Abstract
Objective
To assess the effect of telehealthcare compared with usual practice in patients with chronic obstructive pulmonary disease (COPD).
Design
A cluster-randomised trial with 26 municipal districts that were randomly assigned either to an intervention group whose members received telehealthcare in addition to usual practice or to a control group whose members received usual practice only (13 districts in each arm).
Setting
Twenty-six municipal districts in the North Denmark Region of Denmark.
Participants
Patients who fulfilled the Global Initiative for COPD guidelines and one of the following criteria: COPD Assessment Test score ≥10; or Medical Research Dyspnoea Council Scale ≥3; or Modified Medical Research Dyspnoea Council Scale ≥2; or ≥2 exacerbations during the past 12 months.
Main outcome measures
Health-related quality of life (HRQoL) assessed by the physical component summary (PCS) and mental component summary (MCS) scores of the Short Form 36-Item Health Survey, Version 2. Data were collected at baseline and at 12 month follow-up and analysed according to the intention-to-treat principle with complete cases, n=574 (258 interventions; 316 controls) and imputed data, n=1225 (578 interventions, 647 controls) using multilevel modelling.
Results
In the intention-to-treat analysis (n=1225), the raw mean difference in PCS from baseline to 12 month follow-up was −2.6 (SD 12.4) in the telehealthcare group and −2.8 (SD 11.9) in the usual practice group. The raw mean difference in MCS scores in the same period was −4.7 (SD 16.5) and −5.3 (SD 15.5) for telehealthcare and usual practice, respectively. The adjusted mean difference in PCS and MCS between groups at 12 months was 0.1 (95% CI −1.4 to 1.7) and 0.4 (95% CI −1.7 to 2.4), respectively.
Conclusions
The overall sample and all subgroups demonstrated no statistically significant differences in HRQoL between telehealthcare and usual practice.
Trial registration number
NCT01984840; Results.
Keywords: effectiveness, COPD, telemedicine, RCT, Denmark, quality of life, telehealth, telemonitoring, outcome Assessment (Health Care)
Strengths and limitations of this study.
This is the first large-scale trial in Denmark established to remedy the lack of international evidence on health-related quality of life (HRQoL) in patients with chronic obstructive pulmonary disease (COPD) who are receiving telehealthcare.
The study is a large-scale, pragmatic, two-level, cluster-randomised trial with 12 month follow-up, which produces results applicable to clinical practice.
The trial succeeded in establishing a fruitful intersectoral and interinstitutional cooperation towards a common goal; the implementation of telehealthcare to improve COPD patients’ HRQoL.
Short Form 36-Item Health Survey, Version 2 was used as a quality-of-life instrument, but may be less sensitive to change related to telehealthcare. It would have been desirable to employ a combination of generic and disease-specific questionnaires in this study.
A considerable high attrition rate (651/1225 (53%) patients being incomplete cases or lost-to-follow-up) was present, which could have introduced bias and affected the strength of the trial’s findings.
Introduction
Chronic obstructive pulmonary disease (COPD) is a significant cause of impaired quality of life (QoL), disability, morbidity and mortality in industrialised countries.1 2 Moreover, it constitutes a considerable burden on the affected patients and places an important socioeconomic burden on society due to the growing number of patients requiring care. COPD and other chronic diseases challenge the healthcare systems in ways that call for changes in management and delivery of patient care.3 4
Telehealthcare has the potential to facilitate timely transmission of clinical and physiological data and allows patients to be followed by clinicians more frequently and from a distance.5 It may therefore also facilitate early intervention and improve clinical and patient-related outcomes.6 Systematic reviews conclude that there is a potential for demonstrating that telehealthcare improves health-related outcomes or is at least as good as conventional treatment, but more research is needed.7–9 Some reviews10–12 raise concerns about the quality of the available evidence that is presented in heterogeneous pilot projects which are small, incomparable and difficult to appraise in relation to QoL.13–17 A recent systematic review18 indicates only limited evidence for a positive effect of telehealth interventions on QoL in COPD. This situation has given rise to a demand for large-scale studies; and a large-scale study, The Whole System Demonstrator Project (WSD) conducted in the UK, has attempted to establish a robust evidence base for telehealth.19–23 In the WSD, Cartwright and colleagues24 concluded that the effect of telehealth was clinically insignificant as a supplement to usual care, and telehealth did not improve psychological outcomes and QoL in patients with COPD, heart failure or diabetes.24 In a recent randomised controlled trial (RCT), a more extensive assessment of QoL and psychological outcomes was performed on the COPD cohort of the WSD.25 The findings from the RCT25 are consistent with the above conclusion made by Cartwright and colleagues.24 However, the RCT found no reductions in patients’ QoL in the longer term. In contrast, there was a trend towards improved QoL and mood in the telehealth group at longer-term follow-up, but not at the short-term follow-up, as observed through disease-specific measures.25
In Denmark, the lack of evidence for telehealthcare was discussed among healthcare decision-makers who agreed to strengthen the evidence base by conducting a large-scale study as part of ‘The National Danish Action Plan for Dissemination of Telemedicine’.26 In 2012, the Danish government decided to launch the Action Plan to disseminate telemedicine nationally.26 The action plan included, among others, the TeleCare North trial, the purpose of which was to contribute to the generation of valuable knowledge about the use of telehealthcare for patients with COPD in the North Denmark Region. The TeleCare North trial was designed based on experiences from two Danish pilot studies, the TeleKat Study27 28 and the Nursing Consultations Study,29 30 which had both demonstrated positive effects of telehomecare and teleconsultations.
The present study is embedded in the Danish TeleCare North trial. Its objective was to assess the effectiveness of telehealthcare compared with usual practice based on an assessment of health-related QoL (HRQoL) in patients with COPD. It was hypothesised that adding telehealthcare to usual practice would significantly enhance patients’ HRQoL.31
Methods
Study design
This study was conducted in accordance with the study protocol for the TeleCare North trial,31 which we describe briefly in this section. The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT checklist) and the Consolidated Standards of Reporting Trials (CONSORT checklist) extension were followed in designing the TeleCare North trial (see supplementary material 1 and 2).
The TeleCare North trial was a large-scale, pragmatic, two-level, cluster-randomised trial with 12 month follow-up. The trial was based on the collaborative efforts of the North Denmark Region, all municipalities in the Region, the Region’s general practitioners (GPs) and Aalborg University. The municipalities were organised into 26 districts with 13 clusters in each arm.
Participants
The trial targeted all patients with COPD in the North Denmark Region who fulfilled the inclusion criteria. All GPs from the Region recruited the patients with COPD from a list of suitable patients attending their practices. The selection of participants followed identical guidelines and instructions at all practices. All patients who accepted to participate and were deemed suitable for participation were included. The identification and recruitment of patients took place prior to random allocation of clusters in order to minimise biased recruitment. Assigned to the intervention or to usual practice were 1225 (578 interventions, 647 controls) patients representing different COPD stages, Global Initiative for COPD (GOLD I-IV).32
Inclusion and exclusion criteria
All patients with COPD who may benefit from telehealthcare were considered for inclusion. The following inclusion criteria were used: patients were required to have COPD as their primary disease and be diagnosed by spirometry, and they should receive or be motivated for treatment corresponding to the GOLD guidelines.32 One of the following criteria should also be met: a Medical Research Dyspnoea Council scale (MRC) score ≥3; or a modified Medical Research Dyspnoea Council scale (MMRC) score ≥2; or a COPD Assessment Test score ≥10; or ≥2 exacerbations during the past 12 months.
In addition, on the basis of a health professional’s qualified estimate and assessment, the patients should also have a telephone connection, have permanent residence and be on the list of a GP in the North Denmark Region. Patients should also be able to speak Danish or they should be living with Danish-speaking relatives who were able to support them in their use of the telehealthcare system and to provide assistance in situations involving issues of comprehension of the Danish language.
Patients were excluded if they were cognitively impaired, had no phone line or GSM coverage, or were unable to understand Danish to the extent allowing them to complete the study questionnaires.
Intervention
The intervention of the TeleCare North trial was based on the concept and logic of the TeleKat study.28 Its key concept and primary logic was empowerment achieved by engaging patients with COPD in their illness and increasing their coping abilities through self-monitoring. The study introduced extended monitoring with store-and-forward data connected to healthcare providers to facilitate detection of exacerbations and rapidly initiate preventive antibiotic therapy.
Intervention arm: telehealthcare
Patients in the intervention group received telehealthcare in addition to usual practice. The telehealthcare system coined ‘Telekit’ was used in the TeleCare North trial. It consists of a Samsung Galaxy Tab2 (10.1) with associated devices: a digital blood pressure monitor (UA-767, plus BT-C, Nonin Medical, Minnesota, USA), a fingertip pulse oximeter (Nonin, Onyx II% SpO2) and a health precision scale (UC-321PBT-C, A&D Medical, Tokyo, Japan). The devices can collect and wirelessly transmit relevant disease-specific data consisting of answers to questions related to COPD exacerbations, symptoms and patients’ vital signs: systolic and diastolic blood pressure, heart rate, weight and oxygen saturation (figure 1). The patients were instructed to measure their vital signs, which were then sent asynchronously to municipality healthcare personnel who subsequently established if these data deviated from the normal threshold values. The communication between the healthcare personnel and the patient was one-way only. The patients were contacted if there were adverse changes in their values and responses. Patients were also contacted if the measurements were not carried out as agreed or the measurements were not received as expected.
Figure 1.
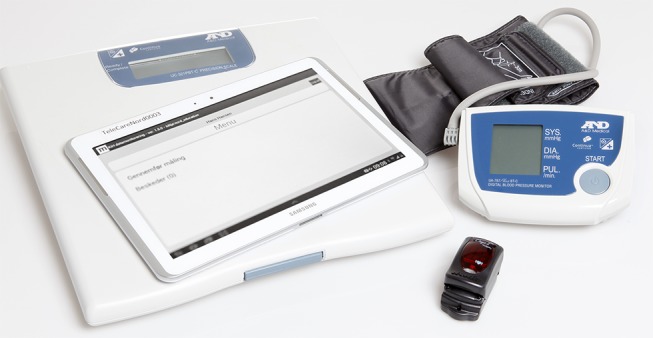
The Telekit system consists of a tablet, a blood pressure monitor, a fingertip pulse oximeter and a health precision scale.
Comparative arm: usual practice
Patients in the control group received their existing usual practice. This involved treatment, monitoring and care throughout the study period. The patients’ GPs provided this treatment and monitoring, and the municipalities held responsibility for the practical help and care provided. The patients in the control group had not received any form of telehealthcare system; but at the end of the 12 month study period, they were offered the same Telekit system as the intervention group for ethical reasons.
Randomisation
On 4 November 2013, the municipality districts (n=26) were randomised so that patients residing in the same district received the same type of care—either telehealthcare in addition to usual practice or usual practice only. The municipality districts were matched 1:1 by the following variables: the total population size of the districts, the proportion of people with a higher education, the sum of the district’s total income, unemployment and the estimated number of patients with COPD.31 The districts were distributed randomly by a blinded volunteer with no relation to the trial, who performed the randomisation by throwing a dice. The volunteer had no knowledge of the distribution of districts on intervention or control group, respectively. The randomisation was recorded by the trial administration secretariat to ensure that the procedure was performed randomly.
Outcome measures
Upon inclusion at the GP’s office, patients were handed a questionnaire comprising the Short Form 36-Item Health Survey , Version 2 (SF-36v2)33 and questions concerning their baseline demographic characteristics such as gender, age, education, comorbidities, smoking status, marital status and job status. The SF-36v2 consists of 36 questions and is one of the most commonly used generic, validated questionnaires for measuring general HRQoL. It captures patients’ perceptions of physical, social, mental and emotional domains, and overall summary scores of physical component summary (PCS) and mental component summary (MCS) are derived from domain scores using a norm-based scoring method.34 The scores are standardised to fall between 0 and 100 with a higher score indicating ‘better health’.33 After 12 months, a similar patient questionnaire was sent to the included patients to compare baseline data with follow-up data. The outcomes of this study were the patients’ mean differences in HRQoL at baseline and at the 12 month follow-up assessed with SF-36v2. The primary outcome measure was the adjusted mean differences in PCS summary scores between treatment groups at 12 month follow-up.
Sample size
The sample size calculation was based on the study protocol’s31 primary outcome measure, PCS. Based on results from a previous Norwegian study,35 it was estimated that eligible patients with COPD had a mean baseline PCS score of 38 with an SD of 10. The average cluster size was assumed to be 50 with a coefficient of variation of 0.5. A sample size of 350 patients from at least seven municipality districts (clusters) in each arm (two-sided significance level, α=0.05, power=80%) was needed to detect minimal, clinically important differences (change equal to 5) and intracluster correlation ((ICC) equal to 0.05) between the intervention group and the control group.35 The total required sample size was estimated to be around 800 patients with an expected lost-to-follow-up-rate of 10%.
Statistical analysis
All analyses of the cluster-randomised trial were conducted according to the intention-to-treat principle. A post hoc subgroup analysis was also performed as a secondary analysis. The analyses were undertaken in STATA 12.1.
SF-36v2 standardised scores for each patient were produced using software provided by QualityMetric (http://www.sf-36.org/), which converts all scores to a single metric (norm-based scoring) based on 2009 US general population norms.34 An analysis of covariance analysis strategy was applied.36 Two separate linear mixed models for continuous outcomes were used to assess PCS and MCS scores at 12 month follow-up controlling for treatment arm, respective baseline score, age, gender, baseline forced expiratory volume in one second (FEV1%), marital status, diabetes status, cancer status and clustering at the municipality district level. The clusters were assumed to be represented as random effects, and the models had robust covariance structures. ICC estimates of patient-reported outcome variables were calculated for measurement of the variability within and across the clusters. The subgroup analyses applied the same statistical models and covariates as above, but with added treatment-by-covariate interaction for each subgroup.
Missing data were assumed missing at random and were handled in coordination with the health economic evaluation of the same trial as described in the trial protocol31 and followed good practices for handling missing data in cost-effectiveness research.37 Missing PCS and MCS scores and baseline characteristics were imputed using multiple imputation and were estimated separately by treatment group to allow for differential covariance structures in treatment group means. Imputation models included PCS and MCS scores, predictors for these scores at both time points, predictors for missing observations in the individual variables and all baseline characteristics. Continuous variables were imputed by predictive mean matching and categorical variables by multinominal logistic or logistic regression. The variables included were non-missing HRQoL (PCS and MCS scores), measures of disease status (FEV1%, forced vital capacity (FVC%)), diastolic and systolic blood pressure, smoking status, duration of COPD, potential comorbidities (diabetes, cardiovascular disease, mental illness, musculoskeletal disorders or cancer), socio-demographic variables (age, gender, marital status, education, employment status) and clustering. The imputation models involved the generation of 30 complete datasets combined by Rubin’s rule. Single imputation was performed on subjects that died during the 12 months by assigning their summary scores values of 0.38 39
The primary analysis and subgroup analysis were based on imputed data, but a complete case analysis was also included as a sensitivity analysis to check the robustness of the main trial findings.
Results
Descriptive characteristics
The CONSORT diagram is shown in figure 2. Twenty-six municipal districts (13 intervention clusters; 13 control clusters) were randomised in 2013, and the TeleCare North trial was completed after the 12 month assessment in 2015. At baseline, 1225 (578 interventions, 647 controls) patients were enrolled in the TeleCare North trial. At 12 months, 109 (18.86%) intervention patients were lost to follow-up (50 were dead, 59 did not respond on questionnaires) and 116 (17.93%) control patients (53 were dead, 63 did not respond on questionnaires after baseline). In total, 101 (17.47%) patients in the intervention group and 61 (9.43%) patients in the control group withdrew their consent. Reasons for withdrawing from the TeleCare North trial included complicated technology, concomitant health problems, not interested, leaving local geographical area, does not trust the equipment or disappointed over not being a part of the telehealth intervention. None of the 26 clusters was lost to follow-up. At 12 months, 264 (110 interventions, 154 controls) patients had incomplete data (patients that were not lost-to-follow-up but had missing values on items in either PCS or MCS at baseline or follow-up). Complete data (patients with non-missing values on MCS and PCS score at baseline and follow-up) were available for 574 (258 interventions, 316 controls) of the 1225 patients at 12 month follow-up, giving an attrition rate of 53% (figure 2).
Figure 2.
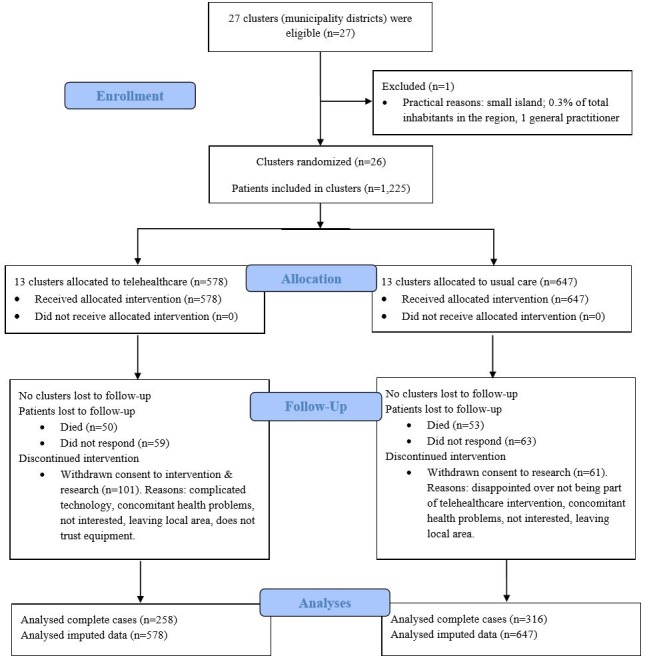
CONSORT diagram of the TeleCare North trial.
At baseline, we assessed socio-demographic factors (gender, age, marital status) and health characteristics (smoking status, duration of COPD, FEV1%, FVC%, comorbidities, SF-36). Statistical comparisons of the participants’ baseline characteristics demonstrated that the two study groups were similar, except for statistically significant differences in FVC% (p<0.05). The control group’s mean FVC% (74.34%) was slightly higher than the intervention group’s mean FVC% (70.38%) (table 1).
Table 1.
Baseline characteristics, at baseline and at 12 month follow-up
| Characteristics | All participants at baseline |
Complete cases at 12 month follow-up* |
Lost-to-follow-up at 12 months† |
Incomplete cases at 12 month follow-up‡ | ||||||||
| THC n=578 | UP n=647 | Diff raw |
THC n=258 | UP n=316 | Diff raw |
THC n=210 | UP n=177 | Diff raw |
THC n=110 | UP n=154 |
Diff raw |
|
| Age (years)§ | 69.6 (9.4) | 70.3 (9.1) | −0.8 | 68.2 (8.8) | 69.5 (9.1) | −1.3 | 70.5 (10.2) | 71.8 (9.5) | −1.4 | 70.9 (8.5) | 70.3 (8.6) | −0.6 |
| Men (%)§ | 48.3 (n=279) | 43.7 (n=283) | 4.5 | 53.5 (n=138) | 44.6 (n=141) | 8.9¶ | 45.2 (n=95) | 45.8 (n=81) | −0.5 | 41.8 (n=46) | 39.6 (n=61) | 2.2 |
| Marital status (%) | ||||||||||||
| Married/in a relationship | 55.9 (n=323) | 54.3 (n=351) | 1.6 | 70.2 (n=181) | 62.0 (n=196) | 8.1 | 40.0 (n=84) | 45.2 (n=80) | −5.2 | 52.7 (n=58) | 48.7 (n=75) | 4.0 |
| Single | 20.4 (n=118) | 22.1 (n=143) | −1.7 | 17.4 (n=45) | 22.2 (n=70) | −4.7 | 24.8 (n=52) | 23.2 (n=41) | 1.6 | 19.1 (n=21) | 20.8 (n=32) | −1.7 |
| Widow/widower | 16.8 (n=97) | 16.5 (n=107) | 0.2 | 12.0 (n=31) | 15.2 (n=48) | −3.2 | 22.9 (n=48) | 19.2 (n=34) | 3.7 | 16.4 (n=18) | 16.2 (n=25) | 0.1 |
| Missing (%) | 6.9 (n=40) | 7.1 (n=46) | −0.2 | 0.4 (n=1) | 0.6 (n=2) | −0.2 | 12.4 (n=26) | 12.4 (n=22) | −0.1 | 11.8 (n=13) | 14.3 (n=22) | −2.5 |
| Smoking status (%) | ||||||||||||
| Non-smokers | 59.3 (n=343) | 63.1 (n=408) | −3.7 | 66.3 (n=171) | 67.4 (n=213) | −1.1 | 51.0 (n=107) | 61.0 (n=108) | −10.1 | 59.1 (n=65) | 56.5 (n=87) | 2.6 |
| Smokers | 33.9 (n=196) | 29.2 (n=189) | 4.7 | 3.0 (n=85) | 31.0 (n=98) | 1.9 | 36.8 (n=77) | 26.6 (n=47) | 10.1 | 30.9 (n=34) | 28.6 (n=44) | 2.3 |
| Missing (%) | 6.8 (n=39) | 7.7 (n=50) | −1.0 | 0.8 (n=2) | 1.6 (n=5) | −0.8 | 12.4 (n=26) | 12.4 (n=22) | −0.1 | 10.0 (n=11) | 14.9 (n=23) | −4.9 |
| Duration of COPD (years) | 7.8 (6.2) | 7.7 (5.8) | 0.1 | 7.6 (6.5) | 7.5 (5.4) | 0.1 | 8.0 (6.5) | 8.4 (6.4) | −0.4 | 8.0 (5.1) | 7.5 (6.0) | 0.5 |
| Missing (%) | 14.0 (n=81) | 15.1 (n=98) | −1.1 | 7.8 (n=20) | 8.2 (n=26) | −0.5 | 21.4 (n=45) | 22.0 (n=39) | −0.6 | 14.6 (n=16) | 21.4 (n=33) | −6.9 |
| FEV1 (%) | 47.7 (18.1) | 48.4 (18.9) | −0.7 | 48.9 (18.3) | 50.3 (19.8) | −1.4 | 47.7 (18.9) | 45.7 (17.9) | 2.1 | 45.1 (15.6) | 47.7 (17.9) | −2.7 |
| Missing (%) | 18.5 (n=107) | 19.8 (n=128) | −1.3 | 18.2 (n=47) | 19.9 (n=63) | −1.7 | 21.4 (n=45) | 15.8 (n=28) | 5.6 | 13.6 (n=15) | 24.0 (n=37) | −10.4 |
| FVC (%) | 70.4 (20.0) | 73.3 (22.3) | −4.0** | 71.2 (19.1) | 75.4 (21.7) | −3.7 | 70.6 (21.3) | 73.2 (24.6) | −2.6 | 66.4 (19.4) | 73.3 (20.8) | −6.9** |
| Missing (%) | 34.4 (n=199) | 39.4 (n=255) | −5.0 | 31.0 (n=80) | 38.0 (n=120) | −7.0 | 37.1 (n=78) | 38.4 (n=68) | −1.3 | 37.3 (n=41) | 43.5 (n=67) | −6.2 |
| Comorbidities (%) | ||||||||||||
| Diabetes | 10.2 (n=59) | 9.9 (n=64) | 0.3 | 8.9 (n=23) | 9.8 (n=31) | −0.9 | 10.5 (n=22) | 8.5 (n=15) | 2.0 | 12.7 (n=14) | 11.7 (n=18) | 1.0 |
| Coronary heart disease | 32.7 (n=189) | 31.8 (n=206) | 0.9 | 32.6 (n=84) | 32.3 (n=102) | 0.3 | 36.2 (n=76) | 34.5 (n=61) | 1.7 | 26.4 (n=29) | 27.9 (n=43) | −1.6 |
| Mental health problem | 4.8 (n=28) | 4.8 (n=31) | 0.1 | 4.3 (n=11) | 5.1 (n=16) | −0.8 | 7.1 (n=15) | 5.1 (n=9) | 2.1 | 1.8 (n=2) | 3.9 (n=6) | −2.1 |
| Musculoskeletal disorder | 24.9 (n=144) | 29.4 (n=190) | −4.5 | 27.9 (n=72) | 29.1 (n=92) | −1.2 | 22.4 (n=47) | 31.1 (n=55) | −8.7 | 22.7 (n=25) | 27.9 (n=43) | −5.2 |
| Cancer | 6.1 (n=35) | 4.8 (n=31) | 1.3 | 5.8 (n=15) | 4.4 (n=14) | 1.4 | 5.7 (n=12) | 5.7 (n=10) | 0.1 | 7.3 (n=8) | 4.6 (n=7) | 2.7 |
| Missing (%) | 8.1 (n=47) | 7.9 (n=51) | 0.3 | 1.9 (n=5) | 2.2 (n=7) | −0.3 | 13.8 (n=29) | 12.4 (n=22) | 1.4 | 11.8 (n=13) | 14.3 (n=22) | −2.5 |
| Baseline PCS | 37.5 (9.2) | 37.7 (8.9) | −0.2 | 38.2 (9.1) | 38.2 (9.2) | 0.0 | 36.0 (9.10) | 36.2 (8.4) | −0.2 | 37.8 (10.1) | 37.9 (8.4) | −0.1 |
| Missing (%) | 23.9 (n=138) | 25.5 (n=165) | 1.6 | 0.0 (n=0) | 0.0 (n=0) | 0.0 | 31.9 (n=67) | 37.9 (n=67) | −6.0 | 64.6 (n=71) | 63.6 (n=98) | 0.9 |
| Baseline MCS | 48.5 (11.6) | 48.9 (11.2) | −0.4 | 49.9 (11.0) | 50.6 (10.8) | −0.7 | 46.0 (12.3) | 45.1 (11.6) | 0.9 | 48.1 (11.5) | 46.8 (11.0) | 1.3 |
| Missing (%) | 23.9 (n=138) | 25.5 (n=165) | 1.6 | 0.0 (n=0) | 0.0 (n=0) | 0.0 | 31.9 (n=67) | 37.9 (n=67) | −6.0 | 64.6 (n=71) | 63.6 (n=98) | 0.9 |
Data are mean (SD) or proportion (number of patients).
All data are based on norms-based scoring.
*Complete case: a case that have nonmissing values on MCS and PCS score at baseline and follow-up.
†Lost-to-follow-up: cases that died, withdrew consent or did not return any study questionnaires after baseline.
‡Incomplete case: a case that is not lost to follow-up but has missing values on items in either PCS and MCS at baseline or follow-up.
§Variable has no missing values.
¶Fischer’s exact test for differences in proportions of patients in telehealth group and usual practice group (at baseline, complete cases, lost-to-follow-up and incomplete cases), p<0.05.
**Mann-Whitney test for differences in mean in telehealth group and usual practice group (at baseline, complete cases, lost-to-follow-up and incomplete cases), p<0.05.
COPD, chronic obstructive pulmonary disease; Diff, difference; FEV1(%), forced expiratory volume in one second of predicted normal; FVC(%), forced vital capacity; MCS, mental component summary; PCS, physical component summary; THC, telehealthcare; UP, usual practice.
Preliminary descriptive analysis of PCS and MCS scores
Table 2 presents descriptive statistics of PCS and MCS scores over time for the two analysis cohorts, complete cases (n=574) and available cases (n=1225) in each treatment arm.
Table 2.
Descriptive analysis of physical component summary (PCS) and mental component summary (MCS) scores
| Primary analysis (n=1225)† | Complete case analysis (n=574) | |||
| THC | UP | THC | UP | |
| PCS at follow-up | 34.6 (13.9) | 34.7 (13.8) | 38.3 (9.6) | 38.1 (9.6) |
| MCS at follow-up | 43.4 (17.2) | 43.5 (17.3) | 48.4 (11.2) | 48.6 (11.4) |
| Difference in PCS scores from baseline to follow-up* | −2.6 (12.4) | −2.8 (11.9) | 0.0 (7.1) | −0.1 (6.7) |
| Difference in MCS scores from baseline to follow-up* | −4.7 (16.5) | −5.3 (15.5) | −1.5 (10.6) | −2.0 (8.7) |
Data are mean (SD).
All data are based on norms-based scoring.
*Follow-up score minus baseline score.
†Primary analysis has imputed missing PCS and MCS scores.
THC, telehealthcare; UP, usual practice.
At follow-up, lower scores of mean PCS and MCS were represented in the primary analysis (n=1225) compared with the complete case analysis (n=574). In the primary analysis, the raw mean difference in PCS scores from baseline to follow-up was −2.6 (SD 12.4) in the telehealthcare group and −2.8 (SD 11.9) in the usual practice group. The raw mean difference in MCS scores in the same period were −4.7 (SD 16.5) and −5.3 (SD 15.5) for telehealthcare and usual practice, respectively (table 2).
In the complete case analysis, the raw mean difference in PCS scores over time was 0.0 (SD 7.1) in the telehealthcare group and −0.0 (SD 6.7) in the usual practice group. The raw difference in MCS scores was −1.5 (SD 10.6) and −2.0 (SD 8.7) for telehealthcare and usual practice, respectively. A comparison of the raw differences in PCS and MCS scores between the two analysis cohorts’ indicated that both complete cases and available cases scored lower HRQoL from baseline to follow-up, except for the telehealthcare group’s PCS score from the complete case analysis, whose score increased from baseline to follow-up (table 2).
Primary analysis and complete case analysis
Adjusted outcomes
Table 3 presents adjusted mean difference in summary scores between treatment groups at 12 month follow-up for each analysis cohort.
Table 3.
Adjusted mean difference in physical component summary (PCS) and mental component summary (MCS) scores between groups, 12 month follow-up
| Primary analysis (n=1225)† | ICC | Complete case analysis (n=574) | ICC | |
| PCS (adjusted mean difference)* | 0.1 (−1.4 to 1.7) | 0.0 | 0.2 (−0.9 to 1.3) | 0.0 |
| MCS (adjusted mean difference)* | 0.4 (−1.7 to 2.4) | 0.0 | 0.4 (−1.0 to 1.7) | 0.0 |
Data are mean (95% CI).
All data are based on norms-based scoring.
Differences can be interpreted as the observed extra effect of telehealthcare compared with usual practice when all mentioned covariates and clustering are taken into account.
*Adjusted mean differences are based on multilevel models controlling for all mentioned covariates and clustering.
†Primary analysis has imputed missing PCS and MCS scores.
ICC, intraclass coefficient.
In the primary analysis (n=1225), the adjusted mean differences in summary scores at 12 month follow-up were PCS 0.1 (95% CI −1.4 to 1.7) and MCS 0.4 (95% CI −1.7 to 2.4). The overlapping confidence intervals indicated that differences between groups at 12 month follow-up were non-significant (table 3).
In the complete case analysis (n=574), the adjusted mean differences in summary scores at 12 month follow-up were PCS 0.2 (95% CI −0.9 to 1.3) and MCS 0.4 (95% CI −1.0 to 1.7). The adjusted outcomes indicated no evidence of statistically significant differences between groups at 12 month follow-up (all CIs crossed 0) (table 3).
Secondary analysis
We also performed a posteriori-defined subgroup analysis, which showed no statistically significant effect of the intervention in any of the defined subgroups. Tables 4 and 5 provide estimates of both adjusted mean differences in PCS and MCS summary scores, 95% CIs and p values for the total sample and for subgroups.
Table 4.
Primary outcome and subgroup analyses of the chronic obstructive pulmonary disease patients’ socio-demographic characteristics: adjusted mean differences in physical component summary (PCS) and mental component summary (MCS) scores for the total sample and subgroups, adjusted for respective baseline PCS or baseline MCS scores and age, gender, baseline forced expiratory volume in one second of predicted normal (FEV1), marital status, cancer and diabetes
| Socio-demographic characteristics | ||||||||
| Effectiveness Total sample |
PCS 0.1 |
PCS 95% CI (−1.4 to 1.7) |
Wald test p value |
ICC 0.0 |
MCS 0.4 |
MCS 95% CI (−1.7 to 2.4) |
Wald test p value |
ICC 0.0 |
| Gender | ||||||||
| Female (54%) | −0.3 | (−1.6 to 1.1) | 0.6 | 0.0 | −0.5 | (−2.6 to 1.7) | 0.3 | 0.0 |
| Male (46%) | 0.5 | (−2.1 to 3.2) | −1.3 | (−1.9 to 4.5) | ||||
| Age (years) | ||||||||
| <60 (16%) | −0.5 | (−4.0 to 3.1) | 0.7 | 0.0 | −0.1 | (−4.3to 4.1) | 0.9 | 0.0 |
| 60–69 (33%) | −1.2 | (−3.2 to 0.9) | −0.7 | (−3.7 to 2.3) | ||||
| 70–79 (38%) | 1.0 | (−1.9 to 3.9) | 0.7 | (−2.7 to 4.2) | ||||
| ≥80 (13%) | 1.7 | (−3.6 to 7.0) | 2.2 | (−5.0 to 9.3) | ||||
| Marital status | ||||||||
| Married/relationship (58%) | 0.3 | (−1.8 to 2.4) | 0.7 | 0.0 | 1.0 | (−2.2 to 4.2) | 0.8 | 0.0 |
| Single (23%) | −0.9 | (−3.8 to 2.0) | −0.6 | (−4.9 to 3.6) | ||||
| Widow/widower (19%) | 0.9 | (−2.6 to 4.3) | −0.5 | (−4.9 to 4.0) | ||||
| Smoking status | ||||||||
| Non-smokers (66%) | 0.4 | (−1.6 to 2.5) | 0.6 | 0.0 | 1.3 | (−1.6 to 4.3) | 0.2 | 0.0 |
| Smoker (34%) | −0.4 | (−2.8 to 1.9) | −1.5 | (−4.3 to 1.4) | ||||
| Job status | ||||||||
| Full-time job (5%) | −1.2 | (−6.1 to 3.8) | 0.8 | 0.0 | 6.0 | (−12.6 to 0.6) | 0.2 | 0.0 |
| Part-time job (7%) | −0.8 | (−5.0 to 3.3) | 0.8 | (−5.2 to 6.9) | ||||
| No job (88%) | 0.3 | (−1.4 to 2.0) | 0.7 | (−1.7 to 3.2) | ||||
| Education | ||||||||
| Elementary school, 7th–10th grade (48%) | 0.1 | (−1.6 to 1.8) | 1.0 | 0.0 | 0.7 | (−2.0 to 3.4) | 0.7 | 0.0 |
| High school (2%) | 0.6 | (−7.9 to 9.0) | 4.7 | (−8.0 to 17.4) | ||||
| Skilled worker (34%) | 0.8 | (−1.8 to 3.4) | 1.2 | (−2.5 to 4.9) | ||||
| Short-term education (2–3 years) (8%) | −1.6 | (−5.7 to 2.5) | −2.4 | (−8.1 to 3.3) | ||||
| Middle-term education (3–5 years) (7%) | −0.5 | (−6.4 to 5.5) | −4.3 | (−10.2 to 1.6) | ||||
| Long-term education (5–8 years) (1%) | −0.8 | (−18.7 to 17.1) | 0.4 | (−22.6 to 23.4) | ||||
All data are based on norms-based scoring.
Multilevel linear models controlling for baseline PCS or MCS score and age, gender, baseline FEV1, marital status, cancer and diabetes and clustering. Priori hypothesis was that adding telehealthcare to usual practice would improve patients’ health-related quality of life relative to usual practice.
Mean difference; 95% CIs.
ICC, intraclass coefficient.
Table 5.
Primary outcome and subgroup analyses of the chronic obstructive pulmonary disease (COPD) patients’ health characteristics: adjusted mean differences in physical component summary (PCS) and mental component summary (MCS) scores for the total sample and subgroups, adjusted for respective baseline PCS or baseline MCS and age, gender, baseline forced expiratory volume in one second of predicted normal (FEV1), marital status, cancer and diabetes
| Health characteristics | ||||||||
| Effectiveness Total sample |
PCS 0.1 |
PCS 95% CI (−1.4 to 1.7) |
Wald test p value |
ICC 0.0 |
MCS 0.4 |
MCS 95% CI (−1.7 to 2.4) |
Wald test p value |
ICC 0.0 |
| COPD severity (GOLD 1–4) | ||||||||
| Mild, 1 (6%) | 1.7 | (−4.7 to 8.2) | 0.7 | 0.0 | 0.6 | (−6.8 to 8.1) | 0.8 | 0.0 |
| Moderate, 2 (38%) | −0.7 | (−3.0 to 1.5) | −0.7 | (−3.8 to 2.4) | ||||
| Severe, 3 (39%) | 1.0 | (−1.7 to 3.7) | 1.5 | (−2.1 to 5.1) | ||||
| Very severe, 4 (17%) | −0.6 | (−4.8 to 3.5) | −0.2 | (−5.2 to 4.8) | ||||
| COPD duration (years) | ||||||||
| <3.5 (25%) | −0.7 | (−2.9 to 1.6) | 0.3 | 0.0 | −1.0 | (−4.4 to 2.4) | 0.1 | 0.0 |
| 3.5–6 (26%) | −1.6 | (−4.6 to 1.4) | −2.0 | (−5.7 to 1.6) | ||||
| 7–10 (26%) | 0.5 | (−2.8 to 3.8) | 0.5 | (−3.6 to 4.7) | ||||
| >10 (23%) | 2.5 | (−0.7 to 5.7) | 4.0 | (−0.2 to 8.2) | ||||
| Diabetes | ||||||||
| No (89%) | 0.3 | (−1.2 to 1.9) | 0.4 | 0.0 | 0.3 | (−1.8 to 2.5) | 0.9 | 0.0 |
| Yes (11%) | −1.4 | (−5.3 to 2.4) | 0.7 | (−4.9 to 6.4) | ||||
| Heart disease | ||||||||
| No (65%) | 0.1 | (−1.5 to 1.6) | 0.9 | 0.0 | 0.7 | (−1.7 to 3.2) | 0.6 | 0.0 |
| Yes (35%) | 0.3 | (−2.6 to 3.2) | −0.3 | (−3.7 to 3.1) | ||||
| Mental health problem | ||||||||
| No (95%) | 0.3 | (−1.3 to 1.9) | 0.3 | 0.0 | 0.3 | (−1.8 to 2.4) | 0.8 | 0.0 |
| Yes (5%) | −3.1 | (−8.7 to 2.5) | 1.5 | (−6.2 to 9.2) | ||||
| Musculoskeletal disease | ||||||||
| No (70%) | 0.1 | (−1.7 to 1.8) | 0.9 | 0.0 | 0.5 | (−1.6 to 2.6) | 0.7 | 0.0 |
| Yes (30%) | 0.2 | (−2.0 to 2.5) | −0.1 | (−3.5 to 3.3) | ||||
| Cancer | ||||||||
| No (94%) | 0.0 | (−1.4 to 1.5) | 0.5 | 0.0 | 0.2 | (−1.85 to 2.28) | 0.6 | 0.0 |
| Yes (6%) | 2.1 | (−4.7 to 8.9) | 2.7 | (−5.4 to 10.7) | ||||
| Number of comorbidities | ||||||||
| Yes (33%) | 0.3 | (−2.1 to 2.6) | 0.1 | 0.0 | 0.3 | (−2.8 to 3.4) | 0.7 | 0.0 |
| No (41%) | 1.4 | (−1.0 to 3.8) | 1.0 | (−2.2 to 4.2) | ||||
| Two or more (26%) | −2.0 | (−4.9 to 1.0) | −0.7 | (−4.3 to 2.9) | ||||
| Hypertension | ||||||||
| Yes (71%) | 0.6 | (−1.3 to 2.5) | 0.3 | 0.0 | 0.4 | (−2.1 to 2.9) | 1.0 | 0.0 |
| No (29%) | −1.1 | (−3.7 to 1.4) | 0.3 | (−3.3 to 3.9) | ||||
| Tachycardia | ||||||||
| Yes (70%) | 0.3 | (−1.4 to 1.9) | 0.8 | 0.0 | 0.1 | (−2.0 to 2.2) | 0.7 | 0.0 |
| No (30%) | −0.2 | (−3.3 to 3.0) | 1.0 | (−2.8 to 4.9) | ||||
| BMI | ||||||||
| <25 (44%) | 0.4 | (−2.1 to 2.8) | 1.0 | 0.0 | 0.5 | (−2.9 to 3.7) | 1.0 | 0.0 |
| 25–30 (34%) | 0.2 | (−2.5 to 2.9) | −0.1 | (−3.8 to 3.6) | ||||
| >30 (22%) | −0.4 | (−4.5 to 3.6) | 0.8 | (−4.0 to 5.6) | ||||
All data are based on norms-based scoring.
Multilevel linear models controlling for baseline PCS or MCS score and age, gender, baseline FEV1, marital status, cancer and diabetes and clustering. Priori hypothesis was that adding telehealthcare to usual practice would improve patients’ health-related quality of life relative to usual practice.
Mean difference; 95% CIs.
GOLD, Global Initiative for COPD; ICC, intraclass coefficient.
Discussion
The present study hypothesised that adding telehealthcare to usual practice would significantly increase patients’ HRQoL.31 This hypothesis was rejected. We found no statistical QoL differences between groups in either the primary analysis, the complete case analysis or in any of the subgroups.
Interpretation of findings
Despite the non-significant differences, the mean differences in PCS and MCS scores at 12 month follow-up were larger for the control group than for the intervention group, which could indicate a faster deterioration over time for the controls than for the intervention patients. The largest mean difference was seen in MCS. If this is the case, it might be explained by the difficulty associated with affecting the physical QoL compared with the mental QoL. The slower decline in MCS for the telehealthcare group may be interpreted as a psychological benefit derived from using the Telekit.
Although the subgroup analysis indicated no statistically significant effects of the intervention in any of the posteriorly defined subgroups, there was an indication of some positive effects on HRQoL within certain subgroups of the intervention group compared with usual practice. These trends towards positive effects on HRQoL should be further investigated.
Strengths and weaknesses
The present study was the first Danish large-scale trial established to remedy the lack of international evidence on HRQoL in patients with COPD who are receiving telehealthcare. A total of 1225 participants from 26 municipality districts in the North Denmark Region were included in the analysis. The trial succeeded in establishing a fruitful cooperation between many stakeholders with different interests in the trial. The trial hence demonstrated the feasibility of intersectoral and interinstitutional collaboration towards a shared end, viz. the implementation of telehealthcare to improve COPD patients’ HRQoL.
In contrast to the WSD,22–25 the TeleCare North trial compared the effects of telehealthcare with the effects of usual practice in patients with COPD only. The design of the WSD was characterised by variability in terms of the employed technologies, the recruited sample, the type of intervention and defined care pathways. Contrary to the WSD,40 the TeleCare North trial used a ‘clean’ control group of patients with COPD who received usual practice and no other forms of care.
The TeleCare North trial was based on the same concept as in previous Danish pilot studies27–30 41 namely to increase patient empowerment and to detect disease deterioration through self-monitoring. In the present study, we attended to clarify the mechanisms that were supposed to provide effects. Organisational initiatives to further this concept, for example, ensuring that patients had functional telehealthcare equipment, instructing patients how to use this equipment and by gearing the organisation to rapidly respond to reported measures to prevent COPD exacerbations. That no significant effects of HRQoL were found therefore cannot be attributed to patients’ lack of equipment, a lack of instructions or inadequate operational equipment.
In the trial, we found a considerable high attrition rate of 53% among participants with over half of the sample not providing follow-up data due to incomplete cases with missing data or loss to follow-up. Conducting a sensitivity analysis as a complete case analysis was therefore relevant in order to explore differences among complete and available cases. The high attrition rate may be attributed to disease progression and may have affected the findings of the trial. However, the consistency of results indicates that conclusions on findings are robust in spite of the high attrition rate. Further research is required to find explanations for high attrition rate among patients with COPD.
The subgroup analysis was not prespecified at the outset of the trial but was undertaken after the data collection of the trial. Because of the limitation noted, the findings of the post hoc subgroup analysis should be interpreted with caution irrespective of their significance.
We cannot rule out the influence of non-specific effects like a Hawthorne effect42 or ‘natural history effects’,43 both of which could have influenced the intervention and the control group to some extent. The potential presence of any such effects may explain why differences in HRQoL between the groups were difficult to detect.
The baseline variables used in the TeleCare North trial were not exhaustive; nor were all relevant variables included. At baseline, the FVC% indicated that the patients in the intervention group were poorer than the patients in the control group. It is widely known that QoL deteriorates with increasing severity of COPD.44 This may also contribute to explaining why no significant differences in HRQoL were found between the groups. It would have been desirable to supplement the baseline variables with other clinical characteristics such as the MRC dyspnoea score and with activities-of-daily-living measures to determine if the groups differed from each other in relation to their state of health. The number of selected baseline variables made it possible to classify the patients only according to the old GOLD classification (I–V). It would have been desirable to classify the patients according to the new GOLD classification (A–D) which is based on symptoms, airflow obstruction and exacerbation history. Use of the new GOLD classification would probably have made it possible to establish more relevant subgroups.32
The SF-36v2 was selected as an appropriate outcome measure because it is a useful, generic and validated questionnaire for comparing differences between populations. It is possible, however, that generic questionnaires do not adequately measure the QoL issues that different groups of patients experience. It has been shown that the SF-36 is susceptible to ceiling and floor effects as it is applicable to a wide population of both healthy and sick individuals. It is possible that the SF-36v2 was not sufficiently sensitive to changes and to identifying outcome differences in patients with COPD.45 This was also confirmed by Rixon et al, 25 who suggest that generic instruments are less sensitive to change related to telehealth than disease-specific instruments are. COPD is associated with symptoms that might have an impact on the patients’ QoL, which makes it uncertain whether the generic questionnaires capture these aspects. Another alternative for measuring QoL could have been a more disease-specific questionnaire for patients with COPD, such as the St. George’ Respiratory Questionnaire46 and other QoL instruments such as the Chronic Respiratory Questionnaire,47 the EQ-5D48 or the Hospital Anxiety and Depression Scale .49 A study by Engström and colleagues50 illustrated that a combination of generic and disease-specific questionnaires was the most suitable choice for measuring differences in COPD patients’ HRQoL following an intervention. They argued that both disease-specific effects and the overall burden of the disease on everyday functioning and mental well-being should be considered. This was also confirmed in a review by Chen, who recommended the use of both generic and disease-specific questionnaires in combination.45
Another relevant consideration is whether QoL measures should be expected to change by implementation of telehealthcare. Two recent systematic reviews18 51 found that the impact of telehealth on QoL in patients with COPD is limited. However, the review suggested that active interventions may improve QoL outcomes in the telehealth group compared with usual care.18 Based on this study’s results and the literature,7 18 24 25 telehealthcare is not assumed to be convincing when looking at QoL as an isolated factor. However, QoL improvements may be expected over time25 in active telehealthcare interventions where some kind of self-management skills training is an integrated part of the intervention.18
The strategy of offering inclusion to all patients with COPD who may benefit from telehealthcare in the whole region of North Denmark strengthens the generalisability of the findings. So does the use of minimal inclusion criteria and the 12-month-long continuous assessment of the patients. Given the significant differences between COPD and other chronic diseases, the findings should, however, not be applied to other chronic diseases.
The North Denmark Region is fairly representative of the whole country of Denmark in terms of population and healthcare system. The findings are therefore generally applicable to the whole of Denmark and, at least partly, also to countries with similar healthcare systems such as the other Nordic countries.
Comparison with other studies
Our results demonstrate a further lack of any improvements in QoL following implementation of telehealthcare in COPD. The WSD is the only large-scale study of telehealthcare in COPD that we have come across. The findings from the WSD study by Cartwright and colleagues24 indicated no improvement in HRQoL from telehealth, but some significant differences suggested that the telehealth group had a slower rate of deterioration over time compared with the control group.
Similarly, no statistical differences in the Telescot study52 or the ‘The Virtual Hospital’ trial53 were identified between the control and intervention groups. The results of our study are consistent with the findings from these studies.
The review and meta-analysis by McLean et al 7 is also relevant to consider; in contrast, their findings indicated possible impact of COPD patients’ QoL. Another recent review by Cruz et al 10 indicated inconsistency in HRQoL findings with most of the studies reporting no significant changes in HRQoL. However, the studies included in the reviews used different HRQoL instruments; and it has therefore been recommended to use similar HRQoL instruments in future studies to enable comparisons.7 10
Nevertheless, the benefits in relation to QoL may be debated although telehealthcare seems unlikely to reduce QoL. One of our previous findings from the TeleCare North trial indicated that the intervention patients experienced enhanced control, freedom, security and greater awareness of their COPD symptoms when using the telehealthcare system.54 These benefits are not underpinned by the present study’s findings on HRQoL.
Implications for practice
Our findings indicate that adding telehealthcare to usual practice does not improve HRQoL in patients with COPD. We did not succeed in achieving the HRQoL effects we had hoped for, and the reduced HRQoL in both groups means that it is doubtful whether telehealthcare benefits patients’ QoL. Therefore, policymakers and healthcare professionals should consider whether telehealthcare should be implemented to achieve other objectives than improving patients’ HRQoL, that is, saving costs, reducing mortality, affecting other outcome variables, and so on.
Furthermore, it is relevant to explore other domains such as physical activity, psychological symptoms and different modes of telehealth application and interventions, which may be important in improving QoL. In addition, more research should be considered within more specific subgroups of patients with COPD to assess whether telehealthcare has a particularly beneficial effect on QoL in some groups. It is possible that patients’ QoL varies between subgroups. Knowledge of such variation is useful and may inform future implementation of telehealthcare allowing for targeting of specific patient subgroups.
Future directions
In the future, more research is needed into the underlying mechanisms behind this lack of an identifiable effect. More qualitative research is required to gain a deeper understanding of the mechanism and preconditions needed to improve patients’ HRQoL by use of telehealthcare. A greater effort should be dedicated to studying the specific subgroups instead of the population as whole because telehealthcare systems likely fit some patients better than others. Furthermore, the patients with COPD in the present trial were recruited from different municipalities, some of which might have been better at organising telehealthcare than others. Large-scale studies are therefore not recommended until these underlying mechanisms have been further investigated.
Future studies should recognise telehealthcare as a complex intervention. Such studies should therefore be designed as a mix of randomised controlled trials and other research designs to fully assess complex interventions. It is possible that we have jumped too quickly to large-scale operational trials. Furthermore, research on the causal relationship between QoL and patients’ socio-demographic and health characteristics is limited, indicating that a number of exploratory studies need to be performed within the TeleCare North trial in the future.
In conclusion, the findings of the present study indicate that the potential of telehealthcare for improving COPD patients’ HRQoL is limited. However, it is assumed on the basis of these results that telehealthcare as an additional service alongside the existing clinical care does not lead to poorer QoL.
bmjopen-2016-014587supp001.pdf (387.4KB, pdf)
bmjopen-2016-014587supp002.pdf (175.5KB, pdf)
Supplementary Material
Acknowledgments
The participation of the Trial Administration Office, the general practitioners, hospitals, healthcare providers and patients in the North Denmark Region is acknowledged. Without their commitment to the implementation of the TeleCare North trial, it would not have been possible to do this study.
Footnotes
Detailed description of intervention and comparator: The study protocol (DOI: 10.1186/1745-6215-15-178) is freely available and can be downloaded from: http://www.trialsjournal.com/content/15/1/178.
Contributors: PHL, FWU, OKH and LHE conceived and designed the study and were responsible for its conduct. PHL and FWU managed the study and the data collection. The trials Administration Office provided administrative support. FWU undertook the data analysis. PHL, FWU, OKH, and LHE interpreted the data. PHL prepared the first draft of the manuscript. OKH, FWU, and LHE revised and contributed to the manuscript. All authors read and approved the final manuscript.
Funding: This paper was funded by the North Denmark Region, the 11 municipalities in the North Denmark Region and by the Obel Family Foundation, the Danish Agency for Digitalization Policy and Strategy and the European Social Fund.
Funding: The funding agencies had no influence on the design of the study, the analysis and interpretation, dissemination of results, or the shaping of the article. The article is based on the authors’ views and not those of the sponsors.
Competing interests: None delared.
Ethics approval: The trial is conducted in accordance with the Helsinki Declaration. The trial was presented to the Danish Ethics Committee for Medical Research in the North Denmark Region and no ethical approval was needed. The trial has also been accepted by the Danish Data Protection Agency.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
References
- 1. Fletcher MJ, Upton J, Taylor-Fishwick J, et al. . COPD uncovered: an international survey on the impact of chronic obstructive pulmonary disease (COPD) on a working age population. BMC Public Health 2011;11:1–13. 10.1186/1471-2458-11-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology 2016;21:14–23. 10.1111/resp.12660 [DOI] [PubMed] [Google Scholar]
- 3. Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765–73. 10.1016/S0140-6736(07)61380-4 [DOI] [PubMed] [Google Scholar]
- 4. Adeloye D, Chua S, Lee C, et al. . Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health 2015;5:1–17. 10.7189/jogh.05.020415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goodridge D, Marciniuk D. Rural and remote care: overcoming the challenges of distance. Chron Respir Dis 2016;13:21:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Annandale J, Lewis KE. Can telehealth help patients with COPD? Nurs Times 2005;107:12–14. [PubMed] [Google Scholar]
- 7. McLean S, Nurmatov U, Liu JL, et al. . Telehealthcare for chronic obstructive pulmonary disease: cochrane Review and meta-analysis. Br J Gen Pract 2012;62:739–49. 10.3399/bjgp12X658269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lundell S, Holmner Å, Rehn B, et al. . Telehealthcare in COPD: a systematic review and meta-analysis on physical outcomes and dyspnea. Respir Med 2015;109:11–26. 10.1016/j.rmed.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 9. Calvo GS, Hernández AS, Padilla DL, et al. . Clinical outcomes of Home Telemonitoring in severe COPD. iMedPub Journals 2016;1:1–7. [Google Scholar]
- 10. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract 2014;68:369–78. 10.1111/ijcp.12345 [DOI] [PubMed] [Google Scholar]
- 11. Bolton CE, Waters CS, Peirce S, et al. . Insufficient evidence of benefit: a systematic review of home telemonitoring for COPD. J Eval Clin Pract 2011;17:1216–22. 10.1111/j.1365-2753.2010.01536.x [DOI] [PubMed] [Google Scholar]
- 12. Bowles KH, Baugh AC. Applying research evidence to optimize telehomecare. J Cardiovasc Nurs 2007;22:5–15. 10.1097/00005082-200701000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Polisena J, Tran K, Cimon K, et al. . Home telehealth for chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Telemed Telecare 2010;16:120–7. 10.1258/jtt.2009.090812 [DOI] [PubMed] [Google Scholar]
- 14. Franek J. Home telehealth for patients with chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser 2012;12:1–58. [PMC free article] [PubMed] [Google Scholar]
- 15. Kitsiou S, Paré G, Jaana M. Systematic reviews and meta-analyses of home telemonitoring interventions for patients with chronic diseases: a critical assessment of their methodological quality. J Med Internet Res 2013;15:e150 10.2196/jmir.2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ekeland AG, Bowes A, Flottorp S. Effectiveness of telemedicine: a systematic review of reviews. Int J Med Inform 2010;79:736–71. 10.1016/j.ijmedinf.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 17. Pedone C, Lelli D. Systematic review of telemonitoring in COPD: an update. Pneumonol Alergol Pol 2015;83:476–84. 10.5603/PiAP.2015.0077 [DOI] [PubMed] [Google Scholar]
- 18. Gregersen TL, Green A, Frausing E, et al. . Do telemedical interventions improve quality of life in patients with COPD? A systematic review. Int J Chron Obstruct Pulmon Dis 2016;11:809 10.2147/COPD.S96079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bower P, Cartwright M, Hirani SP, et al. . A comprehensive evaluation of the impact of telemonitoring in patients with long-term conditions and social care needs: protocol for the whole systems demonstrator cluster randomised trial. BMC Health Serv Res 2011;11:184 10.1186/1472-6963-11-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hirani SP, Beynon M, Cartwright M, et al. . The effect of telecare on the quality of life and psychological well-being of elderly recipients of social care over a 12-month period: the Whole Systems Demonstrator cluster randomised trial. Age Ageing 2014;43:334–41. 10.1093/ageing/aft185 [DOI] [PubMed] [Google Scholar]
- 21. Steventon A, Bardsley M, Billings J, et al. . Effect of telecare on use of health and social care services: findings from the Whole Systems Demonstrator cluster randomised trial. Age Ageing 2013;42:501–8. 10.1093/ageing/aft008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steventon A, Bardsley M, Billings J, et al. . Effect of telehealth on use of secondary care and mortality: findings from the Whole System Demonstrator cluster randomised trial. BMJ 2012;344:e3874 10.1136/bmj.e3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henderson C, Knapp M, Fernández JL, et al. . Cost effectiveness of telehealth for patients with long term conditions (Whole Systems Demonstrator Telehealth Questionnaire Study): nested economic evaluation in a pragmatic, cluster randomised controlled trial. BMJ 2013;346:f1035 10.1136/bmj.f1035 [DOI] [PubMed] [Google Scholar]
- 24. Cartwright M, Hirani SP, Rixon L, et al. . Effect of telehealth on quality of life and psychological outcomes over 12 months (Whole Systems Demonstrator telehealth questionnaire study): nested study of patient reported outcomes in a pragmatic, cluster randomised controlled trial. BMJ 2013;346:f653 10.1136/bmj.f653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rixon L, Hirani SP, Cartwright M, et al. . A RCT of telehealth for COPD patient’s quality of life: the whole system demonstrator evaluation. Clin Respir J 2015:2–25. 10.1111/crj.12359 [DOI] [PubMed] [Google Scholar]
- 26. Fonden for velfærdsteknologi. national handlingsplan for udbredelse af telemedicin. 2012. http://www.digst.dk/Digital-velfaerd/~/media/Files/Velfærdsteknologi/Telemedicinskhandlingsplan/Telemedicinskhandlingsplan-web.pdf
- 27. Haesum LK, Soerensen N, Dinesen B, et al. . Cost-utility analysis of a telerehabilitation program: a case study of COPD patients. Telemed J E Health 2012;18:688–92. 10.1089/tmj.2011.0250 [DOI] [PubMed] [Google Scholar]
- 28. Huniche L, Dinesen B, Grann O, et al. . Empowering patients with COPD using Tele-Homecare Technology. 2010:48–54. [PubMed]
- 29. Sorknaes AD, Madsen H, Hallas J, et al. . Nurse tele-consultations with discharged COPD patients reduce early readmissions--an interventional study. Clin Respir J 2011;5:26–34. 10.1111/j.1752-699X.2010.00187.x [DOI] [PubMed] [Google Scholar]
- 30. Sorknaes AD, Bech M, Madsen H, et al. . The effect of real-time teleconsultations between hospital-based nurses and patients with severe COPD discharged after an exacerbation. J Telemed Telecare 2013;19:466–74. 10.1177/1357633X13512067 [DOI] [PubMed] [Google Scholar]
- 31. Udsen FW, Lilholt PH, Hejlesen O, et al. . Effectiveness and cost-effectiveness of telehealthcare for chronic obstructive pulmonary disease: study protocol for a cluster randomized controlled trial. Trials 2014;15:178 10.1186/1745-6215-15-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. GOLD: global Initiative for Chronic Obstructive lung disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, 2015. www.goldcopd.org
- 33. Davenport TE, Stevens SR, Baroni K, et al. . Reliability and validity of short form 36 version 2 to measure health perceptions in a sub-group of individuals with fatigue. Disabil Rehabil 2011;33:2596–604. 10.3109/09638288.2011.582925 [DOI] [PubMed] [Google Scholar]
- 34. Kosinski M, Dewy J. How to score version two of the SF-36 Health Survey. 3rd ed: Quality Metric, 2001. [Google Scholar]
- 35. Bentsen SB, Rokne B, Wahl AK. Comparison of health-related quality of life between patients with chronic obstructive pulmonary disease and the general population. Scand J Caring Sci 2013;27:905–12. 10.1111/scs.12002 [DOI] [PubMed] [Google Scholar]
- 36. Vickers AJ, Altman DG. Statistics notes: analysing controlled trials with baseline and follow up measurements. BMJ 2001;323:1123–4. 10.1136/bmj.323.7321.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Faria R, Gomes M, Epstein D, et al. . A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics 2014;32:1157–70. 10.1007/s40273-014-0193-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ware JE, et al. . Differences in 4-Year Health Outcomes for Elderly and Poor, chronically III patients treated in HMO and Fee-for-Service Systems. JAMA 1996;276:1039 10.1001/jama.1996.03540130037027 [DOI] [PubMed] [Google Scholar]
- 39. Bjørner JB, Damsgaard MT, Watt T, et al. . Dansk manual til SF-36 København: Lif. 1997.
- 40. Tim E. The Whole System Demonstrator Programme. York, UK: 2009. [Google Scholar]
- 41. Dinesen B, Seeman J, Gustafsson J. Development of a program for tele-rehabilitation of COPD patients across sectors : co-innovation in a network presentation of the Telekat Case Study. Int J Integr Care 2011;11:e012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Konstantinou GN. Pragmatic trials: how to adjust for the ‘Hawthorne effect’? Thorax 2012;67:562.1–562. 10.1136/thoraxjnl-2011-200657 [DOI] [PubMed] [Google Scholar]
- 43. Bouchet C, Guillemin F, Briançon S. Nonspecific effects in longitudinal studies: impact on quality of life measures. J Clin Epidemiol 1996;49:15–20. 10.1016/0895-4356(95)00540-4 [DOI] [PubMed] [Google Scholar]
- 44. Zamzam M, Azab N, El WR, et al. . Quality of life in COPD patients. Egypt J Chest Dis Tuberc 2013;5:36–40. [Google Scholar]
- 45. Chen TH, Li L, Kochen MM. A systematic review: how to choose appropriate health-related quality of life (HRQOL) measures in routine general practice? J Zhejiang Univ Sci B 2005;6:936–40. 10.1631/jzus.2005.B0936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Swigris JJ, Brown KK, Behr J, et al. . The SF-36 and SGRQ: validity and first look at minimum important differences in IPF. Respir Med 2010;104:296–304. 10.1016/j.rmed.2009.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guyatt GH, Berman LB, Townsend M, et al. . A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987;42:773–8. 10.1136/thx.42.10.773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. The EuroQol Group. A new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 49. Aylard PR, Gooding JH, McKenna PJ, et al. . A validation study of three anxiety and depression self-assessment scales. J Psychosom Res 1987;31:261–8. 10.1016/0022-3999(87)90083-3 [DOI] [PubMed] [Google Scholar]
- 50. Engström CP, Persson LO, Larsson S, et al. . Health-related quality of life in COPD: why both disease-specific and generic measures should be used. Eur Respir J 2001;18:69–76. 10.1183/09031936.01.00044901 [DOI] [PubMed] [Google Scholar]
- 51. Liu L, Stroulia E, Nikolaidis I, et al. . Smart homes and home health monitoring technologies for older adults: a systematic review. Int J Med Inform 2016;91:44–59. 10.1016/j.ijmedinf.2016.04.007 [DOI] [PubMed] [Google Scholar]
- 52. Pinnock H, Hanley J, McCloughan L, et al. . Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease: researcher blind, multicentre, randomised controlled trial. BMJ 2013;347:f6070 10.1136/bmj.f6070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jakobsen AS, Laursen LC, Rydahl-Hansen S, et al. . Home-based telehealth hospitalization for exacerbation of chronic obstructive pulmonary disease: findings from "the virtual hospital" trial. Telemed J E Health 2015;21:364–73. 10.1089/tmj.2014.0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lilholt PH, Hæsum LK, Hejlesen OK. Exploring user experience of a Telehealth System for the Danish TeleCare North Trial. Stud Health Technol Inform 2015;210:301–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-014587supp001.pdf (387.4KB, pdf)
bmjopen-2016-014587supp002.pdf (175.5KB, pdf)


