Abstract
Objective
HLA-B27 associated spondyloarthropathies are associated with an altered intestinal microbiota and bowel inflammation. Therefore, we sought to identify B27-dependent changes in both host and microbial metabolites in the HLA-B27/β2m rat and whether microbiota-derived metabolites could impact disease in this major model of spondyloarthropathy.
Methods
Cecal contents were collected from 6wk (pre-diseased) and 16wk (diseased) Fischer 344 HLA-B27/β2m transgenic rats and WT controls. Metabolomic profiling was performed by high-throughput gas- and liquid-chromatography-based mass spectrometry. HLA-B27/β2m rats were treated with microbial metabolites propionate or butyrate in drinking water for 10wks and disease activity subsequently assessed.
Results
Our screen identified 582 metabolites, of which over half were significantly altered by B27 expression at 16wks. Both microbial and host metabolites were altered, with multiple pathways including amino acid, carbohydrate, xenobiotic and medium chain fatty acid metabolism affected. Differences were even observed at 6wks, with upregulation of histidine, tyrosine, spermidine, N-acetylmuramate and glycerate in HLA-B27/β2m rats. Administration of the short chain fatty acid propionate significantly attenuated B27-associated inflammatory disease, albeit was not associated with increased FoxP3+ T cell induction, or altered expression of cytokines IL-10, IL-33 or tight junction protein ZO-1. HLA-B27 expression was also associated with altered host expression of microbial metabolite receptor genes FFAR2, FFAR3 and NIACR1.
Conclusion
HLA-B27 expression profoundly impacts the intestinal metabolome, with changes evident in rats even at 6wks of age. Critically, we demonstrate a microbial metabolite, propionate attenuates development of B27-associated inflammatory disease. These and other microbiota-derived bioactive mediators may provide novel treatment modalities in B27-associated spondyloathropathies.
INTRODUCTION
The intestinal microbiota mediates several core functions essential to host fitness including digestion and metabolite provision, colonization resistance to gut pathogens and signals that promote immune function. A disturbed, or ‘dysbiotic’, gut microbiota is implicated in the pathogenesis of both local inflammatory diseases of the intestine including Crohn’s disease (CD) and ulcerative colitis (UC), but also extra-intestinal inflammatory diseases including arthritis, diabetes and multiple sclerosis (reviewed in [1, 2]. Recently it has been established that dysbiosis may be accompanied by a profound change to the metabolic profile of the gut, including alterations in microbial metabolites such as short chain fatty acids (SCFAs) and trimethylamine N-oxide (TMAO) which are bioactive mediators that modulate host physiology (reviewed in [3]).
The HLA-B27/β2m transgenic rat is a foremost translational model of spondyloarthropathy (SpA). These animals express multiple copies of human HLA-B27 in association with the β2 microglobulin heavy chain [4]. HLA-B27 is the gene with the strongest known genetic association with SpA. These animals model human SpA, with the development of peripheral arthritis, psoriasis and bowel inflammation to varying degrees contingent on the copy number of this transgene in addition to rat background [5]. We have shown previously that HLA-B27 expression significantly alters the intestinal microbiota in this model [6, 7], mirroring parallel reports that SpA patient populations exhibit a dysbiotic gut phenotype [8–10]. These findings complement prior animal and clinical studies that strongly implicate the intestinal microbiota in SpA pathogenesis (reviewed by [11]).
In the present study, we assessed the impact of HLA-B27 expression on the gut metabolome in the rat model of spondyloarthropathy. We found multiple metabolite families were significantly altered by HLA-B27 expression, including amino acids, lipids, carbohydrates and xenobiotics. Amongst the most significantly altered were medium chain and short chain fatty acids. Strikingly, treatment of HLA-B27/β2m transgenic rats with the SCFA propionate significantly attenuated HLA-B27-associated inflammatory disease. These data establish a functional link between HLA-B27 expression, an altered gut metabolome and the pathogenesis of SpA-like disease.
METHODS
Ethical Statement
All animal experiments were performed under the experimental and ethical standards of the Association of Assessment and Accreditation of Laboratory Animal Care International and the Oregon Health and Science University Institutional Animal Care and Use Committee.
Animals
Our colony of Fischer 33-3 HLA-B27/β2m hemizygous rats were maintained as described previously [7]. In brief, hemizygous females were crossed with WT Fischer 344 males to produce HLA-B27/β2m rats and littermate controls. Animals were singly housed and maintained under specific-pathogen free conditions. All animals were fed a diet of standard laboratory chow (LabDiet, St. Louis, MO). All animal groups included males and females, with age/sex matching between groups.
Sample collection
Animals were euthanized at the indicated ages/time points by CO2 asphyxiation and cervical dislocation. Cecal contents (~ 1ml) and cecal and colonic tissue (~50mg) were snap frozen and stored at −80°C prior to subsequent analysis. Snap frozen colonic tissue was collected from mid colon. For cell isolation experiments, intestinal tissue, mesenteric lymph nodes and spleen were collected into PBS/0.1% Bovine Serum Albumin and stored on ice prior to subsequent processing. For histology, longitudinal sections of cecum, and ~1cm cross-sections of proximal-, mid- and distal colon were fixed in 10% neutral buffered formalin.
Metabolomic profiling of the gut
Cecal content samples were analyzed using the METABOLON HD4 platform (Metabolon Inc, Durham, NC). In brief (see Supplementary methods for further detail), samples were prepared using the automated MicroLab STAR® system from Hamilton Company (Reno, NV). To remove protein and dissociate small molecules bound to protein, samples were precipitated with methanol, centrifuged and extract was divided for ultra-performance liquid chromatography tandem mass-spectrometry (UPLC/MS) analysis and gas chromatography/mass spectrometry (GC/MS) analysis.
The GC/MS metabolite screen was performed using a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer (Thermo Electron Corporation, Austin, TX) using electron impact ionization (EI) and operated at unit mass resolving power. The instrument’s scan range was 50–750 m/z.
SCFA analysis
Short chain fatty acids were measured by GC-MS at the BSR/PK core facility at OHSU. See Supplementary methods.
SCFA treatment
Six week old HLA-B27/β2m rats or WT controls were administered either sodium propionate (150mM) or sodium butyrate (150mM) ad libitum in drinking water throughout the experiment. Animals were necropsied at 16wks of age. These doses and route of administration were selected based upon previously published in vivo studies [12, 13].
Histology and disease scoring
Formalin-fixed intestinal tissues were paraffin embedded, sectioned to 5μm and stained with hematoxylin and eosin. Scoring of intestinal inflammation was performed using a semi-quantitative scoring system (0–12) as described previously [7]. In brief, this four parameter scoring system includes epithelial hyperplasia, goblet cell numbers, leukocytic infiltrate and markers of severe inflammation (e.g. submucosal inflammation, abscesses or ulceration). Each parameter is scored 0–3 with total cecum score and mean colon score (for the three colon sections collected) shown for each animal. By the final time point of 16wks used in our study, animals had not presented with either psoriasis nor arthritis which typically develop at >20wks in the Fischer 344 33-3 HLA-B27/β2m transgenic line.
Analysis of intestinal gene expression
For further details (including primer details) see Supplementary methods. In brief, RNA was extracted from intestinal tissue using TRIZOL reagent (Ambion, Waltham, MA) and reverse transcribed into cDNA using High Capacity cDNA Reverse Transcription kit (Life Technologies, Waltham, MA). For RT-qPCR analysis of IL-17A, IFNγ and IL-1β we used published primers sequences and RT2 SYBR Green qPCR Mastermix (Qiagen, Gaithersburg, MD) as described previously [7]. For all other RT-qPCR analyses we used Taqman Gene Expression Assays (Life Technologies) in conjunction with Maxima Probe qPCR mastermix (Thermo Scientific, Waltham, MA). Samples’ HPRT controls were prepared for both RT-qPCR chemistries for the purpose of normalization.
Analysis of immune cells by flow cytometry
Suspensions of spleen and mesenteric lymph node (MLN) cells were prepared as described previously [14]. Cecal and colonic lamina propria lymphocytes were isolated by EDTA digest, collagenase/DNAase treatment and Percoll gradient exactly as previously [7]. Cells were then surface stained for 20mins on ice with AQUA Live/Dead stain (Invitrogen, Waltham, MA), anti-rat CD4 PerCP-Cy5.5 and anti-rat TcRβ PE (BD Biosciences, San Jose, CA), and then FoxP3 staining was performed using the eBioscience FoxP3 staining kit (San Diego, CA) as per manufacturer’s instructions using anti rat/mouse FoxP3 APC (eBioscience). Cells were acquired on a BD Fortessa instrument (BD Biosciences) and analyzed using FlowJo software (FlowJo LLC, Ashland, OR).
Statistical Analysis
For detailed statistical analysis used for our metabolomic screen see Supplementary methods. For analysis of individual metabolite levels (Figs 2 & 3), data were normalized to an equivalent of 2mg dry weigh of cecal material per sample and log transformed prior to application of Welch’s unpaired t test (that does not assume equal variances) with subsequent Benjamani-Hochberg correction to account for multiple comparisons and a false discovery rate of 0.2 was applied. For 6wk old samples, the least abundant 20% of metabolites (median abundance for each metabolite across the dataset irrespective of genotype or fold difference) were removed prior to this analysis. For comparison of histological scores, gene expression, immune cell frequencies and cecal short chain fatty acid concentrations between untreated and SCFA treated animals, or WT and HLA-B27/β2m transgenic animals, the non-parametric Mann Whitney U test was used unless specified otherwise. All statistical analysis was performed using the R package or PRISM software (GraphPad, San Diego, CA).
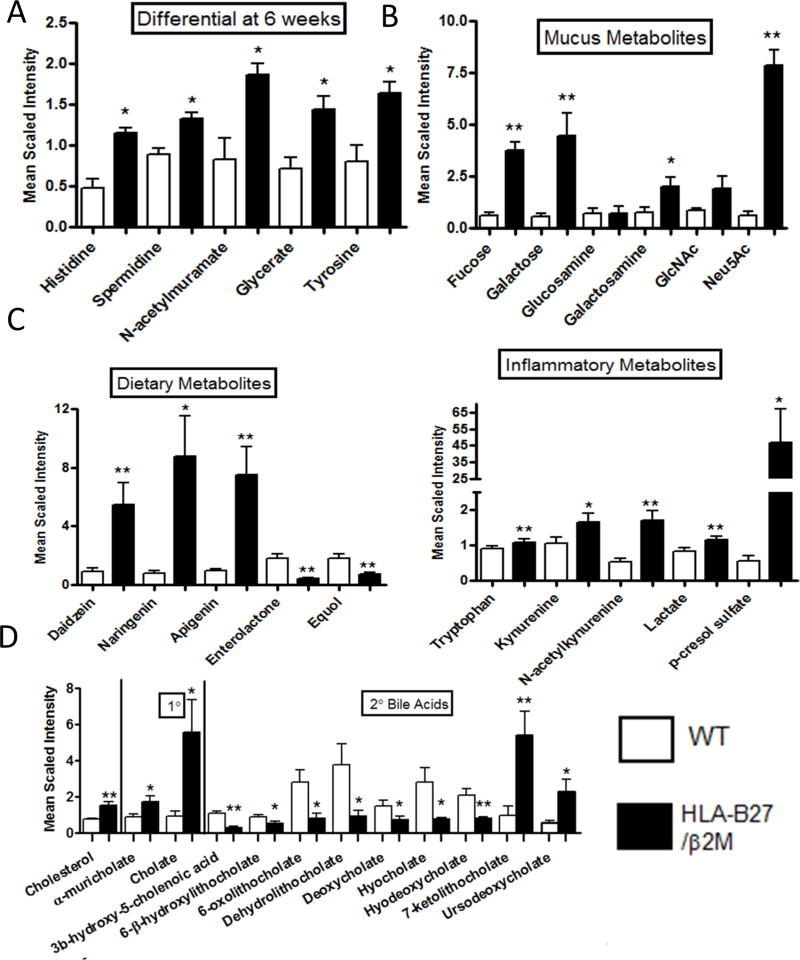
Figure 2. HLA-B27 expression significantly alters amino acids, carbohydrates and xenobiotics.
The abundance of distinct metabolites classes in WT (white bars) vs HLA-B27/β2m transgenic rats (black bars) is shown as determined by UPLS/MS and GC/MS. A) Significantly altered cecal metabolites in pre-disease (6wk old animals) B–E Metabolite levels of B) Mucus components; C) Dietary metabolites; D) Inflammatory metabolites and E) bile acids in 16wk animals are shown (for 6wk animals see Supp Fig 2). Bars represent group means +/− SEM. Symbols represent Benjamini-Hochberg corrected p values with a false discovery rate of 0.2 rather than conventional p values (* < 0.2, ** <0.1). N=8/group.
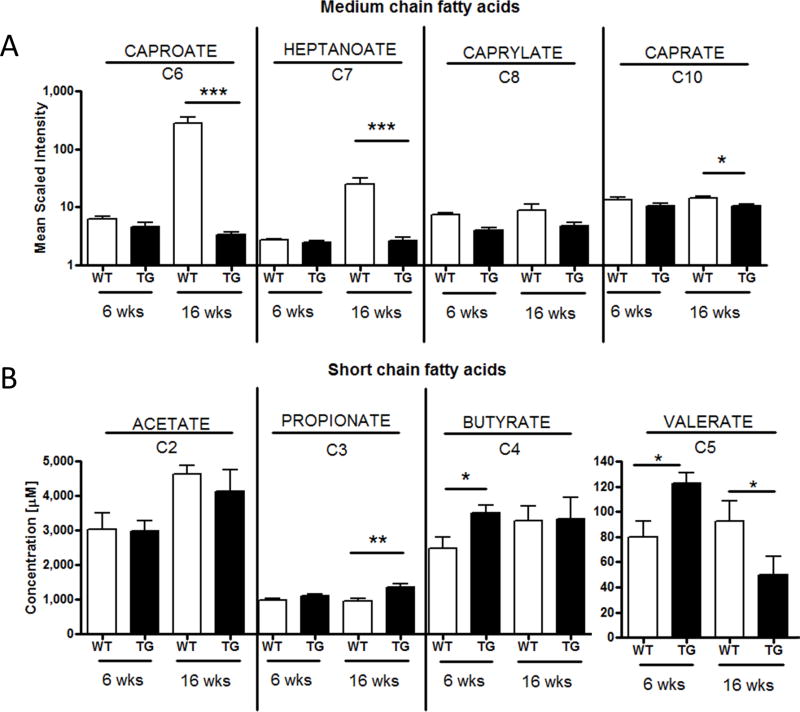
Figure 3. HLA-B27 expression significantly impacts intestinal MCFA and SCFA levels.
A) Medium chain fatty acid fatty acid levels in cecal contents of WT (white bars) vs HLA-B27/β2m transgenic rats (black bars) were measured by LC/MS metabolomic screen. Significance symbols represent Benjamani-Hochberg correct p values as described in Fig 2 (* = p < 0.2, *** = p < 0.05). N=8/group B) Cecal concentration (micromolar) of acetate, propionate, butyrate and valerate as determined by GC-MS. * = p < 0.05, ** = p < 0.01 by Mann Whitney U test. N= 6–8/group. Bars represent mean +/− SEM.
RESULTS
HLA-B27 expression significantly alters the intestinal metabolome
In order to establish the impact of HLA-B27/β2m expression on the intestinal metabolome, we performed metabolomic profiling of cecal contents from HLA-B27/β2m transgenic rats and WT controls at 6 weeks and at 16 weeks of age. These time points were selected to represent pre-diseased animals and those with established disease respectively. Our metabolite screen identified 582 named biochemicals in cecal contents. Using ANOVA with a p value threshold of 0.05 to initially compare groups, we found 188 metabolites were upregulated in 16wk old HLA-B27/β2m animals relative to WT littermate controls, and 66 were downregulated (Supp data), indicating HLA-B27/β2m rats have a profoundly altered intestinal metabolome. Further to these changes with established disease, 52 metabolites were upregulated, and 11 metabolites downregulated in 6 week old transgenic rats relative to age-matched controls. These differences were visualized by hierarchical clustering analysis (Fig 1A) which demonstrates the global metabolic shifts in 16wk old HLA-B27/β2m animals, in addition to more moderate changes in pre-diseased animals (6wks).
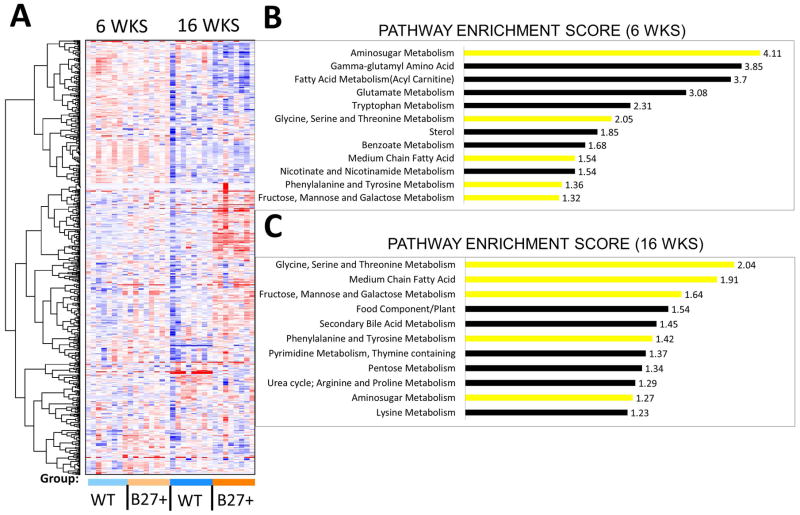
Figure 1. HLA-B27 expression significantly alters the intestinal metabolome.
LC/MS analysis was used to analyze the cecal metabolome of HLA-B27/β2m rats and WT littermate controls either pre-disease (6 weeks) or in animals with active bowel inflammation (16wks). A) Hierarchical clustering analysis of WT and HLA-B27/β2m transgenic rats at each time point. N=8/genotype/age group (B–C) Pathway enrichment analysis of HLA-B27/β2m trangenic rats vs controls at 6 weeks (B) and 16 weeks (C). Yellow bars represent enrichment at both time points. Pathway enrichment scores are shown (see supplementary methods).
To further examine metabolic shifts driven by HLA-B27 expression, we also performed pathway enrichment analysis [15], to identify biochemical pathways that were strongly influenced by HLA-B27 expression (Fig 1B–C). Notably, HLA-B27 expression impacted metabolites involved in numerous biochemical pathways including those associated with amino acid, carbohydrate, lipid and xenobiotic metabolism. Examining both 6 week (Fig 1B) and 16 week time points (Fig 1C), a number of these enriched pathways were shared at both time points including glycine, serine and threonine metabolism; aminosugar metabolism; medium chain fatty acid metabolism; fructose, mannose and galactose metabolism; and phenylalanine and tyrosine metabolism. We noted however that some affected metabolite pathways were only observed at one time point, for instance Gamma-glutamyl amino acids at the 6wk time point (Fig 1B& C, Supp Fig 1) or food component/plant and secondary bile metabolism at the 16wk time point (Fig 1B&C). These may point to evolving disturbances of the intestinal metabolome over time induced by HLA-B27 expression.
Microbial fermentation products are significantly impacted by HLA-B27 expression
In light of the large number of metabolites detected in our screen of cecal contents, and relatively restricted sample size (n = 8 animals/genotype/age group), we were unable to identify any individual metabolites that exhibited differential abundance in 6 week old animals that attained statistical significance when stringently adjusting for multiple comparisons across the entire dataset. After removal of low abundance metabolites however (lowest 20%), we observed five metabolites that exhibited a significantly higher abundance in HLA-B27/β2m transgenic animals vs WT controls (Fig 2A). We did not observe any significantly downregulated individual metabolites at this time point. Namely these upregulated metabolites (Fig 2A) were histidine and tyrosine (amino acids), spermidine (a polyamine), N-acetylmuramate (a microbial cell wall component) and glycerate (a sugar). Histidine, spermidine and glycerate were also elevated at 16wks (Supp Fig 2A) indicating metabolomic changes detected with established disease may also be detected in the pre-disease state. Interestingly however, whereas N-acetyl muramate and tyrosine were elevated at 6 wks in HLA-B27/β2m animals, they were significantly decreased once disease had established at 16wks (Fig 2A and Supp. Fig 2).
Our subsequent analysis focused on the 16wk dataset which had a large number of individual metabolites that were significantly impacted by HLA-B27 expression (Supp Table 1). We have shown previously that HLA-B27 expression is accompanied by a marked expansion of mucin-degrading bacterium Akkermansia muciniphila [6, 7]. We therefore examined the abundance of mucus components identified in our metabolomic screen. Strikingly, mucus carboydrates fucose and galactose were dramatically increased in the cecum of B27 animals (Fig 2B). Mucus aminosugar galactosamine and sialic acids N-acetylneuraminate (Neu5Ac) and N-acetyl-glucosamine (GlcNAc) were also increased in HLA-B27/β2m animals (Fig 2B). Mucus aminosugar glucosamine by contrast was unchanged. Further to this major host-derived microbial energy source, we also examined dietary components and their microbially derived metabolites (Fig 2C). Isoflavones daizein, narigenin and apigenin are flavonoid phytophenols present in soy and other plants and were significantly increased in B27+ animals (Fig 2C). By contrast, equol, a microbial derivative of daidzein was significantly decreased. Enterolactone, a mammalian lignin formed by the action of intestinal bacteria on dietary plant lignans was also significantly decreased (Fig 2C). Together the liberation of mucus components and decrease in some plant derived metabolites (but with increased precursors) may indicate markedly shifted metabolic function by the intestinal microbiota in B27+ animals.
We also found a number of metabolites indicative of an active inflammatory response that were significantly upregulated in intestinal contents of HLA-B27/β2m animals. These included the tryptophan derivatives kynurenine and N-acetylkurenine which are downstream metabolites of indoleamine 2,3-dioxygenase (IDO) activity (Fig 2C). Elevated lactate levels are also a hallmark of intestinal inflammation and were likewise significantly increased at 16wks in HLA-B27/β2m animals (Fig 2C). Interestingly, microbial metabolite p-Cresol sulfate, a product of proteolytic fermentation was also highly increased in HLA-B27/β2m animals. This metabolite is associated with inflammation and oxidative stress.
Bile acid metabolism, in particular the conversion of primary to secondary bile acids is another major functional role of the intestinal microbiota. Examination of bile acids in our metabolomic analysis identified that cholesterol and several bile acids were significantly impacted by HLA-B27 expression (Fig 2D). Interestingly cholesterol and primary bile acids alpha-muricholate and cholate were significantly increased in HLA-B27/β2m animals, whereas the secondary bile acid metabolites 3B-hydroxy-5-cholenoic acid, 6-β-hydroxylithocholate, 6-oxolithocholate, dehyrolithocholate, deoxycholate, hyocholate, and hyodeoxycholate were all significantly decreased. Only 2 bile acids, 7-ketolithocholate and ursodeoxycholate were significantly increased in B27+ animals. Together these findings further indicate that HLA-B27 expression broadly impacts the levels of microbially-derived metabolites in the gut.
HLA-B27 expression is associated with altered Medium and Short Chain Fatty Acid levels
Our prior pathway enrichment analysis had identified medium chain fatty acids (MCFAs) as one of the metabolite pathways most impacted by HLA-B27 expression in 16wk old HLA-B27/β2m animals (Fig 1C). In light of this, we further examined MCFA levels in transgenic rats and WT controls (Fig 3A). Strikingly, MCFAs caproic (hexanoic) acid and enthanoic (hepatonic) acid were significantly reduced in diseased (16wk) HLA-B27/β2m animals. Of note, MCFAs capric (decanoic) and caprylic (octanoic) acid levels also trended in the same direction (the former significantly decreased prior to Benjamani-Hochberg adjustment for multiple comparisons). This trend towards lower MCFA levels was also seen in 6 wk old animals (Fig 3A).
Short chain fatty acids (SCFAs) are soluble, low molecular weight metabolites that are major microbial fermentation of dietary fiber and include acetate, propionate, butyrate and valerate. Since these small molecules were not readily detected by our metabolomic analysis, we performed targeted GC/MS on cecal contents from HLA-B27/β2m transgenic and control rats to measure levels of these metabolites (Fig 3B). Validating this approach, we also confirmed that production of these metabolites was critically dependent on the gut microbiota since antibiotic depletion of the gut microbiota with Vancomycin dramatically reduced cecal SCFA levels (Supp. Fig 3). Interestingly, SCFAs also showed B27-dependent differences, although with more variability than MCFAs. For instance, valerate was significantly increased in pre-disease (6wk) HLA-B27/β2m animals vs controls, albeit was significantly lower at the 16wk time point (Fig 3B). Other changes observed were a significant increase in butyrate concentration at 6wks and a significant increase in propionate concentration at 16wks (Fig 3B). Together these findings indicate HLA-B27 expression leads to altered metabolism of several intestinal fatty acids.
Microbial metabolite propionate functionally impacts B27-associated inflammation
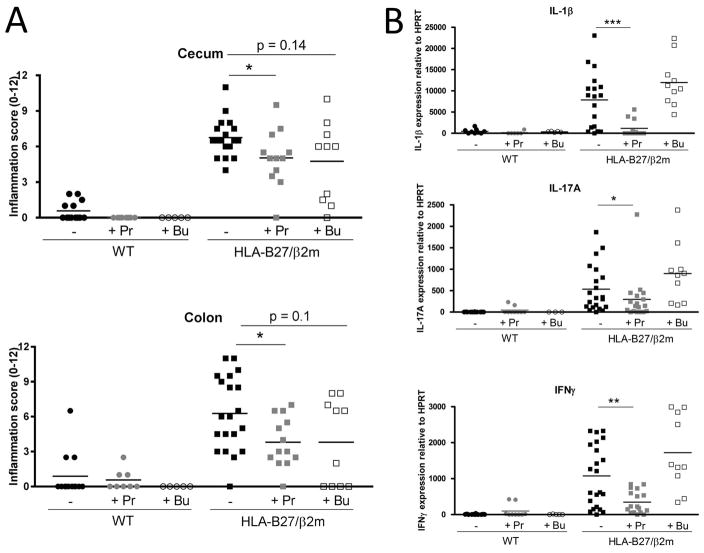
Since HLA-B27 expression clearly impacted the intestinal metabolome, we were eager to determine whether microbial metabolites specifically could modify SpA pathogenesis. In light of the large number of B27-dependent metabolites, we selected SCFAs propionate and butyrate for in vivo functional analysis since they have low toxicity, are commercially available and have reported immune modulatory functions. We therefore administered propionate or butyrate (150mM) in drinking water to pre-diseased (6wk old) rats for 10wks and examined markers of B27-associated bowel inflammation (Fig 4). Strikingly, propionate significantly reduced both cecal and colonic intestinal inflammation in HLA-B27/β2m rats as assessed both histologically and by the mRNA expression of inflammatory mediators IL-1β, IL-17A and IFNγ (Fig 4A–D). By contrast, the effect of butyrate was more subtle, with a trend toward reduced histology scores but with no impact on inflammatory cytokine expression (Fig 4A–D). SCFA treatment had no significant impact on body mass (Supp. Fig 4).
Figure 4. Administration of SCFA sodium propionate significantly attenuates HLA-B27 associated immune pathology.
Six week old HLA-B27/β2m transgenic rats or WT littermate controls were treated with either sodium propionate (150mM) or sodium butyrate (150mM) for 12 weeks in drinking water. At necropsy (16 wks), cecal and colon were harvested to analyze intestinal inflammation. A) Histological assessment of intestinal inflammation in Cecum and Colon by semi-quantitative scoring system (0–12). B) Relative mRNA expression of IL-1β, IL-17A and IFNγ in colon tissue. Bars represent group means and each symbol represents an individual animal. Data is representative of 3 pooled independent experiments. Legend: ‘−’ = untreated controls, ‘P’ = propionate treated, ‘B’ = butyrate treated. * = p < 0.05, ** = p < 0.01.
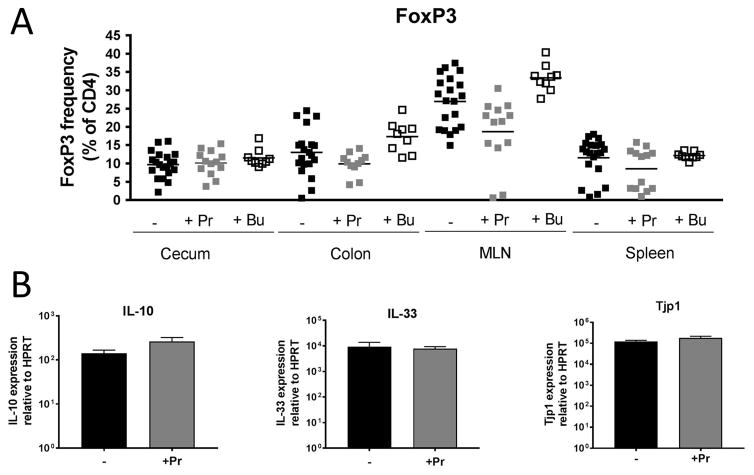
The reported immune modulatory properties of SCFAs include the induction of FoxP3+ve regulatory T cells and the induction of immune regulatory cytokines in the intestine. We therefore determined the frequency of CD4+ FoxP3+ T cells in cecum, colon, the gut-draining mesenteric lymph node (MLN) and spleen following SCFA administration in HLA-B27/β2m rats (Fig 5A). However, we did not observe a higher Treg frequency at any of these sites following SCFA treatment. This effect was also not seen in non-inflamed WT animals (Supp. Fig 5). We did observe a trend towards reduced Treg frequency in the MLN of propionate-treated HLA-B27/β2m animals (Fig 5A), an observation we ascribe to the reported accumulation of Treg at this site with intestinal inflammation (see Asquith et al., 2016) being diminished due to less bowel inflammation in propionate-treated animals. Further to lack of Foxp3+ Treg induction, we also did not observe an effect of SCFA administration on the intestinal mRNA expression of either immune modulatory cytokines IL-10 or IL-33 (Fig 5B) or tight junction protein zona occudulin-1 (ZO-1) in HLA-B27/β2m rats (Fig 5C). We also did not find an obvious anti-microbial effect of SCFA administration that might lower microbial load in the gut and attenuate disease (Supp. Figure 6).
Figure 5. SCFA administration does not significantly impact FoxP3+ve Treg induction, expression of immune regulatory cytokines or tight junction components.
Six week old HLA-B27/β2m transgenic rats or WT littermate controls were treated with either sodium propionate (150mM) or sodium butyrate (150mM) for 12 weeks in drinking water. A) The frequency of CD4+ FoxP3+ ve T cells in cecum, colon, mesenteric lymph node (MLN) and spleen in B27+ animals is shown (see sup fig 5 for WT animals). Each symbol represent an individual animals and horizontal bars represent group means. B) Relative colonic mRNA expression of IL-10 and IL-33. C) Relative colonic mRNA expression of tight junction protein ZO-1/Tight junction protein 1. Bars represent group mean +/− SEM. All mRNA expression data normalized to HPRT. N=8–11 animals/group. Statistical significance (p < 0.05) was not observed by Mann Whitney U test between treated and untreated groups for the parameters shown.
HLA-B27 expression alters host expression of microbial metabolite receptors
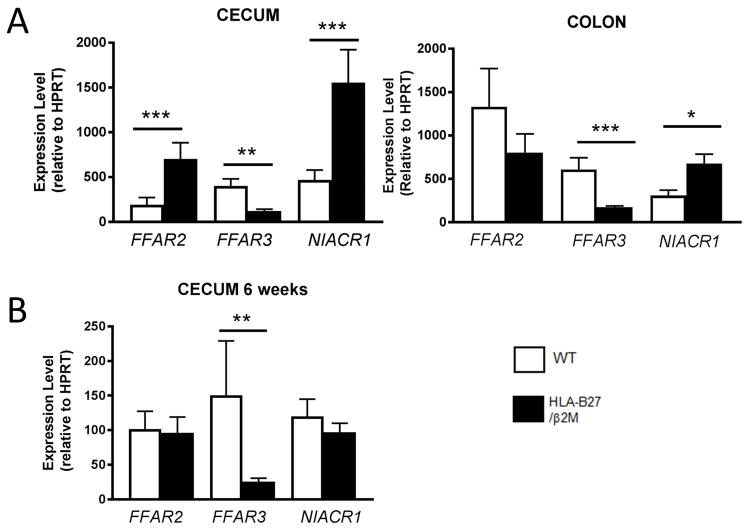
In light of the beneficial effects of propionate on B27-associated inflammatory disease, despite the observation that this specific metabolite was not depleted in unmanipulated HLA-B27/β2m animals vs healthy controls (Fig 3), we hypothesized that HLA-B27 expression may alter host expression of SCFA receptors instead and hence disrupt this homeostatic feedback loop. We therefore examined intestinal expression of FFAR2/Gpr41 (a receptor which binds C2–C4 SCFAs with similar affinity), FFAR3/Gpr43 (a receptor with high affinity for propionate) and butyrate receptor NIACR1/Gpr109 (Fig 6A–B). Strikingly we found that HLA-B27 expression impacted intestinal mRNA expression levels of several SCFA receptors, with significantly decreased cecal expression of propionate receptor FFAR3, in contrast to increased expression of FFAR2 and NIACR1 at 16 wks of age (Fig 6A). This specific reduction in FFAR3 expression was also observed in 6 wk old pre-diseased animals (Fig 6B). Significantly reduced mRNA expression of FFAR3 was also observed in colon (Supp Fig 7). These findings indicate that HLA-B27 expression not only profoundly alters the intestinal metabolome, but is also associated with altered expression of host receptors that bind microbial metabolites.
Figure 6. Intestinal Short Chain Fatty Acid Receptor Expression is significantly altered in HLA-B27/β2m transgenic rats.
Cecal mRNA expression of short chain fatty acid receptors FFAR2, FFAR3 and NIACR1 was determined in unmanipulated WT (white bars) and HLA-B27/β2m transgenic rats at 16 wks of age (A) and 6wks of age (B) by qRT-PCR, Bars represent group mean +/− SEM. All mRNA expression data normalized to HPRT. N = 10–19 animals/group. * = p < 0.05, ** = p < 0.01, *** = p < 0.005 by Mann Whitney U test.
DISCUSSION
Mounting evidence indicates that the intestinal microbiota may play a significant role in the pathogenesis of spondyloarthritic disease. A dysbiotic or altered intestinal microbiota is observed in several forms of spondyloarthropathy including psoriatic arthritis, ankylosing spondylitis and juvenile enthesitis related arthritis [8–10] and subclinical bowel inflammation is observed in over half of AS patients [16]. Reactive arthritis following enteric infection is another prototypical SpA family member. A dysbiotic microbiota is also observed in both the HLA-B27/β2m rat spondyloarthritis model and the murine model of curdlan-induced inflammation in ZAP70 mice [6, 7, 17]. Depletion of the microbiota with antibiotics or the germ free state also attenuates spondyloarthritic disease in both models [17, 18].
It is important to note that evidence of an altered microbiota (for example the relative abundance or presence/absence of microbial species) does not necessarily mean the functional potential of the microbiota is altered [19]. Indeed, healthy humans exhibit a high inter-individual variability in the relative abundance of microbial species in the gut but appear to select species with similar enzymatic and functional potential [19]. Our study provides direct evidence however that the previously described dysbiotic changes observed in spondyloarthropathy are accompanied by functional changes in the metabolic output of the gut, including that of microbe-derived bioactive mediators such as SCFAs.
One of the major changes in the gut metabolic landscape appears to be the shift in nutrient utilization by the intestinal microbiota. For instance, microbial derivatives of plant fiber and isoflavones (such as enterolactone and equol) were decreased in HLA-B27/β2m animals indicative of perturbed microbial fermentation. Interestingly these specific microbial metabolites are estrogenic and thought to have anti-inflammatory properties (reviewed in [20]). By contrast, we observed several mucus components were increased. This is consistent with the increased mucus production previously reported in the HLA-B27/β2m rat [21], but also with the B27-dependent expansion of Akkermansia muciniphila [7] or other microbes which catabolize mucus. Future studies which further address the impact of altered mucus metabolism on both host immunity and the intestinal microbiota would be highly interesting in the context of SpA pathogenesis.
Strikingly, we found that even pre-disease multiple metabolic pathways were impacted by HLA-B27 expression and found a number of individual metabolites were significantly upregulated vs controls at the 6wk time point. This suggests that metabolic shifts in the gut may be a preceding event in SpA pathogenesis, rather than merely secondary to active bowel inflammation. Increased levels of tyrosine and histidine (Fig 2A) and differential usage of amino acid pathways (Fig 2A, Fig 1B) could reflect early changes in colonic protein fermentation or utilization by the host [22]. Polyamines such as spermidine are derived from amino acids (both by the host and the microbiota) and modulate enterocyte tight junction formation [23, 24], secretory IgA production [25] and mucosal maturation and repair from injury [26] and thus may strongly impact intestinal homeostasis [27]. Gamma-glutamyl amino acids also appeared upregulated at this time point (Fig 1B, Supp. Fig 1), which are a product of gamma-glutamyl transferase that may be a marker of oxidative stress [28]. Of note, HLA-B27 misfolding triggers an unfolded protein response (UPR), which may be upregulated in the HLA-B27/β2m rat and is intimately related to oxidative stress responses [29, 30].
Beyond amino acid metabolites, we also observed altered levels of other biochemical classes even in pre-diseased animals. We observed significantly increased glycerate levels at both 6 and 16wks of age (Fig 2A, Supp Fig 2). Glycerate is a simple sugar, derivatives of which are used in a panoply of metabolic pathways including glycolysis. N-acetylmuramate is a component of microbial cell wall component peptidoglycan, and its increase may be indicative of early HLA-B27 dependent dysbiosis. Interestingly, peptidoglycan is a potent adjuvant of the innate immune system and its systemic administration can induce both arthritis and uveitis [31].
In light of our findings that MCFAs are reduced in the HLA-B27/β2m SpA model, it is notable Scher et al. reported reduced fecal levels of the MCFAs hepatanoate and hexanoate in the feces of psoriatic arthritis patients vs healthy controls [8], an observation also made in Crohn’s Disease [32]. Indeed, the microbiota has been reported to regulate absorption of MCFAs by enterocytes and is strongly implicated in the pathogenesis of all these diseases (reviewed in [11, 33]. Interestingly, neither this nor the current study found decreased levels of SCFAs, rather we found cecal propionate (at 16wks) and butyrate (at 6 wks) concentrations were moderately elevated in B27+ animals (Scher et al found no change in their study [8]). This is also consistent with a previous report showing a positive correlation between cecal propionate levels and the extent of intestinal inflammation in HLA-B27/β2m rats [34]. This lies in contrast to CD in which several groups have reported a reduction in intestinal SCFA concentrations [35–37] and indicates canonical B27-associated spondyloarthropathies may have a distinct metabolomic signature compared to IBD despite the degree of clinical overlap in bowel disease with these entities.
Despite the lack of reduced SCFA production however in B27+ rats vs controls, we nonetheless found SCFA propionate significantly attenuated B27-dependent intestinal inflammation. This is broadly consistent with metabolic studies in which dietary supplementation with prebiotics or dietary fiber, which increase total intestinal SCFA levels, reduces intestinal inflammation in HLA-B27/β2m rats [34, 38, 39]. However, the surprising finding that propionate was elevated in luminal contents of B27+ rats vs controls in our study (Fig 3) indicates a considerably complex picture with respect to SCFA production by the microbiota and their metabolism by the host. One possibility is that higher propionate levels in the gut lumen may reflect reduced SCFA absorption or consumption by colonic enterocytes during active bowel inflammation in HLA-B27/β2m rats, with a switch to MCFA utilization instead due to altered energy demand [40]. Alternatively (or in parallel), the specific inflammatory environment of the gut in SpA may create a selective pressure favoring propionate producing bacteria that prevent the further exacerbation of local inflammation.
Reported actions of propionate include the induction of intestinal Treg [12, 13], induction of regulatory cytokines [13, 41] and improvement of barrier function [42]. However, we did not find evidence that these mechanisms were significantly impacted following propionate (or butyrate) treatment of HLA-B27/β2m rats, albeit intestinal inflammation was significantly reduced. One possibility is that this may reflect species-specific differences in propionate activity or uptake. We note however that doses used in our study were highly comparable to those used in murine studies. Another is that regulation of colonic cytokines and tight junction proteins is post-translational, although unfortunately we were unable to obtain sufficient material for protein assays in the current study since intestinal tissue was used for other assays. A third possibility is that these mechanisms have largely been reported in healthy animals or in cell lines and therefore they may have less effect in the context of B27-dependent inflammation. However, we did not find a significant induction of Treg by SCFA treatment even in WT animals (Supp. Fig 4). Alternative mechanisms to identify the therapeutic mechanism of propionate administration hence warrant further scrutiny. We note that an examination of dose responses to SCFAs, their bioavailability and tissue absorption was not performed in the current study and would no doubt be of interest for further investigation. We recognize this limitation and therefore cannot exclude the possibility that other SCFAs, such as butyrate, may also modulate B27-dependent inflammation at other doses or other routes of administration.
In summary, we find HLA-B27 expression dramatically alters the intestinal metabolome. We also provide pivotal data that microbial metabolites significantly impact the development of HLA-B27 associated inflammatory disease. While our study examined B27-associated bowel inflammation, it would obviously be valuable to ascertain whether microbial metabolites can attenuate spondylitic and arthritic disease (an active area of research by our own group and others). Moreover, our current data set was not large enough to correlate levels of B27 associated metabolites with either changes in microbiota composition or disease activity, which likely will continue be a fruitful avenue of future research. Nonetheless, our data provide a compelling rationale to investigate whether further targeting gut microbes or gut metabolites themselves to alter the intestinal metabolome may functionally impact SpA pathogenesis and offer innovative treatment modalities for future clinical management of SpA disease.
Supplementary Material
Supp. Figure 1: Cecal abudance of gamma-glutamyl amino acids in WT and HLA-B27/β2m rats.
The abundance of gamma-glutamyl amino acids in cecal contents of WT (white bars) and HLA-B27/β2m rats (black bars) at 6wks (A) and 16wks (B). Bars represent group means +/− SEM. * = p<0.2, ** = p < 0.1 (Benjamani-Hochberg corrected p values, with a FDR threshold of 0.2).
Supp. Figure 2. Cecal abundance of select metabolites in WT and HLA-B27/β2m rats. This figure is a companion figure to Figure 2 in main text. A) Metabolite levels in 16wk old animals that were found to be significant at 6wks (also see Fig 2A). ** adjusted p value < 0.1, * adjusted p value < 0.2. B–E Metabolite levels of B) Mucus components; C) Dietary metabolites; D) Inflammatory metabolites and E) Bile acids in 6wk old animals are shown.
Supp. Figure 3. Short chain fatty acid production is dependent on the intestinal microbiota.
Six week old WT or HLA-B27/β2m transgenic rats were treated with Vancomycin ad libitum in drinking water (50mg/kg/day). At 12 wks, animals were necropsied, cecal lumen contents collected and SCFA concentrations determined as for Figure 3 in main text. ** p < 0.01, *** p < 0.005. ND – Not Detected. N = 5–9/treatment group. Each symbol represents an individual animal and horizontal bars represent group means.
Supp. Figure 4. Body mass of HLA-B27/β2m rats treated with short chain fatty acids.
Six week old HLA-B27/β2m transgenic rats were administered sodium propionate (150mM) or sodium butyrate (150mM) ad libitum in drinking water or water alone. Percent body mass from original start weight (6 wks) was recorded at the indicated time points. Green = water control, blue = propionate and red = butyrate treatment groups. N = 8–11 rats per group. Values represent groups means +/− SEM.
Supp. Figure 5. The frequency of FoxP3+ CD4+ T cells in SCFA-treated WT animals.
Six week old WT or HLA-B27/β2m transgenic rats were treated with SCFAs propionate and butyrate as in Figure 5, main text. The frequency of CD4+ FoxP3+ve T cells in cecum, colon, mesenteric lymph node (MLN) and spleen in B27+ animals is shown. Each symbol represents an individual animal, horizontal bars represent group means. Legend: ‘−’ = untreated controls, ‘P’ = propionate treated, ‘B’ = butyrate treated.
Supp. Figure 6. SCFA treatment does not lead to gross changes in microbial load.
DNA was extracted from cecal luminal contents from animals treated with short chain fatty acids (as in Figure 4 main text) or age-matched untreated controls. Total quantity of 16s rRNA DNA was quantitated by real-time quantitative PCR, using E. coli DNA as standard.
Supp. Figure 7. Colonic short chain fatty acid receptor expression in WT and HLA-B27/β2m transgenic rats. Colonic mRNA expression of short chain fatty acid receptors FFAR2, FFAR3 and NIACR1 was determined in unmanipulated WT (white bars) and HLA-B27/β2m transgenic rats at 16 wks of age by qRT-PCR, bars represent group mean +/− SEM. All mRNA expression data normalized to HPRT. N = 8–20 animals/group. * = p < 0.05, *** = p < 0.005 by Mann Whitney U test.
Acknowledgments
Supported by grants from the Collins Medical Trust and the Medical Research Foundation of Oregon to M.A. This work was also supported by a Jane Bruckel Award from the Spondylitis Association of America to M.A. PL is supported by a grant from the NIH (K08EY022948) and an individual Research to Prevent Blindness Career Development Award. JTR is supported by the Stan and Madelle Rosenfeld Family Trust and the William and Mary Bauman Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no commercial interests for this study.
References
- 1.Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014;16:1024–1033. doi: 10.1111/cmi.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scher JU, Littman DR, Abramson SB. Review: Microbiome in Inflammatory Arthritis and Human Rheumatic Diseases. Arthritis & rheumatology. 2016;68:35–45. doi: 10.1002/art.39259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharon G, Garg N, Debelius J, et al. Specialized metabolites from the microbiome in health and disease. Cell metabolism. 2014;20:719–730. doi: 10.1016/j.cmet.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammer RE, Maika SD, Richardson JA, et al. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63:1099–1112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- 5.Taurog JD, Maika SD, Satumtira N, et al. Inflammatory disease in HLA-B27 transgenic rats. Immunol Rev. 1999;169:209–223. doi: 10.1111/j.1600-065x.1999.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 6.Lin P, Bach M, Asquith M, et al. HLA-B27 and Human beta2-Microglobulin Affect the Gut Microbiota of Transgenic Rats. PLoS One. 2014;9:e105684. doi: 10.1371/journal.pone.0105684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asquith M, Stauffer P, Davin S, et al. Perturbed mucosal immunity and dysbiosis accompany clinical disease in a rat model of spondyloarthritis. Arthritis & rheumatology. 2016 doi: 10.1002/art.39681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis & rheumatology. 2015;67:128–139. doi: 10.1002/art.38892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costello ME, Ciccia F, Willner D, et al. Intestinal dysbiosis in ankylosing spondylitis. Arthritis & rheumatology. 2014 doi: 10.1002/art.38967. [DOI] [PubMed] [Google Scholar]
- 10.Stoll ML, Kumar R, Morrow CD, et al. Altered microbiota associated with abnormal humoral immune responses to commensal organisms in enthesitis-related arthritis. Arthritis research & therapy. 2014;16:486. doi: 10.1186/s13075-014-0486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asquith M, Elewaut D, Lin P, Rosenbaum JT. The role of the gut and microbes in the pathogenesis of spondyloarthritis. Best practice & research Clinical rheumatology. 2014;28:687–702. doi: 10.1016/j.berh.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uhlig HH, Coombes J, Mottet C, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown DG, Rao S, Weir TL, et al. Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer & metabolism. 2016;4:11. doi: 10.1186/s40170-016-0151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Vos M, Cuvelier C, Mielants H, et al. Ileocolonoscopy in seronegative spondylarthropathy. Gastroenterology. 1989;96:339–344. doi: 10.1016/0016-5085(89)91557-6. [DOI] [PubMed] [Google Scholar]
- 17.Rehaume LM, Mondot S, Aguirre de Carcer D, et al. ZAP-70 Genotype Disrupts the Relationship Between Microbiota and Host, Leading to Spondyloarthritis and Ileitis in SKG Mice. Arthritis & rheumatology. 2014;66:2780–2792. doi: 10.1002/art.38773. [DOI] [PubMed] [Google Scholar]
- 18.Taurog JD, Richardson JA, Croft JT, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaut M, Clavel T. Metabolic diversity of the intestinal microbiota: implications for health and disease. The Journal of nutrition. 2007;137:751S–755S. doi: 10.1093/jn/137.3.751S. [DOI] [PubMed] [Google Scholar]
- 21.Faure M, Moennoz D, Mettraux C, et al. The chronic colitis developed by HLA-B27 transgenic rats is associated with altered in vivo mucin synthesis. Dig Dis Sci. 2004;49:339–346. doi: 10.1023/b:ddas.0000017462.75257.70. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Wu J, Li JV, et al. Gut microbiota composition modifies fecal metabolic profiles in mice. Journal of proteome research. 2013;12:2987–2999. doi: 10.1021/pr400263n. [DOI] [PubMed] [Google Scholar]
- 23.Guo X, Rao JN, Liu L, et al. Polyamines are necessary for synthesis and stability of occludin protein in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1159–1169. doi: 10.1152/ajpgi.00407.2004. [DOI] [PubMed] [Google Scholar]
- 24.Guo X, Rao JN, Liu L, et al. Regulation of adherens junctions and epithelial paracellular permeability: a novel function for polyamines. Am J Physiol Cell Physiol. 2003;285:C1174–1187. doi: 10.1152/ajpcell.00015.2003. [DOI] [PubMed] [Google Scholar]
- 25.Buts JP, De Keyser N, Kolanowski J, et al. Maturation of villus and crypt cell functions in rat small intestine. Role of dietary polyamines Dig Dis Sci. 1993;38:1091–1098. doi: 10.1007/BF01295726. [DOI] [PubMed] [Google Scholar]
- 26.Lux GD, Marton LJ, Baylin SB. Ornithine decarboxylase is important in intestinal mucosal maturation and recovery from injury in rats. Science. 1980;210:195–198. doi: 10.1126/science.6774420. [DOI] [PubMed] [Google Scholar]
- 27.Dixon LJ, Kabi A, Nickerson KP, McDonald C. Combinatorial effects of diet and genetics on inflammatory bowel disease pathogenesis. Inflamm Bowel Dis. 2015;21:912–922. doi: 10.1097/MIB.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee DH, Blomhoff R, Jacobs DR., Jr Is serum gamma glutamyltransferase a marker of oxidative stress? Free radical research. 2004;38:535–539. doi: 10.1080/10715760410001694026. [DOI] [PubMed] [Google Scholar]
- 29.DeLay ML, Turner MJ, Klenk EI, et al. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009;60:2633–2643. doi: 10.1002/art.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wells A, Pararajasegaram G, Baldwin M, et al. Uveitis and arthritis induced by systemic injection of streptococcal cell walls. Investigative ophthalmology & visual science. 1986;27:921–925. [PubMed] [Google Scholar]
- 32.De Preter V, Joossens M, Ballet V, et al. Metabolic profiling of the impact of oligofructose-enriched inulin in Crohn’s disease patients: a double-blinded randomized controlled trial. Clinical and translational gastroenterology. 2013;4:e30. doi: 10.1038/ctg.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semova I, Carten JD, Stombaugh J, et al. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe. 2012;12:277–288. doi: 10.1016/j.chom.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koleva P, Ketabi A, Valcheva R, et al. Chemically defined diet alters the protective properties of fructo-oligosaccharides and isomalto-oligosaccharides in HLA-B27 transgenic rats. PLoS One. 2014;9:e111717. doi: 10.1371/journal.pone.0111717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huda-Faujan N, Abdulamir AS, Fatimah AB, et al. The impact of the level of the intestinal short chain Fatty acids in inflammatory bowel disease patients versus healthy subjects. The open biochemistry journal. 2010;4:53–58. doi: 10.2174/1874091X01004010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takaishi H, Matsuki T, Nakazawa A, et al. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int J Med Microbiol. 2008;298:463–472. doi: 10.1016/j.ijmm.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 37.Marchesi JR, Holmes E, Khan F, et al. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. Journal of proteome research. 2007;6:546–551. doi: 10.1021/pr060470d. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez-Cabezas ME, Galvez J, Camuesco D, et al. Intestinal anti-inflammatory activity of dietary fiber (Plantago ovata seeds) in HLA-B27 transgenic rats. Clinical nutrition. 2003;22:463–471. doi: 10.1016/s0261-5614(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 39.Hoentjen F, Welling GW, Harmsen HJ, et al. Reduction of colitis by prebiotics in HLA-B27 transgenic rats is associated with microflora changes and immunomodulation. Inflamm Bowel Dis. 2005;11:977–985. doi: 10.1097/01.mib.0000183421.02316.d5. [DOI] [PubMed] [Google Scholar]
- 40.Jorgensen JR, Clausen MR, Mortensen PB. Oxidation of short and medium chain C2–C8 fatty acids in Sprague-Dawley rat colonocytes. Gut. 1997;40:400–405. doi: 10.1136/gut.40.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park J, Kim M, Kang SG, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr. 2008;100:297–305. doi: 10.1017/S0007114508888733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp. Figure 1: Cecal abudance of gamma-glutamyl amino acids in WT and HLA-B27/β2m rats.
The abundance of gamma-glutamyl amino acids in cecal contents of WT (white bars) and HLA-B27/β2m rats (black bars) at 6wks (A) and 16wks (B). Bars represent group means +/− SEM. * = p<0.2, ** = p < 0.1 (Benjamani-Hochberg corrected p values, with a FDR threshold of 0.2).
Supp. Figure 2. Cecal abundance of select metabolites in WT and HLA-B27/β2m rats. This figure is a companion figure to Figure 2 in main text. A) Metabolite levels in 16wk old animals that were found to be significant at 6wks (also see Fig 2A). ** adjusted p value < 0.1, * adjusted p value < 0.2. B–E Metabolite levels of B) Mucus components; C) Dietary metabolites; D) Inflammatory metabolites and E) Bile acids in 6wk old animals are shown.
Supp. Figure 3. Short chain fatty acid production is dependent on the intestinal microbiota.
Six week old WT or HLA-B27/β2m transgenic rats were treated with Vancomycin ad libitum in drinking water (50mg/kg/day). At 12 wks, animals were necropsied, cecal lumen contents collected and SCFA concentrations determined as for Figure 3 in main text. ** p < 0.01, *** p < 0.005. ND – Not Detected. N = 5–9/treatment group. Each symbol represents an individual animal and horizontal bars represent group means.
Supp. Figure 4. Body mass of HLA-B27/β2m rats treated with short chain fatty acids.
Six week old HLA-B27/β2m transgenic rats were administered sodium propionate (150mM) or sodium butyrate (150mM) ad libitum in drinking water or water alone. Percent body mass from original start weight (6 wks) was recorded at the indicated time points. Green = water control, blue = propionate and red = butyrate treatment groups. N = 8–11 rats per group. Values represent groups means +/− SEM.
Supp. Figure 5. The frequency of FoxP3+ CD4+ T cells in SCFA-treated WT animals.
Six week old WT or HLA-B27/β2m transgenic rats were treated with SCFAs propionate and butyrate as in Figure 5, main text. The frequency of CD4+ FoxP3+ve T cells in cecum, colon, mesenteric lymph node (MLN) and spleen in B27+ animals is shown. Each symbol represents an individual animal, horizontal bars represent group means. Legend: ‘−’ = untreated controls, ‘P’ = propionate treated, ‘B’ = butyrate treated.
Supp. Figure 6. SCFA treatment does not lead to gross changes in microbial load.
DNA was extracted from cecal luminal contents from animals treated with short chain fatty acids (as in Figure 4 main text) or age-matched untreated controls. Total quantity of 16s rRNA DNA was quantitated by real-time quantitative PCR, using E. coli DNA as standard.
Supp. Figure 7. Colonic short chain fatty acid receptor expression in WT and HLA-B27/β2m transgenic rats. Colonic mRNA expression of short chain fatty acid receptors FFAR2, FFAR3 and NIACR1 was determined in unmanipulated WT (white bars) and HLA-B27/β2m transgenic rats at 16 wks of age by qRT-PCR, bars represent group mean +/− SEM. All mRNA expression data normalized to HPRT. N = 8–20 animals/group. * = p < 0.05, *** = p < 0.005 by Mann Whitney U test.