Abstract
AKI is associated with high morbidity and mortality, and it predisposes to the development and progression of CKD. Novel strategies that minimize AKI and halt the progression of CKD are urgently needed. Normal kidney function involves numerous different cell types, such as tubular epithelial cells, endothelial cells, and podocytes, working in concert. This delicate balance involves many energy-intensive processes. Fatty acids are the preferred energy substrates for the kidney, and defects in fatty acid oxidation and mitochondrial dysfunction are universally involved in diverse causes of AKI and CKD. This review provides an overview of ATP production and energy demands in the kidney and summarizes preclinical and clinical evidence of mitochondrial dysfunction in AKI and CKD. New therapeutic strategies targeting mitochondria protection and cellular bioenergetics are presented, with emphasis on those that have been evaluated in animal models of AKI and CKD. Targeting mitochondrial function and cellular bioenergetics upstream of cellular damage may offer advantages compared with targeting downstream inflammatory and fibrosis processes.
Keywords: diabetic nephropathy, fibrosis, mitochondria, reactive oxygen species, metabolism, ischemia-reperfusion
AKI is associated with high morbidity and mortality, and it predisposes to the development of CKD. The primary causes of AKI include ischemia, sepsis, and nephrotoxicity. There is, at present, no effective treatment for AKI, and therapeutic interventions targeting CKD progression are largely limited to control of hypertension and hyperglycemia, agents that interrupt the renin-angiotensin system, and the possible salutary effects of reduced salt and protein intake.1 Novel strategies that minimize AKI and halt the progression of CKD are urgently needed.
Normal kidney function involves numerous different cell types, such as tubular epithelial cells, endothelial cells, and podocytes, working in concert. This delicate balance involves many energy-intensive processes, particularly sodium transport, necessitating close coordination between energy supply and demand.2 Proximal tubular (PT) epithelial cells are rich in mitochondria and rely on oxidative phosphorylation for ATP generation. The importance of mitochondrial function as energy providers in the kidney is evident in inherited mitochondrial diseases with renal impairments.3,4
The overwhelming evidence for mitochondrial dysfunction in AKI and CKD has led to a search for mitochondria-protective drugs. This review summarizes our current understanding of ATP production and energy demands in the kidney and available evidence of mitochondrial dysfunction in AKI and CKD. New therapeutic strategies targeting mitochondria protection and cellular bioenergetics are presented, with emphasis on those that have been evaluated in animal models of AKI and CKD.
ATP Production in the Kidney
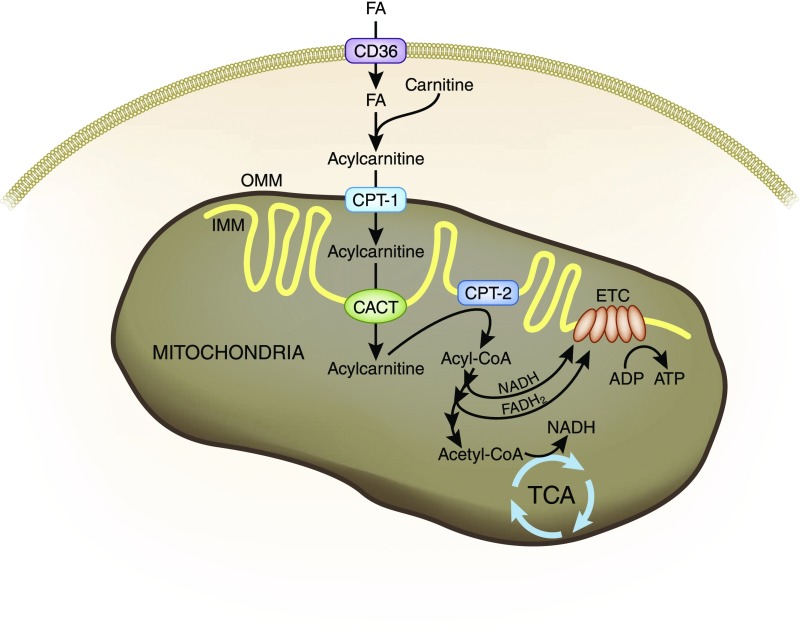
Fatty acid (FA) oxidation is the preferred energy source for highly metabolic tissues, because it generates three times more ATP compared with glucose oxidation.5–7 FA β-oxidation fuels the tricarboxylic acid cycle and oxidative phosphorylation (Figure 1). The uptake of long-chain FAs is facilitated by specific proteins, such as CD36.8 In the cytosol, FAs are activated to acyl-CoA by acyl-CoA synthetases. Because the mitochondrial membrane is impermeable to acyl-CoAs, the carnitine shuttle is needed for their import into mitochondria.5 Carnitine palmitoyltransferase-1 (CPT-1) on the outer mitochondrial membrane (OMM) catalyzes the transesterification of acyl-CoA to acylcarnitine. This complex then enters the mitochondrial matrix via facilitated diffusion by carnitine-acylcarnitine translocase (CACT). CPT-2 on the inner mitochondrial membrane (IMM) reconverts the acylcarnitine into an acyl-CoA. CPT-1 is the rate-limiting enzyme in FA oxidation. Deficiency of CPT-1 results in energy failure and kidney disease.9
Figure 1.
FA uptake and mitochondrial FA β-oxidation. FAs are the preferred energy substrates for the kidney. Uptake of FAs into kidney cells is facilitated by CD36. In the cytosol, FAs are activated to acyl-CoA and transported to mitochondria by the carnitine shuttle. CPT-1 on the OMM catalyzes the transesterification acyl-CoA to acylcarnitine. The complex then enters the mitochondrial matrix via facilitated diffusion by carnitine-acylcarnitine translocase (CACT). CPT-2 on the IMM reconverts the acylcarnitine into an acyl-CoA. In the mitochondrial matrix, acyl-CoAs undergo β-oxidation to generate acetyl-CoA to fuel the tricarboxylic acid cycle (TCA) and also NADH and FADH2 that serve as electron donors to the ETC on the IMM for ATP production. NADH is also produced by the tricarboxylic acid.
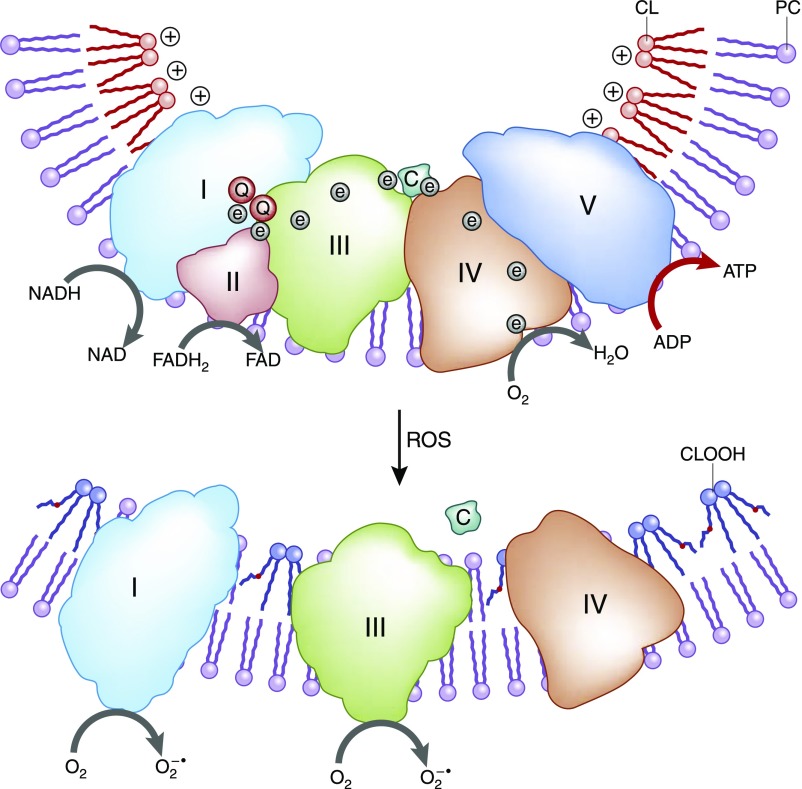
After they are in the mitochondrial matrix, acyl-CoAs undergo β-oxidation to generate acetyl-CoA to fuel the tricarboxylic acid cycle and also NADH (reduced nicotinamide adenine dinucleotide) and FADH2 (reduced flavin adenine dinucleotide), which serve as electron donors to the electron transport chain (ETC) on the IMM (Figure 2). The respiratory protein complexes of the ETC reside on cristae membranes in the IMM. The transfer of electrons from complex 1 to complex 4 via redox reactions is coupled with the transfer of protons into the intermembrane space. This creates an electrochemical proton gradient across the IMM that drives the production of ATP by the F0F1-ATPase. All of these complexes must be assembled properly for efficient electron transfer to take place. The unique conical structure of cardiolipin on the IMM promotes membrane curvature and allows the respiratory complexes to form supercomplexes that facilitates more efficient electron transfer by reducing the distance between electron donor and electron acceptor.10,11 Cardiolipin also serves to anchor cytochrome c to the IMM via electrostatic interaction to facilitate electron transfer from complex 3 to complex 4 and as a proton trap on the outer leaflet of the IMM.
Figure 2.
Role of cardiolipin (CL) in cristae structure and supercomplex formation on the IMM. The protein complexes of the ETC reside on cristae membranes, and CL provides curvatures on the IMM to increase surface area for the respiratory complexes. CL helps to organize the respiratory complexes into supercomplexes to facilitate electron transfer among the redox partners. CL also anchors the highly cationic cytochrome c (c) via electrostatic interaction to bring it in close proximity to complex 3 and complex 4 for efficient electron transfer. In addition, CL serves as a proton trap to optimize the proton gradient for complex 5 (F0,F1-ATPase). CL is particularly vulnerable to oxidative damage because of its high content of unsaturated FAs. Oxidized cardiolipin (CLOOH) destabilizes CL microdomains, reduces cristae curvatures, and disrupts supercomplex formation. Peroxidation of CL also reduces its affinity for cytochrome c and sets the stage for cytochrome c release into the cytosol and apoptosis. e, electron; FAD, flavin adenine dinucleotide; FADH2, reduced flavin adenine dinucleotide; NAD, nicotinamide adenine dinucleotide; NADH, reduced nicotinamide adenine dinucleotide; PC, phosphatidylcholine; Q, coenzyme Q.
Cellular Consequences of Mitochondrial Dysfunction
Inhibition of electron transfer in the ETC leads to electron leak at complex 1 and complex 3 and the formation of reactive oxygen species (ROS). Oxidative damage to the respiratory complexes further inhibits enzyme activity, setting up a feed-forward cycle of mitochondrial oxidative stress and progressive energy deficiency. Cardiolipin is particularly vulnerable to oxidative damage, and oxidized cardiolipin disturbs cardiolipin microdomains on the IMM, causing loss of cristae curvature and inhibition of electron transfer (Figure 2). Oxidized cardiolipin has lower affinity for cytochrome c and frees cytochrome c from the IMM.12 Oxidized cardiolipin also breaks the Met80-Fe ligation on its heme to turn cytochrome c into an oxygenase that can oxidize cardiolipin without ROS.13,14
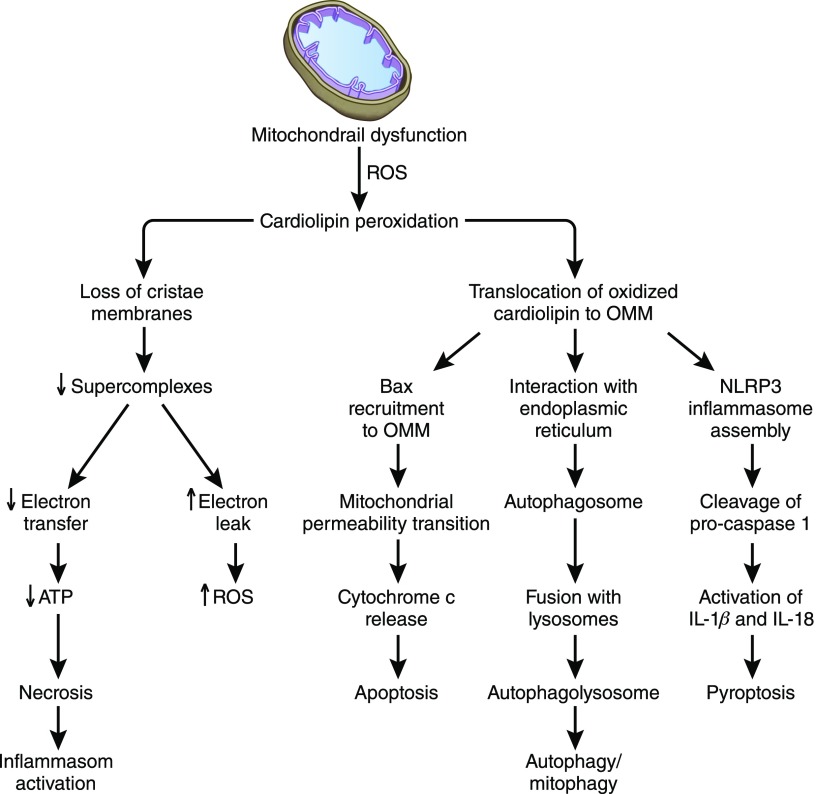
All enzymes for FA oxidation reside in the mitochondrial matrix, and tight cristae membranes serve to concentrate enzymes and substrates close to the respiratory complexes. Defective FA oxidation decreases the supply of reducing equivalents to the ETC. Furthermore, cardiolipin peroxidation can signal a number of cellular events (Figure 3). (1) Oxidized cardiolipin damages cristae curvatures, effectively reducing respiratory complexes. (2) If oxidative stress is mild, oxidized cardiolipin can translocate to the OMM, where it triggers mitophagy to selectively eliminate damaged mitochondria. Damaged mitochondria are enveloped by endoplasmic reticulum membranes to form autophagosomes, which then fuse with lysosomes to form autophagolysosomes that degrade mitochondrial content.15,16 (3) Oxidized cardiolipin on the OMM can also recruit Bax to trigger mitochondrial permeability transition, which releases cytochrome c from mitochondria to initiate apoptosis via activation of caspase-9.17 (4) Oxidized cardiolipin on OMM can also serve as a docking station for NLRP3 (NLR family pyrin domain containing 3) inflammasome assembly, and mitochondrial ROS triggers activation of caspase-1 to cleave pro–IL-1β and pro–IL-18 to cause an inflammatory cell death called pyroptosis.18,19 (5) Cells will undergo necrosis in the event of profound ATP depletion, and danger molecules released from necrotic cells can also trigger inflammasome activation.20
Figure 3.
Cellular consequences of mitochondrial dysfunction. Mitochondrial dysfunction increases ROS and causes cardiolipin peroxidation. Oxidized cardiolipin can signal a number of cellular events. (1) Oxidized cardiolipin damages cristae curvatures, effectively decreasing substrate concentration and delivery of NADH and FADH2 to the respiratory complexes. (2) If oxidative stress is mild, oxidized cardiolipin can translocate to the OMM, where it triggers mitophagy to selectively eliminate damaged mitochondria. Damaged mitochondria are enveloped by endoplasmic reticulum membranes to form autophagosomes, which then fuse with lysosomes to form autophagolysosomes that degrade mitochondrial content. (3) Oxidized cardiolipin on the OMM can also recruit Bax to trigger mitochondrial permeability transition, which releases cytochrome c from mitochondria to initiate apoptosome activation, activation of caspase-3, and apoptosis. (4) Oxidized cardiolipin on OMM can serve as a docking station for NLRP3 inflammasome assembly, and mitochondrial ROS triggers inflammasome activation to activate caspase-1 to cleave pro–IL-1β and pro–IL-18 to cause chronic inflammation and tissue remodeling. (5) Cells will undergo necrosis in the event of profound ATP depletion, and danger molecules released from necrotic cells can also trigger inflammasome activation. FADH2, reduced flavin adenine dinucleotide; NADH, reduced nicotinamide adenine dinucleotide; NLRPE, NLR family pyrin domain containing 3.
Mitochondrial Dysfunction in AKI
Under ischemic conditions, the deficiency of substrates and oxygen inhibits mitochondrial respiration, and the kidney must switch to glycolytic metabolism, which greatly reduces the amount of ATP that can be produced. Mitochondrial respiration is significantly inhibited during ischemia, resulting in dramatic loss of ATP within 5–10 minutes.21,22 In ischemia, Na+-K+-ATPase activity is inhibited within a few minutes, and intracellular Na+ increases three- to fourfold, causing cell swelling.23 ATP is also required for actin polymerization, and cytoskeleton breakdown leads to detachment of endothelial cells,23 loss of brush border and cell detachment in PT cells,23,24 and podocyte effacement.25 Ischemia can result in either apoptotic or necrotic cell death, because ATP is needed for activation of the caspase cascade.26 The S3 segment of the PT is more susceptible to necrosis than S1 and S2 segments due to much lower blood flow in the outer medulla. The distal tubule has greater capacity for glycolytic metabolism and is more likely to undergo apoptosis.27
Rapid recovery of ATP is essential for cell survival, and this is dependent on recovery of mitochondrial function. However, ischemic damage to mitochondria structure limits the ability of these cells to regenerate ATP rapidly on reperfusion. Cell swelling caused by ATP depletion increases the osmotic gradient that drives water into the mitochondrial matrix.28 Matrix swelling is passive and can increase until the IMM becomes completely unfolded into a spherical configuration.29 Matrix swelling has been described in tubular cells in ischemia,22–24 sepsis,30,31 and drug-induced nephrotoxicity.32,33 The loss of cristae membranes during ischemia together with dilution of substrate concentration due to matrix swelling serve to inhibit mitochondrial respiration and delay ATP recovery on reperfusion.22,24,29
Mitochondrial Dysfunction in CKD
There is evidence that mitochondrial damage can persist long after acute ischemia. Atrophic PTs that fail to differentiate 2 weeks after ischemia show greatly reduced mitochondria number and large autophagolysosomes containing degenerated mitochondria.34 Matrix swelling and loss of cristae membranes are evident in both podocytes and PT mitochondria 1 month after ischemia,23 and mitochondria remain small, with only remnants of cristae membranes even 9 months later.35 The persistent accumulation of damaged mitochondria suggests impaired mitochondrial quality control. Autophagosomes containing mitochondria and autophagolysosomes can be seen in podocytes and PTs 9 months after original injury.35 However, many mitochondria show evidence of proteolytic degradation, suggesting that mitophagy might be impaired, and damaged proteins are eliminated by mitochondrial proteases. Degenerative mitochondria were also reported in aged podocytes.36
Defects in FA oxidation have been reported in experimental CKD. High-fat diet causes lipid accumulation in tubular cells and downregulation of CPT-1.37,38 Defective FA oxidation is also seen in patients with diabetic nephropathy. Kidney biopsy samples revealed heavy lipid deposition and increased intracellular lipid droplets.39 Lipid deposition was associated with downregulation of several key genes involved in FA oxidation, linking inhibition of FA oxidation to lipid accumulation. Genome-wide transcriptomic analysis of a large cohort of normal and fibrotic human kidney samples revealed lower expression of CPT-1, CPT-2, and other mitochondrial genes in the fibrotic samples.6 The inhibition of FA oxidation in CKD causes a switch to glucose metabolism and reduced ATP production.
Mitochondria-Targeted Approaches for Kidney Diseases
Given the abundant evidence that mitochondrial dysfunction triggers cellular injury and inflammatory and fibrotic responses, protecting mitochondria may be more effective than targeting individual downstream events40 (Figure 3). A number of novel mitochondria-targeted approaches are currently in development, including compounds known to specifically target mitochondria and others that promote mitochondrial biogenesis or FA oxidation. These are presented below, with emphasis on those that have been evaluated in kidney disease models (a summary is in Table 1).
Table 1.
Mitochondria-targeted agents in animal models of AKI and CKD
| Kidney Disease Model | Species | Experimental Compound | Major Findings | Refs. |
|---|---|---|---|---|
| Warm ischemia-reperfusion | Rat | MitoQ | ↓Oxidative damage, ↑renal function | 43 |
| Warm ischemia-reperfusion | Rat | SkQR1 | ↓Oxidative damage, ↑renal function | 44 |
| Warm ischemia-reperfusion | Rat | SS-31 | ↓Mitochondrial swelling, protect cristae membranes, ↑mitochondrial function, ↓renal pathology, ↑renal function, ↓inflammation and fibrosis | 22–24 |
| Warm ischemia-reperfusion | Rat | MA-5 | ↑Renal function | 61 |
| Sepsis (cecal ligation/puncture) | Mice | SS-31 | ↑ATP, ↓apoptosis, ↓renal pathology | 55 |
| Sepsis (LPS) | Rat | MitoQ | ↑Mitochondrial potential, ↑renal function | 46 |
| Pyelonephritis | Rat | SkQR1 | ↓Oxidative damage, ↓inflammation, ↓mortality | 45 |
| Cisplatin toxicity | Mice | MA-5 | ↓Renal pathology, ↑renal function | 61 |
| Ureteral obstruction | Rat | SS-31 | ↓Oxidative damage, ↓apoptosis, ↓inflammation and fibrosis | 54 |
| Aging | Mice | SS-31 | Protected mitochondria in podocytes, ↓senescent markers, ↓glomerulosclerosis | 36 |
| Renal artery stenosis | Pigs | SS-31 | Restored cardiolipin content, ↓oxidative damage, ↑vascular density, ↓fibrosis | 57 |
| Postischemic CKD | Rat | SS-31 | Protected mitochondrial structure in all kidney cells, ↓inflammasome activation, ↓glomerulosclerosis and fibrosis | 35 |
| Obesity-related nephropathy (high-fat diet) | Mice | SS-31 | Protected mitochondrial structure in all kidney cells, prevent lipid accumulation, ↓mesangial expansion, ↓glomerulosclerosis | 38 |
| Diabetic nephropathy (streptozotocin) | Mice | SS-31 | ↓Mesangial expansion, ↓tubular apoptosis | 58 |
| Diabetic nephropathy (Ins2±Akita) | Mice | MitoQ | ↓Mesangial expansion, ↓interstitial fibrosis, ↓microalbuminuria | 50 |
| Diabetic nephropathy (db/db) | Mice | MitoQ | ↓ROS, ↓apoptosis, ↓glomerular hypertrophy, ↓mesangial expansion, ↓basement membrane thickening | 51 |
Mitochondria-Targeted Antioxidants
Mitochondrial oxidative stress is common in many kidney diseases. The disappointing clinical results with conventional antioxidants led to the development of mitochondria-targeted antioxidants.41 The initial approach involved the use of triphenylphosphonium ion conjugated to lipophilic antioxidant molecules, such as CoQ (MitoQ), TEMPO (MitoTEMPO), or plastoquinone (SkQR1). The positive charge drives the transport of these lipophilic molecules into the mitochondrial matrix in a potential-dependent manner.42 In warm ischemia-reperfusion, pretreatment with MitoQ 15 minutes before ischemia significantly reduced tissue carbonyl content and reduced serum creatinine in rats.43 Pretreatment with SkQR1 before ischemia reduced tissue ROS and lipid peroxidation and improved renal function, but it had no effect when given after ischemia.44 In addition, MitoQ increased mitochondrial potential and improved renal function in a sepsis model using LPS, whereas SkQR1 reduced oxidative damage and renal inflammation in a model of pyelonephritis.45,46 However, there is little direct evidence that these compounds protect mitochondria structure or function, although MitoTEMPO did protect mitochondrial respiratory complex activity and improved renal function in a sepsis model.47 The requirement of mitochondrial potential for uptake may limit their use in some disease states. Furthermore, lipophilic cations can depolarize mitochondria, inhibit oxidative phosphorylation, or become pro-oxidants,48,49 which may account for loss of efficacy at higher doses.47
Hyperglycemia is thought to be associated with increased mitochondrial ROS production. Oral administration of MitoQ in Ins2±AkitaJ mice for 12 weeks attenuated glomerular basement membrane thickening, mesangial expansion, interstitial fibrosis, and albuminuria.50 Because renal mitochondrial function is normal in Ins2±AkitaJ mice, the mechanism of action of MitoQ is unclear. MitoQ also ameliorated glomerular hypertrophy and mesangial expansion in db/db mice and partially reversed mitochondrial fragmentation in tubular cells.51 Notably, MitoQ had no effect on hyperglycemia in either model. These promising results suggest that more research is warranted in mitochondria-targeted antioxidants for kidney diseases.
Cardiolipin-Targeting Peptides That Protect Cristae Structure
The Szeto–Schiller (SS) peptides are cell-permeable tetrapeptides that selectively target mitochondria but concentrate on the IMM rather than penetrate into the mitochondrial matrix.52 Despite their 3+ net charge, their mitochondrial uptake is potential independent, and they do not depolarize mitochondria. These peptides were originally described as mitochondria-targeted antioxidants because of their electron scavenging ability.52 Subsequent research revealed that these peptides interact selectively with cardiolipin to stabilize cristae curvatures.24 After they are bound to cardiolipin, these peptides penetrate deep into the cytochrome c heme environment to promote electron transfer and prevent the conversion of cytochrome c to a peroxidase.24,53 As a result, these peptides promote ATP synthesis, reduce electron leak and ROS production, and inhibit cardiolipin peroxidation.10,13,24,53 By preventing cardiolipin peroxidation, the SS peptides prevent apoptosis, inflammation, and NLRP3 inflammasome activation.22–24,35
SS-31 has been studied extensively in models of AKI. When given before ischemia in rats, SS-31 prevented matrix swelling and preserved cristae structure in tubular epithelial cells and endothelial cells, and it accelerated ATP recovery on reperfusion.22–24 Cell polarity and brush border in PT cells were restored rapidly, and apoptosis and necrosis were ameliorated.24 Protection of endothelial mitochondria by SS-31 mitigated capillary damage and prevented microvascular rarefaction, interstitial inflammation, and fibrosis.23 SS-31 also prevented TGF-β upregulation and tubulointerstitial fibrosis in unilateral ureteral obstruction.54
SS-31 may be best suited for clinical situations where AKI is anticipated, such as sepsis, partial nephrectomy, and transplantation. When administered immediately after cecal ligation and puncture in mice, SS-31 normalized kidney ATP content, decreased apoptosis, reduced histology score, and reduced serum creatinine and BUN.55 Delayed graft function after kidney transplantation reduces long-term graft survival. A recent study of human tissue biopsies showed that grafts with delayed graft function failed to recover mitochondrial respiration.56 Kidney biopsies subjected to warm ischemia resulted in profound reduction of oxygen consumption and complex 1 activity, but these changes were prevented when SS-31 was included in the incubation medium.56
In addition to protecting mitochondria structure from injury, there is evidence that SS-31 can repair damaged mitochondria and restore mitochondria structure in CKD models. Two months of SS-31 treatment restored cristae structure in podocytes of aged mice, decreased senescent markers, and reduced glomerulosclerosis.36 SS-31 treatment starting 1 month after acute ischemia restored normal mitochondria structure in podocytes and prevented mitophagy in PT, and it reduced NLRP3 inflammasome activation, glomerulosclerosis, and tubulointerstitial fibrosis.35 Surprisingly, the kidneys continued to be protected even 6 months after terminating SS-31 treatment, suggesting that restoration of mitochondrial bioenergetics can provide long-term protection.35
Atherosclerotic occlusion of the renal artery is an important cause of CKD. Renal artery stenosis causes oxidative stress, microvascular rarefaction, and fibrosis.57 Treatment with SS-31 blunted oxidative stress, restored cardiolipin content, improved microvascular density, and diminished fibrosis.57
Diabetes and obesity are other major causes of CKD. SS-31 has no effect on blood glucose in diabetic or obese mice.58,59 However, SS-31 significantly reduced glomerular hypertrophy and mesangial expansion and decreased apoptosis in streptozotocin mice.58 Treatment with SS-31 during high-fat diet protected mitochondria structure in all kidney cell types and prevented intracellular lipid accumulation.38 SS-31 prevented loss of glomerular endothelial cells and podocytes, inflammation, mesangial expansion, and glomerulosclerosis.38 These findings suggest that excess lipids in the kidney cause cardiolipin peroxidation and loss of cristae membranes. These damaged mitochondria limit FA β-oxidation and result in further lipid accumulation. By promoting mitochondrial function and preventing cardiolipin peroxidation, SS-31 can break this feed-forward loop that amplifies renal injury.
Mitochondria-Homing Agents
Mitochonic acid 5 (MA-5) belongs to the newest class of mitochondria-targeted agents. It is a derivative of the plant hormone indole-3-acetic acid that was discovered in a screen for compounds that can increase cellular ATP content. MA-5 increased cellular ATP and improved the survival of fibroblasts established from patients with inherited mitochondrial diseases.60 When administered to mice 3 hours before bilateral renal ischemia, MA-5 reduced tubular necrosis, improved renal function on reperfusion. MA-5 also significantly decreased plasma BUN and tubular necrosis after cisplatin administration.61
The mechanism of action of MA-5 remains unclear. It was reported to increase ATP synthesis independently of electron transport or oxidative phosphorylation.61 MA-5 was proposed to target the mitochondrial protein mitofilin/Mic60 at the cristae junction of the IMM and facilitates oligomerization of the ATP synthase and supercomplex formation.61,62 More research is needed to fully understand how interaction with mitofilin/Mic60 improves bioenergetics and protects the kidney.
Agents That Promote FA Oxidation
Carnitine or acetyl-l-carnitine supplements may be useful in assisting mitochondrial FA uptake. Peroxisome proliferator–activated receptor α (PPARα) is a transcriptional factor that regulates CPT-1 expression and FA oxidation, and PPARα is highly expressed in PT and mesangial cells. Pharmacologic activation of PPARα with fenofibrate reduced kidney injury and fibrosis in model of obstructive nephropathy.6 Fenofibrate also reduced urinary albumin excretion and interstitial fibrosis in diabetic nephropathy models by suppressing NF-κB and TGF-β signaling pathways.63–65 Despite clear evidence that combination use of fibrates and statins can prevent cardiovascular disease in patients with diabetes, the use of fenofibrate for diabetic nephropathy is uncertain, because its use can cause a rapid increase in serum creatinine.66
Agents That Promote Mitochondrial Biogenesis
Mitochondria are constantly being renewed through the processes of biogenesis and mitophagy. PGC-1α is a cotranscriptional regulation factor that induces mitochondrial biogenesis by activating mitochondrial transcription factor A.67 PGC-1α is highly expressed in kidney and capable of driving all aspects of mitochondrial biogenesis, thus making it a pharmacologic target for kidney diseases.68 The PPARγ agonist, pioglitazone, activates PGC-1α and ameliorates age-related renal injury.69 Formoterol, a β2-adrenergic receptor agonist, stimulates PGC-1α expression and mitochondrial respiration in PT cells and mouse renal cortex.70 Administration of formoterol 24 hours after ischemic AKI in mice accelerated the return of mitochondrial and renal function by restoring mitochondrial protein expression and respiration via upregulation of PGC-1α.71 Interestingly, 5-HT2 agonists also seem to induce mitochondrial biogenesis in PT cells via PGC-1α.72 However, the signaling pathways by which these G protein–coupled receptor ligands increase mitochondrial biogenesis remain unclear, and current efforts in developing novel compounds are limited to high-throughput physiologic-based screening.68
Activators of AMP Kinase
AMP kinase (AMPK), the major energy-sensing enzyme, is inhibited in metabolic kidney diseases.37,38,73,74 Reduced AMPK activity can result in decreased FA oxidation via decreased CPT-1 expression,37 and AMPK activation by 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside increased FA oxidation and reduced lipid accumulation and renal pathology in mice.74 However, 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside also improved systemic metabolic parameters74; thus, the role of renal AMPK activation is unclear. Metformin, already widely used in diabetes treatment, can activate AMPK, but it also reduces blood glucose.75 In contrast, SS-31 restores renal AMPK activity without affecting blood glucose or insulin sensitivity.38 AMPK activation without protecting mitochondrial structure is not likely to restore FA oxidation. The relationship between mitochondrial protection, AMPK activity, and FA oxidation needs to be studied further.
Summary and Perspective
Defects in FA oxidation and mitochondria function are key players in AKI and CKD. Strategies aimed at stimulating mitochondrial FA uptake and oxidation can only be successful if mitochondria structure is intact. Targeting mitochondrial function and cellular bioenergetics upstream of cellular damage may be more effective than counteracting downstream inflammatory and fibrosis processes (Figure 4). Some of the mitochondria-targeted agents mentioned here are in early clinical development. A phase 2a trial with SS-31 (elamipretide) during renal angioplasty with stenting (NCT01755858) showed increased renal blood flow and improved cortical perfusion.76 A phase 4 trial with MitoQ as a dietary supplement for CKD (NCT02364648) is not yet open for recruitment. In preclinical studies, these mitochondria-targeted approaches seem to protect renal structure and function without affecting systemic metabolic parameters, suggesting that it may be possible to combine these novel targeted agents with standard therapies that target BP, glucose, or lipids.
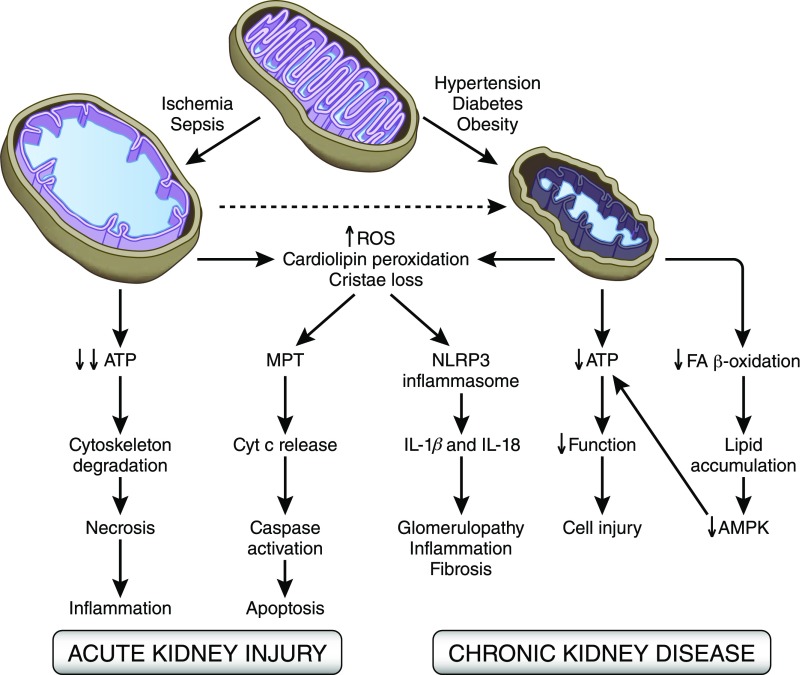
Figure 4.
Mitochondrial changes in animal models of AKI and CKD. Acute stressors, such as ischemia, sepsis, and toxins, cause mitochondrial matrix swelling and loss of cristae membrane. These structural changes are associated with impaired ATP production, oxidative stress, cellular demise, and chronic tissue remodeling. Cardiolipin peroxidation sets the stage for mitochondrial permeability transition, cytochrome c release, and apoptosis. In CKD, mitochondria are small, with reduced matrix density and loss of cristae membranes. Mitochondrial dysfunction causes a drop in ATP levels and cell injury, whereas inhibition of FA oxidation results in cellular lipid accumulation and downregulation of AMPK activity, further contributing to bioenergetics failure. In both AKI and CKD, cardiolipin peroxidation causes the translocation of cardiolipin to the OMM, where it serves as a docking station for NLRP3 inflammasome assembly. Mitochondrial ROS then triggers inflammasome activation to produce IL-1β and IL-18 that sustain tissue remodeling. MPT, mitochondrial permeabiltiy transition; NLRPE, NLR family pyrin domain containing 3.
Disclosures
H.H.S. is the inventor of SS-31 (elamipretide) and the Scientific Founder of Stealth Biotherapeutics, a clinical stage biopharmaceutical company that has licensed the SS peptides technology from the Cornell Research Foundation for commercial research and development.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63: 713–735, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Mount PF, Power DA: Balancing the energy equation for healthy kidneys. J Pathol 237: 407–410, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Emma F, Montini G, Parikh SM, Salviati L: Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat Rev Nephrol 12: 267–280, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Toole JF: Renal manifestations of genetic mitochondrial disease. Int J Nephrol Renovasc Dis 7: 57–67, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houten SM, Violante S, Ventura FV, Wanders RJ: The biochemistry and physiology of mitochondrial fatty acid β-oxidation and its genetic disorders. Annu Rev Physiol 78: 23–44, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, Park AS, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K: Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 21: 37–46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sieber J, Jehle AW: Free fatty acids and their metabolism affect function and survival of podocytes. Front Endocrinol (Lausanne) 5: 186, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glatz JF, Luiken JJ, Bonen A: Membrane fatty acid transporters as regulators of lipid metabolism: Implications for metabolic disease. Physiol Rev 90: 367–417, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Saudubray JM, Martin D, de Lonlay P, Touati G, Poggi-Travert F, Bonnet D, Jouvet P, Boutron M, Slama A, Vianey-Saban C, Bonnefont JP, Rabier D, Kamoun P, Brivet M: Recognition and management of fatty acid oxidation defects: A series of 107 patients. J Inherit Metab Dis 22: 488–502, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Szeto HH: First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol 171: 2029–2050, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szeto HH, Birk AV: Serendipity and the discovery of novel compounds that restore mitochondrial plasticity. Clin Pharmacol Ther 96: 672–683, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orrenius S, Zhivotovsky B: Cardiolipin oxidation sets cytochrome c free. Nat Chem Biol 1: 188–189, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Birk AV, Chao WM, Liu S, Soong Y, Szeto HH: Disruption of cytochrome c heme coordination is responsible for mitochondrial injury during ischemia. Biochim Biophys Acta 1847: 1075–1084, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG: Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol 1: 223–232, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, Oomori H, Noda T, Haraguchi T, Hiraoka Y, Amano A, Yoshimori T: Autophagosomes form at ER-mitochondria contact sites. Nature 495: 389–393, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Qiang Wang KZ, Zhu J, Klein-Seetharaman J, Balasubramanian K, Amoscato AA, Borisenko G, Huang Z, Gusdon AM, Cheikhi A, Steer EK, Wang R, Baty C, Watkins S, Bahar I, Bayır H, Kagan VE: Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol 15: 1197–1205, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paradies G, Petrosillo G, Paradies V, Ruggiero FM: Role of cardiolipin peroxidation and Ca2+ in mitochondrial dysfunction and disease. Cell Calcium 45: 643–650, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Tschopp J, Schroder K: NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 10: 210–215, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, Eisenbarth SC, Nauseef WM, Cassel SL, Sutterwala FS: Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39: 311–323, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, Sutterwala FS: Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A 106: 20388–20393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegel NJ, Avison MJ, Reilly HF, Alger JR, Shulman RG: Enhanced recovery of renal ATP with postischemic infusion of ATP-MgCl2 determined by 31P-NMR. Am J Physiol 245: F530–F534, 1983 [DOI] [PubMed] [Google Scholar]
- 22.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV: Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol 22: 1041–1052, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, Soong Y, Seshan SV, Szeto HH: Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. Am J Physiol Renal Physiol 306: F970–F980, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH: The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol 24: 1250–1261, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner MC, Rhodes G, Wang E, Pruthi V, Arif E, Saleem MA, Wean SE, Garg P, Verma R, Holzman LB, Gattone V, Molitoris BA, Nihalani D: Ischemic injury to kidney induces glomerular podocyte effacement and dissociation of slit diaphragm proteins Neph1 and ZO-1. J Biol Chem 283: 35579–35589, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsujimoto Y: Apoptosis and necrosis: Intracellular ATP level as a determinant for cell death modes. Cell Death Differ 4: 429–434, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaasik A, Safiulina D, Zharkovsky A, Veksler V: Regulation of mitochondrial matrix volume. Am J Physiol Cell Physiol 292: C157–C163, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Szeto HH, Liu S, Soong Y, Birk AV: Improving mitochondrial bioenergetics under ischemic conditions increases warm ischemia tolerance in the kidney. Am J Physiol Renal Physiol 308: F11–F21, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, David S, Burstein D, Karumanchi SA, Stillman IE, Arany Z, Parikh SM: PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest 121: 4003–4014, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parikh SM, Yang Y, He L, Tang C, Zhan M, Dong Z: Mitochondrial function and disturbances in the septic kidney. Semin Nephrol 35: 108–119, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi YM, Kim HK, Shim W, Anwar MA, Kwon JW, Kwon HK, Kim HJ, Jeong H, Kim HM, Hwang D, Kim HS, Choi S: Mechanism of cisplatin-induced cytotoxicity is correlated to impaired metabolism due to mitochondrial ROS generation. PLoS One 10: e0135083, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui J, Bai XY, Sun X, Cai G, Hong Q, Ding R, Chen X: Rapamycin protects against gentamicin-induced acute kidney injury via autophagy in mini-pig models. Sci Rep 5: 11256, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lan R, Geng H, Singha PK, Saikumar P, Bottinger EP, Weinberg JM, Venkatachalam MA: Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. J Am Soc Nephrol 27: 3356–3367, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szeto HH, Liu S, Soong Y, Seshan SV, Cohen-Gould L, Manichev V, Feldman LC, Gustafsson T: Mitochondria protection after acute ischemia prevents prolonged upregulation of IL-1β and IL-18 and arrests CKD. J Am Soc Nephrol 28: 1437–1449, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sweetwyne MT, Pippin JW, Eng DG, Hudkins KL, Chiao YA, Campbell MD, Marcinek DJ, Alpers CE, Szeto HH, Rabinovitch PS, Shankland SJ: The mitochondrial-targeted peptide, SS-31, improves glomerular architecture in mice of advanced age. Kidney Int 91: 1126–1145, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kume S, Uzu T, Araki S, Sugimoto T, Isshiki K, Chin-Kanasaki M, Sakaguchi M, Kubota N, Terauchi Y, Kadowaki T, Haneda M, Kashiwagi A, Koya D: Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J Am Soc Nephrol 18: 2715–2723, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Szeto HH, Liu S, Soong Y, Alam N, Prusky GT, Seshan SV: Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int 90: 997–1011, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U: Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res 55: 561–572, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Declèves AE, Sharma K: Novel targets of antifibrotic and anti-inflammatory treatment in CKD. Nat Rev Nephrol 10: 257–267, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson KJ, Weinberg JM: Postischemic renal injury due to oxygen radicals. Curr Opin Nephrol Hypertens 2: 625–635, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Murphy MP, Smith RA: Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol 47: 629–656, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Dare AJ, Bolton EA, Pettigrew GJ, Bradley JA, Saeb-Parsy K, Murphy MP: Protection against renal ischemia-reperfusion injury in vivo by the mitochondria targeted antioxidant MitoQ. Redox Biol 5: 163–168, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plotnikov EY, Chupyrkina AA, Jankauskas SS, Pevzner IB, Silachev DN, Skulachev VP, Zorov DB: Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochim Biophys Acta 1812: 77–86, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Plotnikov EY, Morosanova MA, Pevzner IB, Zorova LD, Manskikh VN, Pulkova NV, Galkina SI, Skulachev VP, Zorov DB: Protective effect of mitochondria-targeted antioxidants in an acute bacterial infection. Proc Natl Acad Sci U S A 110: E3100–E3108, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lowes DA, Thottakam BM, Webster NR, Murphy MP, Galley HF: The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic Biol Med 45: 1559–1565, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Patil NK, Parajuli N, MacMillan-Crow LA, Mayeux PR: Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: Mitochondria-targeted antioxidant mitigates injury. Am J Physiol Renal Physiol 306: F734–F743, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fink BD, Herlein JA, Yorek MA, Fenner AM, Kerns RJ, Sivitz WI: Bioenergetic effects of mitochondrial-targeted coenzyme Q analogs in endothelial cells. J Pharmacol Exp Ther 342: 709–719, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reily C, Mitchell T, Chacko BK, Benavides G, Murphy MP, Darley-Usmar V: Mitochondrially targeted compounds and their impact on cellular bioenergetics. Redox Biol 1: 86–93, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chacko BK, Reily C, Srivastava A, Johnson MS, Ye Y, Ulasova E, Agarwal A, Zinn KR, Murphy MP, Kalyanaraman B, Darley-Usmar V: Prevention of diabetic nephropathy in Ins2(+/)−(AkitaJ) mice by the mitochondria-targeted therapy MitoQ. Biochem J 432: 9–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao L, Xu X, Zhang F, Wang M, Xu Y, Tang D, Wang J, Qin Y, Liu Y, Tang C, He L, Greka A, Zhou Z, Liu F, Dong Z, Sun L: The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol 11: 297–311, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH: Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279: 34682–34690, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Birk AV, Chao WM, Bracken C, Warren JD, Szeto HH: Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol 171: 2017–2028, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mizuguchi Y, Chen J, Seshan SV, Poppas DP, Szeto HH, Felsen D: A novel cell-permeable antioxidant peptide decreases renal tubular apoptosis and damage in unilateral ureteral obstruction. Am J Physiol Renal Physiol 295: F1545–F1553, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li G, Wu J, Li R, Yuan D, Fan Y, Yang J, Ji M, Zhu S: Protective effects of antioxidant peptide SS-31 against multiple organ dysfunctions during endotoxemia. Inflammation 39: 54–64, 2016 [DOI] [PubMed] [Google Scholar]
- 56.Wijermars LG, Schaapherder AF, de Vries DK, Verschuren L, Wüst RC, Kostidis S, Mayboroda OA, Prins F, Ringers J, Bierau J, Bakker JA, Kooistra T, Lindeman JH: Defective postreperfusion metabolic recovery directly associates with incident delayed graft function. Kidney Int 90: 181–191, 2016 [DOI] [PubMed] [Google Scholar]
- 57.Eirin A, Ebrahimi B, Zhang X, Zhu XY, Woollard JR, He Q, Textor SC, Lerman A, Lerman LO: Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc Res 103: 461–472, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hou Y, Li S, Wu M, Wei J, Ren Y, Du C, Wu H, Han C, Duan H, Shi Y: Mitochondria-targeted peptide SS-31 attenuates renal injury via an antioxidant effect in diabetic nephropathy. Am J Physiol Renal Physiol 310: F547–F559, 2016 [DOI] [PubMed] [Google Scholar]
- 59.Alam NM, Mills WC 4th, Wong AA, Douglas RM, Szeto HH, Prusky GT: A mitochondrial therapeutic reverses visual decline in mouse models of diabetes. Dis Model Mech 8: 701–710, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki T, Yamaguchi H, Kikusato M, Matsuhashi T, Matsuo A, Sato T, Oba Y, Watanabe S, Minaki D, Saigusa D, Shimbo H, Mori N, Mishima E, Shima H, Akiyama Y, Takeuchi Y, Yuri A, Kikuchi K, Toyohara T, Suzuki C, Kohzuki M, Anzai J, Mano N, Kure S, Yanagisawa T, Tomioka Y, Toyomizu M, Ito S, Osaka H, Hayashi K, Abe T: Mitochonic Acid 5 (MA-5), a derivative of the plant hormone indole-3-acetic acid, improves survival of fibroblasts from patients with mitochondrial diseases. Tohoku J Exp Med 236: 225–232, 2015 [DOI] [PubMed] [Google Scholar]
- 61.Suzuki T, Yamaguchi H, Kikusato M, Hashizume O, Nagatoishi S, Matsuo A, Sato T, Kudo T, Matsuhashi T, Murayama K, Ohba Y, Watanabe S, Kanno S, Minaki D, Saigusa D, Shinbo H, Mori N, Yuri A, Yokoro M, Mishima E, Shima H, Akiyama Y, Takeuchi Y, Kikuchi K, Toyohara T, Suzuki C, Ichimura T, Anzai J, Kohzuki M, Mano N, Kure S, Yanagisawa T, Tomioka Y, Toyomizu M, Tsumoto K, Nakada K, Bonventre JV, Ito S, Osaka H, Hayashi K, Abe T: Mitochonic Acid 5 binds mitochondria and ameliorates renal tubular and cardiac myocyte damage. J Am Soc Nephrol 27: 1925–1932, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsuhashi T, Sato T, Kanno SI, Suzuki T, Matsuo A, Oba Y, Kikusato M, Ogasawara E, Kudo T, Suzuki K, Ohara O, Shimbo H, Nanto F, Yamaguchi H, Saigusa D, Mukaiyama Y, Watabe A, Kikuchi K, Shima H, Mishima E, Akiyama Y, Oikawa Y, Hsin-Jung HO, Akiyama Y, Suzuki C, Uematsu M, Ogata M, Kumagai N, Toyomizu M, Hozawa A, Mano N, Owada Y, Aiba S, Yanagisawa T, Tomioka Y, Kure S, Ito S, Nakada K, Hayashi KI, Osaka H, Abe T: Mitochonic Acid 5 (MA-5) facilitates ATP synthase oligomerization and cell survival in various mitochondrial diseases. EBioMedicine 20: 27–38, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park CW, Zhang Y, Zhang X, Wu J, Chen L, Cha DR, Su D, Hwang MT, Fan X, Davis L, Striker G, Zheng F, Breyer M, Guan Y: PPARalpha agonist fenofibrate improves diabetic nephropathy in db/db mice. Kidney Int 69: 1511–1517, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Sohn M, Kim K, Uddin MJ, Lee G, Hwang I, Kang H, Kim H, Lee JH, Ha H: Delayed treatment with fenofibrate protects against high-fat diet-induced kidney injury in mice: The possible role of AMPK autophagy. Am J Physiol Renal Physiol 312: F323–F334, 2017 [DOI] [PubMed] [Google Scholar]
- 65.Li L, Emmett N, Mann D, Zhao X: Fenofibrate attenuates tubulointerstitial fibrosis and inflammation through suppression of nuclear factor-κB and transforming growth factor-β1/Smad3 in diabetic nephropathy. Exp Biol Med (Maywood) 235: 383–391, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kouroumichakis I, Papanas N, Zarogoulidis P, Liakopoulos V, Maltezos E, Mikhailidis DP: Fibrates: Therapeutic potential for diabetic nephropathy? Eur J Intern Med 23: 309–316, 2012 [DOI] [PubMed] [Google Scholar]
- 67.Scarpulla RC, Vega RB, Kelly DP: Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab 23: 459–466, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whitaker RM, Corum D, Beeson CC, Schnellmann RG: Mitochondrial biogenesis as a pharmacological target: A new approach to acute and chronic diseases. Annu Rev Pharmacol Toxicol 56: 229–249, 2016 [DOI] [PubMed] [Google Scholar]
- 69.Yang HC, Deleuze S, Zuo Y, Potthoff SA, Ma LJ, Fogo AB: The PPARgamma agonist pioglitazone ameliorates aging-related progressive renal injury. J Am Soc Nephrol 20: 2380–2388, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wills LP, Trager RE, Beeson GC, Lindsey CC, Peterson YK, Beeson CC, Schnellmann RG: The β2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. J Pharmacol Exp Ther 342: 106–118, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jesinkey SR, Funk JA, Stallons LJ, Wills LP, Megyesi JK, Beeson CC, Schnellmann RG: Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J Am Soc Nephrol 25: 1157–1162, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harmon JL, Wills LP, McOmish CE, Demireva EY, Gingrich JA, Beeson CC, Schnellmann RG: 5-HT2 receptor regulation of mitochondrial genes: Unexpected pharmacological effects of agonists and antagonists. J Pharmacol Exp Ther 357: 1–9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, Andreyev A, Quach T, Ly S, Shekhtman G, Nguyen W, Chepetan A, Le TP, Wang L, Xu M, Paik KP, Fogo A, Viollet B, Murphy A, Brosius F, Naviaux RK, Sharma K: AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest 123: 4888–4899, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Declèves AE, Zolkipli Z, Satriano J, Wang L, Nakayama T, Rogac M, Le TP, Nortier JL, Farquhar MG, Naviaux RK, Sharma K: Regulation of lipid accumulation by AMP-activated kinase [corrected] in high fat diet-induced kidney injury. Kidney Int 85: 611–623, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hawley SA, Gadalla AE, Olsen GS, Hardie DG: The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 51: 2420–2425, 2002 [DOI] [PubMed] [Google Scholar]
- 76.Saad A, Hermann S, Lerman LO, Textor SC: Phase 2a clinical trial of mitochondrial protection (elamipretide) during stent revascularization in patients with atherosclerotic renal artery stenosis. Presented at the American Society of Nephrology Kidney Week, Chicago, November 15–20, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]