine waters and marine bivalves. Sci. Total Environ. 2014;476:317–326. doi: 10.1016/j.scitotenv.2013.12.123. [DOI] [PubMed] [Google Scholar]
- 30.Borga K, Fjeld E, Kierkegaard A, McLachlan MS. Consistency in trophic magnification factors of cyclic methyl siloxanes in pelagic freshwater food webs leading to brown trout. Environ. Sci. Technol. 2013;47(24):14394–14402. doi: 10.1021/es404374j. [DOI] [PubMed] [Google Scholar]
- 31.Du B, Haddad SP, Luek A, Scott WC, Saari GN, Kristofco LA, Connors KA, Rash C, Rasmussen JB, Chambliss CK, Brooks BW. Bioaccumulation and trophic dilution of human pharmaceuticals across trophic positions of an effluent-dependent wadeable stream. Philos. Trans. R. Soc. London, Ser. B. 2014;369(1656):1–10. doi: 10.1098/rstb.2014.0058. DOI Artn 2014005810.1098/Rstb.2014.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia HL, Zhang ZF, Wang CQ, Hong WJ, Sun YQ, Li YF. Trophic transfer of methyl siloxanes in the marine food web from coastal area of Northern China. Environ. Sci. Technol. 2015;49(5):2833–2840. doi: 10.1021/es505445e. [DOI] [PubMed] [Google Scholar]
- 33.Klopman G, Stefan LP, Saiakhov RD. ADME evaluation: 2. A computer model for the prediction of intestinal absorption in humans. Eur. J. Pharm. Sci. 2002;17(4–5):253–263. doi: 10.1016/S0928-0987(02)00219-1. [DOI] [PubMed] [Google Scholar]
- 34.Meshi T, Sato Y. Studies on sulfamethoxazole/trimethoprim: absorption, distribution, excretion and metabolism of trimethoprim in rat. Chem. Pharm. Bullet. 1972;20(10):2079–2090. doi: 10.1248/cpb.20.2079. [DOI] [PubMed] [Google Scholar]
- 35.Touraki M, Niopas I, Kastritsis C. Bioaccumulation of trimethoprim, sulfamethoxazole and N-acetyl-sulfamethoxazole in Artemia nauplii and residual kinetics in seabass larvae after repeated oral dosing of medicated nauplii. Aquaculture. 1999;175(1–2):15–30. doi: 10.1016/S0044-8486(99)00036-8. [DOI] [Google Scholar]
- 36.Connors KA, Du BW, Fitzsimmons PN, Hoffman AD, Chambliss CK, Nichols JW, Brooks BW. Comparative pharmaceutical metabolism by rainbow trout (Oncorhynchus mykiss) liver S9 fractions. Environ. Toxicol. Chem. 2013;32(8):1810–1818. doi: 10.1002/etc.2240. [DOI] [PubMed] [Google Scholar]
- 37.Nouws JFM, et al. Pharmacokinetics, hydroxylation and acetylation of sulphadimidine in mammals, birds, fish, reptiles and molluscs. In: Van Miert ASJPAM, Bogaert MG, Debackere M, editors. Comparative Veterinary Pharmacology, Toxicology and Therapy. MTP Press; Lancaster, England: 1985. pp. 301–318. [Google Scholar]
- 38.Chair M, Nelis HJ, Leger P, Sorgeloos P, de Leenheer AP. Accumulation of trimethoprim, sulfamethoxazole, and N-acetylsulfamethoxazole in fish and shrimp fed medicated Artemia franciscana. Antimicrob. Agents Chemother. 1996;40(7):1649–1652. doi: 10.1128/aac.40.7.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly BC, Ikonomou MG, Blair JD, Morin AE, Gobas FAPC. Food web-specific biomagnification of persistent organic pollutants. Science. 2007;317(5835):236–239. doi: 10.1126/science.1138275. [DOI] [PubMed] [Google Scholar]
- 40.Mackintosh CE, Maldonado J, Jing HW, Hoover N, Chong A, Ikonomou MG, Gobas FAPC. Distribution of phthalate esters in a marine aquatic food web: Comparison to polychlorinated biphenyls. Environ. Sci. Technol. 2004;38(7):2011–2020. doi: 10.1021/es034745r. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi J, Imuta Y, Komorita T, Yamada K, Ishibashi H, Ishihara F, Nakashima N, Sakai J, Arizono K, Koga M. Trophic magnification of polychlorinated biphenyls and polybrominated diphenyl ethers in an estuarine food web of the Ariake Sea, Japan. Chemosphere. 2015;118:201–206. doi: 10.1016/j.chemosphere.2014.08.066. [DOI] [PubMed] [Google Scholar]
- 42.Khairy MA, Weinstein MP, Lohmann R. Trophodynamic behavior of hydrophobic organic contaminants in the aquatic food web of a tidal river. Environ. Sci. Technol. 2014;48(21):12533–12542. doi: 10.1021/es502886n. [DOI] [PubMed] [Google Scholar]
- 43.Zheng GMWY, Hu JY. Intrinsic clearance of xenobiotic chemicals by liver microsomes: Assessment of trophic magnification potentials. Environ. Sci. Technol. 2016;50(12):6343–6353. doi: 10.1021/acs.est.6b01178. [DOI] [PubMed] [Google Scholar]
- 44.Erickson RJ, McKim JM, Lien GJ, Hoffman AD, Batterman SL. Uptake and elimination of ionizable organic chemicals at fish gills: II. Observed and predicted effects of pH, alkalinity, and chemical properties. Environ. Toxicol. Chem. 2006;25(6):1522–1532. doi: 10.1897/05-359R.1. [DOI] [PubMed] [Google Scholar]
- 45.Fu WJ, Franco A, Trapp S. Methods for estimating the bioconcentration factor of ionizable organic chemicals. Environ. Toxicol. Chem. 2009;28(7):1372–1379. doi: 10.1897/08-233.1. [DOI] [PubMed] [Google Scholar]
- 46.Meredith-Williams M, Carter LJ, Fussell R, Raffaelli D, Ashauer R, Boxall ABA. Uptake and depuration of pharmaceuticals in aquatic invertebrates. Environ. Pollut. 2012;165:250–258. doi: 10.1016/j.envpol.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 47.Liu JC, Lu GH, Ding JN, Zhang ZH, Wang YH. Tissue distribution, bioconcentration, metabolism, and effects of erythromycin in crucian carp (Carassius auratus) Sci. Total Environ. 2014;490:914–920. doi: 10.1016/j.scitotenv.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 48.Liu JC, Lu GH, Wang YH, Yan ZH, Yang XF, Ding JN, Jiang Z. Bioconcentration, metabolism, and biomarker responses in freshwater fish Carassius auratus exposed to roxithromycin. Chemosphere. 2014;99:102–108. doi: 10.1016/j.chemosphere.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y, Hermens JLM, Jonker MTO, Arnot JA, Armitage JM, Brown T, Nichols JW, Fay KA, Droge STJ. Environ. Sci. Technol. ASAP; 2016. Which molecular features affect the intrinsic hepatic clearance rate of ionizable organic chemicals in fish? [DOI] [PubMed] [Google Scholar]
- 50.Search fishbase. [Access date: Jun 2016]; http://www.Fishbase.Se/search.php.
- 51.Tian SY, Zhu LY, Liu M. Bioaccumulation and distribution of polybrominated diphenyl ethers in marine species from Bohai Bay, China. Environ. Toxicol. Chem. 2010;29(10):2278–2285. doi: 10.1002/etc.275. [DOI] [PubMed] [Google Scholar]
- 52.Jin J, Liu WZ, Wang Y, Tang XY. Levels and distribution of polybrominated diphenyl ethers in plant, shellfish and sediment samples from Laizhou Bay in China. Chemosphere. 2008;71(6):1043–1050. doi: 10.1016/j.chemosphere.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 53.U.S. Environmental Protection Agency. Guidance for assessing chemical contaminant data for use in fish advisories-Risk assessment and fish consumption limits. 3. Office of Water; Washington, DC: 2000. [Google Scholar]
- 54.National Burean of Statistics of China. [Access date: July 2016]; http://data.stats.gov.cn.
- 55.Vragovic N, Bazulic D, Njari B. Risk assessment of streptomycin and tetracycline residues in meat and milk on Croatian market. Food Chem. Toxicol. 2011;49(2):352–355. doi: 10.1016/j.fct.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Zhang RJ, Zhang G, Zheng Q, Tang JH, Chen YJ, Xu WH, Zou YD, Chen XX. Occurrence and risks of antibiotics in the Laizhou Bay, China: Impacts of river discharge. Ecotox. Environ. Safe. 2012;80:208–215. doi: 10.1016/j.ecoenv.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Zhang RJ, Tang JH, Li J, Zheng Q, Liu D, Chen YJ, Zou YD, Chen XX, Luo CL, Zhang G. Antibiotics in the offshore waters of the Bohai Sea and the Yellow Sea in China: Occurrence, distribution and ecological risks. Environ. Pollut. 2013;174:71–77. doi: 10.1016/j.envpol.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 58.Hu XG, Zhou QX, Luo Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, Northern China. Environ. Pollut. 2010;158(9):2992–2998. doi: 10.1016/j.envpol.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi T, Okihashi M, Harada K, Konishi Y, Uchida K, Do MHN, Bui HDT, Nguyen TD, Nguyen PD, Chau VV, Dao KTV, Nguyen HTN, Kajimura K, Kumeda Y, Bui CT, Vien MQ, Le NH, Hirata K, Yamamoto Y. Antibiotic residue monitoring results for pork, chicken, and beef samples in Vietnam in 2012–2013. J. Agric. Food Chem. 2015;63(21):5141–5145. doi: 10.1021/jf505254y. [DOI] [PubMed] [Google Scholar]
- 60.Li YB, Xu J, Wang F, Wang B, Liu LQ, Hou WL, Fan H, Tong YQ, Zhang J, Lu ZX. Overprescribing in China, driven by financial incentives, results in very high use of antibiotics, injections, and corticosteroids. Health Affair. 2012;31(5):1075–1082. doi: 10.1377/hlthaff.2010.0965. [DOI] [PubMed] [Google Scholar]
- 61.Leung HW, Jin L, Wei S, Tsui MMP, Zhou BS, Jiao LP, Cheung PC, Chun YK, Murphy MB, Lam PKS. Pharmaceuticals in tap water: Human health risk assessment and proposed monitoring framework in China. Environ. Health Persp. 2013;121(7):839–846. doi: 10.1289/ehp.1206244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamscher G, Pawelzick HT, Sczesny S, Nau H, Hartung J. Antibiotics in dust originating from a pig-fattening farm: A new source of health hazard for farmers? Environ. Health Persp. 2003;111(13):1590–1594. doi: 10.1289/ehp.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Callan HE, Jenkins RE, Maggs JL, Lavergne SN, Clarke SE, Naisbitt DJ, Park BK. Multiple adduction reactions of nitroso sulfamethoxazole with cysteinyl residues of peptides and proteins: Implications for hapten formation. Chem. Res. Toxicol. 2009;22(5):937–948. doi: 10.1021/tx900034r. [DOI] [PubMed] [Google Scholar]
- 64.Gill HJ, Hough SJ, Naisbitt DJ, Maggs JL, Kitteringham NR, Pirmohamed M, Park BK. The relationship between the disposition and immunogenicity of sulfamethoxazole in the rat. J. Pharmacol. Exp. Ther. 1997;282(2):795–801. [PubMed] [Google Scholar]
- 65.Komuro M, Higuchi T, Hirobe M. Application of chemical cytochrome P-450 model systems to studies on drug metabolism: VIII. Novel metabolism of carboxylic acids via oxidative decarboxylation. Bioorg. Med. Chem. 1995;3(1):55–65. doi: 10.1016/0968-0896(94)00141-O. [DOI] [PubMed] [Google Scholar]
- 66.Jung JY, Kim Y, Kim J, Jeong DH, Choi K. Environmental levels of ultraviolet light potentiate the toxicity of sulfonamide antibiotics in Daphnia magna. Ecotoxicol. 2008;17(1):37–45. doi: 10.1007/s10646-007-0174-9. [DOI] [PubMed] [Google Scholar]
- 67.Gust M, Fortier M, Garric J, Fournier M, Gagné F. Effects of short-term exposure to environmentally relevant concentrations of different pharmaceutical mixtures on the immune response of the pond snail Lymnaea stagnalis. Sci. Total Environ. 2013;445–446:210–218. doi: 10.1016/j.scitotenv.2012.12.057. [DOI] [PubMed] [Google Scholar]
- 68.Wang HL, Che BG, Duan AL, Mao JW, Dahlgren RA, Zhang MH, Zhang HQ, Zeng AB, Wang XD. Toxicity evaluation of β-diketone antibiotics on the development of embryo-larval zebrafish (Danio rerio) Environ. Toxicol. 2014;29(10):1134–1146. doi: 10.1002/tox.21843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.