Abstract
Background & Aims
When the glial fibrillary acidic protein (GFAP) promoter is used to express cellular toxins that eliminate glia in mice, intestinal epithelial permeability and proliferation increase; this led to the concept that glia are required for maintenance of the gastrointestinal epithelium. Many enteric glia, however, particularly in the mucosa, do not express GFAP. In contrast, virtually all enteric glia express proteolipid protein 1 (PLP1). We investigated whether elimination of PLP1-expressing cells compromises epithelial maintenance or gastrointestinal motility.
Methods
We generated mice that express tamoxifen-inducible Cre recombinase under control of the Plp1 promoter and carry the diptheria toxin subunit A (DTA) transgene in the Rosa26 locus (Plp1CreER;Rosa26DTA mice). In these mice, PLP1-expressing glia are selectively eliminated without affecting neighboring cells. We measured epithelial barrier function and gastrointestinal motility in these mice and littermate controls, and analyzed epithelial cell proliferation and ultrastructure from their intestinal tissues. To compare our findings with those from previous studies, we also eliminated glia with ganciclovir in GfapHSV-TK mice.
Results
Expression of DTA in PLP1-expressing cells selectively eliminated enteric glia from the small and large intestines, but caused no defects in epithelial proliferation, barrier integrity, or ultrastructure. In contrast, administration of ganciclovir to GfapHSV-TK mice eliminated fewer glia but caused considerable non-glial toxicity and epithelial cell death. Elimination of PLP1-expressing cells did not reduce survival of neurons in the intestine, but altered gastrointestinal motility in female, but not male, mice.
Conclusions
Using the Plp1 promoter to selectively eliminate glia in mice, we found that enteric glia are not required for maintenance of the intestinal epithelium but are required for regulation of intestinal motility in females. Previous observations supporting the concept that maintenance of the intestinal epithelium requires enteric glia can be attributed to non-glial toxicity in GfapHSV-TK mice and epithelial-cell expression of GFAP. Contrary to widespread notions, enteric glia are therefore not required for epithelial homeostasis. However, they regulate intestinal motility in a sex-dependent manner.
Keywords: enteric nervous system, epithelial barrier, sex differences
Introduction
The enteric nervous system (ENS) is a large network of neurons and glia intrinsic to the alimentary tract that is essential for gastrointestinal homeostasis1. While it is now well recognized that glial dysfunction contributes to neurological disorders2, the role of glia in digestive disorders remains unclear. Understanding normal enteric glial function is essential for identifying glial contributions to digestive disease. Enteric glia are distributed throughout the laminar structure of the gastrointestinal tract and closely appose neurons, immune cells, blood vessels and the intestinal epithelium3–5. Mucosal glia in the lamina propria directly underlie the epithelium and encircle crypts of Lieberkühn, which contain the epithelial stem cell niche6. This close proximity raised the possibility that enteric glia play a role in regulating epithelial functions such as cellular proliferation and barrier maintenance.
A series of studies that investigated the consequences of eliminating GFAP-expressing glia in mice suggested that enteric glia may be essential for epithelial maintenance and offered clues to glial functions in the bowel. Initial studies used mice that express herpes simplex virus thymidine kinase (HSV-TK) under the control of the Gfap promoter (GfapHSV-TK mice). Upon systemic treatment of GfapHSV-TK mice with ganciclovir, GFAP-expressing enteric glia were eliminated and the animals developed fulminant inflammation selectively in the mid-to-distal small intestine7. Intestinal epithelial permeability and crypt cell proliferation were increased even before histological evidence of inflammation was detected8. These observations were interpreted to mean that enteric glia are required for normal regulation of barrier function and epithelial turnover, and that elimination of enteric glia causes epithelial dysregulation, leading to translocation of luminal bacteria and intestinal inflammation7. Subsequent studies using mice engineered to manifest autoimmune reactions against GFAP-expressing cells also reported intestinal inflammation and premature death9. In vitro, permeability of Caco-2 cell monolayers and their incorporation of [3H]-thymidine both decrease when these cells are co-cultured with enteric glia8, 10, further suggesting that enteric glia can regulate epithelial permeability and proliferation. These experiments have led to the concept, now widely accepted, that enteric glia directly regulate important bowel functions independently of their interactions with neurons and to speculation that enteric glial dysfunction contributes to the pathogenesis of inflammatory bowel disease11.
More recent studies have shown that enteric glia are heterogeneous and that GFAP expression is limited to a subset12, 13. More than half of mucosal and submucosal glia in the ileum and colon do not express GFAP13. In contrast, S100β, SOX10 and PLP1 are expressed by virtually all enteric glia and the Plp1 promoter can be used to manipulate enteric glia in vivo13. To explore enteric glial functions, we targeted PLP1-expressing cells for elimination in mice using inducible expression of diphtheria toxin subunit A (DTA). We generated Plp1CreER;Rosa26DTA mice that express tamoxifen-inducible Cre recombinase (Cre) under the control of the Plp1 promoter and carry the lox-stop-lox-DTA transgene in the Rosa26 locus. Administration of tamoxifen induces DTA expression, selectively eliminating PLP1-expressing glia without affecting neighboring cells. The robust elimination of enteric glia induced in tamoxifen-treated Plp1CreER;Rosa26DTA mice revealed that enteric glia are dispensable for all epithelial functions examined but are required for normal intestinal motility.
Methods
Mouse lines
Plp1CreER, Rosa26DTA, GfapCreER, Rosa26TdTomato, Rosa26LacZ, GfapCre and GfapHSV-TK mice are detailed in Supplemental Table 1. Plp1CreER mice were on a FVB background and others were on a C57BL6 background. Plp1CreER;Rosa26DTA (Cre+, experimental) and Rosa26DTA (Cre−, control) mice used for all experiments were littermates on a mixed FVBN:C57BL6 background. Mice were housed in a facility with a 12h dark cycle and handled in accordance with Columbia University IACUC guidelines.
Induction of glial loss
Plp1CreERT;Rosa26DTA (Cre+) and Rosa26DTA mice (Cre−) were gavaged once at 5–6 weeks of age with 8mg of tamoxifen (Toronto Research Chemicals T006000) solubilized in corn oil. All measurements were obtained at 7–9dpt unless noted. GFAPHSV-TK mice were treated with ganciclovir via SQ pump or gavaged with 50mg/kg of valganciclovir (Cayman Chemical 13875) twice daily for up to 15 days starting at 5–6 weeks of age. We observed no difference between these two induction methods and the data presented are from mice treated with valganciclovir.
Epithelial permeability and proliferation
For permeability measurements, mice were fasted overnight and gavaged with 600mg/kg FITC-dextran (4 kDa, Sigma FD40S) or 200mg/kg fluorescein-5-(and-6)-sulfonic acid (478 Da, Invitrogen F-1130). Four hours later, serum was collected and fluorescence intensity was measured as previously described8. For dextran sodium sulfate (DSS) colitis, mice were treated with 2.5% DSS (Affymetrix 14489) in drinking water for 7 days. Body weight and disease activity index (composite of stool consistency and hematochezia) were assessed daily. For measurement of bacterial penetration into mesenteric lymph nodes (MLNs), RNA was extracted from MLNs and reverse-transcribed. Quantitative PCR (SYBR green) was performed for mouse Gapdh and universal primers for bacterial 16S rRNA14. ΔCT=CT(16S)-CT(Gapdh). For analysis of epithelial proliferation at 7dpt, villus-to-crypt ratios were measured in hematoxylin and eosin-stained paraffin sections (5μm), and represent maximum villous length divided by maximum crypt length. Thirty villus-crypt units were measured from each animal per intestinal segment. Ki67 immunoreactivity was quantified in 5μm paraffin sections. At least 50 crypts/intestinal segment were measured per animal.
Immunocytochemistry, microscopy and quantification
Immunostaining was performed as previously described13. Primary antibodies are detailed in Supplemental Table 2. Anti-goat and anti-donkey secondary antibodies used were conjugates of AlexaFluor 594, 568 or 488 (Invitrogen). Nuclei were counterstained with DAPI (Vector Labs H-1200) or TO-PRO3 (Molecular Probes T3605). For glial density measurement, segments of ileum and colon were immunostained for S100β and NF-H as previously described13, and single planar images were captured at the level of the myenteric plexus or mucosa (5–6 fields per segment identified based on NF-H staining alone). Mucosal glia were quantified relative to number of glands. Images from all animals were coded, randomized and quantified using ImageJ or Volocity 6.3 (PerkinElmer) by a blinded investigator. To quantify neuronal density, segments of ileum and colon were immunostained for ANNA-1 or nNOS and quantified as above. For transmission electron microscopy (TEM), tissues were fixed in a mixture of 4% formaldehyde and 2.5% glutaraldehyde, phosphate-buffered to pH7.4 and post-fixed with 1% OsO4. Fixed tissue was stained with 1% uranyl acetate in maleate buffer (0.5%; pH 6.2), dehydrated with ethanol, cleared with propylene oxide, and embedded in an epoxy resin (Epon812). Thin sections were cut with an ultramicrotome, placed on formvar-coated nickel slot grids, stained with uranyl acetate (1%) and Reynolds lead citrate, and viewed with a JEOLCO TEM (JEM 1200).
Gastrointestinal motility
Total gastrointestinal transit time, gastric emptying, colonic motility, and small intestinal transit were measured as previously described15. Small intestinal transit was estimated by the position of the geometric center of rhodamine-dextran 15 minutes after gavage16; values are distributed between 1 (minimal motility) and 10 (maximal motility). For fecal composition measurements, mice were transferred to empty cages at 9AM and stool was collected for 1 hour to measure “wet” stool weight per mouse. Stool was dried at 45°C for 48 hours and then “dry” weight was recorded. % water content=[(“wet”-“dry”)/# pellets produced in 1 hour]*100.
Statistics
Student’s unpaired t tests were used to compare two means. Two-way ANOVA with Sidak’s multiple comparisons testing was used to compare multiple means in DSS experiment. Graphs display mean ± standard error of the mean (SEM) with all individual data points shown. Each data point represents an individual mouse. A p value of less than 0.05 was considered significant and denoted with *. P values less than 0.005 are noted with ** and less than 0.0005 with ***.
Results
DTA expression in PLP1+ cells provides an inducible, non-inflammatory method to eliminate enteric glia
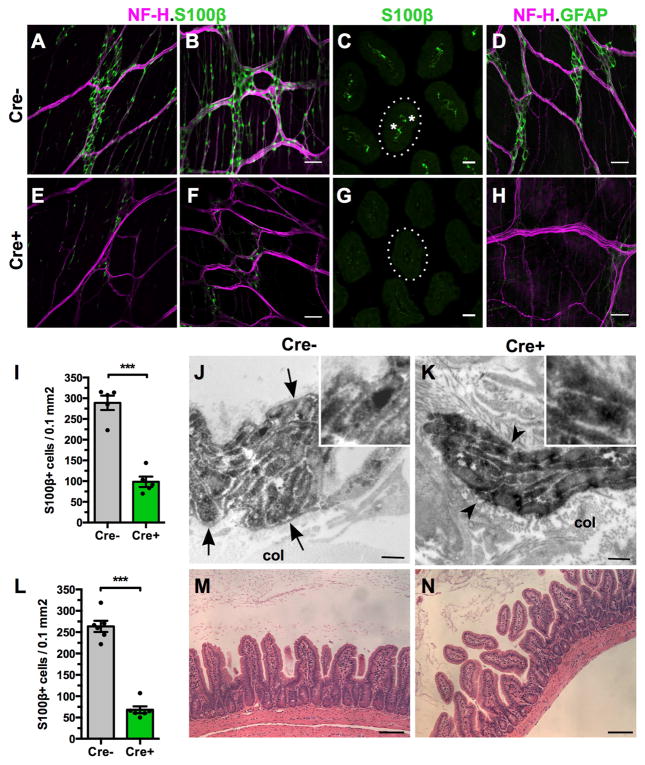
Administration of a single dose of tamoxifen by oral gavage to Plp1CreER;Rosa26DTA mice (annotated as Cre+) led to a 66% loss of S100β+ myenteric and intramuscular glia throughout the small intestine and a 74% loss in the large intestine, relative to Rosa26DTA littermates (annotated as Cre−) within 7 days (Figure 1). All known types of enteric glia were depleted in Cre+ mice, including myenteric, intramuscular, submucosal and mucosal (Figure 1E–G). Mucosal glia were most severely affected with Cre+ mice showing 88% fewer mucosal glia in the small intestine than Cre− controls (p<0.0001, n=6–7mice/group). DTA poisons cells by interfering with protein synthesis. To ensure that DTA induction caused loss of glia and not just loss of glial marker expression, ENS ultrastructure was examined. In Cre+ mice, the glial sheath that normally separates axons within nerve fiber bundles from each other and from surrounding connective tissue was lost, enabling collagen fibers to penetrate the enteric plexuses and directly contact axons (Figure 1J–K). Enteric glial elimination was tamoxifen-dependent; Cre+ mice treated with vehicle exhibited no glial loss and were indistinguishable from Cre− littermates (Figure S1). These data suggest that Plp1CreER;Rosa26DTA mice provide a robust, inducible, cell-specific mouse model for studying enteric glial function in vivo. Schwann cells in the peripheral nervous system (PNS) and oligodendrocytes in the central nervous system (CNS) also express PLP117, 18 and mice with inducible expression of DTA within PLP1-expressing cells have been reported to model CNS demyelination19. Consistent with that report, the Plp1CreER;Rosa26DTA mice utilized in our study also developed hindlimb weakness and coordination deficits starting at 14 days post-tamoxifen induction (dpt); therefore, we limited all analysis of gastrointestinal function to the first 10dpt. Glial loss was similar at 5, 7, 9 and 14dpt in Plp1CreER;Rosa26DTA mice, suggesting that the enteric glial deficit was stable during the period of analysis.
Figure 1. DTA expression in PLP1-expressing cells leads to enteric glial loss without associated inflammation.
Neurofilament-H (NF-H) and S100β immunoreactivities in the ilea (A, E) and colons (B, F) of Rosa26DTA (Cre−, control) and Plp1CreER;Rosa26DTA mice (Cre+, experimental) reveal Cre-dependent loss of myenteric and intramuscular glia.
(C, G) Ileal S100β immunoreactivity, imaged at the level of the villous mucosa, highlights the loss of mucosal glia (*) in the lamina propria of Cre+ mice. Cross-sections of single villi are outlined.
(D, H) NF-H and GFAP immunoreactivities show the Cre-dependent loss of ileal GFAP+ myenteric glia.
(I, L) Quantitation of S100β-expressing cells in ileum (I) and colon (L).
(J–K) Electron microscopic visualization of ileal PGP9.5 immunoreactivity (neuronal marker). In the Cre− control, a glial sheath (arrows) separates neuronal processes from the surrounding collagen (col). Thin glial partitions are seen between bundles of immunoreactive axons (see inset). In contrast, in Cre+ tissue there is no glial sheath around axon bundles or glial partitions separating axons. Collagen fibers thus come into direct contact with axons (arrowheads).
(M–N) Hematoxylin and eosin stained cross-sections of ilea from Cre− (M) and Cre+ mice (N) reveal no evidence of inflammation.
Scale bars A, C, D, F=50μm; B, E=25μm; H–I=500nm; K–L=100μm.
Remarkably, the fulminant inflammation in the mid-to-distal small intestine that occurs when GFAP-expressing cells are eliminated7 did not occur when PLP1-expressing cells were eliminated. Although PLP1 is expressed by far more enteric glia than GFAP and GFAP-expressing glia were clearly eliminated in Plp1CreER;Rosa26DTA mice (Figure 1H), no gross or histological evidence of inflammation in the small or large intestine was observed at any time point examined from 48h to 14dpt (Figures 1M–N, S2). These data suggest that inflammation is not an inevitable consequence of enteric glial elimination.
Enteric glia do not regulate epithelial permeability or homeostasis
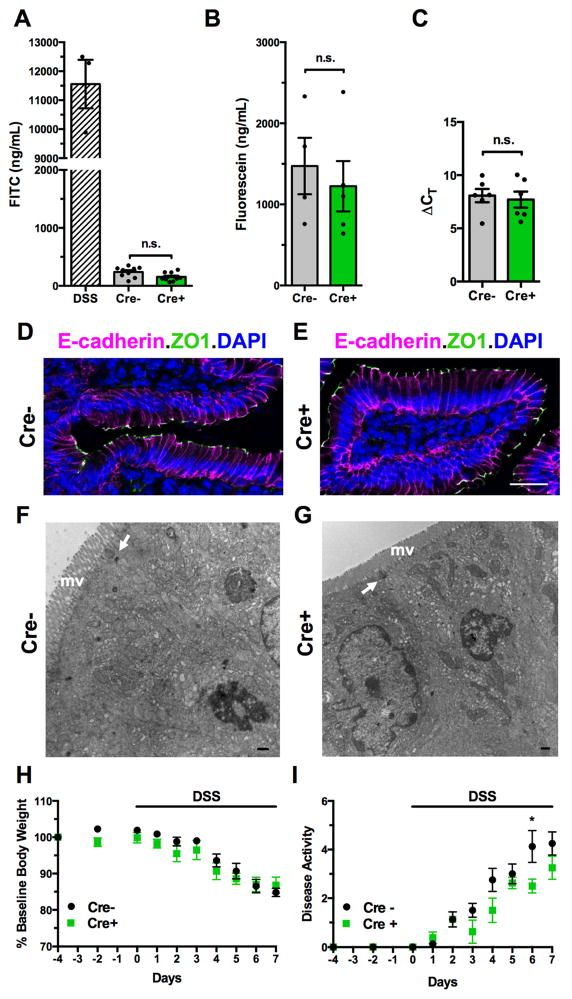
Studies based on elimination of GFAP-expressing cells led to the idea that enteric glia regulate intestinal permeability and epithelial turnover8, 20. Because mice in these studies developed intestinal inflammation, which can independently compromise the epithelial barrier and alter epithelial cell proliferation, a direct link between glial elimination and epithelial dysfunction was not tested. Plp1CreER;Rosa26DTA mice exhibit robust glial loss in the absence of inflammation, providing a means to investigate the role of enteric glia in epithelial homeostasis without confounding effects. To assess whether glial elimination alters macromolecular intestinal permeability, 4.4kDa FITC-dextran or a 478Da fluorescein conjugate of 5, 6-sulfonic acid was administered to Cre+ and Cre− mice by oral gavage; absorption of the tracers was then measured 4 hours later. Serum levels of both tracers did not differ between control and glial-depleted mice (Figure 2A–B). In contrast, when measured in wildtype mice with acute chemical colitis induced with dextran sodium sulfate (DSS) as a positive control, serum FITC-dextran was markedly increased (Figure 2A). Enteric glial elimination in Plp1CreER;Rosa26DTA mice, moreover, did not change 16S rRNA transcript levels in mesenteric lymph nodes (Figure 2C) indicating that microbial translocation through the intestinal epithelial barrier did not increase. Localization of the proteins zonulin-1 and E-cadherin, which are essential for normal intercellular junctions between enterocytes, was also unchanged (Figure 2D–E). Ultrastructural analysis revealed no differences between Cre+ and Cre− mice in enterocyte appearance. Apical regions, brush borders and intercellular junctions of enterocytes remained intact (Figure 2F–G). To determine whether glial-depleted mice might have subtle barrier defects that sensitize them to inflammatory stress, acute DSS colitis was induced in Cre+ and Cre− mice starting at 4dpt. All mice developed colitis over the course of 7 days, and there was no difference in weight loss or disease activity between Cre+ and Cre− mice (Figure 2H–I), implying that glial loss does not sensitize mice to intestinal inflammation. In sum, these data suggest that enteric glia are not required for maintaining the integrity of the intestinal epithelial barrier or inhibiting inflammation.
Figure 2. Enteric glial elimination does not alter intestinal barrier permeability.
(A) Serum FITC levels measured 4 hours after oral gavage of 4.4kDa FITC-dextran into Cre− and Cre+ mice, suggests that glial elimination does not alter macromolecular intestinal permeability. Serum from wildtype mice treated with dextran sodium sulfate (DSS) was used as a positive control.
(B) Serum FITC levels measured 4 hours after oral gavage of a 478Da fluorescein conjugate into Cre− and Cre+ mice. The smaller FITC-conjugate permeated the intestinal epithelium more than 4.4kDa FITC-dextran, yet again no difference was observed with glial elimination.
(C) Bacterial16S rRNA transcript levels in mesenteric lymph nodes were similar in Cre− and Cre+ mice.
(D–E) Localization of E-cadherin and zonulin-1 (ZO1) immunoreactivities is not altered by glial elimination. DAPI marks nuclei. Scale bar=25μm.
(F–G) The ultrastructure of small intestinal enterocytes does not differ in Cre− and Cre+ mice. Note the regular appearance of the microvillus border (mv), equivalent area of terminal web, intact junctional complexes (arrows), and non-vacuolated mitochondria in the cells of both animals. Scale bar=500nm.
(H–I) Cre− and Cre+ mice with acute DSS colitis experience similar degrees of weight loss and disease activity. All mice received a single dose of tamoxifen at day -4 and then were exposed to 2.5% DSS in drinking water from day 0 onwards (n=4/group). * denotes p <0.05 by 2-way ANOVA for Day 5 only. All other comparisons were not significant.
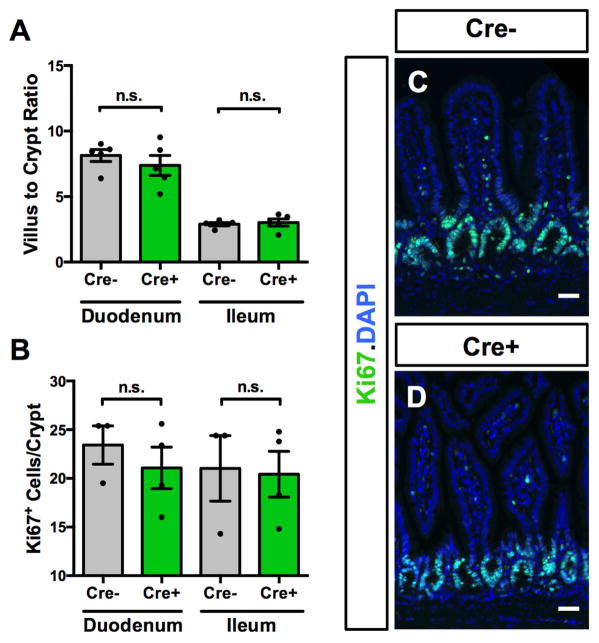
To test the role of enteric glia in epithelial proliferation, we measured the ratio of villus height to crypt depth (V/C ratio) and Ki67-immunoreactive cells/crypt in Cre+ and Cre− mice. Distinct V/C ratios are characteristic of different parts of the small intestine, and V/C ratios are altered by conditions that affect epithelial stem cell function, such as radiation injury, starvation, and inflammatory insults21. There was no change in V/C ratio upon induction of glial elimination, and regional distinctions in V/C ratio were maintained (Fig. 3A). Quantitation of Ki67+ cells revealed that crypt cell mitosis was also not altered (Fig. 2B–D). Elimination of the majority of enteric glia throughout the intestine thus triggers no change in epithelial barrier integrity or proliferation.
Figure 3. Enteric glial elimination does not alter epithelial proliferation.
(A) Villus-to-crypt ratios in Cre− and Cre+ mice were not different, and expected differences between ratios in duodenum and ileum were maintained.
(B–D) Ki67 immunoreactivity was not different in the epithelium of Cre− and Cre+ mice. DAPI marks nuclei. Scale bar=50μm
GFAPHSV-TK mice exhibit off-target effects leading to epithelial damage
To reconcile our observations with those previously reported in mice in which GFAP expression was used to target enteric glia, we attempted to generate GfapCreER;Rosa26DTA mice for direct comparison. We first characterized Gfap promoter-driven Cre expression by breeding GfapCreER mice with a Rosa26LacZ reporter strain22. Minimal reporter expression was detected in the ENS upon tamoxifen treatment despite robust CNS reporter expression, consistent with previous reports of low enteric recombination efficiency in other GfapCreER mice23. LacZ enzymatic activity was, however, detected within occasional stripes of epithelial cells (Figure 4A), suggesting that the Gfap promoter is transiently active in some intestinal epithelial stem cells. Isolated TdTomato-expressing cells were also observed in the small intestinal epithelium of GfapCre;Rosa26TdTomato reporter mice (Figure 4B)24. Consistent with these findings, rare epithelial cells in the distal small intestine were GFAP-immunoreactive (Figure 4C).
Figure 4. GFAP is expressed in rare epithelial cells in the distal small intestine.
(A) X-Gal staining of ileum from GfapCreER;Rosa26LacZ mouse at 15dpt. A stripe of X-Gal labeled epithelial cells ranging from crypt base to villous tip in the small intestine suggests that Cre recombinase was expressed in an epithelial stem cell at the time of induction.
(B) TdTomato expression in the ileal mucosa of a 6-week-old GFAPCre;Rosa26TdTomato mouse. Arrow highlights TdTomato+ epithelial cell.
(C) GFAP immunoreactivity in the ileal mucosa of a wild-type mouse. Arrow highlights GFAP-expressing epithelial cell.
Scale bars A-B=50μm; C=25μm
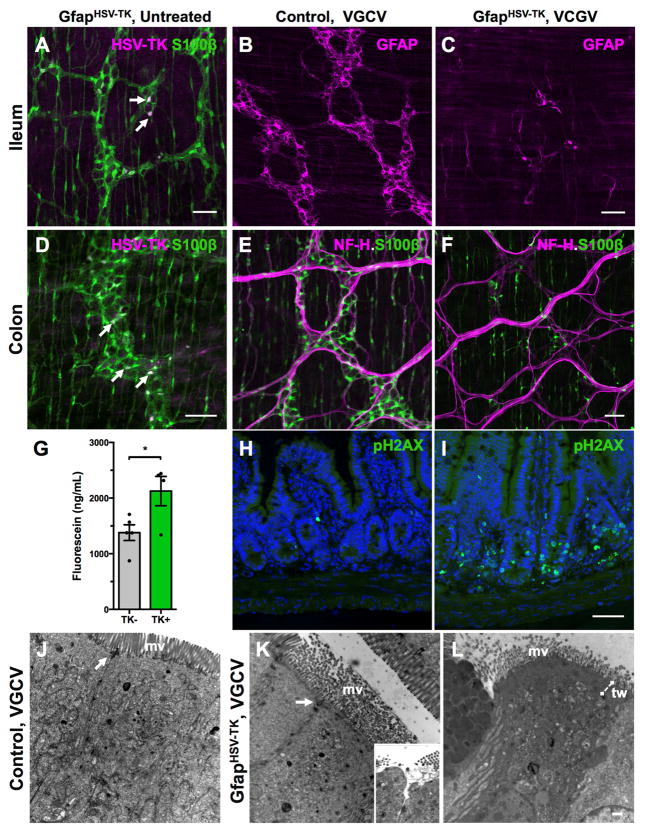
Because we were unable to identify a GfapCreER line that selectively expressed Cre in enteric glia, we characterized the original GfapHSV-TK mouse line used in prior studies7. A subset of enteric glia, mainly within myenteric ganglia, exhibited TK immunoreactivity in GfapHSV-TK mice (Figure 5A, D). Upon systemic treatment with subcutaneous ganciclovir or the oral pro-drug valganciclovir, GFAP-expressing enteric glia were depleted throughout the gastrointestinal tract (Figure 5B–C). Following treatment of TK+ mice with valganciclovir, the ilea and colons contained respectively, 53% and 65% fewer S100β+ myenteric and intramuscular glia than their TK− littermates (Figure 5E–F; n=5 mice/group). Consistent with prior reports, valganciclovir treatment led to hemorrhagic inflammation in the mid-to-distal small intestine of TK+ mice evident between 6–15 days of treatment, as well as altered macromolecular epithelial permeability (Figure 5G). Prior reports attributed the restricted nature of inflammation in these mice to a presumed lack of transgene expression in the large intestine and regional differences in rate of glial turnover, but these presumptions were never verified. We detected both TK transgene expression and valganciclovir -dependent glial loss in the large intestines of GfapHSV-TK mice (Figure 5D–F), suggesting that the transgene expression pattern does not explain the regional specificity of intestinal inflammation in these mice.
Figure 5. GfapHSV-TK mice exhibit transgene expression throughout the intestine and signs of off-target injury to the epithelium.
(A, D) Immunostaining for S100β and HSV-TK shows that only a subset of myenteric glia expresses TK (arrows) in the ileum and colon of untreated GFAPHSV-TK mice. Intramuscular glia are S100β-immunoreactive but none express TK.
(B–F) Valganciclovir (VGCV) treatment eliminates both GFAP+ (compare B and C) and S100β+ (compare E and F) enteric glia in the small and large intestine of GFAPHSV-TK mice. NF-H immunostaining highlights nerve fiber bundles in the myenteric plexus.
(G) Serum FITC levels 4 hours after oral gavage of 478Da fluorescein conjugate into TK− and TK+ mice treated with VGCV shows increased macromolecular intestinal permeability in TK+ mice.
(H–I) Immunoreactivity of the apoptotic marker, phosphorylated histone 2AX (pH2AX) in cross-sections of ilea from TK− (H) and TK+ (I) mice treated with VGCV. Nuclei counterstained with DAPI. VGCV treatment increased apoptosis primarily in crypt cells, but also in the walls of the villi and in the lamina propria of the TK+ mouse.
(J) The ultrastructure of enterocytes in the small intestine of a control TK− mouse treated with VGCV appears normal. The microvillus (mv) border was intact, and their core of actin filaments formed a terminal web and inserted into the adherens junction component of junctional complexes (arrow).
(K) The ultrastructure of some enterocytes in the small intestines of VGCV-treated TK+ mice was abnormal. The microvilli were disorganized, lacked microfilament cores, and displayed variable diameters suggesting swelling. The area of the terminal web was expanded and appeared as an amorphous electron-dense mat. Occasionally, adherens junctions were disrupted although tight junctions remained intact. In rare cells, the underlying cytoplasm appeared to push the microvillus border aside and protrude into the lumen (see inset). In these regions, tight junctions were disrupted, opening a channel to the intercellular space.
(L) In an evident later stage of abnormality in a VGCV-treated TK+ mouse, the cytoplasm of some enterocytes was electron dense, the terminal web (tw) region was expanded, and the mitochondrial matrix was vacuolated.
Scale bars A–I=50μm; J–L=500nm
Unlike DTA, the cellular toxin generated by HSV-TK from ganciclovir can diffuse into and poison neighboring cells. To determine whether non-glial cells were affected in GfapHSV-TK mice, we analyzed expression of phosphorylated-histone 2AX (pH2AX), a marker of double-stranded DNA breaks that are an early step in the apoptotic cascade25. Phosphorylated-H2AX immunoreactivity was widespread in small intestinal crypts in TK+ mice treated with valganciclovir (Figure 5H–I). Extensive pH2AX immunoreactivity was also detected in non-TK expressing cells in the muscularis externa and myenteric plexus, suggesting that the toxicity of metabolites generated by HSV-TK is not limited to enteric glia in GfapHSV-TK mice (Figure S3A–D). In contrast, in Plp1CreER;Rosa26DTA mice treated with tamoxifen, there was no difference in epithelial pH2AX immunoreactivity between Cre− and Cre+ animals despite more extensive glial loss in Cre+ than in TK+ mice (Figure S3E–F). Ultrastructural analysis of small intestines from GfapHSV-TK mice treated with valganciclovir revealed abnormal appearing, vacuolated epithelial cells with disorganized microvilli and intercellular junctions, even in regions with intact mucosal glia (Figures 5J–L). These data suggest that the GfapHSV-TK model of glial elimination causes toxicity to non-glial cells. GFAP expression in rare ileal epithelial cells likely contributes to these off-target effects. This inadvertent toxicity might explain why inflammation occurs in GfapHSV-TK mice but not in Plp1CreER;Rosa26DTA mice, in which glial elimination is cell-autonomous.
Enteric glia regulate colonic motility in a sex-dependent manner
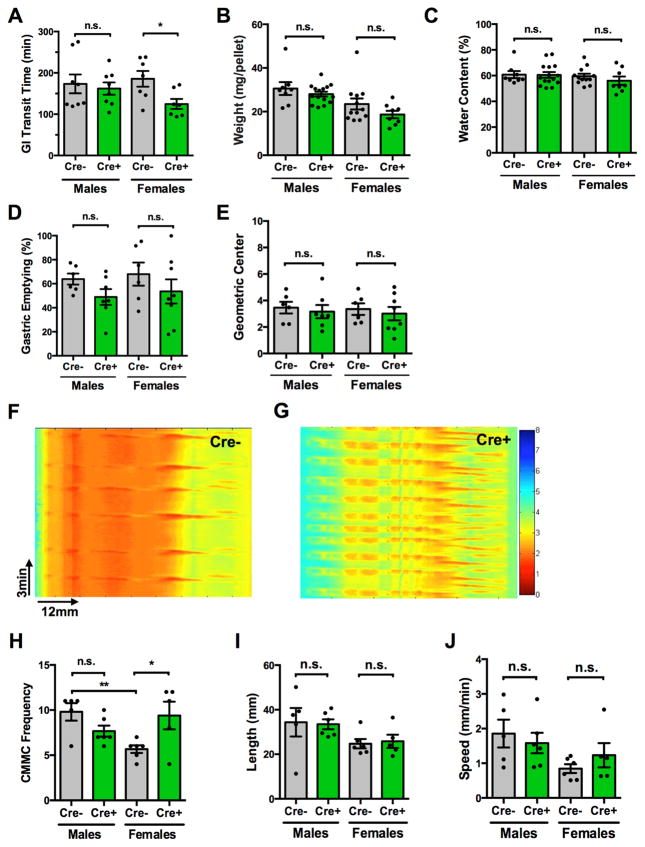
Although elimination of PLP1-expressing cells did not affect epithelial homeostasis, it altered gastrointestinal motility selectively in females. Total gastrointestinal transit time (GITT) was shorter in female Plp1CreER;Rosa26DTA mice than in Cre− littermates after treatment with tamoxifen; but no different in males (Figure 6A). Fecal pellet size and water content were unchanged by glial loss in both sexes (Figure 6B–C), suggesting that fecal composition was unlikely to explain the difference in transit. GITT is a composite of the time required for gastric emptying, small intestinal and colonic transit, each of which is dependent on intrinsic enteric neuronal activity and modified by CNS input. When measured separately, enteric glial elimination did not alter the rates of gastric emptying or small intestinal transit (Figure 6D–E), suggesting that the effects of enteric glial elimination are most prominent in the colon. We therefore examined colonic motility in more detail and excluded the effects of extrinsic nerves by studying the propagation of colonic migrating motor complexes (CMMCs; an ENS-dependent manifestation of the peristaltic reflex) in an ex vivo preparation15, 26. Colons were isolated from Cre− and Cre+ mice, suspended in oxygenated Krebs solution and perfused through the lumen at an intraluminal pressure sufficient to induce CMMCs. Intrinsic microcircuits of the ENS drive CMMCs under these conditions, which can be recorded and analyzed. All CMMCs propagated in an oral to anal direction; no retrograde propagation was observed. In males, there was no difference between Cre− and Cre+ mice in CMMC frequency, velocity or length of propagation (Figure 6H–J). In contrast, CMMC frequency in female Cre+ mice was twice that of their Cre− littermates (Figure 6F–H). Notably, CMMC frequency in Cre− controls was higher in males than females (Figure 6H). Glial disruption selectively altered the peristaltic reflex in female mice, increasing CMMC frequency to male levels (Figure 6H). Velocity and length of CMMC propagation were both unchanged in female glial-depleted mice (Figure 6I–J), suggesting that enteric glia are specifically important for regulating CMMC frequency.
Figure 6. Enteric glial elimination leads to a sex-dependent acceleration of colonic motility.
(A) Total gastrointestinal transit time (GITT) in Rosa26DTA (Cre−, control) and Plp1CreER;Rosa26DTA (Cre+, experimental) mice was compared at 5dpt. GITT was accelerated in female mice upon glial elimination, but was not altered in males.
(B–C) Stool mass per pellet and water content are unchanged by glial elimination in both sexes.
(D) Gastric emptying in male and female Cre+ mice was no different than in Cre− littermates.
(E) Small intestinal transit time (geometric center is a proxy) was not affected by glial elimination in either sex.
(F–G) Spatiotemporal maps derived from video recordings of contractions of colons from Cre− (F) and Cre+ (G) female mice. The maps show the patterns of contractions of the colonic musculature as a function of time. Colonic width is measured as a proxy for contraction, and is depicted on a colorimetric scale (red is maximal contraction). Colonic migrating motor complexes (CMMCs) can be recognized as repeating proximal-distal contractions. X-axis = distance (mm), y-axis = time (min).
(H) In Cre− controls, CMMC frequency was lower in females than males. Glial elimination increased female CMMC frequency to levels seen in males (n=5–6mice/group).
(I–J) Length and velocity of CMMC propagation are not different between Cre− and Cre+ mice.
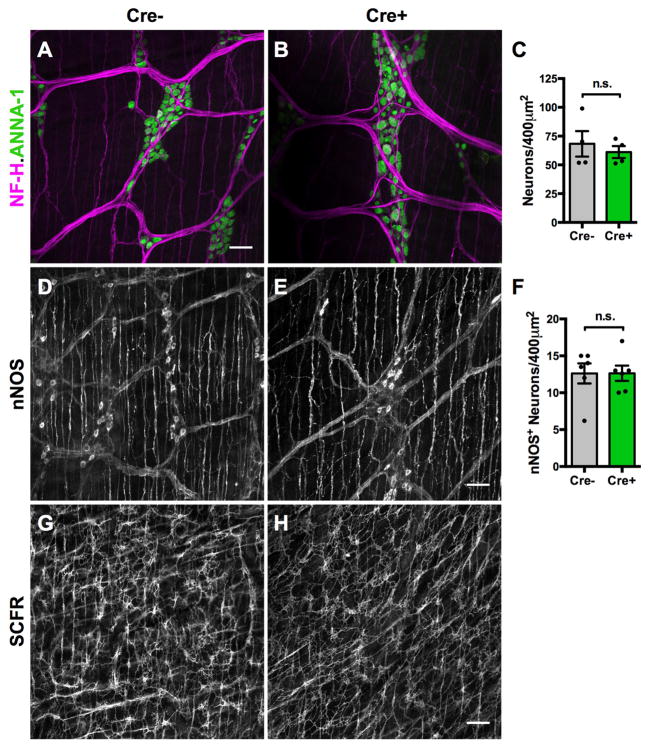
The integrity of cell types important for peristaltic reflexes was examined next. Enterochromaffin cells trigger peristaltic reflexes through the release of serotonin (5-HT)27. There was no difference in colonic enterochromaffin cell number between Cre− and Cre+ mice, in males (p=0.62, n=4/group) or females (p=0.5, n=4/group). Total neuronal density and the density of nitrergic inhibitory motor neurons were also not different in Cre− and Cre+ mice (Figure 7A–F), suggesting that altered motility was not due to an effect on neuronal survival. The nerve fiber network, furthermore, appeared grossly normal (Figure 4A–B). The network of interstitial cells of Cajal (ICCs), the pacemaker cells of the gut resident in the muscularis externa28, also appeared intact (Figure 7G–H). Enteric glia are thus not necessary for the maintenance of enteric neurons, enterochromaffin cells or ICCs in the myenteric plexus, and the role of glia in gastrointestinal motility is probably more complex. In summary, our findings suggest that glia play an essential and sex-dependent role in the ENS regulation of gastrointestinal motility but are not required for epithelial maintenance or neuronal survival.
Figure 7. Enteric glial elimination does not alter neuronal survival or the myenteric networks of nitrergic neurons and interstitial cells of Cajal.
(A–C) Immunoreactivities of neurofilament-H (NF-H) and the pan-neuronal marker, ANNA-1, in colons of Cre− (A) and Cre+ (B) mice revealed no difference in myenteric neuron number or the pattern of interganglionic connectives.
(D–F) Neuronal nitric oxide synthase (nNOS) immunostaining revealed no difference in nitrergic neuron density in the myenteric plexus of Cre− and Cre+ mice.
(G–H) SCFR immunoreactivity, a marker of interstitial cells of Cajal (ICC), revealed no difference in the ICC-MP network in Cre− and Cre+ mice.
Scale bars A–B=25μm; D–G=50μm
Discussion
Enteric glia and epithelial homeostasis
The current understanding of enteric glia is heavily influenced by studies that used the Gfap promoter to target cells for elimination in mice and reported intestinal inflammation. We show that using a cell-autonomous toxin to target PLP1-expressing cells eliminates an even wider population of glia, including those that express GFAP, without causing intestinal inflammation. These findings suggest that the method of disrupting enteric glia, rather than glial disruption itself, explains the previous observations of inflammation. We find that GFAPHSV-TK mice exhibit extensive signs of non-glial toxicity, especially in the intestinal epithelium. This is probably due to GFAP expression in rare epithelial cells of the distal small intestine and non-cell-autonomous effects of the toxin generated by HSV-TK. Findings from transgenic mouse models that utilize the Gfap promoter to study gastrointestinal function must be interpreted with the possibility of epithelial promoter activity in mind.
Elimination of PLP1-expressing cells using the cell-autonomous DTA approach triggered widespread, selective loss of enteric glia, yet did not induce changes in intestinal permeability, epithelial intercellular junctions, microbial translocation, enterocyte ultrastructure, or crypt cell proliferation. These observations led us to conclude that enteric glia are not required for epithelial maintenance in vivo. In the only previous study in which enteric glia were eliminated without selectively targeting GFAP-expressing cells (using the gliotoxin 6-aminonicotinamide), the ultrastructure of myenteric neurons and the colonic epithelium appeared normal despite extensive glial loss29. Similarly, studies in which investigators disrupted glial function without cell elimination also support the idea that glial dysfunction does not directly cause epithelial dysfunction (Supplemental Table 3)30, 31. The chemical and genetic lesions in prior studies probably disrupted some subset(s) of enteric glia more than others; moreover, none of the studies examined epithelial proliferation or in vivo barrier permeability. Plp1CreER;Rosa26DTA mice exhibit robust, inducible elimination of all types of enteric glia throughout the intestine without any evidence of epithelial defects. These data show that intestinal epithelial integrity does not depend on the presence or normal function of enteric glia in healthy mice.
Enteric glia can secrete a number of signaling molecules that modulate epithelial function in vitro8, 10, 32–36 yet glia seem unnecessary for epithelial integrity in vivo. This difference could occur because other cell types secrete these signaling molecules in the absence of glia, or because enteric glia have different capabilities in vitro and in vivo. Such differential capabilities have been reported with regard to their neurogenic potential23, 37. Enteric glia, like other glia, are probably dynamic and highly sensitive to their microenvironment. Removing them from the intestinal milieu might enable them to secrete signals that they do not normally secrete in vivo; moreover, cultured glia might approximate some subset(s) of enteric glia better than others. Enteric glia express toll-like receptors38, require the microbiome for migration39, and communicate with immune cells5. It is thus conceivable that although enteric glia are not required for epithelial homeostasis in healthy mice, they might influence the epithelium when “activated” by certain infectious or immunological conditions. Plp1CreER;Rosa26DTA mice represent a new tool to test this possibility in vivo. Glia can adopt pro- or anti-inflammatory phenotypes depending on the context40, and enteric glia play a role in the neurotoxic effects of intestinal inflammation41. Further investigations of how infectious, dietary, or genetic insults modulate glial phenotype are necessary to better understand the role of glia in epithelial physiology and pathophysiology.
Glial regulation of gastrointestinal motility
Although glial elimination in Plp1CreER;Rosa26DTA mice does not alter the intestinal epithelium, it does cause specific defects in gastrointestinal motility. Glial-depleted female mice, unexpectedly, had accelerated total gastrointestinal transit time and increased CMMC frequency; similarly, mice treated with 6-aminonicotinamide develop diarrhea29. Studies of disrupted enteric glial function also support a role for glia in the regulation of intestinal motility, although the region of intestine affected and the direction of change vary across studies (Supplemental Table 3)30, 42, 43 Together with our findings, these observations show that altering glial function affects gastrointestinal motility; however, the magnitude and direction of the effects are dependent on the nature of the lesion. This variability might reflect the differential effects of each of these lesions on enteric glial subtypes and on glia outside the ENS. Identifying molecular markers for enteric glial subtypes and developing approaches to disrupt enteric glia without altering extra-enteric glia will be essential to better define the role of glia in the regulation of gastrointestinal motility.
Remarkably, we found that the effects of glial elimination on intestinal motility in Plp1CreER;Rosa26DTA mice were sex-dependent. Sex-dependent phenotypes were observed in both in vivo and ex vivo measurements of motility, suggesting that these differences cannot simply be attributed to extrinsic inputs. There were no sex differences in extent of glial elimination or in any other phenotype examined, suggesting that this finding is highly specific to glial regulation of motility. Sex differences were not examined in previous studies of enteric glial disruption; however, functional gastrointestinal disorders exhibit prominent sex differences in both prevalence and phenotype that remain largely unexplained44. Differences in glial regulation of motility could explain some of these observations.
Many cell types in the gut play crucial roles in motility, including enterochromaffin cells, enteric neurons, muscularis macrophages, sympathetic inputs, and the 3 cell types that comprise the SIP syncytium (smooth muscle cells, ICCs, and platelet-derived growth factor receptor α+ cells)45. The sex-dependent defect in gastrointestinal motility observed upon glial elimination could be a direct effect resulting from sex differences in enteric glia themselves, or an indirect effect arising from sexual dimorphism in any of the other cell types that interact with glia. Enterochromaffin cells vary in number over the course of the estrus cycle26 and enteric glia closely appose enteroendocrine cells3. Although glial elimination in Plp1CreER;Rosa26DTA mice did not affect enterochromaffin cell density in either sex, it remains possible that glia modulate 5-HT release or other aspects of enterochromaffin cell function that are sex-dependent. Some enteric neurons express estrogen receptors46 and intestinal smooth muscle cells express androgen receptors47, suggesting that sex hormones signal to cell types important for motility. Plp1CreER;Rosa26DTA mice offer a robust tool to interrogate these intercellular interactions. Glia are considered essential for neuronal health and yet enteric neurons in Plp1CreER;Rosa26DTA mice appeared remarkably intact and gastrointestinal motility in male mice was unchanged. While glia may not be essential for enteric neuronal survival in healthy mice, their absence may have other consequences for neurons. Electrophysiological studies of Plp1CreER;Rosa26DTA mice as well as development of longer-term models of glial loss will be helpful to analyze these consequences.
In summary, genetic ablation of the majority of enteric glia does not alter intestinal epithelial proliferation, barrier function or ultrastructure but does lead to a sex-dependent defect in colonic motility. Previous findings using GFAPHSV-TK mice to support a role for glial regulation of the epithelium were probably confounded by toxicity to neighboring non-glial cells. These findings change the current paradigm for understanding the role of enteric glia in digestive physiology and pathophysiology.
Supplementary Material
Acknowledgments
This work was supported by the NASPGHAN George Ferry Young Investigator Award (M.R.), NIH DK098903 (M.R.), the AGA-Takeda Research Scholar Award (M.R.), Paul Marks Scholar Award (M.R.), philanthropic support from Ivan and Phyllis Seidenberg (M.R.), NIH NS15547 (M.D.G.), Einhorn Family Charitable Trust (M.D.G.), and NIH R01NS35884 (G.C.). We thank V. Lennon for ANNA-1 antibody, S. Wahan for technical assistance, and both M. Rutlin and A. Chalazonitis for helpful discussions.
Footnotes
Conflict of Interest Statement
Authors have no relevant conflicts of interest.
Author Contributions
M.R. designed and performed experiments, analyzed data and wrote the manuscript. D.D.R., S.C., L.D. and W.S. performed experiments and analyzed data. G.C. and M.D.G. designed experiments, analyzed data and revised the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Author names in bold designate shared co-first authorship.
- 1.Furness JB. The enteric nervous system. Malden, Mass: Blackwell Pub; 2006. [Google Scholar]
- 2.Zuchero JB, Barres BA. Glia in mammalian development and disease. Development. 2015;142:3805–9. doi: 10.1242/dev.129304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohorquez DV, Samsa LA, Roholt A, et al. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PLoS One. 2014;9:e89881. doi: 10.1371/journal.pone.0089881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu YY, Peng SJ, Lin HY, et al. 3-D imaging and illustration of mouse intestinal neurovascular complex. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1–11. doi: 10.1152/ajpgi.00209.2012. [DOI] [PubMed] [Google Scholar]
- 5.Ibiza S, Garcia-Cassani B, Ribeiro H, et al. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature. 2016;535:440–3. doi: 10.1038/nature18644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan DW, Barker N. Intestinal stem cells and their defining niche. Curr Top Dev Biol. 2014;107:77–107. doi: 10.1016/B978-0-12-416022-4.00003-2. [DOI] [PubMed] [Google Scholar]
- 7.Bush TG, Savidge TC, Freeman TC, et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 8.Savidge TC, Newman P, Pothoulakis C, et al. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344–58. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 9.Cornet A, Savidge TC, Cabarrocas J, et al. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn’s disease? Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13306–11. doi: 10.1073/pnas.231474098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neunlist M, Aubert P, Bonnaud S, et al. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway. American journal of physiology Gastrointestinal and liver physiology. 2007;292:G231–41. doi: 10.1152/ajpgi.00276.2005. [DOI] [PubMed] [Google Scholar]
- 11.Ochoa-Cortes F, Turco F, Linan-Rico A, et al. Enteric Glial Cells: A New Frontier in Neurogastroenterology and Clinical Target for Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2016;22:433–49. doi: 10.1097/MIB.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boesmans W, Lasrado R, Vanden Berghe P, et al. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia. 2014 doi: 10.1002/glia.22746. [DOI] [PubMed] [Google Scholar]
- 13.Rao M, Nelms BD, Dong L, et al. Enteric glia express proteolipid protein 1 and are a transcriptionally unique population of glia in the mammalian nervous system. Glia. 2015 doi: 10.1002/glia.22876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamouse-Smith ES, Tzeng A, Starnbach MN. The intestinal flora is required to support antibody responses to systemic immunization in infant and germ free mice. PLoS One. 2011;6:e27662. doi: 10.1371/journal.pone.0027662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margolis KG, Li Z, Stevanovic K, et al. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. J Clin Invest. 2016;126:2221–35. doi: 10.1172/JCI84877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller MS, Galligan JJ, Burks TF. Accurate measurement of intestinal transit in the rat. Journal of pharmacological methods. 1981;6:211–7. doi: 10.1016/0160-5402(81)90110-8. [DOI] [PubMed] [Google Scholar]
- 17.Mallon BS, Shick HE, Kidd GJ, et al. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci. 2002;22:876–85. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duregotti E, Negro S, Scorzeto M, et al. Mitochondrial alarmins released by degenerating motor axon terminals activate perisynaptic Schwann cells. Proc Natl Acad Sci U S A. 2015;112:E497–505. doi: 10.1073/pnas.1417108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traka M, Arasi K, Avila RL, et al. A genetic mouse model of adult-onset, pervasive central nervous system demyelination with robust remyelination. Brain: a journal of neurology. 2010;133:3017–29. doi: 10.1093/brain/awq247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aube AC, Cabarrocas J, Bauer J, et al. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut. 2006;55:630–7. doi: 10.1136/gut.2005.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong WM, Wright NA. Cell proliferation in gastrointestinal mucosa. J Clin Pathol. 1999;52:321–33. doi: 10.1136/jcp.52.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casper KB, Jones K, McCarthy KD. Characterization of astrocyte-specific conditional knockouts. Genesis. 2007;45:292–9. doi: 10.1002/dvg.20287. [DOI] [PubMed] [Google Scholar]
- 23.Joseph NM, He S, Quintana E, et al. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J Clin Invest. 2011;121:3398–411. doi: 10.1172/JCI58186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia AD, Doan NB, Imura T, et al. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–41. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 25.Rogakou EP, Nieves-Neira W, Boon C, et al. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem. 2000;275:9390–5. doi: 10.1074/jbc.275.13.9390. [DOI] [PubMed] [Google Scholar]
- 26.Balasuriya GK, Hill-Yardin EL, Gershon MD, et al. A sexually dimorphic effect of cholera toxin: rapid changes in colonic motility mediated via a 5-HT3 receptor-dependent pathway in female C57Bl/6 mice. J Physiol. 2016;594:4325–38. doi: 10.1113/JP272071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bülbring E, Crema A. The release of 5-hydroxytryptamine in relation to pressure exerted on the intestinal mucosa. J Physiol (Lond) 1959;146:18–28. doi: 10.1113/jphysiol.1959.sp006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward SM, Sanders KM. Physiology and pathophysiology of the interstitial cell of Cajal: from bench to bedside. I. Functional development and plasticity of interstitial cells of Cajal networks. Am J Physiol Gastrointest Liver Physiol. 2001;281:G602–11. doi: 10.1152/ajpgi.2001.281.3.G602. [DOI] [PubMed] [Google Scholar]
- 29.Aikawa H, Suzuki K. Enteric gliopathy in niacin-deficiency induced by CNS glio-toxin. Brain Res. 1985;334:354–6. doi: 10.1016/0006-8993(85)90231-8. [DOI] [PubMed] [Google Scholar]
- 30.Nasser Y, Fernandez E, Keenan CM, et al. Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function. Am J Physiol Gastrointest Liver Physiol. 2006;291:G912–27. doi: 10.1152/ajpgi.00067.2006. [DOI] [PubMed] [Google Scholar]
- 31.Grubisic V, Gulbransen BD. Enteric glial activity regulates secretomotor function in the mouse colon but does not acutely affect gut permeability. J Physiol. 2017 doi: 10.1113/JP273492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinkamp M, Geerling I, Seufferlein T, et al. Glial-derived neurotrophic factor regulates apoptosis in colonic epithelial cells. Gastroenterology. 2003;124:1748–57. doi: 10.1016/s0016-5085(03)00404-9. [DOI] [PubMed] [Google Scholar]
- 33.Xiao W, Wang W, Chen W, et al. GDNF is involved in the barrier-inducing effect of enteric glial cells on intestinal epithelial cells under acute ischemia reperfusion stimulation. Mol Neurobiol. 2014;50:274–89. doi: 10.1007/s12035-014-8730-9. [DOI] [PubMed] [Google Scholar]
- 34.Flamant M, Aubert P, Rolli-Derkinderen M, et al. Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: a role for S-nitrosoglutathione. Gut. 2011;60:473–84. doi: 10.1136/gut.2010.229237. [DOI] [PubMed] [Google Scholar]
- 35.Cheadle GA, Costantini TW, Lopez N, et al. Enteric glia cells attenuate cytomix-induced intestinal epithelial barrier breakdown. PLoS One. 2013;8:e69042. doi: 10.1371/journal.pone.0069042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pochard C, Coquenlorge S, Jaulin J, et al. Defects in 15-HETE Production and Control of Epithelial Permeability by Human Enteric Glial Cells From Patients With Crohn’s Disease. Gastroenterology. 2016;150:168–80. doi: 10.1053/j.gastro.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 37.Laranjeira C, Sandgren K, Kessaris N, et al. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest. 2011;121:3412–24. doi: 10.1172/JCI58200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turco F, Sarnelli G, Cirillo C, et al. Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut. 2014;63:105–15. doi: 10.1136/gutjnl-2012-302090. [DOI] [PubMed] [Google Scholar]
- 39.Kabouridis PS, Lasrado R, McCallum S, et al. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron. 2015;85:289–95. doi: 10.1016/j.neuron.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zamanian JL, Xu L, Foo LC, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown IA, McClain JL, Watson RE, et al. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell Mol Gastroenterol Hepatol. 2016;2:77–91. doi: 10.1016/j.jcmgh.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McClain JL, Grubisic V, Fried D, et al. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology. 2014;146:497–507e1. doi: 10.1053/j.gastro.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McClain JL, Fried DE, Gulbransen BD. Agonist-evoked Ca2+ signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell Mol Gastroenterol Hepatol. 2015;1:631–645. doi: 10.1016/j.jcmgh.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:991–1000. doi: 10.1038/ajg.2012.131. [DOI] [PubMed] [Google Scholar]
- 45.Sanders KM, Koh SD, Ro S, et al. Regulation of gastrointestinal motility--insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012;9:633–45. doi: 10.1038/nrgastro.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell-Thompson M, Reyher KK, Wilkinson LB. Immunolocalization of estrogen receptor alpha and beta in gastric epithelium and enteric neurons. J Endocrinol. 2001;171:65–73. doi: 10.1677/joe.0.1710065. [DOI] [PubMed] [Google Scholar]
- 47.Winborn WB, Sheridan PJ, McGill HC., Jr Sex steroid receptors in the stomach, liver, pancreas, and gastrointestinal tract of the baboon. Gastroenterology. 1987;92:23–32. doi: 10.1016/0016-5085(87)90835-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.