Abstract
Perifibrillar adapter proteins, interconnecting collagen fibrils, and linking the collagen network with the aggrecan matrix seem to play a crucial role in the pathogenesis of osteoarthritis (OA). Therefore, we examined immunohistochemically the extracellular distribution of collagen II and the main perifibrillar adapter proteins—collagen IX, decorin, cartilage oligomeric matrix protein (COMP), and matrilin-3—in human samples of healthy (n=4) and OA (n=42) knee joint cartilage. Histopathology assessment was performed using an OA score. Staining patterns were evaluated in relation to the disease stage. The perifibrillar adapter proteins were uniformly distributed in the upper zones of healthy cartilage. In moderate OA (n=8; score 14.3 ± 4.7), all proteins analyzed were locally absent in the fibrillated area or the superficial and upper mid zone. In advanced OA (n=20; score 18.9 ± 5.3), they were uniformly distributed in these zones and accumulated pericellularly. Perifibrillar adapter proteins are important for the stabilization of the collagen network in the upper zones of healthy cartilage. Their degradation might be a critical event in early OA. In advanced OA, there are indications for an increased synthesis in an attempt to regenerate the lost tissue and to protect the remaining cartilage from further destruction.
Keywords: cartilage, collagen, COMP, decorin, extracellular matrix, immunohistological staining, knee joint, matrilin, osteoarthritis, perifibrillar adapter proteins
Introduction
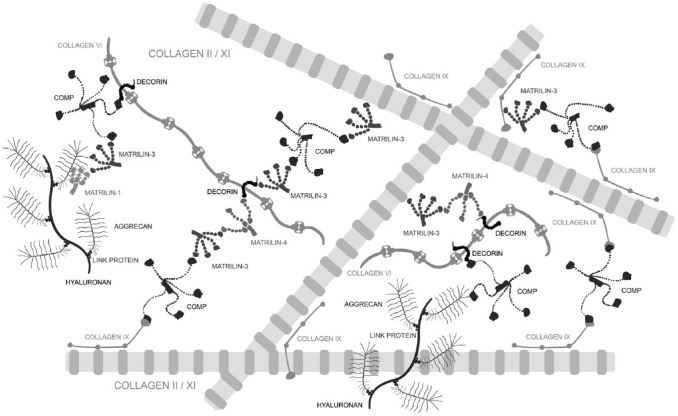
The extracellular matrix (ECM) of articular cartilage consists of two supramolecular compartments, the fibrillar collagen network and the extrafibrillar matrix, mainly composed of the proteoglycan aggrecan.1 The collagen network provides tensile strength for cartilage tissue, and it further restricts the swelling of the tissue caused by the high water binding capacity of aggrecan. The resulting swelling pressure makes the cartilage resistant against compression.2 The interface between the two compartments is constituted by a highly complex fibrillar periphery.1 Perifibrillar adapter proteins, including fibril associated collagens with interrupted triple helices (FACIT), small leucine-rich proteoglycans (SLRP), and other noncollagenous matrix proteins, interconnect the collagen fibrils and mediate interactions between the collagen network and the aggrecan matrix (Fig. 1).
Figure 1.
Schematic illustration of the supramolecular network formation (not to scale). Interactions between the collagenous fibrillar structures and the aggrecan matrix are mediated by the perifibrillar adapter proteins matrilin-1, -3, and -4,17 decorin,13 COMP,9 and collagen IX.8 The adapter molecules interconnect collagen II/XI fibrils and link them to collagen VI microfibrils70 or aggrecan.13,16 Modified from Klatt et al.17 Abbreviation: COMP, cartilage oligomeric matrix protein.
Collagen fibrils, consisting of fibril-forming collagens II (>90%) and XI (~3%), are decorated with collagen IX (~1%), a member of the FACIT collagens.3 The N-terminal short arm of collagen IX projects into the perifibrillar space and, thus, provides binding sites for other matrix proteins while its long arm, oriented parallel to the fibril, allows covalent interactions with collagen II as well as other collagen IX molecules.4–8 Matrilins and the cartilage oligomeric matrix protein (COMP) are members of noncollagenous matrix protein families. In immature cartilage, the pentameric glycoprotein COMP9,10 catalyzes collagen fibril formation11 while in adult cartilage with little collagen fibrillogenesis, it primarily seems to cross-link and stabilize the collagen network12,13 through binding to the mature collagen fibril via collagen IX,14 matrilin-3, and -4.4,15 In addition, COMP interconnects the collagen network with the extrafibrillar matrix by binding to aggrecan.16 Matrilin-3, a homotetramer, forms complexes or even heterooligomers with matrilin-1 and -4 that are linked via the SLRPs biglycan and decorin to collagen VI microfibrils.17 While the core protein of decorin is associated with collagen VI, its glycosaminoglycan side chain can interact with other extrafibrillar molecules.18–20
Perifibrillar adapter proteins are not only fundamental for the mechanical stabilization and interconnection of ECM components, but also for the regulation of the collagen fibril diameter during fibrillogenesis, thus playing a key role in proper collagen network formation.18,21–24 Therefore, it is not surprising that mutations or a lack of distinct perifibrillar adapter proteins cause a broad spectrum of skeletal disorders.1 A severe form of chondrodysplasia, the pseudoachondroplasia, is caused exclusively by mutations in COMP,25,26 whereas a milder form, the multiple epiphyseal dysplasia, has, in addition, been linked to mutations in collagen IX27,28 and matrilin-3.29 Patients with mutations in these genes often develop premature OA. In knockout models, collagen IX and matrilin-3 deficient mice have shown OA-like alterations with proteoglycan depletion and a loss of intact collagen II as well as a higher OA incidence and severity.30–32
In summary, perifibrillar adapter proteins are essential for healthy articular cartilage because they play a crucial role in proper collagen fibril formation, collagen network stabilization, and interconnection of the fibrillar and extrafibrillar matrix.
Concerning the pathogenesis of OA, several authors state that the characteristic swelling of cartilage appearing in early OA may rather result from degradation of molecules in the fibrillar periphery than the collagen network itself.13,33,34 However, current knowledge is limited to just a few studies that analyzed only a few perifibrillar adapter proteins in human articular cartilage. These studies observed an increased expression and altered extracellular distribution of adapter molecules in OA cartilage.35–41 To extend the understanding of OA, an important next step is to make coordinated observations of the distribution patterns of all participating perifibrillar adapter proteins according to stages of disease progression. To our knowledge, we are the first that analyzed simultaneously the extracellular distribution of four major perifibrillar adapter proteins, namely, collagen IX, decorin, matrilin-3, and COMP, and of collagen II, as primary component of the fibrillar matrix, in healthy and osteoarthritic human knee joint cartilage. For the first time, distribution patterns of collagen II and the four perifibrillar adapter proteins were described in OA cartilage and related to the disease stage by using an OA histopathology score.
Materials and Methods
Tissue Samples
A total of 42 cartilage samples from the tibial plateau and the underlying subchondral bone were collected from 11 OA patients undergoing total knee replacement (Table 1). Due to different surgical techniques, the number of suitable samples ranged from two to six per donor (mean: 3.8 ± 1.3).
Table 1.
Demographic and Anthropometric Variables of All OA Patients.
| N | Age (Years) | Body Mass (kg) | Body Height (m) | BMI (kg/m²) | |
|---|---|---|---|---|---|
| Females | 7 | 65.0 ± 7.6 (55–76) | 87.6 ± 17.6 (70–114) | 1.60 ± 0.05 (1.51–1.69) | 34.0 ± 5.8 (27.0–44.0) |
| Males | 4 | 73.0 ± 4.7 (69–78) | 88.8 ± 16.7 (64–100) | 1.77 ± 0.05 (1.70–1.80) | 28.2 ± 4.2 (22.1–31.2) |
| Total | 11 | 67.9 ± 7.6 (55–78) | 88.0 ± 16.4 (64–114) | 1.66 ± 0.10 (1.51–1.80) | 31.9 ± 5.8 (22.1–44.0) |
Values are presented as mean ± SD (min. – max.). Abbreviations: OA, osteoarthritis; BMI, body mass index; SD = standard deviation.
Four macroscopically normal tibial cartilage/bone samples were gained from a 24-year-old male accident victim (86.4 kg, 1.86 m, body mass index 24.9 kg/m2). The study was approved by the local ethics committee, and informed written consent was obtained from tissue donors. Samples were fixed in 4% paraformaldehyde (24 h), decalcified with 20% EDTA (6 weeks), embedded in paraffin, and cut in 10-µm-thick sections.
IHC
Endogenous peroxidase was blocked with methanol and 1% H2O2. For the detection of collagen II, the sections were digested with pepsin (0.5% in 0.01N Hydrochloric Acid; pH 1.85) for 1 h at RT while all other sections were incubated with chondroitinase (40 mU/ml in TBS containing 0.01% BSA) for 1 h at 37C. Afterward, the sections were blocked for 30 min with TBS containing 0.01% BSA. Sections were incubated with primary antibodies overnight at 4C. A mouse monoclonal antibody against collagen II (1:500; Calbiochem, Darmstadt, Germany) and rabbit polyclonal antibodies against collagen IX (α1/NC4; 1:1000),4 decorin (1:500),42 matrilin-3 (1:500),43 and COMP (1:1000)44 were used. Horseradish peroxidase conjugated secondary antibodies, a swine polyclonal antibody against rabbit IgG, and a rabbit polyclonal antibody against mouse IgG (both 1:100; Dako, Glostrup, Denmark) were applied for 1 h at RT. Finally, the sections were stained using 3-amino-9-ethylcarbazole (Sigma Aldrich, St. Louis, MI). To exclude unspecific binding of the secondary antibodies, control stainings were carried out without primary antibody (data not shown). In addition, we performed stainings of the not commercially primary antibodies using isotype controls and preimmunesera, respectively. All these controls were negative (Supplemental Fig. 1A). Furthermore, the specificity of not commercially available primary antibodies was validated on respective knockout extracts and/or tissue sections in earlier studies (supplemental Table 1A).
Osteoarthritis Research Society International (OARSI)-Score
The OARSI OA histopathology grading system was applied for all samples.45 Scoring was performed by two independent observers on Safranin-O stained sections that were made according to standard histochemical protocols. The score (0–24) is the product of the OA severity (six grades) and the horizontal extent of the involved cartilage surface (four stages). Values are presented as mean ± standard deviation (SD).
Histological Analysis
Sections were divided into a superficial, a mid, and a deep zone.46 In addition, an area that corresponded to grade 2 (surface discontinuity), grade 3 (vertical fissures), and grade 4 (erosion) of the OARSI histopathology assessment45 was determined as fibrillated area. The ECM was further divided into pericellular, territorial, and interterritorial regions.46 All analyses were conducted with a light microscope (Nikon ECLIPSE 80i, Nikon Instruments Inc., New York, NY).
Results
OA cartilage samples had a mean OA score of 17.5 ± 5.4. As expected, the score of the control samples (the “healthy samples”) was zero. If the distribution pattern of a protein appeared in more than 10 OA cartilage samples, it was designated as a staining pattern. In OA cartilage, three different staining patterns (I, II, and III) were classified for each of the five ECM proteins analyzed. These patterns are described below. The frequency of occurrence together with the mean OA score of the corresponding cartilage samples are summarized in Table 2. Beyond that, two general distribution patterns (I + II), simultaneously describing the staining patterns of all five ECM proteins, were detected. Please note that the Roman numerals of the staining patterns are just for their differentiation and that there is neither a correlation between the numerals and the OA score, nor between the numerals of the single and the general staining patterns. Furthermore, it has to be taken into account that dependent on the location, several samples showed more than one staining pattern.
Table 2.
Distribution Pattern I to III of Collagen II, Collagen IX, Decorin, COMP, and Matrilin-3 and the Frequency of Occurrence (N) Together With the OA Score (mean ± SD) of the Corresponding OA Cartilage Samples.
| OA Staining Patterns |
||||||
|---|---|---|---|---|---|---|
| I |
II |
III |
||||
| N | OA Score | N | OA Score | N | OA Score | |
| Collagen II | 28 | 16.6 ± 4.9 | 35 | 17.6 ± 4.9 | 32 | 17.9 ± 5.4 |
| Collagen IX | 18 | 16.4 ± 5.4 | 10 | 14.6 ± 4.2 | 38 | 17.8 ± 5.4 |
| Decorin | 17 | 17.1 ± 4.9 | 21 | 16.8 ± 5.7 | 19 | 18.3 ± 5.4 |
| COMP | 17 | 16.6 ± 5.0 | 20 | 15.8 ± 6.4 | 18 | 20.0 ± 3.6 |
| Matrilin-3 | 14 | 15.0 ± 3.6 | 20 | 15.2 ± 5.5 | 16 | 22.0 ± 2.5 |
Please note that several samples showed more than one staining pattern. Abbreviations: COMP, cartilage oligomeric matrix protein; OA, osteoarthritis; SD = standard deviation.
Collagen II
Healthy cartilage showed an even distribution of collagen II (Fig. 2A and E). In the mid and deep zone, the pericellular staining was locally pronounced. However, all zones also had areas with a lack of pericellular staining.
Figure 2.
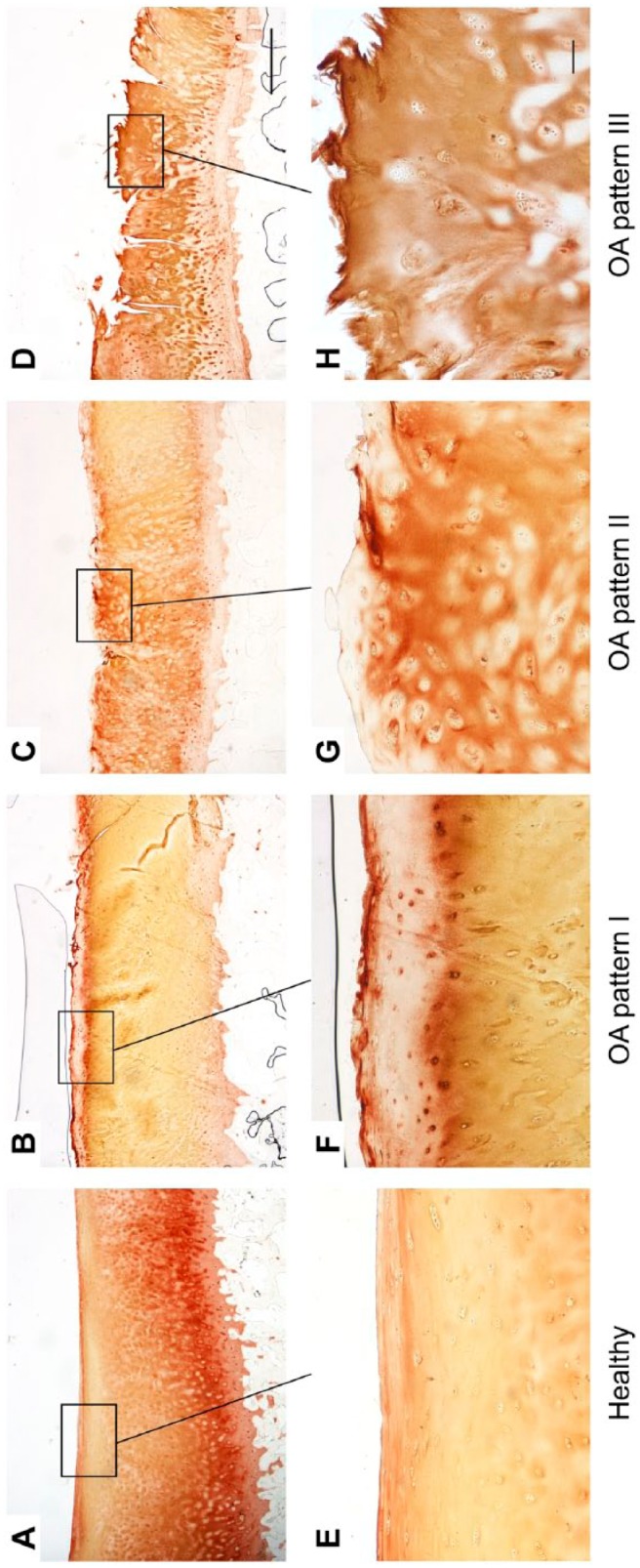
Immunohistological staining patterns of collagen II in healthy (A, E) and OA cartilage (B–D, F–H). Long bar = 1000 µm, short bar = 100 µm. Abbreviation: OA, osteoarthritis.
In OA pattern I, collagen II was locally absent in the superficial zone and fibrillated area, respectively (Fig. 2B and F). OA pattern II showed a uniform collagen II staining in the fibrillated area, superficial, and upper mid zone with a lack of pericellular staining (Fig. 2C and G). In OA pattern III, collagen II was detectable in the fibrillated area or superficial and upper mid zone while in the lower mid and deep zone, there was only a pericellular staining, and almost no staining in the interterritorial matrix (Fig. 2D and H).
Collagen IX
Healthy cartilage showed a uniform staining in the superficial and partly in the upper mid zone with a locally increased pericellular staining (Fig. 3A and E). In the mid zone, collagen IX was located in the pericellular matrix.
Figure 3.
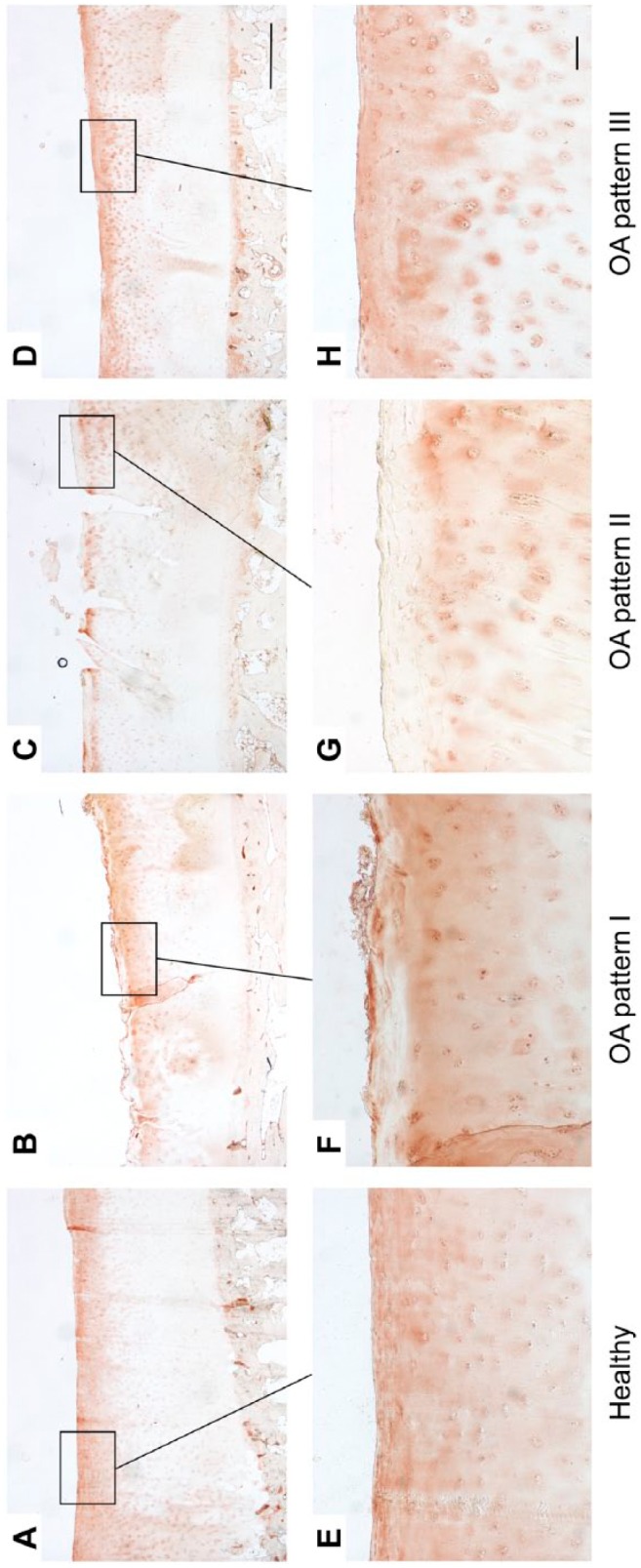
Immunohistological staining patterns of collagen IX in healthy (A, E) and OA cartilage (B–D, F–H). Long bar = 1000 µm, short bar = 100 µm. Abbreviation: OA, osteoarthritis.
OA pattern I was characterized by a lack of collagen IX in the superficial zone and fibrillated area, respectively (Fig. 3B and F). Characteristic for OA pattern II was a collagen IX staining that was exclusively detectable in the pericellular matrix in the superficial and upper mid zone or fibrillated area (Fig. 3C and G). OA pattern III showed a collagen IX staining in the superficial and upper mid zone or fibrillated area that was in part increased in the pericellular matrix. In the lower zones, the staining was limited to the pericellular matrix (Fig. 3D and H).
Decorin
In healthy cartilage, decorin was localized in the superficial and upper mid zone (Fig. 4A and E). In the upper mid zone, the staining in the pericellular matrix was locally increased while it was missing in the interterritorial matrix. Two of four samples also showed a slight staining in the deep zone.
Figure 4.
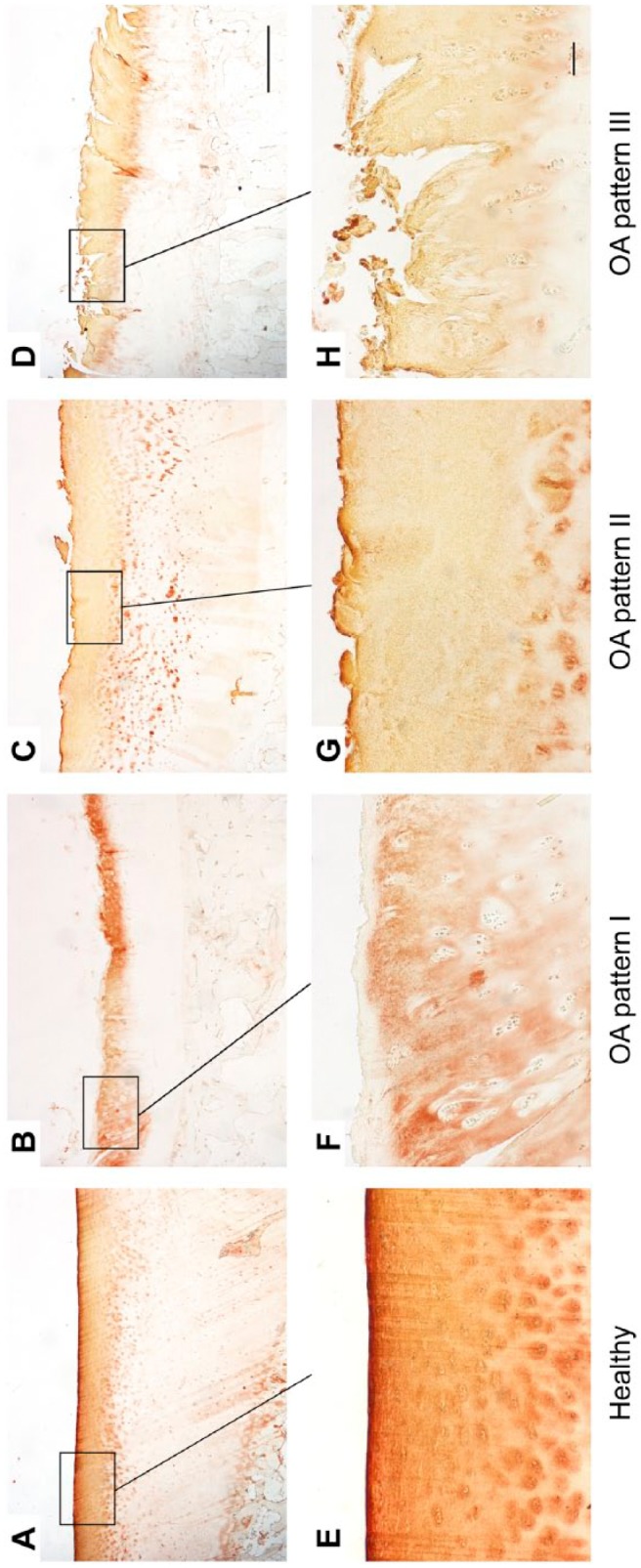
Immunohistological staining patterns of decorin in healthy (A, E) and OA cartilage (B–D, F–H). Long bar = 1000 µm, short bar = 100 µm. Abbreviation: OA, osteoarthritis.
In OA pattern I, decorin was absent in the superficial zone and fibrillated area, respectively (Fig. 4B and F). OA pattern II was characterized by a uniform decorin staining in the fibrillated area, superficial and upper mid zone, and a pericellular staining in the lower zones (Fig. 4C and G). OA pattern III was similar to pattern II, but there was no staining of decorin in the lower mid and deep zone (Fig. 4D and H).
COMP
In healthy cartilage, COMP was detectable in the superficial and upper mid zone (Fig. 5A and E). Compared with the interterritorial and territorial matrix, the staining in the pericellular matrix was sometimes slightly increased.
Figure 5.
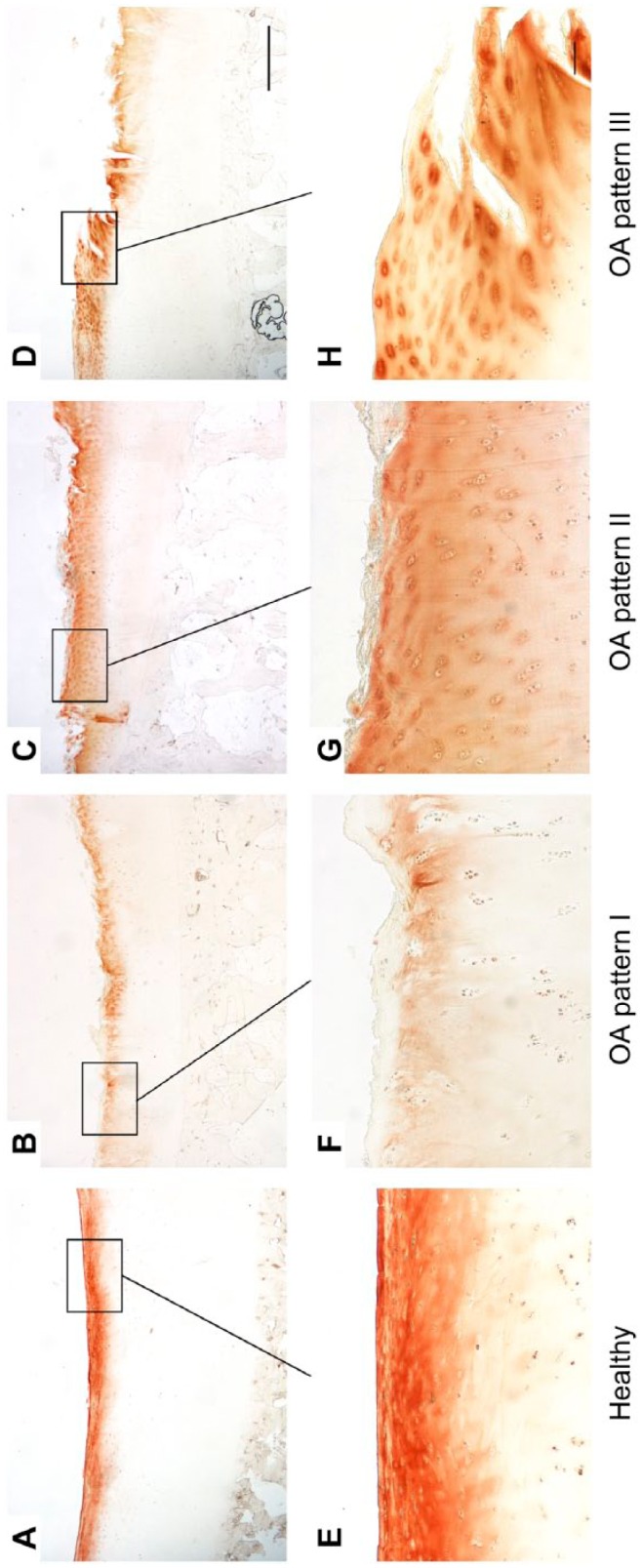
Immunohistological staining patterns of COMP in healthy (A, E) and OA cartilage (B–D, F–H). Long bar = 1000 µm, short bar = 100 µm. Abbreviations: COMP, cartilage oligomeric matrix protein; OA, osteoarthritis.
In OA cartilage, COMP could be detected in the fibrillated area, superficial, and upper mid zone. In OA pattern I, there was a lack of COMP in the superficial zone and fibrillated area, respectively (Fig. 5B and F). Contrary to healthy cartilage, OA pattern II was characterized by a reduced or no staining of COMP in the interterritorial matrix and a pericellular accumulation of COMP (Fig. 5C and G). OA pattern III was similar to pattern II but the pericellular staining was much more pronounced (Fig. 5D and H). In all samples, there was no staining in the deeper zones.
Matrilin-3
In healthy cartilage, a uniform staining in the superficial and upper mid zone and, in part, a pericellular staining that extended to the mid zone could be observed (Fig. 6A and E). In two of four samples, the staining in the superficial zone was locally reduced.
Figure 6.
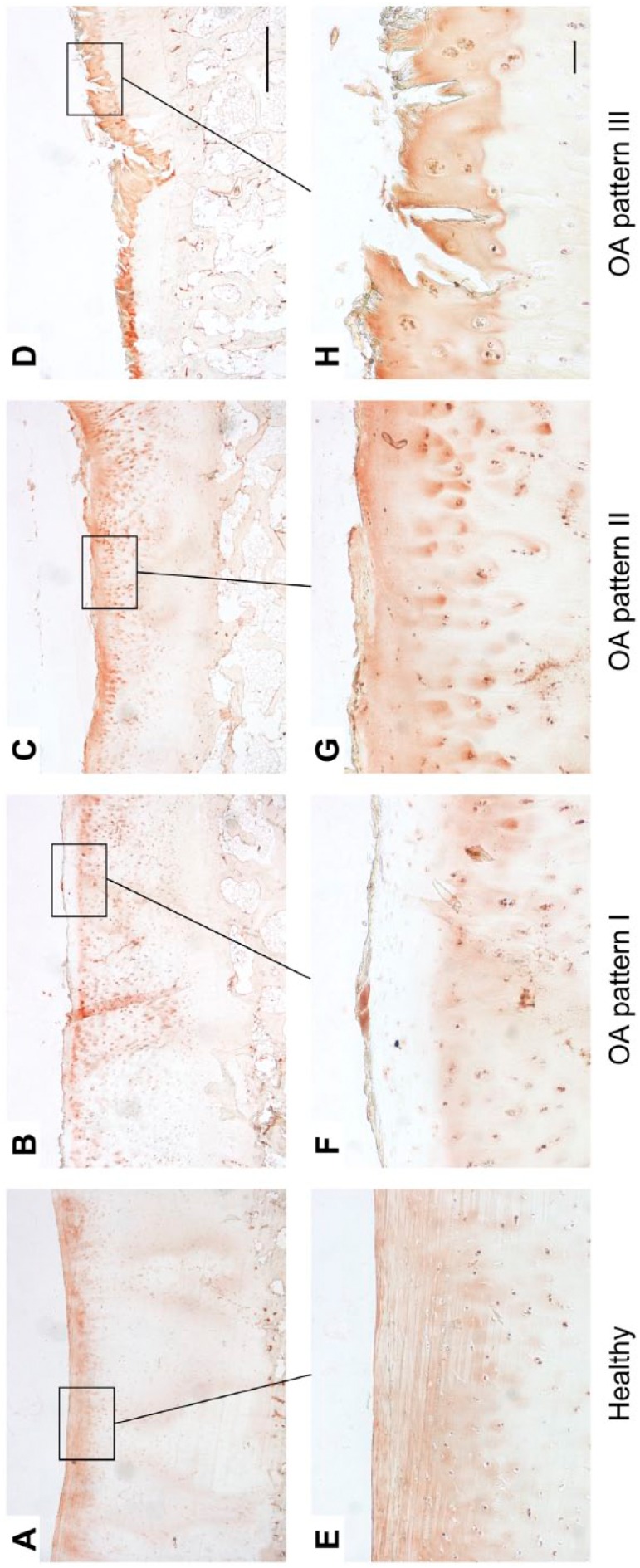
Immunohistological staining patterns of matrilin-3 in healthy (A, E) and OA cartilage (B–D, F–H). Long bar = 1000 µm, short bar = 100 µm. Abbreviation: OA, osteoarthritis.
OA pattern I was characterized by a loss of staining in the superficial zone and fibrillated area, respectively (Fig. 6B and F). In the lower zones, a matrilin-3 staining was found in the interterritorial, territorial, and pericellular matrix with the latter showing the most intense staining. OA pattern II showed a uniform staining of matrilin-3 in the superficial zone and fibrillated area as well as an increased pericellular staining in the lower zones (Fig. 6C and G). In OA pattern III, matrilin-3 was located in the fibrillated area and the staining in the pericellular matrix was increased (Fig. 6D and H).
General Distribution Patterns
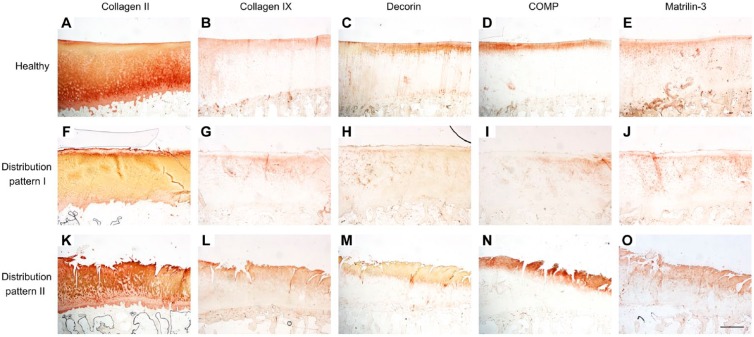
To clarify the changes in the extracellular distribution of the analyzed proteins that occur in parallel in OA cartilage, the staining patterns of all proteins were compared, and two general distribution patterns could be described in 28 OA cartilage samples. In the remaining 14 OA samples, the staining patterns of the five proteins analyzed could not be assigned consistently to a distinct distribution pattern.
Distribution pattern I was found in eight OA cartilage samples (OA score 14.3 ± 4.7; Fig. 7F–J). In contrast with healthy cartilage (Fig. 7A–E), this pattern was characterized by a local distinct lack of collagen II and IX, decorin, matrilin-3, and COMP in the fibrillated area, superficial, and upper mid zone.
Figure 7.
Distribution of collagen II, collagen IX, decorin, COMP, and matrilin-3 in healthy (A–E) and OA cartilage according to pattern I (F–J) and II (K–O). The microscopic images (magnification × 20, bar = 1000 µm) for each of the three distribution patterns originate from the same samples and, thus, same location on the tibia. Abbreviations: COMP, cartilage oligomeric matrix protein; OA, osteoarthritis.
Distribution pattern II was found in 20 OA cartilage samples (OA score 18.9 ± 5.3; Fig. 7K–O). It was characterized by a uniform collagen II staining in the fibrillated area, superficial, and upper mid zone with a lack of pericellular staining. In the deep and lower mid zone, collagen II was localized in the pericellular matrix, whereas it was absent interterritorially. In the fibrillated area, superficial, and upper mid zone, all four perifibrillar adapter proteins were distributed uniformly. In addition, collagen IX, matrilin-3, and COMP showed a partly increased pericellular staining in these zones. In the lower zones, the staining of collagen IX, matrilin-3, and decorin was limited to the pericellular matrix.
Discussion
Perifibrillar adapter proteins are of great importance for proper collagen fibril formation, collagen network stabilization, and interconnection of the fibrillar and extrafibrillar matrix in articular cartilage. Although they seem to play a crucial role in the pathogenesis of OA, studies analyzing their localization in human articular cartilage during the progression of OA are rare. For the first time, we analyzed in the same sample the extracellular distribution of collagen II as main representative of the fibrillar matrix and of collagen IX, decorin, COMP, and matrilin-3 as main perifibrillar adapter proteins in healthy and osteoarthritic human knee joint cartilage. We identified specific distribution patterns for each of these proteins, and show a correlation with defined disease stages using an OA score.
In healthy cartilage, the perifibrillar adapter proteins were localized exclusively in the upper zones, whereas collagen II could be detected across all zones and in every matrix compartment. This is in accordance with previous studies that described the localization of COMP,36 matrilin-3,37 and collagen II47 in healthy human articular cartilage. However, Koelling et al.35 reported that collagen IX was found throughout all cartilage layers in healthy articular cartilage. The authors applied a monoclonal antibody D1-9 against the α1 chain of collagen IX whereas in our study a rabbit polyclonal antibody against collagen IX (α1/NC4) was used. This apparent discrepancy might be explained by the fact that the NC4 domain of collagen IX could be released from cartilage by matrix metalloproteinase-13.48 The tangential collagen fibrils in the superficial zone have to resist tensile and shear stresses. They transfer compressive loads from directly loaded areas to adjacent tissue and, thus, recruit a larger area of cartilage in the deep zone to carry compressive loads.49–51 Perifibrillar adapter proteins are not only vital for proper formation and stabilization of the collagen network in the upper zones of articular cartilage, but also seem to be of high importance for the mechanical functionality of cartilage as a whole.31,52
Compared with healthy cartilage, all proteins analyzed showed distinct alterations in their extracellular distribution in OA cartilage, especially collagen II and COMP. A pericellular loss of collagen II in the fibrillated area and upper mid zone could be identified as the most common feature confirming previous studies.47,53 Chondrocytes can release collagenases and, therefore, might be responsible for the destruction of collagen II in the pericellular matrix themselves.54,55 As indicated by COMP pattern II and III, showing a pericellular accumulation of the protein, it is also possible that too high COMP concentrations relative to collagen II caused the pericellular lack of collagen. COMP catalyzes the fibrillogenesis by binding five collagen molecules at the same time and bringing them in close proximity.11 However, in case of too high concentrations, COMP can act as an inhibitor by saturation of sites with single molecules precluding cross-bridging and, thus, may lead to a defective fibrillogenesis and impaired repair.12 The distribution patterns of collagen II and COMP provided first indications of a mutual interference of the individual ECM proteins. In addition, we could identify three main staining patterns for each of the perifibrillar adapter proteins supporting the notion that OA is not a uniform disease entity. OA is more an end-stage description of various joint degeneration phenotypes with most likely slightly different pathomechanisms. As the disease is multifactorial, the complex and diverse pathomechanisms might strongly depend on the individual patient and their genetic, mechanical, and/or injury history. However, the detailed description of these varying pathomechanisms is essential for the development of new diagnostic and treatment options. Future experiments should, for example, analyze if the staining patterns correlate with biomarkers of cartilage metabolism in blood, urine, or synovial fluid. This would help to validate biomarkers for early diagnosis and progress of OA in personalized therapeutic approaches.
However, coordinated observations of the distribution patterns of all participating perifibrillar adapter proteins according to stages of disease progression have still not been carried out. In our study, we were able to describe two general distribution patterns of all analyzed perifibrillar adapter proteins that provide indications of important events in the course of disease.
With a mean OA score of 14.3 ± 4.7, lying in the middle third of the total score range, distribution pattern I can be seen as typical for moderate OA. The key characteristic of this pattern was a local lack of all proteins analyzed in the fibrillated area, superficial, and upper mid zone. Studies analyzing collagen II revealed that degradation and cleavage is initiated around chondrocytes near the articular surface, extending with progressive disease to the territorial and interterritorial matrix and the lower zones.53–55 Distribution pattern I confirms the observation that tissue degradation starts near the articular surface and, in addition, shows that besides collagen II, also the molecules in the fibrillar periphery are depleted. There are indications that the depletion of one adapter molecule has effects on its binding partners. The association of matrilin-3 with collagen fibrils depends on the presence of collagen IX and to some extent of COMP.4 Thus, a lack of collagen IX leads to a dramatic reduction in COMP and matrilin-3.56 Although we cannot draw any conclusions about the exact time course of ECM degradation, it is very likely that in our OA cartilage samples, the perifibrillar adapter proteins were depleted first. Studies inducing tissue breakdown by factors triggering chondrocytes to secrete proteolytic enzymes have shown an initial degradation of aggrecan, followed by fragmentation of molecules such as COMP and later collagen IX, and a final depletion of the collagen network.12,34,48,57 A loss of perifibrillar adapter proteins might lead to a reduced mechanical stabilization of the collagen network in the upper zones and, thus, to an impaired functionality of the cartilage as a whole. This, in turn, could alter the loading of the chondrocytes, increase chondrocyte death, and diminish the capacity of cartilage to maintain and repair the ECM.13,58–60 In addition, distribution pattern I provides clear indications that in early or moderate OA, a marked degradation of the proteins analyzed took place in the upper zones of human knee joint cartilage. In this way, our study supports the approach to use molecules such as COMP,61 matrilin-3,62 collagen IX,48 and collagen II63 that are degraded in OA and whose fragments can be assayed in body fluids such as synovial fluid, blood, or urine, as biomarkers of cartilage metabolism.12 In conclusion, the loss of perifibrillar adapter proteins in the upper zones of articular cartilage seems to be a critical event in early OA.
With a mean OA score of 18.9 ± 5.3, distribution pattern II can be seen as a characteristic pattern for advanced OA. The main feature was a uniform distribution of all proteins analyzed in the fibrillated area. This is an interesting observation because distribution pattern II includes samples with advanced OA that are characterized by erosion of cartilage,45 and, thus, perifibrillar adapter proteins were detectable in zones in which their evidence in healthy cartilage was missing. This might be explained by the mechanosensitivity of chondrocytes, enabling them to remodel the ECM in response to altered mechanical loading.60,64,65 A loss of perifibrillar adapter proteins in the upper zones of cartilage, as shown in distribution pattern I, and, thus, a change in the mechanical properties of the cartilage in these zones could lead to such an altered loading environment and consequently changed biosynthetic activity of the chondrocytes, explaining the unusual occurrence of perifibrillar adapter proteins in the lower zones of cartilage. The pericellular accumulation of all proteins analyzed in distribution pattern II provides further evidence for an increased anabolic activity. Several authors could show up to five times higher mRNA levels of decorin, collagen IX, and COMP in areas adjacent to the main defect compared with macroscopically intact areas35,36,38 and significantly increased matrilin-3 mRNA levels in cartilage with severe compared with minor OA.37 Increased collagen II mRNA levels66–68 and an elevated content of type II procollagen in the mid and deep zone of OA cartilage69 were found. In conclusion, distribution pattern II indicates an increased anabolic activity of the chondrocytes in the remaining cartilage in advanced OA. This might be seen as an attempt to regenerate the lost tissue and to protect the remaining cartilage from further destruction.
Nevertheless, the current study also has limitations. We did not analyze the protein amounts (e.g., western blotting) or expression levels (e.g., PCR) of the perifibrillar adapter proteins, which could give further important insight into the relationship between catabolic and anabolic reactions or reexpression of cartilage proteins. The immunohistological analysis can only provide information about the status quo regarding the localization of distinct proteins so that we can merely speculate on prior pathological processes. In addition, we cannot draw any conclusions if the immunohistochemically stained proteins are intact, full length, and/or partially degraded. Due to the difficulty to get healthy human articular cartilage, we had a limited number of control samples.
In summary, we detected a uniform distribution of perifibrillar adapter proteins in the superficial and upper mid zone of healthy, mature human knee joint cartilage. This confirms their importance in the mechanical stabilization of the collagen network in the upper zones. In moderate OA, this integrity and, thus, functionality seem to be impaired due to a local lack of the collagen network and the molecules in the fibrillar periphery in the fibrillated area, superficial, and upper mid zone. In cartilage with advanced OA, there are indications for an increased anabolic activity. This could result from an altered loading of the chondrocytes due to the loss of integrity in the upper zones, and it might be seen as an attempt to restore the lost tissue or to stabilize and protect the remaining cartilage from further destruction.
Supplementary Material
Acknowledgments
We gratefully acknowledge Joachim Schmidt for recruiting participants and collecting samples. We would also like to thank Mark Sander for realizing Figure 1; Nina Hamann and Cynthia H. Fantini Pagani for their help in sample preparation; Nina Hamann, Judith Bleuel, and Elena Nebot for their assistance in histochemical analyses; Christiane Gonska for her help with the microscopic analyses; and Sean Walsh for proofreading.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: AN, FZ, G-PB, and SF designed the study. PE, JM, JD, MAR, and K-HS-B recruited participants, collected samples, and made substantial contributions to the acquisition of data. SF performed the analyses and drafted the manuscript. AN, FZ, G-PB, JH, and SF contributed to the manuscript. All authors read and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by a grant from the European Community’s Seventh Framework Programme under grant agreement no. 602300 (SYBIL) to FZ.
Contributor Information
Sara Firner, Institute of Biomechanics and Orthopaedics, German Sport University Cologne, Cologne, Germany.
Frank Zaucke, Dr. Rolf M. Schwiete Research Unit for Osteoarthritis, Orthopaedic University Hospital Friedrichsheim gGmbH, Frankfurt, Germany; Cologne Center for Musculoskeletal Biomechanics (CCMB), Medical Faculty, University of Cologne, Cologne, Germany.
Joern Michael, Department of Orthopaedic and Trauma Surgery, University Hospital Cologne, Cologne, Germany.
Jens Dargel, Department of Orthopaedic and Trauma Surgery, University Hospital Cologne, Cologne, Germany.
Karl-Heinz Schiwy-Bochat, Institute of Legal Medicine, Medical Faculty, University of Cologne, Cologne, Germany.
Juliane Heilig, Cologne Center for Musculoskeletal Biomechanics (CCMB), Medical Faculty, University of Cologne, Cologne, Germany.
Markus Alexander Rothschild, Institute of Legal Medicine, Medical Faculty, University of Cologne, Cologne, Germany.
Peer Eysel, Department of Orthopaedic and Trauma Surgery, University Hospital Cologne, Cologne, Germany; Cologne Center for Musculoskeletal Biomechanics (CCMB), Medical Faculty, University of Cologne, Cologne, Germany.
Gert-Peter Brüggemann, Institute of Biomechanics and Orthopaedics, German Sport University Cologne, Cologne, Germany; Cologne Center for Musculoskeletal Biomechanics (CCMB), Medical Faculty, University of Cologne, Cologne, Germany.
Anja Niehoff, Institute of Biomechanics and Orthopaedics, German Sport University Cologne, Cologne, Germany; Cologne Center for Musculoskeletal Biomechanics (CCMB), Medical Faculty, University of Cologne, Cologne, Germany.
Literature Cited
- 1. Zaucke F, Grassel S. Genetic mouse models for the functional analysis of the perifibrillar components collagen IX, COMP and matrilin-3: implications for growth cartilage differentiation and endochondral ossification. Histol Histopathol. 2009;24(8):1067–79. [DOI] [PubMed] [Google Scholar]
- 2. Poole AR, Kobayashi M, Yasuda T, Laverty S, Mwale F, Kojima T, Sakai T, Wahl C, El-Maadawy S, Webb G, Tchetina E, Wu W. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann Rheum Dis. 2002;61(Suppl. 2):ii78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eyre DR, Weis MA, Wu J. Articular cartilage collagen: an irreplaceable framework? Eur Cell Mater. 2006;12:57–63. [DOI] [PubMed] [Google Scholar]
- 4. Budde B, Blumbach K, Ylostalo J, Zaucke F, Ehlen HWA, Wagener R, Ala-Kokko L, Paulsson M, Bruckner P, Grassel S. Altered integration of matrilin-3 into cartilage extracellular matrix in the absence of collagen IX. Mol Cell Biol. 2005;25(23):10465–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pihlajamaa T, Lankinen H, Ylostalo J, Valmu L, Jaalinoja J, Zaucke F, Spitznagel L, Gosling S, Puustinen A, Mörgelin M, Peranen J, Maurer P, Ala-Kokko L, Kilpelainen I. Characterization of recombinant amino-terminal NC4 domain of human collagen IX: interaction with glycosaminoglycans and cartilage oligomeric matrix protein. J Biol Chem. 2004;279(23):24265–73. [DOI] [PubMed] [Google Scholar]
- 6. Eyre DR, Pietka T, Weis MA, Wu J. Covalent cross-linking of the NC1 domain of collagen type IX to collagen type II in cartilage. J Biol Chem. 2004;279(4):2568–74. [DOI] [PubMed] [Google Scholar]
- 7. Olsen BR. Collagen IX. Int J Biochem Cell Biol. 1997;29(4):555–8. [DOI] [PubMed] [Google Scholar]
- 8. Wu JJ, Woods PE, Eyre DR. Identification of cross-linking sites in bovine cartilage type IX collagen reveals an antiparallel type II-type IX molecular relationship and type IX to type IX bonding. J Biol Chem. 1992;267(32):23007–14. [PubMed] [Google Scholar]
- 9. Mörgelin M, Heinegard D, Engel J, Paulsson M. Electron microscopy of native cartilage oligomeric matrix protein purified from the Swarm rat chondrosarcoma reveals a five-armed structure. J Biol Chem. 1992;267(9):6137–41. [PubMed] [Google Scholar]
- 10. Hedbom E, Antonsson P, Hjerpe A, Aeschlimann D, Paulsson M, Rosa-Pimentel E, Sommarin Y, Wendel M, Oldberg A, Heinegard D. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem. 1992;267(9):6132–6. [PubMed] [Google Scholar]
- 11. Halasz K, Kassner A, Mörgelin M, Heinegard D. COMP acts as a catalyst in collagen fibrillogenesis. J Biol Chem. 2007;282(43):31166–73. [DOI] [PubMed] [Google Scholar]
- 12. Heinegard D. Fell-Muir lecture: proteoglycans and more—from molecules to biology. Int J Exp Pathol. 2009;90(6):575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heinegard D, Bayliss M, Lorenzo P. Biochemistry and metabolism of normal and osteoarthritic cartilage. In: Brandt KD, Doherty M, Lohmander S, editors. Osteoarthritis. Oxford: Oxford University Press; 1998. p. 74–84. [Google Scholar]
- 14. Holden P, Meadows RS, Chapman KL, Grant ME, Kadler KE, Briggs MD. Cartilage oligomeric matrix protein interacts with type IX collagen, and disruptions to these interactions identify a pathogenetic mechanism in a bone dysplasia family. J Biol Chem. 2001;276(8):6046–55. [DOI] [PubMed] [Google Scholar]
- 15. Mann HH, Ozbek S, Engel J, Paulsson M, Wagener R. Interactions between the cartilage oligomeric matrix protein and matrilins. Implications for matrix assembly and the pathogenesis of chondrodysplasias. J Biol Chem. 2004;279(24):25294–8. [DOI] [PubMed] [Google Scholar]
- 16. Chen FH, Herndon ME, Patel N, Hecht JT, Tuan RS, Lawler J. Interaction of cartilage oligomeric matrix protein/thrombospondin 5 with aggrecan. J Biol Chem. 2007;282(34):24591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klatt AR, Becker AA, Neacsu CD, Paulsson M, Wagener R. The matrilins: modulators of extracellular matrix assembly. Int J Biochem Cell Biol. 2011;43(3):320–30. [DOI] [PubMed] [Google Scholar]
- 18. Hagg R, Bruckner P, Hedbom E. Cartilage fibrils of mammals are biochemically heterogeneous: differential distribution of decorin and collagen IX. J Cell Biol. 1998;142(1):285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wiberg C, Klatt AR, Wagener R, Paulsson M, Bateman JF, Heinegard D, Mörgelin M. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J Biol Chem. 2003;278(39):37698–704. [DOI] [PubMed] [Google Scholar]
- 20. Bidanset DJ, Guidry C, Rosenberg LC, Choi HU, Timpl R, Hook M. Binding of the proteoglycan decorin to collagen type VI. J Biol Chem. 1992;267(8):5250–6. [PubMed] [Google Scholar]
- 21. Bastiaansen-Jenniskens YM, de Bart ACW, Koevoet W, Jansen KMB, Verhaar JAN, van Osch GJVM, DeGroot J. Elevated levels of cartilage oligomeric matrix protein during in vitro cartilage matrix generation decrease collagen fibril diameter. Cartilage. 2010;1(3):200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nicolae C, Ko Y, Miosge N, Niehoff A, Studer D, Enggist L, Hunziker EB, Paulsson M, Wagener R, Aszodi A. Abnormal collagen fibrils in cartilage of matrilin-1/matrilin-3-deficient mice. J Biol Chem. 2007;282(30):22163–75. [DOI] [PubMed] [Google Scholar]
- 23. Blumbach K, Bastiaansen-Jenniskens YM, DeGroot J, Paulsson M, van Osch GJVM, Zaucke F. Combined role of type IX collagen and cartilage oligomeric matrix protein in cartilage matrix assembly: cartilage oligomeric matrix protein counteracts type IX collagen-induced limitation of cartilage collagen fibril growth in mouse chondrocyte cultures. Arthritis Rheum. 2009;60(12):3676–85. [DOI] [PubMed] [Google Scholar]
- 24. Rühland C, Schönherr E, Robenek H, Hansen U, Iozzo RV, Bruckner P, Seidler DG. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis. FEBS J. 2007;274(16):4246–55. [DOI] [PubMed] [Google Scholar]
- 25. Hecht JT, Nelson LD, Crowder E, Wang Y, Elder FF, Harrison WR, Francomano CA, Prange CK, Lennon GG, Deere M. Mutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia. Nat Genet. 1995;10(3):325–9. [DOI] [PubMed] [Google Scholar]
- 26. Briggs MD, Hoffman SM, King LM, Olsen AS, Mohrenweiser H, Leroy JG, Mortier GR, Rimoin DL, Lachman RS, Gaines ES. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat Genet. 1995;10(3):330–6. [DOI] [PubMed] [Google Scholar]
- 27. Czarny-Ratajczak M, Lohiniva J, Rogala P, Kozlowski K, Perala M, Carter L, Spector TD, Kolodziej L, Seppanen U, Glazar R, Krolewski J, Latos-Bielenska A, Ala-Kokko L. A mutation in COL9A1 causes multiple epiphyseal dysplasia: further evidence for locus heterogeneity. Am J Hum Genet. 2001;69(5):969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muragaki Y, Mariman EC, van Beersum SE, Perala M, van Mourik JB, Warman ML, Olsen BR, Hamel BC. A mutation in the gene encoding the alpha 2 chain of the fibril-associated collagen IX, COL9A2, causes multiple epiphyseal dysplasia (EDM2). Nat Genet. 1996;12(1):103–5. [DOI] [PubMed] [Google Scholar]
- 29. Chapman KL, Mortier GR, Chapman K, Loughlin J, Grant ME, Briggs MD. Mutations in the region encoding the von Willebrand factor A domain of matrilin-3 are associated with multiple epiphyseal dysplasia. Nat Genet. 2001;28(4):393–6. [DOI] [PubMed] [Google Scholar]
- 30. van der Weyden L, Wei L, Luo J, Yang X, Birk DE, Adams DJ, Bradley A, Chen Q. Functional knockout of the matrilin-3 gene causes premature chondrocyte maturation to hypertrophy and increases bone mineral density and osteoarthritis. Am J Pathol. 2006;169(2):515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu K, Xu L, Cao L, Flahiff CM, Brussiau J, Ho K, Setton LA, Youn I, Guilak F, Olsen BR, Li Y. Pathogenesis of osteoarthritis-like changes in the joints of mice deficient in type IX collagen. Arthritis Rheum. 2006;54(9):2891–900. [DOI] [PubMed] [Google Scholar]
- 32. Fassler R, Schnegelsberg PN, Dausman J, Shinya T, Muragaki Y, McCarthy MT, Olsen BR, Jaenisch R. Mice lacking alpha 1 (IX) collagen develop noninflammatory degenerative joint disease. Proc Natl Acad Sci U S A. 1994;91(11):5070–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hosseini SM, Veldink MB, Ito K, van Donkelaar CC. Is collagen fiber damage the cause of early softening in articular cartilage? Osteoarthritis Cartilage. 2013;21(1):136–43. [DOI] [PubMed] [Google Scholar]
- 34. Heathfield TF, Onnerfjord P, Dahlberg L, Heinegård D. Cleavage of fibromodulin in cartilage explants involves removal of the N-terminal tyrosine sulfate-rich region by proteolysis at a site that is sensitive to matrix metalloproteinase-13. J Biol Chem. 2004;279(8):6286–95. [DOI] [PubMed] [Google Scholar]
- 35. Koelling S, Kruegel J, Klinger M, Schultz W, Miosge N. Collagen IX in weight-bearing areas of human articular cartilage in late stages of osteoarthritis. Arch Orthop Trauma Surg. 2008;128(12):1453–9. [DOI] [PubMed] [Google Scholar]
- 36. Koelling S, Clauditz TS, Kaste M, Miosge N. Cartilage oligomeric matrix protein is involved in human limb development and in the pathogenesis of osteoarthritis. Arthritis Res Ther. 2006;8(3):R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pullig O, Weseloh G, Klatt AR, Wagener R, Swoboda B. Matrilin-3 in human articular cartilage: increased expression in osteoarthritis. Osteoarthritis Cartilage. 2002;10(4):253–63. [DOI] [PubMed] [Google Scholar]
- 38. Bock HC, Michaeli P, Bode C, Schultz W, Kresse H, Herken R, Miosge N. The small proteoglycans decorin and biglycan in human articular cartilage of late-stage osteoarthritis. Osteoarthritis Cartilage. 2001;9(7):654–63. [DOI] [PubMed] [Google Scholar]
- 39. Cs-Szabó G, Melching LI, Roughley PJ, Glant TT. Changes in messenger RNA and protein levels of proteoglycans and link protein in human osteoarthritic cartilage samples. Arthritis Rheum. 1997;40(6):1037–45. [DOI] [PubMed] [Google Scholar]
- 40. Poole AR, Rosenberg LC, Reiner A, Ionescu M, Bogoch E, Roughley PJ. Contents and distributions of the proteoglycans decorin and biglycan in normal and osteoarthritic human articular cartilage. J Orthop Res. 1996;14(5):681–9. [DOI] [PubMed] [Google Scholar]
- 41. Di Cesare PE, Carlson CS, Stolerman ES, Hauser N, Tulli H, Paulsson M. Increased degradation and altered tissue distribution of cartilage oligomeric matrix protein in human rheumatoid and osteoarthritic cartilage. J Orthop Res. 1996;14(6):946–55. [DOI] [PubMed] [Google Scholar]
- 42. Heinegard D, Bjorne-Persson A, Coster L, Franzen A, Gardell S, Malmstrom A, Paulsson M, Sandfalk R, Vogel K. The core proteins of large and small interstitial proteoglycans from various connective tissues form distinct subgroups. Biochem J. 1985;230(1):181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Klatt AR, Nitsche DP, Kobbe B, Mörgelin M, Paulsson M, Wagener R. Molecular structure and tissue distribution of matrilin-3, a filament-forming extracellular matrix protein expressed during skeletal development. J Biol Chem. 2000;275(6):3999–4006. [DOI] [PubMed] [Google Scholar]
- 44. DiCesare PE, Mörgelin M, Mann K, Paulsson M. Cartilage oligomeric matrix protein and thrombospondin 1. Purification from articular cartilage, electron microscopic structure, and chondrocyte binding. Eur J Biochem. 1994;223(3):927–37. [DOI] [PubMed] [Google Scholar]
- 45. Pritzker KPH, Gay S, Jimenez SA, Ostergaard K, Pelletier J, Revell PA, Salter D, van den Berg WB. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. [DOI] [PubMed] [Google Scholar]
- 46. Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dodge GR, Poole AR. Immunohistochemical detection and immunochemical analysis of type II collagen degradation in human normal, rheumatoid, and osteoarthritic articular cartilages and in explants of bovine articular cartilage cultured with interleukin 1. J Clin Invest. 1989;83(2):647–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Danfelter M, Onnerfjord P, Heinegard D. Fragmentation of proteins in cartilage treated with interleukin-1: specific cleavage of type IX collagen by matrix metalloproteinase 13 releases the NC4 domain. J Biol Chem. 2007;282(51):36933–41. [DOI] [PubMed] [Google Scholar]
- 49. Hosseini SM, Wu Y, Ito K, van Donkelaar CC. The importance of superficial collagen fibrils for the function of articular cartilage. Biomech Model Mechanobiol. 2014;13(1):41–51. [DOI] [PubMed] [Google Scholar]
- 50. Bevill SL, Thambyah A, Broom ND. New insights into the role of the superficial tangential zone in influencing the microstructural response of articular cartilage to compression. Osteoarthritis Cartilage. 2010;18(10):1310–8. [DOI] [PubMed] [Google Scholar]
- 51. Wong M, Carter DR. Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone. 2003;33(1):1–13. [DOI] [PubMed] [Google Scholar]
- 52. Kamper M, Hamann N, Prein C, Clausen-Schaumann H, Farkas Z, Aszodi A, Niehoff A, Paulsson M, Zaucke F. Early changes in morphology, bone mineral density and matrix composition of vertebrae lead to disc degeneration in aged collagen IX -/- mice. Matrix Biol. 2016;49:132–43. [DOI] [PubMed] [Google Scholar]
- 53. Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96(6):2859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dejica VM, Mort JS, Laverty S, Antoniou J, Zukor DJ, Tanzer M, Poole AR. Increased type II collagen cleavage by cathepsin K and collagenase activities with aging and osteoarthritis in human articular cartilage. Arthritis Res Ther. 2012;14(3):R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu W, Billinghurst RC, Pidoux I, Antoniou J, Zukor D, Tanzer M, Poole AR. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheum. 2002;46(8):2087–94. [DOI] [PubMed] [Google Scholar]
- 56. Brachvogel B, Zaucke F, Dave K, Norris EL, Stermann J, Dayakli M, Koch M, Gorman JJ, Bateman JF, Wilson R. Comparative proteomic analysis of normal and collagen IX null mouse cartilage reveals altered extracellular matrix composition and novel components of the collagen IX interactome. J Biol Chem. 2013;288(19):13481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Spirito S, Doughty J, O’Byrne E, Ganu V, Goldberg RL. Metalloprotease inhibitors halt collagen breakdown in IL-1 induced bovine nasal cartilage cultures. Inflamm Res. 1995;44(Suppl. 2):S131–2. [DOI] [PubMed] [Google Scholar]
- 58. Horisberger M, Fortuna R, Valderrabano V, Herzog W. Long-term repetitive mechanical loading of the knee joint by in vivo muscle stimulation accelerates cartilage degeneration and increases chondrocyte death in a rabbit model. Clin Biomech (Bristol, Avon). 2013;28(5):536–43. [DOI] [PubMed] [Google Scholar]
- 59. Hashimoto S, Takahashi K, Amiel D, Coutts RD, Lotz M. Chondrocyte apoptosis and nitric oxide production during experimentally induced osteoarthritis. Arthritis Rheum. 1998;41(7):1266–74. [DOI] [PubMed] [Google Scholar]
- 60. Bleuel J, Zaucke F, Brüggemann G, Niehoff A. Effects of cyclic tensile strain on chondrocyte metabolism: a systematic review. PLoS ONE. 2015;10(3):e0119816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Saxne T, Heinegard D. Cartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and blood. Br J Rheumatol. 1992;31(9):583–91. [DOI] [PubMed] [Google Scholar]
- 62. Vincourt J, Gillet P, Rat A, Guillemin F, Netter P, Mainard D, Magdalou J. Measurement of matrilin-3 levels in human serum and synovial fluid using a competitive enzyme-linked immunosorbent assay. Osteoarthritis Cartilage. 2012;20(7):783–6. [DOI] [PubMed] [Google Scholar]
- 63. Poole AR, Ha N, Bourdon S, Sayre EC, Guermazi A, Cibere J. Ability of a urine assay of type II collagen cleavage by collagenases to detect early onset and progression of articular cartilage degeneration: results from a population-based cohort study. J Rheumatol. 2016;43(10):1864–70. [DOI] [PubMed] [Google Scholar]
- 64. Wong M, Siegrist M, Cao X. Cyclic compression of articular cartilage explants is associated with progressive consolidation and altered expression pattern of extracellular matrix proteins. Matrix Biol. 1999;18(4):391–9. [DOI] [PubMed] [Google Scholar]
- 65. Bleuel J, Zaucke F, Brüggemann G, Heilig J, Wolter M, Hamann N, Firner S, Niehoff A. Moderate cyclic tensile strain alters the assembly of cartilage extracellular matrix proteins in vitro. J Biomech Eng. 2015;137(6):061009. [DOI] [PubMed] [Google Scholar]
- 66. Aigner T, Vornehm SI, Zeiler G, Dudhia J, von der Mark K, Bayliss MT. Suppression of cartilage matrix gene expression in upper zone chondrocytes of osteoarthritic cartilage. Arthritis Rheum. 1997;40(3):562–9. [DOI] [PubMed] [Google Scholar]
- 67. Pullig O, Kladny B, Weseloh G, Swoboda B. Metabolische Aktivierung der Chondrozyten bei der humanen Arthrose. Expression von Kollagen Typ II [Metabolic activation of chondrocytes in human osteoarthritis. Expression of type II collagen]. Z Orthop Ihre Grenzgeb. 1999;137(1):67–75. [DOI] [PubMed] [Google Scholar]
- 68. Aigner T, Stöss H, Weseloh G, Zeiler G, von der Mark K. Activation of collagen type II expression in osteoarthritic and rheumatoid cartilage. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62(6):337–45. [DOI] [PubMed] [Google Scholar]
- 69. Nelson F, Dahlberg L, Laverty S, Reiner A, Pidoux I, Ionescu M, Fraser GL, Brooks E, Tanzer M, Rosenberg LC, Dieppe P, Robin Poole A. Evidence for altered synthesis of type II collagen in patients with osteoarthritis. J Clin Invest. 1998;102(12):2115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Maass T, Bayley CP, Mörgelin M, Lettmann S, Bonaldo P, Paulsson M, Baldock C, Wagener R. Heterogeneity of collagen VI microfibrils: structural analysis of non-collagenous regions. J Biol Chem. 2016;291(10):5247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dreier R, Opolka A, Grifka J, Bruckner P, Grässel S. Collagen IX-deficiency seriously compromises growth cartilage development in mice. Matrix Biol. 2008;27(4):319–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.