Abstract
Background
Tuberculosis (TB) is an important cause of illness and death in HIV‐positive children living in areas of high TB prevalence. We know that isoniazid prophylaxis prevents TB in HIV‐negative children following TB exposure, but there is uncertainty related to its role in TB preventive treatment in HIV‐positive children.
Objectives
To summarise the effects of TB preventive treatment versus placebo in HIV‐positive children with no known TB contact on active TB, death, and reported adverse events.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE/PubMed, Embase and two trial registers up to February 2017.
Selection criteria
We included trials of HIV‐positive children with and without known TB exposure, randomized to receive TB preventive treatment or placebo.
Data collection and analysis
Two review authors independently used the study selection criteria, assessed risk of bias, and extracted data. We assessed effects using risk, incidence rate and hazard ratios and assessed the certainty of evidence using GRADE.
Main results
We included three trials, involving 991 participants, below the age of 13 years, from South Africa and Botswana. Children were randomized to isoniazid prophylaxis or placebo, given daily or three times weekly. The median length of follow‐up ranged from 5.7 to 34 months; some were on antiretroviral therapy (ART).
In HIV‐positive children not on ART, isoniazid prophylaxis may reduce the risk of active TB (hazard ratio (HR) 0.31, 95% confidence interval (CI) 0.11 to 0.87; 1 trial, 240 participants, low certainty evidence), and death (HR 0.46, 95% CI 0.22 to 0.95; 1 trial, 240 participants, low certainty evidence). One trial (182 participants) reported number of children with laboratory adverse events, which was similar between the isoniazid prophylaxis and placebo groups. No clinical adverse events were reported.
In HIV‐positive children on ART, we do not know if isoniazid prophylaxis reduces the risk of active TB (risk ratio (RR) 0.76, 95% CI 0.50 to 1.14; 3 trials, 737 participants, very low certainty evidence) or death (RR 1.45, 95% CI 0.78 to 2.72; 3 trials, 737 participants, very low certainty evidence). Two trials (714 participants) reported number of clinical adverse events and three trials (795 participants) reported number of laboratory adverse events; for both categories, the number of adverse events were similar between the isoniazid prophylaxis and placebo groups.
Authors' conclusions
Isoniazid prophylaxis given to all children diagnosed with HIV may reduce the risk of active TB and death in HIV‐positive children not on ART in studies from Africa. For children on ART, no clear benefit was detected. .
12 April 2019
Up to date
All studies incorporated from most recent search
All eligible published studies found in the last search (17 Feb, 2017) were included
Plain language summary
Isoniazid prophylaxis for preventing active tuberculosis and death in HIV‐positive children
What was the aim of this review?
To summarise the effects of isoniazid prophylaxis on TB, death, and adverse effects in HIV‐positive children.
Key messages
In areas of high tuberculosis endemicity, isoniazid prophylaxis prevents active TB and death in HIV‐positive children who are not on ART.
We conducted a review to assess the effect of TB medication on active TB or death and its safety in HIV‐positive children.
What was studied in the review?
TB is a common cause of severe lung disease and death in HIV‐positive children. Childhood TB is common in poor countries, especially those with a coexisting burden of HIV/AIDS disease. HIV‐positive children have a higher risk of developing TB than HIV‐negative children. Isoniazid prevents TB in HIV‐positive adults and is currently used in children who are at high risk of developing TB disease after exposure to someone with TB. However, there is limited information on the effect of isoniazid medication in reducing active TB or death if given to HIV‐positive children without known TB contact.
We searched for studies up to 17 February 2017, and found three studies published between 2007 and 2014 that addressed the effect of isoniazid medication compared to no medication on active TB and death in 991 HIV‐positive children, below the age of 13 years. Most of the children were on antiretroviral therapy (ART) and the studies were conducted in South Africa and Botswana. The median length of follow‐up ranged from 5.7 to 34 months.
What are the main results of the review?
In HIV‐positive children not taking ART, isoniazid medication reduced the number of children developing active TB by 69% (low certainty evidence), and death by 54% (low certainty evidence).
One trial was conducted in HIV‐positive children taking ART, and this did not detect any benefit or harm of isoniazid (very low certainty evidence).
The number of children with adverse effects were similar in children receiving isoniazid medication as the control group in both children on ART and not on ART.
How up to date is the review?
The review authors searched for studies published up to February 2017.
Summary of findings
Summary of findings for the main comparison. Isoniazid prophylaxis compared to placebo for HIV‐positive children not on antiretroviral therapy (ART).
| Isoniazid prophylaxis compared to placebo for HIV‐positive children not on antiretroviral therapy (ART) | ||||||
| Patient or population: HIV‐positive children not taking ART Settings: any setting Intervention: isoniazid prophylaxis daily or three times weekly Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comment | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Isoniazid prophylaxis | |||||
| Active TB | 10 per 100 | 3 per 100 (1 to 9) |
HR 0.31 (95% CI 0.11 to 0.87) |
240 (1 trial) |
⊕⊕⊝⊝
low1,2,3,4,5 due to serious indirectness and imprecision |
Isoniazid prophylaxis may reduce active TB |
| Death | 17 per 100 | 8 per 100 (8 per 17) |
HR 0.46 (95% CI 0.22 to 0.95) |
240 (1 trial) |
⊕⊕⊝⊝
low1,2,3,4,5 due to serious indirectness and imprecision |
Isoniazid prophylaxis may reduce death |
| The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; HR: hazard ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1No serious risk of bias: this trial was at low risk of selection bias, and adequately blinded study participants and personnel. However, the study was stopped early on the recommendation of the data safety monitoring board after only 277 of the planned 432 were enrolled. Not downgraded. 2No serious inconsistency: a single trial. 3Downgraded by 1 for serious indirectness: this single trial is from a single setting in South Africa. Broad generalization of this result to other settings is difficult given the variation in isoniazid resistance worldwide. 4Downgraded by 1 for serious imprecision: there were very few events in this trial and as such the finding is fragile. The original paper reports the result using a hazard ratio and the result reached standard levels of statistical significance. 5We reported the study authors' data.
Summary of findings 2. Isoniazid prophylaxis compared to placebo for HIV‐positive children on antiretroviral therapy (ART).
| Isoniazid prophylaxis compared to placebo for HIV‐positive children on antiretroviral therapy (ART) | ||||||
| Patient or population: HIV‐positive children on ART Settings: any setting Intervention: isoniazid prophylaxis daily or three times weekly Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Isoniazid prophylaxis | |||||
| Active TB | 13 per 100 | 9 per 100 (7 to 15) | RR 0.76 (0.50 to 1.14) | 737 (3 trials) | ⊕⊝⊝⊝
very low1,2,3,4 due to serious indirectness and imprecision |
We don't know if Isoniazid prophylaxis reduce active TB |
| Death | 4 per 100 | 6 per 100 (3 to 11) |
RR 1.45 (0.78 to 2.72) |
737 (3 trials) | ⊕⊝⊝⊝
very low1,2,3,5 due to serious indirectness and imprecision |
We don't know if Isoniazid prophylaxis reduce death |
| The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1No serious risk of bias: trials were at low risk of selection bias. Both studies adequately blinded study participants and personnel. 2No serious inconsistency: statistical heterogeneity was low. 3Downgraded by 1 for serious indirectness: all trials were conducted in South Africa. Given the variation in isoniazid resistance globally it is difficult to generalize this result to all settings. 4Downgraded by 2 for serious imprecision: to confidently detect a 25% relative reduction in active TB would require a sample size of nearly 3000 participants. This meta‐analysis is therefore underpowered, and the 95% CI includes both appreciable benefit and no effect. 5Downgraded by 2 for serious imprecision: there were few events and the 95% CI includes both appreciable harm and no effect.
Background
Description of the condition
Tuberculosis (TB) is an important cause of childhood morbidity and death especially in HIV‐positive children (Kochi 1991; Donald 2002). Infection is caused by Mycobacterium tuberculosis and most commonly results in acute and chronic respiratory disease (Foster 2003). In 1994, the incidence of childhood TB increased in low‐ and middle‐income countries (Nelson 2004). This resurgence was partly attributed to the coexisting burden of HIV disease (WHO 2012), which is most pronounced in sub‐Saharan Africa. At the end of 2012, an estimated 3.3 million children under 15 years of age, were living with HIV (UNAIDS/WHO 2013), and 260,000 new HIV infections in children in low‐ and middle‐income countries were reported the same year (UNAIDS/WHO 2013). The World Health Organization (WHO) estimates that, of the 8.7 million incident cases of TB in 2011, approximately 500,000 occurred in children under 15 years of age (WHO 2012). However, the burden of childhood TB disease is not as well documented as in adults, partly because of the difficulty of confirming the diagnosis.
Dual infection with TB has an important impact on HIV disease. TB accelerates the progression of HIV disease by increasing viral replication (Whalen 1995; Goletti 1996). TB is a common cause of acute pneumonia in African HIV‐positive children (Zar 2001; Jeena 2002), and may lead to chronic lung disease, including bronchiectasis (Ikeogu 1997; Jeena 1998). TB is a common cause of death in HIV‐positive children (Ikeogu 1997; Chintu 2005). Antimycobacterial drugs, such as rifampicin, have deleterious drug interactions with antiretroviral therapy (ART), complicating treatment. Rifampicin, an inducer of cytochrome P450 CYP3A, decreases the concentration of both the protease inhibitors and non‐nucleoside reverse transcriptase inhibitors, leading to sub‐therapeutic levels, and increasing the risk for inadequate viral suppression and consequent drug resistance (Ren 2008; Ren 2009). The large pill burden of two multiple drug regimens increases the risk of adverse events, such as liver toxicity and the likelihood of poorer adherence (Burman 2005).
Conversely, HIV infection has an impact on TB disease. HIV‐positive children have a higher risk of developing primary TB than HIV‐negative children (Mukadi 1997; Hesseling 2009). The diagnosis of TB is more difficult in HIV‐positive children, as other infections or HIV disease may mimic TB. Furthermore, tuberculin skin testing is less sensitive due to immunosuppression, and chest radiography is less specific (Berggren Palme 2002; Chintu 2005). The outcome of HIV‐positive versus HIV‐negative children with TB co‐infection is poorer, with six‐fold increased death in HIV‐positive children not on ART (Berggren Palme 2002; Hesseling 2005). The cure rate of TB in HIV‐positive children not on ART is significantly lower than in HIV‐negative children (Mukadi 1997; Berggren Palme 2002), with a higher rate of recurrence (Schaaf 2005). HIV‐positive children may therefore require a longer course of TB treatment (Perriens 1995; Schaaf 1998). HIV‐positive children, stable on ART, are less likely to develop TB disease and have a better outcome than those not on ART (Walters 2008; Edmonds 2009; Martinson 2009). However, the initiation of ART in the setting of TB co‐infection can lead to a paradoxical worsening of TB from the 'immune reconstitution syndrome' (Narita 1998; Puthanakit 2006; Zampoli 2007; Smith 2009). Moreover, the risk of TB in HIV‐positive children on ART is still higher than that of HIV‐negative children (Madhi 2011).
Description of the intervention
Isoniazid preventive treatment (IPT) is a secondary prevention strategy, whereby isoniazid prevents progression of latent TB to active TB in those at high‐risk of developing active TB (Cohen 2006). The WHO currently recommends that HIV/AIDS programmes include IPT as part of their package of care for HIV‐positive people. Current guidelines recommend six months of IPT for HIV‐positive children aged one year and above, without TB disease, even in the absence of a known TB contact; IPT for up to 3 years of use is recommended in HIV‐positive adolescents or adults (WHO 2010; WHO 2011).
How the intervention might work
Preventing TB infection and disease in HIV‐positive children is potentially an important public health intervention. Isoniazid has been used successfully as preventive treatment in HIV‐negative children following TB exposure (Smieja 1999), and in HIV‐infected adults with a positive tuberculin skin test (Akolo 2010), where treatment reduced TB disease by 62%.This benefit was found for all preventive drug regimens: isoniazid alone, isoniazid with rifampicin, rifampicin with pyrazinamide and isoniazid, rifampicin and pyrazinamide. No reduction in death was found. Isoniazid offers a further 40% reduction in active TB among HIV‐positive adults on ART (Rangaka 2014). Studies showed that IPT reduces active TB at the community and population level (Grant 2005 ). However, the strategy may be compromised by low adherence over the many months necessary to complete a single course of IPT, a problem worsened in HIV‐TB co‐infected individuals for whom IPT durations are prolonged. In contrast to adults, preventive treatment in children is aimed at preventing primary infection rather than reactivation of disease (WHO 2011). In settings of high TB prevalence, the rate of TB re‐infection is high (Verver 2005), and short‐course prophylaxis is potentially inadequate. Longer‐term prophylaxis or repeat courses may be necessary. Preventive treatment can lead to adverse events, mostly liver toxicity, although this is uncommon and rarely requires cessation of antimycobacterial medication in HIV‐positive children (Gray 2009a; Donald 2000; Gray 2009a; Le Roux 2013).The benefits of isoniazid outweigh the risk of liver damage for HIV‐infected people (Getahun 2010).
Why it is important to do this review
In high‐TB prevalence areas, TB preventive treatment may be effective in preventing infection and development of disease in HIV‐positive children. The efficacy of preventive treatment may, however, be limited by adherence difficulties, adverse events, and cost implications. The true efficacy of TB preventive treatment in HIV‐positive children must be clearly established and balanced against the occurrence of these events. We decided to undertake a Cochrane Review on the efficacy and safety of TB preventive treatment in HIV‐positive children with prophylaxis started in all children diagnosed with HIV infection.
Objectives
To summarise the effects of TB preventive treatment versus placebo in HIV‐positive children with no known TB contact on active TB, death, and reported adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) that evaluated the efficacy of TB preventive treatment in participants randomly allocated to preventive treatment for TB and placebo.
Types of participants
We included HIV‐positive children, below 13 years, without TB disease currently (irrespective of prior history of TB treatment, infection or prophylaxis). We included children on antiretroviral therapy (ART). For studies that included eligible and non‐eligible participants, we included only effect estimates of eligible participants.
Types of interventions
Intervention group: any TB drug or drug combination.
Comparison group: inactive placebo.
We did not impose any restrictions on study interventions, such as dose, duration of treatment, timing of outcome measurement, or co‐interventions.
Types of outcome measures
Primary outcomes
Active TB
We defined definite TB by a microbiological or histological identification of Mycobacterium tuberculosis. We based the diagnosis of probable TB on a combination of typical clinical symptoms and signs, tuberculin skin testing, chest radiography, a history of close TB contact, and a documented response to antimycobacterial treatment.
Secondary outcomes
Death
-
Grade 3 or higher clinical adverse events
Peripheral neuropathy (defined as sensory alteration or paraesthesia causing inability to perform usual social and functional activities or disabling sensory alteration or paraesthesia causing inability to perform age appropriate basic self‐care functions) (NIAID 2014)
Other clinical adverse events
-
Grade 3 or higher laboratory adverse events
Haematological abnormalities (defined as neutrophil count of 500/mm3 to 749/mm3 or < 500/mm3; platelet count of 25,000 to 49,999/mm3 or < 25,000/mm3; haemoglobin of 6.5 g/dL to 7.5 g/dL or < 6.5 g/dL) (NIAID 2014)
Liver enzyme abnormalities (raised alanine aminotransferase and aspartate aminotransferase of 5.1 to 10 or > 10, the upper limit of normal) (NIAID 2014)
Other laboratory adverse events
We graded adverse events according to the standardized paediatric AIDS clinical trial grading system of severity of adverse events (NIAID 2014).
Search methods for identification of studies
Electronic searches
We searched:
MEDLINE/PubMed (1946 to 17 February 2017) Appendix 1;
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library (17 February 2017) Appendix 2; and
Embase (1974 to 17 February 2017) Appendix 3.
We also searched for ongoing RCTs of chemoprophylaxis in HIV‐positive children in:
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp; to 17 February 2017); and
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; to 17 February 2017).
Searching other resources
We handsearched reference lists of identified articles and review articles. We screened abstracts and proceedings of the International Union Against Tuberculosis and Lung Disease (IUATLD) World Congress, the American Thoracic Society International Congress, and the European Respiratory Society World Congress conferences. We contacted investigators of identified trials and other content experts to locate information on any further trials that may not have been included in the electronic databases or presented at conferences, to find out whether these were completed, ongoing, or published. We scrutinized non‐Cochrane reviews for any additional relevant studies or unpublished data.
Data collection and analysis
Selection of studies
MZ and TY scrutinized studies independently for eligibility. We retrieved the full text of relevant articles and independently examined them for eligibility. We included studies if they met the prespecified eligibility criteria. Authors assessing study eligibility were not blinded to the names of the trial investigators, their institutions, journals of publication or results of study. Reasons for excluding studies are summarized in Characteristics of excluded studies. We record the selection process in sufficient detail to complete a PRISMA flow diagram Liberati 2009.
Data extraction and management
MZ and a research assistant (OA) extracted the data. The following study characteristics and outcomes were independently extracted in duplicate, under the following subheadings:
Methods (study design, study duration, methodological quality).
Participants (inclusion and exclusion criteria, number of patients randomized, setting).
Interventions (allocated treatment and dosage regimen, treatment duration)
Outcomes (active TB, death, clinical and laboratory adverse events).
Study results (number of participants with event of interest and the total number in that study arm for dichotomous outcomes; number lost to follow‐up, and how incomplete data were addressed)
Review authors who authored an included study did not participate in data extraction and assessment of risk of bias of included studies to maintain independence of the review. We summarized data in the Characteristics of included studies tables.
Assessment of risk of bias in included studies
We used Cochrane's 'Risk of bias' tool to assess the included studies (Deeks 2008; Higgins 2011). MZ and OA independently assessed the methodological quality of the included studies. We assessed the following domains.
The adequacy of generation of allocation sequence and allocation concealment in preventing selection bias.
The presence or absence of blinding of the participant and personnel to reduce performance bias.
The method of outcome assessment: whether the same method of assessment was used in both groups and presence or absence of blinding of outcome assessor in the prevention of detection bias.
Attrition bias by looking at the percentage of participants included in final analyses and the description of those not included. We also assessed whether or not an intention‐to‐treat analysis was performed.
We compared protocols of included studies with published articles to assess for risk of selective reporting bias.
Other bias.
We assigned the following judgements for risk of bias for each domain: low risk, high risk, and unclear risk of bias. We resolved disagreements on eligibility and methodological quality of trials by discussion and by obtaining further information from trial authors. We summarized data in a 'Risk of bias' table and graph, which shows our judgements on risk of bias in each domain, for each study separately, and as a percentage across all included studies.
Measures of treatment effect
For binary outcomes, we calculated risk ratios (RRs) and associated 95% confidence intervals (CIs). We assessed the effect of the intervention (isoniazid prophylaxis) compared to control (placebo) on the time to event (death and developing TB) with the hazard and incidence rate ratios (95% CI) presented in study reports.
Dealing with missing data
Two included trials used an intention‐to‐treat analysis to address missing data from participant dropout and the attrition rate was low in the third trial. We contacted the primary author of one included study for missing summary statistics, however, the author did not respond.
Assessment of heterogeneity
We used the Chi2 test and I2 statistic to assess statistical heterogeneity (Deeks 2008). We considered statistical heterogeneity to be substantial when the Chi2 test, which assesses if the observed differences in results are due to chance alone, had a P value of < 0.10 and when the I2 statistic, describing the percentage of variability in effect estimates due to heterogeneity, was > 50%.
Assessment of reporting biases
There were too few studies to enable investigation of publication bias using funnel plots.
Unit of analysis issues
All included trials followed a simple parallel group design, where children were individually randomized to either isoniazid prophylaxis or placebo group and a single measurement for each outcome (i.e. active TB or death) was collected and analyzed.
Data synthesis
We used a fixed‐effect meta‐analysis incorporating the Mantel‐Haenszel method for dichotomous outcomes and the inverse‐variance method for time‐to‐event outcomes to pool results across studies with no significant heterogeneity (Deeks 2008). Where meta‐analysis was inappropriate, we reported individual study results separately. We used Review Manager 5 for analysis (Review Manager 2014). We stratified the analysis by whether the children were receiving ART or not.
Assessment of overall certainty of evidence
We used GRADE methodology to assess the overall certainty of evidence (Guyatt 2008). In evaluating the certainty of RCT evidence, we considered the following in whether to downgrade the certainty of evidence: methodological limitations, unexplained heterogeneity or inconsistency of study results, indirectness of evidence, imprecision of results, and risk for publication bias. We rated the certainty across studies as high, moderate, low, or very low.
We used GRADEpro software to create two 'Summary of findings' tables for the comparisons 'isoniazid prophylaxis compared to placebo for HIV‐positive children not on ART' and 'isoniazid prophylaxis compared to placebo for HIV‐positive children on ART' (GRADEpro GDT 2014). We included active TB and death outcomes in the 'Summary of findings' tables.
MZ and OA independently assessed the certainty of the evidence. We resolved disagreements on certainty ratings by discussion and provided justification for decisions to down‐ or upgrade the ratings using footnotes in the ‘Summary of findings’ table and made comments to aid readers' understanding of the review, where necessary. We used plain language statements to report these findings in the review.
Subgroup analysis and investigation of heterogeneity
We considered a subgroup analysis based on background TB prevalence, but all studies were from high TB prevalence settings. We will consider subgroup analysis by background TB prevalence in subsequent review updates.
Sensitivity analysis
We could not perform sensitivity analysis due to limited number of trials included in the review.
Results
Description of studies
Results of the search
In the updated search (17 February 2017) we identified an additional two RCTs with 714 participants. We found no ongoing studies in the updated search (Figure 1).
1.
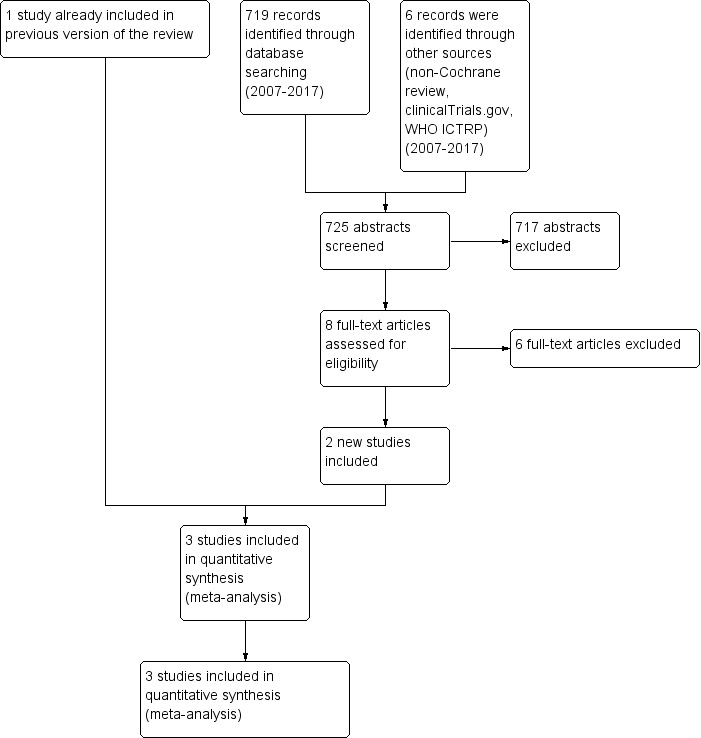
Study flow diagram.
Included studies
Design
We included three randomized controlled trials (RCTs) with a total of 991 participants in the review (Characteristics of included studies). Two trials were conducted in South Africa (Gray 2014; Zar 2007), and one was a multicentre trial that was conducted in South Africa and Botswana; however, enrolment in Botswana began shortly before the study was terminated (Madhi 2011).
Participants
HIV‐positive children in Zar 2007 and Gray 2014 were older (median age 24.7 to 38 months) than those in Madhi 2011 (median age 3.2 months). Zar 2007 and Gray 2014 included children who had a previous history of TB treatment or prophylaxis, 16% and 12%, respectively, compared to Madhi 2011, that excluded all children who had a history of TB or known exposure to a microbiologically confirmed case of TB. Compared to Madhi 2011 and Gray 2014, children in Zar 2007 were severely malnourished at enrolment: median weight‐for‐age z‐score −0.88 versus ‐1.56. Children in Zar 2007 (88% with a Centers for Disease Control and Prevention (CDC) category B or C) and Gray 2014 (92% with World Health Organization (WHO) classification Stage 3 or 4) were more likely to be severely immuno‐compromised compared to children in Madhi 2011 (8% with a CDC category B or C).
Participant inclusion and exclusion criteria are described in the Characteristics of included studies tables.
Interventions
Included studies randomly assigned children to isoniazid prophylaxis or placebo group. The dose of isoniazid prophylaxis (10 mg/kg with a variability of 8 mg/kg to 12 mg/kg) and frequency of treatment (either daily or three times weekly) were similar in Zar 2007 and Gray 2014 compared to Madhi 2011, where children received 10 mg/kg to 20 mg/kg of isoniazid daily. Children assigned to the control groups received placebo with identical appearance to isoniazid prophylaxis tablets, and administered in a similar way to isoniazid prophylaxis in the respective studies.
In Zar 2007, ART was initiated in 9% of children at baseline and in 22% during the trial. In Madhi 2011, ART was initiated at baseline in approximately 20% of HIV‐positive children and in 98.9% within the first year. All children in Gray 2014 were on ART. The analysis was stratified as such. In addition, only HIV‐positive children in Zar 2007 and Madhi 2011 received 5 mg/kg of cotrimoxazole.
Primary outcome
Active TB was similarly defined across all three trials (see the 'Types of outcome measures' section).
Excluded studies
We excluded six studies on review of full articles, see Characteristics of excluded studies tables for details.
Risk of bias in included studies
We have presented the risk of bias for each included trial in the 'Risk of bias' table in the 'Characteristics of included studies' tables. The 'Risk of bias' summary presents the review authors’ judgements on the risk of bias in each domain, for each trial separately (Figure 2), while the 'Risk of bias' graph presents the risk of bias in each domain as a percentage across all included trials (Figure 3). A summary of our findings on study methodological quality for each domain follows below.
2.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
3.
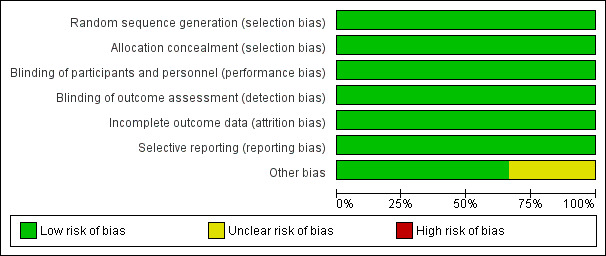
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
Allocation
Variable block random lists were used by Zar 2007 and Gray 2014 for sequence generation; Madhi 2011 created the random list using permuted blocks. Treatment groups were centrally allocated in all three trials (Zar 2007; Madhi 2011; Gray 2014). Methods of sequence generation were random and adequate for the three studies (Zar 2007; Madhi 2011; Gray 2014). Treatment allocation was adequately concealed in all three trials (Zar 2007; Madhi 2011; Gray 2014).
Blinding
In Zar 2007, participants, personnel, and investigators assessing the outcome, including an independent outcome assessor, were blinded to study treatment allocation. Participants, caregivers, investigators, and end point review committee were unaware of study group assignment in Madhi 2011. Participants and personnel were blinded in Gray 2014. The radiologist and the clinician (outcomes assessors) were blinded to the prophylactic regimen to which the child was allocated (Gray 2014). We assessed the three studies as having low risk of participant and personnel performance bias and detection bias (Zar 2007; Madhi 2011; Gray 2014).
Incomplete outcome data
The three studies were at low risk of attrition bias, because the attrition rate was below 20% in one study (Zar 2007), and all participants randomized were included in analysis in two trials (Madhi 2011; Gray 2014).
Selective reporting
We judged risk of reporting bias to be low in all three trials (Zar 2007; Madhi 2011; Gray 2014). All outcomes stated in the study protocols were reported in the published manuscripts.
Other potential sources of bias
The risk of other bias was unclear in one study; the data safety monitoring board recommended randomization into the placebo group to be stopped after 277 of the planned 432 participants were enrolled (Zar 2007). Two studies were at low risk of other potential sources of bias (Madhi 2011; Gray 2014).
Effects of interventions
Isoniazid versus placebo
HIV‐positive children not on antiretroviral therapy (ART)
Active tuberculosis (TB)
One trial from South Africa assessed the effects of isoniazid prophylaxis in HIV‐positive children not taking ART. This trial was stopped early when 263 of 432 (61%) planned participants had been recruited, due to a result that had reached standard levels of statistical significance. Isoniazid prophylaxis reduce the number of children developing active TB by 69% (hazard ratio (HR) 0.31, 95% confidence interval (CI) 0.11 to 0.87; 1 trial, 240 participants, (review authors own figures), low certainty evidence). The number of events in this trial was very low. Therefore, we judged the result to be of low certainty, when downgraded for serious indirectness and imprecision (Table 1).
Death
In children not taking ART, isoniazid prophylaxis reduces death by more than 50% (risk ratio (RR) 0.46, 95% CI 0.22 to 0.95; 1 trial, 240 participants, (review authors own figures), low certainty evidence; Table 1). We downgraded the trial for serious indirectness and imprecision.
Clinical adverse events (grade 3 or higher)
One trial (Zar 2007) reported no clinical adverse events in children not on ART (Table 3).
1. Number of children with adverse events and number of adverse events (of grade 3 or higher) in HIV‐positive children on antiretroviral therapy (ART) and not on ART, by study.
| Number of children with adverse events | Number of adverse events | |||||||
| Zar 2007 | Madhi 2011 | Gray 2014 | ||||||
| Children not on ART | Children on ART | Children on ART | Children on ART | |||||
| Isoniazid prophylaxis group N = 91 |
Placebo group N = 91 |
Isoniazid prophylaxis group N = 41 |
Placebo group N = 40 |
Isoniazid prophylaxis group N = 273 |
Placebo group N = 274 |
Isoniazid prophylaxis group N= 85 |
Placebo group N = 82 |
|
| Clinical adverse events | ||||||||
| Peripheral neuropathy | Not reported | Not reported | Not reported | Not reported | 3 | 2 | Not reported | Not reported |
| Other clinical adverse events | Not reported | Not reported | Not reported | Not reported | 14 | 23 | 1 | 1 |
| Laboratory adverse events | ||||||||
| Haematological (neutropenia, thrombocytopenia, anaemia) | 5 | 6 | 0 | 0 | 10 | 9 | Not reported | Not reported |
| Liver enzyme abnormalities | 0 | 2 | 0 | 0 | 12 | 12 | 3 | 1 |
| Other laboratory adverse events | Not reported | Not reported | 0 | 0 | Not reported | Not reported | Not reported | Not reported |
Abbreviations: ART: antiretroviral therapy; N: number of participants.
Laboratory adverse events (grade 3 or higher)
One trial (182 participants) reported the number of children not on ART with laboratory adverse events (Zar 2007) (Table 3). Five and six children had haematological abnormalities (RR 0.83, 95% CI 0.26 to 2.63) in the isoniazid prophylaxis and placebo groups, respectively. None of the children in the isoniazid prophylaxis group, but two in the placebo group had liver enzyme abnormalities.
HIV‐positive children on antiretroviral therapy (ART)
Active TB
In HIV‐positive children on ART, isoniazid prophylaxis conferred neither benefit nor harm on active TB (RR 0.76, 95% CI 0.50 to 1.14; 3 trials, 737 participants, very low certainty evidence; Analysis 1.1; Figure 4; Table 2). The trials and the meta‐analysis were underpowered to detect clinically important effects and consequently the 95% CI is wide (Table 4). The vast majority of data were from South Africa, limiting the broad generalization of the findings to other settings.
1.1. Analysis.
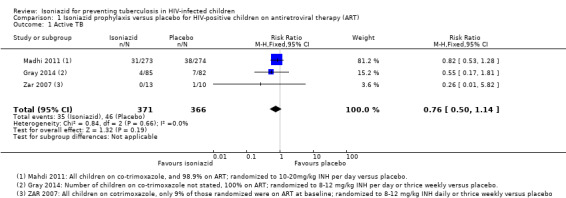
Comparison 1 Isoniazid prophylaxis versus placebo for HIV‐positive children on antiretroviral therapy (ART), Outcome 1 Active TB.
4.
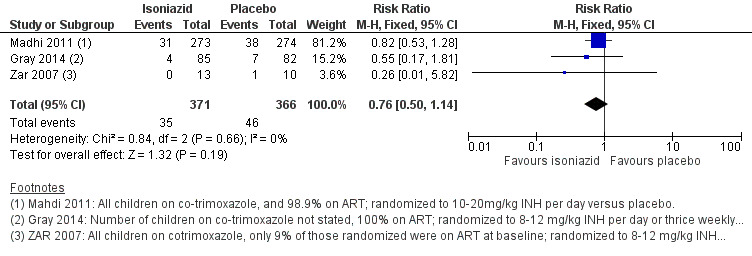
Forest plot of comparison: 1 Isoniazid prophylaxis versus placebo, outcome: 1.1 Active TB, HIV‐positive children on ART.
2. Optimal information size calculations.
| Outcome | Assumed risk | Source | Clinically important relative reduction | Sample size required1,2 |
| Active TB | 46/366 (13%) | Analysis 1.1 | 25% | 2990 |
| Death | 15/366 (4%) | Analysis 1.2 | 50% | 2282 |
1We based all calculations on: 2‐sided tests, with a ratio of 1:1, power of 0.8, and confidence level of 0.05. 2We performed all calculations using: sealedenvelope.com/power/binary‐superiority
Death
Isoniazid prophylaxis conferred neither benefit nor harm on death (RR 1.45, 95% CI 0.78 to 2.72; 3 trials, 737 participants, very low certainty evidence; Analysis 1.2; Figure 5; Table 2). The trials were underpowered to detect clinically important effects of isoniazid prophylaxis on death (Table 4). We downgraded the trials for indirectness and serious imprecision.
1.2. Analysis.
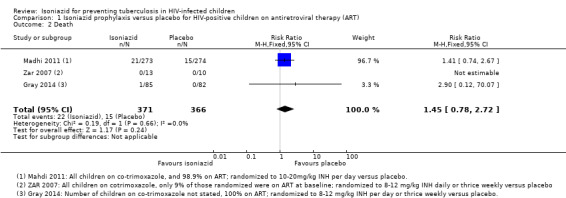
Comparison 1 Isoniazid prophylaxis versus placebo for HIV‐positive children on antiretroviral therapy (ART), Outcome 2 Death.
5.
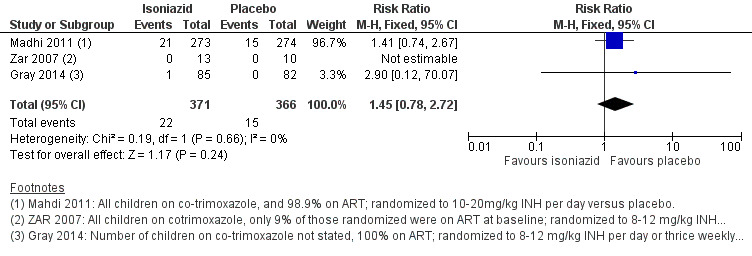
Forest plot of comparison: 1 Isoniazid prophylaxis versus placebo, outcome: 1.2 Death, HIV‐positive children on ART.
Clinical adverse events (grade 3 or higher)
Two trials (714 participants) reported number of clinical adverse events (Table 3). Three and two peripheral neuropathy abnormalities occurred in the isoniazid prophylaxis and placebo groups, respectively (Madhi 2011); 15 and 24 other clinical adverse events respectively, occurred in the isoniazid prophylaxis and placebo groups (Madhi 2011; Gray 2014).
Laboratory adverse events (grade 3 or higher)
Three trials (795 participants) reported number of laboratory adverse events (Table 3). There were 10 and 9 haematological abnormalities in the isoniazid prophylaxis and placebo groups (Madhi 2011); and 15 and 13 liver enzyme abnormalities in the isoniazid prophylaxis and placebo groups, respectively (Madhi 2011; Gray 2014).
Discussion
Summary of main results
In HIV‐positive children not taking antiretroviral therapy (ART), isoniazid prophylaxis may reduce active TB disease (low certainty evidence), and death (low certainty evidence). In HIV‐positive children without known exposure to a TB source case, and on ART, isoniazid prophylaxis conferred neither benefit nor harm for active TB (very low certainty evidence) or death (very low certainty evidence). HIV treatment may modify the effect of TB preventive treatment. Clinical adverse events were similar between the isoniazid prophylaxis and placebo groups, both in children not on ART and those on ART. Laboratory adverse events were similar between the isoniazid prophylaxis and placebo groups, both in children not on ART and those on ART. In a secondary analysis of Zar 2007 data, Le Roux 2013 showed that 16 (5.4%) out of 297 children developed severe liver injury while receiving isoniazid, but that only 1.7% of these cases were related to isoniazid prophylaxis.
Overall completeness and applicability of evidence
Isoniazid preventive therapy is currently recommended for preventing TB infection in HIV‐positive children. We included three trials (n = 991) in this review, which enrolled both young and older HIV‐positive children, living in areas of high TB prevalence. Most of the data were from South Africa, limiting the generalizability of the review findings to other settings, given the variation in isoniazid resistance globally. The trials were underpowered to detect clinically important effects of isoniazid prophylaxis on active TB and death in HIV‐positive children taking ART. ART is known to protect against TB disease, and adequately powered randomized controlled trials (RCTs) are therefore required to assess the possibility of isoniazid prophylaxis efficacy added to ART. Further data on isoniazid prophylaxis efficacy at varying levels of immunosuppression (HIV disease) are required.
Quality of the evidence
We used the GRADE approach to rate the certainty of the evidence GRADEpro GDT 2014. In evaluating the certainty of RCT evidence, we considered the following in whether to downgrade the certainty of the evidence: methodological limitations, inconsistency in study results, indirectness, imprecision, and publication bias. We presented the evidence in two 'Summary of findings' tables for isoniazid prophylaxis efficacy; one for HIV‐positive children not on ART (Table 1), and the other for HIV‐positive children on ART (Table 2). The evidence on the effect of isoniazid prophylaxis for active TB and death in HIV‐positive children not on ART was of low certainty. The effect of isoniazid prophylaxis for active TB and death in HIV‐positive children on ART was inconclusive and the evidence was of very low certainty. We downgraded the trials for serious indirectness and imprecision because all were conducted in one setting and were underpowered with wide confidence intervals.
Potential biases in the review process
We conducted an extensive and comprehensive search of electronic databases, with no language restrictions, to identify published and unpublished trials. MZ and TY independently performed study selection. MZ and OA independently extracted the data and assessed the methodological quality of included studies. Review authors who authored an included study, were not involved in extraction of data and assessment of methodological quality of any included studies, to maintain independence of the review.
Agreements and disagreements with other studies or reviews
The results of this review were similar to those from a review of isoniazid prophylaxis in adults (Akolo 2010). Isoniazid prophylaxis had a greater protective effect on TB disease in HIV‐positive children not on ART, reducing the chance of developing active childhood TB disease by 69%, similar to the 62% reduction in risk of active disease, found in the adult review (Akolo 2010). Evidence on impact of isoniazid prophylaxis on death in HIV‐positive children not on ART, showed a 54% reduction in death (Zar 2007). In contrast, isoniazid prophylaxis had no significant effect on TB disease and death in HIV‐positive children on ART; these findings were consistent with the Akolo 2010 review in HIV‐positive adults, reporting no effect on death, in a comparison of any drug for TB versus placebo. These findings may be consistent with the protective effect of ART, in reducing the risk of TB disease and death in HIV‐positive children.
Authors' conclusions
Implications for practice.
Isoniazid prophylaxis may reduce active tuberculosis (TB) disease and death in HIV‐positive children not on antiretroviral therapy (ART). The WHO 2013 guidelines recommend ART for all HIV‐positive children, which has to be started soon after diagnosis (WHO 2013). However, many children have delayed access to ART and coverage is not universal in most sub‐Saharan settings, suggesting that any child awaiting ART in a high TB prevalence area, should have isoniazid prophylaxis until the child is virally suppressed and immune reconstituted. Baseline risk of HIV‐positive children on ART, benefits and harms should be considered when making treatment decisions.
Implications for research.
The risk of TB is substantially higher in HIV‐positive children on ART compared to HIV‐negative children from the same community. In Madhi 2011, children on ART had a higher rate of TB disease, 121 cases per 1000 child‐years than HIV‐negative children, 41 per 1000 child‐years. Adequately powered trials assessing the impact of TB preventive therapy in HIV‐positive children on ART are required. A target sample size of 848 HIV‐positive children on ART will be required to have an 80% chance of detecting a 43% relative reduction, as significant at the 5% level, in the intervention group assuming a 14% baseline risk in active TB disease outcome. Trials that assess the long‐term effects of TB prophylaxis are also needed to better assess the length of benefit in different settings. The three included trials investigated isoniazid prophylaxis versus placebo. There are no data on the efficacy of other TB preventive treatment regimens. Studies in adults included multiple‐drug combination preventive treatment (Akolo 2010). However adverse events leading to discontinuation of treatment were more common for multiple‐drug therapy, as opposed to isoniazid treatment alone (Akolo 2010).
Although most cases of definite TB in the included studies were sensitive to isoniazid prophylaxis, long‐term data on the impact of isoniazid prophylaxis on Mycobacterium tuberculosis sensitivity are needed.
What's new
| Date | Event | Description |
|---|---|---|
| 18 August 2017 | New citation required and conclusions have changed | Search updated and new studies added. |
| 17 February 2017 | New search has been performed | Search updated and new studies added. A new author joined the team. |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 24 June 2009 | Amended | Added author whose name had inadvertently been omitted. |
| 21 March 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank Paul Garner, Co‐ordinating Editor of the Cochrane Infectious Diseases Group (CIDG), for his guidance. We thank Olatunji Adetokunboh (OA) who assisted with data extraction and assessment of risk of bias.
Moleen Zunza and Taryn Young are partly supported by the Effective Health Care Research Consortium. This Consortium and the CIDG editorial base are funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
Appendices
Appendix 1. MEDLINE search strategy
| #7 | Search (#5 AND #6) |
| #6 | Search (infant[mh] OR infant[tiab] OR infants[tiab] OR infancy[tiab] OR toddler*[tiab] OR preterm*[tiab] OR prematur*[tiab] OR postmatur*[tiab] OR baby[tiab] OR babies[tiab] OR neonat*[tiab] OR newborn[tiab] OR preschool*[tiab] OR pre‐school*[tiab] OR child[mh] OR child*[tiab] OR kindergar*[tiab] OR pupil*[tiab] OR schoolchild*[tiab] OR teen*[tiab] OR youth[tiab] OR youths[tiab] OR youngster*[tiab] OR young person*[tiab] OR young people[tiab] OR minors[mh] OR minors[tiab] OR puberty[mh] OR puberty[tiab] OR pubescen*[tiab] OR prepubescen*[tiab] OR paediatric*[tiab] OR pediatric*[tiab] OR peadiatric*[tiab] OR schools[mh:noexp] OR school*[tiab] OR kid[tiab] OR kids[tiab] OR boy*[tiab] OR girl*[tiab] OR creche*[tiab] OR highschool*[tiab] OR juvenil*[tiab] OR adolescent[mh] OR adolescen*[tiab] OR under ag*[tiab] OR underag*[tiab]) |
| #5 | Search (#1 AND #2 AND #3 AND #4) |
| #4 | Search (chemoprevention[mh] OR chemoprevention[tiab] OR chemoprophylaxis[tiab] OR prophylaxis[tiab] OR antitubercular agents[mh] OR antitubercular agents[tiab] OR antitubercular drugs[tiab] OR antituberculosis drugs[tiab] OR tuberculostatic agents[tiab] OR preventive therapy[tiab]) |
| #3 | Search (tuberculosis[mh] OR tuberculosis[tiab] OR tb[tiab] OR tuberculoses[tiab]) |
| #2 | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) |
| #1 | Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp]) |
Appendix 2. Cochrane library search strategy
| #10 | #6 and #7 and #8 and #9, in Trials |
| #9 | [mh infant] or infant:ti,ab,kw or infants:ti,ab,kw or infancy:ti,ab,kw or toddler*:ti,ab,kw or preterm*:ti,ab,kw or prematur*:ti,ab,kw or postmatur*:ti,ab,kw or baby:ti,ab,kw or babies:ti,ab,kw or neonat*:ti,ab,kw or newborn:ti,ab,kw or preschool*:ti,ab,kw or "pre‐school*":ti,ab,kw or [mh child] or child*:ti,ab,kw or kindergar*:ti,ab,kw or pupil*:ti,ab,kw or schoolchild*:ti,ab,kw or teen*:ti,ab,kw or youth:ti,ab,kw or youths:ti,ab,kw or youngster*:ti,ab,kw or (young next person*):ti,ab,kw or (young next people):ti,ab,kw or [mh minors] or minors:ti,ab,kw or [mh puberty] or puberty:ti,ab,kw or pubescen*:ti,ab,kw or prepubescen*:ti,ab,kw or paediatric*:ti,ab,kw or pediatric*:ti,ab,kw or peadiatric*:ti,ab,kw or [mh ^schools] or school*:ti,ab,kw or kid:ti,ab,kw or kids:ti,ab,kw or boy*:ti,ab,kw or girl*:ti,ab,kw or creche*:ti,ab,kw or highschool*:ti,ab,kw or juvenil*:ti,ab,kw or [mh adolescent] or adolescen*:ti,ab,kw or under ag*:ti,ab,kw or underag*:ti,ab,kw (Word variations have been searched) |
| #8 | [mh chemoprevention] or chemoprevention:ti,ab,kw or chemoprophylaxis:ti,ab,kw or prophylaxis:ti,ab,kw or [mh "antitubercular agents"] or (antitubercular agents):ti,ab,kw or (antitubercular drugs):ti,ab,kw or (antituberculosis drugs):ti,ab,kw or (tuberculostatic agents):ti,ab,kw or (preventive therapy):ti,ab,kw (Word variations have been searched) |
| #7 | [mh tuberculosis] or tuberculosis:ti,ab,kw or tuberculoses:ti,ab,kw or tb:ti,ab,kw |
| #6 | #1 or #2 or #3 or #4 or #5 |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) |
| #2 | MeSH descriptor: [HIV] explode all trees |
| #1 | MeSH descriptor: [HIV Infections] explode all trees |
Appendix 3. EMBASE search strategy
| #12 | #10 AND #11 |
| #11 | 'infant'/exp OR infant:ab,ti OR infants:ab,ti OR infancy:ab,ti OR toddler*:ab,ti OR preterm*:ab,ti OR prematur*:ab,ti OR postmatur*:ab,ti OR baby:ab,ti OR babies:ab,ti OR neonat*:ab,ti OR newborn:ab,ti OR preschool*:ab,ti OR pre+school*:ab,ti OR 'child'/exp OR child*:ab,ti OR kindergar*:ab,ti OR pupil*:ab,ti OR schoolchild*:ab,ti OR teen*:ab,ti OR youth:ab,ti OR youths:ab,ti OR youngster*:ab,ti OR 'young person':ab,ti OR 'young persons':ab,ti OR 'young people':ab,ti OR 'minors'/exp OR minors:ab,ti OR 'puberty'/exp OR puberty:ab,ti OR pubescen*:ab,ti OR prepubescen*:ab,ti OR paediatric*:ab,ti OR pediatric*:ab,ti OR peadiatric*:ab,ti OR 'schools'/exp OR school*:ab,ti OR kid:ab,ti OR kids:ab,ti OR boy*:ab,ti OR girl*:ab,ti OR creche*:ab,ti OR highschool*:ab,ti OR 'juvenile'/exp OR juvenil*:ab,ti OR 'adolescent'/exp OR adolescen*:ab,ti OR (under NEXT/1 ag*):ab,ti OR underag*:ab,ti |
| #10 | #1 AND #7 AND #8 AND #9 |
| #9 | 'chemoprevention'/de OR chemoprevention:ab,ti OR 'chemoprophylaxis'/de OR chemoprophylaxis:ab,ti OR prophylaxis:ab,ti OR (antitubercular NEXT/1 (agent* OR drug*)):ab,ti OR ('anti tubercular' NEXT/1 (agent* OR drug*)):ab,ti OR (antituberculosis NEXT/1 (agent* OR drug*)):ab,ti OR 'tuberculostatic agent'/de OR (tuberculostatic NEXT/1 agent*):ab,ti OR 'preventive therapy':ab,ti |
| #8 | 'tuberculosis'/exp OR tuberculosis:ab,ti OR tuberculoses:ab,ti OR tb:ab,ti |
| #7 | #2 NOT #6 |
| #6 | #3 NOT #5 |
| #5 | #3 AND #4 |
| #4 | 'human'/de OR 'normal human'/de OR 'human cell'/de |
| #3 | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de |
| #2 | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR (doubl* NEAR/3 blind*):ab,ti OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR (cross NEXT/1 over*):ab,ti |
| #1 | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus':ab,ti OR 'human immuno+deficiency virus':ab,ti OR 'human immunedeficiency virus':ab,ti OR 'human immune+deficiency virus':ab,ti OR hiv:ab,ti OR 'hiv‐1':ab,ti OR 'hiv‐2':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno+deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti OR 'acquired immune+deficiency syndrome':ab,ti |
Data and analyses
Comparison 1. Isoniazid prophylaxis versus placebo for HIV‐positive children on antiretroviral therapy (ART).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Active TB | 3 | 737 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.50, 1.14] |
| 2 Death | 3 | 737 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.78, 2.72] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gray 2014.
| Methods | Trial design: double‐blind, randomized placebo‐controlled trial. Follow‐up: full blood count, liver function tests, urea and electrolyte tests, percentage of CD4 cells and viral load were measured at baseline and 6‐monthly. CXR was performed at baseline, and additional CXRs were taken if clinically indicated. Adverse events: symptoms of adverse reactions to INH were recorded at each study visit. |
|
| Participants | Number of participants: 167 Median (IQR) age at baseline: 35 months (15 to 65) Inclusion criteria: age > 8 weeks, on ART for greater than 2 months, weight > 2.5 kg, adherence to ART of > 90%, prior history of TB treatment or prophylaxis, informed consent, resident in the area, access to transport. Exclusion criteria: chronic diarrhoea, currently using isoniazid prophylaxis, exposure to a TB contact, history of prior isoniazid hypersensitivity, severe anaemia (haemoglobin less than 7 gm/dL), neutropenia (absolute neutrophil count less than 400 cells/µL), thrombocytopenia (platelet count less than 50 000/µL), non‐reversible renal failure. |
|
| Interventions |
All children were on ART and had adherence of at least 90% at baseline. |
|
| Outcomes |
Not included in this review
|
|
| Notes | Definitions: ‐ Definite TB: a microbiological or histological identification of Mycobacterium tuberculosis. ‐ Probable TB: based on a combination of typical clinical symptoms and signs, tuberculin skin testing, chest radiography, a history of close TB contact, and a documented response to antimycobacterial therapy. Country: South Africa Prevalence of isoniazid resistance: 0% Positive tuberculin test: 16% Funding: The study was funded by the Medical Research Council, South Africa; the National Research Foundation, Department of Health, South Africa; and the Discovery Foundation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization list was created using permuted blocks, stratified by HIV infection status and balanced by study site. |
| Allocation concealment (selection bias) | Low risk | Treatment groups were centrally allocated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Placebo had an identical appearance to INH tablets and was administered in a double blind matter". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The chest radiographs were reported by a radiologist blinded to the prophylactic regimen to which the child was allocated. Diagnosis of TB was independently reviewed by an experienced clinician blinded to study randomisation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was followed. All participants randomized were included in analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes stated in the protocol were included in the published manuscript. |
| Other bias | Low risk | None suspected. |
Madhi 2011.
| Methods | Trial design: multicentre, phase 2–3, randomized, double‐blind, placebo‐controlled trial. Follow‐up: TB disease‐free survival and incidence of TB disease at 96 weeks after randomization. Adverse events: liver enzyme tests, blood counts, clinical neurologic evaluations for peripheral neuropathy, at scheduled visits every 3 months. |
|
| Participants | Number of participants: 547 Median (IQR) age at baseline: 3 months (3 to 4) Inclusion criteria: age of 91 to 120 days, received Bacille Calmette‐Guerin (BCG) vaccine, no history of TB in the infant, known exposure to a microbiological confirmed case of TB, or active anti‐TB treatment in the mother at the time of the infant's birth, no evidence of failure to thrive, recurrent pneumonia, chronic diarrhoea, or immunosuppressive conditions other than HIV infection. Exclusion criteria: previous diagnosis of TB infection, previous receipt of isoniazid, contact with a known acid fast bacilli (AFB) sputum smear or culture‐positive case of TB before study entry, current acute or recurrent (3 or more prior episodes) lower respiratory tract disease, chronic persistent diarrhoea, significant drop in weight or failure to gain weight appropriately during a 2‐ to 3‐month period, contraindications for use of isoniazid or SMX/TMP, require certain medications, known or suspected immune system diseases other than HIV, current or previous diagnosis of or treatment for cancer, current immunosuppressive therapy greater than 1 mg/kg/day of prednisone or equivalent, anticipated long‐term oral or intravenous corticosteroid therapy (greater than 3 weeks), those receiving nonsteroidal anti‐inflammatory agents and inhaled corticosteroids were not excluded, grade 3 or greater AST/SGOT, ALT/SGPT, ANC, haemoglobin, platelet count, rash, neuropathy, or myopathy at screening, any grade 4 clinical or laboratory toxicity within 14 days prior to study entry, other acute or chronic conditions that, in the opinion of the investigator, may interfere with the study. |
|
| Interventions |
At baseline, 98.7% of the children were on ART. ART mainly included stavudine, lamivudine, and lopinavir‐ritonavir or zidovudine, lamivudine, and lopinavir– ritonavir, following per country‐specific guidelines. |
|
| Outcomes |
Not included in this review
|
|
| Notes | Definitions: ‐ Definite TB: a microbiological or histological identification of Mycobacterium tuberculosis. ‐ Probable TB: based on a combination of typical clinical symptoms and signs, tuberculin skin testing, chest radiography, a history of close TB contact, and a documented response to antimycobacterial therapy. All infants were enrolled in the first 6 months of life. The study included HIV‐uninfected children with outcomes defined differently for this subgroup, however, analysis of HIV‐uninfected children was not included in this review. All HIV‐infected children also received trimethoprim–sulfamethoxazole prophylaxis 5 mg/kg according to WHO guidelines. TB disease‐free survival was defined as the first occurrence of death from any cause or TB disease, 96 weeks after randomization. HIV disease progression, was defined as the first occurrence of worsening of the Centers for Diseases Control and Prevention (CDC) clinical categorization of HIV infection or death. Country: South Africa, Botswana Prevalence of isoniazid resistance (95% CI): 26% (9 to 51) Positive tuberculin test: not reported Funding: The study was supported by the Statistical and Data Analysis Center at the Harvard School of Public Health, under NIAID cooperative agreements with the Pediatric AIDS Clinical Trials Group (5 U01 AI41110) and the IMPAACT Group (1 U01 AI068616); NIAID and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network (NICHD contract number N01‐DK‐9‐001/HHSN267200‐800001C); Secure the Future Fund, a philanthropy program sponsored by Bristol‐Myers Squibb. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization list was created using permuted blocks". |
| Allocation concealment (selection bias) | Low risk | Treatment groups were centrally allocated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, caregivers and investigators were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "End point review committee was unaware of study‐group assignments". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was followed, however, its not clear if all enrolled participants were included in analysis. |
| Selective reporting (reporting bias) | Low risk | All important outcomes stated in the study protocol were reported in the published manuscript. |
| Other bias | Low risk | None suspected. |
Zar 2007.
| Methods | Trial design: double‐blind, placebo‐controlled trial. Follow‐up: children underwent a tuberculin skin test and chest radiography if clinically indicated. Adverse events: alanine transaminase were measured one and three months after randomization and thereafter six‐monthly or more frequently if clinically indicated. |
|
| Participants | Number of participants: 277 Median (IQR) age at baseline: 25 months (9 to 52) Inclusion criteria: age > 8 weeks, weight > 2.5 kg, access to transport, informed consent, children stable on ART for two to three months. Exclusion criteria: chronic diarrhoea, current use of or need for isoniazid prophylaxis, previous hypersensitivity to isoniazid or sulphur containing drugs, haemoglobin < 70 g/L, neutrophil count < 400 cell/uL, platelet count < 50,000 x109/L, non‐reversible renal failure. |
|
| Interventions |
ART was not widely available. Some children obtained treatment through participation in pharmaceutical trials or charitable donations. 23 of 263 (9%) were on ART at enrolment and 58 (22%) started treatment during the trial. |
|
| Outcomes |
Not included in this review: none |
|
| Notes | Definitions: ‐ Definite TB: a microbiological or histological identification of Mycobacterium tuberculosis. ‐ Probable TB: based on a combination of typical clinical symptoms and signs, tuberculin skin testing, chest radiography, a history of close TB contact, and a documented response to antimycobacterial therapy. Cotrimoxazole (5 mg/kg/dose of the trimethoprim component): Given to all children < 12 months and those older with clinical CDC category B or C disease, in those with severe immunological impairment (CD4 count of < 15% of total lymphocyte count), or in those with previous episode of Pneumocystis jirovecii pneumonia. Study was planned to run for 2 years, however, the placebo arm was terminated early on the recommendation of the data safety monitoring board on the basis of the results of interim analyses. About 30% of the children received ART during the trial with similar percentages in isoniazid and placebo groups. Adverse events were graded 1 to 4 according to the toxicity criteria of the National Institutes of Health's division of AIDS (DAIDS). Grade 3 and 4 events were reported. Country: South Africa Prevalence of isoniazid resistance: 0% Positive tuberculin test: 9% Funding: The study was supported by Rockefeller Foundation, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A variable block random list was used. Generation of allocation sequence was achieved by variable blocked randomization lists prepared by the trial statistician and sent to each trial site pharmacist in a sealed opaque envelope. |
| Allocation concealment (selection bias) | Low risk | Central allocation (pharmacists labelled trial drugs with sequential numbers). The participants were allocated study numbers sequentially by the study nurse at enrolment. They were then sequentially allocated to treatment group by the pharmacist according to the prepared list. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was manufactured to have an identical appearance to isoniazid, participants and personnel were blinded to study assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators assessing the outcome and were blinded, the diagnosis of probable TB was subject to independent review by a blinded investigator. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate was very low, < 20%. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the study protocol were the same as those in the published manuscript. |
| Other bias | Unclear risk | The data safety monitoring board recommended randomization into placebo to be stopped after 277 of the planned 432 were enrolled. The placebo arm was ended on 17 May 2004 on the recommendation of the data safety monitoring board on the basis of the results of interim analyses. |
Abbreviations:
ALT/SGPT: Alanine aminotransferase (serum glutamic pyruvic transaminase) ANC: Absolute neutrophil count ART: Antiretroviral therapy AST/SGOT: Aspartate aminotransferase (serum glutamic‐oxaloacetic transaminase) CDC: Centers for Diseases Control and Prevention CXR: Chest x‐rays INH: Isoniazid SMX/TMP: Trimethoprim/Sulfamethoxazole TB: Tuberculosis WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Frigati 2011 | A cohort study. |
| Hesseling 2012 | Secondary analysis of Madhi 2011. |
| Iro 2013 | Commentary. |
| le Roux 2009 | Not addressing review outcomes; secondary analysis of Zar 2007. |
| Le Roux 2013 | Cohort study. Secondary analysis of Zar 2007. |
| Marais 2013 | Commentary. |
Differences between protocol and review
This is an update of the 2009 version Gray 2009b of this review. Gray 2009b included one completed and two ongoing RCTs. The latter two RCTs are complete and now included in the update. An updated search identified no other studies. The methods now include the latest Cochrane risk of bias assessment tool, GRADE and Summary of findings tables. Findings are stratified into HIV positive children on ART and not on ART. Moleen Zunza joined the author team.
Contributions of authors
DG was the lead author for the first version of the review.
DG and HZ conducted the search and scrutinized identified studies for eligibility.
DG and TY assessed the methodological quality of included studies.
All authors critically reviewed the manuscript before submission (initial review).
MZ was the lead author of the review update.
MZ and TY screened the search results and assessed potential studies for eligibility.
MZ and a research assistant assessed the methodological quality of included studies.
DG, MC, and HZ critically reviewed the manuscript for content (review update). All authors approved the final version of the review.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
HIV/AIDS mentoring programme, South African Cochrane Centre, South Africa.
Cochrane Child Health Field Bursary, Canada.
-
Department for International Development, UK. Research Programme Grant, UK.
Grant: 5242
Declarations of interest
MZ: MZ is supported by the Effective Health Care Research Consortium funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
DG: Author of an included study
TY: TY is supported by the Effective Health Care Research Consortium funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
MC: Author of an included study
HZ: Author of an included study
To avoid potential bias, authors who were investigators on included studies did not do data extraction and methodological quality assessment of included studies.
Unchanged
References
References to studies included in this review
Gray 2014 {published data only}
- Gray DM, Workman LJ, Lombard CJ, Jennings T, Innes S, Grobbelaar CJ, et al. Isoniazid preventive therapy in HIV infected children on antiretroviral therapy: a pilot study. International Journal of Tuberculosis and Lung Disease 2014;18(3):322‐7. [DOI] [PubMed] [Google Scholar]
Madhi 2011 {published data only}
- Madhi SA, Nachman S, Violari A, Kim S, Cotton MF, Bobat R, et al. Primary isoniazid prophylaxis against tuberculosis in HIV‐exposed children. New England Journal of Medicine 2011;365(1):21‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zar 2007 {published data only}
- Zar HJ, Cotton MF, Strauss S, Karpakis J, Hussey G, Schaaf HS, et al. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomised controlled trial. Lancet 2007;334:136‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Frigati 2011 {published data only}
- Frigati LJ, Kranzer K, Cotton MF, Schaaf HS, Lombard CJ, Zar HJ. The impact of isoniazid preventive therapy and antiretroviral therapy on tuberculosis in children infected with HIV in a high tuberculosis incidence setting. Thorax 2011;66(6):496‐501. [DOI] [PubMed] [Google Scholar]
Hesseling 2012 {published data only}
- Hesseling AC, Kim S, Madhi S, Nachman S, Schaaf HS, Violari A, et al. High prevalence of drug resistance amongst HIV‐exposed and ‐infected children in a tuberculosis prevention trial. International Journal of Tuberculosis and Lung Disease 2012;16(2):192‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iro 2013 {published data only}
- Iro MA, Brown N. Isoniazid prophylaxis started at 3‐4 months of life does not prevent tuberculosis disease or infection in both HIV‐infected and uninfected children. Archives of Disease in Childhood: Education and Practice Edition 2013;98(1):40. [DOI] [PubMed] [Google Scholar]
le Roux 2009 {published data only}
- Roux SM, Cotton MF, Golub JE, Roux DM, Workman L, Zar HJ. Adherence to isoniazid prophylaxis among HIV‐infected children: a randomized controlled trial comparing two dosing schedules. BMC Medicine 2009;7(67):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Le Roux 2013 {published data only}
- Roux SM, Cotton MF, Myer L, Roux DM, Schaaf HS, Lombard CJ, et al. Safety of long‐term isoniazid preventive therapy in children with HIV: a comparison of two dosing schedules. International Journal of Tuberculosis and Lung Disease 2013;17(1):26‐31. [DOI] [PubMed] [Google Scholar]
Marais 2013 {published data only}
- Marais BJ, Graham SM, Maeurer M, Zumla A. Progress and challenges in childhood tuberculosis. Lancet. Infectious Diseases 2013;13(4):287‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Akolo 2010
- Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent infection in HIV infected persons. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD000171.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berggren Palme 2002
- Berggren Palme I, Gudetta B, Bruchfield J, Muhe L, Giesecke J. Impact of human immunodeficiency virus 1 infection on clinical presentation, treatment outcome and survival in a cohort of Ethiopian children with tuberculosis. Pediatric Infectious Disease Journal 2002;21:1053‐61. [DOI] [PubMed] [Google Scholar]
Burman 2005
- Burman WJ. Issues in the management of HIV‐related tuberculosis. Clinics in Chest Medicine 2005;26:283‐94. [DOI] [PubMed] [Google Scholar]
Chintu 2005
- Chintu C, Mwaba P. Tuberculosis in children with human immunodeficiency virus infection. The International Journal of Tuberculosis Lung Disease 2005;9(5):477‐84. [PubMed] [Google Scholar]
Cohen 2006
- Cohen T, Lipsitch M, Walensky RP, Murray M. Beneficial and perverse effects of isoniazid preventive therapy for latent tuberculosis infection in HIV‐tuberculosis co‐infected populations. Proceeding of the National Academy of Sciences of the United States of America 2006;103(18):7042‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2008
- Deeks J, Higgins JP, Altman D. Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2008:243‐96. [Google Scholar]
Donald 2000
- Donald PR. Childhood tuberculosis. Current Opinion in Pulmonary Medicine 2000;6:187‐92. [DOI] [PubMed] [Google Scholar]
Donald 2002
- Donald PR. Childhood tuberculosis: out of control?. Current Opinion in Pulmonary Medicine 2002;8:178‐82. [DOI] [PubMed] [Google Scholar]
Edmonds 2009
- Edmonds A, Lusiama J, Napravnik S, Kitetele F, Rie A, Behets F. Anti‐retroviral therapy reduces incident tuberculosis in HIV‐infected children. International Journal of Epidemiology 2009;38:1612‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foster 2003
- Foster K, Alton H. Chronic lung infection in children. Paediatric Respiratory Reviews 2003;4:225‐9. [PubMed] [Google Scholar]
Getahun 2010
- Getahun H, Granich R, Sculier D, Gunneberg C, Blanc L, Nunn P, et al. Implementation of isoniazid preventive therapy for people living with HIV worldwide: barriers and solutions. AIDS 2010;24(5):S57‐65. [DOI] [PubMed] [Google Scholar]
Goletti 1996
- Goletti D, Weisman D, Jackson RW, Graham NM, Vlahov D, Klein RS, et al. Effect of mycobacterium tuberculosis on HIV replication: role of immune activation. Journal of Immunology 1996;157:1271‐8. [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Grant 2005
- Grant AD, Charalambous S, Fielding KL, Day JH, Corbertt EL, Chaisson RE, et al. Effect of routine isoniazid preventive therapy on tuberculosis incidence among HIV‐infected men in South Africa. JAMA 2005;293(22):2719‐25. [DOI] [PubMed] [Google Scholar]
Gray 2009a
- Gray D, Nuttall J, Lombard C, Davies MA, Workman L, Apolles P, et al. Low rates of hepatotoxicity in HIV‐infected children on anti‐retroviral therapy with and without isoniazid prophylaxis. Journal of Tropical Pediatrics 2010;56(3):159‐65. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hesseling 2005
- Hesseling AC, Westra AE, Werschkull H, Donald PR, Beyers N, Hussey GD, et al. Outcome of HIV infected children with culture confirmed tuberculosis. Archives of Disease in Childhood 2005;90:1171‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hesseling 2009
- Hesseling AC, Cotton MF, Jennings T, Whitelaw A, Johnson LF, Eley B, et al. High incidence of tuberculosis among HIV‐infected infants: evidence from a South African population‐based study highlights the need for improved tuberculosis control strategies. Clinical Infectious Diseases 2009;48:108–14. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ikeogu 1997
- Ikeogu Mo, Wolf B, Mathe S. Pulmonary manifestations in HIV seropositivity and malnutrition in Zimbabwe. Archives of Disease in Childhood 1997;76:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeena 1998
- Jeena PM, Coovadia HM, Thula SA, Blythe D, Buckels NJ, Chetty R. Persistent and chronic lung disease in HIV‐1‐infected and uninfected African children. AIDS 1998;12:1185‐93. [DOI] [PubMed] [Google Scholar]
Jeena 2002
- Jeena PM, Pillay P, Pillay T, Coovadia HM. Impact of HIV‐1 co‐infection on presentation and hospital‐related mortality in children with culture proven pulmonary tuberculosis in Durban, South Africa. The International Journal of Tuberculosis and Lung Disease 2002;6:672‐8. [PubMed] [Google Scholar]
Kochi 1991
- Kochi A. The global tuberculosis situation and the new strategy of the World Health Organisation. Tubercle 1991;72:1‐6. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinson 2009
- Martinson NA, Moultrie H, Niekerk R, Barry G, Coovadia A, Cotton M, et al. HAART and risk of tuberculosis in HIV‐infected South African children: a multi‐site retrospective cohort. International Journal of Tuberculosis and Lung Disease 2009;13(7):862–7. [PMC free article] [PubMed] [Google Scholar]
Mukadi 1997
- Mukadi YD, Wiktor Z, Coulibaly IM, Coulibaly D, Mbengue A, Folquet AM, et al. Impact of HIV infection on the development, clinical presentation and outcome of tuberculosis among children in Abidjan, Cote d'Ivoire. AIDS 1997;11(9):1151‐8. [DOI] [PubMed] [Google Scholar]
Narita 1998
- Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. American Journal of Respiratory and Critical Care Medicine 1998;158(1):157‐61. [DOI] [PubMed] [Google Scholar]
Nelson 2004
- Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. The International Journal of Tuberculosis and Lung Disease 2004;8(5):636‐47. [PubMed] [Google Scholar]
NIAID 2014
- U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, 2014. https://rsc.tech‐res.com/docs/default‐source/safety/daids_ae_grading_table_v2_nov2014.pdf?sfvrsn=8 (accessed 03 July 2017).
Perriens 1995
- Perriens JH, Louis ME, Mukadi YB, Brown C, Prignot J, Pouthier F, et al. Pulmonary tuberculosis in HIV‐infected patients in Zaire. A controlled trial of treatment for either 6 or 12 months. New England Journal of Medicine 1995;332(12):779‐84. [DOI] [PubMed] [Google Scholar]
Puthanakit 2006
- Puthanakit T, Oberdorfer P, Akarathum N, Wannarit P, Sirisanthana T, Sirianthana V. Immune reconstitution syndrome after highly active antiretroviral therapy in human immunodeficiency virus‐infected Thai children. Pediatric Infectious Disease Journal 2006;25(1):53‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rangaka 2014
- Rangaka MX, Wilkinson RJ, Boulle A, Glynn JR, Fielding K, Cutsem G, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis:a randomised double‐blind, placebo‐controlled trial. Lancet 2014;384(9944):682‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ren 2008
- Ren Y, Nuttall JJ, Egbers C, Eley BS, Meyers TM, Smith PJ, et al. Effect of rifampicin on lopinavir pharmacokinetics in HIV‐infected children with tuberculosis. Journal of Acquired Immune Deficiency Syndromes 2008;47:566–9. [DOI] [PubMed] [Google Scholar]
Ren 2009
- Ren Y, Nuttall JJ, Egbers C, Eley BS, Meyers TM, Smith PJ, et al. Effect of rifampicin on efavirenz pharmacokinetics in HIV‐infected children with tuberculosis. Journal of Acquired Immune Deficiency Syndromes 2009;50:439–43. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schaaf 1998
- Schaaf HS, Geidenduys A, Gie RP, Cotton MF. Culture‐positive tuberculosis in human immunodeficiency virus type‐1‐infected children. Pediatric Infectious Disease Journal 1998;7:599‐604. [DOI] [PubMed] [Google Scholar]
Schaaf 2005
- Schaaf HS, Krook S, Hollemans DW, Warren RM, Donald PR, Hesseling AC. Recurrent culture‐confirmed tuberculosis in human immunodeficiency virus‐infected children. Pediatric Infectious Disease Journal 2005;24(8):685‐91. [DOI] [PubMed] [Google Scholar]
Smieja 1999
- Smieja MJ, Marchettu CA, Cook DJ, Smaill FM. Isoniazid for preventing tuberculosis in non‐HIV infected persons. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD001363] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2009
- Smith K, Kuhn L, Coovadia A, Meyers T, Hub CC, Reitz C, et al. Immune reconstitution inflammatory syndrome among HIV infected South African infants initiating antiretroviral therapy. AIDS 2009;23(9):1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
UNAIDS/WHO 2013
- UNAIDS/WHO. AIDS epidemic update. September. www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/201309_epi_core_en.pdf (accessed 25 November 2013).
Verver 2005
- Verver S, Warren RM, Beyers N, Richardson M, Spuy GD, Borgdorff MW, et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. American Journal of Respiratory and Critical Care Medicine 2005;171:1430–5. [DOI] [PubMed] [Google Scholar]
Walters 2008
- Walters E, Cotton MF, Rabie H, Schaaf HS, Walters LO, Marais BJ. Clinical presentation and outcome of tuberculosis in human immunodeficiency virus infected children on anti‐retroviral therapy. BMC Pediatrics 2008;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whalen 1995
- Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. American Journal of Respiratory and Critical Care Medicine 1995;151:129‐35. [DOI] [PubMed] [Google Scholar]
WHO 2010
- World Health Organization. Antiretroviral therapy for HIV infection in infants and children: towards universal access. Recommendations for a public health approach: 2010 revision. www.who.int/hiv/pub/paediatric/infants2010/en/index.html (accessed 16 November 2015). [PubMed]
WHO 2011
- World Health Organization. Guidelines for intensified tuberculosis case‐finding and isoniazid preventive therapy for people living with HIV in resource‐constrained settings. www.whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf (accessed 16 November 2015).
WHO 2012
- World Health Organization. Global Tuberculosis Report, 2012. apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf (accessed June 2013).
WHO 2013
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, 2013. www.who.int/hiv/pub/guidelines/arv2013/download/en/ (accessed prior to 6 June 2017). [PubMed]
Zampoli 2007
- Zampoli M, Kilborn T, Eley B. Tuberculosis during early antiretroviral‐induced immune reconstitution in HIV‐infected children. International Journal of Tuberculosis and Lung Disease 2007;11(4):417‐23. [PubMed] [Google Scholar]
Zar 2001
- Zar HJ, Manslo D, Tannebaum E, Klein M, Argent A, Eley B, et al. Aetiology and outcome of pneumonia in human immunodeficiency virus‐infected children hospitalized in South Africa. Acta Paediatrica 2001;90(2):119‐25. [PubMed] [Google Scholar]
References to other published versions of this review
Gray 2007
- Gray DM, Zar HJ, Cotton M. The impact of tuberculosis preventive therapy on tuberculosis and mortality in HIV‐infected children. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD006418] [DOI] [PubMed] [Google Scholar]
Gray 2009b
- Gray DM, Young T, Zar H, Cotton M. Impact of tuberculosis preventive therapy on tuberculosis and mortality in HIV‐infected children. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006418.pub2] [DOI] [PubMed] [Google Scholar]


