Abstract
Background
Through investigating antimicrobial susceptibility patterns of Enterobacteriaceae in community-acquired urinary tract infection (CA-UTI), we provide basic evidence for the use of empirical antibiotics in CA-UTI.
Materials and Methods
We retrospectively reviewed the medical records of patients over the age of 19 years who visited a hospital in Seoul between January 2012 and December 2016 for a CA-UTI. Urine cultures were used to identify causative organisms. We investigated extended-spectrum β-lactamase (ESBL) production and the antimicrobial susceptibility of Enterobactereiaceae. We evaluated recommended empirical antibiotics numerically by calculating the syndrome-specific likelihood of inadequate therapy (LIT) for the last 2 years (interpretation of the LIT A value: 1 out of A people is likely to receive inadequate empirical antibiotics).
Results
Urine cultures were performed in 1,605 out of 2,208 patients who were diagnosed with CA-UTI, and causative pathogens were identified in 1,134 (70.7%) cases. There were 998 (88.0%) cases of Enterobacteriaceae and Escherichia coli was the most common pathogen, accounting for 80.3% of cases (911 cases). The overall resistance rates to trimethoprim-sulfamethoxazole, fluoroquinolones, and cefotaxime were 31.7%, 23.2%, and 13.5%, respectively. There were 128 (10.8%) cases of ESBL-producing Entererobacteriaceae with an increasing but non-significant trend (P = 0.255). The LIT for CA-UTI in the past two years was highest for ertapenem and imipenem. Fluoroquinolones ranked 11th, with a LIT of 8.2, and cefotaxime ranked higher, at 10.5. In ESBL-producing Enterobacteriaceae, except for carbapenems, amikacin and piperacillin-tazobactam showed the highest susceptibility rates at 99.2% and 94.3%, respectively.
Conclusion
Empiric treatment with fluoroquinolones in CA-UTI should be carefully considered, given the high resistance rate. The proportion of ESBL-producing Entererobacteriaceae in CA-UTI has increased to a high level in Korea. Amikacin and piperacillin-tazobactam could be considered for empiric treatment in patients at risk for ESBL-producing Entererobacteriaceae when considering alternatives to carbapenems.
Keywords: Urinary tract infections, Community-acquired infection, Susceptibility, Extended-spectrum β-lactamase
Introduction
As antibiotic use has increased, antibiotic resistance has become a concern in the community. Due to resistance, it is difficult to choose early empiric antibiotics for urinary tract infections (UTI), which are a common infection in the community. In particular, extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-PE) are co-resistant to other non-β-lactam antimicrobials [1,2]. As a result, treatment of community-acquired UTI (CA-UTI) has becoming challenging for clinicians [3,4].
The domestic UTI guideline, which was revised in 2011, recommended fluoroquinolones as a primary choice, and this reflected local community resistance until 2008. A study published after the revision of the guideline showed a resistance rate to fluoroquinolones over 20%, which is the limit of the local resistance rate recommended by the Infectious Diseases Society of America (IDSA) for empirical treatment [5,6]. That study also showed that ESBL-PE accounted for 8.7% of CA-UTI cases in 2011 [5]. Given the global trend of increasing ESBL-PE, the proportion is likely much higher recently in Korea [7]. Thus, there is a need to re-evaluate empiric antibiotics for CA-UTIs.
Despite of the need for data on the current antibiotic susceptibility pattern for empirical treatment choices, there is a lack of up-to-date susceptibility data for CA-UTIs in Korea. Furthermore, data on antibiotics that can replace carbapenems in ESBL-PE are needed in cases where carbapenems should be conserved, due to concern for carbapenem-resistant Enterobacteriaceae (CRE). We investigated resistance trends for Enterobacteriaceae in CA-UTI over a five-year period to identify suitable first-line antibiotics. In addition, we evaluated triable alternatives to carbapenems in ESBL-PE.
Materials and Methods
1. Study design and study population
This was a retrospective study to assess the antimicrobial susceptibility of CA-UTI causative pathogens. This study was conducted in a 578-bed university hospital in Seoul from January 2012 to December 2016. We reviewed the medical records of patients diagnosed with UTIs based on the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) codes (urinary tract infection, N390; acute cystitis, N300; acute pyelonephritis, N10.03; and acute prostatitis, N410) in the emergency room and outpatient clinic. Among patients with that diagnosis, patients that received treatment in the outpatient clinics and emergency room and were treated in the ward were all included. We included adults over 19 with urine culture from a voided mid-stream urine specimen. Causative organisms from urine cultures were identified. Only those pathogens identified for the first time were included.
2. Definition and data collection
Among cases diagnosed within the first 48 hours of hospitalization, we excluded patients if any of the following criteria were present: (1) The patient received intravenous therapy, wound care, or specialized nursing care at home or in an outpatient clinic, or received renal dialysis in the previous 30 days before infection, including urinary or digestive tract endoscopy or other invasive procedures, (2) The patient attended a hospital or hemodialysis clinic within 30 days before infection, or (3) The patient was hospitalized for >2 days in an acute care hospital or the patient resided in a nursing home or long-term care facility within the previous 90 days [8,9].
Likelihood of inadequate therapy (LIT) is the frequency of inadequately treated patients for each antibiotic and drug-resistant strain. This score calculates the numerically recommended rank of empiric antibiotics, considering the frequency of pathogens and resistance rates. Organism-specific LIT is the percentage of drug resistance for each bacterium in a local pool of patients divided by 100. For example, if 50% of Escherichia coli were resistant to cefepime, then the E-coli-specific LIT for cefepime is two (100/50%). This value implies that two patients with E. coli infection would need to be treated with cefepime for one to be inadequately treated. For pathogens with 0% resistance, a LIT of 100 is assigned. Syndrome-specific LIT is the frequency of inadequately treated patients with the syndrome. The LIT of each pathogen is multiplied by its frequency of occurrence in a given indication. These multiplied LIT values are summed and then divided by the cumulative percentage rate of the pathogen [10,11].
3. Microbiologic data
Etiologic pathogens were determined when ≥105 colony-forming units/mL of organisms were cultured from midstream urine. Microbial identification was carried out using the Vitek-2 system (bioMerieux, Inc., Durham, NC, USA). Vitek-2 cards containing an ESBL test were used. Antimicrobial susceptibility to amikacin, ampicillin, amoxicillin-clavulanic acid, aztreonam, cefepime, cefotaxime, cefoxitin, ceftazidime, fluoroquinolones, ertapenem, gentamicin, imipenem, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole (TMP/SMX) was tested in Enterobacteriaceae. Susceptibility was determined by the broth microdilution test, according to the guidelines prescribed by Clinical and Laboratory Standards Institute [12,13]. Isolates showing intermediate antimicrobial susceptibility were considered to be resistant. Data was analyzed dividing into patients treated in the outpatient clinic and in admission.
4. Statistical analysis
Statistical significance was assessed via the Chi-square test or Fisher's exact test for the categorical variables. Linear regression was used to assess the trends of the yearly antimicrobial resistance and UTI of ESBL-PE. A P-value of <0.05 was considered statistically significant. Statistical analysis was performed with IBM SPSS ver. 20 (IBM SPSS Inc., Armonk, NY, USA).
5. Ethics statement
The study was approved by the Institutional Review Board of Hallym University Kangnam Sacred Heart Hospital in Korea (IRB#: 2016-12-177).
Results
During the study period, 2,208 patients were diagnosed with a CA-UTI and 1,605 urine specimens were analyzed. 471 specimens in which the causative pathogen could not be identified were excluded. Thus 1,134 (70.7%, 1,134/1,605) cases were included in this study. Among study population, 807 patients (71.2%) were treated in outpatient clinic and 327 patients (28.8%) were in admission.
The most common causative pathogen was E. coli, which caused 911 (80.3%) cases (Table 1). Gram negative bacteria accounted for 89.0% (n = 1,009) and 998 (88.0%) cases were Enterobacteriaceae. After E. coli, Klebsiella species (n = 38, 3.4%) was the most common pathogen, followed by Enterobacter species (n = 27, 2.4%) and Proteus species (n = 11, 1.0%) among gram negative bacteria. Among gram positive bacteria (n = 125, 11.0%), Streptococcus species accounted for the highest proportion at 7.8% (n = 89), followed by Enterococcus species (n = 27, 2.4%) and Staphylococcus aureus (n = 6, 0.5%).
Table 1. Causative pathogens of community-acquired urinary tract infection.
| Pathogens | Community-acquired urinary tract infection, n (%) |
|---|---|
| Gram negative bacteria | 1,009 (89.0) |
| Escherichia coli | 911 (80.3) |
| Klebsiella species | 38 (3.4) |
| Enterobacter species | 27 (2.4) |
| Proteus species | 11 (1.0) |
| Citrobacter species | 4 (0.4) |
| Pseudomonas species | 8 (0.7) |
| Serracia marcescens | 3 (0.3) |
| Others | 7 (0.6) |
| Gram positive bacteria | 125 (11.0) |
| Streptococcus species | 89 (7.8) |
| Enterococcus species | 27 (2.4) |
| Staphylococcus aureus | 6 (0.5) |
| Staphylococcus saprophyticus | 3 (0.3) |
| Total | 1,134 (100) |
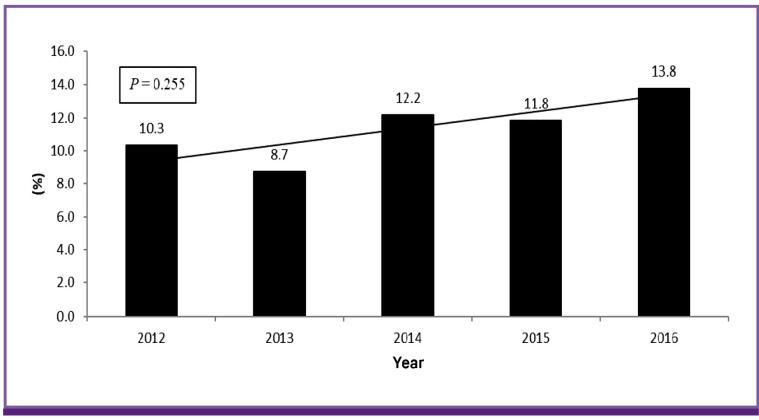
Table 2 shows mean resistance rates of Enterobacteriaceae to antimicrobial agents. Overall, amikacin, ertapenem and imipenem showed the lowest resistance rates at 0.1%, 0.2% and 0.3%, respectively. Cefepime (2.4%), piperacillin-tazobactam (3.5%), ceftazidime (3.9%), and aztreonam (6.0%) had lower than 10% resistance rates. Resistance rates to cefoxitin and cefotaxime were 10.1% and 13.5%, respectively. Gentamicin (20.1%), amoxicillin-clavulanic acid (20.6%), and fluoroquinolones (23.2%) showed resistance rates higher than 20%. TMP/SMX and ampicillin showed the highest resistance rates, at 31.7% and 64.6%. Outpatients accounted for 69.5% (n = 694) and hospitalized patients accounted for 30.5% (n = 304) in Enterobacteriaceae. Resistance rates were similar between hospitalized and outpatient patients (Table 2). Resistance rates to piperacillin-tazobactam, ertapenem and TMP/SMX were higher in admitted patients with significance (P = 0.040, 0.024 and 0.020, respectively). On the other hand, imipenem showed a higher resistance rate in outpatients with significance (P = 0.024). The number of resistant cases in ertapenem, imipenem and piperacillin-tazobactam was quite small. Therefore it was difficult to compare resistance rates between outpatients and hospitalized patients. The resistance rate of cefotaxime was higher in hospitalized patients and that of fluoroquinolone was lower in outpatients but without significance (P = 0.706 and 0.082). The changes in the antimicrobial resistance rates of Enterobacteriaceae over the five-year period are shown in Table 3. Resistance rates of ertapenem, imipenem and piperacillin-tazobactam showed a decreasing tendency with significant according to year (P <0.001, <0.001 and <0.001). However, resistance cases for ertapenem and imipenem were only found in 2014 and the number of that for piperacillin-tazobactam was quite small, it was difficult to assess the trend during five years. Although amoxicillin-clavulanic acid, cefotaxime, and aztreonam also showed increasing trends, there was no statistical significance (P = 0.298, 0.220, and 0.362, respectively). Resistance rates to ampicillin and fluoroquinolones showed a decreasing pattern, but without statistical significance (P = 0.222 and 0.546, respectively). During a five-year period, a total of 118 ESBL-PE (11.3%) were identified. The proportion of ESBL-PE was 10.3% in 2012, 8.7% in 2013, 12.2% in 2014, 11.8% in 2015, and 13.8% in 2016 (Fig. 1). Overall, this tended to increase during the study period, but there was no statistical significance (P = 0.255).
Table 2. Mean antimicrobial resistance rates of Enterobacteriaceae according to outpatient clinic and hospitalization during a five-year period.
| Resistant antibiotics | Total (n = 998) n (%) |
Outpatient clinic (n = 694) n (%) |
Hospitalization (n = 304) n (%) |
P-value |
|---|---|---|---|---|
| AMP | 647 (64.6) | 446 (64.3) | 199 (65.5) | 0.716 |
| AMC | 207 (20.7) | 146 (21.0) | 61 (20.1) | 0.728 |
| TZP | 33 (3.5) | 20 (2.9) | 13 (4.3) | 0.040 |
| FOX | 102 (10.2) | 69 (9.9) | 33 (10.9) | 0.661 |
| CTX | 135 (13.5) | 92 (13.3) | 43 (14.1) | 0.706 |
| CAZ | 39 (3.9) | 28 (4.0) | 11 (3.6) | 0.755 |
| FEM | 24 (2.4) | 15 (2.2) | 9 (3.0) | 0.448 |
| ATM | 60 (6.0) | 43 (6.2) | 17 (5.6) | 0.712 |
| EPM | 2 (0.2) | 1 (0.1) | 1 (0.3) | 0.024 |
| IPM | 4 (0.3) | 4 (0.4) | 0 (0) | 0.028 |
| FQa | 232 (23.2) | 172 (24.8) | 60 (19.7) | 0.082 |
| GEN | 201 (20.1) | 137 (19.7) | 64 (21.1) | 0.634 |
| AMK | 1 (0.1) | 1 (0.1) | 0 (0) | 1.000 |
| TMP/SMX | 316 (31.7) | 204 (29.4) | 112 (36.8) | 0.020 |
aCiprofloxacin and/or levofloxacin
AMP, ampicillin; AMC, amoxicillin-clavulanic acid; TZP, piperacillin-tazobactam; FOX, cefoxitin; CTX, cefotaxime; CAZ, ceftazidime; FEP, cefepime; ATM, aztreonam; EPM, ertapenem; IPM, imipenem; FQ, fluoroquinolones; GEN, gentamicin; AMK, amikacin; TMP/SMX, trimethoprime-sulfamethoxazole
Table 3. Trends in antimicrobial resistance rates of Enterobacteriaceae during the study period (n = 998).
| Antibiotics | Susceptibility rates, n (%) | P-value | ||||
|---|---|---|---|---|---|---|
| 2012 (n = 181) | 2013 (n = 216) | 2014 (n = 184) | 2015 (n = 216) | 2016 (n = 201) | ||
| AMP | 122 (67.4) | 144 (66.7) | 119 (64.7) | 132 (61.1) | 128 (63.7) | 0.222 |
| AMC | 32 (17.7) | 44 (23.9) | 51 (23.6) | 40 (19.9) | 0.298 | |
| TZP | 5 (4.1) | 6 (2.8) | 6 (3.3) | 6 (2.8) | 10 (5.0) | <0.001 |
| FOX | 16 (8.8) | 22 (10.2) | 30 (16.3) | 19 (8.8) | 15 (7.5) | 0.498 |
| CTX | 19 (10.5) | 27 (12.5) | 31 (16.8) | 27 (12.5) | 31 (15.4) | 0.220 |
| CAZ | 3 (1.7) | 6 (2.8) | 6 (7.1) | 7 (3.2) | 10 (5.0) | 0.117 |
| FEM | 0 (0) | 4 (1.9) | 7 (3.8) | 6 (2.8) | 7 (3.5) | 0.027 |
| ATM | 8 (4.4) | 11 (5.1) | 16 (8.7) | 11 (5.1) | 14 (7.0) | 0.362 |
| EPM | 0 (0) | 0 (0) | 2 (1.1) | 0 (0) | 0 (0) | <0.001 |
| IPM | 0 (0) | 0 (0) | 3 (1.6) | 0 (0) | 0 (0) | <0.001 |
| FQa | 43 (23.8) | 48 (22.2) | 52 (28.3) | 46 (21.3) | 43 (21.4) | 0.546 |
| GEN | 40 (22.1) | 42 (19.4) | 49 (26.6) | 32 (14.8) | 38 (18.9) | 0.214 |
| AMK | 0 (0) | 1 (0.5) | 0 (0) | 0 (0) | 0 (0) | 0.458 |
| TMP/SMX | 58 (32.0) | 74 (34.3) | 60 (32.6) | 62 (28.7) | 62 (30.8) | 0.419 |
aCiprofloxacin and/or levofloxacin
AMP, ampicillin; AMC, amoxicillin-clavulanic acid; TZP, piperacillin-tazobactam; FOX, cefoxitin; CTX, cefotaxime; CAZ, ceftazidime; FEP, cefepime; ATM, aztreonam; EPM, ertapenem; IPM, imipenem; FQ, fluoroquinolones; GEN, gentamicin; AMK, amikacin; TMP/SMX, trimethoprime-sulfamethoxazole
Figure 1.
Yearly proportion of extended-spectrum β-lactamase-producing Enterobacteriaceae during the study period.
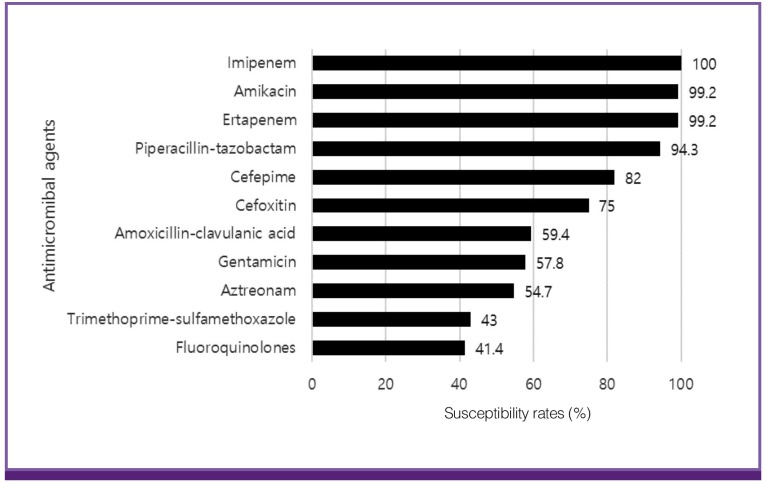
Antimicrobial susceptibility rates in ESBL-PE are shown in Figure 2. Imipenem and ertapenem had high susceptibility rates at 100% and 99.2%, respectively. Amikacin showed the same susceptibility rate as ertapenem. Piperacillin-tazobactam showed a relatively high susceptibility rate of 94.3%, while the susceptibility rate to cefepime was 82.0%. Other antimicrobial agents such as cefoxitin, amoxicillin-clavulanic acid, gentamicin, aztreonam, TMP/SMX, and fluoroquinolones, had over 20% resistance rates.
Figure 2.
Mean antimicrobial susceptibility rates of Extended spectrum β-lactamase-producing Enterobacteriaceae during the study period (n = 118).
To reflect more recent trends, the LIT was calculated with data from the past two years from Enterobacteriaceae in CA-UTIs (Table 4). Organism-specific LIT was different by pathogens. For E. coli, resistance was 0% for ertapenem, imipenem and amikacin and thus represented by an organism-specific LIT of 100. Ampicillin, TMP/SMX and fluoroquinolones showed the lowest organism-specific LIT as 2, 3.2 and 4.4. For Klebsiella species, organism-specific LIT was 100 for ertapenem, imipenem and amikacin and the lowest for ampicillin as 1. Overall, UTI LIT was ranked first for ertapenem, imipenem, and amikacin, with LITs of 100. Except carbapenems, cefepime and piperacillin-tazobactam were ranked highest, with LITs of 34.3 and 31.7, respectively, followed by ceftazidime, with a LIT of 27.0. Aztreonam, cefoxitin, and cefotaxime formed an intermediate group with LITs of 18.1, 16.7, and 10.5, respectively. The LIT value of fluoroquinolones and TMP/SMX was 8.2 and 7.0, respectively. The lowest rank was ampicillin, with a LIT of 1.9. In the outpatient clinic, cefoxitin (LIT = 23.5) ranked higher than aztreonam (LIT = 16.9) followed by cefotaxime, amoxicillin-clavulanic acid and gentamicin with LITs of 11.5, 9.8 and 9.8 respectively. The LIT value of fluoroquinolones, TMP/SMX and ampicillin was 9.2, 8.6 and 2.0 fell into the lower group. There were some differences within hospitalized patients. Aztreonam was ranked higher than outpatients and the LIT value was same with ceftazidime as 24.5. Amoxicillin-clavulanic acid was ranked lower than outpatients with LIT of 4.8.
Table 4. Likelihood of inadequate therapy (LIT) among Enterobacteriaceae in community-acquired urinary tract infections (CA-UTI) over the past two years.
| Rank | Total | Outpatient clinic | Hospitalization | |||
|---|---|---|---|---|---|---|
| Antimicrobial agents | LITa | Antimicrobial agents | LITa | Antimicrobial agents | LITa | |
| 1 | Ertapenem, Imipenem, Amikacin | 100 | Ertapenem, Imipenem, Amikacin | 100 | Ertapenem, Imipenem, Amikacin | 100 |
| 2 | Cefepime | 34.3 | Cefepime | 38.9 | Cefepime | 33.3 |
| 3 | Piperacillin-tazobactam | 31.7 | Piperacillin-tazobactam | 34.1 | Piperacillin-tazobactam | 29.0 |
| 4 | Ceftazidime | 27.0 | Ceftazidime | 30.7 | Ceftazidime, aztreonam | 24.5 |
| 5 | Aztreonam | 18.1 | Cefoxitin | 23.5 | Cefoxitin | 12.5 |
| 6 | Cefoxitin | 16.7 | Aztreonam | 16.9 | Cefotaxime | 9.4 |
| 7 | Cefotaxime | 10.5 | Cefotaxime | 11.5 | Gentamicin | 8.1 |
| 8 | Gentamicin | 8.7 | Amoxicillin-Clavulanic acid, Gentamicin | 9.8 | Fluoroquinolonesb | 7.1 |
| 9 | Fluoroquinolonesb | 8.2 | Fluoroquinolonesb | 9.2 | Amoxicillin-Clavulanic acid | 4.8 |
| 10 | Trimethoprim-Sulfamethoxazole | 7.0 | Trimethoprim-Sulfamethoxazole | 8.6 | Trimethoprim-Sulfamethoxazole | 4.7 |
| 11 | Amoxicillin-Clavulanic acid | 6.4 | Ampicillin | 2.0 | Ampicillin | 1.6 |
| 12 | Ampicillin | 1.9 | ||||
aLIT of Enterobacteriaceae in CA-UTI: The LIT c of each pathogen is multiplied by its frequency of occurrence in a given indication. These multiplied LIT values are summed and then divided by the cumulative percentage rate of the pathogen (n = 89.9%)
bCiprofloxacin and/or levofloxacin
cPercentage of drug resistance for each bacterium in a local pool of patients divided by 100; For pathogens with 0% resistance, a LIT of 100 was assigned.
Discussion
CA-UTIs caused by resistant strains have been increasing worldwide, including ESBL-PE [3,7,14]. Common empiric antibiotics are not effective against resistant pathogens and are likely to lead treatment failure. In order to select appropriate antibiotics, it is necessary to understand community resistance rates. Korean CA-UTI epidemiology data are only up to date until 2011; therefore, there is a need to understand more recent trends. In this study, we analyzed changes in resistance rates of Enterobacteriaceae from the community over the past five years.
IDSA recommends TMP/SMX as an empiric antibiotic treatment for UTI [6]. However, it was not recommended as an empiric treatment in Korea due to a resistance rate over 30% [15]. In our study, the resistance rate to TMP/SMX was 31.7%, which supports the Korean UTI guidelines; these recommend fluoroquinolones as a primary choice. From 2012 to 2016, the mean antimicrobial resistance rate of fluoroquinolones was 23.2% in this study. One study examined the epidemiology of CA-acute pyelonephritis from 2010 to 2011 and reported that the resistance rate to fluoroquinolones was 22.1% [5]. Resistance rates have been relatively stable since then; in addition, except for 2014, resistance rates to fluoroquinolones decreased during this period, and the resistance rate was 21.4% in 2016. IDSA recommends that empiric antibiotics not be used in communities with resistance rates >20% [6]. Considering overall resistance rates, fluoroquinolone use may be limited for primary therapy. However, due to high urinary tract concentrations and relatively stable resistance rates, this antibiotic class is unlikely to be completely excluded from primary treatment [16]. Nevertheless, we should be careful about the use of fluoroquinolones as a primary choice. Cefotaxime showed a resistance rate of 13.5%. Simply considering resistance rates, cefotaxime would be a primary choice for empiric antibiotics for CA-UTIs in Korea. However, there is concern for an increased resistance rate due to increasing use of cefotaxime. The resistance rate to cefotaxime was increased, compared to a rate of 8.3% from 2010 to 2011 [5]. In this study, resistance to cefotaxime increased from 10.5% in 2012 to 15.4% in 2016. Assessment of adequate antibiotic use through continuous monitoring of resistance rates is needed.
ESBL-PE showed an overall increasing pattern in CA-UTIs. In 2016, the rate of ESBL-PE infection was 13.8%, compared to 8.7% from 2010 to 2011 [5]. The proportion of ESBL-PE in the community is high, and this is a global trend [7]. Therefore, it is important to determine who should be treated initially for ESBL-producing pathogens. Although factors such as recent admission history, residence in a long-term care facility, and urethral catheterization were suggested as risk factors, most factors were correlated with healthcare centers. In the community, previous antibiotics use should be considered for treating ESBL-PE especially quinolones or second generation cephalosporins [17]. The male sex also showed an increased risk for ESBL-PE infections in some studies. Advanced age, diabetes mellitus and prostatic disease was considered as risk factors in community-acquired pathogens [2,18,19]. Interestingly, recreational freshwater swimming the past year was considered as a risk factor in one study [20]. Although there are no definite guidelines for selecting patients for treating ESBL-PE, using susceptible antimicrobials for ESBL-producing pathogens in patients with more risk factors will hit the mark the pathogen. There is a conflict about the prognosis of inappropriate empirical therapy. Some studies reported that inadequate empiric therapy did not affect mortality [21]. However, recent studies showed the association between inadequate empiric therapy and higher mortality [22,23]. There are some concerns about toxicity, cost and resistance for using empiric therapy to ESBL-PE such as carbapenem. An appropriate empiric therapy could reduce the length of stay and duration of symptoms [24]. Further study of empiric therapy for UTI is needed because most studies were performed in blood stream infection. Although the result of inadequate empiric therapy was contradictory, we should keep in mind that adequate empiric therapy could be critical for elderly or severely ill patients. There need to be effort to determine appropriate empirical antibiotics.
The first-line therapy for ESBL-PE is carbapenems [25]. The risk of CRE has increased with carbapenem use; therefore, attention to carbapenem alternatives has been growing. Some observational studies have examined the use of non-carbapenem alternatives for treatment of ESBL-PE [26,27,28,29,30]. We also investigated the susceptibility rates of non-carbapenem antibiotics in ESBL-PE. Amikacin showed a high in vitro susceptibility rate of 99.2%, and piperacillin-tazobactam also showed a high susceptibility rate of 94.3%. One study reported that susceptible non-carbapenems had a similar clinical improvement rate for ESBL-PE in UTIs [31]. We could treat with amikacin and piperacillin-tazobactam empirically in cases with risk factors for ESBL-PE infection. Although amikacin showed the same level of susceptibility as ertapenem, clinicians avoid aminoglycosides due to concern for nephrotoxicity. The therapeutic efficacy of amikacin in ESBL-PE has not been investigated sufficiently and should be examined. It may be challenging to use piperacillin-tazobactam in severe cases with a high inoculum effect [25]. Susceptibility to piperacillin-tazobactam ranged from 58.6% to 94.2% in CA-UTIs due to ESBL-PE UTI in Korea [5,30,32]. The reason for this wide range is unclear, but may reflect different regional rates. Therefore, the use of piperacillin-tazobactam as an empiric antibiotic in patients at risk for ESBL-PE infection should take regional susceptibility rates into account.
We analyzed the LIT with data from the past two years. LIT was a helpful marker to determine whether empiric treatment reflects resistance rates and the frequency of pathogens in rank order [10,11]. Carbapenems were the highest ranked. Fluoroquinolones were 11th out of 14 antibiotics, with a LIT value of 8.2; therefore, fluoroquinolones should be cautiously used as empiric CA-UTI treatment. Cefotaxime was ranked higher than fluoroquinolones, with a LIT of 10.5. Cefotaxime would be considered as a primary treatment for CA-UTI, reflecting its rank in this study. In the outpatient clinic, fluoroquinolones should be cautious due to relatively lower ranking. Third generation cephalosporin could be a best choice reflecting highest ranking among antimicrobial agents which could be used orally. Amoxicillin-clavulanic acid could be an alternative. Cefotaxime was also a good choice in hospitalized patients with a LIT value of 9.4. However, amoxicillin-clavulanic acid showed a lower rank than outpatient clinic at 12th and the value of LIT was at 4.8. Considering the rank of fluoroquinolones in hospitalized patients, choosing fluoroquinolones as an empiric therapy also should be careful in admitting patients.
There are some limitations to our study. First, we retrospectively collected data through electronic medical records and chart review. It was difficult to obtain all characteristics for analyzing risk factors such as comorbidity, previous antibiotics use and history of recurrent UTI due to unrecorded information. Also, in defining CA-UTI, healthcare-associated UTI might be classified as CA-UTI. Second, this study was conducted only in a single center. Therefore, it is difficult to reflect the overall characteristics of Korea. This study suggests the need for further large-scale nationwide surveillance on the epidemiology of CA-UTI. Finally, resistance rates are likely overestimated because this study was performed at a university hospital. However, as this study was conducted at a secondary hospital which could be visited without referral, it might reflect the community well.
In conclusion, early empirical treatment with fluoroquinolones in CA-UTI should be carefully considered due to the high resistance rate. The proportion of ESBL-PE in CA-UTI has increased to a high level in Korea. Amikacin and piperacillin-tazobactam can be considered for empiric treatment as alternatives to carbapenems in patients at risk for ESBL-PE .
Footnotes
Conflict of Interest: No conflicts of interest.
References
- 1.Rodríguez-Baño J, Navarro MD, Romero L, Martínez-Martínez L, Muniain MA, Perea EJ, Pérez-Cano R, Pascual A. Epidemiology and clinical features of infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in nonhospitalized patients. J Clin Microbiol. 2004;42:1089–1094. doi: 10.1128/JCM.42.3.1089-1094.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azap OK, Arslan H, Serefhanoğlu K, Colakoğlu S, Erdoğan H, Timurkaynak F, Senger SS. Risk factors for extended-spectrum beta-lactamase positivity in uropathogenic Escherichia coli isolated from community-acquired urinary tract infections. Clin Microbiol Infect. 2010;16:147–151. doi: 10.1111/j.1469-0691.2009.02941.x. [DOI] [PubMed] [Google Scholar]
- 3.Pitout JD, Nordmann P, Laupland KB, Poirel L. Emergence of Enterobacteriaceae producing extended-spectrum β-lactamases (ESBLs) in the community. J Antimicrob Chemother. 2005;56:52–59. doi: 10.1093/jac/dki166. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez‐Baño J, Navarro M. Extended‐spectrum β‐lactamases in ambulatory care: a clinical perspective. Clin Microbiol Infect. 2008;14(Suppl 1):104–110. doi: 10.1111/j.1469-0691.2007.01866.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim B, Kim J, Seo MR, Wie SH, Cho YK, Lim SK, Lee JS, Kwon KT, Lee H, Cheong HJ, Park DW, Ryu SY, Chung MH, Ki M, Pai H. Clinical characteristics of community-acquired acute pyelonephritis caused by ESBL-producing pathogens in South Korea. Infection. 2013;41:603–612. doi: 10.1007/s15010-013-0441-z. [DOI] [PubMed] [Google Scholar]
- 6.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE, Infectious Diseases Society of America European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 7.Lob SH, Nicolle LE, Hoban DJ, Kazmierczak KM, Badal RE, Sahm DF. Susceptibility patterns and ESBL rates of Escherichia coli from urinary tract infections in Canada and the United States, SMART 2010-2014. Diagn Microbiol Infect Dis. 2016;85:459–465. doi: 10.1016/j.diagmicrobio.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 9.Park SH, Choi SM, Lee DG, Kim J, Choi JH, Kim SH, Kwon JC, Yoo JH. Emergence of extended-spectrum beta-lactamase-producing escherichia coli as a cause of community-onset bacteremia in South Korea: risk factors and clinical outcomes. Microb Drug Resist. 2011;17:537–544. doi: 10.1089/mdr.2011.0072. [DOI] [PubMed] [Google Scholar]
- 10.Burgmann H, Stoiser B, Heinz G, Schenk P, Apfalter P, Zedtwitz-Liebenstein K, Frass M, Carmeli Y. Likelihood of inadequate treatment: a novel approach to evaluating drug-resistance patterns. Infect Control Hosp Epidemiol. 2009;30:672–677. doi: 10.1086/598245. [DOI] [PubMed] [Google Scholar]
- 11.Davis ME, Anderson DJ, Sharpe M, Chen LF, Drew RH. Constructing unit-specific empiric treatment guidelines for catheter-related and primary bacteremia by determining the likelihood of inadequate therapy. Infect Control Hosp Epidemiol. 2012;33:416–420. doi: 10.1086/664756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing, 24th informational supplement. Wayne, PA: CLSI; 2014. p. M100-S24. [Google Scholar]
- 13.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing, 21st informational supplement. Wayne, PA: CLSI; 2011. p. M100-S21. [Google Scholar]
- 14.Rodriguez-Baño J, Paterson DL. A change in the epidemiology of infections due to extended-spectrum β-lactamase-producing organisms. Clin Infect Dis. 2006;42:935–937. doi: 10.1086/500945. [DOI] [PubMed] [Google Scholar]
- 15.The Korean Society of Infectious Diseases, The Korean Society for Chemotherapy, Korean Association of Urogenital Tract Infection and Inflammation, The Korean Society of Clinical Microbiology Clinical guideline for the diagnosis and treatment of urinary tract infections: asymptomatic bacteriuria, uncomplicated & complicated urinary tract infections, bacterial prostatitis. Infect Chemother. 2011;43:1–25. [Google Scholar]
- 16.Wright DH, Brown GH, Peterson ML, Rotschafer JC. Application of fluoroquinolone pharmacodynamics. J Antimicrob Chemother. 2000;46:669–683. doi: 10.1093/jac/46.5.669. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz E, Akalin H, Özbey S, Kordan Y, Sinirtaş M, Gürcüoglu E, Özakin C, Heper Y, Mistik R, Helvaci S. Risk factors in community-acquired/onset urinary tract infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae . J Chemother. 2008;20:581–585. doi: 10.1179/joc.2008.20.5.581. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez-Baño J, Picón E, Gijón P, Hernández JR, Ruíz M, Peña C, Almela M, Almirante B, Grill F, Colomina J, Giménez M, Oliver A, Horcajada JP, Navarro G, Coloma A, Pascual A, Spanish Network for Research in Infectious Diseases (REIPI) Community-onset bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: risk factors and prognosis. Clin Infect Dis. 2010;50:40–48. doi: 10.1086/649537. [DOI] [PubMed] [Google Scholar]
- 19.Gopal Rao G, Batura D, Batura N, Nielsen PB. Key demographic characteristics of patients with bacteriuria due to extended spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae in a multiethnic community, in North West London. Infect Dis (Lond) 2015;47:719–724. doi: 10.3109/23744235.2015.1055588. [DOI] [PubMed] [Google Scholar]
- 20.Søraas A, Sundsfjord A, Sandven I, Brunborg C, Jenum PA. Risk factors for community-acquired urinary tract infections caused by ESBL-producing enterobacteriaceae–a case–control study in a low prevalence country. PLoS One. 2013;8:e69581. doi: 10.1371/journal.pone.0069581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frakking FN, Rottier WC, Dorigo-Zetsma JW, van Hattem JM, van Hees BC, Kluytmans JA, Lutgens SP, Prins JM, Thijsen SF, Verbon A, Vlaminckx BJ, Cohen Stuart JW, Leverstein-van Hall MA, Bonten MJ. Appropriateness of empirical treatment and outcome in bacteremia caused by extended-spectrum β-lactamase producing bacteria. Antimicrob Agents Chemother. 2013;57:3092–3099. doi: 10.1128/AAC.01523-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gradel KO, Jensen US, Schønheyder HC, Østergaard C, Knudsen JD, Wehberg S, Søgaard M, Danish Collaborative Bacteraemia Network (DACOBAN) Impact of appropriate empirical antibiotic treatment on recurrence and mortality in patients with bacteraemia: a population-based cohort study. BMC Infect Dis. 2017;17:122. doi: 10.1186/s12879-017-2233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esparcia A, Artero A, Eiros JM, Balaguer M, Madrazo M, Alberola J, Nogueira JM. Influence of inadequate antimicrobial therapy on prognosis in elderly patients with severe urinary tract infections. Eur J Intern Med. 2014;25:523–527. doi: 10.1016/j.ejim.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Shorr AF, Micek ST, Welch EC, Doherty JA, Reichley RM, Kollef MH. Inappropriate antibiotic therapy in Gram-negative sepsis increases hospital length of stay. Crit Care Med. 2011;39:46–51. doi: 10.1097/CCM.0b013e3181fa41a7. [DOI] [PubMed] [Google Scholar]
- 25.Harris PN, Tambyah PA, Paterson DL. β-lactam and β-lactamase inhibitor combinations in the treatment of extended-spectrum β-lactamase producing Enterobacteriaceae: time for a reappraisal in the era of few antibiotic options? Lancet Infect Dis. 2015;15:475–485. doi: 10.1016/S1473-3099(14)70950-8. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez-Baño J, Navarro MD, Retamar P, Picón E, Pascual Á, Extended-Spectrum Beta-Lactamases–Red Española de Investigación en Patología Infecciosa/Grupo de Estudio de Infección Hospitalaria Group β-Lactam/β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase–producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin Infect Dis. 2012;54:167–174. doi: 10.1093/cid/cir790. [DOI] [PubMed] [Google Scholar]
- 27.Trivedi M, Patel V, Soman R, Rodriguez C, Singhal T. The outcome of treating ESBL infections with carbapenems vs. non carbapenem antimicrobials. J Assoc Physicians India. 2012;60:28–30. [PubMed] [Google Scholar]
- 28.Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother. 2012;67:2793–2803. doi: 10.1093/jac/dks301. [DOI] [PubMed] [Google Scholar]
- 29.Doi A, Shimada T, Harada S, Iwata K, Kamiya T. The efficacy of cefmetazole against pyelonephritis caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae . Int J Infect Dis. 2013;17:e159–e163. doi: 10.1016/j.ijid.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Park SH, Choi SM, Chang YK, Lee DG, Cho SY, Lee HJ, Choi JH, Yoo JH. The efficacy of non-carbapenem antibiotics for the treatment of community-onset acute pyelonephritis due to extended-spectrum beta-lactamase-producing Escherichia coli . J Antimicrob Chemother. 2014;69:2848–2856. doi: 10.1093/jac/dku215. [DOI] [PubMed] [Google Scholar]
- 31.Seo YB, Kim YK, Lee J, Song W. Use of non-carbapenem antibiotics in patients with urinary tract infection caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae . Korean J Healthc Assoc Infect Control Prev. 2016;21:50–56. [Google Scholar]
- 32.Cho YH, Jung SI, Chung HS, Yu HS, Hwang EC, Kim SO, Kang TW, Kwon DD, Park K. Antimicrobial susceptibilities of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in health care-associated urinary tract infection: focus on susceptibility to fosfomycin. Int Urol Nephrol. 2015;47:1059–1066. doi: 10.1007/s11255-015-1018-9. [DOI] [PubMed] [Google Scholar]