TRX and NTRC coordinate the stability and activity of Mg-protoporphyrin IX methyltransferase.
Abstract
In chloroplasts, thioredoxin (TRX) isoforms and NADPH-dependent thioredoxin reductase C (NTRC) act as redox regulatory factors involved in multiple plastid biogenesis and metabolic processes. To date, less is known about the functional coordination between TRXs and NTRC in chlorophyll biosynthesis. In this study, we aimed to explore the potential functions of TRX m and NTRC in the regulation of the tetrapyrrole biosynthesis (TBS) pathway. Silencing of three genes, TRX m1, TRX m2, and TRX m4 (TRX ms), led to pale-green leaves, a significantly reduced 5-aminolevulinic acid (ALA)-synthesizing capacity, and reduced accumulation of chlorophyll and its metabolic intermediates in Arabidopsis (Arabidopsis thaliana). The contents of ALA dehydratase, protoporphyrinogen IX oxidase, the I subunit of Mg-chelatase, Mg-protoporphyrin IX methyltransferase (CHLM), and NADPH-protochlorophyllide oxidoreductase were decreased in triple TRX m-silenced seedlings compared with the wild type, although the transcript levels of the corresponding genes were not altered significantly. Protein-protein interaction analyses revealed a physical interaction between the TRX m isoforms and CHLM. 4-Acetoamido-4-maleimidylstilbene-2,2-disulfonate labeling showed the regulatory impact of TRX ms on the CHLM redox status. Since CHLM also is regulated by NTRC (Richter et al., 2013), we assessed the concurrent functions of TRX m and NTRC in the control of CHLM. Combined deficiencies of three TRX m isoforms and NTRC led to a cumulative decrease in leaf pigmentation, TBS intermediate contents, ALA synthesis rate, and CHLM activity. We discuss the coordinated roles of TRX m and NTRC in the redox control of CHLM stability with its corollary activity in the TBS pathway.
The regulation of the redox status of plastid-localized processes modulates protein activity, folding, and stability via reversible disulfide bond formation between the thiol groups of two Cys residues (Richter and Grimm, 2013; Kang and Wang, 2016; Nikkanen et al., 2016). These thiol switches are controlled by three redox regulation systems in chloroplasts, namely, the ferredoxin-thioredoxin (Fd-TRX) system, the NADPH-dependent TRX-reductase (NTR) system, and the glutathione/glutaredoxin (GRX) system (Meyer et al., 2009; Dietz and Pfannschmidt, 2011; Dietz, 2016).
Thioredoxins (TRXs) are small (approximately 12 kD), ubiquitous oxidoreductases possessing the conserved Cys-containing peptide motif WC(G/P)PC at their active sites (Holmgren, 1985; Baumann and Juttner, 2002; Schürmann and Buchanan, 2008). TRXs can be reduced to dithiol by ferredoxin and subsequently serve as electron donors. Reduced TRXs, in turn, can decrease disulfide bridge formation in target proteins, thereby modulating their functions and stability (Dietz and Pfannschmidt, 2011; Dietz, 2016).
In higher plants, the plastid-localized TRX family can be classified into five subgroups, including two f-type (TRX f1 and f2), four m-type (TRX m1–m4), one x-type (TRX x), two y-type (TRX y1 and y2), and one z-type (TRX z) TRX isoforms (Michalska et al., 2009; Michelet et al., 2013; Serrato et al., 2013; Balsera et al., 2014). Numerous in vitro and in vivo studies have shown that TRX f and TRX m are required for multiple metabolic processes in chloroplasts, including the Calvin-Benson cycle (Collin et al., 2003; Michelet et al., 2013; Okegawa and Motohashi, 2015; Yoshida et al., 2015), ATP synthesis (Schwarz et al., 1997) and NADPH export (Wolosiuk et al., 1979; Lara et al., 1980), starch metabolism (Fu et al., 1998; Mikkelsen et al., 2005; Seung et al., 2013; Thormählen et al., 2013), fatty acid synthesis (Sasaki et al., 1997), amino acid synthesis (Balmer et al., 2003), and chlorophyll (Chl) synthesis (Ikegami et al., 2007; Luo et al., 2012). Functional genetic analyses have shown that TRX m4 knockout mutants were affected in cyclic electron flow around PSI (Courteille et al., 2013), while a TRX m3 knockout mutant was affected in meristem development (Benitez-Alfonso et al., 2009). Arabidopsis (Arabidopsis thaliana) lines with combined silencing of TRX m1, m2, and m4 were defective in the biogenesis of PSII (Wang et al., 2013). A recent study suggested that TRX m plays a more important role than TRX f in the activation of Fru-1,6-bisphosphatase (FBPase) and sedoheptulose-1,7-bisphosphatase during carbon assimilation (Okegawa and Motohashi, 2015). TRXs also function in the dynamic acclimation of photosynthesis in fluctuating light (Thormahlen et al., 2017).
In addition to the Fd-TRX system, plants use NTR as a reductant. This enzyme contains a FAD-binding domain and a double Cys peptide motif in its catalytic center. Arabidopsis has three NTR isoforms. While NTRA and NTRB are found in the cytoplasm or mitochondria, NTRC is localized to chloroplasts and, in contrast to the other two isoforms, contains both an NTR and a TRX domain. Only a few targets were shown to be controlled by NTRC. NTRC functions in the abiotic oxidative stress response (Serrato et al., 2004), starch synthesis (Michalska et al., 2009), Chl synthesis (Richter et al., 2013; Pérez-Ruiz et al., 2014), the Calvin-Benson cycle (Thormählen et al., 2015), lateral root formation in Arabidopsis (Kirchsteiger et al., 2012), nonphotochemical quenching (Naranjo et al., 2016), as a holdase chaperone function enhancing thermotolerance to Arabidopsis (Chae et al., 2013), and as an electron donor of 2-Cys-peroxiredoxin, thus playing a key role in the detoxification of reactive oxygen species (ROS; Pérez-Ruiz et al., 2006; Spínola et al., 2008; Kirchsteiger et al., 2009). Recent research suggested that cooperative control of chloroplast functions via the ferredoxin-thioredoxin reductase (FTR)/TRX and NTRC pathways is essential for plant viability. The mutual inactivation of the catalytic subunit of FTR (FTRb) and NTRC leads to the drastic loss of photosynthetic performance and causes a lethal phenotype (Yoshida and Hisabori, 2016). Last but not least, glutaredoxins utilize the reducing power of glutathione to catalyze disulfide reductions in the presence of NADPH and glutathione reductase and operate, like TRXs, as dithiol reductants (Meyer et al., 2012).
Tetrapyrrole biosynthesis (TBS) in plants leads to the production of Chl, heme, phytochromobilin, and siroheme (Tanaka et al., 2011; Brzezowski et al., 2015). This pathway begins with the synthesis of 5-aminolevulinic acid (ALA), the universal precursor of all tetrapyrroles (Tanaka et al., 2011; Brzezowski et al., 2015). Like most prokaryotes, plants synthesize ALA from Glu, which is coupled to tRNAGlu by glutamyl-tRNA synthetase. The first committed enzyme of TBS is glutamyl-tRNA reductase (GluTR), which catalyzes the conversion of activated glutamyl-tRNA to Glu-1-semialdehyde. Glu-1-semialdehyde is then converted to ALA by Glu-1-semialdehyde aminotransferase (GSAAT). ALA synthesis determines the flow of metabolites through the pathway and is accepted to be the rate-limiting step of TBS (Mochizuki et al., 2010; Tanaka et al., 2011). Eight molecules of ALA are converted (in a series of enzymatic steps) to protoporphyrin IX. Protoporphyrin IX is allocated into two branches of the pathway: the heme-synthesizing iron branch and the Chl-synthesizing Mg branch. ATP-dependent, heterotrimeric Mg chelatase (MgCh) is the first enzyme of the Mg branch, which catalyzes the insertion of Mg2+ into protoporphyrin IX. MgCh consists of the subunits CHLI, CHLD, and CHLH (Gibson et al., 1995; Jensen et al., 1996; Petersen et al., 1998; Luo et al., 1999). Mg porphyrin methyltransferase (CHLM) catalyzes the methylation of Mg porphyrin (MgP) to Mg porphyrin monomethylester (MgPMME) prior to the formation of divinyl protochlorophyllide (Pchlide) by MgPMME oxidative cyclase. Protochlorophyllide oxidoreductase (POR) synthesizes chlorophyllide (Chlide) in a strictly light-dependent reaction. Divinyl Pchlide and/or Chlide are converted to the monovinyl forms by divinyl reductase prior to their ultimate conversion to Chl a by Chl synthase (CHLG) and the formation of Chl b by Chl a oxygenase (CAO; Tanaka et al., 2011).
TBS is precisely controlled at the transcriptional and posttranslational levels (Mochizuki et al., 2010; Tanaka et al., 2011; Czarnecki and Grimm, 2012). Recent work has provided the first indication that thiol-based redox regulation belongs to one of the essential posttranslational mechanisms that affect the catalytic activity and stability of TBS enzymes. The regulatory functions of both TRX and NTRC have been reported (Stenbaek and Jensen, 2010; Luo et al., 2012; Richter et al., 2013).
The Arabidopsis ntrc mutant displays pale-green pigmentation and a retarded growth phenotype, which resembles other Chl-deficient mutants. Therefore, the involvement of NTRC in redox-regulated Chl synthesis has been tentatively proposed (Serrato et al., 2004). Subsequent biochemical analysis has provided experimental evidence for NTRC-dependent control of GluTR and CHLM (Richter et al., 2013, 2016). Arabidopsis CHLM contains three conserved Cys residues at positions 111, 115, and 177 (Richter et al., 2013). Recently, x-ray crystallography revealed the structure of CHLM, suggesting that Cys-111 and Cys-177 are buried inside this protein, while Cys-115 might be positioned in the protein to enable its conformational change (Chen et al., 2014). Recombinant CHLM proteins with single and double substitution of the Cys residues showed decreased enzymatic activity, and the CHLM (Cys-177Ser) substitution mutant was not activated by DTT, indicating that Cys-177 is involved in the redox control of CHLM (Richter et al., 2016).
Recombinant TRX m and TRX f reduced intramolecular disulfide bond formation at the C terminus of the MgCh subunit CHLI and stimulated the ATPase activity of CHLI (Ikegami et al., 2007; Luo et al., 2012) as well as MgCh activity in vitro (Luo et al., 2012). The physical interaction of TRX f with CHLI was confirmed by yeast two-hybrid and bimolecular fluorescence complementation (BiFC) assays (Luo et al., 2012). In addition, a recent study indicated that CHLI activation is altered by NTRC-dependent redox modification (Pérez-Ruiz et al., 2014), implying the functional coordination between TRX and NTRC in the redox regulation of TBS.
Early studies using thiol-dependent affinity chromatography suggested that GSAAT, ALA dehydratase (ALAD), uroporphyrinogen decarboxylase, and protoporphyrinogen oxidase1 (PPOX1) interact with TRX f and TRX m (Balmer et al., 2003; Marchand et al., 2006), implying that both TRX m and TRX f are involved in the redox control of TBS. However, Arabidopsis mutants with a deficiency of TRX f and pea (Pisum sativum) plants with TRX f gene silencing did not exhibit macroscopic changes in leaf pigmentation, whereas a pale-green phenotype was observed when both the TRX f and TRX m genes were silenced simultaneously (Luo et al., 2012). Interestingly, we previously found that Arabidopsis plants with virus-induced TRX m1, TRX m2, and TRX m4 silencing (VIGS-TRX m2m4/m1) had pale-green leaves and a strongly reduced Chl content compared with control plants (Wang et al., 2013). This observation led to the suggestion that these TRX m variants play an important role in the Arabidopsis TBS pathway.
The objective of this study was to dissect the regulatory roles of TRX ms in TBS in the presence and absence of NTRC and to identify TRX m-dependent target TBS enzymes. We silenced the three genes TRX m1, TRX m2, and TRX m4 in the wild type and the ntrc mutant background to examine the role of TRX ms in redox regulation of TBS by analyzing the steady-state levels of Chl intermediates as well as protein contents and Chl biosynthesis enzyme activities. Our biochemical studies revealed direct interactions of the three TRX m isoforms with CHLM and the mutually coordinated action of the m-type TRXs and NTRC in the control of the redox status of CHLM. The results of this study provide further evidence for the cooperative interdependence of plastid-localized reductants in the control of the stability and activity of single TBS enzymes.
RESULTS
Inactivation of Three m-Type TRX Genes Reduces Pigment Content in Arabidopsis
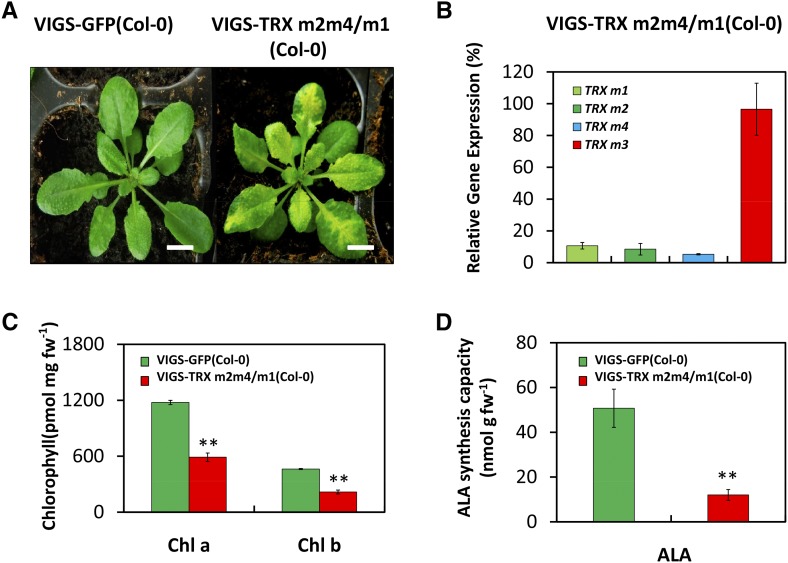
Using tobacco rattle virus-based virus-induced gene silencing (VIGS), we generated Arabidopsis seedlings with inactivated TRX ms expression (Wang et al., 2013) to examine the physiological impact of TRX ms deficiency on Chl biosynthesis. Due to the functional redundancy of the three TRX m genes, only triple TRX m-silencing plants showed a pale-green phenotype compared with the VIGS-GFP(Col-0) control plants (Fig. 1A). Analysis of gene expression by qRT-PCR confirmed the inactivation of TRX m1, TRX m2, and TRX m4 expression by approximately more than 90% in VIGS-TRX m2m4/m1(Col-0) plants relative to control plants (Fig. 1B). TRX m3 also is a fourth homologous gene of the TRX m family, but TRX m3 expression was not modified in VIGS-TRX m2m4/m1(Col-0) plants compared with control plants (Fig. 1B).
Figure 1.
Characterization of Arabidopsis VIGS-TRX m(Col-0) plants. A, Representative images of VIGS-GFP(Col-0) and VIGS-TRX m2m4/m1(Col-0) plants, which were infected via agroinfiltration with the pTRV2-GFP and pTRV2-TRX m2m4 vectors in the wild-type background, respectively. The plants were observed 3 weeks after infiltration. At least three biological replicates were performed, and similar results were obtained. Bars = 1 cm. B, The suppression of TRX m gene expression in VIGS-TRX m(Col-0) plants was analyzed by qRT-PCR. Relative transcript levels of TRX m1, TRX m2, TRX m4, and TRX m3 in VIGS-TRX m2m4/m1(Col-0) plants were normalized to the level in VIGS-GFP(Col-0) plants (100%). The data represent means ± sd of three biological replicates. C, Chl a and b contents of 4-week-old VIGS-GFP(Col-0) and VIGS-TRX m2m4/m1(Col-0) plants. D, ALA-synthesizing capacity was determined with VIGS plants. In C and D, all data are given as means ± sd of three biological replicates. Statistical significance compared with VIGS-GFP(Col-0) plants is indicated by asterisks (**, P ≤ 0.01, Student’s t test). fw, Fresh weight.
We observed pale-green leaves in VIGS-TRX m2m4/m1(Col-0) plants at 2 weeks after infiltration. Chl a and Chl b contents were decreased to 50% of control levels (Fig. 1C). VIGS-TRX m2m4/m1(Col-0) plants showed a significantly decreased ALA-synthesizing capacity of 80% compared with VIGS-GFP(Col-0) plants (Fig. 1D). These results suggest that the deficiency of TRX ms significantly impairs ALA synthesis and reduces Chl content.
Analysis of the contents of tetrapyrrole intermediates revealed that all of the steady-state levels of MgP and MgPMME, Pchlide, and Chlide were reduced significantly in VIGS-TRX m2m4/m1(Col-0) seedlings compared with VIGS-GFP(Col-0) seedlings (Fig. 2, A–C). The heme content also was reduced in VIGS-TRX m2m4/m1(Col-0) seedlings (Fig. 2D), indicating that the reduced flow of early metabolic intermediates compromises not only the Chl branch but also the heme/bilin branch of the pathway. The reduced activity or accumulation of ALA synthesis enzymes might be attributed to TRX m deficiency, which would affect the flow of metabolites through the TBS pathway.
Figure 2.
Contents of intermediates and heme in VIGS-TRX m(Col-0) plants. A and B, Mg P (A) and MgPMME (B) contents in VIGS-GFP(Col-0) and VIGS-TRX m2m4/m1(Col-0) plants. C, Pchlide and Chlide contents in VIGS-GFP(Col-0) and VIGS-TRX m2m4/m1(Col-0) plants. D, Heme contents of VIGS-GFP(Col-0) and VIGS-TRX m2m4/m1(Col-0) plants. All data are given as means ± sd of three biological replicates. Statistical significance compared with VIGS-GFP(Col-0) plants is indicated by asterisks (**, P ≤ 0.01, Student’s t test). fw, Fresh weight.
Accumulation of Enzymes in the TBS Pathway Is Impaired in Triple TRX m-Silenced Plants
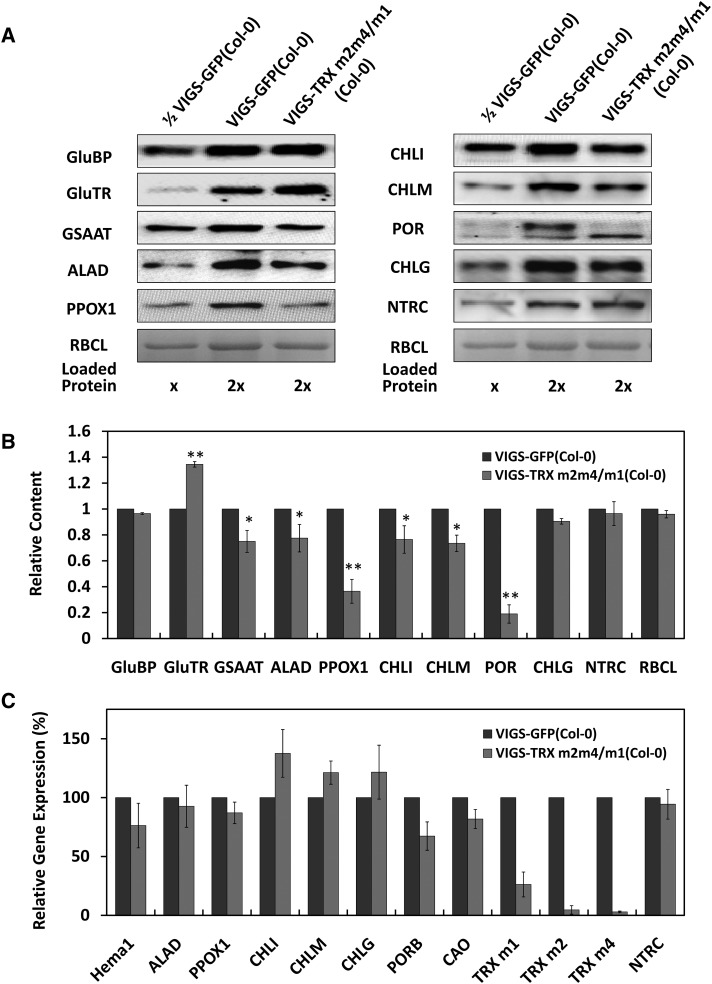
To further explore the influence of deficient expression of TRX ms, we performed immunoblot analyses using antibodies against several enzymatic steps of TBS. Western blotting of total protein extracts of VIGS-GFP(Col-0) and VIGS-TRX m2m4/m1(Col-0) plants revealed decreased contents of PPOX1, CHLI, CHLM, POR, GSAAT, and ALAD due to TRX m deficiency (Fig. 3, A and B). The levels of GluTR-binding protein (Czarnecki et al., 2011), a regulatory protein of GluTR (Apitz et al., 2016), CHLG, and NTRC were not affected significantly in VIGS-TRX m2m4/m1(Col-0) plants relative to the VIGS-GFP control. Surprisingly, the levels of GluTR increased upon TRX ms silencing. Similar results were reported for pea VIGS-TRX f/TRX m plants (Luo et al., 2012).
Figure 3.
Contents of tetrapyrrole synthesis enzymes and transcript analysis of VIGS-TRX m(Col-0) plants. A, Contents of TBS enzymes in VIGS-TRX m(Col-0) plants. Protein names not defined in the text are as follows: GluBP, GluTR-binding protein; RBCL, Rubisco large subunit (Coomassie staining). For loaded proteins, x = 25 μg of total proteins. B, Semiquantitative analysis of TBS proteins presented in immunoblots in A. Means of three biological replicates were analyzed with the Tanon Gis software. Protein amounts of the VIGS-TRX ms plants are shown relative to the amount of VIGS-GFP plants. Data are given as means ± sd of three biological replicates. Statistical significance compared with VIGS-GFP(Col-0) plants is indicated by asterisks (*, P ≤ 0.05 > 0.01 and **, P ≤ 0.01, Student’s t test). C, Transcript analysis of VIGS-TRX m(Col-0) plants. The transcript levels were normalized to those of VIGS-GFP plants (100%). The data represent means ± sd of three independent infiltrations. Transcripts encoding the following enzymes are depicted: HemA1 (glutamyl-tRNA reductase [GluTR1]), ALAD, PPOX1, CHLI, CHLM, CHLG, PORB, POR, CAO, TRX m1, TRX m2, TRX m4, and NTRC. Data are given as means ± sd of three biological replicates.
To investigate whether the significantly decreased contents of these TBS enzymes resulted from impaired transcription of TBS genes, we performed qRT-PCR to analyze the mRNA levels of genes encoding representative TBS enzymes. Although the contents of PPOX1, CHLI, CHLM, and ALAD were reduced substantially in VIGS-TRX m2m4/m1(Col-0) plants, the levels of the corresponding transcripts were not altered significantly compared with VIGS-GFP(Col-0) plants (Fig. 3C). However, the levels of transcripts encoding some other TBS enzymes were decreased, such as HemA1 and PORB (Fig. 3C). These results suggest that the decreased protein contents of several TBS enzymes did not result from the regulatory adaptation of transcriptional activity in TRX m-silencing plants but instead could be explained by changes in posttranscriptional control.
The m-Type TRXs Interact with CHLM
The decreased content of intermediates and the lower abundance of TBS enzymes in VIGS-TRX m2m4/m1(Col-0) plants imply that m-type TRX plays a role in the redox status of the TBS pathway. To test this hypothesis, we examined the physical interaction of TRX m1, TRX m2, and TRX m4 with TBS enzymes. We performed a pull-down experiment with recombinant N-terminal 6×His-tagged TRX m proteins. His-TRX m1, His-TRX m2, His-TRX m3, and His-TRX m4 fusion proteins were used as baits to trap potential target proteins of the chloroplast extracts (Supplemental Fig. S1). Our results show that TRX m variants interact directly with CHLM but not with GSAAT, ALAD, or POR in vitro (Fig. 4A).
Figure 4.
Evidence for the interaction of TRX ms with CHLM. A, In vitro pull-down assay. A total of 200 μg of recombinant His-TRX m1, His-TRX m2, His-TRX m3, and His-TRX m4 recombinant proteins was immobilized on cyanogen bromide-activated Sepharose 4B resin and incubated with 140 μg of Chl total chloroplast proteins; empty cyanogen bromide-activated Sepharose 4B resin without coupled His-TRX m protein was used as the negative control. B, Demonstration of the interaction of TRX m1, TRX m2, and TRX m4 with CHLM by BiFC assays in vivo. CHLM fused with the N terminus of YFP (YN) and TRX m1, TRX m2, TRX m3, and TRX m4 fused with the C terminus of YFP (YC) were cotransfected into protoplasts and visualized using confocal microscopy. bZIP63 fused with YN and bZIP63 fused with YC were cotransfected into protoplasts as positive controls. All experiments were repeated three times with similar results. Bars = 10 μm.
To further confirm the interaction of TRX m1, TRX m2, TRX m3, and TRX m4 with CHLM, we performed BiFC analysis using a transient expression system of Arabidopsis protoplasts. Cotransformation of Arabidopsis protoplast cells with bZIP63-YN and bZIP63-YC constructs was used as a positive control (Fig. 4B). The combined expression of CHLM-YN with each TRX m-YC variant separately revealed that only TRX m1, TRX m2, and TRX m4 interact with CHLM in the chloroplasts of Arabidopsis protoplast cells (Fig. 4B; Supplemental Fig. S2). Furthermore, our immunoblot analyses showed that TRX m3-YC fusion proteins are detectable in protoplast cells (Supplemental Fig. S2B). Therefore, we speculate that the interaction of TRX m3 with CHLM in pull-down assays was caused by the abundance of both proteins in the in vitro pull-down assay. Taken together, the results of pull-down and BiFC experiments demonstrate that TRX m1, TRX m2, and TRX m4 interact with CHLM, which is a prerequisite for TRX m-dependent posttranslational modifications in tetrapyrrole metabolism.
The m-Type TRXs Catalyze the Intramolecular Disulfide Bonds of CHLM in Vivo and in Vitro
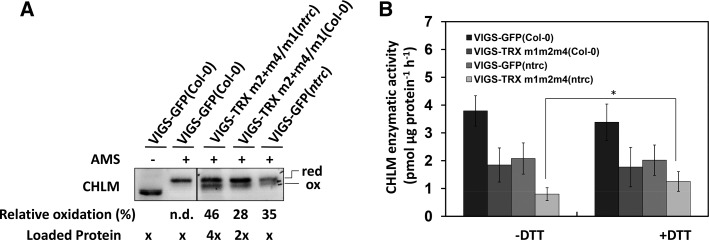
We were also interested to directly determine the redox status of CHLM as a result of TRX m deficiency. The redox status of intramolecular disulfide bridges in CHLM was examined via treatment with 4-acetoamido-4-maleimidylstilbene-2,2-disulfonate (AMS), which binds to the thiol groups of Cys residues. In contrast, oxidized proteins with disulfide bridges or bound sulfenic acids cannot bind AMS. AMS-labeled proteins migrate more slowly on nonreducing SDS-PAGE gels than unlabeled proteins (Kobayashi et al., 1997; Ikegami et al., 2007; Karamoko et al., 2011; Richter et al., 2013; Wang et al., 2013). CHLM has three Cys residues (Cys-111, Cys-115, and Cys-177). When Cys-177 was substituted by Ser, no disulfide bond could be formed, leading to continuous activation of the protein. It was proposed that Cys-177 forms a disulfide bond with Cys-111 and/or Cys-115 (Richter et al., 2016). To comprehensively analyze the exchange of intramolecular disulfide bonds in CHLM, we grew VIGS-GFP(Col-0) and VIGS-TRX m2m4/m1(Col-0) plants for another 3 weeks after infiltration and obtained total protein extracts under nonreducing conditions from leaves treated with AMS or without AMS. More oxidized CHLM accumulated in VIGS-TRX m2m4/m1(Col-0) plants compared with VIGS-GFP(Col-0) plants (Fig. 5A), suggesting that deficiency of TRX ms disturbs the maintenance of the CHLM redox status in vivo.
Figure 5.
TRX ms catalyze intramolecular disulfide bonds of CHLM in vivo and in vitro. A, Redox state of CHLM in VIGS-TRX m2m4/m1(Col-0) plants. Total proteins were extracted from leaves of VIGS-GFP(Col-0) and VIGS-TRX m2m4/m1(Col-0) plants under nonreducing conditions, which grew for 3 weeks after infiltration, treated with (+) or without (−) AMS. In vivo CHLM was detected by western-blot analysis using the anti-CHLM antibody. A total of 100 μg of protein was loaded per lane. Samples were separated on a nonreducing SDS-polyacrylamide gel. red, The reduced form; ox, the oxidized form. B, Determination of the recombinant MBP-CHLM redox state using AMS labeling. A total of 20 μg of recombinant His-CHLM was incubated with different concentrations of DTT in the absence (left) or presence (right) of 14 μg of TRX ms (TRX m1, TRX m2, and TRX m4), precipitated by TCA, and then reacted in the presence or absence of AMS. Protein mobility was visualized using Coomassie Brilliant Blue staining after nonreducing SDS-PAGE.
To confirm further that TRX ms control intramolecular disulfide bonds in CHLM, we examined the reduction of the intramolecular disulfide bonds in purified recombinant His-tagged CHLM protein (Supplemental Fig. S1B) in the presence or absence of TRX ms. The redox state of His-CHLM was visualized upon binding of AMS and by modified mobility in the nonreducing SDS-PAGE. This approach allowed us to distinguish between oxidized and reduced His-CHLM. The applied amount of recombinant His-CHLM was reduced upon incubation with a minimum concentration of 0.5 mm DTT alone (Fig. 5B, left). In the presence of TRX ms, His-CHLM was reduced by a minimum concentration of 0.1 mm DTT (Fig. 5B, right), suggesting that TRX ms catalyzed the reduction of the intramolecular disulfide bond of CHLM in vitro. Taken together, our results confirm the control of the redox status of CHLM in vivo and in vitro by TRX ms.
Combined Inactivation of TRX m1, TRX m2, TRX m4, and NTRC Leads to a Severe Growth Phenotype and an Enhanced Defect in the TBS Pathway
Combining our current results with previous findings (Richter et al., 2013), the redox status of CHLM appears to be controlled by NTRC and TRX ms. It is hypothesized that TRX m and NTRC are involved cooperatively in the posttranslational control of tetrapyrrole metabolism. To examine this idea, we infected ntrc mutants with pTRV2-TRX m2m4 vectors via agroinfiltration to generate quadruple silencing plants, which we designated as VIGS-TRX m2m4/m1(ntrc) plants. Infected ntrc mutants expressing the pTRV2-GFP vector were used as a control and designated as VIGS-GFP(ntrc) plants. Analysis of gene expression by qRT-PCR revealed that the expression of TRX m1, TRX m2, and TRX m4 was decreased by approximately 85%, 95%, and 93%, respectively, in VIGS-TRX m2m4/m1(ntrc) plants versus the control (Fig. 6B). The quadruple silencing plants showed a severe pale-green and dwarf phenotype compared with VIGS-GFP(ntrc) and VIGS-TRX m2m4/m1(Col-0) plants (Fig. 6A), which argues for the functional nonredundancy of both redox systems.
Figure 6.
Characterization of Arabidopsis VIGS plants. A, Representative images of VIGS-GFP(Col-0), VIGS-TRX m2m4/m1(Col-0), VIGS-GFP(ntrc), and VIGS-TRX m2m4/m1(ntrc) plants, which were infected with the pTRV2-GFP and pTRV2-TRX m2m4 vectors in the Columbia-0 and ntrc mutant backgrounds, respectively, via agroinfiltration. The plants were observed at 3 weeks after infiltration. At least three biological replicates were performed, and similar results were obtained. Bar = 1 cm. B, TRX ms gene expression in VIGS plants was analyzed by qRT-PCR. Relative transcript levels of TRX m1, TRX m2, and TRX m4 in VIGS-TRX m2m4/m1(Col-0), VIGS-GFP(ntrc), and VIGS-TRX m2m4/m1(ntrc) plants were normalized to the level in VIGS-GFP(Col-0) plants (100%). The data represent means ± sd of three biological replicates. C, Chl a and b contents of 5-week-old VIGS-GFP(Col-0), VIGS-TRX m2m4/m1(Col-0), VIGS-GFP(ntrc), and VIGS-TRX m2m4/m1(ntrc) plants. Data are given as means ± sd of three biological replicates. Statistical significance compared with VIGS-TRX m2m4/m1(Col-0), VIGS-GFP(ntrc), and VIGS-TRX m2m4/m1(ntrc) plants is indicated by asterisks (**, P ≤ 0.01, Student’s t test). D, ALA-synthesizing capacity was determined using VIGS-GFP(Col-0), VIGS-TRX m2m4/m1(Col-0), VIGS-GFP(ntrc), and VIGS-TRX m2m4/m1(ntrc) plants. All data are given as means ± sd of three biological replicates. Statistical significance compared with VIGS-TRX m2m4/m1(Col-0), VIGS-GFP(ntrc), and VIGS-TRX m2m4/m1(ntrc) plants is indicated by asterisks (*, P ≤ 0.05 > 0.01 and **, P ≤ 0.01, Student’s t test). E, Heme contents of VIGS-GFP(Col-0), VIGS-TRX m2m4/m1(Col-0), VIGS-GFP(ntrc), and VIGS-TRX m2m4/m1(ntrc) plants. All data are given as means ± sd of three biological replicates. Statistical significance compared with VIGS-TRX m2m4/m1(Col-0), VIGS-GFP(ntrc), and VIGS-TRX m2m4/m1(ntrc) plants is indicated by asterisks (**, P ≤ 0.01, Student’s t test). fw, Fresh weight.
The yellow-green leaves of the quadruple silencing plants were paler than those of the triple TRX m-VIGS plants, and the Chl a content of quadruple silencing plants was decreased by 35% and 20% compared with VIGS-GFP(ntrc) and VIGS-TRX m2m4/m1(Col-0) plants, respectively (Fig. 6C). The ALA-synthesizing capacity in the quadruple silencing plants was strongly diminished compared with that of VIGS-GFP(ntrc) and VIGS-TRX m2m4/m1(Col-0) plants (Fig. 6D). Quantitative analysis of tetrapyrrole intermediates revealed decreased steady-state levels of MgP and MgPMME in VIGS-TRX m2m4/m1(ntrc) seedlings (Supplemental Fig. S4). In addition, the heme level also was decreased upon inactivated expression of NTRC and triple TRX m genes but was similar to that of triple TRX m-silencing plants (Fig. 6E). Moreover, simultaneous silencing of TRX ms and NTRC led to reduced PSII activity (Supplemental Fig. S7). The ratio of variable fluorescence to maximum fluorescence, an indicator of the maximum efficiency of PSII photochemistry, was reduced significantly in VIGS-TRX m2m4/m1(ntrc) plants compared with VIGS-TRX m2m4/m1(Col-0) and VIGS-GFP(ntrc) plants (Supplemental Fig. S7A). By contrast, nonphotochemical quenching was higher in VIGS-TRX m2m4/m1(ntrc) plants than in VIGS-TRX m2m4/m1(Col-0) and VIGS-GFP(ntrc) plants (Supplemental Fig. S7B).
Combined Deficiency of TRX m1, TRX m2, TRX m4, and NTRC Strongly Impairs the Redox Status, Stability, and Enzymatic Activity of CHLM
The above results suggest that inactivated synthesis of three TRX m proteins in the wild-type or ntrc mutant background impairs Chl biosynthesis, as indicated by the reduced ALA synthesis and contents of tetrapyrrole intermediates. Subsequently, we analyzed the mutual impact of deficient TRX m and NTRC on the redox status of CHLM. We subjected total protein extracts from VIGS-GFP(Col-0), VIGS-TRX m2m4/m1(Col-0), VIGS-GFP(ntrc), and VIGS-TRX m2m4/m1(ntrc) plants to AMS treatment under nonreducing conditions. AMS-treated protein extracts from VIGS-TRX m2m4/m1(Col-0), VIGS-GFP(ntrc), and VIGS-TRX m2m4/m1(ntrc) plants again produced two immunoreactive CHLM bands, which represent the reduced CHLM and oxidized CHLM variants, respectively (Fig. 7A). The quantification of the oxidized and reduced CHLM variants (Fig. 7A) indicates that both redox systems, the TRX m isoforms and NTRC, are at least partially cooperatively involved in maintaining the redox status of CHLM. Notably, the CHLM content in VIGS-TRX m2m4/m1(ntrc) plants was lower compared with that in control and VIGS-TRX m2m4/m1(Col-0) plants. We loaded 4 times more total protein on the gel to gain a better visualization of the redox status of CHLM in VIGS-TRX m2m4/m1(ntrc) plants (Fig. 7A). The simultaneous lack of TRX ms and NTRC also is proposed to impair the stability of CHLM in vivo.
Figure 7.
In vivo redox state of CHLM in VIGS plants and CHLM enzymatic activity assay. A, Total protein extracts from leaves of VIGS-GFP(Col-0), VIGS-TRX m2m4/m1(Col-0), VIGS-GFP(ntrc), and VIGS-TRX m2m4/m1(ntrc) plants under nonreducing conditions. Plants grew for 3 weeks after infiltration. Extracts were treated with (+) or without (−) AMS. In vivo CHLM was detected by immunoblot analysis using the anti-CHLM antibody. For loaded proteins, x = 50 μg of total proteins. Samples were separated on a nonreducing SDS-polyacrylamide gel. red, The reduced form; ox, the oxidized form. Relative oxidation (%) represents quantification of the oxidized form of CHLM, in which the immunoreacting CHLM protein bands were quantified by using the Tanon Gis software and the content of the oxidized variant was determined from the ratio between the oxidized form and the total amount of both the reduced and oxidized forms of each sample. We repeated it three times and got the similar results. n.d., Nondetectable. B, Plastid CHLM activity was measured with isolated total proteins from 5-week-old VIGS-GFP(Col-0), VIGS-TRX m2m4/m1(Col-0), VIGS-GFP(ntrc), and VIGS-TRX m2m4/m1(ntrc) plants. The extracted total proteins were treated with (+) or without (−) DTT. The data represent means ± sd of three biological replicates. Statistical significance compared with VIGS-TRX m2m4/m1(ntrc) plants treated with DTT and VIGS-TRX m2m4/m1(ntrc) plants without DTT is indicated by the asterisk (*, P ≤ 0.05 > 0.01, Student’s t test).
To assess the possible combined control of TRX m isoforms and NTRC on CHLM activity, we extracted total proteins from VIGS-GFP(Col-0), VIGS-TRX m2m4/m1(Col-0), VIGS-GFP(ntrc), and VIGS-TRX m2m4/m1(ntrc) plants and assayed them for CHLM catalytic activity. Total proteins extracted from VIGS-TRX m2m4/m1(Col-0) and VIGS-GFP(ntrc) plants displayed 51% and 45% reductions in CHLM activity, respectively, compared with VIGS-GFP(Col-0) plants (Fig. 7B). Notably, we observed an additive negative effect on CHLM activity by simultaneous deficiency of NTRC and the three TRX m variants in VIGS-TRX m2m4/m1(ntrc) plants (Fig. 7B). We propose that this observation also is explained by the decreased stability of CHLM in VIGS-TRX m2m4/m1(ntrc) plants compared with VIGS-TRX m2m4/m1(Col-0) and VIGS-GFP(ntrc) plants (Fig. 7A; Supplemental Fig. S8). These findings of lower CHLM activity as a result of a lower CHLM content also are consistent with the previous report on CHLM activity in ntrc (Richter et al., 2013).
DTT treatment of total protein extracts from the control VIGS-GFP(Col-0) lines did not stimulate CHLM activity (Fig. 7B) and confirmed the reduced state of CHLM (Fig. 7A). The comparison of CHLM activities without and with supplemented DTT in extracts from VIGS-TRX m2m4/m1(Col-0) and VIGS-GFP(ntrc) indicates that the decreased enzyme activity in these samples is explained mainly by the decreased CHLM content (Fig. 7B; Supplemental Fig. S8). DTT treatment of total protein extracted from VIGS-TRX m2m4/m1(ntrc) plants resulted in an elevated CHLM activity compared with the DTT-untreated samples (Fig. 7B). The impact of DTT on CHLM activity in VIGS-TRX m2m4/m1(ntrc) plants suggests that, in addition to the lower CHLM content, the increasing level of the oxidized CHLM variant (Fig. 7A) contributed proportionately to the decreased activity of the enzyme. While the lack of either the m-type TRXs or NTRC mainly compromised the content of CHLM, the additionally decreased CHLM activity in VIGS-TRX m2m4/m1(ntrc) extracts correlated with a combined effect of degraded and oxidized CHLM protein. Reduction of the oxidized CHLM by DTT treatment leads to an almost 2 times higher activity of the remaining CHLM in the protein extract (Fig. 7B).
It is likely that CHLM degradation is eased through the oxidized state of the protein. The concurrent lack of TRX m variants and NTRC caused a higher level of the oxidized form as well as additional degradation of CHLM and, in consequence, decreased CHLM activity by more than 50% in comparison with the VIGS plants, which lack either only TRX m isoforms or NTRC. Taken together, our results indicate an additive effect of both m-type TRXs and NTRC on the redox status and stability of CHLM and, thereby, on CHLM activity.
DISCUSSION
Several different thiol-reducing mechanisms function in plant chloroplasts. Two of these involve Fd-TRXs and NTR, which participate in multiple physiological processes. Although certain target proteins are reduced by a single reductant, other targets may be served by different reducing mechanisms. The latter option allows plants to have a flexible response to varying endogenous and environmental stimuli to select an appropriate reductant for diverse functions (Meyer et al., 2009). Here, we addressed the property of TRX ms and NTRC to act cooperatively and specifically in the redox control of TBS. We provided evidence (to our knowledge for the first time in the TBS pathway) that TRX ms and NTRC participate in the redox regulation of CHLM in a mutually dependent manner.
TRX m1, TRX m2, and TRX m4 Are Essential Posttranslational Regulators of the TBS Pathway in Arabidopsis
Plants require rapid posttranslational regulation of their primary metabolism and organellar gene expression to adapt to varying environmental conditions. Considering the light-, temperature-, and drought-sensitive processes of photosynthesis and pigment biosynthesis in the chloroplast, redox-dependent regulation is adequate for rapidly modulating protein activity, folding, and stability (Cook et al., 2013; Richter and Grimm, 2013; Kang and Wang, 2016; Nikkanen et al., 2016). Several enzymes of TBS are redox controlled by different reductants (Ikegami et al., 2007; Luo et al., 2012; Pérez-Ruiz et al., 2014). In vivo and in vitro analyses revealed the requirement of NTRC for the stability and activity of GluTR and CHLM (Richter et al., 2013).
Here, we demonstrated that triple inactivation of three TRX m genes affected several enzymatic steps of the Chl biosynthesis pathway. The triple TRX m-silencing plants displayed pale-green leaves, which contained 40% lower Chl contents than VIGS-GFP(Col-0) control plants (Fig. 1, A and C). This observation is consistent with our previous results (Wang et al., 2013). Here, we extended this analysis by showing that the loss of Chl correlated with an up to 80% reduction in ALA-synthesizing capacity in VIGS-TRX m2m4/m1(Col-0) seedlings (Fig. 1D) and decreased steady-state levels of porphyrin intermediates of TBS (Fig. 2). Interestingly, inactivation of TRX m1, TRX m2, and TRX m4 also compromised the heme content (Fig. 2D), which was likely due to the decreased supply of ALA for Chl and heme biosynthesis. These results suggest that TRX m deficiency impairs the activity and/or stability of several key regulatory steps, including enzymes of ALA synthesis.
ALA synthesis is controlled by posttranslational feedback cues and plastid-derived retrograde signaling (Apitz et al., 2016). GluTR acts as a rate-limiting enzyme and requires a posttranslational adjustment of ALA supply in response to rapid and instantaneous environmental changes (Tanaka and Tanaka, 2007). The ntrc mutant has decreased ALA synthesis capacity, which is attributed to the decreased stability of GluTR resulting from modified redox status (Richter et al., 2013). In TRX-deficient plants, increased levels of GluTR accumulated despite the reduced levels of corresponding mRNA and the reduced ALA synthesis rate (Figs. 1D and 3). These findings point to a complex regulatory mechanism that occurs in the absence of TRX m or NTRC. Elevated GluTR contents also were reported in VIGS-TRX f/TRX m plants (Luo et al., 2012). The Arabidopsis GluTR level also is regulated by the Clp protease (Nishimura et al., 2013, 2015; Nishimura and van Wijk, 2015). Both GluTR and the subunits of the Clp protease, such as CplS1, ClpF, ClpP6, and ClpT2, contain conserved Cys residues. It is not excluded that TRXs are involved in the redox regulation of the Clp protease. Thus, the elevated GluTR content in VIGS-TRX m2m4/m1(Col-0) plants and VIGS-TRX f/TRX m pea plants also could result from a disturbed proteolytic activity of the Clp protease. Further studies are needed to explore the effect of redox control of GluTR inactivation or proteolysis in response to oxidizing conditions.
Balmer et al. (2003) introduced GSAAT and ALAD as targets for TRX activity. However, we did not observe any interaction between TRX ms and GSAAT as well as ALAD in the in vitro pull-down experiments (Fig. 4A). Nevertheless, the contents of GSAAT, ALAD, POR, and PPOX1 were reduced in VIGS-TRX m2m4/m1(Col-0) plants. Thus, we do not exclude the possibility that the stability of these proteins was disturbed during TRX m deficiency.
Furthermore, we demonstrated that the redox status of CHLM was compromised in TRX m-deficient plants. First, our pull-down and BiFC assays showed that TRX m1, TRX m2, and TRX m4 interact with CHLM in plastids (Fig. 4). Second, the complete reduction of CHLM was prevented upon TRX m deficiency, as demonstrated using AMS-conjugated CHLM. A substantial amount of CHLM was oxidized in triple TRX m-deficient plants (Figs. 5A and 7A). Finally, plastids isolated from VIGS-TRX m2m4/m1(Col-0) plants displayed reduced CHLM activity compared with plastids of VIGS-GFP(Col-0) plants (Fig. 7B). These results are consistent with a previous study showing that CHLM activity is decreased in ntrc mutants, which also suffer from elevated oxidizing conditions (Richter et al., 2013). The lower CHLM activity correlated with lower CHLM contents in VIGS-TRX m2m4/m1(Col-0) plants compared with VIGS-GFP(Col-0) plants (Fig. 3, A and B). As the levels of transcripts encoding Chl synthesis enzymes were not modified in triple TRX m-silencing plants compared with VIGS-GFP(Col-0) plants (Fig. 3C), the lower CHLM contents were ascribed to the interrupted posttranslational redox-dependent regulation. Thus, we conclude that the TRX m variants are required for the redox state and the stability of CHLM for optimal enzyme activity and conformational integrity.
TRX ms and NTRC Cooperatively Participate in the Regulation of the TBS Pathway
Biochemical analysis revealed that both NTRC (Richter et al., 2013) and TRX ms can mutually control CHLM. While TRX ms and NTRC appear to cooperatively participate in the redox control of several sites in tetrapyrrole metabolism (see western blot in Fig. 3A; Richter et al., 2013), we provided, to our knowledge, the first indication of their cooperative action on CHLM by analyzing newly generated quadruple silencing plants for three TRX m genes in the ntrc background. These mutant lines displayed paler yellow-green leaves than VIGS-TRX m2m4/m1(Col-0) and VIGS-GFP(ntrc) plants, which is indicative of a stronger reduction in Chl contents. The ntrc mutant with induced TRX m silencing was even more negatively affected in its ALA-synthesizing capacity than original ntrc and the triple TRX m-silencing control plants (Fig. 6D). Analysis of the contents of intermediates in the TBS pathway revealed that the steady-state levels of MgP and MgPMME were reduced significantly in quadruple silencing seedlings compared with VIGS-GFP(ntrc) and VIGS-TRX m2m4/m1(Col-0) seedlings (Supplemental Fig. S4). Heme levels also were reduced in VIGS-TRX m2m4/m1(ntrc) seedlings compared with VIGS-GFP(ntrc) seedlings (Fig. 6E), which likely derived from the lower levels of ALA-synthesizing capacity (Fig. 6D). These results suggest that both redox systems, TRX ms and NTRC, cooperatively function in tetrapyrrole metabolism.
We found that TRX m1, TRX m2, TRX m4, and NTRC were colocalized in chloroplasts (Supplemental Fig. S3), highlighting the biological relevance of their cooperative function (Nikkanen et al., 2016). AMS-labeling experiments suggested that the combined inactivation of TRX m1, TRX m2, TRX m4, and NTRC correlates with both more accumulation of the oxidized form of CHLM (Fig. 7A) and the decreased CHLM content (Fig. 7A; Supplemental Fig. S8), resulting in an additive negative effect on CHLM activity compared with VIGS-TRX m2m4/m1(Col-0) and VIGS-GFP(ntrc) plants (Fig. 7B). Although oxidized CHLM variants also were observed in VIGS-TRX m2m4/m1(Col-0) and VIGS-GFP(ntrc) plants (Fig. 7A), a DTT-stimulated CHLM enzymatic activity was not observed (Fig. 7B). We propose that the proportion of additional activity by DTT-reduced CHLM was either low or undetectable. Moreover, the explanation that oxidized CHLM was reduced during the enzyme assay by cellular reductants in the total extract cannot entirely be excluded. However, the triple TRX m-silencing and ntrc plants exhibited only half of the control CHLM activity, as a result of the decreased CHLM content, and twice as much activity as VIGS-TRX m2m4/m1(ntrc) plants, which contained even less CHLM. It is proposed that the remaining reductants in the plastids are supportive for the preservation of the remaining CHLM activity.
These results of decreased CHLM activity and content were obtained even though the CHLM transcript levels were not altered significantly compared with those of VIGS-GFP(Col-0) plants (Supplemental Fig. S5). The higher extent of oxidation and the lower content of CHLM are due to disturbed and insufficient posttranslational redox-dependent regulation ultimately affecting CHLM stability and activity.
These findings on CHLM are in accordance with previous reports showing that different redox regulatory factors share common target proteins. TRX f and TRX m stimulate the ATPase activity of CHLI in vitro (Ikegami et al., 2007), and pea TRX f and TRX m cooperatively maintain the reduced state of CHLI in vivo (Luo et al., 2012). Combined knockout of TRX f1 and NTRC strongly inhibited light-dependent changes in FBPase redox transition, thereby impairing Calvin-Benson cycle activity (Thormählen et al., 2015). In continuation, FBPase activity and redox state were drastically and contributively compromised in both ntrc-trxx double mutants as well as ntrc-trxf1f2 triple mutants in comparison with the single mutants and the wild type (Ojeda et al., 2017). The low FBPase activity in these mutants was explained by the decreased stability and the additional high ratio of the oxidized to the reduced form of FBPase.
Impact of Insufficient Content of TRX Isoforms and NTRC on Other Biological Processes
Plant chloroplasts perform photosynthesis and many other biochemical reactions, which are well adjusted in response to the environment, plant development, and plastid biogenesis via mutual communication between the nucleus and the organelles. Thus, nuclear gene expression also is controlled by retrograde signals derived from the chloroplast (Chi et al., 2013, 2015; Estavillo et al., 2013; Yoshida et al., 2015). Recent studies have suggested that plastid redox signals, such as ROS, act as one of the important retrograde signals that affect the expression of the photosynthesis-associated nuclear genes (PhANGs), and the redox signaling is mediated by the photosynthetic electron transport chain and generated by photosynthesis-coupled redox-active compounds, such as TRX, NTRC, or GRX (Pfannschmidt et al., 2001; Wagner et al., 2004; Fey et al., 2005a, 2005b; Nott et al., 2006; Dietz and Pfannschmidt, 2011).
In VIGS-TRX m2m4/m1(ntrc) plants, the transcription of almost all genes examined in the TBS pathway, such as HemA1, ALAD, PPOX1, PORB, and CAO, was down-regulated significantly (Supplemental Fig. S5). These expression profiles differ from those of the same genes in the triple TRX-silencing plants (Supplemental Fig. S5). In addition, ROS accumulated in the leaf tissue of quadruple silencing plants (Supplemental Fig. S6). Similar results were reported for pea VIGS-TRX f/TRX m-silencing plants (Luo et al., 2012). These results suggest that redox-dependent control was hampered as a result of TRX ms and NTRC deficiency. The lack of these reductants and the formation of ROS appear to be responsible for the down-regulation of PhANG transcription in VIGS-TRX m2m4/m1(ntrc) plants.
Moreover, the inactivation of both thiol redox regulators, TRX ms and NTRC, led to more severely inhibited growth (Fig. 6A) and decreased PSII activity (Supplemental Fig. S7). The severe growth phenotype also can likely be explained by the defect in plastid redox status, which also controls photosynthetic electron transport, biogenesis of PSII, activation of Calvin cycle enzymes, and starch synthesis in quadruple silencing plants (Michalska et al., 2009; Lepistö et al., 2013; Wang et al., 2013; Okegawa and Motohashi, 2015; Thormählen et al., 2015). Moreover, a double mutant lacking FTRb and NTRC shows a lethal phenotype on soil and is only viable on Murashige and Skoog medium supplemented with Suc. Under these conditions, the mutant displays retarded growth, a weak yellow-green pigmentation, and dramatically decreased photosynthetic activity (Yoshida and Hisabori, 2016). This phenotype resembles the TRX ms and NTRC quadruple silencing plants (Fig. 6A). However, it is not excluded that other redox regulators (e.g. other TRX isoforms) can partially compensate for the dysfunction of TRX m variants and NTRC.
In conclusion, chloroplast redox regulation is essential for intraorganellar processes, including metabolic pathways such as Chl synthesis. The elucidation of the plastid-localized TRX- and NTRC-dependent redox control of enzymes of the TBS pathway in Arabidopsis is just beginning. Our findings indicate that the regulation of the CHLM redox state involves different reductants. The collective redox control by TRX m variants and NTRC maintains the reduced level of CHLM as well as affects its stability and, thus, the enzymatic activity. In addition, we obtained initial hints about the important contributions of TRX m and NTRC to other biological processes, such as the maintenance of ROS levels and the modulation of PhANG expression.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) wild-type (ecotype Columbia-0) and ntrc knockout (At2g41680 [SALK 096776]) plants were grown in soil in a growth chamber (110 μmol photons m−2 s−1, 16 h of light, 21°C–23°C).
VIGS Assay
Plasmids pTRV1 and pTRV2 are VIGS vectors based on Tobacco rattle virus. To prepare the pTRV2-TRX m vectors, TRX m1, TRX m2, and TRX m4 cDNAs were generated by PCR using the primers described in Supplemental Table S1 and inserted into the pTRV2 vector. The pTRV2-GFP vector was utilized as a negative control. The pTRV1 and pTRV2 derivatives were introduced into Arabidopsis plants by Agrobacterium tumefaciens infiltration as described (Wang et al., 2013).
Determination of Pigments and Chl Precursors by HPLC
Light-exposed leaves from various VIGS plants were homogenized under frozen conditions, suspended in ice-cold acetone:0.2 m NH4OH (9:1, v/v) buffer, and centrifuged. The supernatants were analyzed by reverse-phase chromatography on Agilent HPLC systems as described (Schlicke et al., 2014).
ALA Synthesis Capacity Measurement
ALA-synthesizing capacity was analyzed as described (Richter et al., 2013). Briefly, leaf discs with a diameter of approximately 5 mm from different VIGS plants were incubated in 50 mm Tris-HCl, pH 7.2, and 40 mm levulinic acid for 2 h under growth lights. The absorption of ALA pyrrole was measured at 526, 553, and 720 nm. The ALA content was calculated using a dilution curve with an ALA solution (Sigma-Aldrich) and related to the incubation time and fresh weight of the samples.
Protein Extraction and Western-Blot Analysis
Total proteins were extracted from leaves of VIGS plants in extraction buffer (10 mm HEPES-KOH, pH 7.5, 150 mm NaCl, 5 mm EDTA, 0.5% [v/v] Triton X-100, 56 mm DTT, and Cocktail protein inhibitor). Protein concentrations were determined using the Bio-Rad detergent-compatible colorimetric protein assay according to the manufacturer’s protocol. The proteins were separated by 12% SDS-PAGE, blotted onto polyvinylidene difluoride membranes (Millipore), and probed using antibodies specifically directed against individual enzymes of the TBS pathway. Signals were detected with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific).
Gene Expression Analysis
Total RNA was extracted from VIGS plants with the Plant RNeasy Mini Kit (Omega), and 3-μg samples of total RNA were used for cDNA synthesis with the PrimeScript RT Reagent Kit (Takara). qRT-PCR was performed with SYBR Premix Ex Taq (Takara), and real-time amplification was monitored on the LightCycler480 system (Roche). UBIQUITIN4 (At5g20620) was used as an internal control. Primer sequences are listed in Supplemental Table S1.
Expression and Purification of Recombinant His-Tagged Proteins
Arabidopsis TRX m1, TRX m2, TRX m3, and TRX m4 were cloned into the pET28a expression vector (Novagen) for the expression of N-terminal 6×His-tagged proteins. Supplemental Table S1 shows the primers used in the cloning procedures. The pET28a-TRX m1, pET28a-TRX m2, pET28a-TRX m3, and pET28a-TRX m4 plasmids were transformed into Escherichia coli BL21 (DE3) (Stratagene), and protein expression was induced with 0.4 mm isopropyl β-d-thiogalactoside. Purification of the His-tagged TRX m isoforms was carried out with Ni+ affinity chromatography (GE Healthcare). The imidazole in the elution buffer was removed by fast ultrafiltration using 3-kD-limit Amicon Ultra-15 centrifugal filter units (Millipore). The purification of recombination of His-CHLM was performed essentially as described (Richter et al., 2016).
Pull-Down Assay
The pull-down assay was performed essentially as described by Wang et al. (2013), with some modifications. Purified His-TRX m1, His-TRX m2, His-TRX m3, and His-TRX m4 recombination proteins were immobilized on Sepharose 4B resin (GE Healthcare) and incubated with total chloroplast proteins extracted from the wild type. Empty resin without TRX m protein was used as the negative control. The resins were washed several times with washing buffer containing 0.5 m NaCl. TRX m target proteins were then eluted with elution buffer and analyzed by immunoblotting.
BiFC and Subcellular Localization Studies
The BiFC assay was performed as described (Walter et al., 2004). Full-length cDNAs of CHLM, TRX m1, TRX m2, TRX m3, TRX m4, and bZIP63 were cloned into pUC-SPYNE or pUC-SPYCE, and the plasmids were cotransformed into Arabidopsis mesophyll protoplasts. The protoplasts were transiently transformed with the respective constructs as described (Yoo et al., 2007; Zhang et al., 2011). Fluorescence was observed via confocal laser scanning microscopy (Zeiss 7 DUO NLO).
The subcellular localization of NTRC and TRX m1, TRX m2, and TRX m4 fusion proteins was analyzed using YFP and CFP fluorescence. Fluorophore signals, Chl autofluorescence, and bright-field images were scanned sequentially in channel mode to prevent any cross talk between fluorescence channels (Thormählen et al., 2015).
In Vivo CHLM Enzymatic Assay
The in vivo CHLM enzymatic assay was performed according to Richter et al. (2013, 2016), with minor modifications. The freeze-dried leaf materials from various VIGS plants were suspended in resuspension buffer (0.3 m sorbitol, 20 mm Tricine-KOH, pH 8.4, 2.5 mm EDTA, 5 mm MgCl2 and protease inhibitor cocktail). Obtained leaf extracts were incubated with 10 mm DTT or without DTT for 15 min in darkness at room temperature. All assays began with the addition of 250 mm SAM and 5 mm MgP, followed by incubation at 30°C with 10 mm DTT and without DTT up to 15 min. The enzymatic reactions were stopped by the addition of ice-cold acetone:0.2 m NH4OH (9:1, v/v) buffer. The MgPMME products were determined by HPLC.
Determination of the Redox State of CHLM Protein in Vivo and in Vitro
The in vivo redox states of proteins were determined using protein extracts from various VIGS plants without reducing agents in the extraction buffer as described previously (Hendriks et al., 2003), with minor modifications. For the preparation of the protein extracts, we fixed our samples with liquid nitrogen. Extracts were precipitated with 10% (w/v) TCA and washed twice with 100% acetone before the protein pellet was resuspended in 1% SDS and 50 mm Tris-HCl buffer. One part of the samples was incubated with AMS (Life Technologies) for 20 min at room temperature. Both AMS-treated and untreated samples were separated, blotted, and probed with CHLM antibodies.
The in vitro redox states of CHLM proteins were determined as in a previous study (Ikegami et al., 2007; Richter et al., 2016), with some modifications. Recombinant His-CHLM proteins (20 μg) were incubated with various concentrations of DTT in the absence or presence of 14 μg of TRX ms (TRX m1, TRX m2, and TRX m4) for 45 min, precipitated by 10% (w/v) TCA for 10 min on ice, and then reacted with or without AMS (Life Technologies) for 20 min at room temperature. Protein mobility was visualized using Coomassie Brilliant Blue staining after 10% nonreducing SDS-PAGE.
Measurement of ROS in Leaves
The in situ accumulation of O2− and hydrogen peroxide was measured as described (Wang et al., 2013), with minor modifications. Leaves from various VIGS plants were vacuum infiltrated with a solution containing 1 mg mL−1 DAB (pH 3.8) at room temperature for 1 h, followed by incubation in the same solution under growth light illumination for 2 h. The stained leaves were then boiled in a solution of ethanol:acetic acid:glycerol (3:1:1 [v/v/v]) for 10 min. For O2− detection, the leaves were vacuum infiltrated for 1 h with 50 mm PBS buffer (pH 7.5) containing 1 mg mL−1 NBT and subsequently boiled in 90% (v/v) ethanol for 10 min.
Chl Fluorescence Measurements
Chl fluorescence was measured using Maxi-Imaging PAM (Heinz-Walz Instruments). Arabidopsis leaves were dark adapted for 15 min prior to measurements. The maximum fluorescence yield and nonphotochemical quenching were determined as described (Wang et al., 2013).
Accession Numbers
The tetrapyrrole biosynthesis-related gene accession numbers are as follows: UBQ4 (AT5G20620), TRX m1 (AT1G03680), TRX m2 (AT4G03520), TRX m3 (AT2G15570), TRX m4 (AT3G15360), HemA1 (AT1G58290), ALAD (AT1G44286), PPOX1 (AT4G01690), CHLI-1 (AT4G18480), CHLI-2 (AT5G45930), CHLM (AT4G25080), CHLG (AT3G51820), PORB (AT4G27440), CAO (AT1G44446), and NTRC (AT2G41680).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Purification of 6×His-tagged TRX m1, TRX m2, TRX m3, TRX m4, and CHLM.
Supplemental Figure S2. Negative controls used for the BiFC assay and identification of TRX m3-Flag protein expression in transgenic protoplasts.
Supplemental Figure S3. Suborganellar colocalization of TRX ms and NTRC proteins.
Supplemental Figure S4. Contents of MgP and MgPMME in VIGS plants.
Supplemental Figure S5. Transcript analysis of VIGS plants.
Supplemental Figure S6. Visualization of hydrogen peroxide and O2− in leaves of VIGS plants by histochemical staining.
Supplemental Figure S7. Chl fluorescence parameters in VIGS plants.
Supplemental Figure S8. Content of CHLM in total protein extracts of VIGS plants, which were used for the measurement of CHLM enzymatic activity.
Supplemental Table S1. List of primers used in this study.
Acknowledgments
We thank Yule Liu (Tsinghua University) for sharing TRV-based VIGS vectors and Yee-yung Charng (Agricultural Biotechnology Research Center, Academia Sinica) for the gift of CHLG antibody.
Glossary
- Chl
chlorophyll
- ROS
reactive oxygen species
- TBS
tetrapyrrole biosynthesis
- ALA
5-aminolevulinic acid
- Pchlide
protochlorophyllide
- MgP
Mg porphyrin
- MgPMME
Mg porphyrin monomethylester
- Chlide
chlorophyllide
- BiFC
bimolecular fluorescence complementation
- VIGS
virus-induced gene silencing
- AMS
4-acetoamido-4-maleimidylstilbene-2,2-disulfonate
- PhANG
photosynthesis-associated nuclear gene
Footnotes
This work was supported by The National Science Fund for Distinguished Young Scholars (no. 31425003), The National Science and Technology Major Project Foundation of China (no. 2016ZX08009003-005-005), a grant from the Deutsche Forschungsgemeinschaft (DFG 936 17-1) in the framework of the Priority Program Dynamics of Thiol-based Redox Switches in Cellular Physiology (SPP1710) to B.G, a grant from The Alexander von Humboldt Foundation (to P.W.), and grants from the International Program for Ph.D. Candidates, Sun Yat-Sen University (no. 52114000).
Articles can be viewed without a subscription.
References
- Apitz J, Nishimura K, Schmied J, Wolf A, Hedtke B, van Wijk KJ, Grimm B (2016) Posttranslational control of ALA synthesis includes GluTR degradation by Clp protease and stabilization by GluTR-binding protein. Plant Physiol 170: 2040–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer Y, Koller A, del Val G, Manieri W, Schürmann P, Buchanan BB (2003) Proteomics gives insight into the regulatory function of chloroplast thioredoxins. Proc Natl Acad Sci USA 100: 370–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsera M, Uberegui E, Schürmann P, Buchanan BB (2014) Evolutionary development of redox regulation in chloroplasts. Antioxid Redox Signal 21: 1327–1355 [DOI] [PubMed] [Google Scholar]
- Baumann U, Juttner J (2002) Plant thioredoxins: the multiplicity conundrum. Cell Mol Life Sci 59: 1042–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Alfonso Y, Cilia M, San Roman A, Thomas C, Maule A, Hearn S, Jackson D (2009) Control of Arabidopsis meristem development by thioredoxin-dependent regulation of intercellular transport. Proc Natl Acad Sci USA 106: 3615–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezowski P, Richter AS, Grimm B (2015) Regulation and function of tetrapyrrole biosynthesis in plants and algae. Biochim Biophys Acta 1847: 968–985 [DOI] [PubMed] [Google Scholar]
- Chae HB, Moon JC, Shin MR, Chi YH, Jung YJ, Lee SY, Nawkar GM, Jung HS, Hyun JK, Kim WY, et al. (2013) Thioredoxin reductase type C (NTRC) orchestrates enhanced thermotolerance to Arabidopsis by its redox-dependent holdase chaperone function. Mol Plant 6: 323–336 [DOI] [PubMed] [Google Scholar]
- Chen X, Wang X, Feng J, Chen Y, Fang Y, Zhao S, Zhao A, Zhang M, Liu L (2014) Structural insights into the catalytic mechanism of Synechocystis magnesium protoporphyrin IX O-methyltransferase (ChlM). J Biol Chem 289: 25690–25698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi W, Feng P, Ma J, Zhang L (2015) Metabolites and chloroplast retrograde signaling. Curr Opin Plant Biol 25: 32–38 [DOI] [PubMed] [Google Scholar]
- Chi W, Sun X, Zhang L (2013) Intracellular signaling from plastid to nucleus. Annu Rev Plant Biol 64: 559–582 [DOI] [PubMed] [Google Scholar]
- Collin V, Issakidis-Bourguet E, Marchand C, Hirasawa M, Lancelin JM, Knaff DB, Miginiac-Maslow M (2003) The Arabidopsis plastidial thioredoxins: new functions and new insights into specificity. J Biol Chem 278: 23747–23752 [DOI] [PubMed] [Google Scholar]
- Cook KM, McNeil HP, Hogg PJ (2013) Allosteric control of βII-tryptase by a redox active disulfide bond. J Biol Chem 288: 34920–34929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courteille A, Vesa S, Sanz-Barrio R, Cazalé AC, Becuwe-Linka N, Farran I, Havaux M, Rey P, Rumeau D (2013) Thioredoxin m4 controls photosynthetic alternative electron pathways in Arabidopsis. Plant Physiol 161: 508–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki O, Grimm B (2012) Post-translational control of tetrapyrrole biosynthesis in plants, algae, and cyanobacteria. J Exp Bot 63: 1675–1687 [DOI] [PubMed] [Google Scholar]
- Czarnecki O, Hedtke B, Melzer M, Rothbart M, Richter A, Schröter Y, Pfannschmidt T, Grimm B (2011) An Arabidopsis GluTR binding protein mediates spatial separation of 5-aminolevulinic acid synthesis in chloroplasts. Plant Cell 23: 4476–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz KJ. (2016) Thiol-based peroxidases and ascorbate peroxidases: why plants rely on multiple peroxidase systems in the photosynthesizing chloroplast? Mol Cells 39: 20–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz KJ, Pfannschmidt T (2011) Novel regulators in photosynthetic redox control of plant metabolism and gene expression. Plant Physiol 155: 1477–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estavillo GM, Chan KX, Phua SY, Pogson BJ (2013) Reconsidering the nature and mode of action of metabolite retrograde signals from the chloroplast. Front Plant Sci 3: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey V, Wagner R, Bräutigam K, Pfannschmidt T (2005a) Photosynthetic redox control of nuclear gene expression. J Exp Bot 56: 1491–1498 [DOI] [PubMed] [Google Scholar]
- Fey V, Wagner R, Braütigam K, Wirtz M, Hell R, Dietzmann A, Leister D, Oelmüller R, Pfannschmidt T (2005b) Retrograde plastid redox signals in the expression of nuclear genes for chloroplast proteins of Arabidopsis thaliana. J Biol Chem 280: 5318–5328 [DOI] [PubMed] [Google Scholar]
- Fu Y, Ballicora MA, Leykam JF, Preiss J (1998) Mechanism of reductive activation of potato tuber ADP-glucose pyrophosphorylase. J Biol Chem 273: 25045–25052 [DOI] [PubMed] [Google Scholar]
- Gibson LC, Willows RD, Kannangara CG, von Wettstein D, Hunter CN (1995) Magnesium-protoporphyrin chelatase of Rhodobacter sphaeroides: reconstitution of activity by combining the products of the bchH, -I, and -D genes expressed in Escherichia coli. Proc Natl Acad Sci USA 92: 1941–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks JH, Kolbe A, Gibon Y, Stitt M, Geigenberger P (2003) ADP-glucose pyrophosphorylase is activated by posttranslational redox-modification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol 133: 838–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. (1985) Thioredoxin. Annu Rev Biochem 54: 237–271 [DOI] [PubMed] [Google Scholar]
- Ikegami A, Yoshimura N, Motohashi K, Takahashi S, Romano PG, Hisabori T, Takamiya K, Masuda T (2007) The CHLI1 subunit of Arabidopsis thaliana magnesium chelatase is a target protein of the chloroplast thioredoxin. J Biol Chem 282: 19282–19291 [DOI] [PubMed] [Google Scholar]
- Jensen PE, Gibson LC, Henningsen KW, Hunter CN (1996) Expression of the chlI, chlD, and chlH genes from the cyanobacterium Synechocystis PCC6803 in Escherichia coli and demonstration that the three cognate proteins are required for magnesium-protoporphyrin chelatase activity. J Biol Chem 271: 16662–16667 [DOI] [PubMed] [Google Scholar]
- Kang ZH, Wang GX (2016) Redox regulation in the thylakoid lumen. J Plant Physiol 192: 28–37 [DOI] [PubMed] [Google Scholar]
- Karamoko M, Cline S, Redding K, Ruiz N, Hamel PP (2011) Lumen Thiol Oxidoreductase1, a disulfide bond-forming catalyst, is required for the assembly of photosystem II in Arabidopsis. Plant Cell 23: 4462–4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchsteiger K, Ferrández J, Pascual MB, González M, Cejudo FJ (2012) NADPH thioredoxin reductase C is localized in plastids of photosynthetic and nonphotosynthetic tissues and is involved in lateral root formation in Arabidopsis. Plant Cell 24: 1534–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchsteiger K, Pulido P, González M, Cejudo FJ (2009) NADPH thioredoxin reductase C controls the redox status of chloroplast 2-Cys peroxiredoxins in Arabidopsis thaliana. Mol Plant 2: 298–307 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kishigami S, Sone M, Inokuchi H, Mogi T, Ito K (1997) Respiratory chain is required to maintain oxidized states of the DsbA-DsbB disulfide bond formation system in aerobically growing Escherichia coli cells. Proc Natl Acad Sci USA 94: 11857–11862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara C, de la Torre A, Buchanan BB (1980) A new protein factor functional in the ferredoxin-independent light activation of chloroplast fructose 1,6-bisphosphatase. Biochem Biophys Res Commun 93: 544–551 [DOI] [PubMed] [Google Scholar]
- Lepistö A, Pakula E, Toivola J, Krieger-Liszkay A, Vignols F, Rintamäki E (2013) Deletion of chloroplast NADPH-dependent thioredoxin reductase results in inability to regulate starch synthesis and causes stunted growth under short-day photoperiods. J Exp Bot 64: 3843–3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Weinstein JD, Walker CJ (1999) Magnesium chelatase subunit D from pea: characterization of the cDNA, heterologous expression of an enzymatically active protein and immunoassay of the native protein. Plant Mol Biol 41: 721–731 [DOI] [PubMed] [Google Scholar]
- Luo T, Fan T, Liu Y, Rothbart M, Yu J, Zhou S, Grimm B, Luo M (2012) Thioredoxin redox regulates ATPase activity of magnesium chelatase CHLI subunit and modulates redox-mediated signaling in tetrapyrrole biosynthesis and homeostasis of reactive oxygen species in pea plants. Plant Physiol 159: 118–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand C, Le Maréchal P, Meyer Y, Decottignies P (2006) Comparative proteomic approaches for the isolation of proteins interacting with thioredoxin. Proteomics 6: 6528–6537 [DOI] [PubMed] [Google Scholar]
- Meyer Y, Belin C, Delorme-Hinoux V, Reichheld JP, Riondet C (2012) Thioredoxin and glutaredoxin systems in plants: molecular mechanisms, crosstalks, and functional significance. Antioxid Redox Signal 17: 1124–1160 [DOI] [PubMed] [Google Scholar]
- Meyer Y, Buchanan BB, Vignols F, Reichheld JP (2009) Thioredoxins and glutaredoxins: unifying elements in redox biology. Annu Rev Genet 43: 335–367 [DOI] [PubMed] [Google Scholar]
- Michalska J, Zauber H, Buchanan BB, Cejudo FJ, Geigenberger P (2009) NTRC links built-in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proc Natl Acad Sci USA 106: 9908–9913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelet L, Zaffagnini M, Morisse S, Sparla F, Pérez-Pérez ME, Francia F, Danon A, Marchand CH, Fermani S, Trost P, et al. (2013) Redox regulation of the Calvin-Benson cycle: something old, something new. Front Plant Sci 4: 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen R, Mutenda KE, Mant A, Schürmann P, Blennow A (2005) Alpha-glucan, water dikinase (GWD): a plastidic enzyme with redox-regulated and coordinated catalytic activity and binding affinity. Proc Natl Acad Sci USA 102: 1785–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki N, Tanaka R, Grimm B, Masuda T, Moulin M, Smith AG, Tanaka A, Terry MJ (2010) The cell biology of tetrapyrroles: a life and death struggle. Trends Plant Sci 15: 488–498 [DOI] [PubMed] [Google Scholar]
- Naranjo B, Mignée C, Krieger-Liszkay A, Hornero-Méndez D, Gallardo-Guerrero L, Cejudo FJ, Lindahl M (2016) The chloroplast NADPH thioredoxin reductase C, NTRC, controls non-photochemical quenching of light energy and photosynthetic electron transport in Arabidopsis. Plant Cell Environ 39: 804–822 [DOI] [PubMed] [Google Scholar]
- Nikkanen L, Toivola J, Rintamäki E (2016) Crosstalk between chloroplast thioredoxin systems in regulation of photosynthesis. Plant Cell Environ 39: 1691–1705 [DOI] [PubMed] [Google Scholar]
- Nishimura K, Apitz J, Friso G, Kim J, Ponnala L, Grimm B, van Wijk KJ (2015) Discovery of a unique Clp component, ClpF, in chloroplasts: a proposed binary ClpF-ClpS1 adaptor complex functions in substrate recognition and delivery. Plant Cell 27: 2677–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Asakura Y, Friso G, Kim J, Oh SH, Rutschow H, Ponnala L, van Wijk KJ (2013) ClpS1 is a conserved substrate selector for the chloroplast Clp protease system in Arabidopsis. Plant Cell 25: 2276–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, van Wijk KJ (2015) Organization, function and substrates of the essential Clp protease system in plastids. Biochim Biophys Acta 1847: 915–930 [DOI] [PubMed] [Google Scholar]
- Nott A, Jung HS, Koussevitzky S, Chory J (2006) Plastid-to-nucleus retrograde signaling. Annu Rev Plant Biol 57: 739–759 [DOI] [PubMed] [Google Scholar]
- Ojeda V, Pérez-Ruiz JM, González M, Nájera VA, Sahrawy M, Serrato AJ, Geigenberger P, Cejudo FJ (2017) NADPH thioredoxin reductase C and thioredoxins act concertedly in seedling development. Plant Physiol 174: 1436–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okegawa Y, Motohashi K (2015) Chloroplastic thioredoxin m functions as a major regulator of Calvin cycle enzymes during photosynthesis in vivo. Plant J 84: 900–913 [DOI] [PubMed] [Google Scholar]
- Pérez-Ruiz JM, Guinea M, Puerto-Galán L, Cejudo FJ (2014) NADPH thioredoxin reductase C is involved in redox regulation of the Mg-chelatase I subunit in Arabidopsis thaliana chloroplasts. Mol Plant 7: 1252–1255 [DOI] [PubMed] [Google Scholar]
- Pérez-Ruiz JM, Spínola MC, Kirchsteiger K, Moreno J, Sahrawy M, Cejudo FJ (2006) Rice NTRC is a high-efficiency redox system for chloroplast protection against oxidative damage. Plant Cell 18: 2356–236816891402 [Google Scholar]
- Petersen BL, Jensen PE, Gibson LC, Stummann BM, Hunter CN, Henningsen KW (1998) Reconstitution of an active magnesium chelatase enzyme complex from the bchI, -D, and -H gene products of the green sulfur bacterium Chlorobium vibrioforme expressed in Escherichia coli. J Bacteriol 180: 699–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannschmidt T, Schütze K, Brost M, Oelmüller R (2001) A novel mechanism of nuclear photosynthesis gene regulation by redox signals from the chloroplast during photosystem stoichiometry adjustment. J Biol Chem 276: 36125–36130 [DOI] [PubMed] [Google Scholar]
- Richter AS, Grimm B (2013) Thiol-based redox control of enzymes involved in the tetrapyrrole biosynthesis pathway in plants. Front Plant Sci 4: 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter AS, Peter E, Rothbart M, Schlicke H, Toivola J, Rintamäki E, Grimm B (2013) Posttranslational influence of NADPH-dependent thioredoxin reductase C on enzymes in tetrapyrrole synthesis. Plant Physiol 162: 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter AS, Wang P, Grimm B (2016) Arabidopsis Mg-protoporphyrin IX methyltransferase activity and redox regulation depend on conserved cysteines. Plant Cell Physiol 57: 519–527 [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Kozaki A, Hatano M (1997) Link between light and fatty acid synthesis: thioredoxin-linked reductive activation of plastidic acetyl-CoA carboxylase. Proc Natl Acad Sci USA 94: 11096–11101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicke H, Hartwig AS, Firtzlaff V, Richter AS, Glässer C, Maier K, Finkemeier I, Grimm B (2014) Induced deactivation of genes encoding chlorophyll biosynthesis enzymes disentangles tetrapyrrole-mediated retrograde signaling. Mol Plant 7: 1211–1227 [DOI] [PubMed] [Google Scholar]
- Schürmann P, Buchanan BB (2008) The ferredoxin/thioredoxin system of oxygenic photosynthesis. Antioxid Redox Signal 10: 1235–1274 [DOI] [PubMed] [Google Scholar]
- Schwarz O, Schürmann P, Strotmann H (1997) Kinetics and thioredoxin specificity of thiol modulation of the chloroplast H+-ATPase. J Biol Chem 272: 16924–16927 [DOI] [PubMed] [Google Scholar]
- Serrato AJ, Fernández-Trijueque J, Barajas-López JD, Chueca A, Sahrawy M (2013) Plastid thioredoxins: a “one-for-all” redox-signaling system in plants. Front Plant Sci 4: 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrato AJ, Pérez-Ruiz JM, Spínola MC, Cejudo FJ (2004) A novel NADPH thioredoxin reductase, localized in the chloroplast, which deficiency causes hypersensitivity to abiotic stress in Arabidopsis thaliana. J Biol Chem 279: 43821–43827 [DOI] [PubMed] [Google Scholar]
- Seung D, Thalmann M, Sparla F, Abou Hachem M, Lee SK, Issakidis-Bourguet E, Svensson B, Zeeman SC, Santelia D (2013) Arabidopsis thaliana AMY3 is a unique redox-regulated chloroplastic α-amylase. J Biol Chem 288: 33620–33633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spínola MC, Pérez-Ruiz JM, Pulido P, Kirchsteiger K, Guinea M, González M, Cejudo FJ (2008) NTRC new ways of using NADPH in the chloroplast. Physiol Plant 133: 516–524 [DOI] [PubMed] [Google Scholar]
- Stenbaek A, Jensen PE (2010) Redox regulation of chlorophyll biosynthesis. Phytochemistry 71: 853–859 [DOI] [PubMed] [Google Scholar]
- Tanaka R, Kobayashi K, Masuda T (2011) Tetrapyrrole metabolism in Arabidopsis thaliana. The Arabidopsis Book 9: e0145, doi/10.1199/tab.0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Tanaka A (2007) Tetrapyrrole biosynthesis in higher plants. Annu Rev Plant Biol 58: 321–346 [DOI] [PubMed] [Google Scholar]
- Thormählen I, Meitzel T, Groysman J, Öchsner AB, von Roepenack-Lahaye E, Naranjo B, Cejudo FJ, Geigenberger P (2015) Thioredoxin f1 and NADPH-dependent thioredoxin reductase C have overlapping functions in regulating photosynthetic metabolism and plant growth in response to varying light conditions. Plant Physiol 169: 1766–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thormählen I, Ruber J, von Roepenack-Lahaye E, Ehrlich SM, Massot V, Hümmer C, Tezycka J, Issakidis-Bourguet E, Geigenberger P (2013) Inactivation of thioredoxin f1 leads to decreased light activation of ADP-glucose pyrophosphorylase and altered diurnal starch turnover in leaves of Arabidopsis plants. Plant Cell Environ 36: 16–29 [DOI] [PubMed] [Google Scholar]
- Thormahlen I, Zupok A, Rescher J, Leger J, Weissenberger S, Groysman J, Orwat A, Chatel-Innocenti G, Issakidis-Bourguet E, Armbruster U, et al. (2017) Thioredoxins play a crucial role in dynamic acclimation of photosynthesis in fluctuating light. Mol Plant 10: 168–182 [DOI] [PubMed] [Google Scholar]
- Wagner D, Przybyla D, Op den Camp R, Kim C, Landgraf F, Lee KP, Würsch M, Laloi C, Nater M, Hideg E, et al. (2004) The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306: 1183–1185 [DOI] [PubMed] [Google Scholar]
- Walter M, Chaban C, Schütze K, Batistic O, Weckermann K, Näke C, Blazevic D, Grefen C, Schumacher K, Oecking C, et al. (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40: 428–438 [DOI] [PubMed] [Google Scholar]
- Wang P, Liu J, Liu B, Feng D, Da Q, Wang P, Shu S, Su J, Zhang Y, Wang J, et al. (2013) Evidence for a role of chloroplastic m-type thioredoxins in the biogenesis of photosystem II in Arabidopsis. Plant Physiol 163: 1710–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosiuk RA, Crawford NA, Yee BC, Buchanan BB (1979) Isolation of three thioredoxins from spinach leaves. J Biol Chem 254: 1627–1632 [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Hara S, Hisabori T (2015) Thioredoxin selectivity for thiol-based redox regulation of target proteins in chloroplasts. J Biol Chem 290: 19540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Hisabori T (2016) Two distinct redox cascades cooperatively regulate chloroplast functions and sustain plant viability. Proc Natl Acad Sci USA 113: E3967–E3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Su J, Duan S, Ao Y, Dai J, Liu J, Wang P, Li Y, Liu B, Feng D, et al. (2011) A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]