Abstract
Purpose
Ribociclib (an oral, highly-specific cyclin-dependent kinase 4/6 inhibitor) inhibits tumor growth in preclinical models with intact retinoblastoma protein (Rb+). This first-in-human study investigated the maximum tolerated dose (MTD), recommended dose for expansion (RDE), safety, preliminary activity, pharmacokinetics, and pharmacodynamics of ribociclib in patients with Rb+ advanced solid tumors or lymphomas.
Experimental Design
Patients received escalating doses of ribociclib (3-weeks-on/1-week-off or continuous). Dose escalation was guided by a Bayesian Logistic Regression Model with overdose control principle.
Results
Among 132 patients, 125 received ribociclib 3-weeks-on/1-week-off and 7 were dosed continuously. Nine dose-limiting toxicities were observed among 70 MTD/RDE evaluable patients during Cycle 1, most commonly neutropenia (n = 3) and thrombocytopenia (n = 2). The MTD and RDE were established as 900 and 600 mg/day 3-weeks-on/1-week-off, respectively. Common treatment-related adverse events were (all-grade; grade 3/4) neutropenia (46%; 27%), leukopenia (43%; 17%), fatigue (45%; 2%), and nausea (42%; 2%). Asymptomatic Fridericia’s corrected QT prolongation was specific to doses ≥600 mg/day (9% of patients at 600 mg/day; 33% at doses >600 mg/day). Plasma exposure increases were slightly higher than dose proportional; mean half-life at the RDE was 32.6 hours. Reduced Ki67 was observed in paired skin and tumor biopsies, consistent with ribociclib-mediated antiproliferative activity. There were 3 partial responses and 43 patients achieved a best response of stable disease; 8 patients were progression-free for >6 months.
Conclusion
Ribociclib demonstrated an acceptable safety profile, dose-dependent plasma exposure, and preliminary signs of clinical activity. Phase I–III studies of ribociclib are underway in various indications.
Keywords: cyclin-dependent kinase, CDK4/6 inhibitor, ribociclib (LEE011), advanced solid tumors, lymphomas
Introduction
The cyclin D–cyclin-dependent kinase (CDK) 4/6–inhibitor of CDK4 (INK4)–retinoblastoma (Rb) pathway is disrupted in the majority of human cancers, promoting dysregulated cell cycle progression and the suppression of senescence (1, 2). Although Rb loss is common in some cancers, the majority of cancers retain wild-type Rb and are referred to as Rb-positive (Rb+; refs 1, 2). Rb+ cancers driven by oncogenic signaling pathways upstream of cyclin D are expected to be dependent on CDK4/6 activity for cell proliferation. Increased cyclin D–CDK4/6 activity in Rb+ cancers can occur by: overexpression of D cyclins (e.g. via t(11;14) translocation in mantle cell lymphoma [MCL]; refs. 3, 4), overexpression/mutation of CDK4/6 (e.g. by CDK4 amplification in liposarcoma; refs. 2, 5) or inactivation of CDK4/6-negative regulators (such as deletion/mutation or methylation of CDKN2A [encoding p16INK4]; ref. 6).
Available preclinical evidence suggests that targeting the cyclin D–CDK4/6–INK4–Rb pathway could be effective at inhibiting tumor growth across a variety of Rb+ cancers (4). Ribociclib (LEE011) is an orally bioavailable and selective small-molecule inhibitor of CDK4/6 (7, 8). Ribociclib induces complete dephosphorylation of Rb, resulting in sequestration of the E2F transcription factors and G1 cell cycle arrest in Rb+ cell lines (7, 8). In vitro, treatment of neuroblastoma cell lines with ribociclib led to dose-dependent accumulation of cells in the G0/G1 phase of the cell cycle, particularly marked at concentrations above 100 nmol/L (8). In vivo, ribociclib has demonstrated tumor growth inhibition in xenograft models of Rb+ tumors, including estrogen receptor-positive (ER+) breast cancer (9), liposarcoma (5), neuroblastoma (8), melanoma, and malignant rhabdoid tumor (7), as a single agent and in combination. Notably, ribociclib induced complete regressions in a Jeko-1 MCL xenograft model at doses comparable to those achievable in humans (7), demonstrating a high dependency on intact CDK4/6 activity in this model of direct cyclin D–CDK4/6 complex activation through CCND1 translocation.
The main findings in nonclinical toxicity studies of ribociclib indicated a prolongation of the average QTc, as well as effects on the bone marrow (hypocellularity), lymphoid system (lymphoid depletion) and testes (atrophy; unpublished data). These effects were considered to be related to the pharmacological inhibition of cell replication in these tissues due to CDK4/6 inhibition. The hepatobiliary system was identified as an additional target organ of toxicity. Corresponding hematological and/or biochemistry changes were seen for the effects described in the bone marrow, lymphoid system and liver. Generally all changes demonstrated either reversibility or a clear tendency towards reversibility (unpublished data).
Here we report results from the first-in-human Phase I dose-escalation study of single-agent ribociclib in patients with Rb+ advanced solid tumors or lymphomas (CLEE011X2101/NCT01237236).
Materials and Methods
Study design and dose escalation
This Phase I, open-label, dose-escalation study of single-agent ribociclib was undertaken in patients with Rb+ advanced solid tumors or lymphomas to determine the maximum tolerated dose (MTD)/recommended dose for expansion (RDE) of ribociclib, and to characterize the dose-limiting toxicities (DLTs) associated with ribociclib. The study also aimed to assess the safety, pharmacokinetics (PK), pharmacodynamics (PD), and preliminary activity of ribociclib in patients with solid tumors, including tumors that harbored aberrations in the cyclin D–CDK4/6–INK4–Rb pathway and other cancer-related genes.
Patients received escalating doses of oral ribociclib either on a 3-weeks-on/1-week-off schedule, or a continuous 28-day schedule until disease progression, unacceptable toxicity, death, or consent withdrawal. Ribociclib dose escalation (starting dose: 50 mg/day 3-weeks-on/1-week-off; selected based on 4-week preclinical studies) was guided by the adaptive Bayesian Logistic Regression Model (BLRM) including Escalation With Overdose Control (EWOC) principle (11). DLTs were evaluated during the first treatment cycle (28 days) and were defined according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. as adverse events (AEs) or clinically significant abnormal laboratory values suspected to be related to ribociclib treatment, which prevented the start of a new cycle of treatment within 7 days of the scheduled new cycle start date. In order to be eligible for a second or later cycle of treatment, patients were required to have had an absolute neutrophil count ≥1.0 × 109/L, platelet count ≥75.0 × 109/L, and no current non-hematologic toxicities ≥ CTCAE grade 2. Patients falling outside these criteria were deemed to have a DLT. DLTs also comprised: grade 4 neutropenia lasting ≥7 consecutive days; grade 4 thrombocytopenia; grade 3/4 neutropenia with fever (temperature ≥38.5°C); serum creatinine >2 ×upper limit of normal; grade ≥3 total bilirubin, or grade 2 total bilirubin with grade 2 increase in alanine transaminase (ALT), or aspartate aminotransferase; grade ≥3 ALT increase; QT corrected (QTc) interval ≥501 ms on ≥2 separate electrocardiograms; grade ≥3 nausea or vomiting despite optimal anti-emetic therapy; grade ≥3 diarrhea despite optimal antidiarrhea treatment; any grade 3/4 non-hematologic AE (including biochemical findings) except for brief (<72 hours) grade 3 fatigue; grade 3 alopecia, or grade 3 electrolyte disturbance (supplementation allowed) resolving to grade ≤1 within 7 days of drug interruption.
In order to declare a dose as the MTD, at least six evaluable patients must have been treated at that dose level. The starting dose for the continuous schedule was to be at least one dose level below the MTD established with the 3-weeks-on/1-week-off schedule, and was also determined by available safety and PK data. Upon determination of the MTD/RDE, an expansion phase sought to further evaluate the safety and tolerability, preliminary clinical activity, PK, and PD of ribociclib at the RDE.
Patients
Adults (≥18 years) with Eastern Cooperative Oncology Group (ECOG) performance status ≤1 were eligible for inclusion if they had a histologically/cytologically confirmed diagnosis of a solid tumor or lymphoma, with progression despite standard therapy, and with no further effective standard treatment available. Patients were required to have intact tumor Rb (as determined by a past or recent clinical diagnostic procedure) or tumor types that commonly exhibit aberrant activation of the cyclin D–CDK4/6–INK4–Rb pathway (MCL, liposarcoma, human papillomavirus [HPV]-negative head and neck squamous cell carcinoma [HNSCC], melanoma, ER+ breast cancer, and neuroblastoma). Any number of prior chemotherapies or radiation therapies was allowed. Patients were excluded if they had primary central nervous system tumors or brain metastases, unless treated and stable for ≥3 months with no need for steroids or anti-epileptic medications, or Fridericia’s corrected QT (QTcF) >450 ms (men) or >470 ms (women). Patients in the dose-expansion phase had documented evidence of a genetic aberration in the cyclin D–CDK4/6–INK4–Rb pathway (as determined by local standard clinical diagnostic procedures), or had tumor types that commonly exhibit such aberrant activation (see above).
Approval was obtained from the ethics committees of participating institutions and regulatory authorities. All participating patients provided written informed consent and agreed to comply with the study protocol. The study was conducted in accordance with the Declaration of Helsinki and guidelines for Good Clinical Practice as defined by the International Conference on Harmonization.
Safety and efficacy assessments
Safety assessments, including electrocardiograms, were conducted at baseline and at regular intervals throughout the study; AEs were assessed according to CTCAE version 4.0. Response was assessed by computerized tomography or magnetic resonance imaging according to Response Evaluation Criteria In Solid Tumors (RECIST) version 1.0 (12), or International Working Group (IWG) response criteria for lymphomas (13), at the end of every second cycle for the first six cycles, at the end of every third cycle thereafter, and at the end of treatment.
Pharmacokinetics assessments
PK assessments were performed during dose escalation and expansion. Blood samples were obtained on Days 1, 2, 8, 15, 18/21, 22, 23, and 24 of Cycle 1, and on Days 1 and 8 of Cycle 2 from patients on the non-continuous dosing schedule; or on Days 1, 2, 8, 15, 21, and 22 of Cycle 1, and on Day 1 of Cycles 2 and 3 from patients on the continuous dosing schedule. Plasma concentrations of ribociclib and a clinically significant metabolite (LEQ803) were determined by a previously validated liquid chromatography tandem mass spectrometry (LC-MS/MS) assay (lower limit of quantification 1.0 ng/mL). PK parameters were derived from individual plasma concentration–time profiles using non-compartmental analysis (Phoenix®; Pharsight Corporation, Mountain View, CA) and were summarized using descriptive statistics.
Pharmacodynamic assessments
PD biomarkers (Ki67 and phosphorylated Rb [pRb]) were analyzed by immunohistochemistry in skin (sampled at baseline and on Day 15 of Cycle 2) and tumor biopsies (sampled at baseline and on Day 15 of Cycle 1 or Cycle 2). Immunostaining for Ki67 and phosphorylated Rb (pRb) was performed on 4 µm formalin-fixed, paraffin-embedded tissue sections using mouse monoclonal anti-human Ki67 (MIB-1) antibody (Dako, Carpinteria, CA) and rabbit monoclonal anti-pRb (S780) antibody (Abcam, Cambridge, UK) on an Autostainer Universal Staining system (Dako, Carpinteria, CA). Ki67 and pRb staining were assessed semi-quantitatively by a board-certified pathologist in normal skin (sampled at baseline and on Day 15 of Cycle 2) and tumor biopsies (sampled at baseline and on Day 15 of Cycle 1 or Cycle 2). Scoring was performed using percentage of cells positive for Ki67, or a histo-score (H-score) methodology for pRb, based on staining intensity and percentage of positive cells. Intensity was scored as 0: none; 1: weak; 2: moderate; or 3: strong; and H-scores were calculated as follows: H-score = [fraction of cells with intensity grade 1 (%)] + [fraction of cells with intensity grade 2 (%) × 2] + [fraction of cells with intensity grade 3 (%) × 3]. For skin biopsies, Ki67 and pRb staining were scored as the number of positive cells/mm tissue along the basal keratinocyte layer.
Statistical analysis
No formal hypotheses were tested in this study; analyses were descriptive and exploratory. Patients were included in the analyses if they received at least one dose of ribociclib. Patients who met a minimum exposure criterion (≥75% of planned ribociclib doses administered during Cycle 1), and had sufficient safety evaluations (≥28 days following the first dose), or had experienced a DLT during Cycle 1, were evaluable for the determination of MTD. The data cut-off date was April 24, 2014.
Results
Patient characteristics and disposition
Table 1 summarizes characteristics of the 132 patients enrolled into the study between January 2011 and January 2014, from seven sites in the United States, France, and the Netherlands. More than half of the patients had undergone prior radiation, and nearly three-quarters of the patients received two or more prior systemic therapies. The most common tumor types were liposarcoma (n = 39; 30%), breast cancer (n = 20; 15%), colon cancer (n = 19; 14%), and HNSCC (n = 10; 8%). As of April 24, 2014, 111 patients had discontinued treatment for the following reasons: disease progression (n = 96; 86%); AEs (n = 8; 7%); withdrawal of consent (n = 3; 3%); death (n = 2; 2%); abnormal test procedure results (n = 1; 1%); and loss to follow-up (n = 1; 1%).
Table 1.
Patient characteristics at baseline
| Characteristic | Full analysis set (N = 132) |
|---|---|
|
| |
| Median age, years (range) | 60 (22–84) |
|
| |
| Sex | |
| Male, n (%) | 66 (50) |
|
| |
| ECOG performance status, n (%)a | |
| 0 | 47 (36) |
| 1 | 84 (64) |
|
| |
| Primary cancer, n (%) | |
| Liposarcoma | 39 (30) |
| Breast | 20 (15) |
| Colon | 19 (14) |
| HNSCC | 10 (8) |
| Mantle cell lymphoma | 7 (5) |
| Sarcoma | 5 (4) |
| NSCLC (adenocarcinoma) | 4 (3) |
| Melanoma | 3 (2) |
| Ovarian | 3 (2) |
| NSCLC (SCC) | 2 (2) |
| Other | 20 (15) |
|
| |
| Number of prior systemic antineoplastic therapies, n (%)b | |
| 0/1 | 33 (25) |
| 2/3 | 55 (42) |
| 4+ | 42 (32) |
| Number of patients who received prior surgery, n (%)a | 125 (95) |
| Number of patients who received prior radiotherapy, n (%)c | 68 (52) |
Data cut-off: April 24, 2014.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small cell lung cancer; SCC, squamous cell carcinoma.
Unknown in one patient.
Unknown in two patients.
Unknown in four patients.
MTD/RDE determination
As of April 24, 2014, 132 patients had been treated with ribociclib at doses ranging from 50–1,200 mg/day on a 3-weeks-on/1-week-off schedule (n = 125) or at 600 mg/day on a continuous dosing schedule (n = 7). Among the 70 patients evaluable for MTD/RDE determination, nine DLTs were observed during Cycle 1. The most common DLTs were neutropenia (n = 3) and thrombocytopenia (n = 2; Table 2).
Table 2.
Dose-limiting toxicities during dose escalation
| Dose level | Patients evaluable for DLTs |
Patients experiencing DLTs |
DLT |
|---|---|---|---|
| 3-weeks-on/1-week-off dosing schedule | |||
| 50 mg | 4 | 1 | G3 mucositis |
| 70 mg | 2 | - | - |
| 140 mg | 3 | - | - |
| 260 mg | 4 | - | - |
| 280 mg | 4 | 1 | G3 pulmonary embolism |
| 350 mg | 5 | - | - |
| 400 mg | 4 | 1a | G4 neutropenia |
| 600 mg | 12 | 1 | Prolonged G2 increased creatinine |
| 750 mg | 10 | 1 | G4 thrombocytopenia |
| 900 mg | 13 | 1 | G3 QTcF prolongation |
| 1,200 mg | 3 | 2 | G4 febrile neutropenia (n = 1) G4 thrombocytopenia (n = 1) |
| Continuous dosing schedule | |||
| 600 mg | 6 | 1 | Prolonged G3 neutropenia |
Abbreviations: DLT, dose-limiting toxicity; G, grade; QTcF, QT corrected with Fridericia’s formula.
At the ribociclib 400 mg/day dose level, a case of grade 3 hyponatremia was reported. Per protocol amendment 4, hyponatremia no longer qualifies as a DLT.
The starting dose of daily ribociclib was 50 mg/day on a 3-weeks-on/1-week-off schedule. Despite a DLT of grade 3 mucositis, escalation proceeded through doses of 70, 140, 280, and 400 mg/day. DLTs of grade 3 pulmonary embolism and grade 4 neutropenia were observed at 280 and 400 mg/day, respectively. Following these two DLTs, a decision was made to test a reduced dose of 260 mg/day and a cohort for this dose was opened. After confirming safety at this dose level, escalation resumed through doses of 350, 600, 900, and 1,200 mg/day. At this time, a continuous dose at 600 mg/day was also explored to determine if this schedule was tolerable. Six of the seven patients treated with the 600 mg/day continuous dose required dose interruption, and one of the six MTD/RDE evaluable patients experienced a DLT of prolonged grade 3 neutropenia. Therefore, continuous dosing at 600 mg/day was not further explored. Two of three patients treated at 1,200 mg/day on the 3-weeks-on/1-week-off schedule experienced DLTs of grade 4 febrile neutropenia and grade 4 thrombocytopenia. As it was established that escalation beyond 900 mg/day on a 3-weeks-on/1-week-off schedule was not tolerable, additional patients were treated at 600, 750 and 900 mg/day, to determine the MTD and RDE.
The MTD was established as 900 mg/day on a 3-weeks-on/1-week-off schedule, with only one DLT of grade 3 QTcF prolongation observed in 13 patients. This decision was also based on the BLRM results along with EWOC principle (less than 25% posterior probability of the true DLT rate being >33% at this dose level). One of ten patients treated at 750 mg/day experienced a DLT of grade 4 thrombocytopenia, and one of 12 patients treated at 600 mg/day experienced a DLT (grade 2 creatinine elevation). The RDE was established as 600 mg/day on a 3-weeks-on/1-week-off dosing schedule after the review of all available safety and PK data. The decision to declare an RDE below the MTD was driven primarily by the lower rate of QTcF prolongation observed at 600 mg/day compared with higher doses (Table 3). At the time of data cut-off (April 24, 2014), a total of 67 patients had been treated at the RDE.
Table 3.
Adverse events (≥10% of patients overall) suspected to be related to study treatment
| Adverse event, n (%) |
Grade | 50 mg n = 4 |
70 mg n = 2 |
140 mg n = 4 |
260 mg n = 4 |
280 mg n = 4 |
350 mg n = 5 |
400 mg n = 5 |
600 mga n = 67 |
600 mgb n = 7 |
750 mg n = 14 |
900 mg n = 13 |
1,200 mg n = 3 |
All N = 132 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Hematologic adverse events | ||||||||||||||
|
| ||||||||||||||
| Neutropenia | All | 0 | 0 | 0 | 0 | 1 (25) | 1 (20) | 2 (40) | 31 (46) | 6 (86) | 8 (57) | 9 (69) | 2 (67) | 60 (46) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (20) | 19 (28) | 5 (71) | 5 (36) | 5 (39) | 1 (33) | 36 (27) | |
|
| ||||||||||||||
| Leukopenia | All | 0 | 0 | 0 | 1 (25) | 1 (25) | 2 (40) | 1 (20) | 31 (46) | 6 (86) | 6 (43) | 9 (69) | 0 | 57 (43) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 (19) | 5 (71) | 0 | 4 (31) | 0 | 22 (17) | |
|
| ||||||||||||||
| Thrombocytopenia | All | 0 | 0 | 0 | 0 | 1 (25) | 0 | 1 (20) | 23 (34) | 2 (29) | 5 (36) | 6 (46) | 2 (67) | 40 (30) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 (9) | 0 | 3 (21) | 0 | 1 (33) | 10 (8) | |
|
| ||||||||||||||
| Anemia | All | 0 | 0 | 0 | 0 | 0 | 2 (40) | 2 (40) | 19 (28) | 2 (29) | 3 (21) | 3 (23) | 3 (100) | 34 (26) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (3) | 0 | 1 (7) | 0 | 1 (33) | 4 (3) | |
|
| ||||||||||||||
| Lymphopenia | All | 0 | 0 | 0 | 2 (50) | 1 (25) | 1 (20) | 0 | 15 (22) | 3 (43) | 6 (43) | 4 (31) | 0 | 32 (24) |
| 3/4 | 0 | 0 | 0 | 0 | 1 (25) | 1 (20) | 0 | 12 (18) | 2 (29) | 1 (7) | 4 (31) | 0 | 21 (16) | |
|
| ||||||||||||||
| Non-hematologic adverse events | ||||||||||||||
|
| ||||||||||||||
| Fatigue | All | 2 (50) | 2 (100) | 3 (75) | 4 (100) | 1 (25) | 1 (20) | 1 (20) | 22 (33) | 5 (71) | 5 (36) | 10 (77) | 3 (100) | 59 (45) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (3) | 1 (14) | 0 | 0 | 0 | 3 (2) | |
|
| ||||||||||||||
| Nausea | All | 0 | 1 (50) | 0 | 0 | 2 (50) | 2 (40) | 1 (20) | 30 (45) | 3 (43) | 8 (57) | 6 (46) | 3 (100) | 56 (42) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1) | 0 | 1 (7) | 0 | 0 | 2 (2) | |
|
| ||||||||||||||
| Vomiting | All | 0 | 1 (50) | 0 | 0 | 0 | 2 (40) | 2 (40) | 17 (25) | 2 (29) | 4 (29) | 3 (23) | 3 (100) | 34 (26) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
|
| ||||||||||||||
| Diarrhea | All | 0 | 0 | 0 | 0 | 1 (25) | 1 (20) | 0 | 18 (27) | 0 | 4 (29) | 5 (39) | 2 (67) | 31 (23) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (3) | 0 | 0 | 0 | 0 | 2 (2) | |
|
| ||||||||||||||
| Electrocardiogram QT prolonged | All | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 (9) | 0 | 2 (14) | 4 (31) | 2 (67) | 14 (11) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (15) | 0 | 2 (2) | |
|
| ||||||||||||||
| Blood creatinine increased | All | 0 | 0 | 0 | 0 | 0 | 3 (60) | 1 (20) | 4 (6) | 1 (14) | 3 (21) | 2 (15) | 0 | 14 (11) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
|
| ||||||||||||||
| Appetite decreased | All | 0 | 0 | 1 (25) | 0 | 2 (50) | 1 (20) | 0 | 5 (7) | 0 | 3 (21) | 1 (8) | 0 | 13 (10) |
| 3/4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Data cut-off: April 24, 2014.
3-weeks-on/1-week-off schedule.
Continuous dosing schedule.
Safety and tolerability
Safety data for all 132 patients in the study were combined. Median exposure to study treatment was approximately 8 weeks (range: 1–128 weeks). Table 3 shows treatment-related AEs that occurred at any time during the study with a frequency of at least 10%. The most common hematologic AEs (≥25% all-grade) suspected to be treatment-related were neutropenia, leukopenia, thrombocytopenia, and anemia. The onset of neutropenia (most frequently grade 2) occurred by approximately Day 15. Neutropenia typically resolved 7–14 days after dose interruption and infrequently required growth factor support. The most common non-hematologic treatment-related AEs (≥25% all-grade) were fatigue, nausea, and vomiting. Most treatment-related AEs occurred at similar rates at the RDE to those seen overall (Table 3). Out of the 67 patients treated at the RDE, 16 required dose reduction to 400 mg/day (with/without a dose interruption), and 13 had a dose interruption without reduction; dose reductions/interruptions were commonly for clinically manageable cytopenias. Treatment-related asymptomatic QTcF prolongation occurred at doses of ≥600 mg/day, with 9% of patients treated at the RDE and 33% of patients treated at all doses >600 mg/day experiencing QTcF prolongation; grade 3/4 QTcF prolongation only occurred at the 900 mg/day dose level (n = 2). QTcF prolongation trends followed maximal plasma concentration (Cmax) kinetics (see below), peaking at the time of maximum plasma concentration (Tmax), and were reversible in all patients. No clinically significant arrhythmias were observed.
Pharmacokinetics
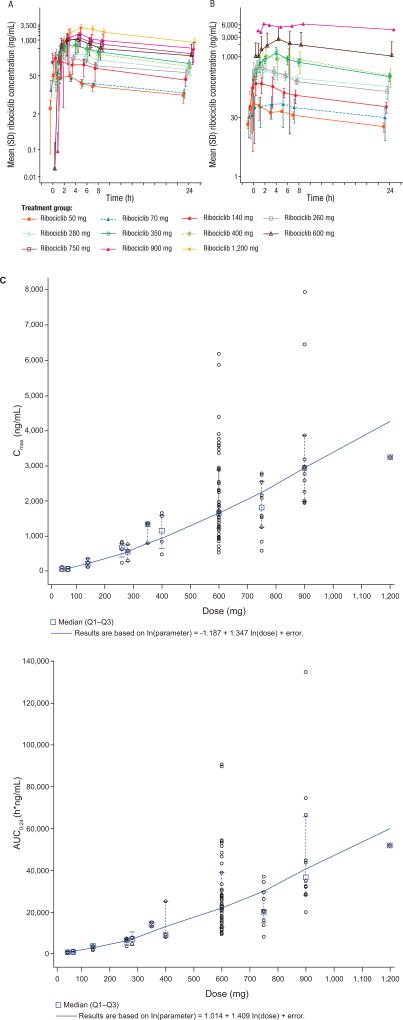
The mean plasma concentration–time profiles for ribociclib on Cycle 1 Day 1 and Cycle 1 Day 18 are shown in Fig. 1A and B. Following oral dosing, ribociclib was rapidly absorbed with median Tmax ranging from 1 to 5 hours. Plasma concentrations increased approximately 2- to 3-fold from Cycle 1 Day 1 to Cycle 1 Day 18/21 due to accumulation, with steady state reached by approximately Day 8 on the basis of trough concentrations after repeated daily dosing. The mean effective half-life based on accumulation ratio (T1/2, acc) was 32.6 hours at the RDE. Dose-proportionality analyses demonstrated that exposure to ribociclib increased with dose, with both Cmax and area under the curve (AUC) increasing slightly more than proportional to dose, over the dose range 50–1,200 mg/day (Fig. 1C). Profiles of LEQ803, the main active metabolite of ribociclib representing approximately 10% of the parent drug, were parallel to that of ribociclib (based on AUC from time zero to 24 hours [AUC0–24] on Days 1 and 18/21 of Cycle 1 for the 600 mg/day dose; data not shown).
Figure 1. Ribociclib pharmacokinetic profile.
(A, B) Mean plasma concentration–time profiles at (A) Cycle 1 Day 1 and (B) Cycle 1 Day 18 for the 128 patients who received ribociclib, and were evaluable for pharmacokinetic analysis. Data cut-off: March 28, 2014. (C) Cmax and AUC0–24 of ribociclib after multiple daily oral doses on Cycle 1 Day 18 or 21 across the dose range (50–1,200 mg). Data cut-off: April 24, 2014. The model ln(Cmax or AUC0–24) = α + β ln(dose) was used to assess the dose proportionality of ribociclib. The solid blue line represents the model estimated regression line; black open circles represent individual values; red filled triangles represent the least square mean; and vertical lines represent the 90% confidence interval of least square mean. The time points for collection of blood samples for PK analysis on Cycle 1 Day 1 and Day 18/21 were: pre-dose, 0.5, 1, 2, 4, 6, 8, and 24 h post-dose.
Abbreviations: AUC0–24, area under the curve from time zero to 24 hours; Cmax, maximal plasma concentration; SD, standard deviation.
Pharmacodynamics
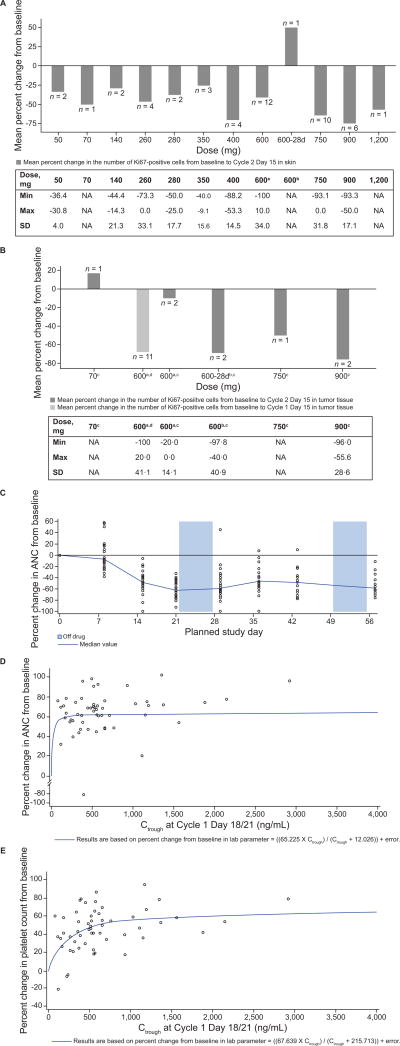
Changes in Ki67 levels, from baseline to Day 15 of Cycle 1 or Cycle 2, were assessed in 48 paired skin biopsies and 19 paired tumor biopsies. In skin biopsies, post-treatment reductions of Ki67 were observed across all dose levels (3-weeks on/1-week off) with a more consistent trend from 400 mg/day onwards (Fig. 2A). In tumor biopsies, reductions of Ki67 from baseline were observed at higher ribociclib doses (Fig. 2B); however, limited samples and varied tumor types prevent drawing conclusions about a dose–pharmacodynamic response relationship. Changes in pRb level from baseline to Day 15 of Cycle 1 or Cycle 2 were assessed in 46 paired skin biopsies and 16 paired tumor biopsies. Mean decreases of pRb in skin samples were observed at all dose levels. In one patient with MCL, a 71% and >92% reduction of pRb level was observed in the skin and tumor sample, respectively (data not shown). However, post-treatment changes in pRb were not significant or consistent in tumor samples, possibly due to varied tumor types and difficulty in preserving phospho-epitopes. Alternatively, ribociclib may have achieved higher local concentrations in skin than it did in tumor tissue, resulting in the absence of consistent pRb modulation in tumor samples. Overall, the decreases in Ki67 and pRb provide evidence for on-target CDK4/6 inhibition with ribociclib treatment; however, the decreases did not appear to be dose-dependent and did not inform the RDE. Strong time- and exposure-dependent myelosuppression, as assessed by neutrophil and platelet levels, was observed (Fig. 2C and E), consistent with other reports of CDK4/6 inhibitors (13).
Figure 2. Ribociclib pharmacodynamics.
(A, B) Mean percentage inhibition of Ki67 in (A) skin and (B) tumor vs. ribociclib dose. Tables provide the minimum and maximum percent change in Ki67 from baseline for each dose group, plus the SD for the mean percent change in Ki67 from baseline. (C) ANC over time (two cycles of ribociclib treatment) in the 600 mg/day ribociclib 3-weeks-on/1-week-off dose group (restricted to patients with a Cycle 1 duration of 28 days or less; n = 31). (D) Absolute neutrophil count by exposure (Ctrough Day 21). (E) Platelet count by exposure (Ctrough Day 21). The relation between the Ctrough and changes in (D) absolute neutrophil count and (E) platelet count were described by the equation:
Data cut-off: April 24, 2014.
Abbreviations: 28d, 28-day continuous dosing schedule; ANC, absolute neutrophil count; C, cycle; Ctrough, trough plasma concentration; D, day; NA, not applicable; SD, standard deviation.
a3-weeks-on/1-week-off schedule. bContinuous dosing schedule. cPost-baseline tumor samples collected at Cycle 2 Day 15. dPost-baseline tumor samples collected at Cycle 1 Day 15.
Clinical activity
All 132 patients were evaluable for radiographic response: three partial responses (PR; 2.3%) per RECIST were observed in patients with the following tumor types: head and neck acinar carcinoma with CDKN2A loss; BRAF/NRAS wild-type, CCND1-amplified melanoma (both 600 mg/day 3-weeks-on/1-week-off); and PIK3CA-mutant, CCND1-amplified ER+ breast cancer (600 mg/day continuous, but received dose interruptions periodically for grade 3/4 neutropenia). Stable disease (SD) was the best overall response in 41 patients by RECIST and two patients per IWG (32.6%; Supplementary Fig. S1). A prolonged duration of stable disease may also be a marker of clinical activity and patient benefit; SD for >6 months was experienced in eight patients (tumor types: liposarcoma [n = 6], teratoma [n = 1], and HNSCC [n = 1; Supplementary Fig. S1 and S2]). One patient with teratoma (400 mg/day 3-weeks-on/1-week-off) was on therapy for 100 weeks, and two patients with liposarcoma were on therapy past the cut-off date, with 131 (280 mg/day 3-weeks-on/1-week-off) and 102 (600 mg/day continuous) weeks on therapy, respectively (Supplementary Fig. S2).
Genetic analysis
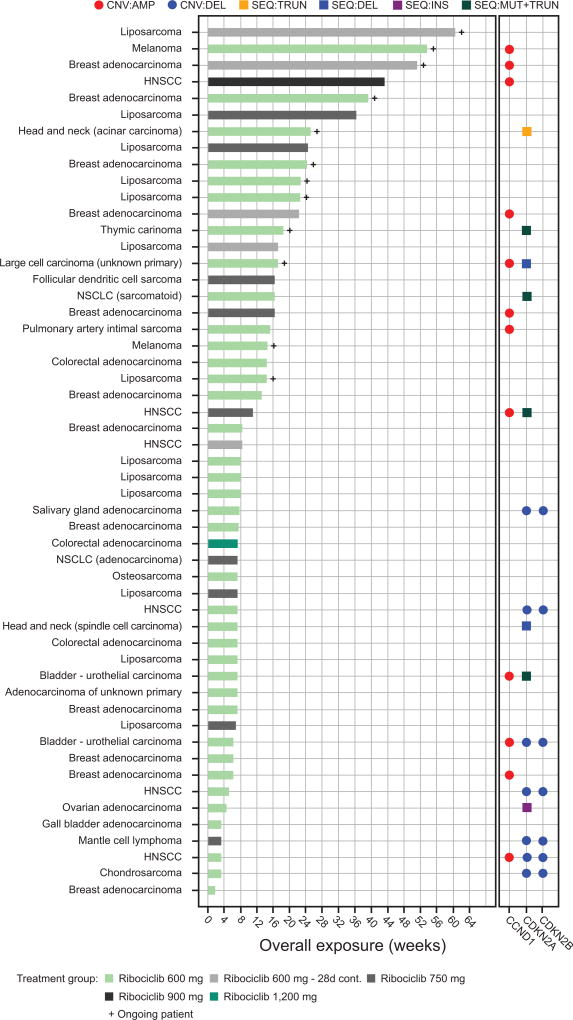
Exploratory genetics data were available for 53 patients from next generation sequencing of tumor samples (Fig. 3; Supplementary Fig. S3). A higher proportion of patients who received ribociclib for ≥8 weeks had tumors with alterations in CCND1 (8/29; 28%), as compared with patients who received ribociclib for <8 weeks (4/24; 17%). Notably, among patients for whom genetics data were available, three of the four patients who remained on therapy for the longest durations harbored CCND1 amplification. In contrast, a lower proportion of patients who received ribociclib for ≥8 weeks had tumors with CDKN2A and CDKN2B co-deletion (0/29; 0%), as compared with patients who received ribociclib for <8 weeks (7/24; 29%). Interestingly, two of four patients with CCDN1-amplified tumors and a treatment duration <8 weeks had CDKN2A and CDKN2B co-deletion.
Figure 3. Duration of exposure vs. observed somatic CDKN2A/B and CCND1 alterations.
Data cut-off: April 24, 2014.
Abbreviations: 28d cont., 28-day continuous dosing schedule; AMP, amplification; CNV, copy number variation; DEL, deletion; HNSCC, head and neck squamous cell carcinoma; INS, insertion; MUT, mutation; NSCLC, non-small cell lung cancer; REA, rearrangement; SEQ, sequence; TRUN, truncation.
Discussion
The findings from this study show that single-agent ribociclib had an acceptable safety profile in patients with advanced solid tumors or lymphomas, with the majority of AEs being mild to moderate. The MTD of ribociclib was established as 900 mg/day on a 3-weeks-on/1-week-off dosing schedule. Based on the tolerability observed during the dose expansion, 600 mg/day on a 3-weeks-on/1-week-off schedule was confirmed as the optimal regimen for future studies.
Similar to other reports of CDK4/6 inhibitors, ribociclib leads to reversible myelosuppression (14). At the RDE (600 mg/day 3-weeks-on/1-week-off), grade 3/4 neutropenia and thrombocytopenia occurred in 28% and 9% of patients, respectively. The observed neutropenia and thrombocytopenia correlated strongly with dose and exposure. Although marrow suppression was usually self-limited and readily reversible, it was the most common cause for dose interruption or modification. Fatigue, nausea, vomiting, and diarrhea were also relatively common but nearly always grade 1/2 and rarely necessitated dose modification. Treatment-related QTcF prolongation, which was always asymptomatic and reversible upon cessation of ribociclib, occurred in 9% of patients treated at the RDE. The effect of ribociclib on the QTcF interval is being further investigated in ongoing clinical trials. Safety information from this study has informed selection criteria, as well as AE and dose modification guidelines for subsequent trials including ribociclib.
Preliminary signs of clinical activity of ribociclib in patients with Rb+ advanced solid tumors and lymphomas were observed, including three PRs. In addition, several patients with little or no change in tumor volume had SD for prolonged periods; while these patients may have had indolent tumors, they may also have derived clinical benefit from cell growth arrest, rather than marked cell death. This is consistent with the known mechanism of action of ribociclib (8). Indeed, indirect evidence of on-target CDK4/6 inhibition with ribociclib was provided by reductions of cellular proliferation in skin and tumor biopsies.
Exploratory genetics data from this study provide insight into the potential underlying genetic aberrations associated with various tumor types and response to treatment. Presence of CCND1 amplification vs. CDKN2A and CDKN2B co-deletion trended towards a longer vs. shorter duration on treatment. Notably, two of four patients with CCDN1-amplified tumors and a treatment duration <8 weeks had CDKN2A/CDKN2B co-deletion. Patient selection based on CCDN1 amplification and/or p16 loss (CDKN2A deletion) did not improve outcomes in a previous study of a CDK4/6 inhibitor plus letrozole in ER+ breast cancer (15); however, it would be interesting to examine the effect of CCDN1 amplification and CDKN2A deletion independently of each other, and in the presence or absence of CDKN2A/CDKN2B co-deletion.
Other oral CDK4/6 inhibitors are also in clinical development, including palbociclib and abemaciclib. In hormone receptor-positive breast cancer, palbociclib demonstrated improved median progression-free survival in combination with letrozole (15) or fulvestrant (16), thereby demonstrating a proof-of-principle for the positive effect of CDK4/6 inhibition in combination with hormone therapy in this tumor type. Ribociclib (this study) and palbociclib (17, 18) have both demonstrated possible clinical activity in liposarcoma and teratoma, suggesting these cancer types might be responsive to CDK4/6 inhibitor-based therapy, either alone or in combination.
Taken together, results from this study demonstrate that ribociclib has an acceptable safety profile and shows preliminary signs of clinical activity in Rb+ tumors, thereby warranting further evaluation. Phase I–III studies of ribociclib are underway in various indications, including breast cancer, melanoma, and teratoma.
Supplementary Material
Statement of translational relevance.
Cyclin-dependent kinases (CDK) 4 and 6 regulate cell cycle progression by controlling the phosphorylation state of the retinoblastoma protein (Rb). Dysregulation of the cyclin D–CDK4/6–inhibitor of CDK4 (INK4)–Rb pathway frequently occurs in many cancer types and results in cell cycle progression and increased proliferation. Preclinical data have demonstrated antiproliferative activity of the CDK4/6 inhibitor, ribociclib (LEE011), in tumors that express functional Rb protein (Rb+). This first-in-human study characterized the maximum tolerated dose, recommended dose for expansion, safety profile, preliminary clinical activity, pharmacokinetics, and pharmacodynamics of ribociclib in patients with Rb+ advanced solid tumors and lymphomas. Ribociclib had acceptable safety and pharmacokinetic profiles, and demonstrated antiproliferative effects in skin and tumor cells, as well as preliminary signs of clinical activity, supporting further investigation of ribociclib either alone or in combination with other agents. Three Phase III clinical trials in patients with hormone receptor-positive breast cancer are ongoing.
Acknowledgments
We would like to thank the patients who took part in this study and their families, as well as the staff that assisted with the study at each site. Ribociclib was discovered by Novartis Institutes for BioMedical Research in collaboration with Astex Pharmaceuticals. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Alex Coulthard BSc (Hons) and Abbie Saunders PhD for editorial assistance.
Financial support: This study was sponsored by Novartis Pharmaceuticals Corporation, who also provided support for third-party writing assistance.
The study was initiated, funded, and sponsored by Novartis Pharmaceuticals. A. Chakraborty, A. Matano, J.R. Dobson, A.S. Crystal, and S. Parasuraman are full time employees of Novartis Pharmaceuticals. A. Chakraborty, A. Matano, A.S. Crystal, and S. Parasuraman own stock in Novartis Pharmaceuticals. J.R. Infante has served as an advisor to Novartis Pharmaceuticals, outside the submitted work. P.A. Cassier received grants, personal fees and non-financial support from Novartis Pharmaceuticals, Roche/Genentech, and Blueprint Medicines, and grants and non-financial support from Bayer, Celgene, Eli Lilly and Company, and AstraZeneca, outside the submitted work. J.F. Gerecitano received personal fees from Samus Therapeutics, Bayer, Roche/Genentech, and Abbvie outside the submitted work. R. Chugh owns stock in Portola Pharmaceuticals and received grants and non-financial support from Novartis Pharmaceuticals, Eli Lilly and Company, Biomarin, Morphotek, MabVax Therapeutics, AADi LLC, Biotronic, and Medtronic, and grants, personal fees and non-financial support from the Sarcoma Alliance for Research through Collaboration (SARC), outside the submitted work. V. Ribrag has served as an advisor and received personal fees from Bristol-Myers Squibb, Servier, Infinity Pharmaceuticals, Eisai, Pharmamar, and Gilead, and has received grants from Argenx, Epizyme, and Servier, outside the submitted work. G.I. Shapiro has served as an advisor to Eli Lilly and Company, G1 Therapeutics, EMD Serono, Vertex Pharmaceuticals, Chugai Pharma, and Millenium/Takeda Oncology, and has received grants from Pfizer and Eli Lilly and Company, outside the submitted work.
Footnotes
Authors’ contributions
Conception and design: J.R. Infante, G.I. Shapiro, A. Matano, S. Parasuraman
Acquisition of data: J.R. Infante, P.A. Cassier, J.F. Gerecitano, P.O. Witteveen, R. Chugh, V. Ribrag, G.I. Shapiro
Analysis and interpretation of data: All authors
Writing, review and/or revision of the manuscript: All authors
Disclosure of Potential Conflicts of Interest: P.O.Witteveen declares no additional competing interests outside the submitted work.
Trial registration ID: NCT01237236
References
- 1.Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov. 2015;6:353–67. doi: 10.1158/2159-8290.CD-15-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24:1770–83. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- 3.Ortega S, Malumbres M, Barbacid M. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta. 2002;1602:73–87. doi: 10.1016/s0304-419x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 4.Baker SJ, Reddy EP. CDK4: a key player in the cell cycle, development, and cancer. Genes Cancer. 2012;3:658–69. doi: 10.1177/1947601913478972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang YX, Sicinska E, Czaplinski JT, Remillard SP, Moss S, Wang Y, et al. Antiproliferative effects of CDK4/6 inhibition in CDK4-amplified human liposarcoma in vitro and in vivo. Mol Cancer Ther. 2014;13:2184–93. doi: 10.1158/1535-7163.MCT-14-0387. [DOI] [PubMed] [Google Scholar]
- 6.Liggett WH, Jr, Sidransky D. Role of the p16 tumor suppressor gene in cancer. J Clin Oncol. 1998;16:1197–206. doi: 10.1200/JCO.1998.16.3.1197. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Loo A, Chopra R, Caponigro G, Huang A, Vora S, et al. LEE011: an orally bioavailable, selective small molecule inhibitor of CDK4/6-- reactivating rb in cancer. Mol Cancer Ther. 2013;12(11 Suppl) Abstract nr PR02 (Oral presentation) [Google Scholar]
- 8.Rader J, Russell MR, Hart LS, Nakazawa MS, Belcastro LT, Martinez D, et al. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin Cancer Res. 2013;19:6173–82. doi: 10.1158/1078-0432.CCR-13-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Brien N, Di Tomaso E, Ayala R, Tong L, Issakhanian S, Linnartz R, et al. In vivo efficacy of combined targeting of CDK4/6, ER and PI3K signaling in ER+ breast cancer [abstract]. Proceedings of the 105th Annual Meeting of the American Association for Cancer Research; 2014 April 5–9; San Diego, CL. Philadelphia (PA): AACR; 2014. Abstract nr 4756. [Google Scholar]
- 10.Montana JG, Dyke HJ. Update on the therapeutic potential of PDE-4 inhibitors. Expert Opinion on Investigational Drugs. 2002;11(1):1–13. doi: 10.1517/13543784.11.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Neuenschwander B, Branson M, Gsponer T. Critical aspects of the Bayesian approach to phase I cancer trials. Stat Med. 2008;27:2420–39. doi: 10.1002/sim.3230. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 14.Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–46. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 16.Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–19. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 17.Dickson MA, Tap WD, Keohan ML, D'Angelo SP, Gounder MM, Antonescu CR, et al. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J Clin Oncol. 2013;31:2024–8. doi: 10.1200/JCO.2012.46.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaughn DJ, Hwang WT, Lal P, Rosen MA, Gallagher M, O'Dwyer PJ. Phase 2 trial of the cyclin-dependent kinase 4/6 inhibitor palbociclib in patients with retinoblastoma protein-expressing germ cell tumors. Cancer. 2015;121:1463–8. doi: 10.1002/cncr.29213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.