Abstract Abstract
Based on numerical analyses of macromorphological characters, scanning electron microscopy observation of leaves and lemma micromorphology, as well as field observations, Stipa pennata subsp. ceynowae was described here as a new taxon from Poland. It differs from the most similar S. pennata subsp. pennata and S. borysthenica mainly by its longer ligules of vegetative shoots. The affinities of this taxon are discussed and a morphological comparison with related species is provided. Illustrations and images of the micromorphological structures, as well as information about its distribution, habitat and conservation status are given.
Keywords: feather grasses, micromorphology, numerical analyses, Poland, taxonomy
Introduction
Stipa Linnaeus (1753) is one of the largest genera in the family Poaceae, subfamily Pooideae (Soreng et al. 2015). In the narrow approach, it comprises over 150 species distributed in open grasslands and steppes, with the highest species diversity in the warm temperate regions of the Old World (Roshevitz 1934, Tzvelev 1968, 1976, Bor 1970, Martinovský 1980, Freitag 1985, Wu and Phillips 2006, Nobis 2013). New species of Stipa continue to be described. For instance, in the last twenty years, over thirty species have been described from such countries as Morocco, Spain, Italy, Turkey, Kazakhstan, Kyrgyzstan, Tajikistan, Mongolia, Bhutan, India and China (e.g. Kotukhov 1998a, 1998b, Noltie 1999, Vázquez and Ramos 2007, Vázquez et al. 2009, Nobis 2013, 2014a, Vázquez and Gutiérrez 2011, Zhao and Guo 2011, Cataldo et al. 2012, Tzvelev 2012, 2014, Nobis et al. 2013, 2016a). At the same time, there are also still many unresolved taxonomic problems within the different sections and taxonomic complexes of this genus.
One of the numerous and taxonomically problematic sections in the genus Stipa is the nominal section, which comprises (depending on the approach) from 15 to 55 species (Smirnov 1925, Martinovský 1965, 1970, 1977, 1980, Klokov and Osychnyuk 1976, Tzvelev 1976, 1986, Freitag 1985, Vázquez and Gutiérrez 2011, Gonzalo et al. 2013, Nobis et al. 2016b). In Central Europe (including Czech Republic, Germany, Poland, Slovakia, Hungary and Austria), the section Stipa is represented by about 10 taxa: S. bavarica Martinovský and Scholz (1968), S. borysthenica Klokov ex Prokudin (1951), S. dasyphylla (Ćernjaev ex Lindemann 1882) Trautvetter (1884), S. eriocaulis Borbás (1878) subsp. eriocaulis, S. eriocaulis subsp. austriaca (Beck von Mannagetta 1890) Martinovský (1965), S. pennata Linnaeus (1753), S. pulcherrima Koch (1848), S. styriaca Martinovský (1970), S. tirsa Steven (1857) and S. zalesskii Wilensky ex Smirnov (1925) (conf. Martinovský 1980, Tzvelev 1986, Conert 1998, Marhold and Hindák 1998, Danihelka et al. 2012). In Poland, there are only four species from the above-mentioned section, namely: S. borysthenica, S. pennata, S. pulcherrima and S. eriocaulis, all of them reaching here the northwestern limit of their general range (Ceynowa-Giełdon 1976, Ceynowa-Giełdon et al. 2014a, 2014b, Nobis 2014b, Nobis et al. 2017). The section Stipa can be divided into many critical groups of closely related and morphologically similar taxa. One example is the group that includes Stipa pennata, a species which over the years has undergone numerous changes. Before Freitag (1985) chose a lectotype from the original material studied by Linnaeus, the name S. pennata was regularly used by various authors to identify different species. The most correct seems to be Mansfeld’s (1939) assumption of the synonymization of S. joannis Čelakovský (1884) with S. pennata. Furthermore, some authors distinguished within the group a number of species and many units of lower rank (Klokov and Osychnyuk 1976, Tzvelev 1976, 2006, Martinovský 1977, 1980), whereas others distinguished a single species and several taxa of lower rank (Freitag 1985).
In our work within the S. pennata group, we included taxa previously classified in the series Penicilliferae Martinovský (1976) and characterized by having leaves with apical tassel, ventral line of hairs terminating below the top of lemma, dorsal line free and longer than the subdorsal ones. Within series Penicilliferae, Martinovský (1977) recognized four species: S. joannis (= S. pennata), S. borysthenica, S. styriaca Martinovský (1970) and S. danubialis Dihoru and Roman (1969). Stipa styriaca and S. danubialis are endemic species (Martinovský 1977) respectively for Austria and Romania. There are five additional taxa from Asia that fit the criteria for incorporation to ser. Penicilliferae (sect. Stipa): S. kirghisorum Smirnov (1925), S. turkestanica Hackel (1906) subsp. turkestanica, S. turkestanica subsp. trichoides (Smirnov 1925) Tzvelev (1974), S. macroglossa Smirnov (1924) subsp. macroglossa and S. macroglossa subsp. kazachstanica (Kotukhov 1994) Nobis (2013). Taxonomic revision as well as macro- and micromorphological variation of those aforementioned taxa have recently been presented by Nobis et al. (2016b).
During the taxonomic revision of the central European representatives of the Stipa pennata group, we came across herbarium specimens from Folusz near Szubin in Poland that greatly differ from the hitherto known species. On the basis of these sheets, Ceynowa-Giełdon (1976) distinguished Stipa joannis var. cujavica. Unfortunately, the name of this taxon was not validly published because the author provided only its brief description in Polish with no references to the type and place of its preservation. The aim of our study was to examine distinctiveness of individuals from Folusz in relation to other Central European taxa from S. pennata group by using multivariate morphometric analysis.
Materials and methods
Over 500 herbarium sheets with specimens from the Stipa pennata group deposited at B, FRU, GAT, GOET, JE, KFTA, KHOR, KRA, KRAM, LE, LECB, M, MSB, MW, NY, PE, POZ, SZUB, PR, PRC, TAD, TASH, TK, TRN, UPS, W, WA, WU were examined (acronyms by Thiers 2016). The morphological characteristics of the vegetative and generative structures were examined on well-developed specimens. For numerical analysis, we selected 177 herbarium sheets (67 of Stipa borysthenica, 104 of Stipa pennata, and 6 of Stipa from Folusz). A list of the morphological characteristics used in analyses is presented in Table 1. Measurements were taken using a Nikon SMZ800 stereo microscope.
Table 1.
Morphological characters used in the present analyses, involving Stipa pennata group.
| Abbreviation | Character |
|---|---|
| AL | length of anthecium (mm) |
| AwnL | length of awn (mm) |
| CL | length of callus (mm) |
| Col1L | length of lower segment of awn (mm) |
| Col2L | length of middle segment of awn (mm) |
| CRL | length of peripheral ring of callus base (mm) |
| CRW | width of peripheral ring of callus base (mm) |
| DDL | distance from the end of dorsal line of hairs to the top of anthecium (mm) |
| DVL | distance from the end of ventral line of hairs to the top of anthecium (mm) |
| LigC | length of ligules of the middle cauline leaves (mm) |
| LigIV | length of ligules of the internal vegetative shoots (mm) |
| LC | length of culm (mm) |
| LCL | length of upper cauline leaves (mm) |
| LP | length of panicle |
| LV | length of vegetative shoots (mm) |
| NF | number of flowers in panicle |
| SL/ColL | ratio length of seta to the sum of length of lower and middle segment of the awn |
| WA | width of anthecium (mm) |
In accordance with the assumption of numerical taxonomy (Sokal and Sneath 1963), each specimen was treated as an Operational Taxonomic Unit (OTU). For testing the normal distribution of each characteristic, the Lilliefors and Shapiro–Wilk statistical tests were performed. Those that did not fulfill the criterion of normality were log-transformed. Next, the Pearson correlation coefficient was calculated; the characteristics in which a strong correlation was found (>0.9) were excluded from further analyses. To illustrate the relationship between the studied taxa and also to select the features that best describe the existing variability, a Principal Component Analysis (PCA) was conducted using all quantitative characteristics. According to the Kaiser criterion, factors with eigenvalues >1 were chosen (Kaiser 1960) and characteristics with the highest factor loadings of the first three principal components (r≥0.60) were determined. Subsequently, descriptive statistics of characters for all recognized taxa were calculated. Levene’s test was using to assess the equality of variances. To reveal significant differences between means of particular characters across all examined taxa, one-way analysis of variance (ANOVA) and nonparametric Kruskal-Wallis test followed by Tukey’s HSD test or multiple comparison test were calculated. All statistical analyses and calculations were performed using Statistica software, version 10 (Statsoft Inc. 2011).
For observations in a scanning electron microscope, samples were coated with gold using a JFC-1100E Ion sputter manufactured by JEOL, then observed and photographed using a Hitachi S-4700 scanning electron microscope (SEM). The methods and terminology were adopted from Thomasson (1978, 1981), Ellis (1979), Snow (1996) and Nobis (2013, 2014a).
Results
Numerical analysis
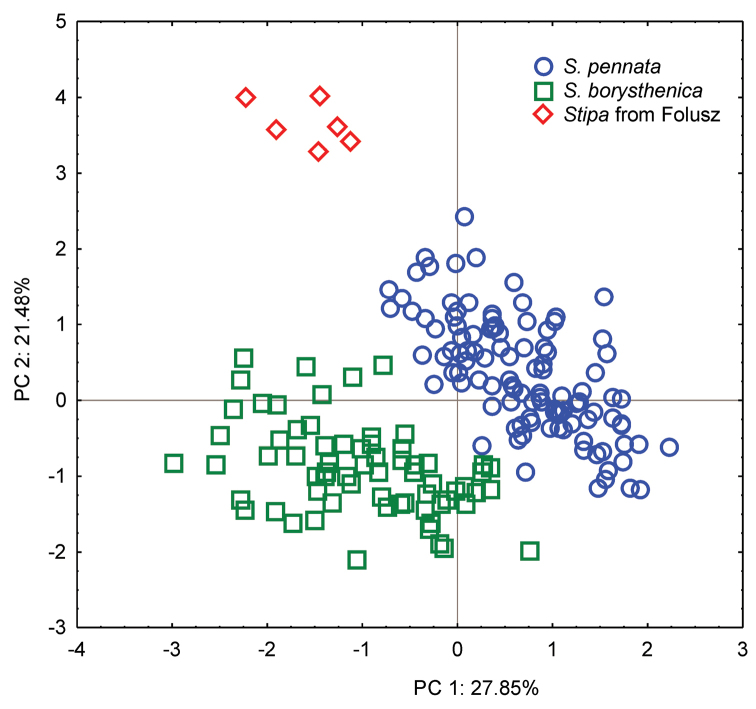
The result of the Principal Component Analysis (PCA) revealed twelve characteristics with high factor loadings (r≥0.6) on the first three principal components. Together, the first three components accounted for 57.71% of the total variation. The first two components explained respectively 27.85% and 21.48% of the total variation (Table 2). The scatterplot of the first two axes showed three group of points (Figure 1). Seven characteristics, including AL, CL, CRL, LCL, LigC, LP, and NF, displayed the highest correlations with the first axis, grouping specimens of Stipa borysthenica on the left and S. pennata on the right side. The remaining characteristics (AwnL, Col1L, LC, LigIV, LV) highly influenced the second axis, separating specimens representing Stipa from Folusz.
Table 2.
Results of numerical analysis involving Stipa pennata group. Principal component analysis (PCA): factor loadings for 18 characters, eigenvalues and percent variation. The highest factor loadings (≥ 0.6) are bolded. One-way ANOVA: F and p values for characters with normal distribution. Kruskal-Wallis test: H and p values for characters with non-normal distribution. The highest F/H values are bolded. For characters abbreviations see Table 1.
| Character abbreviation | PC 1 | PC 2 | PC 3 | ANOVA | |
|---|---|---|---|---|---|
| F/H | p | ||||
| AL | -0.77 | 0.24 | 0.33 | 20.28 | 0.000 |
| AwnL | -0.41 | 0.68 | -0.04 | 19.01 | 0.000 |
| CL | -0.81 | -0.34 | 0.27 | 248.95 | 0.000 |
| Col1L | 0.23 | 0.72 | 0.41 | 47.26 | 0.000 |
| Col2L | -0.24 | 0.58 | 0.45 | 15.27 | 0.001 |
| CRL | -0.62 | -0.07 | 0.21 | 53.73 | 0.000 |
| CRW | 0.55 | 0.37 | -0.16 | 64.72 | 0.000 |
| DDL | -0.50 | 0.48 | 0.13 | 15.29 | 0.001 |
| DVL | -0.21 | 0.19 | 0.38 | 7.20 | 0.027 |
| LC | -0.38 | 0.65 | -0.39 | 64.24 | 0.000 |
| LCL | -0.62 | -0.36 | -0.01 | 17.41 | 0.000 |
| LigC | -0.66 | -0.14 | -0.13 | 113.10 | 0.000 |
| LigIV | -0.37 | 0.60 | -0.16 | 118.02 | 0.000 |
| LP | -0.76 | -0.33 | -0.11 | 100.61 | 0.000 |
| LV | -0.23 | 0.74 | -0.34 | 28.80 | 0.000 |
| NF | -0.67 | -0.35 | -0.03 | 79.41 | 0.000 |
| SL/ColL | -0.57 | -0.16 | -0.57 | 11.76 | 0.001 |
| WA | -0.11 | 0.48 | -0.29 | 21.46 | 0.000 |
| Eigenvalue | 5.01 | 3.87 | 1.51 | ||
| Percent variation (%) | 27.85 | 21.48 | 8.38 | ||
Figure 1.
Biplot of principal component analysis (PCA) performed on 18 characters.
The results of the one-way ANOVA/Kruskal-Wallis test revealed significant differences in all examined characters (Table 2). The results of the post hoc tests (Tukey’s HSD test for variables with normal distribution and multiple comparison tests for characters with non-normal distribution) are presented in Table 3. The ranges of variability of the most important characteristics of the designated morphological groups corresponding to the two examined taxa and the population from Folusz are presented in Table 4.
Table 3.
Results of post-hoc tests. Tukey’s HSD test for characters with normal distribution, multiple comparison tests for characters with non-normal distribution. + – statistically significant, p < 0.05; ns – not significant. Stipa pennata – pe, Stipa borysthenica – bo, Stipa from Folusz – F. For characters abbreviations see Table 1.
| Character | pe-bo | pe-F | bo-F |
|---|---|---|---|
| AL | + | + | ns |
| AwnL | ns | + | + |
| CL | + | + | + |
| Col1L | + | + | + |
| Col2L | ns | + | + |
| CRL | + | + | ns |
| CRW | + | ns | + |
| DDL | ns | + | + |
| DVL | ns | ns | ns |
| LigC | + | + | ns |
| LigIV | ns | + | + |
| LC | ns | + | + |
| LCL | + | + | ns |
| LP | + | ns | ns |
| LV | + | + | + |
| NF | + | ns | ns |
| SL/ColL | + | ns | ns |
| WA | + | + | + |
| Number of significance differences | 12 | 13 | 10 |
Table 4.
Main morphological differences among selected members of Stipa pennata group. Measurements are given in millimeters.
| Taxon Character |
S. borysthenica | S. pennata subsp. ceynowae | S. pennata subsp. pennata |
|---|---|---|---|
| Anthecium length | (15.7–)17.00–18.9(–20.4) | (17.4–)18.1–19.9(–20.0) | (14.25–)15.9–18.0(–19.8) |
| Awn length | (225–)279–334(–396) | (305–)328–412(–442) | (228–)283–340(–408) |
| Callus length | (3.4–)3.7–4.2(–4.6) | (3.1–)3.3–3.8 | (2.4–)2.8–3.25(–3.75) |
| Column length | (43–)57–69(–59) | 81–91(–94) | (55–)64–78(–93) |
| Ligules of the middle cauline leaves length | (1.2–)2.2–4.2(–6.3) | (2.6–)2.8–4.3(–4.4) | (0.4–)1–2.5(–4.0) |
| Ligules of internal vegetative shoots length | (0.9–)1.3–2.2(–3.4) | (3.2–)4.1–5.2(–6.7) | (1.0–)1.3–2.2(–3.6) |
| Uppermost cauline leaves length | (22–)36–62(–125) | (16–)27–38(–69) | (4–)10–22(–40) |
| Shape of callus base | Cuneate | Piriformis | Piriformis |
Scanning microscope observation
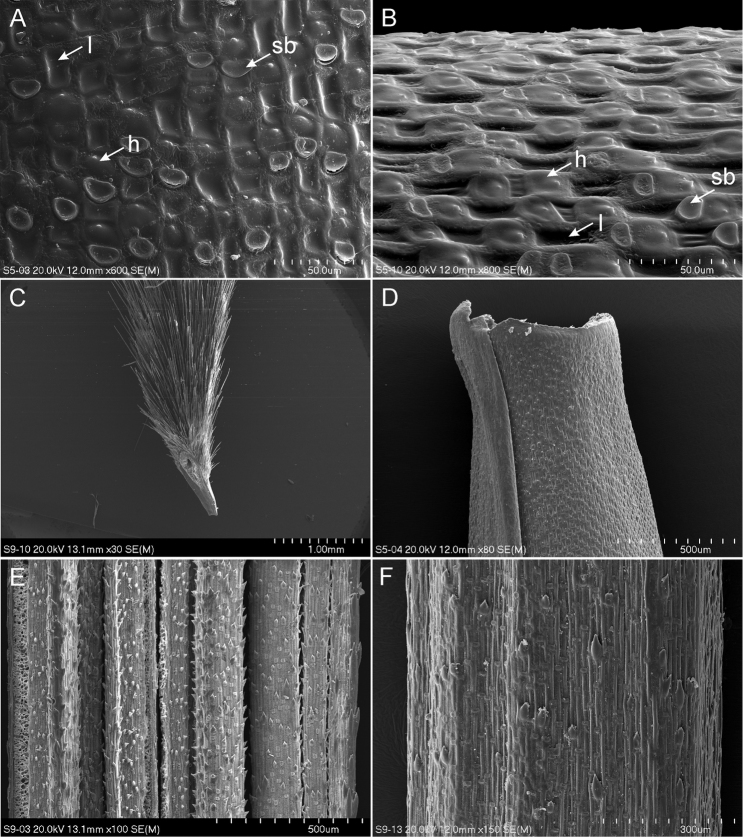
The results of SEM observations showed that the general patterns of the lemma micromorphology of Stipa from Folusz are typical for the genus Stipa (cf. Barkworth and Everett 1987, Nobis 2013, Nobis et al. 2013, 2016b) (Figure 2). Fundamental (long) cells are elongated, rectangular to a more or less square in shape. The side walls of long cells are raised and undulate, but often hidden under a thick layer of wax that hinders observation. Silica bodies are quite common, reniform to oblong or ovate, while cork cells are sparse or absent. Hooks are frequent, oriented towards the lemma apex, whereas prickles are completely absent. Lemma apex is glabrous (Figure 2A–D).
Figure 2.
SEM morphology of Stipa from Folusz. A Structure of lemma – superior view B Structure of lemma – lateral view C Callus D Top of anthecium E Adaxial surface of vegetative leaves F Abaxial surface of vegetative leaves. Abbreviations: h = hook, l = long cell, sb = silica body.
The adaxial surface of leaves of the vegetative shoots is ribbed and densely covered by short prickles, long cells and silica bodies (Figure 2E). Whereas the abaxial surface is dominated by long cells with admixtures of silica bodies and sparsely distributed prickles (Figure 2F).
Taxonomic treatment
Conducted analysis clearly indicated that specimens from Folusz represents a new taxon, which is described below.
Stipa pennata subsp. ceynowae
Klichowska & M.Nobis subsp. nov.
urn:lsid:ipni.org:names: 77164173-1
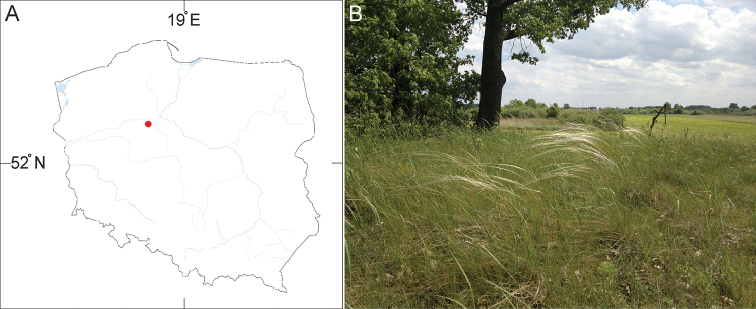
Figure 3.
Stipa pennata subsp. ceynowae from Folusz near Szubin (Poland). A Map of distribution in Poland, red dot – locality of population B Photograph of habitat.
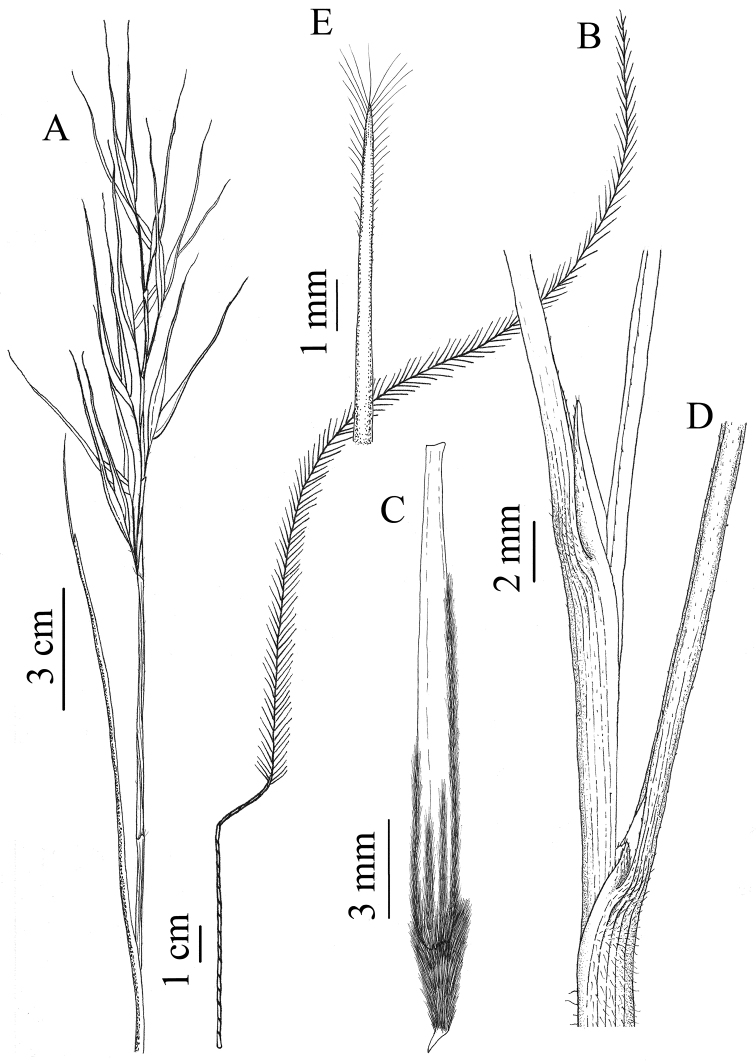
Figure 4.
Stipa pennata subsp. ceynowae based on the holotype. A Panicle with upper cauline leaves B Awn with glabrous column and pilose seta C Anthecium D External (the lower) and internal (the upper) ligules of the vegetative leaves E Apex of juvenile leaves with an apical tassel of hairs.
Figure 5.
Holotype of Stipa pennata subsp. ceynowae.
Diagnosis.
Stipa pennata subsp. ceynowae is most similar to S. pennata subsp. pennata from which differs mainly in longer ligules of internal leaves of vegetative shoots (3.2–)4.1–5.2(–6.7) mm vs. (1.0–)1.3–2.2(–3.6) mm and lemmas with a somewhat longer awn (305–)328–412(–442) mm vs. (228–)283–340(–408) mm respectively.
Type.
POLAND. Folusz koło Szubina, zarośla na wydmie [Folusz near Szubin, scrub on the dune], 5 July 1962, Ceynowa-Giełdon s.n. (holotype TRN!, isotype KRA 0451189!).
Description.
Plant perennial, densely tufted, with a few culms and numerous vegetative shoots. Culms (56–)84–95(–99) cm tall, 3–4-noded, glabrous at nodes and slightly scabrous to more or less densely pubescent below them. Leaves of vegetative shoots: sheaths of external leaves shortly pilose to scabrous, of internal leaves scabrous to almost glabrous; ligules membranous, acute or slightly obtuse, with very short cilia at the apex and shortly setulose on the back, of external leaves (1.0–)1.1–2.1(–2.7) mm long, of internal leaves (3.2–)4.1–5.2(–6.7) mm long; blades convolute, green to pale green, (73–)81–97(–107) cm long, (0.5–)0.7–0.8(–1.0) mm in diameter, abaxial surface from scabrous, covered by short spinules (on external leaves) to slightly scabrous or almost smooth, with spinules almost confined to the margins of leaf blades (on internal leaves), adaxial surface always covered by short prickles less than 0.1 mm long, juvenile leaves usually with an apical tassel of hairs up to 2 mm long. Cauline leaves: sheaths smooth to slightly scabrous (usually in upper part of sheath); ligules of the middle cauline leaves (2.6–)2.8–4.3(–4.4) mm long, slightly acute or obtuse, at the apex with very short cilia, and with short bristles on the back; blades convolute, green or pale green, the uppermost one (1.6–)2.7–3.8(–6.9) cm long, abaxial surface scabrous. Panicle 10–11(–14.5) cm long, contracted, with 8–10 spikelets; branches scabrous or with short hairs 0.2–0.5(–0.8) mm long. Glumes subequal, 56–64 mm long, narrowly lanceolate. Anthecium (17.4–)18.1–20.0 mm long and 1.0–1.25 mm wide; callus (3.1–)3.3–3.8 mm long, with hairs (1.6–)1.7–2.1(–2.4) mm long in ventral part and (1.0–)1.1–1.4 mm long in dorsal; foot of callus curved, peripheral ring flattened 0.9–0.95 × 0.3–0.35 mm; lemma straw-coloured, with 7 lines of hairs, dorsal and subdorsal lines slightly fused at the base, ventral line with (0.5–)0.6–0.8 mm long hairs, terminating at 1/2–2/3 of lemma length about 4.5–5.8(–6.4) mm below the top of lemma; dorsal line with (0.4–)0.5–0.6 mm long hairs, terminating at 1/3 of lemma length, about (9.1–)9.5–11.0(–11.1) mm below the top; awn (305–)328–412(–442) mm long, bigeniculate; column smooth and glabrous, twisted, straw-coloured or slightly green, 0.5–0.6 mm wide near base, 81–91(–94) mm long with the lower segment of column (63–)65–69(–70) mm long and the upper (19–)22–24 mm long; seta (222–)247–318(–354) mm long, pilose, with 5.2–6.0 mm long hairs, gradually decreasing in length towards apex; palea straw-coloured equaling lemma in length. Caryopsis ca.12 mm long.
Etymology.
The name of taxon honors the collector—Prof. Mirosława Ceynowa-Giełdon, who first noted the distinctiveness of Stipa individuals from Folusz.
Distribution and habitat.
Stipa pennata subsp. ceynowae is an endemic taxon, known only from Folusz settlement near Szubin in Kuyavia region (northern Poland). It grows on a dune hill surrounded by wet meadows occurring in the Gąsawka River Valley. The subspecies occurs on small fragment of dry, sandy grassland adjoining oak and pine stands. At the locality, the following species grow together with Stipa: Achillea pannonica Scheele, Asperula tinctoria L., Avenula pratensis (L.) Dumort., Betula pendula Roth, Calamagrostis epigejos (L.) Roth, Carex praecox Schreb., Dianthus carthusianorum L., Euphorbia cyparissias L., Festuca trachyphylla (Hack.) Krajina, Filipendula vulgaris Moench, Galium verum L., Geranium sanguineum L., Peucedanum oreoselinum (L.) Moench, Poa pratensis L., Polygonatum odoratum (Mill.) Druce, Vincetoxicum hirundinaria Medik.
Phenology.
Flowering period: May–June.
Conservation status.
Stipa pennata is a species protected in Poland (Regulation of the Minister of the environment dated October 9, 2014) as well as it was included in the Polish red data book of plants (Ceynowa-Giełdon et al. 2014b). The only known locality of S. pennata subsp. ceynowae was partly destroyed by the extraction of sand (up to the mid-1950s) and the subsequent afforestation of pine and birch trees carried out in the 1990s (Ceynowa-Giełdon 2001, Nienartowicz et al. 2014). Currently, S. pennata subsp. ceynowae should be considered as a critically endangered (CR) species—to date, only several flowering individuals have survived (tufts with 8, 10, 11 and 14 culms), occupying a very small area of dry grassland. Lack of grazing has resulted in increased ground cover by layer of “steppe felt”, which hamper seeds germination and seedlings growth. Also, tree seedlings pose a threat by shading the grasslands. Similarly, as in the case of other dry grassland species— survival depends on the preservation of suitable habitat conditions, which can be achieved through active protection. Due to the extremely small size of the population, it seems reasonable to apply the methods of ex situ conservation, including in vitro propagation.
Additional specimens studied (paratypes). POLAND. Folusz, 16 Jun 1959, Michalska and Bohr s.n. (TRN!); Folusz koło Szubina nad Gąsawką, na wydmie, wśród łąk [Folusz near Szubin on the Gąsawka River, on a dune, among meadows], 13 Jun 1972, Ceynowa-Giełdon s.n. (TRN!)×4; North Poland, Kuyavian-Pomeranian Voivodeship, Folusz near Szubin by the Gąsawka River; xerothermic grassland on a sandy dune, 3 Jun 2014, Klichowska s.n. (KRA 0451190!).
Discussion
Ceynowa-Giełdon (1976) distinguished Stipa joannis var. cujavica (nom. inval.) based on the longer hairless part of the awn, longer vegetative leaves and longer upper cauline leaves than in the case of the typical variety. Although the Principal Component Analysis supports the usefulness of these characteristics (Table 2), their larger size can also be found in individuals of S. pennata subsp. pennata and, after examining a great number of individuals, they seem to be insufficient to distinguish this taxon based on its description. According to our results, the internal vegetative leaves (Figure 4D) in specimens of S. pennata subsp. ceynowae have distinctly longer ligules (usually 4.1–5.2 mm in length) than the other closely related species from Poland, namely S. borysthenica and S. pennata subsp. pennata (in both cases, usually reaching of 1.3–2.2 mm in length; Table 4). Our research carried out on a large number of herbarium specimens (from the geographical range of these taxa), as well as on the findings of other authors (Bor 1970, Tzvelev 1976, Martinovský 1977, 1980, Conert 1998, Gonzalo et al. 2013, Nobis et al. 2016b), confirm that all known taxa closely related with S. pennata do not have such long ligules of their vegetative shoots. Ligules of a similar length or even longer are observed in other species of the section Stipa occurring in Central Asia, namely: S. turkestanica subsp. turkestanica, S. macroglossa subsp. macroglossa and S. macroglossa subsp. kazachstanica (Gonzalo et al. 2013, Nobis et al. 2016b). However, S. pennata subsp. ceynowae cannot be confused with any of them due to its definitely longer anthecium, callus, awn, culm and vegetative leaves, as well as to its distribution, limited only to Central Europe.
Stipa kirghisorum, is another species that is morphologically similar to both S. pennata subsp. pennata and S. pennata subsp. ceynowae. However, S. kirghisorum differs from the two above-mentioned taxa by the strongly scabrous abaxial surface of leaves of the vegetative shoots, shorter anthecium (13.1–)14.5–16.0(–17.8) mm and ventral line of hairs terminating (0.5–)1.4–3.1(–4.6) mm below the top of the lemma, as well as its general range that is limited to the Central Asia (Nobis et al. 2016b).
Stipa pennata subsp. ceynowae is somewhat similar to two other European species from ser. Penicillifera. First is S. styriaca that is also characterized by having long awn up to 445 mm and anthecium 17.5–21.5 mm, but in contrast to S. pennata subsp. ceynowae it has densely pubescent leaf sheaths (with 0.2–0.8 mm hairs) (Martinovský 1977). The second species is S. danubialis that differs from Stipa pennata subsp. ceynowae by having pilose column (lower part of awn) and anthecium 23–25 mm long (Martinovský 1977).
Due to its long awn, Stipa pennata subsp. ceynowae could be also confused with S. pulcherrima that occurs in Central Europe too. However, it can easily be distinguished by its ventral lines of hairs terminating at 1/2–2/3 of lemma length, shorter anthecium (17.4–20.0 mm) and longer ligules on vegetative shoots, while S. pulcherrima is characterized by ventral lines reaching the base of the awn, anthecium 18–25 mm long and ligules of the vegetative shoots not exceeding 2 mm long (Martinovský 1980, Nobis 2014b).
The results of ANOVA and post-hoc tests confirm separateness of the taxon from Folusz (Tables 2, 3). Stipa pennata subsp. ceynowae differs from S. pennata subsp. pennata and from S. borysthenica in a statistically significant way by 13 and 10 characters respectively (Table 3).
A key to identification of feather grasses (Stipa) in Poland
| 1 | Awns scabrous throughout | S. capillata |
| – | Awn smooth in the lower pat and plumose in the upper | 2 |
| 2 | Ventral line of hairs on lemma not reaching the base of awn, ending (1.0–)3.0–6.0(–7.9) mm below the top; dorsal line only in lower 1/4 of its length fused with subdorsal ones | 3 |
| – | Ventral line of hairs on lemma reaching the base of awn; dorsal line at least in 3/4 of its length fused with subdorsal ones | 5 |
| 3 | Blade of uppermost cauline leaf (22–)36–62(–125) mm long; floret callus (3.4–)3.7–4.2(–4.6) mm, straight to slightly curved, callus base cuneate | S. borysthenica |
| – | Blade of uppermost cauline leaf (4–)10–24(–69) mm long; floret callus (2.4–)2.8–3.3(–3.8) mm long, curved, callus base piriform | 4 |
| 4 | Ligules of internal leaves of vegetative shoots (1.0–)1.3–2.2(–3.6) mm long; column of awn (55–)64–78(–93) mm long; blade of uppermost cauline leaves (4–)10–22(–40) mm long | S. pennata subsp. pennata |
| – | Ligules of internal leaves of vegetative shoots (3.2–)4.1–5.2(–6.7) mm long, column of awn 81–91(–94) mm long; blade of uppermost cauline leaves (16–)27–38(–69) mm long | S. pennata subsp. ceynowae |
| 5 | Leaves of the vegetative shoots distinctly scabrous; anthecium (18.1–)20.6–22.8(–24.6) mm long; floret callus (3.7–)4.4–5.1(–5.8) mm long; awn (277–)328–394(–463) mm long | S. pulcherrima |
| – | Leaves of the vegetative shoots glabrous and smooth to very slightly scabrous especially in their lower part; anthecium (15.0–)16.3–18.7(–20.7) mm long; floret callus (3.4–)3.6–4.4(–5.0) mm long; awn (218–)228–269(–312) mm long | S. eriocaulis |
Supplementary Material
Acknowledgements
Authors are grateful to the curators of B, FRU, GAT, GOET, JE, KFTA, KHOR, KRA, KRAM, LE, LECB, M, MSB, MW, NY, PE, POZ, SZUB, PR, PRC, TAD, TASH, TK, TRN, UPS, W, WA, WU for making the collections available for study, as well as Regional Directorates for Environmental Protection in Kielce, Bydgoszcz and Gorzów Wielkopolski for authorization for collection of protected plants (Decisions no. WPN.6400.16.2013.JC.1, WPN.6400.26.2015.JC, WPN. 6400.25.2015.JC, WPN.6400.41.2015.NG, WPN.I.6400.6.2015.AC, WPN.I.6400.17.2015.BD, WPN.I.6400.9.2013.BD, WPN-I.6400.61.2014.AT, WPN-I.6205.25.2015.AI). We thank Anna Łatkiewicz (Institute of Geological Sciences, Jagiellonian University, Poland) for her assistance with the SEM micrographs and Małgorzata Jaźwa (Institute of Botany, Jagiellonian University, Poland) for scanning herbarium sheet of holotype. We are also grateful to Clifford Morden and Robert Soreng for their valuable comments and improvements to the manuscript. This research was supported by the National Science Center (Poland): E. Klichowska grant no. 2014/15/N/NZ8/00340 and M. Nobis grant no. DEC-2013/09/B/NZ8/03287.
Citation
Klichowska E, Nobis M (2017) Stipa pennata subsp. ceynowae (Poaceae, Pooideae), a new taxon from Central Europe. PhytoKeys 83: 75–92. https://doi.org/10.3897/phytokeys.83.12797
References
- Barkworth ME, Everett J. (1987) Evolution in the Stipeae: identification and relationships of its monophyletic taxa. In: Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME. (Eds) Grass systematics and evolution. Smithsonian Institution Press, Washington, DC, 251–264.
- Beck-Mannagetta G. (1890) Flora von Nieder-Österreich. Druck und Verlag von Carl Gerold’s Sohn, Wien, 889 pp. [Google Scholar]
- Bor NL. (1970) Graminae. In: Rechinger KH. (Ed.) Flora Iranica, 70. Academische Druck-und Verlagsanstalt, Graz-Austria, 1–573. [72 tables]
- Borbás V. (1878) Floristicai közlemények a magy. tud. akadémia által támogatott botanikai kutatásaimból. Mathematikai és Természettudományi Közlemények 15: 265–371. [Google Scholar]
- Cataldo D, Giardina SA, Moraldo B, Raimondo FM. (2012) Stipa valdemonensis (Poaceae), a new species from Sicily. Plant Biosystems 146: 658–663. http://dx.doi.org/10.1080/11263504.2012.700961 [Google Scholar]
- Čelakovský LF. (1884) Nachtragliches über Stipa tirsa Steven. Oesterreichische Botanische Zeitschrift. Gemeinnütziges Organ für Botanik 34: 318–321. [Google Scholar]
- Ceynowa-Giełdon M. (1976) Ostnice sekcji Pennatae w Polsce. Rozprawy Uniwersytetu Mikołaja Kopernika, Toruń, 99 pp. [Google Scholar]
- Ceynowa-Giełdon M. (2001) Stipa joannis Čelak. – Ostnica Jana. In: Kaźmierczakowa R, Zarzycki K. (Eds) Polska Czerwona Księga Roślin. Paprotniki i rośliny kwiatowe. W. Szafer Institute of Botany, Polish Academy of Science, Kraków, 260–261.
- Ceynowa-Giełdon M, Nobis M Barańska K. (2014a) Stipa borysthenica Klokov ex Prokudin – Ostnica piaskowa. In: Kaźmierczakowa R, Zarzycki K, Mirek Z. (Eds) Polska czerwona księga roślin: paprotniki i rośliny kwiatowe (ed. 3). Instytut Ochrony Przyrody. Polska Akademia Nauk, Kraków, 656–658.
- Ceynowa-Giełdon M, Nobis M, Rutkowski L. (2014b) Stipa pennata L. – Ostnica piórkowata. In: Kaźmierczakowa R, Zarzycki K, Mirek Z. (Eds) Polska czerwona księga roślin: paprotniki i rośliny kwiatowe (ed. 3). Instytut Ochrony Przyrody. Polska Akademia Nauk, Kraków, 651–654.
- Conert HJ. [Ed.] (1998) Gustav Hegi Illustrierte Flora von Mitteleuropa, 1(3), Spermatophyta: Angiospermae: Monocotyledones 1(2) Poaceae. Parey Buchverlag, Berlin, 897 pp. [Google Scholar]
- Danihelka J, Chrtek Jr J, Kaplan Z. (2012) Checklist of vascular plants of the Czech Republic. Preslia 84: 647–811. [Google Scholar]
- Dihoru GH, Roman N. (1969) Une nouvelle espece du genre Stipa. Revue Roumaine de Biologie, Série Botanique 14(1): 21–27. [Google Scholar]
- Ellis RP. (1979) A procedure for standardizing comparative leaf anatomy in the Poaceae. II. The epidermis as seen in surface view. Bothalia 12: 641–671. https://doi.org/10.4102/abc.v12i4.1441 [Google Scholar]
- Freitag H. (1985) The genus Stipa (Gramineae) in southwest and south Asia. Notes from the Royal Botanical Garden, Edinburgh 42: 355–489. [Google Scholar]
- Gonzalo R, Aedo C, García MA. (2013) Taxonomic revision of the Eurasian Stipa subsections Stipa and Tirsae (Poaceae). Systematic Botany 38: 344–378. https://doi.org/10.1600/036364413X666615 [Google Scholar]
- Hackel E. (1906) Gramineae novae turkestanicae. Trudy Imperatorskago S. -Peterburgskago Botaničeskago Sada 26: 53–60. [Google Scholar]
- Kaiser HF. (1960) The application of electronic computers to factor analysis. Educational and Psychological Measurement 20: 141–151. https://doi.org/10.1177/001316446002000116 [Google Scholar]
- Klokov M, Osychnyuk V. (1976) Stipae Ucrainicae. Novosti Sistematiki Vysshik i nizshikh rastenii Kiev 1975: 7–91. [Google Scholar]
- Koch K. (1848) Beitrage zu einer Flora des Orients. Linnaea 21: 289–736. [Google Scholar]
- Kotukhov YuA. (1994) Novye vidy roda Stipa (Poaceae) iz yuzhnogo Altaya, Saura i Tarbagataya [New species of the genus Stipa (Poaceae) from south Altai, Saur and Tarbagatai]. Botanicheskii Zhurnal 79: 101–106. [Google Scholar]
- Kotukhov YuA. (1998a) New species of grasses (Poaceae) from south Altai, Saur and Tarbagatai. Turczaninowia 1(1): 7–21. [Google Scholar]
- Kotukhov YuA. (1998b) New species of the genus Stipa L. (Poaceae) from western Kazakhstan. Turczaninowia 1(2): 9–15. [Google Scholar]
- Lindemann E. (1882) Flora Chersonensis, 2. Odessae, 329 pp.
- Linnaeus C. (1753) Species Plantarum, 1. L. Salvii, Holmiae, Stockholm, 1–560. http://dx.doi.org/10.5962/bhl.title.669
- Mansfeld R. (1939) Zur Nomenklatur der Farn- und Blütenplflanzen Deutschlands. VII. Feddes Repertorium. Zeitschrift für Botanische Taxonomie und Geobotanik 47: 263–287. [Google Scholar]
- Marhold K, Hindák F. (1998) Zoznam nižších a vyšších rastlín Slovenska - Checklist of non-vascular and vascular plants of Slovakia. Veda, VSAV, Bratislava.
- Martinovský JO. (1965) Kavyly serie Pulcherrimae na Slovensku. Biológia: 498–510.
- Martinovský JO. (1970) Über drei neue Stipa Sippen aus dem Verwandtschaftskreis Stipa joannis s. l. XXII. Beitrag zur Kenntnis der Stipa-Sippen. Oesterreichische Botanische Zeitschrift 118: 171–181. https://doi.org/10.1007/BF01373228 [Google Scholar]
- Martinovský JO. (1976) Neue Stipa–Sippen und einige Ergänzungen der früher beschriebenen Stipa–taxa. Preslia 48: 186–188. [Google Scholar]
- Martinovský JO. (1977) Clavis analytica nec non descriptions breves taxorum generis Stipa in Europa centrali provenientium. Preslia 49: 97–113. [Google Scholar]
- Martinovský JO. (1980) Stipa L. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA. (Eds) Flora Europaea, 5. Cambridge University Press, Cambridge, 247–252.
- Martinovský JO, Scholz H. (1968) Stipa bavarica: Eine Neue Federgrasart. XIII. Beitrag Zur Kenntnis Der Europäischen Federgrassippen (Stipa bavarica: A new species of feather-grass). Willdenowia 4(3): 317–324. [Google Scholar]
- Nienartowicz A, Kamiński D, Kunz M, Deptuła M, Adamska E. (2014) Changes in the plant cover of the dune hill in Folusz near Szubin (NW Poland) between 1959 and 2013: the problem of preservation of xerothermic grasslands in the agricultural landscape. Ecological Questions 20: 23–38. http://dx.doi.org/10.12775/EQ.2014.013 [Google Scholar]
- Nobis M. (2013) Taxonomic revision of the Stipa lipskyi group (Poaceae: Stipa section Smirnovia) in the Pamir Alai and Tian-Shan Mountains. Plant Systematics and Evolution 299: 1307–1354. http://dx.doi.org/10.1007/s00606-013-0799-5 [Google Scholar]
- Nobis M. (2014a) Taxonomic revision of the Central Asian Stipa tianschanica complex (Poaceae) with particular reference to the epidermal micromorphology of the lemma. Folia Geobotanica 49: 283–308. http://dx.doi.org/10.1007/s12224-013-9164-2 [Google Scholar]
- Nobis M. (2014b) Stipa pulcherrima K. Koch. In: Kaźmierczakowa R, Zarzycki K, Mirek Z. (Eds) Polska czerwona księga roślin: paprotniki i rośliny kwiatowe, ed. 3. Instytut Ochrony Przyrody. Polska Akademia Nauk, Kraków, 654–656.
- Nobis M, Erst A, Nowak A, Shaulo D, Olonova M, Kotukhov Yu, Király G, Ebel AL, Kushunina M, Nobis A, Piwowarczyk R, Sukhorukov AP, Verloove F, Zalewska-Gałosz J, Burri JF, Caković D, Jędrzejczak E, Jogan N, Klichowska E, Pliszko A, Popovich AV, Stešević D, Šilc U, Tupitsyna N, Wang W, Werner P, Wolanin MN, Wolanin MM, Xiang KL. (2017) Contribution to the flora of Asian and European countries: new national and regional vascular plant records, 6. Botany Letters 164: 23–45. http://dx.doi.org/10.1080/23818107.2016.1273134 [Google Scholar]
- Nobis M, Klichowska E, Nowak A, Gudkova PD, Rola K. (2016b) Multivariate morphometric analysis of the Stipa turkestanica group (Poaceae). Plant Systematics and Evolution 302: 137–153. http://dx.doi.org/10.1007/s00606-015-1243-9 [Google Scholar]
- Nobis M, Nobis A, Klichowska E, Nowak A, Nowak S, Gudkova PD. (2016a) Stipa dickorei sp. nov. (Poaceae), three new records and a checklist of feather grasses of China. Phytotaxa 267(1): 29–39. http://dx.doi.org/10.11646/phytotaxa.267.1.3 [Google Scholar]
- Nobis M, Nowak A, Nobis A. (2013) Stipa zeravshanica sp. nov. (Poaceae), an endemic species from rocky walls of the western Pamir Alai Mountains (middle Asia). Nordic Journal of Botany 31: 666–675. http://dx.doi.org/10.1111/j.1756-1051.2013.00184.x [Google Scholar]
- Noltie HJ. (1999) Notes relating to the flora of Bhutan: XXXVIII. Gramineae I, tribe Stipeae. Edinburgh Journal of Botany 56: 285–292. https://doi.org/10.1017/S0960428600001141 [Google Scholar]
- Prokudin GN. (1951) Zlaki [Gramineae]. In: Stankov SS. (Ed.) Flora Kryma, 1(4). Gosudarstwennoe Izdatel’stvo Sel’skokhozyaistvennoi literatury, Moscow, 1–153.
- Roshevitz RYu. (1934) Stipa L. In: Komarov VL (Ed.) Flora SSSR, 2. Editio Academiae Scientiarum URSS, Leningrad, 79–112 and 740–741.
- Regulation of the Minister of the Environment (2014) Regulation of the Minister of the Environment dated October 9, 2014, on the protection of the species of plants. Journal of Laws, item 1409.
- Smirnov PA. (1924) Stipa macroglossa P.A. Smirnow sp.n. Botaniceskie Materialy Gerbariya Glavnogo Botanicheskogo Sada RSFSR 5: 47–48. https://doi.org/10.1002/fedr.19250210806 [Google Scholar]
- Smirnov PA. (1925) Die neuen russischen Stipa-Pennata-Arten. Repertorium Novarum Specierum Regni Vegetabilis 21: 231–235. [Google Scholar]
- Snow N. (1996) The phylogenetic utility of lemmatal micromorphology in Leptochloa s.l. and related genera in subtribe Eleusininae (Poaceae, Chloridoideae, Eragrostideae). Annals of the Missouri Botanical Garden 83: 504–529. https://doi.org/10.2307/2399991 [Google Scholar]
- Sokal RR, Sneath PH. (1963) Principles of numerical taxonomy. Freeman WH, San Francisco, 359 pp. [Google Scholar]
- Soreng RJ, Peterson PM, Romschenko K, Davidse G, Zuloaga FO, Judziewicz EJ, Filgueiras TS, Davis JI, Morrone O. (2015) A worldwide phylogenetic classification of the Poaceae (Gramineae). Journal of Systematics and Evolution 53(2): 117–137. https://doi.org/10.1111/jse.12150 [Google Scholar]
- StatSoft Inc. (2011) STATISTICA (data analysis software system), version 10.
- Steven C. (1857) Verzeichniss der auf der taurischen Halbinsel wildwachsenden pflanzen. Bulletin de la Société impériale des naturalistes de Moscou 30(3): 65–133. [Google Scholar]
- Thiers B. (2016) Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/
- Thomasson JR. (1978) Epidermal patterns of the lemma in some fossil and living grasses and their phylogenetic significance. Science 199: 975–977. https://doi.org/10.1126/science.199.4332.975 [DOI] [PubMed] [Google Scholar]
- Thomasson JR. (1981) Micromorphology of the lemma in Stipa robusta and Stipa viridula (Gramineae: Stipeae): taxonomic significance. Southwest Naturalist 26: 211–214. https://doi.org/10.2307/3671126 [Google Scholar]
- Trautvetter E. (1884) Incrementa florae phaenogamae rossicae. Trudy Imperatorskago S. -Peterburgskago Botaniceskago Sada 9: 1–415. [Google Scholar]
- Tzvelev NN. (1968) Zlaki (Gramineae). In: Grubov VI (Ed.) Rastieniya Centralnoi Azii. Po materialam Botanicheskogo Instituta im. Komarova VL (Plantae Asiae Centralis, secus materies Instituti botanici nomine Komarovii VL), 4. Nauka, Leningrad, 1–243 and 12 maps.
- Tzvelev NN. (1974) Zametki o tribe Stipae Dum. semejstva zlakov (Poaceae) v SSSR—Notulae de tribu Stipae Dum. (fam. Poaceae) in URSS. Novosti Sistematiki Vysshikh Rastenii 11: 4–21. [Google Scholar]
- Tzvelev NN. (1976) Zlaki SSSR. Nauka, Leningrad, 1–788.
- Tzvelev NN. (1986) On the feather-grasse (Stipa L., Gramineae) in the Ukraine. Byuletin Moskovskogo Obschestva Ispytatelei Prirody. Otdel Biologicheskii 91(1): 116–124. [Google Scholar]
- Tzvelev NN. (2006) Stipa L. In: Takhtajan AL. (Ed.) Caucasian flora conspectus, 2. Saint-Petersburg University Press, Petersburg, 348–356.
- Tzvelev NN. (2012) Notes on the tribe Stipeae Dumort. (Poaceae). Novosti Sistematiki Vysshikh Rastenii 43: 20–29. [Google Scholar]
- Tzvelev NN. (2014) On some hybridogenous taxa in the genus Stipa L. (Poaceae). Novosti Sistematiki Vysshikh Rastenii 45: 5–8. [Google Scholar]
- Vázquez FM, Ramos S. (2007) Botanical Journal of the Linnean Society 153: 439–444. https://doi.org/10.1111/j.1095-8339.2007.00625.x [Google Scholar]
- Vázquez FM, Gutièrrez M. (2011) Classification of species of Stipa with awns having plumose distal segments. Telopea 13: 155–176. https://doi.org/10.7751/telopea20116012 [Google Scholar]
- Vázquez FM, Perez-Chiscano JL, Gutiérrez M, Ramos S. (2009) A new species of Stipa sect. Leiostipa (Poaceae) from SW Spain. Willdenowia 39: 261–264. http://dx.doi.org/10.3372/wi.39.39204 [Google Scholar]
- Wu ZL, Phillips SM. (2006) Tribe Stipae. In: Wu ZY, Raven PH, Hong DY. (Eds) Flora of China (Poaceae), 22. Science Press, Beijing and Missouri Botanical Garden Press, St. Louis, 188–212.
- Zhao LQ, Guo K. (2011) Stipa albasiensis (Poaceae), a new species from Inner Mongolia, China. Annales Botanici Fennici 48: 522–524. http://dx.doi.org/10.5735/085.048.0615 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.