Abstract
Tomatoes are known to have ameliorative effects on cardiovascular disease and cancer. The nutritional value of tomatoes can be enhanced by increasing flavonoids content through genetic modification. The regulatory gene PAP1 (production of anthocyanin pigment 1) from Arabidopsis is reported to increase initial flavonoid flux and anthocyanin content. The structural gene CHI from Alium cepa increases flavonol content. However, the number of structural genes that can be transferred to plants is limited. To solve this problem, for the first time, we produced gene stacking transgenic tomato, in which Arabidopsis PAP1 (production of anthocyanin pigment 1) was stacked with an onion CHI by crossing. This procedure resulted in increased rutin and total anthocyanin content of as much as 130 and 30 times more, respectively, than the content in wild tomato skin, compared with 2.3 and 3 times more flavonol content, and 1 and 1.5 times more anthocyanin content in unstacked FLS and PAP1 tomatoes, respectively.
Introduction
Flavonoids are a subclass of plant polyphenols1 that are known to have a wide range of health-promoting effects2. Flavonoids are thought to have positive effects on, for example, cardiovascular diseases associated with oxidative stress3, diabetes4,5, and inflammation6. A bioactive flavonol, rutin, is more abundant in tomatoes than other flavonols7, but it is nevertheless present only in trace amounts. It has a range of pharmacological effects that inhibit oxidation, inflammation, and hypertension as well as vasoconstrictive, spasmolytic, and positive inotropic effects8–10. Transgenic tomatoes enriched by rutin have an effect that improves longitudinal bone growth11. Another subclass of flavonoids is anthocyanin, which offers a broad spectrum of potential pharmacological effects that protect against the formation of various cancer cells12,13, chronic obstructive pulmonary disease14, diabetes15 and vascular disease16.
Research work on increasing flavonoid content has been reported17. Genetic engineering is an effective way of increasing the flavonoid content of vegetables and fruits through manipulating structural or regulatory genes along flavonoid synthesis pathways18. Previous studies have attempted to manipulate single or stacking structural or regulatory genes, by either subsequent transformation, crossing, or construction of vectors containing several genes19. For example, ectopic expression of petunia chalcone isomerase (CHI) in tomatoes increased total flavonoid content 78 times20.
A major limiting factor in the flavonoid biosynthetic pathway is the lack of expression of the CHI gene in tomato peel20. The lack of CHI expression in the fruit peel could be caused by a mutation in the promoter21. Reintroducing CHI expression in cultivated tomato fruit can be achieved by interspecific crosses with wild tomato species21 or ectopic expression of CHI isolated from other species, for example petunia20.
Concomitant ectopic expression of CHS (chalcone synthase), CHI, F3H (flavanone 3-hydroxylase), and FLS (flavonol synthase) in tomatoes has been reported22 (Fig. 1). In this study, both the CHI and FLS genes were cloned from onions (Allium cepa L.). FLS is a common enzyme that produces the flavonols quercetin, myricetin and kaempferol23 (Fig. 1). Onions rank highest in quercetin content among vegetables and fruits, containing 20 to 30 times more of this flavonoid than any other crops24. Usually, flavonoids are synthesized in the peel, and negligible levels accumulate in flesh tissues22.
Figure 1.
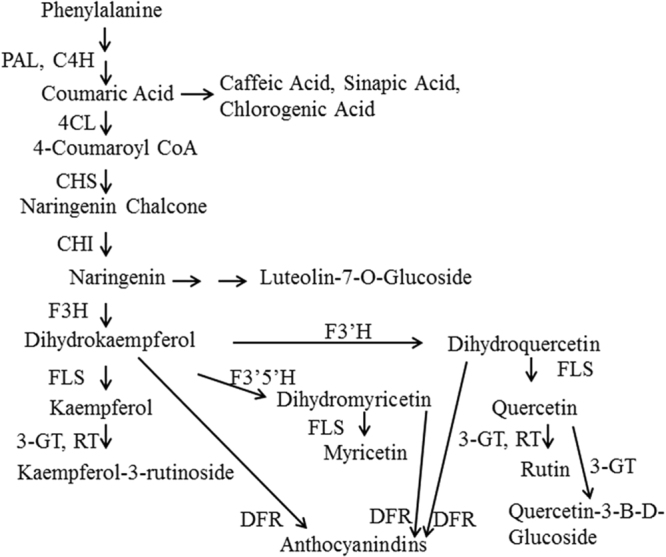
Schematic representation of the flavonoid biosynthetic pathway. PAL: phenylalanine ammonia lyase; 4CL: 4-coumarate:coenzyme A ligase; C4H: cinnamate 4-hydroxylase; C3H: 4-coumarate 3-hydroxylase; CHS: chalcone synthase; CHI: chalcone isomerase; F3H: flavanone-3-hydroxylase; F3′H: flavonoid-3¢-hydroxylase; F3′5′H: flavonoid-3′5′-hydroxylase; FLS: flavonol synthase; DFR: dihydroflavonol reductase; 3-GT: flavonoid 3-O glucosyltransferase; RT: flavonoid 3-O-glucoside-rhamnosyltransferase.
Regulatory genes provide the benefit of upregulating many genes at once25. Regulatory genes have been used with tomato mainly to increase flavonol through anthocyanin. For example, AtMYB12 1 and Lc/C1 26 genes caused flavonol content increased in tomato, while Del/Ros 27 and MYB75/PAP1 17 genes led to anthocyanin content increased. The regulatory gene PAP1 upregulates PAL, CHS and DFR along the flavonoid pathway28. However, PAP1 cannot upregulate all the genes necessary to enhance the flavonoid profile of tomato29. To upregulate genes in anthocyanin pathway,, the PAP1 gene was incorporated into tomato and increased anthocyanin content in shoots, but there was no substantial anthocyanin increase in the fruit17.
The onion CHI 30 and Del/Ros genes were reported to express both in the peel and flesh of tomato fruit. To enhance the flavonoid profile, the staking of structural genes and regulatory genes was first reported by Lim et al.31. By stacking the regulatory gene Del/Ros and the structural gene onion CHI, the content of both flavonol and anthocyanin increased. And the taste of the modified tomato did not differ from that of the wild30. The PAP1 encodes the MYB75 transcription factor32. The MYB75 gene expresses in tomato fruit and upregulatesDFR expression in various organs such as stems, leaves, sepals, and fruits17.
The MYB75 upregulates FLS, F3H, F3′H, CHS and PAL in Arabidopsis 33. Regulation is typically facilitated by an R2R3 MYB and/or a basic helix–loop–helix (bHLH) transcription factor33. Ectopic expression of genes of MYB transcription factors such as PAP1 in various plant species has confirmed that these regulatory elements are conserved across species34. Since the CHI is a major rate-limiting factor26 in flavonol synthesis, our hypothesis is that the insufficient increase in anthocyanin in tomato fruit might be caused by insufficient expression of CHI 17. In this study, we demonstrated that regulatory gene function was enhanced by stacking PAP1 and CHI genes. Consequently, a regulatory gene can be used more effectively in combination with a structural gene than when used alone. This result demonstrated significantly improved tomato nutritional content, making tomatoes healthier to eat.
Results
Generation of CHI- FLS- and PAP1 -expressing tomato plants
Initially, 15 transgenic lines for each FLS and PAP1 were generated. Among them, 9 morphologically normal and healthy lines were selected and subjected to further analysis. The 15 lines were selected on 100 mg/L kanamycin selection analysis before DNA and RNA amplification. All transgenic tomatoes were confirmed by DNA amplification.
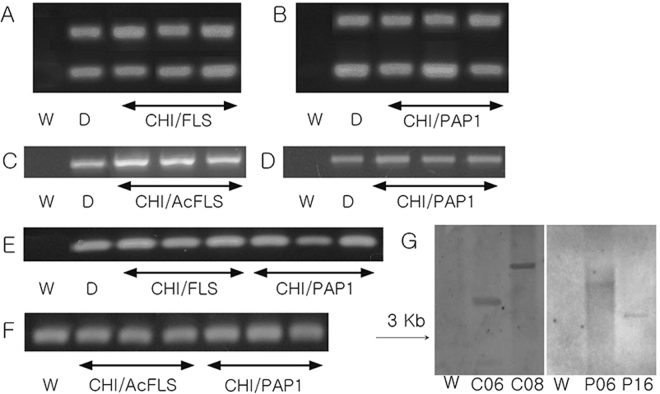
The shapes of all the selected transgenic tomatoes were indistinguishable from those of the wild type (Fig. 2A,B and C). There were no statistical differences for fruit weight and number of fruits per plant between transgenic plants having different transgenes and wild type plants (Table 1). The CHI lines were used from previous work30,31. By crossing, we obtained 4 lines of FLS x CHI and 5 lines of PAP1 x CHI. All stacked lines were confirmed by PCR using genomic DNA (Fig. 3A and B) as the template. The expression of genes was confirmed by RT-PCR (Fig. 3C~F). DNA and RT-PCR were performed by each gene primer for every transgenic plant before further experiments to prevent segregation because each gene was in different vector.
Figure 2.
A ~ C T2 and F2 generations of wild and transgenic tomatoes and whole plants. (A) The fruit harvest at 20 days after the breaker stage. (B) Tomatoes in various ripening states. (C) Whole plants in the greenhouse. (D) Petioles of wild and PAP1 trangenic plants show purple spots in early growth stages: upper: wild; middle: PAP1; bottom: PAP1 x CHI. W: wild type; 1: CHI; 2: FLS; 3: PAP1; 4: FLS x CHI; 5: PAP1 x CHI.
Table 1.
The weight of individual fruits and the number of harvested fruits per plant. Each line has 3–5 replications. The individual plants were pruned to have 3–4 branches.
| Gene | Weight | Number per plant | ||
|---|---|---|---|---|
| Mean | Standard error | Mean | Standard error | |
| Wild | 51.6 | ± 4.4 | 38.2 | ± 6.7 |
| FLS | 53.2 | ± 5.1 | 37.2 | ± 2.6 |
| PAP1 | 48 | ± 4.3 | 41.2 | ± 7.4 |
| CHI | 44.7 | ± 4.6 | 32.6 | ± 4.9 |
| CHI/FLS | 47.2 | ± 5.9 | 41.7 | ± 6.9 |
| CHI/PAP1 | 51.4 | ± 4.8 | 34 | ± 5.3 |
aMature fruits were harvested from T2 for CHI and homozygous F2 populations of three independent transgenic lines. Tomatoes were harvested 20 d after breaker stage. The data represent the mean values (±SD). There were no statistical differences in ANOVA tests.
Figure 3.
Molecular analysis of stacked from tomato peel. (A~B) PCR results derived from genomic DNA; (A) upper bands: amplified FLS gene; lower bands: amplified CHI gene; (B) upper bands: amplified PAP1 gene; lower bands: amplified CHI gene; (C~F) reverse transcription polymerase chain reaction (RT-PCR) amplified by C FLS primer; D PAP1 primer; E CHI primer; F housekeeping gene PP2AS primer; W: wild type. (D) Vector control. (G) Southern blot analysis: C06(CHI-06), C08(CHI-08), P06(PAP1-06), and P16(PAP1-16). Arrows indicate expected fragments larger than 3.0 kb corresponding to the integration of T-DNA into the tomato genomic DNA.
The petioles of approximately 1 month old plants harboring both PAP1 and CHI had dark purple spots and lasted for c.a. 3 weeks (Fig. 2D).
Molecular work
Gene expression was confirmed by RT-PCR (Fig. 3C~F). All the 32 T0 lines of FLS, PAP1, and CHI were confirmed by DNA amplification. All the T1 lines of each gene were checked by DNA and RT-PCR analysis. Consistent with single insertion, each T1 line showed a 3:1 segregation ratio. Over 90% of the F1 generation lines of FLS x CHI and PAP1 x CHI had both genes integrated. Also, this segregation ratio is the same in the F2 generation. All stacked and unstacked genes were stably transmitted to the next generation. Figure 3C~F shows a typical expression pattern of the CHI, FLS, and PAP1 genes in CHI x FLS and CHI x PAP1. They exhibited no distinguishable phenotypes between lines regardless of flavonoid content and inserted genes. The expression of each FLS, CHI and PAP1 was independently strong enough on lines, flavonol and anthocyanin content.
The CHI and PAP1 lines were selected for Southern blot analysis because the stacked lines between them show the highest total flavonol content. Among the five CHI lines, CHI-06 and CHI-08 were selected for Southern blot analysis(Fig. 3G) because they showed the highest content of the major flavonols and total flavonols from previous work30,31 when stacked with the PAP1 and FLS lines. The genomic DNAs from randomly selected CHI and PAP1 transgenic plants were digested with HINDII to include inserted T-DNA and genomic DNA and hybridized with CHI and PAP1 probe. All of the detected fragments with CHI and PAP1 probe had the expected fragment size of larger than 3.0k. These lines demonstrated single copy insertion.
Major flavonoids
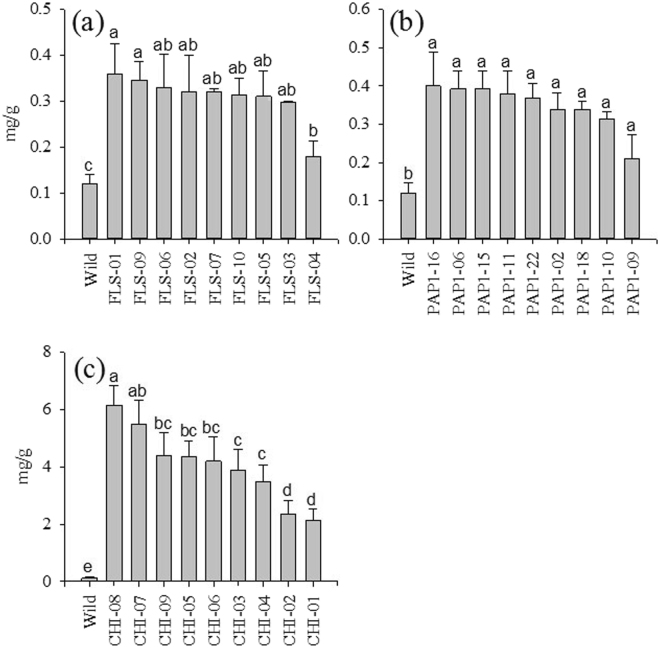
First, we detected the rutin content in CHI, FLS, and PAP1 tomatoes. All the FLS-, CHI-, and PAP1-expressing tomatoes displayed significantly higher rutin content as compared with wild-type tomatoes (Fig. 4). Among these transgenic plants, the CHI-expressing tomatoes exhibited the highest rutin content as compared with other transgenic and wild-type tomatoes (Fig. 4). The CHI lines used for crossing with the FLS and PAP1 lines were selected to compare genetic effects. Rutin concentration in the unstacked T1 generation of CHI-expressing tomatoes exhibited greater variation than that in the FLS- and PAP1-expressing tomatoes. The FLS and CHI lines exhibited significant differences between lines, but PAP1 showed no differences between lines regarding rutin concentration.
Figure 4.
Rutin concentration when selecting T1 generation for crossing (a) FLS, (b) PAP1, and (c) CHI. (c) Screening of transformants that have onion CHI flavonoid levels in the peel of the wild-type fruit and T1 transgenic Rubion tomato fruit determined by HPLC. Fruits were harvested between 15 and 20 days after breaker stage. Values with the same letters are not significantly different at 0.05 using the Tukey test. Tomatoes were harvested 20 d after breaker stage. The data represent the mean values (±SD) derived from 5–7 plants per each line (4 to 6 pooled tomatoes per plant).
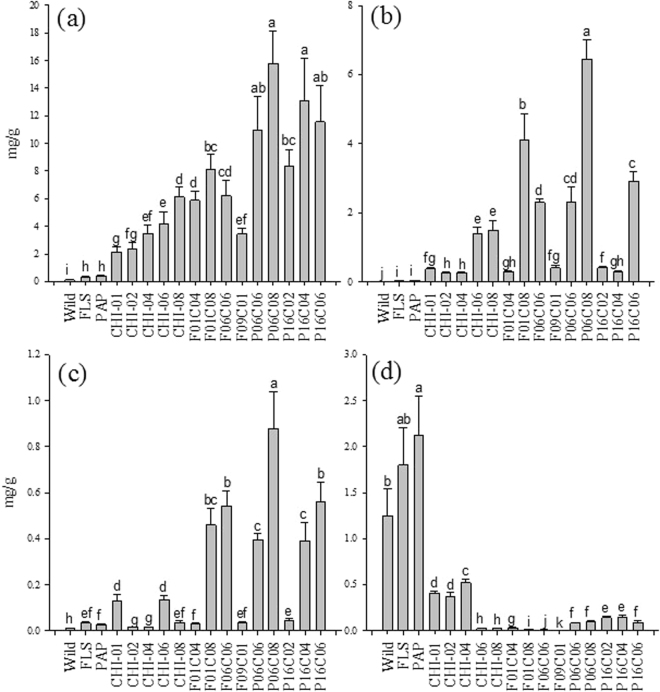
The FLS x CHI and PAP1 x CHI transgenic tomato lines crossed with the CHI-08 line exhibited the highest rutin, quercetin glucoside, and kaempferol rutinoside content (Fig. 5) in each stacked tomato compared with CHI lines. While the wild, FLS, and PAP1 transgenics exhibited unmeasureable traces of quercetin-glucoside, quercetin-glucoside content in the F01C08 and P06C08 lines were 4.11 and 6.44 mg/g, respectively. The CHI lines exhibited the largest difference in production of kaempferol rutinoside when compared with the FLS and PAP1 lines crossed with the CHI lines. Both the CHI 06 and 08 lines crossed with FLS and PAP1 exhibited considerable differences, but the CHI 04 lines exhibited no difference when crossed with FLS. The high flavonol phenotype was maintained in mature fruit of hemizygous T1 and homozygous T2 individuals of the CHI x FLS and CHI x PAP1 lines, indicating that the high-flavonoid phenotype was inherited stably to the next generations (Table 1).
Figure 5.
Major phenolics of F1-crossed generation and T2 parents transgenics: F01C04~F09C01 and P06C06~P16C06 are crossed lines from FLSxCHI and PAP1xCHI, respectively. F and P stand for FLS and PAP1, respectively. (a) Rutin, (b) Quercetin -3-B-D-glucoside, (c) Kaempferol rutinoside, and (d) Naringenin chalcone. The FLS 01, 06, and 09 lines are combined into FLS and the PAP1 06 and 16 lines are combined into PAP1 because there were no statistical differences between the FLS and PAP1 lines. Fruits were harvested between 15 and 20 days after breaker stage. Values with the same letters are not significantly different at 0.05 using the Tukey test. Tomatoes were harvested 20 d after breaker stage. The data represent the mean values (±SD) derived from 5–7 plants per each line (4 to 6 pooled tomatoes per plant).
Naringenin chalcone is a precursor of naringenin converted by CHI (Fig. 1). The content of naringenin chalcone in CHI 06 and 08 was significantly lower than in CHI 01, 02, or 04. It showed an inverse relationship with rutin. When the lines were crossed with the FLS and PAP1 lines, the inverse relationship became less clear. The FLS and PAP1 genes might change the flux of the flavonoids. The variation in CHI lines by themselves and CHI lines crossed with the FLS and PAP1 lines exhibited consistently less variation than the wild, FLS, and PAP1 lines due to overexpression of CHI.
Minor flavonoids
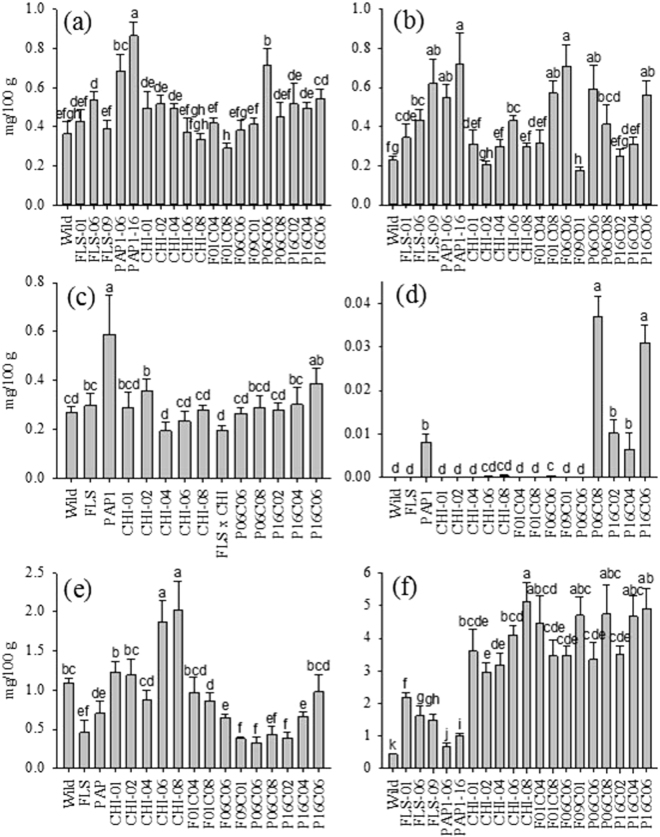
Chlorogenic acid, caffeic acid, cumaric acid, and sinapic acid are upstream of CHI, while luteolin-7-O-glucoside and myricetin are in the downstream (Fig. 1). These minor flavonoids are subject to more complex variation (Fig. 6) than the major flavonoids (Fig. 5). The chlorogenic acid, caffeic acid, and cumaric acid content of all unstacked PAP1 lines increased significantly compared with the wild lines. Sinapic acid increased to an average of 0.008 mg/100 g in the PAP1 line and 0.037 mg/100 g and 0.031 mg/100 g in the P06C08 and P16C06 lines, respectively, from zero in the wild type. The content of luteolin-7-O is highest in the CHI 06 and 08 lines. Neither the unstacked FLS and PAP1 lines nor these lines stacked with CHI had a significant effect on enhancing the luteolin-7-O flavonol content. Regarding myricetin content, all CHI stacked lines with FLS and PAP1 exhibited higher content than the wild, FLS, and PAP1 lines.
Figure 6.
Minor phenolics F1-crossed generation and F2 parents transgenics. Lines of transgenics without statistical differences are merged into one transgenic: (a) Chlorogenic acid, (b) Caffeic acid, (c) Cumaric acid, (d) Sinapic acid, (e) Luteolin-7-O-glucoside, and (f) Myricetin. Fruits were harvested between 15 and 20 days after breaker stage. Values with the same letters are not significantly different at 0.05 using the Tukey test. Tomatoes were harvested 20 d after breaker stage. The data represent the mean values (±SD) derived from 5–7 plants per each line (4 to 6 pooled tomatoes per plant).
Total flavonol content, antioxidant activity, and total anthocyanin content
The lines with highest rutin content in each genotype were selected for analysis. Differences in total flavonol content, anti-oxidant activity, and total anthocyanin content between stacked lines and their parents were measured (Table 2). The methanol- and ethanol (1:1)-extracted antioxidant activity of CHI x PAP1 increased as much as 53 times compared with the wild type. Hence, the most abundant flavonol in tomato peel was rutin, and the total flavonol content was in accordance with the rutin content. Only the CHI x PAP1 lines contributed to the increase in total anthocyanin content.
Table 2.
Total flavonol content, antioxidant activity, and total anthocyanin content in F2 generation.
| Total flavonol content (mg/g) | Antioxdiant activity TEAC mmol Trolox/Kg FW | Total Anthocyanin content ug/g | Lycopene content ug/g | |
|---|---|---|---|---|
| Wild | 1.09 d | 14 d | 8.17 b | 412 a |
| FLS 01 | 2.53 c | 52 c | 8.05 b | 399 a |
| PAP1 06 | 3.22 c | 42 c | 12.3 b | 419 a |
| CHI 08 (published) | 12.7 | 283 | 8.76 | 388 a |
| F01C08 | 16.3 b | 295 b | 9.38 b | 399 a |
| P06C08 | 32.86 a | 754 a | 48.11 a | 402 a |
Fruits were harvested between 15 and 20 days after breaker stage. Values with the same letters are not significantly different at 0.05 using the Tukey test. The data represent the mean values (±SD) derived from 5–7 plants per each line (4 to 6 pooled tomatoes per plant).
Stability of flavonol expression between F1 and F2
Two CHI lines used for crossing and two crossed lines from each genotype were selected to test stability between generations. There was no significant difference between F1 and F2 by t-tests for rutin, quercetin-3-B-D-glucoside, or kaempferol rutinoside (Table 3). The order of flavonol content across lines and genotypes was not changed in any generations.
Table 3.
Genetic stability of the CHI x FLS and CHI x PAP1 expression of phenotypes in F1 and F2 generations of transgenic lines for major flavonols.
| Rutin | Quercetin -3-B-D-glucoside | Kaempferol rutinoside | ||||
|---|---|---|---|---|---|---|
| F1 | F2 | F1 | F2 | F1 | F2 | |
| Wild | 0.12 ± 0.02 | 0.14 ± 0.02 | 0 ± 0 | 0 ± 0 | 0.01 ± 0 | 0.01 ± 0 |
| CHI-06 | 4.2 ± 0.85 | 3.61 ± 0.51 | 1.4 ± 0.18 | 1.01 ± 0.14 | 0.14 ± 0.02 | 0.12 ± 0.01 |
| CHI-08 | 6.15 ± 0.68 | 4.92 ± 0.64 | 1.49 ± 0.28 | 1.91 ± 0.24 | 0.04 ± 0.01 | 0.04 ± 0.01 |
| F01C08 | 8.11 ± 1.1 | 7.62 ± 1.07 | 4.11 ± 0.74 | 3.37 ± 0.58 | 0.46 ± 0.07 | 0.35 ± 0.07 |
| F06C06 | 6.2 ± 1.12 | 5.38 ± 0.96 | 2.3 ± 0.12 | 1.73 ± 0.19 | 0.54 ± 0.07 | 0.39 ± 0.05 |
| P06C06 | 10.99 ± 2.4 | 9.67 ± 1.74 | 2.32 ± 0.42 | 3.02 ± 0.34 | 0.39 ± 0.03 | 0.34 ± 0.02 |
| P06C08 | 15.78 ± 2.37 | 14.84 ± 1.3 | 6.44 ± 0.58 | 4.58 ± 0.45 | 0.88 ± 0.16 | 0.73 ± 0.14 |
Fruits were harvested between 15 and 20 days after breaker stage. Five to seven plants from each independent line were analyzed. From each plant, two or three fruits were pooled. There were no significant differences for all gynotypes between F1 and F1 by t-tests.
Discussion
The ectopic expression of our onion CHI gene resulted in a significant increase in rutin, as expected based on previous work31. The rise in total flavonol accumulation was comprised mainly of increases in the accumulation of rutin, quercetin glucoside, and kaempferol-rutinoside in the peel tissues. This result was consistent with that reported in Verhoeyen et al.22. All these compounds have the same precursor, dihydrokampferol converted by FLS. The ectopic expression of the petunia FLS gene by itself does not increase the flavonol level22. The petunia FLS is effective only when stacked with CHI and CHS. However, our onion FLS increased the rutin content by a factor of as much as 3.5. Our cultivar may have a naringenin chalcone pool, which is the product of CHS, that can supply enough substrate to FLS and CHI.
The onion CHI was used on the assumption that onion has a robust flavonol pathway because onion exhibited the highest reported quercetin content in a survey of 28 vegetables and 9 fruits35. The quercetin constitutes more than 80% of the total flavonoids in onion33. Rutin is the glycoside form of quercetin. In both CHI/PAP1 and CHI/FLS lines, the rutin content increased significantly in comparison with the CHI-only plants of its parent, which indicated that the CHI gene transmitted to the next generation successfully. The line difference was greater than the genotype difference. The order of rutin content was CHI08 > CHI06 > CHI 04. In both CHI/FLS and CHI/PAP1 lines, the order of CHI lines for rutin did not change. It is possible that the effect of CHI is greater than that of FLS or PAP1. The average of rutin content in CHII/PAP1 was greater than in CHI/FLS even though the rutin content in FLS only was almost the same as in PAP1 only. It has been reported that PAP1 upregulates PAL, CHS, and DFR in Arabidopsis 28,32,34,36. The PAP1 overexpression by itself does not fully activate all rate-limiting steps in anthocyanin biosynthesis, requiring further activation of the phenylpropanoid pathway34. The exact function of PAP1 in tomato fruit has not yet been reported. The onion CHI in CHI/PAP1 may relieve the bottleneck, enabling movement of the flavonol flux to naringenin and eventually to rutin.
The content of quercetin-glucoside and kaempferol rutinoside showed more complex variations than that of rutin. However, lines crossed with CHI08 and CHI06 had the highest and second highest content, respectively, of these flavonols in both CHI/PAP1 and CHI/FLS genotypes, and the same content as in the CHI-only tomato. Not all CHI/PAP1 lines showed increased quercetin-glucoside content compared with CHI-only lines. Flavonol content in a stacked genotype depends on how the regulatory gene works to move the flavonoid flux. When CHI is crossed with another regulatory gene, Del/Ros, the content of quercetin-glucoside in CHI/Del/Ros is lower than in CHI-only lines31. The Del/Ros converted the flavonol substrate from flavonol to anthocyanin31. In that case, the CHI08 line showed also the highest anthocyanin content and the CHI06 line shows the second highest31.
The level of naringenin chalcone accumulation was depleted in the high-flavonol fruit when compared with the wild type. The high-flavonol lines, CHI-06 and 08, exhibited the lowest content of naringenin chalcone, which was converted to naringenin by CHI. In high-flavonol transgenic tomato favoring the petunia CHI gene, naringenin and naringenin chalcone have a negative correlation20. The proportional increase in rutin, quercetin-glucoside, and kaempferol rutinoside was much greater than the decrease in naringenin chalcone in the high-flavonol lines. Verhoeyen (2002) suggested that the ectopic expression of CHI utilized the naringenin chalcone pool and that depletion of naringenin chalcone removed a point of negative feedback, in the form of increased flux, from the pathway22.
The PAP1-transferred tomato showed a pale pinkish color on the shoot and some pink spots in the tomato fruit in the green state17. However, the color of the fruit was the same across the genotypes even though there was a 6-fold increase in a CHI/PAP1 line. the typical red color in tomato comes from lycopene31. The color of anthocyanin might be covered by lycopene17 in this experiment. The transcription factors in flavonoid biosynthesis, including PAP1, often work in a complex combinatorial way and can also change the expression of other regulatory factors to enable a cell-specific accumulation of pigments37. Not only regulatory genes, but also structural genes such as CHI and FLS, express in tissue-specific ways38. Most flavonol and anthocyanin are located in tomato peel rather than flesh22. Flavonol alone1 or anthocyanin alone27 increase in both peel and flesh with one and two regulatory genes, respectively. Both flavonol and anthocyanin increased in both flesh with both two regulatory genes and one structural gene31. However, in this report, a single regulatory gene and a single structural gene were used to increase both flavonol and anthocyanin in tomato peel.
Overall, the PAP1 x CHI lines were more effective than the FLS x CHI lines in terms of flavonol production. Even though the PAP1 gene upregulates the DFR gene17, the upregulation of DFR is not active enough to exploit most of the flavonol flux converted by ectopic expression of CHI. For the most part, some minor phenolics, upstream of CHI, exhibited very little increase in the CHI x PAP1 lines. This might be due to the movement of the flavonoid flux by the activation of CHS by PAP1 and the ectopic expression of CHI. There are two groups involved in flavonoid pathways. One is an early biosynthetic gene such as CHS-, CHI- or F3H-regulated R2R3-MYB regulatory genes such as MYB12. The other is a late biosynthetic gene such as DFR, regulated by a ternary transcription factor including PAP1 39. MYB12-inserted tomato increased in flavonol content only1. In this experiment, the onion CHI removed the bottleneck blocking early biosynthetic genes and the PAP1 upregulated late biosynthetic genes. This resulted in both higher flavonol content and higher anthocyanin content. However, the upregulation of late biosynthetic genes was not strong enough to convert all flavonol to anthocyanin. The onion CHI stacked with two regulatory genes, Del/Ros showed the same effect of increasing both flavonol and anthocyanin content31.
We observed strong expression of PAP1 in fruit without environmental stress. In addition to the effect on flavonoid, the PAP1 tends to respond to its environment, with the expression level increasing by exposure to light17. In Arabidopsis, the anthocyanin level increases by osmotic pressure29. Herbivory suppressed the PAP1-induced increase of transcripts of flavonoid biosynthetic genes in tobacco40. However, the PAP1 expressed well in tomato fruit, increasing anthocyanin content without stress17.
In this antioxidant activity test, the main factor affecting the increase of antioxidant activity was the presence of rutin which is the most abundant flavonol in our transgenic unstacked and stacked lines and is a strong antioxidant41–43. The antioxidant activity of rutin increases with any increase in rutin concentration44. Also, the antioxidant activity of plant extracts from ARTEMISIA VULGARIS, of which the main flavonoid is rutin45, increases with the concentration of plant extracts, in the same manner as that of rutin46. In this experiment, the r-square of the regression between rutin content and antioxidant activity is 0.74 (data not shown). Even though total flavonol, rutin and anthocyanin content increased, the lycopene content did not change.
For the first time, we confirmed that combinations of one structural gene CHI and regulatory gene PAP1 enhanced flavonoid production tremendously. There were approximately 130 and 30 times more of the major flavonols, rutin and total anthocyanin, respectively, in tomato peel of CHI/PAP1 compared with the content in wild tomato peel. The research work provides very important information to improve flavonol content in tomato peel, which will add more nutritional value for tomato.
Methods
Vector construction
The FLS and CHI genes were cloned from red onion. RNA was extracted with the RNeasy plant mini kit from QIAGEN (Valencia, CA, U.S.A). cDNA was made with the Advantage RT-for-PCR Kit from Clontech (Mountain View, CA, U.S.A). The primer sequences for CHI and FLS cloning were CHI forward 5′-ATGGAAGCAGTGACAAAGTT-3′, CHI reverse 5′ T CATGAAAGCACCGGTAACT 3′ FLS forward 5′ ATGGAAGTAGAGAGAGTGCAGGCGA 3′, and FLS reverse 5′ TTACTGAGGAAGTTTATTAATTTTG 3′. The joined two vector with pE177547 were transferred to E. coli (DH5α) and Agrobacterium (LBA1775). The PAP1 and FLS vector was constructed following the methods of published reports34,36 with a 35 s promoter. The plasmids containing FLS and CHI were introduced into A. tumefaciens using the freeze–thaw method48. The PAP1 vector harboring PAP1 gene was provided from Vikram et al.36 with 35 s promoter28.
Plant Transformation
Tomato seeds Solanum lycopersicum L. (cv Rubion) were surface-sterilized and germinated on the Murashige and Skoog inorganic salt medium Murashige et al.49. Agrobacterium tumefaciens LBA 4404 was used to generate stable transgenic plants. Tomato transformation was performed via the Agrobacterium-mediated transformation method using cotyledon and hypocotyl explants, as described in Park et al.50. After inoculation with A. tumefaciens, the plant cultures were maintained at 25 °C under a 16-h photoperiod. After 6 to 8 weeks, regenerated shoots were transferred to a rooting medium for 6 more weeks. The temperature of the greenhouse was maintained within a range of 25 °C to 30 °C. All genes mentioned above were transferred to the Rubion tomato cultivar.
Transgenic plant confirmation
Tomato genomic DNA and RNA were extracted from leaf tissue using the Qiagen Plant DNA extraction kit (Germantown, MD, U.S.A). Tomato RNA was extracted from peel using the Qiagen Plant RNA extraction kit (Germantown, MD, U.S.A). cDNA was synthesized by moloney murine leukaemia virus-reverse transciptase (BD Biosciences Clontech, Palo Alto, CA, USA). All the polymerase chain reaction (PCR) was performed with the GoTaq Flexi DNA Polymerase kit (Promega Corporation, Madison, WI, USA) as described in the manual. The initial cycle was 2 min at 94 °C, 10 min at 58 °C, and 2 min at 72 °C. The subsequent 30 cycles were 45 s at 94 °C, 45 s at 58 °C and 30 s min at 72 °C, followed by 10 min at 72 °C for the last cycle.
DNA isolation and Southern blot analysis
The Southern blot procedure was modified from Wu et al.51. Tomato genomic DNA was extracted from leaf tissue using the Qiagen Plant DNA extraction kit. DNA (10 µg) from the CHI lines and the PAP1 lines were digested with XbaI and BamHI, respectively. The digested DNA was separated by electrophoresis and blotted onto a nylon membrane (Zeta-probe GT membrane, Bio-Rad Laboratories, Hercules, CA), following the manufacturer’s instructions. The probe for the CHI and PAP1 genes was isolated from vectors harboring each gene51. The membranes were prehybridized overnight at 65 °C in 7% SDS and 0.25 M Na2HPO4 and then hybridized overnight at 65 °C in the same solution containing the probe labeled by the NEBlot Phototope Kit (New England Biolabs, Ipswich, MA). Membranes were washed twice for 40 min each with 20 mM Na2HPO4 and 5% SDS at 65 °C and then washed twice again for 30 min each with 20 mM Na2HPO4 and 1% SDS at 65 °C. The signal was detected by the Phototope-Star Detection Kit (New England Biolabs, Ipswich, MA, U.S.A).
HPLC analysis
One gram of peel was frozen in liquid nitrogen and macerated in a round-bottom 15 ml tube with a plastic pestle. The samples were extracted with 4.8 ml of 62.5% methanol and 1.2 ml 6 M HCl for 60 min at 45 °C. The extracts were cooled on ice and sonicated at temperature for 45 min. The samples were centrifuged at 13,000 RPM for 20 min. The supernatant was filtered with a 0.45 μm filter. The extraction procedure was based on Muir et al.20.
The HPLC analysis was modified from the published paper52. The HPLC system has an autosampler (SpectraSYSTEM AS1000, Thermo Separation Products, San Jose, CA, USA), a pump (HP 1050, Hewlett Packard, Palo Alto, CA, USA), an integrator (HP 3396, Hewlett Packard, Palo Alto, CA, USA), and an UV/VIS detector (Acutect 500, Thermo Separation Products, San Jose, CA, USA). A 5 μL sample was injected into the HPLC column (Discovery BIO Wide Bore C18, 15 cm × 4.6 mm, 5 μm, Supelco, Inc., Bellefonte, PA, USA) with a guard column (Discovery BIO Wide Bore C18, 2 cm × 4 mm, 5 μm, Supelco, Inc., Bellefonte, PA, USA). The sample was eluted with eluant A [H2O/ CH3COOH (338/1, v/v)] and eluant B [H2O/C4H10O/CH3COOH (330/8/1, v/v/v)] at a flow rate of 1.8 mL/min. The gradient is A 20~20%, B 80~80%, 0~5 min: A 20~0%, B 80~100%, 5~25 min. The peak is determined by UV absorption at 330 nm, compared with standards (5 mg/100 mL), rutin (Sigma–Aldrich, St. Louis, MO, USA), Kaempferol rutinoside (Sigma–Aldrich, St. Louis, MO, USA), Naringenin chalcone (Sigma–Aldrich, St. Louis, MO, USA), chlorogenic acid (Sigma–Aldrich, St. Louis, MO, USA), caffeic acid (Sigma–Aldrich, St. Louis, MO, USA), quercetin-3-O-glucoside (Sigma–Aldrich, St. Louis, MO, USA), cumaric acid (Sigma–Aldrich, St. Louis, MO, USA), sinapic acid (Sigma–Aldrich, St. Louis, MO, USA), luteolin-7-O-glucoside (Indofine Chemical Co., Inc., Hillsborough, NJ, USA), and myricentin (Sigma–Aldrich, St. Louis, MO, USA). The peak is confirmed by a co-chromatograph reference and mass spectrometer.
Stacking genes by crossing
The T2 plants harboring CHI and FLS gene were used as parent. The anthers were removed from unopened flowers one day before anthesis. The next day before noon, the pollens were collected with forceps. The emasculated flowers were pollinated with forceps. After pollination, the forceps were rinsed in a solution of 70% alcohol and wiped with a tissue.
Total flavonoid and anthocyanin content
To record total flavonoid content, the samples before HPLC injection were measured by a 361 nm photospectrometer known as the Nanodrop (Thermo Scientific, Wilmington, DE, USA). Rutin was used as the standard. Anthocyanin content was measured with minor modifications53. Tomato peel was ground in volume HCl 0.5% (v/v) in methanol. One volume of chloroform was added to the extract to remove chlorophylls. The mixture was centrifuged at 14,000 g for 1 min. Anthocyanins containing phase were recovered and absorption was determined spectrophotometrically at 544 nm with the Nanodrop.
Antioxidant activity
The antioxidant capacity of tomato was measured by the modified 2,20 -azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) or ABTS method25,29,30. Antioxidants were extracted with a 5 ml extraction solution [methonal/ethanol (70/29.5/0.5, v/v/v)] from 1 g of tomato peel samples. The extract containing antioxidants was incubated in darkness at −20 °C overnight. Subsequently, the solution was centrifuged at 1,000 rpm for 2 min. ABTS [(2.5 mM) (Roche Diagnostics, Indianapolis, IN, USA)] stock solution was prepared and about 0.4 g of MnO2 (Acros Organics, Belgium) was added to the stock solution to generate ABTS radical cation (ABTS*). Excess MnO2 was removed using a 0.2 mM disk filter (Millipore Corp., Bedford, MA, USA). The ABTS* solution was incubated at 30 °C in a water bath and was diluted to an absorbance of 0.7 (±0.02) at 730 nm using 5 mM phosphate buffer saline [pH 7.4 and ionic strength (150 mM NaCl)]. 100 mL of the extract was added to 1 mL of the ABTS* solution and vortexed for 10 s. The absorbance of the mixture was measured at 730 nm in a spectrophotometer (U-1100, Hitachi Ltd. Japan) after a 1-min reaction period. A Trolox [(6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxyl acid) (Acros Organics, Belgium)] standard curve was prepared using a 0.5-mM stock solution.
Statistical analysis
All data were analyzed using SAS (Version 9.1, Cary, N.C., U.S.A.)54. For mean separation, Tukey’s test was used. Analysis of variance was performed using the GLM procedure. Significant differences were determined at the 95% confidence level (P < 0.05). Each line had 4–6 plants. Two-to-three pooled tomatoes were collected from each plant for every line31.
Data availability
All data generated or analyzed during this study are included in this published article and available from the corresponding author on reasonable request.
Acknowledgements
This research is funded by HIGreen System.
Author Contributions
Wansang Lim designed, performed the experiment and draft the manuscript. Jiarui Li gave advice and idea for this research and wrote this manuscript as a coauthor.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Luo J, et al. AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: expression in fruit results in very high levels of both types of polyphenol. Plant J. 2008;56:316–326. doi: 10.1111/j.1365-313X.2008.03597.x. [DOI] [PubMed] [Google Scholar]
- 2.Wach A, Pyrzynska K, Biesaga M. Quercetin content in some food and herbal samples. Food Chem. 2007;100:699–704. doi: 10.1016/j.foodchem.2005.10.028. [DOI] [Google Scholar]
- 3.He D, et al. Total flavonoids of Flos Chrysanthemi protect arterial endothelial cells against oxidative stress. J Ethnopharmacol. 2012;139:68–73. doi: 10.1016/j.jep.2011.10.043. [DOI] [PubMed] [Google Scholar]
- 4.Zheng XK, et al. Anti-diabetic activity and potential mechanism of total flavonoids of Selaginella tamariscina (Beauv). Spring in rats induced by high fat diet and low dose STZ. J Ethnopharmacol. 2011;137:662–668. doi: 10.1016/j.jep.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Chu JX, Wang ZL, Han SY. The Effects of Total Flavonoids from Buckwheat Flowers and Leaves on Renal Damage and PTP1B Expression in Type 2 Diabetic Rats. IJPR. 2011;10:511–517. [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J. Experimental Study of Antipyretic and Anti-inflammatory Effects of Total Flavonoids in Abelmoschus Manihot. J China Med Univ. 2011;40:763–764. [Google Scholar]
- 7.Colliver S, et al. Improving the nutritional content of tomatoes through reprogramming their flavonoid biosynthetic pathway. Phytochem Rev. 2002;1:113–123. doi: 10.1023/A:1015848724102. [DOI] [Google Scholar]
- 8.Kuntic V, Filipovic I, Vujic Z. Effects of Rutin and Hesperidin and their Al(III) and Cu(II) Complexes on in Vitro Plasma Coagulation Assays. Molecules. 2011;16:1378–1388. doi: 10.3390/molecules16021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La Case C, Willegas I, Alacrcon de la Lastra C, Motilva V, Martin Calero MJ. Evidence for protective and antioxidant properties of rutin, a natural flavones, against ethanol induced gastric lesions. J Ethnoparmacol. 2000;71:45–53. doi: 10.1016/S0378-8741(99)00174-9. [DOI] [PubMed] [Google Scholar]
- 10.Landberg R, et al. Selected dietary flavonoids are associated with markers off inflammation and endothelial dysfunction in U.S. women. J Nutr. 2011;141:618–625. doi: 10.3945/jn.110.133843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choudhary D, et al. Genetically engineered flavonol enriched tomato fruit modulates chondrogenesis to increase bone length in growing animals. Sci Rep. 2016;6:21668. doi: 10.1038/srep21668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seeram NP, et al. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J Agri Food Chem. 2006;54:9329–9339. doi: 10.1021/jf061750g. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava S, et al. Quercetin, a Natural Flavonoid Interacts with DNA, Arrests Cell Cycle and Causes Tumor Regression by Activating Mitochondrial Pathway of Apoptosis. Sci Rep. 2016;6:24049. doi: 10.1038/srep24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flores G, et al. Anthocyanins from Eugenia brasiliensis edible fruits as potential therapeutics for COPD treatment. Food Chem. 2012;134:1256–1262. doi: 10.1016/j.foodchem.2012.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh D, Konishi T. Anthocyanins and anthocyanin-rich extracts: role in diabetes and eye function. Asia Pac J of Clin Nutr. 2007;16:200–208. [PubMed] [Google Scholar]
- 16.Miyazaki K, Makino K, Iwadate E, Deguchi Y, Ishikawa F. Anthocyanins from Purple Sweet Potato Ipomoea batatas Cultivar Ayamurasaki Suppress the Development of Atherosclerotic Lesions and Both Enhancements of Oxidative Stress and Soluble Vascular Cell Adhesion Molecule-1 in Apolipoprotein E-Deficient Mice. J Agri Food Chem. 2008;56:11485–11492. doi: 10.1021/jf801876n. [DOI] [PubMed] [Google Scholar]
- 17.Zuluaga DL, et al. Arabidopsis thaliana MYB75/PAP1 transcription factor induces anthocyanin production in transgenic tomato plants. Funct Plant Biol. 2008;35:606–618. doi: 10.1071/FP08021. [DOI] [PubMed] [Google Scholar]
- 18.Bovy A, Schijlen E, Hall RD. Metabolic engineering of flavonoids in tomato (Solanum lycopersicum): the potential for metabolomics. Metabolomics. 2007;3:399–412. doi: 10.1007/s11306-007-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dietz-Pfeilstetter A. Stability of transgene expression as a challenge for genetic engineering. Plant Sci. 2010;179:164–167. doi: 10.1016/j.plantsci.2010.04.015. [DOI] [Google Scholar]
- 20.Muir SR, et al. Overexpression of petunia chalcone isomerase in tomato results in fruit containing increased levels of flavonols. Nat Biotechonol. 2001;19:470–474. doi: 10.1038/88150. [DOI] [PubMed] [Google Scholar]
- 21.Willits MG, et al. Utilization of the genetic resources of wild species to create a nontransgenic high flavonoid tomato. J Agri Food Chem. 2005;53:1231–1236. doi: 10.1021/jf049355i. [DOI] [PubMed] [Google Scholar]
- 22.Verhoeyen ME, et al. Increasing antioxidant levels in tomatoes through modification of the flavonoid biosynthetic pathway. J Exp Bot. 2002;53:2099–2106. doi: 10.1093/jxb/erf044. [DOI] [PubMed] [Google Scholar]
- 23.Zhang CY, et al. Cloning, characterization and functional analysis of a flavonol synthase from. Vaccinium corymbosum. Trees. 2016;30:1595–1605. doi: 10.1007/s00468-016-1393-6. [DOI] [Google Scholar]
- 24.Aherne SA, O’Brien NM. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition. 2002;18:75–81. doi: 10.1016/S0899-9007(01)00695-5. [DOI] [PubMed] [Google Scholar]
- 25.Martin, C. et al. Combining genomics and metabolomics for the discovery of regulatory genes and their use in metabolic engineering to produce ‘healthy foods’. Acta Hort, 73–84 (2012).
- 26.Bovy A, et al. High-flavonol tomatoes resulting from the heterologous expression of the maize transcription factor genes LC and C1. Plant Cell. 2002;14:2509–2526. doi: 10.1105/tpc.004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butelli E, et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol. 2008;26:1301–1308. doi: 10.1038/nbt.1506. [DOI] [PubMed] [Google Scholar]
- 28.Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C. Activation Tagging Identifies a Conserved MYB Regulator of Phenylpropanoid Biosynthesis. Plant Cell. 2000;12:2383–2393. doi: 10.1105/tpc.12.12.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee WJ, et al. Drastic anthocyanin increase in response to PAP1 overexpression in fls1 knockout mutant confers enhanced osmotic stress tolerance in Arabidopsis thaliana. Plant Cell Rep. 2016;35:2369–2379. doi: 10.1007/s00299-016-2040-9. [DOI] [PubMed] [Google Scholar]
- 30.Lim W, Miller R, Park J, Park S. Consumer Sensory Analysis of High Flavonoid Transgenic Tomatoes. J Food Sci. 2014;79:1212–1217. doi: 10.1111/1750-3841.12478. [DOI] [PubMed] [Google Scholar]
- 31.Lim W, Li J. Co-expression of onion chalcone isomerase in Del/Ros1-expressing tomato enhances anthocyanin and flavonol production. Plant Cell Tissue Organ Cult. 2017;128:113–124. doi: 10.1007/s11240-016-1090-6. [DOI] [Google Scholar]
- 32.Zhou L-L, Zeng H-N, Shi M-Z, Xie D-Y. Development of tobacco callus cultures over expressing Arabidopsis PAP1/MYB75 transcription factor and characterization of anthocyanin biosynthesis. Planta (Berlin) 2008;229:37–51. doi: 10.1007/s00425-008-0809-y. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Jones R, Yoo KS, Pike LM. Gold color in onions (Allium cepa): a natural mutation of the chalcone isomerase gene resulting in a premature stop codon. MoL Gen Genomics. 2004;272:411–419. doi: 10.1007/s00438-004-1076-7. [DOI] [PubMed] [Google Scholar]
- 34.Feng Y, et al. A Three-Component Gene Expression System and Its Application for Inducible Flavonoid Overproduction in Transgenic Arabidopsis thaliana. PLoS One. 2011;6:1–9. doi: 10.1371/journal.pone.0017603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hertog MGL, Hollman PCH, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. Journal of Agricultural and Food Chemistry. 1992;40:2379–2383. doi: 10.1021/jf00024a011. [DOI] [Google Scholar]
- 36.Vikram, M., Feng, Y., Park, S. H., Yoo, K. S. & Koiwa, H. In II International Symposium on Human Health Effects of Fruits and Vegetables: FAVHEALTH 2007, Houston, Texas, USA, 9–13 October 2007. 615–618 (International Society for Horticultural Science (ISHS), 2009).
- 37.Baudry A, Caboche M, Lepiniec L. TT8 controls its own expression in a feedback regulation involving TTG1 and homologous MYB and bHLH factors, allowing a strong and cell-specific accumulation of flavonoids in Arabidopsis thaliana. Plant J. 2006;46:768–779. doi: 10.1111/j.1365-313X.2006.02733.x. [DOI] [PubMed] [Google Scholar]
- 38.Gonzali S, Mazzucato A, Perata P. Purple as a tomato: towards high anthocyanin tomatoes. Trends Plant Sci. 2009;14:237–241. doi: 10.1016/j.tplants.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka Y, Ohmiya A. Seeing is believing: engineering anthocyanin and carotenoid biosynthetic pathways. Curr Opin Biotechnol. 2008;19:190–197. doi: 10.1016/j.copbio.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Mitsunami T, et al. Overexpression of the PAP1 Transcription Factor Reveals a Complex Regulation of Flavonoid and Phenylpropanoid Metabolism in Nicotiana tabacum Plants Attacked by Spodoptera litura. PLoS One. 2014;9:1–9. doi: 10.1371/journal.pone.0108849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.La Casa C, Villegas I, de la Lastra CA, Motilva V, Calero MJM. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J Ethnopharmacol. 2000;71:45–53. doi: 10.1016/S0378-8741(99)00174-9. [DOI] [PubMed] [Google Scholar]
- 42.Alia M, et al. Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2) Eur J Nutr. 2006;45:19–28. doi: 10.1007/s00394-005-0558-7. [DOI] [PubMed] [Google Scholar]
- 43.Torel J, Cillard J, Cillard P. Antioxidant activity of flavonoids and reactivity with peroxy radical. Phytochemistry. 1986;25:383–385. doi: 10.1016/S0031-9422(00)85485-0. [DOI] [Google Scholar]
- 44.Yang JX, Guo J, Yuan JF. In vitro antioxidant properties of rutin. LWT. 2008;41:1060–1066. doi: 10.1016/j.lwt.2007.06.010. [DOI] [Google Scholar]
- 45.Pires JM, Mendes FR, Negri G, Duarte-Almeida JM, Carlini EA. Antinociceptive Peripheral Effect of Achillea millefolium L. and Artemisia vulgaris L.: Both Plants known popularly by Brand Names of Analgesic Drugs. Phytother Res. 2009;23:212–219. doi: 10.1002/ptr.2589. [DOI] [PubMed] [Google Scholar]
- 46.Temraz A, El-Tantawy WH. Characterization of antioxidant activity of extract from Artemisia vulgaris. Pak J Pharm Sci. 2008;21:321–326. [PubMed] [Google Scholar]
- 47.Lee LY, et al. Novel plant transformation vectors containing the superpromoter. Plant Physiol. 2007;145:1294–1300. doi: 10.1104/pp.107.106633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holsters M, et al. Transfection and transformation of Agrobacterium-tumefaciens. Mol Gen Genet. 1978;163:181–187. doi: 10.1007/BF00267408. [DOI] [PubMed] [Google Scholar]
- 49.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 50.Park SH, Morris JL, Park JE, Hirschi KD, Smith RH. Efficient and genotype-independent Agrobacterium – mediated tomato transformation. J Plant Physiol. 2003;160:1253–1257. doi: 10.1078/0176-1617-01103. [DOI] [PubMed] [Google Scholar]
- 51.Wu QY, et al. Ectopic expression of Arabidopsis glutaredoxin AtGRXS17 enhances thermotolerance in tomato. Plant Biotechnol. J. 2012;10:945–955. doi: 10.1111/j.1467-7652.2012.00723.x. [DOI] [PubMed] [Google Scholar]
- 52.Oh M-M, Carey EE, Rajashekar CB. Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol Bioch. 2009;47:578–583. doi: 10.1016/j.plaphy.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006;140:637–646. doi: 10.1104/pp.105.072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim W, Park J, Park S. Re-evaluation of the effects of growth regulators on callus induction and shoot regeneration in Agrobacterium-mediated transformation of lettuce. Acta Physiol Plant. 2011;33:1631–1637. doi: 10.1007/s11738-010-0699-z. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and available from the corresponding author on reasonable request.