Abstract
GN refers to a variety of renal pathologies that often progress to ESRD, but the molecular mechanisms underlying this progression remain incompletely characterized. Here, we determined whether dysregulated expression of the gap junction protein connexin 43, which has been observed in the progression of renal disease, contributes to GN progression. Immunostaining revealed de novo expression of connexin 43 in damaged glomeruli in patients with glomerular diseases as well as in mice after induction of experimental GN. Notably, 2 weeks after the induction of GN with nephrotoxic serum, mice with a heterozygous deletion of the connexin 43 gene (connexin 43+/−) had proteinuria, BUN, and serum creatinine levels significantly lower than those of wild-type animals. Additionally, the connexin 43+/− mice showed less crescent formation, tubular dilation, monocyte infiltration, and interstitial renal fibrosis. Treatment of cultured podocytes with connexin 43–specific blocking peptides attenuated TGF-β–induced cytoskeletal and morphologic changes and apoptosis as did treatment with the purinergic blocker suramin. Finally, therapeutic treatment of GN mice with connexin 43–specific antisense oligodeoxynucleotide improved functional and structural renal parameters. These findings suggest that crosstalk between connexin 43 and purinergic signaling contributes to podocyte damage in GN. Given that this protein is highly induced in individuals with glomerular diseases, connexin 43 may be a novel target for therapeutic treatment of GN.
Keywords: glomerular disease, connexins, podocyte, renal fibrosis

GN encompasses a range of disorders characterized by glomerular damage that subsequently may affect other renal compartments.1,2 Several studies have provided insights showing various crosstalks between inflammatory and renal glomerular cells, both crucial for the progression of the disease.3 Despite these pathophysiologic advances, current treatments for GN remain nonspecific and partially successful.
Rapidly progressive GN is a rare clinical syndrome, which can develop with or without immune deposits and can progress rapidly to terminal renal failure if not treated properly. The main histologic finding of this disease is crescent formation affecting >50% of total glomeruli in humans. Crescent formation presents cellular and noncellular composites interacting through mechanisms that have not been completely mapped. The cellular composition of crescents is controversial; proliferating cells rising from parietal epithelial cells and podocytes, in addition to invading macrophages and myofibroblasts, have been proposed as the main sources.4,5 Recent studies have suggested that podocyte injury leads to dedifferentiation, foot processes effacement followed by detachment, and eventually, podocyte loss and/or migration toward the urinary chamber, contributing to the progression of several glomerular conditions, including rapidly progressive GN.6–8 However, the molecular mechanisms regulating these events are still poorly understood.
Connexins (Cxs) are nowadays considered as emerging proteins playing a crucial role in renal physiopathology, because they control several cell homeostatic processes.9 The Cx family is composed of around 20 different transmembrane proteins.10,11 Six Cx subunits are assembled into hexameric connexons and transported to the plasma membrane, where they can dock with other connexons of adjacent cells to form gap junctions (GJs). These GJs mediate direct intercellular exchange of ions and small metabolites.12,13 In addition, connexons alone can exert their function as hemichannels (HCs) by secreting these small molecules to the extracellular space.14,15
Misregulation of Cx expression, location, and function has been linked to various pathologies.16–18 Moreover, mutations of some Cx subtypes were identified to be responsible for a variety of human genetic diseases.19 Interestingly, few studies reported that increased expression of Cx43 occurred during the progression of renal disease in humans and rodents.20–22 Additionally, we have recently reported an abnormal expression of Cx43 within injured glomeruli of mice after induction of GN by using nephrotoxic serum (NTS-GN).21 However, the pathologic significance of such overexpression remains unknown. In this study, we combined in vivo and in vitro models to assess mechanisms involving Cx43 in the progression of GN. We show that this protein is mainly upregulated in the glomerular endothelial tuft and de novo expressed in podocytes and that it promotes glomerular damage, thus contributing to the progression of the disease. More importantly, we show that inhibiting Cx43 overexpression improves both functional and structural renal parameters.
Results
Cx43 Is Induced in GN
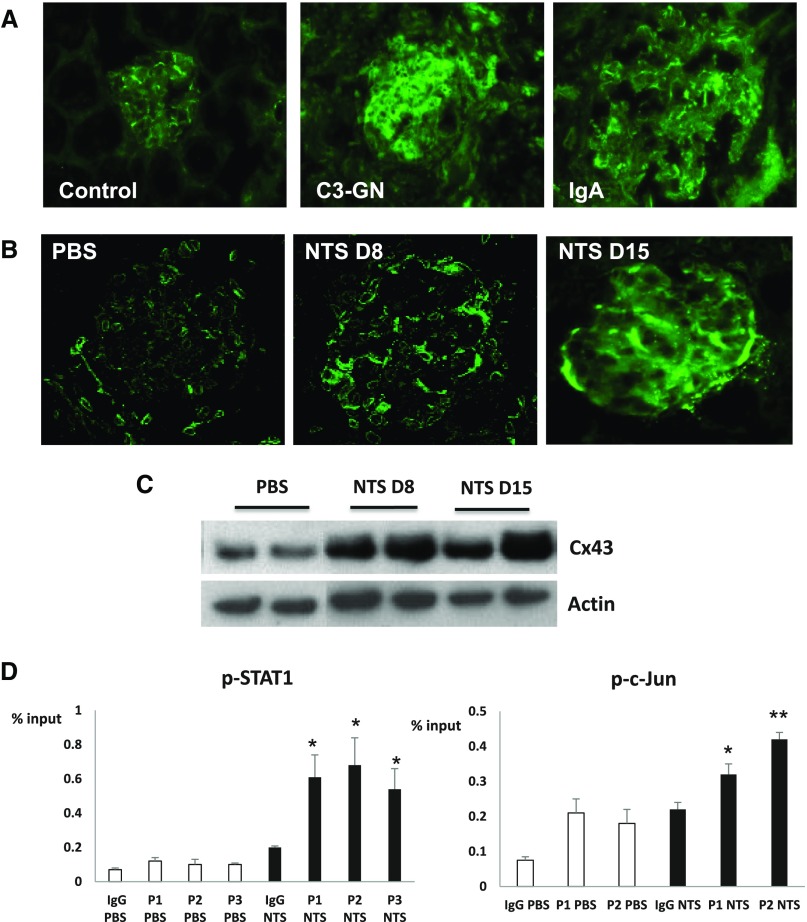
We have previously shown that Cx43 expression was upregulated in glomeruli of mice treated with nephrotoxic serum (NTS).21 Immunostainings in renal biopsies also revealed an abnormal expression of Cx43 within injured glomeruli in patients suffering from IgA and C3 glomerulopathies (Figure 1A). To assess the role of Cx43 in GN, we induced a passive NTS-GN in Cx43+/− and Cx43+/+ mice. Of note, it is impossible to use Cx43−/−, because they die shortly after birth.23 Mice were euthanized 8 or 15 days post-NTS administration, and immunofluorescence for Cx43 indicated a high induction in both time points (Figure 1B). This upregulation was confirmed by Western blotting (Figure 1C). To unravel the mechanism that mediates Cx43 increase, chromatin immunoprecipitation (ChIP) assays were performed with antibodies against several transcription factors chosen after bioinformatics analysis of the Cx43 promoter (Figure 1D). We showed that p-cJUN, which has been reported to upregulate Cx43 in other systems,24–26 and p-STAT1 were bound on the Cx43 promoter after NTS.
Figure 1.
Cx43 is induced in human glomerulopathies and experimental GN. In control human biopsies, faint staining for Cx43 can be observed in tubules, whereas basal expression also occurs in the glomerular endothelium of control biopsies. (A) In C3 and IgA glomerulopathies, Cx43 is highly induced within the injured glomerulus. (B) Cx43 is highly upregulated 8 and 15 days post-NTS administration in mice and mainly localized within the injured glomerulus. (C) This upregulation was confirmed by Western blot. (D) ChIP assays showed that Cx43 upregulation is triggered by p-STAT1 and p-cJun binding on Cx43 promoter. p-STAT1 was enriched in three positions on the Cx43 promoter, whereas p-cJUN was enriched in two. Different positions are indicated by P and sequential numbers. Positions and sequences are given in Table 2. *P<0.05; **P<0.01.
Decreased Cx43 Expression Attenuates NTS-GN
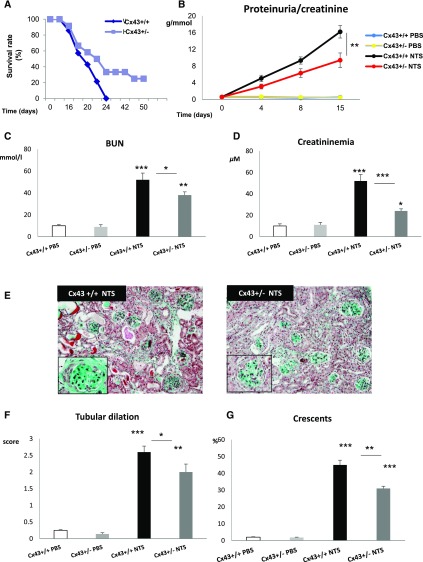
The deleterious role of the Cx43 overexpression was confirmed during the chronic phase of NTS-GN, because Cx43+/− mice were more resistant compared with Cx43+/+ against the disease (Figure 2A). A progressive increase of proteinuria in both NTS-treated groups was observed. However, the increase was less pronounced in Cx43+/− animals at 8 days and significantly smaller at 15 days (Figure 2B). In accordance, at day 15, BUN and serum creatinine levels increased to a lesser extent in Cx43+/− versus wild-type mice (Figure 2, C and D). At the same time point, Masson Trichrome coloration shown that NTS injection induced less histologic damage in Cx43+/− mice (Figure 2E), because both crescent formation and tubular dilation were significantly reduced (Figure 2, F and G). Thus, decreased expression of Cx43 improved renal function and structure during the progression of NTS-GN.
Figure 2.
Cx43+/− mice are protected against NTS-induced GN. (A) Survival curve showed reduced mortality rates in Cx43+/− mice. Renal function was evaluated by proteinuria expressed as (B) grams of protein per 1 mmol creatinine, (C) BUN, and (D) plasma creatinine measurements. All three parameters reveal a slower progress of the disease in Cx43+/− animals. (E) Mason Trichrome staining shows that renal structure was preserved in Cx43+/− versus Cx43+/+, because they developed (F) less tubular dilation and (G) fewer glomerular crescents. Crescents are expressed as the percentage of glomeruli presenting cellular crescents. *P<0.05; **P<0.01; ***P<0.001.
Cx43+/− Mice Show Reduced Inflammation and Fibrosis after NTS Injection
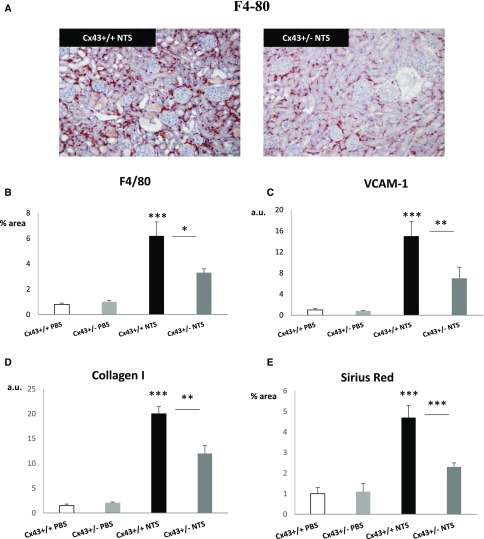
Macrophages play a crucial role in the onset and the progression of crescentic GN.27 F4/80 immunostaining showed limited macrophage infiltration in the renal cortex of Cx43+/− mice after 15 days of NTS administration (Figure 3, A and B). In accordance, quantitative PCR experiments showed that upregulation of VCAM-1, crucial for monocyte adhesion, was blunted in Cx43+/− NTS mice (Figure 3C). Furthermore, collagen I mRNA-increased expression was reduced by one half in Cx43+/− NTS mice 15 days post-NTS injection (Figure 3D). Quantification of Sirius red colorations confirmed that interstitial collagen accumulation was blunted (Figure 3E). Thus, decreased Cx43 expression markedly restricted renal inflammation and interstitial fibrosis in NTS-GN.
Figure 3.
Cx43+/− mice develop less renal inflammation and fibrosis. (A and B) Immunohistochemistry for F4/80 and (C) quantitative PCR for VCAM-1 at day 15 indicate that Cx43+/− mice develop less inflammation compared with wild-type mice. At the same time point, (D) quantitative PCR for collagen I and (E) quantification of Sirius red staining show restriction of the fibrotic response in Cx43+/−mice. Quantitative PCR data are expressed in graphs as arbitrary units (a.u.) that represent the ratio of the target gene to internal control gene (HPRT). *P<0.05; **P<0.01; ***P<0.001.
De Novo Expression of Cx43 in Podocytes Promotes Glomerular Damage
To examine the effect of Cx43 downregulation in the injured glomeruli, electron microscopy images were taken from Cx43+/+ and Cx43+/− mice 4 days after NTS administration. In PBS-treated mice, glomerular basement membrane appeared intact with normal podocytes (Figure 4B, left panel) and foot processes. Cx43+/+ NTS-treated mice showed thickening of the glomerular basement membrane, podocyte necrosis (Figure 4A, center panel), and destruction of the fenestrated endothelium (Figure 4B, center panel). In Cx43+/−, glomerular structure was substantially preserved with intact fenestrated endothelium (Figure 4B, right panel). Cx43 was slightly expressed at basal conditions in the vascular tuft, but no signal was detected in podocytes, because there was no colocalization with nephrin (Figure 5A). Cx43 expression was gradually increased in glomeruli. By day 8, a strong expression was noticed at the periphery of the glomerular tufts and further increased at day 15. Given that nephrin disappeared after day 8, we used nestin, an intermediate filament protein that is highly expressed in podocytes in normal conditions and glomerular disease.28 Cx43 colocalized with nestin at day 15 (Figure 5B). Furthermore, Western blot for nephrin and immunofluorescence for WT-1 (Figure 5, C and D) further confirmed that Cx43 downregulation preserved glomerular structure and protected podocyte integrity. Thus, Cx43 de novo expression in podocytes seems to promote cell damage, leading to proteinuria and podocyte loss.
Figure 4.
Cx43 downregulation preserves glomerular structure. Electron microscopy images from glomeruli of Cx43+/+ and Cx43+/− NTS-treated mice at (A) low and (B) high magnification. PBS-treated mice show intact glomerular basement membrane and podocytes (P) with preserved foot processes (white arrows). (B, center panel) Cx43+/+ NTS mice showed thickening of glomerular basement membrane (white arrows) and disorganization of fenestrated endothelium (black arrow). (B, right panel) In Cx43+/− NTS mice, fenestrated endothelium (black arrow) and foot processes (white arrow) were substantially preserved. Scale bars, 5 μm in A; 2 μm in B.
Figure 5.
Cx43 is expressed by injured podocytes. Double immunofluorescence for Cx43 (green) and the podocyte markers nephrin and nestin (red) in (A) control and (B) damaged podocytes indicate localization of Cx43 in damaged podocytes after 15 days of NTS administration. (C) At the same time point, Cx43+/− mice show preserved expression of nephrin. (D) Immunofluorescence for WT-1 indicates increased number of podocytes per glomerulus in Cx43+/− versus Cx43+/+ mice. Results are expressed as number positive for WT-1 staining nuclei per glomerulus. *P<0.05; **P<0.01; ***P<0.001.
Cx43 Mediates Dedifferentiation and Apoptosis of Podocytes In Vitro
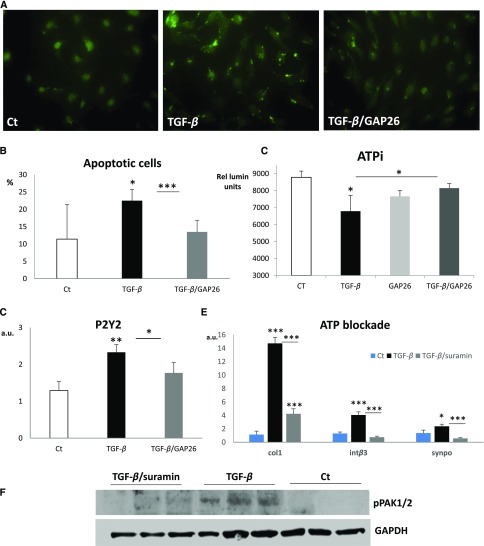
To further investigate the role of Cx43 in podocyte physiopathology, we used the well characterized E.11 podocyte cell line.29 Differentiated podocytes were treated with TGF-β1 for various time intervals (Figure 6A). Results showed a dramatic increase in Cx43 levels after 24 and 48 hours of treatment (Figure 6B). Subsequent experiments were performed at the 24-hour interval. TGF-β1 induced expression of mesenchymal markers, such as collagen I (Figure 6C), and reduced expression of the podocyte marker WT-1 (Figure 6D). Pretreatment with the Cx43-specific blocking peptide GAP26 significantly reduced TGF-β1 effects. Synaptopodin is an actin-associated protein that mediates podocyte migration in vitro,30 whereas integrin-β3 has been linked with foot processes effacement in vivo.31 GAP26 significantly reduced the TGF-β1–induced upregulation of these two markers (Figure 6, E and F), indicating that Cx43 mediates cytoskeletal changes necessary for cell migration and maintenance of cell shape. To this end, phosphorylation levels of focal adhesion kinase, which mediates cytoskeletal rearrangement, and p21-activated kinase1/2 (PAK1/2), a downstream target of integrin-β3 and Cdc42, were highly induced in response to TGF-β1 (Figure 6G). This increase was fully abolished by GAP26 (Figure 6, G–I).
Figure 6.
Cx43 blockade preserves podocyte phenotype in vitro. (A and B) TGF-β (5 ng/ml) increases Cx43 expression in the E.11 cell line at 24- and 48-hour time points. Quantitative PCR for (C) fibrotic, (D) podocyte, and (E and F) migratory markers after treatment with TGF-β in the presence or absence of GAP26. GAP26 alone had no effect on the mRNA levels of the studied genes. Western blot for the phosphorylated forms of kinases involved in (G and H) migration and (G and I) cytoskeletal rearrangement. GAP26 alone had no effect on the phosphorylation of the kinases studied. Quantitative PCR data are expressed in graphs as arbitrary units (a.u.) that represent the ratio of the target gene to internal control gene (HPRT). *P<0.05; **P<0.01; ***P<0.001; #P=0.05.
Dedifferentiating podocytes may also undergo apoptosis.8 Terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) assay 8 days post-NTS administration revealed extensive apoptosis in glomerular and tubular compartments. Cx43+/− mice were significantly protected from apoptosis, especially within damaged glomeruli (Supplemental Figure 1). To investigate whether podocyte death is Cx43 dependent, we evaluated apoptosis in vitro with TUNEL assays and intracellular ATP (ATPi) measurements after pretreatment with GAP26. Figure 7, A and B indicates that TGF-β1 induced apoptosis at 24 hours, whereas blockade of Cx43 significantly reduced the number of apoptotic cells. Apoptosis is accompanied by a rapid decline of ATPi levels and activation of purinergic signaling.32 ATPi levels measurements after 1 hour of TGF-β incubation were significantly reduced but restored to normal when cells were preincubated with GAP26 (Figure 7C).
Figure 7.
Cx43 and purinergic signaling blockade protects podocytes in vitro. (A) TUNEL staining indicates that (B) TGF-β–induced apoptosis is ameliorated by GAP26. (C) Measurements of ATPi levels further confirm the antiapoptotic effect of Cx43 blockade. Results are expressed in relative luminosity units. (D) TGF-β induces activation of P2Y2 receptor mRNA. Blockade of purinergic signaling with suramin (E) limits upregulation of collagen I, integrin-β3, and synaptopodin mRNAs and (F) decreases phosphorylation of pak1/2. Quantitative PCR data are expressed in graphs as arbitrary units (a.u.) that represents the ratio of the target gene to internal control gene (HPRT). *P<0.05; **P<0.01; ***P<0.001.
Purinergic Signaling Blockade Mimics Cx43-Specific Blocking Peptide Effects
ATP release may occur via Cx43 in several cell types.32–34 Because expression of the P2Y2 receptors increased in response to TGF-β1 and returned almost to control levels with GAP26 treatment (Figure 7D), we decided to examine whether purinergic signaling and GJ signaling cooperate to induce phenotypic switch or apoptosis of podocytes. To this end, podocytes were pretreated with the purinergic blocker suramin and then incubated with TGF-β1. Quantitative PCR of mesenchymal and migratory markers collagen I, integrin-β3, and synaptopodin showed that suramin inhibited the TGF-β1–induced phenotypic switch toward a mesenchymal/migratory phenotype (Figure 7E). Suramin effect on PAK1/2 phosphorylation (Figure 7F) was also similar to that of GAP26 (Figure 6G), further pointing toward a crosstalk between Cx43 and purinergic signaling. Consequently, our data suggest that Cx43 deleterious effects on podocyte damage could be mediated via P2Y2 receptors.
Administration of Cx43 Oligodeoxynucleotide Antisense In Vivo Delays the Development of NTS-GN
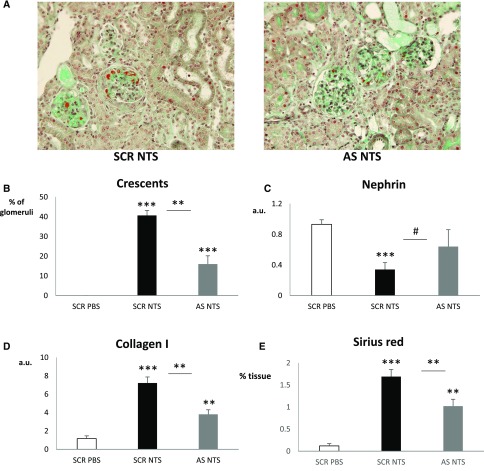
To examine whether Cx43 downregulation could have beneficial effects on renal function after the onset of the disease, we administered Cx43-antisense (AS) and -scrambled (SCR) oligodeoxynucleotide with daily injections beginning 4 days after NTS administration. The specificity of the Cx43-AS has been already validated and tested in other models of experimental nephropathy.22,35 AS, but not SCR, treatment reduced Cx43 protein levels (Figure 8, A and B) and attenuated NTS-induced increase of body weight, proteinuria, creatininemia, and BUN (Figure 8, C–F). This functional protection was accompanied with an overall preservation of renal structure, decreased numbers of crescents, and preserved mRNA levels of nephrin (Figure 9, A–C). Quantitative PCR for collagen I and Sirius red coloration (Figure 9, D and E) further confirmed the beneficial effect of Cx43 downregulation. Thus, targeting Cx43 protects against the progression of the NTS-GN.
Figure 8.
AS ODN against Cx43 preserve renal function after NTS-GN. (A and B) Administration of AS ODN decreases Cx43 expression. The NTS-induced increase of (C) body weight, (D) proteinuria, (E) creatininemia, and (F) BUN is attenuated in wild-type mice treated with Cx43 AS ODN. Body weight increase is presented as weight (grams) gained since the beginning of the protocol. *P<0.05; **P<0.01; ***P<0.001.
Figure 9.
AS ODN against Cx43 preserve renal structure after NTS-GN. (A) Masson Trichrome reveals (B) increased glomerular crescents and fibrin deposits that are significant reduced in AS-treated mice. (C) Quantitative PCR for nephrin indicates preserved glomerular structure after AS treatment. Renal fibrosis, assessed with quantitative PCR for (D) collagen I and (E) Sirius red staining, is diminished after treatment. Quantitative PCR data are expressed in graphs as arbitrary units (a.u.) that represents the ratio of the target gene to internal control gene (HPRT). *P<0.05; **P<0.01; ***P<0.001; #P=0.05.
Discussion
GN currently represents the second cause of end stage renal failure worldwide.1 Because current treatments remain partially efficient, it is crucial to identify molecular insights that will allow histologic diagnosis and treatment. The aim of our study was to investigate the role of Cx43 overexpression in the progression of GN. By combining a model of rapidly progressive nephropathy in mice with in vitro and in vivo Cx43 inhibitory approaches, we showed that Cx43 overexpression in glomeruli modulated the progression of GN.
Colocalization experiments revealed that Cx43 was mainly overexpressed within glomerular capillaries and podocytes (Figure 5B, Supplemental Figure 2). The effect of increased expression of Cx43 in endothelial cells has been already reported.34 Indeed, we showed that Cx43 upregulation in injured endothelium facilitated the recruitment of inflammatory cells during the progression of hypertension-induced and obstructive nephropathy in mice.22 Similarly, we showed in NTS-GN that decreased expression of Cx43 presented reduced VCAM-1 expression, leading to reduced monocyte infiltration within the injured kidneys. Those data were in agreement with studies showing that Cx43 overexpression promoted inflammation in acute and chronic inflammatory diseases.36–42
De novo expression of Cx43 was observed in podocytes after induction of GN, which led to cell damage and deterioration of renal function. More importantly, genetic or pharmacogenetic inhibition of Cx43 delayed renal structural and functional damages in mice suffering from severe GN. Cx43 overexpression in podocytes has been reported in experimental nephropathy, mainly in rats. In the puromycin aminonucleoside model of podocyte injury, Cx43 upregulation was one of the earliest responses in podocytes.43 A possible involvement of an oxidative stress–related mechanism has been reported in this model, because NADPH oxidase–mediated upregulation of Cx43 contributed to podocyte damage. Treatment of podocytes with different GJ inhibitors significantly attenuated the cytotoxicity of puromycin.44 Finally, a recent study provided preliminary evidence that Cx43 upregulation is involved in aldosterone-induced podocyte injury in rats. Silencing Cx43 expression in podocytes attenuated the increased ROS production and upregulation of the Bax-to-Bcl-2 ratio, partially inhibiting the aldosterone-induced apoptosis.45
Our results revealed a novel mechanism involving Cx43 in podocyte injury. Indeed, increased expression of Cx43 from damaged podocytes led to cell dedifferentiation. It is generally accepted that podocyte dedifferentiation implies foot processes effacement, leading to altered renal function. Whether after podocyte injury, there is detachment, podocyte loss, and/or migration toward the urinary chamber is still a matter of debate. In our study, we have considered both possibilities.
A relationship between Cx43 and cell death has been reported in some biologic systems.46 Indeed, Cx43 GJs have been shown to communicate cell death signals from cells transfected with Cx43 cDNA to neighboring cells in vitro, thereby spatially extending apoptosis.18,47 This bystander killing effect was of particular interest in the cancer field, because it has been shown that some therapeutic agents may enhance their toxic effects via Cx43 GJs. Similar effects have been confirmed in a minimal residual disease mouse model, where transplantation of human umbilical cells overexpressing Cx43 induced apoptosis, thus delaying the relapse of leukemia.48 A recent study confirmed that Cx43 may act as a proapoptotic modulator in cisplatin-induced auditory cell death.49 Interestingly, it has also been reported that HC alone can open in some conditions, thereby forming a pore through which molecules can enter or leave the cell, potentially leading to cell death. Cx43 HC inhibitors in astrocytes prevented the regional spread of cortical neuronal death through μ-calpain activation in a rat brain injury model.50 Similar observations were reported in a mouse model of myocardial ischemia-reperfusion injury. Treatment with GAP19 prevented Cx43 HC opening, thus protecting cardiomyocytes against volume overload and cell death and decreasing infarct size.51 Thus, increased expression of Cx43 contributes to the propagation of apoptotic death signals through both Cx43 GJs and HCs.
Progression of GN is associated with podocyte dedifferentiation and migration toward the urinary chamber. Furthermore, parietal epithelial cells can get activated and participate in the formation of glomerular crescents.52 Interestingly, Cx43+/− mice showed reduced parietal epithelial cell activation during the progression of NTS-GN (Supplemental Figure 3). Cx43 was reported to play diverse roles in cell migration in numerous cell types by interfering with receptor signaling and cytosqueletal remodeling mainly via its carboxyl tail.53 High expression of Cx43 was found in migrating neural crest cells involved in heart and brain development but also, migrating endothelial and vascular smooth muscle cells after insult.53,54 Furthermore, depletion of Cx43 inhibited the angiotensin II–induced motility and invasion of glioma cells.55,56 Targeting Cx43 with AS in vitro prevented phenotypic changes and migration of porcine coronary smooth muscle cells after platelet-derived growth factor–BB stimulation.54 Moreover, Cx43+/− hypercholesterolemic mice exhibited restricted migration of vascular smooth muscle cells and limited neointimal hyperplasia after carotid ballooning.38 However, it remains rather unclear whether the reported influence of Cx43 on cell migration is dependent on GJ or HC. Moreover, contrasting effects of Cx43 on cell migration have been reported in various systems. Therefore, Cx43 effect on migration may be cell or environment dependent, affected by compensatory effects of other Cxs.57
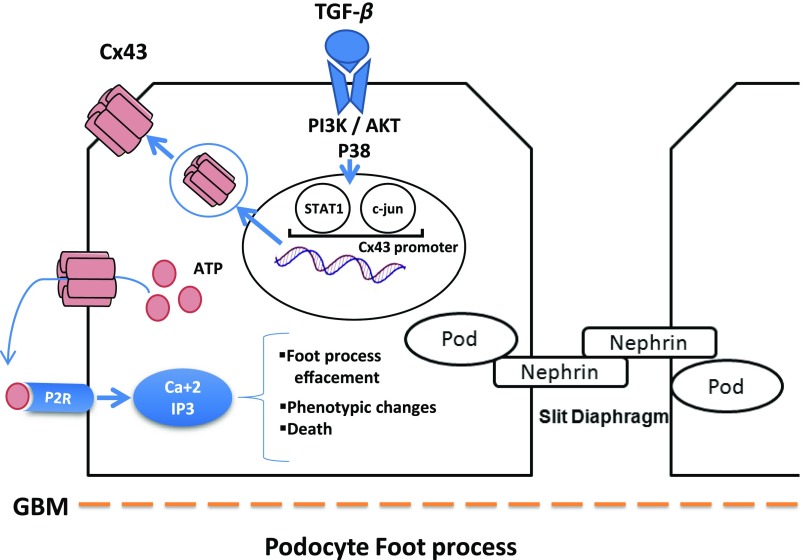
Inflammatory conditions are associated with the extracellular release of ATP, which acts as signaling mediators through the activation of purinergic receptors. Purinergic signaling can propagate proinflammatory and death signals to neighboring cells.58 ATP can be released by Cx43 HCs.34 The robust effect of ATP on podocytes has been reported in vitro, and the expression of the P2 receptors subtypes, such as P2Y2 and P2Y1, has also been described in rat glomeruli.59,60 Burford et al.61 recently set up an elegant method to directly visualize podocyte intracellular Ca2+ ([Ca2+i]) dynamics in vivo by multiphoton electronic microscopy. They showed, in preclinical models of renal fibrosis, that increased podocyte [Ca2+i] resulted in increased capillary permeability, podocyte motility, and glomerulosclerosis. Interestingly, all of the above mentioned damages improved by using inhibitors of purinergic [Ca2+i] or in P2Y2−/− mice, suggesting that purinergic signaling is a robust pathogenic mechanism in podocyte injury. Another study raised the possibility that sustained ATP signaling could contribute to foot process effacement, Ca(2+)-dependent changes in gene expression, and/or detachment of podocytes.62 P2Y2-induced damage was also reported in cardiac fibroblasts, suggesting a crucial role for P2 receptors in the mediation of profibrotic responses.63 All of the above mentioned studies combined with our in vitro data reinforce our hypothesis that Cx43 deleterious effects on podocytes could be mediated via P2 receptors. Consequently, except the deleterious role of Cx43 in other glomerular cell types, this Cx may promote podocyte damage. We propose that, during the progression of experimental GN, proinflammatory and/or profibrotic cytokines activate the expression of Cx43 at the transcriptional level in podocytes, leading to de novo expression of Cx43 HCs and contributing to exacerbated ATP release. ATP, in turn, could mediate, via P2R signaling, podocyte damage, which can be illustrated by either foot process effacement or cell death (Figure 10).
Figure 10.
Proposed model of the Cx43 deleterious effects on podocytes during the progression of GN. Our study shows that de novo expression of Cx43 in podocytes exacerbates ATP release, which in turn, mediates via P2R signaling cell damage. Podocyte damage can be illustrated by either foot process effacement or cell death, thus contributing to the progression of glomerular disease.
Another major finding of our study is that Cx43 downregulation is beneficial in severe models of CKD, because Cx43-AS blunted the progression of NTS-GN. We have recently published that targeting Cx43 expression protected mice against the progression of other models of CKD.22 Consequently, the protective effect of Cx43 blockade on both structural and functional renal parameters can be considered as a more common protective strategy against renal disease. The interest to develop therapeutic devices against Cx43 is strengthened by the overstated expression of this protein in patients with glomerular diseases. To this end, recent promising studies have been reported in mice suffering from myocardial ischemia, where Cx43 has been selectively inhibited by blocking peptides.51
In conclusion, we showed that Cx43 increased expression promotes renal inflammation and glomerular damage, leading to glomerulosclerosis and decline of renal function. In addition, we identified a new mechanism through which this protein mediates podocyte damage in vitro. Given our experimental approaches, global decrease of Cx43 levels blunted the progression of NTS-GN by affecting other renal compartments, such as the renal vasculature, where Cx43 certainly plays a significant role. The in vivo importance of such compartment remains to be evaluated. Nevertheless, we expect that Cx43 inhibitors will considerably supply in delaying the progression of renal diseases.
Concise Methods
Animals
Three-month-old SV129 Cx43+/− female mice and their wild-type littermates (n=8 per group) were used to induce a passive NTS-GN by intravenous administration of 15 μl NTS per gram of body weight over 3 consecutive days (days 1–3). Mice were euthanized 8 or 15 days after NTS administration. Control mice were injected with PBS (n=5 per group). In a separate study exploring mortality rates, experiments were terminated at day 55 (n=10 per group). For electron microscopy experiments, mice were euthanized at day 4 (n=4 per group). In a separate study, wild-type mice were injected with NTS. Four days after injection, administration of Cx43-AS (5′-GTAATTGCGGCAGGAGGAATTGTTTCTGTC-3′) or -SCR (5′-GACAGAAACAATTCCTCCTGCCGCAATTTAC-3′) oligonucleotides at 10 μM began with daily intraperitoneal injections (n=6 per group). Control mice were injected with AS or SCR oligonucleotides and PBS (n=4 per group) as previously reported.22 Mice were euthanized 12 days post-NTS administration. In total, 64 wild-type and 40 Cx43+/− mice were used. All mice were handled in strict accordance with good animal practice as defined by the relevant national animal welfare bodies of France, and all animal work was approved by the appropriate committee of the National Institute for Health and Medical Research and the Pierre and Marie Curie University (Paris, France). Animals were housed at constant temperature with access to water and food ad libitum.
Renal Function
Urine samples were collected on days 0, 4, 8, 12, and 15, and blood samples were collected before euthanasia. Proteinuria was measured with a Konelab automater (Thermo Fisher Scientific, Waltham, MA) and normalized to urine creatinine. BUN and plasma creatinine levels were measured with an enzymatic method (Konelab automater) and expressed in millimoles per liter and micromolar, respectively.
Histology
Kidneys were fixed in 4% formalin solution and embedded in paraffin; 4-μm sections were stained with Masson Trichrome for histologic evaluation. Interstitial fibrosis was assessed semiquantitatively on Sirius red–stained paraffin sections at magnification of ×200 using computer-based morphometric analysis software (Analysis; Olympus) as previously described.22 Crescent formation and tubular dilation were evaluated blinded on coded slides as already reported.64
Immunostainings
Immunofluorescence for Cx43 (Sigma-Aldrich) was performed on methanol-fixed cryosections from human patients with IgA nephropathy and C3 glomerulopathy. Sections were blocked and permeabilized with 5% FBS and 0.2% Triton, and charges were neutralized with 50 mM NH4Cl for 10 minutes at room temperature. Alexa Fluor secondary antibodies were used for detection (1:500). A total of three patient biopsies were used for this set of experiments. Informed consent was given by the patients for use of part of the biopsy for scientific purposes. All procedures and uses of tissue were performed according to the national ethical guidelines and were in accordance with the Declaration of Helsinki. In mice, immunofluorescence for Cx43, nephrin (Santa Cruz Biotechnologies), CD44 (EXBIO Antibodies), αSMA (Sigma-Aldrich), CD146 (a gift of Françoise Dignat-Georges), and nestin (BD Pharmingen) was performed in a similar manner, whereas staining for WT-1 (Santa Cruz Biotechnology) was performed on paraffin sections using standard methods. Immunohistochemistry for F4/80 (Serotec) was performed on paraffin-embedded sections as previously described.22
Total RNA Extraction and Quantitative Real-Time PCR
Total RNA was extracted from renal cortex using TRIzol reagent (Euromedex) and cell lines using the Spin Column Total RNA Mini-Preps Super Kit (Proteogenix). RNA quality was checked by control of OD at 260 and 280 nm. cDNA was synthesized from 1 mg RNA using the Fermentas H Minus First-Strand cDNA Synthesis Kit according to the manufacturer’s instructions. Quantitative PCR experiments were performed as previously described.22 Each sample was run in triplicate, and analysis of relative gene expression was done by using the 2−ΔΔCT method. Results are expressed in graphs as arbitrary units, which represent the ratio of the target gene to the internal control gene (HPRT). Sequences of primers used in our studies are listed in Table 1.
Table 1.
List of primers
| Gene | Sequence |
|---|---|
| Collagen I | FW: GCAGGTTCACCTACTCTGTCCT |
| RV: CTTGCCCCATTCATTTGTCT | |
| VCAM-1 | FW: TGGTGAAATGGAATCTGAACC |
| RV: CCCAGATGGTTTCCTT | |
| WT-1 | FW: CAGATGAACCTAGGAGCTACCTTAAA |
| RV: TGCCCTTCTGTCCATTTCA | |
| Nephrin | FW: TCCTCCCAGAGATGTTCAGC |
| RV: TTCTCCATGTCGTCCAGGTT | |
| Synaptopodin | FW: GTAGCCAGGTGAGCCAAGG |
| RV: TTTTCGGTGAAGCTTGTGC | |
| Integrin-β3 | FW: GTGGGAGGGCAGTCCTCTA |
| RV: CAGGATATCAGGACCCTTGG | |
| HPRT | FW: GGAGCGGTAGCACCTCCT |
| RV: CTGGTTCATCATCGCTAATCAC | |
| P2Y2 | FW: TCTTCCTGTTTCCTGCCTCA |
| RV: TCCAGGTCTGCTGCCATT |
FW, forward; RV, reverse.
Western Blot
Proteins were extracted from renal tissue and cell cultures using RIPA lysis buffer. Western blots for nephrin Cx43 (Abcam), p-focal adhesion kinase (Cell Signaling), and p-PAK1/2 (Cell Signaling) were performed using standard techniques as described elsewhere.22 B-actin (Imgenex) or GAPDH (Sigma-Aldrich) was used as a loading control. Data were expressed as mean values ±SEM of OD.
ChIP
Bioinformatics analysis for mouse Cx43 promoter was performed with the Genomatix genome analyzer platform. Analysis revealed potential binding sites for several proinflammatory and profibrotic transcription factors. ChIP assays were performed with the SimpleChIP Chromatin IP kit (Cell Signaling) with magnetic beads according to the manufacturer’s instructions. Chromatin was prepared from Cx43+/+ mice treated with PBS or NTS. Immunoprecipitation was performed with antibodies for p-STAT1, p-cJUN, and rabbit IgG (Cell Signaling). Quantitative PCR was performed as described above, and results were normalized to 2% input. Lists of primers and corresponding sites to Cx43 promoter are listed in Table 2.
Table 2.
Binding sites on Cx43 promoter and list of primers
| Name | Position | Forward | Reverse |
|---|---|---|---|
| p-STAT1 | |||
| P1 | −24 to 98 | CCAGTTGAGTCAG TGGCTTGA | CTGCTGTTGGGTA CTTCCCTC |
| P2 | −995 to 857 | GCCCACCCTAGGAATGAGGT | CCAATCCTGTCCC TCGGAAG |
| P3 | −1246 to 1168 | TGCTGGGGTCTTAA ACTGTGA | CATTTTCCTGCCGA AGCACC |
| p-cJUN | |||
| P1 | −297 to 193 | GGACATCTTCTCACTGCCCG | TGTACGTCAGCTACTGCGG |
| P2 | −955 to 857 | GCCCACCCTAGGAATGAGGT | CCAATCCTGTCCCTCGGAAG |
Electron Microscopy
Renal cortical slides from Cx43+/+ and Cx43+/− mice were used for electron microscopy. Tissue was collected at day 4, fixed in gluteraldehyde, postfixed in osmium tetroxyde, and embedded in epon using the traditional methods of TEM. Ultrathin (approximately 50-nm) sections, deposited on copper grids, were studied, with or without additional contrast, using a TEM equipped with a CCD camera (Morgagni 268-D).
TUNEL Assay
Apoptosis was evaluated with the DeadEnd Fluorometric TUNEL System (Promega) according to the manufacturer’s instructions. For in vivo experiments, 4-μm paraffin sections were used. The day 8 time point was chosen due to better preserved renal and glomerular structure. For in vitro experiments, cells were cultured in 24-well plates on poly-l-lysine (Sigma-Aldrich)–coated coverslips. A minimum of 500 cells per well was measured, and results were expressed as percentage of TUNEL-positive cells per total counted cells.
In Vitro Assays
The conditionally immortalized mouse podocyte cell line E11 was maintained in RPMI 1640 (Gibco BRL) supplemented with 10% FBS, 100 U/ml penicillin/streptomycin (Gibco BRL), and 10 U/ml recombinant mouse g-IFN (Peprotech, Rocky Hill, NJ) to induce synthesis of the immortalizing Tantigen. Cells were stored in humidified incubators with air and 5% CO2 at 33°C. Subcultures were obtained with trypsin after cells had reached confluence. To initiate differentiation, cells were thermoshifted to 37°C and maintained in medium without g-IFN for 2 weeks. After differentiation, cells were serum starved overnight with 1% FBS culture medium, preincubated with the Cx43-specific inhibitory peptide GAP26 (190 μΜ; Proteogenix) or suramine (30 μg/ml; Sigma-Aldrich) for 1 hour and 10 minutes, respectively, and treated with TGF-β (5 ng/ml; R&D Systems) for 24 hours. For endogenous ATP levels measurements, cells were incubated with TGF-β for 1 hour. ATP/ADP measurements were performed with an ATP monitoring kit according to the manufacturer’s instructions (Enzo Life Sciences). All experiments were performed at least three times, with n≥3 for all conditions.
Statistical Analyses
Values are expressed as mean±SEM. Data were analyzed using one-way ANOVA followed by the protected least significant difference Fisher test of the Statview software package. Error bars represent mean±SEM for in vivo and mean±SD for in vitro data. Results with P<0.05 were considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Prof. Françoise Dignat-Georges for providing us the CD146 antibody. We also thank Chantal Jouanneau and Perrine Frere for excellent technical assistance.
Support for this study was provided by the National Institute for Health and Medical Research Institute (P.K., C.C., and C.E.C.), the University Pierre et Marie Curie (J.-C.D. and C.C.), the Région Île-de-France (C.C. and C.E.C.), French National Research Agency grant ANR-15-CE14-0024 (to C.E.C.), and Fondation du Rein sous égide de la Fondation pour la Recherche Médicale grant R14057DD (to C.E.C.). A.A. is a doctoral fellow of the French Ministry of National Education (Ecole Doctorale de Physiologie and Physiopathologie ED 394). C.P. is a fellow of Fondation pour la Recherche Medicale.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016111211/-/DCSupplemental.
References
- 1.Chadban SJ, Atkins RC: Glomerulonephritis. Lancet 365: 1797–1806, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Mathieson PW: Glomerulonephritis. Semin Immunopathol 29: 315–316, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ: Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Thorner PS, Ho M, Eremina V, Sado Y, Quaggin S: Podocytes contribute to the formation of glomerular crescents. J Am Soc Nephrol 19: 495–502, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smeets B, Uhlig S, Fuss A, Mooren F, Wetzels JF, Floege J, Moeller MJ: Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J Am Soc Nephrol 20: 2604–2615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moeller MJ, Soofi A, Hartmann I, Le Hir M, Wiggins R, Kriz W, Holzman LB: Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J Am Soc Nephrol 15: 61–67, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Bariéty J, Bruneval P, Meyrier A, Mandet C, Hill G, Jacquot C: Podocyte involvement in human immune crescentic glomerulonephritis. Kidney Int 68: 1109–1119, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Tharaux PL, Huber TB: How many ways can a podocyte die? Semin Nephrol 32: 394–404, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Abed AB, Kavvadas P, Chadjichristos CE: Functional roles of connexins and pannexins in the kidney. Cell Mol Life Sci 72: 2869–2877, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Söhl G, Willecke K: Gap junctions and the connexin protein family. Cardiovasc Res 62: 228–232, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Kar R, Batra N, Riquelme MA, Jiang JX: Biological role of connexin intercellular channels and hemichannels. Arch Biochem Biophys 524: 2–15, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sáez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV: Connexin-based gap junction hemichannels: Gating mechanisms. Biochim Biophys Acta 1711: 215–224, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris AL: Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol 94: 120–143, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodenough DA, Paul DL: Beyond the gap: Functions of unpaired connexon channels. Nat Rev Mol Cell Biol 4: 285–294, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Stout C, Goodenough DA, Paul DL: Connexins: Functions without junctions. Curr Opin Cell Biol 16: 507–512, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Scheckenbach KEL, Crespin S, Kwak BR, Chanson M: Connexin channel-dependent signaling pathways in inflammation. J Vasc Res 48: 91–103, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Vinken M, Decrock E, Leybaert L, Bultynck G, Himpens B, Vanhaecke T, Rogiers V: Non-channel functions of connexins in cell growth and cell death. Biochim Biophys Acta 1818: 2002–2008, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Kameritsch P, Khandoga N, Pohl U, Pogoda K: Gap junctional communication promotes apoptosis in a connexin-type-dependent manner. Cell Death Dis 4: e584, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfenniger A, Wohlwend A, Kwak BR: Mutations in connexin genes and disease. Eur J Clin Invest 41: 103–116, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Hillis GS, Duthie LA, Brown PA, Simpson JG, MacLeod AM, Haites NE: Upregulation and co-localization of connexin43 and cellular adhesion molecules in inflammatory renal disease. J Pathol 182: 373–379, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Toubas J, Beck S, Pageaud AL, Huby AC, Mael-Ainin M, Dussaule JC, Chatziantoniou C, Chadjichristos CE: Alteration of connexin expression is an early signal for chronic kidney disease. Am J Physiol Renal Physiol 301: F24–F32, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Abed A, Toubas J, Kavvadas P, Authier F, Cathelin D, Alfieri C, Boffa JJ, Dussaule JC, Chatziantoniou C, Chadjichristos CE: Targeting connexin 43 protects against the progression of experimental chronic kidney disease in mice. Kidney Int 86: 768–779, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J: Cardiac malformation in neonatal mice lacking connexin43. Science 267: 1831–1834, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Geimonen E, Boylston E, Royek A, Andersen J: Elevated connexin-43 expression in term human myometrium correlates with elevated c-Jun expression and is independent of myometrial estrogen receptors. J Clin Endocrinol Metab 83: 1177–1185, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Tacheau C, Fontaine J, Loy J, Mauviel A, Verrecchia F: TGF-beta induces connexin43 gene expression in normal murine mammary gland epithelial cells via activation of p38 and PI3K/AKT signaling pathways. J Cell Physiol 217: 759–768, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Lee GH, Jang B, Choi HS, Kim HJ, Park JH, Jeon YC, Carp RI, Kim YS, Choi EK: Upregulation of connexin 43 expression via C-jun N-terminal kinase signaling in prion disease. J Alzheimers Dis 49: 1005–1019, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Duffield JS, Tipping PG, Kipari T, Cailhier JF, Clay S, Lang R, Bonventre JV, Hughes J: Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol 167: 1207–1219, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perry J, Ho M, Viero S, Zheng K, Jacobs R, Thorner PS: The intermediate filament nestin is highly expressed in normal human podocytes and podocytes in glomerular disease. Pediatr Dev Pathol 10: 369–382, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Schiwek D, Endlich N, Holzman L, Holthöfer H, Kriz W, Endlich K: Stable expression of nephrin and localization to cell-cell contacts in novel murine podocyte cell lines. Kidney Int 66: 91–101, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P: Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol 8: 485–491, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J: Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17: 952–960, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobson KA, Paoletta S, Katritch V, Wu B, Gao ZG, Zhao Q, Stevens RC, Kiselev E: Nucleotides acting at P2Y receptors: Connecting structure and function. Mol Pharmacol 88: 220–230, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jorgensen NR, Geist ST, Civitelli R, Steinberg TH: ATP- and gap junction-dependent intercellular calcium signaling in osteoblastic cells. J Cell Biol 139: 497–506, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M: Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA 95: 15735–15740, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becker DL, McGonnell I, Makarenkova HP, Patel K, Tickle C, Lorimer J, Green CR: Roles for alpha 1 connexin in morphogenesis of chick embryos revealed using a novel antisense approach. Dev Genet 24: 33–42, 1999 [DOI] [PubMed] [Google Scholar]
- 36.D’hondt C, Iyyathurai J, Himpens B, Leybaert L, Bultynck G: Cx43-hemichannel function and regulation in physiology and pathophysiology: Insights from the bovine corneal endothelial cell system and beyond. Front Physiol 5: 348, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parthasarathi K, Ichimura H, Monma E, Lindert J, Quadri S, Issekutz A, Bhattacharya J: Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. J Clin Invest 116: 2193–2200, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chadjichristos CE, Matter CM, Roth I, Sutter E, Pelli G, Lüscher TF, Chanson M, Kwak BR: Reduced connexin43 expression limits neointima formation after balloon distension injury in hypercholesterolemic mice. Circulation 113: 2835–2843, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Mori R, Power KT, Wang CM, Martin P, Becker DL: Acute downregulation of connexin43 at wound sites leads to a reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration. J Cell Sci 119: 5193–5203, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Sarieddine MZ, Scheckenbach KE, Foglia B, Maass K, Garcia I, Kwak BR, Chanson M: Connexin43 modulates neutrophil recruitment to the lung. J Cell Mol Med 13: 4560–4570, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuchida S, Arai Y, Kishida T, Takahashi KA, Honjo K, Terauchi R, Inoue H, Oda R, Mazda O, Kubo T: Silencing the expression of connexin 43 decreases inflammation and joint destruction in experimental arthritis. J Orthop Res 31: 525–530, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Moore K, Bryant ZJ, Ghatnekar G, Singh UP, Gourdie RG, Potts JD: A synthetic connexin 43 mimetic peptide augments corneal wound healing. Exp Eye Res 115: 178–188, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yaoita E, Yao J, Yoshida Y, Morioka T, Nameta M, Takata T, Kamiie J, Fujinaka H, Oite T, Yamamoto T: Up-regulation of connexin43 in glomerular podocytes in response to injury. Am J Pathol 161: 1597–1606, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan Q, Gao K, Chi Y, Li K, Zhu Y, Wan Y, Sun W, Matsue H, Kitamura M, Yao J: NADPH oxidase-mediated upregulation of connexin43 contributes to podocyte injury. Free Radic Biol Med 53: 1286–1297, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Yang M, Wang B, Li M, Jiang B: Connexin 43 is involved in aldosterone-induced podocyte injury. Cell Physiol Biochem 34: 1652–1662, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Krysko DV, Leybaert L, Vandenabeele P, D’Herde K: Gap junctions and the propagation of cell survival and cell death signals. Apoptosis 10: 459–469, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Mesnil M, Piccoli C, Tiraby G, Willecke K, Yamasaki H: Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. Proc Natl Acad Sci USA 93: 1831–1835, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang S, Wen Q, Liu Y, Zhang C, Wang M, Chen G, Gong Y, Zhong J, Chen X, Stucky A, Zhong JF, Zhang X: Increased expression of CX43 on stromal cells promotes leukemia apoptosis. Oncotarget 6: 44323–44331, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim YJ, Kim J, Kim YS, Shin B, Choo OS, Lee JJ, Choung YH: Connexin 43 acts as a proapoptotic modulator in cisplatin-induced auditory cell death. Antioxid Redox Signal 25: 623–636, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Ishii Y, Shintani-Ishida K, Yoshida K: Connexin-43 hemichannels contribute to the propagation of μ-calpain-mediated neuronal death in a cortical ablation injury model. Biochem Biophys Res Commun 441: 457–462, 2013 [PubMed] [Google Scholar]
- 51.Wang N, De Vuyst E, Ponsaerts R, Boengler K, Palacios-Prado N, Wauman J, Lai CP, De Bock M, Decrock E, Bol M, Vinken M, Rogiers V, Tavernier J, Evans WH, Naus CC, Bukauskas FF, Sipido KR, Heusch G, Schulz R, Bultynck G, Leybaert L: Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res Cardiol 108: 309, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moeller MJ, Smeets B: Novel target in the treatment of RPGN: The activated parietal cell. Nephrol Dial Transplant 28: 489–492, 2013 [DOI] [PubMed] [Google Scholar]
- 53.Kameritsch P, Pogoda K, Pohl U: Channel-independent influence of connexin 43 on cell migration. Biochim Biophys Acta 1818: 1993–2001, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Chadjichristos CE, Morel S, Derouette JP, Sutter E, Roth I, Brisset AC, Bochaton-Piallat ML, Kwak BR: Targeting connexin 43 prevents platelet-derived growth factor-BB-induced phenotypic change in porcine coronary artery smooth muscle cells. Circ Res 102: 653–660, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Jia G, Cheng G, Gangahar DM, Agrawal DK: Involvement of connexin 43 in angiotensin II-induced migration and proliferation of saphenous vein smooth muscle cells via the MAPK-AP-1 signaling pathway. J Mol Cell Cardiol 44: 882–890, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bates DC, Sin WC, Aftab Q, Naus CC: Connexin43 enhances glioma invasion by a mechanism involving the carboxy terminus. Glia 55: 1554–1564, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Behrens J, Kameritsch P, Wallner S, Pohl U, Pogoda K: The carboxyl tail of Cx43 augments p38 mediated cell migration in a gap junction-independent manner. Eur J Cell Biol 89: 828–838, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Idzko M, Ferrari D, Eltzschig HK: Nucleotide signalling during inflammation. Nature 509: 310–317, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fischer KG, Saueressig U, Jacobshagen C, Wichelmann A, Pavenstädt H: Extracellular nucleotides regulate cellular functions of podocytes in culture. Am J Physiol Renal Physiol 281: F1075–F1081, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Bailey MA, Turner CM, Hus-Citharel A, Marchetti J, Imbert-Teboul M, Milner P, Burnstock G, Unwin RJ: P2Y receptors present in the native and isolated rat glomerulus. Nephron, Physiol 96: 79–90, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Burford JL, Villanueva K, Lam L, Riquier-Brison A, Hackl MJ, Pippin J, Shankland SJ, Peti-Peterdi J: Intravital imaging of podocyte calcium in glomerular injury and disease. J Clin Invest 124: 2050–2058, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roshanravan H, Dryer SE: ATP acting through P2Y receptors causes activation of podocyte TRPC6 channels: Role of podocin and reactive oxygen species. Am J Physiol Renal Physiol 306: F1088–F1097, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu D, Soleymani S, Madakshire R, Insel PA: ATP released from cardiac fibroblasts via connexin hemichannels activates profibrotic P2Y2 receptors. FASEB J 26: 2580–2591, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kerroch M, Guerrot D, Vandermeersch S, Placier S, Mesnard L, Jouanneau C, Rondeau E, Ronco P, Boffa JJ, Chatziantoniou C, Dussaule JC: Genetic inhibition of discoidin domain receptor 1 protects mice against crescentic glomerulonephritis. FASEB J 26: 4079–4091, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.