Abstract
Lipopolysaccharides or endotoxins elicit an excessive host inflammatory response and lead to life-threatening conditions such as endotoxemia and septic shock. Lipopolysaccharides trigger mobilization and stimulation of leukocytes and exaggerated production of pro-inflammatory molecules including cytokines and proteolytic enzymes. Matrix metalloproteinase-9 (MMP-9) or gelatinase B, a protease stored in the tertiary granules of polymorphonuclear leukocytes, has been implicated in such inflammatory reactions. Moreover, several studies even pinpointed MMP-9 as a potential target molecule to counter excessive inflammation in endotoxemia. Whereas the early effect of lipopolysaccharide-induced inflammation in vivo on the expression of MMP-9 in various peripheral organs has been described, the effects on the bone marrow and during late stage endotoxemia remain elusive. We demonstrate that TIMP-free MMP-9 is a major factor in bone marrow physiology and pathology. By using a mouse model for late-stage endotoxemia, we show that lipopolysaccharides elicited a depletion of neutrophil MMP-9 in the bone marrow and a shift of MMP-9 and MMP-9-containing cells towards peripheral organs, a pattern which was primarily associated with a relocation of CD11bhighGr-1high cells. In contrast, analysis of the tissue inhibitors of metalloproteinases was in line with a natural, systematic upregulation of TIMP-1, the main tissue inhibitor of TIMP-free MMP-9, and a general shift toward control of matrix metalloproteinase activity by tissue inhibitors of metalloproteinases.
Introduction
The regulation of leukocytosis is a balance between the exit of leukocytes into the periphery and the production of these cells in the bone marrow. Within this compartment systemic cytokine levels orchestrate a regulated response to peripheral signals, leading to leukocyte expansion through the action of colony-stimulating factors. At the molecular level this is translated into oligosaccharide-lectin interactions, expression of cell adhesion molecules, balances between proteases and inhibitors, and chemokine-mediated leukocyte recruitment to specific body compartments.1 Interference at any of these levels could represent a possible treatment for acute inflammatory reactions such as shock syndromes.2
An often explored strategy is interference with balances between proteases and inhibitors, for example, matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs).3,4 MMPs are named after their ability to degrade extracellular matrix proteins, namely gelatinases (MMP-2/9), collagenases (MMP-1/8/13), stromelysins (MMP-3/10/11) and others, but are also able to degrade cell-surface and intracellular molecules.5 One particular MMP implicated in cell mobility in and out of the bone marrow is MMP-9 or gelatinase B.5 MMP-9 is primarily produced by neutrophils, which pre-store large quantities of proMMP-9 (zymogen) in their tertiary granules (also called gelatinase granules) for swift release upon encounter with an inflammatory stimulus.6 When released in the bone marrow, activated MMP-9 processes membrane-bound Kit-ligand into soluble Kit-ligand and thereby keeps progenitor cells locally and initiates recruitment of “escaped” hematopoietic stem cells to their proliferative niche.7 In the periphery, MMP-9 contributes to the recruitment of bone marrow-derived neutrophils to the site of an inflammatory stimulus.8 It is not, therefore, surprising that studies involving mouse models for sepsis show beneficial outcomes for MMP-9-deficient mice and/or mice treated with MMP inhibitors.9–12
In the body, MMP proteolytic activity is kept in check by natural tissue inhibitors of metalloproteinases (TIMPs). While all four TIMPs (TIMP-1 to -4) have inhibitory activity on all MMPs, TIMP-1 preferentially binds to MMP-9 and TIMP-2 to MMP-2.13 A unique feature of TIMP-2 is that it can initiate the formation of a MT1-MMP/TIMP-2/proMMP-2 cell surface complex that aids the conversion of the proMMP-2 zymogen into activated MMP-2.14,15 Similar to MMP-9, TIMP-1 is of importance in bone marrow physiology, in particular in the maintenance of hematopoietic stem cell quiescence16 and leukocytosis.17,18
Lipopolysaccharides (LPS) or endotoxins are glycolipids present in the outer membrane of Gram-negative bacteria, and are released upon lysis of bacteria. In the body, LPS are detected as an alarm signal by leukocytes, endothelial cells and parenchymal cells through their LPS-receptor complex composed of myeloid differentiation factor 2 (MD2) and toll-like receptor 4 (TLR4), a process which is facilitated by LPS-binding protein (LBP) and CD14.19 This interaction triggers cytokine production, leukocyte activation and inflammation,20 and excessive stimulation can lead to simultaneous activation of multiple parallel cascades that lead to adult respiratory distress syndrome and shock.21
In this study we used injection of LPS as an animal model to study the mechanisms behind acute inflammation (endotoxemia) caused by Gram-negative bacteria. Whereas the early effects of LPS in vivo on the expression of MMPs in various peripheral organs are known (Online Supplementary Table S1), effects on bone marrow and during late stages remain elusive. This is remarkable, given the importance of MMP-9 in leukocytosis and hematopoietic recovery.7,8 The aim of this study was to evaluate the effects of endotoxemia on bone marrow gelatinases (MMP-2 and MMP-9) and, in parallel, the effect on their natural inhibitors. With the use of real-time polymerase chain reaction (qPCR), gelatin zymography and flow cytometry analysis, we demonstrated that endotoxemia results in depletion of MMP-9 from the bone marrow. In parallel, this MMP-9 shift is complemented by systematic upregulation of TIMP-1. Finally, immunohistochemical analysis confirmed the migration of MMP-9-containing cells from the bone marrow to blood and peripheral organs.
Methods
Mouse model of endotoxemia and sample collection
Endotoxemia was induced in female 8-week old C57BL/6 mice by intraperitoneal (i.p.) injection of LPS (E. coli 0111:B4, Sigma Aldrich, L4391) at a dose of 10 mg/kg.22 Control mice were injected with an equal volume of vehicle (pyrogen-free phosphate-buffered saline, PBS; LPS level below 12.5 pg/mL). Twenty-four hours after injection, mice were sacrificed and organs were collected. Bone marrow cells were collected from femora by flushing the medullary cavity with PBS. Blood samples were collected by cardiac puncture with a heparin-coated needle and syringe, and immediately processed by centrifugation (2000 g for 10 min at 4°C). The supernatant (plasma) was collected and stored for protein analysis and the cell pellet was immediately processed for downstream analysis. Tissues were homogenized and proteins extracted with a Precellys lysing kit (Bertin Technologies), as described in the Online Supplementary Methods. All procedures were conducted in accordance with protocols approved by the local ethics committee (project number P201/2012, KU Leuven, Belgium).
Gelatin zymography
Protein levels of proMMP-9, MMP-9, multimeric MMP-9, proMMP-2 and MMP-2 were determined by gelatin zymography on affinity-purified samples, as previously described.23 and detailed in the Online Supplementary Methods. Prior to zymography analysis, the total protein content was determined using a standard Bradford assay (Bio-Rad).
RNA expression analysis
RNA was extracted and equal amounts of RNA were converted to cDNA. qPCR was performed using TaqMan® fast universal PCR master mix (Applied Biosystems), PrimeTime® predesigned qPCR assays (IDT) and a 7500 Fast Real-Time PCR System (Applied Biosystems). Details, including primer specifications, are provided in the Online Supplementary Methods. The housekeeping gene Tbp was used as a calibrator for the relative quantification of gene expression. Normalization for Tbp and calculation of the relative expression was performed using the ΔΔCT method.24
Immunohistochemistry
Femora were placed in 6% formaldehyde (1 day), decalcified in 7% formic acid (2 days) and embedded in paraffin. Paraffin-embedded bones were sliced into 5 μm sections and dried overnight at 50°C. For immunohistochemical staining the EnVisionTM FLEX kit (DAKO) was used. Goat anti-mouse MMP-9 (R&D Systems) was used as the primary antibody. A detailed protocol is provided in the Online Supplementary Methods.
Flow cytometry analysis
Spleens and bone marrow were passed through cell strainers to obtain single cell suspensions. Red blood cells of spleen, bone marrow and blood cell suspensions were lysed with 0.83% NH4Cl. Cells were incubated with Fc-receptor-blocking antibodies anti-CD16/anti-CD32 (BD Biosciences Pharmingen, San Diego, CA, USA), Zombie aqua BV510 (dead cell staining) (BioLegend, San Diego, CA, USA) and stained with anti-Gr1, anti-F4/80, anti-CD11b, anti-CD3 or anti-CD19 (eBioscience, San Diego, CA, USA). Cells were fixed and analyzed with a FACS Fortessa flow cytometer. Data were processed with the FlowJo software (Becton Dickinson Labware, Franklin Lakes, NJ, USA). A detailed protocol is provided in the Online Supplementary Methods.
Enzyme-linked immunosorbent assays and gelatin degradation assays
TIMP-1 and TIMP-2 concentrations were determined using mouse TIMP-1 (DY980) and TIMP-2 (DY6304-05) DuoSet enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems). To determine the gelatinolytic activity we used a previously described gelatin degradation assay.25 A detailed protocol is provided in the Online Supplementary Methods.
Statistical analysis
Data were analyzed using GraphPad Prism 7 software and are expressed as mean ± standard error of mean (SEM). Differences were determined using a Mann-Whitney test. Data were collected and confirmed over a course of five independent experiments with experimental groups ranging from two to six animals.
Results
The bone marrow compartment contains considerable levels of MMP-9 RNA and protein
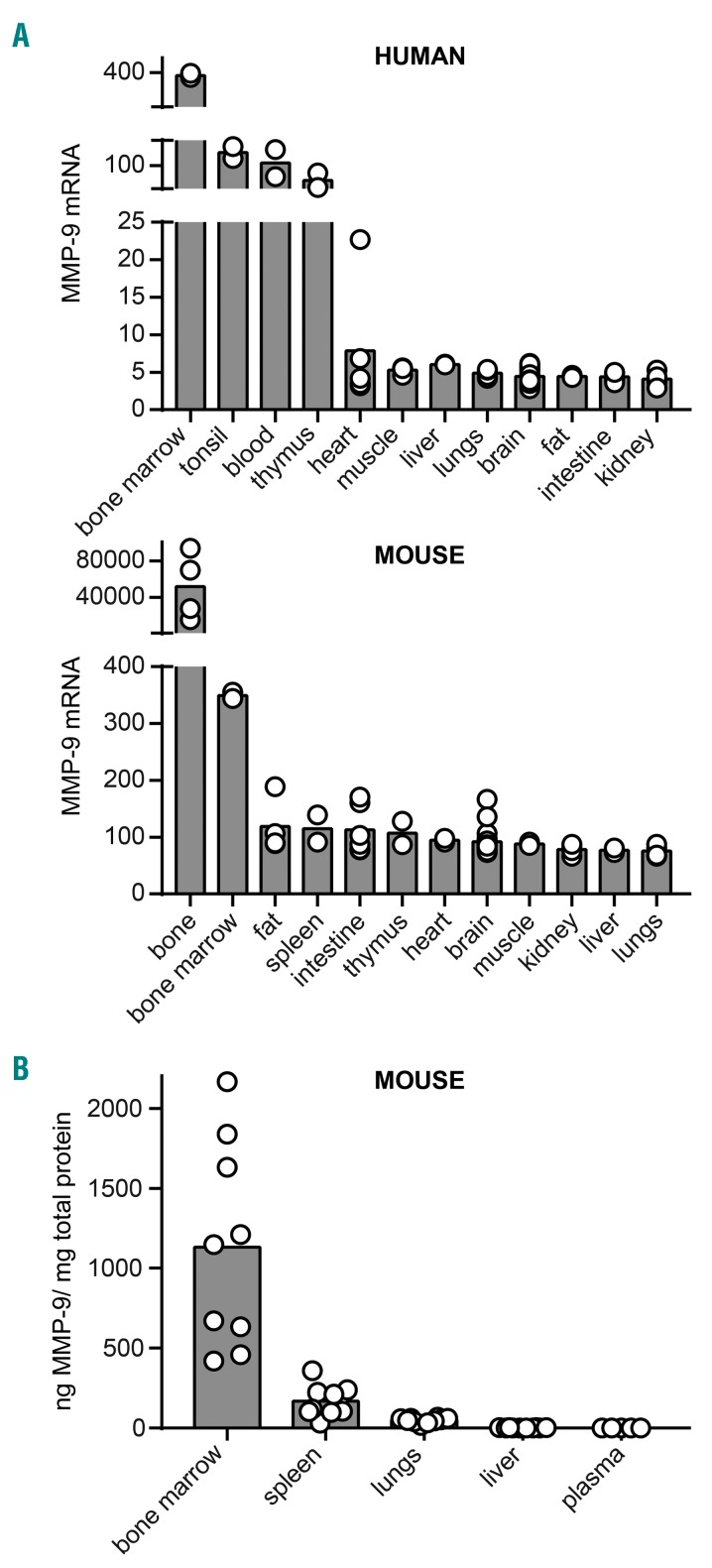
Publicly available mRNA expression data26 of human and mouse tissues reveal that gene expression levels of MMP-9 are highest in bone and bone marrow of humans and mice (Figure 1A). We, therefore, first evaluated the baseline levels of MMP-9 protein in bone marrow, spleen, lungs, liver and plasma by using gelatin zymography. The main source of MMP-9 (here a combination of high-molecular weight proMMP-9 and MMP-9) was indeed the bone marrow (Figure 1B and Online Supplementary Figure S1), with bone marrow MMP-9 representing over 0.1% of the total bone marrow protein. Mean MMP-9 protein levels in bone marrow, spleen, lungs, liver and plasma were respectively 1131±212.8 ng/mg protein, 168.4±29.8 ng/mg protein, 49.1±4.9 ng/mg protein, 2.12±0.26 ng/mg protein and 0.6±0.09 ng/mg protein (mean±SEM, n = 5–10).
Figure 1.
Organ-specific gene and protein expression of MMP-9. (A) Gene expression of MMP-9 in human and mouse tissue based on publicly available array data [human GeneAtlas U133A, gcrma and mouse GeneAtlas GNF1M, gcrma dataset26]. Array data from different locations in one organ were pooled in order to visualize the expression per organ (n = 2–28). (B) MMP-9 protein levels in C57BL/6 mice as determined by gelatin zymography (n = 5–10).
Endotoxemia results in a shift of the Mmp2/9 and Timp1/2/3 expression pattern
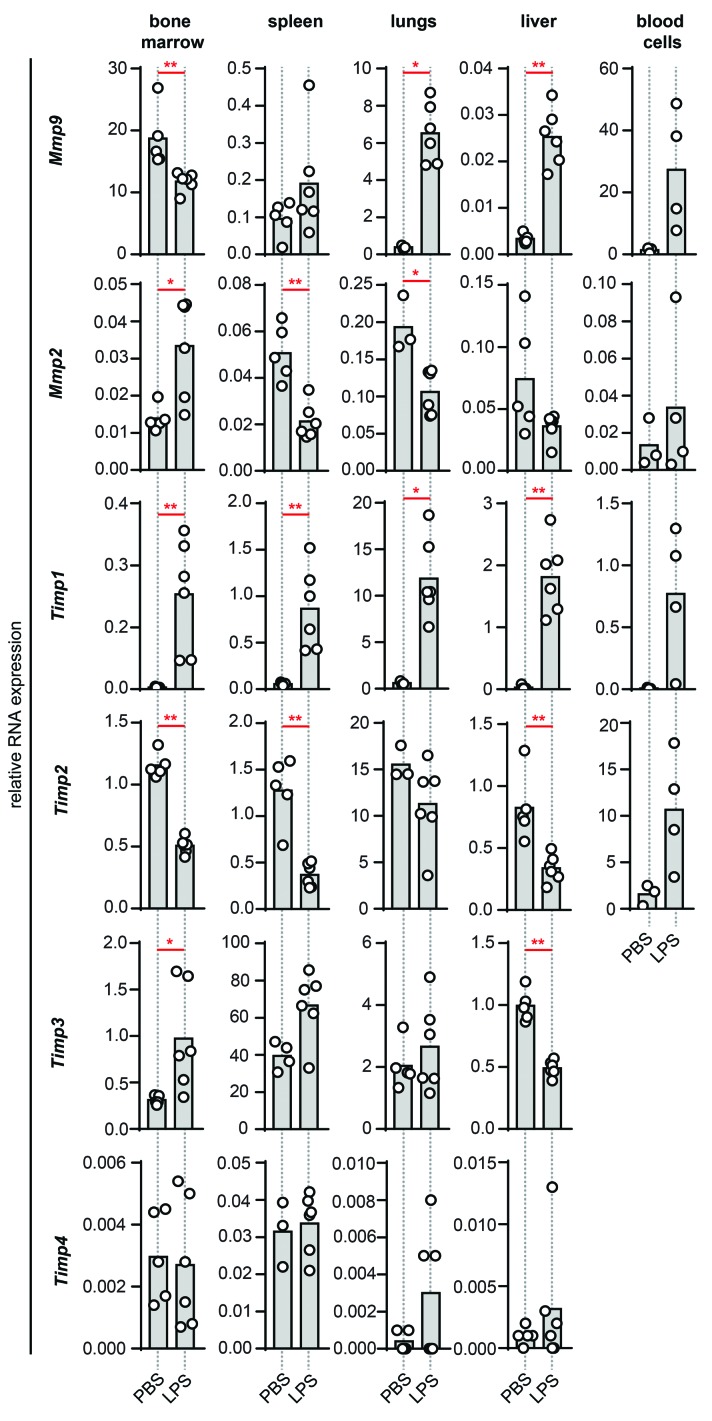
Acute inflammation was induced by i.p. injection of endotoxin. To follow hematologic changes during late stages, samples were collected from bone marrow, blood and spleen 24 h after LPS/PBS injection. Liver and lungs, two organs prone to dysfunction during endotoxemia and sepsis syndromes were also collected.27 Analysis of RNA expression of Mmp9, Mmp2, Timp1, Timp2, Timp3 and Timp4 in these tissues revealed a shift in the expression pattern of both gelatinases and TIMPs (Figure 2). In the bone marrow, the basal expression level of Mmp9 RNA was reduced by endotoxemia, while Mmp9 expression in liver and lungs increased significantly. In blood cells a trend toward increased Mmp9 expression was seen, while Mmp9 mRNA levels in the spleen remained unaltered. Interestingly, although the effect of endotoxemia on Mmp2 mRNA was less pronounced, it generally was opposing the expression pattern of Mmp9. Steady-state Mmp2 mRNA levels were increased in the bone marrow upon induction of endotoxemia, while expression in the spleen and lungs was decreased. While basal Timp1 expression was low, endotoxin triggered a considerable systematic induction. For Timp2 the opposite effect occurred, namely, a general downregulation. A significant alteration in Timp3 expression was found in bone marrow (upregulation) and liver (downregulation) while Timp4 expression remained low and unaltered. All four TIMPs are thus differently regulated in response to LPS.
Figure 2.
The effects of endotoxemia on Mmp9, Mmp2, Timp1, Timp2, Timp3 and Timp4 RNA expression. Relative RNA expression in bone marrow, spleen, lungs, liver and blood cells of LPS-treated mice in comparison with the control group given PBS. Data were normalized for the housekeeping gene Tbp. Histograms represent group medians and individual data points from single animals are shown by dots. *P<0.05, **P<0.01, as determined by the Mann-Whitney test (n = 3–6).
Endotoxemia shifts MMP-9 from bone marrow to the circulation and peripheral organs
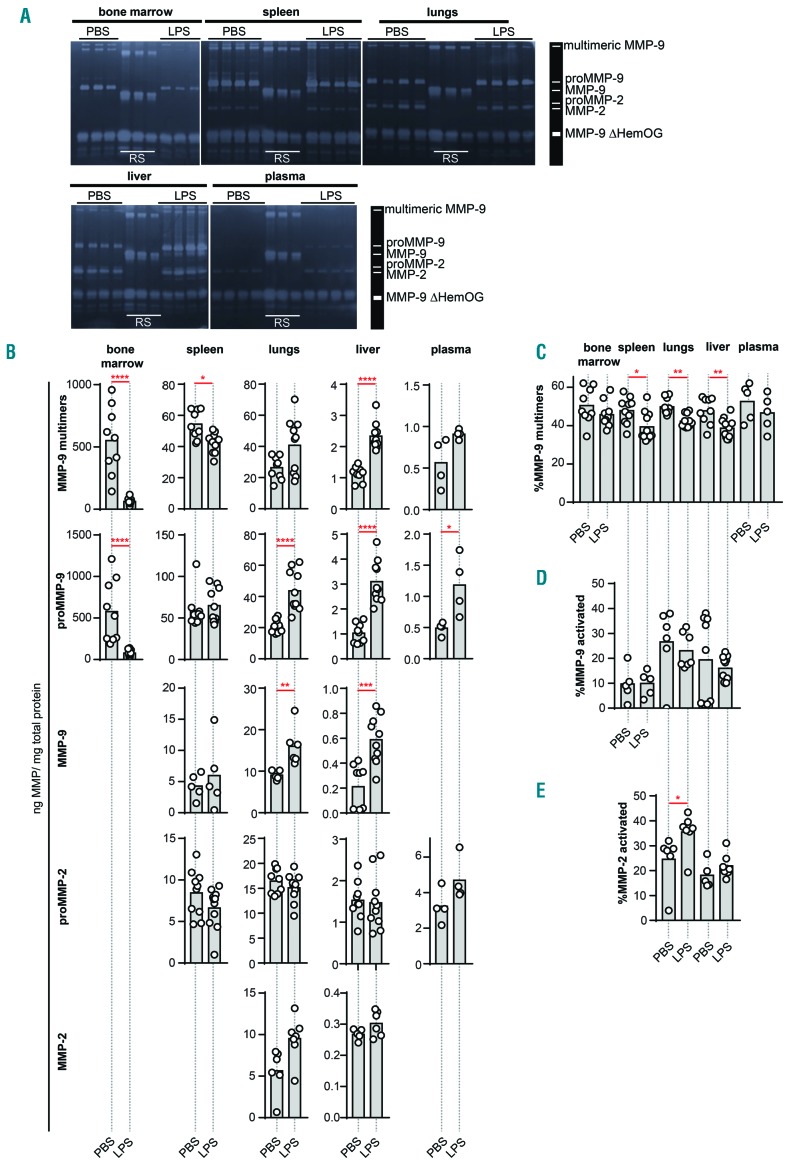
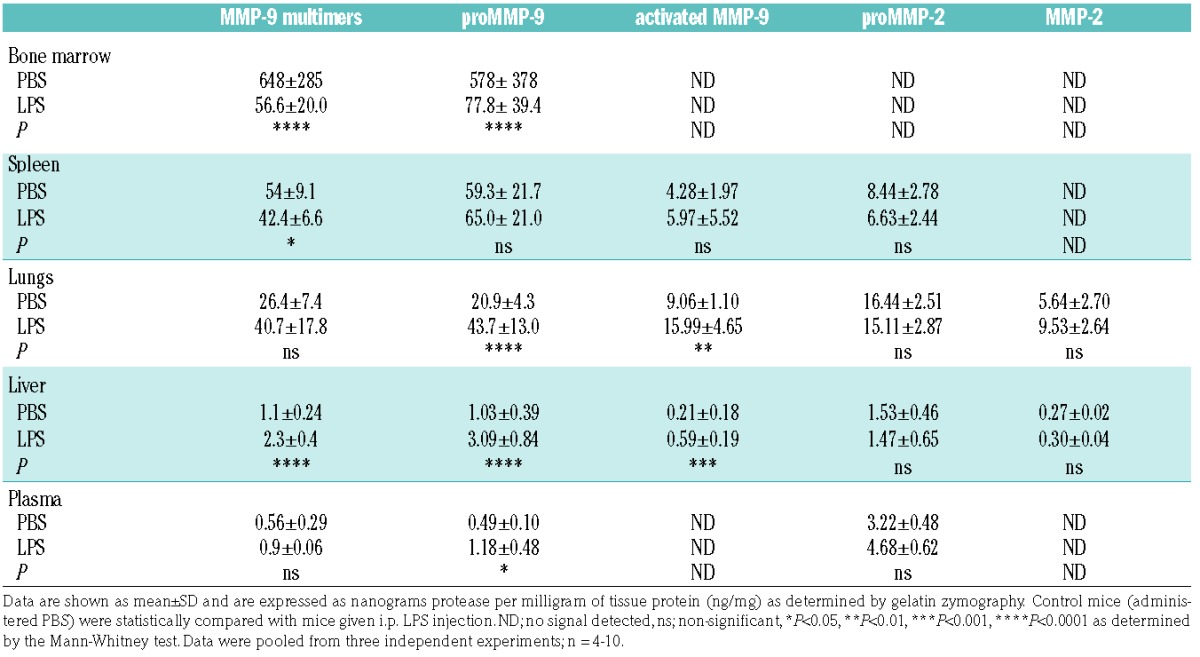
Biological samples contain complex mixtures of forms of MMP-2 and MMP-9 proteins. A first level of complexity lies in the fact that both proteases are produced as inactive pro-enzymes, proMMP-2 and proMMP-9. Activation of these pro-forms into active enzymes requires proteolytic removal of the pro-peptide by other proteases (to form MMP-2 and MMP-9) or chemical modification of the pro-peptide cysteine thereby disturbing the pro-peptide/active site interaction. These processes are fine-tuned and require a local environment favoring MMP activation.5,28,29 A second level of complexity involves the formation of high-molecular weight disulfide linked MMP-9 multimers, including MMP-9 trimers.30 Detailed analysis of these forms provides better insights into the dynamics of protease activity and can be performed using gelatin zymography analysis (Figure 3A).23 In Table 1, protein data for MMP-2 and MMP-9 forms are provided for bone marrow, liver, lungs, spleen and plasma from healthy mice and mice with endotoxemia. As expected, basal levels of all MMP-9 forms were highest in the bone marrow followed by, respectively, spleen, lungs, liver and plasma. Highest levels of (pro)MMP-2 were detected in lungs, followed by spleen and liver. MMP-2 was not detected in bone marrow, due to an overflow effect of the high quantities of MMP-9. Similarly, proteolytically activated MMP-9 could not be detected in bone marrow due to the overflow effect of proMMP-9. While proteolytic activation of MMP-9 probably occurs in this compartment, we could not detect this due to the high levels of proMMP-9 within this compartment. In plasma, MMP-2 levels were below the detection limit but proMMP-2 was detected. Upon induction of endotoxemia, drastic changes occurred in MMP-9 protein levels. The most severe changes were observed in the bone marrow, where protein levels of proMMP-9 and multimeric MMP-9 dropped by approximately 90% or, respectively, around 500 and 600 ng/mL (Table 1 and Figure 3B). This major decrease of bone marrow (pro)MMP-9 coincided with an increase of (pro)MMP-9 in liver and lungs, while less pronounced effects were observed in the spleen. Interestingly, mice with endotoxemia had a significant decrease in the percentage of multimeric MMP-9 in spleen, lungs and liver (Figure 3C). Indeed, downregulation of protein disulfide isomerase in sepsis was previously shown.31
Figure 3.
Gelatin zymography analysis of the protein levels of MMP-9 and MMP-2 forms. (A) Representative gelatin zymography gels of bone marrow, spleen, lungs, liver and plasma of mice treated with LPS versus control mice (PBS). Each lane represents the analysis of the sample from a single mouse (n = 3–4). Each sample was spiked with an internal processing and loading control (MMP-9ΔHemOG – MMP-9 form lacking the C-terminal hemopexin and O-glycosylated domain) and each gel has three lanes of recombinant MMP-9 standard protein (RS), including multimeric, monomeric and MMP-9 ΔHemOG proteins, to serve as a molecular weight marker and standard (10, 5 and 3 pg). The loading quantity of the samples corresponded to, respectively, 0.4 μg (bone marrow), 2 μg (spleen), 4 μg (lungs), 60 μg (liver) and 30 μg (plasma) of total protein. (B) Detailed analysis of protein levels of MMP-9 multimers, proMMP-9 monomers, activated MMP-9 monomers, proMMP-2 and activated MMP-2. Protein quantity was expressed as nanograms of MMP in one milligram of total protein. (C) Percentages of multimeric MMP-9 out of total MMP-9. (D) Percentages of proteolytically activated MMP-9 out of total MMP-9. (E) Percentages of proteolytically activated MMP-2. Histograms represent group medians and individual data points are shown by dots, each representing data from a single mouse. *P<0.05, **P<0.01, ***P<0.001, ***P<0.0001, as determined by the Mann-Whitney test.
Table 1.
Levels of MMP-2 and MMP-9 forms detected in bone marrow, spleen, liver, lungs and plasma.
While Mmp2 RNA expression analysis suggested an opposing effect to MMP-9, this could not be confirmed at the protein level, endorsing the existing notion that during inflammation MMP-2 is less prone to major stimulation, in comparison to the fine-tuned and controlled regulation of MMP-9.32 Nevertheless, one should take into account that non-secreted MMP-2 variants exist, which might shift the balance between intracellular and secreted MMP-2.33
Interestingly, although the total (pro)MMP-2 content remained similar, the percentage of proteolytically activated MMP-2 was increased in the lungs of endotoxemic mice (Figure 3B,E). However, one needs to consider the possibility that proMMP-2 and proMMP-9 might also be activated by other non-proteolytic mechanisms. Many non-proteolytic activation mechanisms of MMPs have been discovered, for example, allosteric activation of proMMPs by substrate binding or ligand binding34,35 and chemical modification of the propeptide cysteine by reactive oxygen/nitrogen species such as peroxynitrite.28,29 To evaluate the possibility that LPS induce different MMP-9 modifications and charge variants, we evaluated the samples by two-dimensional zymography.36 MMP-9 appeared as a wide band of different charge variants, spanning almost the full length of the isoelectric point (pI) gradient (Online Supplementary Figure S2), likely caused by different post-translational modifications. However, no major changes in this pattern were observed in the LPS-stimulated samples. With the limitations of the resolution and sensitivity of this technique, we can conclude that no major charge modifications occurred upon induction of endotoxemia.
Systematic induction of TIMP-1 and reduction of TIMP-2
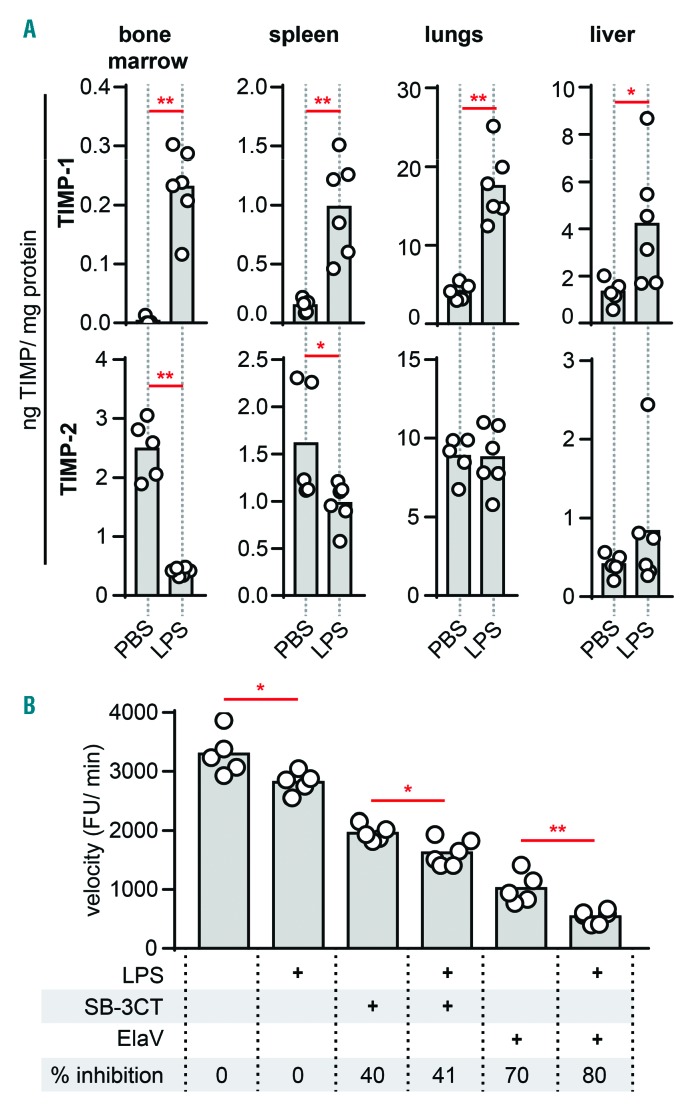
MMP activity is based on a balance between MMPs and their natural inhibitors. We, therefore, analyzed TIMP-1 and TIMP-2 protein levels. Basal levels of TIMP-1 and TIMP-2 were highest in the lungs (Table 2). Interestingly, basal levels of TIMP-1 in bone marrow were low while TIMP-2 was well represented. Next, LPS challenge resulted in a systematic change in TIMP-1 protein levels (Figure 4A). In agreement with RNA expression data, TIMP-2 was significantly decreased by LPS in bone marrow and spleen. In sharp contrast, endotoxemia induced the production of TIMP-1 in all organs, an effect which was most pronounced in the bone marrow compartment. Taken together, with a decrease in MMP-9 of approximately 90% and an almost 8-fold increase of TIMP-1, the effect of endotoxemia on the protease/inhibitor balance in the bone marrow is substantial. Molar ratios of MMP-9/TIMP-1 indeed suggest a basal proteolysis-favoring environment in the bone marrow compartment, which decreases upon induction of endotoxemia (Table 2). It does, however, need to be emphasized that basal gelatinase-B in the bone marrow is primarily in the proMMP-9 form. To confirm this effect, net gelatinolytic activity in the bone marrow was measured using a gelatin degradation assay. As expected, bone marrow samples contained net-proteolytic activity which was significantly reduced upon treatment with LPS (Figure 4B). Moreover, by adding SB-3CT (an inhibitor of MMP-2/MMP-9) and ElaV (an elastase inhibitor) we found that bone marrow proteolysis, here represented by gelatinolysis, is a combination of MMP-9 and elastase activity. MMP-9 proteolysis accounted for approximately 30% of gelatin proteolysis while elastase accounted for the remaining 70%. An interesting observation is that organs with high levels of activated MMP-2/-9 (e.g. lungs and liver) also contained higher levels of TIMPs. Although being paradoxical, this observation might be explained by TIMP-assisted proteolytic activation, as previously shown for MMP-2/TIMP-2.14,15
Table 2.
Levels of TIMP-1 and TIMP-2 detected in bone marrow, spleen, liver and lungs.
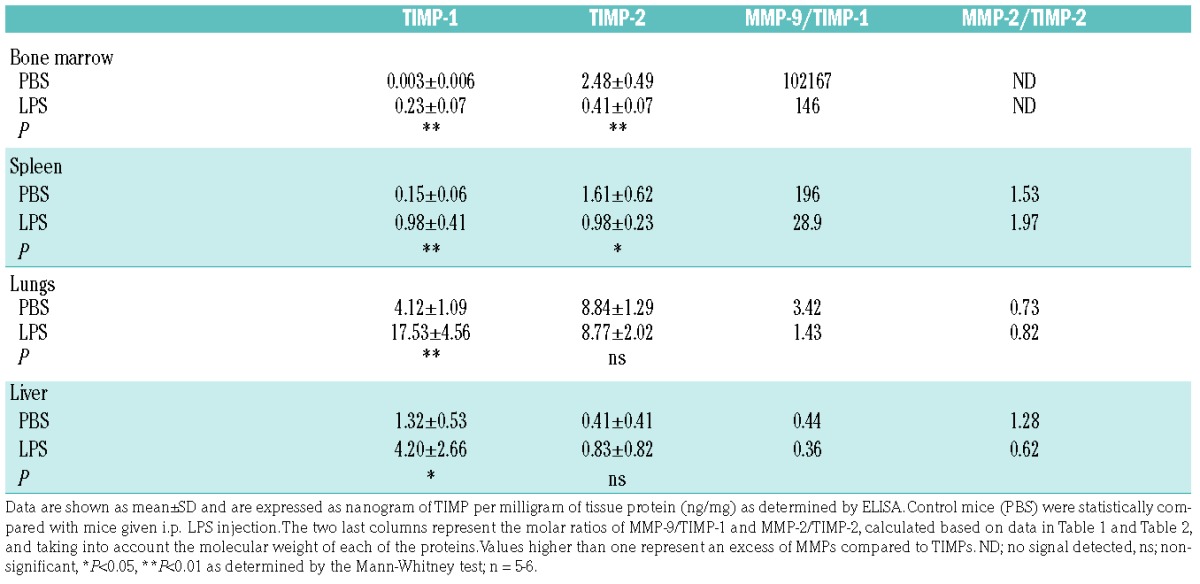
Figure 4.
Systematic induction of TIMP-1 and reduced bone marrow MMP gelatinolytic activity. (A) TIMP-1 and TIMP-2 protein content in bone marrow, spleen, lungs and liver of mice injected i.p. with LPS or control mice (PBS injection) as determined by ELISA. Protein levels detected by ELISA were corrected for the total protein concentration and are presented as nanograms of TIMP in one milligram of total protein. (B) Degradation of fluorogenic gelatin by gelatinases present in bone marrow samples from mice treated with LPS and control mice, in the presence or absence of an inhibitor of MMP-2 and MMP-9 (SB-3CT, 10 μM) or elastase inhibitor (ElaV, 10 μM). The velocity of the gelatin degradation reaction was expressed as fluorescence units (FU) per minute and is indicative of the net proteolytic activity present in the samples. Inhibition percentages are shown. *P<0.05, **P<0.01, as determined by the Mann-Whitney test.
Changes in MMP-9 levels correlate with neutrophil migration patterns
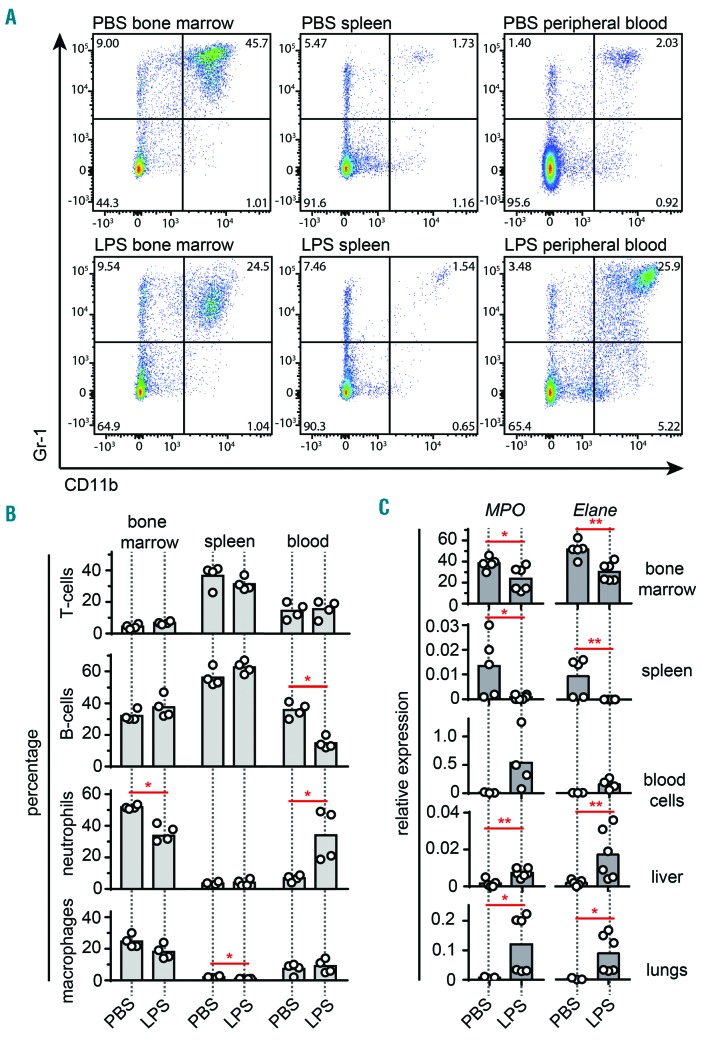
Neutrophils are the main producers of MMP-9 and they are unique because they do not produce TIMP-1.37,38 This concept was elaborated in a pioneering study on angiogenesis in tumor biology,38 but seemingly also has relevance in bone marrow physiology. We, therefore, hypothesized that the TIMP-1/MMP-9 shift during endotoxemia might be due to neutrophil mobilization into the circulation and into peripheral organs. To investigate this, we performed flow-cytometry analysis of bone marrow, spleen and blood cells to study the migration pattern of T cells (CD3+), B cells (CD19+), neutrophils (CD11b+Gr1+) and macrophages (CD11b+F4/80+) in animals with and without LPS injection (Figure 5). Indeed, in the bone marrow a significant decrease (from 52% to 34%) in the CD11b+Gr-1+ population of neutrophils was seen with LPS (Figure 5A,B). In addition, a shift of this population occurred towards the peripheral blood circulation, from 7% to 34% CD11b+Gr-1+ cells. Interestingly, endotoxemia also caused a reduction in splenic macrophages (CD11b+F4/80+ cells: from 2% to 1%) and a decrease in circulating B cells (CD19+ cells: from 36% to 15%). In addition, we evaluated the RNA expression of myeloperoxidase (MPO) and neutrophil elastase (ELANE), two molecules most abundantly expressed by neutrophils and hence functioning as neutrophil markers, in bone marrow, spleen, blood, liver and lungs. Overall, the relative RNA expression of the neutrophil markers Mpo and Elane followed a similar pattern as that for MMP-9, except in the spleen, where a significant decrease was seen in both Mpo and Elane expression upon induction of endotoxemia.
Figure 5.
MMP-9 levels relate with influx or efflux of a population of Gr-1high neutrophils. (A) Bone marrow, spleen and blood neutrophil content of mice not given an LPS injection (PBS, top) and given an LPS injection (bottom). Flow cytometry plots represent the distribution of live cells based on surface Gr-1 and CD11b staining. (B) Analysis of the T-cell (CD3+), B-cell (CD19+), neutrophil (CD11b+Gr1+) and macrophage (CD11b+F4/80+) populations in bone marrow, spleen and blood as determined by flow cytometry. (C) Relative MPO and Elane RNA expression in bone marrow, spleen, blood cells, liver and lungs of mice treated with PBS (control) or LPS. RNA expression data were normalized for the housekeeping gene Tbp. *P<0.05, **P<0.01, as determined by the Mann-Whitney test.
Immunohistochemical analysis of MMP-9
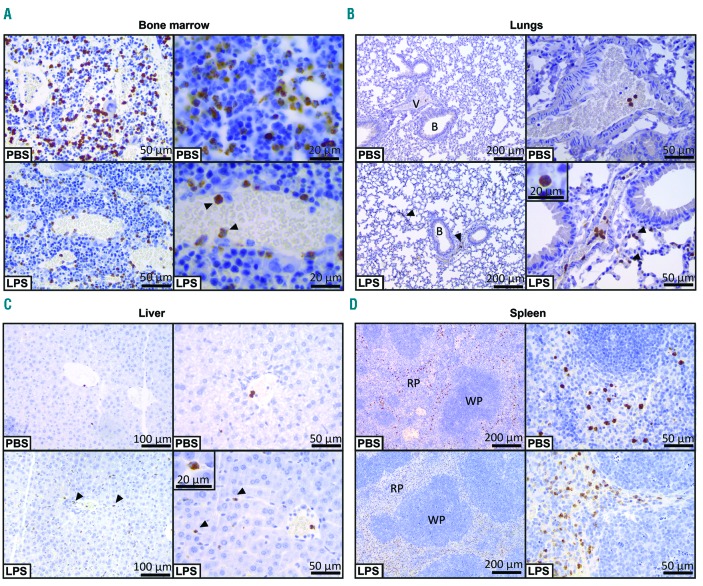
Next, we examined the tissue location of MMP-9 by immunohistochemistry. In the bone marrow, total staining for MMP-9 was markedly reduced in the animals administered LPS (Figure 6A). In addition, the immunoreactive staining was clearly confined to single cells which appeared as polymorphonuclear cells. In control mice (given PBS) these cells were diffusely found across the bone marrow while in endotoxemic conditions more cells were associated with the vasculature. In lungs (Figure 6B) and liver (Figure 6C) the opposite phenomenon occurred. While the baseline level of MMP-9-immunostained cells was low and predominantly restricted to blood vessels, LPS injection caused MMP-9-positive cells to migrate from blood vessels into lung alveoli, bronchioles and liver parenchyma. Again, staining was associated with polymophonuclear cells. Spleen immunohistochemistry revealed a similar staining pattern for both the PBS and LPS conditions (Figure 6D). Generally, MMP-9-positive cells were located in red pulp, while no staining was seen in white pulp. In conclusion, immunohistochemical analysis of bone marrow, spleen, liver and lung sections further reinforced our data, showing decreased staining for MMP-9 in bone marrow, increased staining in liver and lungs, and no changes in the MMP-9 staining in spleen upon systemic in vivo challenge with LPS.
Figure 6.
Immunohistochemical analysis of MMP-9 in bone marrow, lungs, liver and spleen. (A) MMP-9 immunohistochemical analysis of bone marrow from mice with i.p. LPS injection and control mice (PBS). Arrowheads indicate immunoreactivity for MMP-9 in leukocytes associated with a blood vessel wall. (B) Lung MMP-9 immunohistochemical analysis. Increased infiltrations of MMP-9-positive cells from blood vessels (V) to surrounding alveoli and bronchioles (B) in lungs from mice with endotoxemia (LPS), indicated by the arrowheads. The insert shows the typical polymorphonuclear nature of the infiltrating MMP-9-containing cells. (C) Liver MMP-9 histology also shows clear infiltration of MMP-9-positive cells from blood vessels into the surrounding tissue. Infiltrating cells are indicated by arrowheads and the magnification shows the typical polymorphonuclear nature of these cells. (D) Immunohistochemical analysis of the spleens of mice subjected to i.p. LPS or control mice (PBS). MMP-9-positive cells were predominantly associated with the red pulp (RP) and not with white pulp (WP).
Discussion
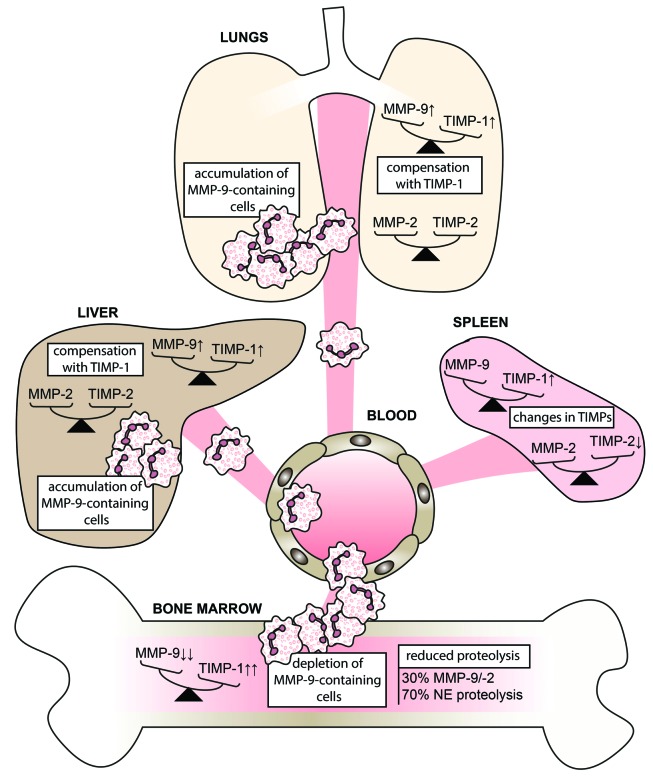
Infection with Gram-negative bacteria accounts for about 60% of the bacterial infections causing sepsis, a disease associated with considerable lethality, even with the most sophisticated medical care.21 Previous studies suggest that MMP inhibition may become a treatment for endotoxin shock, although more in-depth preclinical work is necessary. In our model we used LPS, a Gram-negative bacterial component, to trigger endotoxemia and to study the distribution patterns of the two key gelatinases (MMP-9 and MMP-2) and their inhibitors (TIMPs). Since the time-dependent increase of MMP-9 during early pathology (1–12 h after induction of the syndrome) is well documented9,39,40 (Online Supplementary Table S1), our study was focused at a later stage of endotoxemia (24 h) and involved an in-depth analysis thereof. Whereas the importance of MMP-9 in the development of acute inflammation, such as sepsis syndrome, is evident from the beneficial effects observed with the use of MMP inhibitors,9–12 little is known about bone marrow gelatinases (MMP-9 and MMP-2) or the effects on the balance between MMPs and their natural inhibitors. Here we provide several new insights into this topic (Figure 7).
Figure 7.
Overview of the distribution pattern of MMP-9/TIMP-1 and MMP-2/TIMP-2 balances and MMP-9-positive cells upon challenge with lipopolysaccharide. Induction of endotoxemia results in the migration of MMP-9-positive polymorphonuclear cells out of the bone marrow to accumulate primarily in lungs and liver. This release of MMP-9 is meanwhile counteracted by a systematic upregulation of TIMP-1. In contrast, MMP-2 protein levels remain similar while TIMP-2 is generally reduced. NE; neutrophil elastase.
A first striking observation is the finding that MMP-9 accounts for more than 0.1% of total bone marrow protein, making MMP-9 an important factor in bone marrow physiology and pathology, for example in hematopoiesis. In addition, we show that during endotoxemia, the bone marrow is the major source of MMP-9 and that this MMP-9 is associated with polymorphonuclear cells. A limitation of the present study is that the analysis was performed at the single time-point of 24 h. Previous studies have shown that a peak in plasma MMP-9 occurs as early as 1–12 h after induction of sepsis39,40 and that MMP-9 is predominantly associated with neutrophils6 and late-stage maturing neutrophils such as band cells and segmented cells, present in the bone marrow.32,41 In our model of late endotoxemia (24 h), a significant shift in MMP-9 was still evident. In particular, we observed depletion of bone marrow MMP-9 and significant increases in MMP-9 in lungs and liver, while the spleen remained unaffected. This is in line with previous studies showing that patients suffering from sepsis have increased plasma levels of MMP-942 and that lungs and liver are indeed two highly affected organs during endotoxemia and sepsis syndromes.27 In parallel, we found that LPS induced mobilization of MMP-9-containing cells into the bloodstream. These cells predominantly migrated into lungs and liver, where clear infiltrations of MMP-9-positive cells were observed. We further confirmed this shift by showing a similar pattern for Gr-1high cells, which have been shown to contain large amounts of intracellular MMP-9.43
MMPs are indeed known to aid cell migration because of their ability to degrade extracellular matrix molecules.5,8 This effect relies on direct proteolytic activity which is the result of a fine-tuned balance of protease levels, protease activation (the conversion of proMMP-9 into active MMP-9 by proteolysis of the prodomain or chemical modification of the pro-peptide cysteine) and the presence of inhibitors (e.g. TIMP-1). An important new finding of our work is that, although MMP-9 increases systemically after LPS challenge, a natural anti-proteolytic response occurs by systematically increasing TIMP-1. This is evident from both tissue MMP-9/TIMP-1 ratios and proteolytic activity tests. In addition, the relative expression of Timp1 also considerably increased upon LPS challenge in almost all organs, including the bone marrow. These findings allowed us to address two questions: (i) whether inhibition of proteolysis by MMP-9 still has merit once acute inflammation has progressed (late-stage) and (ii) whether the contribution of TIMP-1 is disease-promoting or disease-limiting. Most animal studies, showing beneficial effects for MMP-9 inhibitors, were performed with inhibitor injections immediately after the induction of endotoxemia10–12 or in MMP-9 knock-out animals.8,9 In addition, higher serum levels of TIMP-1 have been found in non-survivors compared to survivors of severe sepsis.44 This confirms that the fine-tuned regulation of MMPs, including their balance with TIMPs, is crucial for tissue homeostasis.18 A good approach to the treatment of acute inflammations might, therefore, rely on carefully restoring this balance depending on the proteolytic state.
So far, the protease/anti-protease balance has been predominantly investigated in lungs and is key to normal lung physiology. The most studied example in lungs is the elastase/anti-elastase balance. This is exemplified by mouse models in which both induction of elastase and mutations of antitrypsin (elastase inhibitor) lead to emphysema.45 We here show that endotoxemia also drastically affects the lungs by inducing the accumulation of MMP-9-positive cells. Nevertheless, this effect is counterbalanced by induction of TIMP-1. Indeed, it was previously suggested that the pulmonary accumulation of neutrophils in sepsis is due to shedding of platelet-derived surface-expressed CD40L by MMP-9.46
Although TIMP-1 increases in bone marrow, most MMP-9 (>99%) remains TIMP-free and thus potentially catalytically active. Indeed, neutrophils are unique in the fact that they do not secrete TIMP-1.37,38 Moreover, this TIMP-free MMP-9 was shown to have angiogenic capacity,38 in particular when neutrophils infiltrate peripheral tissue or tumor microenvironments.47 In general, we show that bone marrow functioning relies on a combination of proteolysis by MMP-9 and neutrophil elastase and that both activities are reduced during late-stage endotoxemia. Previously it was shown that neutrophil elastase can compensate for MMP-9 deficiency in a model of leukocyte infiltration in experimental peritonitis.48 Neutrophil elastase is a serine protease found in the azurophilic granules of neutrophils. In contrast to MMP-9, neutrophil elastase knock-out mice have impaired host defense against Gram-negative bacterial sepsis.49 Neutrophil elastase can, therefore, also be considered as an important factor in endotoxin shock and requires further investigation.
To date, the management of acute systemic inflammation, such as sepsis in patients, is mostly limited to supportive care. Although targeting MMP-9 in these conditions has been suggested, it has been shown that MMP-9 inhibition has a limited therapeutic time window.50 During late-stage endotoxin shock, the host responds with systematic upregulation of TIMP-1 and shift in the protease/anti-protease balance towards reduced proteolysis. Together with reports showing higher levels of TIMP-1 in non-survivors44 and the present data on profound effects of LPS on MMP-9-laden neutrophils in the bone marrow, successful treatment of sepsis with protease inhibitors might first require detailed analysis of the protease/anti-protease balance in humans. Nevertheless, our study provides new insights, techniques and data to probe critical enzymes and inhibitors. In addition, it shows the technical limitations encountered during in-depth analysis of non-proteolytic MMP activation in a biological setting. A future aim for the treatment of endotoxin shock might be the containment of neutrophils in the bone marrow compartment for the purpose of prohibiting collateral damage in the tissues of peripheral organs.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/10/1671
Funding
The authors would like to thank KU Leuven for C1 grant support (C16/17/010) and the Research Foundation of Flanders (FWO-vlaanderen).
References
- 1.Opdenakker G, Fibbe WE, Van Damme J. The molecular basis of leukocytosis. Immunol Today. 1998;19(4):182–189. [DOI] [PubMed] [Google Scholar]
- 2.Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15(8):551–567. [DOI] [PubMed] [Google Scholar]
- 3.Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6(6):480–498. [DOI] [PubMed] [Google Scholar]
- 4.Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40(6–7):1362–1378. [DOI] [PubMed] [Google Scholar]
- 5.Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit Rev Biochem Mol Biol. 2013;48(3):222–272. [DOI] [PubMed] [Google Scholar]
- 6.Masure S, Proost P, Van Damme J, Opdenakker G. Purification and identification of 91-kDa neutrophil gelatinase. Release by the activating peptide interleukin-8. Eur J Biochem. 1991;198(2):391–398. [DOI] [PubMed] [Google Scholar]
- 7.Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109(5):625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Haese A, Wuyts A, Dillen C, et al. In vivo neutrophil recruitment by granulocyte chemotactic protein-2 is assisted by gelatinase B/MMP-9 in the mouse. J Interferon Cytokine Res. 2000;20(7):667–674. [DOI] [PubMed] [Google Scholar]
- 9.Dubois B, Starckx S, Pagenstecher A, Oord J, Arnold B, Opdenakker G. Gelatinase B deficiency protects against endotoxin shock. Eur J Immunol. 2002;32(8):2163–2171. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg J, Halter J, Schiller HJ, et al. Metalloproteinase inhibition reduces lung injury and improves survival after cecal ligation and puncture in rats. J Surg Res. 2003;111(2):185–195. [DOI] [PubMed] [Google Scholar]
- 11.Lalu MM, Gao CQ, Schulz R. Matrix metalloproteinase inhibitors attenuate endotoxemia induced cardiac dysfunction: a potential role for MMP-9. Mol Cell Biochem. 2003;251(1–2):61–66. [PubMed] [Google Scholar]
- 12.Hu J, Van den Steen PE, Dillen C, Opdenakker G. Targeting neutrophil collagenase/matrix metalloproteinase-8 and gelatinase B/matrix metalloproteinase-9 with a peptidomimetic inhibitor protects against endotoxin shock. Biochem Pharmacol. 2005;70(4):535–544. [DOI] [PubMed] [Google Scholar]
- 13.Murphy G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011;12(11):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward RV, Atkinson SJ, Reynolds JJ, Murphy G. Cell surface-mediated activation of progelatinase A: demonstration of the involvement of the C-terminal domain of progelatinase A in cell surface binding and activation of progelatinase A by primary fibroblasts. Biochem J. 1994;304:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270(10):5331–5338. [DOI] [PubMed] [Google Scholar]
- 16.Rossi L, Ergen AV, Goodell MA. TIMP-1 deficiency subverts cell-cycle dynamics in murine long-term HSCs. Blood. 2011;117 (24): 6479–6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KH, Burkhart K, Chen P, et al. Tissue inhibitor of metalloproteinase-1 deficiency amplifies acute lung injury in bleomycin-exposed mice. Am J Respir Cell Mol Biol. 2005;33(3):271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khokha R, Murthy A, Weiss A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol. 2013;13(9):649–665. [DOI] [PubMed] [Google Scholar]
- 19.Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86(1):9–22. [DOI] [PubMed] [Google Scholar]
- 20.Bohannon JK, Hernandez A, Enkhbaatar P, Adams WL, Sherwood ER. The immunobiology of toll-like receptor 4 agonists: from endotoxin tolerance to immunoadjuvants. Shock. 2013;40(6):451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420(6917):885–891. [DOI] [PubMed] [Google Scholar]
- 22.Zenker S, Panteleev-Ivlev J, Wirtz S, et al. A key regulatory role for Vav1 in controlling lipopolysaccharide endotoxemia via macrophage-derived IL-6. J Immunol. 2014; 192(6):2830–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandooren J, Geurts N, Martens E, Van den Steen PE, Opdenakker G. Zymography methods for visualizing hydrolytic enzymes. Nat Methods. 2013;10(3):211–220. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 25.Vandooren J, Geurts N, Martens E, et al. Gelatin degradation assay reveals MMP-9 inhibitors and function of O-glycosylated domain. World J Biol Chem. 2011;2(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su AI, Wiltshire T, Batalov S, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101(16):6062–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity. 2014;40(4):463–475. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem. 2001;276(31):29596–29602. [DOI] [PubMed] [Google Scholar]
- 29.Viappiani S, Nicolescu AC, Holt A, et al. Activation and modulation of 72kDa matrix metalloproteinase-2 by peroxynitrite and glutathione. Biochem Pharmacol. 2009;77(5):826–834. [DOI] [PubMed] [Google Scholar]
- 30.Vandooren J, Born B, Solomonov I, et al. Circular trimers of gelatinase B/matrix metalloproteinase-9 constitute a distinct population of functional enzyme molecules differentially regulated by tissue inhibitor of metalloproteinases-1. Biochem J. 2015;465(2): 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou M, Jacob A, Ho N, et al. Downregulation of protein disulfide isomerase in sepsis and its role in tumor necrosis factor-alpha release. Crit Care. 2008;12(4):R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit Rev Biochem Mol Biol. 2002;37(6):375–536. [DOI] [PubMed] [Google Scholar]
- 33.Hughes BG, Schulz R. Targeting MMP-2 to treat ischemic heart injury. Basic Res Cardiol. 2014;109(4):424. [DOI] [PubMed] [Google Scholar]
- 34.Bannikov GA, Karelina TV, Collier IE, Marmer BL, Goldberg GI. Substrate binding of gelatinase B induces its enzymatic activity in the presence of intact propeptide. J Biol Chem. 2002;277(18):16022–16027. [DOI] [PubMed] [Google Scholar]
- 35.Geurts N, Martens E, Van Aelst I, Proost P, Opdenakker G, Van den Steen PE. Beta-hematin interaction with the hemopexin domain of gelatinase B/MMP-9 provokes autocatalytic processing of the propeptide, thereby priming activation by MMP-3. Biochemistry. 2008;47(8):2689–2699. [DOI] [PubMed] [Google Scholar]
- 36.Rossano R, Larocca M, Riviello L, et al. Heterogeneity of serum gelatinases MMP-2 and MMP-9 isoforms and charge variants. J Cell Mol Med. 2014;18(2):242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Opdenakker G, Van den Steen PE, Dubois B, et al. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol. 2001;69(6):851–859. [PubMed] [Google Scholar]
- 38.Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci USA. 2007;104(51): 20262–20267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pagenstecher A, Stalder AK, Kincaid CL, Volk B, Campbell IL. Regulation of matrix metalloproteinases and their inhibitor genes in lipopolysaccharide-induced endotoxemia in mice. Am J Pathol. 2000;157(1):197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lalu MM, Csont T, Schulz R. Matrix metalloproteinase activities are altered in the heart and plasma during endotoxemia. Crit Care Med. 2004;32(6):1332–1337. [DOI] [PubMed] [Google Scholar]
- 41.Kjeldsen L, Sengelov H, Lollike K, Nielsen MH, Borregaard N. Isolation and characterization of gelatinase granules from human neutrophils. Blood. 1994;83(6):1640–1649. [PubMed] [Google Scholar]
- 42.Lauhio A, Hastbacka J, Pettila V, et al. Serum MMP-8, -9 and TIMP-1 in sepsis: high serum levels of MMP-8 and TIMP-1 are associated with fatal outcome in a multicentre, prospective cohort study. Hypothetical impact of tetracyclines. Pharmacol Res. 2011;64(6):590–594. [DOI] [PubMed] [Google Scholar]
- 43.Gounko NV, Martens E, Opdenakker G, Rybakin V. Thymocyte development in the absence of matrix metalloproteinase-9/gelatinase B. Sci Rep. 2016;6:29852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann U, Bertsch T, Dvortsak E, et al. Matrix-metalloproteinases and their inhibitors are elevated in severe sepsis: prognostic value of TIMP-1 in severe sepsis. Scand J Infect Dis. 2006;38(10):867–872. [DOI] [PubMed] [Google Scholar]
- 45.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277 (5334):2002–2004. [DOI] [PubMed] [Google Scholar]
- 46.Rahman M, Zhang S, Chew M, Syk I, Jeppsson B, Thorlacius H. Platelet shedding of CD40L is regulated by matrix metalloproteinase-9 in abdominal sepsis. J Thromb Haemost. 2013;11(7):1385–1398. [DOI] [PubMed] [Google Scholar]
- 47.Deryugina EI, Zajac E, Juncker-Jensen A, Kupriyanova TA, Welter L, Quigley JP. Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia. 2014;16(10):771–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolaczkowska E, Grzybek W, van Rooijen N, et al. Neutrophil elastase activity compensates for a genetic lack of matrix metalloproteinase-9 (MMP-9) in leukocyte infiltration in a model of experimental peritonitis. J Leukoc Biol. 2009;85(3):374–381. [DOI] [PubMed] [Google Scholar]
- 49.Belaaouaj A, McCarthy R, Baumann M, et al. Mice lacking neutrophil elastase reveal impaired host defense against Gram negative bacterial sepsis. Nat Med. 1998;4(5):615–618. [DOI] [PubMed] [Google Scholar]
- 50.Qiu Z, Chen J, Xu H, et al. Inhibition of neutrophil collagenase/MMP-8 and gelatinase B/MMP-9 and protection against endotoxin shock. J Immunol Res. 2014; 2014:747426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.