Abstract
Microorganisms continuously monitor their surroundings and adaptively respond to environmental cues. One way to cope with various stress-related situations is through the activation of the stringent stress response pathway. In Pseudomonas aeruginosa this pathway is controlled and coordinated by the activity of the RelA and SpoT enzymes that metabolize the small nucleotide secondary messenger molecule (p)ppGpp. Intracellular ppGpp concentrations are crucial in mediating adaptive responses and virulence. Targeting this cellular stress response has recently been the focus of an alternative approach to fight antibiotic resistant bacteria. Here, we examined the role of the stringent response in the virulence of P. aeruginosa PAO1 and the Liverpool epidemic strain LESB58. A ΔrelA/ΔspoT double mutant showed decreased cytotoxicity toward human epithelial cells, exhibited reduced hemolytic activity, and caused down-regulation of the expression of the alkaline protease aprA gene in stringent response mutants grown on blood agar plates. Promoter fusions of relA or spoT to a bioluminescence reporter gene revealed that both genes were expressed during the formation of cutaneous abscesses in mice. Intriguingly, virulence was attenuated in vivo by the ΔrelA/ΔspoT double mutant, but not the relA mutant nor the ΔrelA/ΔspoT complemented with either gene. Treatment of a cutaneous P. aeruginosa PAO1 infection with anti-biofilm peptides increased animal welfare, decreased dermonecrotic lesion sizes, and reduced bacterial numbers recovered from abscesses, resembling the phenotype of the ΔrelA/ΔspoT infection. It was previously demonstrated by our lab that ppGpp could be targeted by synthetic peptides; here we demonstrated that spoT promoter activity was suppressed during cutaneous abscess formation by treatment with peptides DJK-5 and 1018, and that a peptide-treated relA complemented stringent response double mutant strain exhibited reduced peptide susceptibility. Overall these data strongly indicated that synthetic peptides target the P. aeruginosa stringent response in vivo and thus offer a promising novel therapeutic approach.
Keywords: Stringent response, Anti-biofilm peptides, Pseudomonas, mouse abscess infections
Introduction
Antibiotics are arguably the most successful medical intervention in human history. The lack of effective antibiotics would have devastating effects in medicine such as causing death after major surgeries and even minor injuries. Indeed, resistance to most antibiotics has become a global threat to public health and strategies to overcome this danger are urgently needed. Life-threatening situations with pathogens that are resistant to several classes of antibiotics are on the rise and the widespread distribution of those organisms is of great concern (Ventola, 2015). Finding novel targets to treat bacterial infections has attracted considerable interest, and as such, inhibition of the stringent stress response has been suggested to be a promising alternative approach for disarming pathogens without killing them (Hauryliuk et al., 2015). Moreover, the stringent stress response pathway is a ubiquitous process among bacteria and, importantly, mammalian hosts lack this pathway (Pollard et al., 1980), indicating that the stringent response is an excellent selective drug target in bacteria.
The bacterial stringent response is a crucial survival response to various environmental stress conditions (Potrykus and Cashel, 2008) encountered, for example, during nutrient starvation (Haseltine and Block, 1973), fatty acid or iron limitation (Vinella et al., 2005), heat shock (Vogt et al., 2011), and high density populations such as stationary phase growth (Mansour et al., 2016) and biofilm formation (Strugeon et al., 2016). As a consequence, cells rapidly trigger a cellular reprogramming response (Starosta et al., 2014) that causes bacteria to decelerate energy-consuming processes (such as macromolecular synthesis and growth) and redirect resources toward energy generation, stress coping and expression of biosynthetic genes (Traxler et al., 2008). Activation of the stringent stress response pathway occurs through expression of the genes responsible for the synthesis of the small signaling nucleotide guanosine-pentaphosphate (pppGpp) that is quickly hydrolysed to the active form guanosine-tetraphosphate (ppGpp). In Gram-negative bacteria, two highly conserved homologous proteins, RelA and SpoT, regulate the intracellular concentrations of ppGpp (Pletzer et al., 2016). RelA is a mono-functional synthase that binds to the ribosome and upon entry of an uncharged tRNA molecule that blocks the ribosome, converts GTP and ATP to pppGpp and hence ppGpp. Conversely, SpoT is a bi-functional enzyme that responds to many other types of stresses such as carbon, phosphorus, fatty acid or iron starvation, and triggers the synthesis of ppGpp. Additionally, SpoT possesses another functional domain that allows it to also hydrolyze ppGpp. The second messenger ppGpp functions as a pleiotropic regulator to coordinate the stress response. Rapid accumulation of intracellular ppGpp triggers a switch from cell growth to survival mode through several mechanisms including binding and altering of the specificity of RNA polymerase, interaction with proteins involved in translation, replication and RNA turnover, crosstalk with other second messenger molecules such as c-di-GMP, and by regulation of cellular processes such as quorum sensing (Romling and Balsalobre, 2012; Chua et al., 2015; Hauryliuk et al., 2015; Pletzer and Hancock, 2016).
Although more than 1,000 articles have addressed the stringent response since its discovery 48 years ago (Cashel and Gallant, 1969), only a handful have addressed the bacterial stringent response in the context of vertebrate infection models. Generally speaking, stringent response mutants often exhibit attenuated virulence and persistence as well as enhanced survival rates in a variety of Gram-positive (Kazmierczak et al., 2009; Frank et al., 2014; Mansour et al., 2016; Zhu et al., 2016), mycobacterial (Klinkenberg et al., 2010; Weiss and Stallings, 2013), and Gram-negative bacterial infections (Haralalka et al., 2003; Dean et al., 2009; Vogt et al., 2011; Xu et al., 2016). These animal models provided evidence that the stringent response is crucial during infection, and we recently demonstrated that the stringent response of Gram-positive S. aureus can be effectively directly targeted in vivo [15] by small synthetic cationic anti-biofilm peptides (Pletzer et al., 2016).
Synthetic peptides are short (12–50 amino acids) cationic derivatives of host defense peptides that have potent broad-spectrum activities including antimicrobial, antibiofilm and immunomodulatory properties (de la Fuente-Núñez et al., 2012; Haney and Hancock, 2013). Their distinct mechanism of action against biofilms has been linked to a disruption of the stringent stress response since anti-biofilm peptides act by binding to and triggering the degradation of ppGpp. Disabling the stringent response therefore prevents intracellular accumulation of ppGpp (de la Fuente-Núñez et al., 2014), likely leading to phenotypes similar to those of stringent stress response mutants that completely lack the ability to make ppGpp.
Here, we provide new evidence describing how synthetic cationic peptides target the Pseudomonas aeruginosa stringent response in vitro and in vivo in a murine abscess model. P. aeruginosaΔrelA/ΔspoT double mutants were generated in both strain PAO1 and the clinical epidemic cystic fibrosis isolate LESB58, and these strains were complemented by chromosomal insertion of either relA or spoT under their native promoter. Using these mutants and their complements, we examined, both in vitro and in vivo, the link between the stringent stress response, virulence, and therapeutic treatment with synthetic peptides. Several virulence factors were decreased in expression in stringent response deficient mutants that caused pronounced virulence defects against human cells in vitro, as well as attenuated virulence in a mouse cutaneous abscess model. This also enabled targeting by synthetic cationic anti-biofilm peptides that selectively inhibited the stringent response of P. aeruginosa in vivo.
Materials and Methods
Bacterial Strains and Growth Conditions
Bacterial strains used in this study are listed in Table 1. Plasmids are listed in Supplementary Table S1 in the Supplementary Material. All organisms were cultured at 37°C in LB (Thermo Scientific), double Yeast Tryptone (dYT), King’s B (KB), or modified synthetic cystic fibrosis medium (MSCFM) (Palmer et al., 2007; Yeung et al., 2012). In order to enhance the expression of P. aeruginosa virulence factors (Bains et al., 2012; Gellatly and Hancock, 2013), iron and phosphate were reduced in MSCFM to 0.9 μM and 1 mM, respectively. Liquid cultures were shaken at 250 rpm, 37°C. Cultures harboring individual vectors were supplemented with 15 μg/ml gentamicin (Gm), 100 μg/ml ampicillin (Ap) for Escherichia coli, 50 μg/ml Gm for PAO1, and 500 μg/ml Gm for LESB58. Bacterial growth was monitored using a spectrophotometer at an optical density of 600 nm (OD600).
Table 1.
Bacterial strains used in this study.
| Strain | Relevant characteristics or genotypea | Reference or source |
|---|---|---|
| Escherichia coli | ||
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 (rK- mK+) supE44 relA1 lac,[F′ proAB lacIq ZΔM15Tn10 (Tcr)] | Stratagene |
| ST18 | pro thi hsdR+ Tpr Smr; chromosome::RP4-2 Tc::Mu-Km::Tn7/aaapir ΔhemA | Thoma and Schobert, 2009 |
| Sm17λpir | Tpr Smr recA, thi, pro, hsdR-M+RP4: 2-Tc:Mu: Km, aaapir phage lysogen | Simon et al., 1983 |
| Pseudomonas aeruginosa | ||
| PAO1 | Laboratory wild-type strain | Hancock and Carey, 1979 |
| PAO1.ΔrelA | relA deletion mutant | This study |
| PAO1.ΔrelA/ΔspoT | ΔrelA/ΔspoT double deletion mutant | This study |
| PAO1.ΔrelA/ΔspoT (complement relA) | ΔrelA/ΔspoT deletion mutant chromosomally complemented with the relA gene including its promoter region | This study |
| PAO1.ΔrelA/ΔspoT (complement spoT) | ΔrelA/ΔspoT deletion mutant chromosomally complemented with the spoT gene including the rpoZ-spoT promoter region | This study |
| PAO1.(16S-lux) | Chromosomal insertion of the 16S promoter region fused to the luxABCDEF reporter genes | This study |
| PAO1.(relA-lux) | Chromosomal insertion of the relA promoter region fused to the luxABCDEF reporter genes | This study |
| PAO1.(spoT-lux) | Chromosomal insertion of the rpoZ-spoT promoter region fused to the luxABCDEF reporter genes | This study |
| LESB58 | Liverpool Epidemic Strain isolate | Cheng et al., 1996 |
| LESB58.ΔrelA | relA deletion mutant | This study |
| LESB58.ΔrelA/ΔspoT | ΔrelA/ΔspoT double deletion mutant | This study |
| LESB58.ΔrelA/ΔspoT (complement relA) | ΔrelA/ΔspoT deletion mutant chromosomally complemented with the relA geneincluding its promoter region | This study |
| LESB58.ΔrelA/ΔspoT (complement spoT) | ΔrelA/ΔspoT deletion mutant chromosomally complemented with the spoT gene including its promoter region | This study |
aAntibiotic resistance: Gmr, gentamicin; Tcr, tetracycline; Tpr, trimethoprim; Smr, streptomycin; Km, kanamycin.
PCR Amplifications and DNA Modifications
PCR primers are listed in Supplementary Table S2 and PCR was carried out using the DreamTaq DNA polymerase (Thermo Scientific) in accordance with the manufacturer’s instructions and optimized annealing temperatures for each primer set. For PCR reactions performed with PAO1 or LESB58, bacterial cells were boiled at 98°C (1,000 rpm, 10 min) and subsequently pelleted at 13,000 rpm for 2 min. PCR reactions were supplemented with 5% dimethyl sulfoxide. For high fidelity PCR reactions, the Phusion DNA polymerase (Thermo Scientific) was used.
Restriction digestions were performed using Thermo Scientific FastDigest restriction enzymes according to the manufacturer’s instructions. All ligation reactions were carried out at room temperature using Thermo Scientific T4 DNA ligase. DNA purifications were either performed using the GeneJET PCR purification kit (Thermo Scientific) or the GeneJET Gel extraction kit (Thermo Scientific) following the manufacturer’s instructions.
Peptide Synthesis and In Vivo Application
Peptides 1018 (VRLIVAVRIWRR-NH2) and DJK-5 (VQWRAIRVRVIR-NH2) were synthesized by CPC Scientific using solid-phase 9-flurenylmethoxy carbonyl (Fmoc) chemistry and purified to >95% purity using reverse-phase high-performance liquid chromatography (HPLC). The lyophilized peptide was initially resuspended in endotoxin-free water and further resuspended in saline for in vivo application.
Construction of Unmarked PAO1/LESB58 ΔrelA and ΔrelA/ΔspoT Deletion Mutants
The construction of the knockout vectors was based on the protocol of Zumaquero et al. (2010) and carried out as previously described (Pletzer et al., 2014). Briefly, primers flanking the relA gene, relA-up-F1/relA-up-R1 and relA-down-F2/relA-down_R2 for PAO1, relA-up-F1/relA-upR1LES and relA-down-F2LES/relA-down-R2 for LESB58 were used to amplify the knockout alleles (approximately 500 bp). To amplify the spoT gene knockout alleles, spoT-up-F1/spoT-up-R1 and spoT-down-F2/spoT-down-R2 were used for both strains PAO1 and LESB58. R1 and F2 primers contained homologous overhang sequences. The obtained ‘up’ and ‘down’ fragments were used in an overlapping PCR reaction with primers up-F1/down-R2. Next, each fusion fragment was then sub-cloned using the Zero Blunt TOPO kit (Invitrogen Life Technologies) and verified by sequencing, before further transfer into the suicide vector pEX18Gm (Hoang et al., 1998) via BamHI/PstI.
The generation of the unmarked stringent response deletion mutants in PAO1 and LESB58 was done step-wise, first deleting the relA gene (2.2 kb) and subsequently the spoT gene (2.1 kb), which resulted in a ΔrelA/ΔspoT double mutant. The deletion method was based on the site-specific insertional mutagenesis strategy of Schweizer and Hoang (1995) and carried out as described previously (Pletzer et al., 2014). Briefly, relA deletion was accomplished by transferring the appropriate suicide vector into E. coli Sm17λpir and subsequently conjugating the construct into PAO1 and LESB58, respectively, with the helper plasmid pRK2013. Deletion of spoT was done by transferring the appropriate suicide vector into E. coli ST18 and biparental conjugation into PAO1 and LESB58. Transconjugants were selected on LB/dYT/KB agar plates containing 10% sucrose and gene deletion confirmed by locus-specific primers that bind outside the knockout alleles (relA_out1/relA_out2 and spoT_out1/spoT_out2). The obtained knockout fragments were verified by sequencing.
Single Gene Chromosomal Complementation of PAO1/LESB58 ΔrelA/ΔspoT
Promoter prediction was based on the information available from http://www.pseudomonas.com (Winsor et al., 2016) and the prokaryote promoter prediction tool PEPPER (de Jong et al., 2012). Therefore, primers relA-Pro_fwd/relA_rev were used to amplify relA (2.2 kb) including its 84-bp upstream promoter region. The amplified product was gel-purified, cloned into pUC-mini-Tn7-Gm via ApaI/SpeI, and verified by sequencing. The construct was co-electroporated with the helper plasmid pTNS3 into sucrose-prepared electrocompetent P. aeruginosa PAO1 and LESB58 ΔrelA/ΔspoT cells, respectively. Briefly, Pseudomonas strains were made electrocompetent with 300 mM sucrose in three-washing steps. Next, 500 ng of each plasmid was mixed with competent cells, co-electroporated at 2.5 kV, and recovered for 3 h before plating on the appropriate selection plates. Successful integration onto the chromosome was verified with primers Tn7L/glmS_down and Tn7R/glmS_up, respectively, and subsequent sequencing of the amplified regions. With this strategy, we were able obtain a viable double mutant complemented with relA, reflecting a spoT knockout strain.
For the complementation of spoT, the rpoZ-spoT operon (2.5 kb) including its upstream promoter region using primers rpoZ-Pro_fwd/spoT_rev was amplified. The amplified product was gel purified, digested with SacI/KpnI, and subsequently cloned into the pUC-mini-Tn7 transposon vector. The cloned fragments were verified by sequencing and further transformed in P. aeruginosa as described above.
Drug Susceptibility
The MICs of drugs for P. aeruginosa PAO1 and LESB58 were determined by the broth microdilution assay in 96-well plates (Wiegand et al., 2008) using Mueller-Hinton broth (MHB; DifcoTM). All tests were performed in at least triplicate following the Clinical and Laboratory Standards Institute recommendations. Bacterial growth (37°C) was examined by visual inspection after 16 h (PAO1) to 48 h (LESB58) of incubation. The MIC was defined as the lowest concentration of a compound that completely prevented visible cell growth.
Generation of the PAO1 Promoter-Bioluminescent Reporter Fusions
Transcriptional fusions between the promoter regions of 16S, relA, and spoT, respectively, and the promoterless luxCDABE genes were created on plasmid pUC18T-min-Tn7T-lux (Damron et al., 2013). Briefly, the relevant upstream regions were PCR amplified either with 16S-Pro_fwd/16S-Pro_rev, relA-Pro_fwd/relA-Pro_rev, or rpoZ-Pro_fwd/rpoZ-Pro_rev, which had BamHI/PstI restriction sites incorporated into the primer sequence, and then further subcloned into pTOPO-pCR-BluntII. Amplified regions were subsequently verified by sequencing. Next, the P1 integron promoter was excised from pUCP18T-miniTN7-lux-Gm using BamHI/PstI restriction enzymes and replaced with the amplified promoter regions. Obtained plasmids were transferred onto the PAO1 chromosome as described above.
Cell Culture and Cytotoxicity Assay
The human bronchial epithelial cell line 16HBE14o- (HBE; a gift from D. Gruenert, University of California, San Francisco) was maintained in minimal essential medium (MEM, Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific), L-glutamine (2 mM, Thermo Fisher Scientific) and penicillin/streptomycin (100 U/ml, Thermo Fisher Scientific) at 37°C in 5% CO2.
After overnight growth in MSCFM medium (20 h PAO1; 22 h LESB58), bacterial cells were pelleted at 5000 × g (15 min) and bacterial supernatants (75 μl PAO1, 150 μl LESB58) were used to treat confluent monolayers of HBE cells (8 × 104 cells/well) for 1 h at 37°C (200 μl total reaction volume with MSCFM). HBE cells treated with Triton X-100 (2% v/v) served as a positive control and MSCFM-treated HBE cells as a negative control. Lactate dehydrogenase (LDH) release into the cell culture supernatant was determined by using a cytotoxicity detection kit (Roche) according to the manufacturer’s instructions.
Hemolysis Assay
Human red blood cells (RBCs) from healthy volunteer donors were used to measure the release of hemoglobin after incubation with P. aeruginosa supernatants. Fresh blood was collected in sodium heparin blood collection tubes (BD Vacutainer®, VWR) and RBCs subsequently isolated by centrifugation (500 × g, 10 min). RBCs were washed three times (500 × g, 10 min) using phosphate buffered saline (PBS, Thermo Fisher Scientific) and subsequently resuspended and stored in an equal volume of Alsever’s solution (Sigma–Aldrich) at 4°C for a maximum of 4 weeks.
After overnight growth of P. aeruginosa (20 h PAO1; 22 h LESB58), bacterial cells were pelleted at 5000 × g (15 min), and the supernatants (50 μl PAO1, 100 μl LESB58) further incubated with 1% RBC (washed three times [500 × g, 10 min] with MSCFM prior to usage) in a 200 μl reaction volume with MSCFM. Triton X-100 (2% v/v, Sigma–Aldrich)-treated RBC served as a positive control and MSCFM-treated RBC as a negative control. After incubation for 1 h at 37°C, RBCs were pelleted at 500 × g (10 min), and the hemoglobin content of the supernatants measured at 450 nm (reference 630 nm) using a microplate reader. Hemolysis percentage was calculated by (ΔODsample – ΔODnegative control)/(ΔODpositive control – ΔODnegative control) × 100.
RNA Isolation and Quantitative Real-time (qRT)-PCR
To further investigate their hemolytic activity, PAO1, LESB58, and their corresponding ΔrelA/ΔspoT double mutants were grown on KB plates supplemented with 10% blood. For PAO1, 2-day grown colonies, for LESB58, 3-day grown colonies were scraped from the blood-agar plates, resuspended in RNAprotect Bacteria Reagent (QIAGEN) and further harvested by centrifugation (13,000 rpm, 2 min). Total RNA was isolated using the RNeasy Mini Kit (QIAGEN) following the manufacturer’s instructions. The obtained RNA was DNAse-treated (Ambion/Life Technologies) and subsequently quantified using a Nanodrop ND-2000 spectrophotometer (Thermo Fischer Scientific) and RNA integrity determined by agarose gel electrophoresis.
High quality RNA was reverse transcribed and amplified with a Roche LightCycler 96 instrument, in combination with the qScriptTM One-Step SYBR® Green qRT-PCR Kit (QuantaBio) according to the manufacturer’s protocol. Template RNA (5 ng/sample) was used in a standard 25 μl qRT-PCR reaction with specific primers (Supplementary Table S2). Each sample was analyzed for gene expression in at least triplicate. Quantification of mRNA transcripts was performed by the comparative Ct method (Schmittgen and Livak, 2008) using rpoD as normalizer.
Ethics Statement
Animal experiments were performed in accordance with The Canadian Council on Animal Care (CCAC) guidelines and were approved by the University of British Columbia Animal Care Committee (certificate number A14-0363). Mice used in this study were female outbred CD-1. All animals were purchased from Charles River Laboratories (Wilmington, MA, United States), were 7 weeks of age, and weighed about 25 ± 3 g at the time of the experiments. 1–3% isoflurane was used to anesthetize the mice. Mice were euthanized with carbon dioxide.
Donated human blood was collected from healthy, consenting volunteers using protocols approved by UBCs Clinical Research Ethics Board. A written consent was obtained from all blood donors and the samples subsequently anonymized.
Cutaneous Mouse Infection Model
The abscess infection model was performed as described earlier (Pletzer et al., 2017). Initially, experiments were performed using an inocula of 5 × 107 organisms for PAO1 and LESB58. However, this inoculum caused 20–30% mortality when PAO1 was used. Since we continued to work with this strain, we performed pathogen-dose experiments and found that we could reduce the inoculum to 1 × 107 organisms, which was still responsible for 15% mortality, but significantly reduced fluctuations in abscess sizes. For the experiment, the fur on the backs of the mice was removed by shaving and application of chemical depilatories. P. aeruginosa PAO1 and LESB58, respectively, were grown to an OD600 of 1.0 in dYT broth. Prior to injection, bacterial cells were washed twice with sterile PBS and adjusted to 1–5 × 107 CFU/ml for PAO1 and 5 × 107 CFU/ml for LESB58. A 50 μl bacterial suspension was injected into the right side of the dorsum. All utilized peptides were tested for skin toxicity prior to efficacy testing. Concentrations used were 10 mg/kg for 1018 and 3 mg/kg for DJK-5. Peptides or saline (50 μl) were directly injected subcutaneously into the infected area [intra-abscess (IA) injection] at 1 h post-infection. The progression of the disease/infection was monitored daily and abscess lesion sizes (visible dermonecrosis) on day 3 measured using a caliper. Swelling/inflammation was not considered in the measurements. Skin abscesses were excised (including all accumulated pus), homogenized in sterile PBS using a Mini-Beadbeater-96 (Biospec products) for 5 min, and bacterial counts determined by serial dilution. Experiments were performed at least three times independently with 2–4 animals per group.
Tracking Promoter Activity with Bioluminescence In Vivo
The PAO1 16S gene promoter, fused to a bioluminescence gene, was used to follow disease progress in real-time. Additionally, the 16S promoter fusion served as control for peptide induction studies since it showed constant expression independent of the applied treatment. For induction studies, the relA as well as rpoZ-spoT promoter fused to bioluminescence genes were used. Peptides were injected into the abscess 1 h post-infection as described above and promoter activity measured 1, 24, 48, and 72 h post-treatment. Bioluminescence images were acquired (60 s exposure, medium binning) using the IVIS Lumina system (Perkin Elmer) and analyzed using Living Image software.
Tracking of Reactive Oxygen and Nitrogen Species (ROS/RNS) and Neutrophils In Vivo
Mice were infected with P. aeruginosa LESB58 and subsequently treated with saline (control) or peptide 1018 as described above. To detect the production of ROS/RNS the chemiluminescence probe L-012 (Kielland et al., 2009) was used. To measure and visualize neutrophil chemotaxis and activation, the neutrophil-specific fluorescent NIR dye (Kerafast) was used. Both were applied as described earlier (Pletzer et al., 2017). Representative images, taken at 3, 24, 48, or 72 h, were acquired using the IVIS Lumina system (luminescence: 60 s exposure, medium binning; fluorescence: excitation 745 nm, emission 800 nm) and analyzed using Living Image software. Fluorescent images were subjected to adaptive background subtraction and the fluorescence emission normalized to the incident excitation intensity (radiance of the subject/illumination intensity).
Statistical Analysis
Statistical evaluations were performed using GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, United States). p-values were calculated using one-way ANOVA, Kruskal–Wallis multiple-comparison test followed by the Dunn procedure. Data was considered significant when p-values were below 0.05 or 0.01 as indicated.
Results
Construction and Complementation of the P. aeruginosa PAO1 and LESB58 ΔrelA/ΔspoT Mutants
To enable the study of the stringent response, we created single and double mutants of relA and spoT in the two P. aeruginosa strains PAO1 and LESB58. Several attempts failed to knock out spoT, the ppGpp synthase/hydrolase. Moreover, preliminary experiments to overexpress relA in a PAO1 wild-type showed that a strong induction of the gene is lethal to the strain, indicating that an imbalance of relA expression affect growth. Hence, we concluded that a spoT-deficient strain was not viable in either P. aeruginosa strain, most likely due to the accumulation of ppGpp inside the cell due to the activity of the ppGpp synthase, RelA. Similar observations have been reported in P. aeruginosa (Vogt et al., 2011) and E. coli (Xiao et al., 1991). However, since other non-specific hydrolases have recently been discovered that are capable of degrading ppGpp, such as the nudix pyrophosphatase in Thermus thermophilus (Ooga et al., 2009), we hypothesized that Pseudomonas might be able to survive in the presence of low concentrations of ppGpp. To test this concept, we created a ΔrelA/ΔspoT double knockout strain by successive deletion of the relA gene followed by spoT to obtain ppGpp-deficient mutants in both PAO1 and LESB58.
To complement the ΔrelA/ΔspoT double mutant, we used single chromosomal insertions of either the relA and spoT gene with their corresponding upstream promoter sequences. The prediction tool PEPPER (de Jong et al., 2012) and the information provided at http://www.pseudomonas.com (JBrowse) (Winsor et al., 2016) were used to identify the corresponding promoter regions. Transcription start site prediction indicated that the relA gene from both P. aeruginosa PAO1 and LESB58 was transcribed from two distinct promoters, as previously described for E. coli (Nakagawa et al., 2006). In E. coli, the promoter directly upstream of the relA gene was found to be constitutively active, while the second promoter, found further upstream, was induced during the transition into stationary phase (Nakagawa et al., 2006). To test our hypothesis that P. aeruginosa might survive in the presence of low levels of ppGpp, we used the directly adjacent promoter region upstream of the relA gene to complement the ΔrelA/ΔspoT mutant and found that cells with this construct were indeed viable and thus partly resembled a spoT knockout in both P. aeruginosa PAO1 and LESB58. To complement the ΔrelA/ΔspoT double mutant with the spoT gene, the rpoZ-spoT operon including its upstream promoter region was chromosomally inserted, and the corresponding mutant partially resembled a relA knockout phenotype (Table 1).
P. aeruginosa Stringent Response Mutants Exhibited Attenuated Virulence against Human Bronchial Epithelial Cells
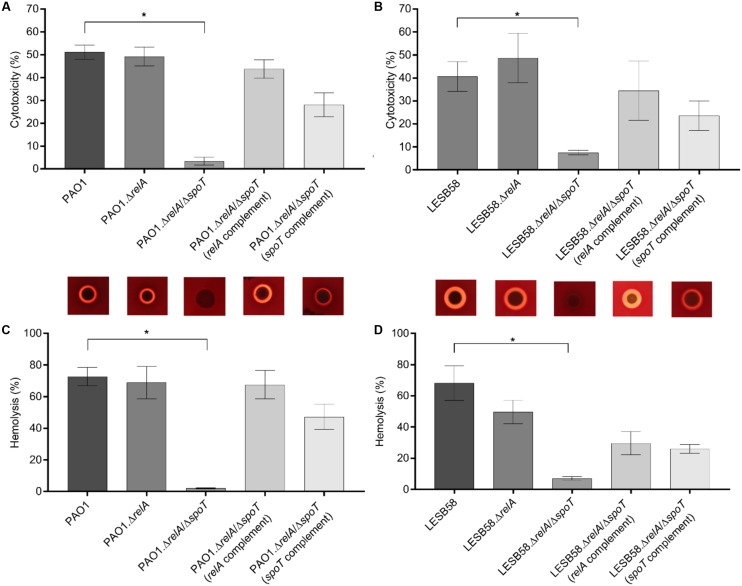
Since the stringent response has been implicated in the regulation of bacterial virulence (Dalebroux et al., 2010; Vogt et al., 2011), we tested whether the P. aeruginosa PAO1 and LESB58 stringent response mutants secreted less cell-damaging virulence factors when compared to the parent strains. Therefore, human bronchial epithelial (HBE) cells were incubated with overnight-grown bacterial supernatants and the release of the cytosolic enzyme LDH from damaged HBE cells was quantified. Our data showed that LESB58 was about 10% less cytotoxic when compared to PAO1 in this experiment (Figures 1A,B).
FIGURE 1.
Cytotoxicity and hemolysis of Pseudomonas aeruginosa PAO1/LESB58, stringent response mutants, and complemented strains. The release of lactate dehydrogenase, from human bronchial epithelial cells incubated for 1 h with supernatants of the indicated strains, into culture supernatants was measured. Hemolytic activity of the strains was determined by either incubating red blood cells with supernatants collected from overnight cultures, or by plating the bacteria onto blood agar plates. (A) Cytotoxicity of PAO1 strains. (B) Cytotoxicity of LESB58 strains. (C) Hemolysis of PAO1 strains on blood agar plates (top) and assessment of hemoglobin release into culture supernatants (bottom). (D) Hemolysis of LESB58 strains on blood agar plates (top) and assessment of hemoglobin release into culture supernatants (bottom). (A–D) Statistical analysis was performed using one-way ANOVA, Kruskal–Wallis test with Dunn’s correction. The asterisk indicates a significant difference to the wild-type (p ≤ 0.01). Error bars, mean ± SEM.
As anticipated, cytotoxicity was reduced in both P. aeruginosa ΔrelA/ΔspoT double mutant strains (PAO1 and LESB58) when compared to their wild-type parents. The PAO1 ΔrelA/ΔspoT mutant showed about 48% less cytotoxicity while the LESB58 ΔrelA/ΔspoT mutant showed a reduction of 33%. Single gene complementation of the double mutant with either relA or spoT increased cytotoxicity levels. Deletion of only relA had no significant impact on cytotoxicity in either in the PAO1 or LESB58 backgrounds (Figures 1A,B).
PAO1/LESB58 Stringent Response Mutants Showed Reduced Hemolytic Activity
Due to the observed decreased cytotoxicity of the ΔrelA/ΔspoT strains, we further evaluated the release of hemoglobin from RBCs when incubated with overnight-grown bacterial supernatants. P. aeruginosa PAO1 and LESB58 showed similar hemolytic activity of approximately 70%. Interestingly, the PAO1 ΔrelA/ΔspoT double mutant showed almost complete reduction in hemolysis (Figure 1C), while a ΔrelA mutant as well as the relA and spoT complemented double mutant strains showed similar hemolysis levels as the wild-type. In LESB58, a reduction in hemolysis was observed for all tested mutants and complemented strains, with a significant reduction (p < 0.01) in the ΔrelA/ΔspoT double mutant (approximately 60%) compared to the wild-type (Figure 1D).
To further elucidate the mechanism behind the decreased hemolytic activity of the stringent response double mutant, we isolated bacterial RNA from cells grown on blood agar plates. As with the previous supernatant assay, PAO1 ΔrelA/ΔspoT showed no hemolytic activity, while LESB58 ΔrelA/ΔspoT was still able to lyse some RBCs (Figures 1C,D, top panel). We compared the ΔrelA/ΔspoT mutant strains to their corresponding wild-type parents and examined gene expression for various virulence-associated factors by qRT-PCR. Interestingly, more virulence-associated genes were dysregulated in the PAO1 stringent response mutant than in LESB58 mutant (Table 2). In particular the aprA gene, encoding an alkaline protease with hemolytic activity (Hong and Ghebrehiwet, 1992), was about 11.2-fold down-regulated in PAO1 ΔrelA/ΔspoT and 4.8-fold down-regulated in LESB58 ΔrelA/ΔspoT, which might explain the loss of cytolytic activity in the stringent response double mutants. Differential hemolytic activity in the two genetic backgrounds might have resulted from the different fold decreases in expression of AprA or from other unique differences. Thus for strain PAO1 there was a >3-fold reduction in expression for genes encoding the secreted hemolysin, phospholipase C (plcB), a phenazine biosynthesis gene (phzB1), rhamnosyltransferase chain B (rhlB), and the galactose binding lectin A gene (pa1L), as well as a >2-fold increased expression of genes encoding the Type III secretion system (exsA, pscF, esxD, and pscI) and a siderophore transporter (pvdE). Conversely, LESB58 ΔrelA/ΔspoT showed reduced expression, by more than twofold, of the genes encoding pyoverdine expression, pvdE, and the type II secretion substrates lipase A (lipA) and exotoxin A (toxA).
Table 2.
Relative fold-changes of P. aeruginosa PAO1 ΔrelA/ΔspoT and LESB58 ΔrelA/ΔspoT mRNA expression compared to their respective wild-type levels of expression.
| Gene | Locus (PAO1/LESB58) | Virulence factor/description | Fold change in ΔrelA/ΔspoT |
|
|---|---|---|---|---|
| PAO1 | LESB58 | |||
| aprA | PA1249/PALES_40631 | Alkaline protease, Type I secretion | -11.2 | -4.8 |
| lasB | PA3724/PALES_12581 | Elastase type II secretion system substrate | -10.6 | 1.8 |
| pa1L | PA2570/PALES_27241 | Galactose binding lectin A | -10.2 | -1.7 |
| phzB1 | PA4211/PALES_07161 | Phenanzine biosynthesis type II secretion | -4.3 | 1.2 |
| rhlB | PA3478/PALES_15341 | Rhamnolipid rhamnosyltransferase chain B | -3.3 | -1.5 |
| plcB | PA0026/PALES_00251 | Phospholipase C, Type II secretion | -3.0 | -1.1 |
| lipA | PA3996/PALES_22021 | Lipase A type II secretion system substrate | -1.4 | -2.7 |
| exoS | PA3841/PALES_11331 | Exoenzyme S type III secretion substrate | -1.4 | n.d. |
| pscC | PA1716/PALES_36131 | Type III secretion outer membrane protein | 1.3 | 1.2 |
| pchB | PA4230/PALES_06971 | Pyochelin siderophore biosynthesis | 1.4 | -1.5 |
| toxA | PA1148/PALES_41711 | Exotoxin A type II secretion system substrate | 1.8 | -2.4 |
| pscI | PA1722/PALES_36071 | Type III secretion export protein | 2.1 | n.d. |
| pvdE | PA2397/PALES_28991 | Pyoverdine siderophore ABC transporter | 2.1 | -2.0 |
| exsD | PA1714/PALES_36151 | Type III secretion system negative regulator | 3.8 | 1.6 |
| pscF | PA1719/PALES_36101 | Type III secretion needle | 4.3 | 1.3 |
| exsA | PA1713/PALES_36161 | Type III secretion system transcriptional activator | 4.6 | 1.2 |
Strains were grown on KB agar plates supplemented with 10% blood for 2 (PAO1) and 3 (LESB58) days, respectively. Transcript abundance was determined by qRT-PCR and represent the means of at least three biological replicates. n.d., not determined.
The Stringent Response Was Required for Cutaneous Abscess Tissue Necrosis in Mice
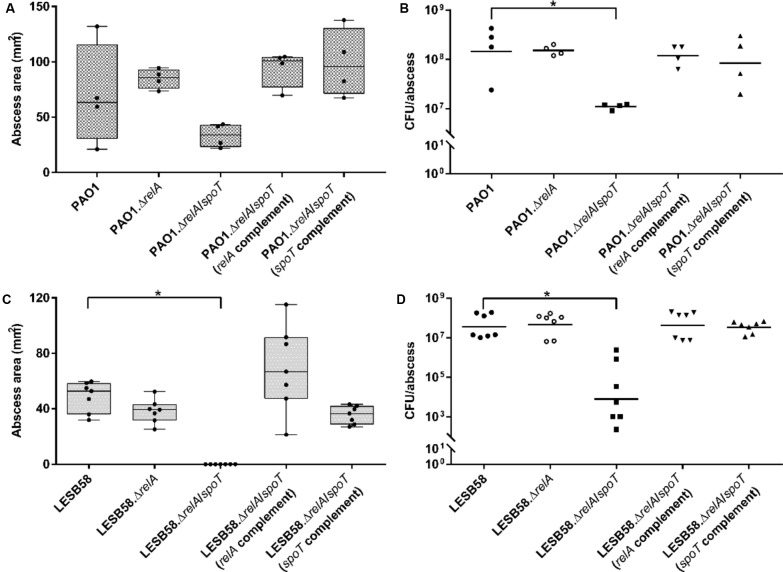
Bacteria found in wounds or chronic skin infections are thought to experience high levels of stress due to the rapid release of reactive oxygen species (i.e., oxidative burst) by host-related defense mechanisms such as macrophages and neutrophils (Slauch, 2011; Dhall et al., 2014). Additional limitations within a wound (e.g., nutrient or iron deprivation due to the high density of organisms) may also contribute to stress-related responses. We hypothesized that these factors are under the control of the bacterial stringent stress response. Therefore, we tested in a murine cutaneous infection model of high-density infections, the effects of inoculation of 5 × 107 CFU of P. aeruginosa strains PAO1 and LESB58 wild-type, ΔrelA, and ΔrelA/ΔspoT mutants with their corresponding complemented strains. These studies investigated their ability to cause abscesses and/or tissue necrosis, the bacterial burden 3 days post-infection, and the survival rate of the infected mice.
The PAO1 ΔrelA/ΔspoT showed visible, but not statistically significant, decreases in abscess sizes of 44% when compared to the wild-type (Figure 2A). However, the relA and spoT complemented ΔrelA/ΔspoT double mutant strains formed significantly greater abscesses by >2-fold compared to the double mutant. PAO1 ΔrelA/ΔspoT further showed a significant 11.6-fold lower recovery of viable cells (CFU) 3 days post-infection, while the relA-deficient strain as well as the complemented double mutants showed similar bacterial counts, comparable to the wild-type (Figure 2B).
FIGURE 2.
Pseudomonas aeruginosa stringent response strains in a cutaneous mouse infection model. CD-1 mice were subcutaneously infected with either PAO1 or LESB58, their corresponding stringent response mutants ΔrelA and ΔrelA/ΔspoT, as well as the ΔrelA/ΔspoT complemented strains. A high bacterial density (5 × 107 CFU) was used. Lesion sizes (Box and whiskers plot) and CFU counts (with geometric mean) were determined 3 days post-infection. (A) PAO1 dermonecrosis measurements. (B) PAO1 CFU counts/abscess. (C) LESB58 dermonecrosis measurements. (D) LESB58 CFU counts/abscess. (A–D) All experiments were done at least three times independently with 2–4 mice/group. Statistical analysis was performed using one-way ANOVA, Kruskal–Wallis test with Dunn’s correction. The asterisk indicates a significant difference to the wild-type (p < 0.05).
In the LESB58 background strain, the ΔrelA/ΔspoT double mutant was unable to cause dermonecrosis while genetic complementation with either relA or spoT restored the ability to cause tissue damage (Figure 2C). Deletion of only the relA synthase showed a similar phenotype as the wild-type. Interestingly, although the double mutant showed no visible tissue dermonecrosis, bacterial cells were still recovered from the injection site 3 days post-infection. However, the LESB58 ΔrelA/ΔspoT double mutant showed 104-fold less CFU when compared to the wild-type (Figure 2D). As previously demonstrated (Pletzer et al., 2017), LESB58 showed no lethality in this model.
Since environmental factors could play a role in the stimulation of ppGpp production, we investigated the expression of relA and spoT using a bioluminescent reporter fusion to track the infection progress in vivo. Previously, we demonstrated that in vivo tracking of bacterial infection using bioluminescence was achievable with LESB58 in this cutaneous abscess model (Pletzer et al., 2017). Here, we created chromosomal promoter–reporter fusions in PAO1 and tracked the infection for 3 days. As shown in Figure 3, the 16S promoter–reporter fusion served as an infection control and was trackable in saline-treated mice with constant expression over 3 days (Figure 3A, left panel). Stringent response gene promoter fusions of relA and spoT to the bioluminescence reporter when administered to saline-treated mice, resulted in luminescence that was detectable throughout the experiment (Figures 3B,C, left panel). Notably, the expression intensity of the 16S construct was about 100-fold higher when compared to relA or spoT expression and although the signal intensity decreased over time it did not influence the experiment.
FIGURE 3.
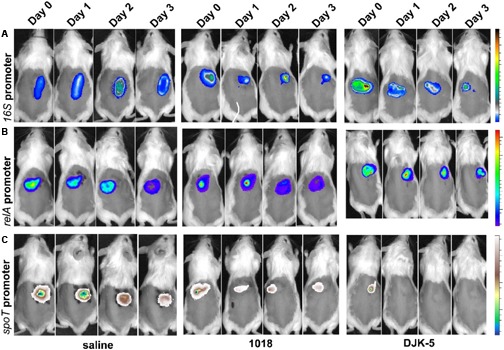
In vivo tracking of P. aeruginosa PAO1 16S, relA, and spoT promoter activity. The infection progress was monitored at the indicated time points. Female CD-1 mice were injected with a high bacterial number (5 × 107 CFU PAO1) and treated with either saline (control, left panel) or peptides 1018 (middle panel) and DJK-5 (right panel) 1 h post-infection. (A) 16S promoter activity as induction/suppression control for peptide treatment. Radiance color scale from 0.5 to 2.5 × 109. (B) relA promoter activity. Radiance color scale from 0.5 to 4.5 × 107. (C) spoT promoter activity. Radiance color scale from 0.5 to 4.5 × 107. (A–C) All experiments were done at least twice with three mice/group.
Targeting the Stringent Response with Peptides 1018 and DJK-5 In Vivo
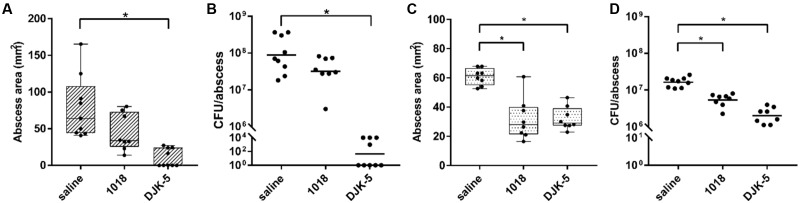
Interestingly, bacterial abscess infections can be treated with synthetic anti-biofilm peptides (Mansour et al., 2016), although biofilm formation and abscess infections are not necessarily related to each other. However, it was previously shown that these peptides target the stringent response in vitro (de la Fuente-Núñez et al., 2014) and that this led to control of the formation of dermonecrotic lesions in vivo for Gram-positive S. aureus cutaneous infections (Mansour et al., 2016). Although there was a therapeutic effect of DJK-5 against P. aeruginosa infections [15], it was not confirmed to be linked with the stringent response. Here, given the impact of the stringent response on tissue dermonecrosis formation and survival within the abscess (Figure 2), we further investigated the treatment of P. aeruginosa abscess infections with anti-biofilm peptides 1018 and DJK-5, which both target the stringent response in vitro (de la Fuente-Núñez et al., 2014). Their respective MIC value against PAO1 in MHB was 25 μg/ml for 1018 and 12.5 μg/ml for DJK-5 and for LESB58 64 μg/ml for 1018 and DJK-5. We separately administered peptides 1018 (10 mg/kg) and DJK-5 (3 mg/kg) directly into the abscess 1 h after the infection was initiated, and found that the peptides significantly reduced tissue dermonecrosis by ∼50% for PAO1 (DJK-5 only; although the appearance of 1018-treated lesions indicate less severe lesions) and LESB58 (Figures 4A,C). The number of recovered bacteria varied between the strains. While single dose treatment with 1018 reduced the bacterial load by approximately 2.8-fold in PAO1 (non-significantly) and 3.2-fold in LESB58 (p < 0.05), DJK-5 almost completely eradicated bacteria (>106-fold reduction) in the case of strain PAO1 and significantly reduced bacteria numbers by about 10-fold for strain LESB58 (Figures 4B,D) at 3 days post-infection. Interestingly, 1018, a profound immune modulator (Mansour et al., 2015), did not significantly affect ROS/RNS levels or the influx of neutrophils (Supplementary Figure S1) that were evident during P. aeruginosa abscess formation (Pletzer et al., 2017).
FIGURE 4.
Therapeutic treatment with synthetic peptides of PAO1 and LESB58 infected mice. CD-1 mice were subcutaneously infected with 5 × 107 CFU P. aeruginosa PAO1 or LESB58 and treated intra-abscess with either saline (control) or synthetic peptides 1018 (10 mg/kg) and DJK-5 (3 mg/kg) 1 h post-infection. Lesion sizes and CFU counts were determined 3 days post-infection. (A) Box and whiskers plot of dermonecrosis measurements for PAO1 infections. (B) CFU counts/abscess with geometric mean for PAO1 infections. (C) Box and whiskers plot of dermonecrosis measurements for LESB58 infections. (D) CFU counts/abscess with geometric mean for LESB58 infections. (A–D) All experiments were done at least three times with 2–4 mice/group. Statistical analysis was performed using one-way ANOVA, Kruskal–Wallis test with Dunn’s correction. The asterisk indicates significant differences to the wild-type (p < 0.05).
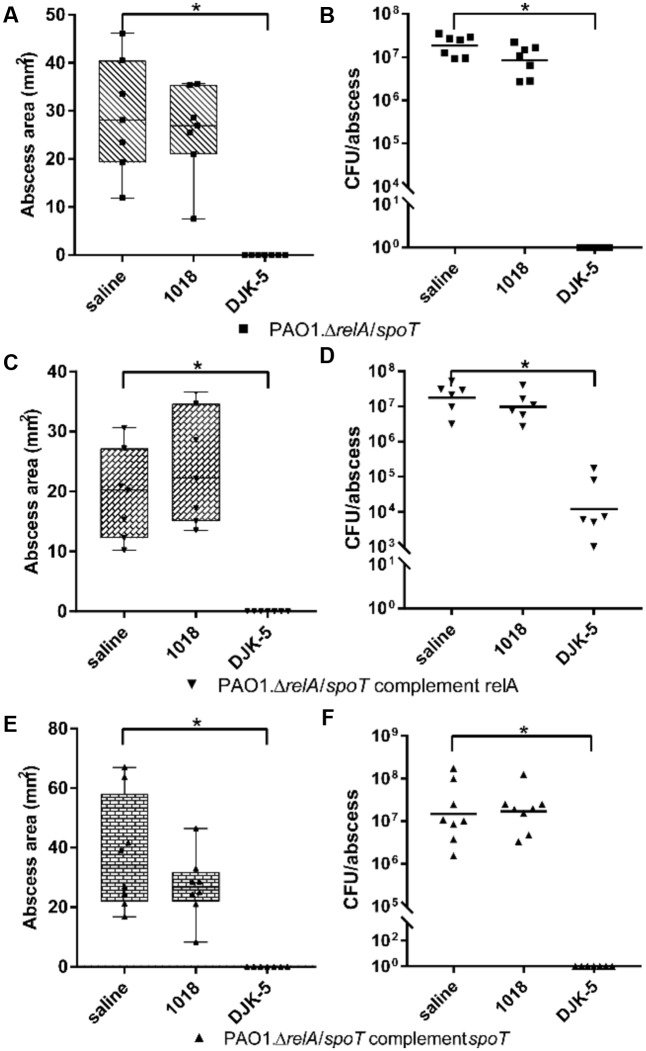
To further elucidate the effect of the peptides on the stringent response in vivo, we used the PAO1 ΔrelA/ΔspoT double mutant, and ΔrelA/ΔspoT complemented with relA or spoT (Figure 5). Interestingly, peptide 1018 was ineffective vs. mice treated with the ΔrelA/ΔspoT double mutant in that there was no visible reduction in abscess lesion sizes and an insignificant reduction of recovered bacteria (Figures 5A,B). However, consistent with its greater potency, treatment with DJK-5 completely eradicated the infections. The ΔrelA/ΔspoT strain complemented with relA, resembling a spoT knockout, showed a large inconsistency in terms of tissue necrosis formation and the amount of recovered CFU (Figures 5C,D). Nevertheless, when we treated the infection with peptides, we found that 1018-treatment showed no significant effect. Intriguingly, DJK-5-treated infections still completely suppressed abscess formation (Figure 5C), but did not completely clear the bacteria from the injection site (Figure 5D), with about 1000-fold less bacteria recovered cf. the saline-treated control groups. In contrast complementation with spoT showed no significant differences compared to the ΔrelA/ΔspoT double mutant (Figures 5E,F).
FIGURE 5.
Therapeutic treatment of PAO1 stringent response mutants and complemented strains with synthetic peptides. CD-1 mice were subcutaneously infected with 1 × 107 CFU P. aeruginosa PAO1, ΔrelA/ΔspoT, and corresponding complemented strains. Intra-abscess administration of either saline (control) or synthetic peptides 1018 (10 mg/kg) and DJK-5 (3 mg/kg) was done 1 h post-infection. Lesion sizes (Box and whiskers plots) and CFU counts (with geometric mean) were determined 3 days post-infection. (A) PAO1.ΔrelA/ΔspoT dermonecrosis measurements. (B) PAO1.ΔrelA/ΔspoT CFU counts/abscess. (C) PAO1.ΔrelA/ΔspoT complemented with relA dermonecrosis measurements. (D) PAO1.ΔrelA/ΔspoT complemented with relA CFU counts/abscess. (E) PAO1.ΔrelA/ΔspoT complemented with spoT dermonecrosis measurements. (F) PAO1.ΔrelA/ΔspoT complemented with spoT CFU counts/abscess. (A–F) All experiments were done at least three times with 2–4 mice/group. Statistical analysis was performed using one-way ANOVA, Kruskal–Wallis test with Dunn’s correction. The asterisk indicates a significant difference to the wild-type (p < 0.05). Data points for mice with no visible abscess or no recovered CFU were set to 1 to perform statistical analysis.
To understand the mechanisms involved, we tested the in vivo influence of the synthetic peptides 1018 and DJK-5 in induction or repression of the expression for ppGpp synthesis genes. Based on light production from the reporter lux operon, treatment of the spoT-reporter construct treated with 1018 led to reduced emitted light (decreased in vivo expression) by approximately twofold at 24 h post-infection (Figure 3C). Even more striking was the finding that DJK-5 completely suppressed spoT promoter activity within 24 h (Figure 3C). There was, however, little change in expression of the relA gene (Figure 3B), or, as a control, the 16S rRNA (Figure 3A).
Taking into account all experiments performed (with 15–24 total animals per strain), the PAO1 wild-type strain was moderately virulent in the cutaneous infection model (15% mortality), while the PAO1 ΔrelA/ΔspoT showed about 12% mortality, the relA complementation 7% mortality and spoT complementation 0% mortality. These variations in mortality rates were not significantly different (as determined by Fisher’s exact test). The direct administration of peptides into abscesses of PAO1-infected mice led to 100% survival of all mice.
Discussion
Adaptation to changing environmental conditions such as those encountered during the establishment of an infection inside a host are hallmarks of many bacteria. A rapid response to cope with limiting factors, such as fluctuations in nutrient availability and other stressors such as host defense mechanisms, can be achieved by various mechanisms including the stringent stress response. The results presented here are consistent with and extend literature studies indicating that the stringent response and its second messenger molecule ppGpp are important in the regulation of adaptive responses and bacterial virulence (Potrykus and Cashel, 2008; Dalebroux et al., 2010), as well as persistence and survival during host invasion and antibiotic resistance (Hauryliuk et al., 2015).
Initially, we found that the deletion of only the ppGpp hydrolase SpoT was lethal in P. aeruginosa, in accordance with other studies (Vogt et al., 2011; Manuel et al., 2012). However, we could partly complement a ΔrelA/ΔspoT double mutant with a chromosomal insertion of relA and its constitutive promoter, which created the equivalent of a viable spoT knockout. We propose that this might be due to the weak expression from the native relA promoter that prevented excessive ppGpp production and the possibility that non-specific hydrolases might be able to degrade the more modest levels of ppGpp produced in this background. The enzymes other than SpoT that appear to influence ppGpp levels in bacteria to date, are the phosphohydrolase MazG in E. coli (Gross et al., 2006) and the nudix pyrophosphatase Ndx8 in Thermus thermophilus (Ooga et al., 2009). However, further studies are needed to explore whether such non-specific hydrolases exist in P. aeruginosa.
Pseudomonas aeruginosa relies on a suite of secreted toxins and virulence factors (including phospholipase C, exotoxin A and Type III secreted exotoxins, rhamnolipids, siderophores, the elastase and alkaline protease, and pyocyanin) to colonize its host and establish an infection (van Delden, 2004; van ’t Wout et al., 2015). Toxin production and secretion during host-microbe interactions causes distinct macroscopic pathological changes including anemia, tissue necrosis, and neural damage (Sadikot et al., 2005). One major weapon involved in cytotoxicity and acute infections in P. aeruginosa is the type III secretion system (Hauser, 2009) that secretes effector proteins to help evading the host tissue (Filloux, 2011). In addition our previous studies indicated that Type III secretion was essential for strain PA14 dermonecrosis (Pletzer et al., 2017). We thus hypothesized that this system might be dysregulated in stringent response mutants that showed reduced cytotoxicity against HBE cells and reduced hemolytic activity against human RBCs (Figure 1). After analyzing the expression of various virulence-associated factors in wild-type and the stringent response mutants (Table 2), we found that Type III secretion was upregulated. Conversely type-I-secreted alkaline protease AprA, a zinc metalloprotease, was the only enzyme down-regulated in both stringent response mutants of PAO1 and LESB58, respectively, and might therefore be a major contributor to the lysis of erythrocytes. AprA has been shown to aid P. aeruginosa survival during lung infection (Kim et al., 2006) and has also been connected to the blockage/degradation of complement proteins that otherwise rapidly detect and promote killing of Gram-negative bacteria (Laarman et al., 2012).
Stringent stress response double mutants exhibited attenuated virulence during PAO1 and LESB58 infections, respectively, with no visible dermonecrosis in LESB58. This could be a result of increased bacterial clearance or a reduced ability to adapt to and grow under the stressful conditions in the abscess. Indeed, our data showed that bacterial loads inside the abscess tissue of mice infected with the double mutant were significantly lower than in mice infected with the wild-type or complemented strains (Figure 2). This is partly consistent with results showing a double mutant was essentially avirulent in an acute P. aeruginosa pulmonary infection model (Xu et al., 2016). On the other hand, reduced virulence might also be connected to a down-regulation of the AprA protease in stringent response mutants, which might otherwise have adverse effects during host colonization. Moreover, Vogt et al. (2011) speculated that a ΔrelA/ΔspoT double mutant would be more susceptible to a variety of different stresses during host interaction, which could be responsible for a reduced ability to survive attacks mediated by the host immune system. We have previously shown that reactive oxygen species are activated during infection in the cutaneous abscess model (Pletzer et al., 2017), and these could conceivably account for increased killing of the double mutant in this model.
Apart from the investigation of the role of the stringent response in virulence during a high-bacterial-density cutaneous abscess infection, we also examined the possibility of specifically targeting the stringent response in vivo. In this context, Wexselblatt et al. (2012) identified a stringent response inhibitor, Relacin, that directly interacted with and completely inhibited purified E. coli RelA enzyme. However, Relacin only reduced ppGpp production in Gram-positive Bacillus subtilis cells, and had no effect on E. coli cells, most likely due to its inability to penetrate the Gram-negative bacterial cell wall (Wexselblatt et al., 2012). Very recently, synthetic ppGpp analogs were investigated and demonstrated a down-regulation of ppGpp concentrations in Mycobacterium smegmatis (Syal et al., 2017). Conversely, we showed that the anti-biofilm peptides 1018 and DJK-5 were able to target and stimulate degradation of ppGpp in Gram-negative P. aeruginosa and Gram-positive S. aureus (de la Fuente-Núñez et al., 2014, 2015). Thus, to our knowledge, only the peptides 1018 and DJK-5 have been shown to provide a possible therapeutic approach against Gram-positive and Gram-negative pathogens in vertebrate infection models (Achtman et al., 2012; Mansour et al., 2016). Recently, the observation that 1018 targets the cellular stress response had been challenged (Andresen et al., 2016). However, the methodological procedures described by Andresen et al. (2016) have serious issues including the use of microtiter plate adherence assays and crystal violet staining (which does not discriminate between live cells and bacterial debris) to assess biofilm formation. Crystal violet experiments show large variations and are quite poor for accurately assessing anti-biofilm activity, especially when set up as a MIC experiment where peptides are added to planktonic cells. Furthermore, Andresen et al. (2016) tested the conditional essentiality of ppGpp on planktonic E. coli cells rather than biofilm cells and they tested the effects of 1018 on bacterial survival on a ppGpp-deficient strain under non-stressed conditions. Ultimately, no studies were included to demonstrate the effects of 1018 on intracellular ppGpp as previously demonstrated (de la Fuente-Núñez et al., 2014).
In the present study, we demonstrated that both peptides were effective in the treatment of cutaneous abscesses caused by P. aeruginosa (Figure 4). Using the moderately virulent PAO1 strain as well as the clinical isolate LESB58, we showed that peptide administration decreased skin lesion sizes and reduced bacterial loads inside the abscess – a phenotype resembling an untreated infection by the stringent response mutants. These observations led us to speculate that synthetic peptides can be used to target the P. aeruginosa stringent response in vivo. In this context, a partially restored spoT knockout (i.e., a relA expressing strain in the ΔrelA/ΔspoT double mutant background) reduced susceptibility to peptide administration, which could be consistent with binding of the peptide to ppGpp, thereby reducing the effect of the peptide. However, it is important to point out that working with intact cells has its limitations and we only demonstrated ex vivo but not in vivo binding of the peptides to ppGpp. Moreover, since a P. aeruginosa spoT knockout is apparently not viable, the use of a partial complementation of the ΔrelA/ΔspoT with the relA gene might have had unknown side effects that could also contribute to increased peptide resistance. The observation that treatment of the ΔrelA/ΔspoT mutant with DJK-5 could still reduce abscess lesion sizes and bacterial loads recovered from the abscess tissue (Figure 5), indicated that the peptide DJK-5 might act not only on the stringent response, but might also target other bacterial cellular mechanisms such as interfering with cell wall synthesis, inhibition of DNA and protein synthesis, or interruption of protein folding and/or enzyme activity. Other possible impacts of peptide administration could be their known immunomodulatory activity as observed for example with 1018 in various animal models (Mansour et al., 2015).
Since an inability to maintain intracellular ppGpp homeostasis, as observed in strains overexpressing relA, leads to cell death, it was very interesting that we observed that synthetic peptides could suppress spoT promoter activity in vivo (Figure 3). Due to the decreased expression of the main enzyme that can hydrolyse ppGpp, we hypothesize that ppGpp homeostasis was disturbed, which might explain in part the attenuated virulence associated with peptide-treated infections. However, spoT is co-transcribed with the ω subunit rpoZ of the RNA polymerase, which could indicate that the observed effect is not spoT-specific. The ω subunit aids in the binding of the sigma factor to the polymerase that further influences gene expression (Gunnelius et al., 2014) and also might directly interact with ppGpp (Chatterji et al., 2007). Chatterji et al. (2007) also proposed that rpoZ has a direct role in the expression of relA (Chatterji et al., 2007) and consequently on ppGpp levels inside the cells, although the physiological role of rpoZ during the stringent response is not well-defined.
Our data invite speculation that cells treated with synthetic peptides that target the stringent response in vivo, share common traits with stringent response ΔrelA/ΔspoT mutants during infection. Furthermore, the observation that synthetic peptides suppressed expression of the rpoZ-spoT operon in vivo enabled us to propose the that the synthetic peptides 1018 and DJK-5 not only bind and degrade ppGpp, but also down-regulate the expression of RNA polymerase ω subunit and spoT, which leads to the down-regulation of relA, consequently shutting down ppGpp production. This would be a response loop that would severely weaken the pathogen, and would be consistent with additional treatment options, e.g., conventional antibiotics that are synergistic with these peptides (Reffuveille et al., 2014; de la Fuente-Núñez et al., 2015). It will be important in the future to better understand the underlying mechanisms.
Conclusion
We have demonstrated that the synthetic cationic peptides 1018 and DJK-5 exhibit excellent promise to treat Gram-negative high-bacterial-density P. aeruginosa infections in a cutaneous abscess mouse model. In vivo, both peptides suppressed spoT promoter activity and reduced tissue lesions and the numbers of bacteria recovered from the infected tissues. Both peptides increased animal welfare and appeared to target the stringent stress response in vivo, while DJK-5 seemed to also act on additional targets, other than ppGpp, during the infection. These additional targets for DJK-5 activity in vivo should be addressed in the future. Given the fact that increasing antibiotic resistance is a global threat to human health, synthetic peptides offer a considerable potential as an alternative and/or adjunctive therapy for the treatment of bacterial infections and merit further research. Future studies should aim to utilize stringent response inhibitors in conjunction with antimicrobial therapy to reduce prescribed antibiotics and this will provide a contribution to the fight against growing antibiotic resistance.
Author Contributions
Conceptualization: RH and DP; formal analysis: DP; funding acquisition: RH and DP; investigation: DP, HW, and MB; methodology: DP, HW, and MB; project administration: RH and DP; resources: RH; supervision: RH; validation: RH and DP; writing – original draft: RH and DP; writing – reviewing and editing: RH and DP.
Conflict of Interest Statement
The peptides described here have been filed for patent protection, assigned to RH’s employer the University of British Columbia, and licensed to ABT Innovations, Inc. in which the University of British Columbia and RH have shares. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Negin Rahanjam for the help with mouse experiments and the technologists at the Modified Barrier Facility at UBC for shaving our mice. Furthermore, we thank Leo Toutatsu Liu for his help in preparing bacterial supernatants and the help with the hemolysis assays.
Footnotes
Funding. Research reported in this publication was supported by a grant from the Canadian Institutes for Health Research MOP-74493, the National Institute of Allergy and Infectious Diseases (NIAID) of the U. S. National Institutes of Health under Award Number R33AI098701, and the Intramural Research Program of the NIAID. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. DP received a Feodor Lynen postdoctoral fellowship from the Alexander von Humboldt Foundation and HW received an Early Postdoc Mobility fellowship from the Swiss National Science Foundation. RH holds a Canada Research Chair in Health and Genomics and a UBC Killam Professorship.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2017.01867/full#supplementary-material
References
- Achtman A. H., Pilat S., Law C. W., Lynn D. J., Janot L., Mayer M. L., et al. (2012). Effective adjunctive therapy by an innate defense regulatory peptide in a preclinical model of severe malaria. Sci. Transl. Med. 4:135ra64 10.1126/scitranslmed.3003515 [DOI] [PubMed] [Google Scholar]
- Andresen L., Tenson T., Hauryliuk V. (2016). Cationic bactericidal peptide 1018 does not specifically target the stringent response alarmone (p)ppGpp. Sci. Rep. 6:36549 10.1038/srep36549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains M., Fernandez L., Hancock R. E. W. (2012). Phosphate starvation promotes swarming motility and cytotoxicity of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 78 6762–6768. 10.1128/AEM.01015-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M., Gallant J. (1969). Two compounds implicated in the function of the RC gene of Escherichia coli. Nature 221 838–841. 10.1038/221838a0 [DOI] [PubMed] [Google Scholar]
- Chatterji D., Ogawa Y., Shimada T., Ishihama A. (2007). The role of the omega subunit of RNA polymerase in expression of the relA gene in Escherichia coli. FEMS Microbiol. Lett. 267 51–55. 10.1111/j.1574-6968.2006.00532.x [DOI] [PubMed] [Google Scholar]
- Cheng K., Smyth R. L., Govan J. R., Doherty C., Winstanley C., Denning N., et al. (1996). Spread of β-lactam-resistant Pseudomonas aeruginosa in a cystic fibrosis clinic. Lancet 348 639–642. 10.1016/S0140-6736(96)05169-0 [DOI] [PubMed] [Google Scholar]
- Choi K. H., Mima T., Casart Y., Rholl D., Kumar A., Beacham I. R., et al. (2008). Genetic tools for select-agent-compliant manipulation of Burkholderia pseudomallei. Appl. Environ. Microbiol. 74 1064–1075. 10.1128/Aem.02430-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K. H., Schweizer H. P. (2006). mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat. Protoc. 1 153–161. 10.1038/nprot.2006.24 [DOI] [PubMed] [Google Scholar]
- Chua S. L., Sivakumar K., Rybtke M., Yuan M. J., Andersen J. B., Nielsen T. E., et al. (2015). C-di-GMP regulates Pseudomonas aeruginosa stress response to tellurite during both planktonic and biofilm modes of growth. Sci. Rep. 5:10052 10.1038/srep10052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalebroux Z. D., Svensson S. L., Gaynor E. C., Swanson M. S. (2010). ppGpp conjures bacterial virulence. Microbiol. Mol. Biol. Rev. 74 171–199. 10.1128/Mmbr.00046-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damron F. H., McKenney E. S., Barbier M., Liechti G. W., Schweizer H. P., Goldberg J. B. (2013). Construction of mobilizable mini-Tn7 vectors for bioluminescent detection of Gram-negative bacteria and single-copy promoter lux reporter analysis. Appl. Environ. Microbiol. 79 4149–4153. 10.1128/Aem.00640-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong A., Pietersma H., Cordes M., Kuipers O. P., Kok J. (2012). PePPER: a webserver for prediction of prokaryote promoter elements and regulons. BMC Genomics 13:299 10.1186/1471-2164-13-299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Núñez C., Korolik V., Bains M., Nguyen U., Breidenstein E. B. M., Horsman S., et al. (2012). Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 56 2696–2704. 10.1128/Aac.00064-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Núñez C., Reffuveille F., Haney E. F., Straus S. K., Hancock R. E. W. (2014). Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLOS Pathog. 10:e1004152 10.1371/journal.ppat.1004152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Núñez C., Reffuveille F., Mansour S. C., Reckseidler-Zenteno S. L., Hernandez D., Brackman G., et al. (2015). D-Enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem. Biol. 22 1280–1282. 10.1016/j.chembiol.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. E., Ireland P. M., Jordan J. E., Titball R. W., Oyston P. C. F. (2009). RelA regulates virulence and intracellular survival of Francisella novicida. Microbiology 155 4104–4113. 10.1099/mic.0.031021-0 [DOI] [PubMed] [Google Scholar]
- Dhall S., Do D., Garcia M., Wijesinghe D. S., Brandon A., Kim J., et al. (2014). A novel model of chronic wounds: importance of redox imbalance and biofilm-forming bacteria for establishment of chronicity. PLOS ONE 9:e109848 10.1371/journal.pone.0109848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. (1979). Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. U.S.A. 76 1648–1652. 10.1073/pnas.76.4.1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filloux A. (2011). Protein secretion systems in Pseudomonas aeruginosa: an essay on diversity, evolution, and function. Front. Microbiol. 2:155 10.3389/fmicb.2011.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank K. L., Colomer-Winter C., Grindle S. M., Lemos J. A., Schlievert P. M., Dunny G. M. (2014). Transcriptome analysis of Enterococcus faecalis during mammalian infection shows cells undergo adaptation and exist in a stringent response state. PLOS ONE 9:e115839 10.1371/journal.pone.0115839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellatly S. L., Hancock R. E. W. (2013). Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog. Dis. 67 159–173. 10.1111/2049-632x.12033 [DOI] [PubMed] [Google Scholar]
- Gross M., Marianovsky I., Glaser G. (2006). MazG - a regulator of programmed cell death in Escherichia coli. Mol. Microbiol. 59 590–601. 10.1111/j.1365-2958.2005.04956.x [DOI] [PubMed] [Google Scholar]
- Gunnelius L., Hakkila K., Kurkela J., Wada H., Tyystjarvi E., Tyystjarvi T. (2014). The omega subunit of the RNA polymerase core directs transcription efficiency in cyanobacteria. Nucleic Acids Res. 42 4606–4614. 10.1093/nar/gku084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. W., Carey A. M. (1979). Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J. Bacteriol. 140 902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney E. F., Hancock R. E. W. (2013). Peptide design for antimicrobial and immunomodulatory applications. Biopolymers 100 572–583. 10.1002/bip.22250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haralalka S., Nandi S., Bhadra R. K. (2003). Mutation in the relA gene of Vibrio cholerae affects in vitro and in vivo expression of virulence factors. J. Bacteriol. 185 4672–4682. 10.1128/Jb.185.16.4672-4682.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Block R. (1973). Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc. Natl. Acad. Sci. U.S.A. 70 1564–1568. 10.1073/pnas.70.5.1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauryliuk V., Atkinson G. C., Murakami K. S., Tenson T., Gerdes K. (2015). Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat. Rev. Microbiol. 13 298–309. 10.1038/nrmicro3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser A. R. (2009). The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat. Rev. Microbiol. 7 654–665. 10.1038/nrmicro2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T. T., Karkhoff-Schweizer R. R., Kutchma A. J., Schweizer H. P. (1998). A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212 77–86. 10.1016/S0378-1119(98)00130-9 [DOI] [PubMed] [Google Scholar]
- Hong Y. Q., Ghebrehiwet B. (1992). Effect of Pseudomonas aeruginosa elastase and alkaline protease on serum complement and isolated components C1q and C3. Clin. Immunol. Immunopathol. 62 133–138. 10.1016/0090-1229(92)90065-V [DOI] [PubMed] [Google Scholar]
- Kazmierczak K. M., Wayne K. J., Rechtsteiner A., Winkler M. E. (2009). Roles of relSpn in stringent response, global regulation and virulence of serotype 2 Streptococcus pneumoniae D39. Mol. Microbiol. 72 590–611. 10.1111/j.1365-2958.2009.06669.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielland A., Blom T., Nandakumar K. S., Holmdahl R., Blomhoff R., Carlsen H. (2009). In vivo imaging of reactive oxygen and nitrogen species in inflammation using the luminescent probe L-012. Free Radic. Biol. Med. 47 760–766. 10.1016/j.freeradbiomed.2009.06.013 [DOI] [PubMed] [Google Scholar]
- Kim S. J., Park R. Y., Kang S. M., Choi M. H., Kim C. M., Shin S. H. (2006). Pseudomonas aeruginosa alkaline protease can facilitate siderophore-mediated iron-uptake via the proteolytic cleavage of transferrins. Biol. Pharm. Bull. 29 2295–2300. 10.1248/bpb.29.2295 [DOI] [PubMed] [Google Scholar]
- Klinkenberg L. G., Lee J. H., Bishai W. R., Karakousis P. C. (2010). The stringent response is required for full virulence of Mycobacterium tuberculosis in guinea pigs. J. Infect. Dis. 202 1397–1404. 10.1086/656524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laarman A. J., Bardoel B. W., Ruyken M., Fernie J., Milder F. J., van Strijp J. A. G., et al. (2012). Pseudomonas aeruginosa alkaline protease blocks complement activation via the classical and lectin pathways. J. Immunol. 188 386–393. 10.4049/jimmunol.1102162 [DOI] [PubMed] [Google Scholar]
- Mansour S. C., de la Fuente-Núñez C., Hancock R. E. W. (2015). Peptide IDR-1018: modulating the immune system and targeting bacterial biofilms to treat antibiotic-resistant bacterial infections. J. Pept. Sci. 21 323–339. 10.1002/psc.2708 [DOI] [PubMed] [Google Scholar]
- Mansour S. C., Pletzer D., de la Fuente-Núñez C., Kim P., Cheung G. Y. C., Joo H. S., et al. (2016). Bacterial abscess formation is controlled by the stringent stress response and can be targeted therapeutically. Ebiomedicine 12 219–226. 10.1016/j.ebiom.2016.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel J., Selin C., Fernando W. G., de Kievit T. (2012). Stringent response mutants of Pseudomonas chlororaphis PA23 exhibit enhanced antifungal activity against Sclerotinia sclerotiorum in vitro. Microbiology 158(Pt 1) 207–216. 10.1099/mic.0.053082-0 [DOI] [PubMed] [Google Scholar]
- Nakagawa A., Oshima T., Mori H. (2006). Identification and characterization of a second, inducible promoter of relA in Escherichia coli. Genes Genet. Syst. 81 299–310. 10.1266/ggs.81.299 [DOI] [PubMed] [Google Scholar]
- Ooga T., Ohashi Y., Kuramitsu S., Koyama Y., Tomita M., Soga T., et al. (2009). Degradation of ppGpp by nudix pyrophosphatase modulates the transition of growth phase in the bacterium Thermus thermophilus. J. Biol. Chem. 284 15549–15556. 10.1074/jbc.M900582200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer K. L., Aye L. M., Whiteley M. (2007). Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 189 8079–8087. 10.1128/JB.01138-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer D., Coleman S. R., Hancock R. E. W. (2016). Anti-biofilm peptides as a new weapon in antimicrobial warfare. Curr. Opin. Microbiol. 33 35–40. 10.1016/j.mib.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer D., Hancock R. E. W. (2016). Antibiofilm peptides: potential as broad-spectrum agents. J. Bacteriol. 198 2572–2578. 10.1128/JB.00017-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer D., Lafon C., Braun Y., Kohler T., Page M. G., Mourez M., et al. (2014). High-throughput screening of dipeptide utilization mediated by the ABC transporter DppBCDF and its substrate-binding proteins DppA1-A5 in Pseudomonas aeruginosa. PLOS ONE 9:e111311 10.1371/journal.pone.0111311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer D., Mansour S. C., Wuerth K., Rahanjam N., Hancock R. E. W. (2017). New mouse model for chronic infections by Gram-negative bacteria enabling the study of anti-infective efficacy and host-microbe interactions. mBio 8:e00140-7 10.1128/mBio.00140-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard J. W., Lam T., Stanners C. P. (1980). Mammalian cells do not have a stringent response. J. Cell. Physiol. 105 313–325. 10.1002/jcp.1041050214 [DOI] [PubMed] [Google Scholar]
- Potrykus K., Cashel M. (2008). (p)ppGpp: still magical? Annu. Rev. Microbiol. 62 35–51. 10.1146/annurev.micro.62.081307.162903 [DOI] [PubMed] [Google Scholar]
- Reffuveille F., de la Fuente-Nunez C., Mansour S., Hancock R. E. W. (2014). A broad-spectrum antibiofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob. Agents Chemother. 58 5363–5371. 10.1128/AAC.03163-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romling U., Balsalobre C. (2012). Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 272 541–561. 10.1111/joim.12004 [DOI] [PubMed] [Google Scholar]
- Sadikot R. T., Blackwell T. S., Christman J. W., Prince A. S. (2005). Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 171 1209–1223. 10.1164/rccm.200408-1044SO [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen T. D., Livak K. J. (2008). Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3 1101–1108. 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- Schweizer H. P., Hoang T. T. (1995). An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 158 15–22. 10.1016/0378-1119(95)00055-B [DOI] [PubMed] [Google Scholar]
- Simon R., Priefer U., Puhler A. (1983). A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio-Technology 1 784–791. 10.1038/nbt1183-784 [DOI] [Google Scholar]
- Slauch J. M. (2011). How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol. Microbiol. 80 580–583. 10.1111/j.1365-2958.2011.07612.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starosta A. L., Lassak J., Jung K., Wilson D. N. (2014). The bacterial translation stress response. FEMS Microbiol. Rev. 38 1172–1201. 10.1111/1574-6976.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strugeon E., Tilloy V., Ploy M. C., Da Re S. (2016). The stringent response promotes antibiotic resistance dissemination by regulating integron integrase expression in biofilms. mBio 7:e00868-16 10.1128/mBio.00868-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syal K., Flentie K., Bhardwaj N., Maiti K., Jayaraman N., Stallings C. L., et al. (2017). Synthetic (p)ppGpp analogue is an inhibitor of stringent response in mycobacteria. Antimicrob. Agents Chemother. 61:e00443-17 10.1128/AAC.00443-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma S., Schobert M. (2009). An improved Escherichia coli donor strain for diparental mating. FEMS Microbiol. Lett. 294 127–132. 10.1111/j.1574-6968.2009.01556.x [DOI] [PubMed] [Google Scholar]
- Traxler M. F., Summers S. M., Nguyen H. T., Zacharia V. M., Hightower G. A., Smith J. T., et al. (2008). The global, ppGpp-mediated stringent response to amino acid starvation in Escherichia coli. Mol. Microbiol. 68 1128–1148. 10.1111/j.1365-2958.2008.06229.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delden C. (2004). “Virulence factors in Pseudomonas aeruginosa,” in Virulence and Gene Regulation ed. Ramos J.-L. (Boston, MA: Springer; ) 3–45. [Google Scholar]
- van ’t Wout E. F. A., van Schadewijk A., van Boxtel R., Dalton L. E., Clarke H. J., Tommassen J., et al. (2015). Virulence factors of Pseudomonas aeruginosa induce both the unfolded protein and integrated stress responses in airway epithelial cells. PLOS Pathog. 11:e1004946 10.1371/journal.ppat.1004946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola C. L. (2015). The antibiotic resistance crisis: part 1: causes and threats. P T 40 277–283. [PMC free article] [PubMed] [Google Scholar]
- Vinella D., Albrecht C., Cashel M., D’Ari R. (2005). Iron limitation induces SpoT-dependent accumulation of ppGpp in Escherichia coli. Mol. Microbiol. 56 958–970. 10.1111/j.1365-2958.2005.04601.x [DOI] [PubMed] [Google Scholar]
- Vogt S. L., Green C., Stevens K. M., Day B., Erickson D. L., Woods D. E., et al. (2011). The stringent response is essential for Pseudomonas aeruginosa virulence in the rat lung agar bead and Drosophila melanogaster feeding models of infection. Infect. Immun. 79 4094–4104. 10.1128/Iai.00193-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L. A., Stallings C. L. (2013). Essential roles for Mycobacterium tuberculosis Rel beyond the production of (p)ppGpp. J. Bacteriol. 195 5629–5638. 10.1128/Jb.00759-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexselblatt E., Oppenheimer-Shaanan Y., Kaspy I., London N., Schueler-Furman O., Yavin E., et al. (2012). Relacin, a novel antibacterial agent targeting the stringent response. PLOS Pathog. 8:e1002925 10.1371/journal.ppat.1002925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand I., Hilpert K., Hancock R. E. W. (2008). Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3 163–175. 10.1038/nprot.2007.521 [DOI] [PubMed] [Google Scholar]
- Winsor G. L., Griffiths E. J., Lo R., Dhillon B. K., Shay J. A., Brinkman F. S. (2016). Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res. 44 D646–D653. 10.1093/nar/gkv1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Kalman M., Ikehara K., Zemel S., Glaser G., Cashel M. (1991). Residual guanosine 3′,5′-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J. Biol. Chem. 266 5980–5990. [PubMed] [Google Scholar]
- Xu X. H., Yu H., Zhang D., Xiong J. Z., Qiu J., Xin R., et al. (2016). Role of ppGpp in Pseudomonas aeruginosa acute pulmonary infection and virulence regulation. Microbiol. Res. 192 84–95. 10.1016/j.micres.2016.06.005 [DOI] [PubMed] [Google Scholar]
- Yeung A. T., Parayno A., Hancock R. E. W. (2012). Mucin promotes rapid surface motility in Pseudomonas aeruginosa. mBio 3:e0073-12. 10.1128/mBio.00073-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Zhang T., Su Z., Li L., Wang D., Xiao R., et al. (2016). (p)ppGpp synthetases regulate the pathogenesis of zoonotic Streptococcus suis. Microbiol. Res. 191 1–11. 10.1016/j.micres.2016.05.007 [DOI] [PubMed] [Google Scholar]
- Zumaquero A., Macho A. P., Rufian J. S., Beuzon C. R. (2010). Analysis of the role of the type III effector inventory of Pseudomonas syringae pv. phaseolicola 1448a in interaction with the plant. J. Bacteriol. 192 4474–4488. 10.1128/JB.00260-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.