Abstract
Purpose
To systematically evaluate and quantify the effects of Tai Chi/Qigong (TCQ) on motor (UPDRS III, balance, falls, Timed-Up-and-Go, and 6-Minute Walk) and non-motor (depression and cognition) function, and quality of life (QOL) in patients with Parkinson’s disease (PD).
Methods
A systematic search on 7 electronic databases targeted clinical studies evaluating TCQ for individuals with PD published through August 2016. Meta-analysis was used to estimate effect sizes (Hedge’s g) and publication bias for randomized controlled trials (RCTs). Methodological bias in RCTs was assessed by two raters.
Results
Our search identified 21 studies, 15 of which were RCTs with a total of 755 subjects. For RCTs, comparison groups included no treatment (n=7, 47%) and active interventions (n=8, 53%). Duration of TCQ ranged from 2 to 6 months. Methodological bias was low in 6 studies, moderate in 7, and high in 2. Fixed-effect models showed that TCQ was associated with significant improvement on most motor outcomes (UPDRS III [ES=-0.444, p<.001], balance [ES=0.544, p<.001], Timed-Up-and-Go [ES=−0.341, p=.005], 6MW [ES=−0.293, p=.06]), falls [ES=−.403, p=.004], as well as depression [ES=−0.457, p=.008] and QOL [ES=−0.393, p<.001], but not cognition [ES= −0.225, p=.477]). I2 indicated limited heterogeneity. Funnel plots suggested some degree of publication bias.
Conclusion
Evidence to date supports a potential benefit of TCQ for improving motor function, depression and QOL for individuals with PD, and validates the need for additional large-scale trials.
Keywords: Meta analysis, Tai Chi, Parkinson disease, Motor activity, Quality of life
Introduction
Parkinson’s disease (PD) is one of the most common progressive neurodegenerative disorders, leading to loss of motor function and reduced quality of life. A growing body of evidence supports the role of exercise in improving both motor and non-motor outcomes in PD[1–3]. In fact, exercise is now considered an integral part of the management of PD [4–6].
Tai Chi and Qigong (TCQ) are two increasingly popular mind-body interventions that have the potential to address a range of motor and non-motor symptoms associated with PD [7]. TCQ share a common history which includes elements of traditional Chinese medicine, martial arts conditioning, and Asian lifestyle philosophy. Both integrate balance, flexibility, and neuromuscular coordination training with a number of cognitive components, including heightened body awareness, focused mental attention, imagery, multi-tasking, and planned and goal-oriented training, which together may result in benefits to PD above and beyond conventional exercise [8]. In contrast to many styles of yoga or seated mind-body meditative practices, TCQ typically places a greater emphasis on standing and dynamic movements (e.g. pushing and lifting gestures linked to martial applications) that have the potential to impact gait, balance, and other functional activities. For these reasons, in the present work TCQ are grouped together and are considered equivalent interventions, paralleling other recent reviews [9–11].
The evidence for TCQ’s effects on PD motor symptoms have been evaluated in a growing number of trials, with prior meta-analyses and systematic reviews generally showing positive support for clinical measures of balance [12–14]. Mixed or inconclusive results for other motor outcomes (e.g., gait, mobility, and falls) have typically been reported, in large part due to the few numbers of studies assessing these outcomes [12–14]. Even less attention has been devoted to systematically evaluating the effects of TCQ on non-motor outcomes [12], and no meta-analyses to date have evaluated key issues such as depression and cognitive function, which have been shown to be strongly linked to overall quality of life [15].
Research to date exploring possible neuromuscular and behavioral mechanisms underlying TCQ’s effect on postural control add credibility to clinical findings, however, these studies have generally not included populations with PD. Studies largely focused on normal aging suggest that enhanced motor performance may be due to various underlying processes, including improved lower extremity strength and flexibility [16–22], proprioception and postural awareness [23–25], neuromuscular coordination and reaction time [26, 27], executive function [28], and reduced fear of falling [29, 30]. Less research has evaluated mechanisms that may contribute to improvements in non-motor function. TCQ related improvements in depression and anxiety have been hypothesized to be related to modest cardiovascular loading, breathing and imagery related changes in autonomic tone, cognitive restructuring leading to reduced rumination and catastrophizing, and indirect effects of enhanced motor confidence and enhanced self-efficacy [11, 31–33]. These mechanisms, as well as multi-tasking and attention shifting training associated with TCQ, have been hypothesized to contribute to enhanced cognitive function [34], and some studies have reported correlations between TCQ-related improvements in cognitive function and brain neural architecture and resting state neural network activity [35, 36]. However, these studies have not been conducted in PD populations.
Using a meta-analytic approach and incorporating a number of recent clinical trials not included in previous reviews[37–41], this study aimed to systematically evaluate and quantify the effects of TCQ on motor and non-motor function and quality of life in patients with PD.
2. Methods
2.1. Literature Search
Electronic literature searches were performed using PubMed, CINAHL, Web of Science, ProQuest Central, Science Direct, Scopus, and Cochrane Library for English language articles published until August 30, 2016. The search terms were Tai Chi, Taiji, Qigong, and Parkinson’s disease. Additional manual searches based on references listed in the retrieved articles were performed to complete the search.
2.2. Eligibility criteria
Randomized controlled trials (RCTs) and prospective non-randomized controlled and observational studies published in English in which Parkinson’s disease was the primary disease and Tai Chi and/or Qigong were the primary interventions were included. Formal meta-analysis was limited to RCTs, with non-RCTs used to qualitatively further inform synthesis of the overall evidence.
2.3. Study selection and Data Extraction
Study eligibility assessment was performed independently by two researchers (WG and KO) who applied eligibility criteria using a standardized protocol. Data were extracted by two reviewers (RS and MP) independently using a standardized template generated in Microsoft Excel. Data related to study design, duration and frequency of the intervention program, type of the control, sample size, and outcome measures were extracted for qualitative analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) [42].
2.4. Risk of bias assessment
Two researchers (RS and MP) independently assessed the methodological quality of RCTs using the 10 item Cochrane Collaboration Tool for assessing risk of bias. Criteria include: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data (attrition rates or ITT), selective reporting, eligibility criteria, groups similar at baseline (primary outcome), rationale for sample size, and other bias [43]. A summed score of 5 points or more was set as the threshold to be excluded in the analysis. The evaluated domains were assessed as 0 (no bias), 1 (minimal bias), or 2 (moderate or severe bias) according to the established criteria [43]. Any discrepancies in the evaluations conducted by two authors were discussed and resolved.
2.5. Safety monitoring
Studies were reviewed to identify if formal protocols for systematically monitoring adverse events were described, and if adverse events reported in the study was associated with the intervention.
2.6. Data analysis
Outcome data for formal meta-analysis using identified 15 RCTs with 755 subjects with PD were systematically extracted and organized in a database. For each outcome, data extracted included the mean and standard deviation (SD) of the pre-test and post-test values for each group, mean and SD of change scores in each group, t score or p-value within groups, and sample size (N) in each group. When these data were not available, data in the form of standard errors, confidence intervals or medians with ranges were converted into mean and SD format using previously suggested statistical formulas [43, 44].
Comprehensive Meta-Analysis software v3 (CMA v3, Biostat, Inc. USA) was used for data synthesis. When an outcome of interest (e.g., depression) was measured with multiple instruments, a pooled effect size was calculated by CMA. For continuous data, Hedge’s g and 95% confidence intervals (CI) using a fixed effect model were calculated to estimate mean differences between groups for all eligible trials. The Q value and I2 statistics were used to assess heterogeneity for the variation in true effect sizes across the included studies; a low p value for Q statistics and/or I2 index above 40% indicated significant heterogeneity [43]. Publication bias was assessed using funnel plots.
Pooled effect size for each outcome variable of motor and non-motor function was calculated. Subgroup analyses were also conducted according to the comparison group; alternative active interventions (e.g., exercise, health education, meditation) or no treatment (e.g., no contact control, wait list).
One study [45] only reported data combining multiple outcomes; the authors for this study were contacted and provided the raw data needed to extract separate scores for each outcome.
3. Results
3.1. Study selection and characteristics
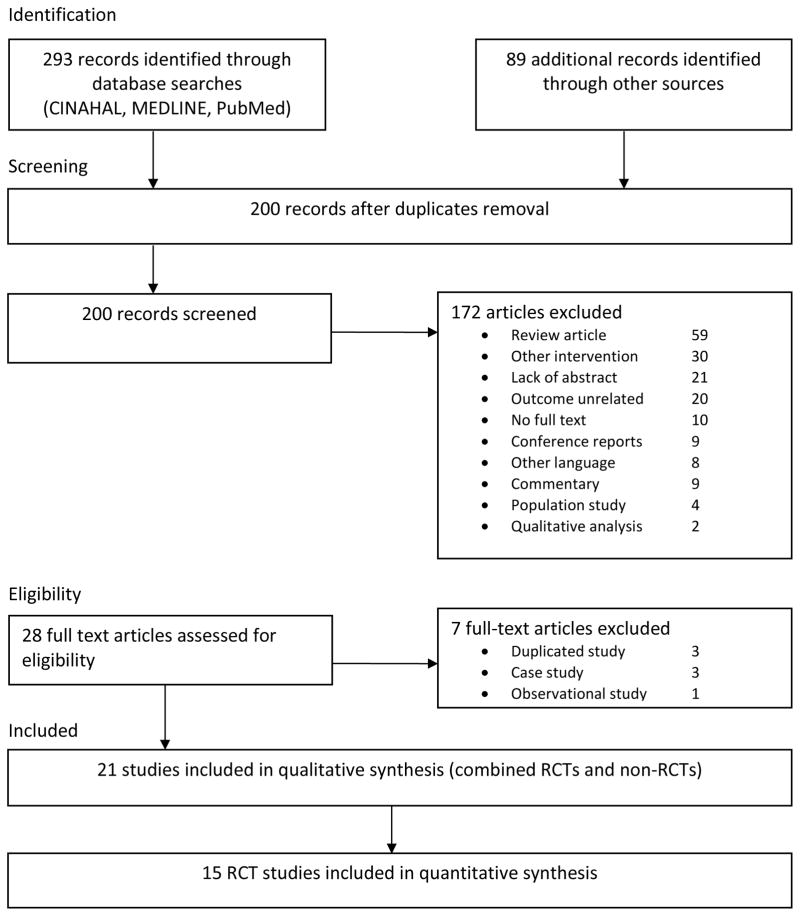
Figure 1 summarizes the flow of the literature search and selection process following PRISMA guidelines. An initial search identified 382 records from multiple databases and manual searches. Removing duplicates resulted in 200 records. The title and abstracts of these records were screened according to the inclusion criteria. A total of 28 full text articles met eligibility criteria and were further reviewed, with an additional 7 being excluded due to duplicated data in separate publications, or inappropriate study design. Of the remaining 21 eligible studies, 15 were RCTs and 6 non-RCTs, of which 11 RCTs and 4 non-RCTs have been included in previous systematic reviews or meta-analysis [37–41].
Figure 1.
Summary of the flow of our literature search according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines
Among the 15 RCTs, Li and colleagues [38, 46, 47] published 3 papers from the same parent trial, with each paper emphasizing different outcome variables. Outcome variables duplicated across publications were excluded in analyses. Li and colleagues [38, 46, 47] also had more than two comparison groups; data for each comparison group were separately entered in the analysis with the sample sizes adjusted (i.e., divided by 2) to minimize over-influence of one study. Amano et al. [48] reported two research studies within one publication, each including unique interventions and control groups; these were treated as separate studies in analyses.
3.1.1. Participants characteristics and study setting
Table 1 summarizes the 21 studies (15 RCTs and 6 non-RCTs) which include a total of 823 subjects. Study participants all had diagnoses of idiopathic Parkinson’s disease with Hoehn & Yahr scores ranging from 1 to 4 with an average of 2.3. The average age of the participants was 67.5 y; 58.4% of participants were male. All RCTs included patients on a stable PD medication regimen. All but two studies conducted testing in the on-medication state. The two studies evaluating outcomes in the off-medication state utilized a 12-hour pre-testing washout period [39, 40].
Table 1.
Summary of Tai Chi/Qigong studies for individuals with Parkinson’s disease
| Main Author (country) | Type of study | Sample (mean age) | Gender (M/F) | Med | Intervention | Frequency (per week) | Duration (weeks) | Control group | Measured outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Burini 2006 (Italy) | RCT with cross-over | 26 (65.2) | 9/16 | on | Qigong | 50m, 3x | 7 | aerobic exercise | UPDRS, 6MW*, MBS, PDQ, BDI, CPET |
| Amano 2013 (USA) | RCT | 21 (66.0) 24 (66.0) |
14/7 14/10 |
on | TaiChi | 1h, 2x | 16 | Qigong meditation | UPDRS, gait |
| Choi 2013 (Korea) | RCT | 22 (60.8) | NS | on | Tai Chi | 1h, 3x | 12 | no treatment | UPDRS, reaction time*, gait, TUG, 6MW, OLS* |
| Kurlan 2015 (USA) | RCT | 44 (72.0) | 27/17 | on | Tai Chi | 1h,1x | 16 | no treatment | UPDRS, S&E, GDS, PDQ, falls |
| Li 2014 (USA) | RCT | 195 (68.7) | 122/73 | on | Tai Chi | 1h, 2x | 24 | resistance training or stretching | UPDRS, 50-ft walk, VPS*, PDQ* |
| Li 2015 (USA) | RCT | 176 (68.7) | 122/73 | on | Tai Chi | 1h, 2x | 24 | resistance training or stretching | Falls*, PDQ*, cost effectiveness* |
| Li 2012 (USA) | RCT | 195 (68.7) | 122/73 | on | Tai Chi | 1h, 2x | 24 | resistance training or stretching | UPDRS*, gait*, TUG*, FRT*, muscle strength* |
| Schmitz-Hubsch 2006 (Germany) | RCT | 56 (63.5) | 43/13 | on | Qigong | 1.5h/ 1x | 8 | no treatment | UPDRS*, MADRS, PDQ |
| Xiao 2015 (China) | RCT | 96 (67.5) | 67/29 | off | Qigong | 3h training + 15m, 4x at home | 24 | walking | UPDRS*, 6MW*, BBS*, PDSS*, TUG*, PFS, gait* |
| Zhang 2015 (China) | RCT | 40 (54.0) | 24/16 | on | Tai Chi | 1h, 2x | 12 | multimodal exercise | UPDRS, BBS, TUG, gait |
| Gao 2014 (China) | RCT | 76 (69.5) | 50/26 | on | Tai Chi | 1h, 3x | 12 | no treatment | UPDRS, TUG, BBS*, falls* |
| Hackney 2008 (USA) | RCT | 26 (63.0) | 21/5 | on | Tai Chi | 1h, 2x | 10 | no treatment | UPDRS*, TUG, TS*, 6MW*, OLS, BBS*, gait |
| Gladfelter 2011 (USA) | RCT | 17 (72.0) | 12/5 | on | Tai Chi | 1h, 2x | 12 | no treatment | PDQ, BBS*, FRT*, TUG |
| Hackney 2009 (USA) | RCT | 71 (66.0) | 45/16 | on | Tai Chi | 1h, 2x | 13 | tango, waltz, no treatment | UPDRS, PDQ |
| Nocera 2014 (USA) | RCT | 21 (66.5) | 11/10 | on | Tai Chi | 1h, 3x | 16 | no treatment | cognition, PDQ*, TFES |
| Cheon 2013 (Korea) | non-RCT | 23 (64.2) | 0/23 | on | Tai Chi | 1h, 3x | 8 | combined exercise or no treatment | UPDRS, QOL, BDI, S&E, functional fitness |
| Li 2007 (USA) | one group pre-post test | 17 (71.5) | 6/11 | on | Tai Chi | 1.5h, 5x | 5 | None | 50-ft walk, TUG, FRT |
| Kim 2014 (Korea) | one group pre-post test | 12 (65.3) | 2/10 | on | Tai Chi | 1h, 3x | 12 | None | COP |
| Kim 2011 (Korea) | one group pre-post test | 10 (78.5) | 1/9 | on | Tai Chi | 1h, 3x | 12 | None | COP |
| Wassom 2015 (USA) | one group pre-post test | 7 (66.9) | 3/4 | off | Qigong | 1h,1x + 40m daily at home | 6 | None | UPDRS, PDSS, PFS, cognition, gait |
| Loftus 2014 (USA) | one group pre-post test | 34 (66.0) | 17/17 | on | Qigong | 1h, 1x | 12 | None | BBS, PDFP, Pull test |
Note. Med, anti-Parkinson medication (‘off’ refers to medication off during measurement). UPDRS, Unified Parkinson’s disease rating scale; 6MW, 6 minute walking; MBS, modified Borg scale; PDQ, Parkinson’s disease questionnaire; BDI, Beck depression inventory; CPET, cardiopulmonary exercise test; TUG, timed up and go; OLS, one leg standing; S&E, Schwab and England activities of daily living scale; GDS, geriatric depression scale; VPS, vitality plus scale; FRT, functional reach test; MADRS, Montgomery-Asperg depression rating scale; BBS, Berg balance scale; PDSS, Parkinson’s disease sleep scale; PFS, Parkinson’s fatigue scale; TS, tandom stance; TFES, Tinetti’s falls efficacy scale; HRQOL, health related quality of life; PDFP, Parkinson’s disease fall profile; MMSE, Mini Mental State Examination.
Indicates outcome significantly improved by Tai Chi/Qigong relative to control group.
3.1.2. Intervention and control group characteristics
Tai Chi was used as an intervention in 16 studies. In nearly all cases, a modified, simplified, or short form of Tai Chi was used. All interventions were delivered in a group setting, but one study [49] utilized home training sessions following only four 45-min group trainings. The duration of interventions ranged from 5 to 24 weeks. Individual intervention sessions ranged from 45 to 90 minutes, and the frequency of classes varied between 1 to 3 times per week. Studies varied in their prescriptions for home practice regimens. Nine studies compared TCQ with other alternative active interventions (e.g., exercise, resistance training, walking or dance); 7 studies employed a no-treatment or wait-list control group, and 5 studies were uncontrolled.
3.1.3. Outcome measures
For motor function, UPDRS Part III was most commonly assessed (14 studies), followed by balance (9 studies), Timed-Up-and-Go (8 studies), 6MW (6 studies), and falls (2 studies). For non-motor outcomes, quality of life was most frequently measured (9 studies), followed by depression (3 studies) and cognition (3 studies).
3.1.4. Adverse effects
None of the 15 RCTs reported protocols for monitoring adverse events. Two studies did mention safety was monitored without providing detail [50, 51], but no safety related results were reported. Three separate studies reported no adverse events related to interventions were observed [41, 47, 52]. No serious adverse events were reported in any study.
3.1.5. Risk of bias assessment
The fifteen RCTs were assessed for risk of bias (Table 2). Randomization procedures were described in all studies, but 6 of them provided no specific details of procedures used. Allocation concealment was not mentioned in 6 studies (60%), while 14 studies (93%) reported blinding of outcome assessment. Seven studies (46%) reported either intention to treat approach or no dropouts. Homogeneity of baseline data was confirmed by most studies, but one study [51] reported significant differences in baseline values of an outcome variable. Twelve studies (80%) did not provide a rationale for the sample size. Overall rating of bias assessment indicated that 6 studies were methodologically strong (0–1 risk), 7 studies moderate (2–3 risk), and 2 studies were weak (4 risk). No studies met the pre-defined threshold for exclusion from the analyses.
Table 2.
Quality assessment of the included studies
| Selection Bias | Performance bias | Attrition bias | Reporting bias | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Random sequence | Allocation concealment | Blinding of participants | Blinding of outcome assessment | Incomplete outcome data (ITT) | Selective reporting | Eligibility criteria | Difference at baseline | Rational for sample size | Other bias | Total |
| Burini 2006 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Amano 2013 | NS | NR | NR | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 3 |
| Choi 2013 | NS | NR | NR | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 |
| Kurlan 2015 | NS | NR | NR | 0 | 1 | 0 | 0 | 0 | 1 | UGS | 3 |
| Li 2014 | 0 | 0 | NR | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Li 2015 | 0 | 0 | NR | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Li 2012 | 0 | 0 | NR | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Schmitz-Hubsch 2006 | 0 | 0 | NR | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Xiao 2015 | NS | NR | NR | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 |
| Zhang 2015 | 0 | 0 | NR | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Gao 2014 | 0 | NR | NR | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Hackney 2008 | 0 | NR | NR | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Gladfelter 2011 | 0 | NR | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Hackney 2009 | NS | NR | 0 | NR | 1 | 0 | 0 | 1 | 1 | 0 | 4 |
| Nocera 2014 | NS | NR | NR | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 4 |
Note. NS (not specified, counted as 1); NR (not reported); UGS (unequal group size).
Higher scores represent more bias.
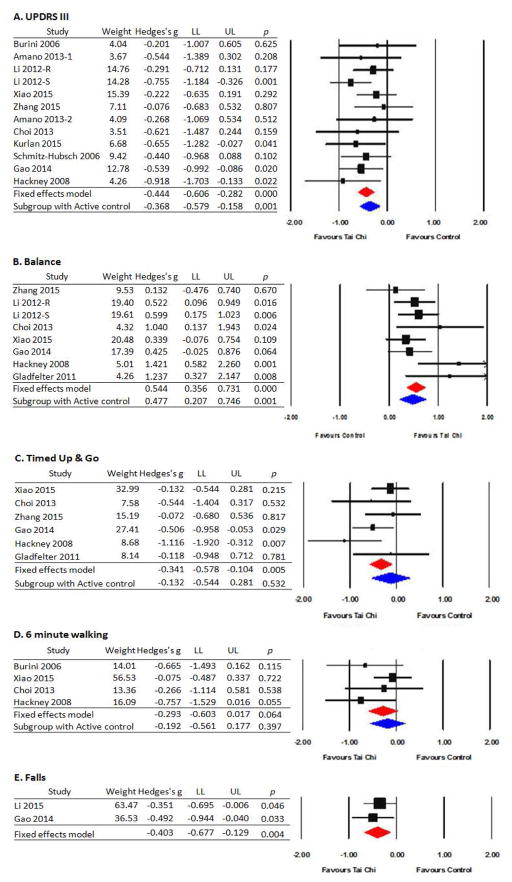
3.2. Effects of TCQ on motor function
3.2.1. Unified Parkinson’s Disease Rating Scale (UPDRS) Part III
Motor symptoms were assessed with the UPDRS III in 13 studies [37, 39, 40, 45, 46, 48, 49, 51, 53–57], including 11 RCTs. Lower scores reflect less severe motor symptoms. Meta-analysis of the 11 RCTs employing a fixed effect model indicated an overall small effect size (Hedges’s g=−0.444, 95% CI −0.606 to −0.282, p< .001) supporting that TCQ significantly improved motor function relative to all control groups. Q-value (p=.732) and I2 (0%) indicate no heterogeneity. The subgroup analysis limited to 6 studies with active control groups revealed a slightly lower but statistically significant effect size (ES=−0.368, p=.001). The effect size limited to the 6 studies with a no-treatment control group was relatively larger (ES=−0.555, p<.001). Lack of heterogeneity persisted in subgroup analyses. Publication bias was not suspected based on the funnel test plot asymmetry. Of note, the two non-RCTs that assessed UPDRS did not report statistically significant within-group improvements [39, 54].
3.2.2. Balance
Balance was reported in 10 studies [40, 41, 45, 46, 49, 50, 55, 58–60], including 7 RCTs. Balance was assessed using the Berg Balance Scale, posturography, or single leg standing time. Results from a fixed effect meta-analysis model indicate that TCQ had greater improvements in balance than control groups with an overall medium effect size (Hedges’s g = 0.544, 95% CI 0.356 to 0.730, p<0.001). Heterogeneity was not substantial based on Q value (p=.156) and I2 (34.12%). Subgroup analysis limited to 3 studies employing active control groups also showed a medium effect size (ES = 0.477, p=0.001), as did subgroup analyses limited to 5 studies using no treatment control groups (ES = 0.544, p < .001). Lack of heterogeneity persisted in subgroup analysis. Three additional non-controlled studies [58–60] also reported within-group improvements in balance.
3.2.3. Timed-Up-and-Go
Timed-Up-and-Go (TUG) was assessed in 7 studies [40, 41, 45, 49, 50, 55, 61], including 6 RCTs. Results of a fixed-effect model of RCTs indicate that TCQ significantly improved TUG compared with control groups, with a small effect size (Hedges’s g=−0.341, 95% CI −0.578 to −0.104, p=.005). Q value (p=.277) and I2 (20.78%) indicate limited heterogeneity. Due to the small number of studies with active control groups, subgroup analysis was not performed. One non-RCT study [61] also reported within-group improvement in TUG.
3.2.4. 6-Minute Walk (6MW)
Four RCTs [40, 45, 53, 55] assessed the effect of TCQ on the 6MW test. A fixed-effect model indicated that TCQ did not significantly improve 6 minute walking speed compared to control groups (Hedges’s g=−0.293, 95% CI −0.603 to 0.017, p=.064), although most studies showed greater improvement in performance in the TCQ group. Q value (p=.356) and I2 (7.49%) showed limited heterogeneity. Due to the small number of studies, subgroup analyses were not performed.
3.2.5. Falls
Only 2 RCTs [38, 49] assessed the effect of TCQ on the number of fall rates. A fixed-effect model indicated that TCQ significantly reduced fall episodes compared to control group (Hedges’s g = −0.403, 95% CI −0.677 to −0.129, p=.004). Q value (p=.623) and I2 (0%) confirms limited heterogeneity. One non-controlled study [60] also reported that the PD patients experienced fewer falls at the beginning vs. at the end of a 6-month Qigong program.
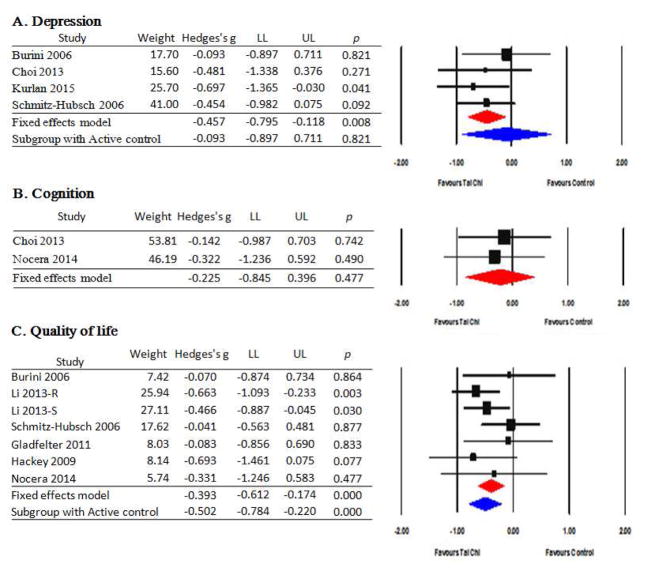
3.3. Effect of TCQ on non-motor function and quality of life
3.3.1. Depression
Depression was assessed in 5 studies [37, 45, 53, 54, 56], including 4 RCTs. Depression was assessed using the Beck Depression Inventory, Geriatric Depression Scale, and Montgomery-Asperg Depression Rating Scale. A fixed-effect model indicated that TCQ significantly reduced depression scores compared to control groups, with an overall medium effect size (Hedges’s g=−0.457, 95% CI −0.795 to −0.118, p=.008). Q value (p=.739) and I2 (0%) indicated limited heterogeneity. Due to the small number of studies, subgroup analyses were not performed. One non-randomized controlled study [54] also reported statistically significant improvements in depression when Tai Chi was compared to a no treatment group.
3.3.2. Cognitive function
Three studies [39, 45, 52], including 2 RCTs, assessed the effect of TCQ on cognition. Cognition in the RCTs was assessed with UPDRS Part I and Trail Making Tests. A fixed-model indicated that TCQ did not significantly improve cognition when compared to control groups (Hedges’s g = −0.225, 95% CI −0.845 to −0.396, p=.477), though both studies reported modest benefits in favor of TCQ. Q value (p=.776) and I2 (0%) indicated limited heterogeneity. One non-controlled study [39] also reported no significant improvements in cognition following 6 weeks of Qigong training.
3.3.3. Quality of life
Quality of life was evaluated in 7 studies [47, 50–54, 56], including 6 RCTs. Five of the 6 RCTs assessed QOL using the Parkinson’s Disease Questionnaire-39 (PDQ-39); 1 used the PDQ-8 short version. A fixed-effect model indicated that TCQ significantly improved quality of life (lower scores) compared with control groups, with an overall small effect size (Hedges’s g= −0.393, 95% CI −0.612 to −0.174, p < .001). Q value (p=.527) and I2 (0%) indicated limited heterogeneity. Subgroup analysis limited to the 3 studies that employed active control groups indicated medium effect size (ES = −0.502, p <.001); the effect size was slightly smaller for the 4 studies that employed a no treatment control group (ES= −0.230, p = .207). Lack of heterogeneity persisted for subgroup analyses. One non-randomized controlled study [54] also reported statistically significant improvements in quality of life when Tai Chi was compared to a no treatment group.
Discussion
Due to recent changes in the understanding of the nature of PD, exercise is now widely recommended to individuals with PD for maintaining balance, mobility and overall quality of life. Nonetheless, few evidence-based guidelines are available to inform optimal exercise regimens. Extending prior meta-analyses and systematic reviews by including 5 recent studies (4 RCTs) not considered in prior reviews, our findings support the idea that TCQ may be effective in reducing multiple motor and non-motor symptoms for people with PD. With respect to motor symptoms, our meta-analyses indicate clinically relevant medium effect sizes in favor of TCQ for Part III of the UPDRS, clinical measures of balance, and fall reduction, and a small effect size for TUG. For non-motor outcomes, meta-analyses indicated clinically relevant small-to-medium effect sizes on quality of life and depression. Of note, for Part III of the UPDRS, balance, and quality of life, significant effect sizes persisted even when comparisons were limited to active controls. This finding suggests that the benefits of TCQ are likely to not solely be due to attention or psychosocial support factors, but instead, are the result of mind-body exercise-specific activities. In fact, the results of two trials support that TCQ may be superior to other conventional exercise interventions, though further comparative effectiveness studies are needed to confirm this observation and inform mechanisms leading to relative superiority [46, 51]. Finally, although the evaluation of TCQ’s safety within most trials that we included was not systematically assessed and was poorly described, there were no serious adverse events reported. This finding suggests that TCQ is likely to be safe for people with PD across multiple stages of disease progression [62].
Gaps in previous reviews
The overall conclusions of our study parallel conclusions reported in other recent reviews, but in addition to including updated evidence, the methods employed in our study differed in important ways. First, a number of prior reviews have been limited to qualitative synthesis and have not also included quantitative meta-analytical methods [63, 64]. For studies that did include meta-analysis, one in 2014 focused only on Tai Chi (excluding Qigong) and included 9 trials in quantitative synthesis (vs. 15 in the present study) [7]. Another 2014 meta-analysis focused only on motor outcomes and included 8 trials [13]. A 2015 review that evaluated both Tai Chi and Qigong included 13 RCTs, however, 4 of these were published in Chinese and thus are not included in our review [14]. Finally, one 2016 review including meta-analytic methods employed broader inclusion criteria, synthesizing results from multiple mind-body modalities, including yoga and dance in addition to Tai Chi [12].
Effects of TCQ on motor function
The UPDRS motor subscale was the most commonly assessed measure of motor function in TCQ trials. This is the most widely used clinical scale to measure disease progression and severity and the response of motor function to therapy [65]. In many pharmacology and exercise studies, it is the primary outcome, thus it is worth discussing this outcome in more depth. For Part III of the UPDRS, we found an overall small effect size of −0.44; when analyses were limited to the 6 trials with active controls, effect size remained statistically significant (−0.37). When we narrowed our focus to the 4 randomized trials deemed to have lowest bias in our review (i.e., bias scores ≤1 out of 10), absolute changes in UPDRS following TCQ ranged from −6.4 to −1.5 (average = −3.7). This average magnitude of change meets the threshold for established clinically important differences (minimum of 2.5 for UPDRS to 3.5 for MDS-UPDRS) with variation in reported meaningful differences due to instrument used (UPDRS vs. MDS-UPDRS) and severity of disease progression [66, 67]. The magnitude of effects of TCQ on Part III of the UPDRS that we observed are similar to those reported for physiotherapy [68], but lower than those reported following training in Argentine Tango [69], or for patients taking dopamine medications [57] or undergoing deep brain stimulation [70].
Because of the significant variability in intervention duration for studies evaluating UPDRS motor subscale, we also conducted an exploratory subgroup analysis based on TCQ exposure length. TCQ protocols varied from 5 to 24 weeks across studies. A comparison of shorter-term (12 weeks or less) vs. longer-term (13 weeks or more) protocols indicated effect sizes of −0.121 (p=.625) and −0.426 (p <.001), respectively. This suggest that longer exposures may be more effective, and emphasizes the need for further dosing studies, including studies that explore multiple domains of dosing, such as combined hours of class and home practice and measures of progressions in proficiency of performance [71].
In addition to overall motor performance assessed with part III of the UPDRS, we also found significant improvements in clinical measures of balance and mobility. The 6MW speed also showed trends towards improvement following TCQ training, but this effect was not statistically significant. Only two studies evaluated fall frequency, but given the public health concerns and impact of falls in PD, the positive finding we observed (ES= −.40) warrants comment. Both included trials had low risks of bias. In the smaller of the two studies (n=76) conducted by Gao and colleagues [49], 12 weeks of Tai Chi training resulted in a significant drop in fall incidence assessed 6 months post training, with 21.6% of Tai Chi group reporting falling at least once, compared to 48.7% in the control group. In the study by Li and colleagues [46] (n=195), 24 weeks of Tai Chi training resulted in an average of 12% monthly fall rates in the Tai Chi group (estimated over a 9-month period); in comparison, resistance training and seated stretching controls exhibited 23% and 38% average fall rates, respectively. A subsequent analysis of the data of Li and colleagues concluded that Tai Chi is a cost effective approach for fall prevention in PD [38].
Effects of TCQ on non-motor outcomes
An important finding from this review is that Tai Chi positively impacts multiple non-motor symptoms, in addition to motor symptoms. The most common non-motor outcome evaluated in TCQ trials was QOL assessed in all RCTs using the PDQ-39 [72]. For QOL, we found an overall effect size of −0.39; when analyses were limited to the 3 trials with active controls, effects size actually increased slightly to −0.50. In the largest trial conducted by Li et al [46], QOL in the Tai Chi group improved by 38%; in comparison, QOL in the resistance training and stretching improved by 15% and <1%, respectfully. This is similar to the magnitude of QOL improvements following deep brain stimulation (14–38%) [70]. Meta-analyses also indicated significant improvements in depression. In contrast, the effects of TCQ on cognition was small and not statistically significant, however this estimate was based on 2 small studies [45, 52]. As cognitive deficits are now recognized as an undisputable and highly prevalent feature of PD, both due to natural disease progression and side effects of some medication [73], additional study of the effects of TCQ in cognition are warranted. In particular, given the demonstrated importance of executive function in gait and balance in PD [74, 75], and the potential of Tai Chi to improve executive function in normal aging [34], studies evaluating the impact of TCQ on executive function and dual task performance may be particularly informative. Other outcomes not systematically addressed in TCQ studies to date, but that warrant further study based on the promise of mind-body interventions in other populations, include sleep [76] and autonomic function [77].
It has been suggested that the broad, multi-system and multi-symptom benefits of TCQ may result from its multi-component approach, incorporating training in motor control, postural awareness, cognition, breathing, and stress reduction [8, 78]. In normal aging, some progress has been made in identifying mechanisms of TCQ that may improve balance and gait, and reduce falls, including improving lower extremity strength and flexibility [16–22], proprioception and postural awareness [23–25], neuromuscular coordination and reaction time [26, 27], executive function [28], and reducing fear of falling [29, 30]. Preliminary studies also support a neural basis for enhancing cognitive and motor processes [35, 36]. The mechanistic exploration of how TCQ impacts motor and non-motor symptoms in PD is a rich area for further research.
Methodological issues and limitations
An important methodological decision in this study was to use fixed- vs. random-effects models for meta-analyses. Our decision was based on a number of factors and follows established guidelines [79]. First, we began with the statistical assumption that the sample of studies we evaluated was similar enough to represent the true effect sizes for each outcome assessed in trials of TCQ for PD. This assumption is supported by the facts that within each motor and non-motor outcome, there was strong similarity in instruments used, and that study populations across studies were similar with respect to disease progression. This assumption is also supported by I2 and Q statistics that indicated low between-study heterogeneity in effect size. Finally, it has been suggested that random-models only be used when > 5 studies are included in analyses [79]. As this was not the case in some subgroup analyses, we chose a fixed-effect model approach. While not reported in our results, exploratory analyses of effect sizes for all outcomes based on random models were found to be either equal to or larger than the fixed-effect results we reported, with only minor qualitative differences in statistical significance.
A general limitation of this review relates to the pluralistic nature of TCQ. Both Tai Chi and Qigong are represented by many different styles (e.g. Chen, Yang, Wu or Sun styles of Tai Chi) and within each style there exist many training protocols (e.g. short- vs. long-forms), with some training regimens developed specifically for research purposes. This heterogeneity limits comparisons between, and generalizations across, studies [71]. Additionally, because of the limited number of studies available evaluating TCQ for PD, as well as the heterogeneity of PD populations evaluated across studies, we did not attempt to quantify or draw general conclusions of effectiveness based on specific styles or regimens. As results of additional larger-scale trials become available, future studies should consider individual patient-data meta-analyses that can evaluate the impact of training regimen while controlling for stage of PD progression and other relevant confounding factors [80].
There are a number of methodological limitations with our study. First, our study only included trials published in the English language. Future studies should include other languages for a better global estimate. Second, because of the small number of relatively small studies available to assess most outcomes, these findings should be considered suggestive, but not definitive. This concern is reinforced by limitations in methodological quality and by the heterogeneity of both TCQ interventions and controls. Third, we did not distinguish outcomes that were assessed on and off-medication, as has been done in prior reviews [7, 14]. Fourth, there were too few studies to compare the effects of TCQ on subgroups of PD patients, such as early vs. later disease progression or tremor dominant vs. postural instability and gait difficulty. Future TCQ trials targeting or stratifying by specific PD subgroups would contribute significantly to the current evidence base. Finally, our conclusion that TCQ is ‘likely to be safe for people with PD’ warrants further research. As is the case in trials of many other non-pharmacological therapies, AE reporting in studies of TCQ for PD is poor [62]. Nevertheless, findings of a comprehensive review of 153 Tai Chi studies found that, when conclusions were limited to studies with valid AE monitoring and reporting protocols, Tai Chi was found to be a safe exercise option [62].
Conclusions
RCT evidence supports a potential benefit of TCQ for improving multiple motor and non-motor outcomes for individuals with PD. Multiple studies and small-to-medium effect sizes support clinically meaningful effects in motor function, balance, and quality of life. More limited evidence also supports positive effects on fall risk and depression. However, all findings must be interpreted cautiously due to limitations in both the quantity and quality of available evidence. Additional large, rigorous trials are warranted to better characterize the effects of TCQ in PD and to guide selection of optimal doses and specific protocols for individuals with different PD subtypes and symptom burdens.
Figure 2.
Effects of Tai Chi/Qigong on motor functions
Note 1.data value indicated weight, effect size (Hedges’s g) and confidence interval of LL(lower limit) to UL(upper limit). 2. Plots to the left of zero indicate negative effect sizes all outcomes in favor of Tai Chi (i.e. less symptoms), except for balance where plots to the right of zero represent a positive effect.
Figure 3.
Effects of Tai Chi/Qigong on non-motor functions and quality of life
Note 1.data value indicated weight, effect size (Hedges’s g) and confidence interval of LL(lower limit) to UL(upper limit). 2. Plots to the left of zero indicate negative effect sizes all outcomes in favor of Tai Chi (i.e. less symptoms), except for quality of life where plots to the right of zero represent a positive effect.
Highlights.
Tai Chi/Qigong is a mind-body intervention that has the potential to address motor and non-motor symptoms associated with Parkinson’s disease.
Mixed results for motor outcomes have been reported, while even less attention has been devoted to systematically evaluating the effects of Tai Chi/Qigong on non-motor outcomes.
Our meta-analyses indicate clinically relevant effect sizes in favor of Tai Chi/Qigong for motor function, balance, and quality of life, and significant effect sizes persisted even when comparisons were limited to active controls.
Acknowledgments
Funding sources
This study was supported by the grants from the Davis Phinney Foundation for Parkinson’s, the Osher Center for Integrative Medicine, and Basic Science Research Program through the National Research Foundation of Korea (2013R-1A-1A-2065536).
The authors would like to thank Dr. Choi, H-J and Dr Kang, H-J for providing the subset of their unpublished data of the clinical trial.
Footnotes
Author’s role
| 1. Research project | A) conception B) organization C) execution |
| 2. Statistical analysis | A) design B) execution C) review and critique |
| 3. Manuscript | A) writing of the first draft B) review and critique |
| Rhayun Song, | 1B, 1C, 2A, 2B, 3A |
| Weronika Grabowska, | 1B, 1C |
| Moonkyoung Park, | 1C, 2B |
| Kamila Osypiuk, | 1B, 1C, 3B |
| Gloria P. Vergara Diaz, | 1A, 3B |
| Paolo Bonato, | 1A, 3B Jeffrey |
| Hausdorff, | 1A, 3B |
| Mike Fox, | 1A, 3B |
| Lewis R. Sudarsky, | 1A, 3B |
| Eric A. Macklin, | 2A, 2C, 3B |
| Peter M. Wayne | 1A, 1B, 2A, 2C, 3A, 3B |
Conflict of interest
Financial disclosure: Peter Wayne is the founder and sole owner of the Tree of Life Tai Chi Center. Peter Wayne’s interests were reviewed and managed by the Brigham and Women’s Hospital and Partner’s HealthCare in accordance with their conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bloem BR, de Vries NM, Ebersbach G. Nonpharmacological treatments for patients with Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2015;30:1504–20. doi: 10.1002/mds.26363. [DOI] [PubMed] [Google Scholar]
- 2.Duchesne C, Lungu O, Nadeau A, Robillard ME, Bore A, Bobeuf F, et al. Enhancing both motor and cognitive functioning in Parkinson's disease: Aerobic exercise as a rehabilitative intervention. Brain and cognition. 2015;99:68–77. doi: 10.1016/j.bandc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch MA, Iyer SS, Sanjak M. Exercise-induced neuroplasticity in human Parkinson's disease: What is the evidence telling us? Parkinsonism & related disorders. 2016;22(Suppl 1):S78–81. doi: 10.1016/j.parkreldis.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 4.Suchowersky O, Gronseth G, Perlmutter J, Reich S, Zesiewicz T, Weiner WJ, et al. Practice Parameter: neuroprotective strategies and alternative therapies for Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:976–82. doi: 10.1212/01.wnl.0000206363.57955.1b. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds GO, Otto MW, Ellis TD, Cronin-Golomb A. The Therapeutic Potential of Exercise to Improve Mood, Cognition, and Sleep in Parkinson's Disease. Movement disorders : official journal of the Movement Disorder Society. 2016;31:23–38. doi: 10.1002/mds.26484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim SY, Tan AH, Fox SH, Evans AH, Low SC. Integrating Patient Concerns into Parkinson's Disease Management. Current neurology and neuroscience reports. 2017;17:3. doi: 10.1007/s11910-017-0717-2. [DOI] [PubMed] [Google Scholar]
- 7.Ni X, Liu S, Lu F, Shi X, Guo X. Efficacy and safety of Tai Chi for Parkinson's disease: a systematic review and meta-analysis of randomized controlled trials. PloS one. 2014;9:e99377. doi: 10.1371/journal.pone.0099377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wayne PM, Manor B, Novak V, Costa MD, Hausdorff JM, Goldberger AL, et al. A systems biology approach to studying Tai Chi, physiological complexity and healthy aging: design and rationale of a pragmatic randomized controlled trial. Contemporary clinical trials. 2013;34:21–34. doi: 10.1016/j.cct.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein PJ, Rivers L. Taiji for individuals with Parkinson disease and their support partners: a program evaluation. Journal of neurologic physical therapy : JNPT. 2006;30:22–7. doi: 10.1097/01.npt.0000282146.18446.f1. [DOI] [PubMed] [Google Scholar]
- 10.Jahnke R, Larkey L, Rogers C, Etnier J, Lin F. A comprehensive review of health benefits of qigong and tai chi. American journal of health promotion : AJHP. 2010;24:e1–e25. doi: 10.4278/ajhp.081013-LIT-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Payne P, Crane-Godreau MA. Meditative movement for depression and anxiety. Frontiers in psychiatry. 2013;4:71. doi: 10.3389/fpsyt.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwok JY, Choi KC, Chan HY. Effects of mind-body exercises on the physiological and psychosocial well-being of individuals with Parkinson's disease: A systematic review and meta-analysis. Complementary therapies in medicine. 2016;29:121–31. doi: 10.1016/j.ctim.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Li XY, Gong L, Zhu YL, Hao YL. Tai Chi for improvement of motor function, balance and gait in Parkinson's disease: a systematic review and meta-analysis. PloS one. 2014;9:e102942. doi: 10.1371/journal.pone.0102942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Qiu WQ, Hao YL, Lv ZY, Jiao SJ, Teng JF. The efficacy of traditional Chinese Medical Exercise for Parkinson's disease: a systematic review and meta-analysis. PloS one. 2015;10:e0122469. doi: 10.1371/journal.pone.0122469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu WM, Lin RJ, Yu RL, Tai CH, Lin CH, Wu RM. The impact of nonmotor symptoms on quality of life in patients with Parkinson's disease in Taiwan. Neuropsychiatric disease and treatment. 2015;11:2865–73. doi: 10.2147/NDT.S88968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Audette JF, Jin YS, Newcomer R, Stein L, Duncan G, Frontera WR. Tai Chi versus brisk walking in elderly women. Age Ageing. 2006;35:388–93. doi: 10.1093/ageing/afl006. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson B, Chen H, Cashel C. The effect of Tai Chi Chuan training on balance, kinesthetic sense, and strength. Percept Mot Skills. 1997;84:27–33. doi: 10.2466/pms.1997.84.1.27. [DOI] [PubMed] [Google Scholar]
- 18.Lan C, Lai J, Wong M. 12-month Tai Chi training in the elderly: its effect on health fitness. Med Sci Sports Exerc. 1998;30:345–51. doi: 10.1097/00005768-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Lan C, Lai J, Chen S, Wong M. Tai Chi Chuan to improve muscular strength and endurance in elderly individuals: a pilot study. Archives of physical medicine and rehabilitation. 2000;81:601–7. doi: 10.1016/s0003-9993(00)90042-x. [DOI] [PubMed] [Google Scholar]
- 20.Lan C, Lai J, Wong M. Cardiorespiratory function, flexibility, and body composition among geriatric Tai Chi Chuan practitioners. Archives of physical medicine and rehabilitation. 1996;77:612–6. doi: 10.1016/s0003-9993(96)90305-6. [DOI] [PubMed] [Google Scholar]
- 21.Li JX, Xu DQ, Hong Y. Changes in muscle strength, endurance, and reaction of the lower extremities with Tai Chi intervention. Journal of biomechanics. 2009;42:967–71. doi: 10.1016/j.jbiomech.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Wu G, Zhao F, Zhou X, Wei L. Improvement of isokinetic knee extensor strength and reduction of postural sway in the elderly from long-term Tai Chi exercise. Archives of physical medicine and rehabilitation. 2002;83:1364–9. doi: 10.1053/apmr.2002.34596. [DOI] [PubMed] [Google Scholar]
- 23.Kerr CE, Shaw JR, Wasserman RH, Chen VW, Kanojia A, Bayer T, et al. Tactile acuity in experienced Tai Chi practitioners: evidence for use dependent plasticity as an effect of sensory-attentional training. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2008;188:317–22. doi: 10.1007/s00221-008-1409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manor B, Lipsitz LA, Wayne PM, Peng CK, Li L. Complexity-based measures inform tai chi's impact on standing postural control in older adults with peripheral neuropathy. BMC complementary and alternative medicine. 2013;13:87. doi: 10.1186/1472-6882-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobson BH, Chen HC, Cashel C, Guerrero L. The effect of T'ai Chi Chuan training on balance, kinesthetic sense, and strength. Percept Mot Skills. 1997;84:27–33. doi: 10.2466/pms.1997.84.1.27. [DOI] [PubMed] [Google Scholar]
- 26.Hass CJ, Gregor RJ, Waddell DE, Oliver A, Smith DW, Fleming RP, et al. The influence of Tai Chi training on the center of pressure trajectory during gait initiation in older adults. Archives of physical medicine and rehabilitation. 2004;85:1593–8. doi: 10.1016/j.apmr.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 27.McGibbon CA, Krebs DE, Parker SW, Scarborough DM, Wayne PM, Wolf SL. Tai Chi and vestibular rehabilitation improve vestibulopathic gait via different neuromuscular mechanisms: preliminary report. BMC neurology. 2005;5:3. doi: 10.1186/1471-2377-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wayne PM, Walsh JN, Taylor-Piliae RE, Wells RE, Papp KV, Donovan NJ, et al. Effect of tai chi on cognitive performance in older adults: systematic review and meta-analysis. Journal of the American Geriatrics Society. 2014;62:25–39. doi: 10.1111/jgs.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sattin RW, Easley KA, Wolf SL, Chen Y, Kutner MH. Reduction in fear of falling through intense tai chi exercise training in older, transitionally frail adults. Journal of the American Geriatrics Society. 2005;53:1168–78. doi: 10.1111/j.1532-5415.2005.53375.x. [DOI] [PubMed] [Google Scholar]
- 30.Logghe IH, Verhagen AP, Rademaker AC, Bierma-Zeinstra SM, van Rossum E, Faber MJ, et al. The effects of Tai Chi on fall prevention, fear of falling and balance in older people: A meta-analysis. Preventive Medicine. 2010;51:222–7. doi: 10.1016/j.ypmed.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Tsang HW, Fung KM. A review on neurobiological and psychological mechanisms underlying the anti-depressive effect of qigong exercise. Journal of health psychology. 2008;13:857–63. doi: 10.1177/1359105308095057. [DOI] [PubMed] [Google Scholar]
- 32.Hall AM, Kamper SJ, Emsley R, Maher CG. Does pain-catastrophising mediate the effect of tai chi on treatment outcomes for people with low back pain? Complementary therapies in medicine. 2016;25:61–6. doi: 10.1016/j.ctim.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Tsang HW, Chan EP, Cheung WM. Effects of mindful and non-mindful exercises on people with depression: a systematic review. The British journal of clinical psychology. 2008;47:303–22. doi: 10.1348/014466508X279260. [DOI] [PubMed] [Google Scholar]
- 34.Wayne PM, Hausdorff JM, Lough M, Gow BJ, Lipsitz L, Novak V, et al. Tai Chi Training may Reduce Dual Task Gait Variability, a Potential Mediator of Fall Risk, in Healthy Older Adults: Cross-Sectional and Randomized Trial Studies. Frontiers in human neuroscience. 2015;9:332. doi: 10.3389/fnhum.2015.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li R, Zhu X, Yin S, Niu Y, Zheng Z, Huang X, et al. Multimodal intervention in older adults improves resting-state functional connectivity between the medial prefrontal cortex and medial temporal lobe. Frontiers in aging neuroscience. 2014;6:39. doi: 10.3389/fnagi.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng Z, Zhu X, Yin S, Wang B, Niu Y, Huang X, et al. Combined cognitive-psychological-physical intervention induces reorganization of intrinsic functional brain architecture in older adults. Neural plasticity. 2015;2015:713104. doi: 10.1155/2015/713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurlan R, Evans R, Wrigley S, McPartland S, Bustami R, Cottor A. Tai Chi in Parkinson's disease: A preliminary randomized, controlled, and rater-blinded study. Advances in Parkinson's Disease. 2015;4:9–12. [Google Scholar]
- 38.Li F, Harmer P. Economic Evaluation of a Tai Ji Quan Intervention to Reduce Falls in People With Parkinson Disease, Oregon, 2008–2011. Preventing chronic disease. 2015;12:E120. doi: 10.5888/pcd12.140413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wassom DJ, Lyons KE, Pahwa R, Liu W. Qigong exercise may improve sleep quality and gait performance in Parkinson's disease: a pilot study. The International journal of neuroscience. 2015;125:578–84. doi: 10.3109/00207454.2014.966820. [DOI] [PubMed] [Google Scholar]
- 40.Xiao CM, Zhuang YC. Effect of health Baduanjin Qigong for mild to moderate Parkinson's disease. Geriatrics & gerontology international. 2016;16:911–9. doi: 10.1111/ggi.12571. [DOI] [PubMed] [Google Scholar]
- 41.Zhang TY, Hu Y, Nie ZY, Jin RX, Chen F, Guan Q, et al. Effects of Tai Chi and Multimodal Exercise Training on Movement and Balance Function in Mild to Moderate Idiopathic Parkinson Disease. American journal of physical medicine & rehabilitation. 2015;94:921–9. doi: 10.1097/PHM.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 42.Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj. 2016;354:i4086. doi: 10.1136/bmj.i4086. [DOI] [PubMed] [Google Scholar]
- 43.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. 2011. [Google Scholar]
- 44.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC medical research methodology. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi HJ. Effects of therapeutic Tai chi on functional fitness and activities of daily living in patients with Parkinson disease. Journal of exercise rehabilitation. 2016;12:499–503. doi: 10.12965/jer.1632654.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li F, Harmer P, Fitzgerald K, Eckstrom E, Stock R, Galver J, et al. Tai chi and postural stability in patients with Parkinson's disease. The New England journal of medicine. 2012;366:511–9. doi: 10.1056/NEJMoa1107911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li F, Harmer P, Liu Y, Eckstrom E, Fitzgerald K, Stock R, et al. A randomized controlled trial of patient-reported outcomes with tai chi exercise in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2014;29:539–45. doi: 10.1002/mds.25787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amano S, Nocera JR, Vallabhajosula S, Juncos JL, Gregor RJ, Waddell DE, et al. The effect of Tai Chi exercise on gait initiation and gait performance in persons with Parkinson's disease. Parkinsonism & related disorders. 2013;19:955–60. doi: 10.1016/j.parkreldis.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao Q, Leung A, Yang Y, Wei Q, Guan M, Jia C, et al. Effects of Tai Chi on balance and fall prevention in Parkinson's disease: a randomized controlled trial. Clinical rehabilitation. 2014;28:748–53. doi: 10.1177/0269215514521044. [DOI] [PubMed] [Google Scholar]
- 50.Gladfelter BA. Evidence-Based Practice Project Reports. Valparaiso, IN, USA: Valparaiso University; 2011. The effect of Tai Chi exercise on balance and falls in persons with Parkinson's. [Google Scholar]
- 51.Hackney ME, Earhart GM. Health-related quality of life and alternative forms of exercise in Parkinson disease. Parkinsonism & related disorders. 2009;15:644–8. doi: 10.1016/j.parkreldis.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nocera JR, Amano S, Vallabhajosula S, Hass CJ. Tai Chi Exercise to Improve Non-Motor Symptoms of Parkinson's Disease. Journal of yoga & physical therapy. 2013:3. doi: 10.4172/2157-7595.1000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burini D, Farabollini B, Iacucci S, Rimatori C, Riccardi G, Capecci M, et al. A randomised controlled cross-over trial of aerobic training versus Qigong in advanced Parkinson's disease. Europa medicophysica. 2006;42:231–8. [PubMed] [Google Scholar]
- 54.Cheon SM, Chae BK, Sung HR, Lee GC, Kim JW. The Efficacy of Exercise Programs for Parkinson's Disease: Tai Chi versus Combined Exercise. Journal of clinical neurology (Seoul, Korea) 2013;9:237–43. doi: 10.3988/jcn.2013.9.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hackney ME, Earhart GM. Tai Chi improves balance and mobility in people with Parkinson disease. Gait & posture. 2008;28:456–60. doi: 10.1016/j.gaitpost.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmitz-Hubsch T, Pyfer D, Kielwein K, Fimmers R, Klockgether T, Wullner U. Qigong exercise for the symptoms of Parkinson's disease: a randomized, controlled pilot study. Movement disorders : official journal of the Movement Disorder Society. 2006;21:543–8. doi: 10.1002/mds.20705. [DOI] [PubMed] [Google Scholar]
- 57.Zhao S, Cheng R, Zheng J, Li Q, Wang J, Fan W, et al. A randomized, double-blind, controlled trial of add-on therapy in moderate-to-severe Parkinson's disease. Parkinsonism & related disorders. 2015;21:1214–8. doi: 10.1016/j.parkreldis.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 58.Kim HD, Jae HD, Jeong JH. Tai Chi Exercise can Improve the Obstacle Negotiating Ability of People with Parkinson's Disease: A Preliminary Study. Journal of physical therapy science. 2014;26:1025–30. doi: 10.1589/jpts.26.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim H-D, Kim T-Y, Jae HD, Son S-T. The effect of Tai Chi based exercise on dynamic postural control of Parkinson's disease patients while initiating gait. Journal of physical therapy science. 2011;23:265–69. [Google Scholar]
- 60.Loftus SL. Qigong to improve postural stability for Parkinson fall prevention. Topics in Geriatric Rehabilitation. 2014;30:58–69. [Google Scholar]
- 61.Li F, Harmer P, Fisher KJ, Xu J, Fitzgerald K, Vongjaturapat N. Tai Chi-based exercise for older adults with Parkinson's disease: a pilot-program evaluation. Journal of aging and physical activity. 2007;15:139–51. doi: 10.1123/japa.15.2.139. [DOI] [PubMed] [Google Scholar]
- 62.Wayne PM, Berkowitz DL, Litrownik DE, Buring JE, Yeh GY. What do we really know about the safety of tai chi?: A systematic review of adverse event reports in randomized trials. Archives of physical medicine and rehabilitation. 2014;95:2470–83. doi: 10.1016/j.apmr.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee MS, Lam P, Ernst E. Effectiveness of tai chi for Parkinson's disease: a critical review. Parkinsonism & related disorders. 2008;14:589–94. doi: 10.1016/j.parkreldis.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 64.Toh SFM. A systematic review on the effectiveness of Tai Chi exercise in individuals with Parkinson's disease from 2003 to 2013. Hong Kong Journal of Occupational Therapy. 2013;23:69–81. [Google Scholar]
- 65.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement disorders : official journal of the Movement Disorder Society. 2008;23:2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 66.Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the unified Parkinson's disease rating scale. Archives of neurology. 2010;67:64–70. doi: 10.1001/archneurol.2009.295. [DOI] [PubMed] [Google Scholar]
- 67.Horvath K, Aschermann Z, Acs P, Deli G, Janszky J, Komoly S, et al. Minimal clinically important difference on the Motor Examination part of MDS-UPDRS. Parkinsonism & related disorders. 2015;21:1421–6. doi: 10.1016/j.parkreldis.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 68.Tomlinson CL, Herd CP, Clarke CE, Meek C, Patel S, Stowe R, et al. Physiotherapy for Parkinson's disease: a comparison of techniques. The Cochrane database of systematic reviews. 2014:CD002815. doi: 10.1002/14651858.CD002815.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lotzke D, Ostermann T, Bussing A. Argentine tango in Parkinson disease--a systematic review and meta-analysis. BMC neurology. 2015;15:226. doi: 10.1186/s12883-015-0484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xie CL, Shao B, Chen J, Zhou Y, Lin SY, Wang WW. Effects of neurostimulation for advanced Parkinson's disease patients on motor symptoms: A multiple-treatments meta-analysas of randomized controlled trials. Scientific reports. 2016;6:25285. doi: 10.1038/srep25285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wayne PM, Kaptchuk TJ. Challenges inherent to t'ai chi research: part II-defining the intervention and optimal study design. Journal of alternative and complementary medicine. 2008;14:191–7. doi: 10.1089/acm.2007.7170b. [DOI] [PubMed] [Google Scholar]
- 72.Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 1995;4:241–8. doi: 10.1007/BF02260863. [DOI] [PubMed] [Google Scholar]
- 73.Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease: a 12-year population study. Neurology. 2008;70:1017–22. doi: 10.1212/01.wnl.0000306632.43729.24. [DOI] [PubMed] [Google Scholar]
- 74.Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson's disease: a review. Journal of neuropsychology. 2013;7:193–224. doi: 10.1111/jnp.12028. [DOI] [PubMed] [Google Scholar]
- 75.Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson's disease: which aspects of gait are attention demanding? The European journal of neuroscience. 2005;22:1248–56. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 76.Wu WW, Kwong E, Lan XY, Jiang XY. The Effect of a Meditative Movement Intervention on Quality of Sleep in the Elderly: A Systematic Review and Meta-Analysis. Journal of alternative and complementary medicine. 2015;21:509–19. doi: 10.1089/acm.2014.0251. [DOI] [PubMed] [Google Scholar]
- 77.Lu WA, Kuo CD. Breathing frequency-independent effect of Tai Chi Chuan on autonomic modulation. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2014;24:47–52. doi: 10.1007/s10286-014-0224-3. [DOI] [PubMed] [Google Scholar]
- 78.Wayne PM, Krebs DE, Wolf SL, Gill-Body KM, Scarborough DM, McGibbon CA, et al. Can Tai Chi improve vestibulopathic postural control? Archives of physical medicine and rehabilitation. 2004;85:142–52. doi: 10.1016/s0003-9993(03)00652-x. [DOI] [PubMed] [Google Scholar]
- 79.Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. International journal of evidence-based healthcare. 2015;13:196–207. doi: 10.1097/XEB.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 80.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. Bmj. 2010;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]