A facile preparation of phase-stable cesium lead triiodide perovskite for high-performance solar cells.
Abstract
Among various all-inorganic halide perovskites exhibiting better stability than organic-inorganic halide perovskites, α-CsPbI3 with the most suitable band gap for tandem solar cell application faces an issue of phase instability under ambient conditions. We discovered that a small amount of two-dimensional (2D) EDAPbI4 perovskite containing the ethylenediamine (EDA) cation stabilizes the α-CsPbI3 to avoid the undesirable formation of the nonperovskite δ phase. Moreover, not only the 2D perovskite of EDAPbI4 facilitate the formation of α-CsPbI3 perovskite films exhibiting high phase stability at room temperature for months and at 100°C for >150 hours but also the α-CsPbI3 perovskite solar cells (PSCs) display highly reproducible efficiency of 11.8%, a record for all-inorganic lead halide PSCs. Therefore, using the bication EDA presents a novel and promising strategy to design all-inorganic lead halide PSCs with high performance and reliability.
INTRODUCTION
In past years, the organic-inorganic hybrid lead halide perovskite solar cells (PSCs) have progressed in an impressive manner approaching commercialization (1–5). However, the instability of organic-inorganic hybrid perovskite, such as CH3NH3PbI3 under thermal stress, might stem from the volatility of the organic cation and has become a challenge for long-term practical deployment. Although organic-inorganic mixed-cation PSCs demonstrating improved efficiency and stabilities have been documented (6–8), all-inorganic lead halide perovskite absorbers are much desired, specifically because the issues related to the release or decomposition of the organic component can be avoided. The most suitable all-inorganic structure could be based on CsPbX3 perovskite because only the Cs is large enough to occupy the A sites with a suitable tolerance factor to fit the ABX3 perovskite (B = Pb, X = halide) configuration. The phase-stable CsPbX3 perovskite is usually based on bromide with a suitable tolerance factor, but the CsPbBr3 perovskite has a too wide band gap to realize the fabrication of high-efficiency solar cells. Unfortunately, the α-CsPbI3 perovskite with a band gap of 1.73 eV, which is suitable for tandem solar cells, is structurally unstable and transforms spontaneously into the unwanted δ-CsPbI3 phase under ambient conditions at room temperature. Currently, cesium has been successfully used as A cation along with formamidinium in high-efficiency PSCs, but at concentrations above 20%, it leads to the formation of nonperovskite δ-CsPbI3 (7–12). Previous reports have suggested that the phase stability of α-CsPbI3 strongly depends on the crystallite size because stability improves by trimming the dimensions of the α-CsPbI3 grains toward the nanoregime (13, 14). Size-dependent phase or thermal stability has been reported in nanomaterials, especially when surfactants were used as growth-controlling agents (15, 16). However, conventional deposition strategies, which do not involve surfactants, mostly yield large-sized CsPbI3 crystallites. Recently, α-CsPbI3 quantum dots with well-controlled size synthesized by hot injection have been fashioned into stable PSCs exhibiting an efficiency of up to 10% (17). In addition, a two-dimensional (2D) interface has been demonstrated as an effective strategy to stabilize the organic-inorganic hybrid perovskite or to increase the thermal stability of CH3NH3PbI3 (18, 19). However, a 2D segment of these structures may impair the electron transport across the device, lowering their efficiency (18). Therefore, it becomes imperative to judiciously design the bication 2D architecture.
Here, we report a new and facile one-step deposition method to obtain highly efficient and stable α-CsPbI3 PSCs (2). We stabilize the α-CsPbI3 perovskite films by introducing ethylenediamine cations (EDA2+) whose terminal NH3+ groups are expected to cross-link the CsPbI3 perovskite crystal units, rendering them less prone to unwanted phase transition to the δ structure. Stable α-CsPbI3 films have been deposited by introducing a small amount of bication 2D perovskite of EDAPbI4 into the CsPbI3 precursor solution. This cross-links the α-CsPbI3 films without impairing the charge-carrier transport. The resulting α-CsPbI3 structures are highly robust at room temperature for months and can retain their phase even after annealing at 100°C for a week. In addition to the high stability, PSCs based on the α-CsPbI3 films showed highly reproducible photoconversion efficiency (PCE) of 11.8%, a record for α-CsPbI3 devices.
RESULTS
We used a typical one-step method to deposit CsPbI3 films using a regular precursor containing stoichiometric CsI and PbI2 dissolved in N,N′-dimethylformamide (DMF). A rough yellowish film was obtained after annealing the precursor film at 150°C. As shown in Fig. 1 (A and B), the absorbance peak located at 414 nm and a strong x-ray diffraction (XRD) peak at 9.78° suggest the formation of the unwanted nonperovskite δ-CsPbI3 phase. This is quite expected because the yellow-to-black phase transformation occurs at 350°C (13, 20). Toward the formation of α-CsPbI3, we develop a low-temperature fabrication method using PbI2·xHI (x > 1.3) and CsI as precursors. The XRD pattern of PbI2·xHI is shown in fig. S1, which exhibits no signature corresponding to the PbI2 phase and is different from the previous reports on HPbI3 (21). The brown perovskite CsPbI3 film was obtained via one-step deposition using the PbI2·xHI + CsI precursor, followed by the low-temperature annealing at 100° to 150°C. It seems that the HI in the PbI2·xHI adduct decreases the crystallization energy barrier for the α-CsPbI3 phase. The as-prepared film shows an absorbance onset at ~718 nm in Fig. 1A, indicating a band gap of 1.73 eV, consistent with the previous reports on α-CsPbI3 (13, 22). The XRD pattern (Fig. 1B) obtained from the brown CsPbI3 film could be indexed to a phase-pure cubic α-CsPbI3 perovskite structure (12, 13). However, the PSC based on this α-CsPbI3 film revealed a modest PCE of 5.59% (Fig. 1C), which is comparable to previous reports (10, 13, 23). Unfortunately, this phase-pure α-CsPbI3 film also suffers from phase instability issues. As shown in Fig. 1D, the brown α-CsPbI3 film transforms into a yellow δ-CsPbI3 film within 12 hours.
Fig. 1. Spectroscopic and structural characterization, photovoltaic performance, and stability test of CsPbI3 films.
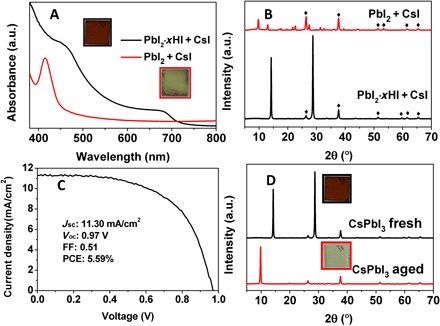
(A) Ultraviolet-visible (UV-vis) spectra of CsPbI3 films prepared from PbI2 + CsI and PbI2·xHI + CsI. a.u., arbitrary units. (B) XRD patterns of CsPbI3 films prepared from PbI2 + CsI and PbI2·xHI + CsI. (C) Photocurrent density–voltage (J-V) curve of α-CsPbI3–based PSCs. FF, fill factor. (D) XRD pattern and photos of fresh and aged (within a day) CsPbI3 films prepared from PbI2·xHI + CsI.
In organic-inorganic or inorganic lead halide perovskite, the cation usually occupies one A site in either 3D ABX3 or 2D A2BX4 perovskites. Here, the two NH3+ groups of EDA can occupy two A sites and cross-link these 2D layers. This bication 2D perovskite has been theoretically predicted, and the copper halide perovskite based on EDA has been previously reported (24–26). The EDAPbI4 perovskite film appears greenish, and its absorption spectrum and XRD pattern are listed in Fig. 2. In the XRD pattern (Fig. 2A), a peak located at 2θ = ~6° is a characteristic feature of 2D perovskites (21, 27). Atomic force microscopy (AFM) images reveal that the EDAPbI4 films are consisted of stacks of layered structure (fig. S2). Furthermore, an absorption peak around 420 nm observed in the UV-vis spectrum (Fig. 2B) suggested that the EDAPbI4 is a wider band-gap material (2, 28). Previously, some reports suggested that the bication 2D perovskite can be either a regular (100) layered perovskite or a corrugated (110) layered perovskite (29–33). Given the band-gap value of EDAPbI4 sample and the short alkyl chain length of the EDA, we assume that it should be the formation of a (110) layered perovskite structure (fig. S3). We introduce a small amount of EDAPbI4 into the CsPbI3 precursor, and these samples are noted as CsPbI3·xEDAPbI4. The x-ray photoelectron spectroscopy (XPS) spectra corresponding to an N element acquired from the CsPbI3·xEDAPbI4 (x = 0.0125 to 0.05) samples establish the presence of EDA cations in these films (fig. S4). XRD patterns and UV-vis spectra of CsPbI3·xEDAPbI4 samples are listed in Fig. 2 (C and D). Irrespective of their EDAPbI4 content (x), all the XRD patterns could be indexed to the standard α-CsPbI3 perovskite phase. No signature (peaks below 2θ = 10°) corresponding to the EDAPbI4 phase was found in the CsPbI3·xEDAPbI4 samples, which suggests either the absence of the EDAPbI4 crystal phase or the formation of extremely thin EDAPbI4 layers or crystallites. Amorphous EDAPbI4 could also be present. This matches the behavior of previously reported 2D/3D perovskite formulations (18).
Fig. 2. Structural characterization and spectroscopic study of EDAPbI4 and CsPbI3·xEDAPbI4 films.
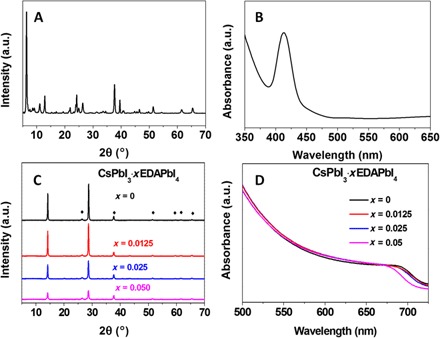
(A) XRD pattern and (B) UV-vis spectrum of EDAPbI4 films. (C) XRD patterns and (D) UV-vis absorption spectra of CsPbI3·xEDAPbI4 (x = 0 to 0.05) perovskites.
Within the detection limit of XRD, no peak indexable to any impurity phase, such as δ-CsPbI3 or PbI2, is present. The absence of a PbI2 impurity peak further indicates that ethylenediamine dihydroiodide (EDAI2) is incorporated in the crystal structure. If we add an excess amount (x) of PbI2 without EDAI2 into the precursor, PbI2 crystallizes as a separate phase (fig. S5). The XRD data indicated that the addition of xEDAPbI4 in CsPbI3 does not form a mixture of 2D EDAPbI4 and 3D CsPbI3 perovskite. Furthermore, by increasing the content (x) of EDAPbI4 (in CsPbI3·xEDAPbI4 samples), the intensity of the XRD peaks decreases, whereas their peak width broadens, indicating the decrease of crystallite size. Such a confinement effect was further manifested by the blueshift of absorption onset (Fig. 2D), which is quite evident for the CsPbI3·0.05EDAPbI4 sample. The photoluminescence (PL) spectra of CsPbI3·xEDAPbI4 samples (fig. S6A) also exhibited blueshift when the content of EDAPbI4 is increased. Moreover, their PL lifetimes also increased with the content of EDAPbI4 (fig. S5B). Such a blueshift has been observed in CsPbI3 quantum dots synthesized using hot-injection solution-based method and other hybrid lead halide perovskite films with the incorporation of 2D perovskite (34). The blueshift in both UV-vis and PL spectra can be ascribed either to the formation of 2D/3D perovskite of CsPbI3·0.05EDAPbI4 or to the decrease in crystal size. In the plausible 2D/3D configuration, we hypothesize that the (110) layered 2D EDAPbI4 component can also function as an interface to separate small CsPbI3 crystal units, as in the regular 2D/3D perovskite. The 2D EDAPbI4 might also work as a blocking layer similar to the surfactant, which can eventually reduce the crystallite size of the CsPbI3·xEDAPbI4 perovskite structures. Previous reports have suggested that the reduced crystal size can lead to the blueshift of optical spectrum even when the crystal size is larger than the Bohr radius (35).
Furthermore, the addition of a small amount of EDAPbI4 also helps reduce the pinholes in the deposited CsPbI3 perovskite films and passivates their surface, similar to a previously reported regular 2D/3D or cross-linked 2D/3D perovskite (18, 28). Figure S6B shows that the PL lifetime of the CsPbI3·xEDAPbI4 film increases with its EDAPbI4 content, suggesting a suppression of radiation-less recombination. The scanning electron microscopy (SEM) and AFM images (Fig. 3, A and B) show that the grain size of CsPbI3·xEDAPbI4 decreases markedly from ~300 nm (x = 0) to ~35 nm (x = 0.025) with increasing EDAPbI4 content. This is consistent with the XRD peak broadening observed in Fig. 2. Note that the pinholes became much less with the addition of EDAPbI4, which is favorable for high-performance PSC fabrication.
Fig. 3. Effect of EDAPbI4 on the evolution of morphology of CsPbI3·xEDAPbI4.
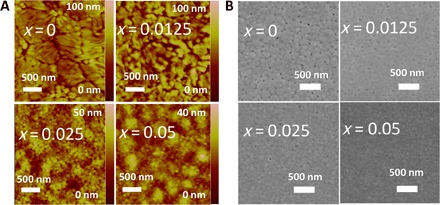
(A) AFM and (B) SEM images of perovskite films obtained from the CsPbI3·xEDAPbI4 additive precursor (x = 0, 0.0125, 0.025, and 0.05).
All these results reveal that the addition of a small content of EDAPbI4 has strongly affected the properties of CsPbI3. To rationalize this observation, we first exclude the possibility of Cs+ substitution by EDA on a single A site of CsPbI3 to form an EDAxCs1−2xPbI3 mixed-cation perovskites on the basis of the large size of EDA (r = 0.31 nm). Moreover, replacing Cs+ by larger cations, such as EDA, should narrow the band gap and induce an XRD peak shift, although we observe a widening of the band gap and no shift in the XRD peak position.
We adopted a planar configuration to fabricate PSCs based on these CsPbI3·xEDAPbI4 perovskites. The photovoltaic parameters extracted from the J-V curves (Fig. 4A) are listed in Table 1. We found that CsPbI3·xEDAPbI4 (x = 0.0125 to 0.05) devices show a much better performance than those based on the pure CsPbI3. The enhancement in all the photovoltaic parameters, that is, JSC, VOC, and FF, can be attributed to less pinholes, desired charge-carrier dynamics, and surface passivation by EDAPbI4. For x ≤ 0.025, the transient photovoltage decay curves (fig. S7) exhibit monotonic increase in the lifetime with the EDAPbI4 incorporation, which is consistent with the PL decay dynamics (fig. S6B). Furthermore, the 2D EDAPbI4 in these CsPbI3·xEDAPbI4 (x = 0.0125 to 0.05) compositions seem to have less impact on the charge transfer because this can be significantly hindered in regular 2D/3D PSCs (18). The best performing cell used the CsPbI3·0.025EDAPbI4 perovskite formulation showing a remarkable PCE of 11.8% under reverse scan. To the best of our knowledge, this is a record for CsPbI3-based PSCs. Hysteresis between different scan directions is also found in our planar cell structure (fig. S8), and the scan rate–independent maximum power tracking (Fig. 4B) indicates an efficiency of 10.5%. The integrated JSC obtained from the incident photon-to-electron conversion efficiency (IPCE) is consistent with the values extracted from the J-V curves. Note that the IPCE value of the champion cell has reached 86% over a wide wavelength range. Device performance was also highly reproducible, as shown in Fig. 4D. The improved reproducibility demonstrated by the CsPbI3·xEDAPbI4 solar cells can be ascribed to the better control on the film formation (less pinholes) (36, 37).
Fig. 4. Device characteristics for CsPbI3·xEDAPbI4 films.
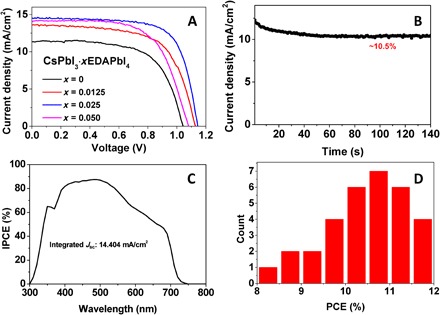
(A) Typical J-V curves of CsPbI3·xEDAPbI4 PSCs. (B) Stable current output at the maximum point of the champion CsPbI3·0.025EDAPbI4 PSC. (C) IPCE of the best solar cell based on the CsPbI3·0.025EDAPbI4 perovskite. (D) Histogram of device efficiencies of CsPbI3·0.025EDAPbI4 PSCs based on 32 cells from three batches.
Table 1. Effect of EDAPbI4 on the metrics of planar CsPbI3 PSCs (12 to 32 cells for each type).
| Precursor type | JSC (mA/cm2) | VOC (V) | FF | η (%) |
| Pure CsPbI3 | 11.33 (11.63 ± 1.55) | 1.04 (0.89 ± 0.09) | 0.65 (0.53 ± 0.06) | 7.66 (5.56 ± 1.16) |
| CsPbI3·0.0125EDAPbI4 | 13.59 (13.31 ± 0.36) | 1.13 (1.09 ± 0.06) | 0.65 (0.56 ± 0.06) | 9.98 (8.22 ± 1.46) |
| CsPbI3·0.025EDAPbI4 | 14.53 (14.05 ± 0.57) | 1.15 (1.13 ± 0.02) | 0.71 (0.64 ± 0.08) | 11.86 (10.42 ± 0.91) |
| CsPbI3·0.05EDAPbI4 | 13.97 (13.17 ± 0.88) | 1.08 (1.06 ± 0.03) | 0.65 (0.61 ± 0.02) | 9.81 (8.58 ± 0.66) |
The best solar cell based on the CsPbI3·0.025EDAPbI4 perovskite showed good stability because it retained ~10% efficiency after storing in a dark dry box for 1 month without any encapsulation (Fig. 5A). All the above results establish that the formation of CsPbI3·0.025EDAPbI4 perovskite significantly improves the performance of the devices. Besides the high efficiency, the thermal stability of CsPbI3·xEDAPbI4 films markedly improved as compared to the pure CsPbI3 (fig. S9). The α-CsPbI3 perovskite phase of the CsPbI3·0.025EDAPbI4 sample can be retained for months at room temperature (fig. S10) and after heating the CsPbI3·0.025EDAPbI4 film at 100°C for 1 week, as shown in Fig. 5B.
Fig. 5. Stability test of CsPbI3·0.025EDAPbI4-based devices and films.
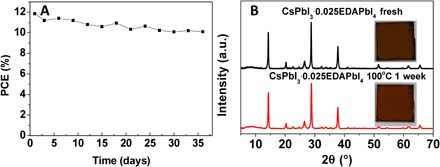
(A) PCE of the champion PSC fabricated from CsPbI3·0.025EDAPbI4 as a function of storage time in a dark dry box. (B) XRD pattern and images of the CsPbI3·0.025EDAPbI4 film heated at 100°C in a dry box for 1 week.
DISCUSSION
All of the above results demonstrate the superior photovoltaic performance of CsPbI3·xEDAPbI4 over pure α-CsPbI3–based PSCs. It appears that the hypothesized EDAPbI4 2D perovskite component not only stabilizes the α-CsPbI3 perovskite phase but also reduces the deterioration of charge-carrier transport across the perovskite film caused by the “insulating” long alkyl spacing layers (18, 38). This improvement in both efficiency and stability is closely related to this unique bifunctional cation perovskite component. We cannot obtain the efficient and stable α-CsPbI3 perovskite films if we merely add EDAI2 instead of EDAI2 + PbI2 into the CsPbI3 precursor solution. In addition, if the EDA is replaced by the monofunctional ethylamine (EA) in the regular 2D/3D CsPbI3·xEA2PbI4 samples, the perovskite phase of CsPbI3·xEA2PbI4 (x = 0.025) deteriorated as markedly as observed in the pure CsPbI3. As shown in fig. S11, the typical sample of brown CsPbI3·0.025EA2PbI4 turns into a yellow phase within a day at room temperature. In contrast, the bication EDA with CH2–CH2 can effectively assemble the CsPbI3 crystal units. Furthermore, all the CsPbI3·xEA2PbI4 perovskite film–based solar cells exhibited poor photovoltaic performance, and the best J-V curve obtained from the PSCs based on CsPbI3·0.025EA2PbI4 is listed in fig. S12. The efficiency is only ~4.4%, which is way too low than that of CsPbI3 PSCs. Such low stability could be due to either the weak steric effect of short alkyl chain containing the EA cation compared to EDA or the lack of bication effect. Not appropriately, we used a longer-chain alkylamine, such as butylamine (BA), with an even larger size to form the CsPbI3·0.025BA2PbI4 perovskite. The CsPbI3·0.025BA2PbI4 samples show some improved stability than those of CsPbI3·0.025EA2PbI4 but still far more unstable than the CsPbI3·0.025EDAPbI4 samples (fig. S13). This result suggested that both the steric effect and bication in the 2D perovskite improve the phase stability, although the latter might be more important.
To further understand the mechanism behind the high phase stability of CsPbI3·xEDAPbI4, we used other two bications, that is, 1,4-diaminobutane (BDA2+) and 2,2′-(ethylenedioxy)bis(ethylammonium) (EDBE2+), which are similar to EDA. The BDAPbI4 perovskite has been demonstrated to be a (100) layered bication 2D perovskite, whereas the EDBEPbI4 has been shown to be a (110) layered 2D perovskite (29–31, 33). Like CsPbI3·0.025EDAPbI4, the CsPbI3·0.025BDAPbI4 samples are composed of smaller perovskite crystallites (fig. S14), exhibiting significantly enhanced thermal stability than pristine CsPbI3. However, after holding at 100°C for 3 days, the CsPbI3·0.025EDAPbI4 film turns into the yellow δ phase, suggesting that its phase stability is lower than that of CsPbI3·0.025EDAPbI4 (fig. S15A). The main difference between EDAPbI4 and BDAPbI4 is that the former is a (110) layered 2D perovskite, whereas the latter is a (100) layered 2D perovskite. It is likely that the higher phase stability of CsPbI3·0.025EDAPbI4 could be ascribed to the unique (110) layered perovskite structure of EDAPbI4. The CsPbI3·0.025EDBEPbI4 sample with the (110) layered EDBEPbI4 shows enhanced thermal stability than CsPbI3; however, as compared to CsPbI3·0.025BDAPbI4, its thermal stability is even poorer, as shown in fig. S15B. It is found that the CsPbI3·0.025EDBEPbI4 perovskite sample shows a larger crystal size than CsPbI3·0.025BDAPbI4 and CsPbI3·0.025EDAPbI4, suggesting that the lower thermal stability of CsPbI3·0.025EDBEPbI4 sample might be due to the presence of large crystallites. All these findings demonstrate that the confluence of reduced crystal size and the unique (110) layered bication 2D perovskite structure enhance the overall phase stability of α-CsPbI3. Specifically, we ascribe the enhanced phase stability of CsPbI3·xEDAPbI4 to the reduced crystallite size and the EDAPbI4 component’s unique (110) layered structure.
In summary, we report a phase-stable α-CsPbI3 film with an EDAPbI4 2D perovskite component prepared via a novel and facile single-step method under ambient conditions for high-efficiency all-inorganic PSCs. By introducing the 2D perovskite of EDAPbI4, the structurally robust α-CsPbI3 perovskite films were obtained even at temperatures several hundred°C below the phase transition point. The addition of a small amount of EDAPbI4 stabilizes the α-CsPbI3. Moreover, these perovskite films can retain the α-CsPbI3 phase even after annealing at 100°C for >150 hours and are also stable at room temperature for months. The EDAPbI4 (x = 0 to 0.05) not only enhances the phase stability of α-CsPbI3 crystallites significantly but also connects them for effective electron transfer and passivates the surface defects. Finally, a champion α-CsPbI3 PSC based on CsPbI3·0.025EDAPbI4 perovskite films showing a PCE of 11.8%, a record for all-inorganic PSCs, was realized. Therefore, the concept of using bication presents a novel and promising strategy for designing all-inorganic lead halide PSCs yielding high performance and reliability. Such a bication 2D perovskite with different oriented layer structure concepts could also be extended to balance high performance and high stability in organic-inorganic hybrid lead halide perovskites with the incorporation of a 2D component for their use in optoelectronic applications.
MATERIALS AND METHODS
Materials
EDAI2 was synthesized by reacting EDA and hydroiodic acid with a molar ratio of 1:2.2 in an ice bath for 2 hours. The precipitate was collected by rotary evaporation, washed three times with diethyl ether, and then vacuum-dried. 1,4-Diaminobutane dihydroiodide (BDAI2), 2,2′-(ethylenedioxy)bis(ethylammonium) dihydroiodide (EDBEI2), ethylammonium iodide (EAI), and butylammonium iodide (BAI) were synthesized following the similar procedure. The PbI2·xHI sample was synthesized as follows: 1 M PbI2 in DMF solution was reacted with 1.5 molar ratio of hydroiodic acid for 1 hour, followed by rotary evaporation, washed three times with diethyl ether, and then vacuum-dried; the concentration of PbI2 in the final product should be within the range of 62 to 65 weight %. All the other materials were purchased from Sigma-Aldrich and used as received without any purification.
The CsPbI3·xEDAPbI4 precursor solution was prepared by dissolving 1 mmol of PbI2·xHI and 1 mmol of CsI (1 mM) in 2 ml of DMF to form a 0.5 M precursor solution mixed with different x ratios of 0.5 M EDAPbI4 solution. The 0.5 M EDAPbI4 solution was obtained by dissolving 0.5 mmol of EDAI2 and 0.5 mmol of PbI2 into 1 ml of DMF.
Device fabrication
A 20-nm-thick compact TiO2 layer was first deposited on the patterned fluorine-doped tin oxide using 0.2 M Ti(IV) bis(ethyl acetoacetato)diisopropoxide in 1-butanol solution at 450°C, followed by annealing at 450°C for 1 hour. The CsPbI3·xEDAI2 precursor solutions were then spin-coated onto the prewarmed c-TiO2–coated substrate (50°C) at 3500 rpm for 30 s, followed by annealing at 150°C for 2 min. After the films were cooled down to room temperature, a layer of hole transport material of 0.1 M spiro-MeOTAD, 0.035 M bis(trifluoromethane)sulfonimide lithium salt, and 0.12 M 4-tert-butylpyridine in chlorobenzene/acetonitrile (10:1, v/v) solution was spin-coated at 4000 rpm for 20 s. Finally, a 100-nm-thick Ag contact layer was thermally evaporated as back contact.
Characterization
The crystal structures of the CsPbI3·xEDAPbI4 films were examined by a Shimadzu XRD-6100 diffractometer with Cu Kα radiation. The morphologies of the CsPbI3·xEDAPbI4 films were observed by a JSM-7800F Prime SEM and a Bruker Multimode Nanoscope IIIa AFM. The absorption spectra of the EDAPbI4 and CsPbI3·xEDAPbI4 perovskite films were taken on a Cary 60 UV-vis spectrophotometer. XPS spectra were acquired with a Kratos Axis Ultra DLD spectrometer (Kratos Analytical-A Shimadzu Group Company) using a monochromatic Al K source (1486.6 eV). Time-integrated PL and time-resolved PL experiments were performed by exciting the samples deposited onto a nonconducting glass using the second harmonic of a picosecond mode–locked Ti-sapphire laser (80.5 MHz) at 420 nm under ambient conditions. The average power was kept around 0.05 μJ/cm2 per pulse. Using a 32-cm focal length monochromator equipped with a charge-coupled device, which has a spectral resolution of >1 meV, and a streak camera with a temporal resolution of >2 ps, the PL data were spectrally and temporally analyzed. The J-V curves of the PSCs were measured by a Keithley 2401 SourceMeter under simulated air mass 1.5-global illumination with a scan rate of 0.05 V/S (100 mW/cm2) (Enlitech SS-F5-3A Class AAA Solar Simulator; the light intensity was calibrated by a stand Si cell before test), equipped with a nonreflective metal mask with an aperture area of 0.12 cm2; the IPCE was measured on a QE-3011 system from Enlitech. All the J-V and IPCE tests were processed in atmosphere with a relative humidity of 30 to 45%.
Supplementary Material
Acknowledgments
We thank G. Jacopin for his help with the PL analysis. Funding: Y.Z. acknowledges the support of the National Natural Science Foundation of China (grant 51372151) and a Huoyingdong grant (151046). M.I.D. and M.G. acknowledge the King Abdulaziz City for Science and Technology and funding from the European Union’s Horizon 2020 Programme, through a Future and Emerging Technologies (FET) Open research and innovation action under grant agreement no. 687008. Author contributions: Y.Z. and M.G. designed and directed the research. T.Z., M.I.D., G.L., N.G., and F.X. fabricated and characterized the perovskite thin films and devices. Y.Z., T.Z., M.I.D., and M.G. wrote the manuscript, with inputs from all authors. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or in the Supplementary Materials. Additional data are available from Y.Z. (yixin.zhao@sjtu.edu.cn) upon request.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/9/e1700841/DC1
fig. S1. Comparative analysis of crystal structures of PbI2·xHI and HPbI3.
fig. S2. Morphology of EDAPbI4 films.
fig. S3. Schematic structure of (110) layered 2D films.
fig. S4. The organic compositions of CsPbI3·xEDAPbI4 films.
fig. S5. Characterization of CsPbI3 + 0.05PbI2 with or without EDAI2.
fig. S6. Effect of EDAPbI4 on the optical properties.
fig. S7. Effect of EDAPbI4 on the transient photovoltage behavior.
fig. S8. Hysteresis behavior of CsPbI3·0.025EDAPbI4-based device.
fig. S9. Effect of EDAPbI4 on the phase stability of CsPbI3·xEDAPbI4 perovskite films.
fig. S10. Phase stability of CsPbI3·0.025EDAPbI4 perovskite film under room temperature.
fig. S11. Phase stability of CsPbI3·0.025EA2PbI4-based films.
fig. S12. Device performance of CsPbI3·0.025EA2PbI4-based solar cell.
fig. S13. Phase stability of CsPbI3·0.025BA2PbI4-based films.
fig. S14. Effect of CsPbI3·0.025BDAPbI4 and CsPbI3·0.025EDBEPbI4 2D perovskite component on the evolution of morphology.
fig. S15. Phase stability of CsPbI3·0.025BDAPbI4 and CsPbI3·0.025EDBEPbI4 films.
REFERENCES AND NOTES
- 1.Park N.-G., Grätzel M., Miyasaka T., Zhu K., Emery K., Towards stable and commercially available perovskite solar cells. Nat. Energy 1, 16152 (2016). [Google Scholar]
- 2.Zhao Y., Zhu K., Organic–inorganic hybrid lead halide perovskites for optoelectronic and electronic applications. Chem. Soc. Rev. 45, 655–689 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Kojima A., Teshima K., Shirai Y., Miyasaka T., Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Kim H.-S., Lee C.-R., Im J.-H., Lee K.-B., Moehl T., Marchioro A., Moon S.-J., Humphry-Baker R., Yum J.-H., Moser J. E., Grätzel M., Park N.-G., Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2, 591 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee M. M., Teuscher J., Miyasaka T., Murakami T. N., Snaith H. J., Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 338, 643–647 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Saliba M., Matsui T., Domanski K., Seo J.-Y., Ummadisingu A., Zakeeruddin S. M., Correa-Baena J.-P., Tress W. R., Abate A., Hagfeldt A., Grätzel M., Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 354, 206–209 (2016). [DOI] [PubMed] [Google Scholar]
- 7.McMeekin D. P., Sadoughi G., Rehman W., Eperon G. E., Saliba M., Hörantner M. T., Haghighirad A., Sakai N., Korte L., Rech B., Johnston M. B., Herz L. M., Snaith H. J., A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 351, 151–155 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Saliba M., Matsui T., Seo J.-Y., Domanski K., Correa-Baena J.-P., Nazeeruddin M. K., Zakeeruddin S. M., Tress W., Abate A., Hagfeldt A., Grätzel M., Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 9, 1989–1997 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi C., Luo J., Meloni S., Boziki A., Ashari-Astani N., Grätzel C., Zakeeruddin S. M., Rӧthlisberger U., Grätzel M., Entropic stabilization of mixed A-cation ABX3 metal halide perovskites for high performance perovskite solar cells. Energy Environ. Sci. 9, 656–662 (2016). [Google Scholar]
- 10.Choi H., Jeong J., Kim H.-B., Kim S., Walker B., Kim G.-H., Young Kim J., Cesium-doped methylammonium lead iodide perovskite light absorber for hybrid solar cells. Nano Energy 7, 80–85 (2014). [Google Scholar]
- 11.Lee J.-W., Kim D.-H., Kim H.-S., Seo S.-W., Cho S. M., Park N.-G., Formamidinium and cesium hybridization for photo- and moisture-stable perovskite solar cell. Adv. Energy Mater. 5, 1501310 (2015). [Google Scholar]
- 12.Li Z., Yang M., Park J.-S., Wei S.-H., Berry J. J., Zhu K., Stabilizing perovskite structures by tuning tolerance factor: Formation of formamidinium and cesium lead iodide solid-state alloys. Chem. Mater. 28, 284–292 (2016). [Google Scholar]
- 13.Eperon G. E., Paterno G. M., Sutton R. J., Zampetti A., Haghighirad A.-A., Cacialli F., Snaith H. J., Inorganic caesium lead iodide perovskite solar cells. J. Mater. Chem. A 3, 19688–19695 (2015). [Google Scholar]
- 14.Protesescu L., Yakunin S., Bodnarchuk M. I., Krieg F., Caputo R., Hendon C. H., Yang R. X., Walsh A., Kovalenko M. V., Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 15, 3692–3696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burda C., Chen X., Narayanan R., El-Sayed M. A., Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 105, 1025–1102 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Fu Y., Wu T., Wang J., Zhai J., Shearer M. J., Zhao Y., Hamers R. J., Kan E., Deng K., Zhu X.-Y., Jin S., Stabilization of the metastable lead iodide perovskite phase via surface functionalization. Nano Lett. 17, 4405–4414 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Swarnkar A., Marshall A. R., Sanehira E. M., Chernomordik B. D., Moore D. T., Christians J. A., Chakrabarti T., Luther J. M., Quantum dot–induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 354, 92–95 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Tsai H., Nie W., Blancon J.-C., Stoumpos C. C., Asadpour R., Harutyunyan B., Neukirch A. J., Verduzco R., Crochet J. J., Tretiak S., Pedesseau L., Even J., Alam M. A., Gupta G., Lou J., Ajayan P. M., Bedzyk M. J., Kanatzidis M. G., Mohite A. D., High-efficiency two-dimensional Ruddlesden–Popper perovskite solar cells. Nature 536, 312–316 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Wang N., Cheng L., Ge R., Zhang S., Miao Y., Zou W., Yi C., Sun Y., Cao Y., Yang R., Wei Y., Guo Q., Ke Y., Yu M., Jin Y., Liu Y., Ding Q., Di D., Yang L., Xing G., Tian H., Jin C., Gao F., Friend R. H., Wang J., Huang W., Perovskite light-emitting diodes based on solution-processed self-organized multiple quantum wells. Nat. Photonics 10, 699–704 (2016). [Google Scholar]
- 20.Stoumpos C. C., Malliakas C. D., Kanatzidis M. G., Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 52, 9019–9038 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Wang F., Yu H., Xu H., Zhao N., HPbI3: A new precursor compound for highly efficient solution-processed perovskite solar cells. Adv. Funct. Mater. 25, 1120–1126 (2015). [Google Scholar]
- 22.Eperon G. E., Stranks S. D., Menelaou C., Johnston M. B., Herz L. M., Snaith H. J., Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 7, 982–988 (2014). [Google Scholar]
- 23.Ripolles T. S., Nishinaka K., Ogomi Y., Miyata Y., Hayase S., Efficiency enhancement by changing perovskite crystal phase and adding a charge extraction interlayer in organic amine free-perovskite solar cells based on cesium. Sol. Energy Mater. Sol. Cells 144, 532–536 (2016). [Google Scholar]
- 24.Li C., Evangelista F. A., Towards numerically robust multireference theories: The driven similarity renormalization group truncated to one- and two-body operators. J. Chem. Phys. 144, 164114 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Arend H., Huber W., Mischgofsky F. H., Richter-Van Leeuwen G. K., Layer perovskites of the (CnH2n+1NH3)2MX4 and NH3(CH2)mNH3MX4 families with M = Cd, Cu, Fe, Mn or Pd and X = Cl or Br: Importance, solubilities and simple growth techniques. J. Cryst. Growth 43, 213–223 (1978). [Google Scholar]
- 26.Skaarup S., Berg R. W., Structural properties and vibrational spectra of the ethylene-diammonium family of perovskite layer-type crystals: [NH3CH2CH2NH3] [MCl4], M = Ni, Pd, Cu, Cd, Mn. J. Solid State Chem. 26, 59–67 (1978). [Google Scholar]
- 27.Cheng Z., Lin J., Layered organic–inorganic hybrid perovskites: Structure, optical properties, film preparation, patterning and templating engineering. CrstEngComm 12, 2646–2662 (2010). [Google Scholar]
- 28.Li G., Zhang T., Guo N., Xu F., Qian X., Zhao Y., Ion-exchange-induced 2D–3D conversion of HMA1−xFAxPbI3Cl perovskite into a high-quality MA1−xFAxPbI3 perovskite. Angew. Chem. Int. Ed. 55, 13460–13464 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Cortecchia D., Neutzner S., Srimath Kandada A. R., Mosconi E., Meggiolaro D., De Angelis F., Soci C., Petrozza A., Broadband emission in two-dimensional hybrid perovskites: The role of structural deformation. J. Am. Chem. Soc. 139, 39–42 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Dohner E. R., Hoke E. T., Karunadasa H. I., Self-assembly of broadband white-light emitters. J. Am. Chem. Soc. 136, 1718–1721 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Dohner E. R., Jaffe A., Bradshaw L. R., Karunadasa H. I., Intrinsic white-light emission from layered hybrid perovskites. J. Am. Chem. Soc. 136, 13154–13157 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Hu T., Smith M. D., Dohner E. R., Sher M.-J., Wu X., Trinh M. T., Fisher A., Corbett J., Zhu X.-Y., Karunadasa H. I., Lindenberg A. M., Mechanism for broadband white-light emission from two-dimensional (110) hybrid perovskites. J. Phys. Chem. Lett. 7, 2258–2263 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Safdari M., Svensson P. H., Hoang M. T., Oh I., Kloo L., Gardner J. M., Layered 2D alkyldiammonium lead iodide perovskites: Synthesis, characterization, and use in solar cells. J. Mater. Chem. A 4, 15638–15646 (2016). [Google Scholar]
- 34.Zhang T., Xie L., Chen L., Guo N., Li G., Tian Z., Mao B., Zhao Y., In situ fabrication of highly luminescent bifunctional amino acid crosslinked 2D/3D NH3C4H9COO(CH3NH3PbBr3)n perovskite films. Adv. Funct. Mater. 27, 1603568 (2017). [Google Scholar]
- 35.D’Innocenzo V., Srimath Kandada A. R., De Bastiani M., Gandini M., Petrozza A., Tuning the light emission properties by band gap engineering in hybrid lead halide perovskite. J. Am. Chem. Soc. 136, 17730–17733 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Yang M., Zhou Y., Zeng Y., Jiang C.-S., Padture N. P., Zhu K., Square-centimeter solution-processed planar CH3NH3PbI3 perovskite solar cells with efficiency exceeding 15%. Adv. Mater. 27, 6363–6370 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Li X., Bi D., Yi C., Décoppet J.-D., Luo J., Zakeeruddin S. M., Hagfeldt A., Grätzel M., A vacuum flash–assisted solution process for high-efficiency large-area perovskite solar cells. Science 353, 58–62 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Cao D. H., Stoumpos C. C., Farha O. K., Hupp J. T., Kanatzidis M. G., 2D homologous perovskites as light-absorbing materials for solar cell applications. J. Am. Chem. Soc. 137, 7843–7850 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/9/e1700841/DC1
fig. S1. Comparative analysis of crystal structures of PbI2·xHI and HPbI3.
fig. S2. Morphology of EDAPbI4 films.
fig. S3. Schematic structure of (110) layered 2D films.
fig. S4. The organic compositions of CsPbI3·xEDAPbI4 films.
fig. S5. Characterization of CsPbI3 + 0.05PbI2 with or without EDAI2.
fig. S6. Effect of EDAPbI4 on the optical properties.
fig. S7. Effect of EDAPbI4 on the transient photovoltage behavior.
fig. S8. Hysteresis behavior of CsPbI3·0.025EDAPbI4-based device.
fig. S9. Effect of EDAPbI4 on the phase stability of CsPbI3·xEDAPbI4 perovskite films.
fig. S10. Phase stability of CsPbI3·0.025EDAPbI4 perovskite film under room temperature.
fig. S11. Phase stability of CsPbI3·0.025EA2PbI4-based films.
fig. S12. Device performance of CsPbI3·0.025EA2PbI4-based solar cell.
fig. S13. Phase stability of CsPbI3·0.025BA2PbI4-based films.
fig. S14. Effect of CsPbI3·0.025BDAPbI4 and CsPbI3·0.025EDBEPbI4 2D perovskite component on the evolution of morphology.
fig. S15. Phase stability of CsPbI3·0.025BDAPbI4 and CsPbI3·0.025EDBEPbI4 films.


