Abstract
Neurofibromatosis type 1 (NF1) is a common, cancer-predisposing disease caused by mutations in the NF1 tumor gene. Patients with NF1 have an increased risk for benign and malignant tumors of the nervous system (e.g., neurofibromas, malignant peripheral nerve sheath tumors, gliomas) and other tissues (e.g., leukemias, rhabdomyosarcoma, etc.) as well as increased susceptibility to learning disabilities, chronic pain/migraines, hypertension, pigmentary changes, and developmental lesions (e.g., tibial pseudoarthrosis). Pigs are an attractive and upcoming animal model for future NF1 studies, but a potential limitation to porcine model research has been the lack of validated reagents for direct translational study to humans. To address that issue, we used formalin-fixed tissues (human and pigs) to evaluate select immunohistochemical markers (activated caspase-3, allograft inflammatory factor-1, beta-tubulin III, calbindin D, CD13, CD20, desmin, epithelial membrane antigen, glial fibrillary acidic protein, glucose transporter-1, laminin, myelin basic protein, myoglobin, proliferating cell nuclear antigen, S100, vimentin, and von Willebrand factor). The markers were validated by comparing known expression and localization in human and pig tissues. Validation of these markers on fixed tissues will facilitate prospective immunohistochemical studies of NF1 pigs, as well as other pig models, in a more efficient, reproducible, and translationally relevant manner.
Keywords: antibody, formalin fixation, humans, immunohistochemistry, markers, neurofibromatosis type 1, pigs, tissues
Introduction
Neurofibromatosis 1 (NF1) is an autosomal dominant disease caused by mutations in the gene encoding neurofibromin, a tumor suppressor protein that negatively regulates multiple proliferative cellular pathways.1–3 The RAS oncogene is the primary target of neurofibromin, a Ras-GAP (GTPase-activating protein) that inhibits Ras signaling by promoting its conversion to the GDP-bound inactive form.4 As a consequence, loss of neurofibromin increases the activity of Ras and its numerous downstream effectors, including the Raf/MEK/ERK and PI3K/Akt/mTOR pathways.2,3,5–13 Hyperactivated Ras drives tumorigenesis through increased cell proliferation and survival.14 In fact, nearly all NF1 patients develop benign neurofibromas while a smaller percentage (roughly 15–20%) develops cancers, including optic gliomas, rhabdomyosarcoma, leukemias, and malignant peripheral nerve sheath tumors (MPNST).15–18 In addition to tumors, NF1 patients are also prone to a spectrum of clinical features including pigmentary changes, skeletal deformities, and cognitive and behavioral disorders, to name a few.16,19
Numerous genetically engineered mouse models targeting the NF1 gene have been generated. Unlike humans, however, mice expressing a single mutant allele of Nf1 fail to develop the classic features of NF1 and are considered inaccurate models of the disease. The Nf1+/− mice do exhibit learning and memory deficits that are common in NF1 patients, but they fail to develop the hallmarks of NF1 including neurofibromas, pigmentation defects, enhanced pain perception, and various malignancies such as MPNSTs.20–22 To successfully model typical NF1 tumor types, conditional inactivation of both Nf1 alleles or combined alteration of other cancer genes, such as p53 or INK4a/ARF, with Nf1+/− mutation is required.23–28 Several of these more recent mouse models have served as valuable preclinical tools for testing novel therapeutics for NF1 tumors. Unfortunately, the animals only replicate select aspects of NF1 and are, thus, limited by their inability to mimic the full spectrum of NF1 lesions. As such, there is an ongoing effort within the NF1 research community to develop new animal models that better recapitulate the many NF1 phenotypes seen in patients.
Over the past few years, genetically modified pig models of human diseases have been increasingly and successfully used to study a broad range of diseases including cystic fibrosis,29–32 muscular dystrophy,33 cancer,34,35 cardiovascular disease,36,37 and ataxia telangiectasia,38 to name a few. There are several reasons for using pig models, including the similarities to humans such as comparable anatomy, physiology, metabolism, and pathology.39,40 However, compared with rodent models, pig models have limited access to validated reagents and techniques for translational research.41 In this article, we validate IHC markers for pig and human tissues that will have translational relevance in the study of NF1 in novel pig models.
Materials and Methods
Tissues
Archival tissues (non-NF1 tissues, i.e., “wild type”) from pigs and humans were acquired from the Comparative Pathology Laboratory (University of Iowa). All tissues had been fixed in 10 percent neutral buffered formalin and processed as previously described.42 Pig tissues were acquired from paraffin-embedded tissue blocks previously used in studies that had received University of Iowa Institutional Animal Care and Use Committee approval. These tissues samples were taken from pigs (Sus scrofa domestica) and included multiple breeds (e.g., Large White, Yucatan, etc.) from commercial farm and/or research sources because (to date) we have not observed variations in immunohistochemical staining between various breeds of pig. Tissues were from pigs less than one year of age and for each marker, a total of three pigs (at least one of each sex) were evaluated. No overt sex-related differences in immunostaining were noted. Human tissues were acquired from de-identified autopsy tissues previously used as IHC control tissues, or through the Cell Culture Core Repository (University of Iowa) that has institutional approval from the University of Iowa Institutional Review Board (IRB #:199507432) for collection of human tissues. Unless otherwise specified, tissues came from individuals that were generally healthy and lacked overt clinical disease. Importantly, we used tissues that had been placed into fixative in a timely manner following harvest to mitigate autolysis, a cellular process that can confound IHC studies.42
IHC
Markers for optimization and validation were selected based on three criteria: (1) prospective relevance to NF1 studies—for example, through identification of normal tissue structure, diagnostic utility, or study of NF1-related pathogenesis; (2) availability of organs/tissues (human and pig) with known marker expression; and (3) distinct microanatomical localization of the marker for morphological corroboration of human and pig expression. We preferentially evaluated select antibodies/techniques from our lab that were previously optimized/validated for human tissues. Markers and their respective techniques that were successfully optimized and validated are shown in Table 1. For these studies, 3,3′-diaminobenzidine (DAB, brown staining) was used as the chromogen, and Harris hematoxylin (basophilic staining) was used as the counterstain.
Table 1.
IHC Protocols for Validated Cellular Markers in Pig and Human Tissues.
| Marker | Primary Antibody | Antigen Retrieval | Secondary Reagents |
|---|---|---|---|
| MBP | Anti-MBP Polyclonal Antibody (LS-C312288, LifeSpan BioSciences, Inc., Seattle, WA) in Diluent; Pig:1:600/1 hr; Human: 1:200/1 hr | HIER, Citrate buffer pH 6.0, 110C for 15 min; 20 min cool down | Dako EnVision+ System- HRP Labeled Polymer Anti-rabbit, 30 min (Dako North America, Inc., Carpentaria, CA) |
| Anti-Calbindin-D28K | Anti-Calbindin-D-28K Monoclonal (C9848, Sigma-Aldrich Co. LLC, St. Louis, MO) in Diluent; Pig: 1:6000/1 hr Human: 1:6000/1 hr |
HIER, Citrate buffer pH 6.0, 110C for 15 min; 20 min cool down | Dako EnVision+ System- HRP Labeled Polymer Anti-mouse, 30 min (Dako North America, Inc., Carpentaria, CA) |
| GFAP | Anti-GFAP Polyclonal Antibody (AB16997, Abcam, Plc., Cambridge, MA) in Diluent; Pig: 1:200/1 hr Human: 1:200/1 hr |
HIER, Citrate buffer pH 6.0, 125C for 5 min; 20 min cool down | Dako EnVision+ System- HRP Labeled Polymer Anti-rabbit, 15 min (Dako North America, Inc., Carpentaria, CA) |
| AIF1 | Anti-AIF1 Polyclonal (#019-19741, Wako Pure Chemical Industries, Ltd., Richmond, VA) in Diluent; Pig: 1:2000/1 hr Human: 1:1000/1 hr | HIER, Citrate buffer pH 6.0, 125C for 5 min; 20 min cool down | Dako EnVision+ System- HRP Labeled Polymer Anti-rabbit, 30 min (Dako North America, Inc., Carpentaria, CA) |
| S100 | Anti-S100 Polyclonal (#Z0311, Dako North America, Inc., Carpentaria, CA) in Diluent; Pig: 1:4000/45 min Human: 1:4000/45 min |
HIER, Citrate buffer pH 6.0, 110C for 15 min; 20 min cool down | Dako EnVision+ System- HRP Labeled Polymer Anti-rabbit, 20 min (Dako North America, Inc., Carpentaria, CA) |
| beta-Tubulin III | Anti-beta-Tubulin III Polyclonal (#ab18207, Abcam, Plc., Cambridge, MA) in Diluent; Pig: 1:500/1.5 hr/37C oven Human: 1:500/1.5 hr/37C oven | HIER, Citrate buffer pH 6.0, 110C for 15 min; 20 min cool down | Dako EnVision+ System- HRP Labeled Polymer Anti-rabbit, 30 min (Dako North America, Inc., Carpentaria, CA) |
| vWF | Anti-vWF Polyclonal (A0082, Dako North America, Inc., Carpentaria, CA) in Diluent; Pig: 1:500/1 hr Human: 1:4000/1 hr | Dako Proteinase K, 5 min (Dako North America, Inc., Carpentaria, CA) | Dako EnVision+ System- HRP Labeled Polymer Anti-rabbit, 15 min (Dako North America, Inc., Carpentaria, CA) |
| Cleaved Caspase-3 | Anti-Cleaved Caspase-3 Polyclonal (#9661, Cell Signaling Company, Danvers, MA) in Diluent; Pig: 1:200/1 hr Human: 1:200/1 hr | HIER, Steamer, Citrate Buffer pH 6.0, incubate for 40 min, 20 min cool down | Dako EnVision+ System- HRP Labeled Polymer Anti-rabbit, 30 min (Dako North America, Inc., Carpentaria, CA) |
| PCNA | Anti-PCNA Monoclonal (Clone PC10, #M0879, Dako North America, Inc., Carpentaria, CA) in Diluent; Pig: 1:4000/1 hr Human: 1:4000/1 hr | HIER, Citrate buffer pH 6.0, 125C for 5 min; 20 min cool down | Dako EnVision+ System- HRP Labeled Polymer Anti-mouse, 15 min (Dako North America, Inc., Carpentaria, CA) |
| EMA | Anti-EMA Polyclonal Antibody (LS-C30532, LifeSpan BioSciences, Inc., Seattle, WA) in Diluent; Pig: 1:400/1 hr Human: 1:400/1 hr | HIER, Citrate buffer pH 6.0, 110C for 15 min; 20 min cool down | Dako EnVision+ System- HRP Labeled Polymer Anti-rabbit, 30 min (Dako North America, Inc., Carpentaria, CA) |
| Glucose transporter 1 (Glut1) | Anti-Glut1 Polyclonal Antibody (LS-C188008, LifeSpan BioSciences, Inc., Seattle, WA) in Diluent; Pig: 1:250/1 hr Human: 1:250/1 hr | HIER, Citrate buffer pH 6.0, 110C for 25 min; 20 min cool down | Dako EnVision+ System- HRP Labeled Polymer Anti-rabbit, 30 min (Dako North America, Inc., Carpentaria, CA) |
| Laminin | Anti-Laminin Polyclonal Antibody (#ab11575, Abcam, Plc., Cambridge, MA) in Diluent; Pig: 1:100/2 hr Human: 1:100/2 hr | Pig—HIER, Tris pH 9.0, 125C for 5 min; 20 min cool down Human-Dako Proteinase K, 5 min (Dako North America, Inc., Carpentaria, CA) |
Dako EnVision+ System- HRP Labeled Polymer Anti-rabbit, 15 min (Dako North America, Inc., Carpentaria, CA) |
| CD20 | Anti-CD20 Polyclonal (ACR 3004 A, B, Biocare Medical, Pacheco, CA) in Diluent; Pig: 1:200/1 hr; Human: 1:200/1 hr | HIER, Citrate buffer pH 6.0, 110C for 15 min; 20 min cool down | Dako EnVision+ System- HRP Labeled Polymer Anti-rabbit, 30 min (Dako North America, Inc., Carpentaria, CA) |
| CD13 | Anti-CD13 SP182 (AM33219PU-N, Acris Antibodies GmbH, San Diego, CA) in Diluent; Pig: 1:1200/1 hr Human: 1:1200/1 hr | HIER, Citrate buffer pH 6.0, 110C for 15 min; 20 min cool down | Dako EnVision+ System- HRP Labeled Polymer Anti-rabbit, 30 min (Dako North America, Inc., Carpentaria, CA) |
| Vimentin | Anti-Vimentin Monoclonal (#M0725, Dako North America, Inc., Carpentaria, CA) in Diluent; Pig: 1:300/15 hr Human: 1:300/15 hr | HIER, Citrate buffer pH 6.0, 110C for 15 min; 20 min cool down | Dako EnVision+ System- HRP Labeled Polymer Anti-mouse, 15 min (Dako North America, Inc., Carpentaria, CA) |
| Desmin | Anti-Desmin Monoclonal (#M0760, Dako North America, Inc., Carpentaria, CA) in Diluent; Pig: 1:100/15 min Human: 1:100/15 min | HIER, Citrate buffer pH 6.0, 110C for 15 min; 20 min cool down | Dako EnVision+ System- HRP Labeled Polymer Anti-mouse, 15 min (Dako North America, Inc., Carpentaria, CA) |
| Myoglobin | Anti-Myoglobin Polyclonal Antibody (#A324, Dako North America, Inc., Carpentaria, CA) in Diluent; Pig: 1:1600/2 hr Human: 1:25,600/2 hr | None | Dako EnVision+ System- HRP Labeled Polymer Anti-rabbit, 15 min (Dako North America, Inc., Carpentaria, CA) |
Abbreviations: MBP, Myelin Basic Protein; HIER, Heat Induced Epitope Retrieval (unless otherwise specified—Decloaking Chamber Plus, Biocare Medical, Concord, CA); HRP = horseradish peroxidase; “Diluent” is, unless otherwise specified, Dako Antibody Diluent (Dako North America, Inc., Carpentaria, CA); GFAP, glial fibrillary acidic protein; AIF1, allograft inflammatory factor 1; vWF, von Willebrand Factor; PCNA, proliferating cell nuclear antigen; EMA, epithelial membrane antigen.
Results
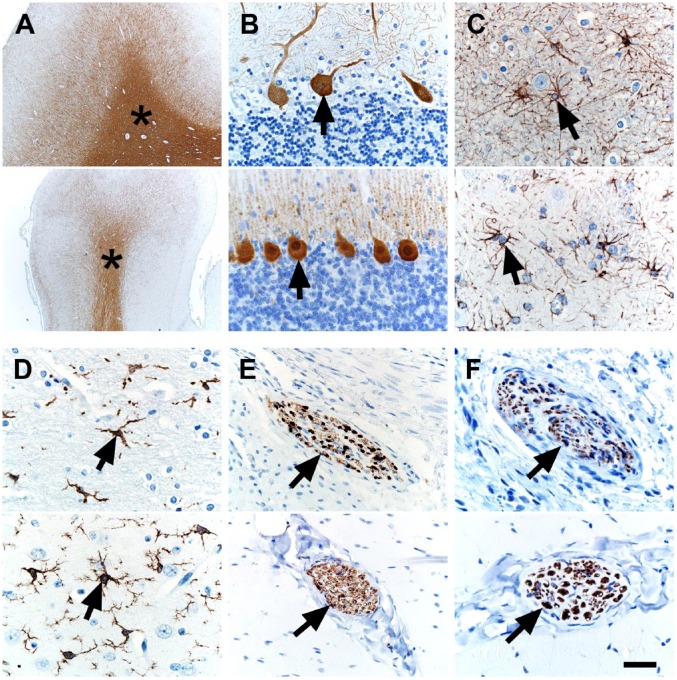
Translational markers of the nervous system would be useful as it is commonly affected in NF1.16 Specifically, markers that allow identification of normal central or peripheral neural structure/organization, or markers that increase the diagnostic sensitivity or specificity for NF1-specific lesions (e.g., neurofibromas) would be useful (see marker selection criteria in section “Materials and Methods”). Myelin basic protein (MBP) was found in myelinated (e.g., white matter) tracts of the brain (Fig. 1A) and can serve as a landmark of brain organization.43 Calbindin-D28K is a marker of Purkinje cells,38 specialized neurons of the cerebellum and serve as a marker of cerebellum organization. We observed Purkinje cell immunostaining in the cerebellum (Fig. 1B). Glial fibrillary antigen protein (GFAP) is a marker of glial cells (e.g., astrocytes) and can be useful to detect astrocyte activation (i.e., astrogliosis) in NF1.44,45 GFAP immunostaining was seen in astrocytes of the brain (Fig. 1C). Microglia are resident macrophages in the brain and may play a role in pathogenesis of optic gliomas (an NF1-related tumor) and the ensuing sexual dimorphism of optic glioma-associated retinal injury.46,47 Allograft inflammatory factor 1 (AIF1, also known as ionized calcium-binding adapter molecule-1) is a marker for microglia and can label alveolar macrophages of the lung of pigs and humans.42 We optimized the AIF1 technique so it immunostained microglia in the brain (Fig. 1D). S100 can be a useful marker in the clinical diagnosis of neurofibromas, intraocular gliomas, and normal peripheral nerves.48–52 We localized S100 in peripheral nerves of muscle tissue (Fig. 1E). Neuronal markers, such as beta-tubulin III,53 can highlight axonal structure within peripheral nerves. We localized beta-tubulin III in axons of peripheral nerves (Fig. 1F).
Figure 1.
IHC of human (top panel) and pig (bottom panel) tissues. (A) MBP immunostaining was localized to white matter (asterisks) in the cerebrum. (B) Calbindin-D28K immunostaining was localized to Purkinje cells (arrows) in the cerebellum. (C) GFAP immunostaining was localized to glial cells (arrows) in the cerebrum. (D) AIF1 immunostaining was localized to microglial cells (arrows) in the cerebrum. (E) S100 immunostaining was localized to peripheral nerves (arrows) in skeletal muscle. (F) beta-tubulin III immunostaining was localized to axons within peripheral nerves (arrows) of skeletal muscle. Scale bar = 26 (C, D, F), 40 (B, E), and 800 (A) µm. Abbreviations: MBP, Myelin basic protein; GFAP, glial fibrillary acidic protein; AIF1, allograft inflammatory factor 1.
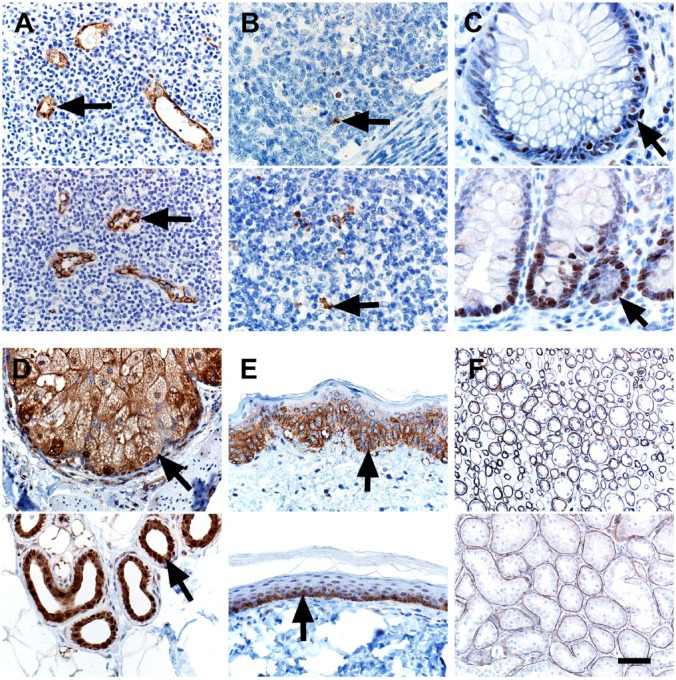
The vasculature is emerging as an important tissue in NF1 pathogenesis. For example, aneurysms and stenoses are vascular anomalies that have been reported in people with NF1.54 Furthermore, Schwann cells from NF1 neurofibromas have been reported to produce midkine, an angiogenic and mitogenic factor.55 We evaluated the endothelial marker CD34 using a rabbit polyclonal antibody (#250591, Abbiotec LLC, San Diego, CA), but the immunostaining in both human and pig tissues did not meet our standards for sensitivity and specificity (data not shown) and, thus, did not meet our threshold for inclusion in this study (see section “Materials and Methods”). So, instead, we used von Willebrand Factor (vWF) as a vascular marker,56 and it distinctly labeled vessels within lymphoid tissues of both species (Fig. 2A). Apoptosis and proliferation are common cellular markers that can be useful in evaluating tumor grade and effects of tumor therapy.57,58 We examined markers of apoptosis (cleaved caspase-3) and proliferation (proliferating cell nuclear antigen, PCNA), and both markers had expected immunostaining that was similar between human and pig tissues (Fig. 2B and C). Epithelial membrane antigen (EMA),10 glucose transporter 1 (GLUT1), and laminin IHC have been used to rule out other tumors (e.g., perineurioma) and help classify soft tissue tumors.59–61 EMA was localized in the glands (e.g., sebaceus) of normal skin (Fig. 2D).62 GLUT1 is known to be highly expressed in the lower (more basal) epidermis in humans and was localized there in both species (Fig. 2E).63 Laminin is expressed in basement membranes and in the perineural tissue, so it can be useful in diagnostics of soft tissue tumors such as neurofibromas and malignant peripheral nerve sheath tumors.60 Laminin was localized in basement membranes of renal tubules (Fig. 2F).
Figure 2.
IHC of human (top panel) and pig (bottom panel) tissues. (A) vWF immunostaining was localized to endothelial cells (arrows) of vessels. (B) Cleaved Caspase-3 immunostaining was localized to apoptotic cells (arrows) and debris in lymphoid tissues. (C) PCNA immunostaining was localized to nuclei of proliferating epithelial cells (arrows) in the crypts of the colon. (D) EMA10 immunostaining was localized to adnexal glands (arrows) of the skin. (E) Glut1 immunostaining was localized to the lower, basal-oriented cells (arrows) of the epidermis. (F) Laminin immunostaining was localized to the tubular basement membranes in the kidney. Scale bar = 26 (B, C), 40 (A, D, E), and 80 (F) µm. Abbreviations: vWF, von Willebrand Factor; PCNA, proliferating cell nuclear antigen; EMA, epithelial membrane antigen; Glut1, glucose transporter 1.
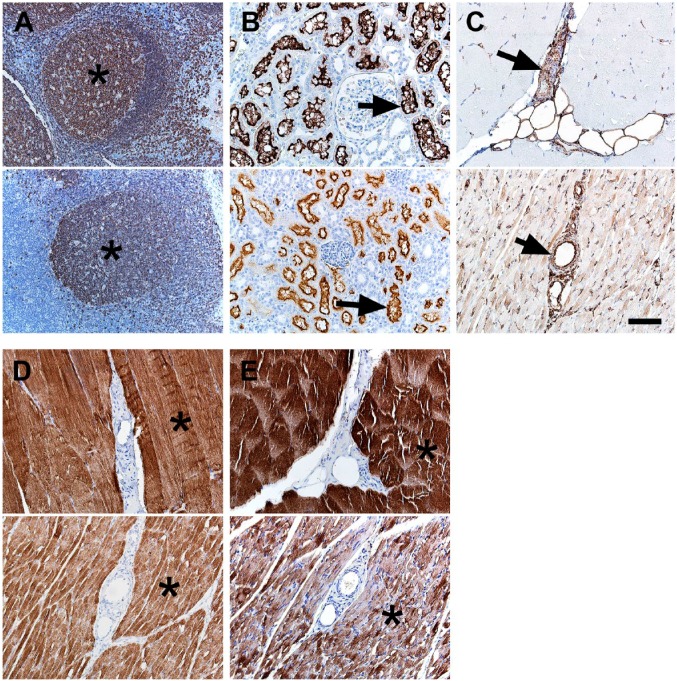
Inflammation (macrophages and lesser numbers of lymphocytes) has been associated with NF1 mutations in glioblastomas.64 Recently, panels of immune cell markers were evaluated in pig and human tissues42; however, the B cell immunostaining in pigs (i.e., CD79a) was not as robust as in humans. We tested another B-cell marker CD20, and it had the expected B-cell distribution in lymphoid tissues (Fig. 3A), but in similar fashion to CD79a, it had less robust staining in pigs than humans. Children with NF1 may be predisposed to myelogenous leukemias,65 and CD13 is a myeloid lineage marker that can aid in its diagnosis.66 CD13 is highly expressed and localized in the renal tubules, and this localization was seen in our tissues (Fig. 3B). Rhabdomyosarcoma is a malignancy of muscle that has increased risks in the NF1 population.15–17 Myoglobin, desmin, and vimentin are potential markers useful for diagnosing skeletal muscle tumors.67,68 In skeletal muscle, these markers exhibited sarcoplasmic-specific staining except for vimentin, which had more sarcoplasmic staining in pigs compared with the scant immunostaining in humans (Fig. 3C–E). In general, vimentin specificity was low as it was seen in other interstitial and mesenchymal tissues (e.g., vascular walls, adipocytes, etc.). The potential for broad or even false positive artifactual immunostaining patterns for vimentin have been recognized69,70 when studying in tissues. Together, these features might limit vimentin usage in situations to corroborate more specific immunostaining (e.g., myoglobin, desmin).
Figure 3.
IHC of human (top panel) and pig (bottom panel) tissues. (A) CD20 immunostaining was localized to B-cell rich regions (e.g., germinal centers, asterisks) in lymphoid tissue. (B) CD13 immunostaining was localized to the tubular brush borders of the kidney. (C) Vimentin immunostaining of muscle tissue was preferentially localized to vascular walls (arrows) and interstitial connective tissue with less intense immunostaining of muscle sarcoplasm (pig > human) and adipocytes. (D) Desmin immunostaining of muscle tissue was localized to muscle sarcoplasm (asterisks). (E) Myoglobin immunostaining of muscle tissue was localized to muscle sarcoplasm (asterisks). Scale bar = 80 µm.
Discussion
In this study, we were able to validate several IHC tissue markers in pig and human formalin-fixed paraffin-embedded tissues. Importantly, we did this using the same reagents (i.e., primary antibodies) with infrequent, minor species variation in incubation/concentration (Table 1). We specifically targeted markers that were applicable for prospective NF1 pig studies and, through this, were able to expand the scope and fill in the gaps of validated markers that are available for translational pig studies.
Study of tissues in the nervous system is vital for NF1 research.16 Tumors of nervous system origin (e.g., neurofibromas) are common in NF1 patients as are cognitive/social deficits, migraines, and chronic pain.71,72 In this study, we were able to validate several markers that would allow for examination of structural organization (e.g., beta-tubulin III, S100) and remodeling (e.g., GFAP, AIF1) of the nervous system in tissues. Markers to study proliferation (e.g., PCNA) and apoptosis (e.g., activated caspase-3), which may be relevant in interrogating the cellular biology of NF,73 were also validated. These tools allow for detection of abnormal neural structure/organization and tissue remodeling that can help to better detect and clarify the progression of lesions in early NF1 pathogenesis.
People with NF1 are prone to various benign and malignant tumors originating from the nervous system as well as from other tissues. Classification of the tumors in terms of grade (e.g., benign to malignant), cell lineage, and exclusion of other tumors with similar morphology can be critical components in understanding NF1 pig model phenotype and comparing with that of NF1 disease in humans. For instance, S100+ immunostaining is a useful marker to corroborate neurofibroma diagnosis, whereas immunostaining for other markers (e.g., EMA, GLUT1) may point toward different diagnoses (e.g., perineurioma) and, thus, should generally be negative in neurofibromas.50,61 We also were able to validate select markers that could be useful for rhabdomyosarcoma (e.g., myoglobin, desmin) and myelogenous leukemia (CD13)—tumors that are preferentially seen in NF.15–17,65,66
Validated IHC markers, such as these demonstrated in this article, offer investigators a resource for simplicity, consistency, and repeatability in studies of pig models of NF1 as well as other human diseases. With the prospective development of NF1 mutant pigs (unpublished), we expect to further validate and expand the scope of markers that can be used to investigate these models. These markers are part of an emerging “toolbox” that are essential for characterization, diagnostics, and phenotyping of current and future pig models.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors contributed to the experimental design, writing, and editing of the manuscript. DKM, GKO-A, MRL, JAG performed tissue handling, immunohistochemistry, tissue analysis, and figure preparation. All authors read and approved the manuscript before submission.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Children’s Tumor Foundation (NF1 Synodos), National Institutes of Health (P01HL051670, P01 HL091842, P30 DK054759), and the Cystic Fibrosis Foundation.
Contributor Information
David K. Meyerholz, Department of Pathology.
Georgina K. Ofori-Amanfo, Department of Pathology
Mariah R. Leidinger, Department of Pathology
J. Adam Goeken, Department of Pathology.
Rajesh Khanna, University of Iowa, Iowa City, Iowa, Departments of Pharmacology and Anesthesiology, College of Medicine, University of Arizona, Tucson, Arizona; Departments of Pharmacology and Anesthesiology, College of Medicine, University of Arizona, Tucson, Arizona.
Jessica C. Sieren, Department of Radiology Department of Biomedical Engineering.
Benjamin W. Darbro, Department of Pediatrics
Dawn E. Quelle, Department of Pathology Department of Pediatrics; Department of Pharmacology.
Jill M. Weimer, Pediatrics and Rare Disease Group, Sanford Research, Sioux Falls, South Dakota Department of Pediatrics, University of South Dakota, Vermillion, South Dakota.
Literature Cited
- 1. Gutmann DH, Wood DL, Collins FS. Identification of the neurofibromatosis type 1 gene product. Proc Natl Acad Sci U S A. 1991;88:9658–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bollag G, Clapp DW, Shih S, Adler F, Zhang YY, Thompson P, Lange BJ, Freedman MH, McCormick F, Jacks T, Shannon K. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in haematopoietic cells. Nat Genet. 1996;12:144–8. [DOI] [PubMed] [Google Scholar]
- 3. Dasgupta B, Yi Y, Chen DY, Weber JD, Gutmann DH. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 2005;65:2755–60. [DOI] [PubMed] [Google Scholar]
- 4. Martin GA, Viskochil D, Bollag G, McCabe PC, Crosier WJ, Haubruck H, Conroy L, Clark R, O’Connell P, Cawthon RM, Innis MA, McCormick F. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990;63:843–9. [DOI] [PubMed] [Google Scholar]
- 5. Banerjee S, Crouse NR, Emnett RJ, Gianino SM, Gutmann DH. Neurofibromatosis-1 regulates mTOR-mediated astrocyte growth and glioma formation in a TSC/Rheb-independent manner. Proc Natl Acad Sci U S A. 2011;108:15996–6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356:713–5. [DOI] [PubMed] [Google Scholar]
- 7. Chang T, Krisman K, Theobald EH, Xu J, Akutagawa J, Lauchle JO, Kogan S, Braun BS, Shannon K. Sustained MEK inhibition abrogates myeloproliferative disease in Nf1 mutant mice. J Clin Invest. 2013;123:335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dasgupta B, Yi Y, Hegedus B, Weber JD, Gutmann DH. Cerebrospinal fluid proteomic analysis reveals dysregulation of methionine aminopeptidase-2 expression in human and mouse neurofibromatosis 1-associated glioma. Cancer Res. 2005;65:9843–50. [DOI] [PubMed] [Google Scholar]
- 9. DeClue JE, Papageorge AG, Fletcher JA, Diehl SR, Ratner N, Vass WC, Lowy DR. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell. 1992;69:265–73. [DOI] [PubMed] [Google Scholar]
- 10. Jessen WJ, Miller SJ, Jousma E, Wu J, Rizvi TA, Brundage ME, Eaves D, Widemann B, Kim MO, Dombi E, Sabo J, Hardiman Dudley A, Niwa-Kawakita M, Page GP, Giovannini M, Aronow BJ, Cripe TP, Ratner N. MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. J Clin Invest. 2013;123:340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005;102:8573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaul A, Toonen JA, Cimino PJ, Gianino SM, Gutmann DH. Akt- or MEK-mediated mTOR inhibition suppresses Nf1 optic glioma growth. Neuro Oncol. 2015;17:843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sherman LS, Atit R, Rosenbaum T, Cox AD, Ratner N. Single cell Ras-GTP analysis reveals altered Ras activity in a subpopulation of neurofibroma Schwann cells but not fibroblasts. J Biol Chem. 2000;275:30740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 15. Crucis A, Richer W, Brugieres L, Bergeron C, Marie-Cardine A, Stephan JL, Girard P, Corradini N, Munzer M, Lacour B, Minard-Colin V, Sarnacki S, Ranchere-Vince D, Orbach D, Bourdeaut F. Rhabdomyosarcomas in children with neurofibromatosis type I: a national historical cohort. Pediatr Blood Cancer. 2015;62:1733–8. [DOI] [PubMed] [Google Scholar]
- 16. Gutmann DH, Ferner RE, Listernick RH, Korf BR, Wolters PL, Johnson KJ. Neurofibromatosis type 1. Nat Rev Dis Primers. 2017;3:17004. [DOI] [PubMed] [Google Scholar]
- 17. Matsui I, Tanimura M, Kobayashi N, Sawada T, Nagahara N, Akatsuka J. Neurofibromatosis type 1 and childhood cancer. Cancer. 1993;72:2746–54. [DOI] [PubMed] [Google Scholar]
- 18. Theos A, Korf BR, American College of Physicians, American Physiological Society. Pathophysiology of neurofibromatosis type 1. Ann Intern Med. 2006;144:842–9. [DOI] [PubMed] [Google Scholar]
- 19. Pasmant E, Vidaud M, Vidaud D, Wolkenstein P. Neurofibromatosis type 1: from genotype to phenotype. J Med Genet. 2012;49:483–9. [DOI] [PubMed] [Google Scholar]
- 20. Costa RM, Yang T, Huynh DP, Pulst SM, Viskochil DH, Silva AJ, Brannan CI. Learning deficits, but normal development and tumor predisposition, in mice lacking exon 23a of Nf1. Nat Genet. 2001;27:399–405. [DOI] [PubMed] [Google Scholar]
- 21. Jacks T, Shih TS, Schmitt EM, Bronson RT, Bernards A, Weinberg RA. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nat Genet. 1994;7:353–61. [DOI] [PubMed] [Google Scholar]
- 22. Silva AJ, Frankland PW, Marowitz Z, Friedman E, Laszlo GS, Cioffi D, Jacks T, Bourtchuladze R. A mouse model for the learning and memory deficits associated with neurofibromatosis type I. Nat Genet. 1997;15:281–4. [DOI] [PubMed] [Google Scholar]
- 23. Bajenaru ML, Hernandez MR, Perry A, Zhu Y, Parada LF, Garbow JR, Gutmann DH. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63:8573–7. [PubMed] [Google Scholar]
- 24. Cichowski K, Shih TS, Schmitt E, Santiago S, Reilly K, McLaughlin ME, Bronson RT, Jacks T. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999;286:2172–6. [DOI] [PubMed] [Google Scholar]
- 25. Dodd RD, Mito JK, Eward WC, Chitalia R, Sachdeva M, Ma Y, Barretina J, Dodd L, Kirsch DG. NF1 deletion generates multiple subtypes of soft-tissue sarcoma that respond to MEK inhibition. Mol Cancer Ther. 2013;12:1906–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gutmann DH. NF GEMMs already! the power and promise of mouse tumor models. Cancer Cell. 2014;26:596–9. [DOI] [PubMed] [Google Scholar]
- 27. Vogel KS, Klesse LJ, Velasco-Miguel S, Meyers K, Rushing EJ, Parada LF. Mouse tumor model for neurofibromatosis type 1. Science. 1999;286:2176–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu J, Williams JP, Rizvi TA, Kordich JJ, Witte D, Meijer D, Stemmer-Rachamimov AO, Cancelas JA, Ratner N. Plexiform and dermal neurofibromas and pigmentation are caused by Nf1 loss in desert hedgehog-expressing cells. Cancer Cell. 2008;13:105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, Kabel AC, Wohlford-Lenane CL, Davis GJ, Hanfland RA, Smith TL, Samuel M, Wax D, Murphy CN, Rieke A, Whitworth K, Uc A, Starner TD, Brogden KA, Shilyansky J, McCray PB, Jr, Zabner J, Prather RS, Welsh MJ. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane C, Dohrn CL, Bartlett JA, Nelson GA, 4th, Chang EH, Taft PJ, Ludwig PS, Estin M, Hornick EE, Launspach JL, Samuel M, Rokhlina T, Karp PH, Ostedgaard LS, Uc A, Starner TD, Horswill AR, Brogden KA, Prather RS, Richter SS, Shilyansky J, McCray PB, Jr, Zabner J, Welsh MJ. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2:29ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ostedgaard LS, Meyerholz DK, Chen JH, Pezzulo AA, Karp PH, Rokhlina T, Ernst SE, Hanfland RA, Reznikov LR, Ludwig PS, Rogan MP, Davis GJ, Dohrn CL, Wohlford-Lenane C, Taft PJ, Rector MV, Hornick E, Nassar BS, Samuel M, Zhang Y, Richter SS, Uc A, Shilyansky J, Prather RS, McCray PB, Jr, Zabner J, Welsh MJ, Stoltz DA. The ΔF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med. 2011;3:74ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stoltz DA, Rokhlina T, Ernst SE, Pezzulo AA, Ostedgaard LS, Karp PH, Samuel MS, Reznikov LR, Rector MV, Gansemer ND, Bouzek DC, Abou Alaiwa MH, Hoegger MJ, Ludwig PS, Taft PJ, Wallen TJ, Wohlford-Lenane C, McMenimen JD, Chen JH, Bogan KL, Adam RJ, Hornick EE, Nelson GA, 4th, Hoffman EA, Chang EH, Zabner J, McCray PB, Jr, Prather RS, Meyerholz DK, Welsh MJ. Intestinal CFTR expression alleviates meconium ileus in cystic fibrosis pigs. J Clin Invest. 2013;123:2685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klymiuk N, Blutke A, Graf A, Krause S, Burkhardt K, Wuensch A, Krebs S, Kessler B, Zakhartchenko V, Kurome M, Kemter E, Nagashima H, Schoser B, Herbach N, Blum H, Wanke R, Aartsma-Rus A, Thirion C, Lochmüller H, Walter MC, Wolf E. Dystrophin-deficient pigs provide new insights into the hierarchy of physiological derangements of dystrophic muscle. Hum Mol Genet. 2013;22:4368–82. [DOI] [PubMed] [Google Scholar]
- 34. Sieren JC, Meyerholz DK, Wang XJ, Davis BT, Newell JD, Jr, Hammond E, Rohret JA, Rohret FA, Struzynski JT, Goeken JA, Naumann PW, Leidinger MR, Taghiyev A, Van Rheeden R, Hagen J, Darbro BW, Quelle DE, Rogers CS. Development and translational imaging of a TP53 porcine tumorigenesis model. J Clin Invest. 2014;124:4052–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schook LB, Collares TV, Hu W, Liang Y, Rodrigues FM, Rund LA, Schachtschneider KM, Seixas FK, Singh K, Wells KD, Walters EM, Prather RS, Counter CM. A genetic porcine model of cancer. PLoS ONE. 2015;10:e0128864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Davis BT, Wang XJ, Rohret JA, Struzynski JT, Merricks EP, Bellinger DA, Rohret FA, Nichols TC, Rogers CS. Targeted disruption of LDLR causes hypercholesterolemia and atherosclerosis in Yucatan miniature pigs. PLoS ONE. 2014;9:e93457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Amuzie C, Swart JR, Rogers CS, Vihtelic T, Denham S, Mais DE. A translational model for diet-related atherosclerosis: effect of statins on hypercholesterolemia and atherosclerosis in a minipig. Toxicol Pathol. 2016;44:442–9. [DOI] [PubMed] [Google Scholar]
- 38. Beraldi R, Chan CH, Rogers CS, Kovács AD, Meyerholz DK, Trantzas C, Lambertz AM, Darbro BW, Weber KL, White KA, Rheeden RV, Kruer MC, Dacken BA, Wang XJ, Davis BT, Rohret JA, Struzynski JT, Rohret FA, Weimer JM, Pearce DA. A novel porcine model of ataxia telangiectasia reproduces neurological features and motor deficits of human disease. Hum Mol Genet. 2015;24:6473–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aigner B, Renner S, Kessler B, Klymiuk N, Kurome M, Wünsch A, Wolf E. Transgenic pigs as models for translational biomedical research. J Mol Med. 2010;88:653–64. [DOI] [PubMed] [Google Scholar]
- 40. Rogers CS, Abraham WM, Brogden KA, Engelhardt JF, Fisher JT, McCray PB, Jr, McLennan G, Meyerholz DK, Namati E, Ostedgaard LS, Prather RS, Sabater JR, Stoltz DA, Zabner J, Welsh MJ. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L240–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Olivier AK, Naumann P, Goeken A, Hochstedler C, Sturm M, Rodgers JR, Gibson-Corley KN, Meyerholz DK. Genetically modified species in research: opportunities and challenges for the histology core laboratory. J Histotechnol. 2012;35:63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meyerholz DK, Lambertz AM, Reznikov LR, Ofori-Amanfo GK, Karp PH, McCray PB, Jr, Welsh MJ, Stoltz DA. Immunohistochemical detection of markers for translational studies of lung disease in pigs and humans. Toxicol Pathol. 2016;44:434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stephenson DT, O’Neill SM, Narayan S, Tiwari A, Arnold E, Samaroo HD, Du F, Ring RH, Campbell B, Pletcher M, Vaidya VA, Morton D. Histopathologic characterization of the BTBR mouse model of autistic-like behavior reveals selective changes in neurodevelopmental proteins and adult hippocampal neurogenesis. Mol Autism. 2011;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nordlund ML, Rizvi TA, Brannan CI, Ratner N. Neurofibromin expression and astrogliosis in neurofibromatosis (type 1) brains. J Neuropathol Exp Neurol. 1995;54:588–600. [DOI] [PubMed] [Google Scholar]
- 45. Rizvi TA, Akunuru S, de Courten-Myers G, Switzer RC, 3rd, Nordlund ML, Ratner N. Region-specific astrogliosis in brains of mice heterozygous for mutations in the neurofibromatosis type 1 (Nf1) tumor suppressor. Brain Res. 1999;816:111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Toonen JA, Solga AC, Ma Y, Gutmann DH. Estrogen activation of microglia underlies the sexually dimorphic differences in Nf1 optic glioma-induced retinal pathology. J Exp Med. 2017;214:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simmons GW, Pong WW, Emnett RJ, White CR, Gianino SM, Rodriguez FJ, Gutmann DH. Neurofibromatosis-1 heterozygosity increases microglia in a spatially and temporally restricted pattern relevant to mouse optic glioma formation and growth. J Neuropathol Exp Neurol. 2011;70:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Albuerne M, Mammola CL, Naves FJ, Levanti B, Germana G, Vega JA. Immunohistochemical localization of S100 proteins in dorsal root, sympathetic and enteric ganglia of several mammalian species, including man. J Peripher Nerv Syst. 1998;3:243–53. [PubMed] [Google Scholar]
- 49. Gonzalez-Martinez T, Perez-Pinera P, Diaz-Esnal B, Vega JA. S-100 proteins in the human peripheral nervous system. Microsc Res Tech. 2003;60:633–8. [DOI] [PubMed] [Google Scholar]
- 50. Karvonen SL, Kallioinen M, Yla-Outinen H, Poyhonen M, Oikarinen A, Peltonen J. Occult neurofibroma and increased S100 protein in the skin of patients with neurofibromatosis type 1: new insight to the etiopathomechanism of neurofibromas. Arch Dermatol. 2000;136:1207–9. [DOI] [PubMed] [Google Scholar]
- 51. Meyerholz DK, Haynes JS. Solitary retinal astrocytoma in a dog. Vet Pathol. 2004;41:177–8. [DOI] [PubMed] [Google Scholar]
- 52. Pusateri A, Margo CE. Intraocular astrocytoma and its differential diagnosis. Arch Pathol Lab Med. 2014;138:1250–4. [DOI] [PubMed] [Google Scholar]
- 53. Hoffman PN, Lopata MA, Watson DF, Luduena RF. Axonal transport of class II and III beta-tubulin: evidence that the slow component wave represents the movement of only a small fraction of the tubulin in mature motor axons. J Cell Biol. 1992;119:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Oderich GS, Sullivan TM, Bower TC, Gloviczki P, Miller DV, Babovic-Vuksanovic D, Macedo TA, Stanson A. Vascular abnormalities in patients with neurofibromatosis syndrome type I: clinical spectrum, management, and results. J Vasc Surg. 2007;46:475–84. [DOI] [PubMed] [Google Scholar]
- 55. Sheela S, Riccardi VM, Ratner N. Angiogenic and invasive properties of neurofibroma Schwann cells. J Cell Biol. 1990;111:645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mesri M, Birse C, Heidbrink J, McKinnon K, Brand E, Bermingham CL, Feild B, Fitzhugh W, He T, Ruben S, Moore PA. Identification and characterization of angiogenesis targets through proteomic profiling of endothelial cells in human cancer tissues. PLoS ONE. 2013;8:e78885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Patel AJ, Liao CP, Chen Z, Liu C, Wang Y, Le LQ. BET bromodomain inhibition triggers apoptosis of NF1-associated malignant peripheral nerve sheath tumors through Bim induction. Cell Rep. 2014;6:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Watson AL, Anderson LK, Greeley AD, Keng VW, Rahrmann EP, Halfond AL, Powell NM, Collins MH, Rizvi T, Moertel CL, Ratner N, Largaespada DA. Co-targeting the MAPK and PI3K/AKT/mTOR pathways in two genetically engineered mouse models of Schwann cell tumors reduces tumor grade and multiplicity. Oncotarget. 2014;5:1502–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hirose T, Tani T, Shimada T, Ishizawa K, Shimada S, Sano T. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol. 2003;16:293–8. [DOI] [PubMed] [Google Scholar]
- 60. d’Ardenne AJ, Kirkpatrick P, Sykes BC. Distribution of laminin, fibronectin, and interstitial collagen type III in soft tissue tumours. J Clin Pathol. 1984;37:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Macarenco RS, Ellinger F, Oliveira AM. Perineurioma: a distinctive and underrecognized peripheral nerve sheath neoplasm. Arch Pathol Lab Med. 2007;131:625–36. [DOI] [PubMed] [Google Scholar]
- 62. Fuertes L, Santonja C, Kutzner H, Requena L. Immunohistochemistry in dermatopathology: a review of the most commonly used antibodies (part I). Actas Dermosifiliogr. 2013;104:99–127. [DOI] [PubMed] [Google Scholar]
- 63. Gherzi R, Melioli G, de Luca M, D’Agostino A, Distefano G, Guastella M, D’Anna F, Franzi AT, Cancedda R. “HepG2/erythroid/brain” type glucose transporter (GLUT1) is highly expressed in human epidermis: keratinocyte differentiation affects GLUT1 levels in reconstituted epidermis. J Cell Physiol. 1992;150:463–74. [DOI] [PubMed] [Google Scholar]
- 64. Rutledge WC, Kong J, Gao J, Gutman DA, Cooper LA, Appin C, Park Y, Scarpace L, Mikkelsen T, Cohen ML, Aldape KD, McLendon RE, Lehman NL, Miller CR, Schniederjan MJ, Brennan CW, Saltz JH, Moreno CS, Brat DJ. Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clin Cancer Res. 2013;19:4951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Miles DK, Freedman MH, Stephens K, Pallavicini M, Sievers EL, Weaver M, Grunberger T, Thompson P, Shannon KM. Patterns of hematopoietic lineage involvement in children with neurofibromatosis type 1 and malignant myeloid disorders. Blood. 1996;88:4314–20. [PubMed] [Google Scholar]
- 66. Estey EH. Acute myeloid leukemia: 2013 update on risk-stratification and management. Am J Hematol. 2013;88:318–27. [DOI] [PubMed] [Google Scholar]
- 67. Kodet R. Rhabdomyosarcoma in childhood. An immunohistological analysis with myoglobin, desmin and vimentin. Pathol Res Pract. 1989;185:207–13. [DOI] [PubMed] [Google Scholar]
- 68. Meyerholz DK, Caston SS, Haynes JS. Congenital fetal rhabdomyoma in a foal. Vet Pathol. 2004;41:518–20. [DOI] [PubMed] [Google Scholar]
- 69. True LD. Quality control in molecular immunohistochemistry. Histochem Cell Biol. 2008;130:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bahrami A, Truong LD, Ro JY. Undifferentiated tumor: true identity by immunohistochemistry. Arch Pathol Lab Med. 2008;132:326–48. [DOI] [PubMed] [Google Scholar]
- 71. Laycock-van Spyk S, Thomas N, Cooper DN, Upadhyaya M. Neurofibromatosis type 1-associated tumours: their somatic mutational spectrum and pathogenesis. Hum Genomics. 2011;5:623–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. DiMario FJ, Jr., Langshur S. Headaches in patients with neurofibromatosis-1. J Child Neurol. 2000;15:235–8. [DOI] [PubMed] [Google Scholar]
- 73. Shapira S, Barkan B, Friedman E, Kloog Y, Stein R. The tumor suppressor neurofibromin confers sensitivity to apoptosis by Ras-dependent and Ras-independent pathways. Cell Death Differ. 2007;14:895–906. [DOI] [PubMed] [Google Scholar]