Abstract
Background
Strategies to reduce the risk of mother‐to‐child transmission of the human immunodeficiency virus (HIV) include lifelong antiretroviral therapy (ART) for HIV‐positive women, exclusive breastfeeding from birth for six weeks plus nevirapine or replacement feeding plus nevirapine from birth for four to six weeks, elective Caesarean section delivery, and avoiding giving children chewed food. In some settings, these interventions may not be practical, feasible, or affordable. Simple, inexpensive, and effective interventions (that could potentially be implemented even in the absence of prenatal HIV testing programmes) would be valuable. Vitamin A, which plays a role in immune function, is one low‐cost intervention that has been suggested in such settings.
Objectives
To summarize the effects of giving vitamin A supplements to HIV‐positive women during pregnancy and after delivery.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Embase, and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) up to 25 August 2017, and checked the reference lists of relevant articles for eligible studies.
Selection criteria
We included randomized controlled trials conducted in any setting that compared vitamin A supplements to placebo or no intervention among HIV‐positive women during pregnancy or after delivery, or both.
Data collection and analysis
At least two review authors independently assessed study eligibility and extracted data. We expressed study results as risk ratios (RR) or mean differences (MD) as appropriate, with their 95% confidence intervals (CI), and conducted random‐effects meta‐analyses. This is an update of a review last published in 2011.
Main results
Five trials met the inclusion criteria. These were conducted in Malawi, South Africa, Tanzania, and Zimbabwe between 1995 and 2005 and none of the participants received ART. Women allocated to intervention arms received vitamin A supplements at a variety of doses (daily during pregnancy; a single dose immediately after delivery, or daily doses during pregnancy plus a single dose after delivery). Women allocated to comparison arms received identical placebo (6601 women, 4 trials) or no intervention (697 women, 1 trial). Four trials (with 6995 women) had low risk of bias and one trial (with 303 women) had high risk of attrition bias.
The trials show that giving vitamin A supplements to HIV‐positive women during pregnancy, the immediate postpartum period, or both, probably has little or no effect on mother‐to‐child transmission of HIV (RR 1.07, 95% CI 0.91 to 1.26; 4428 women, 5 trials, moderate certainty evidence) and may have little or no effect on child death by two years of age (RR 1.06, 95% CI 0.92 to 1.22; 3883 women, 3 trials, low certainty evidence). However, giving vitamin A supplements during pregnancy may increase the mean birthweight (MD 34.12 g, 95% CI −12.79 to 81.02; 2181 women, 3 trials, low certainty evidence) and probably reduces the incidence of low birthweight (RR 0.78, 95% CI 0.63 to 0.97; 1819 women, 3 trials, moderate certainty evidence); but we do not know whether vitamin A supplements affect the risk of preterm delivery (1577 women, 2 trials), stillbirth (2335 women, 3 trials), or maternal death (1267 women, 2 trials).
Authors' conclusions
Antepartum or postpartum vitamin A supplementation, or both, probably has little or no effect on mother‐to‐child transmission of HIV in women living with HIV infection and not on antiretroviral drugs. The intervention has largely been superseded by ART which is widely available and effective in preventing vertical transmission.
2 April 2019
Up to date
All studies incorporated from most recent search
Updated review: all eligible published studies found in the last search (25 Aug, 2017) were included
Plain language summary
Vitamin A supplements for reducing mother‐to‐child transmission of HIV infection
What is the aim of this review?
The main aim of this Cochrane Review was to assess the effects of giving vitamin A supplements to HIV‐positive women, during pregnancy or after delivery, or both, on the risk of mother‐to‐child transmission of HIV infection. Cochrane researchers collected and examined all relevant studies to answer this question and included five trials. This is an update of a review last published in 2011.
What is the key message of this review?
Giving vitamin A supplements to HIV‐positive women, during pregnancy or after delivery, or both, probably makes little or no difference to the risk of mother‐to‐child transmission of HIV (moderate certainty evidence).
What are the main results of the review?
Five trials met the inclusion criteria of the review. Two trials were from South Africa and one trial each from Malawi, Tanzania, and Zimbabwe. The trials compared women receiving vitamin A supplements to women not receiving such supplements. None of the participants received antiretroviral therapy (ART).
The review shows that in women living with HIV infection and not on ART:
‐ giving vitamin A supplements to HIV‐positive women during pregnancy, immediately after delivery, or both, probably has little or no effect on the risk of mother‐to‐child transmission of HIV (moderate certainty evidence) and may have little or no effect on child death by two years of age (low certainty evidence);
‐ giving vitamin A supplements to HIV‐positive women during pregnancy may increase the mean birthweight (low certainty evidence) and probably reduces the number of low birthweight babies (moderate certainty evidence), but it is uncertain whether the intervention has an effect on the number of preterm births, stillbirths, or deaths among the women (very low certainty evidence).
The intervention has largely been superseded by ART, which is widely available and effective in preventing mother‐to‐child transmission of HIV.
How up‐to‐date is this review?
The review authors searched for studies up to 25 August 2017.
Summary of findings
Summary of findings for the main comparison. Effects of giving vitamin A supplements to HIV‐positive women during pregnancy or after delivery.
|
Population: HIV‐positive women during pregnancy and immediate postpartum period
Settings: any setting
Intervention: vitamin A supplements Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with no vitamin A | Corresponding risk with vitamin A supplements | |||||
| HIV infection status of the child | 27 per 100 | 29 per 100 (24 to 34) | RR 1.07 (0.91 to 1.26) |
4428 (5 trials) | ⊕⊕⊕⊝
moderate1 due to imprecision |
Vitamin A supplements probably have little or no effect on mother‐to‐child transmission of HIV. |
| Mean birthweight | 2964 g | 34 g higher (13 g lower to 81 g higher) | MD 34.12 (−12.79 to 81.02) |
2181 (3 trials) | ⊕⊕⊝⊝
low2 due to imprecision |
Vitamin A supplements may increase the mean birthweight |
| Low birthweight | 17 per 100 | 13 per 100 (11 to 17) | RR 0.78 (0.63 to 0.97) | 1819 (3 trials) | ⊕⊕⊕⊝
moderate3 due to imprecision |
Vitamin A supplements probably reduce the incidence of low birthweight babies. |
| Child death by two years of age | 14 per 100 | 15 per 100 (13 to 18) | RR 1.06 (0.92 to 1.22) | 3883 (3 trials) | ⊕⊕⊝⊝
low2 due to imprecision |
Vitamin A supplements may have little or no effect on child death by two years of age. |
| Preterm delivery | 20 per 100 | 17 per 100 (10 to 28) | RR 0.84 (0.52 to 1.37) | 1577 (2 trials) | ⊕⊝⊝⊝
very low2,4 due to imprecision and selective reporting |
It is uncertain whether or not vitamin A supplements have an effect on preterm deliveries. |
| Stillbirth | 3 per 100 | 3 per 100 (2 to 5) | RR 1.13 (0.72 to 1.77) | 2335 (3 trials) | ⊕⊝⊝⊝
very low2,4 due to imprecision and selective reporting |
It is uncertain whether or not vitamin A supplements have an effect on stillbirths. |
| Maternal death | 3 per 100 | 2 per 100 (1 to 4) | RR 0.71 (0.35 to 1.43) | 1267 (2 trials) | ⊕⊝⊝⊝
very low2,4 due to imprecision and selective reporting |
It is uncertain whether or not vitamin A supplements have an effect on maternal deaths. |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; g: gram; MD: mean difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1We downgraded by 1 for imprecision, as the CI ranges from small benefits to a clinically important increase in harm. 2We downgraded by 2 for imprecision, as the CI ranges from clinically important benefits to a substantial increase in harm. 3We downgraded by 1 for imprecision, as the CI ranges from substantial benefits to no effect. 4We downgraded by 1 for possibility of selective reporting, because 1 or more eligible studies did not report this outcome.
Background
Description of the condition
The Global Burden of Disease Study estimates that there were 38.8 million people living with the human immunodeficiency virus (HIV) worldwide in 2015, half of whom were women of childbearing age (Wang 2016). In addition, there are more than 600 new paediatric infections each day worldwide; most of which occur in sub‐Saharan Africa (UNAIDS 2015). Children mostly acquire these infections from their mothers during pregnancy, delivery, or breastfeeding.
The high number of women who are of childbearing age and who are living with HIV makes the prevention of mother‐to‐child transmission of HIV a global health priority (UNAIDS 2015). Current strategies to reduce the risk of transmission include lifelong antiretroviral therapy (ART) to HIV‐positive women, exclusive breastfeeding from birth for six weeks plus nevirapine, or replacement feeding plus nevirapine from birth for four to six weeks (WHO 2015), elective Caesarean section delivery (Read 2005), and avoiding giving children chewed food. Despite their benefits, these interventions are impractical in many resource‐limited countries because they require the determination of the HIV status of pregnant women and are costly, complex, and require skilled personnel. Simple, inexpensive, and effective interventions that could potentially be implemented in the absence of prenatal HIV testing programmes would be valuable. Vitamin A supplementation during pregnancy is one low‐cost intervention that has been suggested (Newell 2000).
Description of the intervention
Vitamin A refers to a group of unsaturated organic compounds that include preformed vitamin A and provitamin A carotenoids (Damodaran 2017). The vitamin exists as preformed retinol in animal food sources, retinyl esters in fortified foods, and provitamin A carotenoids in plant sources. Both preformed vitamin A and provitamin A are metabolized in cells to retinal and retinoic acid, the active forms of vitamin A, to upkeep the vitamin’s multiple biological functions (Thorne‐Lyman 2012).
The biological functions of vitamin A include the regulation and promotion of growth and differentiation of many cells, and maintenance of the integrity of the epithelial cells of the respiratory and digestive tracts. Vitamin A is necessary for formation of the photosensitive visual pigment in the retina, and reproductive functions (Wolf 2001; Tanumihardjo 2011). In the 1920s the vitamin was considered to be an anti‐infective agent (Green 1928), and there is increasing evidence that it is essential for normal immune function (Ross 1996; Semba 1998).
Vitamin A deficiency is most prevalent in areas where diets lack preformed vitamin A, such as in South and Southeast Asia, and Sahel and sub‐Saharan regions of Africa (West 2001). It has been estimated that about 19 million pregnant women in low‐income countries are affected with vitamin A deficiency each year (West 2002; WHO 2009). Vitamin A deficiency in pregnant women is associated with night blindness, severe anaemia, wasting, malnutrition, reproductive and infectious morbidity (Christian 1998a), and increased risk of mortality one to two years following delivery (Christian 2000). About 10 million women suffer from night blindness during pregnancy as a result of Vitamin A deficiency, and this is associated with a constellation of adverse health and nutritional conditions among mothers and their infants (Christian 1998b; Christian 1998c; Christian 2001; WHO 2009).
How the intervention might work
In areas where poor diet and infection coexist, Vitamin A deficiency can increase the severity of infection, which in turn can reduce intake and accelerate body losses of vitamin A to exacerbate deficiency. Vitamin A deficiency and infection in vulnerable groups (notably young children and pregnant or lactating mothers) represent the most compelling consequences of vitamin A deficiency and underlie its significance as a public health problem around the world (WHO 2009).
Observational studies in sub‐Saharan Africa have shown low serum vitamin A levels in HIV‐positive women to be associated with significantly increased rates of mother‐to‐child transmission of HIV (Semba 1994) and infant mortality (Semba 1995). However, three observational studies in the USA provided conflicting results: low serum vitamin A was associated with a higher risk of mother‐to‐child transmission of HIV in one study (Greenberg 1997), but not the other two (Burger 1997; Burns 1999). These studies used different definitions for vitamin A deficiency: two studies used serum retinol levels of less than 30 mg/dL (Greenberg 1997; Burns 1999), and the other study used less than 20 mg/dL (Burger 1997). The studies also had small sample sizes; for example, in Burger 1997, only 4/95 (4.2%) of women had serum retinol levels of less than 20 mg/dL.
Vitamin A was hypothesized to decrease mother‐to‐child transmission of HIV by acting through several maternal, foetal, child risk factors for transmission, or all three. The proposed risk factors were the clinical, immunological, or viral stage of HIV disease among women; the integrity of the epithelial lining of the placenta, maternal lower genital tract, or breast; the occurrence of prematurity and low birthweight; and the status of the systemic and digestive mucosal immune systems of the foetus and the child (Fawzi 1998; Fawzi 2000).
Why it is important to do this review
Even though there have been dramatic reductions in the number of new HIV infections among children (UNAIDS 2015), the magnitude of the paediatric HIV epidemic in resource‐limited countries is still important. The simplicity and low cost of vitamin A supplementation makes the clarification of its role in mother‐to‐child transmission of HIV of considerable importance. We aimed to combine all high‐quality randomized controlled trials (RCTs) conducted to date to estimate the effect of vitamin A supplementation on mother‐to‐child transmission of HIV. This is an update of a Cochrane Review published in 2011 (Wiysonge 2011).
Objectives
To summarize the effects of giving vitamin A supplements to HIV‐positive women during pregnancy and after delivery.
Methods
Criteria for considering studies for this review
Types of studies
RCTs.
Types of participants
Pregnant or breastfeeding women, confirmed HIV‐positive by a validated laboratory test. The women could be of any age, at any clinical stage of HIV disease, and could be living in any setting.
Types of interventions
Intervention
Vitamin A supplementation, irrespective of formulation (retinol with or without beta‐carotene), timing of supplementation (antepartum, postpartum, or both), dosing, or duration of supplementation. We conducted sensitivity analyses to investigate the robustness of the results to the inclusion of trials that used beta‐carotene.
Control
Eligible comparison interventions included placebo or no intervention.
Types of outcome measures
Primary outcomes
HIV infection status of the child.
Secondary outcomes
Child
Mean birthweight.
Low birthweight, defined as birthweight less than 2500 g.
Child death by two years of age.
Preterm delivery, defined as birth at less than 37 weeks of gestation.
Stillbirth.
Mother
Maternal death.
Postpartum CD4 count.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published or unpublished) up to 25 August 2017 (Table 2).
1. Search strategy used on 25 August 2017.
| Search set | CENTRAL | PubMed | Embase | WHO ICTRP | ClinicalTrials.gov |
| #1 | MeSH descriptor: [HIV Infections] explode all trees | Search ((HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp])) | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/de OR 'human immunodeficiency virus' OR 'human immunodeficiency virus:ab,ti' OR hiv:ab,ti OR 'hiv‐1':ab,ti OR 'hiv‐2':ab,ti OR 'human immunodeficiency virus':ab,ti OR 'human immunedeficiency virus':ab,ti OR 'human immune‐deficiency virus':ab,ti OR 'human immuno‐deficiency virus':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno‐deficiency syndrome':ab,ti OR 'acquired immune‐deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti | hiv AND vitamin A OR hiv AND retinol OR hiv AND retinoic OR hiv AND micronutrients OR hiv AND carotene | HIV and "VITAMIN A" | Interventional Studies | Studies received from 05/20/2016 to 08/25/2017 |
| #2 | MeSH descriptor: [HIV] explode all trees | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR "clinical trials as topic"[mesh: noexp] OR randomly [tiab] OR trial [tiab]) NOT (animals [mh] NOT humans [mh]) | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR ((doubl* NEAR/3 blind*):ab,ti) OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR ((cross NEXT/1 over*):ab,ti) | — | — |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) (Word variations have been searched) | Search (infectious disease transmission, vertical[mh] OR vertical transmission[tiab] OR vertical infect*[tiab] OR infection transmission[tiab] OR mother‐to‐child transmission[tiab] OR MTCT[tiab]) | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de | — | — |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only | Search (vitamin A[mh] OR vitamin*[tiab] OR caroten*[tiab] OR retinol[tiab] OR retinoic[tiab] OR micronutrient*[tiab]) | 'human'/de OR 'normal human'/de OR 'human cell'/de | — | — |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only | Search (#1 AND #2 AND #3 AND #4) | #3 AND #4 | — | — |
| #6 | #1 or #2 or #3 or #4 or #5 | Search (((#1 AND #2 AND #3 AND #4))) AND ("2016/05/20"[Date ‐ Publication] : "2017/08/25"[Date ‐ Publication]) | #3 NOT #5 | — | — |
| #7 | [mh "infectious disease transmission, vertical"] or "vertical transmission":ti,ab,kw or vertical next infect*:ti,ab,kw or "infection transmission":ti,ab,kw or "mother‐to‐child transmission":ti,ab,kw or MTCT:ti,ab,kw (Word variations have been searched) | — | #2 NOT #6 | — | — |
| #8 | [mh "vitamin A"] or vitamin*:ti,ab,kw or caroten*:ti,ab,kw or retinol:ti,ab,kw or retinoic:ti,ab,kw or micronutrient*:ti,ab,kw (Word variations have been searched) | — | 'vertical transmission'/de OR 'vertical transmission':ab,ti OR 'infectious disease transmission':ab,ti OR 'mother+to+child transmission':ab,ti OR mtct:ab,ti | — | — |
| #9 | #6 and #7 and #8 | — | caroten*:ab,ti OR retinoic:ab,ti OR 'retinol'/de OR retinol:ab,ti OR vitamin*:ab,ti OR 'vitamin a'/de OR micronutrient*:ab,ti | — | — |
| #10 | #6 and #7 and #8 Publication Year from 2016 to 2017 | — | #1 AND #7 AND #8 AND #9 | — | — |
| #11 | — | — | #1 AND #7 AND #8 AND #9 AND [20‐5‐2016]/sd NOT [25‐8‐2017]/sd | — | — |
The HIV Information Specialist, Joy Oliver, searched the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Embase, Clinicaltrials.gov, and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) using the search strategies shown in Table 2 and Table 3. We have also provided the search strategy for previous editions of the review in Table 4 and Table 5.
2. Search strategy used on 20 May 2016.
| Search set | CENTRAL | PubMed | Embase |
| #1 | HIV Infections | HIV Infections OR HIV OR hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR hiv infect* OR human immunodeficiency virus OR human immunedeficiency virus OR human immuno‐deficiency virus OR human immune‐deficiency virus OR (human immun* AND deficiency virus) OR acquired immunodeficiency syndrome OR acquired immunedeficiency syndrome OR acquired immuno‐deficiency syndrome OR acquired immune‐deficiency syndrome OR (acquired immun* AND deficiency syndrome) OR "sexually transmitted diseases, Viral" | human immunodeficiency virus infection OR human immunodeficiency virus infection OR human immunodeficiency virus infection OR human immunodeficiency virus OR human immunodeficiency virus OR human immunodeficiency virus OR human immunodeficiency virus OR hiv OR hiv‐1 OR hiv‐2 OR human immunodeficiency virus OR human immunedeficiency virus OR human immune‐deficiency virus OR human immuno‐deficiency virus OR acquired immunodeficiency syndrome OR acquired immuno‐deficiency syndrome OR acquired immune‐deficiency syndrome OR acquired immunedeficiency syndrome |
| #2 | HIV | randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR "clinical trials as topic" OR randomly OR trial) NOT (animals NOT humans) | randomized controlled trial OR randomized controlled trial OR random* OR trial OR allocat* OR factorial* OR placebo* OR assign* OR volunteer* OR crossover procedure OR crossover procedure OR double‐blind procedure OR double‐blind procedure OR single‐blind procedure OR single‐blind procedure OR doubl* NEAR/3 blind* OR singl* AND blind* OR crossover* OR cross+over* OR cross NEXT/1 over* |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or hiv near infect* or human immunodeficiency virus or human immunedeficiency virus or human immune‐deficiency virus or human immuno‐deficiency virus or human immune deficiency virus or human immuno deficiency virus or acquired immunodeficiency syndrome or acquired immunedeficiency syndrome or acquired immuno‐deficiency syndrome or acquired immune‐deficiency syndrome or acquired immun* deficiency syndrome | infectious disease transmission, vertical OR vertical transmission OR vertical infect* OR infection transmission OR mother‐to‐child transmission OR MTCT | animal OR animal experiment OR invertebrate OR animal tissue OR animal cell OR nonhuman |
| #4 | Lymphoma, AIDS‐Related | vitamin A OR vitamin* OR caroten* OR retinol OR retinoic OR micronutrient* | human OR normal human OR human cell |
| #5 | Sexually Transmitted Diseases, Viral | 1‐4/AND | #3 AND #4 |
| #6 | 1‐5/OR | 5 AND (2010/06/01 NOT 2016/05/20) | #3 NOT #5 |
| #7 | infectious disease transmission, vertical or vertical transmission or vertical next infect* or infection transmission or mother‐to‐child transmission or MTCT | — | #2 NOT #6 |
| #8 | vitamin A or vitamin* or caroten* or retinol or retinoic or micronutrient* | — | vertical transmission OR vertical transmission OR infectious disease transmission OR mother+to+child transmission OR mtct |
| #9 | 6‐8/AND | — | caroten* OR retinoic OR retinol OR retinol OR vitamin* OR vitamin a OR micronutrient* |
| #10 | Limit 9 to publication date 2010‐2016 | — | #1 AND #7 AND #8 AND #9 |
| #11 | — | — | #10 AND (2010/06/01 NOT 2016/05/20) |
3. Search strategy used in June 2010.
| Search set | CENTRAL | PubMed | Embase |
| #1 | MeSH descriptor HIV Infections explode all trees | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MESH:NoExp] | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/de OR 'human immunodeficiency virus'OR hiv:ti OR hiv:ab OR 'hiv‐1':ti OR 'hiv‐1':ab OR 'hiv‐2':ti OR 'hiv‐2':ab OR 'human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab OR 'human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab OR 'human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab OR 'human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab OR 'acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab OR 'acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab OR 'acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab OR 'acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab |
| #2 | MeSH descriptor HIV explode all trees | Search (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) | random*:ti OR random*:ab OR factorial*:ti OR factorial*:ab OR cross?over*:ti OR cross?over*:ab OR crossover*:ti OR crossover*:ab OR placebo*:ti OR placebo*:ab OR (doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab) OR (singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab) OR assign*:ti OR assign*:ab OR allocat*:ti OR allocat*:ab OR volunteer*:ti OR volunteer*:ab OR 'crossover procedure'/exp OR 'crossover procedure'/de OR 'crossover procedure'OR 'double‐blind procedure'/exp OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/exp OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR 'randomised controlled trial'/exp OR 'randomised controlled trial'/de OR 'randomised controlled trial' |
| #3 | hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR HIV INFECT* OR HUMAN IMMUNODEFICIENCY VIRUS OR HUMAN IMMUNEDEFICIENCY VIRUS OR HUMAN IMMUNE‐DEFICIENCY VIRUS OR HUMAN IMMUNO‐DEFICIENCY VIRUS OR HUMAN IMMUN* DEFICIENCY VIRUS OR ACQUIRED IMMUNODEFICIENCY SYNDROME OR ACQUIRED IMMUNEDEFICIENCY SYNDROME OR ACQUIRED IMMUNO‐DEFICIENCY SYNDROME OR ACQUIRED IMMUNE‐DEFICIENCY SYNDROME OR ACQUIRED IMMUN* DEFICIENCY SYNDROME | Search mother‐to‐child‐transmission OR MTCT OR infectious disease transmission, vertical | 'mother‐to‐child transmission' OR 'mother to child transmission' OR mtct OR 'vertical transmission'/de OR 'vertical transmission' |
| #4 | MeSH descriptor Lymphoma, AIDS‐Related, this term only | Search caroten* OR retinoic OR retinol OR vitamin* OR vitamin A OR micronutrient* | caroten* OR retinoicOR 'retinol'/de OR retinolOR vitamin*OR 'vitamin a'/de OR 'vitamin a'OR micronutrient* |
| #5 | MeSH descriptor Sexually Transmitted Diseases, Viral, this term only | Search #1 AND #2 AND #3 AND #4 Limits: Publication Date from 2007/01/01 to 2010/06/08 | #1 AND #2AND #3AND #4 |
| #6 | (#1 OR #2 OR #3 OR #4 OR #5) | — | #1 AND #2AND #3AND #4AND [humans]/lim AND [embase]/lim AND [1‐1‐2007]/sd NOT [8‐6‐2010]/sd |
| #7 | mother‐to‐child‐transmission OR MTCT | — | — |
| #8 | MeSH descriptor Infectious Disease Transmission, Vertical, this term only | — | — |
| #9 | (#7 OR #8) | — | — |
| #10 | caroten* OR retinoic OR vitamin* OR vitamin A OR micronutrient* | — | — |
| #11 | (#6 AND #9 AND #10) | — | — |
| #12 | (#6 AND #9 AND #10), from 2007 to 2010 | — | — |
4. Search strategy used in February 2008.
| Search set | PubMed | Embase | AIDSearch | GATEWAY |
| #1 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MH] | (('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection')) OR ((('human immunodeficiency virus'/exp OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'))) OR ((('b cell lymphoma'/exp OR 'b cell lymphoma') OR ('b cell lymphoma'/exp OR 'b cell lymphoma'))) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL) | Search: (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw]) AND (acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MH]) |
| #2 | Search randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR (comparative study) OR (comparative studies) OR (evaluation studies) OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) | (random*:ti OR random*:ab) OR (factorial*:ti OR factorial*:ab) OR (cross?over*:ti OR cross?over:ab OR crossover*:ti OR crossover*:ab) OR (placebo*:ti OR placebo*:ab) OR (((doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab))) OR (((singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab))) OR (assign*:ti OR assign*:ab) OR (volunteer*:ti OR volunteer*:ab) OR (((('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/exp OR 'crossover procedure')) OR (('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/exp OR 'crossover procedure')))) OR (((('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/exp OR 'double‐blind procedure')) OR (('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/exp OR 'double‐blind procedure')))) OR (((('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/exp OR 'single‐blind procedure')) OR (('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/exp OR 'single‐blind procedure')))) OR (((('randomised controlled trial'/exp OR 'randomised controlled trial') OR ('randomised controlled trial'/exp OR 'randomised controlled trial')) OR (('randomised controlled trial'/exp OR 'randomised controlled trial') OR ('randomised controlled trial'/exp OR 'randomised controlled trial')))) OR (allocat*:ti OR allocat*:ab) AND [2003‐2008]/py | ((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR ("CLINICAL TRIAL") OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL* AND (MASK* OR BLIND* )) OR PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*)) NOT (ANIMALS NOT HUMAN ) | Search: (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw]))) OR (( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR (comparative study) OR (comparative studies) OR (evaluation studies) OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh])) |
| #3 | Search (DISEASE TRANSMISSION, VERTICAL) OR MTCT OR (MOTHER‐TO‐CHILD TRANSMISSION) | 'mother‐to‐child transmission' OR mtct OR 'vertical disease transmission' AND [2003‐2008]/py | (MOTHER‐TO‐CHILD TRANSMISSION) OR MTCT OR (VERTICAL DISEASE TRANSMISSION) | Search: (DISEASE TRANSMISSION, VERTICAL) OR MTCT OR (MOTHER‐TO‐CHILD TRANSMISSION) |
| #4 | Search CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* | caroten* OR retinoic OR ('retinol'/exp OR 'retinol') OR vitamin* OR micronutrient* AND [2003‐2008]/py | CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* | Search: CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* |
| #5 | Search PREGNANT OR PREGNANCY OR ANTEPARTUM OR PRENATAL OR ANTE‐PARTUM OR PRE‐NATAL OR PREPART* | pregnant OR ('pregnancy'/exp OR 'pregnancy') OR antepartum OR ('ante partum') OR antenatal OR ('ante natal') OR prenatal OR ('pre natal') AND [2003‐2008]/py | PREGNANT OR PREGNANCY OR ANTEPARTUM OR (ANTE‐PARTUM) OR ANTENATAL OR (ANTE‐NATAL) OR PRENATAL OR (PRE‐NATAL) | Search: PREGNANT OR PREGNANCY OR ANTEPARTUM OR PRENATAL OR ANTE‐PARTUM OR PRE‐NATAL OR PREPART* |
| #6 | Search #1 AND #2 AND #3 AND #4 AND #5 Limits: Publication Date from 2003 to 2008 | #1 AND #2 AND #3 AND #4 AND #5 | #1 AND #2 AND #3 AND #4 AND #5 | #1 and #2 and #3 and #4 and #5 Limit: 2003:2008 |
Data collection and analysis
Two review authors evaluated study eligibility, assessed risk of bias, and extracted data in duplicate. The two authors resolved any disagreements by discussion and consensus. The review authors involved in evaluating study eligibility, assessing risk of bias, and extracting data were not blinded to the names of the trial authors, their institutions, or journals of publication. We reported the data collection and analysis procedures of previous editions of this Cochrane Review in the previous published versions of this review (Wiysonge 2002; Wiysonge 2005; Kongnyuy 2009; Wiysonge 2011).
Selection of studies
Two review authors (either CSW and VNN or CSW and EJK) screened the literature search results by title and abstract for potentially relevant trials and retrieved the full‐text articles as required. The two review authors then independently assessed trial eligibility and resolved differences by discussion and consensus. We considered a trial with multiple publications as one trial. We contacted the corresponding authors of two potentially eligible trials to obtain unpublished data (Chikobvu 2000; Friis 2004). We listed all studies that we excluded after full‐text assessment and the reasons for exclusion in the ʽCharacteristics of excluded studies' table. We constructed a PRISMA flow diagram to illustrate the study selection process.
Data extraction and management
Two review authors (CSW and VNN, CSW and EJK, or CSW and MSS) extracted information on study methods, participants, interventions, and outcomes from each included trial, and reported this information in the ʽCharacteristics of included studies' table.
Assessment of risk of bias in included studies
For each included trial, two review authors (CSW and VNN, CSW and EJK, or CSW and MSS) independently assessed the risk of bias by addressing seven prespecified domains (Higgins 2011): generation of the randomization sequence, concealment of the allocation of interventions, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, completeness of outcome reporting, and any other concerns.
We described what the trial authors reported that they did for each domain and then decided the risk of bias for that domain by assigning a judgement of ʽlow', ʽhigh', or ʽunclear' risk of bias. We summarized the risk of bias for each trial as either low or high. We classified any trial that had a high risk of bias linked to allocation concealment, blinding of outcome assessment, or completeness of outcome data as having a high risk of bias. We considered all other trials to have low risk of bias.
Measures of treatment effect
We expressed each study result as the risk ratio (RR) for dichotomous data or the mean difference (MD) for continuous data, with 95% confidence intervals (CIs).
Unit of analysis issues
There were no unit of analysis issues in this Cochrane Review, as all eligible trials were individually randomized.
Dealing with missing data
We conducted a complete‐case analysis such that we included all participants with a recorded outcome in the analyses. We have reported all missing data and trial dropouts (see the ʽCharacteristics of included studies' table). One trial reported results as means with their standard errors (SE) instead of standard deviations (SD) (Kumwenda 2002). We calculated the SD using the following formula: SD = (square root of N) x (SE).
Assessment of heterogeneity
We assessed the heterogeneity of effects across included trials by visually inspecting the graphical display of data in forest plots and using the Chi² test of homogeneity. In the presence of significant statistical heterogeneity, defined as P < 0.1, we first checked data accuracy to exclude data capturing errors. We quantified observed heterogeneity using the Higgins I² statistic.
Assessment of reporting biases
If we had 10 or more trials included in a meta‐analysis, we would have used funnel plots to assess the risk of publication bias. In such circumstances, we would have assessed the funnel plot visually, followed by formal statistical tests to assess any observed funnel plot asymmetry (Egger 1997; Harbord 2006). Apart from reporting biases, potential causes of funnel plot asymmetry may include high risk of bias, true heterogeneity of effects, and chance (Higgins 2011).
Data synthesis
We used both unpublished (Chikobvu 2000), and published data (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002; Humphrey 2006), to analyse trial participants in groups to which they were randomized, regardless of which or how much treatment they actually received.
Two trials used two‐by‐two factorial designs (Fawzi 2002; Humphrey 2006).
In the first trial, women and their newborns were recruited within four days of delivery and randomly assigned to four treatment groups: Aa, Ap, Pa, and Pp. “A” was maternal vitamin A supplementation, “P” was maternal placebo, “a” was infant vitamin A supplementation, and “p” was infant placebo (Humphrey 2006). In this Cochrane Review, we only used data from Ap (intervention) versus Pp (placebo).
In the second trial with a two‐by‐two factorial design, pregnant women were randomly assigned to receive either "vitamin A alone", "vitamin A plus multivitamins", "multivitamins without vitamin A", or "placebo" (Fawzi 2002). Although the trial authors stated that there was no evidence of clinical interaction between vitamin A and multivitamins, our plan was to consider only the "vitamin A alone" arm as the intervention. However, in the multiple publications from this trial, separate data for "vitamin A alone" were only available for low birthweight and maternal deaths. Thus, for the other outcomes we used data as reported by the trial authors, that is, vitamin A (a combination of "vitamin A alone" and"vitamin A plus multivitamins" arms) versus no vitamin A (consisting of "multivitamins without vitamin A" and "placebo" arms).
We conducted random‐effects meta‐analyses in RevMan 5 because of the diversity of study designs, type of intervention (that is, preformed vitamin A with or without beta‐carotene), timing of intervention (that is, antepartum supplementation, postpartum supplementation, or both), dosing of the supplements, and comparison groups (that is, placebo or no intervention) (RevMan 2014).
We also calculated the optimal information size for the outcomes and presented this information in Table 6. In addition, we used the GRADE approach to assess the certainty of the evidence for the effect of vitamin A supplementation on each key outcome (Guyatt 2008). We constructed a ʽSummary of findings' table using GRADEpro software (GRADEpro GDT 2015).
5. Optimal information size calculation.
| Outcome | Assumed risk | Source | Clinically important relative improvement | Sample size required |
| HIV infection in child | 27/100 | Analysis 1.1 | 25% | 1236 |
| Mean birthweight | 2964 | Analysis 1.2 | 25% | 6178 |
| Low birthweight | 17/100 | Analysis 1.3 | 25% | 2194 |
| Still birth | 3/100 | Analysis 1.4 | 25% | 14,264 |
| Preterm birth | 20/100 | Analysis 1.5 | 25% | 1806 |
| Child death | 14/100 | Analysis 1.6 | 25% | 2748 |
| Maternal death | 3/100 | Analysis 1.7 | 25% | 14,264 |
We based the sample size calculations: 2‐sided tests, with ratio of 1:1, power of 0.8 and confidence level of 0.05. We performed the sample size calculations using http://www.sealedenvelope.com/power/binary‐superiority/
Subgroup analysis and investigation of heterogeneity
For the primary outcome, we conducted a subgroup analysis based on the timing of vitamin A supplementation, that is antepartum supplementation (Chikobvu 2000; Kumwenda 2002), postpartum supplementation (Humphrey 2006), or both (Coutsoudis 1999; Fawzi 2002).
Sensitivity analysis
We included trials that provided supplements containing only preformed vitamin A (Chikobvu 2000; Kumwenda 2002; Humphrey 2006), and trials that used both preformed vitamin A (retinol) and a provitamin A carotenoid (beta‐carotene) (Coutsoudis 1999; Fawzi 2002).
Beta‐carotene is easily converted to retinol, but also has antioxidative properties (Thorne‐Lyman 2012). We therefore conducted sensitivity analyses to investigate the robustness of the results on the primary outcome to the type of intervention (that is, we omitted trials that used beta‐carotene).
Results
Description of studies
Results of the search
Figure 1 shows the search and study selection process for this Cochrane Review, in line with the PRISMA statement (Moher 2009).
1.
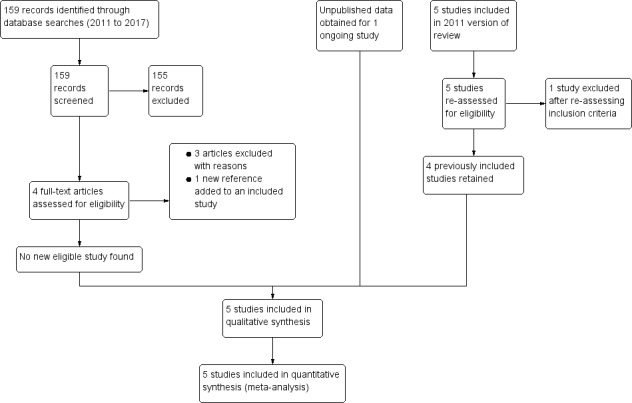
PRISMA flow diagram
Of the five trials included in the previous version of this review, Wiysonge 2011, we retained four of the previously included trials (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002; Humphrey 2006), and excluded one trial because further assessment revealed that it did not meet our inclusion criteria (Friis 2004). We obtained unpublished data for one ongoing trial (Chikobvu 2000). Of the 159 records identified from the updated literature search, we excluded 158 and identified one new article to add to an already included study.
Included studies
Five trials met the inclusion criteria (see Figure 1 and the ʽCharacteristics of included studies' table). These include a trial that we classified as ongoing in previous published versions of this review (Chikobvu 2000). The trial was conducted from September 1997 to December 2000 in South Africa, and the principal investigator of the trial has provided us with outcome data on mother‐to‐child transmission of HIV (Chikobvu 2000). We briefly describe below the designs, participants, interventions, comparisons, and outcome measures of the five included trials.
Period of study
These five included trials were conducted between 1995 and 2005, at the height of the HIV epidemic in sub‐Saharan Africa.
Design
Three of the included trials were randomized trials (Fawzi 2002; Kumwenda 2002; Humphrey 2006). Two trials were described as randomized trials, but the trial authors did not describe the methods used to generate the randomization sequence (Coutsoudis 1999; Chikobvu 2000). Two trials had two‐by‐two factorial designs (Fawzi 2002; Humphrey 2006). All trials used participants as units of randomization. The proportion of participants lost to follow‐up ranged from 3.2% (Humphrey 2006), to more than 48% (Chikobvu 2000). All trial authors excluded mother‐infant pairs lost to follow‐up from their analyses.
Location
The five trials were conducted in four countries in sub‐Saharan Africa: Malawi (one trial; Kumwenda 2002), South Africa (two trials; Coutsoudis 1999; Chikobvu 2000), Tanzania (one trial; Fawzi 2002), and Zimbabwe (one trial; Humphrey 2006).
Population
In trials with antepartum vitamin A supplementation, participants consisted of HIV‐positive pregnant women recruited during their first antenatal visit (Chikobvu 2000), 12 to 27 weeks' gestation (Fawzi 2002), 18 to 28 weeks' gestation (Kumwenda 2002), and 17 to 39 weeks' gestation (Coutsoudis 1999). For the single trial of postpartum vitamin A supplementation, HIV‐positive women were recruited within 96 hours of delivery (Humphrey 2006).The prevalence of vitamin A deficiency among the women at baseline was 30.6% in Coutsoudis 1999, 34% in Fawzi 2002, and 51% in Kumwenda 2002; but was not reported in two trials (Chikobvu 2000; Humphrey 2006).
Interventions
Vitamin A supplements
Three trials used supplements that contained retinol alone (Chikobvu 2000; Kumwenda 2002; Humphrey 2006), and two trials used both retinol and beta‐carotene (Coutsoudis 1999; Fawzi 2002).
Two trials provided vitamin A supplements to women only during pregnancy (Chikobvu 2000; Kumwenda 2002). One trial provided vitamin A during pregnancy, but did not report information on the type or dose of vitamin A used (Chikobvu 2000). The second trial provided pregnant women in the intervention arm with 10,000 IU daily oral doses of vitamin A (Kumwenda 2002).
One trial provided vitamin A supplements only after delivery (Humphrey 2006). This trial had a factorial design and the interventions consisted of a single oral dose of 400,000 IU vitamin A to the mother only; 50,000 IU single oral dose to the newborn only; or 400,000 IU to the mother and 50,000 IU to the newborn (Humphrey 2006). In our analyses, we considered only the group in which the mother alone received vitamin A supplements as the intervention arm.
In two trials, women received vitamin A supplements both during pregnancy and immediately after delivery (Coutsoudis 1999; Fawzi 2002). In the first trial, women in the intervention arm received 5000 IU retinyl palmitate and 30 mg beta‐carotene daily during pregnancy. In addition, at delivery, women in the intervention arm received 200,000 IU of retinyl palmitate (Coutsoudis 1999). The second trial employed a two‐by‐two factorial design (Fawzi 2002). Women in the intervention arms received daily doses of vitamin A (30 mg beta carotene plus 5000 IU retinyl palmitate) alone; vitamin A plus multivitamins (20 mg vitamin B1, 20 mg vitamin B2, 25 mg vitamin B6, 100 mg niacin, 50 µg vitamin B12, 500 mg vitamin C, 30 mg vitamin E, and 0.8 mg folic); or multivitamins alone. At delivery, women receiving any vitamin A were given an additional 200,000 IU oral dose of vitamin A (Fawzi 2002).
Concomitant interventions
Two trials did not provide information on any concomitant interventions (Coutsoudis 1999; Chikobvu 2000). In the remaining three trials, the pregnant women received daily oral doses of iron and folic acid (Fawzi 2002; Kumwenda 2002; Humphrey 2006). One trial also reported providing weekly doses of chloroquine (Fawzi 2002), and another trial provided all women with oral vitamin A (100,000 IU) at six weeks postpartum, according to national policy (Kumwenda 2002). In one trial all children, regardless of whether they were in the intervention or comparison arm, received 100,000 IU vitamin A at six months of age and 200,000 IU of vitamin A every six months afterwards (Fawzi 2002).
Antiretroviral therapy (ART)
None of the five included trials reported giving ART to participants.
Comparison interventions
In four trials, the comparison intervention was a placebo (Coutsoudis 1999; Chikobvu 2000; Fawzi 2002; Humphrey 2006). The fifth study used a "no intervention" comparison group (Kumwenda 2002). All women in this trial received iron and folic acid, and half of them were randomly assigned to receive vitamin A. Supplements that contained vitamin A, iron, and folic acid were indistinguishable from the ones that contained only iron and folic acid (Kumwenda 2002).
Outcome measures
Primary outcomes
HIV infection status of the child
We obtained data on children's HIV infection status at three months (Coutsoudis 1999; Chikobvu 2000), and at 24 months (Fawzi 2002; Kumwenda 2002; Humphrey 2006). A child was determined to be HIV‐positive when a blood specimen tested positive using polymerase chain reaction (PCR) at any point or a plasma specimen obtained at 18 months of age or older tested positive using enzyme‐linked immunosorbent assay (ELISA).
Secondary outcomes
Birthweight
Three trials reported data on mean birthweight (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002).
Child death by two years of age
Three trials reported data on child death by 24 months of age (Fawzi 2002; Kumwenda 2002; Humphrey 2006).
Low birthweight
Three trials reported data on the occurrence of low birthweight, that is, children born with birthweight less than 2500g (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002).
Preterm delivery
Two trials reported data on preterm deliveries, that is, births at less than 37 weeks of gestation (Coutsoudis 1999; Fawzi 2002).
Stillbirth
Three trials reported data on stillbirths (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002).
Maternal death
One trial reported data on maternal deaths from any cause by three months after delivery (Coutsoudis 1999). The second trial reported data on maternal deaths from AIDS‐related causes at two and four years (Fawzi 2002). We have used the two‐year data in this review.
Postpartum CD4 count
One trial reported postpartum CD4 cell count at two and four years (Fawzi 2002). We have used the two‐year data in this review.
Excluded studies
We included Friis 2004 in the previous version of this review (Wiysonge 2011), but excluded the study from this review update because the intervention consisted of multivitamins (including vitamin A) rather than vitamin A alone. We identified 159 records from literature searches. We excluded 155 records after screening by title/abstract. Of the four remaining studies, we excluded three studies after full‐text assessment (Duggan 2012; Olofin 2016; Locks 2017), and identified one new reference to an already included study. We excluded the remaining three studies because they assessed the effects of multivitamins (and not vitamin A) and had as participants, children born to HIV‐positive women (rather than the women themselves) (Duggan 2012; Olofin 2016; Locks 2017).
Risk of bias in included studies
We have provided detailed ʽRisk of bias' assessments of the included trials in the ʽCharacteristics of included studies' table, and provided a summary in Figure 2 and Figure 3.
2.
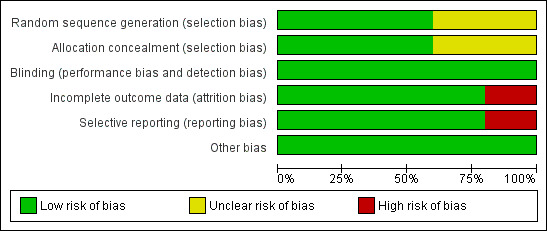
ʽRisk of bias' graph: review authors' judgements about each ʽRisk of bias' item presented as percentages across all included trials
3.
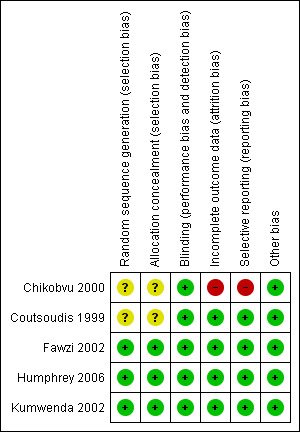
ʽRisk of bias' summary: review authors' judgements about each ʽRisk of bias' item for each included trial
Allocation
Sequence generation
Regarding sequence generation, three trials were at low risk of bias (Fawzi 2002; Kumwenda 2002; Humphrey 2006), and the risk of bias in two trials was unclear (Coutsoudis 1999; Chikobvu 2000). The authors of the latter trials did not clearly describe the methods used to generate the randomization sequence (Coutsoudis 1999; Chikobvu 2000).
Allocation concealment
Three trials were at low risk of bias as per allocation concealment (Fawzi 2002; Humphrey 2006; Kumwenda 2002). We judged two trials to have an unclear risk of bias (Coutsoudis 1999; Chikobvu 2000), as the trial authors did not describe the methods used to conceal allocation to intervention and comparison groups.
Blinding
All five trials were at low risk of performance bias because participants and care providers were blinded to treatment allocation.
The five included trials performed blinding of outcome assessors, thus we judged them to be at low risk of detection bias.
Incomplete outcome data
Four trials had low risk of bias in relation to completeness of outcome data (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002; Humphrey 2006), but we judged one trial to be at high risk of bias (Chikobvu 2000). The proportion of participants lost to follow‐up was 3.2% in Humphrey 2006, 5.0% in Fawzi 2002, 7.8% in Coutsoudis 1999, 9.0% in Kumwenda 2002, and more than 48% in Chikobvu 2000. Therefore, the proportion of randomized participants with evaluable data ranged from less than 52% (Chikobvu 2000), to 96.8% (Humphrey 2006).
Selective reporting
We judged the risk of reporting bias to be low in four trials (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002; Humphrey 2006). However, we observed evidence of selective reporting in one trial, which we judged to be at high risk of reporting bias (Chikobvu 2000). The trial did not report data on mother‐to‐child transmission of HIV because of high loss to follow‐up (Chikobvu 2000).
Other potential sources of bias
We observed no other potential sources of bias in included studies.
Summary of 'Risk of bias' assessment
Overall, four trials (with 6995 women) were at low risk of bias (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002; Humphrey 2006), and one trial (with 303 women) was at high risk of bias (Chikobvu 2000).
Effects of interventions
See: Table 1
We have summarized the effects of vitamin A supplementation of HIV‐positive women during pregnancy, immediately after delivery, or both, on key outcomes in the ʽSummary of findings' table (Table 1).
Primary outcome
HIV infection status of the child
The risk ratio for the effect of vitamin A supplementation during pregnancy on mother‐to‐child transmission of HIV was 0.85 (95% CI 0.67 to 1.09; 650 women, 2 trials) and for vitamin A supplementation immediately after delivery was 1.11 (95% CI 0.98 to 1.09; 2248 women, 1 trial). In the two trials that provided vitamin A supplementation to women during pregnancy and immediately after delivery, the RR was 1.17 (95% CI 0.86 to 1.59; 1530 women, 2 trials).
Overall, the trials show that vitamin A supplementation of HIV‐positive women during pregnancy or the immediate postpartum period, or both, probably has little or no effect on the risk of mother‐to‐child transmission of HIV (RR 1.07, 95% CI 0.91 to 1.26; 4428 women, 5 trials, moderate certainty evidence). There were no substantial differences in effects across the three subgroups (heterogeneity P = 0.15, I² statistic = 48.1%; Analysis 1.1).
1.1. Analysis.
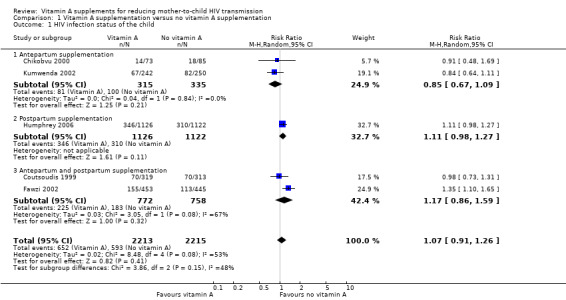
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 1 HIV infection status of the child.
The effects were similar between studies that provided supplements containing only retinol (RR 1.00, 95% CI 0.81 to 1.22; 2898 women, 3 trials) and those that provided both retinol and beta‐carotene (RR 1.17, 95% CI 0.86 to 1.59; 1530 women, 2 trials).
Secondary outcomes
Child
Birthweight
Vitamin A supplementation of HIV‐positive women during pregnancy may increase the mean birth weight of babies (MD 34.12 g, 95% CI −12.79g to 81.02; 2181 women, 3 trials, low certainty evidence). The effect was fairly consistent across the three trials (heterogeneity P = 0.34, I² statistic 8%; Analysis 1.2).
1.2. Analysis.
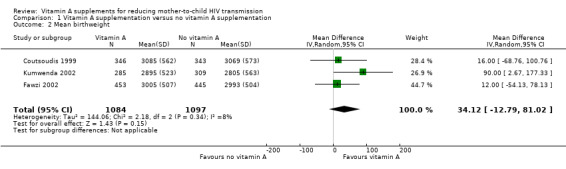
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 2 Mean birthweight.
Low birthweight
Vitamin A supplementation of HIV‐positive pregnant women probably reduces the incidence of low birthweight (RR 0.78, 95% CI 0.63 to 0.97; 1819 women, 3 trials, moderate certainty evidence). The effect was homogenous across the three included trials (heterogeneity P = 0.56; I² statistic = 0%; Analysis 1.3).
1.3. Analysis.
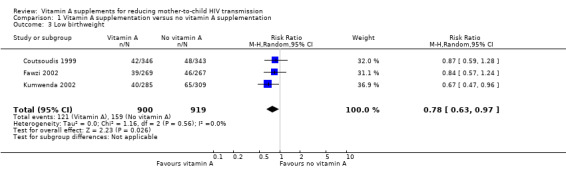
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 3 Low birthweight.
Child death by two years of age
Vitamin A supplementation of HIV‐positive women during pregnancy or the immediate postpartum period, or both, may have little or no effect on child death by two years of age (RR 1.06, 95% CI 0.92 to 1.22; 3883 women, 3 trials, low certainty evidence). This finding was consistent across the three studies (heterogeneity P = 0.49, I² statistic = 0%; Analysis 1.4).
1.4. Analysis.
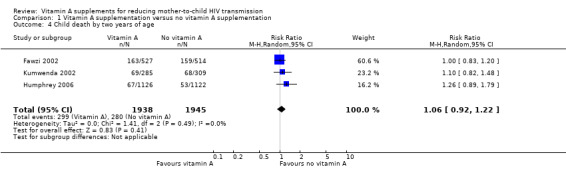
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 4 Child death by two years of age.
Preterm delivery
It is uncertain whether vitamin A supplementation of HIV‐positive pregnant women has an effect on the risk of preterm deliveries (RR 0.84, 95% CI 0.52 to 1.37; 1577 women, 2 trials, very low certainty evidence). There was unexplained heterogeneity of effects between the two studies (heterogeneity P = 0.03, I² statistic = 79%; Analysis 1.5).
1.5. Analysis.
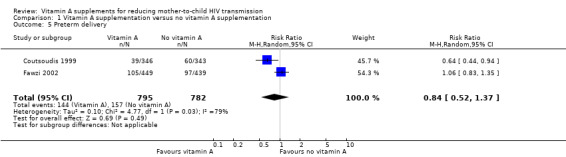
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 5 Preterm delivery.
Stillbirth
It is uncertain whether vitamin A supplementation of HIV‐positive pregnant women has an effect on the incidence of stillbirths (RR 1.13, 95% CI 0.72 to 1.77; 2335 women, 3 trials, very low certainty evidence). This uncertainty was consistent across the three trials (heterogeneity P = 0.80, I² statistic = 0%; Analysis 1.6).
1.6. Analysis.
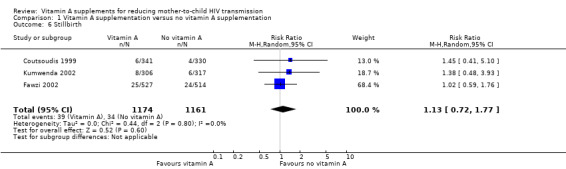
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 6 Stillbirth.
Mother
Maternal death
It is uncertain whether vitamin A supplementation of HIV‐positive women during pregnancy and immediate postpartum period has an effect on maternal deaths, as the certainty of the evidence was very low (RR 0.71, 95% CI 0.35 to 1.43; 1267 women, 2 trials). This finding was consistent between the two trials (heterogeneity P = 0.75, I² statistic = 0%; Analysis 1.7).
1.7. Analysis.
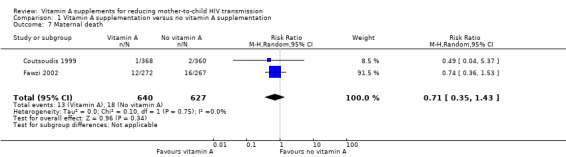
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 7 Maternal death.
Postpartum CD4 count
It is uncertain whether vitamin A supplementation of HIV‐positive women during pregnancy and immediate postpartum period has an effect on postpartum maternal CD4 levels, as the certainty of the evidence was very low (mean difference −13.00, 95% CI −60.46 to 34.46; 511 women, 1 trial; Analysis 1.8).
1.8. Analysis.
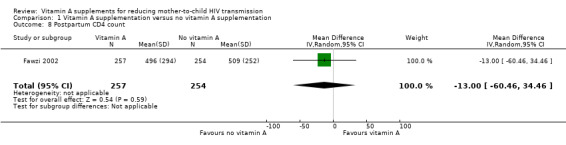
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 8 Postpartum CD4 count.
Discussion
Summary of main results
Five randomized trials, which were conducted between 1995 and 2005 and included 7298 HIV‐positive women in sub‐Saharan Africa, met the inclusion criteria of this Cochrane Review. The included trials assessed the effects of vitamin A supplementation during pregnancy, immediately after delivery, or both. A synthesis of the results of the trials shows that vitamin A supplementation probably has little or no effect on mother‐to‐child transmission of HIV (moderate certainty evidence) and may have little or no effect on child death by two years of age (low certainty evidence). However, vitamin A supplements may increase the mean birthweight (low certainty evidence) and probably reduce the incidence of low birthweight (moderate certainty evidence). Due to very low certainty evidence it is uncertain whether vitamin A supplements have an effect on preterm delivery, stillbirth, or maternal death.
Overall completeness and applicability of evidence
By the end of the 20th century, HIV had produced a global epidemic that was far more extensive than was predicted when the infection emerged less than two decades earlier. Antenatal seroprevalence of HIV was more than 10% in many countries in sub‐Saharan Africa, the risk of mother‐to‐child transmission of HIV was about 30% to 45% in the region, and the region was contributing more than 90% of the nearly 2000 new HIV infections in children each day worldwide (De Cock 2000; UNAIDS 2001).
In this context, it was estimated that even with a 3% reduction in transmission, vitamin A supplementation of HIV‐positive women would be a cost‐effective method of preventing mother‐to‐child transmission of HIV. Vitamin A supplements are easily provided through existing health services (Wiysonge 2006). It was thus necessary to clarify the effect of the vitamin on mother‐to‐child transmission of HIV. Such clarification was judged to be important to decision‐makers considering affordable options for reducing mother‐to‐child transmission of HIV in resource‐limited settings (Wiysonge 2002).
Despite our comprehensive search, we found only six potentially eligible trials on the topic, of which five trials met our inclusion criteria. Our review shows that vitamin A supplementation of HIV‐positive women during pregnancy, after delivery, or both, probably has little or no effect on mother‐to‐child transmission of HIV.
Our data suggest that the association between low serum vitamin A levels and increased risk of mother‐to‐child transmission of HIV seen in observational studies (Semba 1994; Greenberg 1997), could have other explanations. Given the observational design of such studies, residual confounding by advanced HIV infection or other factors may explain the seemingly protective association (Fawzi 1998).
In high‐income countries, the combination of (1) antiretroviral prophylaxis, (2) elective Caesarean section delivery, and (3) formula feeding in clinical practice, coupled with increased prenatal HIV counselling and testing, has essentially eliminated mother‐to‐child transmission of HIV in those settings (Mofenson 2000; Navér 2006; UNAIDS 2015). Due to cost and other constraints, many countries in sub‐Saharan Africa had difficulties implementing these interventions (McIntyre 2002). However, in the last decade, there have been dramatic improvements. Antiretroviral drugs are now widely available in sub‐Saharan Africa and other resource‐constrained settings (UNAIDS 2015; WHO 2015; Wang 2016).
Vitamin A is teratogenic when consumed at high doses during early pregnancy, but none of the trials included in this review reported information on adverse events. However, the doses of vitamin A used in the trials were within the recommended safe low doses (WHO 1998).
Quality of the evidence
Due to the inconsistency of the effect of vitamin A supplementation on mother‐to‐child of HIV across included trials, we downgraded the certainty of this evidence to moderate. The GRADE Working Group classifies research evidence as being of moderate certainty if the true effect of the intervention is likely to be close to what was found in the research but there is a possibility that it is substantially different (Guyatt 2008). Therefore, this review does not completely exclude the possibility of a small beneficial or harmful effect of vitamin A supplementation on the risk of mother‐to‐child of HIV.
For most of the other outcomes assessed, we judged the certainty of the evidence to be very low (Table 1), implying that we are uncertain about the true effect of vitamin A supplementation on these outcomes. Our main concerns with the evidence were imprecision (as the CIs ranged from large benefits to clinically important increases in harm) and the possibility of publication bias (because two or more eligible trials did not report the outcomes).
Potential biases in the review process
We minimized bias in the process of conducting and reporting the review by adhering to the Methodological Expectations of Cochrane Intervention Reviews (MECIR) (Higgins 2016).
The ultimate goal of this review was to determine whether vitamin A supplementation of HIV‐positive women could be recommended as a public health policy to reduce mother‐to‐child of HIV. We therefore considered overall mother‐to‐child of HIV, whether occurring during pregnancy, during delivery, or after birth. As such we pooled data from all studies, subgrouped by the timing of supplementation. We do not think that pooling data from these trials has introduced bias to the review.
Agreements and disagreements with other studies or reviews
We found that vitamin A supplements probably make little or no difference to the risk of mother‐to‐child transmission of HIV. This finding is consistent with the findings of previous reviews of maternal vitamin A supplementation by our team (Wiysonge 2002; Wiysonge 2005; Kongnyuy 2009; Wiysonge 2011), and others (Thorne‐Lyman 2012).
Our review also shows that giving vitamin A supplements during pregnancy or the immediate postpartum period, or both, may have little or no effect on child death by two years of age. This finding is consistent with that of other authors (Gorgia 2010; Thorne‐Lyman 2012). In a previous systematic review, Gorgia 2010 pooled data from six randomized trials and found little or no effect on infant mortality of giving synthetic vitamin A supplements to seemingly healthy mothers after delivery.
In another review, Thorne‐Lyman 2012 pooled data from 17 trials among women of any HIV status and found little or no effect of antepartum retinol and beta‐carotene supplements on infant mortality. As in this Cochrane Review, Thorne‐Lyman 2012 found that antepartum supplementation was protective against low birthweight among HIV‐positive women.
Vitamin A is important for embryogenesis; playing a vital role in the development of the foetal heart, embryonal circulatory system, and the regulation of heart asymmetry (Zile 1998). This role could explain the substantial reduction in low birthweight in the vitamin A group compared to placebo or no intervention.
Authors' conclusions
Implications for practice.
Antepartum or postpartum vitamin A supplementation, or both, probably makes little or no difference to the risk of mother‐to‐child transmission of HIV. The evidence from this Cochrane Review suggests that giving vitamin A supplements to pregnant HIV‐positive women may have beneficial effects on birthweight.
Implications for research.
Given that the currently available randomized trial data do not exclude the possibility that vitamin A supplementation could have small beneficial or harmful effects on mother‐to‐child transmission of HIV, there may be need for an appropriately powered randomized trial to assess the additive effect of the intervention in antiretroviral‐treated women. However, with the current widespread use and success of antiretroviral therapy (ART) in reducing mother‐to‐child transmission of HIV, further research on the use of vitamin A supplements for this indication may not be warranted.
What's new
| Date | Event | Description |
|---|---|---|
| 7 September 2017 | New search has been performed | One new trial met the inclusion criteria of this review update. We excluded one trial that was included in the previous edition of the review, Wiysonge 2011, from this review update because it did not meet our inclusion criteria, and we re‐extracted birthweight data. We amended the number of child‐related and maternal secondary outcomes. In addition, there were changes to the review author team. |
| 7 September 2017 | New citation required but conclusions have not changed | This is an update to a review published in 2011. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 2, 1995
| Date | Event | Description |
|---|---|---|
| 18 January 2011 | Amended | External source of support added. |
| 7 September 2010 | New citation required but conclusions have not changed | Review expanded to include postpartum supplementation; SOF table added. |
| 7 September 2010 | New search has been performed | Updated, with GRADE Summary of Findings table. |
| 14 May 2008 | Amended | Converted to new review format. |
| 14 May 2008 | New search has been performed | Update of review. |
| 11 January 2008 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank Paul Garner, George Rutherford, and Harshi Sachdev for comments on earlier versions of this edition of the Cochrane Review, which helped to substantially improve the quality of the conduct and reporting of the review. We acknowledge the substantial contributions of Jonathan Sterne and Peter Brocklehurst to previous editions of this Cochrane Review. We are grateful to Henrik Friis and Perpetual Chikobvu for sharing unpublished trial data with us.
Charles S Wiysonge's work is supported by the South African Medical Research Council, the National Research Foundation of South Africa (Grant: 108571), and the Effective Health Care Research Consortium. The Effective Health Care Research Consortium and the CIDG editorial base are funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
Data and analyses
Comparison 1. Vitamin A supplementation versus no vitamin A supplementation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HIV infection status of the child | 5 | 4428 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.91, 1.26] |
| 1.1 Antepartum supplementation | 2 | 650 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.67, 1.09] |
| 1.2 Postpartum supplementation | 1 | 2248 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.98, 1.27] |
| 1.3 Antepartum and postpartum supplementation | 2 | 1530 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.86, 1.59] |
| 2 Mean birthweight | 3 | 2181 | Mean Difference (IV, Random, 95% CI) | 34.12 [‐12.79, 81.02] |
| 3 Low birthweight | 3 | 1819 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.63, 0.97] |
| 4 Child death by two years of age | 3 | 3883 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.92, 1.22] |
| 5 Preterm delivery | 2 | 1577 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.52, 1.37] |
| 6 Stillbirth | 3 | 2335 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.72, 1.77] |
| 7 Maternal death | 2 | 1267 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.35, 1.43] |
| 8 Postpartum CD4 count | 1 | 511 | Mean Difference (IV, Random, 95% CI) | ‐13.0 [‐60.46, 34.46] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chikobvu 2000.
| Methods | Randomized controlled trial (RCT) | |
| Participants | 303 HIV‐positive pregnant women from metropolitan Bloemfontein, South Africa. Most participants (56%) lived in informal settlements and all attended public health facilities. For the trial, women were asked to volunteer for HIV testing during their first antenatal visit. Pretest counselling was done in groups, and post‐test counselling was done individually. Women who were seropositive for HIV were asked to participate in the trial. | |
| Interventions | Vitamin A supplementation versus placebo | |
| Outcomes | Mother‐to‐child transmission (MTCT) of HIV | |
| Notes | All trial participants gave separate informed consent for the trial. All patients were recruited by one study physician and received verbal or written information (Sesotho, English, or Afrikaans information sheets). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not mention the method used to generate the randomization sequence. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method used to conceal the treatment allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial used an identical placebo; HIV diagnosis was done in the laboratory. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 48% of women were lost to follow‐up and we do not know whether this was related to outcomes. |
| Selective reporting (reporting bias) | High risk | Not all prespecified outcomes were reported in various publications from this trial. |
| Other bias | Low risk | We do not believe that other biases were introduced, over and above the high loss to follow‐up and the selective reporting. |
Coutsoudis 1999.
| Methods | Described as randomized double‐blind. The trial authors lost eight per cent of mother‐infant pairs during follow‐up and excluded them from the analysis. | |
| Participants | 728 HIV‐positive women enrolled at 17 to 39 weeks' gestation in KwaZulu‐Natal Province of South Africa; 30.6% of whom had serum retinol levels < 20 µg/dL. | |
| Interventions | Daily oral vitamin A (5000 IU retinyl palmitate and 30 mg beta‐carotene) or placebo. At delivery, women in the vitamin A group received a dose of 200,000 IU of retinyl palmitate while the placebo arm received an identical placebo. | |
| Outcomes | Stillbirths, HIV infection in the child, neonatal deaths, preterm birth, birthweight, low birthweight. | |
| Notes | No woman received any antiretroviral therapy (ART). It is not stated in the trial reports whether the women also received iron or folic acid, or both. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method used to generate the randomization sequence. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method used to conceal the treatment allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Use of identical placebo; diagnosis of HIV was done in the laboratory. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% of women were lost to follow‐up and we do not believe that this was related to the outcome. We do not believe this introduced bias. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in various publications from this trial. |
| Other bias | Low risk | We do not believe that other biases were introduced that could have affected study findings. |
Fawzi 2002.
| Methods | Randomized, placebo‐controlled, double‐blind. The trial authors lost five per cent of mother‐infant pairs during follow‐up and excluded them from the analysis. | |
| Participants | 1075 pregnant HIV‐positive women enrolled at 12 to 27 weeks' gestation in Dar es Salam, Tanzania. Of 1085 women initially randomized, 10 were eventually excluded for either being HIV‐negative (n = 7) or not pregnant (n = 3). The prevalence of vitamin A deficiency (< 0.70 µmol/L) was about 34% during the second trimester. | |
| Interventions | Daily oral dose of one of: vitamin A (30 mg beta carotene + 5000 IU retinyl palmitate) alone, multivitamins (20 mg vitamin B1, 20 mg vitamin B2, 25 mg vitamin B6, 100 mg niacin, 50 µg vitamin B12, 500 mg vitamin C, 30 mg vitamin E, and 0.8 mg folic) plus vitamin A, multivitamins without vitamin A, or placebo. At delivery, women receiving any vitamin A were given an additional 200,000 IU oral dose of vitamin A while the others received an extra dose of placebo. In this review, we used only data from the vitamin A only (intervention) and the placebo arms. |
|
| Outcomes | Stillbirths, HIV infection in child, preterm delivery, low birthweight, postpartum CD4 levels. | |
| Notes | It is not mentioned in this trial whether any woman received ART. All women were given daily oral doses of iron and folic acid, and weekly doses of chloroquine. All children, regardless of which intervention group they were in, received 100,000 IU vitamin A at six months of age, and 200,000 IU of vitamin A every six months afterwards. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used computer‐generated randomization sequence. |
| Allocation concealment (selection bias) | Low risk | Block randomization; blocks of 20. At enrolment, the investigators assigned each eligible woman the next numbered bottle of study drug. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | At enrolment, each eligible woman was assigned the next numbered bottle of study drug. Active tablets and placebo were indistinguishable, so that neither the participants nor the investigators could identify which participants were randomized to the which regimen. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fifty‐four women (5.0%) were lost to follow‐up, and we do not believe that this was related to the outcome. We do not believe this introduced bias. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported on all outcomes specified in the goals of the study articles. |
| Other bias | Low risk | We do not believe that other biases were introduced that could have affected the study findings. |
Humphrey 2006.
| Methods | Randomized, placebo‐controlled trial. | |
| Participants | 4495 mother‐infants pairs who were part of the Zimbabwe Vitamin A for Mothers and Babies (ZVITAMBO) trial in Harare Zimbabwe. Mother‐infant pairs were enrolled at 96 hours (or less) after delivery. | |
| Interventions | A 2‐by‐2 factorial design with 4 treatment groups Aa, Ap, Pa, and Pp; where “A” was maternal vitamin A supplementation (400,000 IU), “P” was maternal placebo, “a” was infant vitamin A supplementation (50,000 IU), and “p” was infant placebo. In this review, we used only data from Ap (intervention) versus Pp (placebo). |
|
| Outcomes | Primary outcome: breastfeeding–associated MTCT and HIV‐free survival. Secondary outcome: adverse effects in HIV‐positive women or their infants. |
|
| Notes | All but 4 mothers initiated breastfeeding, no information on ART or cotrimoxazole prophylaxis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors performed randomization using computer‐generated blocks of 12. |
| Allocation concealment (selection bias) | Low risk | Treatment assignment was concealed by pre‐packing study supplements in sequentially numbered series assigned to study identification numbers. Concealed envelopes with study number; link between number and treatment assignment kept at central location. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, care providers, and outcome assessors were blinded to the treatment allocation. Mothers were assigned an original study identification number at enrolment and were given the next sequentially numbered opaque bottle with supplements. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One hundred and forty‐three (3.2%) mother‐infant pairs were lost to follow‐up. We do not believe that the loss to follow‐up was related to the outcome. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported outcomes that were prespecified in the protocol (NCT00198718). |
| Other bias | Low risk | We do not believe that other biases were introduced that could have affected the study findings. |
Kumwenda 2002.
| Methods | RCT. Participants were assigned to treatment using computer‐generated random numbers, and treatment was concealed by pre‐packing study supplements in sequentially numbered series assigned to study identification numbers. Sixty‐three women (9%) were lost to follow‐up before delivery and excluded from the analyses. The 14 pairs of twins in the study were excluded from the birth weight and mortality analyses because twins are known to have lower birth weights and higher mortality rates. | |
| Participants | 697 pregnant HIV‐positive women enrolled at 18 to 28 week's gestation in Blantyre, Malawi. The prevalence of vitamin A deficiency (< 0.70 µmol/L) was 51% during the second trimester. | |
| Interventions | All women received orally administered daily doses of iron (30 mg of elemental iron) and folate (400 µg) from enrolment until delivery. One‐half of the women were randomized to receive daily doses of orally administered vitamin A (10,000 IU). | |
| Outcomes | Stillbirths, HIV infection in child, preterm delivery, low birthweight, postpartum CD4 levels. | |
| Notes | All women received oral vitamin A (100,000 IU) at 6 weeks postpartum, as per policy of the Malawi Ministry of Health. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors determined treatment assignment by use of a computer random‐number generator. |
| Allocation concealment (selection bias) | Low risk | Treatment assignment was concealed by pre‐packing study supplements in sequentially numbered series assigned to study identification numbers. Mothers were assigned an original study identification number at enrolment and were given the next sequentially numbered opaque bottle with supplements. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Supplements containing vitamin A, iron, and folate were identical in appearance to the supplements containing iron and folate. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Nine per cent of women were lost to follow‐up and we do not believe that this was related to the outcome. We do not believe this introduced bias. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported on all outcomes specified in the goals of the study articles. |
| Other bias | Low risk | We do not believe that other biases were introduced that could have affected the study findings. |
Abbreviations: ART: antiretroviral therapy; MTCT: mother‐to‐child transmission; RCT: randomized controlled trial.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Duggan 2012 | The intervention consisted of multiple multivitamins (not vitamin A) and the participants were children born to HIV‐positive women (rather than the women themselves). |
| Friis 2004 | The intervention consisted of multiple multivitamins and not vitamin A. |
| Locks 2017 | The intervention consisted of multiple multivitamins (not vitamin A) and the participants were children born to HIV‐positive women (rather than the women themselves). |
| Olofin 2016 | The intervention consisted of multiple multivitamins (not vitamin A) and the participants were children born to HIV‐positive women (rather than the women themselves). |
Differences between protocol and review
Differences between 2011 review and this review update
Authorship
The 2011 review had five authors (Wiysonge CS, Shey MS, Kongnyuy EJ, Sterne JA, and Brocklehurst P), but the current update has four authors (Wiysonge CS, Ndze VN, Kongnyuy EJ, and Shey MS).
Primary outcome
There are no differences between the two versions of the review, as both have an identical primary outcome (HIV infection status of the child).
Secondary outcomes
The 2011 review had 12 secondary outcomes linked to the child (infant death, stillbirth, neonatal sepsis, neonatal admission to neonatal unit, death by 24 months of age, side effects in the child, preterm delivery, very preterm delivery, birth weight, low birth weight, very low birthweight, and long‐term side effects in survivors) and five maternal secondary outcomes (maternal death, postpartum infection, side effects in the mother, cost of the intervention, and acceptability of the intervention). The current update has five child‐related secondary outcomes (mean birthweight, low birthweight, child death by two years of age, preterm delivery, and stillbirth) and two secondary outcomes linked to the mother (maternal death and postpartum CD4 count).
Methods
In Wiysonge 2011, we used a fixed‐effect method as our default method for meta‐analysis, and only used a random‐effects model when there was substantial statistical heterogeneity (P < 0.1). However, due to clinical heterogeneity, we used the random‐effects method for all meta‐analyses in this review update.
Included studies
We included five studies in the 2011 review (Coutsoudis 1999; Fawzi 2002; Kumwenda 2002; Friis 2004; Humphrey 2006), and five reviews in the current update (Coutsoudis 1999; Chikobvu 2000; Fawzi 2002; Kumwenda 2002; Humphrey 2006). We included Friis 2004 in the 2011 review but excluded it from this review update because further assessment revealed that the study did not meet our inclusion criteria. In addition, we included a new study in this update (Chikobvu 2000).
Contributions of authors
Charles S Wiysonge led the conduct and writing of this update of the Cochrane Review, with substantial intellectual contributions from Valantine N Ndze, Eugene J Kongnyuy, and Muki S Shey. All review authors approved the final version of the review for submission.
Sources of support
Internal sources
South African Medical Research Council, South Africa.
Liverpool School of Tropical Medicine, UK.
External sources
-
Effective Health Care Research Consortium, UK.
Grant: 5242
-
National Research Foundation of South Africa, South Africa.
Grant: 108571
Declarations of interest
Charles S Wiysonge has no known conflicts of interest. Valantine N Ndze has no known conflicts of interest. Eugene J Kongnyuy has no known conflicts of interest. Muki S Shey has no known conflicts of interest.
Unchanged
References
References to studies included in this review
Chikobvu 2000 {published data only (unpublished sought but not used)}
- Chikobvu P, Steinberg WJ, Joubert G, Viljoen JI, Coetzee M, Kriel J, et al. Lessons learned in establishing a randomised controlled trial to investigate the effect of vitamin A on vertical transmission of HIV. South African Journal of Epidemiology and Infection 2000;15(1):19–22. [Google Scholar]
- Joubert G, Steinberg H, Ryst E, Chikobvu P. Consent for participation in the Bloemfontein vitamin A trial: how informed and voluntary?. American Journal of Public Health 2003;93(4):582‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coutsoudis 1999 {published data only}
- Coutsoudis A, Bobat RA, Coovadia HM, Kuhn L, Tsai WY, Stein ZA. The effects of vitamin A supplementation on the morbidity of children born to HIV‐infected women. American Journal of Public Health 1995;85(8 Pt 1):1076‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutsoudis A, Moodley D, Pillay K, Harrigan R, Stone C, Moodley J, et al. Effects of vitamin A supplementation on viral load in HIV‐1‐infected pregnant women. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 1997;15(1):86‐7. [DOI] [PubMed] [Google Scholar]
- Coutsoudis A, Pillay K, Kuhn L, Spoonera E, Tsaic WY, Coovadiaa HM, South African Vitamin A Study Group. Method of feeding and transmission of HIV‐1 from mothers to children by 15 months of age: prospective cohort study from Durban, South Africa. AIDS 2001;15(3):379‐87. [DOI] [PubMed] [Google Scholar]
- Coutsoudis A, Pillay K, Spooner E, Coovadia HM, Pembrey L, Newell ML. Morbidity in children born to women infected with human immunodeficiency virus in South Africa: does mode of feeding matter?. Acta Paediatrica 2003;92(8):890‐5. [PubMed] [Google Scholar]
- Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. Randomized trial testing the effect of vitamin A supplementation on pregnancy outcomes and early mother‐to‐child transmission of HIV‐1 in Durban, South Africa. AIDS 1999;13(12):1517‐24. [DOI] [PubMed] [Google Scholar]
- Filteau S, Rollins NC, Coutsoudis A, Sullivan KR, Willumsen JF, Tomkins AM. The effect of antenatal vitamin A and beta‐carotene supplementation on gut integrity of infants of HIV‐infected South African women. Journal of Pediatric Gastroenterology and Nutrition 2001;32(4):464‐70. [DOI] [PubMed] [Google Scholar]
- Kennedy CM, Coutsoudis A, Kuhn L, Pillay K, Mburu A, Stein Z, et al. Randomized controlled trial assessing the effect of vitamin A supplementation on maternal morbidity during pregnancy and postpartum among HIV‐infected women. Journal of Acquired Immune Deficiency Syndromes 2000;24(1):37‐44. [DOI] [PubMed] [Google Scholar]
Fawzi 2002 {published data only}
- Baylin A, Villamor E, Rifai N, Msamanga G, Fawzi WW. Effect of vitamin supplementation to HIV‐infected pregnant women on the micronutrient status of their infants. European Journal of Clinical Nutrition 2005;59(8):960‐8. [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Msamanga G, Hunter D, Urassa E, Renjifo B, Mwakagile D, et al. Randomized trial of vitamin supplements in relation to vertical transmission of HIV‐1 in Tanzania. Journal of Acquired Immune Deficiency Syndromes 2000;23(3):246‐54. [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Msamanga GI, Hunter D, Renjifo B, Antelman G, Bang H, et al. Randomized trial of vitamin supplements in relation to transmission of HIV‐1 through breastfeeding and early child mortality. AIDS 2002;16(14):1935‐44. [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, Hunter DJ. Rationale and design of the Tanzania vitamin and HIV infection trial. Controlled Clinical Trials 1999;20(1):75‐90. [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, McGrath N, Mwakagile D, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV‐infected women in Tanzania. Lancet 1998;351(9114):1477‐82. [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Msamanga GI, Spiegelman D, Wei R, Kapiga S, Villamor E, et al. A randomized trial of multivitamin supplements and HIV disease progression and mortality. New England Journal of Medicine 2004;351(1):23‐32. [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Msamanga GI, Wei R, Spiegelman D, Antelman G, Villamor E, et al. Effect of providing vitamin supplements to human immunodeficiency virus–infected, lactating mothers on the child’s morbidity and CD4+ cell counts. Clinical Infectious Diseases 2003;36(8):1053‐62. [DOI] [PubMed] [Google Scholar]
- Kawai K, Msamanga G, Manji K, Villamor E, Bosch RJ, Hertzmark E, et al. Sex differences in the effects of maternal vitamin supplements on mortality and morbidity among children born to HIV‐infected women in Tanzania. British Journal of Nutrition 2010;103(12):1784‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamor E, Saathoff E, Bosch R, Hertzmark E, Baylin A, Manji K, et al. Vitamin supplementation of HIV‐infected women improves postnatal child growth. American Journal of Clin Nutrition 2005;81(4):880‐8. [DOI] [PubMed] [Google Scholar]
- Webb AL, Aboud S, Furtado J, Murrin C, Campos H, Fawzi WW, et al. Effect of vitamin supplementation on breast milk concentrations of retinol, carotenoids and tocopherols in HIV‐infected Tanzanian women. European Journal of Clinical Nutrition 2009;63(3):332‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Humphrey 2006 {unpublished data only}
- Humphrey JH. Vitamin A supplementation of breast feeding mothers and their neonates at delivery: impact on mother to child transmission of HIV during lactation, HIV infection among women during the postpartum year, and infant mortality. Available at https://clinicaltrials.gov/show/NCT00198718 (accessed 26 August 2017).
- Humphrey JH, Iliff PJ, Marinda ET, Mutasa K, Moulton LH, Chidawanyika H, et al. Effects of a single large dose of vitamin A, given during the postpartum period to HIV‐positive women and their infants, on child HIV infection, HIV‐free survival, and mortality. Journal of Infectious Diseases 2006;193(6):860‐71. [DOI] [PubMed] [Google Scholar]
- Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV‐1 transmission and increases HIV‐free survival. AIDS 2005;19(7):699‐708. [DOI] [PubMed] [Google Scholar]
- Malaba LC, Iliff PJ, Nathoo KJ, Marinda E, Moulton LH, Zijenah LS, et al. Effect of postpartum maternal or neonatal vitamin A supplementation on infant mortality among infants born to HIV‐negative mothers in Zimbabwe. American Journal of Clinical Nutrition 2005;81(2):454‐60. [DOI] [PubMed] [Google Scholar]
- Marinda E, Humphrey JH, Iliff PJ, Mutasa K, Nathoo KJ, Piwoz EG, et al. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatric Infectious Diseases Journal 2007;26(6):519‐26. [DOI] [PubMed] [Google Scholar]
- Miller MF, Soltzfus RJ, Mbuya NV, Malaba LC, Iliff PJ, Humphrey JH, ZVITAMBO Study Group. Total body iron in HIV‐positive and HIV‐negative Zimbabwean newborns strongly predicts anemia throughout infancy and is predicted by maternal hemoglobin concentration. Journal of Nutrition 2003;133(11):3461‐8. [DOI] [PubMed] [Google Scholar]
- Zijenah LS, Moulton LH, Iliff P, Nathoo K, Munjoma MW, Mutasa K, et al. Timing of mother‐to‐child transmission of HIV‐1 and infant mortality in the first 6 months of life in Harare, Zimbabwe. AIDS 2004;18(2):273‐80. [DOI] [PubMed] [Google Scholar]
Kumwenda 2002 {published data only}
- Kumwenda N, Miotti PG, Taha TE, Broadhead R, Biggar RJ, Jackson JB, et al. Antenatal vitamin A supplementation increases birth weight and decreases anemia among infants born to human immunodeficiency virus‐infected women in Malawi. Clinical Infectious Diseases 2002;35(5):618‐24. [DOI] [PubMed] [Google Scholar]
- Semba RD, Kumwenda N, Taha ET, Mtimavalye L, Broadhead R, Miotti PG, et al. Plasma and breast milk vitamin A as indicators of vitamin A status in pregnant women. International Journal for Vitamin and Nutrition Research 2000;70(6):271‐7. [DOI] [PubMed] [Google Scholar]
- Semba RD, Kumwenda N, Taha TE, Mtimavalye L, Broadhead R, Garrett E, et al. Impact of vitamin A supplementation on anaemia and plasma erythropoietin concentrations in pregnant women: a controlled trial. European Journal of Haematology 2001;66(6):389‐95. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Duggan 2012 {published data only}
- Duggan C, Manji KP, Kupka R, Bosch RJ, Aboud S, Kisenge R, et al. Multiple micronutrient supplementation in Tanzanian infants born to HIV‐infected mothers: a randomized, double‐blind, placebo‐controlled clinical trial. American Journal of Clinical Nutrition 2012;96(6):1437‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friis 2004 {published and unpublished data}
- Friis H, Gomo E, Nyasema N, Ndhlovu P, Krarup H, Kaestel P, et al. Effect of multinutrient supplementation on gestational length and birth size: a randomized, placebo‐controlled, double‐blind effectiveness trial in Zimbabwe. American Journal of Clinical Nutrition 2004;80(1):178‐84. [DOI] [PubMed] [Google Scholar]
Locks 2017 {published data only}
- Locks LM, Manji KP, Kupka R, Liu E, Kisenge R, McDonald CM, et al. High burden of morbidity and mortality but not growth failure in infants exposed to but uninfected with human immunodeficiency virus in Tanzania. Journal of Pediatrics 2017;180:191‐9.e.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olofin 2016 {published data only}
- Olofin IO, Liu E, Manji KP, Danaei G, Duggan C, Aboud S, et al. Active tuberculosis in HIV‐exposed Tanzanian children up to 2 years of age: early‐life nutrition, multivitamin supplementation and other potential risk factors. Journal of Tropical Pediatrics 2016;62(1):29‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Burger 1997
- Burger H, Kovacs A, Weiser B, Grimson R, Nachman S, Tropper P, et al. Maternal serum vitamin A levels are not associated with mother‐to‐child transmission of HIV‐1 in the United States. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 1997;14(4):321‐6. [DOI] [PubMed] [Google Scholar]
Burns 1999
- Burns DN, FitzGerald G, Semba RD, Hershow R, Zorrilla C, Pitt J, et al. Vitamin A deficiency and other nutritional indices during pregnancy in human immunodeficiency virus infection: prevalence, clinical correlates, and outcome. Clinical Infectious Diseases 1999;29(2):328‐34. [DOI] [PubMed] [Google Scholar]
Christian 1998a
- Christian P, Schulze K, Stoltzfus RJ, West KP Jr. Hyporetinolemia, illness symptoms, and acute phase protein response in pregnant women with and without night blindness. American Journal of Clinical Nutrition 1998;67(6):1237‐43. [DOI] [PubMed] [Google Scholar]
Christian 1998b
- Christian P, West KP Jr, Khatry SK, Katz J, LeClerq S, Pradhan EK, et al. Vitamin A or beta‐carotene supplementation reduces but does not eliminate maternal night blindness in Nepal. Journal of Nutrition 1998;128(9):1458–63. [DOI] [PubMed] [Google Scholar]
Christian 1998c
- Christian P, West KP Jr, Khatry SK, Katz J, Shrestha SR, Pradhan EK, et al. Night blindness of pregnancy in rural Nepal – nutritional and health risks. International Journal of Epidemiology 1998;27(2):231–7. [DOI] [PubMed] [Google Scholar]
Christian 2000
- Christian P, West KP Jr, Khatry SK, Kimbrough‐Pradhan E, LeClerq SC, Katz J, et al. Night blindness during pregnancy and subsequent mortality among women in Nepal: effects of vitamin A and beta‐carotene supplementation. American Journal of Epidemiology 2000;152(6):542‐7. [DOI] [PubMed] [Google Scholar]
Christian 2001
- Christian P, West KP Jr, Khatry SK, LeClerq SC, Kimbrough‐Pradhan E, Katz J, et al. Maternal night blindness increases risk of mortality in the first 6 months of life among infants in Nepal. Journal of Nutrition 2001;131(5):1510–2. [DOI] [PubMed] [Google Scholar]
Damodaran 2017
- Damodaran S, Parkin KL. Fennema's Food Chemistry. 5th Edition. Florida: CRC Press, 2017. [Google Scholar]
De Cock 2000
- Cock KM, Fowler MG, Mercier E, Vincenzi I, Saba J, Hoff E, et al. Prevention of mother to‐child HIV transmission in resource‐poor countries: translating research into policy and practice. JAMA 2000;283(9):1175‐82. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fawzi 1998
- Fawzi WW, Hunter DJ. Vitamins in HIV disease and vertical transmission. Epidemiology 1998;9(4):457‐66. [PubMed] [Google Scholar]
Fawzi 2000
- Fawzi WW. Nutritional factors and vertical transmission of HIV‐1. Epidemiology and potential mechanisms. Annals of the New York Academy of Sciences 2000;918:99‐114. [DOI] [PubMed] [Google Scholar]
Gorgia 2010
- Gogia S, Sachdev HS. Maternal postpartum vitamin Asupplementation for the prevention of mortality and morbidity in infancy: a systematic review of randomized controlled trials. International Journal of Epidemiology 2010;39(5):1217‐26. [DOI: 10.1093/ije/dyq080] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 10 August 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Green 1928
- Green HN, Mellanby E. Vitamin A as an anti‐infective agent. BMJ 1928;2(3537):691‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenberg 1997
- Greenberg BI, Semba RD, Vink, Farley JJ, Sivapalasingam M, Steketee RW, et al. Vitamin A deficiency and maternal‐infant transmission of HIV in two metropolitan areas in the United States. AIDS 1997;11(3):325‐32. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small study effects in meta‐analysis of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2016
- Higgins JPT, Lasserson T, Chandler J, Tovey D, Churchill R. Methodological Expectations of Cochrane InterventionReviews. Cochrane: London, 2016. http://methods.cochrane.org/sites/default/files/public/uploads/mecir_printed_booklet_final.pdf (accessed 10 August 2017).
McIntyre 2002
- McIntyre J, Gray G. What can we do to reduce mother to child transmission of HIV?. BMJ 2002;324(7331):218‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mofenson 2000
- Mofenson LM, McIntyre JA. Advances and research directions in the prevention of mother‐to‐child HIV‐1 transmission. Lancet 2000;355(9222):2237‐44. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Navér 2006
- Navér L, Lindgren S, Belfrage E, Gyllensten K, Lidman K, Gisslén M, et al. Children born to HIV‐1‐infected women in Sweden in 1982‐2003: trends in epidemiology and vertical transmission. Journal of Acquired Immune Deficiency Syndromes 2006;42(4):484‐9. [DOI] [PubMed] [Google Scholar]
Newell 2000
- Newell ML. Vertical transmission of HIV‐1 infection. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(1):1‐2. [DOI] [PubMed] [Google Scholar]
Read 2005
- Read JS, Newell ML. Efficacy and safety of cesarean delivery for prevention of mother‐to‐child transmission of HIV‐1. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005479] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ross 1996
- Ross AC, Stephensen CB. Vitamin A and retinoids in antiviral responses. FASEB Journal 1996;10(9):979‐85. [PubMed] [Google Scholar]
Semba 1994
- Semba RD, Miotti PG, Chiphanwi JD, Saah AJ, Canner JK, Dallabetta GA, et al. Maternal vitamin A deficiency and mother to child transmission of HIV‐1. Lancet 1994;343(8913):1593‐7. [DOI] [PubMed] [Google Scholar]
Semba 1995
- Semba RD, Miotti PG, Chiphangwi JD, Liomba G, Yang LP, Saah AJ, et al. Infant mortality and vitamin A deficiency during human immunodeficiency virus infection. Clinical Infectious Diseases 1995;21(4):966‐72. [DOI] [PubMed] [Google Scholar]
Semba 1998
- Semba RD. The role of vitamin A and related carotenoids in immune function. Nutrition Reviews 1998;56(1 Pt 2):S38‐48. [DOI] [PubMed] [Google Scholar]
Tanumihardjo 2011
- Tanumihardjo SA. Vitamin A: biomarkers of nutrition for development. American Journal of Clinical Nutrition 2011;94(2):658S–65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorne‐Lyman 2012
- Thorne‐Lyman AL, Fawzi WW. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta‐analysis. Paediatric and Perinatal Epidemiology 2012;26(Suppl 1):36‐54. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
UNAIDS 2001
- Joint United Nations Programme on HIV/AIDS (UNAIDS), World Health Orgnanization. AIDS epidemic update. http://www.who.int/hiv/facts/en/isbn9291731323.pdf (accessed 10 August 2017).
UNAIDS 2015
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Fact sheet 2016. http://www.unaids.org/sites/default/files/media_asset/20150901_FactSheet_2015_en.pdf (accessed 15 February 2016).
Wang 2016
- Wang H, Wolock TM, Carter A, Nguyen G, Kyu HH, Gakidou E, et al. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980‐2015: the Global Burden of Disease Study 2015. Lancet HIV 2016;3(8):e361‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2001
- West K, Darnton‐Hill I. Vitamin A deficiency. In: Semba R, Bloem M editor(s). Nutrition and Health in Developing Countries. Totowa, NJ: Humana Press, Inc, 2001:267‐306. [Google Scholar]
West 2002
- West KP Jr. Extent of vitamin A deficiency among preschool children and women of reproductive age. Journal of Nutrition 2002;132(9 Suppl):2857S–66S. [DOI] [PubMed] [Google Scholar]
WHO 1998
- World Health Organization. Safe vitamin A dosage during pregnancy and lactation: Recommendations and report of a consultation. 1998. http://apps.who.int/iris/bitstream/10665/63838/1/WHO_NUT_98.4_eng.pdf?ua=1 (accessed 10 August 2017).
WHO 2009
- WHO. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. WHO Global Database on Vitamin A deficiency. 2009. http://apps.who.int/iris/bitstream/10665/44110/1/9789241598019_eng.pdf. Geneva: World Health Organization, (accessed 10 August 2017).
WHO 2015
- World Health Organization. Guideline on when to start antiretroviral therapy and on pre‐exposure prophylaxis for HIV. September 2015. Available at http://www.who.int/hiv/pub/guidelines/earlyrelease‐arv/en/. WHO, (accessed 10 August 2017). [PubMed]
Wiysonge 2006
- Wiysonge CS, Nomo E, Mawo JN, Ticha JM. Accelerated measles control in sub‐Saharan Africa. Lancet 2006;367(9508):394‐5. [DOI] [PubMed] [Google Scholar]
Wolf 2001
- Wolf G. The discovery of the visual function of vitamin A. Journal of Nutrition 2001;131(6):1647–50. [DOI] [PubMed] [Google Scholar]
Zile 1998
- Zile MH. Vitamin A and embryonic development: an overview. Journal of Nutrition 1998;128(2 Suppl):455S‐8S. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kongnyuy 2009
- Kongnyuy EJ, Wiysonge CS, Shey MS. A systematic review of randomized controlled trials of prenatal and postnatal vitamin A supplementation of HIV‐infected women. International Journal of Gynaecology and Obstetrics 2009;104(1):5‐8. [DOI] [PubMed] [Google Scholar]
Wiysonge 2002
- Shey Wiysonge C, Brocklehurst P, Sterne JA. Vitamin A supplementation for reducing the risk of mother‐to‐child transmission of HIV infection. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003648] [DOI] [PubMed] [Google Scholar]
Wiysonge 2005
- Wiysonge CS, Shey MS, Sterne JAC, Brocklehurst P. Vitamin A supplementation for reducing the risk of mother‐to‐child transmission of HIV infection. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003648.pub2] [DOI] [PubMed] [Google Scholar]
Wiysonge 2011
- Wiysonge CS, Shey M, Kongnyuy EJ, Sterne JA, Brocklehurst P. Vitamin A supplementation for reducing the risk of mother‐to‐child transmission of HIV infection. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD003648.pub3] [DOI] [PubMed] [Google Scholar]


