Abstract
Despite the popularity of anterior cruciate ligament (ACL) reconstruction procedures, the ideal graft for reconstruction remains a matter of controversy. The ideal graft for ACL reconstruction should have histologic and biomechanical characteristics similar to those of the native ACL; should be quickly and fully incorporated within the bony tunnels; should maintain its viscoelastic properties for a long time; should have minimal donor-site morbidity; should be of sufficient length and diameter; should have minimal adverse effects on the extensor mechanism; should have no risk of rejection or disease transmission; and should be cost-effective and readily available. Synthetic grafts are not widely accepted because of their dangerous complications. The main sources of grafts for ACL reconstruction are allografts and autografts. Each type of graft has its own relative advantages and disadvantages. Allografts are not available in every country, besides being expensive, and there are many concerns regarding disease transmission. Autografts, particularly bone–patellar tendon–bone (BPTB), and hamstring tendon grafts have been the standard for ACL reconstruction. The main advantage of autogenous BPTB grafts is the direct bone-to-bone healing in the tunnel, whereas the main disadvantages of such grafts are related to donor-site morbidity, anterior knee pain, and extensor mechanism dysfunction. The popularity of autogenous hamstring tendon grafts for ACL reconstruction is increasing, but there are still concerns regarding the slow soft tissue–to–bone healing, with delayed healing and incorporation of the graft. We describe a technique for ACL reconstruction with autogenous hamstring-bone graft, aiming to produce a type of graft that combines the main advantages of BPTB and hamstring grafts, with avoidance of the main disadvantages of these 2 most commonly used graft types in ACL reconstruction.
Anterior cruciate ligament (ACL) reconstruction is a common surgical procedure performed by orthopaedic surgeons. It is considered the sixth most common orthopaedic procedure in orthopaedic surgery, with approximately 125,000 cases performed annually in the United States.1, 2 The aim of ACL reconstruction is to restore normal knee stability especially in sports activities that require cutting and pivoting motions, as well as to protect the knee cartilage and menisci against subsequent injury and development of knee arthritis.3
Despite the popularity of the procedure, the ideal graft for reconstruction remains a matter of controversy. The ideal graft for ACL reconstruction should have histologic and biomechanical characteristics similar to those of the native ACL; should be quickly and fully incorporated within the bony tunnels; should maintain its viscoelastic properties for a long time; should have minimal donor-site morbidity; should be of sufficient length and diameter; should have minimal adverse effects on the extensor mechanism; should have no risk of rejection or disease transmission; and should be cost-effective and readily available.4, 5, 6
Many factors, such as patient age, patient activity level, patient occupation, isolated versus multiligament knee instability, graft availability, patient surgical history, existing tendinopathy, and surgeon experience and preference, should be considered and discussed with the patient before ACL reconstruction.7 The use of synthetic ligament substitutes has been attempted to develop an ideal graft without donor-site morbidity, with proper mechanical strength, and without any risk of disease transmission. Unfortunately, a high rate of complications was associated with the use of these synthetic substitutes, making their use not widely accepted.8, 9
Currently, the main sources of grafts for ACL reconstruction are autografts and allografts. Bone–patellar tendon–bone (BPTB), hamstring tendon, and quadriceps tendon–bone grafts are the most common autograft choices, whereas BPTB, hamstring tendon, tibialis anterior, tibialis posterior, and Achilles tendon grafts are the most common allograft choices. Each type of graft has its own relative advantages and disadvantages.
We describe a technique for ACL reconstruction using autogenous hamstring-bone graft. This type of graft is superior to the traditional hamstring graft because the bone attached to one side of the graft will accelerate its incorporation in the bony tunnels with direct bone-to-bone healing; in addition, it avoids tunnel widening, which is a common disadvantage encountered with traditional hamstring tendon grafts.
Surgical Technique
This article describes the step-by-step autogenous hamstring-bone graft harvest and preparation for anatomic single-bundle ACL reconstruction (Video 1). A comparison between allografts and autografts is shown in Table 1. The biomechanical properties of different grafts available for ACL reconstruction are shown in Table 2. The advantages and disadvantages of different types of available autogenous grafts in ACL reconstruction are shown in Table 3. The advantages and limitations of the hamstring-bone graft technique are summarized in Table 4.
Table 1.
Comparison Between Allografts and Autografts
| Autografts | Allografts | |
|---|---|---|
| Origin | Hamstring tendon | BPTB composites |
| BPTB composites | Achilles tendon | |
| Quadriceps tendon | Tibialis anterior | |
| Tibialis posterior | ||
| Fascia lata | ||
| Hamstring | ||
| Advantages | Heal more quickly with long-term viability | No donor-site morbidity |
| Not involved in disease transmission or initiation of host's immune reaction | Less postoperative and long-term pain | |
| Inexpensive | Decreased operative time | |
| No special instrumentation for preservation | Better cosmetic appearance | |
| Lower failure rate | No functional impairment | |
| Lower infection rate | Large variety of graft sizes and shapes | |
| Fewer ethical and religious concerns | ||
| Disadvantages | Limited availability | Expensive |
| Increased operative time | Disease transmission | |
| Donor-site morbidity | Healing concerns | |
| Functional impairment (e.g. muscle weakness) | Unclear long-term viability | |
| Concerns about immune response and rejection | ||
| Lack of availability | ||
| Ethical and religious concerns |
BPTB, bone–patellar tendon–bone.
Table 2.
Biomechanical Properties of Different Grafts Available for ACL Reconstruction
| Graft | Ultimate Tensile Load, N | Stiffness, N/mm | Cross-Sectional Area, mm2 |
|---|---|---|---|
| Intact ACL10 | 2,160 | 242 | 44 |
| BPTB (10 mm) autograft and allograft11 | 2,977 | 455 (autograft) 620 (allograft) |
32 (autograft) 35 (allograft) |
| Quadrupled hamstring autograft and allograft12 | 4,090 | 776 | 53 |
| Quadriceps tendon (10-mm) autograft13 | 2,174 | 463 | 62 |
| Achilles tendon14 | 4,617 | 685 | 67 |
| Tibialis anterior allograft14 | 4,122 | 460 | 48 |
| Tibialis posterior allograft14 | 3,594 | 379 | 44 |
ACL, anterior cruciate ligament; BPTB, bone–patellar tendon–bone.
Table 3.
Advantages and Disadvantages of Different Types of Available Autogenous Grafts in ACL Reconstruction
| Advantages | Disadvantages | |
|---|---|---|
| BPTB | Structural similarity to ACL | Anterior knee pain |
| Most physiological reconstruction because of natural insertion site of tendon preserved on bone plug | Patellar fracture | |
| Bone-to-bone healing with secure fixation | Patellar tendon tendinopathy and rupture | |
| Allows for early vigorous rehabilitation | Increased joint stiffness | |
| Less stretching | Weakness of quadriceps | |
| Proper ultimate strength and stiffness | Higher incidence of thigh muscle atrophy | |
| Reduced rate of rerupture Lower incidence of tunnel widening |
More technical challenges for surgeon such as graft-tunnel mismatch | |
| Hamstring tendon | Less postoperative pain | Slower soft tissue–graft tunnel healing capacity |
| Less quadriceps muscle weakness Less frequent patellar tendon rupture or patellar fracture |
Potential for tunnel widening, graft laxity, and less secure fixation to bone | |
| Less thigh muscle atrophy | ||
| High load to failure | ||
| Greater cross-sectional area | ||
| Easier passage | ||
| Small harvest incision | ||
| Less difficult graft preparation | ||
| Replicates nonisometric behavior of intact ACL | ||
| No need for aggressive postoperative rehabilitation | ||
| Hamstring tendon–bone graft | Same as traditional hamstring tendon plus bone-to-bone healing on one side of graft | Does not afford advantage of bone-to-bone healing on both sides of graft |
| Quadriceps tendon–bone graft | Large cross-sectional area Allows for bone-to-bone healing Ultimate tensile load similar to that of BPTB graft |
Potential morbidity of disrupting extensor mechanism Cosmetically less pleasant Does not afford advantage of bone-to-bone healing on both sides of graft |
ACL, anterior cruciate ligament; BPTB, bone–patellar tendon–bone.
Table 4.
Advantages and Limitations of Hamstring-Bone Graft
| Advantages | Limitations |
|---|---|
| The technique is easy and reproducible. | Only an open-type stripper is suitable for this technique. |
| No special instruments are needed. No additional operative steps are needed, so the operative time is not prolonged. No special precautions are needed postoperatively. There is low donor-site morbidity in comparison with BPTB graft. The technique allows faster and stronger (bone-to-bone) healing in comparison with the traditional hamstring tendon graft with slower (soft tissue to bone) healing. The graft is biomechanically strong: The tripled 6-strand preparation of the graft produces a strong graft with a large cross-sectional area and a graft diameter >9 mm. |
The technique is not suitable for skeletally immature patients for fear of development of a cross bar at the physis. This is a limitation of all types of bone-tendon graft preparations, and it is not specific for this type of graft preparation. The technique does not afford the advantage of bone-to-bone healing on both sides of the graft. This limitation can be overcome in the preparation of the graft in a future work. Perfect tubularization of the graft on the bone shell side can be refined in a future work. |
| The technique is more biological given that the natural continuity between the bone shell and the tendons is preserved. | |
| The technique is cost-effective. | |
| In contrast to the single-strand patellar tendon graft, the nonisometric characteristic of the native ACL is reproduced. | |
| Tibial fixation can rely only on the larger size of the graft on the bone shell side and thus can be achieved without any implants. | |
| There are no concerns regarding graft–tunnel length mismatch. |
ACL, anterior cruciate ligament; BPTB, bone–patellar tendon–bone.
Patient Position and Surgical Landmarks
After induction of anesthesia, the patient is placed in the supine position. Landmarks for arthroscopic work are drawn. The patient is examined under anesthesia. A high-thigh, nonsterile padded tourniquet is then applied. The patient is prepared and draped in the usual manner.
Graft Harvesting and Preparation
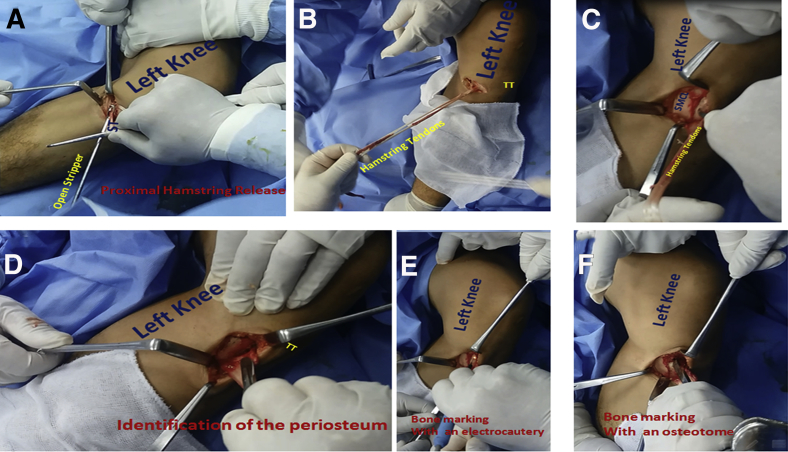
While the patient is supine, the limb is placed in the figure-of-4 position. A small incision (2-3 cm) is made at the medial aspect of the proximal tibia. The semitendinosus and gracilis tendons are identified after the sartorial fascia is incised. The 2 tendons are harvested with an open-type stripper (Arthrex, Naples, FL). The 2 tendons are released from their proximal muscular attachment, whereas their distal tibial attachment is left intact (Fig 1 A and B). The superficial medial collateral ligament is identified (Fig 1C). The periosteum at the attachment bed of the semitendinosus and gracilis tendons to the proximal tibia is also identified (Fig 1D). An electrocautery device is used to mark the bone on the proximal tibial cortex between the superficial medial collateral ligament and the periosteum of the bed of the distal tibial attachment of the hamstring tendons (Fig 1E). The site at which bone cutting will begin is marked with an osteotome (Fig 1F).
Fig 1.
Hamstring tendon harvest in a left knee while the patient is supine. (A) An open stripper is used to release the hamstring tendons (semitendinosus [ST] and gracilis) from their proximal muscular attachment. (B) The 2 hamstring tendons (semitendinosus and gracilis) are released from their proximal muscular attachment, whereas their distal tibial attachment is left intact. (TT, tibial tuberosity.) (C) The tibial cortex between the superficial medial collateral ligament (SMCL) and the bed of the hamstring attachment to the proximal tibia (forceps tip) is shown. (D) The periosteum at the bed of the hamstring tendons is shown at their distal tibial attachment (scalpel blade). (TT, tibial tuberosity.) (E) The tibial cortex is marked with an electrocautery device to define the site at which bone cutting will begin. (F) The tibial cortex is marked with an osteotome to define the site at which bone cutting will begin.
Creation of the bone shell is started by making the horizontal and vertical edges of the shell with an osteotome (Fig 2 A and B). Advancement in creating the bone shell is performed with an osteotome. The osteotome is directed from proximal to distal in line with the direction of the hamstring tendons while an assistant pulls the tendons medially and distally. The osteotome is directed away from the tibial tuberosity. At all times, the osteotome is applied tangentially to the proximal-medial tibial cortex to avoid unnecessary deepening of the bone cut (Fig 2C). With the help of a scalpel blade, the distal release of the hamstring-bone construct is finished by cutting its soft-tissue attachment to the bone while the tendons are pulled laterally by an assistant (Fig 2D).
Fig 2.
Steps for distal release of the hamstring tendons from their distal tibial attachment with a corticocancellous shell of bone from the left leg. The patient is supine. (A) An osteotome is used to cut the horizontal part of the bone shell at the previously determined markings; the hamstring tendons are pulled medially and distally by an assistant. (G, gracilis; ST, semitendinosus; TT, tibial tuberosity.) (B) An osteotome is used to cut the vertical part of the bone shell. The hamstring tendons are pulled medially and distally by an assistant. (G, gracilis; ST, semitendinosus.) (C) An osteotome is used to advance cutting and creation of the bone shell from the proximal-medial tibial cortex. Bone cutting is performed from proximal to distal in line with the direction of the hamstring tendons away from the tibial tuberosity (TT). (G, gracilis; ST, semitendinosus.) (D) The osteotome is applied tangentially to the proximal-medial tibial cortex to avoid unnecessary deepening of the bone cut. With the help of a scalpel blade, distal release of the hamstring-bone construct is finished by cutting its soft-tissue attachment to the bone while the tendons are pulled laterally by an assistant. (TT, tibial tuberosity.)
The muscle fibers are removed from the tendons (Fig 3A). The length of the tendons and dimensions of the bone shell are determined (Fig 3 B-D). The 2 hamstring tendons (semitendinosus and gracilis) are connected at different sites with No. 1 Vicryl stitches (Ethicon, Somerville, NJ) (Fig 4A). The edges of the bone shell are trimmed (Fig 4B).
Fig 3.
Clearing and measuring of harvested hamstring tendons. (A) The harvested hamstring tendons (semitendinosus and gracilis) are cleared from any muscle fibers. The arrow points to the bone shell that is taken from the proximal tibia in continuity with the hamstring tendons, with preservation of the natural attachment between the tendons and bone. (B) The length of the harvested hamstring tendons (semitendinosus and gracilis) is measured. (C, D) The dimensions of the corticocancellous bone shell are measured.
Fig 4.
Initials steps of graft preparation. (A) The 2 hamstring tendons (semitendinosus and gracilis) are connected with Vicryl stitches at different sites. (B) The edges of the bone shell are trimmed with scissors.
A K-wire is used to drill multiple holes in the bone shell (Fig 5A). Ethibond (Ethicon) strands are passed through these holes to stabilize the bone shell to the tendons and periosteum. In addition, the Ethibond strands facilitate manipulation of the bone shell while preparing the graft (Fig 5 B and C).
Fig 5.
Additional steps of graft preparation. (A) A K-wire is used to drill multiple holes in the bone shell for passage of Ethibond strands into the bone shell. The Ethibond strands are passed to well fix the bone shell to the tendons and periosteum, as well as to manipulate the bone shell while the graft is being stitched. (B) Passage of Ethibond strands for stabilization of bone shell to tendons and periosteum. (C) Stabilization of bone shell to tendons and periosteum at different points.
The hamstring tendons are tripled to obtain a 6-strand hamstring-bone construct. The bone shell is positioned in the graft construct in a way that allows for exposure of most of its cancellous surface to the tubular tunnel walls to enhance bone-to-bone healing (Fig 6A). The 6 strands are connected with running sutures with No. 1 Vicryl. The graft diameter is measured. The bone shell is then broken while maintaining its natural connection with the soft tissue. Breaking the bone shell facilitates passage of the graft into the tubular tunnel and increases the contact surface area between the cancellous face of the bone shell and the tubular walls of the bony tunnel (Fig 6B). The diameter of the graft on the bone shell side is then determined.
Fig 6.
Final steps of graft preparation. (A) Tripled hamstring-bone graft construct. The graft is tripled to make a 6-strand graft, which in most cases is more than 9 mm in diameter. The bone shell is well localized in the graft such that its cancellous surface is exposed. (B) Tripled hamstring-bone graft construct after being stitched with running sutures. An instrument is used to break the bone shell to facilitate graft passage into the tunnel, as well as to increase the contact surface area of the bone shell to the walls of the tubular bone tunnel.
Arthroscopic ACL Reconstruction
Three arthroscopic portals are created: a high anterolateral portal, a high anteromedial portal, and an accessory anteromedial portal. Routine knee arthroscopy is performed. Any chondral or meniscal pathology is managed.
A tibial guide pin is inserted in the anatomic tibial footprint of the ACL by using a tip aimer ACL tibial guide (Acufex; Smith & Nephew, Andover, MA). The anatomic femoral ACL attachment point is determined. The femoral tunnel is created in an outside-in manner with a drill bit of the same diameter as the graft. Then, the tibial tunnel is created using a drill bit of the same diameter as the graft. The tibial tunnel is enlarged for a distance of about 15 to 20 mm with a drill bit of the same diameter as the graft on the bone shell side; this allows for press-fit fixation of the graft on the tibial side. A wire loop is used to shuttle the Ethibond traction sutures for graft passage (Fig 7A). The graft is fixed on the tibial side with the press-fit technique; bio-screws or staples can be used as another method of fixation (Fig 7B).
Fig 7.
Steps of graft passage and fixation in a left knee with the patient supine. (A) With the knee flexed 30°, a wire loop is used to shuttle the traction sutures of the hamstring graft for graft passage into the joint. (B) With the knee flexed 30°, the graft is fixed in the tibial tunnel with a bio-screw. (C) Bone wax is applied to the bed of the bone shell for hemostasis.
A drain is inserted into the joint and into the graft harvest incision for 24 hours. For hemostasis, bone wax is applied to the raw area at the bed of the bone shell (Fig 7C). The wounds are closed, and an ice bag is applied around the knee.
Discussion
Allografts have been widely used in ACL reconstruction with acceptable outcomes.15, 16 The main advantages offered by allografts are that there is no donor-site morbidity, with less subsequent postoperative and long-term pain; the operative time is decreased; a better cosmetic appearance is achieved; there is no functional impairment; and there are a large variety of graft sizes and shapes.15, 16, 17, 18 There are many disadvantages and limitations with the use of allografts, including that they are expensive; there are concerns about disease transmission (e.g. hepatitis and human immunodeficiency virus); there are healing concerns; their long-term viability is not clear; there are concerns about immune response and rejection; their availability is limited; and there are ethical and religious concerns19, 20, 21, 22, 23 (Table 1).
Autografts, particularly BPTB, hamstring tendon, and quadriceps tendon–bone grafts, have been the standard for ACL reconstruction.24 The advantages of autografts over allografts are as follows: Autografts heal more quickly with long-term viability; they are not involved in disease transmission or initiation of the host's immune reaction; they are inexpensive; they do not require special instrumentation for preservation, they have a lower failure rate; there are fewer ethical and religious concerns20, 21, 25, 26; and they have a lower infection rate.27 The main disadvantages and limitations of autografts are their limited availability and increased operative time, as well as donor-site morbidity, with functional impairment at the donor site (e.g. muscle weakness) (Table 1).
When one is deciding on the type of graft to use in ACL reconstruction, it is important to understand the biological healing of the graft. The incorporation process of the graft in the joint involves many phases.4 In the first phase the graft undergoes degeneration as the fibroblasts undergo cell death and so the graft acts as a scaffold for host cell migration. The second phase (from the third week to 3-6 months postoperatively) is revascularization of the graft and fibroblast migration into the graft. During and after vascularization of the graft, a process of ligamentization occurs as the fibroblasts lay down a matrix into the graft.28 The final phase of graft healing is the remodeling phase, during which the collagen fibrils are arranged in a more organized pattern with improvement of the graft strength.4
The biological characteristics at the graft insertion site are very important factors that can affect graft healing. There are 2 types of graft healing at the insertion site (femoral and tibial tunnels): bone-to-bone healing (a graft with a bone plug) and tendon-to-bone healing (soft-tissue grafts). It is widely believed that bone-to-bone healing occurs with creeping substitution, which is much faster, stronger, and more reliable than the spot welds of soft tissue–to–bone healing. Bone-to-bone healing of an autogenous graft occurs within 4 to 6 weeks, similar to fracture healing, whereas tendon-to-bone healing of an autogenous graft occurs at 8 to 12 weeks after surgery.4, 29, 30, 31
The magnitude of graft motion in the tunnel is inversely proportional to graft healing. Graft motion and tunnel widening are more frequently found with soft-tissue grafts.26, 32, 33, 34
The bony insertion of the native ACL into the femur and tibia is characterized by the presence of a transitional zone that is characterized by 4 layers: ligament, fibrocartilage, mineralized fibrocartilage, and bone. The collagen fibers of the ligament extend into both the fibrocartilage and mineralized fibrocartilage. The 4 tissue types at the native ACL–bone insertion exhibit an increase in stiffness from the ligament proper to bone that allows for the effective load transfer from ligament to bone, thereby minimizing stress concentration and preventing failure. This increase in stiffness is closely related to the transition in the chemical composition that is closely correlated with the concentration of calcium phosphate in tissues. The natural insertion site of the tendon preserved on the bone plug is closer in structure to the native ACL.35
From a biomechanical point of view, many studies have reported and compared the biomechanical properties of the native ACL and those of the available grafts for reconstruction (Table 2). The strength of all the available grafts is superior to that of the native ACL. All these tests were performed on the unimplanted graft, and therefore the subsequent weakening that takes place in the graft after implantation and during healing should be taken into consideration.4
For the past few decades, patellar tendon autograft has been the gold standard for ACL reconstruction for many reasons, including the following: structural similarity to the ACL, providing of the most physiological reconstruction because of the natural insertion site of the tendon being preserved on the bone plug, bone-to-bone healing with secure fixation that allows for early vigorous rehabilitation, less stretching, proper ultimate strength and stiffness of the tissues, reduced rate of rerupture, and lower incidence of tunnel widening.6, 36, 37 The disadvantages are predominantly related to the donor site and include anterior knee pain, patellar fracture, patellar tendon tendinopathy, patellar tendon rupture, increased joint stiffness, weakness of the quadriceps, and higher incidence of thigh muscle atrophy; in addition, the use of such grafts can present technical challenges for the surgeon such as graft-tunnel mismatch6, 38, 39 (Table 3).
The use of hamstring tendons for ACL reconstruction has increased in popularity, with more surgeons selecting it as their graft of choice. Hamstring tendon use has the following advantages: there is less postoperative pain, less quadriceps muscle weakness, less frequent patellar tendon rupture or patellar fracture, less thigh muscle atrophy, a high load to failure and stiffness, a greater cross-sectional area of the tendon, easier passage of the graft, and a small harvest incision; graft preparation is less difficult technically than preparation of the BPTB graft; the nonisometric behavior of the intact ACL (with its anteromedial and posterolateral bundles) is more closely replicated than with a single-stranded graft; and aggressive postoperative rehabilitation is not required after hamstring tendon harvest because the strength and function of the leg are not compromised. At 3 years after hamstring harvest for ACL reconstruction, hamstring strength has been reported to be 95% of the preoperative value.4, 6, 40, 41
Despite their increasing popularity, hamstring tendon grafts have potential limitations, such as slower soft tissue–graft tunnel healing capacity in comparison with BPTB graft, potential for tunnel widening and graft laxity, and less secure fixation to bone42, 43 (Table 3). Autogenous quadriceps-bone graft is used by some surgeons. It has the advantage of having a larger cross-sectional area than BPTB graft; it provides a large tendinous graft with a bone plug on one side of it, allowing for bone-to-bone healing; and its ultimate tensile load is similar to that of BPTB graft. The main disadvantages include that it has the potential morbidity of disrupting the extensor mechanism, it is cosmetically less pleasant, and it does not afford the advantage of bone-to-bone healing on both sides of the graft6, 44, 45 (Table 3).
Many reports regarding bone harvest from the proximal tibia have shown that it is a safe procedure with very low donor-site morbidity. The medial approach would have fewer serious structures in harm's way compared with the lateral approach.46, 47
In our technique we tried to use 6-strand hamstring tendon–bone graft for ACL reconstruction. The 6-strand hamstring graft construct enables us to have a graft diameter of more than 9 mm in all cases, which is a biomechanical advantage. The hamstring tendons are released distally from the proximal tibia with a corticocancellous bone shell, so the natural attachment of these tendons to bone is left intact. In the traditional press-fit technique for ACL reconstruction, a bone plug is placed beside or within the tendons and there is no natural continuity between the bone plug and the tendons, so the problem of soft tissue–to–bone healing still exists.
We think that the described type of graft can combine the advantages of hamstring tendon graft, with its low donor-site morbidity as compared with BPTB graft, and the advantages of BPTB graft regarding tunnel bone-to-bone healing, as well as secure graft fixation with avoidance of tunnel dilatation (Table 4). Further modification, refinement, and instrumentation for this technique are to be considered in a future work.
Acknowledgment
The authors thank the first author's parents, Rania Ali Moharam, Ahmed Assem, and Shady Assem, for their great help and support in editing this article.
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Step-by-step harvest and preparation of autogenous hamstring-bone graft for anterior cruciate ligament reconstruction in left knee. The graft is first released proximally with an open stripper. Then, the distal release of the graft is performed with an osteotome to obtain a corticocancellous shell of bone from the proximal tibial cortex in continuity with the tendons, with preservation of the natural attachment between the tendons and bone. The bone shell is trimmed. The graft is tripled to produce a 6-strand graft. The diameter of the graft is measured on both sides of the graft. The bone shell is broken while maintaining its soft-tissue attachment to facilitate graft passage and to increase the contact surface area to the walls of the tubular tunnel.
References
- 1.Irarrázaval S., Kurosaka M., Cohen M., Fu F.H. Anterior cruciate ligament reconstruction (state of the art) J ISAKOS. 2016;1:38–52. [Google Scholar]
- 2.Kim S., Bosque J., Meehan J.P., Jamali A., Marder R. Increase in outpatient knee arthroscopy in the United States: A comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93:994–1000. doi: 10.2106/JBJS.I.01618. [DOI] [PubMed] [Google Scholar]
- 3.Struewer J., Frangen T.M., Ishaque B. Knee function and prevalence of osteoarthritis after isolated anterior cruciate ligament reconstruction using bone-patellar tendon-bone graft: Long-term follow-up. Int Orthop. 2012;36:171–177. doi: 10.1007/s00264-011-1345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.West R.V., Harner C.D. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13:197–207. doi: 10.5435/00124635-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Prodromos C.C., Fu F.H., Howell S.M. Controversies in soft-tissue anterior cruciate ligament reconstruction: Grafts, bundles, tunnels, fixation, and harvest. J Am Acad Orthop Surg. 2008;16:376–384. doi: 10.5435/00124635-200807000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Reinhardt K.R., Hetsroni I.F., Marx R.G. Graft selection for anterior cruciate ligament reconstruction: A level I systematic review comparing failure rates and functional outcomes. Orthop Clin North Am. 2010;41:249–262. doi: 10.1016/j.ocl.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Mehran N., Moutzouros V., Bedi A. A review of current graft options for anterior cruciate ligament reconstruction. JBJS Rev. 2015;3:1–11. doi: 10.2106/JBJS.RVW.O.00009. [DOI] [PubMed] [Google Scholar]
- 8.Zoltan D.J., Reinecke C., Indelicato P.A. Synthetic and allograft anterior cruciate ligament reconstruction. Clin Sports Med. 1988;7:773–784. [PubMed] [Google Scholar]
- 9.Makisalo S., Skutnabb K., Holmstrom J. Reconstruction of anterior cruciate ligament with carbon fiber: An experimental study on pigs. Am J Sports Med. 1988;16:589–593. doi: 10.1177/036354658801600606. [DOI] [PubMed] [Google Scholar]
- 10.Noyes F.R., Butler D.L., Grood E.S. Biomechanical analysis of human ligament grafts used in knee ligament repairs and reconstructions. J Bone Joint Surg Am. 1984;66:344–352. [PubMed] [Google Scholar]
- 11.Cooper D.E., Deng X.H., Burstein A.L., Warren R.F. The strength of the central third patellar tendon graft. A biomechanical study. Am J Sports Med. 1993;21:818–823. doi: 10.1177/036354659302100610. [DOI] [PubMed] [Google Scholar]
- 12.Hamner D.L., Brown C.H., Jr., Steiner M.E., Hecker A.T., Hayes W.C. Hamstring tendon grafts for reconstruction of the anterior cruciate ligament: Biomechanical evaluation of the use of multiple strands and tensioning techniques. J Bone Joint Surg Am. 1999;81:549–557. doi: 10.2106/00004623-199904000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Staubli H.U., Schatzmann L., Brunner P. Mechanical tensile properties of the quadriceps tendon and patella ligament in young adults. Am J Sports Med. 1999;27:27–34. doi: 10.1177/03635465990270011301. [DOI] [PubMed] [Google Scholar]
- 14.Wren T.A., Yerby S.A., Beaupré G.S., Carter D.R. Mechanical properties of the human Achilles tendon. Clin Biomech (Bristol, Avon) 2001;16:245–251. doi: 10.1016/s0268-0033(00)00089-9. [DOI] [PubMed] [Google Scholar]
- 15.Noyes F.R., Barber S.D., Mangine R.E. Bone-patellar ligament bone and fascia lata allografts for reconstruction of the anterior ligament. J Bone Joint Surg Am. 1990;72:1125–1136. [PubMed] [Google Scholar]
- 16.Peterson R.K., Shelton W.R., Bomboy A.L. Allograft versus autograft PT anterior cruciate ligament reconstruction: A 5-year followup. Arthroscopy. 2001;17:9–13. doi: 10.1053/jars.2001.19965. [DOI] [PubMed] [Google Scholar]
- 17.Andrews M., Noyes F.R., Barber-Westin S.D. Anterior cruciate allograft reconstruction in the skeletally immature athlete. Am J Sports Med. 1994;22:48–54. doi: 10.1177/036354659402200109. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs R., Wheatley W., Uribe J.W. Intra-articular anterior cruciate ligament reconstruction using PT allograft in the skeletally immature patient. Arthroscopy. 2002;18:824–828. doi: 10.1053/jars.2002.36136. [DOI] [PubMed] [Google Scholar]
- 19.Pallis M., Svoboda S.J., Cameron K.L. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States military academy. Am J Sports Med. 2012;40:1242–1246. doi: 10.1177/0363546512443945. [DOI] [PubMed] [Google Scholar]
- 20.Barrera Oro F., Sikka R.S., Wolters B. Autograft versus allograft: An economic cost comparison of anterior cruciate ligament reconstruction. Arthroscopy. 2011;27:1219–1225. doi: 10.1016/j.arthro.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Rice R.S., Waterman B.R., Lubowitz J.H. Allograft versus autograft decision for anterior cruciate ligament reconstruction: An expected-value decision analysis evaluating hypothetical patients. Arthroscopy. 2012;28:539–547. doi: 10.1016/j.arthro.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Soon M.Y., Hassan A., Hui J.H. An analysis of soft tissue allograft anterior cruciate ligament reconstruction in a rabbit model: A short-term study of the use of mesenchymal stem cells to enhance tendon osteointegration. Am J Sports Med. 2007;35:962–971. doi: 10.1177/0363546507300057. [DOI] [PubMed] [Google Scholar]
- 23.Buck B.E., Malinin T.I., Brown M.D. Bone transplantation and human immunodeficiency virus. Clin Orthop Relat Res. 1989;240:129–136. [PubMed] [Google Scholar]
- 24.Mariscalco M.W., Magnussen R.A., Mehta D., Hewett T.E., Flanigan D.C., Kaeding C.C. Autograft versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: A systematic review. Am J Sports Med. 2014;42:492–499. doi: 10.1177/0363546513497566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aune A.K., Holm I., Risberg M.A. Four-strand HT autograft compared with PT-bone autograft for anterior cruciate ligament reconstruction: A randomized study with two-year follow-up. Am J Sports Med. 2001;29:722–728. doi: 10.1177/03635465010290060901. [DOI] [PubMed] [Google Scholar]
- 26.Gulotta L.V., Rodeo S.A. Biology of autograft and allograft healing in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26:509–524. doi: 10.1016/j.csm.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Crawford C., Kainer M., Jernigan D. Investigation of postoperative allograft-associated infections in patients who underwent musculoskeletal allograft implantation. Clin Infect Dis. 2005;41:195–200. doi: 10.1086/430911. [DOI] [PubMed] [Google Scholar]
- 28.Falconiero R.P., DiStefano V.J., Cook T.M. Revascularization and ligamentization of autogenous anterior cruciate ligament grafts in humans. Arthroscopy. 1998;14:197–205. doi: 10.1016/s0749-8063(98)70041-6. [DOI] [PubMed] [Google Scholar]
- 29.Kim S.J., Kumar P., Oh K.S. Anterior cruciate ligament reconstruction: Autogenous quadriceps tendon-bone compared with bone-patellar tendon-bone grafts at 2-year follow-up. Arthroscopy. 2009;25:137–144. doi: 10.1016/j.arthro.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Han H.S., Seong S.C., Lee S., Lee M.C. Anterior cruciate ligament reconstruction: Quadriceps versus patellar autograft. Clin Orthop Relat Res. 2008;466:198–204. doi: 10.1007/s11999-007-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park M.J., Lee M.C., Seong S.C. A comparative study of the healing of tendon autograft and tendon-bone autograft using patellar tendon in rabbits. Int Orthop. 2001;25:35–39. doi: 10.1007/s002640000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eriksson E. Vascular ingrowth into ACL grafts. Knee Surg Sports Traumatol Arthrosc. 2008;16:341. doi: 10.1007/s00167-008-0514-1. [DOI] [PubMed] [Google Scholar]
- 33.Weiler A., Hoffmann R.F., Bail H.J. Tendon healing in a bone tunnel. Part II: Histologic analysis after biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy. 2002;18:124–135. doi: 10.1053/jars.2002.30657. [DOI] [PubMed] [Google Scholar]
- 34.Nebelung W., Becker R., Urbach D. Histological findings of tendon-bone healing following anterior cruciate ligament reconstruction with hamstring grafts. Arch Orthop Trauma Surg. 2003;123:158–163. doi: 10.1007/s00402-002-0463-y. [DOI] [PubMed] [Google Scholar]
- 35.Baxter F.R., Bach S.J., Detrez F. Augmentation of bone tunnel healing in anterior cruciate ligament grafts: Application of calcium phosphates and other materials. J Tissue Eng. 2010;2010:712370. doi: 10.4061/2010/712370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartlett R.J., Clatworthy M.G., Nguyen T.N. Graft selection in reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br. 2001;83:625–634. doi: 10.1302/0301-620x.83b5.12308. [DOI] [PubMed] [Google Scholar]
- 37.Deehan D.J., Salman L.J., Webb V.J. Endoscopic reconstruction of the anterior cruciate ligament with an ipsilateral patellar tendon autograft: A prospective longitudinal five-year study. J Bone Joint Surg Br. 2000;82:984–991. doi: 10.1302/0301-620x.82b7.10573. [DOI] [PubMed] [Google Scholar]
- 38.Feller J.A., Webster K.E. A randomized comparison of patellar tendon and hamstring tendon anterior cruciate ligament reconstruction. Am J Sports Med. 2003;31:564–573. doi: 10.1177/03635465030310041501. [DOI] [PubMed] [Google Scholar]
- 39.Mohammadi F., Salavati M., Akhbari B., Mazaheri M., Mohsen Mir S., Etemadi Y. Comparison of functional outcome measures after ACL reconstruction in competitive soccer players: A randomized trial. J Bone Joint Surg Am. 2013;95:1271–1277. doi: 10.2106/JBJS.L.00724. [DOI] [PubMed] [Google Scholar]
- 40.Corry I.S., Webb J.M., Clingeleffer A.J. Arthroscopic reconstruction of the anterior cruciate ligament: A comparison of PT autograft and four-strand HT autograft. Am J Sports Med. 1999;27:444–454. doi: 10.1177/03635465990270040701. [DOI] [PubMed] [Google Scholar]
- 41.Janssen R.P., van der Wijk J., Fiedler A. Remodelling of human hamstring autografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19:1299–1306. doi: 10.1007/s00167-011-1419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bizzini M., Gorelick M., Munzinger U., Drobny T. Joint laxity and isokinetic thigh muscle strength characteristics after anterior cruciate ligament reconstruction: Bone PT bone versus quadrupled hamstring autografts. Clin J Sport Med. 2006;16:4–9. doi: 10.1097/01.jsm.0000188040.97135.43. [DOI] [PubMed] [Google Scholar]
- 43.Hollis R., West H., Greis P. Autologous bone effects on femoral tunnel widening in hamstring anterior cruciate ligament reconstruction. J Knee Surg. 2009;22:114–119. doi: 10.1055/s-0030-1247735. [DOI] [PubMed] [Google Scholar]
- 44.Bourke H.E., Gordon D.J., Salmon L.J. The outcome at 15 years of endoscopic anterior cruciate ligament reconstruction using HT autograft for ‘isolated’ anterior cruciate ligament rupture. J Bone Joint Surg Br. 2012;94:630–637. doi: 10.1302/0301-620X.94B5.28675. [DOI] [PubMed] [Google Scholar]
- 45.Schatzmann L., Brunner P., Staubli H.U. Effect of cyclic preconditioning on the tensile properties of human quadriceps tendons and patellar ligaments. Knee Surg Sports Traumatol Arthrosc. 1998;6:S56–S61. doi: 10.1007/s001670050224. [DOI] [PubMed] [Google Scholar]
- 46.Benninger B., Ross A., Delamarter T. Approaches to proximal tibial bone harvest techniques. J Oral Maxillofac Res. 2012;3:e2. doi: 10.5037/jomr.2012.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jia T.Y., Gurmeet S., Asni A., Ramanathan R. Proximal tibia bone graft: An alternative donor source especially for foot and ankle procedures. Malays Orthop J. 2015;9:14–17. doi: 10.5704/MOJ.1503.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Step-by-step harvest and preparation of autogenous hamstring-bone graft for anterior cruciate ligament reconstruction in left knee. The graft is first released proximally with an open stripper. Then, the distal release of the graft is performed with an osteotome to obtain a corticocancellous shell of bone from the proximal tibial cortex in continuity with the tendons, with preservation of the natural attachment between the tendons and bone. The bone shell is trimmed. The graft is tripled to produce a 6-strand graft. The diameter of the graft is measured on both sides of the graft. The bone shell is broken while maintaining its soft-tissue attachment to facilitate graft passage and to increase the contact surface area to the walls of the tubular tunnel.