Abstract
Despite the success of combination antiretroviral therapy (cART) for suppressing HIV and improving patients’ quality of life, HIV persists in cART-treated patients and remains an incurable disease. Financial burdens and health consequences of lifelong cART treatment call for novel HIV therapies that result in a permanent cure. Cellular immunity is central in controlling HIV replication. However, HIV adopts numerous strategies to evade immune surveillance. Engineered immunity via genetic manipulation could offer a functional cure by generating cells that have enhanced antiviral activity and are resistant to HIV infection. Recently, encouraging reports from several human clinical trials using an anti-CD19 chimeric antigen receptor (CAR) modified T-cell therapy for treating B-cell malignancies have provided valuable insights and generated remarkable enthusiasm in engineered T-cell therapy. In this review, we discuss the development of HIV-specific chimeric antigen receptors and the use of stem cell based therapies to generate lifelong anti-HIV immunity.
Keywords: : Chimeric antigen receptors, engineered immunity; HIV cytotoxic T lymphocytes (CTL); HIV infection; HIV therapy; stem cell-based gene therapy
Chimeric antigen receptors & lessons from cancer immune therapies
The primary purpose of a chimeric antigen receptor (CAR) is to direct or redirect a T cell to specifically target an antigen of interest. CARs are hybrid receptors consisting of an extracellular antigen-binding domain linked to an intracellular T-cell receptor (TCR) stimulatory domain, most commonly CD3-ζ [1–4]. The target-binding domain is often made up of a single chain variable fragment (scFv) derived from a monoclonal antibody. However, the target-binding domain is not restricted to scFv as long as it offers recognition and ligand binding to target cells. Examples of these non-scFv target-binding domains include the use of the CD4 molecule, which is the primary receptor for HIV envelope protein, gp120 binding [5,6] and the use of an IL-13 mutein that can bind to the IL-13 receptor on the target cell [7,8].
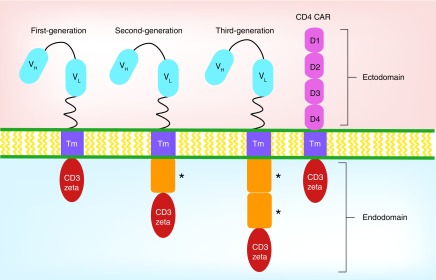
Genetic modification of T cells with a synthetic chimeric TCR was first reported in 1989. Cytotoxic T cells were made to express a receptor containing an antibody portion that recognized the 2,4,6-trinitrophenyl hapten, enabling these T cells to become activated and kill 2,4,6-trinitrophenyl expressing target cells in an MHC-independent manner [2]. This paved the way for generating T cells with a desired specificity using chimeric receptors and testing their efficacy in the clinical setting, such as, cancer and HIV. Today, there are three generations of CARs that have been developed so far (Figure 1). The first generation consists of an extracellular antigen recognition ectodomain derived from an scFv, a transmembrane domain and an intracellular signaling endodomain containing the signaling portion of the CD3-ζ chain [1,3,9–10]. Upon engagement of the ectodomain to its ligand, the signal is transmitted to the intracellular signaling domain of the CD3-ζ chain. The addition of a costimulatory molecule, such as, CD28 or CD137 (4–1BB) created the second generation of CARs and this proved to enhance CAR T-cell responses [11,12]. The third generation of CARs carry more than one costimulatory molecule in the endodomain [3] and have shown robust antitumor efficacy in preclinical models [13–15]. Third generation CARs have begun clinical trials testing for safety and therapeutic efficacy [16,17]. Whether second or third generation of anti-HIV CARs will have any antiviral efficiency in clinical trials remains to be determined.
Figure 1. . Design of chimeric antigen receptor.
CAR design consists of an ectodomain, transmembrane domain and an endodomain. Ectodomain is derived from either a single-chain variable fragment of heavy and light chains of a monoclonal antibody or the extracellular portion of the CD4 molecule for the CD4-based CAR. The endodomain contains the intracellular signaling portion of the receptor that is derived from the CD3-ζ chain of the T-cell receptor and either one (second generation) or two (third generation) costimulatory domains (*).
CAR: Chimeric antigen receptor.
An important benefit of using a CAR to redirect immunity, as compared with molecularly cloned TCR, is that CAR-expressing T cells have the ability to become activated and kill targeted cells upon binding to its target surface antigen independent of the MHC [1,3,9]. Thus, an advantage of CAR T-cell therapy is that it is less susceptible to escape mechanisms, such as, downregulation of MHC class I molecules, commonly used by tumor cells [18] and HIV-infected cells [19,20]. Because MHC class I recognition is not required for the engagement of CAR receptor, CAR T-cell therapy can be widely applied to patients of various HLA subtypes.
Early studies in treatment of B-cell malignancy with first-generation CARs targeting the CD19 molecule, a common B-cell antigen that is expressed on both malignant and nonmalignant B cells, showed limited antitumor effects [10]. Second-generation CD28-containing CD19 CARs had better success in the clinic [21] as did a 4–1BB-containing second-generation CD19 CAR that was used to treat relapsed B-cell chronic lymphocytic leukemia [22,23]. The 4–1BB-containing CAR T cells had a greater than 1000-fold expansion, trafficked to bone marrow, and continued to express functional CARs at high levels for at least 6 months. As a result, impressive clinical outcomes were obtained, with two long-term complete remissions and one prolonged remission despite large tumor burdens. However, CD19 CAR T-cell therapy has reported significant adverse events in patients, including cytokine release syndrome, macrophage activation syndrome and B-cell aplasia [9–10,24]. The mechanism(s) by which CAR mediates optimal T-cell activation remains incompletely elucidated and it is conceivable that optimal CAR design in different clinical settings may require different combinations of ligand binding, transmembrane, stimulatory and costimulatory domains to optimize efficacy and minimize side effects [3,9].
The addition of a costimulatory domain in the design of the CAR has dramatically improved the efficacy of certain CARs when expressed in T cells. More recently, a focus has been on exploring other ways to enhance CAR T-cell responses in vivo. The utilization of specific T-cell effector/memory subsets with better inherent abilities for proliferation and persistence can also enhance efficacy. A recent study using a murine model showed that CD19 CAR T cells derived from CD4+ naive and CD8+ central memory subsets conferred the strongest antitumor effects compared with CD19 CAR T cells derived from peripheral blood mononuclear cells (PBMC) or from effector memory subsets [25]. These results led to a Phase I clinical trial using CD19 CAR T cells of defined CD4+ and CD8+ subset composition that resulted in promising clinical outcomes with 93% of adults with B-cell acute lymphoblastic leukemia achieving bone marrow remission [26]. Moreover, using a subset composition of a defined CD4+:CD8+ T-cell ratio at a single low dose of 2 × 105 T cells/kg was effective for patients with high tumor burden to undergo complete remission without high toxicity. It was discovered that patients who showed disease relapse due to loss of CAR-expressing T cells in the blood generated an immune response against the CD19 CAR, thus providing a possible mechanism for loss of CAR T cells seen in other clinical trials. This is the first clinical trial to report the use of defined T-cell subsets to manufacture CD19 CAR T cells and this is likely to be an important aspect of CAR-based therapy in the future.
Another subset of T cells that has shown great promise with in vivo persistence and antitumor potency are the ‘T memory stem cells’ (TSCM) [27]. Ex vivo culturing conditions using the cytokines, IL-7 and IL-15 in cultures of patient PBMCs have found to provide better expansion than IL-2 alone and generate T cells with a more stem/central memory phenotype [28–32]. In a clinical setting utilizing CD19 CAR T cells cultured in IL-7 and IL-15, it was shown that the frequency of CD8+ T cells that phenotypically resembled TSCM correlated with CAR T-cell expansion in patients with relapsed B-cell malignancies [28]. It still remains to be determined whether these TSCM and their effectiveness to expand in vivo can lead to greater clinical outcome, but it is likely that further characterization of the use of different T-cell subsets in CAR-based therapy will optimize therapeutic strategies. Whether TSCM will be an important subset to generate potent anti-HIV CAR T-cell responses for HIV still needs to be evaluated. However, it has recently been shown that CD4+ TSCM are permissive to HIV infection and can support long-term HIV persistence even during suppressive antiretroviral therapy (ART) [33,34]. In addition, it has been recently found that HIV-1 specific CD8 TSCM populations have shown to be compromised during chronic HIV infection, but restored during ART [35]. Moreover, HIV-1 specific CD8 TSCM retained ability to produce IL-2 in response to viral antigen, however, there was no association between frequency of HIV-1 specific CD8 TSCM and CD4 T-cell counts or viral load during untreated HIV infection, suggesting that they are not directly involved in antiviral immune defense [35]. Nevertheless, the use of CD8 TSCM in CAR T-cell therapy for HIV could be a beneficial subset to utilize in order to promote and maintain a memory pool of redirected CD8+ anti-HIV CAR T cells for lifelong control of viral replication and perhaps eradication of residual reservoirs.
CAR T-cell therapy for HIV infection: lessons from CD4-ζ CAR T-cell therapy
The development of CARs for HIV was first reported more than 20 years ago [5,6]. These studies initially created and characterized two different CARs, one containing an scFv derived from the anti-gp41 monoclonal antibody clone 98–6, while the other one containing a CAR composed of the extracellular and transmembrane domains of a CD4 receptor fused to a CD3-ζ chain (termed the CD4-ζ CAR). Upon binding to HIV envelope protein, these CARs were capable of triggering T-cell activation, proliferation and cytokine production in vitro. Most importantly, CAR-modified CD8+ T cells showed similar killing ability of infected target cells as compared with other HIV-specific cytotoxic T lymphocyte clones and were capable of suppressing diverse HIV-1 strains in primary lymphocytes [5–6,36].
Gammaretroviral vector-based CD4-ζ CAR T-cell clinical trials were carried out to evaluate the efficacy of CD4-ζ CAR expressed in autologous CD4+ and CD8+ T cells in patients with active viremia or in patients who were on combination ART (cART) that had undetectable plasma viral RNA [37–39]. Importantly, these studies determined that CD4-ζ CAR therapy had minimal toxicities and no serious adverse events were reported. However, the infusion of gene-modified T cells resulted in no or very modest reductions of HIV plasma RNA or blood HIV DNA levels. Interestingly, decade-long follow-up studies of PBMCs collected from 43 subjects revealed that the transfused CD4-ζ CAR modified T cells had remarkably long half-lives (>16 years) [40]. This is in contrast to other first-generation CARs, which tended to have poor persistence of the gene marked transfused T cells. To account for this long-term, stable persistence of CD4-ζ CAR T cells in these patients, it was speculated that these cells periodically encountered HIV envelope during bursts of viral replication and received continuous, low-level stimulation by binding to its low-affinity ligand, the MHC class II molecule [40].
Despite the persistent presence of CD4-ζ CAR T cells in the subjects, it was reported that there was no significant expansion of CD4-ζ CAR T cells upon infusion, even in viremic patients [37,38]. This likely explains the lack of antiviral efficacy. There remains a variety of ways to improve on these results to make this type of approach more efficacious. Inclusion of costimulatory domains and the development of the CD4-ζ CAR into second- and third-generation CARs may enhance proliferation and killing activity of these cells [3,9]. Also, better cell-handling procedures utilized today in the genetic modification of PBMCs would likely increase antiviral efficacy of these modified cells (see above). Interestingly, one overlooked issue with these studies was the expression of the CD4-ζ CAR on activated T cells can render them highly susceptible to HIV infection and subsequent viral-mediated elimination [41–43]. This may have been one of the major reasons for the limited clinical efficacy observed in these studies. One strategy to solve this problem is to include anti-HIV reagents to protect CD4-ζ CAR T cells from HIV-1 infection. For example, the use of protective anti-HIV shRNAs can be engineered to be coexpressed from the same lentiviral vector expressing the CAR, resulting in protection of the cell from direct infection through the CD4 extracellular domain [41,44]. shRNA-mediated knockdown of the HIV coreceptor, CCR5 can also effectively be used to reduce CCR5 expression on CD4-ζ CAR T cells, therefore protecting the cells from infection with R5 tropic HIV-1 viruses [41,43]. Other anti-HIV reagents, such as, anti-HIV shRNAs and coexpression of the anti-HIV fusion peptide, C46 can also be used in combination with the anti-CCR5 shRNA to prevent infection by X4 tropic or dual tropic viruses [41,45–46].
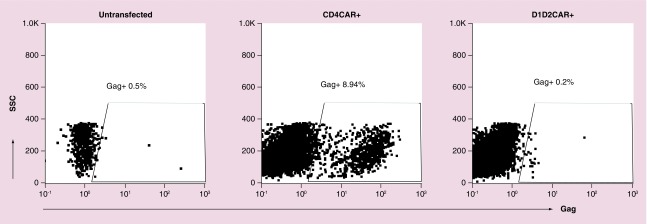
Another strategy to prevent infection of CD4-ζ CAR T cells is to modify the antigen recognition part of the CAR molecule to prevent it from allowing HIV infection. To initiate entry, HIV envelope protein first attaches to the CD4 on the host cell, which leads to conformational changes in envelope that are necessary for coreceptor binding and subsequent viral fusion [45,47]. The first two domains of the CD4 extracellular domains: D1D2 domain, primarily mediate HIV envelope binding to the CD4 molecule [47]. We found that simply deleting D3–D4 domains of CD4-ζ CAR can prevent CD4-mediated HIV infection of CD4-ζ CAR T cells but still allows envelope binding and signaling through the CD4 D1D2 domain (Figure 2). A recent report by Liu et al. [42] described a novel CD4-based bispecific CAR that consists of CD4D1D2 domain linked to an scFv of the 17b anti-HIV envelope neutralizing human monoclonal antibody that recognized a highly conserved epitope on gp120. The authors tested different lengths of linker peptide between the CD4D1D2 and 17b and found that a ten-peptide linker (CD4–10–17b) CAR has the highest potency. Interestingly, both CD4–17b CARs tested were devoid of the unwanted property of CD4-ζ CAR rendering the transduced CD8+ T cells susceptible to HIV infection [42]. However, in contrast to our results, they found the standard CD4D1D2 CAR was susceptible to HIV infection. Further studies need to be done to verify the susceptibility or resistance of a truncated CD4-based CAR to HIV infection in vivo.
Figure 2. . D1D2 chimeric antigen receptor does not allow infection of HIV in HOS CXCR4 cells.
HOS CXCR4 cells were transfected with either CD4CAR, CD4D1D2CAR or left untransfected for 2 days and infected by NL4–3 (X4 tropic) for 4 days. Cells were intracellularly stained with anti-Gag (KC57 PE) for detection of HIV-infected cells.
Another way to improve a CAR approach to HIV infection is the modification of the ectodomain of CAR, which mediates antigen recognition. T cells expressing CARs containing high-affinity scFv appear to elicit superior effector functions than those containing low-affinity scFvs [9]. HIV-specific broad neutralizing antibodies, identified from HIV-infected nonprogressors, can elicit potent neutralizing responses against a variety of HIV strains [48]. These HIV-specific broad neutralizing antibodies can be engineered into single-chain antibodies and then fused with the ζ domain with or without second- and third-generation CAR costimulatory domains to target HIV infection. The growing number of HIV broadly neutralizing antibodies (bNAbs) offers alternative opportunities for creating novel CARs that can be potentially more active and effective. A recent report by Ali et al. constructed and tested seven HIV-specific CARs based on well-defined HIV-1 bNAbs. The authors found that each novel CAR conferred potent antiviral activities to transduced CD8+ T cells against HIV-infected cells in vitro [49]. A VRC01 HIV specific bNAb-based third-generation CAR not only conferred antiviral activity to transduced CD8 T cells but also effectively induced cytolysis of reactivated latently infected CD4+ T cells isolated from infected individuals on cART treatment [50]. This demonstrates the potential use of the CAR therapy for the eradication of reactivated latent HIV-1 reservoir by latency-reversing agents, which is also under intensive investigations.
Stem cell based CAR therapy for redirecting anti-HIV immunity
Hematopoietic stem cell (HSC) based therapy offers a promising alternative to adoptive T-cell therapies as it can provide long-term treatment that is crucial for achieving a ‘functional cure’ for HIV infection. When engrafted successfully, modified HSCs can offer long-term, stable and continuous production of genetically modified cells. Combination of two different approaches has been applied utilizing HSC-based therapies targeted at eradicating HIV. One approach modifies developing immune cells to produce cells that are resistant to HIV infection while another redirects cells to target and kill HIV-infected cells. Multiple studies have attempted to modify HSCs and disrupt CCR5 expression in order to block HIV/SIV infection [43,51–54]. When transplanted, the modified HSCs can differentiate into multiple lineages, including both CD4 and CD8 T cells that lack or have decreased expression of CCR5 receptor. This renders them resistant to R5 tropic HIV infection. Autologous transplant of these HSCs can lead to decreased or controlled HIV-1 viral replication as well as a selection and expansion/reconstitution of HIV-resistant cells in a humanized mouse model of HIV infection [43].
To generate engineered immunity from HSCs, we and others showed that HSCs modified with a molecular clone of an HIV-specific TCR can successfully differentiate into functional T cells that recognize HIV-infected cells in the humanized mouse model [54–56]. In addition to achieving successful engraftment and T-cell development, introduction of a cloned exogenous TCR could shut down endogenous TCR rearrangement during thymopoiesis, thereby eliminating the risk of TCR mispairing between endogenous and exogenous TCRs and generation of self-activating T cells [56].
Recently, we found that anti-HIV immunity can be derived from HSCs modified with a protective CD4-ζ CAR that contains shRNAs against CCR5 and HIV-1 LTR (Triple CD4-ζ CAR) [41]. Triple CD4-ζ CAR modified stem cells engrafted successfully in humanized mice and differentiated into multiple hematopoietic lineages, including both CD4+ and CD8+ T cells, NK cells, B cells and monocytes. Naive Triple CD4-ζ CAR T cells that differentiated from modified HSCs are functional and resistant to HIV infection. When primed properly, Triple CD4-ζ CAR T cells proliferate and suppress HIV infection in vivo and develop into effector and memory T cells [41]. Similar to TCR-modified HSCs, introduction of CD4-ζ CAR resulted in suppression of endogenous TCR recombination in some cells during thymopoiesis. This is likely caused by the extracellular CD4 domain interaction with HLA class II that is expressed by the thymic stroma, which triggers positive selection of the cells. We found that cells that express the highest levels of CD4-ζ CAR have the lowest level of CD3 and lower levels of the TCR excision circles than cells having lower or no CAR levels. Since CD3 expression is dependent on the expression of a functional TCR, this strongly suggests that the cells that had the strongest signaling via the ζ chain turned off endogenous TCR arrangement and thus lacked TCR and CD3 expression on the surface. However, lack of CD3 expression on CAR-bearing T cells did not affect cytokine production of these cells, suggesting that signaling through the ζ was sufficient for the CAR T cells to function [41].
In addition to the development of CD4-ζ CAR T cells, we detected CD4-ζ CAR NK cells that might have also contributed to viral suppression [41]. NK cells, in addition to T cells, express the intracellular signaling components to allow functional signaling through the ζ chain of the CAR molecule. Previous studies have shown that CD4-ζ CAR modified NK cells can be used to kill HIV-infected cells in vitro [57]. Therefore, the development of CD4-ζ CAR NK cells can provide a constant source of rapid innate responses against HIV-infected cells and further augment the anti-HIV immunity.
In summary, CAR-bearing T cells have been developed from genetically modified HSCs through natural thymopoiesis, which eliminated self-reactive T cells and limited off-target effects. Importantly, because naive CD4-ζ CAR T cells have a high ability to proliferate under priming and antigen stimulation, it is not necessary to reconstitute the bone marrow with high levels of HSCs to achieve viral suppression. This is in contrast to HSC-based gene therapy protecting cells from infection, in which high level of HSCs replacement and ultimate peripheral cell reconstitution with modified HSCs are necessary to have significant effects on viral replication and allow better immune reconstitution [53].
Conclusion & future perspective
Since the description of the CAR concept 25 years ago for cell-based therapy, many iterative modifications have been made based on the ‘first-generation’ CAR. This includes adding one or two costimulatory domain(s) (termed second- and third-generation CAR) to the ζ chain to improve the signaling intensity of the CAR molecule and the replicative and persistence of CAR-modified T cells [9,58]. However, the effect(s) of a co-stimulatory domain for CD4ζ CAR engineered stem cells remains to be tested. CD4-ζ CAR expressing T cells, which is the first-generation of CAR design, does not target MHC class II expressing cells due to the weak binding between CD4 and MHC II molecule. However, inclusion of costimulatory domains may lead to targeting of CD4-ζ CAR to MHC class II expressing cells, such as, APCs. In addition, having too strong of a signal may also impede CD4-ζ CAR modified stem cells from normal thymocyte development. T cells with extremely high avidity and binding for self-MHCs are eliminated from the thymus during negative selection. Thus, the addition of costimulatory domains for CD4-ζ CAR may lead to strong signaling from CD4 binding to MHC II molecule, resulting in loss of CD4-ζ CAR T cells during negative selection. Therefore, thorough testing is necessary when constructing second and third generation of CD4-ζ CAR for stem cell-based gene therapy.
bNAb-based CARs are potential alternatives to CD4-ζ CARs for stem cell based therapy. Since they do not bind to human natural antigens, the expression of the bNAb-based CAR will unlikely affect thymopoiesis. In addition, the expression of the bNAb-based CAR does not mediate infection on CD8 cells, unlike CD4-ζ CAR (Zhen, Carrillo, Kitchen; Unpublished data), making them potentially superior candidates for CAR development against HIV infection. Problems with the use of antibody CAR include possible immunogenicity of antibody CAR and the development of anti-idiotype antibodies that may inhibit its activity [9]. Second, since single bNAbs readily select HIV escape mutants [59,60], certain combination of various CARs based on distinct individual bNAbs targeting various region of viral envelope may be necessary to fully suppress viral replication.
HSCs have two important properties: the ability to self-renew and the ability to differentiate into multiple hematopoietic lineages, including those that have the capacity to kill HIV-infected cells (T cells and NK cells). Although these features are very important and beneficial from a regenerative standpoint, they do create certain safety concerns. One particular concern is whether stem cells can lead to tumor formation themselves. When gamma retrovirus vector was used for modification and therapeutic expression of genes to autologous hematopoietic cells, the approach suffered from adverse effect caused by the vector integration of the cell genome [61,62]. Gamma retroviruses have a preference of transcriptional active sites for integration, and this increases the likelihood of activation of nearby proto-oncogenes by the powerful enhancers contained in the gamma virus [63]. Lentivirus-based vectors, on the other hand, transduce human HSCs effectively and display a superior safety profile [64,65]. However, careful clonal tracking of the lentivirus-modified HSCs should be included in preclinical and clinical studies to carefully document transplanted HSC behaviors [65]. Inclusion of suicide gene in addition to cellular therapeutics can allow selective ablation of gene-modified cells and improve the safety of cell-based therapy [1,66]. Introducing features like cell type-specific expression of the CAR molecule in T cells and NK cells will likely improve the clinical safety of this approach.
Although cART has been very successful in controlling HIV replication, this lifelong treatment can have its own challenges including drug-related toxicities and high cost of treatment [67,68]. CAR-based stem cell therapy for HIV infection can have beneficial clinical outcome for patients. It can potentially elicit a lifelong cellular immunity to generate complete viral suppression without antiretroviral drug treatment. CAR T-cell therapy for HIV will likely be more successful without treatment of cART, as this will allow viral production and therefore elevated levels of HIV envelope antigen to elicit effective CAR T-cell signaling to generate a robust anti-HIV CAR response and promote clearance of virus. It will also give an opportunity to see whether anti-HIV CAR cells can remain uninfected during an active HIV infection if given protection through knockdown of CCR5 for instance. In addition, utilizing latency reactivators with CAR T-cell therapy can potentially enable CAR T cells to target latent reservoirs. A possible obstacle in CAR-based therapy for HIV infection is expression of very low levels of viral envelope on the surface of HIV-infected cells. The development of TCR-like CARs that target and bind to peptide/MHC complexes much like natural TCRs can offer an alternative approach to target surface antigen limited cells [69]. TCR-like CARs combine the binding properties of TCR-like antibodies fused with costimulatory and TCR-activating domains, however, unlike scFv-based CARs, TCR-like CARs are HLA restricted [69,70]. Whether these TCR-like CARs can have any therapeutic effect in HIV infection remains to be determined.
Unlike CD19 CARs, it is yet to be determined whether CD4-based CARs may come with some risks, such as, cytokine storms and neurologic toxicities. Early CD4-based CARs in clinical trials did not report any severe cases of off-target toxicities, in fact, these studies showed CD4-based CARs to be safe and persist for a very long time [37–40]. Another potential risk could be immune escape and generating HIV resistance to CAR T-cell therapy. Whether there is viral escape against the CD4-based CAR receptor, is not yet well known. It would be difficult for HIV virus to escape a CD4-based CAR due to the necessity of virus to bind to the CD4 molecule. If there are escape mutants, those mutants would likely render the virus less fit to replicate, because the mutations acquired would possibly compromise the effectiveness of the virus to bind to CD4.
The use of preclinical animal models for HIV infection is critical in testing the safety and efficacy of CD4 and antibody-based CAR T-cell therapy. The humanized mouse model provides an in vivo analysis of human immune responses to HIV infection and other human diseases [71,72]. Although stem cell based CAR T-cell therapy against HIV has been proven feasible and successful in a humanized mouse model [41], there are limitations to the use of this model that include deficits in lymphoid structures and graft versus host disease [73]. An alternative approach to preclinical evaluation of CAR T-cell therapy in vivo is the use of nonhuman primates where infection with SIV and hybrid SHIV strains mimic HIV infection of humans [74]. Stem cell based CAR T-cell therapy in response to SHIV infection has already been tested in a nonhuman primate model and showed to be safe without any significant or life threatening side effects (manuscript in preparation). Thus, the feasibility and safety of stem cell based CAR T-cell therapy shown in preclinical animal models will likely translate to the clinic.
The persistence of HIV reservoir despite long-term combined ART remains as a major barrier to curing HIV infection. In addition to being a standalone therapy to suppress HIV replication, the CAR-based therapy can be potentially given alongside latency reactivators as a more effective way to eradicate latently infected cells as compared with patients’ own immunity. In summary, CAR stem cell therapy offers a novel and promising alternative approach to T-cell-based adoptive therapies to redirect and generate long-term and effective anti-HIV immunity.
Executive summary.
Chimeric antigen receptors & lessons from cancer immune therapies
Chimeric antigen receptors (CARs) have been successfully employed for the treatment of several malignancies.
Purification and specific utilization of certain T-cell subsets and refinements in cell handling and genetic modification procedures have resulted in markedly improved CAR T-cell responses against tumors.
The lessons learned from CAR T-cell therapy in the treatment of cancer may be highly applicable toward the treatment of HIV.
CAR T-cell therapy for HIV infection: lessons from CD4-ζ CAR T-cell therapy
Initial clinical trials for treatment of HIV infection with CD4 CAR modified T cells resulted in limited antiviral efficacy.
CD4-ζ CAR T-cell treatment was safe and resulted in long-term engraftment.
Strategies have been developed to improve CD4-based CARs for better therapeutic efficacy in the clinic.
Stem cell based CAR therapy for redirecting anti-HIV immunity
Modified hematopoietic stem cells with CARs can potentially offer a functional cure for HIV infection by providing unlimited production of immune cells redirected to target and kill HIV-infected cells in vivo.
We highlight key preclinical studies that have shown the feasibility and therapeutic efficacy of this approach to generate immune cells protected from HIV infection and, in turn, directed toward HIV-infected cells.
Footnotes
Financial & competing interests disclosure
This work was funded by grants from the NIH, grant nos. AI078806, AI110306–01 (SG Kitchen), the UCLA Center for AIDS Research (CFAR), grant no. P30AI28697, the California Center for Regenerative Medicine, grant no. TR4–06845, and the UC Multi-campus Research Program and Initiatives, California Center for Antiviral Drug discovery (CCADD) and NIH T32 AI060567 (to A Zhen), and NIH F32 A1118565 (to A Zhen) and UCLA AIDS Institute and UCLA Center for AIDS Research (AI28697) (to A Zhen), and NIH T32 Al60567–12 (to M Carrillo). The flow cytometry machine used in the study was purchased from a Pendleton Foundation grant. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annu. Rev. Med. 2014;65:333–347. doi: 10.1146/annurev-med-060512-150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl Acad. Sci. USA. 1989;86(24):10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4):388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuwana Y, Asakura Y, Utsunomiya N, et al. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 1987;149(3):960–968. doi: 10.1016/0006-291x(87)90502-x. [DOI] [PubMed] [Google Scholar]
- 5.Yang OO, Tran AC, Kalams SA, Johnson RP, Roberts MR, Walker BD. Lysis of HIV-1-infected cells and inhibition of viral replication by universal receptor T cells. Proc. Natl Acad. Sci. USA. 1997;94(21):11478–11483. doi: 10.1073/pnas.94.21.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts MR, Qin L, Zhang D, et al. Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors. Blood. 1994;84(9):2878–2889. [PubMed] [Google Scholar]
- 7.Kong S, Sengupta S, Tyler B, et al. Suppression of human glioma xenografts with second-generation IL13R-specific chimeric antigen receptor-modified T cells. Clin. Cancer Res. 2012;18(21):5949–5960. doi: 10.1158/1078-0432.CCR-12-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krebs S, Chow KK, Yi Z, et al. T cells redirected to interleukin-13Ralpha2 with interleukin-13 mutein – chimeric antigen receptors have anti-glioma activity but also recognize interleukin-13Ralpha1. Cytotherapy. 2014;16(8):1121–1131. doi: 10.1016/j.jcyt.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol. Rev. 2014;257(1):107–126. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramos CA, Savoldo B, Dotti G. CD19-CAR trials. Cancer J. 2014;20(2):112–118. doi: 10.1097/PPO.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo . Mol. Ther. 2009;17(8):1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66(22):10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 13.Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl Acad. Sci. USA. 2009;106(9):3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4–1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol. Ther. 2010;18(2):413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Jensen M, Lin Y, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum. Gene Ther. 2007;18(8):712–725. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- 16.Till BG, Jensen MC, Wang J, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4–1BB domains: pilot clinical trial results. Blood. 2012;119(17):3940–3950. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang XY, Sun Y, Zhang A, et al. Third-generation CD28/4–1BB chimeric antigen receptor T cells for chemotherapy relapsed or refractory acute lymphoblastic leukaemia: a non-randomised, open-label Phase I trial protocol. BMJ Open. 2016;6(12):e013904. doi: 10.1136/bmjopen-2016-013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Igney FH, Krammer PH. Immune escape of tumors: apoptosis resistance and tumor counterattack. J. Leukoc. Biol. 2002;71(6):907–920. [PubMed] [Google Scholar]
- 19.Wonderlich ER, Leonard JA, Collins KL. HIV immune evasion disruption of antigen presentation by the HIV Nef protein. Adv. Virus Res. 2011;80:103–127. doi: 10.1016/B978-0-12-385987-7.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391(6665):397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 21.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sommermeyer D, Hudecek M, Kosasih PL, et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo . Leukemia. 2016;30(2):492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 2016;126(6):2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Restifo NP, Gattinoni L. Lineage relationship of effector and memory T cells. Curr. Opin. Immunol. 2013;25(5):556–563. doi: 10.1016/j.coi.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y, Zhang M, Ramos CA, et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014;123(24):3750–3759. doi: 10.1182/blood-2014-01-552174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cieri N, Camisa B, Cocchiarella F, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. 2013 doi: 10.1182/blood-2012-05-431718. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Gomez-Eerland R, Nuijen B, Heemskerk B, et al. Manufacture of gene-modified human T-cells with a memory stem/central memory phenotype. Hum. Gene Ther. Methods. 2014;25(5):277–287. doi: 10.1089/hgtb.2014.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cha E, Graham L, Manjili MH, Bear HD. IL-7 + IL-15 are superior to IL-2 for the ex vivo expansion of 4T1 mammary carcinoma-specific T cells with greater efficacy against tumors in vivo . Breast Cancer Res. Treat. 2010;122(2):359–369. doi: 10.1007/s10549-009-0573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gargett T, Brown MP. Different cytokine and stimulation conditions influence the expansion and immune phenotype of third-generation chimeric antigen receptor T cells specific for tumor antigen GD2. Cytotherapy. 2015;17(4):487–495. doi: 10.1016/j.jcyt.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Chahroudi A, Silvestri G, Lichterfeld M. T memory stem cells and HIV: a long-term relationship. Curr. HIV/AIDS Rep. 2015;12(1):33–40. doi: 10.1007/s11904-014-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buzon MJ, Sun H, Li C, et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat. Med. 2014;20(2):139–142. doi: 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vigano S, Negron J, Ouyang Z, et al. Prolonged antiretroviral therapy preserves HIV-1-Specific CD8 T cells with stem cell-like properties. J. Virol. 2015;89(15):7829–7840. doi: 10.1128/JVI.00789-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romeo C, Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell. 1991;64(5):1037–1046. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- 37.Mitsuyasu RT, Anton PA, Deeks SG, et al. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood. 2000;96(3):785–793. [PubMed] [Google Scholar]
- 38.Deeks SG, Wagner B, Anton PA, et al. A Phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol. Ther. 2002;5(6):788–797. doi: 10.1006/mthe.2002.0611. [DOI] [PubMed] [Google Scholar]
- 39.Walker RE, Bechtel CM, Natarajan V, et al. Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection. Blood. 2000;96(2):467–474. [PubMed] [Google Scholar]
- 40.Scholler J, Brady TL, Binder-Scholl G, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 2012;4(132):132ra153. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhen A, Kamata M, Rezek V, et al. HIV-specific immunity derived from chimeric antigen receptor-engineered stem cells. Mol. Ther. 2015;23(8):1358–1367. doi: 10.1038/mt.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu L, Patel B, Ghanem MH, et al. Novel CD4-based bispecific chimeric antigen receptor designed for enhanced anti-HIV potency and absence of HIV entry receptor activity. J. Virol. 2015;89(13):6685–6694. doi: 10.1128/JVI.00474-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimizu S, Hong P, Arumugam B, et al. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood. 2010;115(8):1534–1544. doi: 10.1182/blood-2009-04-215855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamata M, Kim PY, Ng HL, et al. Ectopic expression of anti-HIV-1 shRNAs protects CD8(+) T cells modified with CD4zeta CAR from HIV-1 infection and alleviates impairment of cell proliferation. Biochem. Biophys. Res. Commun. 2015;463(3):216–221. doi: 10.1016/j.bbrc.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilen CB, Tilton JC, Doms RW. HIV: cell binding and entry. Cold Spring Harb. Perspect. Med. 2012;2(8) doi: 10.1101/cshperspect.a006866. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Younan PM, Polacino P, Kowalski JP, et al. Positive selection of mC46-expressing CD4+ T cells and maintenance of virus specific immunity in a primate AIDS model. Blood. 2013;122(2):179–187. doi: 10.1182/blood-2013-01-482224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393(6686):648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwong PD, Mascola JR, Nabel GJ. Broadly neutralizing antibodies and the search for an HIV-1 vaccine: the end of the beginning. Nat. Rev. Immunol. 2013;13(9):693–701. doi: 10.1038/nri3516. [DOI] [PubMed] [Google Scholar]
- 49.Ali A, Kitchen SG, Chen IS, Ng HL, Zack JA, Yang OO. HIV-1-specific chimeric antigen receptors based on broadly neutralizing antibodies. J. Virol. 2016;90(15):6999–7006. doi: 10.1128/JVI.00805-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu B, Zou F, Lu L, et al. Chimeric antigen receptor T cells guided by the single-chain Fv of a broadly neutralizing antibody specifically and effectively eradicate virus reactivated from latency in CD4+ T lymphocytes isolated from HIV-1-infected individuals receiving suppressive combined antiretroviral therapy. J. Virol. 2016;90(21):9712–9724. doi: 10.1128/JVI.00852-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ringpis GE, Shimizu S, Arokium H, et al. Engineering HIV-1-resistant T-cells from short-hairpin RNA-expressing hematopoietic stem/progenitor cells in humanized BLT mice. PLoS ONE. 2012;7(12):e53492. doi: 10.1371/journal.pone.0053492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burke BP, Levin BR, Zhang J, et al. Engineering cellular resistance to HIV-1 infection in vivo using a dual therapeutic lentiviral vector. Mol. Ther. Nucleic Acids. 2015;4:e236. doi: 10.1038/mtna.2015.10. [DOI] [PubMed] [Google Scholar]
- 53.Kiem HP, Jerome KR, Deeks SG, Mccune JM. Hematopoietic-stem-cell-based gene therapy for HIV disease. Cell stem cell. 2012;10(2):137–147. doi: 10.1016/j.stem.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kitchen SG, Bennett M, Galic Z, et al. Engineering antigen-specific T cells from genetically modified human hematopoietic stem cells in immunodeficient mice. PLoS ONE. 2009;4(12):e8208. doi: 10.1371/journal.pone.0008208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vatakis DN, Koya RC, Nixon CC, et al. Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells. Proc. Natl Acad. Sci. USA. 2011;108(51):E1408–E1416. doi: 10.1073/pnas.1115050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vatakis DN, Arumugam B, Kim SG, Bristol G, Yang O, Zack JA. Introduction of exogenous T-cell receptors into human hematopoietic progenitors results in exclusion of endogenous T-cell receptor expression. Mol. Ther. 2013;21(5):1055–1063. doi: 10.1038/mt.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ni Z, Knorr DA, Bendzick L, Allred J, Kaufman DS. Expression of chimeric receptor CD4ζ by natural killer cells derived from human pluripotent stem cells improves in vitro activity but does not enhance suppression of HIV infection in vivo . Stem Cells. 2014;32(4):1021–1031. doi: 10.1002/stem.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125(26):4017–4023. doi: 10.1182/blood-2014-12-580068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature's pathways. Immunol. Rev. 2013;254(1):225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liao HX, Lynch R, Zhou T, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496(7446):469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Howe SJ, Mansour MR, Schwarzwaelder K, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 2008;118(9):3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kohn DB, Sadelain M, Glorioso JC. Occurrence of leukaemia following gene therapy of X-linked SCID. Nat. Rev. Cancer. 2003;3(7):477–488. doi: 10.1038/nrc1122. [DOI] [PubMed] [Google Scholar]
- 63.Persons DA. Lentiviral vector gene therapy: effective and safe? Mol. Ther. 2010;18(5):861–862. doi: 10.1038/mt.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biffi A, Bartolomae CC, Cesana D, et al. Lentiviral vector common integration sites in preclinical models and a clinical trial reflect a benign integration bias and not oncogenic selection. Blood. 2011;117(20):5332–5339. doi: 10.1182/blood-2010-09-306761. [DOI] [PubMed] [Google Scholar]
- 65.Kim S, Kim N, Presson AP, et al. Dynamics of HSPC repopulation in nonhuman primates revealed by a decade-long clonal-tracking study. Cell stem cell. 2014;14(4):473–485. doi: 10.1016/j.stem.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones BS, Lamb LS, Goldman F, Di Stasi A. Improving the safety of cell therapy products by suicide gene transfer. Front. Pharmacol. 2014;5:254. doi: 10.3389/fphar.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fernandez-Montero JV, Eugenia E, Barreiro P, Labarga P, Soriano V. Antiretroviral drug-related toxicities – clinical spectrum, prevention, and management. Expert Opin. Drug Saf. 2013;12(5):697–707. doi: 10.1517/14740338.2013.806480. [DOI] [PubMed] [Google Scholar]
- 68.Hill AM, Cho M, Mrus JM. The costs of full suppression of plasma HIV RNA in highly antiretroviral-experienced patients. AIDS Rev. 2011;13(1):41–48. [PubMed] [Google Scholar]
- 69.Zhang G, Wang L, Cui H, et al. Anti-melanoma activity of T cells redirected with a TCR-like chimeric antigen receptor. Sci. Rep. 2014;4:3571. doi: 10.1038/srep03571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oren R, Hod-Marco M, Haus-Cohen M, et al. Functional comparison of engineered T cells carrying a native TCR versus TCR-like antibody-based chimeric antigen receptors indicates affinity/avidity thresholds. J. Immunol. 2014;193(11):5733–5743. doi: 10.4049/jimmunol.1301769. [DOI] [PubMed] [Google Scholar]
- 71.Brainard DM, Seung E, Frahm N, et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J. Virol. 2009;83(14):7305–7321. doi: 10.1128/JVI.02207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ito R, Takahashi T, Katano I, Ito M. Current advances in humanized mouse models. Cell. Mol. Immunol. 2012;9(3):208–214. doi: 10.1038/cmi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akkina R, Allam A, Balazs AB, et al. Improvements and limitations of humanized mouse models for HIV research: NIH/NIAID ‘Meet the Experts’ 2015 Workshop Summary. AIDS Res. Hum. Retroviruses. 2016;32(2):109–119. doi: 10.1089/aid.2015.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hatziioannou T, Evans DT. Animal models for HIV/AIDS research. Nat. Rev. Microbiol. 2012;10(12):852–867. doi: 10.1038/nrmicro2911. [DOI] [PMC free article] [PubMed] [Google Scholar]