Abstract
Conjunctival biopsies constitute a fairly large number of cases in a typical busy ophthalmic pathology practice. They range from a single biopsy through multiple mapping biopsies to assess the extent of a particular pathological process. Like most anatomical sites, the conjunctiva is subject to a very wide range of pathological processes. This article will cover key, commonly encountered nonneoplastic and neoplastic entities. Where relevant, sections will include recommendations on how best to submit specimens to the ophthalmic pathology laboratory and the relevance of up-to-date molecular techniques.
Keywords: Conjunctiva, histopathology, pathology
Nonneoplastic Pathology
Ultraviolet-related pathology[1]
The most common lesions in this category are pinguecula and pterygium. Pinguecula does not grow onto the corneal surface.
Histopathology
Pingueculae are usually well-defined nodular lesions, whereas pterygia are the sheets of tissue that curl up in the formalin pot and often have forceps marks on them. The epithelium of both can be thinned or hyperplastic, and in patients with brown or black skin, it is common to see epithelial racial melanosis. The key thing to look out for is epithelial dysplasia. The substantia propria exhibits elastotic degeneration, comprising random tangles of elastic fibers [Fig. 1a], sometimes associated with calcified globules. In a pinguecula, the elastotic degeneration is nodular; however, in pterygia, it is often diffuse and patchy. In pterygia, other features such as established stromal fibrosis, neovascularization, vascular congestion, and patchy chronic inflammation are also common.
Figure 1.
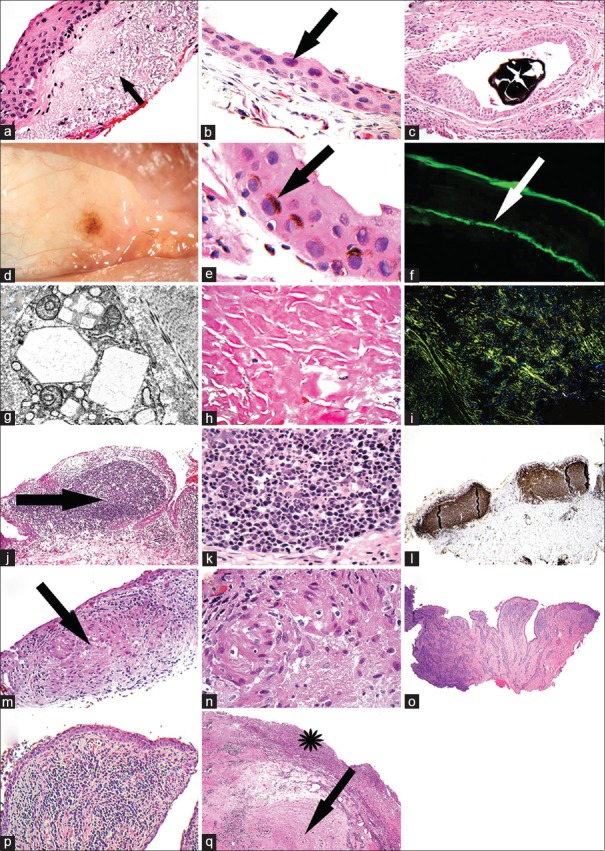
(a) Section of a pinguecula. The arrow points to bluish elastotic degeneration (H and E). (b) Section of a postmitomycin C-treated conjunctiva showing random, superficial, hyperchromatic nuclei with smudgy chromatin (H and E). (c) Section showing a black minocycline concretion in a conjunctival retention cyst (H and E). (d) A clinical photograph of the right eye showing conjunctival chlorpromazine-induced melanosis. (e) Section of a biopsy from the patient shown in plate d. The arrow points to supranuclear melanin caps in the epithelial cells (H and E). (f) Direct immunofluorescence photograph showing linear immunoglobulin A deposits (arrow) along the epithelial basement membrane in a case of mucous membrane pemphigoid. (g) Transmission electron micrograph showing the geometric crystals of cystine in lysosomes of fibroblasts in the conjunctiva. (h) Section of amyloid (H and E). (i) Apple green birefringence of the amyloid with polarized optics. (j) Section showing a reactive lymphoid follicle (arrow) in a case of follicular conjunctivitis (H and E). (k) Section of a higher power of plate j showing tangible body macrophages among the lymphocytes. Tingible body macrophages tend to be a feature of a benign lymphoid follicle (H and E). (l) A case of follicular conjunctivitis stained immunohistochemically with a B-cell marker, CD20. This shows that the follicles are composed of B-cells and have a nondestructive, well-defined architecture. (m) Section of a case of sarcoid of the conjunctiva showing a granuloma (arrow) (H and E). (n) Higher power of plate m showing the epithelioid histiocytes that comprise this noncaseating granuloma. (o) Section of a case of papillary conjunctivitis. Note the finger-like extension of the stroma (H and E). (p) Section higher power of plate o showing the fibrovascular core of a papilla, containing chronic inflammatory cells (H and E). (q) Section of a case of ligneous conjunctivitis. The arrow points to the fibrinous material and the asterisk shows the superficial inflamed granulation-like tissue (H and E)
Drugs[2,3,4]
Topical and systemic therapeutic drugs can cause a variety of acute and chronic conjunctival changes.
Histopathology
Random epithelial squamous cell atypia is very often seen after treating the ocular surface with mitomycin C drops for conjunctival melanoma or squamous carcinoma. The nuclei become enlarged, hyperchromatic, with a smudgy chromatin quality; the cytoplasm similarly expands in size/volume thus preserving the nuclear-to-cytoplasmic ratio (N/C), which distinguishes this from neoplastic cells where there is a reduction in the N/C [Fig. 1b].
Conjunctival pigment deposition is classically attributable to chlorpromazine and minocycline and drugs in the same family. Minocycline metabolites accumulate as brown concretions [Fig. 1c], and chlorpromazine causes an increased amount of melanin production by the melanocytes [Fig. 1d and e].
Practice point
If a drug-related conjunctival effect is suspected, it is important to inform the pathologist of this or mention it on the pathology request form. This will save much time and effort when discussing etiologies/causation of unexplained findings.
Autoimmune[5,6,7,8]
Biopsies are often taken in suspected cases of ocular cicatricial pemphigoid/mucous membrane pemphigoid (OCP/MMP). These are exposed to direct immunofluorescence (DIF) to look for the presence of immunoglobulin G (IgG), IgA, IgM, and complement.
Histopathology
The hematoxylin and eosin appearances are not specific for OCP/MMP. Changes that are often seen include squamous metaplasia of the epithelium with reduction in goblet cell numbers, chronic inflammation of the substantia propria (comprising neutrophils [depending on disease activity] lymphocytes, plasma cells, macrophages, mast cells, and sometimes eosinophils), and varying degrees of substantia propria fibrosis. However, all of these changes can be seen in a variety of other chronic ocular surface disorders that cause cicatrization, such as topical drugs, allergic, and rosacea keratoconjunctivitis.
The DIF will show linear immunoreactants (IgG, IgA, IgM, and complement 3) along the epithelial basement membrane zone and is characteristic for OCP/MMP [Fig. 1f]. Not all of the immunoreactants have to be present at one time. The absence of immunoreactants does not exclude the diagnosis of OCP/MMP as disease activity affects the deposition of Igs, especially as inflammation effaces the basement membrane. Other conditions can result in linear basement membrane zone immunoreactants, such as bullous pemphigoid, linear IgA disease, paraneoplastic syndromes, and drug-induced conjunctival cicatrization. In pemphigus involving the conjunctiva, the classic pattern of immunoreactant distribution is intraepithelial, intercellular, and like a “fishnet.”
Practice point
Biopsies that require DIF should be placed in Michel's aqueous medium and not formalin. Consideration should be given to sampling the buccal mucosa first for DIF as this is less invasive than conjunctival sampling. If the buccal biopsy is negative, both conjunctival surfaces (preferably the superior bulbar conjunctiva because of upper lid protection) should be sampled for DIF along with biopsies placed in formalin to assess for neoplasia, granulomas, and inflammatory cell types. Both conjunctivae are sampled because DIF can be unilaterally positive (inflammation can destroy the epithelium basement membrane where the Igs are concentrated).
Metabolic[9,10,11,12]
Cystinosis
While rare, it is possible that the ophthalmic pathologist will encounter a conjunctival biopsy from a patient with confirmed or suspected cystinosis. Cystinosis is an autosomal recessive lysosomal disorder leading to the accumulation of cystine in lysosomes. The mutation is in the CTNS gene that encodes for a lysosomal membrane transporter protein. Needle-like crystals accumulate in the cornea and conjunctiva.
Histopathology
This will show intralysosomal polygonal crystals that appear membrane bound with electron dense material [Fig. 1g].
Practice point
As cystine crystals are water soluble, placing a conjunctival biopsy in formalin will wash them out and render the material useless. Liaison with the pathology laboratory will allow the acquisition of absolute alcohol, which is the fixative of choice. Tissue also placed into 2% buffered glutaraldehyde will allow transmission electron microscopy to be carried out.
Amyloid
Amyloid deposits are not uncommonly biopsied. They can occur in any conjunctival location, are often yellow, can be nodular or diffuse, and can be mistaken for a neoplasm clinically. Conjunctival amyloidosis is most often of primary localized type.
Histopathology
Eosinophilic, homogeneous, acellular material accumulates around conjunctival vessels and interstitially. The deposits stain red [Fig. 1h] for Congo red, and when the latter stained tissue sections are observed under special polarized light, the red becomes a bright green [birefringence – Fig. 1i].
Practice point
It is important not to miss a case of systemic amyloidosis. Amyloid can be typed using immunohistochemistry, but the most up-to-date method that is utilized in some centers is mass spectrometry for typing.
Noninfectious inflammatory[13,14,15,16,17,18,19]
Most cases of conjunctivitis are not biopsied – an ophthalmologist, armed with clinical acumen and with various microbiological techniques, can make a quick and accurate diagnosis. If the inflammation becomes chronic, the ocular pathologist is likely to encounter a biopsy. Under such circumstances, the conjunctiva commonly reveals two reaction patterns: follicular and papillary.
Follicular conjunctivitis
This has a number of cases that include viral infection, chlamydial infection, topical drug-induced, Parinaud oculo-glandular disease, and idiopathic. Papillary conjunctivitis causes include allergic/atopic, topical drugs, and chronic mechanical irritation.
Histopathology
Follicular conjunctivitis comprises nodules of lymphocytes, comprising reactive germinal centers, composed of immature large B-cells, surrounded by a mantle of smaller mature B-cells. These nodules are present in the substantia propria and cause a smooth bulge of the overlying epithelium. Papillary conjunctivitis comprises polygonal distortion of the epithelium. Each elevation is usually polygonal, larger than a follicle and contains vertically orientated vessels around which are many inflammatory cells. The nature of the inflammatory cells can suggest etiology. For example, if mast cells and eosinophils are seen, it points to an allergic/atopic etiology [Fig. 1j–l].
Granulomatous conjunctivitis
Common causes include foreign material (suture or traumatic foreign body) and sarcoid.
Histopathology
A granuloma is when two or more epithelioid histiocytes cluster with or without other features such as giant cells (various types) and necrosis, necrobiosis, and suppuration. In foreign body granulomas, lymphocytes, plasma cells, and epithelioid histiocytes swarm around the foreign material. The epithelioid histiocytes have the most cytoplasm and have slipper- or foot-print-shaped nuclei. When the histiocytes merge, they form a foreign body-type giant cell.
In sarcoid, yellowish nodules are seen on the tarsus and fornix usually. These comprise collections of epithelioid histiocytes without necrosis with or without giant cells. While classically the granulomas are described as naked (minimal accompanying lymphocytes), this is not always the case [Fig. 1m and n].
Practice point
A random conjunctival biopsy for suspected sarcoid is likely to yield no result. While obvious, it is always worth targeting the biopsy to nodules on the conjunctival surface as this will yield granulomas in a high number of cases.
Papillary conjunctivitis
These are elevations, often polygonal and affecting the tarsal conjunctiva and are larger than follicles.
Histopathology
They comprise a fibrovascular core with a variety of inflammatory cells, and the surface is often covered in metaplastic squamous epithelium. Papillary conjunctivitis is often caused by allergies (atopic, vernal, seasonal, or perennial), topical preparations, and chronic irritation (dry eyes, superior limbic conjunctivitis). The papillae in allergic type disorders are often packed full of eosinophils and mast cells [Fig. 1o and p].
Ligneous conjunctivitis
This is an autosomal recessive inherited disease caused by mutations in the plasminogen type-1 gene. Patients show low levels of plasmatic plasminogen and low plasminogen functional activity. Patients present with recurrent, bilateral lesions on many mucous membranes of body. In the eye, it results in a recurrent pseudomembranous conjunctivitis that evolves to a wood-like consistency.
Histopathology
Tarsal conjunctiva histology usually shows eroded, inflamed epithelium and granulation tissue over clotted fibrin (hyaline-like material). The histology reflects arrested wound healing at granulation tissue and fibrin clot stage. The fibrin clot reflects a lack of plasmin-mediated extracellular fibrinolysis [Fig. 1q].
Cysts[20,21,22,23]
Lymphatic dilation
This common entity is not a true cyst (not epithelial lining) and comprises dilated lymphatics with or without surrounding chronic inflammation and fibrosis. It is often found in postinflammatory states. They can contain blood (due to communication with conjunctival veins) which leads to an erroneous interpretation of hemangioma.
Histopathology
They are made up of dilated, geographic-shaped channels, lined by endothelium and often contain eosinophilic material and sometimes surrounded by clusters of lymphocytes. Some lymphangiectasias are associated with Turner's syndrome and Nonne-Milroy-Meige disease [Fig. 2a].
Figure 2.
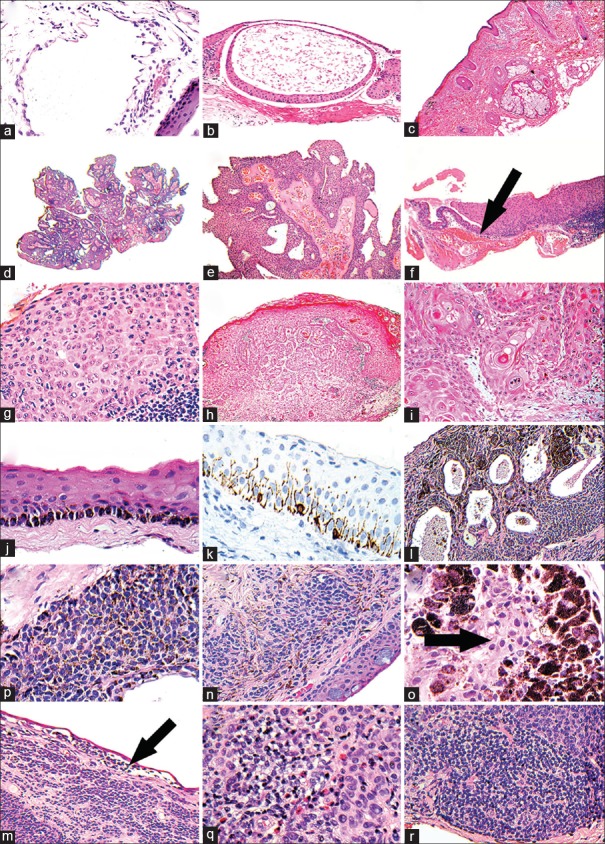
(a) Section showing an irregular space lined by attenuated lymphatic endothelial cells (H and E). (b) Section showing a conjunctival retention cyst lined by conjunctival type epithelium (H and E). (c) Section showing a limbal dermoid composed of skin type epidermis, dermis, and adnexal structures (H and E). (d) Section at scanning power of a viral squamous papilloma – note the finger-like projections (H and E). (e) Plate d at higher power showing the typical fibrovascular cores, draped in hyperplastic epithelium. (f) Section at scanning power. The thin epithelium to the left of the arrow is normal; that to the right is thickened and raised. This is the typical low power appearance of a squamous dysplasia of the conjunctival epithelium-abrupt transition (H and E). (g) Higher power of plat f with atypical squamous cells occupying the full-thickness of the epithelium. (h) Section showing, at scanning power, invasive, well-differentiated squamous cell carcinoma – note the central irregular tongues of cells invading the stroma (H and E). (i) Higher power of plate h showing the atypical, invasive squamous cells with keratin whorls/pearls. (j) Section showing basal epithelial melanin pigmentation in a case of racial melanosis (H and E). (k) Immunohistochemical staining with melan A of the case shown in plate j showing basally orientated, dendritic melanocytes. (l) Section scanning power showing numerous cysts surrounded by cells, in a case of a cystic melanocytic nevus (H and E). (m) Higher power of plate l showing the regular, tightly packed nevus cells, some of which contain melanin pigment. (n) Section showing an example of a combined nevus; the conventional component is similar to the nevus cells in plate m; the blue nevus component corresponds to the drawn-out brown cells in between (H and E). (o) Section of a cellular blue nevus. Note arrows points to the blue nevus cells, which are encased in heavily melanized melanophages. (p) Section at scanning power. The arrow points to a discohesion of cells just beneath the epithelium in a case of an inflamed juvenile nevus. (q) Higher power of plate p that shows eosinophils in the inflammatory component. (r) Higher power of plate p showing the lymphoid aggregates at the base of an inflamed juvenile nevus
Retention cysts
These are mostly acquired and often posttraumatic.
Histopathology
They are lined by conjunctival epithelium with or without goblet cells and with or without squamous metaplasia of the epithelium. They can contain nothing, mucin, cellular debris, or hemorrhage [Fig. 2b].
Choristomas[24,25]
These are congenital lesions and comprise mature, normal tissue at an abnormal anatomical location. The two most common conjunctival choristomas are epibulbar dermoids and dermolipomas.
Epibulbar dermoids are classically located at the corneoscleral limbus in the pediatric age group.
Histopathology
They look identical to skin with stratified squamous epithelium, dermal collagen, and deeper adipose tissue and contain skin adnexae such as pilosebaceous units and sweat glands. Some more complex lesions (called complex choristomas) can contain cartilage and bone. Syndromes associated with epibulbar dermoids include Goldenhar syndrome, linear nevus sebaceous syndrome, and Treacher–Collins syndrome [Fig. 2c].
Dermolipomas are often detected in adults and are detected superotemporally.
Histopathology
They comprise stratified squamous epithelium, dermal type collagen, and deeper mature adipose tissue to all intents similar to a dermoid but minus the skin adnexal structures.
Neoplastic Pathology
Epithelial tumors
Viral squamous papillomas[26,27,28,29]
These broadly occur in the pediatric age group and in adults; the former are often more pedunculated and classically occur at the inner canthus and fornix, whereas the latter tend to be more placoid and often located at the corneoscleral limbus. Both have a strong association with human papillomavirus (HPV) 6, 11, and 16.
Histopathology
The childhood viral papillomas are exophytic, frond-like and composed of fibrovascular cores draped in nonkeratinizing squamous epithelium with a variable number of goblet cells, attached to a pedicle. It is very common to see intraepithelial neutrophils. The corneoscleral limbal papillomas are more placoid, with a broad sessile base, composed of thickened stratified squamous epithelium, with or without keratinization. The under surface of the epithelium is undulating. Some cytological atypia can be seen amounting to dysplasia. Unlike skin viral lesions, it is rare to see HPV cytopathic sequelae such as koilocytosis, hypergranulosis, parakeratosis, abrupt keratinization, and in turning of the outer epithelium [Fig. 2d and e].
In situ and invasive squamous carcinoma[30,31,32,33,34,35,36,37,38,39]
This occurs mainly in the adult population in countries that are equatorial and is associated with chronic ultraviolet exposure and immunocompromised states (HIV and posttransplantation drug induced). Patients with xeroderma pigmentosum are affected at an earlier age. Depending on the population studied, there is some association with HPV 16 and 18. Most lesions are limbal based with or without corneal involvement. In patients with atopic conditions (eczema and asthma), lesions can be fornix based and bilateral.
Histopathology
Intraepithelial disease is sharply demarcated from the surrounding nondysplastic epithelium. It comprises atypical squamous epithelial cells, with nuclear chromatin abnormality (hyperchromasia, clearing, irregular nuclear profile), prominent nucleoli, although some cases can show smaller hyperchromatic nuclei or spindle-shaped nuclei. It is common to see mitotic activity, dyskeratosis, and sometimes HPV cytopathic effect (koilocytosis, parakeratosis, and hyperkeratosis). The atypical nuclei can partly involve the epithelial thickness or involve the full-thickness. The extent of thickness involvement can be graded as mild (lower third), moderate (half of epithelium to two-thirds), or severe (full thickness involvement equating to in-situ squamous carcinoma). A papillary in-situ variant also exists. Invasive squamous carcinomas tend to be well differentiated and keratinizing and are associated with the same risk factors as in-situ squamous carcinoma. They tend to have well-defined eosinophilic cytoplasm with abrupt keratinization, atypical nuclei often with prominent nucleoli. The tumor is disposed as infiltrating lobules or cords of variable thickness, often with a surrounding stromal desmoplastic response. Perineural, lymphatic, or blood vessel invasion may be seen. The background flat epithelium may show squamous dysplasia or in-situ squamous carcinoma. Rarely, intraocular invasion can occur in the limbal region [Fig. 2f–i].
Recent developments
Telomerase reverse transcriptase promoter (TERT) mutations are found in almost half of ocular surface squamous neoplasia. They have a mutation profile supporting ultraviolet mutagenesis, leading to aberrant overexpression of telomerase which is thought to be a pathogenetic factor in conjunctival squamous carcinoma. Furthermore, multiple DNA copy number alterations including frequent 8p11.22 amplification in conjunctival squamous carcinoma have recently been identified.
Benign melanocytic lesions
Racial melanosis[40,41,42]
There is increased melanin production with or without melanocyte hyperplasia along the basal layer; the melanosomes are taken up by the neighboring squamous epithelial cells at all layers that often form supra-nuclear caps. There is thought to be no malignant potential [Fig. 2j and k].
Primary melanosis without atypia[43]
Histopathology
The first pattern is an increase in melanin production without an increase in melanocytes. The second pattern is hyperplasia of melanocytes, similar to that seen in racial melanosis with or without an increase in melanosome production. The exclusion of atypia can only be made histologically. It is rare for this lesion to progress to melanoma.
Conventional nevus[44,45]
These are the most common benign tumor of the conjunctiva and have a variety of clinical appearances. Many have intralesional cysts. Some can also transform to malignant melanoma in around 1% of cases. They can be congenital or acquired.
Histopathology
Junctional nevi (a diagnosis only to be made in children) are composed of nests of nevus cells, at the epithelium–substantia propria interface. These nests can be of variable size and can become confluence. Melanization can be variable from case to case. Sometimes, clefts appear between the epithelium and the junctional nests – a feature that can worry pathologists into thinking that malignant discohesion is present. There is no pagetoid spread.
Compound nevus has a junctional and subepithelial component, often associated with down-growths of solid epithelial nests, many of which become cysts. The cysts usually contain many goblet cells, similar to the surface conjunctival epithelium. The surface junctional component does not shoulder beyond the subepithelial component. The junctional activity is very prevalent along the outer edges of the epithelial nests/cysts. The subepithelial component shows maturation of the nevus cells with depth (the melanocytes get smaller with stromal depth, which represents cellular senescence) and can be associated with inflammation – lymphocytes, plasma cells, and notable eosinophils in younger patients. Subepithelial nevus is composed of bland nevus cells purely present in the substantia propria and is very similar to intradermal skin nevi [Fig. 2l and m].
Practice point
A diagnosis of a junctional nevus should never be made in an adult as these lesions are usually in-situ melanoma.
Combination nevi[46,47]
It is common to see a combination of an acquired subepithelial or compound nevus with a more superficial blue nevus component.
Histopathology
The latter tends to show a fibrotic stroma and composed of pigmented dendritic and spindle melanocytes parallel to the epithelium. Cellular blue nevi are composed of a cluster of plump, epithelioid melanocytes, with central open nuclei, often with nucleoli, and lightly pigmented cytoplasm. The melanocytes tend to be wrapped by a dense population of melanophages [Fig. 2n and o].
Inflamed juvenile nevus[43,48]
This nevus causes clinical and diagnostic concern because of its rapid growth and pigmentary changes. It occurs in children, adolescents, and young adults and is strongly associated with allergies (atopy).
Histopathology
It can cause great concern to pathologists unfamiliar with these lesions. Prominent junctional activity with nests of varying size and confluence, discohesion, and shouldering beyond the subepithelial component is seen (these can all be features of malignancy in other contexts). The lesion contains epithelial cysts and the melanocytes are arranged in solid sheets or nests, sometimes with minimal maturation. The striking feature is the heavy inflammation at the base, featuring lymphocytes, reactive lymphoid follicles, plasma cells, and eosinophils, which cluster through the entire lesion and can be seen in the surface epithelium. The differential diagnosis is with a regressing melanoma. However, attention to detect an in-situ component and residual surviving islands of invasive melanoma and the age of the patient should allow a clear distinction. These lesions can rarely recur if incompletely excised [Fig. 2p–r].
Premalignant and malignant melanocytic lesions
Primary acquired melanosis with atypia/conjunctival melanocytic intraepithelial neoplasia/in-situ melanoma[49,50,51,52,53,54,55,56,57,58,59,60,61,62]
The terminology used by most ophthalmic pathologists is primary acquired melanosis (PAM) with atypia although some use the conjunctival melanocytic intraepithelial neoplasia (CMIN) terminology alongside the PAM with atypia terminology. No grading scheme is perfect.
Histopathology
PAM with atypia shows cytological and architectural atypical features. There are melanocytes that tend to be larger than the size of the basal keratinocyte and show clefts between them and the neighboring keratinocytes. Their shape can be round/oval, spindle, dendritic, and epithelioid. The melanocytes can show a linear basal pattern, nested, and pagetoid growth patterns. The epithelium can be completely consumed by atypical melanocytes. Melan A immunohistochemical stain (or equivalent) is very useful in showing the architectural pattern of the disease.
Grading of PAM with atypia is subjective; some have used mild, moderate, and severe with the latter equating to in-situ melanoma. Some use low risk and high risk where the risk relates to the chances of it transforming to invasive melanoma. A high-risk lesion has epithelioid cells and/or shows a suprabasilar proliferation, whereas a low-risk lesion is composed of cells other than epithelioid and is confined to the basal layer [Fig. 3a and b].
Figure 3.
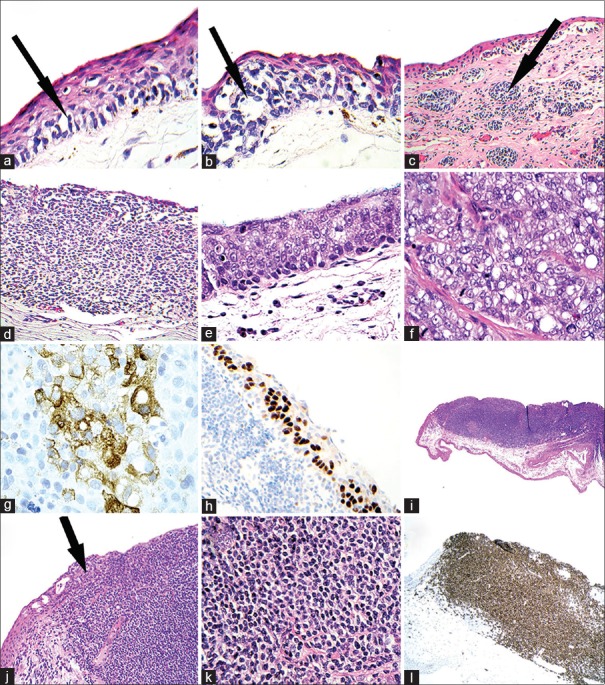
(a) Section showing melanosis with atypia (mild grade). Note the discohesive atypical melanocytes occupying the basal aspect of the epithelium (arrow) (H and E). (b) Section showing a more extensive lesion of melanosis with atypia, amounting to in-situ melanoma (arrow points to discohesive atypical melanocytes) (H and E). (c) Section at scanning power showing “nevoid” invasive melanoma. The arrow points to ovoid nests in the stroma with an aberrant architecture. Note the atypical, discohesive junctional component just beneath the epithelium (H and E). (d) Section showing a more solid invasive melanoma, compared to plate c (H and E). (e) Section showing at scanning power, the epithelium replaced by cells with some cytoplasm clearing (H and E). (f) Higher power of plate e showing atypical epithelial cells with bubbly, lipid laden macrophages. This is the typical cytology for sebaceous carcinoma. (g) Immunohistochemical staining of the cytoplasm of the sebaceous carcinoma with epithelial membrane antigen. Note the bubbly brown positive signal. (h) Immunohistochemical staining of the nuclei of intraepithelial sebaceous carcinoma with antibodies to androgen receptor. (i) Section scanning power showing a diffuse tumor mass in the substantia propria (H and E). (j) Higher power of plate i showing that the mass impinges on the epithelium and consumes it (arrow). (k) Higher power of plate i showing the mass composed of small lymphoid cells, with clear cytoplasm (monocytoid cells). (l) Immunohistochemical staining of the lymphoid mass with CD20 shows. This confirms a B-cell lymphoma and this particular case represents MALToma (extranodal marginal zone lymphoma of MALT-type)
Practice point
No histological grading scheme for severity of intraepithelial melanocytic neoplastic disease is perfect, and all of the systems that have been devised to date have their flaws with regard to intra- and inter-personal variation. Future classifications will incorporate molecular genetic classifications to help delineate what is a benign or malignant melanocyte.
Invasive melanoma[49,50,51,54,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82]
This accounts for approximately 5% of all ocular melanomas, affecting mainly adults of an average age of around 50 years. It affects white Caucasian patients mainly. It is unilateral, can be pigmented or amelanotic, unifocal and multifocal and does not only occur most commonly at the limbus in the interpalpebral bulbar conjunctiva but also affect the palpebral conjunctiva and fornix as well as the plica and caruncle. Invasive melanoma of the conjunctiva can arise from PAM with atypia/in-situ melanoma/CMIN, from longstanding nevi or de novo. Invasive melanoma metastasizes to the local conjunctiva (local conjunctiva metastases), regional lymph nodes, and systemic organs in 20/30% of cases.
Histopathology
Melanoma cells can be nevoid (small round cells), epithelioid, and spindled. There is no dermatopathological concept of radial and vertical growth phase as any melanoma cells that have invaded the substantia propria are invasive melanomas. Invasive cells can be pigmented or amelanotic, show nuclear pleomorphism, with atypical chromatin, and often show prominent nucleoli. Mitotic figures can be seen but are often quite sparse unless it is a rapidly growing lesion. The presence of inflammation and pigmentation toward the base can also be seen. The invasive cells can be arranged as nests, trabecular, and diffuse sheets – it is usual to see more than one growth pattern in any one case. Lymphatic and blood vessel channel invasion is detected in a small number of cases. When the nesting pattern is observed, it can look very nevoid, but attention to the spacing of the nests (which is wider for melanoma than nevus) and the cell morphology will allow a correct diagnosis [Fig. 3c and d].
Prognostic factors[70,71,72,73,74,75,76,77,78,79,80,81,82]
Primary conjunctival melanoma located at “unfavorable” sites (fornix, palpebral conjunctiva, medial bulbar conjunctiva, caruncle, plica semilunaris, and corneal stroma) is associated with a higher recurrence rate and metastatic death, compared with favorable sites (such as the bulbar and limbal conjunctiva). Melanomas larger than 10 mm in maximum extent that are stage pT3 or above and are multifocal recur locally and have a higher metastatic rate.
The thickness predicts prognosis. The current AJCC 7th edition and TNM 7th edition now qualify the TNM staging by mentioning whether the melanoma occurs on the bulbar, palpebral, fornix, or caruncle, and the depth of invasion categories are <0.5 mm, between 0.5 mm and 1.5 mm, and >1.5 mm. Tumors with lymphovascular space invasion are associated with a higher rate of metastasis, and tumors with contaminated histological margins are associated with local recurrence, metastasis, and higher mortality.
Recent genetic and molecular developments[83,84,85,86,87,88,89,90]
Fluorescence in-situ hybridization has been effectively utilized on histologically challenging conjunctival lesions where there is uncertainty about whether a lesion is benign or malignant. Using probes against CCND1, RREB1, and MYB has allowed a distinction between benign and malignant to be made in significant number of cases. BRAF, NRAS, and TERT mutations are found in conjunctival invasive melanoma. The presence of BRAF mutations allows some patients to benefit from vemurafenib therapy.
Sebaceous carcinoma[91,92,93,94,95,96,97,98,99,100]
This is an ocular adnexal carcinoma but is mentioned here as there is a variant called primary intraepithelial sebaceous carcinoma that arises from the epithelium in the absence of an adnexal primary nidus.
Histopathology
The tumor cells exhibit atypical nuclei (misshapen, hyperchromatic, nucleoli), often with conspicuous mitotic activity, apoptosis, and subepithelial chronic inflammation. The cytoplasm of the tumor cells is often vacuolated due to the presence of lipid. The cells frequently have a buck-shot scatter distribution in the epithelium (pagetoid spread) or fill the entire epithelium. Useful immunohistochemical markers include antibodies to epithelial membrane antigen, adipophilin, and androgen receptor with negative profiling with MNF116 to help confirm sebaceous differentiation [Fig. 3e–h]. Some cases are associated with Muir–Torre syndrome, due to mutations in the DNA mismatch repair protein genes.
Practice point
The absence of mismatch repair protein immunohistochemical staining can indicate Muir–Torre syndrome.
Conjunctival lymphoma[101,102,103,104,105,106]
The conjunctiva is part of the ocular adnexa. Non-Hodgkin's lymphomas commonly affect the conjunctiva and account for around 2% of all lymphomas. The World Health Organization classification of lymphoma permits each non-Hodgkin's lymphoma to be subcategorized into distinct clinico-pathological-molecular entities. This classification is critical as each category has a distinct prognosis and treatment options. The most common non-Hodgkin's lymphomas to affect the conjunctiva are extranodal marginal zone lymphoma of MALT type (ENMZL/MALToma), followed by follicular lymphoma and diffuse large B-cell lymphoma. These are all tumors of the B-cell lymphoid lineage.
Histopathology
MALToma is composed of small lymphocytes with sparse, eosinophilic, or clear cytoplasm (monocytoid cells), either disposed as nodules or diffuse sheets. It is not uncommon to see reactive lymphoid aggregates in the background as MALToma is thought to arise from a backdrop of chronic antigen stimulation, leading to oligoclonal and then monoclonal proliferations of B-lymphocytes. Sometimes, tumor cells enter the conjunctival epithelium-called a lymphoepithelial lesion. It is not uncommon to observe plasmacytoid cells, especially at the edges of the MALToma. Immunohistochemistry staining shows a nodular or diffuse mass of CD20 positive B-cells [Fig. 3i–l]. Other immunohistochemical markers are utilized to distinguish MALToma from follicular lymphoma, chronic lymphocytic leukemia, and mantle cell lymphoma. Space considerations do not permit a comprehensive histological description of all conjunctival lymphomas.
Recent developments
Some evidence suggests that Chlamydia psittaci infection may be associated with MALToma, but this does not hold true in all regions of the world. Some centers have therefore utilized anti-Chlamydia antibiotic therapy as the first-line treatment, although the outcomes are somewhat variable. Chromosomal translocations are common in MALToma, targeting the MALT1, BCL10, and FOXP1 genes.
Summary
This review article has summarized briefly the wide range of pathologies that can affect the conjunctiva and the awareness that is required from the specialized ophthalmic and general pathologists to secure a firm diagnosis. Since the ocular surface is an easily accessible anatomical site, biopsies from this location are likely to continue to constitute a large proportion of an ophthalmic pathologist's caseload. With a revolution occurring in cancer genetics and epigenetics, it is likely that a full understanding of the genetics and epigenetics of ocular surface tumors will be reached, leading to topical, individualized therapies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Spencer WH. Ophthalmic Pathology. An Atlas and Textbook. 4th ed. Vol. 1. Philadelphia: W.B. Saunders; 1996. p. 101. [Google Scholar]
- 2.Salomão DR, Mathers WD, Sutphin JE, Cuevas K, Folberg R. Cytologic changes in the conjunctiva mimicking malignancy after topical mitomycin C chemotherapy. Ophthalmology. 1999;106:1756–60. doi: 10.1016/S0161-6420(99)90355-X. [DOI] [PubMed] [Google Scholar]
- 3.Messmer E, Font RL, Sheldon G, Murphy D. Pigmented conjunctival cysts following tetracycline/minocycline therapy. Histochemical and electron microscopic observations. Ophthalmology. 1983;90:1462–8. doi: 10.1016/s0161-6420(83)34377-3. [DOI] [PubMed] [Google Scholar]
- 4.Cameron ME. Ocular melanosis with special reference to chlorpromazine. Br J Ophthalmol. 1967;51:295–305. doi: 10.1136/bjo.51.5.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan LS, Ahmed AR, Anhalt GJ, Bernauer W, Cooper KD, Elder MJ, et al. The first international consensus on mucous membrane pemphigoid: Definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. 2002;138:370–9. doi: 10.1001/archderm.138.3.370. [DOI] [PubMed] [Google Scholar]
- 6.Michel B, Milner Y, David K. Preservation of tissue-fixed immunoglobulins in skin biopsies of patients with lupus erythematosus and bullous diseases – Preliminary report. J Invest Dermatol. 1972;59:449–52. doi: 10.1111/1523-1747.ep12627611. [DOI] [PubMed] [Google Scholar]
- 7.Grau AE, Setterfield J, Saw VP. How to do buccal and conjunctival biopsies to investigate cicatrizing conjunctivitis: Improving the diagnosis of ocular mucous membrane pemphigoid. British J Ophthalmol. 2013;97:530–1. doi: 10.1136/bjophthalmol-2012-302963. [DOI] [PubMed] [Google Scholar]
- 8.Bernauer W, Dart JK, Elder MJ. Developments in Ophthalmology. Vol. 28. Basel: Karger; 1997. Cicatrising Conjunctivitis; pp. 102–10. [DOI] [PubMed] [Google Scholar]
- 9.Cogan DG, Kuwabara Y. Ocular pathology of cystinosis; with particular reference to the elusiveness of the corneal crystals. Arch Ophthalmol. 1960;63:51–7. doi: 10.1001/archopht.1960.00950020053008. [DOI] [PubMed] [Google Scholar]
- 10.Town M, Jean G, Cherqui S, Attard M, Forestier L, Whitmore SA, et al. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet. 1998;18:319–24. doi: 10.1038/ng0498-319. [DOI] [PubMed] [Google Scholar]
- 11.Richlin JJ, Kuwabara T. Amyloid disease of the eyelid and conjunctiva. Arch Ophthalmol. 1962;67:138–42. doi: 10.1001/archopht.1962.00960020140006. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez FJ, Gamez JD, Vrana JA, Theis JD, Giannini C, Scheithauer BW, et al. Immunoglobulin derived depositions in the nervous system: Novel mass spectrometry application for protein characterization in formalin-fixed tissues. Lab Invest. 2008;88:1024–37. doi: 10.1038/labinvest.2008.72. [DOI] [PubMed] [Google Scholar]
- 13.Dawson CR, Jones BR, Tarizzo ML. Who Guide to Trachoma Control in Programs for the Prevention of Blindness. Geneva: WHO; 1981. [Google Scholar]
- 14.Grayston JT, Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975;132:87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- 15.Henriquez AS, Kenyon KR, Allansmith MR. Mast cell ultrastructure. Comparison in contact lens-associated giant papillary conjunctivitis and vernal conjunctivitis. Arch Ophthalmol. 1981;99:1266–72. doi: 10.1001/archopht.1981.03930020140019. [DOI] [PubMed] [Google Scholar]
- 16.Nichols CW, Eagle RC, Jr, Yanoff M, Menocal NG. Conjunctival biopsy as an aid in the evaluation of the patient with suspected sarcoidosis. Ophthalmology. 1980;87:287–91. doi: 10.1016/s0161-6420(80)35236-6. [DOI] [PubMed] [Google Scholar]
- 17.Hidayat AA, Riddle PJ. Ligneous conjunctivitis. A clinicopathologic study of 17 cases. Ophthalmology. 1987;94:949–59. doi: 10.1016/s0161-6420(87)33341-x. [DOI] [PubMed] [Google Scholar]
- 18.Mingers AM, Heimburger N, Zeitler P, Kreth HW, Schuster V. Homozygous type I plasminogen deficiency: Ligneous conjunctivitis and additional lesions in other mucous membranes as important clinical manifestations. Semin Thromb Hemost. 1997;23:259–69. doi: 10.1055/s-2007-996099. [DOI] [PubMed] [Google Scholar]
- 19.Schuster V, Mingers AM, Seidenspinner S, Nüssgens Z, Pukrop T, Kreth HW, et al. Homozygous mutations in the plasminogen gene of two unrelated girls with ligneous conjunctivitis. Blood. 1997;90:958–66. [PubMed] [Google Scholar]
- 20.Perry HD, Cossari AJ. Chronic lymphangiectasis in Turner's syndrome. Br J Ophthalmol. 1986;70:396–9. doi: 10.1136/bjo.70.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jampol LM, Nagpal KC. Haemorrhagic lymphangiectasia of the cornea. Am J Ophthalmol. 1978;85:419–20. [Google Scholar]
- 22.Goldstein JH. Conjunctival cysts following strabismus surgery. J Pediatr Ophthalmol Strabismus. 1968;5:204–6. [Google Scholar]
- 23.Gloor P, Horio B, Klassen M, Eagle RC., Jr Conjunctival cyst. Arch Ophthalmol. 1996;114:1020–1. doi: 10.1001/archopht.1996.01100140228026. [DOI] [PubMed] [Google Scholar]
- 24.Cunha RP, Cunha MC, Shields JA. Epibulbar tumors in children: A survey of 282 biopsies. J Pediatr Ophthalmol Strabismus. 1987;24:249–54. doi: 10.3928/0191-3913-19870901-13. [DOI] [PubMed] [Google Scholar]
- 25.Elsas FJ, Green WR. Epibulbar tumors in childhood. Am J Ophthalmol. 1975;79:1001–7. doi: 10.1016/0002-9394(75)90685-6. [DOI] [PubMed] [Google Scholar]
- 26.Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Surv Ophthalmol. 2004;49:3–24. doi: 10.1016/j.survophthal.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Sjö N, Heegaard S, Prause JU. Conjunctival papilloma. A histopathologically based retrospective study. Acta Ophthalmol Scand. 2000;78:663–6. doi: 10.1034/j.1600-0420.2000.078006663.x. [DOI] [PubMed] [Google Scholar]
- 28.Sjö NC, Heegaard S, Prause JU, von Buchwald C, Lindeberg H. Human papillomavirus in conjunctival papilloma. Br J Ophthalmol. 2001;85:785–7. doi: 10.1136/bjo.85.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lass JH, Jenson AB, Papale JJ, Albert DM. Papillomavirus in human conjunctival papillomas. Am J Ophthalmol. 1983;95:364–8. doi: 10.1016/s0002-9394(14)78307-2. [DOI] [PubMed] [Google Scholar]
- 30.Lee GA, Hirst LW. Ocular surface squamous neoplasia. Surv Ophthalmol. 1995;39:429–50. doi: 10.1016/s0039-6257(05)80054-2. [DOI] [PubMed] [Google Scholar]
- 31.Sun EC, Fears TR, Goedert JJ. Epidemiology of squamous cell conjunctival cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:73–7. [PubMed] [Google Scholar]
- 32.Newton R, Ferlay J, Reeves G, Beral V, Parkin DM. Effect of ambient solar ultraviolet radiation on incidence of squamous-cell carcinoma of the eye. Lancet. 1996;347:1450–1. doi: 10.1016/s0140-6736(96)91685-2. [DOI] [PubMed] [Google Scholar]
- 33.Erie JC, Campbell RJ, Liesegang TJ. Conjunctival and corneal intraepithelial and invasive neoplasia. Ophthalmology. 1986;93:176–83. doi: 10.1016/s0161-6420(86)33764-3. [DOI] [PubMed] [Google Scholar]
- 34.McDonnell JM, Mayr AJ, Martin WJ. DNA of human papillomavirus type 16 in dysplastic and malignant lesions of the conjunctiva and cornea. N Engl J Med. 1989;320:1442–6. doi: 10.1056/NEJM198906013202202. [DOI] [PubMed] [Google Scholar]
- 35.Goedert JJ, Coté TR. Conjunctival malignant disease with AIDS in USA. Lancet. 1995;346:257–8. doi: 10.1016/s0140-6736(95)91309-2. [DOI] [PubMed] [Google Scholar]
- 36.Waring GO, 3rd, Roth AM, Ekins MB. Clinical and pathologic description of 17 cases of corneal intraepithelial neoplasia. Am J Ophthalmol. 1984;97:547–59. doi: 10.1016/0002-9394(84)90371-4. [DOI] [PubMed] [Google Scholar]
- 37.Fraunfelder FT, Wallace TR, Farris HE, Watkins J, 3rd, Hendrickson R, Smead WJ, et al. The role of cryosurgery in external ocular and periocular disease. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83:713–24. [PubMed] [Google Scholar]
- 38.Scholz SL, Thomasen H, Reis H, Möller I, Darawsha R, Müller B, et al. Frequent TERT promoter mutations in ocular surface squamous neoplasia. Invest Ophthalmol Vis Sci. 2015;56:5854–61. doi: 10.1167/iovs.15-17469. [DOI] [PubMed] [Google Scholar]
- 39.Asnaghi L, Alkatan H, Mahale A, Othman M, Alwadani S, Al-Hussain H, et al. Identification of multiple DNA copy number alterations including frequent 8p11.22 amplification in conjunctival squamous cell carcinoma. Invest Ophthalmol Vis Sci. 2014;55:8604–13. doi: 10.1167/iovs.14-14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmerman LE. The histogenesis of conjunctival melanomas: The First Algernon B. Reese Lecture. In: Jakobiec FA, editor. Ocular and Adnexal Tumors. Birmingham AL: Aesculapius Publishing Co; 1978. pp. 600–30. [Google Scholar]
- 41.Henkind P, Benjamin JV. Conjunctival melanocytic lesions. Natural history. Trans Ophthalmol Soc U K. 1977;97:373–7. [PubMed] [Google Scholar]
- 42.Jakobiec FA. The ultrastructure of conjunctival melanocytic tumors. Trans Am Ophthalmol Soc. 1984;82:599–752. [PMC free article] [PubMed] [Google Scholar]
- 43.Folberg R, Jakobiec FA, Bernardino VB, Iwamoto T. Benign conjunctival melanocytic lesions. Clinicopathologic features. Ophthalmology. 1989;96:436–61. doi: 10.1016/s0161-6420(89)32878-8. [DOI] [PubMed] [Google Scholar]
- 44.Gerner N, Nørregaard JC, Jensen OA, Prause JU. Conjunctival naevi in Denmark 1960-1980. A 21-year follow-up study. Acta Ophthalmol Scand. 1996;74:334–7. doi: 10.1111/j.1600-0420.1996.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 45.Jakobiec FA, Zuckerman BD, Berlin AJ, Odell P, MacRae DW, Tuthill RJ, et al. Unusual melanocytic nevi of the conjunctiva. Am J Ophthalmol. 1985;100:100–13. doi: 10.1016/s0002-9394(14)74991-8. [DOI] [PubMed] [Google Scholar]
- 46.Blicker JA, Rootman J, White VA. Cellular blue nevus of the conjunctiva. Ophthalmology. 1992;99:1714–7. doi: 10.1016/s0161-6420(92)31742-7. [DOI] [PubMed] [Google Scholar]
- 47.Crawford JB, Howes EL, Jr, Char DH. Combined nevi of the conjunctiva. Arch Ophthalmol. 1999;117:1121–7. [PubMed] [Google Scholar]
- 48.Zamir E, Mechoulam H, Micera A, Levi-Schaffer F, Pe'er J. Inflamed juvenile conjunctival naevus: Clinicopathological characterisation. Br J Ophthalmol. 2002;86:28–30. doi: 10.1136/bjo.86.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Folberg R, McLean IW, Zimmerman LE. Conjunctival melanosis and melanoma. Ophthalmology. 1984;91:673–8. doi: 10.1016/s0161-6420(84)34245-2. [DOI] [PubMed] [Google Scholar]
- 50.Jakobiec FA, Folberg R, Iwamoto T. Clinicopathologic characteristics of premalignant and malignant melanocytic lesions of the conjunctiva. Ophthalmology. 1989;96:147–66. doi: 10.1016/s0161-6420(89)32920-4. [DOI] [PubMed] [Google Scholar]
- 51.Folberg R, McLean IW. Primary acquired melanosis and melanoma of the conjunctiva: Terminology, classification, and biologic behavior. Hum Pathol. 1986;17:652–4. doi: 10.1016/s0046-8177(86)80175-7. [DOI] [PubMed] [Google Scholar]
- 52.Folberg R, Jakobiec FA, McLean IW, Zimmerman LE. Is primary acquired melanosis of the conjunctiva equivalent to melanoma in situ? Mod Pathol. 1992;5:2–5. [PubMed] [Google Scholar]
- 53.Folberg R, McLean IW, Zimmerman LE. Primary acquired melanosis of the conjunctiva. Hum Pathol. 1985;16:129–35. doi: 10.1016/s0046-8177(85)80061-7. [DOI] [PubMed] [Google Scholar]
- 54.Folberg R, McLean IW, Zimmerman LE. Malignant melanoma of the conjunctiva. Hum Pathol. 1985;16:136–43. doi: 10.1016/s0046-8177(85)80062-9. [DOI] [PubMed] [Google Scholar]
- 55.Shields JA, Shields CL, Mashayekhi A, Marr BP, Benavides R, Thangappan A, et al. Primary acquired melanosis of the conjunctiva: Risks for progression to melanoma in 311 eyes. The 2006 Lorenz E. Zimmerman lecture. Ophthalmology. 2008;115:511–9. doi: 10.1016/j.ophtha.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Ackerman AB. Primary acquired melanosis (letter) Hum Pathol. 1985;16:1077. doi: 10.1016/s0046-8177(85)80292-6. [DOI] [PubMed] [Google Scholar]
- 57.Ackerman AB, Sood R, Koenig M. Primary acquired melanosis of the conjunctiva is melanoma in situ. Mod Pathol. 1991;4:253–63. [PubMed] [Google Scholar]
- 58.Damato B, Coupland SE. Conjunctival melanoma and melanosis: A reappraisal of terminology, classification and staging. Clin Exp Ophthalmol. 2008;36:786–95. doi: 10.1111/j.1442-9071.2008.01888.x. [DOI] [PubMed] [Google Scholar]
- 59.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. pp. 539–46. [Google Scholar]
- 60.Sobin LH, Gospodarowicz MK, Wittekind DM. TNM Classification of Malignant Tumours. 7th ed. Oxford: Blackwell Publishing Ltd; 2010. pp. 279–83. [Google Scholar]
- 61.Singh AD, De Potter P, Fijal BA, Shields CL, Shields JA, Elston RC, et al. Lifetime prevalence of uveal melanoma in white patients with oculo (dermal) melanocytosis. Ophthalmology. 1998;105:195–8. doi: 10.1016/s0161-6420(98)92205-9. [DOI] [PubMed] [Google Scholar]
- 62.Sugiura M, Colby KA, Mihm MC, Jr, Zembowicz A. Low-risk and high-risk histologic features in conjunctival primary acquired melanosis with atypia: Clinicopathologic analysis of 29 cases. Am J Surg Pathol. 2007;31:185–92. doi: 10.1097/01.pas.0000213339.32734.64. [DOI] [PubMed] [Google Scholar]
- 63.Char DH. The management of lid and conjunctival malignancies. Surv Ophthalmol. 1980;24:679–89. doi: 10.1016/0039-6257(80)90127-7. [DOI] [PubMed] [Google Scholar]
- 64.Osterlind A. Trends in incidence of ocular malignant melanoma in Denmark 1943-1982. Int J Cancer. 1987;40:161–4. doi: 10.1002/ijc.2910400206. [DOI] [PubMed] [Google Scholar]
- 65.Scotto J, Fraumeni JF, Jr, Lee JA. Melanomas of the eye and other noncutaneous sites: Epidemiologic aspects. J Natl Cancer Inst. 1976;56:489–91. doi: 10.1093/jnci/56.3.489. [DOI] [PubMed] [Google Scholar]
- 66.Seregard S, Kock E. Conjunctival malignant melanoma in Sweden 1969-91. Acta Ophthalmol (Copenh) 1992;70:289–96. doi: 10.1111/j.1755-3768.1992.tb08566.x. [DOI] [PubMed] [Google Scholar]
- 67.Norregaard JC, Gerner N, Jensen OA, Prause JU. Malignant melanoma of the conjunctiva: Occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234:569–72. doi: 10.1007/BF00448801. [DOI] [PubMed] [Google Scholar]
- 68.Jakobiec FA, Rini FJ, Fraunfelder FT, Brownstein S. Cryotherapy for conjunctival primary acquired melanosis and malignant melanoma. Experience with 62 cases. Ophthalmology. 1988;95:1058–70. doi: 10.1016/s0161-6420(88)33058-7. [DOI] [PubMed] [Google Scholar]
- 69.Griffith WR, Green WR, Weinstein GW. Conjunctival malignant melanoma originating in acquired melanosis sine pigmento. Am J Ophthalmol. 1971;72:595–9. doi: 10.1016/0002-9394(71)90857-9. [DOI] [PubMed] [Google Scholar]
- 70.Paridaens AD, McCartney AC, Minassian DC, Hungerford JL. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520–8. doi: 10.1136/bjo.78.7.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shields CL, Shields JA, Gündüz K, Cater J, Mercado GV, Gross N, et al. Conjunctival melanoma: Risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Arch Ophthalmol. 2000;118:1497–507. doi: 10.1001/archopht.118.11.1497. [DOI] [PubMed] [Google Scholar]
- 72.Paridaens AD, McCartney AC, Lavelle RJ, Hungerford JL. Nasal and orbital recurrence of conjunctival melanoma 21 years after exenteration. Br J Ophthalmol. 1992;76:369–71. doi: 10.1136/bjo.76.6.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paridaens AD, Minassian DC, McCartney AC, Hungerford JL. Prognostic factors in primary malignant melanoma of the conjunctiva: A clinicopathological study of 256 cases. Br J Ophthalmol. 1994;78:252–9. doi: 10.1136/bjo.78.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crawford JB. Conjunctival melanomas: Prognostic factors a review and an analysis of a series. Trans Am Ophthalmol Soc. 1980;78:467–502. [PMC free article] [PubMed] [Google Scholar]
- 75.Fuchs U, Kivelä T, Liesto K, Tarkkanen A. Prognosis of conjunctival melanomas in relation to histopathological features. Br J Cancer. 1989;59:261–7. doi: 10.1038/bjc.1989.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jeffrey IJ, Lucas DR, McEwan C, Lee WR. Malignant melanoma of the conjunctiva. Histopathology. 1986;10:363–78. doi: 10.1111/j.1365-2559.1986.tb02490.x. [DOI] [PubMed] [Google Scholar]
- 77.Jay B. Naevi and melanomata of the conjunctiva. Br J Ophthalmol. 1965;49:169–204. doi: 10.1136/bjo.49.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Werschnik C, Lommatzsch PK. Long-term follow-up of patients with conjunctival melanoma. Am J Clin Oncol. 2002;25:248–55. doi: 10.1097/00000421-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 79.Lommatzsch PK, Lommatzsch RE, Kirsch I, Fuhrmann P. Therapeutic outcome of patients suffering from malignant melanomas of the conjunctiva. Br J Ophthalmol. 1990;74:615–9. doi: 10.1136/bjo.74.10.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jakobiec FA. Conjunctival melanoma. Arch Ophthalmol. 1980;98:1378–84. doi: 10.1001/archopht.1980.01020040230003. [DOI] [PubMed] [Google Scholar]
- 81.Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: Implications for sentinel lymph node biopsy. Ophthalmology. 2004;111:816–21. doi: 10.1016/j.ophtha.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 82.Anastassiou G, Heiligenhaus A, Bechrakis N, Bader E, Bornfeld N, Steuhl KP, et al. Prognostic value of clinical and histopathological parameters in conjunctival melanomas: A retrospective study. Br J Ophthalmol. 2002;86:163–7. doi: 10.1136/bjo.86.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gear H, Williams H, Kemp EG, Roberts F. BRAF mutations in conjunctival melanoma. Invest Ophthalmol Vis Sci. 2004;45:2484–8. doi: 10.1167/iovs.04-0093. [DOI] [PubMed] [Google Scholar]
- 84.Spendlove HE, Damato BE, Humphreys J, Barker KT, Hiscott PS, Houlston RS, et al. BRAF mutations are detectable in conjunctival but not uveal melanomas. Melanoma Res. 2004;14:449–52. doi: 10.1097/00008390-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 85.Griewank KG, Westekemper H, Murali R, Mach M, Schilling B, Wiesner T, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143–52. doi: 10.1158/1078-0432.CCR-13-0163. [DOI] [PubMed] [Google Scholar]
- 86.Dratviman-Storobinsky O, Cohen Y, Frenkel S, Pe'er J, Goldenberg-Cohen N. Lack of oncogenic GNAQ mutations in melanocytic lesions of the conjunctiva as compared to uveal melanoma. Invest Ophthalmol Vis Sci. 2010;51:6180–2. doi: 10.1167/iovs.10-5677. [DOI] [PubMed] [Google Scholar]
- 87.Griewank KG, Murali R, Schilling B, Scholz S, Sucker A, Song M, et al. TERT promoter mutations in ocular melanoma distinguish between conjunctival and uveal tumours. Br J Cancer. 2013;109:497–501. doi: 10.1038/bjc.2013.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lake SL, Jmor F, Dopierala J, Taktak AF, Coupland SE, Damato BE, et al. Multiplex ligation-dependent probe amplification of conjunctival melanoma reveals common BRAF V600E gene mutation and gene copy number changes. Invest Ophthalmol Vis Sci. 2011;52:5598–604. doi: 10.1167/iovs.10-6934. [DOI] [PubMed] [Google Scholar]
- 89.Busam KJ, Fang Y, Jhanwar SC, Pulitzer MP, Marr B, Abramson DH, et al. Distinction of conjunctival melanocytic nevi from melanomas by fluorescence in situ hybridization. J Cutan Pathol. 2010;37:196–203. doi: 10.1111/j.1600-0560.2009.01488.x. [DOI] [PubMed] [Google Scholar]
- 90.Mudhar HS, Smith K, Talley P, Whitworth A, Atkey N, Rennie IG, et al. Fluorescence in situ hybridisation (FISH) in histologically challenging conjunctival melanocytic lesions. Br J Ophthalmol. 2013;97:40–6. doi: 10.1136/bjophthalmol-2012-302261. [DOI] [PubMed] [Google Scholar]
- 91.Margo CE, Lessner A, Stern GA. Intraepithelial sebaceous carcinoma of the conjunctiva and skin of the eyelid. Ophthalmology. 1992;99:227–31. doi: 10.1016/s0161-6420(92)31988-8. [DOI] [PubMed] [Google Scholar]
- 92.Margo CE, Grossniklaus HE. Intraepithelial sebaceous neoplasia without underlying invasive carcinoma. Surv Ophthalmol. 1995;39:293–301. doi: 10.1016/s0039-6257(05)80106-7. [DOI] [PubMed] [Google Scholar]
- 93.Jakobiec FA, Werdich X. Androgen receptor identification in the diagnosis of eyelid sebaceous carcinomas. Am J Ophthalmol. 2014;157:687–960. doi: 10.1016/j.ajo.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 94.Rajan Kd A, Burris C, Iliff N, Grant M, Eshleman JR, Eberhart CG, et al. DNA mismatch repair defects and microsatellite instability status in periocular sebaceous carcinoma. Am J Ophthalmol. 2014;157:640–70. doi: 10.1016/j.ajo.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 95.Jakobiec FA, Mendoza PR. Eyelid sebaceous carcinoma: Clinicopathologic and multiparametric immunohistochemical analysis that includes adipophilin. Am J Ophthalmol. 2014;157:186–208. doi: 10.1016/j.ajo.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 96.Gaskin BJ, Fernando BS, Sullivan CA, Whitehead K, Sullivan TJ. The significance of DNA mismatch repair genes in the diagnosis and management of periocular sebaceous cell carcinoma and muir-torre syndrome. Br J Ophthalmol. 2011;95:1686–90. doi: 10.1136/bjophthalmol-2011-300612. [DOI] [PubMed] [Google Scholar]
- 97.Orta L, Klimstra DS, Qin J, Mecca P, Tang LH, Busam KJ, et al. Towards identification of hereditary DNA mismatch repair deficiency: Sebaceous neoplasm warrants routine immunohistochemical screening regardless of patient's age or other clinical characteristics. Am J Surg Pathol. 2009;33:934–44. doi: 10.1097/PAS.0b013e318199edca. [DOI] [PubMed] [Google Scholar]
- 98.Muthusamy K, Halbert G, Roberts F. Immunohistochemical staining for adipophilin, perilipin and TIP47. J Clin Pathol. 2006;59:1166–70. doi: 10.1136/jcp.2005.033381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnson JS, Lee JA, Cotton DW, Lee WR, Parsons MA. Dimorphic immunohistochemical staining in ocular sebaceous neoplasms: A useful diagnostic aid. Eye (Lond) 1999;13(Pt 1):104–8. doi: 10.1038/eye.1999.19. [DOI] [PubMed] [Google Scholar]
- 100.Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: A sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426–31. doi: 10.1097/00000372-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 101.Rath S, Connors JM, Dolman PJ, Rootman J, Rootman DB, White VA, et al. Comparison of American Joint Committee on cancer TNM-based staging system (7th edition) and Ann Arbor classification for predicting outcome in ocular adnexal lymphoma. Orbit. 2014;33:23–8. doi: 10.3109/01676830.2013.842257. [DOI] [PubMed] [Google Scholar]
- 102.Ferry JA, Fung CY, Zukerberg L, Lucarelli MJ, Hasserjian RP, Preffer FI, et al. Lymphoma of the ocular adnexa: A study of 353 cases. Am J Surg Pathol. 2007;31:170–84. doi: 10.1097/01.pas.0000213350.49767.46. [DOI] [PubMed] [Google Scholar]
- 103.Ferreri AJ, Guidoboni M, Ponzoni M, De Conciliis C, Dell'Oro S, Fleischhauer K, et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst. 2004;96:586–94. doi: 10.1093/jnci/djh102. [DOI] [PubMed] [Google Scholar]
- 104.Collina F, De Chiara A, De Renzo A, De Rosa G, Botti G, Franco R, et al. Chlamydia psittaci in ocular adnexa MALT lymphoma: A possible role in lymphomagenesis and a different geographical distribution. Infect Agent Cancer. 2012;7:8. doi: 10.1186/1750-9378-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhu D, Ikpatt OF, Dubovy SR, Lossos C, Natkunam Y, Chapman-Fredricks JR, et al. Molecular and genomic aberrations in chlamydophila psittaci negative ocular adnexal marginal zone lymphomas. Am J Hematol. 2013;88:730–5. doi: 10.1002/ajh.23490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferreri AJ, Ponzoni M, Guidoboni M, Resti AG, Politi LS, Cortelazzo S, et al. Bacteria-eradicating therapy with doxycycline in ocular adnexal MALT lymphoma: A multicenter prospective trial. J Natl Cancer Inst. 2006;98:1375–82. doi: 10.1093/jnci/djj373. [DOI] [PubMed] [Google Scholar]